Abstract
Pulmonary atelectasis is common in the perioperative period. Physiologically, it is produced when collapsing forces derived from positive pleural pressure and surface tension overcome expanding forces from alveolar pressure and parenchymal tethering. Atelectasis impairs blood oxygenation and reduces lung compliance. It is increasingly recognized that it can also induce local tissue biological responses, such as inflammation, local immune dysfunction, and damage of the alveolar-capillary barrier, with potential loss of lung fluid clearance, increased lung protein permeability and susceptibility to infection, factors that can initiate or exaggerate lung injury. Mechanical ventilation of a heterogeneously-aerated lung (e.g., in the presence of atelectatic lung tissue) involves biomechanical processes that may precipitate further lung damage: concentration of mechanical forces, propagation of gas-liquid interfaces, and remote overdistension. Knowledge of such pathophysiological mechanisms of atelectasis and their consequences in the healthy and diseased lung should guide optimal clinical management.
Introduction
The term atelectasis derives from the Greek words atelez meaning “imperfect”, and ektasiz, “expansion”. Pulmonary atelectasis, thus, refers to the incomplete expansion of alveoli and terminal bronchioles. In its paradigmatic form, atelectasis is represented by complete deaeration of lung units. Atelectasis is pervasive in anesthesia practice, and already in 1963 Bendixen et al. demonstrated that general anesthesia with mechanical ventilation resulted in deterioration of intraoperative oxygenation and compliance in patients with normal preoperative lung function.1 Brismar et al. subsequently demonstrated that such deterioration was associated with pulmonary densities revealed by computed tomography.2 Besides physiological impairment, pulmonary atelectasis could contribute to perioperative lung injury.3 The clinical presentation of significant atelectasis in surgical patients is variable from no sequalae to prolonged oxygen requirement to hypoxemia requiring endotracheal intubation and ventilation to even acute respiratory distress syndrome (ARDS). This article focuses on the perioperative period and aims to review the etiology of pulmonary atelectasis and provide a pathophysiological discussion including biological as well as biomechanical processes.
Physiological principles of bronchiolar and alveolar expansion
Bronchioles and alveoli walls are composed of cells and extracellular matrix, and covered by a liquid film on their luminal side containing surfactant. Each of these elements are exposed to expanding and collapsing forces.
Stresses acting on bronchioles and alveolar walls
Normal stress is the force per unit of area (A) perpendicular to the surface where the force is exerted. Three main components of the normal stresses acting on bronchioles and alveolar walls determine their expansion (Fig. 1)4: fluid pressure, tethering stress and surface tension. A conceptual note on the physical meaning of these mechanical components is that, while related, they are not equivalent as pressure is a scalar (a physical quantity having only magnitude) while stress is a vector (a physical quantity with direction and magnitude).
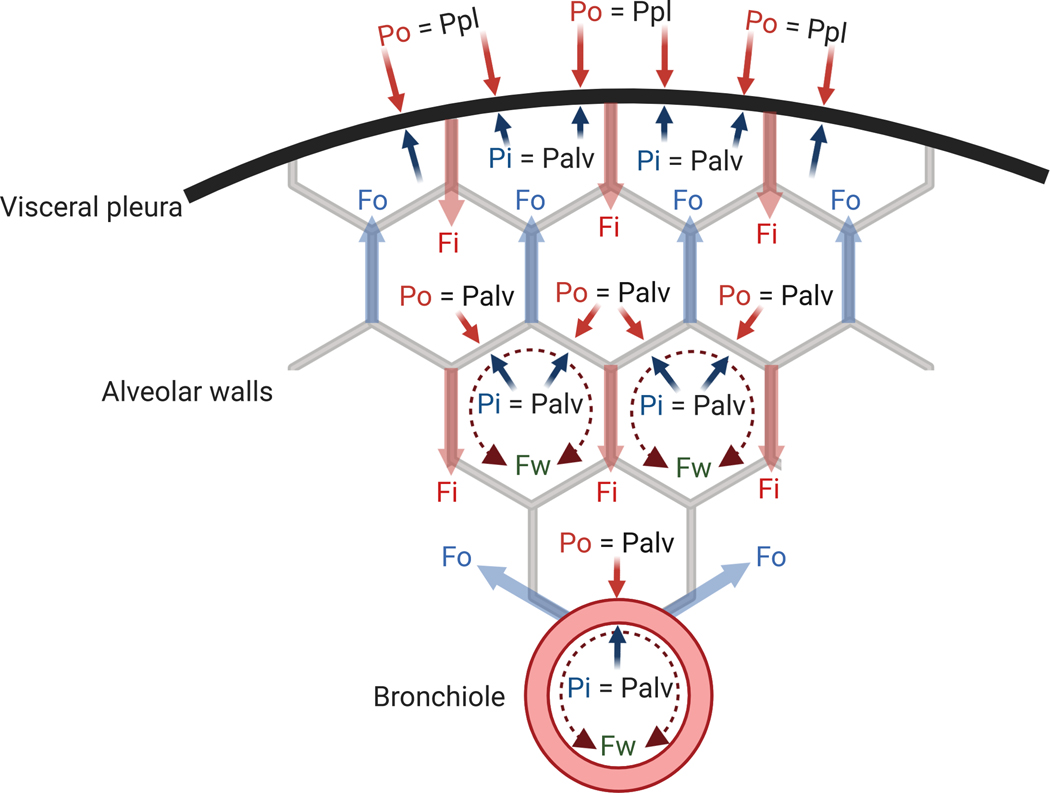
Fig. 1: Pressures and forces acting on alveolar and bronchiolar walls and visceral pleura surface.
Fi = inward tethering; Fo = outward tethering; Fw = circumferential component of force applied by the layer of surface-active fluid; Palv = alveolar pressure; Pi = inside pressure; Po = outside pressure; Ppl = pleural pressure.
- Fluid pressure: represents the pressure applied by fluids (gas or liquid) to the surface of the alveolar or bronchiolar wall. The net result of the fluid pressures derives from the difference of inside (Pi) and outside (Po) pressures, expressed by the formulation of transmural pressure:
- Tethering stress: represents radial stresses due to attachments of bronchioles and alveolar walls to adjacent structures through the tissue matrix. The radial tethering stress is mechanically transmitted to alveoli, bronchioles and pleural surface through a network of collagen and elastin fibers composing the extracellular matrix in the pulmonary septa. These fibers are the force-bearing elements. The parenchymal cells themselves (epithelial and endothelial cells) have a lower mechanical contribution. Preserved lung interstitial architecture, thus, ensures the transmission of the tethering stress inside the lung parenchyma.5 The net effect of those stresses applied inside (Fi/A) and outside (Fo/A) the bronchioles or alveolar walls can be expressed as (Fig. 1):
-
Surface Tension: represents the inward-acting radial stress arising from the circumferential components of forces applied by the thin layer of fluid lining the bronchioloalveolar walls resulting from the effect of the surface tension (T). For a spherical structure with radius R (alveoli), the Young-Laplace pressure equation expresses the relationship between the pressure difference across the fluid interface (Pw) and the surface tension as:For a cylindrical structure of radius R (bronchioles), the relationship is:
Accordingly, the pressure difference across the fluid interface becomes substantial for small R. The surface tension is likely more important in the cylindric bronchioles than in alveoli, which are not strictly spherical.6 Pulmonary surfactant, a lipoprotein complex secreted by type II alveolar epithelial cells, is a critical biomechanical stabilizer to bronchioles and alveoli expansion.7 Its presence at the air-liquid interface strongly reduces surface tension, decreasing the magnitude of this collapsing contribution.
The balance of these forces and pressures allow for quantitative relationships in specific conditions (Fig. 1):
- In equilibrium, the balance of the expanding and collapsing radial stresses acting on bronchioles or alveolar walls should be zero4:
- No external tissue attachments are present at the pleural surface. Consequently, Fo=0 and transmural pressure is determined by Pi (=alveolar pressure, Palv) and Po (=pleural pressure, Ppl). Radial stresses corresponding to the sum of inward-acting tissue and surface forces are balanced by the transmural pressure (Palv-Ppl) acting on the pleural surface area (Apl). As the radius of the pleural surface curvature is large, the effect of surface tension is negligible (Pw≈0). Accordingly, the balance of forces at the pleural surface is:
- Within the lung, if all airways are patent, pressures on the two sides of alveolar walls (i.e., Pi and Po) equal the same alveolar pressure (Palv). Therefore, transmural pressure between adjacent alveoli is null, and the outward-acting tethering force (Fo) counteracts inward-acting tissue and surface forces:
The elastic recoil of the lung
Lung elastic recoil represents the propensity of lung tissue to shrink and is the main physiological mechanism of passive exhalation. It results from the combined effects of: (1) extracellular matrix elastic fibers (contributing to Fi); and (2) bronchioloalveolar surface tension.4,7 Accordingly, degradation of the elastic fibers in the extracellular matrix, as during emphysema, reduces elastic recoil, thus, reducing the expiratory capacity and acting against alveolar collapse.8 Conversely, diseases leading to quantitative or qualitative surfactant impairment increase surface tension and facilitate alveolar collapse.7
The interdependent lung expansion
Pulmonary interdependence represents the interplay of mechanical forces amongst lung tissue components – alveolar units, airways, vasculature, and extracellular matrix. For instance, interdependence during lung expansion transmits tethering stress to traction airway walls outwards.5,9–11 Interdependence relies on normal lung architecture, including the extracellular matrix fibers.12 In a homogeneous lung, outward tethering stresses (ΣFo/Apl) are transmitted from the visceral pleura surface to the innermost lung regions. These stresses are determined by the elastic recoil pressure of the lung Pel(L),13 equal to the transmural pressure at the pleural surface:
A positive Pel(L), transmitted to the inner lung through interdependence, is the primary determinant of lung expansion. During awake spontaneous breathing, Ppl is negative throughout the pleural space leading to a positive Pel(L) as Palv=0 (=atmospheric pressure). In contrast, Pel(L)≤0 is associated with unphysiological conditions resulting in lung collapse such as open-chest or general anesthesia with mechanical ventilation.
The transpulmonary pressure (PL) has been advanced as a surrogate of the elastic recoil pressure of the lung when the alveolar pressure (Palv) can be approximated by the pressure at the airway opening (Pao). This occurs when respiratory flows are zero (usually at end-expiration and end-inspiration) and no gas trapping exists:13
Hence, a positive transpulmonary pressure throughout the respiratory cycle is required to maintain alveolar expansion.
Mechanisms of atelectasis in the perioperative period
General anesthesia, mechanical ventilation and surgical interventions produce several biophysical factors promoting lung tissue collapse (Fig. 2). Three major interrelated collapsing factors influence the balance of forces discussed above and contribute to perioperative atelectasis: increased pleural pressure, low alveolar pressure and surfactant impairment. As a result of these factors, continuous or intermittent airway and alveolar closure occur, presumably more commonly the first than the latter.14,15
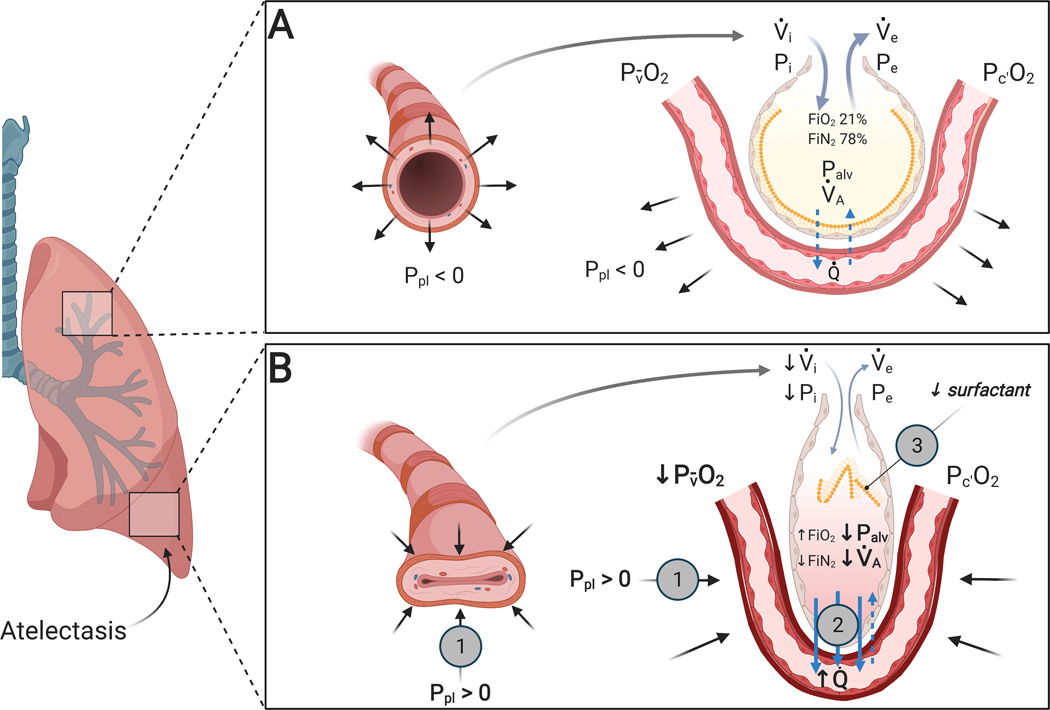
Fig. 2: Mechanisms producing atelectasis in the perioperative period.
(A) Normal lung unit in awake conditions: adequate inspiratory (Pi) and expiratory (Pe) intraluminal pressure and bronchiolar or alveolar tethering stress associated with negative pleural pressure (Ppl) allow for the normal opening of the bronchiole and a normal alveolar ventilation (⩒A). Alveolar gas absorption is physiological due to physiological ⩒A/Q̇ and atmospheric FIO2. Normal surfactant reduces alveolar surface tension. (B) Lung unit exposed to perioperative atelectasis: increase in pleural pressure (Ppl) due to extrinsic or intrinsic compression ( ) is responsible for loss of expansion and reduced alveolar ventilation (⩒A). Increased alveolar gas absorption (
) is responsible for loss of expansion and reduced alveolar ventilation (⩒A). Increased alveolar gas absorption ( ) reduces intraluminal alveolar pressure (Palv). Low ⩒A/Q̇, high FIO2 and low mixed venous oxygen partial pressure (P O2) may participate in such gas exchange imbalance. Quantitative or qualitative surfactant impairment leads to higher surface tension and facilitates alveolar collapse (
) reduces intraluminal alveolar pressure (Palv). Low ⩒A/Q̇, high FIO2 and low mixed venous oxygen partial pressure (P O2) may participate in such gas exchange imbalance. Quantitative or qualitative surfactant impairment leads to higher surface tension and facilitates alveolar collapse ( ). Pc’O2 = end-capillary oxygen partial pressure.
). Pc’O2 = end-capillary oxygen partial pressure.
Increased pleural pressure
Pleural pressure is the pressure within the pleural cavity. It varies regionally across the pleural space depending on anatomical and physiological interactions between the lung parenchyma, chest wall and gravity.16 General anesthesia affects such interactions increasing regional pleural pressure (e.g., dorso-caudal in supine patients), resulting in negative transpulmonary pressure and compressive atelectasis (Fig. 2).
Functional changes of the diaphragm and additional chest wall components
The chest wall can be understood as composed by two functional portions: an elastic portion represented by the rib cage and abdominal wall and a constant weight component exerting a hydrostatic pressure represented by the abdomen. Changes in these portions will affect pleural pressure and lung expansion in the perioperative period, in line with the previously discussed equilibration of forces throughout the lung. The diaphragm is the primary muscle of lung ventilation and, consequently, significantly contributes to lung expansion and atelectasis development during anesthesia. E.g., in anesthetized intubated patients without cardiopulmonary disease, phrenic nerve stimulation to produce diaphragm contraction reduces atelectatic area by approximately 33% as compared to mechanical ventilation with equal tidal volumes.17
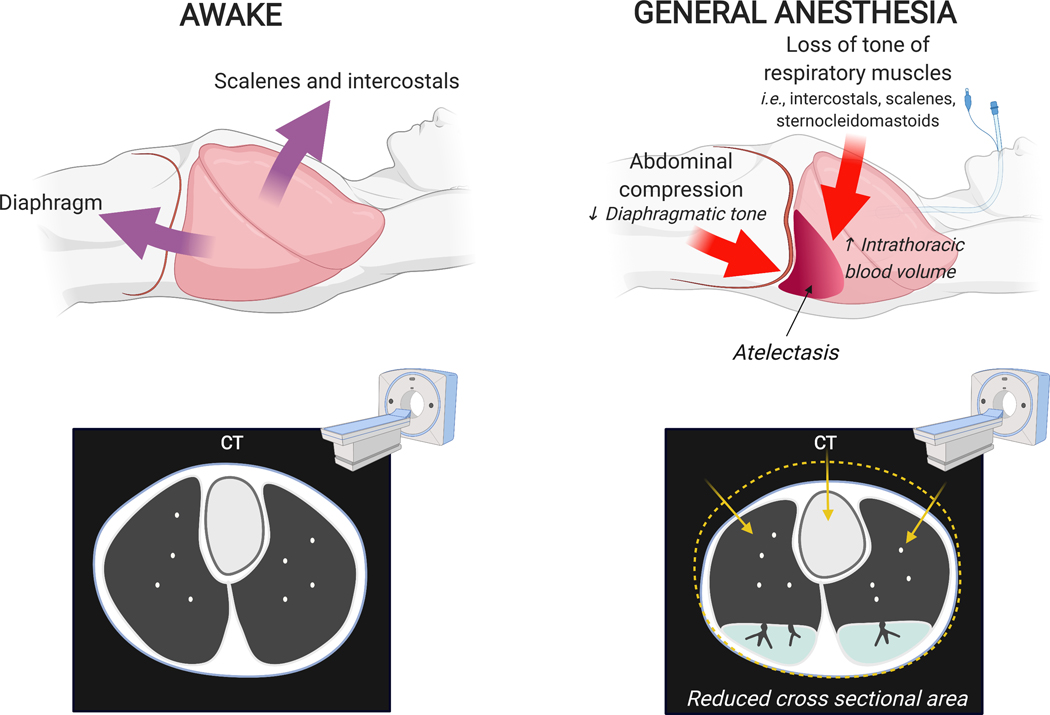
In supine spontaneously breathing humans, diaphragmatic displacement and lung expansion are larger in dependent than nondependent lung regions.18,19 This is due to the diaphragm dome shape with a smaller dependent radius of curvature, higher dependent stretch producing more favorable dorsal length-tension relationships, and possibly larger number of muscle fibers20 and higher compliance of the crural than costal diaphragm18. Diaphragm tension reduces the transmission of abdominal pressure to the lungs.21 Reduction or loss of such diaphragmatic tone during anesthesia, thus, affects the net balance of stresses acting on the lungs not only reducing the preferential dependent displacement of the diaphragm but also facilitating the transmission of abdominal pressure to the lungs. This results in a cephalad shift of the dependent diaphragm with dependent lung compression and atelectasis,22,23 and no change or caudad shift of nondependent regions.19,23 Relaxation of accessory respiratory muscles such as intercostals, scalenes and sternocleidomastoids further contribute to reduction in cross-section chest area and lung aeration (Fig. 3). In spontaneously breathing normal subjects receiving volatile anesthesia the activity of parasternal muscles is abolished and phasic expiratory activity in abdominal and lateral ribcage muscles enhanced,24 contributing to caudad dependent atelectasis.23 Muscle paralysis compounds to the dependent cephalad shift of the diaphragm and atelectasis during general anesthesia23, as the balance between alveolar pressure and the gravity dependent hydrostatic pressure of abdominal contents becomes the main determinant of diaphragm motion.19 Ultimately, atelectasis that is preferentially dependent and caudad is detected in 90% of a broad population of patients without cardiopulmonary disease undergoing general anesthesia,25 with up to 20–25% of initially normal lung either atelectatic or poorly aerated in transverse computed tomography during anesthesia.26
Fig. 3: Changes in chest wall shape due to general anesthesia in a supine patient.
During awake spontaneous breathing, contraction of diaphragm and accessory muscles of respiration maintain lung expansion. Loss of muscular tone during anesthesia is associated with cephalad motion of the dependent diaphragm, reduction in cross-sectional chest area, and generation of non-gravitational compressive forces (i.e., cephalocaudal gradients). Together with gravitational forces and potential increase in intrathoracic blood volume, these factors contribute to reduction of lung volume and lung collapse particularly on the dorsal and basal lung regions.
Increased abdominal pressure as present with pneumoperitoneum, obesity, abdominal compartment syndrome, peritonitis, or abdominal shift of intrathoracic blood27,28 produces further imbalance of net stresses on the lung, as it exposes the dorso-caudal lung to higher pleural pressure and susceptibility to atelectasis,2,29 with cephalad shift of both diaphragm and intra-abdominal organs.19,23,27 Of note, cephalad-outward movement of the lower ribs potentially produced by those factors can increase the cross section of the lower chest and partially compensate for the loss of lung volume.21
While gravity has been frequently cited as a key determinant of lung expansion, cephalocaudal gradients of lung expansion present in large animals30 and humans22,31 indicate the relevance of factors other than gravity. These include the matching of the lung to the thoracic cavity and the partially independent displacement of lobes, which are relevant determinants of regional lung expansion in supine and prone positions beyond gravitational factors.30,32 Perioperative chest wall reshaping is influenced by body position, with proning allowing for recruitment of dorso-caudal lung.33
Postoperative respiratory muscle dysfunction, particularly diaphragm dysfunction, has been documented after abdominal,34 thoracic,35 and cardiac surgery.36 It facilitates the development of atelectasis as demonstrated by the significantly larger fraction of patients with atelectasis 24h after thoracic surgery in the presence of postoperative ultrasound-diagnosed diaphragmatic dysfunction (35%) than in its absence (13%).35 Diaphragm dysfunction can persist from a day to a week,37,38 and even a year.39 It can occur due to direct injury to the diaphragm36 or phrenic nerve,40 or to indirect factors such as phrenic nerve dysfunction37,41 and impaired thoracoabdominal mechanics.42 These could compound with previous diaphragmatic compromise, e.g., as present in neuromuscular disorders, sepsis,43 abdominal hernias44 and potentially obesity.45 Of note, diaphragmatic function could conversely affect regional lung inflammation by producing local high transpulmonary pressures as shown by the observation that spontaneously breathing lung injured pigs exposed to low positive end-expiratory pressure (PEEP) present more dependent lung inflammation than those receiving high PEEP.46
While anesthetics (e.g., isoflurane, sevoflurane and propofol) can compromise diaphragmatic function47–49 they do not affect contractility.50 The diaphragmatic electromyographic activity can also be impaired by unwarranted administration of cholinesterase inhibitors, e.g., neostigmine followed by sugammadex in humans51 or neostigmine administered after full recovery from neuromuscular block in rats52.
Intrapulmonary gravity gradient
The weight of the non-dependent lung compresses the dependent lung and pleural space determining pleural pressure increases along the vertical axis11 with transpulmonary pressure reduction in dependent lung regions.53,54 Pulmonary edema increases the weight of the lung tissues increasing the risk of dependent atelectasis due to the superimposed hydrostatic pressure.54,55 Body position influences the effect of intrapulmonary compression by modulating the volume of dependent lung. For instance, the triangular shape of the lungs with the large dorsal base results in a greater volume of dependent lung in the supine than in the prone position.55,56
Compression by intrathoracic elements
In supine patients, the mediastinal weight, particularly the heart, has been associated with pleural space compression and preferential retrocardiac lung collapse.57 Pleural effusions may also compress the lung. However, the effusion volume does not entirely translate into compression58 due to the compliance of the chest wall. For instance, in ARDS patients, chest wall expansion in the presence of a pleural effusion accommodates ~70% of the effusion volume.58,59
Low alveolar pressure
The concept of critical opening pressure has been introduced as the minimal alveolar pressure (Palv in the previously described formulation) required to counteract the regional effect of collapsing forces.60 Accordingly, lung units are expanded when alveolar pressure is higher than the critical opening pressure. A parallel concept is that of critical closing pressure, i.e., the alveolar pressure below which open lung units collapse. Mean closing pressure has been estimated as 6 cmH2O in a small number of anesthetized mechanically ventilated patients,61 an interesting value to compare to the usual initial clinical setting of 5 cmH2O. As determined experimentally in animal and computational models,62,63 the critical closing pressure is lower than the critical opening pressure due to lung hysteresis, i.e., the difference between the inspiratory and expiratory components of the pulmonary pressure-volume curve, produced by opening of previously nonaerated peripheral airspaces.64 Due to the vertical dependence of pleural pressures, critical opening pressures are higher in dependent regions as lung regions exposed to positive pleural pressure require alveolar pressures higher than these pleural pressures to achieve positive transpulmonary pressure and expansion.
The rationale for the use of PEEP derives from such a concept, ultimately aiming to keep alveolar pressures above critical closing pressures at end-exhalation to prevent lung collapse. Local variation in critical opening and closing pressures conditions the regions expanded and kept inflated throughout the breathing cycle at a given PEEP. Even normal lungs, when mechanically ventilated without PEEP for many hours, will progressively lose aeration preferentially in dorsal regions.65 Of note, lung hysteresis imply that the PEEP required to keep lung regions open is lower than that required to open them (Fig. 4).66 This provides support to the practice of recruiting the lungs at pressures higher than those used during steady state mechanical ventilation.
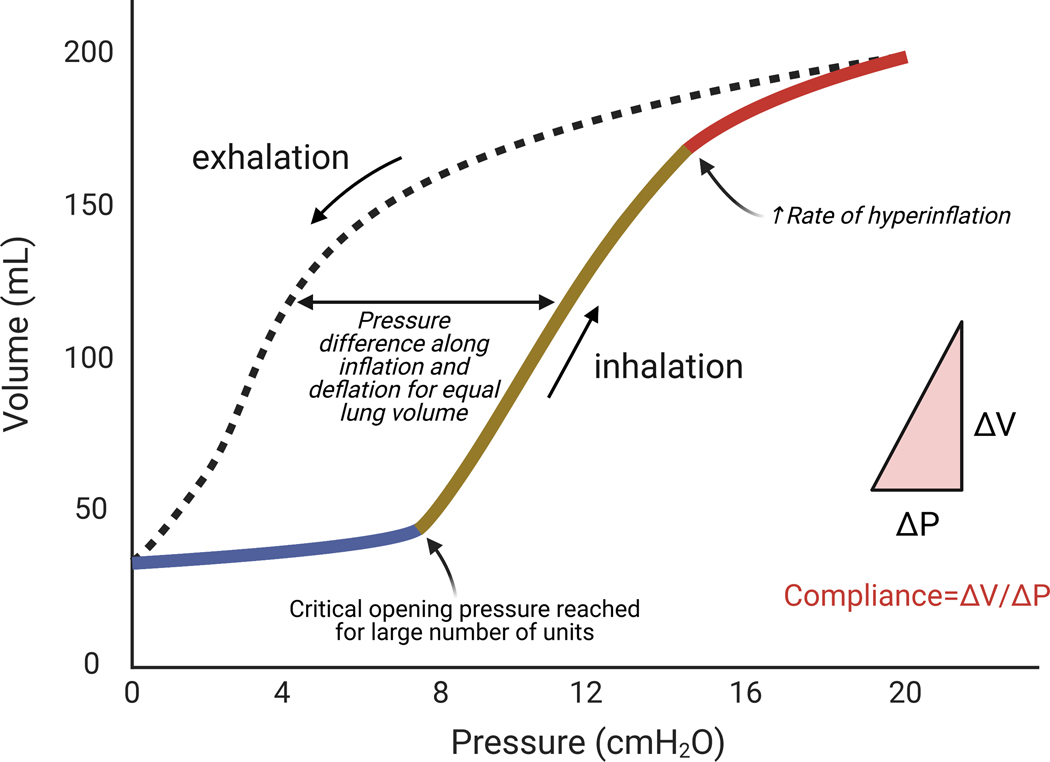
Fig. 4: Pulmonary pressure-volume curve during inhalation and exhalation showing lung hysteresis.
The shape of the lung pressure-volume curve is sigmoidal. There are three main portions of the curve. The initial portion (blue) of lung recruitment at low pressures and volumes is related to low compliance (i.e., the change in volume divided by the change in pressure is low). This is followed by a portion with linear relationship between volume and pressure (ochre) with higher compliance. Finally, hyperinflation ensues at high pressures and volumes with return of lower compliance (red). The transition between the first and second portions indicate that critical opening pressures for a large number of bronchoalveolar regions has been reached (lower inflection point). Note the higher pressure to reach the same lung volume during inhalation than exhalation. Modifed from Radford EP Jr.66
Resistance of upstream airways
The transmission of upper airway pressures to distal lung regions, i.e., the proximity between Palv and Pao, depends on the patency of regional airways (Fig. 2). Increased airway resistance, secondary to airway constriction, obstruction, or compression13 determines a pressure drop in the distal lung with local alveolar pressures potentially lower than tracheal pressures (Palv<Pao). Obstructive atelectasis is the term used to describe lung collapse resulting from airway obstruction, usually caused by mucous plugs and retention of secretions superimposed or not to airway constriction. Anesthetics dose-dependently compromise ciliary motility of respiratory epithelial cells (isoflurane, ketamine, thiopental) potentially facilitating obstruction, and this compromise seems weaker with sevoflurane,67 fentanyl or propofol.68 Mucociliary clearance appears to be more compromised by cuffed endotracheal tubes than laryngeal mask airways.69
Balance of alveolar gas exchange
Lung units presenting larger alveolar gas outflow than inflow will ultimately collapse. This is the basic concept of absorption atelectasis.70 Such an imbalance is mostly determined by low local alveolar ventilation-to-perfusion (⩒A/Q̇) ratios and high FIO2. Low ⩒A/Q̇ determines low inflow of fresh air into alveoli in relation to the local perfusion associated with O2 absorption. The lowest ⩒A/Q ratios are found in the most gravity dependent regions,71 where intraoperative atelectasis is typically present.72,73 High FIO2 facilitates outflow as alveolar gas absorption by capillary blood is higher with O2 than with gases presenting lower blood:gas solubility as nitrogen.74–77 Lower mixed venous oxygen content further increases the rate of oxygen absorption (Fig. 2).
Surfactant impairment
Several factors during general anesthesia could impair surfactant function and contribute to the development of atelectasis: inflammatory response, mechanical ventilation, high oxygen concentration, anesthetics, and pulmonary edema. While these factors produce minimal compromise in short uncomplicated cases, they could become relevant as the surgical insult and patient compromise increase.
Inflammatory lung injury resulting from endotoxemia and bacteremia lead to reduced production of surfactant phospholipids,78 increased surfactant turnover,79 decreased tubular myelin80 and altered surfactant protein A gene expression.81 Inflammation with surfactant dysfunction is also present during severe respiratory failure and pneumonia.82 This dysfunction can be mediated by inflammatory cytokines,83 proteases secreted from immune cells,84 and increased proteins in alveoli.85 In line with these findings, in vivo lung neutrophilic inflammation developing within few hours of mechanical ventilation in poorly-aerated areas was reported in association with surfactant dysfunction86, with inflammation worsened by endotoxemia.87 The translational relevance of such data is suggested by the quantitative and qualitative surfactant impairment observed in surgeries associated with large systemic and pulmonary inflammatory response (e.g., on-pump cardiac surgery) or lung injury.88,89
Mechanical ventilation impairs surfactant production by type II alveolar cells 90,91 through alveolar overstretching92, under-stretching93 and monotonic stretching94. Conversely, deep breaths95 and biologically variable ventilation96 increase the release of active surfactant. Short-term exposure to high oxygen concentrations (100%) adversely affects surfactant function by increasing its susceptibility to rupture.97 Suggestion that inhaled anesthetics could compromise surfactant biosynthesis in time- and dose-dependent patterns comes to date essentially from in vitro studies and is rapidly reversible after discontinuation.98 Pulmonary edema, e.g., from fluid overload, can change surfactant activity and increase surface tension, potentially by the loss of surfactant into the edema fluid.99 For permeability pulmonary edema, surfactant function can be inactivated by proteins in alveoli secondary to barrier disruption,82,100 via increasing its conversion to non-surface-active forms.85
Pathophysiological effects of pulmonary atelectasis
Global Physiological Effects
Respiratory mechanics and lung volumes
Functional residual capacity is reduced in the supine position, and further by general anesthesia and muscle paralysis. Such decrease is associated with loss of muscle tone, and related reduction of the cross-sectional area of the thorax23, cephalad displacement of the diaphragm,19 increased curvature of the vertebral column,24 and increased intrathoracic blood volume.22 Of note, change in diaphragm displacement, thorax cross-section or functional residual capacity during general anesthesia were found not to correlate to the magnitude of atelectasis22,23 suggesting the relevance of other factors for ultimate lung collapse, such as intrathoracic blood volume and decreased volume of the aerated lung.
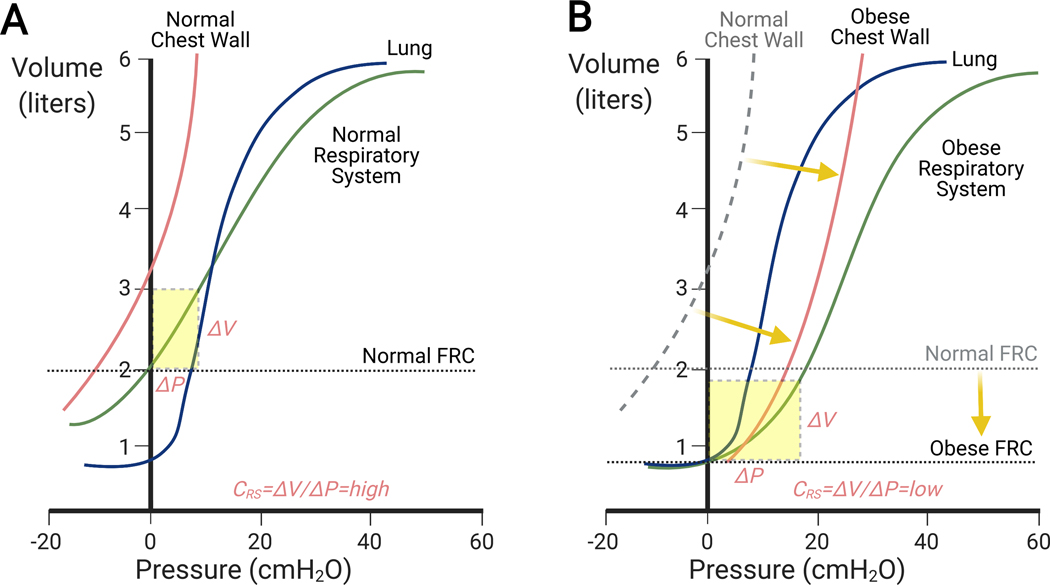
Atelectasis is associated with lower respiratory system and lung compliances. This is because closure of alveoli and small airways corresponds to the low-volume portion of the pressure-volume curve associated with smaller change in lung volumes in relation to applied pressures, i.e., low compliance (Fig. 4).101,102 Indeed, in mechanically-ventilated dogs, the respiratory system compliance is linearly related to lung volume.103 Alveolar and airway closure is affected by supine position and may be further influenced by obesity (Fig. 5).104 Higher driving pressures (Pplateau-PEEP = tidal volume/respiratory system compliance) consequently also ensue.105 End-expiratory lung volumes can be lower than closing capacity during mechanical ventilation likely due to loss of radial traction from parenchymal interdependence.10,106 The same mechanism may contribute to the increased lung and respiratory system resistance associated with atelectasis in obese patients.107 Of note, once atelectasis settles, the lung pressure-volume curve also becomes abnormal, further compounding the loss of lung volume.
Fig. 5: Pressure-volume relationship for the chest wall, lungs and their combination (respiratory system) in normal (A) and obese (B) subjects in supine positions.
(A) The shape of the respiratory system pressure-volume curve reflects the balance of the forces from the chest wall and lung parenchyma. In normals, functional residual capacity is reduced as compared to the upright position but large enough to locate an operating range of the respiratory system pressure-volume curve within a region of high compliance (dashed yellow rectangle). (B) In obese subjects, increased weight of the chest wall and abdomen shift the chest wall pressure-volume curve to the right at similar chest wall compliances. Combined with the substantial reduction of functional residual capacity (FRC), the operating range of the respiratory system pressure-volume curve moves to a region of low compliance (dashed yellow rectangle). This occurs even in the presence of the same normal pressure-volume curve of the lungs. Modifed from Behazin et al.104
Gas exchange
The most clinically evident pathophysiological effect of pulmonary atelectasis is hypoxemia.1,102 Impaired blood oxygenation has been described during routine general anesthesia with both controlled108 and spontaneous ventilation,109 and correlated with the degree of atelectasis.22,110 The mechanisms are low ⩒A/Q̇ ratios and intrapulmonary right-to-left shunt.109,111 In experimental and clinical studies, alveolar recruitment promptly reverses gas exchange dysfunction.1,101,112 Chronic obstructive pulmonary disease patients are less susceptible to oxygen absorption atelectasis113 and may develop less atelectasis during mechanical ventilation after cardiopulmonary bypass,114 presumably due to a combination of loss of elastic recoil and high airway resistance.
Hypoxic pulmonary vasoconstriction
Hypoxic pulmonary vasoconstriction is a reflex constriction particularly of distal pulmonary arteries but also venules in response to hypoxia.115,116 Oxygen sensing occurs at the alveolo-capillary level,117 with endothelial cell depolarization and retrograde propagation of the signal via gap junctions to upstream arterioles, where it is transmitted to pulmonary arterial smooth muscle cells to produce vasoconstriction.117 In health, the onset of the reflex to hypoxic gas mixtures occurs within seconds, and progresses into a plateau period (phase 1) of at least 20 min, and then a further rise (phase 2) starting after ~43 min and reaching a peak at ~2 h.118 This pattern is consistent with the response times to atelectasis observed in a dog model.119
Hypoxic pulmonary vasoconstriction mitigates right-to-left shunt diverting blood away from poorly oxygenated lung areas, thus, supporting adequate arterial oxygenation during general anesthesia.120,121 The more effective the hypoxic pulmonary vasoconstriction, the lower the effect of atelectasis on right-to-left shunt and consequent hypoxemia. In normal humans, 47% of blood flow is diverted from a hypoxemic lobe within 5 min.121 Factors that impair hypoxic pulmonary vasoconstriction accentuate hypoxemia produced by atelectasis: pneumonia,122 ARDS,123 endotoxemia,124 sepsis,125 calcium channel blockers,126 atelectatic size,127 or volatile agents (isoflurane, sevoflurane, desflurane) above 1 minimum alveolar concentration.128–130 Mechanical factors associated with atelectasis could also impair hypoxic vasoconstriction, e.g., unstable alveoli in the collapsed large animal lung could stent open pulmonary corner vessels through outward radial traction, reducing the hypoxic constriction.131 Intravenous anesthetics present a discrete to nonexistent effect on hypoxic vasoconstriction, as no blunting has been found with propofol,132 ketamine,133 or fentanyl,134 while others found even oxygenation improvement with propofol.133,135 During general anesthesia, the significant correlation between shunt and atelectatic area calculated from computed tomography in patients without significant lung disease indicates that hypoxic pulmonary vasoconstriction is not complete and contributes to hypoxemia even in normal lungs.110 In disease conditions, a strong correlation between shunt and atelectatic volume suggests more compromised hypoxic pulmonary vasoconstriction such as observed after mitral valve surgery when compared to coronary artery bypass graft surgery.136
In normal humans, increases in mean pulmonary artery pressure of ~10 mmHg are observed due to hypoxic vasoconstriction with arterial oxygen saturation (Sao2) changes from >95% to 85–90% and of ~16mmHg with Sao2=75–80%, corresponding to a duplication and more of the pulmonary vascular resistance.137 In severe cases, right ventricular dysfunction can ensue as a consequence of the afterload elevation during atelectasis.138,139 Accordingly, alveolar recruitment reduces pulmonary vascular resistance and increases pulmonary blood flow in patients after cardiopulmonary bypass140 and in dogs with healthy or injured lungs,141,142 subsequently improving right ventricular function.
Regional molecular and cellular response
Molecular (e.g., cytokine, chemokine and pathways) and cellular (e.g., alveolar endothelial, epithelial and inflammatory) changes during atelectasis imply significant local biological responses and allow for insights into the mechanisms of lung injury. Atelectasis-associated lung injury could relate to direct or indirect, independent or synergistic local biological effects such as inflammation, structural damage, hypoxic injury, surfactant dysfunction, and susceptibility to infection (Fig. 6).
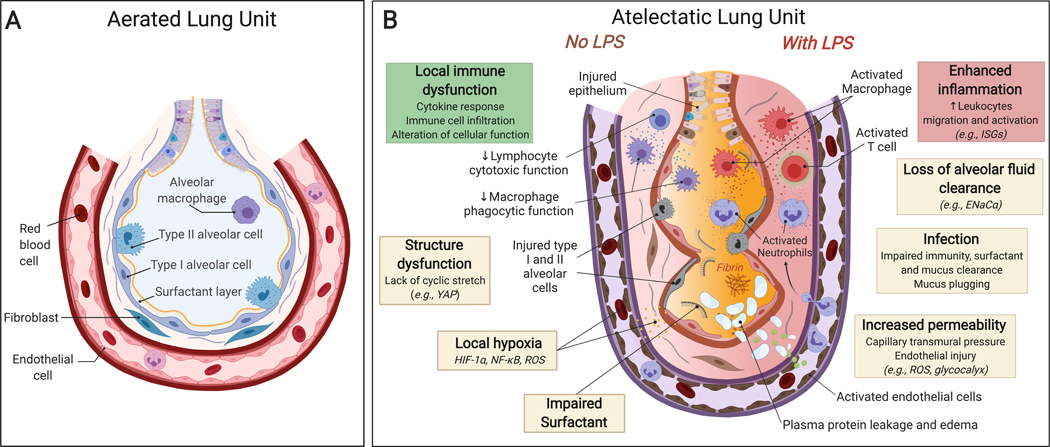
Fig. 6: Atelectasis-associated regional biological injury.
(A) Normally aerated lung. (B) Atelectatic Lung. There is local immune dysfunction with dysregulated cytokine secretion and impaired function of immune cell and surfactant, in part associated with local hypoxia and lack of cyclic stretch. Endotoxemia (=systemic lipopolysaccharide, LPS) enhances inflammatory responses characterized by marked immune cell infiltration and activation. In addition, atelectasis leads to structure dysfunction accompanied by loss of alveolar fluid clearance and increased protein permeability, flooding of the airspace with protein-rich pulmonary edema fluid, and potentially increased susceptibility to infection.
Inflammatory response
Links between atelectasis and inflammation have been indicated by clinical and experimental studies. Indeed, normal lungs exposed to 16 hours of atelectasis and mechanical ventilation with low tidal volumes presented metabolic changes suggestive of incipient inflammation in large animals with aeration heterogeneity comparable to that of humans.65 The hallmarks of atelectasis-related inflammatory response include the presence of cytokines and immune cells, and their functional alteration (Table 1).
Table 1.
Atelectasis associated inflammatory response in clinical and animal studies.
| Inflammatory response | Subject | Model/Design | Mechanical ventilation | Samples | Results | Reference |
|---|---|---|---|---|---|---|
| Cytokine production | C57BL/6 mice | Ex vivo IPL model | VT=7 mL/kg, PEEP=0 cmH2O, no recruitment | BALF | ↑ KC | Wakabayashi et al. 2014148 |
| Sprague-Dawley rats | OLV model | VT=8 ml/kg, PEEP=4 cmH2O, TLV and OLV | Lung homogenates |
↑ MPO ↓ CCL-2 |
Tojo et al. 2015146 | |
| Patients, lung resection surgery | Prospective, observational | TLV: VT=8 ml/kg, PEEP=3–5 cmH2O; OLV: VT=6 ml/kg, PEEP=5 cmH2O | BALF | ↑ IL-1, IL-2, IL-6, TNF-α, NO, CO and MMP-2 | de la Gala et al. 2015143 | |
| Patients, lung resection surgery | Prospective, observational | VT=10 ml/kg, RR to maintain normal PaCO2 | ELF | ↑ IL-8 | Komatsu et al. 2012144 | |
| ICU patients with atelectasis and no cardiopulmonary disease | Prospective controlled study | VT=9–11 ml/kg with PEEP=3–5 cmH2O | BALF | ↑ PAF | Nakos et al. 2003147 | |
| Sheep | Left lung collapse and right lung ventilated model with or without systemic LPS | VT=10 ml/kg with PEEP=2 cmH2O | Lung tissue mRNA |
↓ CXCL-8 and F2RL1 (without systemic LPS) ↑ CXCL-9, CXCL-10, CCL-5, IL-12B, MX1 and MX2 (with systemic LPS) |
Zeng et al. 2020154 | |
| Cell infiltration | Dogs | Lobar atelectasis | BALF | ↑ Neutrophils | Nguyen et al. 1991149 | |
| ICU patients with atelectasis and no cardiopulmonary disease | Prospective controlled study | VT=9–11 ml/kg with PEEP=3–5 cmH2O | BALF | ↑ Neutrophils | Nakos et al. 2003147 | |
| Wistar albino rats | Pneumothorax model | Lung tissue | ↑ Neutrophils | Sivrikoz et al. 2002156 | ||
| Cellular function | Sprague-Dawley rats | Left main stem bronchus ligation | VT=2.5 ml/kg, RR=90 breaths/min | Alveolar macrophages | ↑ Activation with increased IL-1 and TNF release | Kisala et al. 1993152 |
| Yorkshire swine | Right upper lobe atelectasis | Alveolar macrophages | ↓ In vitro phagocytosis against Pseudomonas aeruginosa | Shennib et al. 1984153 | ||
| Dogs | Lobar atelectasis | Broncho-alveolar lymphocytes | ↓ Cytotoxic activity | Nguyen et al. 1991149 |
IPL: isolated, buffer-perfused lungs; VT: tidal volume; KC: keratinocyte-derived chemokine; TNF: tumor necrosis factor; PEEP: positive end-expiratory pressure; BALF: bronchoalveolar lavage fluid; IL: interleukin; NO: nitric oxide; CO: carbon monoxide; MMP-2: matrix metalloproteinase 2; OLV: one-lung ventilation; TLV: two-lung ventilation; RR: respiratory rate; ELF: epithelial lining fluid; ICU: intensive care units; PaCO2: partial tension of carbon dioxide; PAF: platelet-activating factor; CCL: chemokine (C-C motif) ligand; LPS: lipopolysaccharides; CXCL: chemokine (C-X-C motif) ligand; F2RL1: coagulation factor II (thrombin) receptor-like 1; MX: Interferon-induced GTP-binding protein; MPO: myeloperoxidase.
Cytokine response
Atelectasis is often associated with local production of inflammatory cytokines. Multiple clinical studies of one-lung ventilation143–145 have reported increased levels of pro-inflammatory cytokines in the atelectatic lung, such as interleukin (IL)-1, IL-6, IL-8 and tumor necrosis factor (TNF)-a, all involved in inflammatory injury. These cytokine levels were directly related to the duration of atelectasis144,145 and potentially increase the susceptibility to postoperative pulmonary complications in patients undergoing lung resection surgery.143 Moreover, atelectasis could result in significant concentrations of chemotactic cytokines, including chemokine (C-X-C motif) ligand (CXCL)-1,146 a potent neutrophil chemoattractant; platelet-activating factor,147 a mediator of platelet aggregation and degranulation, and leukocyte chemotaxis; and keratinocyte-derived chemokine,148 another immune cell chemoattractant particularly for neutrophils. As a result, cytokines increased in atelectatic areas may cause direct injury, and additionally act as homing molecules recruiting cells (e.g., neutrophils) into these regions that could further magnify damage, e.g., by releasing injurious cytokines.
Inflammatory cell response
Atelectasis contributes to inflammatory cell infiltration, at least in part through the inflammatory cytokines described above. For example, neutrophils, key immune cells in the inflammatory response and tissue damage, are increased in bronchoalveolar lavage fluid of atelectatic regions in mechanically ventilated patients or spontaneously breathing dogs when compared to fluid obtained before atelectasis.147,149,150 Those inflammatory infiltrates related to atelectasis duration150 and were further magnified by systemic endotoxemia.151 Additionally, atelectasis by itself can alter cellular immune function, e.g., enhance alveolar macrophage cytokine secretion in rats,152 impair macrophage phagocytosis against bacteria in vitro in piglets,153 and reduce local bronchoalveolar lymphocyte function in dogs.149
Current evidence reinforces the concept that atelectasis produces inflammatory response with pathophysiological mechanisms different from those occurring in aerated lung regions. Indeed, different transcriptomic patterns in immunity have been documented recently in the atelectatic versus ventilated sheep lung, with less NF-κB-related genes in sterile lungs and higher interferon stimulated genes in the presence of systemic endotoxemia.154 Such regional differences have also been found in a one-lung ventilation rat model showing increased myeloperoxidase, a neutrophil marker, in atelectasis and CCL2, a macrophage chemoattractant, in aerated lung regions.146 Accordingly, findings reporting the similarity of inflammatory injury between atelectatic and ventilated lung either in humans143,145,155 or in animals146,156 may actually derive from different underlying cytokine and genomics responses in aerated and atelectatic areas. Understanding such regional responses to atelectasis could help to identify potential treatments beyond the usual ventilatory interventions. For example, nanoparticle delivery of microRNAs (i.e., miR-146a) mitigated mouse lung injury during mechanical ventilation.157
Structural dysfunction
Pulmonary structure disruption is a hallmark of lung injury. Immobility (lack of cyclic stretch) associated with atelectasis could contribute to structural damage potentially by disorganization of actin networks,158 loss of adherens junction,159 and impairment of barrier properties.160,161 A recent sheep study also provided genomic support to these findings by revealing in initially healthy atelectatic lungs dysfunction of the lung tissue transcriptome related to structural components: endothelium, epithelium, and actin cytoskeleton.154
Other factors potentially present during atelectasis such as inflammation and ischemia can also lead to structural dysfunction, i.e., impairment of sodium and chloride channels (i.e., ENaCα),162 an ATP dependent process involved in alveolar fluid clearance; and injury of the endothelial glycocalyx layer,163 a critical component for lung barrier homeostasis. Re-expansion of atelectatic lung, a common process after one-lung ventilation, could be another contributing factor to structural damage due to oxidative stress and inflammation.164,165 Increased capillary transmural pressure from reversal of hypoxic pulmonary vasoconstriction following re-expansion and decreased pulmonary vascular resistance could mechanically injure the basement membrane of the alveolar–capillary lining.166
Yes-associated protein 1 (YAP) signaling is a key pathway in the control of cell proliferation, apoptosis and fate,167 and related to regulation of actin cytoskeleton dynamics168 and alveolar epithelium repair and regeneration.169 YAP signaling has been reported to be less activated in static pulmonary epithelial cells170 and in atelectatic lung than in normally-ventilated lung.154 Together these experimental studies suggest the potential role of YAP signaling and its possible use as a treatment target in structural dysfunction during atelectasis.
In line with those findings, microvascular endothelial disruption has been documented in atelectatic rat lung.138 Such structural compromise could lead to lung edema,156 microvascular protein leakage,147,171 and even bacterial translocation to the blood stream,172 suggesting an additional mechanism contributing to increased lung permeability and decompartmentalization of the lung inflammatory response, increasing the risk for multiorgan dysfunction.173 Potential therapies for structural dysfunction, e.g., β2 agonists directed at accelerating fluid clearance attempted in an acute lung injury trial (ALTA), are examples of treatment targets derived from basic knowledge,174 which illustrate the relevance of advancing the area.
Ultraprotective ventilation (limiting stress and strain with limited tidal volume and pressure) during extracorporeal membrane oxygenation (ECMO) for respiratory failure175–177 is a recently discussed strategy in which the biological effects of static and dynamic stretch may be relevant. The expected advantages of “lung rest” could benefit from barrier (e.g., epithelial and endothelial cells) protective effects of low cyclic stretch,178,179 and yet conflict with the damaging effects of lung immobility as well as the injurious effects of large static stretch on alveolar epithelial cells and extracellular matrix.180,181 A recent trial (LIFEGARDS) reported no association of mechanical ventilation settings during the first two days of ECMO with survival of patients with severe lung injury.182 A possible explanation is that the severity of the inflammatory response in these patients is so high that the ventilatory intervention would not be able to generate a biological response. Thus, optimal ventilatory settings and length of their application for best lung recovery strategies in ECMO patients, including the best balance between immobility and cyclic load, remains an open question.
Hypoxic injury
Lung collapse results in local hypoxia, a potent inducer of lung inflammation183 and microvascular injury.184 Attenuating hypoxia or eliminating atelectasis by lung recruitment reverses lung injury induced by alveolar hypoxia.171 Experimental data suggest that such hypoxia-related lung injury may be associated with NF-κB-dependent CXCL1 secretion from lung epithelial cells;146 macrophage recruitment and activation;183 decreased expression of lung neprilysin (a neutral endopeptidase);184 and excess reactive oxygen and nitrogen species (superoxide anion radical O2•- and nitric oxide NO•).185
The hypoxia activated transcription factor hypoxia-inducible factor (HIF)-1 could be another important regulator in atelectatic tissue associated both with pro- and anti-inflammatory mechanisms.186 HIF-1α is increased and activated in nonventilated atelectatic rat lungs,146 with distinct cellular effects. In myeloid cells, HIF-1α promotes acute inflammatory response through the regulation of glycolytic capacity.187 In endothelial cells, HIF-1α reduces mitochondrial respiratory capacity and activates vascular inflammation by promoting glycolysis.188,189 In contrast, in lung epithelial cells, HIF-1 contributes to anti-inflammation146 and barrier protection.190
Surfactant dysfunction
While surfactant dysfunction produces atelectasis as discussed above, conversely, atelectasis can lead to surfactant dysfunction. Classic studies reported that surfactant compression beyond 50% of its initial area, as potentially present during atelectasis, could result in film rupture on re-expansion and loss of function,191,192 in line with increased surface forces associated with low PEEP (i.e., deaerated lung) during in vitro ventilation.193 High surface forces in the alveoli causes transudation of proteinaceous fluid from capillaries into alveoli further contributing to surfactant dysfunction.194 Such dysfunction following atelectasis has been reinforced by clinical data from patients without cardiopulmonary disease showing that surfactant phospholipids in bronchoalveolar lavage of atelectatic regions were lower after onset of atelectasis, and remained low even after its resolution.147
Atelectasis-related pneumonia
Pneumonia is a major postoperative pulmonary complication. Its incidence has been reported as 1.8% in ASA 3 patients undergoing non-cardiothoracic predominantly abdominal and pelvic surgery,195 3.5% following cardiac surgery,196 and up to 25% after major lung resection.197
Atelectasis has been often suggested as associated with pneumonia. The biological compromise of the atelectatic lung immune defenses could facilitate the development of pneumonia, as detailed in the Inflammatory response section above. Local depletion and dysfunction of surfactant secondary to significant atelectasis or following pulmonary edema could further compromise the anti-infectious response as surfactant possesses antimicrobial properties198 and enhances macrophage phagocytosis and bacterial clearance.199 Additionally, mucus plugging or impaired mucus clearance following long periods of atelectasis can increase the risk of infection by compromising mucociliary clearance against organisms entering the lung and trapping them within collapsed regions.200,201
Experimental studies support that atelectasis was associated with larger bacterial growth and pneumonia when bacteria were present or instilled into collapsed lungs.172,200,201 Similarly, mechanical ventilation settings facilitating atelectasis (PEEP=0 cmH2O) increased lung bacterial burden in rabbits following tracheal bacterial instillation when compared with spontaneously breathing controls.202 Also, following the systemic intravenous injection of bacteria, the susceptibility to bacterial infection of atelectatic regions was greater than that of aerated regions.200 Of note, such effects of atelectasis might not be present when collapsed lung tissue is not exposed to any infectious agent, as atelectasis did not increase the incidence of pneumonia in dogs with non-infected lungs in a historical study.200
Clinical evidence has been more conflicting than such experimental studies. A large trial during major abdominal surgery indicated the high incidence of atelectasis and pneumonia in patients with ventilation settings predisposing to atelectasis (PEEP=0 cmH2O).3 However, the specific role of atelectasis or even an association could not be determined as large tidal volumes were combined with PEEP=0 cmH2O, and subsequent trials comparing high vs low PEEP in patients at different risk for alveolar collapse in similar settings did not show an effect of PEEP on postoperative pneumonia.203,204 A potential explanation for those findings could be that the short-lasting intraoperative reduction of intraoperative atelectasis might not be enough for a longer lasting effect in preventing infections through the first 5–7 days following surgery. Indeed, use of interventions addressing not only intra- but also immediate postoperative lung expansion resulted in less postoperative atelectasis and infectious complications in patients receiving lung expansion suggesting a clinical effect.205 Such hypothesis generating clinical results together with the basic science and translational findings suggest that interventions to at least minimize atelectasis could be relevant, and require further investigation.
Regional mechanical injury
Lung tissues are continuously subjected to different mechanical forces associated with lung inflation during spontaneous and mechanical ventilation, as discussed above (section “Physiological principles of bronchiolar and alveolar expansion”). During atelectasis, lung mechanical forces contributing significantly to lung injury might be ascribed to different biomechanical processes, including cyclic opening and closing, stress concentration, and over-distension of the non-atelectatic lung (Fig. 7).
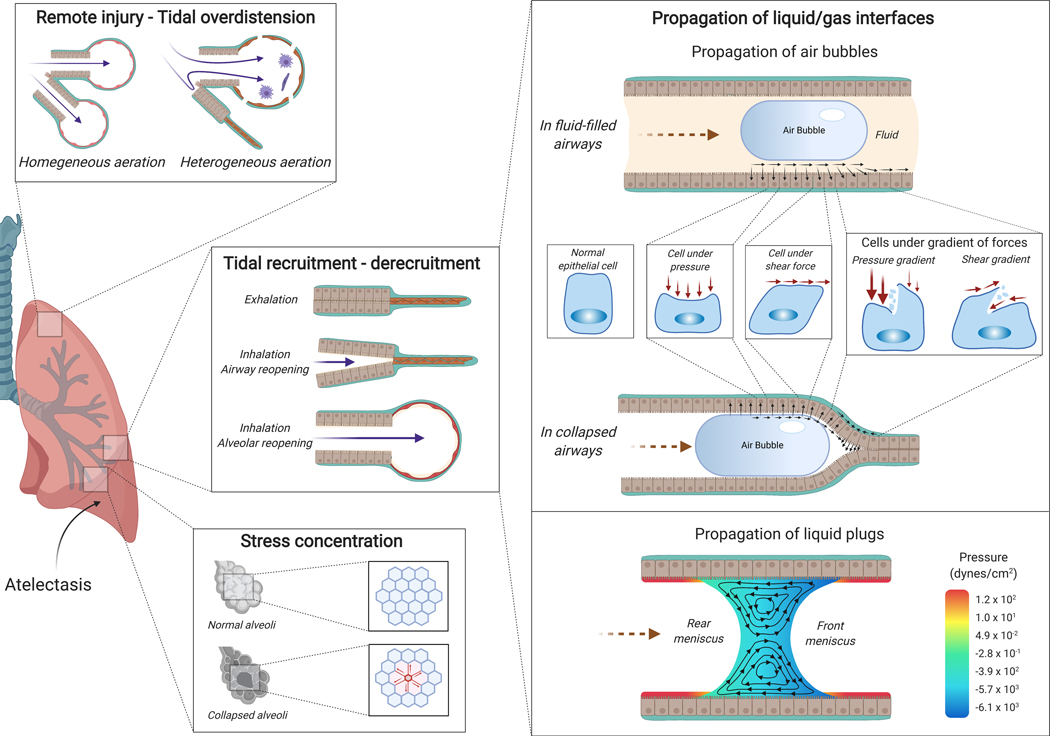
Fig. 7: Atelectasis-associated regional mechanical injury.
Airway or alveolar injury can occur during tidal recruitment-derecruitment. Propagation of gas-liquid interfaces is a potential mechanism. During inhalation, air propagates into a fluid-filled airway (right upper panel) or a collapsed airway (right middle panel) generating mechanical forces at the interface of air bubble and airway with resulting cell deformations due to normal pressure, shear stress, and their gradients. The pressure gradient is likely the major determinant of injury. Propagation of liquid plugs and rupture of the liquid menisci also generate abnormally large mechanical forces in the area of smallest film thickness where the front meniscus converges to a precursor film (right lower panel). Stress concentration (left bottom panel) is another potential mechanism for atelectasis-related lung injury. In normally expanded lungs, the alveoli are ventilated homogeneously. During atelectasis, however, collapsed areas may result in stress concentration, locally multiplying the stress around the atelectatic regions. Atelectasis also leads to the redistribution of tidal volume from atelectatic to aerated areas resulting in remote tidal overdistension (left upper panel).
Cyclic opening and closing
Cyclic opening and closing of lung units (i.e., bronchioles and alveoli), presumably resulting from the unfavorable balance of forces acting on airways and alveoli, is a frequently cited but still incompletely understood mechanism for lung injury associated with atelectasis. Different processes potentially present during repeated opening and closing have been studied to explain the resulting injury, such as cyclic airway and/or alveolar reopening, the propagation of air-liquid interfaces with production of injurious longitudinal gradients of pressure, as well as shear stress.
Airways and/or alveoli reopening
Histological injury in lungs ventilated with PEEP below the inflection point of the pressure-volume curve (Fig. 4), representative of PEEP insufficient to maintain lung units open,206 lead to the concept of mechanical trauma due to cyclic opening and closing. The critical opening pressure of an airway depends directly on airway fluid surface tension (γ) and inversely on airway radius (R) (Pcrit=8.3γ/R).207 Such relationship suggests a distribution of opening pressures along the airway tree with higher critical pressures and presumably injury from tidal recruitment at smaller airways.
Airway closure has been documented in vivo by computed tomography imaging in injured experimental lung models.208,209 Cyclic opening and closing of airways results in bronchiolar injury as reported in animal models ventilated with zero210 or negative end-expiratory pressure.211 In addition, repetitive alveolar collapse and expansion, directly visualized in surfactant-deactivated lung using in vivo microscope,212 produces histologic injury with thickened alveolar walls, significant intra-alveolar edema, and numerous neutrophils.212,213 Experiments document significant regional ventilation heterogeneity in poorly aerated regions in healthy lungs comparable to those of humans compatible with intermittent airway closure and reversible with PEEP.214 The extent to which cyclic opening and closing occurs in human lungs and contributes to injury remains to be defined.
Surface forces during propagation of gas-liquid interfaces
Surface forces can importantly contribute to epithelial injury during ventilation of atelectatic regions and associated opening-closing of airways and alveoli.215,216 Secretions, surfactant dysfunction, and alveolar edema affect the fluid lining the airway and can lead to the formation of liquid plugs or liquid bridges in the airway lumen in association with lung collapse. Mechanical or spontaneous ventilation of such airways and underlying alveoli produces the propagation of these gas-liquid interfaces, i.e., movement of the liquid plugs/bridges by the incoming air (Fig. 7). The mechanical forces acting on the epithelium lining of airways and alveoli resulting from this movement have been advanced as a key mechanism of cell injury in mechanical ventilation of atelectatic regions.215,216 The relevance of this mechanism has been experimentally supported in large tidal volume ventilation of normal rat lungs by observation of more wounded epithelial cells when gas-liquid interfaces were present (partial lung instillation of normal saline) than when lungs were either exclusively overdistended or completely saline-filled (i.e., no gas-liquid interface).217
Cellular injury produced by propagation of gas-liquid interfaces include cell detachment and necrosis,218 cell membrane fracture,219 impairment of cell-cell adhesion220 and deterioration of cytoskeletal structure221. Such damage increase with reduced airway compliance222 and diameter.218 The rupture of liquid plugs can also lead to epithelial cell injury due to fluid mechanical stresses in the vicinity and downstream of plug rupture216 with associated inflammatory response.223 Different mechanical forces generated during interface propagation and acting on the epithelial cells lining opening airways include pressure and pressure gradients, shear stress and shear stress gradients (Fig. 7), the pressure gradient likely being the primary determinant of mechanical damage.215,224
Surfactant treatment reduces the mechanical stress imparted by the propagation of gas-liquid interfaces.225 This is consistent with its success in neonatal use.226 In adults, failure of surfactant trials may have resulted from inadequate surfactant delivery to the distal airways and alveoli due to low instilled dose volume.227
Shear stress
Shear stress is defined as the force divided by the area parallel to the applied force (Fig. 7). Cyclic opening and closing of the small airways or alveolar ducts could generate shear stress, acting on the collapsed and surrounding lung.215,218 While frequently mentioned as a common cause of injury during repeated opening and closing, no studies directly assessed shear stress in vivo. During the propagation of gas-liquid interfaces in vitro, shear stress at the air bubble cap was estimated as far greater than that in the regions upstream or downstream of the bubble tip.215 However, theoretical investigations as detailed above suggested that shear stress is less important than the longitudinal pressure gradient in producing cell injury.215,224
Stress concentration
Atelectasis-related mechanical injury can also be produced by “stress concentration”, first proposed by Mead et al.4 It is due to the distribution of mechanical forces in the three-dimensional lung structure around a region whose initially surrounding area is reduced by atelectasis. Stress concentration occurs in normal regions at the interface between open and closed lung, which are thus exposed to exaggerated stress (e.g., tethering stress described in physiology section) during ventilation (Fig. 7).228,229
This mechanism could explain the injury observed around the atelectatic lung tissues, as presented in an ex vivo isolated, perfused rat lung model.230 Acting as a stress concentrator, atelectasis can generate structural alveolar injury and inflammation in the surrounding lung tissue.231 Even microatelectasis can lead to histological epithelial injury due to stress concentration as reported in a bleomycin injured rat lung when ventilated with low PEEP and large tidal volume for 3h.232 In addition, such stress concentration around atelectasis helps explain the clinical phenomenon of increased local neutrophilic activation at the interface between inflated and non-inflated tissue in patients detected by positron emission tomography.233
Remote injury-tidal overdistension
Atelectasis leads to loss of aerated lung volume with redistribution of tidal lung volume during ventilation to the remaining smaller aerated lung (Fig. 7).151 Thus, regional strain increases in such ventilated areas, with susceptibility to hyperinflation detectable by computed tomography.234–236 This hyperinflation of aerated regions could promote higher lung inflammation than atelectasis at comparable low tidal volume and lower driving pressure.237 Consistent with these considerations, experimental findings in a rat lavage model of dependent atelectasis showed the coexistence of dependent atelectasis and remote nondependent lung injury characterized by distal airway injury and increased alveolar epithelial mRNA expression of inflammatory cytokines (e.g., IL-6, IL-1 and MIP2).238 Also, in initially healthy sheep with lung size and heterogeneity comparable to that of humans receiving protective ventilation in the presence of mild systemic inflammation progressive atelectasis was associated with lung strain increased to areas of high aeration.151 Of note, these regions showed increased inflammation as assessed by positron emission tomography both in large animals151 and in patients with inflamed lungs,239 suggesting their contribution to ultimate clinical lung injury.
Closing remarks
The perioperative period is associated with a profound imbalance of the physical forces that maintain, in the awake conditions, the physiological expansion of the lung. Accordingly, pulmonary atelectasis, most frequently located in the dorso-caudal regions, represents an almost constant feature of general anesthesia. Hypoxemia and lowered respiratory system compliance are classical presentations of atelectasis at the bedside. Prolonged lung collapse and the associated biomechanical processes secondary to the ventilation of a heterogeneously-aerated lung may actively participate in significant lung injury. The biological response associated with atelectasis, before and after re-expansion, could further compound with the injurious process. The impact of intraoperative pulmonary atelectasis on postoperative outcomes such as pneumonia and acute lung injury while presumed is still in need of high level evidence. The presented information is expected to provide a basis for future inquire and physiological-based clinical practice. Although the current focus on preventing postoperative pulmonary complications lies on using ventilator strategies to prevent atelectasis or overexpansion of atelectatic lungs, future approaches may take advantage of common or novel perioperative medications, which would address some of the sequalae of significant atelectasis at the cellular and molecular levels.
Summary statement:
Up-to-date information on the pathophysiological mechanisms producing atelectasis and its functional, biological and biomechanical consequences are reviewed. The mechanistical understanding aims to provide a solid basis for critical assessment of clinical management.
Acknowledgments:
Figures created with BioRender.com.
Funding statement:
This work was funded by NIH-NHLBI grants R01 HL121228 and UH3 HL140177 to MFVM. DL received research grants from SFAR, EACTA and Fondation Monahan. JWL received support from NHLBI grants R01 HL113022 and HL148781.
Footnotes
Conflicts of Interest: none.
REFERENCES
- 1.Bendixen HH, Hedley-Whyte J, Laver MB: Impaired oxygenation in surgical patients during general anesthesia with controlled ventilation. A concept of atelectasis. N Engl J Med 1963; 269:991–6 [DOI] [PubMed] [Google Scholar]
- 2.Brismar B, Hedenstierna G, Lundquist H, Strandberg A, Svensson L, Tokics L: Pulmonary densities during anesthesia with muscular relaxation--a proposal of atelectasis. Anesthesiology 1985; 62:422–8 [DOI] [PubMed] [Google Scholar]
- 3.Futier E, Constantin J-M, Paugam-Burtz C, Pascal J, Eurin M, Neuschwander A, Marret E, Beaussier M, Gutton C, Lefrant J-Y, Allaouchiche B, Verzilli D, Leone M, De Jong A, Bazin J-E, Pereira B, Jaber S: A Trial of Intraoperative Low-Tidal-Volume Ventilation in Abdominal Surgery. N Engl J Med 2013; 369:428–37 [DOI] [PubMed] [Google Scholar]
- 4.Mead J, Takishima T, Leith D: Stress distribution in lungs: a model of pulmonary elasticity. J Appl Physiol 1970; 28:596–608 [DOI] [PubMed] [Google Scholar]
- 5.Burgstaller G, Oehrle B, Gerckens M, White ES, Schiller HB, Eickelberg O: The instructive extracellular matrix of the lung: basic composition and alterations in chronic lung disease. Eur Respir J 2017; 50 [DOI] [PubMed] [Google Scholar]
- 6.Prange HD: Laplace’s law and the alveolus: a misconception of anatomy and a misapplication of physics. Adv Physiol Educ 2003; 27:34–40 [DOI] [PubMed] [Google Scholar]
- 7.Albert RK: The role of ventilation-induced surfactant dysfunction and atelectasis in causing acute respiratory distress syndrome. Am J Respir Crit Care Med 2012; 185:702–8 [DOI] [PubMed] [Google Scholar]
- 8.Sciurba FC, Rogers RM, Keenan RJ, Slivka WA, Gorcsan J, Ferson PF, Holbert JM, Brown ML, Landreneau RJ: Improvement in pulmonary function and elastic recoil after lung-reduction surgery for diffuse emphysema. N Engl J Med 1996; 334:1095–9 [DOI] [PubMed] [Google Scholar]
- 9.Anafi RC, Wilson TA: Airway stability and heterogeneity in the constricted lung. J Appl Physiol (1985) 2001; 91:1185–92 [DOI] [PubMed] [Google Scholar]
- 10.Paré PD, Mitzner W: Airway-parenchymal interdependence. Compr Physiol 2012; 2:1921–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tawhai MH, Nash MP, Lin C-L, Hoffman EA: Supine and prone differences in regional lung density and pleural pressure gradients in the human lung with constant shape. J Appl Physiol (1985) 2009; 107:912–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ito S, Ingenito EP, Brewer KK, Black LD, Parameswaran H, Lutchen KR, Suki B: Mechanics, nonlinearity, and failure strength of lung tissue in a mouse model of emphysema: possible role of collagen remodeling. J Appl Physiol (1985) 2005; 98:503–11 [DOI] [PubMed] [Google Scholar]
- 13.Loring SH, Topulos GP, Hubmayr RD: Transpulmonary Pressure: The Importance of Precise Definitions and Limiting Assumptions. Am J Respir Crit Care Med 2016; 194:1452–7 [DOI] [PubMed] [Google Scholar]
- 14.Hedenstierna G, McCarthy G, Bergström M: Airway closure during mechanical ventilation. Anesthesiology 1976; 44:114–23 [DOI] [PubMed] [Google Scholar]
- 15.Rothen HU, Sporre B, Engberg G, Wegenius G, Hedenstierna G: Airway closure, atelectasis and gas exchange during general anaesthesia. Br J Anaesth 1998; 81:681–6 [DOI] [PubMed] [Google Scholar]
- 16.Lai-Fook SJ, Rodarte JR: Pleural pressure distribution and its relationship to lung volume and interstitial pressure. J Appl Physiol (1985) 1991; 70:967–78 [DOI] [PubMed] [Google Scholar]
- 17.Hedenstierna G, Tokics L, Lundquist H, Andersson T, Strandberg Å, Brismar B: Phrenic Nerve Stimulation during Halothane Anesthesia. Anesthesiology 1994; 80:751–60 [DOI] [PubMed] [Google Scholar]
- 18.Krayer S, Rehder K, Vettermann J, Didier EP, Ritman EL: Position and motion of the human diaphragm during anesthesia-paralysis. Anesthesiology 1989; 70:891–8 [DOI] [PubMed] [Google Scholar]
- 19.Froese AB, Bryan AC: Effects of anesthesia and paralysis on diaphragmatic mechanics in man. Anesthesiology 1974; 41:242–55 [DOI] [PubMed] [Google Scholar]
- 20.Boriek AM, Rodarte JR: Inferences on passive diaphragm mechanics from gross anatomy. J Appl Physiol (1985) 1994; 77:2065–70 [DOI] [PubMed] [Google Scholar]
- 21.Hubmayr RD, Sprung J, Nelson S: Determinants of transdiaphragmatic pressure in dogs. J Appl Physiol (1985) 1990; 69:2050–6 [DOI] [PubMed] [Google Scholar]
- 22.Warner DO, Warner MA, Ritman EL: Atelectasis and chest wall shape during halothane anesthesia. Anesthesiology 1996; 85:49–59 [DOI] [PubMed] [Google Scholar]
- 23.Reber A, Nylund U, Hedenstierna G: Position and shape of the diaphragm: implications for atelectasis formation. Anaesthesia 1998; 53:1054–61 [DOI] [PubMed] [Google Scholar]
- 24.Warner DO, Warner MA, Ritman EL: Human chest wall function while awake and during halothane anesthesia. I. Quiet breathing. Anesthesiology 1995; 82:6–19 [DOI] [PubMed] [Google Scholar]
- 25.Hedenstierna G, Tokics L, Reinius H, Rothen HU, Östberg E, Öhrvik J: Higher age and obesity limit atelectasis formation during anaesthesia: an analysis of computed tomography data in 243 subjects. Br J Anaesth 2020; 124:336–44 [DOI] [PubMed] [Google Scholar]
- 26.Reber A, Engberg G, Sporre B, Kviele L, Rothen HU, Wegenius G, Nylund U, Hedenstierna G: Volumetric analysis of aeration in the lungs during general anaesthesia. Br J Anaesth 1996; 76:760–6 [DOI] [PubMed] [Google Scholar]
- 27.Hedenstierna G, Strandberg A, Brismar B, Lundquist H, Svensson L, Tokics L: Functional residual capacity, thoracoabdominal dimensions, and central blood volume during general anesthesia with muscle paralysis and mechanical ventilation. Anesthesiology 1985; 62:247–54 [DOI] [PubMed] [Google Scholar]
- 28.Mutoh T, Lamm WJ, Embree LJ, Hildebrandt J, Albert RK: Volume infusion produces abdominal distension, lung compression, and chest wall stiffening in pigs. J Appl Physiol (1985) 1992; 72:575–82 [DOI] [PubMed] [Google Scholar]
- 29.Lundquist H, Hedenstierna G, Strandberg A, Tokics L, Brismar B: CT-assessment of dependent lung densities in man during general anaesthesia. Acta Radiol 1995; 36:626–32 [PubMed] [Google Scholar]
- 30.Rodarte JR, Hubmayr RD, Stamenovic D, Walters BJ: Regional lung strain in dogs during deflation from total lung capacity. J Appl Physiol (1985) 1985; 58:164–72 [DOI] [PubMed] [Google Scholar]
- 31.Puybasset L, Cluzel P, Chao N, Slutsky AS, Coriat P, Rouby JJ: A computed tomography scan assessment of regional lung volume in acute lung injury. The CT Scan ARDS Study Group. Am J Respir Crit Care Med 1998; 158:1644–55 [DOI] [PubMed] [Google Scholar]
- 32.Hubmayr RD, Rodarte JR, Walters BJ, Tonelli FM: Regional ventilation during spontaneous breathing and mechanical ventilation in dogs. J Appl Physiol (1985) 1987; 63:2467–75 [DOI] [PubMed] [Google Scholar]
- 33.Xin Y, Cereda M, Hamedani H, Martin KT, Tustison NJ, Pourfathi M, Kadlecek S, Siddiqui S, Amzajerdian F, Connell M, Abate N, Kajanaku A, Duncan I, Gee JC, Rizi RR: Positional Therapy and Regional Pulmonary Ventilation. Anesthesiology 2020; 133:1093–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ayoub J, Cohendy R, Prioux J, Ahmaidi S, Bourgeois JM, Dauzat M, Ramonatxo M, Préfaut C: Diaphragm movement before and after cholecystectomy: a sonographic study. Anesth Analg 2001; 92:755–61 [DOI] [PubMed] [Google Scholar]
- 35.Spadaro S, Grasso S, Dres M, Fogagnolo A, Dalla Corte F, Tamburini N, Maniscalco P, Cavallesco G, Alvisi V, Stripoli T, De Camillis E, Ragazzi R, Volta CA: Point of Care Ultrasound to Identify Diaphragmatic Dysfunction after Thoracic Surgery. Anesthesiology 2019; 131:266–78 [DOI] [PubMed] [Google Scholar]
- 36.Laghlam D, Lê MP, Srour A, Monsonego R, Estagnasié P, Brusset A, Squara P: Diaphragm Dysfunction After Cardiac Surgery: Reappraisal. J Cardiothorac Vasc Anesth 2021. doi: 10.1053/j.jvca.2021.02.023 [DOI] [PubMed] [Google Scholar]
- 37.Ford GT, Whitelaw WA, Rosenal TW, Cruse PJ, Guenter CA: Diaphragm function after upper abdominal surgery in humans. Am Rev Respir Dis 1983; 127:431–6 [DOI] [PubMed] [Google Scholar]
- 38.Simonneau G, Vivien A, Sartene R, Kunstlinger F, Samii K, Noviant Y, Duroux P: Diaphragm dysfunction induced by upper abdominal surgery. Role of postoperative pain. Am Rev Respir Dis 1983; 128:899–903 [DOI] [PubMed] [Google Scholar]
- 39.Merino-Ramirez MA, Juan G, Ramón M, Cortijo J, Rubio E, Montero A, Morcillo EJ: Electrophysiologic evaluation of phrenic nerve and diaphragm function after coronary bypass surgery: prospective study of diabetes and other risk factors. J Thorac Cardiovasc Surg 2006; 132:530–6, 536.e1–2 [DOI] [PubMed] [Google Scholar]
- 40.Canbaz S, Turgut N, Halici U, Balci K, Ege T, Duran E: Electrophysiological evaluation of phrenic nerve injury during cardiac surgery--a prospective, controlled, clinical study. BMC Surg 2004; 4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Easton PA, Fitting JW, Arnoux R, Guerraty A, Grassino AE: Recovery of diaphragm function after laparotomy and chronic sonomicrometer implantation. J Appl Physiol (1985) 1989; 66:613–21 [DOI] [PubMed] [Google Scholar]
- 42.Duggan J, Drummond GB: Activity of lower intercostal and abdominal muscle after upper abdominal surgery. Anesth Analg 1987; 66:852–5 [PubMed] [Google Scholar]
- 43.Demoule A, Jung B, Prodanovic H, Molinari N, Chanques G, Coirault C, Matecki S, Duguet A, Similowski T, Jaber S: Diaphragm dysfunction on admission to the intensive care unit. Prevalence, risk factors, and prognostic impact-a prospective study. Am J Respir Crit Care Med 2013; 188:213–9 [DOI] [PubMed] [Google Scholar]
- 44.Koo P, Gartman EJ, Sethi JM, McCool FD: Physiology in Medicine: physiological basis of diaphragmatic dysfunction with abdominal hernias-implications for therapy. J Appl Physiol (1985) 2015; 118:142–7 [DOI] [PubMed] [Google Scholar]
- 45.Lal S: Apomorphine in the evaluation of dopaminergic function in man. Prog Neuropsychopharmacol Biol Psychiatry 1988; 12:117–64 [DOI] [PubMed] [Google Scholar]
- 46.Morais CCA, Koyama Y, Yoshida T, Plens GM, Gomes S, Lima CAS, Ramos OPS, Pereira SM, Kawaguchi N, Yamamoto H, Uchiyama A, Borges JB, Vidal Melo MF, Tucci MR, Amato MBP, Kavanagh BP, Costa ELV, Fujino Y: High Positive End-Expiratory Pressure Renders Spontaneous Effort Noninjurious. Am J Respir Crit Care Med 2018; 197:1285–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ide T, Kochi T, Isono S, Mizuguchi T: Diaphragmatic activity during isoflurane anaesthesia in dogs. Acta Anaesthesiol Scand 1993; 37:253–7 [DOI] [PubMed] [Google Scholar]
- 48.Jalde FC, Jalde F, Sackey PV, Radell PJ, Eksborg S, Wallin MKEB: Neurally adjusted ventilatory assist feasibility during anaesthesia: A randomised crossover study of two anaesthetics in a large animal model. Eur J Anaesthesiol 2016; 33:283–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rocco M, Maggi L, Ranieri G, Ferrari G, Gregoretti C, Conti G, DE Blasi RA: Propofol sedation reduces diaphragm activity in spontaneously breathing patients: ultrasound assessment. Minerva Anestesiol 2017; 83:266–73 [DOI] [PubMed] [Google Scholar]
- 50.Nishina K, Mikawa K, Kodama S, Kagawa T, Uesugi T, Obara H: The effects of enflurane, isoflurane, and intravenous anesthetics on rat diaphragmatic function and fatigability. Anesth Analg 2003; 96:1674–8 [DOI] [PubMed] [Google Scholar]
- 51.Cammu G, Schepens T, De Neve N, Wildemeersch D, Foubert L, Jorens PG: Diaphragmatic and intercostal electromyographic activity during neostigmine, sugammadex and neostigmine-sugammadex-enhanced recovery after neuromuscular blockade: A randomised controlled volunteer study. Eur J Anaesthesiol 2017; 34:8–15 [DOI] [PubMed] [Google Scholar]
- 52.Eikermann M, Fassbender P, Malhotra A, Takahashi M, Kubo S, Jordan AS, Gautam S, White DP, Chamberlin NL: Unwarranted administration of acetylcholinesterase inhibitors can impair genioglossus and diaphragm muscle function. Anesthesiology 2007; 107:621–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gattinoni L, Caironi P, Cressoni M, Chiumello D, Ranieri VM, Quintel M, Russo S, Patroniti N, Cornejo R, Bugedo G: Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med 2006; 354:1775–86 [DOI] [PubMed] [Google Scholar]
- 54.Pelosi P, D’Andrea L, Vitale G, Pesenti A, Gattinoni L: Vertical gradient of regional lung inflation in adult respiratory distress syndrome. Am J Respir Crit Care Med 1994; 149:8–13 [DOI] [PubMed] [Google Scholar]
- 55.Gattinoni L, Caironi P: Prone positioning: beyond physiology. Anesthesiology 2010; 113:1262–4 [DOI] [PubMed] [Google Scholar]
- 56.Musch G, Layfield JDH, Harris RS, Melo MFV, Winkler T, Callahan RJ, Fischman AJ, Venegas JG: Topographical distribution of pulmonary perfusion and ventilation, assessed by PET in supine and prone humans. J Appl Physiol (1985) 2002; 93:1841–51 [DOI] [PubMed] [Google Scholar]
- 57.Neves FH, Carmona MJ, Auler JOC, Rodrigues RR, Rouby JJ, Malbouisson LMS: Cardiac compression of lung lower lobes after coronary artery bypass graft with cardiopulmonary bypass. PLoS One 2013; 8:e78643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chiumello D, Marino A, Cressoni M, Mietto C, Berto V, Gallazzi E, Chiurazzi C, Lazzerini M, Cadringher P, Quintel M, Gattinoni L: Pleural effusion in patients with acute lung injury: a CT scan study. Crit Care Med 2013; 41:935–44 [DOI] [PubMed] [Google Scholar]
- 59.Melo MFV, Bates JHT: Pleural effusion in acute respiratory distress syndrome: water, water, everywhere, nor any drop to drain. Crit Care Med 2013; 41:1133–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bates JHT, Irvin CG: Time dependence of recruitment and derecruitment in the lung: a theoretical model. J Appl Physiol (1985) 2002; 93:705–13 [DOI] [PubMed] [Google Scholar]
- 61.Hedenstierna G, McCarthy GS: Airway closure and closing pressure during mechanical ventilation. Acta Anaesthesiol Scand 1980; 24:299–304 [DOI] [PubMed] [Google Scholar]
- 62.Massa CB, Allen GB, Bates JHT: Modeling the dynamics of recruitment and derecruitment in mice with acute lung injury. J Appl Physiol (1985) 2008; 105:1813–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gil J, Weibel ER: Morphological study of pressure-volume hysteresis in rat lungs fixed by vascular perfusion. Respir Physiol 1972; 15:190–213 [DOI] [PubMed] [Google Scholar]
- 64.Cereda M, Xin Y, Emami K, Huang J, Rajaei J, Profka H, Han B, Mongkolwisetwara P, Kadlecek S, Kuzma NN, Pickup S, Kavanagh BP, Deutschman CS, Rizi RR: Positive end-expiratory pressure increments during anesthesia in normal lung result in hysteresis and greater numbers of smaller aerated airspaces. Anesthesiology 2013; 119:1402–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tucci MR, Costa ELV, Wellman TJ, Musch G, Winkler T, Harris RS, Venegas JG, Amato MBP, Melo MFV: Regional Lung Derecruitment and Inflammation during 16 Hours of Mechanical Ventilation in Supine Healthy Sheep: Anesthesiology 2013; 119:156–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Radford EP Jr: Static mechanical properties of mammalian lungs, Handbook of physiology. Edited by Bethesda Fenn W.: American Physiological Society, 1964, pp 429–49 [Google Scholar]
- 67.Matsuura S, Shirakami G, Iida H, Tanimoto K, Fukuda K: The effect of sevoflurane on ciliary motility in rat cultured tracheal epithelial cells: a comparison with isoflurane and halothane. Anesth Analg 2006; 102:1703–8 [DOI] [PubMed] [Google Scholar]
- 68.Feldman KS, Kim E, Czachowski MJ, Wu Y, Lo CW, Zahid M: Differential effect of anesthetics on mucociliary clearance in vivo in mice. Sci Rep 2021; 11:4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Keller C, Brimacombe J: Bronchial mucus transport velocity in paralyzed anesthetized patients: a comparison of the laryngeal mask airway and cuffed tracheal tube. Anesth Analg 1998; 86:1280–2 [DOI] [PubMed] [Google Scholar]
- 70.Rehder K, Knopp TJ, Sessler AD, Didier EP: Ventilation-perfusion relationship in young healthy awake and anesthetized-paralyzed man. J Appl Physiol Respir Environ Exerc Physiol 1979; 47:745–53 [DOI] [PubMed] [Google Scholar]
- 71.West JB: Distribution of blood and gas in lungs. Phys Med Biol 1966; 11:357–70 [DOI] [PubMed] [Google Scholar]
- 72.Glenny RW: Determinants of regional ventilation and blood flow in the lung. Intensive Care Med 2009; 35:1833–42 [DOI] [PubMed] [Google Scholar]
- 73.Tokics L, Hedenstierna G, Svensson L, Brismar B, Cederlund T, Lundquist H, Strandberg A: V/Q distribution and correlation to atelectasis in anesthetized paralyzed humans. J Appl Physiol (1985) 1996; 81:1822–33 [DOI] [PubMed] [Google Scholar]
- 74.Dantzker DR, Wagner PD, West JB: Proceedings: Instability of poorly ventilated lung units during oxygen breathing. J Physiol 1974; 242:72P. [PubMed] [Google Scholar]
- 75.Rothen HU, Sporre B, Engberg G, Wegenius G, Högman M, Hedenstierna G: Influence of gas composition on recurrence of atelectasis after a reexpansion maneuver during general anesthesia. Anesthesiology 1995; 82:832–42 [DOI] [PubMed] [Google Scholar]
- 76.Rothen HU, Sporre B, Engberg G, Wegenius G, Reber A, Hedenstierna G: Prevention of atelectasis during general anaesthesia. Lancet 1995; 345:1387–91 [DOI] [PubMed] [Google Scholar]
- 77.Joyce CJ, Williams AB: Kinetics of absorption atelectasis during anesthesia: a mathematical model. J Appl Physiol (1985) 1999; 86:1116–25 [DOI] [PubMed] [Google Scholar]
- 78.Oldham KT, Guice KS, Stetson PS, Wolfe RR: Bacteremia-induced suppression of alveolar surfactant production. J Surg Res 1989; 47:397–402 [DOI] [PubMed] [Google Scholar]
- 79.Davidson KG, Bersten AD, Barr HA, Dowling KD, Nicholas TE, Doyle IR: Endotoxin induces respiratory failure and increases surfactant turnover and respiration independent of alveolocapillary injury in rats. Am J Respir Crit Care Med 2002; 165:1516–25 [DOI] [PubMed] [Google Scholar]
- 80.Castiello A, Paterson JF, Shelley SA, Haller EM, Balis JU: Depletion of surfactant tubular myelin with pulmonary dysfunction in a rat model for acute endotoxemia. Shock 1994; 2:427–32 [DOI] [PubMed] [Google Scholar]
- 81.George CLS, White ML, O’Neill ME, Thorne PS, Schwartz DA, Snyder JM: Altered surfactant protein A gene expression and protein metabolism associated with repeat exposure to inhaled endotoxin. Am J Physiol Lung Cell Mol Physiol 2003; 285:L1337–1344 [DOI] [PubMed] [Google Scholar]
- 82.Günther A, Siebert C, Schmidt R, Ziegler S, Grimminger F, Yabut M, Temmesfeld B, Walmrath D, Morr H, Seeger W: Surfactant alterations in severe pneumonia, acute respiratory distress syndrome, and cardiogenic lung edema. Am J Respir Crit Care Med 1996; 153:176–84 [DOI] [PubMed] [Google Scholar]
- 83.Arias-Díaz J, Vara E, García C, Balibrea JL: Tumor necrosis factor-alpha-induced inhibition of phosphatidylcholine synthesis by human type II pneumocytes is partially mediated by prostaglandins. J Clin Invest 1994; 94:244–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liau DF, Yin NX, Huang J, Ryan SF: Effects of human polymorphonuclear leukocyte elastase upon surfactant proteins in vitro. Biochim Biophys Acta 1996; 1302:117–28 [DOI] [PubMed] [Google Scholar]
- 85.Ueda T, Ikegami M, Jobe A: Surfactant subtypes. In vitro conversion, in vivo function, and effects of serum proteins. Am J Respir Crit Care Med 1994; 149:1254–9 [DOI] [PubMed] [Google Scholar]
- 86.Prost N de Costa EL, Wellman T, Musch G, Winkler T, Tucci MR, Harris RS, Venegas JG, Vidal Melo MF: Effects of surfactant depletion on regional pulmonary metabolic activity during mechanical ventilation. J Appl Physiol (1985) 2011; 111:1249–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.de Prost N, Feng Y, Wellman T, Tucci MR, Costa EL, Musch G, Winkler T, Harris RS, Venegas JG, Chao W, Vidal Melo MF: 18F-FDG kinetics parameters depend on the mechanism of injury in early experimental acute respiratory distress syndrome. J Nucl Med 2014; 55:1871–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Govender M, Bihari S, Bersten AD, De Pasquale CG, Lawrence MD, Baker RA, Bennetts J, Dixon D-L: Surfactant and lung function following cardiac surgery. Heart Lung 2019; 48:55–60 [DOI] [PubMed] [Google Scholar]
- 89.Griese M, Wilnhammer C, Jansen S, Rinker C: Cardiopulmonary bypass reduces pulmonary surfactant activity in infants. J Thorac Cardiovasc Surg 1999; 118:237–44 [DOI] [PubMed] [Google Scholar]
- 90.Veldhuizen RA, Tremblay LN, Govindarajan A, Rozendaal BA van, Haagsman HP, Slutsky AS: Pulmonary surfactant is altered during mechanical ventilation of isolated rat lung. Crit Care Med 2000; 28:2545–51 [DOI] [PubMed] [Google Scholar]
- 91.Veldhuizen RA, Slutsky AS, Joseph M, McCaig L: Effects of mechanical ventilation of isolated mouse lungs on surfactant and inflammatory cytokines. Eur Respir J 2001; 17:488–94 [DOI] [PubMed] [Google Scholar]
- 92.Verbrugge SJ, Böhm SH, Gommers D, Zimmerman LJ, Lachmann B: Surfactant impairment after mechanical ventilation with large alveolar surface area changes and effects of positive end-expiratory pressure. Br J Anaesth 1998; 80:360–4 [DOI] [PubMed] [Google Scholar]
- 93.Letsiou E, Kitsiouli E, Nakos G, Lekka ME: Mild stretch activates cPLA2 in alveolar type II epithelial cells independently through the MEK/ERK and PI3K pathways. Biochim Biophys Acta 2011; 1811:370–6 [DOI] [PubMed] [Google Scholar]
- 94.Arold SP, Bartolák-Suki E, Suki B: Variable stretch pattern enhances surfactant secretion in alveolar type II cells in culture. Am J Physiol Lung Cell Mol Physiol 2009; 296:L574–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hildebran JN, Goerke J, Clements JA: Surfactant release in excised rat lung is stimulated by air inflation. J Appl Physiol Respir Environ Exerc Physiol 1981; 51:905–10 [DOI] [PubMed] [Google Scholar]
- 96.Arold SP, Suki B, Alencar AM, Lutchen KR, Ingenito EP: Variable ventilation induces endogenous surfactant release in normal guinea pigs. Am J Physiol Lung Cell Mol Physiol 2003; 285:L370–375 [DOI] [PubMed] [Google Scholar]
- 97.Smallwood CD, Boloori-Zadeh P, Silva MR, Gouldstone A: High Oxygen Concentrations Adversely Affect the Performance of Pulmonary Surfactant. Respir Care 2017; 62:1085–90 [DOI] [PubMed] [Google Scholar]
- 98.Molliex S, Crestani B, Dureuil B, Rolland C, Aubier M, Desmonts JM: Differential effects of isoflurane and i.v. anaesthetic agents on metabolism of alveolar type II cells. Br J Anaesth 1999; 82:767–9 [DOI] [PubMed] [Google Scholar]
- 99.Said SI, Avery ME, Davis RK, Banerjee CM, El-Gohary M: Pulmonary surface activity in induced pulmonary edema. J Clin Invest 1965; 44:458–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nakos G, Pneumatikos J, Tsangaris I, Tellis C, Lekka M: Proteins and phospholipids in BAL from patients with hydrostatic pulmonary edema. Am J Respir Crit Care Med 1997; 155:945–51 [DOI] [PubMed] [Google Scholar]
- 101.Egbert LD, Laver MB, Bendixen HH: Intermittent deep breaths and compliance during anesthesia in man. Anesthesiology 1963; 24:57–60 [Google Scholar]
- 102.Laver MB, Morgan J, Bendixen HH, Radford EP: Lung volume, compliance, and arterial oxygen tensions during controlled ventilation. J Appl Physiol 1964; 19:725–33 [DOI] [PubMed] [Google Scholar]
- 103.Mead J, Collier C: Relation of volume history of lungs to respiratory mechanics in anesthetized dogs. J Appl Physiol 1959; 14:669–78 [Google Scholar]
- 104.Behazin N, Jones SB, Cohen RI, Loring SH: Respiratory restriction and elevated pleural and esophageal pressures in morbid obesity. J Appl Physiol (1985) 2010; 108:212–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Neto AS, Hemmes SNT, Barbas CSV, Beiderlinden M, Fernandez-Bustamante A, Futier E, Gajic O, El-Tahan MR, Ghamdi AAA, Günay E, Jaber S, Kokulu S, Kozian A, Licker M, Lin W-Q, Maslow AD, Memtsoudis SG, Reis Miranda D, Moine P, Ng T, Paparella D, Ranieri VM, Scavonetto F, Schilling T, Selmo G, Severgnini P, Sprung J, Sundar S, Talmor D, Treschan T, Unzueta C, Weingarten TN, Wolthuis EK, Wrigge H, Amato MBP, Costa ELV, de Abreu MG, Pelosi P, Schultz MJ, PROVE Network Investigators: Association between driving pressure and development of postoperative pulmonary complications in patients undergoing mechanical ventilation for general anaesthesia: a meta-analysis of individual patient data. Lancet Respir Med 2016; 4:272–80 [DOI] [PubMed] [Google Scholar]
- 106.Ma B, Bates JHT: Mechanical interactions between adjacent airways in the lung. J Appl Physiol (1985) 2014; 116:628–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bonatti G, Robba C, Ball L, Silva PL, Rocco PRM, Pelosi P: Controversies when using mechanical ventilation in obese patients with and without acute distress respiratory syndrome. Expert Rev Respir Med 2019; 13:471–9 [DOI] [PubMed] [Google Scholar]
- 108.Nunn JF, Bergman NA, Coleman AJ: Factors influencing the arterial oxygen tension during anaesthesia with artificial ventilation. Br J Anaesth 1965; 37:898–914 [DOI] [PubMed] [Google Scholar]
- 109.Bendixen HH, Bullwinkel B, Hedley-Whyte J, Laver MB: Atelectasis and shunting during spontaneous ventilation in anesthetized patients. Anesthesiology 1964; 25:297–301 [DOI] [PubMed] [Google Scholar]
- 110.Tokics L, Hedenstierna G, Strandberg A, Brismar B, Lundquist H: Lung collapse and gas exchange during general anesthesia: effects of spontaneous breathing, muscle paralysis, and positive end-expiratory pressure. Anesthesiology 1987; 66:157–67 [DOI] [PubMed] [Google Scholar]
- 111.Magnusson L, Zemgulis V, Wicky S, Tydén H, Thelin S, Hedenstierna G: Atelectasis is a major cause of hypoxemia and shunt after cardiopulmonary bypass: an experimental study. Anesthesiology 1997; 87:1153–63 [DOI] [PubMed] [Google Scholar]
- 112.Magnusson L, Zemgulis V, Tenling A, Wernlund J, Tydén H, Thelin S, Hedenstierna G: Use of a vital capacity maneuver to prevent atelectasis after cardiopulmonary bypass: an experimental study. Anesthesiology 1998; 88:134–42 [DOI] [PubMed] [Google Scholar]
- 113.Wagner PD, Dantzker DR, Dueck R, Clausen JL, West JB: Ventilation-perfusion inequality in chronic obstructive pulmonary disease. J Clin Invest 1977; 59:203–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Carvalho AR, Ichinose F, Schettino IA, Hess D, Rojas J, Giannella-Neto A, Agnihotri A, Walker J, MacGillivray TE, Vidal Melo MF: Tidal lung recruitment and exhaled nitric oxide during coronary artery bypass grafting in patients with and without chronic obstructive pulmonary disease. Lung 2011; 189:499–509 [DOI] [PubMed] [Google Scholar]
- 115.Nagasaka Y, Bhattacharya J, Nanjo S, Gropper MA, Staub NC: Micropuncture measurement of lung microvascular pressure profile during hypoxia in cats. Circ Res 1984; 54:90–5 [DOI] [PubMed] [Google Scholar]
- 116.Euler US v, Liljestrand G: Observations on the Pulmonary Arterial Blood Pressure in the Cat. Acta Physiol Scand 1946; 12:301–20 [Google Scholar]
- 117.Wang L, Yin J, Nickles HT, Ranke H, Tabuchi A, Hoffmann J, Tabeling C, Barbosa-Sicard E, Chanson M, Kwak BR, Shin H-S, Wu S, Isakson BE, Witzenrath M, Wit C de, Fleming I, Kuppe H, Kuebler WM: Hypoxic pulmonary vasoconstriction requires connexin 40-mediated endothelial signal conduction. J Clin Invest 2012; 122:4218–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Talbot NP, Balanos GM, Dorrington KL, Robbins PA: Two temporal components within the human pulmonary vascular response to approximately 2 h of isocapnic hypoxia. J Appl Physiol (1985) 2005; 98:1125–39 [DOI] [PubMed] [Google Scholar]
- 119.Glasser SA, Domino KB, Lindgren L, Parcella P, Marshall C, Marshall BE: Pulmonary blood pressure and flow during atelectasis in the dog. Anesthesiology 1983; 58:225–31 [DOI] [PubMed] [Google Scholar]
- 120.Lumb AB, Slinger P: Hypoxic pulmonary vasoconstriction: physiology and anesthetic implications. Anesthesiology 2015; 122:932–46 [DOI] [PubMed] [Google Scholar]
- 121.Morrell NW, Nijran KS, Biggs T, Seed WA: Magnitude and time course of acute hypoxic pulmonary vasoconstriction in man. Respir Physiol 1995; 100:271–81 [DOI] [PubMed] [Google Scholar]
- 122.McCormack DG, Paterson NA: Loss of hypoxic pulmonary vasoconstriction in chronic pneumonia is not mediated by nitric oxide. Am J Physiol 1993; 265:H1523–1528 [DOI] [PubMed] [Google Scholar]
- 123.Pandolfi R, Barreira B, Moreno E, Lara-Acedo V, Morales-Cano D, Martínez-Ramas A, Olaiz Navarro B de, Herrero R, Lorente JÁ, Cogolludo Á, Pérez-Vizcaíno F, Moreno L: Role of acid sphingomyelinase and IL-6 as mediators of endotoxin-induced pulmonary vascular dysfunction. Thorax 2017; 72:460–71 [DOI] [PubMed] [Google Scholar]
- 124.Wepler M, Beloiartsev A, Buswell MD, Panigrahy D, Malhotra R, Buys ES, Radermacher P, Ichinose F, Bloch DB, Zapol WM: Soluble epoxide hydrolase deficiency or inhibition enhances murine hypoxic pulmonary vasoconstriction after lipopolysaccharide challenge. Am J Physiol Lung Cell Mol Physiol 2016; 311:L1213–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fischer LG, Freise H, Hilpert J-H, Wendholt D, Lauer S, Van Aken H, Sielenkämper AW: Modulation of hypoxic pulmonary vasoconstriction is time and nitric oxide dependent in a peritonitis model of sepsis. Intensive Care Med 2004; 30:1821–8 [DOI] [PubMed] [Google Scholar]
- 126.Simonneau G, Escourrou P, Duroux P, Lockhart A: Inhibition of hypoxic pulmonary vasoconstriction by nifedipine. N Engl J Med 1981; 304:1582–5 [DOI] [PubMed] [Google Scholar]
- 127.Marshall BE, Marshall C, Benumof J, Saidman LJ: Hypoxic pulmonary vasoconstriction in dogs: effects of lung segment size and oxygen tension. J Appl Physiol Respir Environ Exerc Physiol 1981; 51:1543–51 [DOI] [PubMed] [Google Scholar]
- 128.Pagel PS, Fu JL, Damask MC, Davis RF, Samuelson PN, Howie MB, Warltier DC: Desflurane and isoflurane produce similar alterations in systemic and pulmonary hemodynamics and arterial oxygenation in patients undergoing one-lung ventilation during thoracotomy. Anesth Analg 1998; 87:800–7 [DOI] [PubMed] [Google Scholar]
- 129.Kerbaul F, Bellezza M, Guidon C, Roussel L, Imbert M, Carpentier JP, Auffray JP: Effects of sevoflurane on hypoxic pulmonary vasoconstriction in anaesthetized piglets. Br J Anaesth 2000; 85:440–5 [DOI] [PubMed] [Google Scholar]
- 130.Benumof JL, Augustine SD, Gibbons JA: Halothane and isoflurane only slightly impair arterial oxygenation during one-lung ventilation in patients undergoing thoracotomy. Anesthesiology 1987; 67:910–5 [DOI] [PubMed] [Google Scholar]
- 131.McCann UG, Schiller HJ, Gatto LA, Steinberg JM, Carney DE, Nieman GF: Alveolar mechanics alter hypoxic pulmonary vasoconstriction. Crit Care Med 2002; 30:1315–21 [DOI] [PubMed] [Google Scholar]
- 132.Van Keer L, Van Aken H, Vandermeersch E, Vermaut G, Lerut T: Propofol does not inhibit hypoxic pulmonary vasoconstriction in humans. J Clin Anesth 1989; 1:284–8 [DOI] [PubMed] [Google Scholar]
- 133.Nakayama M, Murray PA: Ketamine preserves and propofol potentiates hypoxic pulmonary vasoconstriction compared with the conscious state in chronically instrumented dogs. Anesthesiology 1999; 91:760–71 [DOI] [PubMed] [Google Scholar]
- 134.Bjertnaes L, Hauge A, Kriz M: Hypoxia-induced pulmonary vasoconstriction: effects of fentanyl following different routes of administration. Acta Anaesthesiol Scand 1980; 24:53–7 [DOI] [PubMed] [Google Scholar]
- 135.Abe K, Shimizu T, Takashina M, Shiozaki H, Yoshiya I: The effects of propofol, isoflurane, and sevoflurane on oxygenation and shunt fraction during one-lung ventilation. Anesth Analg 1998; 87:1164–9 [DOI] [PubMed] [Google Scholar]
- 136.Tenling A, Hachenberg T, Tydén H, Wegenius G, Hedenstierna G: Atelectasis and gas exchange after cardiac surgery. Anesthesiology 1998; 89:371–8 [DOI] [PubMed] [Google Scholar]
- 137.Kiely DG, Cargill RI, Lipworth BJ: Acute hypoxic pulmonary vasoconstriction in man is attenuated by type I angiotensin II receptor blockade. Cardiovasc Res 1995; 30:875–80 [PubMed] [Google Scholar]
- 138.Duggan M, McCaul CL, McNamara PJ, Engelberts D, Ackerley C, Kavanagh BP: Atelectasis causes vascular leak and lethal right ventricular failure in uninjured rat lungs. Am J Respir Crit Care Med 2003; 167:1633–40 [DOI] [PubMed] [Google Scholar]
- 139.Schmitt JM, Vieillard-Baron A, Augarde R, Prin S, Page B, Jardin F: Positive end-expiratory pressure titration in acute respiratory distress syndrome patients: impact on right ventricular outflow impedance evaluated by pulmonary artery Doppler flow velocity measurements. Crit Care Med 2001; 29:1154–8 [DOI] [PubMed] [Google Scholar]
- 140.Longo S, Siri J, Acosta C, Palencia A, Echegaray A, Chiotti I, Parisi A, Ricci L, Natal M, Suarez-Sipmann F, Tusman G: Lung recruitment improves right ventricular performance after cardiopulmonary bypass: A randomised controlled trial. Eur J Anaesthesiol 2017; 34:66–74 [DOI] [PubMed] [Google Scholar]
- 141.Canada E, Benumof JL, Tousdale FR: Pulmonary vascular resistance correlates in intact normal and abnormal canine lungs. Crit Care Med 1982; 10:719–23 [DOI] [PubMed] [Google Scholar]
- 142.Hughes JM, Glazier JB, Maloney JE, West JB: Effect of extra-alveolar vessels on distribution of blood flow in the dog lung. J Appl Physiol 1968; 25:701–12 [DOI] [PubMed] [Google Scholar]
- 143.Gala F de la, Piñeiro P, Garutti I, Reyes A, Olmedilla L, Cruz P, Duque P, Casanova J, Rancan L, Benito P, Vara E: Systemic and alveolar inflammatory response in the dependent and nondependent lung in patients undergoing lung resection surgery: A prospective observational study. Eur J Anaesthesiol 2015; 32:872–80 [DOI] [PubMed] [Google Scholar]
- 144.Komatsu Y, Yamamoto H, Tsushima K, Furuya S, Yoshikawa S, Yasuo M, Kubo K, Yamazaki Y, Hasegawa J, Eguchi T, Kondo R, Yoshida K, Koizumi T: Increased interleukin-8 in epithelial lining fluid of collapsed lungs during one-lung ventilation for thoracotomy. Inflammation 2012; 35:1844–50 [DOI] [PubMed] [Google Scholar]
- 145.Sugasawa Y, Yamaguchi K, Kumakura S, Murakami T, Suzuki K, Nagaoka I, Inada E: Effects of sevoflurane and propofol on pulmonary inflammatory responses during lung resection. J Anesth 2012; 26:62–9 [DOI] [PubMed] [Google Scholar]
- 146.Tojo K, Nagamine Y, Yazawa T, Mihara T, Baba Y, Ota S, Goto T, Kurahashi K: Atelectasis causes alveolar hypoxia-induced inflammation during uneven mechanical ventilation in rats. ICMx 2015; 3:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nakos G, Tsangaris H, Liokatis S, Kitsiouli E, Lekka ME: Ventilator-associated pneumonia and atelectasis: evaluation through bronchoalveolar lavage fluid analysis. Intensive Care Med 2003; 29:555–63 [DOI] [PubMed] [Google Scholar]
- 148.Wakabayashi K, Wilson MR, Tatham KC, O’Dea KP, Takata M: Volutrauma, but not Atelectrauma, Induces Systemic Cytokine Production by Lung-Marginated Monocytes. Crit Care Med 2014; 42:e49–57 [DOI] [PubMed] [Google Scholar]
- 149.Nguyen DM, Mulder DS, Shennib H: Altered cellular immune function in the atelectatic lung. Ann Thorac Surg 1991; 51:76–80 [DOI] [PubMed] [Google Scholar]
- 150.De Conno E, Steurer MP, Wittlinger M, Zalunardo MP, Weder W, Schneiter D, Schimmer RC, Klaghofer R, Neff TA, Schmid ER, Spahn DR, Z’graggen BR, Urner M, Beck-Schimmer B: Anesthetic-induced improvement of the inflammatory response to one-lung ventilation. Anesthesiology 2009; 110:1316–26 [DOI] [PubMed] [Google Scholar]
- 151.Motta-Ribeiro GC, Hashimoto S, Winkler T, Baron RM, Grogg K, Paula LFSC, Santos A, Zeng C, Hibbert K, Harris RS, Bajwa E, Vidal Melo MF: Deterioration of Regional Lung Strain and Inflammation during Early Lung Injury. Am J Respir Crit Care Med 2018; 198:891–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kisala JM, Ayala A, Stephan RN, Chaudry IH: A model of pulmonary atelectasis in rats: activation of alveolar macrophage and cytokine release. Am J Physiol 1993; 264:R610–614 [DOI] [PubMed] [Google Scholar]
- 153.Shennib H: The Effects of Pulmonary Atelectasis and Reexpansion on Lung Cellular Immune Defenses. Arch Surg 1984; 119:274. [DOI] [PubMed] [Google Scholar]
- 154.Zeng C, Motta-Ribeiro GC, Hinoshita T, Lessa MA, Winkler T, Grogg K, Kingston NM, Hutchinson JN, Sholl LM, Fang X, Varelas X, Layne MD, Baron RM, Vidal Melo MF: Lung Atelectasis Promotes Immune and Barrier Dysfunction as Revealed by Transcriptome Sequencing in Female Sheep. Anesthesiology 2020; 133:1060–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gao W, Liu D-D, Li D, Cui G: Effect of Therapeutic Hypercapnia on Inflammatory Responses to One-lung Ventilation in Lobectomy Patients. Anesthesiology 2015; 122:1235–52 [DOI] [PubMed] [Google Scholar]
- 156.Sivrikoz MC, Tunçözgür B, Cekmen M, Bakir K, Meram I, Koçer E, Cengiz B, Elbeyli L: The role of tissue reperfusion in the reexpansion injury of the lungs. Eur J Cardiothorac Surg 2002; 22:721–7 [DOI] [PubMed] [Google Scholar]
- 157.Bobba CM, Fei Q, Shukla V, Lee H, Patel P, Putman RK, Spitzer C, Tsai M, Wewers MD, Lee RJ, Christman JW, Ballinger MN, Ghadiali SN, Englert JA: Nanoparticle delivery of microRNA-146a regulates mechanotransduction in lung macrophages and mitigates injury during mechanical ventilation. Nat Commun 2021; 12:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bartolák-Suki E, Imsirovic J, Parameswaran H, Wellman TJ, Martinez N, Allen PG, Frey U, Suki B: Fluctuation-driven mechanotransduction regulates mitochondrial-network structure and function. Nat Mater 2015; 14:1049–57 [DOI] [PubMed] [Google Scholar]
- 159.Birukova AA, Rios A, Birukov KG: Long-term cyclic stretch controls pulmonary endothelial permeability at translational and post-translational levels. Experimental Cell Research 2008; 314:3466–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Birukov KG, Jacobson JR, Flores AA, Ye SQ, Birukova AA, Verin AD, Garcia JGN: Magnitude-dependent regulation of pulmonary endothelial cell barrier function by cyclic stretch. Am J Physiol Lung Cell Mol Physiol 2003; 285:L785–97 [DOI] [PubMed] [Google Scholar]
- 161.Birukova AA, Moldobaeva N, Xing J, Birukov KG: Magnitude-dependent effects of cyclic stretch on HGF- and VEGF-induced pulmonary endothelial remodeling and barrier regulation. Am J Physiol Lung Cell Mol Physiol 2008; 295:L612–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lee JW, Fang X, Dolganov G, Fremont RD, Bastarache JA, Ware LB, Matthay MA: Acute lung injury edema fluid decreases net fluid transport across human alveolar epithelial type II cells. J Biol Chem 2007; 282:24109–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Mulivor AW, Lipowsky HH: Inflammation- and ischemia-induced shedding of venular glycocalyx. Am J Physiol Heart Circ Physiol 2004; 286:H1672–1680 [DOI] [PubMed] [Google Scholar]
- 164.Jackson RM, Veal CF, Alexander CB, Brannen AL, Fulmer JD: Re-expansion pulmonary edema. A potential role for free radicals in its pathogenesis. Am Rev Respir Dis 1988; 137:1165–71 [DOI] [PubMed] [Google Scholar]
- 165.Goldman G, Welbourn R, Rothlein R, Wiles M, Kobzik L, Valeri CR, Shepro D, Hechtman HB: Adherent neutrophils mediate permeability after atelectasis. Ann Surg 1992; 216:372–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sue RD, Matthay MA, Ware LB: Hydrostatic mechanisms may contribute to the pathogenesis of human re-expansion pulmonary edema. Intensive Care Med 2004; 30:1921–6 [DOI] [PubMed] [Google Scholar]
- 167.Varelas X: The Hippo pathway effectors TAZ and YAP in development, homeostasis and disease. Development 2014; 141:1614–26 [DOI] [PubMed] [Google Scholar]
- 168.Kim J, Kim YH, Kim J, Park DY, Bae H, Lee D-H, Kim KH, Hong SP, Jang SP, Kubota Y, Kwon Y-G, Lim D-S, Koh GY: YAP/TAZ regulates sprouting angiogenesis and vascular barrier maturation. J Clin Invest 2017; 127:3441–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.LaCanna R, Liccardo D, Zhang P, Tragesser L, Wang Y, Cao T, Chapman HA, Morrisey EE, Shen H, Koch WJ, Kosmider B, Wolfson MR, Tian Y: Yap/Taz regulate alveolar regeneration and resolution of lung inflammation. J Clin Invest 2019; 129:2107–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Liu Z, Wu H, Jiang K, Wang Y, Zhang W, Chu Q, Li J, Huang H, Cai T, Ji H, Yang C, Tang N: MAPK-Mediated YAP Activation Controls Mechanical-Tension-Induced Pulmonary Alveolar Regeneration. Cell Reports 2016; 16:1810–9 [DOI] [PubMed] [Google Scholar]
- 171.Duggan M, McNamara PJ, Engelberts D, Pace-Asciak C, Babyn P, Post M, Kavanagh BP: Oxygen Attenuates Atelectasis-induced Injury in the In Vivo Rat Lung: Anesthesiology 2005; 103:522–31 [DOI] [PubMed] [Google Scholar]
- 172.Kaam AH van Lachmann RA, Herting E, De Jaegere A, Iwaarden F van, Noorduyn LA, Kok JH, Haitsma JJ, Lachmann B: Reducing Atelectasis Attenuates Bacterial Growth and Translocation in Experimental Pneumonia. Am J Respir Crit Care Med 2004; 169:1046–53 [DOI] [PubMed] [Google Scholar]
- 173.Gando S, Fujishima S, Saitoh D, Shiraishi A, Yamakawa K, Kushimoto S, Ogura H, Abe T, Mayumi T, Sasaki J, Kotani J, Takeyama N, Tsuruta R, Takuma K, Yamashita N, Shiraishi S-I, Ikeda H, Shiino Y, Tarui T, Nakada T-A, Hifumi T, Otomo Y, Okamoto K, Sakamoto Y, Hagiwara A, Masuno T, Ueyama M, Fujimi S, Umemura Y, Japanese Association for Acute Medicine (JAAM) Focused Outcomes Research in Emergency Care in Acute Respiratory Distress Syndrome, Sepsis and Trauma (FORECAST) Study Group: The significance of disseminated intravascular coagulation on multiple organ dysfunction during the early stage of acute respiratory distress syndrome. Thromb Res 2020; 191:15–21 [DOI] [PubMed] [Google Scholar]
- 174.Heart National, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network, Matthay MA, Brower RG, Carson S, Douglas IS, Eisner M, Hite D, Holets S, Kallet RH, Liu KD, MacIntyre N, Moss M, Schoenfeld D, Steingrub J, Thompson BT: Randomized, placebo-controlled clinical trial of an aerosolized β₂-agonist for treatment of acute lung injury. Am J Respir Crit Care Med 2011; 184:561–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Marhong JD, Telesnicki T, Munshi L, Del Sorbo L, Detsky M, Fan E: Mechanical Ventilation during Extracorporeal Membrane Oxygenation. An International Survey. Annals ATS 2014; 11:956–61 [DOI] [PubMed] [Google Scholar]
- 176.Brodie D, Bacchetta M: Extracorporeal membrane oxygenation for ARDS in adults. N Engl J Med 2011; 365:1905–14 [DOI] [PubMed] [Google Scholar]
- 177.Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, Hibbert CL, Truesdale A, Clemens F, Cooper N, Firmin RK, Elbourne D, CESAR trial collaboration: Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009; 374:1351–6319762075 [Google Scholar]
- 178.Birukova AA, Chatchavalvanich S, Rios A, Kawkitinarong K, Garcia JGN, Birukov KG: Differential regulation of pulmonary endothelial monolayer integrity by varying degrees of cyclic stretch. Am J Pathol 2006; 168:1749–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ke Y, Karki P, Zhang C, Li Y, Nguyen T, Birukov KG, Birukova AA: Mechanosensitive Rap1 activation promotes barrier function of lung vascular endothelium under cyclic stretch. Mol Biol Cell 2019; 30:959–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Tschumperlin DJ, Oswari J, Margulies AS: Deformation-induced injury of alveolar epithelial cells. Effect of frequency, duration, and amplitude. Am J Respir Crit Care Med 2000; 162:357–62 [DOI] [PubMed] [Google Scholar]
- 181.Jesudason R, Black L, Majumdar A, Stone P, Suki B: Differential effects of static and cyclic stretching during elastase digestion on the mechanical properties of extracellular matrices. J Appl Physiol (1985) 2007; 103:803–11 [DOI] [PubMed] [Google Scholar]
- 182.Schmidt M, Pham T, Arcadipane A, Agerstrand C, Ohshimo S, Pellegrino V, Vuylsteke A, Guervilly C, McGuinness S, Pierard S, Breeding J, Stewart C, Ching SSW, Camuso JM, Stephens RS, King B, Herr D, Schultz MJ, Neuville M, Zogheib E, Mira J-P, Rozé H, Pierrot M, Tobin A, Hodgson C, Chevret S, Brodie D, Combes A: Mechanical Ventilation Management during Extracorporeal Membrane Oxygenation for Acute Respiratory Distress Syndrome. An International Multicenter Prospective Cohort. Am J Respir Crit Care Med 2019; 200:1002–12 [DOI] [PubMed] [Google Scholar]
- 183.Madjdpour C, Jewell UR, Kneller S, Ziegler U, Schwendener R, Booy C, Kläusli L, Pasch T, Schimmer RC, Beck-Schimmer B: Decreased alveolar oxygen induces lung inflammation. Am J Physiol Lung Cell Mol Physiol 2003; 284:L360–367 [DOI] [PubMed] [Google Scholar]
- 184.Carpenter TC, Stenmark KR: Hypoxia decreases lung neprilysin expression and increases pulmonary vascular leak. Am J Physiol Lung Cell Mol Physiol 2001; 281:L941–948 [DOI] [PubMed] [Google Scholar]
- 185.Heerdt PM, Stowe DF: Single-lung ventilation and oxidative stress: a different perspective on a common practice. Curr Opin Anaesthesiol 2017; 30:42–9 [DOI] [PubMed] [Google Scholar]
- 186.Vohwinkel CU, Hoegl S, Eltzschig HK: Hypoxia signaling during acute lung injury. J Appl Physiol (1985) 2015; 119:1157–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Cramer T, Yamanishi Y, Clausen BE, Förster I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Nizet V, Firestein GS, Gerber HP, Ferrara N, Johnson RS: HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell 2003; 112:645–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Feng S, Bowden N, Fragiadaki M, Souilhol C, Hsiao S, Mahmoud M, Allen S, Pirri D, Ayllon BT, Akhtar S, Thompson AAR, Jo H, Weber C, Ridger V, Schober A, Evans PC: Mechanical Activation of Hypoxia-Inducible Factor 1α Drives Endothelial Dysfunction at Atheroprone Sites. Arterioscler Thromb Vasc Biol 2017; 37:2087–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wu D, Huang R-T, Hamanaka RB, Krause M, Oh M-J, Kuo C-H, Nigdelioglu R, Meliton AY, Witt L, Dai G, Civelek M, Prabhakar NR, Fang Y, Mutlu GM: HIF-1α is required for disturbed flow-induced metabolic reprogramming in human and porcine vascular endothelium. Elife 2017; 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Olson N, Hristova M, Heintz NH, Lounsbury KM, Vliet A van der: Activation of hypoxia-inducible factor-1 protects airway epithelium against oxidant-induced barrier dysfunction. Am J Physiol Lung Cell Mol Physiol 2011; 301:L993–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Brown ES, Johnson RP, Clements JA: Pulmonary surface tension. J Appl Physiol 1959; 14:717–20 [DOI] [PubMed] [Google Scholar]
- 192.Levine BE, Johnson RP: Effects of atelectasis on pulmonary surfactant and quasi-static lung mechanics. J Appl Physiol 1965; 20:859–64 [DOI] [PubMed] [Google Scholar]
- 193.Faridy EE, Permutt S, Riley RL: Effect of ventilation on surface forces in excised dogs’ lungs. J Appl Physiol 1966; 21:1453–62 [DOI] [PubMed] [Google Scholar]
- 194.Taeusch HW: Treatment of acute (Adult) respiratory distress syndrome. The holy grail of surfactant therapy. Biol Neonate 2000; 77 Suppl 1:2–8 [DOI] [PubMed] [Google Scholar]
- 195.Fernandez-Bustamante A, Frendl G, Sprung J, Kor DJ, Subramaniam B, Martinez Ruiz R, Lee J-W, Henderson WG, Moss A, Mehdiratta N, Colwell MM, Bartels K, Kolodzie K, Giquel J, Vidal Melo MF: Postoperative Pulmonary Complications, Early Mortality, and Hospital Stay Following Noncardiothoracic Surgery: A Multicenter Study by the Perioperative Research Network Investigators. JAMA Surg 2017; 152:157–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Allou N, Allyn J, Snauwaert A, Welsch C, Lucet JC, Kortbaoui R, Desmard M, Augustin P, Montravers P: Postoperative pneumonia following cardiac surgery in non-ventilated patients versus mechanically ventilated patients: is there any difference? Crit Care 2015; 19:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Schussler O, Alifano M, Dermine H, Strano S, Casetta A, Sepulveda S, Chafik A, Coignard S, Rabbat A, Regnard J-F: Postoperative pneumonia after major lung resection. Am J Respir Crit Care Med 2006; 173:1161–9 [DOI] [PubMed] [Google Scholar]
- 198.Wu H, Kuzmenko A, Wan S, Schaffer L, Weiss A, Fisher JH, Kim KS, McCormack FX: Surfactant proteins A and D inhibit the growth of Gram-negative bacteria by increasing membrane permeability. J Clin Invest 2003; 111:1589–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.LeVine AM, Kurak KE, Bruno MD, Stark JM, Whitsett JA, Korfhagen TR: Surfactant protein-A-deficient mice are susceptible to Pseudomonas aeruginosa infection. Am J Respir Cell Mol Biol 1998; 19:700–8 [DOI] [PubMed] [Google Scholar]
- 200.Shields RT: Pathogenesis of postoperative pulmonary atelectasis; an experimental study. Arch Surg 1949; 58:489–503 [DOI] [PubMed] [Google Scholar]
- 201.Drinkwater DC, Wittnich C, Mulder DS, Richards GK, Chiu RC: Mechanical and cellular bacterial clearance in lung atelectasis. Ann Thorac Surg 1981; 32:235–43 [DOI] [PubMed] [Google Scholar]
- 202.Charles PE, Martin L, Etienne M, Croisier D, Piroth L, Lequeu C, Pugin J, Portier H, Chavanet P: Influence of positive end-expiratory pressure (PEEP) on histopathological and bacteriological aspects of pneumonia during low tidal volume mechanical ventilation. Intensive Care Med 2004; 30:2263–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.PROVE Network Investigators for the Clinical Trial Network of the European Society of Anaesthesiology, Hemmes SNT, Gama de Abreu M, Pelosi P, Schultz MJ: High versus low positive end-expiratory pressure during general anaesthesia for open abdominal surgery (PROVHILO trial): a multicentre randomised controlled trial. Lancet 2014; 384:495–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Writing Committee for the PROBESE Collaborative Group of the PROtective VEntilation Network (PROVEnet) for the Clinical Trial Network of the European Society of Anaesthesiology, Bluth T, Serpa Neto A, Schultz MJ, Pelosi P, Gama de Abreu M: Effect of Intraoperative High Positive End-Expiratory Pressure (PEEP) With Recruitment Maneuvers vs Low PEEP on Postoperative Pulmonary Complications in Obese Patients: A Randomized Clinical Trial. JAMA 2019; 321:2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ferrando C, Soro M, Unzueta C, Suarez-Sipmann F, Canet J, Librero J, Pozo N, Peiró S, Llombart A, León I, India I, Aldecoa C, Díaz-Cambronero O, Pestaña D, Redondo FJ, Garutti I, Balust J, García JI, Ibáñez M, Granell M, Rodríguez A, Gallego L, de la Matta M, Gonzalez R, Brunelli A, García J, Rovira L, Barrios F, Torres V, Hernández S, Padrón O, Colás A, Puig J, Azparren G, Tusman G, Villar J, Belda J; Individualized PeRioperative Open-lung VEntilation (iPROVE) Network: Individualised perioperative open-lung approach versus standard protective ventilation in abdominal surgery (iPROVE): a randomised controlled trial. Lancet Respir Med 2018; 6:193–203 [DOI] [PubMed] [Google Scholar]
- 206.Muscedere JG, Mullen JB, Gan K, Slutsky AS: Tidal ventilation at low airway pressures can augment lung injury. Am J Respir Crit Care Med 1994; 149:1327–34 [DOI] [PubMed] [Google Scholar]
- 207.Naureckas ET, Dawson CA, Gerber BS, Gaver DP, Gerber HL, Linehan JH, Solway J, Samsel RW: Airway reopening pressure in isolated rat lungs. J Appl Physiol (1985) 1994; 76:1372–7 [DOI] [PubMed] [Google Scholar]
- 208.Broche L, Pisa P, Porra L, Degrugilliers L, Bravin A, Pellegrini M, Borges JB, Perchiazzi G, Larsson A, Hedenstierna G, Bayat S: Individual Airway Closure Characterized In Vivo by Phase-Contrast CT Imaging in Injured Rabbit Lung. Crit Care Med 2019; 47:e774–81 [DOI] [PubMed] [Google Scholar]
- 209.Derosa S, Borges JB, Segelsjö M, Tannoia A, Pellegrini M, Larsson A, Perchiazzi G, Hedenstierna G: Reabsorption atelectasis in a porcine model of ARDS: regional and temporal effects of airway closure, oxygen, and distending pressure. J Appl Physiol (1985) 2013; 115:1464–73 [DOI] [PubMed] [Google Scholar]
- 210.D’Angelo E, Pecchiari M, Gentile G: Dependence of lung injury on surface tension during low-volume ventilation in normal open-chest rabbits. J Appl Physiol (1985) 2007; 102:174–82 [DOI] [PubMed] [Google Scholar]
- 211.D’Angelo E, Koutsoukou A, Della Valle P, Gentile G, Pecchiari M: Cytokine release, small airway injury, and parenchymal damage during mechanical ventilation in normal open-chest rats. J Appl Physiol (1985) 2008; 104:41–9 [DOI] [PubMed] [Google Scholar]
- 212.Steinberg JM, Schiller HJ, Halter JM, Gatto LA, Lee H-M, Pavone LA, Nieman GF: Alveolar instability causes early ventilator-induced lung injury independent of neutrophils. Am J Respir Crit Care Med 2004; 169:57–63 [DOI] [PubMed] [Google Scholar]
- 213.Musch G, Venegas JG, Bellani G, Winkler T, Schroeder T, Petersen B, Harris RS, Melo MFV: Regional gas exchange and cellular metabolic activity in ventilator-induced lung injury. Anesthesiology 2007; 106:723–35 [DOI] [PubMed] [Google Scholar]
- 214.Wellman TJ, Winkler T, Costa EL, Musch G, Harris RS, Venegas JG, Vidal Melo MF: Effect of regional lung inflation on ventilation heterogeneity at different length scales during mechanical ventilation of normal sheep lungs. J Appl Physiol (1985) 2012; 113:947–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Bilek AM, Dee KC, Gaver DP: Mechanisms of surface-tension-induced epithelial cell damage in a model of pulmonary airway reopening. J Appl Physiol (1985) 2003; 94:770–83 [DOI] [PubMed] [Google Scholar]
- 216.Huh D, Fujioka H, Tung Y-C, Futai N, Paine R, Grotberg JB, Takayama S: Acoustically detectable cellular-level lung injury induced by fluid mechanical stresses in microfluidic airway systems. Proc Natl Acad Sci U S A 2007; 104:18886–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Hussein O, Walters B, Stroetz R, Valencia P, McCall D, Hubmayr RD: Biophysical determinants of alveolar epithelial plasma membrane wounding associated with mechanical ventilation. Am J Physiol Lung Cell Mol Physiol 2013; 305:L478–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Yalcin HC, Perry SF, Ghadiali SN: Influence of airway diameter and cell confluence on epithelial cell injury in an in vitro model of airway reopening. J Appl Physiol (1985) 2007; 103:1796–807 [DOI] [PubMed] [Google Scholar]
- 219.Chen Z-L, Song Y-L, Hu Z-Y, Zhang S, Chen Y-Z: An estimation of mechanical stress on alveolar walls during repetitive alveolar reopening and closure. J Appl Physiol (1985) 2015; 119:190–201 [DOI] [PubMed] [Google Scholar]
- 220.Jacob A-M, Gaver DP: Atelectrauma disrupts pulmonary epithelial barrier integrity and alters the distribution of tight junction proteins ZO-1 and claudin 4. J Appl Physiol (1985) 2012; 113:1377–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Yalcin HC, Hallow KM, Wang J, Wei MT, Ou-Yang HD, Ghadiali SN: Influence of cytoskeletal structure and mechanics on epithelial cell injury during cyclic airway reopening. Am J Physiol Lung Cell Mol Physiol 2009; 297:L881–891 [DOI] [PubMed] [Google Scholar]
- 222.Higuita-Castro N, Mihai C, Hansford DJ, Ghadiali SN: Influence of airway wall compliance on epithelial cell injury and adhesion during interfacial flows. J Appl Physiol (1985) 2014; 117:1231–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Song JW, Paek J, Park K-T, Seo J, Huh D: A bioinspired microfluidic model of liquid plug-induced mechanical airway injury. Biomicrofluidics 2018; 12:042211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Kay SS, Bilek AM, Dee KC, Gaver DP: Pressure gradient, not exposure duration, determines the extent of epithelial cell damage in a model of pulmonary airway reopening. J Appl Physiol (1985) 2004; 97:269–76 [DOI] [PubMed] [Google Scholar]
- 225.Muradoglu M, Romanò F, Fujioka H, Grotberg JB: Effects of surfactant on propagation and rupture of a liquid plug in a tube. J Fluid Mech 2019; 872:407–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Engle WA, American Academy of Pediatrics Committee on Fetus and Newborn: Surfactant-replacement therapy for respiratory distress in the preterm and term neonate. Pediatrics 2008; 121:419–32 [DOI] [PubMed] [Google Scholar]
- 227.Filoche M, Tai C-F, Grotberg JB: Three-dimensional model of surfactant replacement therapy. Proc Natl Acad Sci U S A 2015; 112:9287–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Denny E, Schroter RC: A model of non-uniform lung parenchyma distortion. J Biomech 2006; 39:652–63 [DOI] [PubMed] [Google Scholar]
- 229.Makiyama AM, Gibson LJ, Harris RS, Venegas JG: Stress concentration around an atelectatic region: a finite element model. Respir Physiol Neurobiol 2014; 201:101–10 [DOI] [PubMed] [Google Scholar]
- 230.Wu Y, Kharge AB, Perlman CE: Lung ventilation injures areas with discrete alveolar flooding, in a surface tension-dependent fashion. J Appl Physiol (1985) 2014; 117:788–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Retamal J, Bergamini BC, Carvalho AR, Bozza FA, Borzone G, Borges JB, Larsson A, Hedenstierna G, Bugedo G, Bruhn A: Non-lobar atelectasis generates inflammation and structural alveolar injury in the surrounding healthy tissue during mechanical ventilation. Crit Care 2014; 18:505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Albert K, Krischer J-M, Pfaffenroth A, Wilde S, Lopez-Rodriguez E, Braun A, Smith BJ, Knudsen L: Hidden Microatelectases Increase Vulnerability to Ventilation-Induced Lung Injury. Front Physiol 2020; 11:530485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Cressoni M, Chiumello D, Chiurazzi C, Brioni M, Algieri I, Gotti M, Nikolla K, Massari D, Cammaroto A, Colombo A, Cadringher P, Carlesso E, Benti R, Casati R, Zito F, Gattinoni L: Lung inhomogeneities, inflation and [18F]2-fluoro-2-deoxy-D-glucose uptake rate in acute respiratory distress syndrome. Eur Respir J 2016; 47:233–42 [DOI] [PubMed] [Google Scholar]
- 234.Cereda M, Emami K, Xin Y, Kadlecek S, Kuzma NN, Mongkolwisetwara P, Profka H, Pickup S, Ishii M, Kavanagh BP, Deutschman CS, Rizi RR: Imaging the interaction of atelectasis and overdistension in surfactant-depleted lungs. Crit Care Med 2013; 41:527–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Beda A, Carvalho AR, Carvalho NC, Hammermüller S, Amato MBP, Muders T, Gittel C, Noreikat K, Wrigge H, Reske AW: Mapping Regional Differences of Local Pressure-Volume Curves With Electrical Impedance Tomography. Crit Care Med 2017; 45:679–86 [DOI] [PubMed] [Google Scholar]
- 236.Paula LF, Wellman TJ, Winkler T, Spieth PM, Güldner A, Venegas JG, Gama de Abreu M, Carvalho AR, Vidal Melo MF: Regional tidal lung strain in mechanically ventilated normal lungs. J Appl Physiol (1985) 2016; 121:1335–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Güldner A, Braune A, Ball L, Silva PL, Samary C, Insorsi A, Huhle R, Rentzsch I, Becker C, Oehme L, Andreeff M, Vidal Melo MF, Winkler T, Pelosi P, Rocco PRM, Kotzerke J, Gama de Abreu M: Comparative Effects of Volutrauma and Atelectrauma on Lung Inflammation in Experimental Acute Respiratory Distress Syndrome. Crit Care Med 2016; 44:e854–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Tsuchida S, Engelberts D, Peltekova V, Hopkins N, Frndova H, Babyn P, McKerlie C, Post M, McLoughlin P, Kavanagh BP: Atelectasis Causes Alveolar Injury in Nonatelectatic Lung Regions. Am J Respir Crit Care Med 2006; 174:279–89 [DOI] [PubMed] [Google Scholar]
- 239.Bellani G, Guerra L, Musch G, Zanella A, Patroniti N, Mauri T, Messa C, Pesenti A: Lung Regional Metabolic Activity and Gas Volume Changes Induced by Tidal Ventilation in Patients with Acute Lung Injury. Am J Respir Crit Care Med 2011; 183:1193–9 [DOI] [PMC free article] [PubMed] [Google Scholar]