Abstract
Introduction:
The goal of this paper was to determine the impact of calcium hydroxide (CH) and nano-calcium hydroxide (NCH) on the push-out bond strength of an epoxy resin-based sealer.
Materials and Methods:
A total of 48 mandibular premolars were decoronated in vitro and instrumented by a ProTaper rotary system up to F4. The specimens then were randomly allocated into 3 groups (n=16). The two intervention groups were treated with either CH or NCH and one control group which did not receive any intracanal medicament. After one week of medicament placement, the specimens were irrigated by 10 mL 17% EDTA, followed by 10 mL 2.5% NaOCl and an ultimate flushing by 5 mL sterile saline. The samples were obturated using AH-Plus Jet sealer and gutta-percha by lateral condensation technique. Push-out test was done by a universal test machine to evaluate the bond strength among the sealer and the root canal dentin. Repeated measurement analysis followed by Tukey’s HSD and Bonferroni post-hoc tests were used for data analysis.
Results:
The control group showed a higher push-out bond strength in comparison to the specimens in the CH and NCH groups (P<0.05). In spite of the greater push-out bond strength of the samples treated with NCH in comparison to those in CH group, no statistically notable difference was found among them (P>0.05). Additionally, irrespective of the kind of medicament, the bonding at the apical level of the root canal was stronger compared to the coronal third (P<0.001).
Conclusion:
According to the present in vitro study, application of CH and NCH resulted in a significant reduction of the bond strength between the epoxy resin-based sealer and dentinal walls. Although, application of these medicaments resulted in reduction of bonding of epoxy resin-based sealers root canal dentin significantly.
Key Words: Calcium Hydroxide, Epoxy Resin-based Root Canal Sealer, Nanoparticles, Root Canal Therapy
Introduction
The objective of root canal therapy is to wipe out bacterial pathogens colonized in the root canals and to hinder bacterial regrowth through the chemo-mechanical treatment of the root canals [1, 2]. Additionally, intracanal medication has been greatly recommended to optimize root canal debridement [3]. Calcium hydroxide (CH) stands in the most widely used intracanal medicaments owing to its antimicrobial efficacy against most root canal bacteria, biocompatibility, tissue-dissolving capability, potency to inhibit inflammation and osteoclastic activity, and ability to induce mineralized tissue formation and lipopolysaccharides inactivation [4]. Despite the aforementioned benefits of CH, it cannot inhibit a number of resistant pathogens [5]. Another disadvantage of CH is its effect on the root canal dentin microhardness [6-8].
A research interest has been sparked within dental and medical sciences by the emergence of nanotechnology regarding their potential applications and advantages compared to the conventionally used materials [9]. The constituent components of nanomaterials are less than 100 nm in at least one dimension [10]. They improve the physicochemical, mechanical, and biological properties due to their larger surface area and quantum effects [10, 11]. Compared with the commonly used CH, nano-calcium hydroxide (NCH) exhibits a higher antibacterial efficacy, superior penetration depth into the dentinal tubules, and higher alkalization capacity in a shorter time [12-14]. Interestingly, the microhardness value of dentin was not affected after employing NCH [15]. In addition, NCH was powerful in eliminating most root canal endotoxins at the depth of almost 300 μm of the root canal dentin and in removing Enterococcus (E.) faecalis from the dentinal tubules [16, 17]. Furthermore, placement of the NCH sealer resulted in a remarkably lower leakage compared to conventional zinc oxide eugenol (ZOE) sealers [18]. However, NCH had a remarkable influence on the chemical structure of radicular dentin through collagen degradation or demineralization after four weeks of usage [19].
As mentioned, CH has some shortcomings such as its limited effect to eliminate E. faecalis and its role in reducing root fracture resistance [20-22]. Besides, the major defect of CH is that its residues on the root canal walls can lead to reactions with the root canal sealers and alter their features. For instance, these residues can increase the viscosity, reduce the flowability, and alter the setting time of root canal sealers. Consequently, they can hamper the sealer penetration into the dentinal tubules and its adhesion to them [2, 23]. These changes can have an adverse impact on the bond strength among the sealer and dentin [23-25]. Physical and chemical properties of materials might be influenced by alterations of pH [26]. It is worth mentioning that the high alkalinity of CH can result in the denaturation of proteins and a subsequent weakening of root dentin structure can occur [7, 8]. The nano-size of particles of NCH may penetrate and remain more on canal walls and may weaken the bond of canal walls to the sealers more than the conventional CH. To the best of the authors’ knowledge, no other studies in the literature have compared the push-out bond strengths of NCH and CH. Accordingly, the current research was aimed to investigate the influence of CH and NCH on the push-out bond strength of AH-Plus jet sealer. The null hypothesis was defined as no difference would be detected regarding push-out bond strength following usage of CH and NCH.
Materials and Methods
The outline of the present in vitro study was approved by the Ethics Committee of Shiraz University of Medical Sciences, Shiraz, Iran (Ethical code: IR.SUMS.DENTAL.REC.1399.218).
Sample preparation
Forty eight mandibular premolars which had been extracted due to periodontal problems or orthodontic reasons were gathered. The written informed consent was taken from the patients that their teeth will be used for researches. The samples were placed in 0.5% chloramine-T solution (Merck, Darmstadt, Germany) for a 48-h period to become disinfected and then they were kept in distilled water until the time of experiment. After removal of the debris and calculus, radiographic evaluation was performed to exclude the teeth possessed more than 1 root canal. The exclusion criteria encompassed multi-rooted teeth, teeth with multiple apical foramina, teeth with previous endodontic therapy, root resorption, immature root apices, root caries, root cracks, and/or root canal curvature of greater than ten degrees. The teeth were inspected under 40× magnification using Dino-Lite USB Digital Microscope AM3111-0.3MP (Dino-Lite, Hanoi city, Vietnam) to choose the teeth meeting inclusion criteria. The cementoenamel junction (CEJ) to the root apex were considered to ensure the selected premolars possess identical root lengths. Finally, 48 mandibular premolars were chosen (according to Ackay et al. [27]) and decoronated to reach homogenized root length of 15 mm via a diamond disk. Study the root canal preparation was done by a single operator. The canal patency was reassessed by insertion of #10 K-file (Dentsply Maillefer, Ballaigues, Switzerland) into the root canals and its visualization at apical foramen. The working length was ascertained by deducting one mm from the file length inserted in each canal. We used Dentsply Maillefer rotary instruments to prepare the root canal systems from F1 to F4 (size 40/0.06 ProTaper) and the root canal rinsing was done with 2 mL of 2.5% sodium hypochlorite solution (NaOCl) (ImidentMed, Konya, Turkey) amid each change of instrument. Ultimately, the samples were rinsed with 17% EDTA (5 mL) for one min, 1% NaOCl (5 mL) for one min, and the final rinsing of the samples was done with normal saline [27]. Afterward, drying of the root canals were conducted by absorbent paper points (Dentsply Maillefer, Ballaigues, Switzerland) and based on the intracanal medicament used, the premolars were allocated randomly into three groups (n=16): control group containing teeth without treatment of any intracanal medicament, CH group, and NCH group. The whole procedures of root canal preparation were performed by a single operator.
Characterization of CH
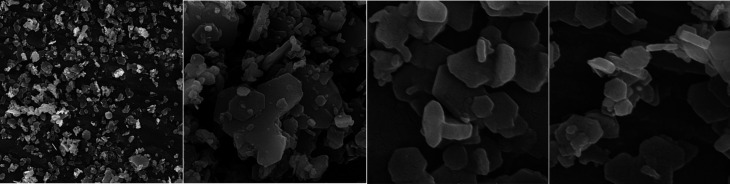
X-ray diffraction analysis (XRD), Fourier transform infrared spectroscopy (FTIR), and field emission scanning electron microscopy (FESEM) were performed for characterization of NCH (Figure 1). XRD pattern and analysis of the phase and crystallinity of NCH was achieved by X-ray diffractometer (D8 DISCOVER; Bruker, Billerica, MA, USA) with Cu Kα radiation (k=1.54 A◦) and scanning rate of 1 step/s with step size of 0.1◦/step. FTIR was applied for characterization of the molecular interactions in the NCH with FTIR spectrometer (Spectrum One FTIR spectrometer; PerkinElmer, Waltham, MA, USA) using KBr method [28]. And ultimately, field emission scanning electron microscopy (FESEM; JSM5410; JEOL, Tokyo, Japan) was applied for observation of the size and morphology of NCH.
Figure 1.
Field emission scanning electron microscopy micrographs of nano-calcium hydroxide
According to the protocols provided by Hoshino et al. and Naseri et al. [15, 29], medicament pastes were produced through mixing CH (Merck, Darmstadt, Germany) and NCH (Polymer and Petrochemical Institute, Tehran, Iran) powder with normal saline in a powder to liquid ratio of 3:1, separately [15, 29]. By employing a #40 Lentulo spiral (Dentsply Maillefer, Ballaigues, Switzerland), the medicaments were introduced into the root canal system and the coronal openings were sealed temporarily with small pellets of cotton and provisional filling material (Cavizol, Aria Dent, Iran) in order to prevent any coronal leakage. The specimens were kept at the temperature of 37°C and humidity of 100% for 7 days to stimulate the clinical conditions. Following a week of incubation [24], the medicaments were removed by an F4 ProTaper rotary instrument (Dentsply Maillefer, Ballaigues, Switzerland), followed by 10 mL 17% EDTA, then by 10 mL 2.5% NaOCl and a final flushing was performed by 5 mL sterile saline. After drying the root canals with paper points, the working length was filled by insertion of F4 size gutta-percha cones (F4, Dentsply Maillefer) into root canals which were sparingly dipped in sealer (AH-Plus Jet; Dentsply Maillefer, Ballaigues, Switzerland)[single cone technique]. Radiographs of the mesiodistal and buccolingual views of each tooth were taken to affirm integral obturation of the root canals. After obturation, a temporary filling material (Cavizol, Aria Dent, Iran) was placed to seal the coronal opening and a week incubation with 100% humidity and temperature of 37°C was applied for the samples. Transparent polyester was poured in plastic cubes and each tooth was placed into the cubes in order to mount the samples in blocks for facilitating sectioning process.
Push-out bond strength evaluation
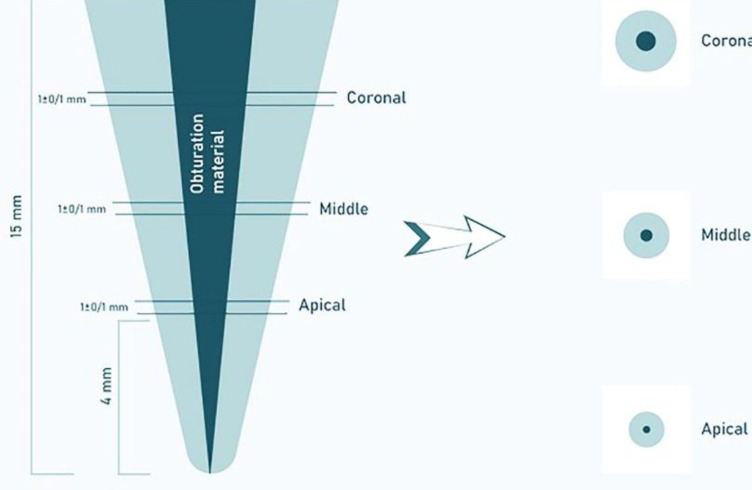
One week after obturation a Buehler IsoMet precision saw (1000; Buehler IsoMet, Lake Bluff, IL, USA) was used to deliver perpendicular cut at each tooth to the long axis using cooling water at low speed. For each specimen, 3 sections were made at depths of 4 mm apical, 7 mm middle, and 10 mm coronal thirds and near to 1±0.1 mm width, giving a total of 48 specimen (Figure 2). With the aid of a magnification device, the diameter of each hole was measure from the coronal and apical aspects. We used a universal test machine to measure the strength of the push-out bond at a crosshead speed of 1 mm/min (AGS-X; Shimadzu Corp, Kyoto,, Japan). Three sets of cylindrical pluggers of diameter 0.6-, 0.7-, and 0.8-mm that matched each canal third diameter were used for the push-out test of each tooth. The pluggers’ diameter conformed to a minimum 80% of the canal diameter. The greatest force applied prior failure of the obturation material was first noted in Newtons and later transmuted to megapascals (MPa) based on the ensuing formula: push-out bond strength (MPa)=greatest load (N)/adhesion area of root filling (A) (mm2). The subsequent equation computed the area occupied by the root canal filling: A=()XL, where , r1 stands for the minor radius, r2 point to the major radius of the canal diameter (mm), h is the the root section thickness (mm), and is the constant 3.14 [27].
Figure 2.
Schematic representation of preparation of dentin disks for the push-out bond strength testing
Statistical analysis
By usage of the SPSS software (SPSS version 25; Chicago, IL, USA) statistical analysis was done. The Kolmogrov-Smirnov statistical test was implemented to test the assumption of normality. Repeated measurement analysis followed by Tukey’s HSD and Bonferroni post-hoc tests were used to determine whether significant differences in push-out bond strength values existed intergroup and within groups. For statistical analyses, we considered P-values lower than than 0.05 as statistically significant.
Results
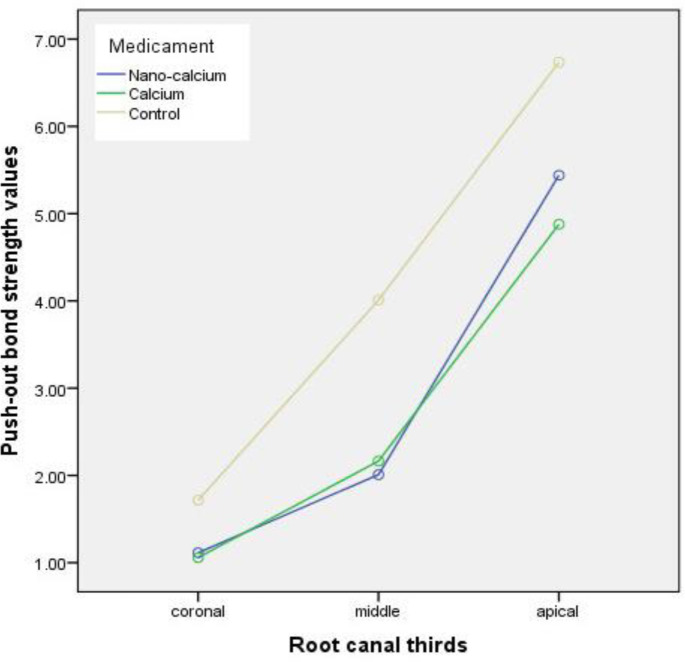
Based on the repeated measurement analysis, no significant interaction effect was detected between the type of the medicament and the root canal thirds, regarding the bond strength values (P=0.283). The push-out bond strength of the sealer to the root canal dentin in accordance to the type of intracanal dressing in the root canal thirds is presented in mean and standard deviation in Table 1. The push-out bond strength values were significantly affected by the intracanal medicaments (P˂0.001). The control group which received no medicament revealed the maximum bond strength in comparison to the CH and NCH groups (Figure 3). However, the samples treated with NCH demonstrated a greater push out bond strength than those in CH group, no statistically noticeable difference was detected between NCH and CH group (P>0.05) (Table 2). Under controlling the effect of the type of medicament, a remarkable difference was found among the root canal thirds (P˂0.001) and the push-out bond strength had increased in a coronoapically direction and the apical third exhibited the highest bond strength values.
Table 1.
Mean (SD) of push-out bond strengths (MPa) following placement of medicaments in accordance to root canal thirds
| Root canal third | Type of medicament | ||
|---|---|---|---|
| Nano-calcium hydroxide | Calcium hydroxide | Control (without medicament) | |
| Coronal | 1.11 (0.63) | 1.05 (0.38) | 1.71 (0.57) |
| Middle | 2.00 (0.89) | 2.16 (0.73) | 4.00 (1.98) |
| Apical | 5.43 (2.44) | 4.87 (2.33) | 6.73 (2.09) |
Figure 3.
Push-out bond strengths of studied groups at each of the root canal thirds
Table 2.
Comparison of the push out bond strength values of the experimental intracanal dressings by Tukey’s HSD test
| P -value | ||
|---|---|---|
| Nano-calcium hydroxide | Calcium hydroxide | 0.895 |
| Nano-calcium hydroxide | Control | 0.001 |
| Calcium hydroxide | Control | ˂0.001 |
Discussion
By the improvement of various properties of CH medicament when it is implemented in its nano-sized particles, we evaluated the push-out bond strength of epoxy-resin based sealer to root canal dentin (AH-Plus jet) under influence of CH and NCH. The null hypothesis of the current study was that no difference would be detected regarding push-out bond strength values following usage of CH and NCH. Although, the results demonstrated that usage of CH and NCH as intracanal medicament, adversely affected on the push-out bond strength of AH-Plus jet sealer, and there was no statistically signigicant difference between them. Hence, our null hypothesis has been approved.
In accordance to Kenee et al. [30] study, which recommended the additional use of rotary or ultrasonic instrumentation after hand filing and irrigation to enhance the elimination of CH from the canals, we used a F4 ProTaper file with 10 mL of 17% EDTA solution and 10 mL of 2.5% NaOCl solution to irrigate CH and the NCH from each canal [30]. Various irrigation solutions and devices including endodontic hand files and brushes, nickel-titanium (Ni-Ti) rotary instruments, sonic and ultrasonic activated irrigation solution, and laser activated irrigation solution have been advocated to wipe out CH from root canal system [31, 32]. However, till date, neither any technique nor any agent has been able to sufficiently eliminate intracanal CH from the root canals. Conventionally, the removal of CH present in the root canals was performed by canal irrigation with sodium hypochlorite to the working length and EDTA [33].
One of the most preferred techniques to evaluate bond strength and adhesion is the push-out testing method [34, 35]. The push-out test measures the shear stress between the sealer and the dentin which is similar to the stress produced under clinical conditions [35]. This method is efficient and reproducible, and is measurable even in conditions where the bond strength is low [34, 36]. The strength of push-out bonding relies on the sample thickness, diameter of the plugger, the orientation of the specimens, and the sealing ability of the sealer to the root canal walls [34]. The design of current study was developed in accordance to Ackay et al. study to obtain the most favorable findings during the preparation of the samples and the test performance [27]. Three different sizes of pluggers were considered for the test performance, 0.6, 0.7, and 0.8 mm, each of which conformed to the diameter of a canal third such that most diameter of the gutta-percha was covered with the plugger at the point of contact.
The sealer of choice in the current study was AH-Plus jet. According to some studies, epoxy resin-based sealers like AH-Plus sealers are reported to cause higher strength in bonding compared to other endodontic sealers [36-40]. Probably, following the opening of the epoxide rings of the sealer, stronger covalent bonds are formed among the epoxy groups of the sealer and the amino groups of the collagen in dentin thus creating stronger linkage between the sealer and dentinal walls [41, 42].
Our survey in the literature did not show any study assessing the push-out bonding of epoxy resin-based sealers following NCH application. However, some previous reports have investigated the efficacy of CH on the adhesion strength of sealers with basis of epoxy resin [3, 24, 27, 38, 43]. The findings from the current study indicated that the control group possesses the highest mean strength of push-out bonding compared to that of premolars treated with CH or NCH groups irrespective of the root canal position (thirds). This is in consistent with the results of the Guiotti et al. [24] study, which highlighted that the negative impact of CH result in weaker push-out bond strength of AH-Plus sealer [24]. Also, Ghabraei et al. [44] the bond strength of AH-26 (Dentsply, Tulsa Dental, Tulsa, OK, USA) and BC sealers (Brasseler, Savannah, GA, USA) were negatively impacted by the remnants of CH [44]. The current research’s authors infer that CH residue in the root canal system may have hindered adequate interlocking and bond formation between the sealer and the dentinal tubules, hence, the bond strength has reduced in premolars treated with CH and NCH. However, further investigation is required to evaluate the correlation between the penetration depth of NCH and the sealing capacity of the root canal material. Furthermore, the high alkalinity of CH and NCH can negatively influence on the structure of root canal dentin and a consequent disturbance of proper adhesion between root canal walls and sealer may occur [6-8].
In the current research, the push-out bond strength has elevated regionally from coronal to apical irrespective to the type of the medicaments used. Other studies have reported similar regional push-out bond strength results for AH plus sealer that are in line to our results [45, 46]. Ackay et al. [27] on the contrary reported that root canal thirds have not affected the push out bond strength [27]. Since the samples had similar lengths and the diameter of the root canals declined from the coronal portion to the apical, may the greater push-out bond strength at apical region be more related to the higher frictional resistance among the sealer and root canal walls at apical third in narrower parts of canal rather than an improved bond strength. Also, the higher bond strength of AH-Plus jet sealer in the apical third may be related to some other factors. For instance, apical third of root canals own more prominent anatomical irregularities such as accessory root canals, resorption or repaired resorption areas, and irregular secondary dentin [24, 27].
Conclusion
According to the results of the present in vitro study, when epoxy resin-based sealers are used during root canal therapy, if it’s not necessary to use CH and NCH as intracanal medicaments, their application may result in reduction of push-out bond strength and is not recommended.
Acknowledgements
The authors thank the Vice-Chancellery of research of Shiraz University of Medical Sciences for supporting this research and Biomaterial Research Center of Shiraz Dental School for testing the samples. Also, the authors thank Dr. Mehrdad Vossoughi and Dr. Parisa Chamanpara from the Dental Research Development Center, for the statistical analysis.
Conflict of Interest:
‘None declared’.
References
- 1.Agrawal P, Garg G, Bavabeedu SS, Arora S, Moyin S, Punathil S. Evaluation of intracanal calcium hydroxide removal with different techniques: a scanning electron microscope study. J Contemp Dent Pract. 2018;19(12):1463–8. [PubMed] [Google Scholar]
- 2.Turkaydin D, Basturk F, Goker S, Tarcin B, Berker YG, Ovecoglu HS. Efficacy of Endoactivator, CanalBrush, and passive ultrasonic irrigation in the removal of cal-cium hydroxide paste with iodoform and pchloro-phenol from root canals. Niger J Clin Pract. 2020;23(9):1237–42. doi: 10.4103/njcp.njcp_710_19. [DOI] [PubMed] [Google Scholar]
- 3.Üstün Y, Arslan S, Aslan T. Effects of calcium hydroxide and propolis intracanal medicaments on bond strength of resin-based endodontic sealer as assessed by push-out test. Dent Mater J. 2013 doi: 10.4012/dmj.2013-094. [DOI] [PubMed] [Google Scholar]
- 4.Kim D, Kim E. Antimicrobial effect of calcium hydroxide as an intracanal medicament in root canal treatment: a literature review-Part I In vitro studies. Restor Dent Endod. 2014;39(4):241–52. doi: 10.5395/rde.2014.39.4.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbizam JVB, Trope M, Teixeira ÉC, Tanomaru-Filho M, Teixeira FB. Effect of calcium hydroxide intracanal dressing on the bond strength of a resin-based endodontic sealer. Braz Dent J. 2008;19:224–7. doi: 10.1590/s0103-64402008000300009. [DOI] [PubMed] [Google Scholar]
- 6.Moazami F, Sahebi S, Jamshidi D, Alavi A. The long-term effect of calcium hydroxide, calcium-enriched mixture cement and mineral trioxide aggregate on dentin strength. Iran Endod J. 2014;9(3) [PMC free article] [PubMed] [Google Scholar]
- 7.Sahebi S, Nabavizadeh M, Dolatkhah V, Jamshidi D. Short term effect of calcium hydroxide, mineral trioxide aggregate and calcium-enriched mixture cement on the strength of bovine root dentin. Iran Endod J. 2012;7(2) [PMC free article] [PubMed] [Google Scholar]
- 8.Sahebi S, Sobhnamayan F, Naghizade S. The effects of various endodontic irrigants on the push-out bond strength of calcium-enriched mixture cement and mineral trioxide aggregate. Iran Endod J. 2016;11(4) doi: 10.22037/iej.2016.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.AlKahtani RN. The implications and applications of nanotechnology in dentistry: A review. Saudi Dent j. 2018;30(2):107–16. doi: 10.1016/j.sdentj.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhardwaj A, Bhardwaj A, Misuriya A, Maroli S, Manjula S, Singh AK. Nanotechnology in dentistry: Present and future. J Int Oral Health. 2014;6(1) [PMC free article] [PubMed] [Google Scholar]
- 11.Raura N, Garg A, Arora A, Roma M. Nanoparticle technology and its implications in endodontics: A review. Biomater Res. 2020;24(1):1–8. doi: 10.1186/s40824-020-00198-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dianat O, Saedi S, Kazem M, Alam M. Antimicrobial activity of nanoparticle calcium hydroxide against Enterococcus faecalis: an in vitro study. Iran Endod J. 2015;10(1) [PMC free article] [PubMed] [Google Scholar]
- 13.Tanomaru-Filho M, Guerreiro-Tanomaru JM, Faria G, Aguiar AS, Leonardo RT. Antimicrobial activity and ph of calcium hydroxide and zinc oxide nanoparticles intracanal medication and association with chlorhexidine. J Contemp Dent Pract. 2015;16(8):624–9. doi: 10.5005/jp-journals-10024-1732. [DOI] [PubMed] [Google Scholar]
- 14.Zand V, Mokhtari H, Hasani A, Jabbari G. Comparison of the penetration depth of conventional and nano-particle calcium hydroxide into dentinal tubules. Iran Endod J. 2017;12(3) doi: 10.22037/iej.v12i3.16421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naseri M, Eftekhar L, Gholami F, Atai M, Dianat O. The effect of calcium hydroxide and nano–calcium hydroxide on microhardness and superficial chemical structure of root canal dentin: an ex vivo study. J Endod. 2019;45(9):1148–54. doi: 10.1016/j.joen.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 16.Louwakul P, Saelo A, Khemaleelakul S. Efficacy of calcium oxide and calcium hydroxide nanoparticles on the elimination of Enterococcus faecalis in human root dentin. Clin Oral Investig. 2017;21(3):865–71. doi: 10.1007/s00784-016-1836-x. [DOI] [PubMed] [Google Scholar]
- 17.Lungkapinth P, Louwakul P, editors. Reduction of Endotoxin from Human Root Canals by Calcium Hydroxide Nanoparticles. IOP Conference Series: Materials Science and Engineering; 2019: IOP Publishing. [Google Scholar]
- 18.Desouky AA, Negm MM, Ali MM. Sealability of Different Root Canal Nanosealers: Nano Calcium Hydroxide and Nano Bioactive Glass. Open Dent J. 2019;13:1. [Google Scholar]
- 19.Devaraj S, Jagannathan N, Neelakantan P. Antibiofilm efficacy of photoactivated curcumin, triple and double antibiotic paste, 2% chlorhexidine and calcium hydroxide against Enterococcus fecalis in vitro. Sci Rep. 2016;6(1):1–6. doi: 10.1038/srep24797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim D, Kim EJRd, endodontics Antimicrobial effect of calcium hydroxide as an intracanal medicament in root canal treatment: a literature review-Part II. in vivo studies. 2015;40(2):97–103. doi: 10.5395/rde.2015.40.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Markan S, Lehl G, Kapoor S. Recent advances of nanotechnology in endodontics, conservative and preventive dentistry-a review. J Dent Oral Biol. 2017;2(10):1–4. [Google Scholar]
- 22.Sahebi S, Moazami F, Abbott P. The effects of short‐term calcium hydroxide application on the strength of dentine. Dent Traumatol. 2010;26(1):43–6. doi: 10.1111/j.1600-9657.2009.00834.x. [DOI] [PubMed] [Google Scholar]
- 23.Denna J, Shafie LA, Alsofi L, Al-Habib M, AlShwaimi E. Efficacy of the rotary instrument XP-Endo Finisher in the removal of calcium hydroxide intracanal medicament in combination with different irrigation techniques: a microtomographic study. Materials. 2020;13(10) doi: 10.3390/ma13102222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guiotti FA, Kuga MC, Duarte MAH, Sant'Anna A, Faria G. Effect of calcium hydroxide dressing on push-out bond strength of endodontic sealers to root canal dentin. Braz Oral Res. 2014;28:1–7. doi: 10.1590/S1806-83242014.50000002. [DOI] [PubMed] [Google Scholar]
- 25.Pabel A-K, Hülsmann M. Comparison of different techniques for removal of calcium hydroxide from straight root canals: an in vitro study. Odontology. 2017;105(4):453–9. doi: 10.1007/s10266-017-0293-6. [DOI] [PubMed] [Google Scholar]
- 26.Ghasemi N, Reyhani MF, Milani AS, Mokhtari H, Khoshmanzar F. Effect of calcium hydroxide on the push-out bond strength of endodontic biomaterials in simulated furcation perforations. Iran Endod J. 2016;11(2) doi: 10.7508/iej.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akcay M, Arslan H, Topcuoglu HS, Tuncay O. Effect of calcium hydroxide and double and triple antibiotic pastes on the bond strength of epoxy resin–based sealer to root canal dentin. J Endod. 2014;40(10):1663–7. doi: 10.1016/j.joen.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 28.Sireesha A, Jayasree R, Vidhya S, Mahalaxmi S, Sujatha V, Kumar TS. Comparative evaluation of micron-and nano-sized intracanal medicaments on penetration and fracture resistance of root dentin–An in vitro study. Int J Biol Macromol. 2017;104:1866–73. doi: 10.1016/j.ijbiomac.2017.05.126. [DOI] [PubMed] [Google Scholar]
- 29.Hoshino E, Kurihara‐Ando N, Sato I, Uematsu H, Sato M, Kota K, Iwaku M. In‐vitro antibacterial susceptibility of bacteria taken from infected root dentine to a mixture of ciprofloxacin, metronidazole and minocycline. Int Endod J. 1996;29(2):125–30. doi: 10.1111/j.1365-2591.1996.tb01173.x. [DOI] [PubMed] [Google Scholar]
- 30.Kenee DM, Allemang JD, Johnson JD, Hellstein J, Nichol BK. A quantitative assessment of efficacy of various calcium hydroxide removal techniques. J Endod. 2006;32(6):563–5. doi: 10.1016/j.joen.2005.10.065. [DOI] [PubMed] [Google Scholar]
- 31.Bhuyan A, Seal M, Pendharkar K. Effectiveness of four different techniques in removing intracanal medicament from the root canals: An in vitro study. Contemp Clin Dent. 2015;6(3):309. doi: 10.4103/0976-237X.161860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamdan R, Michetti J, Pinchon D, Diemer F, Georgelin-Gurgel M. The XP-Endo Finisher for the removal of calcium hydroxide paste from root canals and from the apical third. J Clin Exp Dent. 2017;9(7):e855. doi: 10.4317/jced.53962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Faria G, Kuga MC, Ruy AC, Aranda-Garcia AJ, Bonetti-Filho I, Guerreiro-Tanomaru JM, Leonardo RT. The efficacy of the self-adjusting file and ProTaper for removal of calcium hydroxide from root canals. J Appl Oral Sci. 2013;21:346–50. doi: 10.1590/1679-775720130034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Al-Hiyasat AS, Alfirjani SA. The effect of obturation techniques on the push-out bond strength of a premixed bioceramic root canal sealer. J Dent. 2019;89:103169. doi: 10.1016/j.jdent.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 35.Moinuddin MK, Prasad LK, Ramachandruni N, Kamishetty S, Cherkupalli RC. Comparison of push-out bond strength of three different obturating systems to intraradicular dentin: An In vitro study. Contemp Clin Dent. 2019;10(4):631. doi: 10.4103/ccd.ccd_640_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ungor M, Onay E, Orucoglu H. Push‐out bond strengths: the Epiphany–Resilon endodontic obturation system compared with different pairings of Epiphany, Resilon, AH Plus and gutta‐percha. Int Endod J. 2006;39(8):643–7. doi: 10.1111/j.1365-2591.2006.01132.x. [DOI] [PubMed] [Google Scholar]
- 37.Ali N, Saha SG, Vijayvargiya P, Bhardwaj A, Shrivastava S, Sharma V, Sachdeva HS. Comparative evaluation of push-out bond strength of gutta-percha using different sealers with lateral condensation and thermoplasticized obturation technique: An in vitro study. J Conserv Dent. 2019;22(6):593. doi: 10.4103/JCD.JCD_553_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amin SAW, Seyam RS, El-Samman MA. The effect of prior calcium hydroxide intracanal placement on the bond strength of two calcium silicate–based and an epoxy resin–based endodontic sealer. J Endod. 2012;38(5):696–9. doi: 10.1016/j.joen.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 39.Dem K, Wu Y, Kaminga AC, Dai Z, Cao X, Zhu B. The push out bond strength of polydimethylsiloxane endodontic sealers to dentin. BMC oral health. 2019;19(1):1–6. doi: 10.1186/s12903-019-0867-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tedesco M, Chain MC, Felippe WT, Alves AMH, Garcia LdFR, Bortoluzzi EA, Cordeiro MR, Teixeira CS. Correlation between bond strength to dentin and sealers penetration by push-out test and CLSM analysis. Braz Dent J. 2019;30:555–62. doi: 10.1590/0103-6440201902766. [DOI] [PubMed] [Google Scholar]
- 41.Donnermeyer D, Dornseifer P, Schäfer E, Dammaschke T. The push-out bond strength of calcium silicate-based endodontic sealers. Head Face Med. 2018;14(1):1–7. doi: 10.1186/s13005-018-0170-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee K-W, Williams MC, Camps JJ, Pashley DH. Adhesion of endodontic sealers to dentin and gutta-percha. J Endod. 2002;28(10):684–8. doi: 10.1097/00004770-200210000-00002. [DOI] [PubMed] [Google Scholar]
- 43.Carvalho CN, Bauer J, Ferrari PHP, Souza SFC, Soares SP, Loguercio AD, Bombana AC. Influence of calcium hydroxide intracanal medication on bond strength of two endodontic resin‐based sealers assessed by micropush‐out test. Dent Traumatol. 2013;29(1):73–6. doi: 10.1111/j.1600-9657.2011.01109.x. [DOI] [PubMed] [Google Scholar]
- 44.Ghabraei S, Bolhari B, Yaghoobnejad F, Meraji N. Effect of intra-canal calcium hydroxide remnants on the push-out bond strength of two endodontic sealers. Iran Endod J. 2017;12(2):168. doi: 10.22037/iej.2017.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cakici F, Cakici EB, Ceyhanli KT, Celik E, Kucukekenci FF, Gunseren AO. Evaluation of bond strength of various epoxy resin based sealers in oval shaped root canals. BMC oral health. 2016;16(1):1–5. doi: 10.1186/s12903-016-0301-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mahdi AA, Bolanos-Carmona V, Gonzalez-Lopez S. Bond strength to root dentin and fluid filtration test of AH Plus/gutta-percha, EndoREZ and RealSeal systems. J Appl Oral Sci. 2013;21:369–75. doi: 10.1590/1679-775720130114. [DOI] [PMC free article] [PubMed] [Google Scholar]