Abstract
Introduction
Exercise-induced laryngeal obstruction (EILO) is a common cause of exertional breathing problems in young adults. Current management generally consists of breathing advice, speech therapy, inspiratory muscle training or supraglottoplasty in highly motivated subjects with supraglottic collapse. Inhaled ipratropium bromide (IB) is a muscarinic receptor antagonist used to treat asthma that is suggested in a few reports to improve EILO symptoms. The aim of the present study was to investigate effects of inhaled IB in EILO diagnosed by continuous laryngoscopy exercise (CLE) test and classified by CLE scores.
Methods
A randomised crossover trial was conducted at Haukeland University Hospital, Bergen, Norway, enrolling participants diagnosed with EILO defined by characteristic symptoms and CLE score ≥3 (range 0–12). Two consecutive CLE tests were performed within 2 weeks, one test with and one test without prior administration of inhaled IB in a randomised order. Main outcomes were the CLE score, dyspnoea measured using a modified BORG scale (range 0–10) and cardiopulmonary exercise data provided by the CLE test.
Results
20 participants (14 females) aged 12–25 years participated, and all ran to exhaustion on both tests. Mean CLE score, BORG score and peak oxygen consumption were similar in tests performed with and without IB; mean differences (95% confidence interval) were 0.08 (−0.28–0.43), 0.35 (−0.29–0.99) and −0.4 (−1.9–1.1) mL·kg−1·min−1, respectively.
Conclusion
Inhaled IB did not improve CLE score, dyspnoea or exercise capacity in subjects with EILO. The study does not support the use of inhaled IB to treat EILO.
Short abstract
Use of inhaled ipratropium bromide to improve exercise-induced laryngeal obstruction cannot be recommended https://bit.ly/3VVrQ1L
Introduction
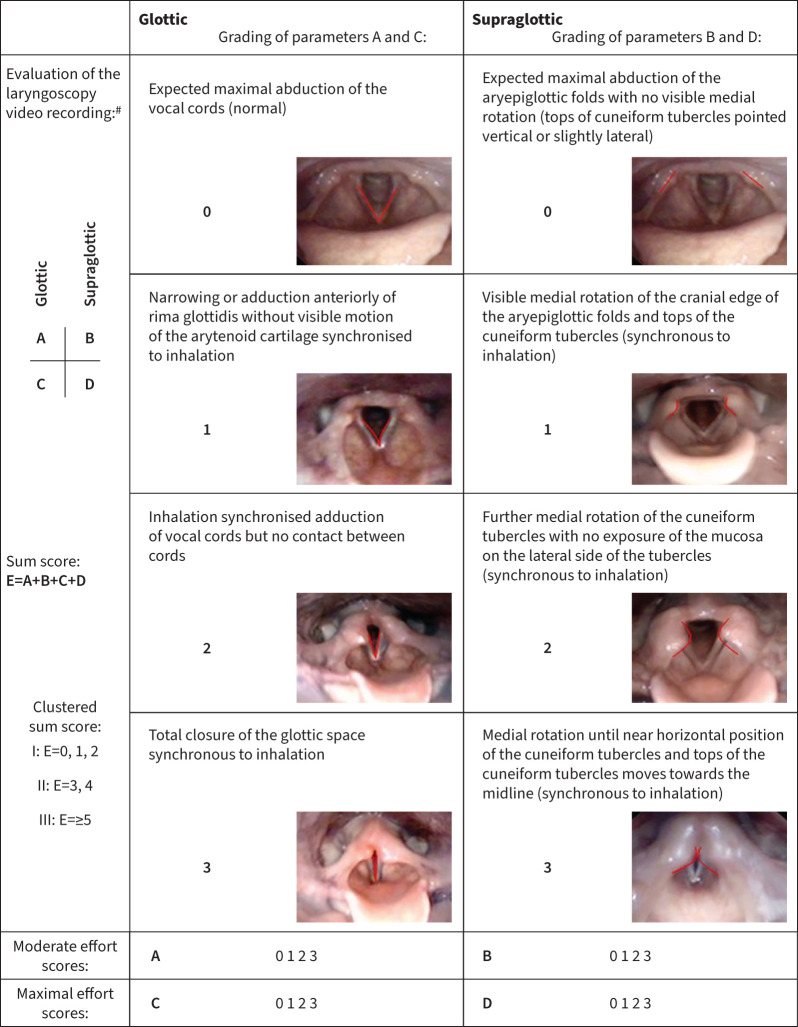
Exercise-induced laryngeal obstruction (EILO) is caused by paradoxical dynamic adduction of laryngeal structures during exercise, resulting in dyspnoea and chest tightness, with or without stridor [1, 2]. Participants with EILO are diverse, ranging from sedentary youngsters affected in daily activities, to top athletes limited during competitions [3]. The condition has been reported to affect 5–7% of otherwise healthy young people [4, 5]. Symptoms typically peak towards the end of exercise or immediately after, with increasing ventilation paralleled by increasing respiratory distress [6]. Symptoms of EILO can be misinterpreted as asthma/exercise-induced bronchoconstriction (EIB), a situation that may lead to mismanagement of both conditions [7–9]. Asthma/EIB and EILO can also coexist, further challenging treatment decisions [10, 11]. EILO is diagnosed by continuously visualising the larynx with a flexible laryngoscope during maximal exercise (continuous laryngoscopy exercise (CLE) test) [12]. Changes in the size of the laryngeal inlet during exercise is visually evaluated and graded (CLE score), where higher scores correlate to more severe adduction (figure 1) [2, 6, 13].
FIGURE 1.
Continuous laryngoscopy exercise (CLE) scoring system. Reproduced and modified from [13] with permission. #: the scores at each level, glottic (A and C) and supraglottic (B and D), were given at moderate (A and B) and maximal effort (C and D), and the sum of all four scores constitutes the sum score (E) for each test/subject.
Treatment of EILO is mostly based on empirical data and case reports [2, 6, 14, 15], and we lack evidence-based data from randomised controlled studies on treatment modalities in regular use. Current treatments generally focus on making patients aware of their inappropriate breathing patterns, and to provide structured breathing advice, biofeedback, speech therapy or inspiratory muscle training [9, 15–20]. Questions have been raised on whether pharmacological treatment can improve EILO, and anecdotal reports have suggested that inhaled ipratropium bromide (IB) may be beneficial [21, 22]. Our hospital functions as a third line national reference centre for EILO, and we have experienced that IB is relatively often prescribed for patients with suspected EILO. IB is a muscarinic receptor antagonist, used to treat bronchoconstriction in asthma/EIB by inhibiting acetylcholine release from efferent parasympathetic nerves travelling in the vagal nerve [23–25]. If effective also in EILO, this would simplify treatment in patients with coexisting EIB and EILO, which is not uncommon. The theoretical basis for using inhaled IB to treat EILO is weak, resting on studies reporting effects on the positioning of the vocal folds from vagal nerve electrical stimulation [26, 27]. Although the mechanisms behind these observations are unclear, one may speculate if local application of IB may prevent laryngeal adduction by blocking vagal innervation [28]. In this randomised crossover trial in patients with confirmed EILO, we aimed to examine if inhaled IB reduced laryngeal obstruction during exercise, reduced perceived dyspnoea or increased maximal exercise capacity.
Methods
This descriptive randomised crossover trial included 20 participants (males and females) enrolled from the outpatient clinic at Haukeland University Hospital in Bergen, Norway. Inclusion criteria were age 12–25 years, being otherwise healthy, complaints of inspiratory breathing difficulties during exercise and verified EILO with CLE score ≥3 (range 0–12, see figure 1) [13]. Participants were excluded if they had a previous history of IB intolerance, were pregnant or were unable to perform two additional CLE tests due to lack of motivation and/or perceived discomfort at the diagnostic test. Asthma (previous or current) was an exclusion criterion, defined by the medical history provided by the patient and by the judgement of the paediatrician who attended the diagnostic CLE test. If in doubt, patients were not enrolled.
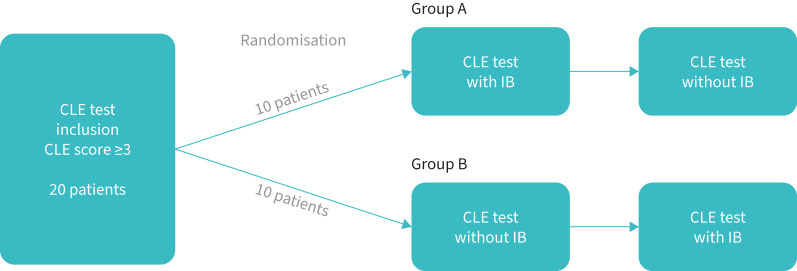
All participants performed three CLE tests in total, one diagnostic test followed by two additional tests to investigate the effects of IB. The two latter CLE tests were performed with an in-between washout period of at least 1 day and a maximum of 2 weeks. A study nurse otherwise not involved in the testing randomised participants 1:1 by 20 closed coloured envelopes, so that half of them (Group A) were pre-treated with inhaled IB before the CLE test on test Day 1 and no medication on test Day 2, whereas the order was reversed in the other half (Group B) (figure 2).
FIGURE 2.
Study design and randomisation of ipratropium bromide (IB). CLE: continuous laryngoscopy exercise.
The first test was performed within 1 month after diagnosis.
Cardiopulmonary exercise test
Peak exercise capacity defined as peak oxygen consumption (peak V′O2) was determined using an incremental treadmill (Woodway PPS 55 Med; Woodway, Weil am Rhein, Germany) exercise test according to a modified Bruce protocol [29] using a Vyntus CPX unit powered by SentrySuite software (Vyaire Medical GmbH, Hoechberg, Germany). Speed and elevation increased every minute, starting from a slow walking phase, until the participants reached their maximum intensity level. The test was stopped when the participant indicated severe exhaustion or was unable to continue due to EILO symptoms, preferably supported by a respiratory exchange ratio (RER) exceeding 1.05 or heart rate exceeding 95% of maximally predicted [30]. Airflow and gas exchange parameters was measured breath-by-breath through a modified face mask (Hans Rudolph Inc., Kansas City, MO, USA) and averaged over 10 s. The cardiopulmonary exercise test (CPET) parameters recorded at maximal exhaustion are listed in table 1.
TABLE 1.
Continuous laryngoscopy exercise (CLE) score, BORG score and cardiopulmonary exercise test (CPET) variables obtained at maximum intensity during the two CLE tests (n=20)
|
CLE test without IB,
mean±sd |
CLE test with IB,
mean±sd |
Mean difference:
without IB – with IB (95% CI) |
|
| CLE score# | 3.98±1.18 | 3.90±1.33 | 0.08 (−0.28–0.43) |
| BORG score ¶ | 7.85±1.93 | 7.50±2.09 | 0.35 (−0.29–0.99) |
| Distance m | 833.4±175.4 | 825.3±185.4 | −8.1 (−43–27.3) |
| Peak V′O2 mL·min−1·kg−1 | 46.1±6.73 | 45.7±7.77 | −0.4 (−1.9–1.1) |
| HR beats·min−1 | 187.1±9.1 | 185.9±7.72 | 1.2 (−1.6–3.9) |
| BF breaths·min−1 | 50.4±7.9 | 49.8±9.8 | 0.6 (−2.4–3.6) |
| V′E L·min−1 | 104.4±24.3 | 100.6±27.4 | 3.8 (−1.6–9.1) |
| Tidal volume L | 2.11±0.49 | 2.05±0.46 | 0.06 (−0.02–0.15) |
| t I /t tot | 49.7±2.05 | 50.1±2.06 | −0.4 (−1.36–0.66) |
All values other than CLE score are obtained at peak intensity and based on a linear model. IB: ipratropium bromide; peak V′O2: peak oxygen consumption; HR: heart rate; BF: breathing frequency; V′E: minute ventilation; tI: inspiratory time of breath (in seconds); ttot: total time of one breath (in seconds). #: CLE score represents the mean CLE score of both moderate and maximum intensity during the test, range 0–12. ¶: BORG score is used for self-assessed symptoms of dyspnoea during the test, range 0–10.
CLE test
Continuous laryngoscopy was performed during the CPET using a transnasal flexible video-laryngoscopy (ENF TYPE V2, video processor CV-170; OLYMPUS, Tokyo, Japan) as described previously [31]. Laryngeal obstruction during treadmill running was assessed and rated according to a modified version of the classification described by Maat et al. (Figure 1) [13]. Laryngeal obstruction on either glottic or supraglottic level at moderate and maximum exercise was rated from 0 to 3 at each level, giving a score ranging from 0 to 12 (CLE score). CLE test recordings were evaluated blinded in retrospect by two experienced reviewers (O.D. Røksund and H.H. Clemm), and disagreements were resolved by consensus.
BORG dyspnoea scale
We used a modified version of the BORG dyspnoea scale (range 0–10) to evaluate the participants' perceived dyspnoea. Participants were familiarised with the BORG scale before the CLE test and were asked to rate their own perceived dyspnoea every minute during the CLE test. The BORG dyspnoea scale is considered a valid and reliable assessment tool for dyspnoea [32].
Ipratropium bromide
IB (muscarinic receptor antagonist) aerosol was administered through a plastic spacer (Optichamber®; Philips Medical Systems Nederland B.V., Best, The Netherlands). A similar dose as used for asthma (40 µg) was given 20–30 min before the CLE test.
Spirometry
Spirometry was performed three times each test day; i.e., before and 20 min after the administration of IB but before the CLE test, and after the CLE test. Vyntus® PNEUMO spirometer (Vyaire Medical GmbH, Hoechberg, Germany) was used to perform spirometry according to guidelines [33]. Forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1) and FEV1/FVC were recorded.
Statistical methods
The main outcome was the difference in CLE score between tests performed with and without administration of inhaled IB prior to the CLE test. We have previously argued that a mean group difference exceeding 0.5 in glottic or supraglottic CLE score observed at moderate or peak exercise is of clinical interest, which transforms into a mean difference of 2 on the scale used in this study, that encompasses all these four elements. The secondary outcomes were the difference in BORG scores, peak V′O2 (mL·min−1·kg−1) and the completed distance on the treadmill.
Further, we report the number of participants with better, worse or identical CLE or BORG scores when IB was administered prior to the CLE test. To estimate the effect of IB on CLE and BORG scores, mixed-effects models were fitted with an intervention and a period (time) effect. Participant was included as a random effect. As the scores are ordinal and with a narrow range, our main and most robust analyses were based on ordinal (proportional odds cumulative logit) models. However, for ease of interpretation, the mean scores from a similar linear model (roughly equivalent to a paired t-test, but taking into account any period effect) are also reported. For other variables, standard deviations and 95% CI for the difference of means are reported, in addition to paired t-tests, i.e., ignoring any period effect (which was balanced across treatments).
All analyses were performed using SPSS version 26, or R version 4.0.4 [34] with the “ordinal” package (version 2019.12–10) and the “lme4” package (version 1.1-27.1).
Ethics
The study was approved by the regional ethical committee of Western Norway (REK 2014-01885) and by the Norwegian Medicines Agency (EudraCTnr 2014-000302-34). Informed written consent was obtained from each participant or from both parents/guardians if the participant was younger than 16 years. The study complied with the Declaration of Helsinki, the International Conference on Harmonization/Good Clinical Practice and applicable regulatory requirements. Monitoring was provided by Department of Research and Development, Haukeland University Hospital, Bergen, Norway.
Results
20 otherwise healthy participants, 14 females and six males with verified EILO, aged 12–25 years, were included between 2016 and 2020. All participants presented with complaints of inspiratory problems during exercise at inclusion. Symptoms were verified as EILO by the baseline diagnostic CLE test, with a CLE score of at least 3 (range 3–7). One patient reported exercising only 1 h per week; the rest exercised 4 to >7 h per week.
Spirometry
As evident from table 2, there were no differences regarding data on lung function obtained at the different test days, and there was no reversibility to inhaled IB on a group level. On an individual level, four participants had a clinically relevant improvement of FEV1 (>5%) after IB administration [35].
TABLE 2.
Spirometry results sorted by visit (n=20)
| Visit | FEV1 baseline# |
FEV1
after IB |
FEV1
after CLE |
FVC baseline |
FVC
after IB |
FVC
after CLE |
| Day of EILO diagnosis | 3.56±0.64 | - | - | 4.06±0.76 | - | - |
| Day without IB | 3.53±0.61 | - | 3.52±0.62 | 4.07±0.75 | - | 3.94±0.78 |
| Day with IB | 3.58±0.62 | 3.64±0.65 | 3.65±0.70 | 4.12±0.75 | 4.07±0.79 | 4.02±0.78 |
Data are presented as mean±sd. CLE: continuous laryngoscopy exercise; IB: ipratropium bromide; EILO: exercise-induced laryngeal obstruction. All FEV1 values are litres during first second of forced expiration. All FVC are total forced vital capacity. #: at the day of inclusion, only baseline spirometry was performed; an additional spirometry was performed 20 min after receiving IB before the CLE test.
CLE and BORG score
Tests performed with IB versus without IB were quite similar regarding CLE scores, BORG scores and CPET data (table 1). Mean CLE score was 3.98 without IB and 3.90 with IB (mean difference 0.08; 95% CI: −0.28–0.43; p=0.69). The corresponding odds ratio for the CLE score using an ordinal model was 0.64 (95% CI: 0.16–2.54, p=0.52). This OR represents a relatively small change in the numerical score (a small difference in mean scores of 0.5 correspond to an OR of ∼0.35). The mean BORG scores were 7.85 without IB and 7.50 with IB (mean difference 0.35; 95% CI: −0.29–0.99; p=0.30).
When comparing individual CLE tests with versus without IB, four (20%) participants had less laryngeal obstruction with IB, of whom three had a 1 point lower CLE score, and one had a 2 point lower score. Three (15%) participants had higher CLE score with IB (0.5, 1 and 2 points, respectively), whereas 13 (65%) had identical scores. Regarding BORG scores, seven (35%) participants had lower, five (25%) had higher and eight (40%) had identical scores. The differences in BORG scores were generally small, typically 1 or 2, except for one participant who had a score of 4 with IB compared to 9 without IB.
Reproducibility
As there is a lack of data on reproducibility of CLE scores in EILO, and as IB did not seem to influence any of the outcome measures (table 3), reproducibility was estimated by comparing the two CLE tests performed with versus without IB. The pairwise mean difference between the CLE scores obtained at maximum effort was negligible. For 90% of the participants, the absolute difference was ≤1, and for 100% of the participants, it was ≤2.
TABLE 3.
CLE scores at maximum effort, including the diagnostic baseline assessment (n=20)
| Paired sample t-test | Mean±sd | Mean difference±sd | 95% CI | p-value |
|
CLE score inclusion versus
CLE score without IB |
3.95±1.28 | −0.03±0.95 | −0.5–0.4 | 0.91 |
| 3.98±1.18 | ||||
|
CLE score inclusion versus
CLE score with IB |
3.95±1.28 | 0.05±0.94 | −0.4–0.5 | 0.82 |
| 3.90±1.33 | ||||
|
CLE score without IB versus
CLE score with IB |
3.98±1.18 | 0.08±0.80 | −0.3–0.4 | 0.68 |
| 3.90±1.33 |
This table shows the mean CLE score. Excluding the individual being reversible on IB only made the differences smaller. CLE: continuous laryngoscopy exercise test; IB: ipratropium bromide.
Reversibility for inhaled IB versus changes in CLE scores
Although asthma was an exclusion criterion, one participant was marginally reversible in FEV1 for inhaled IB on the test day, with FEV1 increasing from 3.62 L to 4.01 L (11%), and if compared to FEV1 obtained at diagnosis 4 days before (3.52 L), the difference was 14%, suggesting a clinically relevant bronchial lability for inhaled IB [36]. In this participant, the CLE score changed from 5 on the day with inhaled IB to 4 on the day with no IB. Removing this participant from the statistical analyses did not change the mean CLE score. However, for the BORG score, the difference between tests performed with versus without inhaled IB was reduced from 0.35 to 0.12, with a narrower 95% CI (−0.36–0.59).
There was no clear association between reversibility for IB versus changes in CLE scores. Four participants had lower CLE score after IB, of whom two had >5% improvement in FEV1, which has been considered a clinically relevant difference [35]. Two additional participants had >5% improvement in FEV1 after IB, of whom one had similar CLE scores, and one had higher (worse) CLE score after IB.
Discussion
To our knowledge, this is the first randomised study to evaluate the effect of IB as treatment for EILO where all participants were diagnosed and examined with a CLE test. We did not reveal any influence from IB on the grade of the obstruction at the laryngeal inlet (CLE scores), self-assessed symptoms of dyspnoea during the test (BORG score) or CPET performance.
Effects of IB in EILO
The finding that IB does not improve EILO is contrary to previous reports. Doshi et al. [22] reported that six of 29 participants did not develop EILO when pre-treated with IB. In a case report by Weinberger and Doshi [28], a participant with inspiratory stridor was shown to have vocal fold adduction on laryngoscopy performed immediately after exercise. Suspecting a vagal mechanism, IB was administered, and the participant was relieved from the symptoms. However, there was no information if laryngoscopy had been performed after IB was administered, nor information on lung function changes. The weakness of these reports is the lack of testing with versus without IB treatment and lack of validated outcome measures, like CLE scores and BORG scores.
EILO and asthma
From previous studies, we know that EILO and asthma can coexist [4, 11, 37]. In our study, one participant had a tendency for bronchial lability to inhaled IB. This participant had a better BORG score but a worse CLE score when pre-treated with IB. We speculate that IB, by relieving an ongoing bronchoconstriction, has led to these improvements. Based on this case, one may further speculate whether previous reports of effects from inhaled IB on EILO may be due to asthma comorbidity in athletes, with IB functioning more as a bronchodilator than having a direct effect on the obstructed larynx. This underlines the importance of proper testing of both asthma and EILO when a patient suffers from breathing problems during exercise.
Reproducibility
Each participant performed a total of three CLE tests, and their CLE scores remained similar throughout the three tests. The CLE score is ordinal from 0 to 12. The number is meant to be an integer, but since the degree of closure in the laryngeal inlet is continuous, it can be difficult for the physician to differentiate between 0 and 1, 1 and 2, or 2 and 3 on each level [38]. Although following defined criteria (figure 1), the CLE score inevitably must involve some degree of subjectivity, as pointed out by Walsted et al. [39]. This study supports what Maat et al. [13] have reported previously, that CLE scores are reproducible measures that can be used confidently by experienced assessors to grade EILO. As time had elapsed between the diagnostic CLE test and the two tests performed afterwards (table 3), this suggests that the laryngeal response patterns in these participants were correspondingly stable.
Strengths and limitations
The random crossover design, where participants performed test with versus without pre-treatment with inhaled IB, minimised the risk of confounding factors. Further, participants were their own controls which reduced the effects of potential inter-individual differences [40]. Evaluations based on CLE tests provided verifiable outcome data based on a direct assessment of the laryngeal responses. The results of this study support previous findings, indicating that the CLE scores can reliably be used to grade EILO [13]. The protocol used for treadmill exercise was computerised and identical in all participants at all tests, as was the use of the BORG score to evaluate symptoms every minute. The time gap between the two tests was kept relatively short, reducing the risk of influence from external factors. By measuring CPET data, we could confirm that both tests were completed with similar intensity, and that the scoring was conducted at similar levels of exertion (table 1).
The participants were all included from the same university hospital, which is a nationwide reference centre for EILO. This could introduce a referral bias, as participants with only minor breathing problems will not be seen at this clinic. However, as the participants served as their own controls, and as the study was designed to include participants with a defined degree of EILO, the results are representative for this particular EILO population. Asthma was not formally tested within the frame of this particular study. Precautions were taken to avoid enrolling patients with asthma based on the referral letter, medical story and the judgements by the attending paediatricians highly experienced with young people with breathing difficulties.
We made calculations on reproducibility based on two tests that were performed under different conditions, i.e., performed with versus without pre-treatment with inhaled IB, which obviously was not an optimal situation. However, since doing this will in general overestimate the magnitude of the differences one would have obtained under identical conditions, the results do indicate that agreement between two CLE tests performed within a time-frame of maximum 2 weeks is likely to be ≤2 on a scale with the range 0–12.
Power calculations were not performed in this explorative study, because essential parameters such as effect size and distributions were unknown. A placebo inhaler was not used in the group receiving no pre-treatment, which was a weakness. However, we reasoned that a potential placebo effect, induced simply by the use of an inhaler, was likely to be positive, i.e. that this most likely would have led to better (not worse) outcomes after IB pre-treatment when compared to no pre-treatment. We therefore made an “escalation plan” prior to this study, where we planned to introduce a placebo inhaler in a controlled next-level study, if any effect from IB was discovered. Finally, we did not attempt to distinguish between sub-categories of EILO, nor did we particularly penetrate the mental status of the participants, as the relevance of such issues in EILO is debated and as our patient population in general reflects the background population [41]. Although further studies with a larger sample size may be helpful to conclude, our study suggests that IB has no clinical effects on EILO.
Conclusion
This randomised crossover trial gave no indications of clinical effects from inhaled IB in participants with EILO on laryngeal inlet obstruction, self-perceived dyspnoea or exercise capacity. Therefore, we would not recommend the use of IB in EILO, but encourage performing objective tests for both asthma and EILO in individuals with exercise-related breathing problems.
Footnotes
Provenance: Submitted article, peer reviewed.
This study is registered at https://eudract.ema.europa.eu/ with identifier number 2014-000302-34 and at https://rekportalen.no/ with identifier number 2014-01885. In accordance with the approvals granted for this study by The Regional Committee on Medical Research Ethics and The Norwegian Data Inspectorate, the data files are stored securely and in accordance with the Norwegian Law of Privacy Protection. The data file cannot be made publicly available as this might compromise the respondents' privacy. A subset of the data file with anonymised data can be made available to interested researchers upon reasonable request to Hege Clemm (hsyh@helse-bergen.no), providing Norwegian privacy legislation and GDPR are respected, and that permission is granted from The Norwegian Data Inspectorate and the data protection officer at Haukeland University Hospital.
Author contributions: P. Muralitharan and P. Carlsen organised the data, carried out the analyses, drafted the initial manuscript and revised the manuscript. I. Delestre-Levai collected data, carried out initial analyses and critically reviewed the manuscript for important intellectual content. M. Vollsæter and M. Hilland conceptualised and designed the study, and critically reviewed the manuscript for important intellectual content. K.O. Hufthammer gave advice on the analysis of data, performed the statistical analyses on the main outcomes, participated in the interpretation of the data and has critically reviewed the manuscript for important intellectual content. M. Engan gave advice on the analysis of data, performed statistical analyses, participated in the interpretation of the data and has critically reviewed the manuscript for important intellectual content. D. Røksund conceptualised and designed the study, collected data and critically reviewed the manuscript for important intellectual content. T. Halvorsen conceptualised and designed the study, provided funding, supervised data collection and critically reviewed the study manuscript for important intellectual content. H.H. Clemm has conceptualised and designed the study, designed the data collection instruments, coordinated and collected data, and critically reviewed the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work.
Conflict of interest: The authors have no conflicts of interest to disclose.
Support statement: Major funding institutions: Western Norway Regional Health Authority, Haukeland University Hospital and University of Bergen, and Medicines for Children Network, Norway. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Christensen PM, Heimdal JH, Christopher KL, et al. ERS/ELS/ACCP 2013 international consensus conference nomenclature on inducible laryngeal obstructions. Eur Respir Rev 2015; 24: 445–450. doi: 10.1183/16000617.00006513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halvorsen T, Walsted ES, Bucca C, et al. Inducible laryngeal obstruction: an official joint European Respiratory Society and European Laryngological Society statement. Eur Respir J 2017; 50: 1602221. doi: 10.1183/13993003.02221-2016 [DOI] [PubMed] [Google Scholar]
- 3.Mirza KK, Walsted ES, Backer V. Ergospirometry with concurrent fibre optic laryngoscopy: a randomised crossover study. Eur Clin Respir J 2017; 4: 1399033. doi: 10.1080/20018525.2017.1399033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christensen PM, Thomsen SF, Rasmussen N, et al. Exercise-induced laryngeal obstructions: prevalence and symptoms in the general public. Eur Arch Otorhinolaryngol 2011; 268: 1313–1319. doi: 10.1007/s00405-011-1612-0 [DOI] [PubMed] [Google Scholar]
- 5.Johansson H, Norlander K, Berglund L, et al. Prevalence of exercise-induced bronchoconstriction and exercise-induced laryngeal obstruction in a general adolescent population. Thorax 2015; 70: 57–63. doi: 10.1136/thoraxjnl-2014-205738 [DOI] [PubMed] [Google Scholar]
- 6.Liyanagedera S, McLeod R, Elhassan HA. Exercise induced laryngeal obstruction: a review of diagnosis and management. Eur Arch Otorhinolaryngol 2017; 274: 1781–1789. doi: 10.1007/s00405-016-4338-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rundell KW, Spiering BA. Inspiratory stridor in elite athletes. Chest 2003; 123: 468–474. doi: 10.1378/chest.123.2.468 [DOI] [PubMed] [Google Scholar]
- 8.Shembel AC, Sandage MJ, Abbott KV. Episodic laryngeal breathing disorders: literature review and proposal of preliminary theoretical framework. J Voice 2017; 31: 125.e7–125.e16. doi: 10.1016/j.jvoice.2015.11.027 [DOI] [PubMed] [Google Scholar]
- 9.Buchvald F, Phillipsen LD, Hjuler T, et al. Exercise-induced inspiratory symptoms in school children. Pediatr Pulmonol 2016; 51: 1200–1205. doi: 10.1002/ppul.23530 [DOI] [PubMed] [Google Scholar]
- 10.Clemm HH, Olin JT, McIntosh C, et al. Exercise-induced laryngeal obstruction (EILO) in athletes: a narrative review by a subgroup of the IOC Consensus on ‘acute respiratory illness in the athlete’. Br J Sports Med 2022; 56: 622–629. doi: 10.1136/bjsports-2021-104704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hammer J, Halvorsen I, Vollsaeter T, et al. Conundrums in the breathless athlete; exercise-induced laryngeal obstruction or asthma? Scand J Med Sci Sports 2022; 32: 1041–1049. doi: 10.1111/sms.14137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heimdal JH, Roksund OD, Halvorsen T, et al. Continuous laryngoscopy exercise test: a method for visualizing laryngeal dysfunction during exercise. Laryngoscope 2006; 116: 52–57. doi: 10.1097/01.mlg.0000184528.16229.ba [DOI] [PubMed] [Google Scholar]
- 13.Maat RC, Roksund OD, Halvorsen T, et al. Audiovisual assessment of exercise-induced laryngeal obstruction: reliability and validity of observations. Eur Arch Otorhinolaryngol 2009; 266: 1929–1936. doi: 10.1007/s00405-009-1030-8 [DOI] [PubMed] [Google Scholar]
- 14.Haines J, Hull JH, Fowler SJ. Clinical presentation, assessment, and management of inducible laryngeal obstruction. Curr Opin Otolaryngol Head Neck Surg 2018; 26: 174–179. doi: 10.1097/MOO.0000000000000452 [DOI] [PubMed] [Google Scholar]
- 15.Roksund OD, Heimdal JH, Clemm H, et al. Exercise inducible laryngeal obstruction: diagnostics and management. Paediatr Respir Rev 2017; 21: 86–94. doi: 10.1016/j.prrv.2016.07.003 [DOI] [PubMed] [Google Scholar]
- 16.Newsham KR, Klaben BK, Miller VJ, et al. Paradoxical vocal-cord dysfunction: management in athletes. J Athl Train 2002; 37: 325–328. [PMC free article] [PubMed] [Google Scholar]
- 17.Shaffer M, Litts JK, Nauman E, et al. Speech-language pathology as a primary treatment for exercise-induced laryngeal obstruction. Immunol Allergy Clin North Am 2018; 38: 293–302. doi: 10.1016/j.iac.2018.01.003 [DOI] [PubMed] [Google Scholar]
- 18.Bikov A, Hull JH, Kunos L. Exhaled breath analysis, a simple tool to study the pathophysiology of obstructive sleep apnoea. Sleep Med Rev 2016; 27: 1–8. doi: 10.1016/j.smrv.2015.07.005 [DOI] [PubMed] [Google Scholar]
- 19.Rameau A, Foltz RS, Wagner K, et al. Multidisciplinary approach to vocal cord dysfunction diagnosis and treatment in one session: a single institutional outcome study. Int J Pediatr Otorhinolaryngol 2012; 76: 31–35. doi: 10.1016/j.ijporl.2011.09.017 [DOI] [PubMed] [Google Scholar]
- 20.Johnston KL, Bradford H, Hodges H, et al. The Olin EILOBI breathing techniques: description and initial case series of novel respiratory retraining strategies for athletes with exercise-induced laryngeal obstruction. J Voice 2018; 32: 698–704. doi: 10.1016/j.jvoice.2017.08.020 [DOI] [PubMed] [Google Scholar]
- 21.Weinberger M, Abu-Hasan M. Pseudo-asthma: when cough, wheezing, and dyspnea are not asthma. Pediatrics 2007; 120: 855–864. doi: 10.1542/peds.2007-0078 [DOI] [PubMed] [Google Scholar]
- 22.Doshi DR, Weinberger M. Long-term outcome of vocal cord dysfunction. Ann Allergy Asthma Immunol 2006; 96: 794–799. doi: 10.1016/S1081-1206(10)61341-5 [DOI] [PubMed] [Google Scholar]
- 23.Chapman KR. Anticholinergic bronchodilators for adult obstructive airways disease. Am J Med 1991; 91: 4, Suppl. 1, S13–S16. doi: 10.1016/0002-9343(91)90256-W [DOI] [PubMed] [Google Scholar]
- 24.Hahn HL. Role of the parasympathetic nervous system and of cholinergic mechanisms in bronchial hyperreactivity. Bull Eur Physiopathol Respir 1986; 22: Suppl. 7, 112–142. [PubMed] [Google Scholar]
- 25.Lundy DS, Casiano RR, Landy HJ, et al. Effects of vagal nerve stimulation on laryngeal function. J Voice 1993; 7: 359–364. doi: 10.1016/S0892-1997(05)80259-0 [DOI] [PubMed] [Google Scholar]
- 26.Vassilyadi M, Strawsburg RH. Delayed onset of vocal cord paralysis after explantation of a vagus nerve stimulator in a child. Childs Nerv Syst 2003; 19: 261–263. doi: 10.1007/s00381-003-0722-4 [DOI] [PubMed] [Google Scholar]
- 27.Zalvan C, Sulica L, Wolf S, et al. Laryngopharyngeal dysfunction from the implant vagal nerve stimulator. Laryngoscope 2003; 113: 221–225. doi: 10.1097/00005537-200302000-00005 [DOI] [PubMed] [Google Scholar]
- 28.Weinberger M, Doshi D. Vocal cord dysfunction: a functional cause of respiratory distress. Breathe (Sheff) 2017; 13: 15–21. doi: 10.1183/20734735.019316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cumming GR, Everatt D, Hastman L. Bruce treadmill test in children: normal values in a clinic population. Am J Cardiol 1978; 41: 69–75. doi: 10.1016/0002-9149(78)90134-0 [DOI] [PubMed] [Google Scholar]
- 30.Nes BM, Janszky I, Wisloff U, et al. Age-predicted maximal heart rate in healthy subjects: The HUNT fitness study. Scand J Med Sci Sports 2013; 23: 697–704. doi: 10.1111/j.1600-0838.2012.01445.x [DOI] [PubMed] [Google Scholar]
- 31.Engan M, Jansrud Hammer I, Bekken M, et al. Reliability of maximum oxygen uptake in cardiopulmonary exercise testing with continuous laryngoscopy. ERJ Open Res 2021; 7: 00825–2020. doi: 10.1183/23120541.00825-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kendrick KR, Baxi SC, Smith RM. Usefulness of the modified 0–10 Borg scale in assessing the degree of dyspnea in patients with COPD and asthma. J Emerg Nurs 2000; 26: 216–222. doi: 10.1016/S0099-1767(00)90093-X [DOI] [PubMed] [Google Scholar]
- 33.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005; 26: 319–338. doi: 10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- 34.R core team . R: A language and environment for statistical computing. 2021. https://www.r-project.org/
- 35.Jones PW, Beeh KM, Chapman KR, et al. Minimal clinically important differences in pharmacological trials. Am J Respir Crit Care Med 2014; 189: 250–255. doi: 10.1164/rccm.201310-1863PP [DOI] [PubMed] [Google Scholar]
- 36.Reddel HK, Bacharier LB, Bateman ED, et al. Global Initiative for Asthma: Pocket guide for asthma management and prevention. 2021, pp. 8–12. Available from: http://ginasthma.org/ [Google Scholar]
- 37.Nielsen EW, Hull JH, Backer V. High prevalence of exercise-induced laryngeal obstruction in athletes. Med Sci Sports Exerc 2013; 45: 2030–2035. doi: 10.1249/MSS.0b013e318298b19a [DOI] [PubMed] [Google Scholar]
- 38.Norlander K, Christensen PM, Maat RC, et al. Comparison between two assessment methods for exercise-induced laryngeal obstructions. Eur Arch Otorhinolaryngol 2016; 273: 425–430. doi: 10.1007/s00405-015-3758-7 [DOI] [PubMed] [Google Scholar]
- 39.Walsted ES, Hull JH, Hvedstrup J, et al. Validity and reliability of grade scoring in the diagnosis of exercise-induced laryngeal obstruction. ERJ Open Res 2017; 3: 00070–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Armitage P, Hills M. The two-period crossover trial. Br J Clin Pharmacol 1982; 31: 119–131. [Google Scholar]
- 41.Benestad MR, Drageset J, Clemm H, et al. Self-reported health in adolescents with exercise-induced laryngeal obstruction; a cross-sectional study. Front Pediatr 2021; 9: 617759. doi: 10.3389/fped.2021.617759 [DOI] [PMC free article] [PubMed] [Google Scholar]