Abstract
Metabolic-associated fatty liver disease (MAFLD) is a highly prevalent disease with increasing prevalence worldwide. Currently, no universal screening methods have been standardized, even when this disease poses a major health burden. MAFLD as a spectrum of diseases can range from simple steatosis, and steatohepatitis to fibrosis and hepatocellular carcinoma. Its extra-hepatic manifestations are vast and include cardiovascular diseases, extra-hepatic malignancies, cognitive and respiratory alterations. Given its extensive damage targets as well as its high prevalence, timely identification of the high-risk population presenting metabolic dysfunction should undergo universal non-invasive screening methods, which can be carried out through blood tests, easy and effective imaging techniques, such as ultrasound, score calculation and general clinical information brought together from primary patient–physician contact.
Keywords: diagnosis, fatty liver, screening, MAFLD
Introduction
Metabolic-associated fatty liver disease (MAFLD) is a highly prevalent disease, which affects more than one-third of the global population,1 according to a 2022 meta-analysis and systematic review. It is defined as the presence of liver steatosis (diagnosed histologically, through blood biomarkers or scores, or through imaging studies) along with the presence of either one of the following conditions: overweight or obesity (body mass index, BMI > 25 kg/m2), metabolic dysfunction (which covers hypertriglyceridemia or hypercholesterolemia, increased waist circumference, insulin resistance and systemic hypertension).2 Even though the previous term NAFLD (non-alcoholic fatty liver disease) was estimated to have a prevalence of 25% in the global population,3 only recent studies are starting to show the MAFLD prevalence, considering its specific diagnostic criteria.
MAFLD consists not of a single entity, but rather a spectrum of progressive stages of liver disease, starting from simple steatosis, leading into steatohepatitis, fibrosis, cirrhosis and hepatocellular carcinoma (HCC). This range of progressive diseases has the characteristic of not necessarily going through every stage before skipping to the terminal, irreversible conditions. MAFLD carries an important number of associated comorbidities, which ultimately lead to increased mortality owing to cardiovascular disease (CVD), non-liver malignancies, lung diseases, chronic kidney disease (CKD), cognitive impairment and complications of type 2 diabetes mellitus (T2DM), being CVDs the principal cause of death in these patients.4 The novel MAFLD diagnostic criteria better predict the mentioned hepatic and extra-hepatic outcomes. As in the case of gestational diabetes mellitus (GDM), redefinition of the disease based on current knowledge could lead to increase in incidence (because of timely diagnosis), and also improved primary health outcomes.5
MAFLD carries the risk of being underdiagnosed, given lack of general population and even hospital-staff knowledge regarding this public health issue. This disease entity was previously known as NAFLD, which was a diagnosis that had to be established on the basis of the exclusion of other possible causes of liver disease, including not only alcoholic liver disease (ALD), but also any other cause of chronic liver disease (including haemochromatosis, Wilson’s disease and inborn errors of metabolism).6 This inevitably leads to ineffective diagnosis in the larger portion of the population, which leads to the late diagnosis once the disease has progressed. Given this issue, and after multiple years of research leading to the insights on MAFLD pathogenesis, the term was proposed to be changed to the current MAFLD, which diagnoses a larger part of the population based on the factors that lead to fatty liver, which are the components of metabolic dysfunction.7,8 This new definition not only leads to early detection of the disease by making diagnosis more straightforward, but also targets the underlying conditions that lead to its progression, making it possible to treat the disease precisely. Even though there has been important articles analysing the pros and cons of the nomenclature change, such as a possibility of premature change, it is also important to establish that while MAFLD- and NAFLD-diagnosed patients do not encompass the same population, it is true that the underlying component between MAFLD and NAFLD pathogenesis is metabolic dysfunction, which does not necessarily require overweight or obesity to be present.9 In addition, as some voices have already expressed, integrating the term ‘metabolic’ to the new nomenclature of the disease eases physician–patient communication, given that the name of the disease establishes in itself its cause, instead of describing what the disease is not. Understanding of the disease by the patient is crucial for appropriate diagnosis, treatment and favourable prognosis.10
Having said this, we must understand that for prompt diagnosis and treatment to take place, screening plays a pivotal role in the clinical scene. This article reviews the most relevant points to take into consideration when making a presumptive diagnosis of MAFLD, as well as the process we propose to be taken for appropriate screening in the general population.11
Methodology: literature search
For this article, we used a series of published articles focussed on diagnosis of MAFLD, although we also included papers that gave an important perspective on the diagnosis of fatty liver in general, as well as important topics for the understanding of the aetiology and risk factors for the disease. For the purpose of this overview, and given the relatively large amount of articles written using the NAFLD definition, we based our search on the fact that there is a high concordance rate between MAFLD and NAFLD, according to Dr Targher, with a Cohen’s kappa coefficient of 0.92.12 We performed a database search on PubMed selecting papers published between January 2000 and August 2022 written in English. To this purpose, we considered the following keywords and MESH terms during the PubMed search: ((“‘fatty liver’ [Mesh Terms] AND ‘fatty liver’ [All Fields] AND ‘diagnosis’ [MeSH Terms] AND ‘Risk Factors’ [MeSH Terms] AND ‘MAFLD’ [All Fields] OR ‘NAFLD’ [All Fields])). This search yielded 35,465 results, from which we excluded papers that were not pertinent to the specifics of fatty liver screening or clear information regarding its diagnosis.
WHO: understanding the basic characteristics of population at risk
If we wanted to describe the genesis of MAFLD in the simplest way possible, the most appropriate words to do so would be ‘metabolic dysfunction’. Understanding the multitudinous ways in which metabolic dysfunction presents on different organs is the key to understanding the development of liver steatosis in patients without any other apparent cause of liver disease, such as alcohol consumption, infections or chronic diseases. This is true to the point where absence of metabolic health worsens progression in every existing liver disease, even when that was not the primary cause, as in the case of MAFLD.
What is exactly metabolic dysfunction? Metabolic health has been defined differently by multiple authors by observing how counterintuitively obese people occasionally had less disease burden compared with the non-obese.13 Based on the findings, it was decided to categorize obesity into metabolic healthy obesity (MHO) and metabolic unhealthy obesity (MUO). The initial approach to describing metabolic healthy, but obese people was established by Karelis et al.14 in 2004, after which multiple definitions later arose. In their study, metabolic health was defined base on lipid profile, which measured four specific variables (including total cholesterol, triglycerides, high density lipoprotein (HDL)- and low density lipoprotein (LDL)-cholesterol levels) and the level of insulin sensitivity, quantified through the homeostatic model assessment (HOMA). Thus, the presence of metabolic dysfunction implies any mechanism that impairs lipid and glucose metabolism, ultimately leading to increased cardiovascular risk.15 On the clinical level, measuring laboratory parameters could indicate the presence of metabolic dysfunction, that is, the presence of insulin resistance, T2DM, dyslipidaemia, and so on. However, for the total serum values of specific markers to be altered, smaller physiological processes on each cell are the ones that occur at the earlier stages. This leads to difficulty measuring the presence of metabolic dysfunction in the initial phase. Therefore, evaluation of risk factors for metabolic dysfunction in the scenario we just illustrated plays the essential puzzle piece on appropriate MAFLD screening in the general population.
The risk factors for developing metabolic dysfunction include overweight or obesity, body fat distribution, dietary and lifestyle habits, comorbidities and genetic risk factors according to family history and ethnicity. A recent exploration into what is known as ‘lean MAFLD’ (i.e. patients with fatty liver but normal weight) shed light into the actual factors that induce fatty liver. It was see that patients with MAFLD, both lean and non-lean, have similar natural histories, including liver-related events and the resulting mortality.15,16 In a 2020 meta-analysis, the overall prevalence of non-obese MAFLD was calculated to be 40.8% among the MAFLD population and 12.1% in the general population.17 Interestingly, the same study showed that the overall liver- and cardiovascular-specific mortality rates were 12.1, 4.1 and 4 per 1000 person-years, respectively, in patients with lean MAFLD. By contrast, the rates were 7.5, 2.4 and 2.4 per 1000 person-years, respectively, among patients with obesity and MAFLD.17 Does this mean that lean MAFLD carries a higher mortality compared with non-lean MAFLD? Even though data might point towards one side or the other, the question is still far from being answered.
We are obligated to wonder the extent to which metabolic dysfunction leads to worse outcomes on patients diagnosed with MAFLD compared with NAFLD. It has been demonstrated that MAFLD defines a group of patients with greater disease burden and higher risk of progression to fibrosis, especially when compared with non-NAFLD MAFLD individuals. The presence of diabetes as a metabolic trait is a very significant factor associated with increased mortality, increased risk of cirrhosis and HCC development.18,19 Diabetes mellitus also had the strongest predictive factor (odds ratio (OR) of 2.49) for advanced fibrosis in a 424-biopsy-proven MAFLD patients.20
As previously mentioned, the leading cause of death in the MAFLD population is related to CVD, followed by extra-hepatic cancers (principally colorectal cancer), CKD and complications associated with liver cirrhosis.21 Therefore, when thinking about who to screen for MAFLD, we are obligated to consider people at increased risk and family history of CVD, as well as any type of malignancy.
Another point to consider for evaluation of screening in the general population is the prevalence of MAFLD depending on the sex of the patients. Oestrogen’s role in fatty liver development has been deeply studied, with interesting results suggesting a protective effect of oestrogens on women, as suggested by a study carried out in premenopausal, postmenopausal and women with polycystic ovarian syndrome (PCOS).22 The mechanism has been discovered to a certain degree, finding that by acting through the oestrogen receptors (ERs) ERα, ERβ and GPER (G-protein-coupled oestrogen receptor), oestrogens limit dietary-induced de novo lipogenesis (DNL) and reduce free fatty acid (FFA) uptake, thus restricting the influx of these into the liver, decreasing steatosis development in the liver.23 Furthermore, increased oestrogen supplementation in rats was shown to decrease the hepatic expression of lipogenic enzyme synthesis through decreased hepatic expression of SREBP-1c.24,25 Based on the mentioned facts, men and women in lower oestrogen states would have increased risk and therefore more reason for MAFLD screening, when evaluated with other risk factors.
Finally, ethnicity plays an important role in the stratification of patients at risk of developing MAFLD. This occurs as a result of genetic variations as well as the diversity of gut microbiome among different populations. As a broad example, the I148M (rs738409 C/G) mutation on the patatin-like phospholipase domain-containing 3 gene (PNPLA3) determines hepatic fat accumulation and progression of simple steatosis into steatohepatitis and fibrosis.26,27 Consequently, populations with increased expression of this variant have higher susceptibility to the development of MAFLD, such as the Hispanic population, while African-Americans constitute the group with least susceptibility and lowest PNPLA3 variant expression.28,29 Thus, ethnic-specific screening protocols must be developed based on the community susceptibility. As higher understanding is achieved, we might be able to identify other important risk factors, such as the recently uncovering of air pollution in the development of MAFLD.30
An important comment on the population at risk regards younger individuals, due to the recent increase in obesity and overweight in lower age groups. As we mentioned, metabolic dysfunction is the known basis of metabolic fatty liver; thus, naturally, the presence of obesity in younger age groups leads to higher incidence of fatty liver, especially when considering the MAFLD criteria opposed to the NAFLD ones. Furthermore, studies have shown significant mortality risk in individuals <25 years old who presented with hepatic steatosis in younger ages compared with the people who presented steatosis in their later years of life.31
WHEN: optimizing time and resources for appropriate diagnosis
Risk factors for metabolic dysfunction are multiple and require appropriate tailoring to both the population and the individual. Thus, we must consider the differences in affected age groups according to the risk factors. For instance, MAFLD is mainly a disease of middle and old age, with increased mortality in >60 years old.32 In contrast, ALD presents in young-middle-aged groups, between the ages of 40 and 50 years old, starting at ages as young as 19 years old.33 Thus, higher age groups should be prioritized when screening for MAFLD.
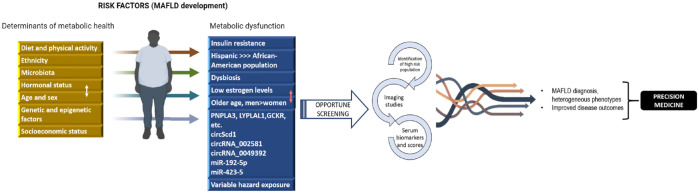
Second, when considering the appropriate time to start screening for metabolic fatty liver, we must consider the fact that during regular physician check-ups, non-specific blood tests and somatometry could point towards the need of further workup to approach a possible MAFLD diagnosis. Metabolic syndrome markers, including lipid levels, fasting glucose and possible glycated haemoglobin (HbA1c) values can suggest metabolic dysfunction and possible insulin resistance, T2DM, or dyslipidaemias, which constitute the backbone of fatty liver. When discovering abnormalities in any of the said parameters as well as abnormal somatometry measurements, screening for MAFLD is a must, which can be carried out through imaging or specific blood tests (Figure 1).
Figure 1.
Identification of MAFLD cases in the general population.
We propose a schematic of the main factors involved in MAFLD pathogenesis, that is, the contributing factors in the process of metabolic health turning into metabolic dysfunction, as well as the steps that should be carried out universally to appropriately identify high-risk population. The figure also briefly portrays the expected final outcomes of appropriate and cost-effective MAFLD screening in patient care.
Finally, as a preponderant number of findings in medicine, presumptive diagnosis of MAFLD can be incidental. During imaging tests, which can go from ultrasound to computed tomography (CT) scans or magnetic resonance imaging (MRI), special attention must always be paid to liver morphology and gross structure. Incidental imaging findings constitute an important source of fatty liver diagnosis in the primary care or hospital setting.34
HOW: steps towards appropriate screening in at-risk population
Universal screening for MAFLD has not reached a consensus, while the European guidelines have supported screening for MAFLD in high-risk patients with obesity (BMI > 30 kg/m2) or metabolic syndrome,35 the American Association for the Study of Liver Disease (AASLD) still argues the utility of routine screening for MAFLD in these high-risk subjects, due to the lack of cost-effective tests and an established effective pharmacologic treatment for the disease.36 However, new guidelines from the Asian-Pacific Association for the study of the Liver (APASL) developed new guidelines for the diagnosis and management for MAFLD, considering the new definition and criteria.37
However, ongoing research is constantly yielding new targets for pharmacologic therapy for MAFLD, including resveratrol, SRT1720, and NAD+ precursors (nicotinamide riboside, nicotinic acid and nicotinamide mononucleotide), which target sirtuins (SIRT1).38 These drugs have anti-oxidative properties and induce molecular mechanisms that alter lipid and glucose metabolism in the liver.39,40 However, results on the treatment for MAFLD are still heterogeneous.41–45 Thus, the lack of current effective pharmacologic treatment should not be a limitation for universal screening in high-risk patients, especially taking into consideration that the initial stages of MAFLD (i.e. simple steatosis and even steatohepatitis) can regress or hinder progression with adequate lifestyle modifications and treatment of comorbidities.46
We already discussed that identification of high-risk groups is the toughest and often overlooked part of the diagnosis. Once suspicion is established, a number of steps can be taken towards appropriate non-invasive cost-effective screening, through either imaging or blood test analysis.
To date, ultrasound is recommended as the first-line screening tool for defining steatosis in a selected population,47 since it offers 67–94% sensitivity (depending of the degree of damage) and up to 97% specificity for hepatic steatosis, especially through bright liver echo pattern.48,49 In addition, it is readily available in most hospitals and has low cost.50 Alternative imaging techniques involve higher cost; however, in case of stronger incertitude these lead to more reliable results. These include the use of vibration-controlled transient elastography (VCTE, commercialized as Fibroscan) as well as magnetic resonance spectroscopy (MRS) or regular MRI scanners applying a magnetic resonance proton density fat fraction (MR-PDFF) protocol.51–53 These yield more specific results; however, ultrasound is the way to go for general screening in patients at risk.
On the other side, serum biochemical markers also represent an important screening tool in the general population. The use of serum biomarkers is based on their quantification and on the biological properties of liver tissue to assess damage; that is, they can come directly either from the turnover of the extracellular matrix (ECM) and fibrogenic cell changes or from an altered function of the liver. The advantage of using serum biomarkers is the fact that they are more reproducible than transient elastography use and the assays can be standardized in the laboratory setting.54,55 Biomarkers, including total cholesterol, triglycerides, measure of insulin resistance through HOMA and C-peptide, have been used for many years. With time, novel biomarkers, including apolipoprotein A1, apolipoprotein B, leptin, adiponectin, ghrelin and tumour necrosis factor-alpha (TNF-α), have been proposed as valuable complementary tools to be analysed along traditional biomarkers.56 Recently, the role of PRO-C3 (a marker of type III collagen formation) was studied as a biomarker for advanced fibrosis in MAFLD, this parameter is now part of the ADAPT algorithm to evaluate disease progression.57 From the diagnostic panels, including collagen turnover and ECM remodelling parameters, only Pro-C3 and the ADAPT algorithm (which stands for: age, presence of diabetes, PRO-C3, and platelet count) was validated in the MAFLD population (in this case, Asian patients) to exclude advanced fibrosis.58 New biomarkers to yield more specific non-invasive diagnosis are constantly arising in different age groups, as seen with the finding of serum osteocalcin levels as important marker of MAFLD progression in children with obesity.59 Even though novel biomarkers could lead in the future for universal screening methods for MAFLD, the most important tests that should be carried out in high-risk patients include periodic liver function tests (LFTs), given that MAFLD is the main reason for unexpectedly elevated liver enzymes in developed countries,35 as well as total cholesterol and triglyceride levels, as well as HbA1C monitoring. Screening for metabolic diseases should unquestionably lead to simultaneous screening of MAFLD, and vice versa.
Apart from the mentioned parameters, there are specific scores that combine different variables for the evaluation of MAFLD in an individual. These scores range from the diagnosis of fatty liver to quantification of fibrosis in a patient with MAFLD. The fatty liver index (FLI) incorporates the patient’s BMI, waist circumference, gamma-glutamyl transferase (GGT) and serum triglyceride levels.54 Liver fibrosis is considered.60 Liver fibrosis is the principal determinant of liver-related morbidity and mortality in patients with MAFLD.61 Therefore, non-invasive fibrosis scores in patients with MAFLD have been developed, mainly to exclude the presence of advanced fibrosis. These scores include the NAFLD fibrosis score (NFS) that considers fasting glucose and albumin, the aspartate aminotransferase (AST)-to-platelet ratio index (APRI) and the fibrosis-4 index (FIB-4), which takes into account age, alanine transaminase (ALT), aspartate aminotransferase (AST) and platelet count. Comparison of the non-invasive markers of fibrosis showed the superiority of the FIB-4 score over other commonly used scores.62 Of the non-invasive blood tests, FIB-4 and NFS mostly outperformed the other scoring systems (namely, APRI and BARD, which stands for BMI, AST/ALT ratio and presence of T2DM) for both MAFLD and NAFLD.63,64 Even though these two tests can be used with confidence in overweight, obese and severely obese patients, their performance in lean and morbidly obese patients was found to be questionable.65
Even though we described many aspects regarding the diagnostic tests, these tests alone are not able to define the patients at high risk of advanced fibrosis adequately. Therefore, for the NAFLD patients, a further diagnostic method, such as Fibroscan following FIB-4 is recommended.66,67 This stepwise approach can significantly lower the unnecessary biopsies.65 ADAPT followed by Fibroscan and FIB-4, succeeded by the Enhanced Liver Fibrosis (ELF) score are other diagnostic options, which were shown to increase the diagnostic rate.65,68
Even though more studies need to be carried out to validate the more effective methods for fibrosis assessment in the MAFLD defined population, these studies show a clear path for starting risk assessment. By measuring simple clinical parameters, diagnosis and damage stratification can be carried out in the population.
Conclusion
In conclusion, MAFLD is a highly prevalent chronic disease that creates an increasing health burden worldwide. Identifying high-risk groups is key to establish effective screening strategies, as both imaging and serum laboratory tests can be tailored to detect the disease based on the specific metabolic alterations most prevalent in a particular population. However, this need for screening must be preceded by studies on the epidemiology of the disease, genetic background and characteristics of the patients on each country. Furthermore, additional research needs to be carried out to define the high-risk group of patients better than using general metabolic health parameters.
Acknowledgments
None.
Footnotes
ORCID iD: Nahum Méndez-Sánchez  https://orcid.org/0000-0001-5257-8048
https://orcid.org/0000-0001-5257-8048
Contributor Information
Shreya C. Pal, Faculty of Medicine, National Autonomous University of Mexico, Mexico City, Mexico Liver Research Unit, Medica Sur Clinic Foundation, Mexico City, Mexico.
Nahum Méndez-Sánchez, Liver Research Unit, Medica Sur Clinic Foundation, Puente de Piedra 150, 14050 Mexico City, Mexico; Faculty of Medicine, National Autonomous University of Mexico, Mexico City, Mexico.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Shreya C. Pal: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Resources; Software; Validation; Visualization; Writing – original draft; Writing – review & editing.
Nahum Méndez-Sánchez: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Resources; Software; Supervision; Validation; Visualization; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of data and materials: Not applicable.
References
- 1. Chan KE, Koh TJL, Tang ASP, et al. Global prevalence and clinical characteristics of metabolic-associated fatty liver disease: a meta-analysis and systematic review of 10 739 607 individuals. J Clin Endocrinol Metab 2022; 107: 2691–2700. [DOI] [PubMed] [Google Scholar]
- 2. Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol 2020; 73: 202–209. [DOI] [PubMed] [Google Scholar]
- 3. Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease – meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016; 64: 73–84. [DOI] [PubMed] [Google Scholar]
- 4. Paik JM, Henry L, De Avila L, et al. Mortality related to nonalcoholic fatty liver disease is increasing in the United States. Hepatol Commun 2019; 3: 1459–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cade TJ, Polyakov A, Brennecke SP. Implications of the introduction of new criteria for the diagnosis of gestational diabetes: a health outcome and cost of care analysis. BMJ Open 2019; 9: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ando Y, Jou JH. Nonalcoholic fatty liver disease and recent guideline updates. Clin Liver Dis 2021; 17: 23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mendez-Sanchez N, Arrese M, Gadano A, et al. The Latin American Association for the Study of the Liver (ALEH) position statement on the redefinition of fatty liver disease. Lancet Gastroenterol Hepatol 2021; 6: 65–72. [DOI] [PubMed] [Google Scholar]
- 8. Méndez-Sánchez N, Bugianesi E, Gish RG, et al. Global multi-stakeholder endorsement of the MAFLD definition. Lancet Gastroenterol Hepatol 2022; 7: 388–390. [DOI] [PubMed] [Google Scholar]
- 9. Kaya E, Zedginidze A, Bechmann L, et al. Metabolic dysfunction-associated fatty liver disease (MAFLD) and non-alcoholic fatty liver disease (NAFLD): distinct fatty liver entities with different clinical outcomes? Hepatobiliary Surg Nutr 2022; 11: 299–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yilmaz Y, Byrne CD, Musso G. A single-letter change in an acronym: signals, reasons, promises, challenges, and steps ahead for moving from NAFLD to MAFLD. Expert Rev Gastroenterol Hepatol 2021; 15: 345–352. [DOI] [PubMed] [Google Scholar]
- 11. Méndez-Sánchez N, Chavez-Tapia NC, Almeda-Valdes P, et al. The management of incidental fatty liver found on imaging. What do we need to do? Am J Gastroenterol 2018; 113: 1274–1276. [DOI] [PubMed] [Google Scholar]
- 12. Targher G. Concordance between MAFLD and NAFLD diagnostic criteria in ‘real-world’ data. Liver Int 2020; 40: 2879–2880. [DOI] [PubMed] [Google Scholar]
- 13. Eslam M, Fan JG, Mendez-Sanchez N. Non-alcoholic fatty liver disease in non-obese individuals: the impact of metabolic health. Lancet Gastroenterol Hepatol 2020; 5: 713–715. [DOI] [PubMed] [Google Scholar]
- 14. Karelis AD, Brochu M, Rabasa-Lhoret R. Can we identify metabolically healthy but obese individuals (MHO)? Diabetes Metab 2004; 30: 569–572. [DOI] [PubMed] [Google Scholar]
- 15. Eslam M, El-Serag HB, Francque S, et al. Metabolic (dysfunction)-associated fatty liver disease in individuals of normal weight. Nat Rev Gastroenterol Hepatol 2022; 19: 638–651. [DOI] [PubMed] [Google Scholar]
- 16. Chen F, Esmaili S, Rogers GB, et al. Lean NAFLD: a distinct entity shaped by differential metabolic adaptation. Hepatology 2020; 71: 1213–1227. [DOI] [PubMed] [Google Scholar]
- 17. Ye Q, Zou B, Yeo YH, et al. Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2020; 5: 739–752. [DOI] [PubMed] [Google Scholar]
- 18. Nguyen VH, Le MH, Cheung RC, et al. Differential clinical characteristics and mortality outcomes in persons with NAFLD and/or MAFLD. Clin Gastroenterol Hepatol 2021; 19: 2172–2181.e6. [DOI] [PubMed] [Google Scholar]
- 19. Yilmaz Y, Yilmaz N, Ates F, et al. The prevalence of metabolic associated fatty liver disease in the Turkish population: a multicenter study. Hepatol Forum 2021; 2: 37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kani HT, Demirtas CO, Keklikkiran C, et al. Evaluation of the impact of metabolic syndrome on fibrosis in metabolic dysfunction-associated fatty liver disease. Turk J Gastroenterol 2021; 32: 661–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lonardo A, Mantovani A, Lugari S, et al. Epidemiology and pathophysiology of the association between NAFLD and metabolically healthy or metabolically unhealthy obesity. Ann Hepatol 2020; 19: 359–366. [DOI] [PubMed] [Google Scholar]
- 22. Gutierrez-Grobe Y, Ponciano-Rodríguez G, Ramos MH, et al. Prevalence of non alcoholic fatty liver disease in premenopausal, posmenopausal and polycystic ovary syndrome women. The role of estrogens. Ann Hepatol 2010; 9: 402–409. [PubMed] [Google Scholar]
- 23. Pal SC, Eslam M, Méndez-Sánchez N. Detangling the interrelations between MAFLD, insulin resistance, and key hormones. Hormones 2022; 21: 573–589. [DOI] [PubMed] [Google Scholar]
- 24. D’Eon TM, Souza SC, Aronovitz M, et al. Estrogen regulation of adiposity and fuel partitioning: evidence of genomic and non-genomic regulation of lipogenic and oxidative pathways. J Biol Chem 2005; 280: 35983–35991. [DOI] [PubMed] [Google Scholar]
- 25. Paquette A, Shinoda M, Lhoret RR, et al. Time course of liver lipid infiltration in ovariectomized rats: impact of a high-fat diet. Maturitas 2007; 58: 182–190. [DOI] [PubMed] [Google Scholar]
- 26. Sookoian S, Pirola CJ. Meta-analysis of the influence of I148M variant of patatin-like phospholipase domain containing 3 gene (PNPLA3) on the susceptibility and histological severity of nonalcoholic fatty liver disease. Hepatology 2011; 53: 1883–1894. [DOI] [PubMed] [Google Scholar]
- 27. Li JZ, Huang Y, Karaman R, et al. Chronic overexpression of PNPLA3 I148M in mouse liver causes hepatic steatosis. J Clin Invest 2012; 122: 4130–4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Romeo S, Kozlitina J, Xing C, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet 2008; 40: 1461–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chinchilla- López P, Ramírez -Pérez O, Cruz-Ramón V, et al. More evidence for the genetic susceptibility of Mexican population to nonalcoholic fatty liver disease through PNPLA3. Ann Hepatol 2018; 17: 250–255. [DOI] [PubMed] [Google Scholar]
- 30. Guo B, Guo Y, Nima Q, et al. Exposure to air pollution is associated with an increased risk of metabolic dysfunction-associated fatty liver disease. J Hepatol 2022; 76: 518–525. [DOI] [PubMed] [Google Scholar]
- 31. Kaya E, Yilmaz Y. Insidious danger for young adults: metabolic (dysfunction)- associated fatty liver disease. Hepatol Forum 2022; 3: 39–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Frith J, Day CP, Henderson E, et al. Non-alcoholic fatty liver disease in older people. Gerontology 2009; 55: 607–613. [DOI] [PubMed] [Google Scholar]
- 33. Mellinger JL. Epidemiology of alcohol use and alcoholic liver disease. Clin Liver Dis 2019; 13: 136–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang XJ, Malhi H. Nonalcoholic fatty liver disease. Ann Intern Med 2018; 169: ITC65-ITC80. [DOI] [PubMed] [Google Scholar]
- 35. Marchesini G, Day CP, Dufour JF, et al. EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 2016; 64: 1388–1402. [DOI] [PubMed] [Google Scholar]
- 36. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018; 67: 328–357. [DOI] [PubMed] [Google Scholar]
- 37. Eslam M, Sarin SK, Wai V, et al. The Asian Pacific Association for the Study of the Liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Hepatol Int 2020; 14: 889–919. [DOI] [PubMed] [Google Scholar]
- 38. Colak Y, Ozturk O, Senates E, et al. SIRT1 as a potential therapeutic target for treatment of nonalcoholic fatty liver disease. Med Sci Monit 2011; 17: HY5–HY9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bordone L, Motta MC, Picard F, et al. Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic beta cells. PLoS Biol 2006; 4: 210–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ponugoti B, Kim DH, Xiao Z, et al. SIRT1 deacetylates and inhibits SREBP-1C activity in regulation of hepatic lipid metabolism. J Biol Chem 2010; 285: 33959–33970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wei S, Yu X. Efficacy of resveratrol supplementation on liver enzymes in patients with non-alcoholic fatty liver disease: a systematic review and meta-analysis. Complement Ther Med 2021; 57: 102635. [DOI] [PubMed] [Google Scholar]
- 42. Darand M, Farrokhzad A, Ghavami A, et al. Effects of resveratrol supplementation on liver enzymes: a systematic review and meta-analysis of randomised controlled trials. Int J Clin Pract 2021; 75: e13692. [DOI] [PubMed] [Google Scholar]
- 43. Elgebaly A, Radwan IAI, Aboelnas MM, et al. Resveratrol supplementation in patients with non-alcoholic fatty liver disease: systematic review and meta-analysis. J Gastrointest Liver Dis 2017; 26: 59–67. [DOI] [PubMed] [Google Scholar]
- 44. Akbari M, Tamtaji OR, Lankarani KB, et al. The effects of resveratrol on lipid profiles and liver enzymes in patients with metabolic syndrome and related disorders: a systematic review and meta-analysis of randomized controlled trials. Lipids Health Dis 2020; 19: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jakubczyk K, Skonieczna-z.ydecka K, Kałduńska J, et al. Effects of resveratrol supplementation in patients with non-alcoholic fatty liver disease – a meta-analysis. Nutrients 2020; 12: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zou TT, Zhang C, Zhou YF, et al. Lifestyle interventions for patients with nonalcoholic fatty liver disease: a network meta-analysis. Eur J Gastroenterol Hepatol 2018; 30: 747–755. [DOI] [PubMed] [Google Scholar]
- 47. Papatheodoridi M, Cholongitas E. Diagnosis of non-alcoholic fatty liver disease (NAFLD): current concepts. Curr Pharm Des 2019; 24: 4574–4586. [DOI] [PubMed] [Google Scholar]
- 48. Saverymuttu SH, Joseph AEA, Maxwell JD. Ultrasound scanning in the detection of hepatic fibrosis and steatosis. Br Med J 1986; 292: 13–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Palmentieri B, de Sio I, La Mura V, et al. The role of bright liver echo pattern on ultrasound B-mode examination in the diagnosis of liver steatosis. Dig Liver Dis 2006; 38: 485–489. [DOI] [PubMed] [Google Scholar]
- 50. Zhu JZ, Hollis-Hansen K, Wan XY, et al. Clinical guidelines of non-alcoholic fatty liver disease: a systematic review. World J Gastroenterol 2016; 22: 8226–8233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Thomas EL, Hamilton G, Patel N, et al. Hepatic triglyceride content and its relation to body adiposity: a magnetic resonance imaging and proton magnetic resonance spectroscopy study. Gut 2005; 54: 122–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Myers RP, Pollett A, Kirsch R, et al. Controlled attenuation parameter (CAP): a noninvasive method for the detection of hepatic steatosis based on transient elastography. Liver Int 2012; 32: 902–910. [DOI] [PubMed] [Google Scholar]
- 53. Tang A, Desai A, Hamilton G, et al. Accuracy of MR imaging-estimated proton density fat fraction for classification of dichotomized histologic steatosis grades in nonalcoholic fatty liver disease. Radiology 2015; 274: 416–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Castera L, Friedrich-Rust M, Loomba R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology 2019; 176: 139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Forsgren MF, Nasr P, Karlsson M, et al. Biomarkers of liver fibrosis: prospective comparison of multimodal magnetic resonance, serum algorithms and transient elastography. Scand J Gastroenterol 2020; 55: 848–859. [DOI] [PubMed] [Google Scholar]
- 56. Neuman MG, Cohen LB, Nanau RM. Biomarkers in nonalcoholic fatty liver disease. Can J Gastroenterol Hepatol 2014; 28: 607–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Daniels SJ, Leeming DJ, Eslam M, et al. ADAPT: an algorithm incorporating PRO-C3 accurately identifies patients with NAFLD and advanced fibrosis. Hepatology 2019; 69: 1075–1086. [DOI] [PubMed] [Google Scholar]
- 58. Tang LJ, Ma HL, Eslam M, et al. Among simple non-invasive scores, Pro-C3 and ADAPT best exclude advanced fibrosis in Asian patients with MAFLD. Metabolism 2022; 128: 154958. [DOI] [PubMed] [Google Scholar]
- 59. Amin S, El Amrousy D, Elrifaey S, et al. Serum osteocalcin levels in children with nonalcoholic fatty liver disease. J Pediatr Gastroenterol Nutr 2018; 66: 117–121. [DOI] [PubMed] [Google Scholar]
- 60. Alharthi J, Eslam M. Biomarkers of metabolic (Dysfunction)-associated fatty liver disease: an update. J Clin Transl Hepatol 2022; 10: 134–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Vilar-Gomez E, Calzadilla-Bertot L, Wai-Sun Wong V, et al. Fibrosis severity as a determinant of cause-specific mortality in patients with advanced nonalcoholic fatty liver disease: a multi-national cohort study. Gastroenterology 2018; 155: 443–457. [DOI] [PubMed] [Google Scholar]
- 62. Shah AG, Lydecker A, Murray K, et al. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2009; 7: 1104–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wu YL, Kumar R, Wang MF, et al. Validation of conventional non-invasive fibrosis scoring systems in patients with metabolic associated fatty liver disease. World J Gastroenterol 2021; 27: 5733–5763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Peleg N, Issachar A, Sneh-Arbib O, et al. AST to platelet ratio index and fibrosis 4 calculator scores for non-invasive assessment of hepatic fibrosis in patients with non-alcoholic fatty liver disease. Dig Liver Dis 2017; 49: 1133–1138. [DOI] [PubMed] [Google Scholar]
- 65. Eren F, Kaya E, Yilmaz Y. Accuracy of fibrosis-4 index and non-alcoholic fatty liver disease fibrosis scores in metabolic (dysfunction) associated fatty liver disease according to body mass index: failure in the prediction of advanced fibrosis in lean and morbidly obese individual. Eur J Gastroenterol Hepatol 2022; 34: 98–103. [DOI] [PubMed] [Google Scholar]
- 66. Younossi ZM, Corey KE, Alkhouri N, et al. Clinical assessment for high-risk patients with non-alcoholic fatty liver disease in primary care and diabetology practices. Aliment Pharmacol Ther 2020; 52: 513–526. [DOI] [PubMed] [Google Scholar]
- 67. Yilmaz Y, Kaya E, Eren F. Letter: the use of fibrosis-4 score in primary care and diabetology practices-Occam’s razor applied to advanced fibrosis screening. Aliment Pharmacol Ther 2020; 52: 1759–1760. [DOI] [PubMed] [Google Scholar]
- 68. Eslam M, Wong GLH, Hashem AM, et al. A sequential algorithm combining ADAPT and liver stiffness can stage metabolic-associated fatty liver disease in hospital-based and primary care patients. Am J Gastroenterol 2021; 116: 984–993. [DOI] [PubMed] [Google Scholar]