Abstract
Objectives
Among advanced multiple myeloma (MM) patients, B-cell maturation antigen (BCMA) specific targets like Belantamab Mafodotin (belamaf) and CAR T-cell therapies have been shown to improve clinical outcomes, but at significant costs. To compare the expected costs per quality-adjusted life years (QALYs) gained among a hypothetical cohort of triple refractory MM patients treated with one of three BCMA-directed therapies: (1) idecabtagene vicleucel (ide-cel), (2) ciltacabtagene autoleucel (cilta-cel), and (3) belamaf for up to 20 months.
Methods
In this cost-effectiveness analysis, we built a Monte Carlo Markov Chain microsimulation model using estimates and parameters from the evidence on MM treatment for 10 000 hypothetical patients between the ages for 40 and 80. We assigned expected years of life remaining and made varying assumptions about survival beyond 5 years
Results
We predicted total cost of treatment for CAR-T therapy to be six times greater than for belamaf, but the QALYs gained from treatment are 6 to 8 times greater. Ide-cel was weakly dominated by cilta-cel and our base-case incremental cost effectiveness ratio (ICER) comparing cilta-cel with belamaf was $109,497 per QALY gained, averaging $123,618 in probabilistic sensitivity analyses.
Conclusions
These findings hinge on the assumption of longer-term survival but suggest that the use of CAR-T therapy is approaching standard ICER thresholds.
Keywords: multiple myeloma, cost effectiveness analysis, Immunotherapy, CAR TCell
Introduction
Multiple Myeloma (MM) is a disorder of clonal plasma cells in the bone-marrow leading to end-organ damage and is responsible for 2% of all cancer related deaths in U.S.1 MM is the second most common blood cancer with 34,920 estimated new cases in 2021 and a 5-year relative survival rate of 55.6%.1
Despite improvement in treatment strategies, there has been no plateau in the survival curves in MM, especially for patients who are “triple class” refractory. Triple-class means the cancer has been resistant to three classes of standard treatment for MM – proteasome inhibitors, immunomodulatory agents, and anti-CD38 monoclonal antibodies.2 B-cell maturation antigen (BCMA)-directed therapies have emerged as a potential game changer for patients with triple refractory MM.3 Until recently, there were two FDA approved BCMA-directed therapies being used – belantamab mafodotin-blmf (“belamaf,” Blenrep™, GlaxoSmithKline), idecabtagene vicleucel (“ide-cel,” Abecma®, Bristol-Myers Squibb and bluebird bio). Ciltacabtagene autoleucel (“cilta-cel,” Janssen and Legend Biotech) is another BCMA directed-therapy that was approved by the FDA in February 2022.
Belamaf is a BCMA-directed antibody and microtubule inhibitor conjugate approved by the FDA in August 2020 for triple-class refractory MM patients.4,5 However, response rates are still relatively low with more than 63% of patients not responding.6 Ide-cel is a chimeric antigen receptor T-cell (CAR T-cell) therapy that has shown great promise in trials and was approved by the FDA in March 2021. In the KarMMa trial, 128 patients received ide-cel with a 73% overall response rate and a 33% complete response rate or better with a median progression-free survival of 19 months (95% CI: 11.3, 22).6 In indirect matching-adjusted comparisons, patients receiving ide-cel had significant improvements in progression free survival and overall survival relative to belamaf.7 Cilta-cel, the most recently approved CAR T-cell therapy for MM, has also had significant response rates, with an overall response rate of 97% of patients in the CARTITUDE-1 trial (n = 97).8
Ide-cel and cilta-cel are considerably more expensive relative to belamaf and other standard treatment regimens for MM patients (eg Selinexor (Xpovio®)). For example, the monthly treatment costs, including inpatient, pharmaceutical and non-drug, of ide-cel and cilta-cel are $442,705 and $465,000, not including the costs of adverse side effects or pre-infusion chemotherapy needed prior to CAR T-cell therapies.9 Belamaf, on the other hand, is only about $23,900 per month of treatment not including the adverse side effects.
The Institute for Clinical and Economic Review conducted a preliminary cost-effectiveness analysis comparing ide-cel and cilta-cel with a CAR-T comparator market basket as well as comparing belamaf with its own comparator market basket.3 The preliminary findings suggest an incremental cost effectiveness ratio (ICER) for belamaf that is within the range of acceptable cost per quality-adjusted life year (QALY) gained, but this analysis does not compare the three treatments with each other directly.10,11
In this study, we extend this analysis by extrapolating data from the single-arm trials for the 3 options (belamaf, ide-cel, and cilta-cel) to simulate how patients might respond when all three options are available. This is distinct from using market basket comparisons. We built a Monte Carlo Markov Chain (MCMC) microsimulation model to compare the expected costs per QALYs gained among a hypothetical cohort of triple class refractory MM patients who are treated with one of three treatments: (1) ide-cel, (2) cilta-cel, and (3) belamaf. We focused on the two CAR-T cell therapies vs belamaf as a recent study found that belamaf was lower cost with higher QALY gains relative to Selinexor.12 In addition, our model allows for partitioning hypothetical patients based on response and we conducted several sensitivity checks.
Methods
Model Structure
We modeled treatment outcomes over a 20-month period assuming monthly treatment cycles. Patients were randomly assigned an age between 40 and 80 where the median age was set to 61 to match the age distribution of patients in the trials. For each treatment, patients were initially probabilistically assigned to one of three response categories: (1) best response (BR) includes complete response, very good partial response, or partial response; (2) minimal response (MR) which includes patients not meeting the criteria for BR and whose disease is not progressive; or (3) no response (NR), based on data from the three trials (DREAMM-2 for belamaf,4 KarMMA for ide-cel,6 and CARTITUDE for cilta-cel8). The categorizations are derived from the International Myeloma Working Group consensus recommendations13 used in the trials and are shown in the second column of Table 1 (% in Category). Among patients in the BR or MR categories, their duration of response was drawn from the distribution of response durations reported in the clinical trials (parameters in Table 1, see Supplement for more details). Once patients stop responding in the model they move to the progressive disease state, where they undergo palliative chemotherapy, the cost for which is based on a market comparison built as a weighted average of the most common treatments.14 The treatment costs for CAR-T cell therapies are incurred once, whereas the belamaf cost is per 3-week cycle. The treatment costs for both ide-cel and cilta-cel include the pre-infusion lymphodepleting chemotherapy costs15 for all patients receiving treatment, but the adverse event (AE) treatment costs are probabilistically assigned based on whether the simulated patients are predicted to have Grade 3 or 4 AEs. Virtually all patients in the KarMMa6 and most in the CARTITUDE trials8 had grade 3 or 4 AEs. We accounted for the higher costs of treating Grade 3 or 4 cytokine release syndrome (CRS) and neurotoxicities, which occurred among 8 and 13% of KarMMa and CARTITUDE study participants, respectively. Median costs of managing CRS and neurotoxicities among these patients have been reported to be between $60,588 and $121,535, depending on the severity.16 We used a weighted average of these costs for patients simulated to experience Grade 3 or 4 CRS at $80,904. Average costs of managing all other Grade 3 or 4 adverse events among CAR-T therapy patients were derived from prevalence rates reported in the trials and expected treatment costs.14 Among patients receiving belamaf, a similar fraction had adverse events; thus, following the trial data, 9% were simulated to stop receiving treatment, about 52% were simulated to delay up to one cycle of treatment, and the 37% would continue treatment after the first cycle.4 We assume AEs affect the first cycle of treatment, except for belamaf patients who could pause one cycle and then still experience them in the next cycle.
Table 1.
Model parameters and assumptions.
| Response category | % In category | Duration of responsiveness to treatment (In months) | State utility | Cycle cost | Grade 3-4 AE prevalence and costs | |
|---|---|---|---|---|---|---|
| PFS (PSA Range) | OS (PSA range | |||||
| Ide-cel6 | ||||||
| BR | 52.34% | 21.5 | 12.5, NE | .8017,18 | $442,7059 + $12,981 for pre-treatment chemotherapy15 (one time) | • 99%; 8% CRS/NE |
| MR | 21.10% | 10.4 | 5.1, 12.2 | .7517,18 | • Mean AE treatment cost for non-CRS/NE = $3928 | |
| NR | 26.56% | n/a moves to PD | • Median treatment cost of CRS/NE = $80,90416,19 | |||
| Cilta-cel8 | ||||||
| BR | 93% | 22.8 | 22.8, NE | .8017,18 | $465,000+ + $12,981 for pre-treatment chemotherapy15 (one time) | • 94%; 13% CRS/NE |
| MR | 4.12% | 10.4 | 5.1, 12.2 | .7517,18 | • Mean AE treatment cost for non-CRS = $1899 | |
| NR | 3.09% | n/a moves to PD | • Median treatment cost of CRS/NE = $80,90416,19 | |||
| Belantamab-mafodotin4 | ||||||
| BR | 18.55% | 13.1 | 4.9, 16 | .8017,18 | $24,435 per 3-week cycle | • 98% |
| MR | 17.53% | 11.7 | 4.2, 16 | .7517,18 | • 58% (9%) delayed (stopped) treatment due to AE | |
| NR | 63.92% | n/a moves to PD | • $1,84114/month | |||
| Patients in all arms, not responding can transition to | ||||||
| PD | 1.85,20 | 1.2, 1.9 | .4517 | $17,94014 | ||
Notes: This table includes all input parameters and assumptions used in the microsimulation model. BR = complete response, very good partial response, or partial response. MR = minimal response. NR = no response. PD = progressive disease state. AE = adverse events. All parameters are from the respective trials, unless otherwise noted: DREAMM-2 for Belantamab mafodotin,4 KarMMA for Ide-cel,6 CARTITUDE for Cilta-cel.8 The duration for patients with MR in the CARTITUDE study were not estimated so we used the values from the KarMMa trial. The cost for patients in the progressive disease (PD) category are based on a weighted average of monthly pharmacy, medical and severe adverse effect costs14 of these 7 monthly treatment combinations frequently used: panobinostat + bortezomib + dexamethasone (16%); bortezomib + dexamethasone (16%); lenalidomide + dexamethasone (16%); lenalidomide + bortezomib + dexamethasone (16%); carfilzomib + lenalidomide + dexamethasone (16%); carfilzomib monotherapy (10%); and pomalidomide + dexamethasone (10%). All cost values have been converted to constant 2020 U.S. dollars. See supplement for more details on costs.
Microsimulation Modeling
In each monthly cycle, we simulated outcomes for 10,000 patients using a MCMC microsimulation where patients could stay on treatment (if disease not progressing), transition to progressive disease, or die. Transitional probabilities to continued receipt of treatment, change to another alternative treatment, remission, or death are based on trajectories for each response category as published in respective clinical trials. Note that these treatments have not been compared head-to-head in a clinical trial setting.
Among those responsive to treatment, they are modeled to continue treatment each month where duration of treatment in each state is drawn from the published estimates of progression free survival rates (shown in Table 1). Those in the progressive disease state were assumed to receive salvage chemotherapy or palliative care until they die.
After 20 monthly cycles, we modeled a longer-term survival projection among the patients still living using age-adjusted life expectancies discounted to generate QALYs remaining: where lifeexp is the population-based remaining years of life expected conditional on being age a; a is the age of the simulated patient at the end of the 20 month cycles; and u represents the utility state of the patient given where they are at the end of the 20-month cycles (eg response, palliative care). is a factor to adjust for the fact that most advanced MM patients do not have a full life expectancy and we allow this range from 0 to 1 with an average value of .5.
We discounted life expectancies using utilities from the literature as shown in Table 1 and a 3% annual discount rate. We used a U.S. healthcare payer perspective and all costs have been converted to 2020 dollars.
Our main outcomes were quality-adjusted life years (QALYs) gained and the incremental cost-effectiveness ratio calculated as the change in costs from one treatment to another divided by the change in QALYs.
No human subjects were enrolled specifically in this study, and thus the study was exempt from regulations guiding protection of human subjects. This is a microsimulation model using only published estimates from previous trials to generate a hypothetical cohort of patients. We followed the CHEERS guidelines.21
Sensitivity Analyses
We conducted several one-way sensitivity analyses to examine how our results change as we changed modeling assumptions. As noted, we randomly drew the long-term survival rate for each simulated patient from 0 to 100% ( above) with an overall population mean of 50%. In addition, we conducted probabilistic sensitivity analyses (PSA) using second-order Monte Carlo simulations (1000 iterations) to model parameter uncertainty. We allowed duration of treatment values to fall within the ranges shown in Table 1 assuming a beta distribution. We allowed the costs to be drawn from a gamma distribution. All transition and adverse event probabilities were allowed to vary from 0 to 1 in the PSA. All analyses were completed in Amua.22
Results
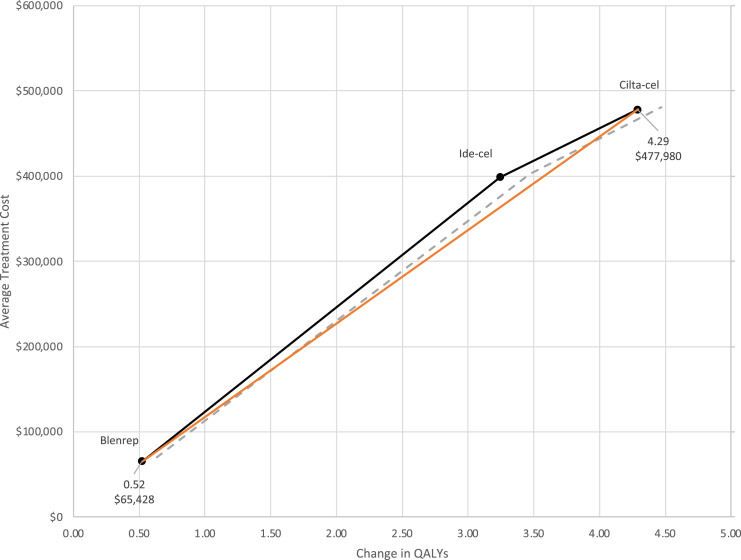
In our base case analysis, we found that CAR-T therapies were significantly more expensive than belamaf, but they also generated significantly more lifetime QALY gains. On average, the cost of belamaf was $65,428, approximately one-seventh the cost of cilta-cel. The additional QALYs gained, however were much lower at .52, on average, for patients simulated to receive belamaf. In Figure 1, we present the average treatment costs for each strategy (y-axis) and the average discounted QALYs gained (x-axis) with 95% confidence intervals shown as dashed lines. Ide-cel is weakly dominated as the marginal cost per QALY gained is lower for cilta-cel (as shown by the orange line). In other words, for the same gain in QALYs, the average treatment cost is lower for cilta-cel. The ICER is calculated as the increased cost for cilta-cel relative to belamaf, divided by the increased QALYs gained: ($477,980 - $65,428)/(4.29 – .52) = $109,497. So, at a Willingness-To-Pay (WTP) threshold greater than $109,497, cilta-cel would be the dominant strategy.
Figure 1.
Average treatment costs and QALYs gained across treatment strategies.
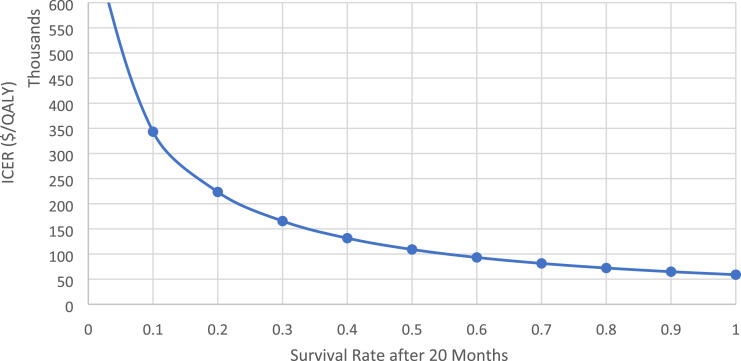
In one-way sensitivity analyses where treatment costs and other assumptions are held constant, the ICER declines as the survival rate increases () (see Figure 2).
Figure 2.
One-way sensitivity of five-year survival rate assumption. Note: This applies to simulated patients who survived until the end of the 20-months of treatment.
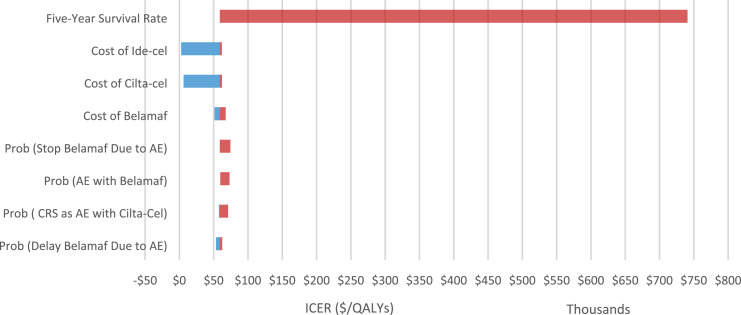
This longer-term survival rate assumption was the most significant in increasing the uncertainty of our estimates. In Figure 3, we show a tornado diagram for the modeling parameters that yielded the greatest changes in our estimates. We show only model parameters that yielded a change of at least $5,000 per QY in our ICER estimate. The blue bars represent estimated ICERs that were lower than our base case estimate ($109,497) and the red bars represent estimated ICERs above the base case. Although varying the assumptions for longer-term survival created the most significant variation in our ICER, several other factors mattered. The costs of CAR-T therapy, which we allowed to range from $100,000 to $500,000 also generated uncertainty in our ICER estimate, but all ICERs were less than $100,000 per QALY gained. The probability of having AEs among belamaf patients and whether their treatment was delayed or stopped due to those AEs yielded ICERs above $100,000 per QALY. An increase in the probability of CRS among patients treated with CAR T-cell therapies also increased the ICER.
Figure 3.
One-way sensitivity analyses – tornado diagram.
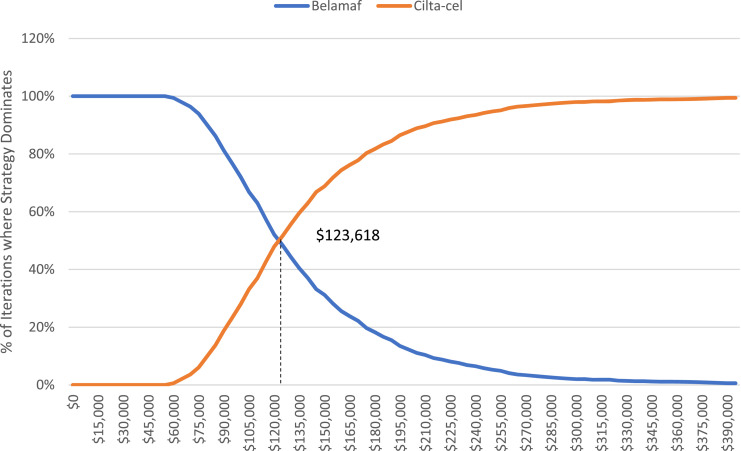
Finally, we conducted PSAs to show how parameter uncertainty affects these estimates and because we know the longer-term survival assumption is critical to our estimates, we highlight these results as our preferred estimates in 1000 Monte Carlo second order iterations, we estimated average total treatment costs as $93,694, $398,529, and $477,900 for belamaf, ide-cel, and cilta-cel, respectively (see Table 2). The average QALYs gained ranged from 1.2 to 4.31 across the three arms. Consistent with our base case analysis, ide-cel was weakly dominated by cilta-cel (the average cost per QALY was higher for ide-cel). Thus, we calculated the ICER between cilta-cel and belamaf, which was an average of $123,618. We also show the distribution of the iterations where each treatment strategy (belamaf vs cilta-cel) dominates in Figure 4. These cost acceptability curves suggest that we should be indifferent between the two strategies at a WTP threshold of $123,618.
Table 2.
Probabilistic sensitivity analysis cost per qaly gained results.
| Total Costs | Total QALYs Gained | Cost/QALY | Delta QALY | Delta Costs | ICER | |
|---|---|---|---|---|---|---|
| Belamaf | ||||||
| Mean | $93,694 | 1.20 | $77,884 | |||
| PSA range | ($4,900, $176,000) | (0.3) | ($30067, $176000) | |||
| Ide-cel | ||||||
| Mean | $398,529 | 2.87 | $138,812 | 1.67 | $304,835 | Weakly dominated |
| PSA range | ($392,000, $450,000) | (1,6) | ($65500, $449000) | |||
| Cilta-cel | ||||||
| Mean | $477,900 | 4.31 | $110,856 | 3.11 | $384,206 | $123,618 |
| PSA range | ($467,000, $543,000) | (1.8) | ($63000, $538000) | ($63148, $775853) | ||
Figure 4.
Cost acceptability curves from probabilistic sensitivity analysis.
Discussion
Although recent trials suggest promising evidence that CAR-T therapy may be an effective treatment for advanced MM patients, there have been no head-to-head comparisons of these different therapies against each other or other less-costly alternatives.
In this study, we developed a Markov model to estimate the cost-effectiveness of two CAR-T therapies – ide-cel and cilta-cel, compared to belamaf. Thus far, cilta-cel has been shown to have a higher response rate than ide-cel resulting in it “weakly dominating” ide-cel. In other words, at the same price, cilta-cel seems to deliver a greater chance of response relative to ide-cel.
Thus, our main ICER is in comparing cilta-cel to belamaf, which is significantly cheaper per treatment. However, our estimates suggest an ICER of $123,618 (as per our probabilistic sensitivity results). Although this is higher than the standard $100,000 threshold,10,23 it is lower than the ICER computed by the Institute for Clinical and Economic Research comparing cilta-cel to a market basket. Their study compares each therapy against a “market basket” (and not to each other) and reported ICERs of $98,000, $253,000, and $319,000 respectively for belamaf, cilta-cel, and ide-cel, respectively. Our total costs differ from the ICER analysis because we include adverse event costs differently (see Supplemental Information) and we assigned “progressed treatment costs” only among patients simulated to have progressive disease. Our calculations of AEs adjust separately for those patients simulated to have cytokine release syndrome or neurotoxicities as the average cost of treating that is significantly greater than most other AEs. We also do not include indirect costs (ie time of work, transportation costs, etc.).
Currently, data are not available for long-term survival for this patient population receiving CAR-T therapies. Current estimates suggest that the median survival period among triple refractory MM patients, on average is around 13.9 months (95% CI: 10.5-16.6), but this does not take into account survival rates from these newer treatments.24 As the recent CAR T-cell trials from which we derived inputs for our model suggest median survival times nearly twice as long (not accounting for censoring), longer-term survival rates are improving significantly.
As CART therapy options increase, we might expect the treatment costs to decline which would increase the ICERs and the likelihood that patients/payers would be willing to cover these costs and the likelihood that providers suggest these options earlier.
We note the following limitations to our analysis. Our model is limited by the parameters that were available for use based on the clinical trials to date, all of which are single-armed. Other studies have suggested that these therapies may not perform as well in the “real world,” which would affect our conclusions. Although we did conduct sensitivity analyses, our results are representative of what we would expect given patient populations that are like those observed in the trials. Notably, as our analysis is based on patients with advanced disease (triple-class refractory patients), the longer-term survival may be higher, on average, among patients treated earlier. This has implications for the cost effectiveness analyses, as more life years gained over which to spread the treatment costs yields more favorable ratios for costly treatments. In addition, there are other challenges with CAR-T therapy that we were not able to include in this analysis, including delays from prolonged manufacturing times, manufacturing failures, and other clinical or logistical challenges for which we do not have costs. Finally, we are unable to examine the extent to which there may be disparities in access to and use of these therapies which may generate additional unintended consequences.
Conclusions
With a constantly evolving treatment landscape in MM, and ongoing clinical trials focused on using CAR T-cell therapies in earlier lines of treatment, our results provide a critical understanding of the cost-effectiveness of novel MM therapies. Importantly, although treatment costs are relatively high for these novel therapies, our work suggests that CAR-T cell therapies can be cost effective at most conventional thresholds for patients with relapsed/refractory MM.
Supplemental Material
Supplemental Material for Cost-Effectiveness Analysis of CAR T-Cell Therapies vs Antibody Drug Conjugates for Patients with Advanced Multiple Myeloma by Kandice A. Kapinos, Ellen Hu, Jigar Trivedi, Praveen Ramakrishnan Geethakumari, Ankit Kansagra in Cancer Control
Acknowledgments
We thank Myriam Hunink, Lyndon James, Gurbakhash Kaur, Amar Kelkar, and Natalia Kunst for helpful comments on the development of the model and earlier versions of this manuscript.
Author Contributions: Conceptualization: AK; methodology: KAK, EH; analysis and interpretation of data: KAK, EH, AK; drafting of the manuscript: KAK; critical revision of the manuscript for important intellectual content: all authors; funding acquisition: AK.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: AK has served as a member of scientific advisory boards for Abbvie, Alynylam, BMS, Cota Health, GSK, Janssen, Oncopeptide, Takeda. AK completed this work while a faculty member at UT Southwestern Medical Center; he is now an employee of Janssen. PR has served in Advisory Boards for Kite Pharma, Rafael Pharma and Pharmacyclics LLC. None of the other authors have any relevant conflicts of interest.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Data sharing: The microsimulation model was built using parameters as delineated in Table 1. Code is available upon request.
Supplemental Material: Supplemental material for this article is available online.
ORCID iD
Kandice A. Kapinos https://orcid.org/0000-0003-1577-4917
References
- 1.National Cancer Institute , SEER Cancer Stat Facts: Myeloma. Bethesda, Maryland, National Cancer Institute, 2017 [Google Scholar]
- 2.Bal S, Malek E, Kansagra A, et al. Treatment outcomes of triple class refractory multiple myeloma: a benchmark for new therapies. Leukemia. 2021, 36(3), 877-880. [DOI] [PubMed] [Google Scholar]
- 3.Lee SJ, McQueen RB, Beinfeld M, et al. Anti B-Cell Maturation Antigen CAR T-Cell and Antibody Conjugate Therapy for Heavily Pre-treated Relapsed and Refractory Multiple Myeloma: FInal Evidence Report; Boston, MA: Institute for Clinical and Economic Review, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lonial S, Lee HC, Badros A, et al. Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): A two-arm, randomised, open-label, phase 2 study. Lancet Oncol. 2020;21(2):207-221. [DOI] [PubMed] [Google Scholar]
- 5.Gandhi UH, Cornell RF, Lakshman A, et al. Outcomes of patients with multiple myeloma refractory to CD38-targeted monoclonal antibody therapy. Leukemia. 2019;33(9):2266-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munshi NC, Anderson LD, Shah N, et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med. 2021;384(8):705-716. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez-Otero P, Ayers D, Cope S, et al. Matching adjusted indirect comparisons of efficacy outcomes for idecabtagene vicleucel (ide-cel, bb2121) versus selinexor + dexamethasone and belantamab mafodotin in relapsed and refractory multiple myeloma. Leuk Lymphoma. 2021, 62(10), 1-10. [DOI] [PubMed] [Google Scholar]
- 8.Berdeja JG, Madduri D, Usmani SZ, et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): A phase 1b/2 open-label study. Lancet. 2021;398(10297):314-324. [DOI] [PubMed] [Google Scholar]
- 9.Lyman GH, Nguyen A, Snyder S, Gitlin M, Chung KC. Economic evaluation of chimeric antigen receptor t-cell therapy by site of care among patients with relapsed or refractory large b-cell lymphoma. JAMA Netw Open. 2020;3(4):e202072-e202072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Institute for Clinical Economic Review . Value Assessment Framework. Boston, MA, USA; Institute for Clinical and Economic Review; 2020. [Google Scholar]
- 11.Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316(10):1093-1103. [DOI] [PubMed] [Google Scholar]
- 12.Nikolaou A, Ambavane A, Shah A, et al. Belantamab mafodotin for the treatment of relapsed/refractory multiple myeloma in heavily pretreated patients: A US cost-effectiveness analysis. Expet Rev Hematol. 2021;14(12):1-9. [DOI] [PubMed] [Google Scholar]
- 13.Rajkumar SV, Harousseau J-L, Durie B, et al. Consensus recommendations for the uniform reporting of clinical trials: Report of the international myeloma workshop consensus panel 1. Blood. 2011;117(18):4691-4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roy A, Kish JK, Bloudek L, et al. Estimating the costs of therapy in patients with relapsed and/or refractory multiple myeloma: A model framework. Am Health Drug Benefits. 2015;8(4):204-215. [PMC free article] [PubMed] [Google Scholar]
- 15.Yang H, Hao Y, Chai X, Qi CZ, Wu EQ. Estimation of total costs in patients with relapsed or refractory diffuse large B-cell lymphoma receiving tisagenlecleucel from a US hospital’s perspective. J Med Econ. 2020;23(9):1016-1024. [DOI] [PubMed] [Google Scholar]
- 16.Hari P, Nguyen A, McGarvey N, et al. Healthcare resource utilization and cost of cytokine release syndrome and neurotoxicity in patients with relapsed and refractory multiple myeloma receiving the bcma-directed chimeric antigen receptor t cell therapy idecabtagene vicleucel (ide-cel, bb2121) in the karmma trial. Inn: Paper presented at: 62nd American Society of Hematology Annual Meeting and Exposition202, December 5, 2020,
- 17.Frödin U, Börjeson S, Lyth J, Lotfi K. A prospective evaluation of patients’ health-related quality of life during auto-SCT: A 3-year follow-up. Bone Marrow Transplant. 2011;46 (10):1345-1352. [DOI] [PubMed] [Google Scholar]
- 18.Delforge M, Shah N, Rodriguez-Otero P, et al. Health state utility valuation in patients with triple-class-exposed relapsed and refractory multiple myeloma treated with the bcma-directed car t cell therapy, idecabtagene vicleucel (ide-cel, bb2121): Results from the karmma trial. Blood. 2020;136:14-15. [Google Scholar]
- 19.Hari P, Nguyen A, Pelletier C, McGarvey N, Gitlin M, Parikh K. Healthcare resource utilization and economic burden of cytokine release syndrome (CRS) and neurologic events (NE) in patients (pts) with relapsed/refractory multiple myeloma (RRMM) receiving idecabtagene vicleucel (ide-cel, bb2121) in KarMMa. J Clin Oncol. 2020;38(29_suppl l):61-61. [Google Scholar]
- 20.Kumar SK, Dimopoulos MA, Kastritis E, et al. Natural history of relapsed myeloma, refractory to immunomodulatory drugs and proteasome inhibitors: A multicenter IMWG study. Leukemia. 2017;31(11):2443-2448. [DOI] [PubMed] [Google Scholar]
- 21.Husereau D, Drummond M, Petrou S, et al. Consolidated health economic evaluation reporting standards (cheers) statement. Value Health. 2013;16(2):e1-e5. [DOI] [PubMed] [Google Scholar]
- 22.Ward ZJ. Amua: An open source modeling framework. 2019. [Google Scholar]
- 23.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness—the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371(9):796-797. [DOI] [PubMed] [Google Scholar]
- 24.Bazarbachi AH, Al Hamed R, Malard F, Harousseau J-L, Mohty M. Relapsed refractory multiple myeloma: a comprehensive overview. Leukemia. 2019;33(10):2343-2357. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Cost-Effectiveness Analysis of CAR T-Cell Therapies vs Antibody Drug Conjugates for Patients with Advanced Multiple Myeloma by Kandice A. Kapinos, Ellen Hu, Jigar Trivedi, Praveen Ramakrishnan Geethakumari, Ankit Kansagra in Cancer Control