Abstract
Background:
Thrombosis and inflammation are crucial elements in the pathogenesis of cardiovascular disease. Hematological parameters elucidate information involving the inflammatory and blood coagulation processes.
Objectives:
The current study explored the association of hematological parameters with EOCAD to identify specific risk factors.
Design:
A single-center retrospective case–control study was conducted with 1693 coronary artery disease patients and 1693 controls.
Methods:
Hematological parameters were examined through an automated analyzer.
Results:
The basophil percentage was significantly reduced in EOCAD (0.43 ± 0.26, p < 0.001) and MI (0.33 ± 0.24, p < 0.001) groups compared with controls (0.54 ± 0.28). The eosinophil percentage was also significantly lower in EOCAD (2.21 ± 1.71, p < 0.001) and MI (1.71 ± 2.44, p < 0.001) groups compared with controls (2.41 ± 1.75). The lymphocyte percentage in patients of EOCAD and MI and controls was 31.65 ± 7.93, 25.48 ± 9.43, and 34.82 ± 7.28, respectively. A significant difference was observed among the groups (p < 0.001). Except for the mean corpuscular hemoglobin (MCH), other red blood cell (RBC) parameters significantly differed between EOCAD patients and controls. The red blood cell distribution width (RDW), hematocrit (HCT), RBC count, mean corpuscular hemoglobin concentration (MCHC), mean corpuscular volume (MCV), and hemoglobin level were associated with EOCAD prevalence after adjusting for baseline differences. Platelet volume distribution width (PDW) also correlated with EOCAD prevalence (ORadjust = 1.087, 95% CI: 1.044–1.131).
Conclusions:
Hematological parameters are closely associated with EOCAD. Moreover, leukocyte parameters correlated with the presence and severity of the disease. In addition, erythrocyte parameters were associated with the disease presence but not with the disease severity. Among the platelet parameters, only PDW was related to the disease presence.
Keywords: cardiovascular diseases, early-onset coronary artery disease, hematological parameters
Introduction
The health status of human societies has been historically associated with their social and economic development. With industrialization, cardiovascular disease (CVD) prevalence seriously compromises the health of populations worldwide. Screening and identifying risk factors are essential to prevent and control CVD.
Generally, routine blood tests help in the early diagnosis of diseases, thereby facilitating the physicians with information regarding inflammatory processes.1 Hematological parameters obtained using blood investigations mainly include classifying and quantifying white blood cells (WBCs), red blood cells (RBCs), and platelets. Modern automated equipment comprehensively evaluates the association between coronary atherosclerotic heart disease (CAD) and hematological factors. Recent studies have shown that RBC distribution width (RDW),2,3 mean platelet volume (MPV),4,5 neutrophil-to-lymphocyte ratio (NLR),6,7 and other parameters are associated with the severity and prognosis of CAD.8,9 Otherwise, CAD is characterized with a high inflammatory burden. Recent studies suggested that MPV was associated with inflammatory conditions, such as type 2 diabetes mellitus (DM), diabetic nephropathy, infections, nasal polyps, vertebral disc opathies, irritable bowel disease, rheumatoid arthritis, obesity, and liver fibrosis.10–18 Similarly, increased RDW has been reported in degenerative vertebral conditions, rheumatoid arthritis, functional bowel conditions, autoimmune diseases, malignancy, autoimmune hepatitis, and even in Covid-19 infections.14–16,19–21 Furthermore, several inflammatory diseases, including thyroiditis, irritable bowel disease, and Covid-19 infection, were reported to be related with increased NLR levels.22–24 All these conditions are associated with high inflammatory burden like cardiac conditions.
Modern lifestyle elevates the percentage of the younger population suffering from CAD. Early-onset coronary artery disease (EOCAD) is defined as CAD occurring among patients aged 50 years or younger. Familial hypercholesterolemia is a significant pathogenesis of EOCAD.25,26 There are differences involving the etiology and risk factors between EOCAD and late-onset CAD.27–29 Although many studies have depicted the relationship between hematological parameters and CAD, only a few studies have focused on EOCAD. Therefore, the current study explored the association of hematological parameters with EOCAD to identify specific risk factors. These findings would help understand the pathophysiological process of EOCAD with special significance toward disease risk assessment and prediction.
Materials and methods
Subjects
A single-center retrospective case–control study was conducted, recruiting EOCAD patients attending the cardiology clinic at the Affiliated Hospital of Qingdao University between January 2013 and December 2021. The 1683 recruited EOCAD patients were aged ⩽ 50 years during their first symptom onset and hospitalization for coronary angiography. Patients with a history of blood disease, chronic infection, tumors, hepatobiliary, or kidney diseases were excluded. Moreover, 1683 healthy controls volunteered from the Health Management Centre. The control group was age- and sex-matched with the disease group with similar geographical backgrounds. The control population had no signs or symptoms of cardiovascular events, no history of CAD, and the possibility of myocardial infarction (MI) was ruled out through electrocardiography. The study was conducted in accordance with the ethical standards of the 1975 Declaration of Helsinki. Moreover, the study was approved by the Research Ethics Committee of the Affiliated Hospital of Qingdao University (QYFYWZLL27293), and written informed consent was obtained from all participants.
Coronary angiography
Coronary angiography was performed by the standard Judkins technique. Coronary angiograms were independently evaluated by two senior cardiologists who were blinded to the clinical data. In case of a dispute, a third cardiologist joined and gave the final decision. To reduce intra-observer variability, assessments were completed in the shortest time possible.
Four major coronary artery branches were assessed, which included the left main coronary artery, left circumflex, left anterior descending, and right coronary artery. In addition, a luminal stenosis degree of 50% or more was characterized as a significant lesion. Based on the number of significantly stenosed vessels, patients were categorized into single-, double-, and triple-vessel subgroups.
For each patient, the Gensini score was calculated from the coronary angiogram by assigning a severity score to each coronary stenosis according to the degree of luminal narrowing and its geographic importance of the lesion position in the coronary arterial tree. In summary, the quantitative assessment was performed angiographically according to the degree of narrowing of the coronary artery lumen, which was defined as 1 for 0−25% stenosis, 2 for 25−50% stenosis, 4 for 50−75% stenosis, 8 for 75−90% stenosis, 16 for 90−99% stenosis, and 32 for 99−100% stenosis, multiplying the geographical importance of its location (score from 0.5 to 5). Thus, the Gensini score provides valuable information on the degree of a vascular lesion in terms of the degree of arterial stenosis and the location of the stenosis.
Clinical parameters
All participants underwent a complete physical evaluation. Baseline data included sex, age at onset of CAD symptoms, height, weight, smoking and drinking habits, body mass index (BMI), hypertension, and DM. Systolic and diastolic blood pressure was measured twice at 30 min intervals through an automated oscillometric device. The average value of the two blood pressure readings was used. Hypertension was defined as blood pressure readings ⩾ 140/90 mmHg or was assumed to be present among patients taking anti-hypertensive drugs. Based on the American Diabetes Association, the criteria for DM depended on venous samples and laboratory methods. A fasting plasma glucose level of ⩾ 7.0 mmol/l, a 2-h plasma glucose value within a 75-g oral glucose tolerance test of ⩾ 11.1 mmol/l, or glycated hemoglobin (HbA1c) of ⩾ 6.5%.
Biochemical measurements
An automatic biochemistry analyzer (Hitachi HCP-7600, Hitachi, Japan) was used to determine the levels of serum alanine aminotransferase (ALT), serum creatinine (SCr), fasting blood glucose (FBG), triglyceride (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C).
Hematological parameters
Hematological parameters, such as WBC and their subpopulations, RBC count and associated parameters, platelet count and related factors, and hemoglobin levels, were analyzed with an automated analyzer (Sysmex XN-9000, Kobe, Japan).
Statistical analysis
Statistical analyses were conducted using GraphPad Prism Software version 8 (GraphPad Software Inc., San Diego, CA, USA) and SPSS 16.0 software (SPSS Inc., Chicago, IL, USA). The data are presented as the mean ± standard deviation (SD). The categorical variables are depicted as numbers and percentages. In all cases, the Kolmogorov–Smirnov (K-S) test was applied for a normal distribution. The comparisons of categorical variables were undergone through the chi-square test or the Fisher’s exact test. An independent sample t-test analyzed the mean comparison of the two independent groups. The one-way analysis of variance (ANOVA) determined whether there were any statistically significant differences among the means of three or more unrelated independent groups. Subsequently, it was followed by a post hoc least significant difference (LSD) test for multiple comparisons. For non-normally distributed variables, the groups were compared using the Mann–Whitney U test or Kruskal–Wallis test. Pearson’s correlation coefficient (r) evaluated the strength of a linear relationship between two variables. Logistic regression assessed the association between the hematological parameters and EOCAD, and the results were represented as odds ratios (ORs) with 95% confidence intervals (CIs). A p value less than 0.05 was considered statistically significant. Power calculations were used to determine the number of subjects required with 0.90 power at an alpha level of 0.05. Estimates were calculated using power analysis and PASS software (version 15.0).
Results
Baseline characteristics
A total of 1683 EOCAD patients (mean age 45.16 ± 4.54; 84.73% men) and 1683 controls (mean age 45.16 ± 4.54; 84.73% men) were recruited for this study. No significant differences were observed between EOCAD patients and controls based on sex, age, and SCr. However, FBG, BMI, TG, TC, LDL-C, and ALT activity/levels were significantly increased in EOCAD patients. Furthermore, the EOCAD group had elevated hypertension, DM, smoking, and drinking rates than the controls. Moreover, in the EOCAD patient group, 604 patients were diagnosed with MI. EOCAD patients included 600 patients with single-diseased, 326 double-diseased, and 167 triple-diseased vessels. The clinical characteristics of all participants are summarized in Table 1.
Table 1.
Demographic and clinical characteristics of CAD patients and controls.
| Variable | CAD (n = 1683) |
Control (n = 1683) |
p |
|---|---|---|---|
| Sex, male n (%)* | 1426 (84.73) | 1426 (84.73) | 1.000 |
| Age, years** | 45.16 ± 4.54 | 45.16 ± 4.54 | 1.000 |
| BMI (kg/m2)** | 26.64 ± 3.74 | 24.47 ± 3.25 | <0.001 |
| Hypertension, n (%)* | 779 (46.29) | 227 (13.49) | <0.001 |
| Diabetes, n (%)* | 220 (13.07) | 102 (6.06) | <0.001 |
| Smoking, n (%)* | 903 (53.65) | 458 (27.21) | <0.001 |
| Drinking, n (%)* | 697 (41.41) | 332 (19.72) | <0.001 |
| FBG, mmol/l** | 5.82 ± 2.14 | 5.26 ± 1.37 | <0.001 |
| TG, mmol/l** | 2.12 ± 2.15 | 1.74 ± 1.39 | <0.001 |
| TC, mmol/l** | 4.52 ± 1.22 | 4.30 ± 0.99 | <0.001 |
| HDL-C, mmol/l** | 1.14 ± 0.29 | 1.31 ± 0.30 | <0.001 |
| LDL-C, mmol/l** | 2.65 ± 0.95 | 2.32 ± 0.82 | <0.001 |
| SCr, μmol/l** | 72.71 ± 18.57 | 79.96 ± 12.13 | 0.650 |
| ALT, U/l** | 27.37 ± 11.64 | 25.10 ± 10.63 | <0.001 |
| Medications | – | – | – |
| Statins, n (%) | 210 (12.47) | 72 (4.27) | <0.001 |
| β-blockers, n (%) | 423 (25.13) | 56 (3.32) | <0.001 |
| Ace-inhibitors, n (%) | 135 (8.02) | 32 (1.90) | <0.001 |
| Clopidogrel, n (%) | 72 (4.28) | 2 (0.12) | <0.001 |
| MI, n (%) | 604 (35.89) | – | – |
| Severity of CAD | – | – | – |
| Single-diseased vessels, n (%) | 600 (35.65) | – | – |
| Double-diseased vessels, n (%) | 326 (19.37) | – | – |
| Triple-diseased vessels, n (%) | 167 (9.92) | – | – |
ALT, alanine aminotransferase; BMI, body mass index; CAD, coronary artery disease; FBG, fasting blood glucose; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; SCr: serum creatinine; TC, total cholesterol; TG, triglyceride.
Categorical variables are expressed as percentages. P values of the categorical variables were calculated by chi-square test.
Continuous variables are expressed as the mean ± SD. P values of the continuous variables were calculated using independent sample t-test or Mann–Whitney U test.
Relationship between WBC parameters and EOCAD
As shown in Table 2, the WBC count, neutrophil, and monocyte counts were significantly increased in EOCAD patients than in controls. However, eosinophil, basophil, and lymphocyte counts were significantly reduced compared with those in the control group.
Table 2.
Hematological parameters in CAD patients and controls.
| Variable | CAD (n = 1683) |
Control (n = 1683) |
p |
|---|---|---|---|
| WBC parameters | |||
| WBC count, (109/l) | 7.49 ± 2.46 | 6.25 ± 1.54 | <0.001 |
| Neutrophil count, (109/l) | 4.69 ± 2.18 | 3.52 ± 1.17 | <0.001 |
| Neutrophil percentage, (%) | 55.78 ± 7.77 | 60.89 ± 10.17 | <0.001 |
| Monocyte count, (109/l) | 0.54 ± 0.24 | 0.40 ± 0.13 | <0.001 |
| Monocyte percentage, (%) | 7.26 ± 2.10 | 6.46 ± 1.60 | <0.001 |
| Basophil count, (109/l) | 0.028 ± 0.020 | 0.033 ± 0.018 | <0.001 |
| Basophil percentage, (%) | 0.40 ± 0.26 | 0.54 ± 0.28 | <0.001 |
| Eosinophil count, (109/l) | 0.14 ± 0.17 | 0.15 ± 0.12 | <0.001 |
| Eosinophil percentage, (%) | 2.03 ± 2.02 | 2.41 ± 1.75 | <0.001 |
| Lymphocyte count, (109/l) | 2.09 ± 0.70 | 2.14 ± 0.58 | <0.001 |
| Lymphocyte percentage, (%) | 29.43 ± 9.00 | 34.82 ± 7.28 | <0.001 |
| RBC parameters | |||
| RBC count, (1012/l) | 4.77 ± 0.47 | 5.04 ± 0.43 | <0.001 |
| Hemoglobin, (g/l) | 144.76 ± 15.02 | 152.93 ± 13.89 | <0.001 |
| Hematocrit, (%) | 42.67 ± 4.18 | 45.58 ± 3.73 | <0.001 |
| Mean corpuscular volume, (fl) | 89.69 ± 4.67 | 90.66 ± 4.84 | <0.001 |
| Mean corpuscular hemoglobin, (pg) | 30.42 ± 1.86 | 30.41 ± 1.97 | 0.196 |
| Mean corpuscular hemoglobin concentration, (g/l) | 339.20 ± 11.85 | 335.32 ± 10.85 | <0.001 |
| RDW (SD), (fl) | 41.46 ± 2.43 | 40.79 ± 2.69 | <0.001 |
| RDW (CV), (%) | 12.57 ± 0.84 | 12.74 ± 0.96 | <0.001 |
| PLT parameters | |||
| PLT count, (109/l) | 234.20 ± 62.51 | 239.08 ± 51.47 | <0.001 |
| Plateletcrit, (%) | 0.231 ± 0.058 | 0.235 ± 0.048 | <0.001 |
| MPV, (fl) | 9.96 ± 1.01 | 9.90 ± 1.03 | 0.027 |
| Platelet distribution width, (fl) | 13.25 ± 2.50 | 12.72 ± 2.17 | <0.001 |
| Platelet large cell ratio | 25.43 ± 7.33 | 25.30 ± 7.25 | 0.520 |
CAD, coronary artery disease; CV, coefficient of variation; PLT, platelet; RBC, red blood cell; SD, standard deviation; WBC, white blood cell.
Continuous variables are expressed as the mean ± SD. P values of the continuous variables were calculated using independent sample t-test or Mann–Whitney U test.
WBC count, neutrophil count, and percentage, and monocyte count and percentage reflected the severity of EOCAD, thereby showing a positive correlation. Moreover, decreased basophil, eosinophil, and lymphocyte percentages associated with the severity of EOCAD (Table 3).
Table 3.
Leukocyte parameters according to patient category.
| Variable | CAD (non-MI) (n = 1079) |
MI (n = 604) |
Control (n = 1683) |
p CAD versus control | p MI versus control | p CAD versus MI |
|---|---|---|---|---|---|---|
| WBC count, (109/l) | 6.82 ± 1.86 | 8.69 ± 2.91 | 6.25 ± 1.54 | <0.001 | <0.001 | <0.001 |
| Neutrophil count, (109/l) | 4.05 ± 1.46 | 5.83 ± 2.73 | 3.52 ± 1.17 | <0.001 | <0.001 | <0.001 |
| Neutrophil percentage, (%) | 58.61 ± 8.91 | 64.97 ± 10.98 | 55.78 ± 7.77 | <0.001 | <0.001 | <0.001 |
| Monocyte count, (109/l) | 0.48 ± 0.17 | 0.64 ± 0.30 | 0.40 ± 0.13 | <0.001 | <0.001 | <0.001 |
| Monocyte percentage, (%) | 7.11 ± 1.89 | 7.52 ± 1.41 | 6.46 ± 1.60 | <0.001 | <0.001 | <0.001 |
| Basophil count, (109/l) | 0.029 ± 0.020 | 0.027 ± 0.020 | 0.033 ± 0.018 | <0.001 | <0.001 | 0.060 |
| Basophil percentage, (%) | 0.54 ± 0.28 | 0.33 ± 0.24 | 0.54 ± 0.28 | <0.001 | <0.001 | <0.001 |
| Eosinophil count, (109/l) | 0.14 ± 0.12 | 0.13 ± 0.24 | 0.15 ± 0.12 | 0.338 | <0.001 | <0.001 |
| Eosinophil percentage, (%) | 2.21 ± 1.71 | 1.71 ± 2.44 | 2.41 ± 1.75 | 0.003 | <0.001 | <0.001 |
| Lymphocyte count, (109/l) | 2.11 ± 0.66 | 2.07 ± 0.77 | 2.14 ± 0.58 | 0.082 | 0.003 | 0.482 |
| Lymphocyte percentage, (%) | 31.65 ± 7.93 | 25.48 ± 9.43 | 34.82 ± 7.28 | <0.001 | <0.001 | <0.001 |
CAD, coronary artery disease; MI, myocardial infarction; WBC, white blood cell.
Continuous variables are expressed as the mean ± SD. P values of the continuous variables were calculated using one-way ANOVA.
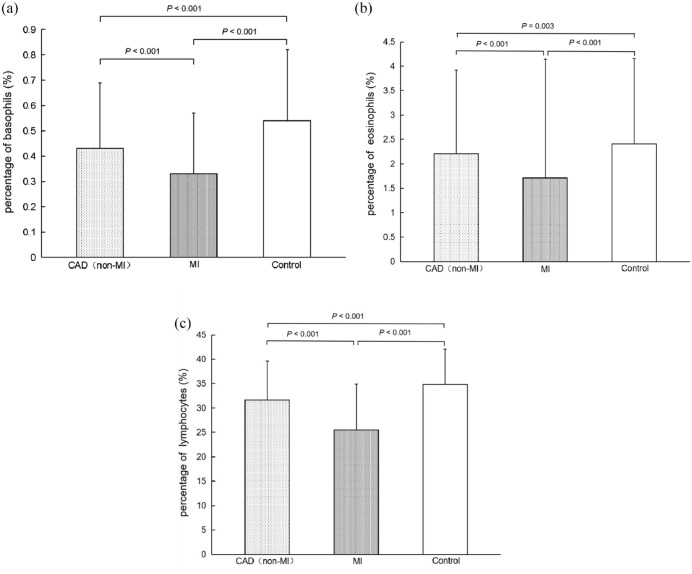
The basophil percentage was significantly reduced in the EOCAD (non-MI) (0.43 ± 0.26, p < 0.001) and MI (0.33 ± 0.24, p < 0.001) groups compared with the control group (0.54 ± 0.28, Figure 1(a)). In addition, a significant negative correlation was observed between basophil percentage and the Gensini scores (r = −0.069, p = 0.005). After adjusting the baseline differences, the percentage of basophils significantly correlated with the presence of EOCAD (ORadjust = 0.142, 95% CI: 0.097–0.208, Table 4), and the cutoff value was 0.37.
Figure 1.
The basophil, eosinophil, and lymphocyte percentage in EOCAD patients and controls. (a) The basophil percentage in patients with MI (0.33 ± 0.24) was significantly lower than those in patients with CAD (without MI) (0.43 ± 0.26, p < 0.001) and controls (0.54 ± 0.28, p < 0.001). The basophil percentage was significantly lower in patients with CAD (without MI) than in healthy controls (< 0.001). (b) The eosinophil percentage was significantly lower in the CAD (non-MI) (2.21 ± 1.71, p = 0.003) and MI (1.71 ± 1.44, p < 0.001) groups than in the controls (2.41 ± 1.75). (c) The lymphocyte percentage in CAD (non-MI) patients, MI patients, and controls was 31.65 ± 7.93, 25.48 ± 9.43, and 34.82 ± 7.28, respectively. There was a significant difference between the groups (p < 0.001).
Table 4.
Associations between WBC parameters and the presence of EOCAD.
| Adjustment models | Basophil percentage | Eosinophil percentage | Lymphocyte percentage | |||
|---|---|---|---|---|---|---|
| OR (95% CI) |
p | OR (95% CI) | p | OR (95% CI) | p | |
| Crude | – | <0.001 | – | <0.001 | – | <0.001 |
| Adjusting for sex, age, BMI, smoking, alcohol consumption, DM. Hypertension, ALT, TC, LDL-C, HDL-C, TG, FBG, and medications. | 0.142 (0.097–0.208) |
<0.001 | 0.876 (0.830–0.925) |
<0.001 | 0.926 (0.918–0.932) |
<0.001 |
ALT, alanine aminotransferase; BMI, body mass index; CI, confidence interval; DM, Diabetes; EOCAD, early-onset coronary artery disease; FBG, fasting blood glucose; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; OR, odds ratios; TC, total cholesterol; TG, triglyceride; WBC, white blood cell.
The eosinophil percentage was also significantly lower in the EOCAD (non-MI) (2.21 ± 1.71, p = 0.003) and MI (1.71 ± 2.44, p < 0.001) groups compared with the control group (2.41 ± 1.75, Figure 1(b)). The eosinophil percentage negatively associated with Gensini score (r = −0.069, p = 0.005). Moreover, the elevated eosinophil percentage was an independent protective factor of EOCAD (ORadjust = 0.876, 95% CI: 0.830–0.925, Table 4), and the cutoff value was 1.47.
The lymphocyte percentage among EOCAD (non-MI) patients, MI patients, and controls was 31.65 ± 7.93, 25.48 ± 9.43, and 34.82 ± 7.28, respectively. A significant difference was observed among the groups (p < 0.001, Figure 1(c) ). The lymphocyte percentage negatively correlated with the Gensini score (r = −0.092, p < 0.001). The lymphocyte percentage significantly associated with EOCAD (ORadjust = 0.926, 95% CI: 0.918–0.932, Table 4) after adjusting for baseline differences. The cutoff value was 30.15. No correlation was observed between the number of diseased vessels and the percentage of eosinophils (p = 0.328), basophils (p = 0.588), and lymphocytes (p = 0.858).
In addition to the monocyte percentage (r = 0.456, p = 0.060) and lymphocyte count (r = −0.021, p = 0.397), WBC count (r = 0.456), neutrophil count (r = 0.498) and percentage (r = 0.412), monocyte count (r = 0.292), basophil count (r = −0.107) and percentage (r = −0.247), eosinophil count (r = −0.115) and percentage (r = −0.198), and lymphocyte percentage (r = −0.400) were significantly correlated with high-sensitive cardiac troponin (hs-cTn) levels (p < 0.001). Moreover, the WBC count (r = 0.383), neutrophil count (r = 0.396) and percentage (r = 0.210), monocyte count (r = 0.337), and lymphocyte percentage (r = −0.229) were significantly correlated with B-type natriuretic peptide (BNP) levels (p < 0.001). WBC count (r = 0.245), neutrophil count (r = 0.270) and percentage (r = 0.238), monocyte count (r = 0.318) and percentage (r = 0.155), basophil count (r = −0.124) and percentage (r = −0.199), eosinophil percentage (r = −0.126), and lymphocyte percentage (r = −0.269) significantly correlated with C-reactive protein (CRP) levels (p < 0.001). The results are presented in Table 5.
Table 5.
Correlation of hematological parameters with hs-cTn, BNP, and CRP.
| Variable | hs-cTn | BNP | CRP | |||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| WBC parameters | ||||||
| WBC count | 0.456 | <0.001 | 0.383 | <0.001 | 0.245 | <0.001 |
| Neutrophil count | 0.498 | <0.001 | 0.396 | <0.001 | 0.270 | <0.001 |
| Neutrophil percentage | 0.412 | <0.001 | 0.210 | <0.001 | 0.238 | <0.001 |
| Monocyte count | 0.292 | <0.001 | 0.337 | <0.001 | 0.318 | <0.001 |
| Monocyte percentage | 0.456 | 0.060 | 0.026 | 0.594 | 0.155 | <0.001 |
| Eosinophil count | −0.115 | <0.001 | −0.040 | 0.410 | −0.072 | 0.052 |
| Eosinophil percentage | −0.198 | <0.001 | −0.063 | 0.202 | −0.126 | <0.001 |
| Basophil count | −0.107 | <0.001 | −0.037 | 0.447 | −0.124 | <0.001 |
| Basophil percentage | −0.247 | <0.001 | −0.097 | 0.047 | −0.199 | <0.001 |
| Lymphocyte count | −0.021 | 0.397 | −0.038 | 0.439 | −0.071 | 0.06 |
| Lymphocyte percentage | −0.400 | <0.001 | −0.229 | <0.001 | −0.269 | <0.001 |
| RBC parameters | ||||||
| RBC count | 0.028 | 0.504 | −0.014 | 0.783 | −0.062 | 0.093 |
| Hemoglobin | 0.052 | 0.221 | −0.028 | 0.574 | −0.061 | 0.101 |
| Hematocrit | 0.057 | 0.180 | −0.019 | 0.702 | −0.078 | 0.034 |
| Mean corpuscular volume | 0.050 | 0.240 | −0.014 | 0.781 | −0.030 | 0.417 |
| Mean corpuscular hemoglobin | 0.046 | 0.280 | −0.021 | 0.673 | 0.004 | 0.924 |
| Mean corpuscular hemoglobin concentration | 0.010 | 0.808 | −0.010 | 0.831 | 0.048 | 0.191 |
| RDW (SD) | 0.040 | 0.474 | 0.057 | 0.249 | 0.012 | 0.738 |
| RDW (CV) | 0.009 | 0.837 | 0.084 | 0.085 | 0.034 | 0.356 |
| PLT parameters | ||||||
| PLT count | 0.031 | 0.467 | 0.098 | 0.046 | 0.049 | 0.182 |
| Plateletcrit | −0.002 | 0.995 | 0.121 | 0.013 | 0.057 | 0.121 |
| MPV | 0.005 | 0.925 | 0.041 | 0.401 | 0.018 | 0.622 |
| Platelet distribution width | 0.056 | 0.190 | 0.066 | 0.179 | 0.024 | 0.510 |
| Platelet large cell ratio | −0.014 | 0.739 | 0.166 | 0.184 | 0.024 | 0.510 |
BNP, B-type natriuretic peptide; CRP, C-reactive protein; CV, coefficient of variation; hs-cTn, high-sensitive cardiac troponin; PLT, platelet; RBC, red blood cell; SD, standard deviation; WBC, white blood cell.
Pearson’s correlation coefficient (r) was used to evaluate the strength of a linear relationship between two variables.
Relationship between RBC parameters and EOCAD
Apart from the mean corpuscular hemoglobin (MCH), other RBC parameters differed significantly between the EOCAD group and the control group (Table 2). In addition, the mean corpuscular hemoglobin concentration (MCHC) was significantly higher in the EOCAD group compared with the control group. RBC count, hemoglobin, hematocrit (HCT), mean corpuscular volume (MCV), the standard deviation of RBC distribution width (RDW-SD), and the coefficient variation of RBC distribution width (RDW-CV) were significantly higher in the control group compared with those in the EOCAD group. After adjusting for baseline differences, RDW-CV (ORadjust = 0.788, 95% CI: 0.704–0.882), RDW-SD (ORadjust = 0.866, 95% CI: 0.833–0.900), HCT (ORadjust = 0.737, 95% CI: 0.715–0.761), RBC count (ORadjust = 0.107, 95% CI: 0.082–0.138), MCHC (ORadjust = 1.023, 95% CI: 1.015–1.032), MCV (ORadjust = 0.945, 95% CI: 0.927–0.963), and hemoglobin (ORadjust = 0.933, 95% CI: 0.926–0.941) were correlated with EOCAD prevalence (Table 6). The cutoff values for EOCAD were as follows: RDW-CV 12.35%, RDW-SD 39.95 fl, HCT 44.55%, RBC 4.865 1012/l, MCHC 339.5 g/l, MCV 89.75 fl, and hemoglobin 151.5 g/l.
Table 6.
Associations between RBC parameters and the presence of EOCAD.
| Adjustment models | RDW-CV | RDW-SD | HCT | RBC count | MCHC | MCV | Hemoglobin | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) |
p | OR (95% CI) |
p | OR (95% CI) |
p | OR (95% CI) |
p | OR (95% CI) |
p | OR (95% CI) |
p | OR (95% CI) |
p | |
| Crude | – | <0.001 | – | <0.001 | – | <0.001 | – | <0.001 | – | <0.001 | – | <0.001 | – | <0.001 |
| Adjusting for sex, age, BMI, smoking, alcohol consumption, DM. Hypertension, ALT, TC, LDL-C, HDL-C, TG, FBG, and medications. | 0.788 (0.704–0.882) |
<0.001 | 0.866 (0.833–0.900) |
<0.001 | 0.737 (0.715–0.761) |
<0.001 | 0.107 (0.082–0.138) |
<0.001 | 1.023 (1.015–1.032) |
<0.001 | 0.945 (0.927–0.963) |
<0.001 | 0.933 (0.927–0.963) |
<0.001 |
ALT, alanine aminotransferase; BMI, body mass index; CI, confidence interval; DM, diabetes mellitus; EOCAD, early-onset coronary artery disease; FBG, fasting blood glucose; HCT, hematocrit; HDLC, high-density lipoprotein cholesterol; LDLC, low-density lipoprotein cholesterol; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; OR, odds ratios; RBC, red blood cell; RDW-CV, red blood cell distribution width-coefficient of variation; RDW-SD, red blood cell distribution width-standard deviation; TC, total cholesterol; TG, triglyceride.
No correlation was observed between RBC parameters and the number of diseased vessels, Gensini score, BNP, and the disease subtype. However, there was a slight association between HCT and CRP (r = −0.078, p = 0.034).
Relationship between platelet parameters and EOCAD
The platelet volume distribution width (PDW), platelet hematocrit (PCT), MPV, and platelet count differed significantly between the EOCAD group and the control group (Table 2). No differences were observed in the platelet-larger cell ratio (P-LCR) between the two groups. After adjusting for sex, age, BMI, smoking, alcohol consumption, DM, hypertension, ALT, TC, LDL-C, HDL-C, TG, FBG, and medication, PDW only associated with EOCAD prevalence (ORadjust = 1.087, 95% CI: 1.044–1.131), and the cutoff value was 15.45 fl.
The platelet serial parameters were not significantly different between the CAD (non-MI) and MI groups. Platelet series parameters were also not associated with the number of diseased vessels. However, a positive correlation was observed between MPV and the Gensini score (r = 0.04, p = 0.004). There was a significant correlation between PCT and BNP (r = 0.121, p = 0.013). However, no correlation was observed between platelet parameters and CRP levels.
Discussion
In the current study, we comprehensively analyzed the relationship between the hematological parameters and EOCAD. The results indicated that (1) WBC count, neutrophil count and percentage, monocyte count and percentage, basophil, eosinophil, and lymphocyte percentages correlated with the occurrence and severity of EOCAD; (2) RDW-CV, RDW-SD, HCT, RBC count, MCHC, MCV, hemoglobin, and PDW were associated with EOCAD but did not reflect disease severity.
The relationship between leukocytes and CAD has been extensively studied. Several studies have considered elevated WBC an independent risk factor for atherosclerotic vascular disease.30,31 Moreover, leukocytes are significant mediators of inflammation.32 Inflammatory activation of the coronary endothelium could lead to thrombus formation and plaque rupture, causing ischemia and infarction.33 Therefore, leukocytes were widely investigated to monitor the disease process and the impact of anti-inflammatory treatments, thus enabling the control of disease activity. CRP is a typical inflammatory marker. Our results indicate that almost all leukocyte parameters (except monocyte percentage and lymphocyte count) correlated with CRP levels in EOCAD patients. Neutrophils are the most abundant type of leukocyte, and one of the first responders among inflammatory cells to migrate toward the inflammation site, developing a hallmark of acute inflammation. The monocyte reflects an elevated immuno-inflammatory activity and is correlated with atherosclerosis.34 In our study, the counts and percentages of neutrophils and monocyte were significantly higher among the EOCAD (non-MI) and MI groups than in the control group. The basophil was the least abundant leukocyte. However, it could play an essential role in immunomodulatory functions.35 Few studies are there on the effect of basophil on CAD. In particular, the association between basophil and EOCAD has not been reported. Our data revealed that basophil count and percentage decreased significantly in the EOCAD group compared with the control group. The MI group observed a further decrease in basophil count and percentage than the CAD (non-MI) group. In addition, basophil percentage associated with EOCAD severity and was an independent factor influencing disease occurrence. However, basophil counts did not reveal these characteristics.
Eosinophils are significant immune cells combating parasites and participating in hypersensitivity or allergic responses.36 Unlike basophils, the association between eosinophils and CAD has been extensively studied. However, the results widely varied. Several studies have demonstrated that an elevated eosinophil count associated with CAD, thereby depicting higher cardiovascular mortality.37,38 However, other studies have reported that CAD patients exhibited lower eosinophil percentages than controls without CAD.39 Our data indicated that the eosinophil count and percentage were significantly reduced in EOCAD patients and were independent factors influencing disease occurrence. The eosinophil percentage in controls, CAD (non-MI), and MI groups gradually reduced, and pairwise comparisons were statistically significant. The eosinophil count was significantly lower in the MI group compared with the control and CAD (non-MI) groups. No differences in eosinophil count were observed between the control and CAD (non-MI) groups. Gao et al. inferred that extensive thrombus formation could have induced the decreased eosinophil count.37 Our results partially support the hypothesis because the difference in eosinophil count was only present after comparing the control and EOCAD (non-MI) groups with the MI group. Our results also echo those of Sincer et al.40 They also found a significant decrease in eosinophils in MI patients compared with patients with unstable angina.
The relationship between lymphocytes and CAD has been straightforward. The lymphocyte is the gatekeeper of the immune system in the body. Experimental studies have indicated that immuno-suppression could accelerate atherosclerosis.41,42 After the development of atherosclerotic plaques, lymphocyte apoptosis is more frequent and influential, causing plaque growth, lipid nucleation, plaque rupture, and thrombosis.43 Clinical studies have indicated that lymphocyte count and percentage significantly decreased in CAD patients.44,45 Moreover, recent studies have linked lymphopenia with a worse MI prognosis, unstable angina, and heart failure.46,47 In our study, lymphocyte count and the percentage were significantly reduced in EOCAD patients and were independent factors influencing disease occurrence. The percentage of lymphocytes can reflect the disease severity, and its decrease was most evident in the MI group and depicted a significant negative correlation with the Gensini score.
We also observed that some leukocyte parameters, including the WBC count, neutrophil count and percentage, monocyte count, and lymphocyte percentage, correlated with post-MI heart failure, evaluated by the correlation study of these indicators with BNP. Therefore, the relationship between leukocytes and EOCAD is good, and the relationship between different types of leukocytes and EOCAD has varied characteristics. Moreover, it is a direction worthy of further studies.
Erythrocyte parameters primarily include RBC count, hemoglobin, HCT, MCV, MCH, MCHC, RDW-SD, and RDW-CV. Numerous clinical trials have explored the relationship between RBC parameters and CAD, with inconsistent results. Moreover, few studies have reported the association between RBC parameters and EOCAD. In this study, erythrocyte parameters other than MCHC were associated with EOCAD without association with disease severity. Mechanistically, erythrocytes could only initiate atherosclerotic plaque formation and play a minor role in its progression. The primary function of the erythrocyte is to and fro transport of oxygen and carbon dioxide. Furthermore, the erythrocyte regulates redox balance and vascular endothelial function by releasing nitric oxide and adenosine triphosphate.48 Experimental studies have indicated that erythrocyte has a fundamental role in cardiovascular homeostasis by regulating vascular function and integrity.49 Therefore, the relationship between erythrocytes and CVD requires further investigation after extrapolating the inconsistency of clinical findings.
Platelets play a crucial role in atherosclerosis and thrombosis. Platelets initiate atherothrombosis through endothelial function, mediating inflammatory stimulation and synthesizing bioactive proteins.50 The widespread use of antiplatelet drugs (aspirin, clopidogrel) in CVD patients slows the formation of atherosclerotic plaques, especially among high-risk populations. Thus, many studies have focused on the relationship between platelet parameters and CVD.
Platelet parameters primarily include platelet count, PCT, MPV, PDW, and P-LCR, among which MPV and PDW are essential. MPV is a measure of platelet size and indicates platelet reactivity. Platelet volume is generally proportional to activity, and elevated platelet activity enhances adhesion and aggregation, causing vascular thromboembolic events.51 Although many studies have evaluated the relationship between MPV and CAD, the results have been conflicting. Due to heterogeneity, meta-analysis outcomes remain uncertain whether MPV is a direct or indirect risk factor for CAD.5 In this study, MPV was significantly elevated in the EOCAD group compared with the control group. However, MPV was not independently associated with EOCAD occurrence. Despite a slight association with the Gensini score, MPV did not reflect disease severity. The relationship between PDW and CVD is still controversial. In several studies, it was suggested that PDW was not related to the extent of CAD, while in other studies, it was determined that PDW is an independent marker of ST-elevation MI, and is related to coronary collateral development in subjects with non-ST-elevation MI.8,52 PDW in the EOCAD group was significantly higher than in the control group and was independently associated with EOCAD occurrence. There were differences in the results of the studies on whether PDW could affect CAD severity.53,54 Our study did not show any correlation between PDW and disease severity.
Although this was one of the few studies on the relationship between hematological parameters and EOCAD, there are some limitations. First, this was a retrospective, single-center study. No follow-up data were collected, and the role of hematological parameters on EOCAD progression could not be described, especially regarding long-term events. Second, we analyzed patients scheduled for coronary angiography. Hence, this hospital-based population might not have been a random population representative. Third, in our study, we did not evaluate other cardiovascular risk factors, including lipoprotein a and homocysteine. Furthermore, in our study, we did not assess folic acid and vitamin B12, which could have affected hematological parameters. Finally, we clear that coronary angiography should be performed in all controls to rule out CAD, but younger people with no symptoms of CVDs rarely undergo coronary angiography. In the study, the selection population in the control group was based only on symptoms and resting electrocardiogram (ECG) as the incidence of ischemia with normal ECG in an asymptomatic active young adult is uncommon but still cannot guarantee 100% that he is not ischemic.
Conclusion
In this study, the association of hematological parameters with EOCAD was systematically evaluated. Leukocyte parameters significantly associated with EOCAD. Apart from eosinophil and basophil counts, other parameters were related to disease occurrence and severity. Erythrocyte parameters (except MCHC) could independently affect EOCAD occurrence, but not disease severity. PDW was associated with EOCAD prevalence, and other platelet parameters were not associated with the occurrence and severity of the disease. In the future, prospective studies are needed to elucidate the exact mechanisms of hematological parameters involved in pathophysiology and prognosis. As complete blood count is a cost-effective, and rapid, test, clinicians could derive information from hematological parameters that would benefit the diagnosis and risk assessment of EOCAD.
Acknowledgments
None.
Footnotes
ORCID iD: Chao Xuan  https://orcid.org/0000-0001-8273-0178
https://orcid.org/0000-0001-8273-0178
Contributor Information
Huan Wang, Department of Clinical Laboratory, The Affiliated Hospital of Qingdao University, Qingdao, China.
Hui Li, Department of Clinical Laboratory, The Affiliated Hospital of Qingdao University, Qingdao, China.
Yan Wang, Health Management Institute, The Affiliated Hospital of Qingdao University, Qingdao, China.
Cong Zhao, Department of Cardiology, The Affiliated Hospital of Qingdao University, Qingdao, China.
Qing-Wu Tian, Department of Clinical Laboratory, The Affiliated Hospital of Qingdao University, Qingdao, China.
Qing Wang, Department of Clinical Laboratory, The Affiliated Hospital of Qingdao University, Qingdao, China.
Guo-Wei He, Department of Cardiovascular Surgery, TEDA International Cardiovascular Hospital, Academy of Medical Sciences & Peking Union Medical College, Tianjin, China; Department of Surgery, Oregon Health and Science University, Portland, OR, USA.
Li-Min Lun, Department of Clinical Laboratory, The Affiliated Hospital of Qingdao University, Qingdao, China.
Chao Xuan, Department of Clinical Laboratory, The Affiliated Hospital of Qingdao University, No. 1677, Wutai Mountain Road, Qingdao 266500, China.
Declarations
Ethics approval and consent to participate: The study was approved by the Ethics Committee of the Affiliated Hospital of Qingdao University (QYFYWZLL27293). Written informed consent was obtained from all participants.
Consent for publication: Not applicable.
Author contributions: Huan Wang: Data curation; Formal analysis; Investigation; Writing – original draft.
Hui Li: Data curation.
Yan Wang: Data curation; Formal analysis.
Cong Zhao: Formal analysis; Investigation.
Qing-Wu Tian: Data curation.
Qing Wang: Formal analysis.
Guo-Wei He: Writing – review & editing.
Li-Min Lun: Writing – review & editing.
Chao Xuan: Formal analysis; Project administration; Writing – original draft.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was supported by grants from the National Natural Science Foundation of China (Grant No. 81672073) and Shandong Provincial Natural Science Foundation, China (Grant Nos. ZR2022MH200 and ZR2020MH034).
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of data and materials: The datasets used and analyzed during this current study are available from the corresponding author on reasonable request.
References
- 1. Kounis NG, Soufras GD, Tsigkas G, et al. White blood cell counts, leukocyte ratios, and eosinophils as inflammatory markers in patients with coronary artery disease. Clin Appl Thromb Hemost 2015; 21: 139–143. [DOI] [PubMed] [Google Scholar]
- 2. Loprinzi PD. Comparative evaluation of red blood cell distribution width and high sensitivity C-reactive protein in predicting all-cause mortality and coronary heart disease mortality. Int J Cardiol 2016; 223: 72–73. [DOI] [PubMed] [Google Scholar]
- 3. Wu TT, Zheng YY, Hou XG, et al. Red blood cell distribution width as long-term prognostic markers in patients with coronary artery disease undergoing percutaneous coronary intervention. Lipids Health Dis 2019; 18: 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sansanayudh N, Numthavaj P, Muntham D, et al. Prognostic effect of mean platelet volume in patients with coronary artery disease. A systematic review and meta-analysis. Thromb Haemost 2015; 114: 1299–1309. [DOI] [PubMed] [Google Scholar]
- 5. Sansanayudh N, Anothaisintawee T, Muntham D, et al. Mean platelet volume and coronary artery disease: a systematic review and meta-analysis. Int J Cardiol 2014; 175: 433–440. [DOI] [PubMed] [Google Scholar]
- 6. Dong CH, Wang ZM, Chen SY. Neutrophil to lymphocyte ratio predict mortality and major adverse cardiac events in acute coronary syndrome: a systematic review and meta-analysis. Clin Biochem 2018; 52: 131–136. [DOI] [PubMed] [Google Scholar]
- 7. Dentali F, Nigro O, Squizzato A, et al. Impact of neutrophils to lymphocytes ratio on major clinical outcomes in patients with acute coronary syndromes: a systematic review and meta-analysis of the literature. Int J Cardiol 2018; 266: 31–37. [DOI] [PubMed] [Google Scholar]
- 8. De Luca G, Venegoni L, Iorio S, et al. Platelet distribution width and the extent of coronary artery disease: results from a large prospective study. Platelets 2010; 21: 508–514. [DOI] [PubMed] [Google Scholar]
- 9. Quan XQ, Wang RC, Zhang Q, et al. The predictive value of lymphocyte-to-monocyte ratio in the prognosis of acute coronary syndrome patients: a systematic review and meta-analysis. BMC Cardiovasc Disord 2020; 20: 338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cakir L, Aktas G, Enginyurt O, et al. Mean platelet volume increases in type 2 diabetes mellitus independent of HbA1c level. Acta Medica Mediterranea 2014; 30: 425–428. [Google Scholar]
- 11. Kodiatte TA, Manikyam UK, Rao SB, et al. Mean platelet volume in type 2 diabetes mellitus. J Lab Physicians 2012; 4: 5–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aktas G, Cakiroglu B, Sit M, et al. Mean platelet volume: a simple indicator of chronic prostatitis. Acta Medica Mediterranea 2013; 29: 551–554. [Google Scholar]
- 13. Aktas G, Sit M, Tekce H, et al. Mean platelet volume in nasal polyps. West Indian Med J 2013; 62: 515–518. [DOI] [PubMed] [Google Scholar]
- 14. Dagistan Y, Dagistan E, Gezici AR, et al. Could red cell distribution width and mean platelet volume be a predictor for lumbar disc hernias. Ideggyogy Sz 2016; 69: 411–414. [DOI] [PubMed] [Google Scholar]
- 15. Aktas G, Alcelik A, Tekce BK, et al. Red cell distribution width and mean platelet volume in patients with irritable bowel syndrome. Prz Gastroenterol 2014; 9: 160–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cakir L, Aktas G, Mercimek OB, et al. Are red cell distribution width and mean platelet volume associated with rheumatoid arthritis? Biomed Res 2016; 27: 292–294. [Google Scholar]
- 17. Aktas G, Kocak MZ, Duman TT, et al. Mean platelet volume (MPV) as an inflammatory marker in type 2 diabetes mellitus and obesity. Bali Med J 2018; 7: 650–653. [Google Scholar]
- 18. Kosekli MA. Mean platelet volume and platelet to lymphocyte count ratio are associated with hepatitis B-related liver fibrosis. Eur J Gastroenterol Hepatol 2022; 34: 324–327. [DOI] [PubMed] [Google Scholar]
- 19. Aktas G, Sit M, Dikbas O, et al. Could red cell distribution width be a marker in Hashimoto’s thyroiditis. Exp Clin Endocrinol Diabetes 2014; 122: 572–574. [DOI] [PubMed] [Google Scholar]
- 20. Aktas G, Sit M, Karagoz I, et al. Could red cell distribution width be a marker of thyroid cancer. J Coll Physicians Surg Pak 2017; 27: 556–558. [PubMed] [Google Scholar]
- 21. Ustaoglu M, Aktas G, Avcioglu U, et al. Elevated platelet distribution width and red cell distribution width are associated with autoimmune liver diseases. Eur J Gastroenterol Hepatol 2021; 33(Suppl. 1): e905–e908. [DOI] [PubMed] [Google Scholar]
- 22. Aktas G. Hematological predictors of novel coronavirus infection. Rev Assoc Med Bras 2021; 67(Suppl. 1): 1–2. [DOI] [PubMed] [Google Scholar]
- 23. Aktas G, Sit M, Dikbas O, et al. Elevated neutrophil-to-lymphocyte ratio in the diagnosis of Hashimoto’s thyroiditis. Rev Assoc Med Bras 2017; 63: 1065–1068. [DOI] [PubMed] [Google Scholar]
- 24. Aktas G, Duman T, Atak B, et al. Irritable bowel syndrome is associated with novel inflammatory markers derived from hemogram parameters. Fam Med Prim Care Rev 2020; 22: 107–110. [Google Scholar]
- 25. Trinder M, Li X, DeCastro ML, et al. Risk of premature atherosclerotic disease in patients with monogenic versus polygenic familial hypercholesterolemia. J Am Coll Cardiol 2019; 74: 512–522. [DOI] [PubMed] [Google Scholar]
- 26. Xuan C, Li H, Li LL, et al. Screening and identification of pregnancy zone protein and leucine-rich alpha-2-glycoprotein as potential serum biomarkers for early-onset myocardial infarction using protein profile analysis. Proteomics Clin Appl 2019; 13: e1800079. [DOI] [PubMed] [Google Scholar]
- 27. Zhang SY, Xuan C, Wang Y, et al. Association between ALMS 1 variants and early-onset coronary artery disease: a case-control study in Chinese population. Biosci Rep 2020; 40: BSR20193637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xuan C, Liu ZF, Wang Q, et al. Increased serum concentrations of asymmetric dimethylarginine (ADMA) in patients with early-onset coronary artery disease. Clin Chim Acta 2017; 464: 195–199. [DOI] [PubMed] [Google Scholar]
- 29. Zhang SY, Xuan C, Zhang XC, et al. Association between MTHFR gene common variants, serum homocysteine, and risk of early-onset coronary artery disease: a case-control study. Biochem Genet 2020; 58: 245–256. [DOI] [PubMed] [Google Scholar]
- 30. Dehghani MR, Rezaei Y, Taghipour-Sani L. White blood cell count to mean platelet volume ratio as a novel non-invasive marker predicting long-term outcomes in patients with non-ST elevation acute coronary syndrome. Cardiol J 2015; 22: 437–445. [DOI] [PubMed] [Google Scholar]
- 31. Zhao X, Jiang L, Xu L, et al. Predictive value of in-hospital white blood cell count in Chinese patients with triple-vessel coronary disease. Eur J Prev Cardiol 2019; 26: 872–882. [DOI] [PubMed] [Google Scholar]
- 32. Fordham JB, Naqvi AR, Nares S. Leukocyte production of inflammatory mediators is inhibited by the antioxidants phloretin, silymarin, hesperetin, and resveratrol. Mediators Inflamm 2014; 2014: 938712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Badimon L, Vilahur G. Thrombosis formation on atherosclerotic lesions and plaque rupture. J Intern Med 2014; 276: 618–632. [DOI] [PubMed] [Google Scholar]
- 34. Yang J, Zhang L, Yu C, et al. Monocyte and macrophage differentiation: circulation inflammatory monocyte as biomarker for inflammatory diseases. Biomark Res 2014; 2: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Santos AF, Alpan O, Hoffmann HJ. Basophil activation test: mechanisms and considerations for use in clinical trials and clinical practice. Allergy 2021; 76: 2420–2432. [DOI] [PubMed] [Google Scholar]
- 36. Long H, Liao W, Wang L, et al. A player and coordinator: the versatile roles of eosinophils in the immune system. Transfus Med Hemother 2016; 43: 96–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gao S, Deng Y, Wu J, et al. Eosinophils count in peripheral circulation is associated with coronary artery disease. Atherosclerosis 2019; 286: 128–134. [DOI] [PubMed] [Google Scholar]
- 38. Tenekecioglu E, Yilmaz M, Bekler A, et al. Eosinophil count is related with coronary thrombus in non ST-elevated acute coronary syndrome. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2015; 159: 266–271. [DOI] [PubMed] [Google Scholar]
- 39. Verdoia M, Schaffer A, Cassetti E, et al. Absolute eosinophils count and the extent of coronary artery disease: a single centre cohort study. J Thromb Thrombolysis 2015; 39: 459–466. [DOI] [PubMed] [Google Scholar]
- 40. Sincer I, Gunes Y, Mansiroglu AK, et al. Differential value of eosinophil count in acute coronary syndrome among elderly patients. Aging Male 2020; 23: 958–961. [DOI] [PubMed] [Google Scholar]
- 41. Zaric BL, Radovanovic JN, Gluvic Z, et al. Atherosclerosis linked to aberrant amino acid metabolism and immunosuppressive amino acid catabolizing enzymes. Front Immunol 2020; 11: 551758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kwiatkowska M, Oldakowska-Jedynak U, Wojtaszek E, et al. Potential effects of immunosuppression on oxidative stress and atherosclerosis in kidney transplant recipients. Oxid Med Cell Longev 2021; 2021: 6660846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sakakura K, Nakano M, Otsuka F, et al. Pathophysiology of atherosclerosis plaque progression. Heart Lung Circ 2013; 22: 399–411. [DOI] [PubMed] [Google Scholar]
- 44. Park JJ, Jang HJ, Oh IY, et al. Prognostic value of neutrophil to lymphocyte ratio in patients presenting with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am J Cardiol 2013; 111: 636–642. [DOI] [PubMed] [Google Scholar]
- 45. Núñez J, Sastre C, D’Ascoli G, et al. Relation of low lymphocyte count to frailty and its usefulness as a prognostic biomarker in patients > 65 years of age with acute coronary syndrome. Am J Cardiol 2020; 125: 1033–1038. [DOI] [PubMed] [Google Scholar]
- 46. Maimaiti A, Li Y, Wang YT, et al. Association of platelet-to-lymphocyte count ratio with myocardial reperfusion and major adverse events in patients with acute myocardial infarction: a two-centre retrospective cohort study. BMJ Open 2019; 9: e25628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Marçula M, de Souza Buto MF, Madaloso BA, et al. Lymphocyte count and prognosis in patients with heart failure. Int J Cardiol 2015; 188: 60–62. [DOI] [PubMed] [Google Scholar]
- 48. Kuhn V, Diederich L, Keller T, et al. Red blood cell function and dysfunction: redox regulation, nitric oxide metabolism, anemia. Antioxid Redox Signal 2017; 26: 718–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pernow J, Mahdi A, Yang J, et al. Red blood cell dysfunction: a new player in cardiovascular disease. Cardiovasc Res 2019; 115: 1596–1605. [DOI] [PubMed] [Google Scholar]
- 50. Koenen RR, Binder CJ. Platelets and coagulation factors: established and novel roles in atherosclerosis and atherothrombosis. Atherosclerosis 2020; 307: 78–79. [DOI] [PubMed] [Google Scholar]
- 51. Barale C, Russo I. Influence of cardiometabolic risk factors on platelet function. Int J Mol Sci 2020; 21: 623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cetin MS, Ozcan Cetin EH, Akdi A, et al. Platelet distribution width and plateletcrit: novel biomarkers of ST elevation myocardial infarction in young patients. Kardiol Pol 2017; 75: 1005–1012. [DOI] [PubMed] [Google Scholar]
- 53. De Luca G, Secco GG, Verdoia M, et al. Combination between mean platelet volume and platelet distribution width to predict the prevalence and extent of coronary artery disease: results from a large cohort study. Blood Coagul Fibrinolysis 2014; 25: 86–91. [DOI] [PubMed] [Google Scholar]
- 54. Bekler A, Ozkan MT, Tenekecioglu E, et al. Increased platelet distribution width is associated with severity of coronary artery disease in patients with acute coronary syndrome. Angiology 2015; 66: 638–643. [DOI] [PubMed] [Google Scholar]