Abstract
Psoriasis increases the risk of cardiovascular disease (CVD). Biomarkers for cardiovascular (CV) risk stratification in psoriasis are lacking, and the effects of psoriasis biologics on CV risk reduction remain unclear. The goal of this study was to identify biomarkers of CV risk in psoriasis blood that are reduced by ustekinumab. We quantified 276 inflammatory and CV-related serum proteins with Olink’s multiplex assay in 10 psoriasis patients (vs. 18 healthy controls) and after 12 weeks of ustekinumab treatment. For each protein down-regulated after treatment, the literature was reviewed for studies assessing the protein’s association with CVD. Data were collected from each study to calculate CV risk thresholds for each protein, which were compared with protein levels in psoriasis patients before and after treatment. Our results showed that 43 out of 276 proteins were down-regulated after treatment, 25 of which were initially up-regulated at baseline (vs. controls, all p-values ≤0.1). 8 down-regulated proteins were initially elevated above thresholds associated with enhanced CV risk in the literature (myeloperoxidase, C-X-C motif chemokine 10, E-selectin, interleukin-6, cystatin B, von Willebrand factor, tumor necrosis factor receptor 1 and N-terminal prohormone brain natriuretic peptide). Treatment lowered these proteins to below their risk thresholds, except for IL-6, which was lowered but remained at its risk threshold despite successful psoriasis skin treatment. In summary, 12 weeks of ustekinumab treatment reduced serum proteins present at levels associated with CV risk in psoriasis patients. Further studies can evaluate these proteins as potential ustekinumab-modulated biomarkers of CV risk in psoriasis and the impact of ustekinumab on CV risk reduction.
Keywords: biologic, biomarker, blood, inflammatory, interleukin-6
1 |. INTRODUCTION
Psoriasis is a chronic inflammatory skin disorder that increases the risk of cardiovascular disease (CVD) upwards of 50%.1,2 Cardiovascular (CV) related abnormalities in psoriasis include vascular inflammation, platelet activation, endothelial dysfunction and elevated lipid profiles.1–6 The 6.2% increased absolute risk of a 10-year major adverse cardiac event (MACE) in severe psoriasis is independent of traditional CV risk factors and is thought to be related to underlying systemic inflammation.7 Both the joint American Academy of Dermatology-National Psoriasis Foundation (AAD-NPF) and the American College of Cardiology-American Heart Association (ACC-AHA) now recognize psoriasis as a CV risk-enhancing condition and the AAD-NPF suggests adapting CV risk score models to include psoriasis.8,9
Despite these recommendations, guidelines rely on a clinical phenotype to calculate CV risk and do not fully take into account the underlying systemic inflammation in psoriasis that might impact CV risk.2,8 Tools such as fluorodeoxyglucose positron emission tomography-computed tomography (FDG-PET/CT) and coronary artery calcium score, can assess vascular inflammation beyond traditional CV risk factors; however, evidence to support inflammatory biomarkers for CV risk stratification and management in psoriasis is lacking.2,4,10,11 In non-psoriasis populations, pro-inflammatory biomarkers such as high-sensitivity C-reactive protein (hs-CRP) aid in CV risk stratification, with upstream targeting of hs-CRP shown to reduce CV events.12,13 Therefore, in psoriasis, proteomic analysis of inflammatory and CV-related serum proteins might help identify new biomarkers to improve CV risk management.
In addition, while recent studies suggest that psoriasis biologics can reduce systemic inflammation associated with CVD, the impact of disease-modifying therapies on CV risk warrants further study, and individual biologics might differentially impact risk.3,14,15
Kim et al. showed that the psoriasis biologics tofacitinib and etanercept reduced inflammatory and CVD-related blood proteins, providing insight into underlying protein changes that are differentially impacted by biologics that target specific pathways.16 A similar study has not been performed for ustekinumab, a widely used psoriasis biologic with a low rate of drug discontinuation, that acts by targeting the shared p40 subunit of interleukin (IL)-12 and IL-23.17 Further, as psoriasis research has focused on larger changes in gene expression in lesional skin, the relatively smaller reductions in blood proteins (range 1.2–2.83 fold change [FCH]) shown by Kim et al. are of unclear significance.16,18 Therefore, we aimed to quantify inflammatory and CV-related blood proteins in psoriasis before and after ustekinumab treatment and to frame the significance of the magnitude of these changes with an extensive review of these proteins in the CV literature. Our goal was to identify biomarkers of CV risk in psoriasis that are modulated by ustekinumab.
2 |. METHODS
2.1 |. Subjects and Samples
The primary objective of the analysis was to identify differentially expressed inflammatory and cardiovascular-related proteins in psoriasis at baseline and after 12 weeks of ustekinumab treatment. Blood samples from ten adults (mean ± standard deviation [SD] age 50.0 ± 8.1) with moderate-to-severe psoriasis before and after 12 weeks of ustekinumab treatment were taken from a phase 3 randomized blinded trial where patients were administered 45 mg or 90 mg ustekinumab on Weeks 0 and 4 (Active Comparator [CNTO 1275/Enbrel] Psoriasis Trial [ACCEPT]; ClinicalTrials.gov identifier, NCT00454584). The study protocol was approved by the institutional review board or ethics committee at each site where the study was conducted. Informed consent was obtained from all patients. Exclusion criteria included history of or concurrent congestive heart failure and signs or symptoms of severe, progressive or uncontrolled cardiac disease. Blood samples from 18 healthy controls were taken. Samples were centrifuged, and serum was analysed for 276 protein concentrations using three panels (Cardiovascular II, Cardiovascular III and Inflammation) from Olink’s high-throughput multiplex assay, as has been previously described (Olink Proteomics, Uppsala, Sweden).16
2.2 |. Statistical analysis
All statistical analysis was performed using publicly available Bioconductor packages (Bioconductor.org, Bioconductor Core Team) in R language (R-project.org, R foundation). Protein expression profiles were estimated under limma package framework using mixed-effect linear models. Condition (Psoriasis/Control) and time point (Baseline/Week 12) were considered as fixed factors while random effect related to each patient was included. Least squared means (LS mean) for each group and fold changes for every comparison were calculated. Proteins were defined as differentially expressed if the average value in psoriasis serum was an increase or decrease relative to control with a p-value ≤0.1. A disease proteome was defined at baseline (psoriasis vs. controls) and a treatment effect was measured after 12 weeks of treatment (Week 12 vs. baseline). A student’s t test was used for the comparisons. FCH at baseline from control and from baseline after treatment was converted to an equivalent percent increase or decrease. Correlations among proteins, body mass index (BMI) and psoriasis area and severity index (PASI) at baseline and posttreatment were assessed using univariate Pearson correlation and Chi-squared test.
2.3 |. Literature review and analysis
The secondary objective was to compare the FCH of each down-regulated protein with its cardiovascular risk threshold found in the literature. For each protein down-regulated with treatment (p ≤ 0.1), we searched the literature for studies assessing the protein’s association with CVD, using the following search terms: “circulating,” “serum,” “plasma,” “blood,” “protein,” “cardiovascular disease,” “cardiovascular risk,” “atherosclerosis” and “biomarker.” From each study, data on blood protein levels in healthy controls and CV patients, or tertile and quartile protein concentrations corresponding to disease, were used to calculate the percent increase over healthy population levels associated with CVD, reported here as the calculated percent increase associated with CV risk in the literature. The calculated percent increases and LS means of protein levels in healthy controls were used to estimate the CV risk threshold for each protein according to its respective study. The LS means of protein levels in the psoriasis cohort at baseline and post-treatment were compared with the calculated percent increase associated with CVD and the CV risk threshold.
3 |. RESULTS
There was no difference in age, gender, BMI and race between patients and controls (Table S1.). The mean ± SD baseline and post-treatment PASI was 21.0 ± 8.3 (range 12.0–37.6) and 0.9 ± 0.6 (range 0–1.7) respectively (Table S1.).
After psoriasis skin treatment with ustekinumab for 12 weeks, 43 serum proteins were down-regulated, 25 of which were initially up-regulated at baseline relative to healthy controls (p ≤ 0.1) (Figure 1, Table S2). Our observed downregulation of IL-17A, as well as IL-17A induced inflammatory factors in psoriasis, including IL-17C, IL-8 and CCL20, indicates successful suppression of the IL-23/IL-17 axis by ustekinumab.19 Ustekinumab’s modulation of serum proteins was also consistent with ustekinumab’s effect on gene transcription in psoriasis skin lesions, where ustekinumab has been shown to suppress IL17-A and IL-17 modulated genes by over 90%.18 Only 10 proteins were significantly increased after treatment (Table S2). The most significant upregulation posttreatment was seen with IL-12-B (FCH 6.74). This was expected due to ustekinumab’s mechanism of action. Ustekinumab binds the IL-12p40 subunit and sequesters this cytokine subunit in the blood, causing an expected elevation in IL-12-B.
FIGURE 1.
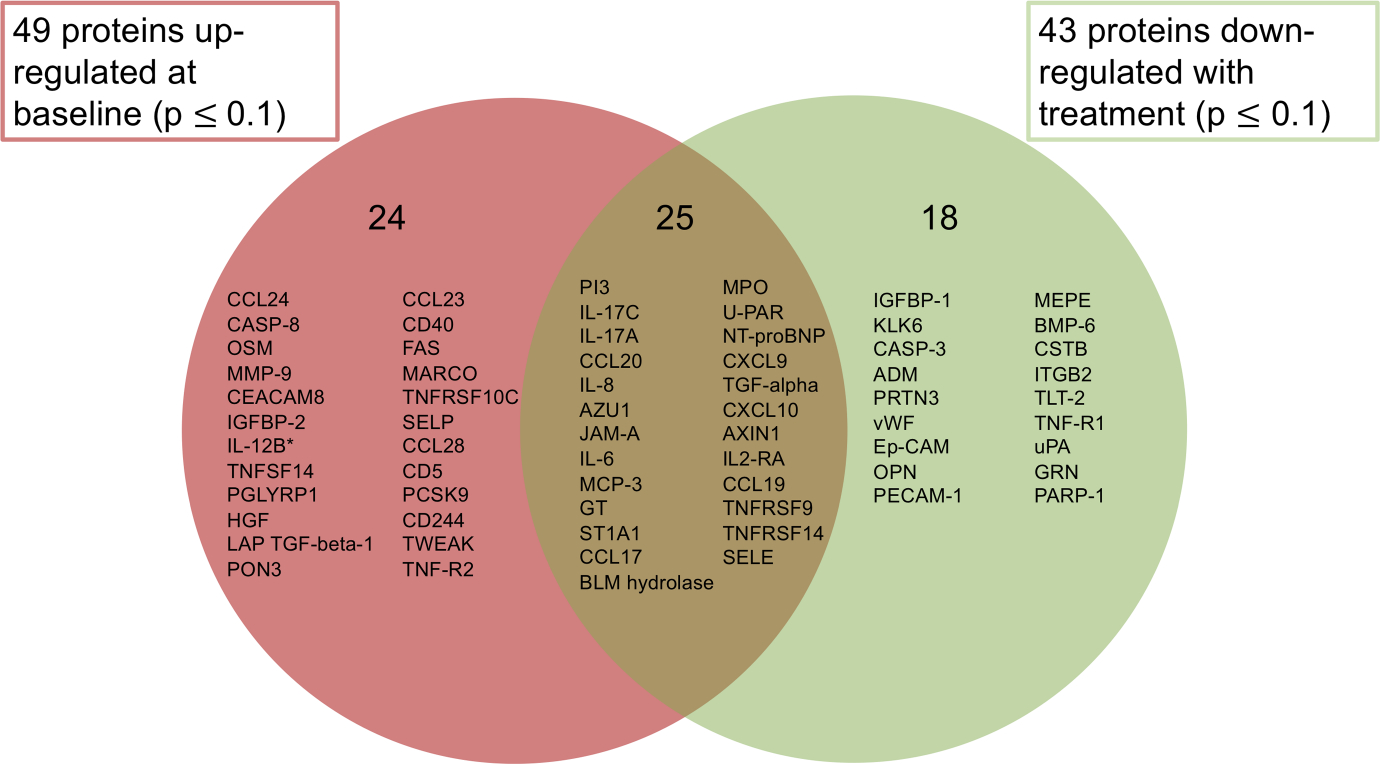
Proteins up-regulated proteins in psoriasis serum at baseline and down-regulated after 12 weeks of ustekinumab treatment. Serum samples from 10 psoriasis patients at baseline and after 12 weeks of ustekinumab treatment, and from 18 healthy controls were analysed for 276 protein concentrations using three panels (Cardiovascular II, Cardiovascular III and Inflammation) from the Olink high-throughput multiplex assay. Protein expression profiles were estimated under limma package framework using mixed-effect linear models. Fold changes (FCH) for condition (baseline/control) and timepoint (baseline/posttreatment Week 12) were calculated. Proteins were defined as differentially expressed if the average value in psoriasis serum was an increase or decrease relative to control with a p-value ≤0.1. Left: 49 proteins were up-regulated in psoriasis patients at baseline (p ≤ 0.1). Right: 43 proteins were down-regulated after 12 weeks of ustekinumab treatment (p ≤ 0.1). Middle: 25 proteins were up-regulated at baseline and down-regulated after treatment. *indicates modulation of IL-12B could not be assessed due to ustekinumab’s interference with the IL-12p40 subunit
We next sought to investigate the relationship between CVD and the 43 proteins that were down-regulated by ustekinumab. Our literature review identified that 28 of these proteins are associated with CVD (Tables S3 and S4).20–79 13 proteins were studied prospectively for their role in predicting future CV risk (Table S3), of which 10 were studied in non-CVD populations, thus allowing a more refined understanding of the protein’s impact on the risk of a primary CV event (IL-6, E-selectin (SELE), myeloperoxidase (MPO), von Willebrand factor (vWF), C-X-C motif chemokine ligand (CXCL)-10, cystatin B (CSTB), N-terminal prohormone brain natriuretic peptide (NT-proBNP), tumor necrosis factor receptor 1 (TNF-R1), IL-8 and urokinase plasminogen activator surface receptor (U-PAR), Table S3).
We compared the calculated percent increase of circulating protein levels associated with CVD in each study, to the percent increase in circulating protein levels in our psoriasis patients at baseline (Tables S3 and S4). Of the ten proteins studied prospectively as primary CV risk proteins in non-CVD populations, eight were elevated in our psoriasis patients by a percent increase that was above the percent increase associated with the CV risk in the literature (MPO, CXCL10, SELE, IL-6, CSTB, vWF, TNF-R1 and NT-proBNP, Table 1). For example, in Ridker et al.’s study, median IL-6 concentrations were approximately 24% higher in apparently healthy men who developed a myocardial infarction (MI) within 6 years compared with men who did not.41 Our psoriasis patients had a 95% increase (1.95 FCH) in circulating IL-6 levels at baseline compared with controls, placing them in the risk range for future MI outlined by this study.
TABLE 1.
Eight ustekinumab-modulated blood proteins in psoriasis are elevated to levels associated with future cardiovascular risk in the literature
| Protein | Fold change at baseline in psoriasis from control [equivalent percent increase] (p-value) | Fold change from baseline after treatment [equivalent percent decrease] (p-value) | Calculated % increase associated with CV risk in study | Study outcome | Study population |
|---|---|---|---|---|---|
| Myeloperoxidase (MPO) | 1.42 [42%] (0.06) |
−1.39 [39%] (0.02) |
10% | CAD development within 8 y | Apparently healthy men and women aged 40–79 without MI or stroke25 |
| C-X-C motif chemokine 10 (CXCL10) | 1.44 [44%] (0.04) |
−1.30 [30%] (0.03) |
34% | MI or SCD within 11 y | Men and women aged 35–74 without CHD39 |
| 26% | Ischemic stroke within 10 y | Asymptomatic men aged 50–59 without CAD or stroke44 | |||
| E-selectin (SELE) | 1.39 [39%] (0.04) |
−1.41 [41%] (0.01) |
22% | CVD event within 6 y | Type 2 diabetes patients of mean age 60.1 without CVD24 |
| Interleukin-6 (IL-6) | 1.95 [95%] (0.03) |
−1.56 [56%] (0.03) |
24% | MI within 6 y | Apparently healthy men aged 40–84 without CVD41 |
| Cystatin-B (CSTB) | 1.09 [9%] (0.55) |
−1.26 [26%] (0.05) |
7% | First MI or CHD related death within 14 y | Men and women aged 45–64 without CVD37 |
| von Willebrand factor (vWF) | 1.32 [32%] (0.20) |
−1.51 [51%] (0.01) |
12% | MI (fatal or non-fatal) within 5 y | Healthy men aged 50–59 with no previous CHD events20 |
| Tumor necrosis factor receptor 1 (TNF-R1) | 1.16 [16%] (0.14) |
−1.17 [17%] (0.05) |
14% | CHD within 8 y | Men and women aged 30–75 without CVD38 |
| N-terminal prohormone brain natriuretic peptide (NT-proBNP) | 1.42 [42%] (0.06) |
−1.37 [37%] (0.03) |
37% | First major CVD event within 5 ya | Men and women aged 30–59 without CVD36 |
The percent increases in protein levels of the psoriasis cohort at baseline were compared to the calculated percent increase in protein levels associated with future CV risk in the literature. Eight proteins were elevated in the psoriasis cohort at baseline by a percent increase that was above the percent increase associated with CV risk, and were significantly down-regulated after 12 weeks of ustekinumab treatment.
Abbreviations: AP, angina pectoris; CAD, coronary artery disease; CHD, coronary heart disease; CV, cardiovascular; CVD, cardiovascular disease; HF, heart failure; MI, myocardial infarction; SCD, sudden cardiac death; y, year.
This study measured BNP levels, which have been shown to correlate linearly with NT-proBNP.47
For the CV risk proteins in Table 1, we compared baseline and posttreatment protein levels to the protein’s CV risk threshold in a given study, both by individual patient (P)1–10, and by the LS means of the psoriasis group as a whole. For the CV risk proteins in Table 1, we compared LS mean and individual protein levels to the protein’s CV risk threshold in its respective study. Ustekinumab brought LS mean protein levels from above CV risk threshold at baseline to below risk threshold for CXCL10 (Figure 2A), SELE (Figure 2C), TNF-R1, CSTB, NT-proBNP, MPO and vWF (Figure S1.). For IL-6, ustekinumab lowered levels to the risk threshold but not below it (Figure 2B). Baseline protein levels varied widely between patients for all 8 proteins, showing heterogeneity in protein elevations between individuals. For example, only four of the ten patients (P1-P3 and P5) had baseline CXCL10 levels above the risk threshold, two of which were brought to below the risk threshold with treatment (Figure 2A).
FIGURE 2.
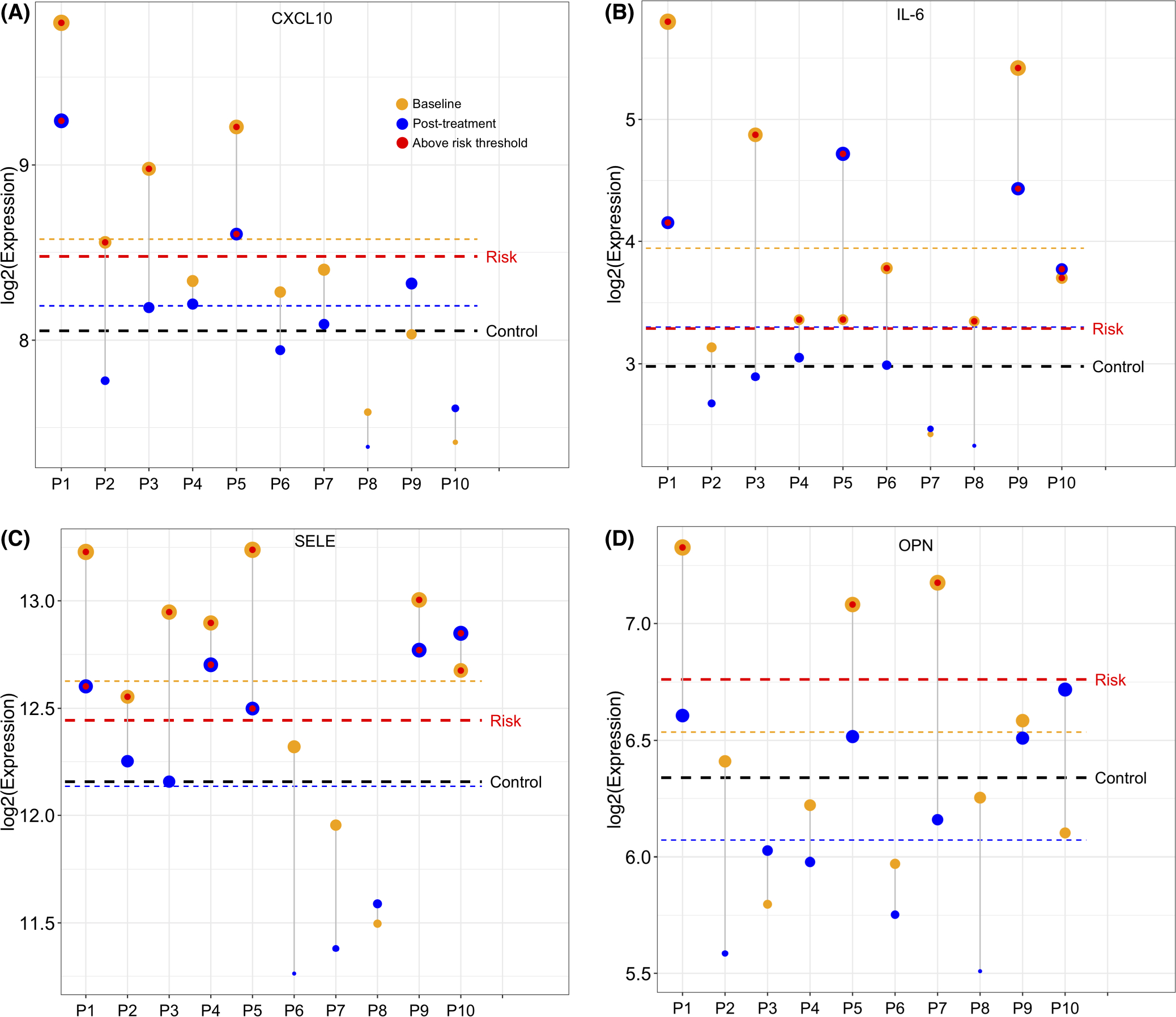
Mean and individual protein levels in psoriasis patients before and after ustekinumab treatment compared with the protein’s cardiovascular risk threshold. Red line indicates the calculated cardiovascular (CV) risk threshold for each protein according to its respective study. Yellow line indicates least squared (LS) mean protein level of psoriasis cohort at baseline. Blue line indicates LS mean protein level in psoriasis cohort after treatment. Black line indicates LS mean protein level in controls. P1-P10 indicates Patient 1—Patient 10. Yellow circles indicate individual patient’s protein level before treatment and blue circles indicate patient’s protein level after treatment. Red circle indicates protein level in individual is above CV risk threshold. CXCL10 = C-X-C motif chemokine 10; IL-6 = interleukin-6; SELE = E-selectin; OPN = osteopontin. (A) LS mean and individual CXCL10 levels in psoriasis cohort at baseline and after treatment in comparison with the CV risk threshold conferred by CXCL10 in Herder et al.’s study.39 (B–D) LS mean and individual protein levels at baseline and after treatment in comparison to CV risk threshold for IL-6 according to Ridker et al.41(B), for SELE according to Matsumoto et al.24(C), for OPN according to Kato et al.32(D)
Our complete literature review showed that many ustekinumab-modulated proteins in our patients at baseline were at levels associated with a CVD outlined in a study (vWF, SELE, U-PAR, NT-proBNP, CSTB, TNF-R1, IL-6, CXCL10, CXCL9, C-C motif chemokine ligand 17, insulin-like growth factor-binding protein 1, junctional adhesion molecule A, platelet endothelial cell adhesion molecule, IL-2 receptor alpha, bleomycin hydrolase and IL-8, Tables S2 and S3). When the LS mean protein level in our patients did not pass the CV risk threshold of a particular protein established in a given study, select individuals were still often found to fall within risk range, due to the heterogeneity of protein levels across individuals. For example, while the LS mean baseline elevation of osteopontin (OPN) in our patients did not meet the risk threshold for MACE within 3 years according to Kato et al.’s study, three patients (P1, P5 and P7) had OPN levels above the risk threshold, and treatment lowered their levels to below risk threshold (Figure 2D).32
Observing a heterogeneity in protein levels between patients, we sought to frame the CV risk in individual patients. For this analysis, we selected the 12 studies assessing 10 unique proteins as CV risk proteins in non-CVD populations (IL-6, SELE, MPO, vWF, CXCL10, CSTB, NT-proBNP, TNF-R1, IL-8 and U-PAR, Table S3) and measured the number of studies identifying a CV risk in each patient according to each patient’s protein levels, both before and after treatment. Patients differed in number of studies for which they were above the CV risk threshold (Figure 3). For example, P1’s baseline protein levels passed the risk threshold for the greatest number of studies, fulfilling risk criteria for all 12 studies. After treatment, P1 passed the CV risk threshold in 10 studies, indicating a slight reduction of risk factors. In contrast, P6 fulfilled risk criteria for 6 studies before treatment but no studies after treatment, indicating potential elimination of risk factors. An elevation of risk factors after treatment was suggested only in P10.
FIGURE 3.
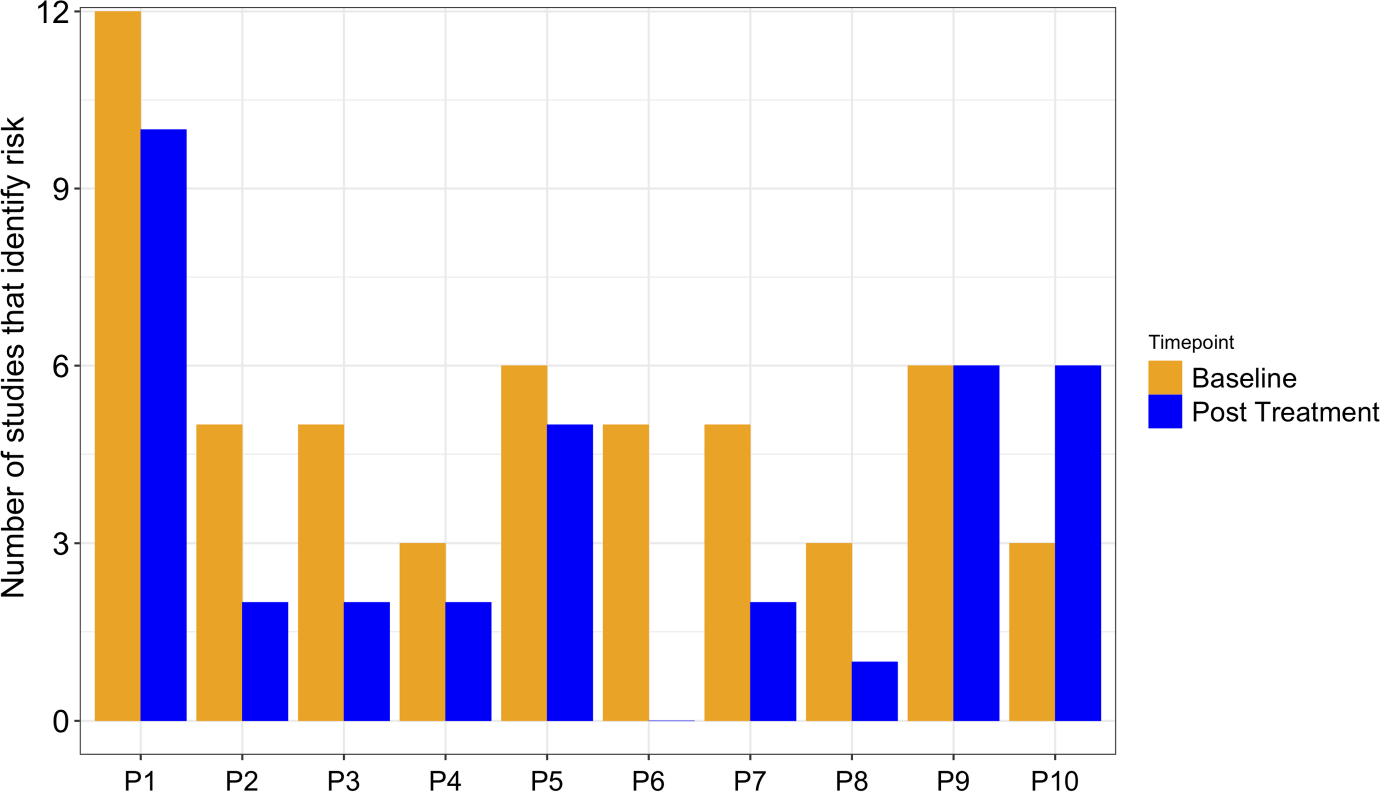
Number of protein studies that identify a cardiovascular risk in each patient. 12 studies (Ridker et al.,41 Matsumoto et al.,24 Meuwese et al.,25 Morange et al.,20 Canoui-Poitrine et al.,44 Gonçalves et al.,37 Wang et al.,36 Herder et al.,39 Pai et al.,38 Doi et al.,34 Herder et al.,39 Persson et al.31) assessing the association of ten proteins (IL-6, SELE, MPO, vWF, CXCL10, CSTB, TNF-R1, NT-proBNP, IL-8 and U-PAR) with primary cardiovascular (CV) risk in non-CVD populations were included in the analysis. The CV risk threshold for each protein was calculated from its respective study in the literature, and compared with each patient’s protein levels at baseline and after treatment. The number of studies per patient that identify a protein level above a risk threshold is shown here. P1-P10 indicates patient 1—patient 10
Using the same 10 proteins studied as risk proteins in non-CVD populations, we identified the most frequently elevated proteins in our patients (Figure 4). In 80% of our patients, IL-6 levels were above the level associated with MI within 6 years according to Ridker et al.41 Additional ustekinumab-modulated proteins that were elevated beyond a risk level according to their respective studies in ≥50% of our patients includes SELE, MPO, vWF, CXCL10 and CSTB.
FIGURE 4.
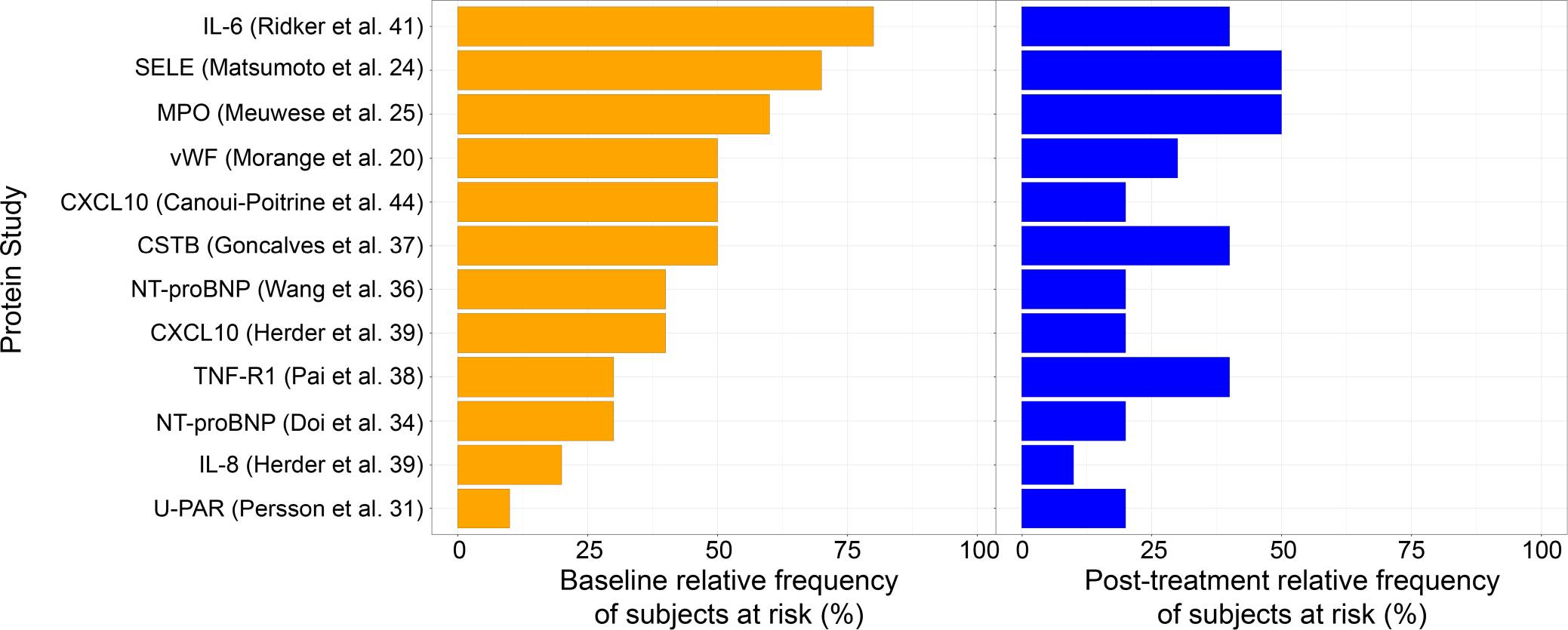
Relative frequency of patients that meet cardiovascular risk threshold for each protein study. 12 studies (Ridker et al.,41 Matsumoto et al.,24 Meuwese et al.,25 Morange et al.,20 Canoui-Poitrine et al.,44 Gonçalves et al.,37 Wang et al.,36 Herder et al.,39 Pai et al.,38 Doi et al.,34 Herder et al.,39 Persson et al.31) assessing the association of ten proteins (IL-6, SELE, MPO, vWF, CXCL10, CSTB, TNF-R1, NT-proBNP, IL-8, U-PAR) with primary cardiovascular (CV) risk in non-CVD populations were included in the analysis. The CV risk threshold for each protein was calculated from its respective study in the literature, and compared with each patient’s protein levels at baseline and after treatment. The frequency of patients with protein levels above a CV risk threshold in a given study, both before and after treatment, is shown here
We measured the correlations between the proteins studied as risk proteins in non-CVD populations (Figure S2). NT-proBNP and vWF were not correlated with other proteins. Therefore, these proteins might represent at least 3 independent risk pathways in psoriasis. SELE (0.93, p < 0.001) and TNF-R1 (0.71, p < 0.05) correlated with BMI, but still showed reductions posttreatment. While the 10 primary CV risk proteins did not correlate with PASI, overall, we identified 25 proteins that correlated with PASI at baseline and 26 that correlated with PASI change at Week 12 (p ≤ 0.05), which includes three ustekinumab-modulated proteins (IL-17A, integrin beta-2 and matrix extracellular phosphoglycoprotein) (Table S5).
4 |. DISCUSSION
In this study, 12 weeks of ustekinumab treatment reduced 43 inflammatory and CV-related proteins in psoriasis. Mean elevation of many of the ustekinumab-modulated proteins in our psoriasis patients was at levels associated with clinical CVD in the literature, and 8 proteins were present at levels shown to confer enhanced CV risk in non-CVD populations, specifically MPO, CXCL10, SELE, IL-6, CSTB, vWF, TNF-R1 and NT-proBNP. We showed a heterogeneity in risk protein levels between individuals, and identified that IL-6, SELE, MPO, CXCL10, CSTB and vWF were commonly elevated proteins, as they were above the risk threshold in >50% of our patients. Except for IL-6, ustekinumab lowered these 8 proteins to below risk threshold, suggesting that ustekinumab is an important modulator of CV risk proteins in psoriasis. These proteins may aid in CV risk stratification and management and warrant further study into their prognostic value as ustekinumab-modulated CV risk biomarkers in psoriasis.
IL-6 was increased by 95% compared with controls and was above the CV risk threshold in 80% of our patients. IL-6 is known to be highly expressed in lesional skin and might be linked to impaired vascular health in psoriasis via inflammasome signalling (IL-1B/IL-6 axis) and endothelial activation, a key early step in atherosclerosis development.80,81 A link between IL-6 and CV risk has been established, as reduction of IL-6 via IL-1B modulation reduced MACE by 32% and CV mortality by 52% in atherosclerosis patients.12 In our psoriasis patients, IL-6 was significantly reduced with treatment, but was not lowered to below the CV risk threshold, suggesting that while ustekinumab can mitigate some risk conferred by IL-6, patients treated with ustekinumab might have residual IL-6 related CV risk despite successful psoriasis skin treatment.
Previous studies have used varying approaches to evaluate how psoriasis biologics modulate systemic inflammation and CV risk, and the data on ustekinumab remains unclear.82 While one study showed that ustekinumab decreased MACE over 4 years, conflicting outcome studies showed no effect on MACE.83 Studies using imaging techniques suggest that biologics can improve vascular inflammation underlying CVD. For example, a prospective study using coronary computed tomography (CT) showed reduction of coronary artery disease progression with biologics. This study included ustekinumab, but did not differentiate ustekinumab’s effects from other biologics studied.84 In a recent study that included ustekinumab, coronary CT angiography showed modification of a high-risk coronary plaque feature with 1 year of biologic therapy.15 The vascular inflammation in psoriasis-ustekinumab (VIP-U) trial showed reduced aortic vascular inflammation measured by FDG-PET/CT in psoriasis after 12 weeks of ustekinumab use; however, this reduction was transient. A secondary endpoint in VIP-U confirmed an enduring reduction of inflammatory blood biomarkers associated with atherosclerotic CV disease at 52 weeks, specifically tumor necrosis factor (TNF)-α, IL-1B, IL-17A and IL-6.3
Our study, covering a large number of potential biomarkers, expands on the observations made in the VIP-U trial and contributes to a better understanding of ustekinumab’s effects on CVD risk factors. Proteomic studies provide an additional means to assess systemic inflammation and CV risk in psoriasis, and are more cost-effective and safer than imaging studies which require radiation. However, with fewer blood proteomic studies in psoriasis, it has been difficult to interpret and appreciate the significance of smaller changes in circulating protein levels compared with the larger transcriptome changes that occur in lesional skin, where proteins have been shown to be increased by over 1000 FCH (S100A12). For example, a study showed that SELE is increased by 7.77 FCH in lesional psoriatic skin, and decreased by 5.04 FCH with ustekinumab treatment. By contrast, in our study, SELE was only increased by 1.39 FCH in the serum, and was decreased by 1.41 FCH after treatment; however, we show that these smaller changes are significant for CV risk.18 Overall, our study shows that relatively modest elevations in blood proteins in psoriasis, compared with elevations in lesional skin, are potentially important for conferring CV risk. Our literature review provides a framework for future biomarker studies to interpret the risks associated with elevations in psoriasis blood proteins.
We compared our findings with a similar Olink study that assessed protein modulation with tofacitinib and etanercept.16 We found an overlap of 9 proteins modulated by ustekinumab, tofacitinib (a Janus Tyrosine Kinase [JAK]1/JAK3 inhibitor) and etanercept (a TNF-α antagonist). These proteins might represent common pathways contributing to the psoriasis phenotype that improve in the skin with an effective treatment and are then reflected in the circulation (kallikrein-6 [KLK-6], CCL20, IL-17C, IL-17A, CXCL9, TNF-R1, SELE, IL-6 and CXCL10, Figure S3). By contrast, 34 proteins were reduced by ustekinumab but not by tofacitinib or etanercept, some of which are primary CV risk proteins in the literature (MPO, U-PAR, CSTB, IL-8, NT-proBNP and vWF).
These differences might be due to differences in follow-up time and significance parameters between studies and analysis of slightly different protein panel. These differences might also reflect mechanistic differences between biologics. Future studies comparing multiple biologics across a common panel of proteins can lend further insight into the differential impact of biologics on inflammatory pathways and CV risk proteins in psoriasis. The differential modulation of risk proteins by biologics, in addition to the heterogeneity in protein elevation between patients, supports an individualized approach to CV risk stratification and management in psoriasis, where a specific biologic is selected for its ability to reduce select risk proteins in an individual. Our study can provide a first step towards a future patient-tailored approach.
Our study has limitations. We studied a small number of patients; however, the sample size is comparable with other studies.85 The Olink methodology provides a relative quantification, rather than an absolute quantification of proteins. However, Olink is an ultra-sensitive method that allowed us to study and analyse a large panel of proteins using relatively small amount of serum which would not have been possible with other methodologies including ELISA. In addition, we do not have data to characterize the exact cardiovascular phenotype of our patients including cardiovascular comorbidities; however, enrolled patients were relatively healthy and patients with signs or symptoms of severe cardiac disease or history of heart failure were excluded. While our patients might have CV comorbidities, they represent the general psoriasis population which we intended to study. We do not have data on the breakdown of treatment doses (45 mg vs 90 mg) which might account for heterogeneity in responses. While Kim et al. found that IL-6, CXCL10 and IL-8 correlated with PASI, we did not find correlations between PASI and these proteins, which might be due to sample size. Future studies can clarify the relationship between PASI and these proteins. We cannot confirm whether ustekinumab causes long-term changes in protein levels beyond 12 weeks. Our analysis is limited by the studies included in our literature review, which were not randomized control trials, and thus do not establish a direct link between protein and CV risk. Finally, we have not followed patients longitudinally for CV outcomes; so, we cannot conclude that reduction of these proteins with ustekinumab impacts cardiac events.
In conclusion, 12 weeks of ustekinumab treatment reduced inflammatory and CV-related proteins that were present at levels associated with CV risk in psoriasis patients. We identified potential CV risk biomarkers that are modulated by ustekinumab and may be implicated in improving patient-tailored CV risk stratification and management in psoriasis; however, additional studies are needed to confirm our findings.
Supplementary Material
Figure S1. Protein elevations in psoriasis group and in individual patients before and after treatment in comparison to each protein’s cardiovascular risk threshold in the literature for the remaining Table 1 proteins. Red line indicates the calculated cardiovascular (CV) risk thresholds for each protein according to its respective study. Yellow line indicates least squared (LS) mean protein level of psoriasis cohort at baseline. Black line indicates LS mean protein level in controls. Blue line indicates LS mean protein level in psoriasis cohort after treatment. P1-P10 indicates Patient 1 –Patient 10. Yellow circles indicate individual patient’s protein level before treatment and blue circles indicate patient’s protein level after treatment. Red circle indicates patient’s protein level is above CV risk threshold. P = patient; TNF-R1 = Tumor necrosis factor receptor 1 (TNF-R1); CSTB = Cystatin B; N-terminal prohormone brain natriuretic peptide = NT-proBNP; CXCL10 = C-X-C motif chemokine 10; MPO = myeloperoxidase; vWF = von Willebrand Factor. (A) TNF-R1 levels in psoriasis cohort and in individual patients before and after treatment in comparison to CV risk threshold conferred by TNF-R1 in Pai et al’s study.38 (B–F) For CSTB according to Gonçalves et al.37(B); for NT-proBNP according to Wang et al.36(C); for CXCL10 according to Canoui-Poitrine et al.44(D), for MPO according to Meuwese et al.25(E); for vWF according to Morange et al.20(E).
Figure S2. Correlations between primary cardiovascular risk proteins and clinical parameters. Correlations among proteins, body mass index (BMI), psoriasis area and severity index at baseline (PASI-BL), PASI change after treatment (PASI-CHANGE), and PASI improvement (PASI-IMPR), were assessed using univariate Pearson correlation and Chi-squared test. Cor indicates correlation coefficient, CAR indicates cardiovascular II/III panel, INF indicates inflammation panel.
Figure S3. Protein modulation by ustekinumab compared to etanercept and tofacitinib. Left: Cardiovascular and inflammatory proteins modulated by tofacitinib and etanercept (FCH > 1.2, FDR < 0.05)16, *indicates etanercept modulated a subset of the tofacitinib-modulated proteins, plus CCL4. Right: cardiovascular and inflammatory proteins modulated by ustekinumab in our study (p ≤ 0.1), **indicates proteins associated with primary CV risk in our analysis. vWF and OPN were not included in Kim et al’s study16.
Table S1. Characteristics of patients and controls
Table S2. Differentially expressed proteins before and after ustekinumab treatment.
Table S3. Ustekinumab-modulated proteins with prospective studies assessing protein’s association with future cardiovascular risk.
Table S4. Ustekinumab-modulated proteins with cross-sectional studies assessing protein’s association with cardiovascular disease.
Table S5. Correlations with Psoriasis Area and Severity Index (PASI) at baseline and after treatment.
ACKNOWLEDGEMENTS
We thank Carrie Brodmerkel, Katherine Li and Kim Campbell for providing serum samples used in this study and careful reading of the manuscript. Financial support was provided, in part, by an American Heart Association Career Development Grant 18CDA34080540, National Psoriasis Foundation Bridge Grant and K23 Career Development Award (K23 HL152013) to Michael Garshick.
Footnotes
CONFLICT OF INTEREST
Dr. Krueger has received grants paid to institution from Novartis, Pfizer, Amgen, Lilly, Boehringer, Innovaderm, BMS, Janssen, Abbvie, Paraxel, Leo Pharma, Vitae, Akros, Regeneron, Allergan, Novan, Biogen MA, Sienna, UCB, Celgene, Botanix, Incyte, Avillion, Exicure; has received personal fees from Novartis, Pfizer, Amgen, Lilly, Boehringer, BiogenIdec, Abbvie, Leo Pharma, Escalier, Valeant, Aurigne, Allergan, Asana, UCB, Sienna, Celgene, Nimbus, Menlo, Aristea, Sanofi, Sun Pharma, Almirall, Arena, BMS, Ventyx, Aclaris, Galapagos.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the supplementary material of this article.
REFERENCES
- 1.Naik HB, Natarajan B, Stansky E, et al. Severity of psoriasis associates with aortic vascular inflammation detected by FDG PET/CT and neutrophil activation in a prospective observational study. Arterioscler Thromb Vasc Biol. 2015;35(12):2667–2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garshick Michael S, Ward Nicole L, Krueger James G, Berger JS. Cardiovascular risk in patients with psoriasis. J Am Coll Cardiol. 2021;77(13):1670–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gelfand JM, Shin DB, Alavi A, et al. A phase IV, randomized, double-blind, placebo-controlled crossover study of the effects of Ustekinumab on vascular inflammation in psoriasis (the VIP-U trial). J Invest Dermatol. 2020;140(1):85–93.e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dey AK, Joshi AA, Chaturvedi A, et al. Association between skin and aortic vascular inflammation in patients with psoriasis: A case-cohort study using positron emission tomography/computed tomography. JAMA Cardiol. 2017;2(9):1013–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garshick Michael S, Tawil M, Barrett Tessa J, et al. Activated platelets induce endothelial cell inflammatory response in psoriasis via COX-1. Arterioscler Thromb Vasc Biol. 2020;40(5):1340–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coimbra S, Oliveira H, Reis F, et al. Psoriasis therapy and cardiovascular risk factors: a 12-week follow-up study. Am J Clin Dermatol. 2010;11(6):423–432. [DOI] [PubMed] [Google Scholar]
- 7.Mehta NN, Yu Y, Pinnelas R, et al. Attributable risk estimate of severe psoriasis on major cardiovascular events. Am J Med. 2011;124(8):775.e771–775.e776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80(4):1073–1113. [DOI] [PubMed] [Google Scholar]
- 9.Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary: A report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. 2019;74(10):1376–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mansouri B, Kivelevitch D, Natarajan B, et al. Comparison of coronary artery calcium scores between patients with psoriasis and type 2 diabetes. JAMA Dermatol. 2016;152(11):1244–1253. [DOI] [PubMed] [Google Scholar]
- 11.Mehta NN, Yu Y, Saboury B, et al. Systemic and vascular inflammation in patients with moderate to severe psoriasis as measured by [18F]-fluorodeoxyglucose positron emission tomography-computed tomography (FDG-PET/CT): a pilot study. Arch Dermatol. 2011;147(9):1031–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ridker PM, Libby P, MacFadyen JG, et al. Modulation of the interleukin-6 signalling pathway and incidence rates of atherosclerotic events and all-cause mortality: analyses from the canakinumab anti-inflammatory thrombosis outcomes study (CANTOS). Eur Heart J. 2018;39(38):3499–3507. [DOI] [PubMed] [Google Scholar]
- 13.Choi S The potential role of biomarkers associated with ASCVD risk: risk-enhancing biomarkers. J Lipid Atheroscler. 2019;8(2):173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gelfand JM, Shin DB, Duffin KC, et al. A randomized placebo-controlled trial of Secukinumab on aortic vascular inflammation in moderate-to-severe plaque psoriasis (VIP-S). J Invest Dermatol. 2020;140(9):1784–1793.e1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi H, Uceda DE, Dey AK, et al. Treatment of psoriasis with biologic therapy is associated with improvement of coronary artery plaque lipid-rich necrotic Core. Circulation. Cardiovasc Imaging. 2020;13(9):e011199. [DOI] [PubMed] [Google Scholar]
- 16.Kim J, Tomalin L, Lee J, et al. Reduction of inflammatory and cardiovascular proteins in the blood of patients with psoriasis: differential responses between tofacitinib and etanercept after 4 weeks of treatment. J Invest Dermatol. 2018;138(2):273–281. [DOI] [PubMed] [Google Scholar]
- 17.Lin P-T, Wang S-H, Chi C-C. Drug survival of biologics in treating psoriasis: a meta-analysis of real-world evidence. Sci Rep. 2018;8(1):16068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brodmerkel C, Li K, Garcet S, et al. Modulation of inflammatory gene transcripts in psoriasis vulgaris: differences between ustekinumab and etanercept. J Allergy Clin Immunol. 2019;143(5):1965–1969. [DOI] [PubMed] [Google Scholar]
- 19.Martin DA, Towne JE, Kricorian G, et al. The emerging role of IL-17 in the pathogenesis of psoriasis: preclinical and clinical findings. Journal of Investigative Dermatology. 2013;133(1):17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morange PE, Simon C, Alessi MC, et al. Endothelial cell markers and the risk of coronary heart disease: the prospective epidemiological study of myocardial infarction (PRIME) study. Circulation. 2004;109(11):1343–1348. [DOI] [PubMed] [Google Scholar]
- 21.Haines AP, Howarth D, North WR, et al. Haemostatic variables and the outcome of myocardial infarction. Thromb Haemost. 1983;50(4):800–803. [PubMed] [Google Scholar]
- 22.Wiman B, Andersson T, Hallqvist J, Reuterwall C, Ahlbom A, de-Faire U. Plasma levels of tissue plasminogen activator/plasminogen activator Inhibitor-1 complex and von Willebrand factor are significant risk markers for recurrent myocardial infarction in the Stockholm heart epidemiology program (SHEEP) study. Arterioscler Thromb Vasc Biol. 2000;20(8):2019–2023. [DOI] [PubMed] [Google Scholar]
- 23.Thompson SG, Kienast J, Pyke SD, Haverkate F, van de Loo JC. Hemostatic factors and the risk of myocardial infarction or sudden death in patients with angina pectoris. European concerted action on thrombosis and disabilities angina pectoris study group. N Engl J Med. 1995;332(10):635–641. [DOI] [PubMed] [Google Scholar]
- 24.Matsumoto K, Fujishima K, Moriuchi A, Saishoji H, Ueki Y. Soluble adhesion molecule E-selectin predicts cardiovascular events in Japanese patients with type 2 diabetes mellitus. Metabolism. 2010;59(3):320–324. [DOI] [PubMed] [Google Scholar]
- 25.Meuwese MC, Stroes ES, Hazen SL, et al. Serum myeloperoxidase levels are associated with the future risk of coronary artery disease in apparently healthy individuals: the EPIC-Norfolk prospective population study. J Am Coll Cardiol. 2007;50(2):159–165. [DOI] [PubMed] [Google Scholar]
- 26.Heslop CL, Frohlich JJ, Hill JS. Myeloperoxidase and C-reactive protein have combined utility for long-term prediction of cardiovascular mortality after coronary angiography. J Am Coll Cardiol. 2010;55(11):1102–1109. [DOI] [PubMed] [Google Scholar]
- 27.Brennan M-L, Penn MS, Van Lente F, et al. Prognostic value of myeloperoxidase in patients with chest pain. N Engl J Med. 2003;349(17):1595–1604. [DOI] [PubMed] [Google Scholar]
- 28.Morrow DA, Sabatine MS, Brennan ML, et al. Concurrent evaluation of novel cardiac biomarkers in acute coronary syndrome: myeloperoxidase and soluble CD40 ligand and the risk of recurrent ischaemic events in TACTICS-TIMI 18. Eur Heart J. 2008;29(9):1096–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jalkanen V, Vaahersalo J, Pettilä V, et al. The predictive value of soluble urokinase plasminogen activator receptor (SuPAR) regarding 90-day mortality and 12-month neurological outcome in critically ill patients after out-of-hospital cardiac arrest. Data from the prospective FINNRESUSCI study. Resuscitation. 2014;85(11):1562–1567. [DOI] [PubMed] [Google Scholar]
- 30.Lyngbæk S, Marott JL, Sehestedt T, et al. Cardiovascular risk prediction in the general population with use of suPAR, CRP, and Framingham risk score. Int J Cardiol. 2013;167(6):2904–2911. [DOI] [PubMed] [Google Scholar]
- 31.Persson M, Östling G, Smith G, et al. Soluble urokinase plasminogen activator receptor: a risk factor for carotid plaque, stroke, and coronary artery disease. Stroke. 2014;45(1):18–23. [DOI] [PubMed] [Google Scholar]
- 32.Kato R, Momiyama Y, Ohmori R, et al. Prognostic significance of plasma osteopontin levels in patients undergoing percutaneous coronary intervention. Circ J. 2009;73(1):152–157. [DOI] [PubMed] [Google Scholar]
- 33.Rudolf H, Mügge A, Trampisch HJ, Scharnagl H, März W, Kara K. NT-proBNP for risk prediction of cardiovascular events and all-cause mortality: the getABI-study. Int J Cardiol Heart Vasc. 2020;29:100553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Doi Y, Ninomiya T, Hata J, et al. N-terminal pro-brain natriuretic peptide and risk of cardiovascular events in a Japanese community. Arterioscler Thromb Vasc Biol. 2011;31(12):2997–3003. [DOI] [PubMed] [Google Scholar]
- 35.deFilippi CR, Christenson RH, Gottdiener JS, Kop WJ, Seliger SL. Dynamic cardiovascular risk assessment in elderly people. The role of repeated N-terminal pro-B-type natriuretic peptide testing. J Am Coll Cardiol. 2010;55(5):441–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang TJ, Larson MG, Levy D, et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;350(7):655–663. [DOI] [PubMed] [Google Scholar]
- 37.Gonçalves I, Hultman K, Dunér P, et al. High levels of cathepsin D and cystatin B are associated with increased risk of coronary events. Open Heart. 2016;3(1):e000353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pai JK, Pischon T, Ma J, et al. Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med. 2004;351(25):2599–2610. [DOI] [PubMed] [Google Scholar]
- 39.Herder C, Baumert J, Thorand B, et al. Chemokines and incident coronary heart disease: results from the MONICA/KORA Augsburg case-cohort study, 1984–2002. Arterioscler Thromb Vasc Biol. 2006;26(9):2147–2152. [DOI] [PubMed] [Google Scholar]
- 40.Inoue T, Komoda H, Nonaka M, Kameda M, Uchida T, Node K. Interleukin-8 as an independent predictor of long-term clinical outcome in patients with coronary artery disease. Int J Cardiol. 2008;124(3):319–325. [DOI] [PubMed] [Google Scholar]
- 41.Ridker Paul M, Rifai N, Stampfer Meir J, Hennekens CH. Plasma concentration of Interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101(15):1767–1772. [DOI] [PubMed] [Google Scholar]
- 42.Fan ZX, Hua Q, Li YP, Liu RK, Yang Z. Interleukin-6, but not soluble adhesion molecules, predicts a subsequent mortality from cardiovascular disease in patients with acute ST-segment elevation myocardial infarction. Cell Biochem Biophys. 2011;61(2):443–448. [DOI] [PubMed] [Google Scholar]
- 43.Simon T, Taleb S, Danchin N, et al. Circulating levels of interleukin-17 and cardiovascular outcomes in patients with acute myocardial infarction. Eur Heart J. 2013;34(8):570–577. [DOI] [PubMed] [Google Scholar]
- 44.Canouï-Poitrine F, Luc G, Mallat Z, et al. Systemic chemokine levels, coronary heart disease, and ischemic stroke events: the PRIME study. Neurology. 2011;77(12):1165–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ng LL, Khan SQ, Narayan H, Quinn P, Squire IB, Davies JE. Proteinase 3 and prognosis of patients with acute myocardial infarction. Clin Sci (Lond). 2011;120(6):231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harrela M, Qiao Q, Koistinen R, et al. High serum insulin-like growth factor binding protein-1 is associated with increased cardiovascular mortality in elderly men. Horm Metab Res. 2002;34(3):144–149. [DOI] [PubMed] [Google Scholar]
- 47.Willeit P, Kaptoge S, Welsh P, et al. Natriuretic peptides and integrated risk assessment for cardiovascular disease: an individual-participant-data meta-analysis. Lancet Diab Endocrinol. 2016;4(10):840–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ye Y, Yang X, Zhao X, et al. Serum chemokine CCL17/thymus activation and regulated chemokine is correlated with coronary artery diseases. Atherosclerosis. 2015;238(2):365–369. [DOI] [PubMed] [Google Scholar]
- 49.Banach J, Gilewski W, Słomka A, et al. Bone morphogenetic protein 6—a possible new player in pathophysiology of heart failure. Clin Exp Pharmacol Physiol. 2016;43(12):1247–1250. [DOI] [PubMed] [Google Scholar]
- 50.Zheng W, Lai Y, Jin P, Gu W, Zhou Q, Wu X. Association of Circulating IGFBP1 level with the severity of coronary artery lesions in patients with unstable angina. Dis Markers. 2017;2017:1917291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaplan RC, McGinn AP, Pollak MN, et al. High insulinlike growth factor binding protein 1 level predicts incident congestive heart failure in the elderly. Am Heart J. 2008;155(6):1006–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ipek E, Yolcu M, Yildirim E, et al. A novel marker of inflammation: Azurocidin in patients with ST segment elevation myocardial infarction. Int J Mol Sci. 2018;19(12):3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cavusoglu E, Kornecki E, Sobocka MB, et al. Association of plasma levels of F11 receptor/junctional adhesion molecule-A (F11R/JAM-A) with human atherosclerosis. J Am Coll Cardiol. 2007;50(18):1768–1776. [DOI] [PubMed] [Google Scholar]
- 54.Matulevicius S, Rohatgi A, Khera A, et al. The association between plasma caspase-3, atherosclerosis, and vascular function in the Dallas heart study. Apoptosis. 2008;13(10):1281–1289. [DOI] [PubMed] [Google Scholar]
- 55.Khadir A, Madhu D, Kavalakatt S, et al. PR3 levels are impaired in plasma and PBMCs from Arabs with cardiovascular diseases. PLoS One. 2020;15(1):e0227606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ndrepepa G, Braun S, Mehilli J, von Beckerath N, Schömig A, Kastrati A. Myeloperoxidase level in patients with stable coronary artery disease and acute coronary syndromes. Eur J Clin Invest. 2008;38(2):90–96. [DOI] [PubMed] [Google Scholar]
- 57.Eapen DJ, Manocha P, Ghasemzadeh N, et al. Soluble urokinase plasminogen activator receptor level is an independent predictor of the presence and severity of coronary artery disease and of future adverse events. J Am Heart Assoc. 2014;3(5):e001118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abdel-Azeez HA, Al-Zaky M. Plasma osteopontin as a predictor of coronary artery disease: association with echocardiographic characteristics of atherosclerosis. J Clin Lab Anal. 2010;24(3):201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koshikawa M, Aizawa K, Kasai H, et al. Elevated osteopontin levels in patients with peripheral arterial disease. Angiology. 2009;60(1):42–45. [DOI] [PubMed] [Google Scholar]
- 60.Ohmori R, Momiyama Y, Taniguchi H, et al. Plasma osteopontin levels are associated with the presence and extent of coronary artery disease. Atherosclerosis. 2003;170(2):333–337. [DOI] [PubMed] [Google Scholar]
- 61.Fang L, Wei H, Chowdhury SH, et al. Association of Leu125Val polymorphism of platelet endothelial cell adhesion molecule-1 (PECAM-1) gene & soluble level of PECAM-1 with coronary artery disease in Asian Indians. Indian J Med Res. 2005;121(2):92–99. [PubMed] [Google Scholar]
- 62.Soeki T, Tamura Y, Shinohara H, Sakabe K, Onose Y, Fukuda N. Increased soluble platelet/endothelial cell adhesion molecule-1 in the early stages of acute coronary syndromes. Int J Cardiol. 2003;90(2–3):261–268. [DOI] [PubMed] [Google Scholar]
- 63.Serebruany VL, Murugesan SR, Pothula A, Semaan H, Gurbel PA. Soluble PECAM-1, but not P-selectin, nor osteonectin identify acute myocardial infarction in patients presenting with chest pain. Cardiology. 1999;91(1):50–55. [DOI] [PubMed] [Google Scholar]
- 64.Sakamoto A, Ishizaka N, Saito K, et al. Serum levels of IgG4 and soluble interleukin-2 receptor in patients with coronary artery disease. Clin Chim Acta. 2012;413(5):577–581. [DOI] [PubMed] [Google Scholar]
- 65.Wang Y, Liu CL, Lindholt JS, Shi GP, Zhang J. Plasma cystatin B association with abdominal aortic aneurysms and need for later surgical repair: A sub-study of the VIVA trial. Eur J Vasc Endovasc Surg. 2018;56(6):826–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Memon AA, Sundquist K, PirouziFard M, et al. Identification of novel diagnostic biomarkers for deep venous thrombosis. Br J Haematol. 2018;181(3):378–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou RH, Shi Q, Gao HQ, Shen BJ. Changes in serum interleukin-8 and interleukin-12 levels in patients with ischemic heart disease in a Chinese population. J Atheroscler Thromb. 2001;8(1):30–32. [DOI] [PubMed] [Google Scholar]
- 68.Hashmi S, Zeng QT. Role of interleukin-17 and interleukin-17-induced cytokines interleukin-6 and interleukin-8 in unstable coronary artery disease. Coron Artery Dis. 2006;17(8):699–706. [DOI] [PubMed] [Google Scholar]
- 69.Rothenbacher D, Müller-Scholze S, Herder C, Koenig W, Kolb H. Differential expression of chemokines, risk of stable coronary heart disease, and correlation with established cardiovascular risk markers. Arterioscler Thromb Vasc Biol. 2006;26(1):194–199. [DOI] [PubMed] [Google Scholar]
- 70.Ardigo D, Assimes TL, Fortmann SP, et al. Circulating chemokines accurately identify individuals with clinically significant atherosclerotic heart disease. Physiol Genomics. 2007;31(3):402–409. [DOI] [PubMed] [Google Scholar]
- 71.Wainstein MV, Mossmann M, Araujo GN, et al. Elevated serum interleukin-6 is predictive of coronary artery disease in intermediate risk overweight patients referred for coronary angiography. Diabetol Metab Syndr. 2017;9(1):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liang J, Zheng Z, Wang M, et al. Myeloperoxidase (MPO) and Interleukin-17 (IL-17) plasma levels are increased in patients with acute coronary syndromes. J Int Med Res. 2009;37(3):862–866. [DOI] [PubMed] [Google Scholar]
- 73.Salem MK, Butt HZ, Choke E, et al. Gene and Protein expression of chemokine (C-C-motif) ligand 19 is upregulated in unstable carotid atherosclerotic plaques. Eur J Vasc Endovasc Surg. 2016;52(4):427–436. [DOI] [PubMed] [Google Scholar]
- 74.Damås JK, Smith C, Øie E, et al. Enhanced expression of the homeostatic chemokines CCL19 and CCL21 in clinical and experimental atherosclerosis: possible pathogenic role in plaque destabilization. Arterioscler Thromb Vasc Biol. 2007;27(3):614–620. [DOI] [PubMed] [Google Scholar]
- 75.Altara R, Gu YM, Struijker-Boudier HA, Thijs L, Staessen JA, Blankesteijn WM. Left ventricular dysfunction and CXCR3 ligands in hypertension: from animal experiments to a population-based pilot study. PLoS One. 2015;10(10):e0141394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.He Y, Ao DH, Li XQ, et al. Increased soluble CD137 levels and CD4+ T-cell-associated expression of CD137 in acute atherothrombotic stroke. Clin Transl Sci. 2018;11(4):428–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Safa A, Rashidinejad HR, Khalili M, et al. Higher circulating levels of chemokines CXCL10, CCL20 and CCL22 in patients with ischemic heart disease. Cytokine. 2016;83:147–157. [DOI] [PubMed] [Google Scholar]
- 78.Nishikimi T, Saito Y, Kitamura K, et al. Increased plasma levels of adrenomedullin in patients with heart failure. J Am Coll Cardiol. 1995;26(6):1424–1431. [DOI] [PubMed] [Google Scholar]
- 79.Pousset F, Masson F, Chavirovskaia O, et al. Plasma adrenomedullin, a new independent predictor of prognosis in patients with chronic heart failure. Eur Heart J. 2000;21(12):1009–1014. [DOI] [PubMed] [Google Scholar]
- 80.Grossman RM, Krueger J, Yourish D, et al. Interleukin 6 is expressed in high levels in psoriatic skin and stimulates proliferation of cultured human keratinocytes. Proc Natl Acad Sci USA. 1989;86(16):6367–6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Garshick Michael S, Barrett Tessa J, Wechter T, et al. Inflammasome signaling and impaired vascular health in psoriasis. Arterioscler Thromb Vasc Biol. 2019;39(4):787–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hugh J, Van Voorhees AS, Nijhawan RI, et al. From the medical Board of the National Psoriasis Foundation: the risk of cardiovascular disease in individuals with psoriasis and the potential impact of current therapies. J Am Acad Dermatol. 2014;70(1):168–177. [DOI] [PubMed] [Google Scholar]
- 83.Reich K, Papp KA, Griffiths CE, et al. An update on the long-term safety experience of ustekinumab: results from the psoriasis clinical development program with up to four years of follow-up. J Drugs Dermatol. 2012;11(3):300–312. [PubMed] [Google Scholar]
- 84.Hjuler KF, Bøttcher M, Vestergaard C, Bøtker HE, Iversen L, Kragballe K. Association between changes in coronary artery disease progression and treatment with biologic agents for severe psoriasis. JAMA Dermatol. 2016;152(10):1114–1121. [DOI] [PubMed] [Google Scholar]
- 85.Elnabawi YA, Garshick MS, Tawil M, et al. CCL20 in psoriasis: a potential biomarker of disease severity, inflammation, and impaired vascular health. J Am Acad Dermatol. 2021;84(4):913–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Protein elevations in psoriasis group and in individual patients before and after treatment in comparison to each protein’s cardiovascular risk threshold in the literature for the remaining Table 1 proteins. Red line indicates the calculated cardiovascular (CV) risk thresholds for each protein according to its respective study. Yellow line indicates least squared (LS) mean protein level of psoriasis cohort at baseline. Black line indicates LS mean protein level in controls. Blue line indicates LS mean protein level in psoriasis cohort after treatment. P1-P10 indicates Patient 1 –Patient 10. Yellow circles indicate individual patient’s protein level before treatment and blue circles indicate patient’s protein level after treatment. Red circle indicates patient’s protein level is above CV risk threshold. P = patient; TNF-R1 = Tumor necrosis factor receptor 1 (TNF-R1); CSTB = Cystatin B; N-terminal prohormone brain natriuretic peptide = NT-proBNP; CXCL10 = C-X-C motif chemokine 10; MPO = myeloperoxidase; vWF = von Willebrand Factor. (A) TNF-R1 levels in psoriasis cohort and in individual patients before and after treatment in comparison to CV risk threshold conferred by TNF-R1 in Pai et al’s study.38 (B–F) For CSTB according to Gonçalves et al.37(B); for NT-proBNP according to Wang et al.36(C); for CXCL10 according to Canoui-Poitrine et al.44(D), for MPO according to Meuwese et al.25(E); for vWF according to Morange et al.20(E).
Figure S2. Correlations between primary cardiovascular risk proteins and clinical parameters. Correlations among proteins, body mass index (BMI), psoriasis area and severity index at baseline (PASI-BL), PASI change after treatment (PASI-CHANGE), and PASI improvement (PASI-IMPR), were assessed using univariate Pearson correlation and Chi-squared test. Cor indicates correlation coefficient, CAR indicates cardiovascular II/III panel, INF indicates inflammation panel.
Figure S3. Protein modulation by ustekinumab compared to etanercept and tofacitinib. Left: Cardiovascular and inflammatory proteins modulated by tofacitinib and etanercept (FCH > 1.2, FDR < 0.05)16, *indicates etanercept modulated a subset of the tofacitinib-modulated proteins, plus CCL4. Right: cardiovascular and inflammatory proteins modulated by ustekinumab in our study (p ≤ 0.1), **indicates proteins associated with primary CV risk in our analysis. vWF and OPN were not included in Kim et al’s study16.
Table S1. Characteristics of patients and controls
Table S2. Differentially expressed proteins before and after ustekinumab treatment.
Table S3. Ustekinumab-modulated proteins with prospective studies assessing protein’s association with future cardiovascular risk.
Table S4. Ustekinumab-modulated proteins with cross-sectional studies assessing protein’s association with cardiovascular disease.
Table S5. Correlations with Psoriasis Area and Severity Index (PASI) at baseline and after treatment.
Data Availability Statement
The data that supports the findings of this study are available in the supplementary material of this article.


