Abstract
Cytolethal distending toxins (CDTs) are multisubunit proteins produced by a variety of bacterial pathogens that cause enlargement, cell cycle arrest, and apoptosis in mammalian cells. While their function remains uncertain, recent studies suggest that they can act as intracellular DNases in mammalian cells. Here we establish a novel yeast model for understanding CDT-associated disease. Expression of the CdtB subunit in yeast causes a G2/M arrest, as seen in mammalian cells. CdtB toxicity is not circumvented in yeast genetically altered to lack DNA damage checkpoint control or that constitutively promote cell cycle progression via mutant Cdk1, because CdtB causes a permanent type of damage that results in loss of viability. Finally, we establish that CDTs are likely to be potent genotoxins, as indicated by in vivo degradation of chromosomal DNA associated with expression of CdtB—suggesting that the varied distribution of CDT in bacteria implicates many human pathogens as possessors of genotoxic activity.
Cytolethal distending toxins (CDTs) are highly related, bacterially encoded proteins associated with gastrointestinal disease (34) and, perhaps, the unrelated diseases periodontitis (46) and chancroid (7). Intoxication with CDT causes cells to exhibit nuclear and cytoplasmic enlargement accompanied by G2 arrest associated with invocation of the DNA damage checkpoint and eventual apoptotic cell death (9, 20, 42, 50). Campylobacter jejuni, a producer of CDT, is the most common bacterial cause of food-borne infectious illness in the United States and is responsible for approximately 2 million cases of gastrointestinal illness each year (47). Infections with this organism are also an antecedent to the paralytic disorder Guillain-Barre syndrome (26; B. Speed, J. Kaldor, and P. Cavanagh, Letter, J. Infect. 8:85–86, 1984).
Opportunities for CDT to intoxicate humans are abundant. By association with C. jejuni alone, CDT is prevalent in the majority of uncooked store-bought chicken carcasses (18). Moreover, CDT is present in a number of other common human pathogens, including Campylobacter coli, Campylobacter fetus (23, 37), various Escherichia coli isolates (24, 36, 41), Haemophilus ducreyi (7, 8), enterohepatic Helicobacter spp. (5, 53, 54), Actinobacillus actinomycetemcomitans (31, 43, 46), and Shigella spp. (34).
The mechanism by which CDT acts is uncertain, but recent findings have suggested that it may act as an intracellular DNase (12, 17, 28). The toxin is encoded by three conserved genes: cdtA, cdtB, and cdtC (36). These gene products exhibit weak homologies to proteins outside the CDT family. CdtA bears homology to the ricin B chain, CdtC is most similar to CdtA (C. Pickett, unpublished data), and CdtB exhibits similarity to a broad class of enzymes sharing phosphoesterase activity, including nucleases, protein phosphatases, inositol polyphosphate phosphatases, and sphingomyelinases (12). The similarity of CdtB to DNase I, in particular, has attracted attention and led to the demonstration of in vitro DNase activity by CDT, but not by CDT containing CdtB subunits mutated in residues predicted to be critical for catalysis based on the DNase I mechanism (17). Furthermore, ectopic expression in mammalian cells of the CdtB subunit alone, but not the CdtA or CdtC subunits, recapitulates the toxic effects obtained when cells are treated with CDT (28). These results readily explain the CDT-induced G2 cell cycle arrest that has been noted in multiple studies (6, 8, 35, 42, 44, 46, 50) as resulting from elicitation of the cell's DNA damage checkpoint response. Contrary to this model, however, CDT-induced DNA damage in vivo was specifically rejected as a potential mechanism by Sert et al. (42) and the rather weak in vitro CdtB DNase activity (28) seems inconsistent with the potency of CDT as a toxin. Moreover, the critical residues of CdtB predicted to be important for its putative DNase activity are similarly predicted to be important for any potential phosphoesterase activity based on the similarities noted by Dlakić (12). Any of these other potential activities could conceivably result in the activation of a G2 checkpoint response.
In an effort to clarify some of these issues and gain further understanding of how CDT causes disease, we have explored the use of a more tractable model system to study the in vivo mechanism of CDT toxicity. In this study, we show that expression of CdtB—but not CdtA, CdtC, or a CdtB mutant in a residue predicted to be essential for phosphoesterase activity—in the yeast Saccharomyces cerevisiae induces an irreversible G2 cell cycle arrest accompanied by degradation of the chromosomal DNA.
MATERIALS AND METHODS
Strains, medium, and cell culture methods.
Yeast strains used in this study are outlined in Table 1. S. cerevisiae strains Y300 and Y610 were generous gifts from S. J. Elledge (Baylor University, Houston, Tex.). Y477 and Y510 were generous gifts from D. Lew (Duke University, Durham, N.C.). Where appropriate, yeast was grown in either standard YPD medium or SD medium at 30°C lacking the appropriate amino acid with either 2% glucose, sucrose, or galactose as a carbon source. Broth cultures were shaken at 250 rpm. Semisolid medium contained 1.5% agar (Difco, Detroit, Mich.).
TABLE 1.
Strains and plasmids used in this study
| Strain | Relevant genotype or markers | Reference |
|---|---|---|
| Strains | ||
| E. coli Trc18CDT | cdtABC | 37 |
| S. cerevisiae | ||
| EY957 | MATa leu2-3,112 | 13 |
| Y300 | MATa leu2-3,112 | 11 |
| Y610 | Y300 mecl::HIS3 + pBAD79 | 11 |
| DCH6041 | EY957 + pDCH-CdtA | This study |
| DCH6042 | EY957 + pDCH-CdtB | This study |
| DCH6043 | EY957 + pDCH-CdtC | This study |
| DCH6044 | EY957 + pDCH-CdtBD222A | This study |
| DCH6045 | EY957 + pMDM00333 | This study |
| DCH3002 | Y300 + pDCH-CdtB | This study |
| DCH3005 | Y300 + pMDM00333 | This study |
| DCH6102 | Y610 + pDCH-CdtB | This study |
| DCH6105 | Y610 + pMDM00333 | This study |
| Y477 | HA-Cdk1 | 45 |
| Y510 | HA-Cdk1-AF | 45 |
| DCH4772 | Y477 + pDCH-CdtB | This study |
| DCH4775 | Y477 + pMDM00333 | This study |
| DCH5102 | Y510 + pDCH-CdtB | This study |
| DCH5105 | Y510 + pMDM00333 | This study |
| Plasmids | ||
| pTrc18CDT | AprcdtABC | 37 |
| pDCH-CdtA | AprLEU2 CEN4 GAL1-cdtA | This study |
| pDCH-CdtB | AprLEU2 CEN4 GAL1-cdtB | This study |
| pDCH-CdtC | AprLEU2 CEN4 GAL1-cdtC | This study |
| pDCH-CdtBD222A | AprLEU2 CEN4 GAL1-cdtBD222A | This study |
| pBAD79 | AprTRP1 2μ GAP-RNR3 | 11 |
| pMDM00333 | AprLEU2 CEN4 GAL1p | This study |
Plasmids.
The following plasmids were used: pTrc18CDT containing the wild-type cdtA, cdtB, and cdtC genes isolated from C. jejuni 81–176 (50); and pDCH-CdtA, pDCH-CdtB, and pDCH-CdtC, pDCH-CdtBD222A, which are yeast expression plasmids that express cdtA, cdtB, cdtC, and cdtB containing a point mutation changing Asp-222 to an Ala-222, respectively, under the control of the GAL1 promoter. The yeast plasmids in this study are based on the low-copy-number CEN4, LEU2-based yeast expression vector, pMDM00333, which consisted of the GAL1-GAL10 intergenic region of pBM150 (25) cloned into the multiple cloning site of YCplac111 (22). The cdt genes were cloned as XbaI-SalI fragments into the remaining portion of the multiple cloning site. In all yeast plasmids, cdtA, cdtB, or cdtC genes were truncated and mutagenized so as to lack their putative leader sequences and contain a Kozak consensus start codon (27), which was confirmed by sequence analysis.
PCR isolation of cdt genes.
Using pTrc18CDT as a template, the three CDT subunits were individually PCR amplified with Vent DNA polymerase as per the manufacturer's recommendations (New England Biolabs, Beverly, Mass.) with the following subunit-specific primers (Integrated DNA Technologies, Coralville, Iowa): cdtA was amplified with 5′-CGCGTCTAGAACTATGGAAAATGTAAATCCTTTGGGGCGTTCATTTGC-3′ and 5′-GCGGGTCGACTTTTCATCGTACCTCTCCTTGGCG-3′, cdtB was amplified with 5′-CGCGTCTAGAAATATATGGAAAATTTTAATGTTGGCACTTGG-3′ and 5′-GGCGGTCGACTGTCCTAAAATTTTCTAAAATTTACTGG-3′, and cdtC was amplified with 5′-GCGCTCTAGAACAATGGGAGATTTGAAAGATTTTACCGAAAT-3′ and 5′-GGCGGTCGACCAAGATAAAAATCTTATTCTAAAGGGGTAGC-3′.
Directed mutagenesis of cdtB.
Asp-222 was changed to Ala-222 by the ExSite protocol (Stratagene, La Jolla, Calif.) with the mutagenic oligonucleotides 5′-CTCTTGCTTATGCAATTACAGGAAATTC-3′ and 5′-TCCCTCCGCTTGCTTGAGTTGCTGC-3′ against pTrc18CDT template to generate cdtBD222A. Mutants were identified by nucleotide sequence analysis.
Yeast transformation.
Yeast strains were transformed with plasmid DNA according to the EasyComp protocol (Invitrogen, San Diego, Calif.).
Induction of gene expression with galactose.
GAL1-controlled genes were activated by harvesting exponentially growing cells from media containing sucrose, washing them in galactose-containing media, and then shifting them to galactose. Immediately prior to induction with galactose, cells were harvested by centrifugation and resuspended in the appropriate SD medium containing 2% galactose to a final optical density at 600 nm (OD600) of 0.1 to 0.3.
Measurement of yeast DNA content.
Yeast DNA content was measured throughout these experiments by propidium iodide (PI) staining essentially as described previously (39). At least 10,000 events were assessed for PI fluorescence intensity with a FACScan flow cytometer (Becton-Dickinson, Franklin Lakes, N.J.) in each assay.
Microscopy.
Microscopic images were obtained on an AxioPhot microscope (Carl Zeiss, Inc., Thornwood, N.Y.) equipped with a Spot digital camera (Diagnostic Instruments, Sterling Heights, Mich.). Prior to microscopy, cells were sonicated at 45% power for 5 s to separate individual cells. For Nomarski imaging, cells were placed on poly-l-lysine-coated coverslips for 5 min and then mounted onto glass slides. For nuclear visualization, cells were fixed in 70% ethanol for at least 30 min at 4°C and then stained for 5 min in 10 μg of Hoechst 33342 per ml (Molecular Probes, Eugene, Oreg.).
RT-PCR of yeast transcripts.
Equivalent cell numbers were washed and resuspended in a mixture of 40 mM phosphate buffer (pH 6.8) and 1.2 M sorbitol containing 75 U of lyticase per ml. After 30 min of incubation at 30°C, total RNA was extracted from spheroplasts by using Trizol (Life Technologies, Rockville, Maryland) and submitted to reverse transcriptase PCR (RT-PCR) according to the manufacturer's specifications by using primer sets for either RNR2 or GPD1. Band intensities on agarose gels were quantified by using Kodak 1-D Image Software (Rochester, N.Y.). All RNA preparations were checked for genomic DNA contamination by PCR with equivalent amounts of nonreverse-transcribed RNA preparations as a template for 32 cycles with the GPD1 primer set.
PFGE.
Yeast chromosomal DNA was resolved by running contour-clamped homogeneous electric field gels essentially as described previously (21) by using equivalent numbers of yeast cells. Briefly, whole cells were embedded in plugs consisting of 0.7% low-melting-point agarose in the presence of lyticase and 10 mM Tris-HCl (pH 7.5)–0.5 M EDTA for 18 h at 37°C. Plugs were then transferred to a solution containing 1% Sarkosyl, 2 mg of proteinase K per ml, 10 mM Tris-HCl (pH 7.5), and 0.5 M EDTA for 18 to 24 h at 50°C. Afterwards, the agarose plugs were washed and dialyzed against 10 mM Tris-HCl (pH 7.5)–50 mM EDTA (pH 7.5) for 6 h at room temperature. Pulsed-field gel electrophoresis (PFGE) was conducted at 200 V with a 60-s pulse frequency for 20 h. The gel was poststained with ethidium bromide and observed under UV transillumination.
RESULTS
Expression of CdtB unaccompanied by other CDT subunits is toxic in S. cerevisiae.
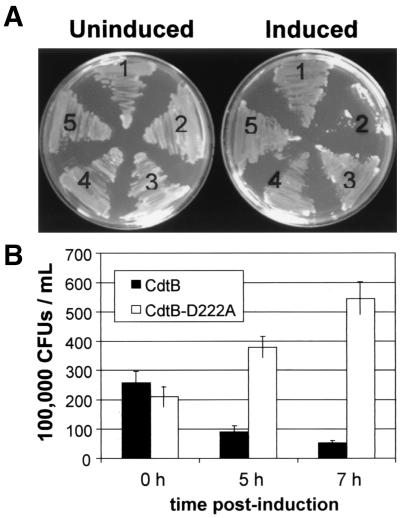
We evaluated whether cdtA, cdtB, or cdtC possesses a toxic activity by ectopically expressing each CDT subunit under the control of the GAL1 promoter in the S. cerevisiae EY957 genetic background (Table 1). All strains grew normally on glucose when compared to the vector control strain (Fig. 1A). However, when expression of the CDT subunits was induced by restreaking colonies onto medium containing galactose, only CdtA, CdtC, and the vector control grew, requiring 2 to 3 days at 30°C to obtain colonies that were clearly visible. In contrast, growth was not detected when yeast expressed CdtB, even when plates were incubated for 5 days. To confirm that the subunits were expressed, we performed Western blots with protein derived from the same yeast strains grown in broth culture in the presence of galactose. Each of these subunit proteins was detected under the inducing conditions (not shown).
FIG. 1.
Loss of viability associated with expression of CdtB on galactose medium. (A) Cells were streaked onto medium containing either glucose (left) or galactose (right). Growth after 72 h at 30°C is shown. The following cell types are shown: 1, CdtA expressing; 2, CdtB expressing; 3, CdtC expressing; 4, vector control; and 5, CdtBD222A expressing. (B) Cell survival of S. cerevisiae strains expressing either CdtB (DCH6072; solid bars) or CdtBD222A (DCH6044; open bars) at 0, 5, and 7 h of growth on galactose. Error bars represent the standard deviation (P < 0.01).
Mutations in phosphoesterase-like residues of CdtB result in loss of toxicity.
To ascertain whether it was a possible phosphoesterase-like activity that was responsible for the CdtB toxicity in yeast cells, we constructed a series of point mutations in residues of CdtB expected to be critical for its putative catalytic activity (12). Among these mutants was an aspartate-to-alanine mutation in position 222 of wild-type CdtB (CdtBD222A). CDT holotoxin in which CdtB was replaced with the CdtBD222A allele was ineffective at intoxicating HeLa cells, while wild-type CDT caused intoxication (Pickett, unpublished). To determine whether CdtBD222A would also fail to intoxicate yeast, we expressed CdtBD222A in S. cerevisiae EY957 under control of the GAL1 promoter as before. Expression of CdtBD222A did not inhibit the growth of yeast on galactose-containing agar plates in contrast to control yeast that expressed wild-type CdtB (Fig. 1A). The presence of CdtBD222A in yeast growing in galactose-containing medium was confirmed by Western blot analysis (not shown). These results indicated that the toxicity was likely to be due to a specific enzymatic activity of CdtB and not just generally to its expression in a heterologous organism.
CdtB expression is associated with an irreversible loss of viability.
Glucose is a potent inhibitor of GAL1-controlled expression (19). To determine whether CdtB was causing a loss of viability, cells were taken out of galactose-containing liquid medium, washed, and plated onto glucose-containing medium (YPD) at 0, 5, and 7 h postinduction of CdtB. After 7 h of growth in galactose-containing medium, the cultures containing yeast that expressed CdtB produced many fewer colonies (∼20% relative to time 0). However, the number of colonies produced from cultures expressing mutant CdtBD222A allele increased (Fig. 1B), thus establishing that the toxic effects of CdtB were irreversibly lethal and dependent upon a phosphoesterase-like residue.
Expression of CdtB produces a large-budded terminal phenotype accompanied by a G2 arrest.
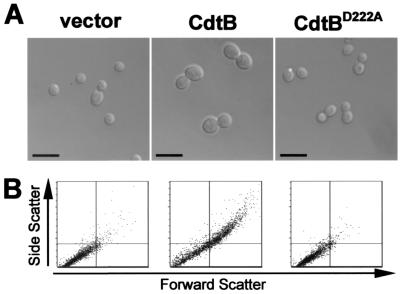
Changes in cellular morphology often reveal the general nature of cell cycle defects in yeast. For example, accumulations of unbudded cells are largely indicative of a G1 arrest, while accumulations of large-budded cells suggest an accumulation of G2 or M-phase cells (29). We determined whether expression of individual CDT subunits could induce morphological changes in yeast. Exponentially growing yeast cells encoding GAL1-controlled CdtA, CdtB, CdtC, or CdtBD222A were shifted into galactose-containing medium to induce gene expression. Cells were then sampled from growing liquid cultures at 0, 5, 7, and 10 h postinduction. The CdtB-expressing strain predominantly exhibited a marked increase in the size of both the mother cell and bud that was absent with expression of CdtBD222A (Fig. 2A). While the majority (∼75%) of CdtB-expressing cells were large-budded, other relatively infrequent aberrant phenotypes included cells with elongated buds or large unbudded cells. Expression of CdtA or CdtC did not produce changes in morphology relative to vector control cells (not shown). The increase in size of the CdtB-expressing strains was confirmed by flow cytometric analysis (Fig. 2B). CdtB expression also caused a profound decrease in cell proliferation rates. While control cells doubled in number every 150 min (following an initial growth lag of approximately 200 min upon shift into galactose medium), cells expressing CdtB demonstrated only a single doubling in a 24-h period.
FIG. 2.
Abnormal budding morphology arising from CdtB expression. (A) Left to right, S. cerevisiae EY957 vector control (vector) compared to CdtB-expressing S. cerevisiae DCH6042 (CdtB), and CdtBD222A-expressing S. cerevisiae DCH6044 (CdtBD222A). Cells are shown under equivalent ×48 magnification after 10 h of growth on galactose. (B) Flow cytometric analysis of yeast cells grown for 10 h in galactose as in panel A. The horizontal and vertical axes indicate forward scatter and side scatter, respectively. Normal populations are primarily represented in the lower left quadrant of each plot. Vector control cells (left) are compared to CdtB-expressing cells (center) and CdtBD222A-expressing cells (right). Equal numbers of events are represented in each dot plot.
Expression of CdtB results in an accumulation of G2-arrested cells.
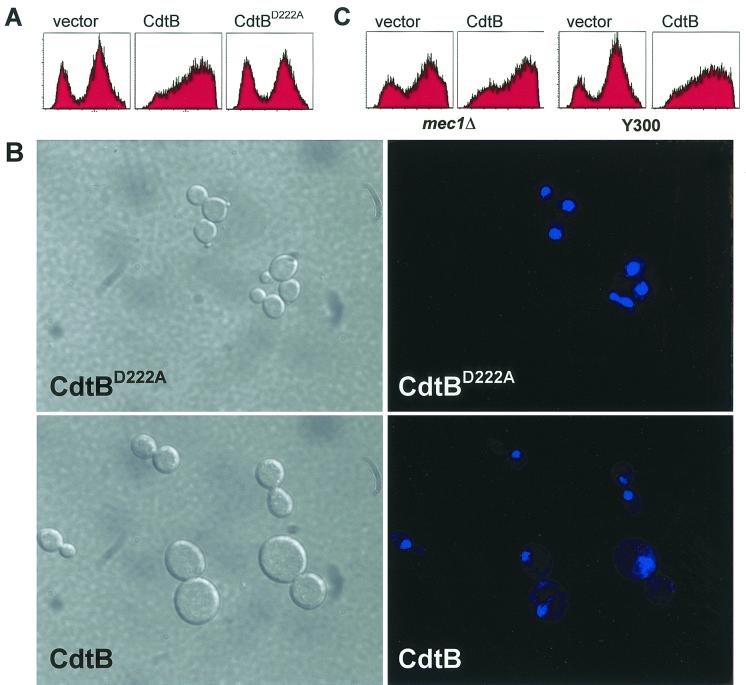
More detailed flow cytometric analysis was conducted to determine the DNA content of cells expressing a CDT subunit relative to vector control. Yeast cells were sampled at 0, 5, 7, 10, and 24 h postinduction to establish whether subunit-expressing yeast demonstrated an aberrant cell cycle distribution. At 7 h postinduction, we observed CdtB-expressing cells noticeably accumulating in G2 phase, while CdtBD222A-expressing yeast did not. At 10 h postinduction, this effect was even more prominent. The distribution of DNA content in cells expressing CdtA or CdtC (not shown) was similar to that of vector control cells at 10 h (Fig. 3A), suggesting that neither CdtA nor CdtC was able to affect cell cycle progression. These findings suggested that cells were arresting primarily at G2/M, as was seen previously in mammalian cells intoxicated with CDT (8, 10, 35, 42, 44, 46, 50, 52), and that this activity could be attributed directly to CdtB in an Asp-222-dependent fashion. This result provided an initial validation of the relevance of our yeast model to CDT-related disease, and we pursued it further.
FIG. 3.
CdtB causes yeast cells to accumulate with G2 DNA content. (A) DNA content of cells grown in galactose for 10 h. Vector control strain (vector, left), CdtB-expressing strain (CdtB; middle), and CdtBD222A-expressing strain DCH6044 (CdtBD222A). The first peak of each histogram indicates cells with G1 DNA content, while the second peak indicates G2/M DNA content. CdtB-expressing strains exhibit predominantly a G2/M peak. (B) Localization and morphology of yeast and their nuclei at ×120 magnification in CdtB-expressing yeast (CdtB; bottom) and CdtBD222A-expressing yeast (CdtBD222A; top). Nomarski images (left) are shown alongside the same cells stained with Hoechst 33342 (right). (C) DNA content of mec1 mutants expressing CdtB (CdtB; second panel) and vector control (vector; first panel) after 10 h of growth on galactose. The syngeneic Mec1-proficient strain, Y300, is shown for comparison expressing CdtB (CdtB; fourth panel) and vector control (vector; third panel).
The nuclear morphology of the arrested cells was examined by Hoechst 33342 staining (Fig. 3B). As expected for a G2/M arrest, the moderately sized cells possessing large buds accumulated with a single nucleus located at the mother-daughter neck. The larger cells, which had presumably sat at the arrest point for an extended period of time, often appeared to have an unequally fragmented nucleus, as if the cells had attempted to segregate chromosomes that were not fully attached to the spindle or that were unable to separate sister chromatids. This phenomenon in which cells bypass a checkpoint after extended arrest and which results in misegregation of chromosomal DNA has been observed previously (40, 48).
CdtB does not act directly at the level of Cdk1 in yeast.
In animal cells, DNA damage results in the inhibitory phosphorylation of Cdk1 on Tyr-15 through the control of a network of protein kinases and phosphatases. Despite the existence of a similar network of regulators controlling Cdk1 phosphorylation in S. cerevisiae, however, DNA damage does not result in inhibitory Cdk1 phosphorylation. These regulators are instead ultimately responsive to disruptions leading to loss of cell polarity, and the DNA damage cell cycle arrest response is accomplished by alternative means (11, 29, 32, 33, 49).
We took advantage of this divergence to determine whether CdtB acted on the pathway near Cdk1, possibly as a protein phosphatase, by expressing CdtB in yeast in which wild-type Cdk1 was replaced with the Cdk1-AF allele, which lacks both sites needed for inhibitory phosphorylation. If phosphorylation of Cdk1 were critical for CDT toxicity, this mutant would not become intoxicated when CdtB is expressed. If it acted through the DNA damage pathway, we expected this mutant to remain sensitive to CdtB. We found that expression of CdtB resulted in toxicity when expressed in yeast containing the Cdk1-AF allele as well as a syngenic control strain. This indicated that CdtB did not act upon Cdk1 or upon the proximal regulators in the pathway leading to Cdk1 inhibitory phosphorylation.
mec1 mutants of S. cerevisiae are susceptible to intoxication with CdtB.
The Mec1 protein of S. cerevisiae is required for both the S-phase and G2 checkpoints responsive to DNA damage and unreplicated DNA (49). Mec1 is a homologue of the mammalian ATM protein, which performs a similar function in mammalian cells (33). The G2/M accumulation of cells associated with CDT intoxication is abolished by caffeine (9, 42), an ATM inhibitor (4), and results from an ATM-dependent checkpoint (9). To determine whether the yeast arrest resulted from the activation of the corresponding checkpoint, CdtB was expressed in both a mec1 null strain and its syngenic counterpart. We then analyzed these CdtB-expressing strains as before alongside vector controls. If CdtB were causing physical damage to S. cerevisiae, we expected that the mec1 null yeast would still lose viability after expressing CdtB, but would not arrest at the G2 checkpoint. Alternatively, if CdtB were merely causing the perception of damage by manipulating the DNA damage sensory machinery at the level of Mec1 or upstream, the CdtB-expressing yeast should survive. All CdtB-expressing strains, including the mec1 strain, failed to grow in galactose-containing media, suggesting physical damage and not manipulation of proteins involved in the checkpoint. Flow cytometric analysis of cellular DNA content indicated that cells continued to arrest with G2/M DNA content despite the absence of the G2 checkpoint (Fig. 3C). It is possible that the G2 arrest in the mec1 background is due to the activation of an independent checkpoint, perhaps that involving spindle attachment to the kinetochores—a possibility currently undergoing investigation.
RNR2 upregulation is associated with CdtB expression.
In light of the Asp-222-dependent toxicity of CdtB, we revisited the possibility of a CdtB-associated DNA damage response by examining RNR2 transcription. Upregulation of RNR2 occurs when yeast cells are exposed to DNA-damaging agents such as uv radiation or hydrogen peroxide (3, 14–16). Specific reporter systems have been described in yeast to identify genotoxins based on this upregulation (1, 2). We used RT-PCR to evaluate RNR2 expression semiquantitatively in yeast expressing either CdtB or CdtBD222A alongside positive and negative controls. CdtB expression resulted in an Asp-222-dependent increase in RNR2 transcription, which was also observed with hydrogen peroxide treatment, but absent in the vector control (Fig. 4). The RNR2 band is evident as early as 21 cycles of PCR in both CdtB-expressing cells and hydrogen peroxide-treated cells. Transcription of another gene unrelated to the DNA damage checkpoint, GPD1, was similar in both vector control yeast and CdtBD222A-expressing yeast. GPD1 transcription was notably reduced by treatment with hydrogen peroxide and modestly reduced during expression of CdtB—possibly the result of nonspecific toxicity or stress associated with these treatments. The augmented RNR2 transcription suggested that CdtB possesses a genotoxic activity, consistent with its putative role as a DNase.
FIG. 4.
Heightened expression of RNR2 in CdtB-expressing yeast and hydrogen peroxide-treated yeast relative to CdtBD222A and vector controls. (A) The bar chart compares net intensities of bands shown on the agarose gel and is representative of three experiments (B) at 21, 25, 28, and 32 PCR cycles. CdtB-expressing yeast cells are represented by solid bars (CdtB), CdtBD222A-expressing yeast cells are represented by hatched bars (D222A), vector control cells are represented by dotted bars (vector), and hydrogen peroxide-treated yeast cells are represented by open bars (H2O2). (B) The agarose gel shows bands arising from RT-PCR of RNA samples using either GPD1-specific or RNR2-specific primers. Intensity of RNR2 bands increases relative to controls in both CdtB-expressing and H2O2-treated yeast, while the intensity of GPD1 bands in similar under all conditions except H2O2 treatment. CdtB-expressing yeast (CdtB), CdtBD222A-expressing yeast (D222A), vector control yeast (vector), and peroxide-treated yeast (H2O2).
CdtB, but not CdtBD222A, causes degradation of chromosomal DNA in vivo.
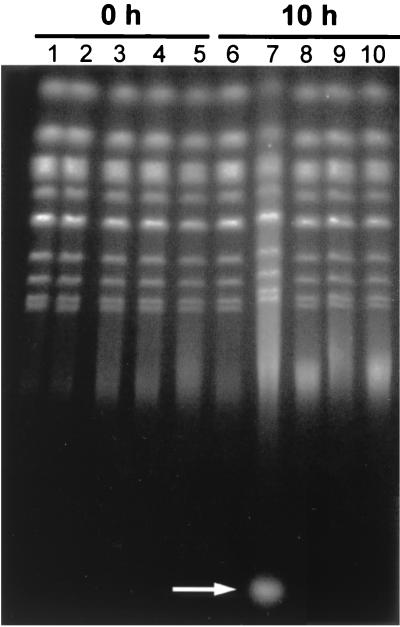
These results encouraged us to look at the integrity of yeast chromosomes by using PFGE to ascertain whether potential DNA damage resulting from CdtB expression could be directly observed. Chromosomes isolated from yeast expressing CdtB were compared to chromosomes from yeast expressing CdtA, CdtC, CdtBD222A, or the vector control at 0, 6, or 10 h postinduction. We found that chromosomes from all yeast obtained at time 0 were entirely intact relative to the vector control (Fig. 5). Initial evidence of CdtB-associated chromosome degradation was apparent by 6 h postinduction (not shown). However, by 10 h postinduction, CdtB-associated degradation was very apparent and consisted of a smear of high-molecular-weight DNA (Fig. 5, lane 7). CdtB expression was also accompanied by production of a relatively low-molecular-weight RNA fragment (Fig. 5, lane 7, white arrow) as determined by sensitivity to RNase A, presumably arising from continued biosynthesis in the absence of cell division. We are further characterizing this observation. In contrast, yeast expressing CdtA, CdtC, or CdtBD222A did not undergo detectable damage (Fig. 5, lanes 6 and 8 to 10). Thus, the degradation was only associated with CdtB and depended absolutely on the DNase-related residue, Asp-222.
FIG. 5.
Chromosome degradation is dependent on a DNase-related residue of CdtB. Yeast chromosomes, ranging in size from 220 to 1,600 kb, are resolved by PFGE. Chromosomes isolated at time 0 (lanes 1 to 5) are compared to chromosomes isolated after 10 h of growth in galactose-containing medium (lanes 6 to 10). The CdtA-expressing strain (DCH6041; lanes 1 and 6), CdtB-expressing strain (DCH6042; lanes 2 and 7), CdtC-expressing strain (DCH6043; lanes 3 and 8), CdtBD222A-expressing strain (DCH6044; lanes 4 and 9), and the vector control strain (DCH6045; lanes 5 and 10) are compared. The white arrow indicates the position of an RNA product associated with expression of CdtB.
DISCUSSION
We have shown here that a yeast model system can be used to analyze the action of the C. jejuni Cdt subunits. The action of CDT on mammalian cells has been shown to cause G2 arrest, likely due to invoking of the DNA damage-incomplete replication checkpoints. Expression of CdtB alone was sufficient to cause a G2 block in S. cerevisiae, and use of S. cerevisiae mutants allowed us to clearly show that CdtB does not directly act on cell cycle machinery, but instead appears to involve direct damage and subsequent activation of the checkpoint pathway. In addition to use of the mutants for examining possible invocation of the checkpoint pathway, we showed that activation of RNR2, a gene known to be upregulated in response to direct DNA damage, occurred as early as 4 h after induction of CdtB expression. At 4 h, this observation occurred several hours before loss of chromosomal integrity could be detected by PFGE and before cells accumulated in G2/M. There is current evidence supporting this hypothesis: an unspecified amount of purified CdtB demonstrates DNase activity (17), albeit weak (28), in vitro, and when CdtB is microinjected into mammalian cells, these cells become distended at low doses, while chromatin collapse visualized by nuclear staining is observed at relatively higher doses (28). The results reported here add further support to the idea that CDT is indeed a nuclease and to the finding that CDT invokes the DNA damage checkpoint reported previously by Cortes-Bratti et al. (9) and Sert et al. (43). Finally, since yeast have relatively small and separable chromosomes compared to mammalian cells, we were able to use PFGE to analyze whether CdtB caused noticeable DNA damage in our system. This is the first time this type of analysis has been done with CDT-affected cells and provides visual confirmation of the action of CdtB in vivo in a way not previously demonstrated. Thus, this study indicates the likelihood that CdtB does indeed have DNase I-like activity.
However, it must be pointed out that under some circumstances, yeasts appear to undergo an apoptotic-like response (30). Therefore, it is important to consider the possibility that the DNA degradation we observed might be the downstream result of such a response induced by CdtB. Since the apoptotic response has been a complicating factor in determining the CdtB mechanism in mammalian cells (28), we have attempted to resolve this issue by determining whether the production of reactive oxygen species (ROS) occurs in response to CdtB, an effect that is independent of nuclear events, but common to both the mammalian and yeast apoptotic-like responses. We found that CdtB-associated DNA damage in yeast occurs in the absence of ROS production, suggesting that the yeast apoptotic-like response is not responsible for the DNA damage associated with CdtB expression (Pickett, unpublished). We are continuing to investigate this possibility further in both our yeast model and in mammalian cells. However, in light of the in vitro DNase activity of CdtB (17), we consider it likely that the chromosome degradation that we are observing in vivo is a direct result of the action of CdtB—demonstrating that CDTs induce cell death as a novel class of bacterial genotoxins.
Our findings contradict a report by Sert et al. (42) in which it was suggested that cells intoxicated with CDT did not undergo DNA damage. Possibly, the extent of DNA damage associated with closer-to-physiological CDT doses is not detectable in the assays used by those authors. In our study, amounts of CdtB per cell (and per unit genome size) are likely to be considerably higher than during intoxication of mammalian cells with the CDT holotoxin. Consequently, the extent of DNA degradation is likely enhanced in our system, making the biological role of CdtB more readily apparent in vivo. This would appear to be another advantage of using this novel yeast model system for the study of a bacterial protein that brings about a cell cycle block apparently through very modest DNA damage. We consider it possible that CdtB preferentially targets a particular DNA site or structure, the damage to which cannot be detected by standard assays at lower doses of CdtB.
The ability of CdtB to recapitulate in yeast the major effects observed with CDT-treated mammalian cells (38) suggests that yeast can serve as an excellent model organism that can be exploited to further understand how CDTs cause disease in vivo. Moreover, the CdtB-expressing yeast developed in this work have potential application as a drug screening system in which CDT-inhibiting compounds are identified by selection for yeast that can grow during expression of CdtB.
ACKNOWLEDGMENTS
We thank Daniel L. Cottle for excellent technical assistance, Gregory Bauman and Jennifer Strange for fluorescence-activated cell sorter assistance, and Mensur Dlakić for sharing data prepublication. We also thank Monica L. Guzman and Craig T. Jordan for assistance and/or useful discussions.
This work was supported in part by NIH grant AI41477 to C.L.P.
REFERENCES
- 1.Afanassiev V, Sefton M, Anantachaiyong T, Barker G, Walmsley R, Wolfl S. Application of yeast cells transformed with GFP expression constructs containing the RAD54 or RNR2 promoter as a test for the genotoxic potential of chemical substances. Mutat Res. 2000;464:297–308. doi: 10.1016/s1383-5718(99)00209-0. [DOI] [PubMed] [Google Scholar]
- 2.Averbeck D, Averbeck S. DNA photodamage, repair, gene induction and genotoxicity following exposures to 254 nm UV and 8-methoxypsoralen plus UVA in a eukaryotic cell system. Photochem Photobiol. 1998;68:289–295. [PubMed] [Google Scholar]
- 3.Averbeck D, Averbeck S. Induction of the genes RAD54 and RNR2 by various DNA damaging agents in Saccharomyces cerevisiae. Mutat Res. 1994;315:123–138. doi: 10.1016/0921-8777(94)90013-2. [DOI] [PubMed] [Google Scholar]
- 4.Blasina A, Price B D, Turenne G A, McGowan C H. Caffeine inhibits the checkpoints kinase ATM. Curr Biol. 1999;9:1135–1138. doi: 10.1016/s0960-9822(99)80486-2. [DOI] [PubMed] [Google Scholar]
- 5.Chien C C, Taylor N S, Ge Z, Schauer D B, Young V B, Fox J G. Identification of cdtB homologues and cytolethal distending toxin activity in enterohepatic Helicobacter spp. J Med Microbiol. 2000;49:525–534. doi: 10.1099/0022-1317-49-6-525. [DOI] [PubMed] [Google Scholar]
- 6.Comayras C, Tasca C, Pérès S Y, Ducommun B, Oswald E, De Rycke J. Escherichia coli cytolethal distending toxin blocks the HeLa cell cycle at the G2/M transition by preventing cdc2 protein kinase dephosphorylation and activation. Infect Immun. 1997;65:5088–5095. doi: 10.1128/iai.65.12.5088-5095.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cope L D, Lumbley S, Latimer J L, Klesney-Tait J, Stevens M K, Johnson L S, Purven M, Munson R S, Jr, Lagergard T, Radolf J D, Hansen E J. A diffusible cytotoxin of Haemophilus ducreyi. Proc Natl Acad Sci USA. 1997;94:4056–4061. doi: 10.1073/pnas.94.8.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cortes-Bratti X, Chaves-Olarte E, Lagergard T, Thelestam M. The cytolethal distending toxin from the chancroid bacterium Haemophilus ducreyi induces cell-cycle arrest in the G2 phase. J Clin Investig. 1999;103:107–115. doi: 10.1172/JCI3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cortes-Bratti X, Karlsson C, Lagergard T, Thelestam M, Frisan T. The Haemophilus ducreyi cytolethal distending toxin induces cell cycle arrest and apoptosis via the DNA damage checkpoint pathways. J Biol Chem. 2001;276:5296–5302. doi: 10.1074/jbc.M008527200. [DOI] [PubMed] [Google Scholar]
- 10.De Rycke J, Sert V, Comayras C, Tasca C. Sequence of lethal events in HeLa cells exposed to the G2 blocking cytolethal distending toxin. Eur J Cell Biol. 2000;79:192–201. doi: 10.1078/S0171-9335(04)70022-9. [DOI] [PubMed] [Google Scholar]
- 11.Desany B A, Alcasabas A A, Bachant J B, Elledge S J. Recovery from DNA replicational stress is the essential function of the S-phase checkpoint pathway. Genes Dev. 1998;12:2956–2970. doi: 10.1101/gad.12.18.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dlakić M. Functionally unrelated signalling proteins contain a fold similar to Mg2+-dependent endonucleases. Trends Biochem Sci. 2000;25:272–273. doi: 10.1016/s0968-0004(00)01582-6. [DOI] [PubMed] [Google Scholar]
- 13.Elion E A, Satterberg B, Kranz J E. FUS3 phosphorylates multiple components of the mating signal transduction cascade: evidence for STE12 and FAR1. Mol Biol Cell. 1993;4:495–510. doi: 10.1091/mbc.4.5.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elledge S J, Davis R W. Identification and isolation of the gene encoding the small subunit of ribonucleotide reductase from Saccharomyces cerevisiae: DNA damage-inducible gene required for mitotic viability. Mol Cell Biol. 1987;7:2783–2793. doi: 10.1128/mcb.7.8.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elledge S J, Davis R W. Identification of the DNA damage-responsive element of RNR2 and evidence that four distinct cellular factors bind it. Mol Cell Biol. 1989;9:5373–5386. doi: 10.1128/mcb.9.12.5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elledge S J, Zhou Z, Allen J B, Navas T A. DNA damage and cell cycle regulation of ribonucleotide reductase. Bioessays. 1993;15:333–339. doi: 10.1002/bies.950150507. [DOI] [PubMed] [Google Scholar]
- 17.Elwell C A, Dreyfus L A. DNase I homologous residues in CdtB are critical for cytolethal distending toxin-mediated cell cycle arrest. Mol Microbiol. 2000;37:952–963. doi: 10.1046/j.1365-2958.2000.02070.x. [DOI] [PubMed] [Google Scholar]
- 18.Eyigor A, Dawson K A, Langlois B E, Pickett C L. Cytolethal distending toxin genes in Campylobacter jejuni and Campylobacter coli isolates: detection and analysis by PCR. J Clin Microbiol. 1999;37:1646–1650. doi: 10.1128/jcm.37.5.1646-1650.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flick J S, Johnston M. Two systems of glucose repression of the GAL1 promoter in Saccharomyces cerevisiae. Mol Cell Biol. 1990;10:4757–4769. doi: 10.1128/mcb.10.9.4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gelfanova V, Hansen E J, Spinola S M. Cytolethal distending toxin of Haemophilus ducreyi induces apoptotic death of Jurkat T cells. Infect Immun. 1999;67:6394–6402. doi: 10.1128/iai.67.12.6394-6402.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gerring S L, Connelly C, Hieter P. Positional mapping of genes by chromosome blotting and chromosome fragmentation. Methods Enzymol. 1991;194:57–77. doi: 10.1016/0076-6879(91)94007-y. [DOI] [PubMed] [Google Scholar]
- 22.Gietz R D, Sugino A. New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene. 1988;74:527–534. doi: 10.1016/0378-1119(88)90185-0. [DOI] [PubMed] [Google Scholar]
- 23.Johnson W M, Lior H. A new heat-labile cytolethal distending toxin (CLDT) produced by Campylobacter spp. Microb Pathog. 1988;4:115–126. doi: 10.1016/0882-4010(88)90053-8. [DOI] [PubMed] [Google Scholar]
- 24.Johnson W M, Lior H. A new heat-labile cytolethal distending toxin (CLDT) produced by Escherichia coli isolates from clinical material. Microb Pathog. 1988;4:103–113. doi: 10.1016/0882-4010(88)90052-6. [DOI] [PubMed] [Google Scholar]
- 25.Johnston M, Davis R W. Sequences that regulate the divergent GAL1-GAL10 promoter in Saccharomyces cerevisiae. Mol Cell Biol. 1984;4:1440–1448. doi: 10.1128/mcb.4.8.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaldor J, Speed B R. Guillain-Barre syndrome and Campylobacter jejuni: a serological study. Br Med J. 1984;288:1867–1870. doi: 10.1136/bmj.288.6434.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kozak M. Possible role of flanking nucleotides in recognition of the AUG initiator codon by eukaryotic ribosomes. Nucleic Acids Res. 1981;9:5233–5262. doi: 10.1093/nar/9.20.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lara-Tejero M, Galan J E. A bacterial toxin that controls cell cycle progression as a deoxyribonuclease I-like protein. Science. 2000;290:354–357. doi: 10.1126/science.290.5490.354. [DOI] [PubMed] [Google Scholar]
- 29.Lew D J, Reed S I. Cell cycle control of morphogenesis in budding yeast. Curr Opin Genet Dev. 1995;5:17–23. doi: 10.1016/s0959-437x(95)90048-9. [DOI] [PubMed] [Google Scholar]
- 30.Madeo F, Frohlich E, Frohlich K U. A yeast mutant showing diagnostic markers of early and late apoptosis. J Cell Biol. 1997;139:729–734. doi: 10.1083/jcb.139.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mayer M P, Bueno L C, Hansen E J, DiRienzo J M. Identification of a cytolethal distending toxin gene locus and features of a virulence-associated region in Actinobacillus actinomycetemcomitans. Infect Immun. 1999;67:1227–1237. doi: 10.1128/iai.67.3.1227-1237.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Minshull J, Straight A, Rudner A D, Dernburg A F, Belmont A, Murray A W. Protein phosphatase 2A regulates MPF activity and sister chromatid cohesion in budding yeast. Curr Biol. 1996;6:1609–1620. doi: 10.1016/s0960-9822(02)70784-7. [DOI] [PubMed] [Google Scholar]
- 33.Morrow D M, Tagle D A, Shiloh Y, Collins F S, Hieter P. TEL1, an S. cerevisiae homolog of the human gene mutated in ataxia telangiectasia, is functionally related to the yeast checkpoint gene MEC1. Cell. 1995;82:831–840. doi: 10.1016/0092-8674(95)90480-8. [DOI] [PubMed] [Google Scholar]
- 34.Okuda J, Kurazono H, Takeda Y. Distribution of the cytolethal distending toxin A gene (cdtA) among species of Shigella and Vibrio, and cloning and sequencing of the cdt gene from Shigella dysenteriae. Microb Pathog. 1995;18:167–172. doi: 10.1016/s0882-4010(95)90022-5. [DOI] [PubMed] [Google Scholar]
- 35.Peres S Y, Marches O, Daigle F, Nougayrede J P, Herault F, Tasca C, De Rycke J, Oswald E. A new cytolethal distending toxin (CDT) from Escherichia coli producing CNF2 blocks HeLa cell division in G2/M phase. Mol Microbiol. 1997;24:1095–1107. doi: 10.1046/j.1365-2958.1997.4181785.x. [DOI] [PubMed] [Google Scholar]
- 36.Pickett C L, Cottle D L, Pesci E C, Bikah G. Cloning, sequencing, and expression of the Escherichia coli cytolethal distending toxin genes. Infect Immun. 1994;62:1046–1051. doi: 10.1128/iai.62.3.1046-1051.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pickett C L, Pesci E C, Cottle D L, Russell G, Erdem A N, Zeytin H. Prevalence of cytolethal distending toxin production in Campylobacter jejuni and relatedness of Campylobacter sp. cdtB genes. Infect Immun. 1996;64:2070–2078. doi: 10.1128/iai.64.6.2070-2078.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pickett C L, Whitehouse C A. The cytolethal distending toxin family. Trends Microbiol. 1999;7:292–297. doi: 10.1016/s0966-842x(99)01537-1. [DOI] [PubMed] [Google Scholar]
- 39.Robinson J P, editor. Current protocols in cytometry. New York, N.Y: John Wiley & Sons, Inc.; 1999. [Google Scholar]
- 40.Sandell L L, Zakian V A. Loss of a yeast telomere: arrest, recovery, and chromosome loss. Cell. 1993;75:729–739. doi: 10.1016/0092-8674(93)90493-a. [DOI] [PubMed] [Google Scholar]
- 41.Scott D A, Kaper J B. Cloning and sequencing of the genes encoding Escherichia coli cytolethal distending toxin. Infect Immun. 1994;62:244–251. doi: 10.1128/iai.62.1.244-251.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sert V, Cans C, Tasca C, Bret-Bennis L, Oswald E, Ducommun B, De Rycke J. The bacterial cytolethal distending toxin (CDT) triggers a G2 cell cycle checkpoint in mammalian cells without preliminary induction of DNA strand breaks. Oncogene. 1999;18:6296–6304. doi: 10.1038/sj.onc.1203007. [DOI] [PubMed] [Google Scholar]
- 43.Shenker B J, Hoffmaster R H, McKay T L, Demuth D R. Expression of the cytolethal distending toxin (Cdt) operon in Actinobacillus actinomycetemcomitans: evidence that the CdtB protein is responsible for G2 arrest of the cell cycle in human T cells. J Immunol. 2000;165:2612–2618. doi: 10.4049/jimmunol.165.5.2612. [DOI] [PubMed] [Google Scholar]
- 44.Shenker B J, McKay T, Datar S, Miller M, Chowhan R, Demuth D. Actinobacillus actinomycetemcomitans immunosuppressive protein is a member of the family of cytolethal distending toxins capable of causing a G2 arrest in human T cells. J Immunol. 1999;162:4773–4780. [PubMed] [Google Scholar]
- 45.Sia R A, Herald H A, Lew D J. Cdc28 tyrosine phosphorylation and the morphogenesis checkpoint in budding yeast. Mol Biol Cell. 1996;7:1657–1666. doi: 10.1091/mbc.7.11.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sugai M, Kawamoto T, Pérès S Y, Ueno Y, Komatsuzawa H, Fujiwara T, Kurihara H, Suginaka H, Oswald E. The cell cycle-specific growth-inhibitory factor produced by Actinobacillus actinomycetemcomitans is a cytolethal distending toxin. Infect Immun. 1998;66:5008–5019. doi: 10.1128/iai.66.10.5008-5019.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tauxe R V. Epidemiology of Campylobacter jejuni infections in the United States and other industrialized nations. In: Nachamkin I, Blaser M J, Tompkins L S, editors. Campylobacter jejuni: current status and future trends. Washington, D.C.: American Society for Microbiology; 1992. pp. 9–19. [Google Scholar]
- 48.Toczyski D P, Galgoczy D J, Hartwell L H. CDC5 and CKII control adaptation to the yeast DNA damage checkpoint. Cell. 1997;90:1097–1106. doi: 10.1016/s0092-8674(00)80375-x. [DOI] [PubMed] [Google Scholar]
- 49.Weinert T A. Dual cell cycle checkpoints sensitive to chromosome replication and DNA damage in the budding yeast Saccharomyces cerevisiae. Radiat Res. 1992;132:141–143. [PubMed] [Google Scholar]
- 50.Whitehouse C A, Balbo P B, Pesci E C, Cottle D L, Mirabito P M, Pickett C L. Campylobacter jejuni cytolethal distending toxin causes a G2-phase cell cycle block. Infect Immun. 1998;66:1934–1940. doi: 10.1128/iai.66.5.1934-1940.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Young V B, Chien C C, Knox K A, Taylor N S, Schauer D B, Fox J G. Cytolethal distending toxin in avian and human isolates of Helicobacter pullorum. J Infect Dis. 2000;182:620–623. doi: 10.1086/315705. [DOI] [PubMed] [Google Scholar]
- 52.Young V B, Knox K A, Schauer D B. Cytolethal distending toxin sequence and activity in the enterohepatic pathogen Helicobacter hepaticus. Infect Immun. 2000;68:184–191. doi: 10.1128/iai.68.1.184-191.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]