Highlights
-
•
Clinically significant reduction in PTSD symptom severity with alpha-down NFB.
-
•
Intervention increased activation in PFC areas involved in top-down emotion control.
-
•
Intervention improved task-irrelevant decoupling with executive areas (dlPFC)
-
•
Intervention improved DMN integration with posterior nodes.
-
•
Better NFB performance linked with increased activity in regions of bodily self-consciousness processing.
Keywords: Post-traumatic stress disorder (PTSD), Neurofeedback (NFB), Functional magnetic resonance imaging (fMRI), DLPFC, Top-down control, Cognition, Emotion regulation
Abstract
Background
Posttraumatic stress disorder (PTSD) has been found to be associated with emotion under-modulation from the prefrontal cortex and a breakdown of the top-down control of cognition and emotion. Novel adjunct therapies such as neurofeedback (NFB) have been shown to normalize aberrant neural circuits that underlie PTSD psychopathology at rest. However, little evidence exists for NFB-linked neural improvements under emotionally relevant cognitive load. The current study sought to address this gap by examining the effects of alpha-down NFB in the context of an emotional n-back task.
Methods
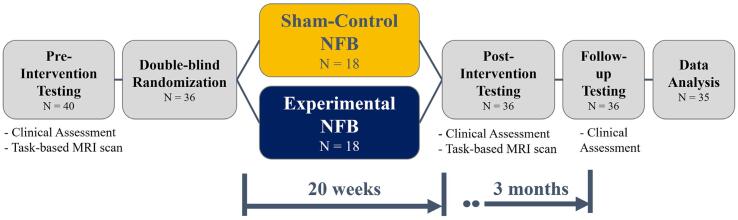
We conducted a 20-week double-blind randomized, sham-controlled trial of alpha-down NFB and collected neuroimaging data before and after the NFB protocol. Participants performed an emotional 1-back and 2-back working memory task, with interleaved trauma-neutral and trauma-relevant cues in the fMRI scanner. Data from 35 participants with a primary diagnosis of PTSD were analyzed in this study (n = 18 in the experimental group undergoing alpha-down NFB, n = 17 in the sham-control group).
Results
Firstly, within-group analyses showed clinically significant reductions in PTSD symptom severity scores at the post-intervention timepoint and 3-month follow-up for the experimental group, and not for the sham-control group. The neuroimaging analyses revealed that alpha-down NFB enhanced engagement of top-down cognitive and emotional control centers, such as the dorsolateral prefrontal cortex (dlPFC), and improved integration of the anterior and posterior parts of the default mode network (DMN). Finally, our results also indicate that increased alpha-down NFB performance correlated with increased activity in brain regions involved in top-down control and bodily consciousness/embodied processing of self (TPJ and posterior insula).
Conclusion
This is the first study to provide mechanistic insights into how NFB may normalize dysfunctional brain activity and connectivity in PTSD under cognitive load with simultaneous symptom provocation, adding to a growing body of evidence supporting the therapeutic neuromodulatory effects of NFB. This preliminary study highlights the benefits of alpha-down NFB training as an adjunctive therapy for PTSD and warrants further investigation into its therapeutic effects on cognitive and emotion control in those with PTSD.
1. Introduction
A wide range of cognitive functions and behaviour have been shown to be impaired among those with post-traumatic stress disorder (PTSD) (Jelinek et al., 2008, Koso and Hansen, 2006, Larsen et al., 2019, Op den Kelder et al., 2018, Scott et al., 2015, Woon et al., 2017). Recent research indicates that difficulties in domains such as inhibitory and emotional control might be critical factors limiting the effectiveness of mainstream evidence-based cognitive therapies for PTSD (Falconer et al., 2013, Scott et al., 2015, Wild and Gur, 2008). It has therefore been proposed that a useful strategy to boost the efficacy of mainstream PTSD therapies, and to reduce attrition rates, may be alternate or adjunctive interventions (Boyd et al., 2018, Watkins et al., 2018), which may serve to directly improve these critical domains. This is of urgent concern to healthcare systems since lifetime prevalence of PTSD among the general population in North-America is around 8–14 % (Kilpatrick et al., 2013, Spottswood et al., 2017) and can be as high as 20–30 % among military members, veterans, public safety personnel and healthcare workers due to occupation-related trauma exposure (Berger et al., 2012, Fulton et al., 2015, Koenigs et al., 2008, Kulka et al., 1990, Li et al., 2021, Petrie et al., 2018, Sendlera et al., 2016, Thomas et al., 2010). Unfortunately, these populations also suffer from high non-response rates (Cusack et al., 2016, Watkins et al., 2018, Watts et al., 2013), with up to two-thirds of patients retaining diagnosis after mainstream PTSD treatments (Steenkamp et al., 2015). Collectively, these problems highlight the need to investigate and adopt novel adjunctive therapies capable of improving the critical domains of cognitive and emotional control.
Neurofeedback (NFB) is one such promising intervention that allows one to voluntarily self-regulate neural circuits that are directly associated with psychopathology and symptom maintenance. Indeed, NFB engages executive processes (da Silva and de Souza, 2021), can improve some of these impaired cognitive domains (da Silva and de Souza, 2021, Hsueh et al., 2016), and is shown to be effective in reducing the clinical symptoms of PTSD while also normalizing associated large-scale brain networks (Bell et al., 2019, du Bois et al., 2021, Kluetsch et al., 2014, Nicholson et al., 2020b, Niv, 2013, Reiter et al., 2016, Ros et al., 2013, van der Kolk et al., 2016, Zotev et al., 2018). For instance, an investigation of NFB in those with chronic PTSD was successful in significantly reducing PTSD symptoms and improving affect regulation (Gapen et al., 2016, van der Kolk et al., 2016). Further work by this group also showed reductions in PTSD symptom severity, especially behavioural and emotional symptoms, alongside improved executive functioning after NFB in a group of treatment-resistant children with a history of neglect and developmental trauma (Rogel et al., 2020), adding to a growing literature showing NFB-based improvement in executive functioning. Furthermore, these NFB-linked improvements are not limited to the more commonly used electroencephalogram-based (EEG) NFB protocols and are also found in functional magnetic resonance imaging (fMRI) based NFB protocols (Nicholson et al., 2017b, Sherwood et al., 2016, Zotev et al., 2016). Of the many NFB protocols available, EEG-based alpha-down NFB has emerged as a particularly useful protocol for ameliorating symptoms in PTSD populations (du Bois et al., 2021, Kluetsch et al., 2014, Nicholson et al., 2020b, Ros et al., 2013). Additionally, the ability of the alpha-down NFB protocol in producing clinically significant reductions in PTSD severity has also been replicated using a low-cost EEG system in Rwanda (du Bois et al., 2021), highlighting the critical ability of neurofeedback interventions in improving treatment accessibility.
The currently implemented alpha-down NFB protocol consists of multiple training sessions during which participants learn to reduce their alpha activity (oscillations in the 8–12 Hz range) at a particular electrode location on their scalp. Notably, alpha-based neurofeedback (NFB) has demonstrated its ability to modulate network activation (Bell et al., 2019, du Bois et al., 2021, Kluetsch et al., 2014, Nicholson et al., 2020b, Nicholson et al., 2016a, Niv, 2013, Reiter et al., 2016, Ros et al., 2013) and also improve PFC-linked working memory (Escolano et al., 2011, Hsueh et al., 2016). More specifically, resting-state fMRI analyses collected from the participants in the current randomized controlled trial of alpha-down NFB demonstrated increased dmPFC connectivity with the anterior DMN community, indicating increased DMN integration (Nicholson et al., 2020b) in those that performed alpha-down NFB, as compared to the sham NFB group. Single-session mechanistic studies from our group (Kluetsch et al., 2014, Nicholson et al., 2016a, Ros et al., 2013) have also shown that PTSD patients after a single 30-minute alpha-down NFB session displayed a therapeutic increase in connectivity between the amygdala and mPFC at rest, which also negatively correlated with PTSD symptom severity scores. Collectively, these findings support the therapeutic ability of alpha-down neurofeedback in reducing PTSD symptoms (du Bois et al., 2021, Nicholson et al., 2020b) while bringing online PFC-centric executive functioning regions at rest, leaving an important open-question. Does alpha-down NFB also result in improved top-down control under conditions with greater cognitive load? Furthermore, given that difficulties in inhibitory and emotion control might affect therapeutic effectiveness (Falconer et al., 2013), it is important to probe the effect of alpha-down NFB on the inhibitory and emotion control centres of the brain. To accomplish this, the current study extends the results of Nicholson et al., 2020b (that characterized the neural changes associated with alpha-down NFB training at rest) by investigating neural changes in PTSD patients while they perform an emotion-based n-back working memory task, before and after they participate in a randomized controlled trial of alpha-down NFB training.
Dysfunctional prefrontal cortex (PFC) activity (both medial and lateral subregions) and connectivity, both at rest and during executive functioning tasks, are one of the hallmark neural biomarkers characterising PTSD (Hopper et al., 2007, Lanius et al., 2001, Liberzon and Abelson, 2016, Sheynin and Liberzon, 2017). More specifically, a plethora of studies have reported reduced dmPFC/dlPFC thickness (Geuze et al., 2008), reduced PFC (multiple subregions) activation during rest and under cognitive load (Aupperle et al., 2012, Hopper et al., 2007, Lanius et al., 2010a, Scott et al., 2015), and hypo-connectivity with an over-active amygdala (Nicholson et al., 2015) in PTSD during rest. Together, this is thought to represent the undermodulation of emotions as well as suboptimal contextualization of trauma memories, thereby leading to PTSD symptomatology, including alterations in cognitions and mood, hyperarousal, and intrusive trauma-related memories (Lanius et al., 2006, Lanius et al., 2001, Liberzon and Abelson, 2016, Sheynin and Liberzon, 2017). Critically, Aupperle and colleagues have shown that higher dlPFC activation during the anticipation of emotionally valent stimuli is associated with lower PTSD symptom severity, in addition to enhanced executive functioning (Aupperle et al., 2012). Collectively, these findings highlight the critical role of the PFC in the top-down control of emotion and detail how abnormal PFC activation and connectivity are associated with dysregulated top-down cognitive and emotional control.
Aberrant PFC functioning is also repeatedly observed in the context of disruptions and imbalances between intrinsic connectivity networks (ICNs) in those with PTSD. In the healthy brain, dorsolateral subregions of the PFC synchronously co-activate with parietal and cerebellar brain regions, forming the central executive network (CEN) (also known as the fronto-parietal network – FPN); and medial PFC regions synchronize with temporo-parietal and medial-temporal regions to form the default mode network (DMN). The CEN plays an important role in executive functioning and working memory (Nejati et al., 2018), while the DMN is involved in social cognition, self-related processing, episodic memory recall, and future thinking (Spreng and Grady, 2010). Yet another behaviourally important ICN is the salience network (SN), comprising of the dorsal anterior cingulate and insular brain regions. The SN plays a major role in integrating sensory inputs, internal states, and emotions (Harricharan et al., 2021, Harricharan et al., 2020, Menon and Uddin, 2010, Uddin, 2015) and is thought to orchestrate switching between the CEN and DMN by co-activating with the task-relevant network (Shaw et al., 2021). Hence, interactions between these ICNs are important for healthy cognitive and behavioural functioning (Dosenbach et al., 2008, Ryali et al., 2016), and these ICNs are found to be dysregulated in a number of psychopathologies (Menon, 2011, Sha et al., 2019, Wang et al., 2020), including PTSD (Daniels et al., 2010, Lanius et al., 2015, Lanius et al., 2010b, Nicholson et al., 2020a).
The CEN displays significant alterations in activity, connectivity, and function among individuals with PTSD, at rest and under cognitive load (Daniels et al., 2010, Lanius et al., 2015, Nicholson et al., 2020a). Specifically, nodes within the CEN, such as the right and left dlPFC, are found to be hypo-connected with each other at rest in those with PTSD (Holmes et al., 2018), in addition to being hypo-connected with limbic structures (Barredo et al., 2018), providing further evidence for the under-modulation of emotional control in PTSD. A recent study also found decreased left CEN connectivity with sections of the left superior/middle temporal gyrus (a brain region implicated in multisensory integration, bodily self-consciousness and executive functioning), indicating diminished control over these faculties (Nicholson et al., 2020a). Importantly, this pattern of CEN hypoconnectivity at rest is in stark contrast to that found under cognitive load. Abnormally high recruitment of the CEN has been observed in those with PTSD during a working memory task, as compared to healthy controls, indicating the need for increased attentional resources during cognitive tasks (Moores et al., 2008). This study further showed abnormal recruitment of working memory updating structures (dlPFC and the inferior parietal lobule – IPL) during working memory maintenance. In addition, increased dlPFC activation has been observed during symptom provocation, suggesting attentional bias towards threat in those with PTSD (Fani et al., 2012). Notably, erroneous recruitment of the DMN instead of the task-appropriate CEN has also been observed in PTSD patients during an n-back working memory task (Daniels et al., 2010).
One of the most prominent PFC-linked ICN dysfunctions observed in those with PTSD at rest is the decreased connectivity of the mPFC within the anterior DMN community (Akiki et al., 2018), and its segregation from the posterior DMN communities (Akiki et al., 2018, Bluhm et al., 2009). Additionally, in the context of PTSD at rest, the SN is found to be hyper-connected with other SN nodes (Harricharan et al., 2020, Lanius et al., 2015, Nicholson et al., 2020b, Nicholson et al., 2020a), amygdala subregions (Nicholson et al., 2016b) and DMN nodes (Sripada et al., 2012), while being hypo-connected with PFC regions (Harricharan et al., 2020). Furthermore, the SN is critically involved in the innate alarm system (IAS) (Lanius et al., 2017) and subserves increased hypervigilance and hyperarousal symptoms in PTSD. The SN is also involved in multisensory integration and facilitates embodiment functions, known to be disrupted in PTSD (Harricharan et al., 2021).
Taken together, these findings suggest that improvements in PFC activity and connectivity may improve the balance between ICNs and also improve PTSD symptomatology, particularly in the context of cognitive tasks, making it a critical target for the treatment of PTSD. Consequently, there is a need for an intervention that can restore PFC disruptions observed in PTSD and improve top-down control of emotions in order to reduce symptom severity. As discussed in detail above, alpha-down NFB is a promising candidate with considerable evidence for improved ICN dynamics and reduced PTSD symptom severity post-intervention. However, despite growing evidence for the benefits of NFB in the treatment of PTSD, there is a poor understanding of how alpha-down NFB changes focal brain activity and large-scale network dynamics under cognitive load, particularly during symptom provocation. To address this gap, we carried out a double-blind randomized controlled trial of alpha-down NFB and assessed changes in brain activity before and after the NFB-intervention, while participants performed an emotional 1-back and 2-back working memory task. Specifically, our paradigm consisted of interleaved trauma-relevant and trauma-neutral cues that engaged emotional processing pathways in the brain to manage their trauma memory while still under cognitive load, necessitating the use of top-down emotional control. Using this data, we aimed to answer three critical questions:
-
1.
Firstly (study aim 1), what are the whole-brain activation effects of alpha-down NFB during this emotional working memory task?
-
2.
Secondly (study aim 2), what are the ICN-based effects of alpha-down NFB during this emotional working memory task?
-
3.
And finally (study aim 3), how are these neural activation/connectivity patterns correlated to metrics of neurofeedback success and reductions in PTSD symptoms?
Firstly, as shown by Nicholson et al. (2020b), the experimental group was expected to show a greater reduction in their alpha power metric and their PTSD symptom severity. Next, based on the discussed literature and prior findings, those undergoing the alpha-down NFB protocol (the experimental group) were expected to show increased recruitment of PFC brain areas involved in top-down control of emotions during the emotional n-back tasks (study aim 1), as compared to the sham-control group. Furthermore, given the normalizing shift in DMN and SN connectivity observed in the experimental group at rest in Nicholson et al. (2020b), we hypothesized that the experimental group in the current study would also show a normalizing shift in the connectivity of ICNs integral to emotion regulation under cognitive load (study aim 2). More specifically, the DMN was expected to be less fractionated in the experimental group, compared to the control group, with increased connectivity of the anterior DMN (aDMN) with posterior nodes involved in embodied processing of emotion, such as the angular gyrus (AG). Additionally, regions involved in the task-relevant process of working memory, such as the dlPFC, were expected to show less connectivity with brain regions and ICNs involved in the processing of emotion in the experimental group, compared to the control group. Lastly, the alpha-down NFB protocol was expected to increase recruitment of regions responsible for emotion processing and control (such as the prefrontal cortex – PFC and the temporo-parietal junction – TPJ) to a greater extent in the participants that were more successful in decreasing alpha activity within the experimental group, but not in the sham-control group.
2. Methods
2.1. Participants
The sample for the currently reported analysis consisted of n = 40 individuals with a primary diagnosis of PTSD. Four participants were excluded from the present neuroimaging analyses due to incomplete fMRI scans. As such, a total of n = 36 participants with PTSD (21–59 years-of-age, see Table 1 for complete clinical and demographic information) were randomly assigned to either the experimental EEG-NFB group (n = 18 participants), or the sham-control EEG-NFB group (n = 18 participants). One additional participant’s data had to be excluded from the analyses after study completion due to partial corruption of their fMRI data during the working memory task, resulting in n = 18 participants in the experimental EEG-NFB group (M = 40.28 ± 12.21 years-of-age, n = 12 females) and n = 17 participants in the sham-control group (M = 45.88 ± 12.63 years-of-age, n = 13 females). The participant cohort analyzed in this study is identical to the PTSD cohort reported in Nicholson et al. (2020b), which examined resting-state intrinsic connectivity network dynamics as a function of neurofeedback in the current RCT, with the notable exclusion of one participant in the sham-control group. The current study also did not have a baseline resting-state healthy control group comparison since the primary goal of this study was to understand the effects of alpha-down NFB in improving emotion processing under cognitive load.
Table 1.
Participant demographics and clinical scores at the pre-intervention timepoint (baseline). Abbreviations: CAPS – Clinician Administered PTSD Scale (Normalized to CAPS-5), CTQ – Childhood Trauma Questionnaire, MDI – Multiscale Dissociation Inventory, MDD – Major Depressive Disorder. Bonferroni-corrected threshold for significant group differences is p = 0.05/5 = 0.01.
| Experimental Group | Sham – Control Group | Group Comparisons | |
|---|---|---|---|
| Number of Participants | 18 | 17 | – |
| Sex | 12 Females | 13 Females | χ2 = 0.412; p = 0.52; φ = 0.11 |
| Age | 40.28 ± 12.21 | 45.88 ± 12.63 | t(33) = -1.33; p = 0.19; dz = 0.225 |
| CAPS – Total | 36.86 ± 10.36 | 39.64 ± 7.97 | t(33) = -0.90; p = 0.37; dz = 0.152 |
| CTQ – Total | 54.61 ± 19.88 | 62.50 ± 19.73 | t(33) = -1.16; p = 0.25; dz = 0.196 |
| MDI – Total | 52.89 ± 14.87 | 67.75 ± 21.46 | t(33) = -2.36; p = 0.02; dz = 0.398 |
| MDD | Current/Past = 5/8 | Current/Past = 6/5 | χ2 = 0.23/0.85; p = 0.63/0.36; φ = 0.08/−0.15 |
| Somatization Disorder | Current/Past = 1/0 | Current/Past = 3/0 | χ2 = 1.26/–; p = 0.26/–; φ = 0.19/– |
| Specific Phobia | Current/Past = 0/0 | Current/Past = 1/0 | χ2 = 1.09/– ; p = 0.30/–; φ = 0.18/– |
| Medication | 12 | 11 | χ2 = 0.015; p = 0.90; φ = -0.02 |
Individuals who participated in the current randomized controlled trial were recruited over a 4-year period through referrals from family physicians, mental healthcare providers, psychiatry clinics, and community advertisements in London, Ontario, Canada. The inclusion criteria for the PTSD group in this RCT included a primary PTSD diagnosis, evaluated using the Clinician-Administered PTSD Scale [CAPS; versions IV (n = 4) and 5 (n = 31)] and the Structured Clinical Interview for DSM-IV (SCID) (First et al., 2002). Any patients with PTSD and with active substance use disorder within 3 months of study onset were excluded, as were those with any lifetime diagnoses of bipolar or psychotic disorders. Those actively engaged in any other trauma-focused psychotherapy treatments were also excluded, along with those that previously received biofeedback therapy. In addition, those who had significant suicidal ideation over the past 3 months, exhibited self-injurious behaviour necessitating medical attention in the past 3 months, had unstable living conditions (such as homelessness), or were currently involved in a violent relationship were excluded from the study. Furthermore, participants were excluded if they were non-compliant with fMRI safety standards, pregnant, suffering from significant medical illnesses, or had a history of developmental or neurological disorder, including prior head injury with loss of consciousness.
Prevalence of psychiatric comorbid conditions did not differ significantly between the experimental and sham-control NFB groups (see Table 1). Additionally, the distribution of criterion A trauma categories did not differ significantly between the two groups. Criterion A traumatic events related to PTSD diagnoses in the experimental NFB group consisted of military occupational trauma (n = 3), first-responder occupational trauma (n = 2), and civilian physical/sexual abuse or neglect (n = 13). Similarly, criterion A traumas related to PTSD diagnoses in the sham-control NFB group consisted of military occupational trauma (n = 3), first-responder occupational trauma (n = 1), and civilian physical/sexual abuse or neglect (n = 13). Of importance, age, biological sex, number of participants receiving psychotropic medication (total n = 23, n = 12 in the experimental group, n = 11 in the sham-control NFB group) as well as psychotropic medication class (which included antidepressants n = 18, atypical antipsychotics n = 6, sedatives n = 8, and stimulants n = 2) did not differ significantly between the experimental and sham-control NFB groups. Participants with PTSD who were receiving psychotropic medication were on a stable dose prior to study onset and were asked to remain on the same medication regime throughout the duration of the study, if possible. Here, average dose within a particular class of medication did not differ significantly between groups at baseline, nor throughout the clinical trial. Notably, results reported below did not differ significantly when psychotropic medication was included as a covariate in the analyses.
The study protocols were ratified by the Western University Research Ethics Board (WREB). All participants gave their informed consent and received financial compensation for participation in this study. None of the participant debrief material contained our hypotheses, and the participants were aware that they would be randomly assigned to either the experimental or the sham-control NFB group. All participants were informed that the goal of the study was to examine whether they could learn to control their brain activity and how they would go about achieving this. Additionally, participants in the sham-control NFB group were offered the alpha-down NFB protocol after the end of the study period. This preliminary investigation was a pilot study and was therefore not pre-registered as a clinical trial; hence, we were highly restrictive with the outcome measures we examined, with the primary outcome measure being PTSD severity scores (CAPS).
2.2. Study design
Recruited participants with PTSD were randomized to either the experimental alpha-desynchronizing NFB group, or the sham-control NFB group, under double-blind conditions. We first conducted baseline assessments (pre-intervention timepoint) on the CAPS and SCID and additionally administered the Multiscale Dissociation Inventory (MDI) (Briere et al., 2005) and the Childhood Trauma Questionnaire (CTQ) (Bernstein et al., 2003) approximately one week prior to the onset of the NFB intervention protocol.
Following this, all participants performed two emotional n-back tasks while inside the fMRI scanner – one asking the participant to remember the word shown 1 word ago (1-back), and the other asking the participant to remember the word shown 2 words ago (2-back). Each emotional n-back task consisted of 48 cued trials: 24 trials of neutral cues (color names) and 24 trials of personalized trauma cues, presented in a randomized order. The cues were personalized to ensure that the neutral cues were emotionally neutral for each participant. This task was conducted during the same scanning session as that of the resting-state scan reported in Nicholson et al. (2020b). Nicholson et al. (2020b) reported on the results of the resting-state scan, while the current study reports on the results of the described emotional n-back task. This task was performed approximately one week prior to the start of the NFB protocol (pre-intervention timepoint), and was repeated approximately one week after the end of the NFB protocol (post-intervention timepoint). In summary, this reflects a 2 (Group: experimental vs sham-control) by 2 (Timepoint: pre vs post-NFB) mixed-effects model, split-plot design for each n-back task (1 and 2-back). Some of the aforementioned clinical assessments (CAPS and MDI) were also repeated after the end of the NFB protocol (post-intervention timepoint), followed by a third assessment session 3-months after the end of the NFB protocol (follow-up timepoint). This is summarized in Fig. 1
Fig. 1.
Schematic of the study design showing the number of participants through each section of the study.
2.3. EEG neurofeedback paradigm
The participants began their respective NFB protocols approximately one week after the pre-intervention fMRI data collection. The protocol consisted of weekly NFB sessions that were 20-minutes in length. The participants had to complete a minimum of 15 NFB sessions, with a maximum of 20 sessions available. All participants completed at least 17 sessions, and the mean number of sessions completed by the participants did not differ between the two groups (M = 19.6 ± 0.98 in the experimental EEG-NFB group, and M = 19.9 ± 0.24 in the sham-control group), nor did the duration of treatment (M = 161.1 days ± 36.3 in the experimental EEG-NFB group, and M = 180.6 days ± 37.9 in the sham-control group).
We implemented the same EEG-NFB protocol reported previously (Nicholson et al., 2020b). During the first NFB session, the participants received an introduction to NFB technology/equipment, psychoeducation, and were encouraged to establish goals for treatment. A baseline EEG recording was also collected during this first NFB session. All NFB sessions following this first session consisted of an initial 3-minute baseline EEG recording without feedback (during which the participants were asked to relax with their eyes open without moving their eyes excessively, while staring at a wall), followed by 20 min of EEG-NFB training using an alpha desynchronizing protocol. Hence, the NFB analyses that assessed training effects in this manuscript relied on alpha-dynamics from session 2 onwards. The alpha desynchronization NFB protocol implemented here is identical to that described previously (Kluetsch et al., 2014, Nicholson et al., 2020b, Ros et al., 2013), using the Pz electrode to provide real-time feedback. After receiving identical instructions, participants in the experimental EEG-NFB group received feedback targeting the desynchronization of alpha rhythms (8–12 Hz), while those in the sham-control group received yoked sham feedback signals corresponding to a successful participant from the experimental group, thereby ensuring balanced motivational states (Sorger et al., 2019). The sham training was implemented using EEGer and gave the impression of real feedback by allowing the NFB signal to remain sensitive to real-time EEG artifacts such as muscular and ocular artifacts. Participants in both groups were not provided with any explicit cognitive strategies for neuromodulation and were asked to explore personal strategies that allowed them to best down-regulate the alpha signal.
The feedback was provided to the participants using a combination of auditory and visual cues, presented as an interactive game. To account for participants’ personal preferences and maintain engagement over the 20-week NFB protocol, the participants were offered two distinct types of visual feedback during the interactive games. This also allowed us to provide the NFB therapy in a trauma-informed manner since the participants could choose an alternate type of visual feedback in case one was emotionally triggering. The two forms of visual feedback included: 1) a photo divided into a grid, with each grid piece appearing as alpha activity was suppressed, and 2) a cartoon character moving across the screen as alpha activity was suppressed. For each of these forms of visual feedback, the participants were tasked with training themselves to either 1) complete the image by making all the pieces appear, or to 2) keep the cartoon character moving across the screen. Regardless of which visual feedback type the participants chose, they received auditory feedback in the form of single beeps when they were successfully suppressing their alpha activity.
Each 20-minute session was divided into 7 training periods (6×3-minute time periods and 1×2-minute time period). The EEG-NFB signal was infinite impulse response (IIR) band-pass filtered between 8 and 12 Hz in order to extract alpha oscillations with an epoch size of 0.5 s. Feedback was updated at the end of each epoch, resulting in an update frequency of 2 Hz. Reward thresholds represent the alpha power below which positive feedback was provided to the participant and were set based on the ratio of positive to negative feedback within each training session. Reward thresholds began at 65 % positive feedback and 35 % negative feedback and were re-adjusted at the start of each training period (using the EEG of the preceding 30 s) to meet the desired ratio when the participants achieved disproportionately higher (>90 %) or lower (<50 %) reward rates. These readjustments were made at the beginning of the next training period and were based on the EEG from the past 30 s. For further details of the NFB protocol, please refer to Nicholson et al. (2020b).
2.4. EEG recording and analyses
All EEG was recorded using the Phoenix A202 2-channel EEG system, with the ground and reference electrodes placed on the right and left earlobes, respectively. The continuous EEG recording was sampled at 250 Hz and was later filtered with a 0.5–40 Hz bandpass filter within EEGLAB toolbox (Delorme and Makeig, 2004) in MATLAB (The Mathworks Inc.). Artifact removal was then performed using the FASTR EEGLAB plugin (Nolan et. al., 2010), followed by removal of segments during which the amplitude and variance of the EEG data fell beyond ± 2 standard deviations of the mean. Absolute alpha amplitudes were then estimated using Welch’s method of power spectral density estimation, with Hanning windows of width 2 s and 50 % overlap.
2.5. fMRI paradigm, image acquisition and pre-processing
2.5.1. fMRI paradigm
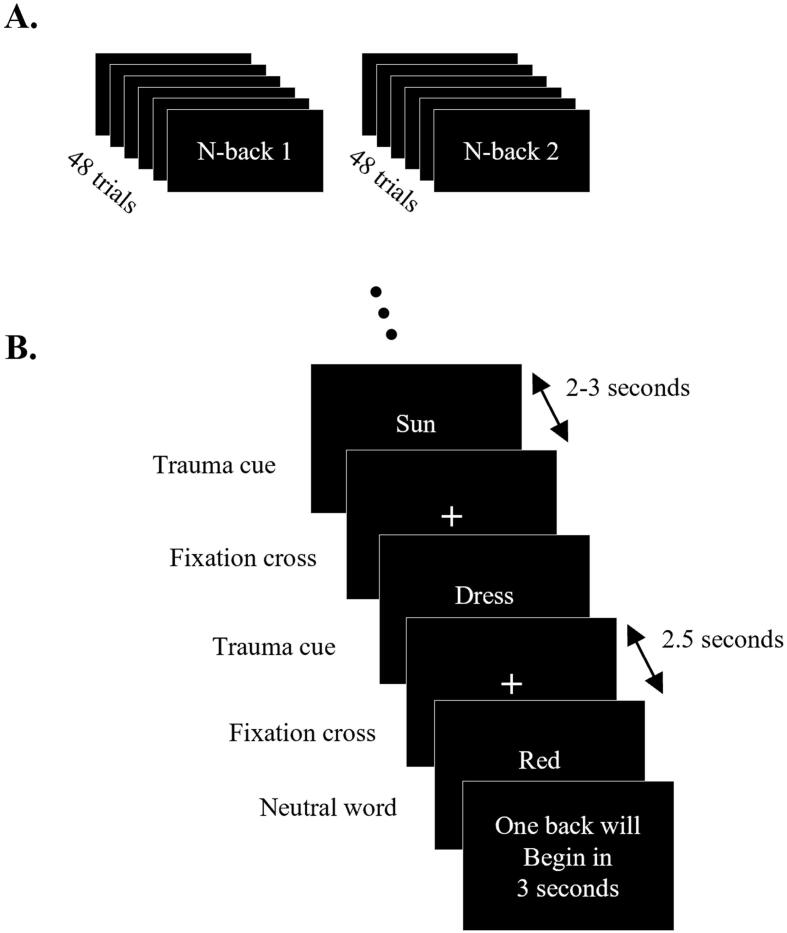
During a pre-imaging session, participants created a traumatic memory script with a trauma-informed clinician. Specifically, individualized trauma scripts were based on vivid trauma memories; participants then extracted trauma cues from this script, which were then used for the trauma-cue trials within the n-back 1 and n-back 2 tasks. Each task consisted of 48 cued trials, each 2.5 s in duration. Each trial was interleaved with a fixation cross that was presented for a variable duration, ranging from 2 to 3 s to avoid expectation-based confounds. Of these 48 trials, 24 trials were neutral words (color names), while the remaining 24 trials were trauma cue words (derived earlier). Trials were presented in a random order, and a 6 s break was provided to the participants halfway through the trials (after 24 trials). The total scan time of the n-back 1 and n-back 2 tasks were identical at 4 min and 15 s, with the instructions to the participant being the only point of difference. The participants were asked to press a button if the current word was identical to the word shown previously (1 word ago) for the n-back 1 task, and if the word was identical to that shown two words ago for the n-back 2 task. A schematic describing both tasks is shown in Fig. 2. The fMRI scans of the n-back 1 and n-back 2 tasks were part of a larger 1-hour long scanning session that also included a high-resolution anatomical scan (MPRAGE) and a 6-minute resting state fMRI scan before the participants performed the above-described tasks.
Fig. 2.
Schematic of the n-back 1 and n-back 2 tasks. A. shows the two tasks, while B. shows the order and timings of the trials within each task. The trauma and neutral word cues were presented in a random order. For the n-back 1 task, the participants were instructed to press a button if the current word was identical to the previous word, while for the n-back 2 task the participants were instructed to press a button if the current word was identical to the word shown two words ago.
2.5.2. Imaging protocol and pre-processing
All fMRI scanning was performed using a 3 Tesla Siemens Trio MRI scanner (Siemens Medical Solutions, Erlangen, Germany), equipped with a 32-channel phased array head coil. A high-resolution anatomical scan was acquired for each participant using the IR prepped axial 3D MPRAGE sequence (TI/TR/TE = 900/2000/4.2 ms, 9° flip angle, 192 slices of size 256×256 and 1 mm thickness). This was followed by two fMRI scans, one for each of the n-back tasks. Each fMRI scan was acquired using a 2D GRE EPI sequence (TR/TE = 3000/20 ms, 90° flip angle, 83 volumes of size 128×128×62 and 2 mm thickness interleaved).
All MRI pre-processing steps were performed using SPM12 and the CONN toolbox (Whitfield-Gabrieli and Nieto-Castanon, 2012). Realignment and unwarping were first performed on the fMRI scans, followed by motion correction, done by adding the participant's estimated motion (12 DOF) as a first-level covariate in a denoising general linear model (GLM). This was followed by frequency-domain phase shift slice timing correction (STC) and ART-based identification of outlier scans to be scrubbed (available at https://www.nitrc.org/projects/artifact_detect). The functional scans were then normalized to the MNI152 atlas and segmented to remove skull, white matter and cerebral spinal fluid (CSF) using the unified segmentation and normalization procedure (Ashburner and Friston, 2005). Physiological confounds were corrected by including the average white matter and CSF signals as first-level covariates in the denoising general linear model (GLM). Spatial smoothing was applied by convolving the BOLD signal with an 8 mm Gaussian kernel. Finally, the BOLD data was band-pass filtered between 0.008 Hz and 0.09 Hz.
2.6. Study aim 1 – Whole-brain activation analyses
To accomplish study aim 1, whole-brain activation analyses were performed using a 2-way full-factorial ANOVA with a between-subjects factor of intervention group (experimental vs sham-control NFB groups) and a within-subjects factor of session (pre- vs post-intervention). This ANOVA was performed separately for each n-back task (1-back and 2-back) and grouped data from all trials (trauma-cue & neutral-cue trials) together. This statistical design was motivated by n-back task differences not being the focus of study aim 1 and by the large number of factors in the 3-way ANOVA relative to our sample size. This was supported by the observation that the n-back task showed no significant effect when a 3-way full-factorial ANOVA (2×2×2) was performed, which included n-back task (1-back vs 2-back) as a within-subjects factor, in addition to intervention group (experimental vs sham-control NFB groups) and session (pre vs post-intervention), validating that significant variance across the n-back task factor was not being missed.
We then conducted a-priori defined contrasts using the 2-way ANOVA model described above, grouping all trials together (i.e., trauma and neutral cues). First, the two intervention groups were compared (experimental vs sham-control NFB groups) at the pre-intervention timepoint to verify that the brain activation did not differ at baseline. Next, experimental vs sham-control NFB groups were compared at the post-intervention timepoint to understand the between-group differences in brain activation. Finally, this between-group difference was examined as a function of the within-group difference in brain activation (group × session interaction: experimental vs sham-control NFB & post vs pre-intervention).
Once these results were established across all trials of the 1-back and 2-back tasks, a follow-up 3-way ANOVA was performed with an additional within-subjects factor of trial type/condition (trauma cue vs neutral cue trials) to further understand which trial type contributed to the observed group differences. The between-group contrasts examined above were then re-examined for each trial type within this 3-way ANOVA.
All results reported followed the guidelines in “Minimum statistical standards for submissions to Neuroimage: Clinical” (Roiser et al., 2016) by setting the voxel discovery threshold at p < 0.001, size at k = 10, and the FDR-corrected cluster discovery threshold at p(FDR) < 0.05. The results were also masked by the subject-specific grey-matter masks.
2.7. Study aim 2 – Large-scale brain network analyses
In addition to the whole-brain activation analyses described above, a connectivity-based analysis was performed to understand the NFB-linked changes in large-scale brain networks while the participants engaged in the n-back tasks (study aim 2). A group independent component analysis (group-ICA) (Calhoun et al., 2009) was performed on the whole-brain denoised fMRI voxel-level data using the iterative FastICA algorithm on data from all participants, across all sessions, conditions and trials. This analysis identified 20 mutually independent spatio-temporal patterns of activity, some of which are known to represent ICNs. This process was repeated three times to ensure the stability and reliability of these ICA components. These group-level ICA components were then back-projected to individual participants' data using GICA back-projection (Erhardt et al., 2011) to obtain the participant-specific first-level maps and timeseries of each ICA component. Finally, the spatial overlaps (Dice coefficients) of the group-ICA components with templates of known ICNs (Shirer et al., 2012) were used to label the ICA components representative of ICNs, specifically identifying the components that corresponded to CEN, DMN or SN sub-networks.
Similar to the whole-brain activation analyses described above, a 2-way full-factorial ANOVA was performed using a between-subjects factor of intervention group (experimental NFB vs sham-control NFB) and a within-subjects factor of session (Pre-intervention vs Post-intervention), while considering data from all trials together. Second-level contrasts between the experimental NFB vs sham-control NFB groups were examined as a function of the within-group difference in large-scale brain network connectivity (group × session interaction: experimental vs sham-control NFB & post vs pre-intervention).
2.8. Clinical data analysis
2.8.1. Baseline group comparisons
The clinical measures (CAPS, CTQ and MDI) and age of the participants were compared across groups (experimental vs sham-control NFB) at the pre-intervention timepoint using independent samples t-tests. Additionally, sex, current major depressive disorder diagnoses and other Axis I disorder diagnoses were also compared between the participants of the two intervention groups using Pearson’s chi-squared and Fisher’s exact tests.
2.8.2. Post-Intervention analyses
Change in the CAPS score was the primary outcome variable in this preliminary EEG-NFB trial and represented the change in PTSD severity. To maximize the sample size, the 4 participants’ data collected with CAPS-IV (prior to the release of CAPS-5) were normalized to the CAPS-5 scale by dividing the CAPS-IV scores with the maximum score available for CAPS-IV, followed by multiplication by the maximum score available for CAPS-5. To assess the change in CAPS as a result of the interventions, a split plot repeated-measures 2-way ANOVA was conducted, with a between-subjects factor of intervention group (experimental vs sham-control NFB group) and a within-subjects factor of session (pre-intervention vs post-intervention vs follow-up). Within-group changes in CAPS were assessed using post-hoc paired-samples t-tests, while between-group differences in CAPS scores were assessed using post-hoc independent samples t-tests. All post-hoc tests were corrected for multiple comparisons using Bonferroni’s method (p = 0.05/6 = 0.0083).
2.9. Study aim 3 – Neuroimaging correlations
Finally, to understand the link between the neuroimaging findings and alpha-down NFB (study aim 3), the first-level, whole-brain activation results of the post-intervention > pre-intervention contrast were regressed against each participants’ alpha power metric. This was defined as the change in alpha power per session, averaged over all NFB sessions, and was represented as a number centered around 1, with values below 1 representing an relative decrease in alpha power per session (e.g. 0.9 represented a 10 % decrease), while a value >1 represented a relative increase in alpha power per session (e.g. 1.1 represented a 10 % increase). This analysis was performed in a within-group format, with separate regressions for each intervention group. The results of these regression analyses were also thresholded using the same thresholding scheme described using above, i.e., cluster discovery p-FDR < 0.05, k = 10, after a voxel discovery threshold of p-uncorrected < 0.001. In order to compare these results with those reported in Nicholson et al. (2020b), these regression analyses were repeated using the alpha power metric used in Nicholson et al. (2020b), i.e., the average correlation coefficient between the alpha power and the seven training periods within each NFB training session, averaged across all NFB sessions. Here, a greater negative correlation coefficient reflected a decrease in the participant’s alpha power as they progressed through the session. These results are included in the supplementary analyses. These alpha power metrics represented the participants’ NFB performance. Since the goal of the NFB training protocol used was to decrease alpha power, a decrease in the alpha power metrics represented greater NFB performance.
3. Results
3.1. Clinical results
3.1.1. Baseline group comparisons
At the pre-intervention timepoint (baseline), no significant between-group differences were observed with respect to participants’ age, sex, and current psychiatric comorbidities, including major depressive disorder. Furthermore, the groups did not differ in terms of their PTSD severity scores (normalized CAPS-5 total), childhood trauma exposure (CTQ), and their dissociative symptom scores (MDI). These values are shown in Table 1.
3.1.2. NFB-induced changes in PTSD symptoms
The primary clinical outcome of the current RCT was PTSD severity (normalized CAPS-5 total scores), which showed a main effect of time [F(1.42, 42.60) = 9.16, p = 0.002, η2 = 0.234]. The group × time interaction did not reach statistical significance [F(1.42, 42.60) = 2.54, p = 0.107, η2 = 0.078]. The reported statistics are Greenhouse-Geisser corrected since Mauchly's Test of Sphericity had been violated [χ2(2) = 15.2, p < 0.0005].
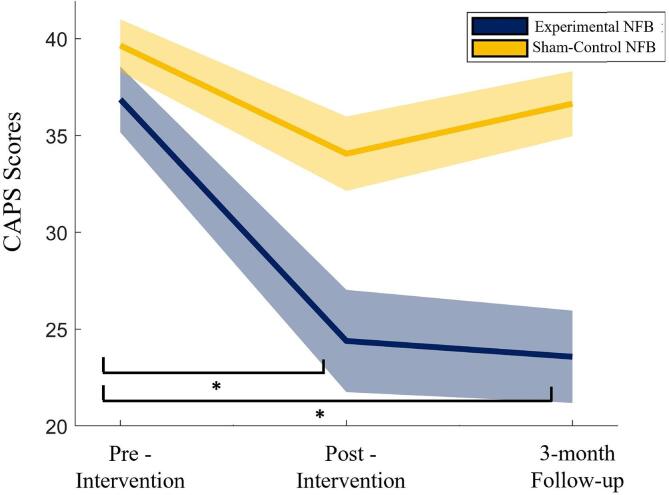
Post-hoc within-group t-tests revealed that only the experimental NFB group showed significant post-intervention decreases in PTSD severity scores [t(17) = 3.00, p = 0.008, dz = 0.71], which persisted at the 3-month follow-up visit [t(17) = 3.24, p = 0.005, dz = 0.77]. This represents a clinically significant (>30 % change; Halvorsen, 2016) reduction in the average PTSD symptom severity score within the experimental group, with a 33.8 % change at the post-intervention timepoint and a 36 % change at the 3-month follow-up visit. In comparison, PTSD symptom severity of participants in the sham-control NFB group was not significantly different at the post-intervention timepoint [t(16) = 2.06, p = 0.06, dz = 0.515] or the 3-month follow-up [t(16) = 2.05, p = 0.06, dz = 0.513], as compared to the pre-intervention timepoint. This represents a clinically non-significant (<30 % change; Halvorsen, 2016) reduction in the average PTSD symptom severity score within the sham-control NFB group, with a 14.2 % change at the post-intervention timepoint and a 17.2 % change at the 3-month follow-up visit. Additionally, between-group post-hoc independent samples t-tests found that the PTSD severity of the experimental and sham-control NFB groups were significantly different at the post-intervention timepoint [t(31.02) = -2.10, p = 0.044, dz = 0.356, unequal variances assumed based on Levine’s test of homogeneity of variance] and 3-month follow-up timepoint [t(33) = -2.05, p = 0.048, dz = 0.346]. The between-group difference in PTSD severity does not survive Bonferroni correction. These results are shown in Fig. 3.
Fig. 3.
CAPS score for the experimental NFB (blue) and Sham (yellow) groups at the pre-intervention, post-intervention, and the 3-month follow-up timepoints. The shaded regions represent ± 1 SE. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Furthermore, no significant main effect of group, time, or group × time interaction effect was observed when comparing the participants’ n-back performance (assessed using percent correct and reaction times as metrics), indicating that the current alpha-down NFB did not have an impact on explicit n-back performance.
3.2. Neurofeedback results
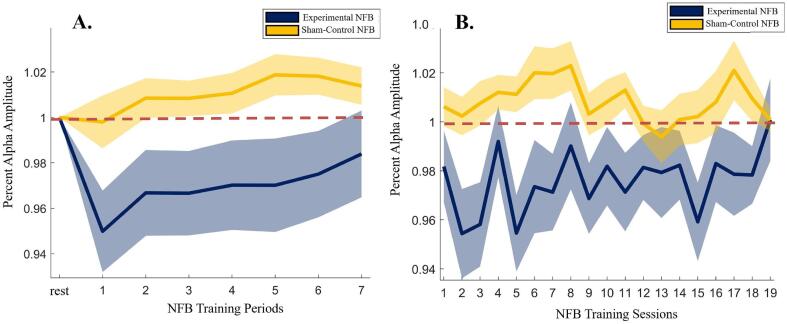
Next, the effectiveness of the alpha-down NFB training protocol in changing the alpha amplitude at the NFB training site (Pz electrode) was assessed by examining the percentage change in the alpha amplitude at the Pz electrode over the course of each 20-min NFB session (6×3-minute training periods and 1×2-minute training period) for the experimental and sham-control NFB groups. Here, we expressed training alpha power (within periods 1–7) as average percent change from baseline alpha power (i.e., the initial rest period of that session) within each respective session.
This was examined using a within-session and between-sessions approach by averaging the percentage change in alpha amplitude across all sessions (retaining period-wise data) and across all periods (retaining session-wise data), respectively. As can be seen from Fig. 4A, showing the results of the within-session analysis, the experimental NFB group was able to reduce their average alpha amplitude throughout the course of the seven NFB training periods, while the sham-control NFB group did not display significant decreases in alpha amplitude. These patterns of average alpha amplitude change were significantly different between the experimental NFB and the sham-control NFB groups, with a 2-way repeated-measures ANOVA showing a group × time interaction [F(7,3332) = 8.65, p < 0.001, η2 = 0.018]. Furthermore, the between-sessions (longitudinal) analysis (shown in Fig. 4B) found that the experimental NFB group showed an overall lower average alpha amplitude change per session, throughout the course of the intervention, as supported by a main effect of group [F(1,20) = 5.77, p = 0.026, η2 = 0.224], without a main effect of time, or interaction effect of group × time.
Fig. 4.
Average alpha amplitude at the site of NFB training (Pz electrode), shown for each of the A. seven NFB training periods within each 20-minute session (averaged across all sessions), and B. NFB training sessions (averaged across all periods). Note that the alpha amplitudes are expressed as percentage change with respect to the 3-minute “rest” period at the start of each session. Therefore, a value < 1 represents a decrease in alpha amplitude relative to the “rest” period, a value > 1 represents an increase in alpha amplitude relative to the “rest” period, and a value of 1 represents no change in the alpha amplitude from the “rest” period (represented by the dashed red line). The shaded regions represent ± 1 SE. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Interestingly, when asked about the cognitive strategy subjects employed to perform the alpha-down NFB training, most participants reported trying to “quiet their minds”, with some focusing on the presented colors, while others imagined themselves in the picture presented, and yet others focused on the auditory feedback.
3.3. Study aim 1: Whole-brain activation analyses
First, the two intervention groups (experimental NFB vs sham-control NFB contrast) were compared at the pre-intervention and post-intervention sessions, using the 2-way ANOVAs for each n-back task with intervention group and session as the between-subject and within-subject factors, respectively, while including data from all trials (trauma-cue & neutral cue). This was followed by comparing the two intervention groups using the 3-way ANOVAs for each n-back task that additionally included trial type/condition (trauma cue vs neutral cue trials) as a within-subjects factor.
As expected, there were no significant clusters that showed significant patterns of activation when comparing the experimental NFB and sham-control NFB groups at the pre-intervention timepoint for any of the ANOVA contrasts, for either n-back tasks, implying that the groups were not different at the pre-intervention timepoint.
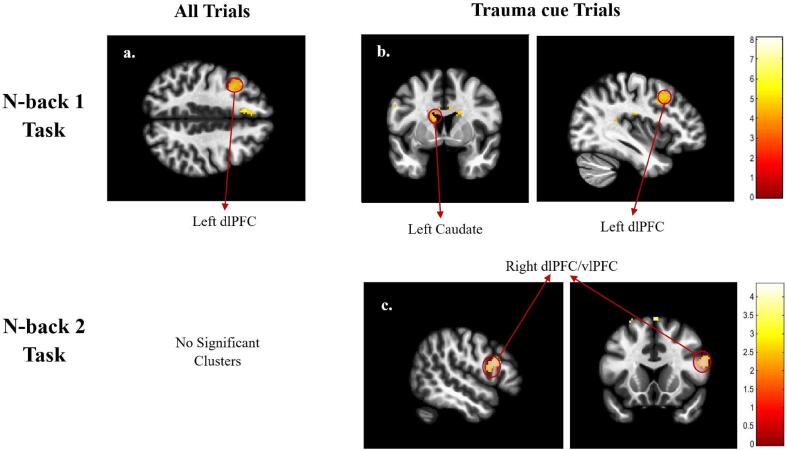
When comparing the intervention groups at the post-intervention timepoint, no significant clusters of activation were observed for the either the n-back 1 or n-back 2 tasks when considering all trials. However, when the group difference at the post-intervention timepoint was assessed separately for each trial type (using the 3-way ANOVA), significant clusters of activation were observed for the trauma cues during the n-back 1 task that were centered at the left thalamus and angular gyrus.
Finally, only one significant cluster of activation was observed when the between-group difference was examined as a function of the within-group difference in brain activation across sessions (group × session interaction: experimental vs sham-control NFB & post vs pre-intervention) while including all trials. This cluster was only observed during the n-back 1 task and was centered at the left dorsolateral prefrontal cortex (dlPFC). When this group × session interaction was examined during each trial-type separately (using the 3-way ANOVA), significant dorsolateral prefrontal cortex (dlPFC) clusters were observed for both the n-back 1 and n-back 2 tasks, while thalamic and caudate clusters were found only for the n-back 1 task. Notably, the dlPFC, angular gyrus and thalamic clusters also survived the FWE correction (see Table 2), indicating that these clusters might represent the most robust findings from this analysis. These results (given in Table 2 and are shown in Fig. 5) suggest a NFB-linked increase in top-down inhibitory control during both emotion-based n-back tasks, potentially reflecting an increase in top-down control of emotion during the ongoing working memory task.
Table 2.
The significantly different clusters for the contrasts between the two intervention groups (experimental NFB vs sham-control NFB) and the two timepoints (Post-intervention vs Pre-intervention). The reverse contrasts (if significant) are shown with negative T-statistic values. Abbreviations: dlPFC - Dorsolateral Prefrontal Cortex, vlPFC - Ventrolateral Prefrontal Cortex.
| Session | Contrast | Brain Region | Cluster Size | T-statistic | p (FDR) | p (FWE) | MNI Coordinates |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||||
| Pre: | Experimental > Sham-control | – | – | – | – | – | – | – | – | |
| Post: | Experimental > Sham-control | |||||||||
| n-back 1 | Trauma cues | L. Angular Gyrus | 159 | 4.63 | 0.04 | 0.034 | −48 | −56 | 38 | |
| L. Thalamus | 204 | 4.20 | 0.03 | 0.011 | −12 | –22 | 20 | |||
| Post > Pre: | Experimental > Sham-control | |||||||||
| n-back 1 | All Trials | L. dlPFC | 170 | 4.61 | 0.035 | 0.031 | −36 | 14 | 46 | |
| Trauma | L. Caudate | 227 | 5.77 | 0.04 | 0.12 | −14 | 6 | 20 | ||
| cues | L. dlPFC | 250 | 4.50 | 0.04 | 0.005 | −36 | 14 | 46 | ||
| n-back 2 | Trauma cues | R. dlPFC/vlPFC | 285 | 4.33 | 0.005 | 0.002 | 52 | 16 | 20 | |
Fig. 5.
Whole-brain activation results of the contrast experimental NFB > sham & post-intervention > pre-intervention, shown for all trials in the first column, and the Trauma cue trials in the following columns). These are shown for the n-back 1 task (first row) and the n-back 2 task (second row). The Neutral cue trials are not shown since no significant clusters were found within these trials. Significant clusters of activation are labeled. The colors represent the T-values of the voxels for the given contrast, as shown in the color bars. Abbreviations: dlPFC - Dorsolateral Prefrontal Cortex, vlPFC - Ventrolateral Prefrontal Cortex.
3.4. Study aim 2: ICA-based network analyses
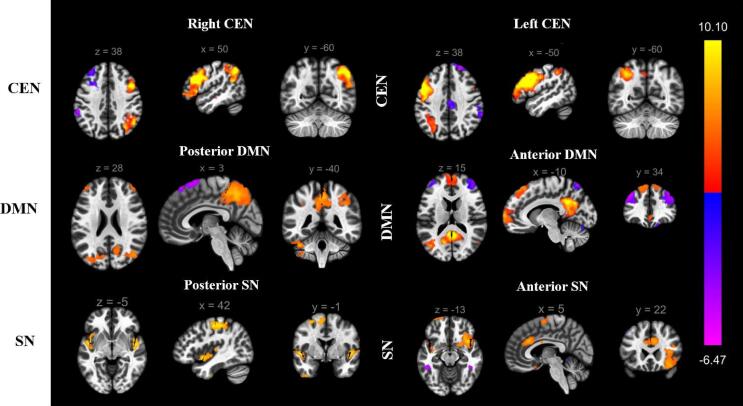
Of the 20 ICA components identified, six ICA components corresponded to sub-networks within the tri-network model (Menon, 2011, Shirer et al., 2012) and were further studied. These included the anterior default mode network (aDMN), posterior default mode network (pDMN), the right central executive network (R.CEN), the left central executive network (L.CEN), the anterior salience network (aSN), and the posterior salience network (pSN). The activation patterns for each of these networks are shown in Fig. 6.
Fig. 6.
Intrinsic Connectivity Networks (ICNs) identified using independent component analysis (ICA). The labels are assigned based on spatial similarity to the networks identified in Shirer et al., 2012. For the sake of consistency with recent literature, the ventral DMN and dorsal DMN defined in Shirer et al. (2012) have been renamed posterior DMN and anterior DMN, respectively. Abbreviations: CEN - Central Executive Network, DMN - Default Mode Network, SN - Salience Network.
To assess between group differences in ICN activity, the two intervention groups (experimental NFB vs sham-control NFB contrast) were compared at the pre-intervention and post-intervention sessions using the 2-way ANOVAs for each n-back task with intervention group and session as the between-subject and within-subject factors, respectively, while including data from all trials (trauma-cue & neutral cue).
Firstly, none of the identified networks showed any between-group differences (experimental NFB vs sham-control NFB) at the pre-intervention timepoint, except for the L.CEN. During the n-back 1 task at the pre-intervention timepoint, the L.CEN showed increased connectivity with left dlPFC and dmPFC in the experimental NFB group as compared to the sham-control NFB group. By contrast, during the n-back 2 task at the pre-intervention timepoint, the L.CEN was more connected with the sub-callousal region in the experimental NFB group as compared to the sham-control NFB group. Due to this difference at the pre-intervention timepoint, the group × session interaction contrast for this network was interpreted with caution.
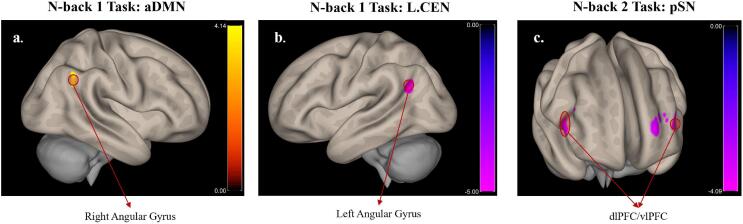
The connectivity patterns of the aDMN, the L.CEN, and the pSN showed significant between-group differences when examined as a function of the within-group difference in brain connectivity across sessions (group × session interaction: experimental vs sham-control NFB & post vs pre-intervention) while including all trials. More specifically, increased connectivity was observed between the aDMN and the right angular gyrus during the n-back 1 task in the experimental NFB group as compared to the sham-control NFB group, post compared to pre-intervention. By contrast, the L.CEN showed decreased connectivity with the left angular gyrus in the experimental NFB as compared to the sham-control NFB group, post compared to pre-intervention. Lastly, post as compared to pre-intervention, decreased connectivity between the pSN and bilateral dlPFC/vlPFC was observed during the n-back 2 task in the experimental NFB as compared to the sham-control NFB group (Fig. 7).
Fig. 7.
Connectivity of ICA networks with other brain regions, shown for the contrast experimental NFB > Sham & Post-intervention > Pre-intervention. The images were created by first thresholding the data at p(uncorr) < 0.001, and then identifying the significant clusters, i.e. p(FDR) < 0.05. Only the significant clusters are shown in this figure. The colors represent the T-values of the voxels for the given contrast, as shown in the color bars. Abbreviations: CEN - Central Executive Network, aDMN - Anterior Default Mode Network, pSN - Posterior Salience Network, dlPFC - Dorsolateral Prefrontal Cortex, vlPFC - Ventrolateral Prefrontal Cortex.
Taken together, these results show that alpha-down NFB may decouple brain regions and networks involved in embodiment (angular gyrus and pSN) from primarily executive processing areas (dlPFC) and networks (CEN), while increasing connectivity with networks responsible for self-related processing (aDMN) under cognitive load. This could in turn help to restore increased connectivity between the posterior and anterior DMN communities while under cognitive load, which have been shown to be segregated in PTSD (Akiki et al., 2018).
3.5. Study aim 3: Regression of whole-brain activation analyses with NFB metric
Only the experimental NFB group showed significant clusters of whole-brain activation that correlated with NFB performance using the post > pre-intervention contrast. Hence, all reported regression results are within the experimental NFB group.
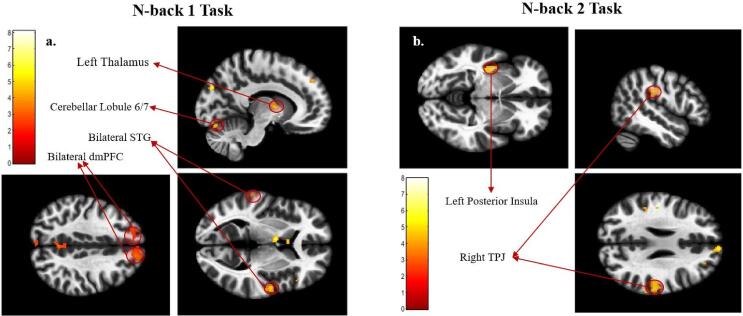
Significant clusters centered at the dorsomedial PFC (dmPFC), the fusiform gyrus, the right superior temporal gyrus (R. STG), the thalamus, the left temporo-parietal junction (L. TPJ), and the cerebellum lobule 6–7 were found to be negatively correlated with their alpha power metric during the n-back 1 task. Interestingly, compared to the results found during the n-back 1 task, a markedly different collection of clusters correlated with their alpha power metric during the n-back 2 task. During this more demanding task, the participants’ alpha power metric was negatively correlated with BOLD activation in the left posterior insula (L. PI) during the trauma-cue trials and with activation of the right temporo-parietal junction (R. TPJ) during the neutral-cue trials. These results are shown in Fig. 8.
Fig. 8.
Results of the regression analysis with alpha power metric within the experimental NFB group (no significant clusters were found within the Sham group). Significant clusters that negatively correlated with alpha power metric are labeled. NOTE: due to the way the alpha power metric is defined (change in alpha power per session averaged over all NFB sessions) for the alpha-down NFB protocol, a successful NFB performer with an overall lower average alpha amplitude would correspond to a lower alpha power metric (decrease in average alpha power). Hence these clusters positively correlate with the participants’ NFB performance. The colors represent the T-values of the voxels for the given contrast, as shown in the color bars. Abbreviations: STG - Superior Temporal Gyrus, dmPFC - Dorsomedial Prefrontal Cortex, TPJ - Temporo-Parietal Junction.
When repeated with the alpha power metric from Nicholson et al. (2020b), the significant clusters during the n-back 1 task showed similar significant clusters centered at the dmPFC, the thalamus and the cerebellum (lobule 6), with additional clusters at the precuneus and the right angular gyrus (R. AG). No significant clusters were found during the more demanding n-back 2 task. These results are shown in Supplementary Fig. 1. Since the goal of the NFB training protocol was to reduce alpha power, a lower (or negative) alpha power metric reflected better NFB performance. Hence, increased activity in these clusters of negative correlation with the alpha power metric represent increased NFB performance.
Taken together, these results suggest that increased activity within regions responsible for social cognition (STG, AG, dmPFC) and working memory (cerebellum 6/7) during the n-back 1 task are associated with more successful alpha-down NFB training, while increased activity within brain areas involved in embodiment (posterior insula and the TPJ) during the n-back 2 task was associated with more successful alpha-down NFB training.
4. Discussion
In this study, we conducted a randomized controlled trial on a 20-week alpha-down NFB training protocol and investigated subsequent neurocognitive changes while the participants engaged in an emotional n-back task engaging top-down control of emotion. Firstly, we found a significant reduction in PTSD symptom severity in those that performed the alpha-down NFB, consistent with previous trials of EEG-NFB in those with PTSD (du Bois et al., 2021, Gapen et al., 2016, Rogel et al., 2020, van der Kolk et al., 2016), although a significant time × group interaction effect was not observed. Interestingly, this reduction in symptom severity persisted even at the 3-month follow-up period (seen in Fig. 3). Next, we found that alpha-down NFB increased activation in PFC regions involved in the top-down control of emotion (dlPFC). The intervention also improved healthy ICN connectivity by decoupling brain regions and networks involved in processing of embodiment (angular gyrus and pSN) from primarily executive processing areas (dlPFC) and networks (CEN), while instead increasing connectivity with networks responsible for self-related processing (aDMN) under cognitive load. Finally, we also found an increase in activity in brain regions involved in top-down control of emotion and embodiment/bodily consciousness (TPJ, posterior insula) that correlated with the extent of the participants’ alpha-amplitude decrease, thus directly linking these neural changes to the alpha-down NFB. This is the first study, to the best of our knowledge, that examined NFB-linked changes under an emotional cognitive task. The study results are discussed in more detail below.
-
i.
Alpha-down NFB enhances activation of top-down control centres during emotional n-back task
The increased activation of the dlPFC (seen in Table 2), along with increased connectivity of the aDMN with the angular gyrus (seen in Fig. 7a), might indicate improved top-down emotion processing within the experimental NFB group. Notably, when examined for each trial type separately, the increase in dlPFC activity was found during the trauma cue trials (Table 2 and Fig. 5 b/c), the condition under which emotion regulation processes would be expected to have been maximally recruited. This result was not observed when examining the neutral cue trials in isolation. Consistent with increased top-down emotion control in the face of trauma triggers, these results highlight the ability of alpha-down NFB to increase top-down control of emotion processing under cognitive load, even in the face of trauma-relevant stimuli.
Interestingly, such engagement of executive brain centres is a well-known strategy of improving emotion regulation (Tornås et al., 2016), with the dlPFC extensively involved in the top-down control of emotion (Comte et al., 2016, Otto et al., 2014), along with cognitive control of attention (Comte et al., 2016) and avoiding distractions during emotion reappraisal processes (Otto et al., 2014). This brain region also works in concert with other key cognitive control and emotion processing centres, such as the anterior cingulate cortex (ACC) and the amygdala, to adequately regulate emotion (Comte et al., 2016, Delgado et al., 2008). These results also directly align with the findings of Aupperle and colleagues that link higher dlPFC activation during emotionally valent stimuli with lower PTSD symptom severity (Aupperle et al., 2012), showing that alpha-down NFB can shift neural activity in a manner that can be directly beneficial for PTSD symptoms. This indicates a step towards NFB-linked resolution of the PFC-based under-modulation seen in those with non-dissociative PTSD (Akiki et al., 2017, Hopper et al., 2007, Lanius et al., 2015).
-
ii.
Alpha-down NFB remedies aberrant increased PFC connectivity with salience regions, while activating brain regions involved in the embodied processing of trauma.
Evident in the results from the more cognitively demanding n-back 2 task, alpha-down NFB seemed to increase activation of top-down emotion control centres (such as the dlPFC, as seen in Table 2 and Fig. 5c), while reducing its connectivity with the pSN (seen in Fig. 7) that is not directly relevant to the working memory demands of the task. Furthermore, the participants’ ability to decrease alpha amplitude correlated with increased activation in these brain regions (posterior insula and TPJ, as seen in Fig. 8b) involved in embodiment/bodily consciousness (Harricharan et al., 2021).
Collectively, alpha-down NFB might promote improved recruitment of executive processing centres under high cognitive load conditions, limit the conscious processing of emotional stimuli during high cognitive load by disengaging the pSN and the executive nodes of the CEN (dlPFC), while also increasing the embodied processing of these emotional stimuli by increasing activation in relevant brain regions (PI and TPJ). Indeed, increased TPJ and PI activity have been implicated in the processing of emotional information during a working memory task (Smith et al., 2017). Collectively, they form a critical node that receives viscerosensory information from the brainstem for exteroceptive and interoceptive sensory processing (Lenggenhager et al., 2015, Lopez and Blanke, 2011), ultimately relaying it to the prefrontal cortex for further multisensory processing and processing of the embodied self (Harricharan et al., 2021, Lenggenhager et al., 2015). The PI is also thought to relay the interoceptive information to the dorsal anterior insula, where it is integrated with affective inputs from the ventral anterior insula (Deen et al., 2011) to inform the switching between CEN and DMN (Menon, 2011, Shaw et al., 2021), and to appropriately mediate the relationship between emotion perception and executive control (Luo et al., 2014).
Given that the dlPFC resides at the top of the chain for processing incoming viscerosensory information (Harricharan et al., 2021), which could be detrimental for working memory processes at higher cognitive loads, the observed disengagement of the dlPFC with the pSN could indicate a shift towards a more efficient allocation of cognitive resources to the task-relevant working memory processing.
Furthermore, the correlation between posterior insula activation and alpha-down NFB performance may represent one mechanism through which alpha-down NFB remedies the subset of PTSD symptoms linked to emotional numbing, blunted sensory and tactile awareness and poor interoception. These symptoms are thought to arise from a hypoactive posterior insula at rest in those with PTSD (Akiki et al., 2017, Harricharan et al., 2021, Hopper et al., 2007).
Collectively, these focal and ICN changes indicate a shift towards a more balanced recruitment of cognitive and emotional control centres in those that performed alpha-down NFB. Individual alpha-down NFB sessions are found to up-regulate salience network structures (Ros et al., 2013) that are important cognitive control centres (Dosenbach et al., 2008), and are critical in task-appropriate gating of CEN and DMN activation (Shaw et al., 2021). In fact, NFB was found to improve SN-based network synchrony subserving the task-appropriate gating of the CEN and DMN (Shaw et al., 2022). Targeted up-regulation of this network using electrical stimulation has also been shown to causally improve cognitive control (Li et al., 2019). Furthermore, a single session of alpha-down NFB was also found to increase posterior insular integration, a region critical for embodied processing of somatosensory information (Kluetsch et al., 2014). Finally, the SN is found to integrate cognitive control processes with emotion perception and processing in the context of emotionally charged working memory tasks (Luo et al., 2014), such as that employed in this study. Hence, repeated upregulation of the SN through alpha-down NFB training could be improving the interaction between cognitive control and emotional control processes, resulting in increased dlPFC activation and more balanced PFC-salience connectivity during emotionally charged working memory tasks, as detailed above. This can also be seen in Nicholson et al. (2020b), where the current randomized controlled trial of alpha-down NFB showed down-regulation of the anterior insula within the SN and greater integration of the posterior insula, a region critical for embodied processing of self, within the aDMN at rest.
When combined with the alpha-down NFB based changes in resting-state ICNs during the same randomized controlled trial reported in Nicholson et al. (2020b) and prior research showing a normalization of dysfunctional ICN activity at rest (Kluetsch et al., 2014, Nicholson et al., 2016a, Ros et al., 2013), it becomes evident that alpha-down NFB can remedy the dysfunctional activation and ICN connectivity seen in PTSD, at rest and under cognitive load. One of the primary neuroimaging findings of Nicholson et al. (2020b) was increased connectivity between the mPFC and aDMN along with decreased connectivity between the posterior DMN nodes and aDMN, showing a remedial increase in aDMN integration and decrease in pDMN integration during rest. This ameliorates the fractionated DMN with an overactive pDMN community, seen in PTSD at rest (Akiki et al., 2018, Bluhm et al., 2009, Holmes et al., 2018). In context of these results, our findings of increased aDMN connectivity with a posterior node of the DMN involved with embodied processing of emotion (angular gyrus), while under emotional and cognitive load, show an overall increase in task-appropriate DMN integration within and between its anterior and posterior communities. This also aligns with the DMN-centered alpha resynchronization observed in EEG data from participants in this randomized controlled trial (Nicholson et al., In Press).
Nicholson et al. (2020b) also found a normalizing shift in SN connectivity with decreased connectivity to anterior insula (AI) at rest, alongside increased posterior insula (PI) connectivity with the aDMN in those with better NFB performance. The current findings add to this body of results by showing greater decoupling between the dlPFC and the pSN, that is not directly relevant to the working memory demands of the task, while under cognitive load and increased activation of brain regions involved in embodied self processing (PI and TPJ) in those with better NFB performance while under cognitive load. Collectively, these findings reflect more cognition-appropriate involvement of somatosensory integration and embodied processing centres post-NFB, adding further evidence for a more balanced SN connectivity profile after alpha-down NFB training.
5. Limitations
This preliminary study was not pre-registered as a clinical trial since the ethics protocol approval was obtained before pre-registering trials was common in the field. Consequently, we were extremely restrictive with our outcome measures to mitigate this limitation. Additionally, the participants choice of visual feedback was not recorded for all participants, preventing us from assessing the effects of different visual feedback stimuli on NFB performance or changes in brain activation/connectivity.
The changes in working memory strategy are inferred from the shift in patterns of neural activity and not through direct feedback from the participants. Hence, it is still unknown if these neural changes lead to cognitive changes that are perceived by the participant.
Additionally, due to limitations on the scanning time for each participants’ imaging session, a working memory task without trauma-related triggers was not performed, precluding any relevant covariates such as NFB-linked changes in working memory capacity in the absence of trauma triggers. Future studies should compare the emotional n-back task with a more traditional n-back task to assess the effects of the additional emotional load added by processing trauma-relevant cues during the emotional n-back task. Furthermore, this study did not include a transfer session. Hence, it is unclear if the participants could supress parietal alpha if they used the learned strategies in the absence of neurofeedback. Future studies should investigate such transfer learning in those with PTSD.
Finally, it is also important to acknowledge that an opposite pattern of emotional-overmodulation is observed in the dissociative subtype of PTSD (PTSD + DS), and this was not studied in this work. Individuals with PTSD + DS show increased PFC volume with greater dissociation severity (Daniels et al., 2016), hyper-connectivity of the PFC with the amygdala (Nicholson et al., 2015) and with other innate alarm system (IAS) regions (Nicholson et al., 2017a), and a generalized pattern of large-scale hyperconnectivity (Shaw. Terpou et al., 2022). This leads to considerable heterogeneity in the pattern of connectivity within a general PTSD population, potentially contributing to the baseline group differences in L.CEN connectivity observed in this study, limiting our ability to draw conclusions on the effect of the alpha-down NFB on L.CEN connectivity. Hence, future investigations into the effect of alpha-down NFB training on a PTSD population should include a PTSD + DS cohort.
6. Conclusions
In conclusion, this preliminary double-blind randomized controlled trial shows the therapeutic utility of alpha-down NFB in reducing PTSD symptom severity while also improving the neural systems underlying top-down control of emotion and embodied processing of emotional stimuli during an emotional n-back task. More specifically, the results indicate that alpha-down NFB increased activation in PFC regions involved in the top-down control of emotion (dlPFC) and improved DMN integration through increased connectivity between the aDMN and the angular gyrus. Finally, the results also indicate that increased alpha-down NFB performance correlated with increased activity in brain regions involved in top-down control and embodied processing of self (TPJ, posterior insula). These mechanistic insights provide the first understanding of how NFB might be normalizing dysfunctional brain activity and connectivity in PTSD under emotional cognitive load.
CRediT authorship contribution statement
Saurabh Bhaskar Shaw: Formal analysis, Software, Writing – original draft. Andrew Nicholson: Conceptualization, Investigation, Data curation, Funding acquisition, Writing – review & editing. Tomas Ros: Conceptualization, Investigation, Methodology. Sherain Harricharan: Methodology, Writing – review & editing. Braeden Terpou: Methodology, Writing – review & editing. Maria Densmore: Conceptualization, Methodology. Jean Theberge: Conceptualization, Methodology. Paul Frewen: Conceptualization, Methodology. Ruth Lanius: Conceptualization, Supervision, Data curation, Funding acquisition, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The current study was funded by the Canadian Institute for Military and Veteran Health Research (CIMVHR; grant number W7716-125624/001/SV), and ANS Research, London, United Kingdom. R. Lanius is supported by the Harris-Woodman Chair in Psyche and Soma at Western University. S.B. Shaw and B. Terpou were supported by MITACS Elevate funding, in partnership with Homewood Research Institute in Guelph, Ontario, Canada. A.A. Nicholson received a Marie Sklodowska-Curie Individual Fellowship (grant number 897709), under the European Unions' Horizon 2020 research and innovation program, and a Banting Research Foundation award (grant number 2021-1424). We are also deeply thankful to Suzy Southwell, Stephanie Nevill, and Barbara Whelan for their involvement in data collection and curation.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2023.103313.
Contributor Information
Saurabh Bhaskar Shaw, Email: sshaw62@uwo.ca.
Ruth A. Lanius, Email: Ruth.Lanius@lhsc.on.ca.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
The data that has been used is confidential.
References
- Akiki T.J., Averill C.L., Abdallah C.G. A Network-Based Neurobiological Model of PTSD: Evidence From Structural and Functional Neuroimaging Studies. Curr Psychiatry Rep. 2017;19 doi: 10.1007/s11920-017-0840-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiki T.J., Averill C.L., Wrocklage K.M., Scott J.C., Averill L.A., Schweinsburg B., Alexander-Bloch A., Martini B., Southwick S.M., Krystal J.H., Abdallah C.G. Default mode network abnormalities in posttraumatic stress disorder: A novel network-restricted topology approach. Neuroimage. 2018;176:489–498. doi: 10.1016/j.neuroimage.2018.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/J.NEUROIMAGE.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Aupperle R.L., Allard C.B., Grimes E.M., Simmons A.N., Flagan T., Behrooznia M., Cissell S.H., Twamley E.W., Thorp S.R., Norman S.B., Paulus M.P., Stein M.B. Dorsolateral prefrontal cortex activation during emotional anticipation and neuropsychological performance in posttraumatic stress disorder. Arch Gen Psychiatry. 2012;69:360–371. doi: 10.1001/archgenpsychiatry.2011.1539. [DOI] [PubMed] [Google Scholar]
- Barredo J., Aiken E., van 't Wout-Frank M., Greenberg B.D., Carpenter L.L., Philip N.S. Network functional architecture and aberrant functional connectivity in post-traumatic stress disorder: A clinical application of network convergence. Brain Connect. 2018;8(9):549–557. doi: 10.1089/brain.2018.0634. [DOI] [PubMed] [Google Scholar]
- Bell A.N., Moss D., Kallmeyer R.J. Healing the neurophysiological roots of trauma: A controlled study examining loreta z-score neurofeedback and HRV biofeedback for chronic PTSD. NeuroRegulation. 2019;6:54–70. doi: 10.15540/nr.6.2.54. [DOI] [Google Scholar]
- Berger W., Coutinho E.S.F., Figueira I., Marques-Portella C., Luz M.P., Neylan T.C., Marmar C.R., Mendlowicz M.V. Rescuers at risk: A systematic review and meta-regression analysis of the worldwide current prevalence and correlates of PTSD in rescue workers. Soc Psychiatry Psychiatr Epidemiol. 2012;47:1001–1011. doi: 10.1007/S00127-011-0408-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein D.P., Stein J.A., Newcomb M.D., Walker E., Pogge D., Ahluvalia T., Stokes J., Handelsman L., Medrano M., Desmond D., Zule W. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl. 2003;27:169–190. doi: 10.1016/S0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- Bluhm R.L., Williamson P.C., Osuch E.A., Frewen P.A., Stevens T.K., Boksman K., Neufeld R.W.J., Théberge J., Lanius R.A. Alterations in default network connectivity in posttraumatic stress disorder related to early-life trauma. J Psychiatry Neurosci. 2009;34:187–194. [PMC free article] [PubMed] [Google Scholar]
- Boyd J.E., Lanius R.A., McKinnon M.C. Mindfulness-based treatments for posttraumatic stress disorder: a review of the treatment literature and neurobiological evidence. J Psychiatry Neurosci. 2018;43(1):7–25. doi: 10.1503/jpn.170021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briere J., Weathers F.W., Runtz M. Is dissociation a multidimensional construct? Data from the Multiscale Dissociation Inventory. J Trauma Stress. 2005;18:221–231. doi: 10.1002/JTS.20024. [DOI] [PubMed] [Google Scholar]
- Calhoun V.D., Liu J., Adalı T. A review of group ICA for fMRI data and ICA for joint inference of imaging, genetic, and ERP data. Neuroimage. 2009;45:S163. doi: 10.1016/j.neuroimage.2008.10.057. S172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comte M., Schön D., Coull J.T., Reynaud E., Khalfa S., Belzeaux R., Ibrahim E.C., Guedj E., Blin O., Weinberger D.R., Fakra E. Dissociating Bottom-Up and Top-Down Mechanisms in the Cortico-Limbic System during Emotion Processing. Cerebral Cortex. 2016;26:144–155. doi: 10.1093/cercor/bhu185. [DOI] [PubMed] [Google Scholar]
- Cusack K., Jonas D.E., Forneris C.A., Wines C., Sonis J., Middleton J.C., Feltner C., Brownley K.A., Olmsted K.R., Greenblatt A., Weil A., Gaynes B.N. Psychological treatments for adults with posttraumatic stress disorder: A systematic review and meta-analysis. Clin Psychol Rev. 2016;43:128–141. doi: 10.1016/j.cpr.2015.10.003. [DOI] [PubMed] [Google Scholar]
- da Silva J.C., de Souza M.L. Neurofeedback training for cognitive performance improvement in healthy subjects: A systematic review. Psychol Neurosci. 2021;14:262–279. doi: 10.1037/pne0000261. [DOI] [Google Scholar]
- Daniels J.K., McFarlane A.C., Bluhm R.L., Moores K.A., Clark C.R., Shaw M.E., Williamson P.C., Densmore M., Lanius R.A. Switching between executive and default mode networks in posttraumatic stress disorder: alterations in functional connectivity. J Psychiatry Neurosci. 2010;35:258–266. doi: 10.1503/jpn.090175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels J.K., Frewen P., Theberge J., Lanius R.A. Structural brain aberrations associated with the dissociative subtype of post-traumatic stress disorder. Acta Psychiatr Scand. 2016;133:232–240. doi: 10.1111/acps.12464. [DOI] [PubMed] [Google Scholar]
- Deen B., Pitskel N.B., Pelphrey K.A. Three Systems of Insular Functional Connectivity Identified with Cluster Analysis. Cerebral Cortex. 2011;21:1498–1506. doi: 10.1093/cercor/bhq186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado M.R., Nearing K.I., LeDoux J.E., Phelps E.A. Neural Circuitry Underlying the Regulation of Conditioned Fear and Its Relation to Extinction. Neuron. 2008;59:829–838. doi: 10.1016/j.neuron.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A., Makeig S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Dosenbach N.U.F., Fair D.A., Cohen A.L., Schlaggar B.L., Petersen S.E. A dual-networks architecture of top-down control. Trends Cogn Sci. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- du Bois N., Bigirimana A.D., Korik A., Kéthina L.G., Rutembesa E., Mutabaruka J., Mutesa L., Prasad G., Jansen S., Coyle D.H. Neurofeedback with low-cost, wearable electroencephalography (EEG) reduces symptoms in chronic Post-Traumatic Stress Disorder. J Affect Disord. 2021;295:1319–1334. doi: 10.1016/J.JAD.2021.08.071. [DOI] [PubMed] [Google Scholar]
- Erhardt E.B., Rachakonda S., Bedrick E.J., Allen E.A., Adali T., Calhoun V.D. Comparison of multi-subject ICA methods for analysis of fMRI data. Hum Brain Mapp. 2011;32:2075–2095. doi: 10.1002/hbm.21170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escolano, C., Aguilar, M., Minguez, J., 2011. EEG-based upper alpha neurofeedback training improves working memory performance. Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS 2327–2330. https://doi.org/10.1109/IEMBS.2011.6090651. [DOI] [PubMed]
- Falconer E., Allen A., Felmingham K.L., Williams L.M., Bryant R.A. Inhibitory neural activity predicts response to cognitive-behavioral therapy for posttraumatic stress disorder. Journal of Clinical Psychiatry. 2013;74:895–901. doi: 10.4088/JCP.12m08020. [DOI] [PubMed] [Google Scholar]
- Fani N., Jovanovic T., Ely T.D., Bradley B., Gutman D., Tone E.B., Ressler K.J. Neural correlates of attention bias to threat in post-traumatic stress disorder. Biol Psychol. 2012;90:134–142. doi: 10.1016/J.BIOPSYCHO.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M.B., Spitzer R.L., Gibbon M., Williams J.B.W. Biometrics Research, New York State Psychiatric Institute; New York: 2002. Structured clinical interview for DSM-IV axis I disorders. [Google Scholar]
- Fulton J.J., Calhoun P.S., Wagner H.R., Schry A.R., Hair L.P., Feeling N., Elbogen E., Beckham J.C. The prevalence of posttraumatic stress disorder in Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF) Veterans: A meta-analysis. J Anxiety Disord. 2015;31:98–107. doi: 10.1016/j.janxdis.2015.02.003. [DOI] [PubMed] [Google Scholar]
- Gapen M., van der Kolk B.A., Hamlin E., Hirshberg L., Suvak M., Spinazzola J. A Pilot Study of Neurofeedback for Chronic PTSD. Applied Psychophysiology Biofeedback. 2016;41:251–261. doi: 10.1007/s10484-015-9326-5. [DOI] [PubMed] [Google Scholar]
- Geuze E., Westenberg H.G.M., Heinecke A., de Kloet C.S., Goebel R., Vermetten E. Thinner prefrontal cortex in veterans with posttraumatic stress disorder. Neuroimage. 2008;41:675–681. doi: 10.1016/j.neuroimage.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Halvorsen J.Ø. Defining clinically significant change. The British Journal of Psychiatry. 2016;209:85. doi: 10.1192/BJP.209.1.85. [DOI] [PubMed] [Google Scholar]
- Harricharan S., Nicholson A.A., Thome J., Densmore M., McKinnon M.C., Théberge J., Frewen P.A., Neufeld R.W.J., Lanius R.A. PTSD and its dissociative subtype through the lens of the insula: Anterior and posterior insula resting-state functional connectivity and its predictive validity using machine learning. Psychophysiology. 2020;57:1–23. doi: 10.1111/psyp.13472. [DOI] [PubMed] [Google Scholar]
- Harricharan S., McKinnon M.C., Lanius R.A. How Processing of Sensory Information From the Internal and External Worlds Shape the Perception and Engagement With the World in the Aftermath of Trauma: Implications for PTSD. Front Neurosci. 2021;15:1–20. doi: 10.3389/fnins.2021.625490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes, S.E., Scheinost, D., DellaGioia, N., Davis, M.T., Matuskey, D., Pietrzak, R.H., Hampson, M., Krystal, J.H., Esterlis, I., 2018. Cerebellar and Prefrontal Cortical Alterations in PTSD: Structural and Functional Evidence. Chronic Stress 2. https://doi.org/10.1177/2470547018786390. [DOI] [PMC free article] [PubMed]
- Hopper J.W., Frewen P.A., van der Kolk B.A., Lanius R.A. Neural correlates of reexperiencing, avoidance, and dissociation in PTSD: Symptom dimensions and emotion dysregulation in responses to script-driven trauma imagery. J Trauma Stress. 2007;20:713–725. doi: 10.1002/jts.20284. [DOI] [PubMed] [Google Scholar]
- Hsueh J.J., Chen T.S., Chen J.J., Shaw F.Z. Neurofeedback training of EEG alpha rhythm enhances episodic and working memory. Hum Brain Mapp. 2016;37:2662–2675. doi: 10.1002/hbm.23201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinek L., Moritz S., Randjbar S., Sommerfeldt D., Püschel K., Seifert D. Does the evocation of traumatic memories confound subsequent working memory performance in posttraumatic stress disorder (PTSD)? Depress Anxiety. 2008;25:175–179. doi: 10.1002/da.20300. [DOI] [PubMed] [Google Scholar]
- Kilpatrick D.G., Resnick H.S., Milanak M.E., Miller M.W., Keyes K.M., Friedman M.J. National Estimates of Exposure to Traumatic Events and PTSD Prevalence Using DSM-IV and DSM-5 Criteria. J Trauma Stress. 2013;26:537–547. doi: 10.1002/jts.21848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluetsch R.C., Ros T., Théberge J., Frewen P.A., Calhoun V.D., Schmahl C., Jetly R., Lanius R.A. Plastic modulation of PTSD resting-state networks and subjective wellbeing by EEG neurofeedback. Acta Psychiatr Scand. 2014;130:123–136. doi: 10.1111/acps.12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M., Huey E.D., Raymont V., Cheon B., Solomon J., Wassermann E.M., Grafman J. Focal brain damage protects against post-traumatic stress disorder in combat veterans. Nat Neurosci. 2008;11:232–237. doi: 10.1038/nn2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koso M., Hansen S. Executive function and memory in posttraumatic stress disorder: a study of Bosnian war veterans. European Psychiatry. 2006;21:167–173. doi: 10.1016/j.eurpsy.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Kulka, R., Schlenger, W., Fairbank, J., Hough, R., 1990. Trauma and the Vietnam war generation: Report of findings from the National Vietnam Veterans Readjustment Study. Brunner/Mazel Psychosocial Stress Series 18.
- Lanius R.A., Bluhm R., Lanius U., Pain C. A review of neuroimaging studies in PTSD: Heterogeneity of response to symptom provocation. J Psychiatr Res. 2006;40:709–729. doi: 10.1016/j.jpsychires.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Lanius R.A., Bluhm R.L., Coupland N.J., Hegadoren K.M., Rowe B., Théberge J., Neufeld R.W.J., Williamson P.C., Brimson M. Default mode network connectivity as a predictor of post-traumatic stress disorder symptom severity in acutely traumatized subjects. Acta Psychiatr Scand. 2010;121:33–40. doi: 10.1111/j.1600-0447.2009.01391.x. [DOI] [PubMed] [Google Scholar]
- Lanius RuthA., Frewen PaulA., Vermetten E., Yehuda R. Fear conditioning and early life vulnerabilities: two distinct pathways of emotional dysregulation and brain dysfunction in PTSD. Eur J Psychotraumatol. 2010;1(1) doi: 10.3402/ejpt.v1i0.5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanius R.A., Williamson P.C., Densmore M., Boksman K., Gupta M.A., Neufeld R.W., Gati J.S., Menon R.S. Neural correlates of traumatic memories in posttraumatic stress disorder: A functional MRI investigation. American Journal of Psychiatry. 2001;158:1920–1922. doi: 10.1176/appi.ajp.158.11.1920. [DOI] [PubMed] [Google Scholar]
- Lanius R.A., Frewen P.A., Tursich M., Jetly R., McKinnon M.C. Restoring large-scale brain networks in PTSD and related disorders: a proposal for neuroscientifically-informed treatment interventions. Eur J Psychotraumatol. 2015;6:27313. doi: 10.3402/ejpt.v6.27313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanius R.A., Rabellino D., Boyd J.E., Harricharan S., Frewen P.A., McKinnon M.C. The innate alarm system in PTSD: conscious and subconscious processing of threat. Curr Opin Psychol. 2017;14:109–115. doi: 10.1016/j.copsyc.2016.11.006. [DOI] [PubMed] [Google Scholar]
- Larsen S.E., Lotfi S., Bennett K.P., Larson C.L., Dean-Bernhoft C., Lee H.J. A pilot randomized trial of a dual n-back emotional working memory training program for veterans with elevated PTSD symptoms. Psychiatry Res. 2019;275:261–268. doi: 10.1016/j.psychres.2019.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenggenhager, B., Lopez, C., Metzinger, T., Windt, J.M., 2015. Vestibular Contributions to the Sense of Body, Self, and Others. https://doi.org/10.15502/9783958570023.
- Li Y., Scherer N., Felix L., Kuper H., Pietschnig J. Prevalence of depression, anxiety and post-traumatic stress disorder in health care workers during the COVID-19 pandemic: A systematic review and meta-analysis. PLoS One. 2021;16(3) doi: 10.1371/JOURNAL.PONE.0246454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L.M., Violante I.R., Leech R., Hampshire A., Opitz A., McArthur D., Carmichael D.W., Sharp D.J. Cognitive enhancement with Salience Network electrical stimulation is influenced by network structural connectivity. Neuroimage. 2019;185:425–433. doi: 10.1016/J.NEUROIMAGE.2018.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon I., Abelson J.L. Context Processing and the Neurobiology of Post-Traumatic Stress Disorder. Neuron. 2016;92:14–30. doi: 10.1016/J.NEURON.2016.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez C., Blanke O. The thalamocortical vestibular system in animals and humans. Brain Res Rev. 2011;67:119–146. doi: 10.1016/J.BRAINRESREV.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Luo Y., Qin S., Fernández G., Zhang Y., Klumpers F., Li H. Emotion perception and executive control interact in the salience network during emotionally charged working memory processing. Hum Brain Mapp. 2014;35:5606–5616. doi: 10.1002/HBM.22573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: A unifying triple network model. Trends Cogn Sci. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Menon V., Uddin L.Q. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214(5-6):655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moores K.A., Clark C.R., McFarlane A.C., Brown G.C., Puce A., Taylor D.J. Abnormal recruitment of working memory updating networks during maintenance of trauma-neutral information in post-traumatic stress disorder. Psychiatry Res Neuroimaging. 2008;163:156–170. doi: 10.1016/J.PSCYCHRESNS.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Nejati V., Salehinejad M.A., Nitsche M.A. Interaction of the Left Dorsolateral Prefrontal Cortex (l-DLPFC) and Right Orbitofrontal Cortex (OFC) in Hot and Cold Executive Functions: Evidence from Transcranial Direct Current Stimulation (tDCS) Neuroscience. 2018;369:109–123. doi: 10.1016/j.neuroscience.2017.10.042. [DOI] [PubMed] [Google Scholar]
- Nicholson, A. A., Densmore, M., Frewen, P. A., Neufeld, R. W. J., Théberge, J., Jetly, R., Lanius, R. A., Ros, T., (In Press). Homeostatic Normalization of Alpha Brain Rhythms within the Default-Mode Network: Reduced Symptoms in Posttraumatic Stress Disorder Following a Double-Blind, Randomized Controlled Trial of EEG Neurofeedback . Brain Communications. [DOI] [PMC free article] [PubMed]
- Nicholson A.A., Densmore M., Frewen P.A., Théberge J., Neufeld R.W.J., McKinnon M.C., Lanius R.A. The Dissociative Subtype of Posttraumatic Stress Disorder: Unique Resting-State Functional Connectivity of Basolateral and Centromedial Amygdala Complexes. Neuropsychopharmacology. 2015;40:2317–2326. doi: 10.1038/npp.2015.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson A.A., Ros T., Frewen P.A., Densmore M., Théberge J., Kluetsch R.C., Jetly R., Lanius R.A. Alpha oscillation neurofeedback modulates amygdala complex connectivity and arousal in posttraumatic stress disorder. Neuroimage Clin. 2016;12:506–516. doi: 10.1016/j.nicl.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson A.A., Sapru I., Densmore M., Frewen P.A., Neufeld R.W.J., Théberge J., McKinnon M.C., Lanius R.A. Unique insula subregion resting-state functional connectivity with amygdala complexes in posttraumatic stress disorder and its dissociative subtype. Psychiatry Res Neuroimaging. 2016;250:61–72. doi: 10.1016/j.pscychresns.2016.02.002. [DOI] [PubMed] [Google Scholar]
- Nicholson A.A., Friston K.J., Zeidman P., Harricharan S., McKinnon M.C., Densmore M., Neufeld R.W.J., Théberge J., Corrigan F., Jetly R., Spiegel D., Lanius R.A. Dynamic causal modeling in PTSD and its dissociative subtype: Bottom-up versus top-down processing within fear and emotion regulation circuitry. Hum Brain Mapp. 2017;38:5551–5561. doi: 10.1002/hbm.23748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson A.A., Rabellino D., Densmore M., Frewen P.A., Paret C., Kluetsch R., Schmahl C., Théberge J., Neufeld R.W.J., McKinnon M.C., Reiss J.P., Jetly R., Lanius R.A. The neurobiology of emotion regulation in posttraumatic stress disorder: Amygdala downregulation via real-time fMRI neurofeedback. Hum Brain Mapp. 2017;38:541–560. doi: 10.1002/hbm.23402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson A.A., Harricharan S., Densmore M., Neufeld R.W.J., Ros T., McKinnon M.C., Frewen P.A., Théberge J., Jetly R., Pedlar D., Lanius R.A. Classifying heterogeneous presentations of PTSD via the default mode, central executive, and salience networks with machine learning. NeuroImage: Clinical. 2020;27:102262. doi: 10.1016/j.nicl.2020.102262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson A.A., Ros T., Densmore M., Frewen P.A., Neufeld R.W.J., Théberge J., Jetly R., Lanius R.A. A randomized, controlled trial of alpha-rhythm EEG neurofeedback in posttraumatic stress disorder: A preliminary investigation showing evidence of decreased PTSD symptoms and restored default mode and salience network connectivity using fMRI. Neuroimage Clin. 2020;28:102490. doi: 10.1016/j.nicl.2020.102490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niv S. Clinical efficacy and potential mechanisms of neurofeedback. Pers Individ Dif. 2013;54:676–686. doi: 10.1016/j.paid.2012.11.037. [DOI] [Google Scholar]
- Op den Kelder R., Van den Akker A.L., Geurts H.M., Lindauer R.J.L., Overbeek G. Executive functions in trauma-exposed youth: a meta-analysis. Eur J Psychotraumatol. 2018;9(1):1450595. doi: 10.1080/20008198.2018.1450595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto B., Misra S., Prasad A., McRae K. Functional overlap of top-down emotion regulation and generation: An fMRI study identifying common neural substrates between cognitive reappraisal and cognitively generated emotions. Cogn Affect Behav Neurosci. 2014;14:923–938. doi: 10.3758/s13415-013-0240-0. [DOI] [PubMed] [Google Scholar]
- Petrie K., Milligan-Saville J., Gayed A., Deady M., Phelps A., Dell L., Forbes D., Bryant R.A., Calvo R.A., Glozier N., Harvey S.B. Prevalence of PTSD and common mental disorders amongst ambulance personnel: a systematic review and meta-analysis. Soc Psychiatry Psychiatr Epidemiol. 2018;53:897–909. doi: 10.1007/S00127-018-1539-5. [DOI] [PubMed] [Google Scholar]
- Reiter K., Andersen S.B., Carlsson J. Neurofeedback treatment and posttraumatic stress disorder: Effectiveness of neurofeedback on posttraumatic stress disorder and the optimal choice of protocol. Journal of Nervous and Mental Disease. 2016;204:69–77. doi: 10.1097/NMD.0000000000000418. [DOI] [PubMed] [Google Scholar]
- Rogel A., Loomis A.M., Hamlin E., Hodgdon H., Spinazzola J., van der Kolk B. The impact of neurofeedback training on children with developmental trauma: A randomized controlled study. Psychol Trauma. 2020;12:918–929. doi: 10.1037/tra0000648. [DOI] [PubMed] [Google Scholar]
- Roiser J.P., Linden D.E., Gorno-Tempinin M.L., Moran R.J., Dickerson B.C., Grafton S.T. Minimum statistical standards for submissions to Neuroimage: Clinical. Neuroimage Clin. 2016;12:1045–1047. doi: 10.1016/j.nicl.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ros T., Théberge J., Frewen P.A., Kluetsch R., Densmore M., Calhoun V.D., Lanius R.A. Mind over chatter: Plastic up-regulation of the fMRI salience network directly after EEG neurofeedback. Neuroimage. 2013;65:324–335. doi: 10.1016/j.neuroimage.2012.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryali S., Supekar K., Chen T., Kochalka J., Cai W., Nicholas J., Padmanabhan A., Menon V., Marinazzo D. Temporal Dynamics and Developmental Maturation of Salience, Default and Central-Executive Network Interactions Revealed by Variational Bayes Hidden Markov Modeling. PLoS Comput Biol. 2016;12(12):e1005138. doi: 10.1371/journal.pcbi.1005138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J.C., Matt G.E., Wrocklage K.M., Crnich C., Jordan J., Southwick S.M., Krystal J.H., Schweinsburg B.C. A quantitative meta-analysis of neurocognitive functioning in posttraumatic stress disorder. Psychol Bull. 2015;141:105–140. doi: 10.1037/a0038039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sendlera D.J., Rutkowskaa A., Makara-Studzinskaa M. How the exposure to trauma has hindered physicians’ capacity to heal: prevalence of PTSD among healthcare workers. Eur. J Psychiatry. 2016;30 [Google Scholar]
- Sha Z., Wager T.D., Mechelli A., He Y. Common Dysfunction of Large-Scale Neurocognitive Networks Across Psychiatric Disorders. Biol Psychiatry. 2019;85:379–388. doi: 10.1016/j.biopsych.2018.11.011. [DOI] [PubMed] [Google Scholar]
- Shaw S.B., McKinnon M.C., Heisz J., Becker S. Dynamic task-linked switching between brain networks – A tri-network perspective. Brain Cogn. 2021;151 doi: 10.1016/j.bandc.2021.105725. [DOI] [PubMed] [Google Scholar]
- Shaw S.B., Levy Y., Mizzi A., Herman G., McKinnon M.C., Heisz J.J., Becker S. Combined Aerobic Exercise and Neurofeedback Lead to Improved Task-Relevant Intrinsic Network Synchrony. Front Hum Neurosci. 2022:341. doi: 10.3389/FNHUM.2022.838614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw, S. B., Terpou, B. A., Densmore, M., Theberge, J., Frewen, P., McKinnon, M. C., Lanius, R. A. Large-Scale Functional Hyperconnectivity Patterns Characterizing Trauma-Related Dissociation: A rs-fMRI Study of PTSD and its Dissociative Subtype., 11 November 2022, PREPRINT (Version 1) available at Research Square. 10.21203/rs.3.rs-2178523/v1. [DOI]
- Sherwood M.S., Kane J.H., Weisend M.P., Parker J.G. Enhanced control of dorsolateral prefrontal cortex neurophysiology with real-time functional magnetic resonance imaging (rt-fMRI) neurofeedback training and working memory practice. Neuroimage. 2016;124:214–223. doi: 10.1016/j.neuroimage.2015.08.074. [DOI] [PubMed] [Google Scholar]
- Sheynin J., Liberzon I. Circuit dysregulation and circuit-based treatments in posttraumatic stress disorder. Neurosci Lett. 2017;649:133–138. doi: 10.1016/J.NEULET.2016.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirer W.R., Ryali S., Rykhlevskaia E., Menon V., Greicius M.D. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb Cortex. 2012;22:158–165. doi: 10.1093/cercor/bhr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R., Lane R.D., Alkozei A., Bao J., Smith C., Sanova A., Nettles M., Killgore W.D.S. Maintaining the feelings of others in working memory is associated with activation of the left anterior insula and left frontal-parietal control network. Soc Cogn Affect Neurosci. 2017;12:848–860. doi: 10.1093/SCAN/NSX011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spottswood M., Davydow D.S., Huang H. The Prevalence of Posttraumatic Stress Disorder in Primary Care: A Systematic Review. Harv Rev Psychiatry. 2017;25(4):159–169. doi: 10.1097/HRP.0000000000000136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng R.N., Grady C.L. Patterns of Brain Activity Supporting Autobiographical Memory, Prospection, and Theory of Mind, and Their Relationship to the Default Mode Network. J Cogn Neurosci. 2010;22:1112–1123. doi: 10.1162/jocn.2009.21282. [DOI] [PubMed] [Google Scholar]
- Sripada R.K., King A.P., Welsh R.C., Garfinkel S.N., Wang X., Sripada C.S., Liberzon I. Neural dysregulation in posttraumatic stress disorder: Evidence for disrupted equilibrium between salience and default mode brain networks. Psychosom Med. 2012;74:904–911. doi: 10.1097/PSY.0b013e318273bf33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenkamp M.M., Litz B.T., Hoge C.W., Marmar C.R., Affiliations A., Cohen A., Cohen Veterans A. Psychotherapy for Military-Related PTSD: A Review of Randomized Clinical Trials. JAMA. 2015;314:489–500. doi: 10.1001/JAMA.2015.8370. [DOI] [PubMed] [Google Scholar]
- Thomas J.L., Wilk J.E., Riviere L.A., McGurk D., Castro C.A., Hoge C.W. Prevalence of mental health problems and functional impairment among active component and national guard soldiers 3 and 12 months following combat in Iraq. Arch Gen Psychiatry. 2010;67:614–623. doi: 10.1001/archgenpsychiatry.2010.54. [DOI] [PubMed] [Google Scholar]
- Tornås S., Løvstad M., Solbakk A.K., Schanke A.K., Stubberud J. Goal Management Training Combined With External Cuing as a Means to Improve Emotional Regulation, Psychological Functioning, and Quality of Life in Patients With Acquired Brain Injury: A Randomized Controlled Trial. Arch Phys Med Rehabil. 2016;97:1841–1852.e3. doi: 10.1016/j.apmr.2016.06.014. [DOI] [PubMed] [Google Scholar]
- Uddin L.Q. Salience processing and insular cortical function and dysfunction. Nat Rev Neurosci. 2015;16:55–61. doi: 10.1038/nrn3857. [DOI] [PubMed] [Google Scholar]
- van der Kolk B.A., Hodgdon H., Gapen M., Musicaro R., Suvak M.K., Hamlin E.d., Spinazzola J., Matsuoka Y.J. A randomized controlled study of neurofeedback for chronic PTSD. PLoS One. 2016;11(12):e0166752. doi: 10.1371/journal.pone.0166752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Gao Y., Tang S., Lu L., Zhang L., Bu X., Li H., Hu X., Hu X., Jiang P., Jia Z., Gong Q., Sweeney J.A., Huang X. Large-scale network dysfunction in the acute state compared to the remitted state of bipolar disorder: A meta-analysis of resting-state functional connectivity. EBioMedicine. 2020;54 doi: 10.1016/j.ebiom.2020.102742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins L.E., Sprang K.R., Rothbaum B.O. Treating PTSD: A review of evidence-based psychotherapy interventions. Front Behav Neurosci. 2018;12:1–9. doi: 10.3389/fnbeh.2018.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts B.V., Schnurr P.P., Mayo L., Young-Xu Y., Weeks W.B., Friedman M.J. Meta-analysis of the efficacy of treatments for posttraumatic stress disorder. Journal of Clinical Psychiatry. 2013;74(06):e541. doi: 10.4088/JCP.12r08225. e550. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2:125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Wild J., Gur R.C. Verbal memory and treatment response in post-traumatic stress disorder. British Journal of Psychiatry. 2008;193:254–255. doi: 10.1192/bjp.bp.107.045922. [DOI] [PubMed] [Google Scholar]
- Woon F.L., Farrer T.J., Braman C.R., Mabey J.K., Hedges D.W. A meta-analysis of the relationship between symptom severity of Posttraumatic Stress Disorder and executive function. Cogn Neuropsychiatry. 2017;22:1–16. doi: 10.1080/13546805.2016.1255603. [DOI] [PubMed] [Google Scholar]
- Zotev, V., Phillips, R., Misaki, M., Wong, C.K., Wurfel, B., Meyer, M., Krueger, F., Feldner, M., Bodurka, J., 2016. Evaluation of real-time fMRI neurofeedback training effects in combat-related PTSD using simultaneous EEG. p. 5. [DOI] [PMC free article] [PubMed]
- Zotev V., Phillips R., Misaki M., Wong C.K., Wurfel B.E., Krueger F., Feldner M., Bodurka J. Real-time fMRI neurofeedback training of the amygdala activity with simultaneous EEG in veterans with combat-related PTSD. Neuroimage Clin. 2018;19:106–121. doi: 10.1016/j.nicl.2018.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that has been used is confidential.