Abstract
Rationale:
Bartonella sp. are the most common causes of culture-negative infective endocarditis (IE) cases in the United States. Although, infection-related glomerulonephritis can frequently mimic primary vasculitis due to pauci-immune pattern, majority of previously reported cases of Bartonella henselae-associated glomerulonephritis have immune-complex deposits on immunofluorescence. We present a rare case of B henselae IE-related pauci-immune necrotizing glomerulonephritis. Timely recognition of this atypical presentation led to appropriately directed medical therapy.
Presenting concerns of the patient:
A 33-year-old Caucasian male with a history of human immunodeficiency virus (HIV) on highly active antiretroviral therapy (HAART), alcohol abuse, previous subarachnoid hemorrhage (SAH), and recent wisdom tooth extraction (on amoxicillin) was transferred from an outside hospital for further evaluation of severe headache. He was diagnosed with an SAH and right anterior cerebral artery mycotic aneurysm. The serum creatinine at the outside hospital was 292 umol/L (3.3 mg/dL) with a previously normal baseline around 2 years ago. The serum creatinine at our institution was 256 umol/L (3.0 mg/dL). The urinalysis demonstrated +100 protein, +3 blood and 29 red blood cells/high power field. The urine protein creatinine ratio (UPC) was 1.7 g/g. Serologic evaluation was positive for a low C4 10.2 mg/dL, elevated rheumatoid factor 40 IU/mL and an elevated proteinase 3 (PR-3) antineutrophilic cytoplasmic antibodies (ANCA Ab) 4.0 U/mL. A transesophageal echocardiogram (TEE) showed echo densities on both mitral and aortic valve. Blood cultures were negative. Further serologic evaluation was positive for B henselae IgG titer of 1:2560 (normal <1:320) with a negative IgM titer.
Diagnoses:
A percutaneous kidney biopsy revealed pauci-immune necrotizing glomerulonephritis, with 14/16 glomeruli globally sclerotic, and 2 glomeruli with active segmental necrotizing lesions. There was no evidence of immune-complex deposition on immunofluorescence or electron microscopy. Clinical findings were consistent with B henselae IE associated mycotic aneurysm and necrotizing glomerulonephritis.
Intervention:
Empiric treatment for an active glomerulonephritis with immunosuppressive agents was deferred on admission, given concern for an underlying infectious process and mycotic aneurysms in an HIV-positive patient. He received antibiotic treatment with doxycycline and ceftriaxone with gentamicin for synergy. Despite this, the mitral and aortic valve regurgitation worsened, and he developed congestive heart failure requiring aortic valve replacement and mitral valve repair. The explanted aortic valve was positive for B henselae by polymerase chain reaction (PCR) confirming the diagnosis of B henselae IE.
Outcomes:
Immunosuppression was deferred due to timely identification of an atypical presentation of B henselae-associated ANCA antibodies-positive, pauci-immune necrotizing glomerulonephritis. A course of antibiotic treatment resulted in improved renal functions along with undetectable B henselae and PR3 Ab titers. The serum creatinine decreased to 176 umol/L (2 mg/dL) and remained stable 12 months after discharge.
Teaching points:
B henselae IE should be suspected in patients with pauci-immune necrotizing glomerulonephritis and culture-negative IE. This is imperative for optimal decision making in the management of such patients. Having high clinical suspicion can avoid unnecessary and potentially deleterious use of immunosuppressive agents.
Keywords: acute kidney injury, B henselae, pauci-immune necrotizing glomerulonephritis, infective endocarditis
Abrégé
Justification:
La bactérie Bartonella sp est la cause la plus fréquente des cas d’endocardite infectieuse (EI) à culture négative aux États-Unis. Bien qu’il arrive souvent que les glomérulonéphrites, en raison de leur schéma auto-immun, puissent ressembler à des vascularites primaires, la majorité des cas précédemment signalés de glomérulonéphrites associées à B. henselae présentent des dépôts de complexes immuns sur immunofluorescence. Nous présentons un cas rare d’endocardite infectieuse à B. henselae associée à une glomérulonéphrite pauci-immune nécrosante. La reconnaissance rapide de cette présentation atypique a conduit à un traitement médical bien dirigé.
Présentation du cas:
Un homme caucasien de 33 ans atteints du virus de l’immunodéficience humaine (VIH) sous traitement antirétroviral hautement actif (HAART) qui avait été transféré d’un autre hôpital pour une évaluation plus approfondie de céphalées intenses. Le patient avait des antécédents d’abus d’alcool, d’une hémorragie sous-arachnoïdienne (HSA) antérieure et d’une récente extraction de dents de sagesse (prise d’amoxicilline). Le patient a reçu un diagnostic d’HSA et d’anévrisme mycotique de l’artère cérébrale antérieure droite. Le taux de créatinine sérique mesuré à l’hôpital externe était de 292 umol/L (3,3 mg/dL); le patient présentait une valeur normale environ 2 ans auparavant. Le taux de créatinine sérique mesuré dans notre établissement était de 256 umol/L (3,0 mg/dL). L’analyze d’urine a révélé un décompte supérieur à 100 pour les protéines et de + 3 pour le sang avec 29 globules rouges/champ à puissance élevée. Le rapport protéine/créatinine urinaire (UPC) était de 1,7 g/g. L’évaluation sérologique était positive pour un faible taux de C4 (10,2 mg/dL), un taux élevé de facteur rhumatoïde (40 UI/mL) et un taux élevé (4,0 U/mL) d’anticorps anti-cytoplasme des neutrophiles (ANCA) anti-protéinase 3 (PR-3). Une échocardiographie transœsophagienne (ÉTO) a montré des végétations sur les valves mitrale et aortique. Les hémocultures étaient négatives. Une évaluation sérologique plus poussée s’est avérée positive pour le titer d’IgG de B. henselae, avec un rapport de 1:2560 (normale = inférieur à 1:320), et négative pour le titer d’IgM.
Diagnostics:
Une biopsie rénale percutanée a révélé une glomérulonéphrite pauci-immune nécrosante avec un taux de 14/16 glomérules sclérotiques et 2 glomérules présentant des lésions segmentaires nécrosantes actives. Aucune preuve de dépôt de complexe immun n’a été observée par immunofluorescence ou par microscopie électronique. Les résultats cliniques correspondaient à une endocardite infectieuse à B. henselae associée à l’anévrisme mycotique et à la glomérulonéphrite nécrosante.
Intervention:
Le traitement empirique d’une glomérulonéphrite active avec des agents immunosuppresseurs a été reporté lors de l’admission, en raison de la crainte d’un processus infectieux sous-jacent et d’anévrismes mycotiques chez un patient séropositif. Le patient a reçu un traitement antibiotique de doxycycline et de ceftriaxone avec gentamicine pour la synergie. Malgré cette intervention, la régurgitation des valves mitrale et aortique s’est aggravée et le patient a développé une insuffisance cardiaque congestive qui a nécessité le remplacement de la valve aortique et la réparation de la valve mitrale. Une analyze par PCR (réaction en chaîne de la polymérase) sur la valve aortique explantée s’est avérée positive pour B. henselae, ce qui a confirmé le diagnostic d’endocardite infectieuse à B. henselae.
Résultats:
Le traitement immunosuppresseur a été reporté en raison de l’identification opportune d’une présentation atypique de glomérulonéphrite pauci-immune nécrosante positive pour les anticorps anti-cytoplasme des neutrophiles (ANCA) associés à B. henselae. Un traitement antibiotique a permis d’améliorer la fonction rénale et a ramené les titres de B. henselae et d’Ac PR3 à des niveaux indétectables. Le taux de créatinine sérique est passé à 176 umol/L (2 mg/dL) et est demeuré stable 12 mois après le congé du patient.
Enseignements tirés:
L’endocardite infectieuse associée à B. henselae doit être suspectée chez les patients atteints d’une glomérulonéphrite pauci-immune nécrosante et d’une endocardite infectieuse à culture négative. Ceci est impératif afin d’assurer une prise de décision optimale pour la prise en charge de ces patients. Dans ce cas particulier, une suspicion clinique importante peut prévenir l’utilization inutile et potentiellement délétère d’agents immunosuppresseurs.
Introduction
Infective endocarditis-associated glomerulonephritis is a well-documented cause of nephritic syndrome.1 The diagnosis of blood culture-negative infective endocarditis can account for 8% of infective endocarditis cases.2 These rare culture-negative cases are often due to fastidious organisms like Bartonella, Coxiella, and various fungi or noninfectious conditions like vasculitis.3 Like other infectious organisms, B henselae-related infective endocarditis has also been associated with glomerulonephritis. Historically, these cases test positive for C-antineutrophilic cytoplasmic antibodies (C-ANCA) and proteinase 3 (PR3) antibodies which commonly leads to an initial misdiagnosis of ANCA-associated small-vessel vasculitis.4 Renal biopsies with immune-complex deposition on immunofluorescence microscopy support the diagnosis of infection-related glomerulonephritis. However, an increasing number of infective endocarditis cases associated with pauci-immune glomerulonephritis have emerged. We present another unique and challenging case of PR3-positive, pauci-immune necrotizing glomerulonephritis found to be secondary to B henselae-related infective endocarditis. This is only the 5th such case reported. We also present a summary of the clinical characteristics, treatment, and outcomes of the previously reported cases with both, B henselae and quintana.
Case Presentation
A 33-year-old Caucasian male with history of human immunodeficiency virus (HIV) on highly active antiretroviral therapy (HAART), alcohol abuse, previous subarachnoid hemorrhage (SAH), and recent wisdom tooth extraction (on amoxicillin) was transferred from an outside hospital for further evaluation of severe headache. He was diagnosed with an SAH with an atypical pattern in suprasellar and basilar cisterns with extension into ventricles. The serum creatinine (SCr) at the outside hospital was 292 umol/L (3.3 mg/dL) with a previously normal baseline around 2 years ago. Serum creatinine at our institution was 256 umol/L (3.0 mg/dL). The urinalysis demonstrated +100 protein, +3 blood and 29 red blood cells/high power field. The urine protein creatinine ratio (UPC) was 1.7 g/g. A kidney ultrasound was unremarkable. Serologic evaluation was positive for a low C4 at 10.2 mg/dL (normal 13-49 mg/dL), elevated rheumatoid factor at 40 IU/mL (normal <35), and a elevated PR-3 ANCA Ab elevated at 4.0 U/mL (normal <3.5). C-reactive protein (CRP) and erythrocyte sedimentation rare were both elevated at 36 mg/dL and 80 mm/hour, respectively. Other pertinent negative investigations included anti-double stranded DNA antibody, antistreptolysin antibody, antiphospholipid antibody, undetectable HIV viral load, hepatitis B antigen, and hepatitis C antibody. Treatment for an active glomerulonephritis with immunosuppressive agents was deferred, given concern for an underlying infectious process and mycotic aneurysms in an HIV-positive patient. Clinical and laboratory characteristics are summarized below in Table 1.
Table 1.
Summary of Clinical and Laboratory Data.
| Demographics: • Age: 32 • Ethnicity: White American • Sex: Male Predisposing state: • History of intravenous drug abuse • HIV positive (viral load undetectable) • History of exposure to cat Laboratory data: • Leukocyte count: 8 × 103/uL with normal differential (normal 4-10 × 103/uL) • Hemoglobin: 100 g/L (normal 130-150 g/L) • Platelet count: 240 × 103/uL (normal 150-450 × 103/uL) • Blood urea nitrogen: 30 mg/dL (6-24 mg/dL) • Creatinine at presentation 292 umol/L (3.3 mg/dL); at biopsy: 256 umol/L (3 mg/dL); at discharge and 12 months follow-up: 176 umol/L (2 mg/dL). (Baseline: 88.42 umol/L [0.6 mg/dL]) • Urinalysis: +100 protein, +3 blood and 29 RBCs/HPF • Urine protein/creatinine ratio: 1.7 g/g • Erythrocyte sedimentation rate: 80 mm/hour (normal 1-13 mm/hour) • C-reactive protein: 36 mg/L (normal <10 mg/L) • Rheumatoid factor: 40 IU/mL (normal <35 IU/mL) • C3: 90 (normal 80-160 mg/dL) • C4: 10.2 mg/dL (normal 13-49 mg/dL) • c-ANCA: Negative • PR-3 Ab: 4.0 U/mL (normal <3.5) • p-ANCA and MPO Ab: Negative • HIV viral load: Undetectable • Rapid plasma reagin: Negative • Hepatitis B surface Ag: Negative • Hepatitis C Antibody: Negative • RPR: Negative • Anti-dsDNA antibody: Negative • Anti-streptolysin antibody: Negative • Serum creatinine at 12 months after biopsy: 176 umol/L (2 mg/dL) |
Note. RBC = red blood cells; HPF = high power field; ANCA = antineutrophilic cytoplasmic antibodies; PR-3 = proteinase 3; MPO = myeloperoxidase; RPR = rapid plasma reagin.
A transesophageal echocardiogram demonstrated multiple mobile echo densities adherent to both atrial and ventricular aspects of mitral valve concerning for endocarditis. A transesophageal echocardiogram revealed a highly mobile vegetation on the mitral valve (0.6 cm × 0.3 cm) along with a vegetation on a bicuspid aortic valve with moderate aortic regurgitation. Blood cultures were negative, but a serologic evaluation revealed B henselae IgG titers of 1:2560 (normal <1:320) with negative IgM titers.
A cerebral angiogram revealed distal right frontopolar artery aneurysm thought to be mycotic in nature and this was embolized. Given the mitral and aortic valve vegetations and concerns for a mycotic aneurysm, a diagnosis of Bartonella-infective endocarditis was made. Further history revealed the patient had exposure to pet cat over for the past 3 months. Treatment was therefore initiated with doxycycline and ceftriaxone along with gentamicin for synergy.
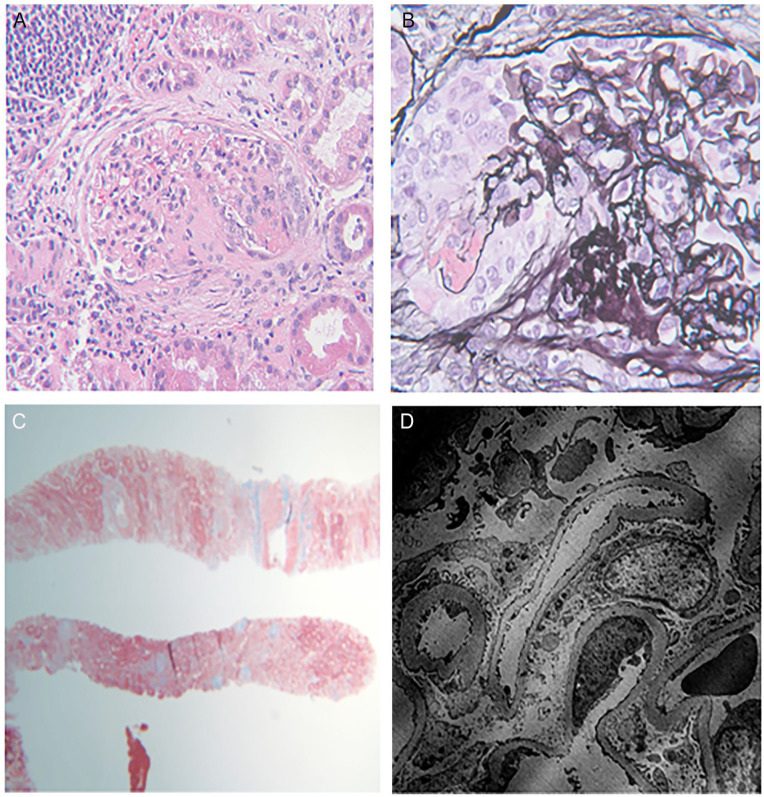
A kidney biopsy was performed to assess the cause of acute kidney injury with proteinuria and active urine sediment. This revealed a pauci-immune necrotizing glomerulonephritis, with 14 of 16 glomeruli globally sclerotic, and 2 glomeruli with active segmental necrotizing lesions with fibrinoid necrosis and karyorrhectic debris (Figure 1A and 1B) and, there was evidence of acute tubular injury. Trichrome stain (Figure 1C) showed around 50% to 60% interstitial fibrosis and tubular atrophy (IFTA) alluding to the chronicity of the underlying process. Staining for IgG, IgA, IgM, C3, C1Q, fibrin, kappa, and lambda on routine immunofluorescence was negative. There were no immune-complex deposits on electron micoscopy either (Figure 1D). He was diagnosed with B henselae endocarditis complicated by pauci-immune necrotizing glomerulonephritis.
Figure 1.
(A) Hematoxylin and eosin stain on light microscopy—Segmental necrotizing crescentic lesion (Asterisk). (B) Silver stain on light microscopy—Cellular crescent with fibrinoid necrosis (Arrow) and break in the glomerular basement membrane. (C) Trichrome stain showing 55% to 60% Interstitial Fibrosis and Tubular Atrophy. (D) Electron microscopy show glomerular basement membrane within normal limits without any electron dense deposits. Partial foot process effacement (25%-50%). No mesangial expansion or deposits with normal endothelium and patent capillary lumen.
Outcome
Despite treatment with antibiotics, he developed decompensated heart failure due to severe mitral and aortic regurgitation. He underwent aortic valve replacement and mitral valve repair with debridement of phlegmonous tissue. The aortic valve was positive for B henselae on polymerase chain reaction (PCR). The kidney functions improved and stabilized with an SCr at 176 umol/L (2 mg/dL). He was discharged on a 6-week course of ceftriaxone and completed doxycycline course for a total of 3 months.
Follow-Up
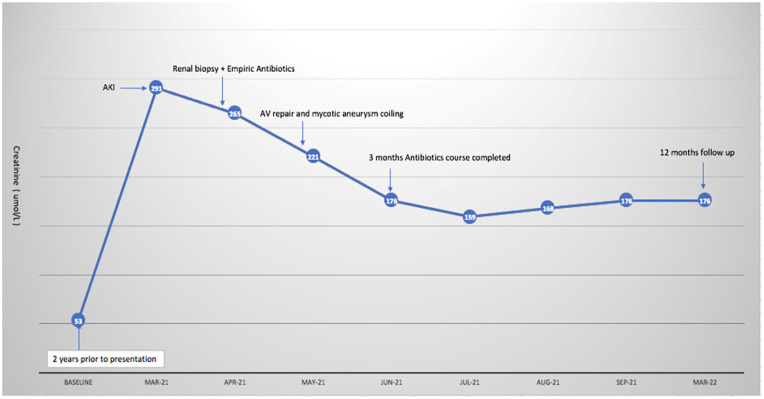
At 12 months follow-up, his kidney functions remained stable with SCr ranging from 167 to 176 umol/L (1.9-2 mg/dL). The timeline of clinical events is summarized below in Graph 1.
Graph 1.
Timeline of events.
Discussion
Despite recent advances in laboratory methods, culture-negative endocarditis is diagnostically challenging and accounts for up to 8% of the overall cases.2 Multiple Bartonella species have been reported to cause culture-negative endocarditis. The most common species, accounting for up to 95% of cases, include B henselae and B Quintana.5 Less common species include B alsatica, B vinsonii, B elizabethae, and B koehlerae.6 Risk factors associated with B henselae and quintana infection include male sex, alcohol abuse, exposure to cats, homelessness, pre-existing valvular disease, and prior louse infections.7 Epidemiologically, men are more likely to be homeless and alcohol abusers which portends the greater risk related to male sex.
While cardiac complications associated with Bartonella endocarditis, such as valvular perforation and heart failure, are worse with prosthetic valves compared to the native valves,8 our patient developed severe mitral and aortic valve regurgitation leading to congestive heart failure due to infection of native valves after failing treatment with antibiotics alone. As a result, he required aortic valve replacement along with mitral valve repair. Evaluation of the aortic valve for B henselae was positive by PCR. While the specificity of PCR is 100%, the sensitivity is poor at 58%, and the test is not always available.9,10 Furthermore, growth in blood culture is hindered by the fastidious nature of the organism and can take up to 21 days for incubation further delaying timely diagnosis and intervention. Therefore, the initial diagnosis is reliant upon evaluation of serum IgM and IgG titers. In cases of recent or early Bartonella infection, an IgM titer of 1:16 is diagnostic11 whereas an IgG titer of 1:256 or greater suggests active or acute infection.11 Our patient’s B henselae IgG titers were 1:2560 (normal range: 1:320) with negative IgM titers and can be considered definitive even if valve PCR had not been available.
Primary systemic vasculitides can manifest with cardiac abnormalities and can be hard to distinguish clinically from infectious endocarditis. Granulomatosis with polyangiitis is the most common form of small vessel vasculitis affecting the heart but rarely presents as valvular vegetations.12 In one cohort, valvular disease was present in only 6% of cases, while majority patients (77%) had either pericarditis, cardiomyopathy or coronary artery disease as the primary cardiac presentation.12 Serological markers like antinuclear antibodies and rheumatoid factor, erythrocyte sedimentation rate (ESR) and CRP can be positive in both infectious endocarditis as well as systemic vasculitides and therefore are nondiagnostic. In general, proteinase-3 antibodies (PR3-ANCA) are deemed very specific for granulomatosis with polyangiitis, but there are increasing cases of these antibodies in infective endocarditis13 with are at least 9 reported cases of Bartonella species prior to our case.4,8,9,14 -19
In all these cases, the exact cause of proteinase-3 antibodies (PR-3 ANCA Ab) is unclear but potential reasons include false-positive test, infection-related phenomena, or C-ANCA production by B lymphocyte in response to PR3 release from neutrophils.1 This association has important implications in cases where empirical treatment with immunosuppressants is considered for systemic vasculitis based on C-ANCA and PR-3 Ab titers. Our patient had low C4 and mildly elevated rheumatoid factor and history of exposure to a pet cat which resulted in suspicion of an underlying infectious process. As a result, we decided against empiric treatment with immunosuppressive agents. The same rationale resulted in the performance of a kidney biopsy to elucidate the cause of acute kidney injury and to differentiate between infection-related glomerulonephritis versus underlying systemic vasculitis. Glomerular involvement in infection-related glomerulonephritis can be focal or diffuse. Based on the largest biopsy-based cohort (n = 49) published by Boils et al in 2015, crescentic glomerulonephritis was the most predominant histological form (53%) followed by diffuse proliferative glomerulonephritis (DGPN) (33%).1 About 44% of patients in this cohort had pauci-immune pattern of involvement on immunofluorescence. In a previous case series of infection-related glomerulonephritis patients (n = 37) published in 2012, 63% to 66% of patients had pauci-immune pattern.20
Previous cases of Bartonella-related infectious endocarditis with glomerular involvement (n = 10) were summarized by Raybould et al4 in 2016. Eight out of 10 cases showed immune-complex deposition (IgG, IgA, IgM, C1Q, or C3) in the mesangium and capillary loops on IF.4 Only 2 cases were pauci-immune, one from B henselae8 and B Quintana4 each. Our case is overall the fifth reported case of B henselae-related pauci-immune necrotizing glomerulonephritis, and we have tabulated all the previous cases comparing their demographics and clinical characteristics in Table 2.
Table 2.
Clinical Characteristics of Previously Reported Cases of Bartonella Endocarditis With Biopsy-Proven Glomerulonephritis.
| Case | Age | Sex | Organism | Light microscopy | Immunoflorescence | EM | ANCA | Immunosuppression | Antibiotics/valve replacement | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Raybould et al4 | 55 | M | B quintana by serology | Focal proliferative necrosis | Pauci-immune | No EDD | c-ANCA+ | IV methyl prednisone, PO prednisone | Doxycycline and rifampin for 24 weeks. Patient refused valve surgery |
Stable renal functions |
| Vikram et al8 | 43 | M | B henselae by PCR | Focal segmental necrosis/crescents | Pauci-immune | N/A | c-ANCA+ and anti-PR3+ | Cyclophosphamide, PO prednisone | Vancomycin + ceftriaxone + gentamicin for 6 weeks, Doxycycline for 1 year AV + MV replaced |
Stable 18 months postop |
| Beydon et al21
Case 1 |
78 | M | B henselae by serology and aortic valve PCR | Crescentic GN | Pauci-immune | N/A | Anti-PR3+ cryoglobulinemia, +RF | Methyl prednisone, CYC, Plasmapheresis | Doxycycline + rifampicin AV replaced |
Lost follow-up |
| Beydon et al21
Case 2 |
54 | M | B henselae by serology and aortic valve PCR | Crescentic GN | Pauci-immune | N/A | Anti-PR3+ cryoglobulinemia, +RF | Methyl prednisone, CYC, Plasmapheresis | Amoxicillin, vibramycin, and gentamicin AV replaced |
Stable renal functions |
| Shah et al22 | 36 | F | B henselae by serology and aortic valve culture | Crescentic GN | Pauci-immune | N/A | Anti-PR3, RF+ | Methyl prednisone, CYC, AZA and MMF | Doxycycline + gentamicin AV replaced |
Stable renal functions |
| Our case | 33 | M | B henselae by PCR | Focal segmental necrosis/crescent | Pauci-immune | No EDD | Anti-PR3+ | None | Ceftriaxone (6 weeks + gentamicin for 2 weeks for synergy). Doxycycline 12 weeks AV replaced, MV repaired |
Stable renal functions Creatinine stable at 2 mg/dL 1 year later |
| Bookman et al 23
Case 1 |
53 | F | B henselae by serology | Diffuse, segmental necrosis/crescents | Strong IgM, C3-capiilary loop and mesangial deposits. Moderate IgG capillary loop and mesangial deposits | EDD in mesangium and sub endothelium | Negative | IV methyl prednisone, PO prednisone | Ceftriaxone + doxycycline for 6 weeks | Deceased after readmission with renal failure |
| Bookman et al 23
Case 2 |
35 | M | B henselae by serology | Focal segmental necrosis/crescents | Strong IgM, C3-capiilary loop and mesangial deposits. Moderate IgG capillary loop and mesangial deposits | EDD in mesangium and sub endothelium | Negative | PO prednisone | Tobramycin + doxycycline for 6 weeks Cardiac valve replaced |
Stable renal functions |
| Bookman et al 23
Case 3 |
46 | M | B henselae by serology | Focal segmental necrosis/crescents with mild endocapillary proliferation | Strong IgM, C3-capiilary loop and mesangial deposits. Moderate IgG capillary loop and mesangial deposits | EDD in mesangium and subendothelium | Negative | None | Azithromycin+ ceftriaxone for 6 weeks Cardiac valve replaced |
Stable renal functions |
| Salvado et al16 | 78 | F | B henselae by serology | Diffuse crescents/diffuse endocapillary hypercellularity and fibrinoid necrosis | IgM, C3, C1q in capillary loops | N/A | C-ANCA and anti-PR3+ | None | Doxycycline for 8 weeks N/A |
Normal renal functions |
| Van Tooren et al24 | 53 | M | B henselae by serology | Diffuse proliferative GN/crescents | IgG/M, C3, C1q in capillary loops | N/A | Negative | None | Ceftazidime + ofloxacin preop and doxycycline for 6 weeks N/A |
Improved renal functions |
| Sugiyama et al9 | 64 | M | B quintana by serology | Focal sclerosis | Complement deposits | N/A | C-ANCA+ | None | Doxycycline + ceftriaxone for 6 weeks N/A |
Normal renal functions |
| Turner et al19 | 58 | M | B henselae by serology | Focal crescents | IgA, mesangial deposits | N/A | C-ANCA and anti-PR3+ | PO prednisone, cyclophosphamide | Gentamicine + doxycycline for 2 weeks followed by 5 weeks of doxycycline No valve replacement needed |
Normal renal functions |
| Khalighi et al14 | 18 | F | B henselae by serology | Diffuse proliferative GN, focal crescents | IgM, C3, C1q, Kappa, lambda and IgG deposits in capillary loops and mesangium | EDD in subendothelium and mesangium | C-ANCA and anti-PR3 Ab+ | IV methyl prednisone and PO prednisone | Rifampin and doxycycline for 15 weeks N/A |
Stable renal functions |
Note. EM = electron microscopy; ANCA = antineutrophilic cytoplasmic antibodies; EDD = electron dense deposits; PCR = polymerase chain reaction; PR3 Ab = proteinase 3 antibodies; AV = aortic valve; MV = mitral valve; GN = glomerulonephritis; RF = rheumatoid factor; N/A = not available; PO = per oral; CYC = cyclophosphamide; AZA = azathioprine; MMF = mycophenolate mofetil.
The treatment of Bartonella endocarditis is as challenging as the diagnosis itself. The mainstay of therapy remains the surgical valvular repair or replacement. Mortality of patients in which infectious endocarditis is associated with heart failure can be significantly high and reported around 51% based on one study.25 Unfortunately, evidence-based data regarding the optimal management of Bartonella-infective endocarditis is scarce due to rarity of the condition itself. Last update by Infectious Diseases Society of America (IDSA) from 2015 recommends treatment with 6 weeks of oral doxycycline with a bactericidal agent like gentamicin for the first 2 weeks of the antibiotics course.26 Our patient was treated with prolonged antibiotic course (ceftriaxone for 6 weeks, gentamicin for initial 2 weeks, and doxycycline for a total of 3 months). The treatment of pauci-immune glomerulonephritis due to infection-related glomerulonephritis is also aimed at treating the underlying infection. However, there have been 3 refractory cases in literature where treatment with steroids resulted in improved kidney functions.27 -29 However, this approach has to be taken with extreme caution since treatment of an actively bacteremic and immunocompromised patient with high-dose steroids can further compromise the immunity resulting in increased morbidity and mortality. The kidney functions of our patient stabilized after the prolonged course of antibiotics as mentioned above. Serum creatinine was improved to 176 umol/L (2 mg/dL) at discharge and remained stable at 167 to 176 umol/L (1.9-2 mg/dL) at 12 months from discharge without requiring any renal replacement therapy throughout the course of his illness. Our case adds to the existing literature of B henselae-related pauci-immune necrotizing glomerulonephritis cases. Through this patient, we have highlighted the importance of keeping a low threshold to rule out subacute bacterial endocarditis as a culprit of glomerulonephritis in patients who have a high clinical likelihood of ANCA-positive vasculitis. Since the overall management of the 2 conditions vary significantly, more work needs to be done to understand the pathophysiologic bases of high ANCA titers in patients with underlying infection. Through our case, we also emphasize the need to develop more sensitive and specific biological markers to differentiate the 2 conditions.
Learning Points
B henselae-associated glomerulonephritis can clinically and serologically mimic myeloperoxidase (MPO) and PR-3 positive vasculitis.
We present overall the fifth case of B henselae-related glomerulonephritis with pauci-immune pattern, adding to the growing literature.
An evaluation for B henselae-infective endocarditis should be pursued in cases of pauci-immune necrotizing glomerulonephritis.
Titers of either PR-3 antibodies or myeloperoxidase (MPO) antibodies are usually not as high in subacute bacterial endocarditis-related pauci-immune glomerulonephritis cases than systemic vasculitis, hence can be a good differentiating factor between the 2 conditions.
This is imperative to guide optimal decision-making in management of such patients.
Footnotes
Ethics Approval and Consent to Participate: Obtained from the concerned department at Rush University Medical Center. Informed consent was obtained from the patient for publication of this case report and any accompanying images.
Consent for Publication: All authors provided their consent for publication.
Availability of Data and Materials: The de-identified data underlying this article will be shared on reasonable request to the corresponding author.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Muhammad Asim Shahzad  https://orcid.org/0000-0003-1729-8520
https://orcid.org/0000-0003-1729-8520
References
- 1. Boils CL, Nasr SH, Walker PD, Couser WG, Larsen CP. Update on endocarditis-associated glomerulonephritis. Kidney Int. 2015;87(6):1241-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fowler VG, Miro JM, Hoen B, et al. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA. 2005;293(24):3012-3021. [DOI] [PubMed] [Google Scholar]
- 3. Brouqui P, Raoult D. Endocarditis due to rare and fastidious bacteria. Clin Microbiol Rev. 2001;14(1):177-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Raybould JE, Raybould AL, Morales MK, et al. Bartonella endocarditis and pauci-immune glomerulonephritis: a case report and review of the literature. Infect Dis Clin Pract. 2016;24(5):254-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Raoult D, Fournier PE, Drancourt M, et al. Diagnosis of 22 new cases of Bartonella endocarditis. Ann Intern Med. 1996;125(8):646-652. [DOI] [PubMed] [Google Scholar]
- 6. Okaro U, Addisu A, Casanas B, Anderson B. Bartonella species, an emerging cause of blood-culture-negative endocarditis. Clin Microbiol Rev. 2017;30(3):709-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fournier PE, Lelievre H, Eykyn SJ, et al. Epidemiologic and clinical characteristics of Bartonella quintana and Bartonella henselae endocarditis: a study of 48 patients. Medicine. 2001;80(4):245-251. [DOI] [PubMed] [Google Scholar]
- 8. Vikram HR, Bacani AK, DeValeria PA, Cunningham SA, Cockerill FR, III. Bivalvular Bartonella henselae prosthetic valve endocarditis. J Clin Microbiol. 2007;45(12):4081-4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sugiyama H, Sahara M, Imai Y, et al. Infective endocarditis by Bartonella quintana masquerading as antineutrophil cytoplasmic antibody-associated small vessel vasculitis. Cardiology. 2009;114(3):208-211. [DOI] [PubMed] [Google Scholar]
- 10. Marín M, Muñoz P, Sánchez M, et al. Molecular diagnosis of infective endocarditis by real-time broad-range polymerase chain reaction (PCR) and sequencing directly from heart valve tissue. Medicine. 2007;86(4):195-202. [DOI] [PubMed] [Google Scholar]
- 11. Hansmann Y, DeMartino S, Piémont Y, et al. Diagnosis of cat scratch disease with detection of Bartonella henselae by PCR: a study of patients with lymph node enlargement. J Clin Microbiol. 2005;43(8):3800-3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McGeoch L, Carette S, Cuthbertson D, et al. Cardiac involvement in granulomatosis with polyangiitis. J Rheumatol. 2015;42(7):1209-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fukasawa H, Hayashi M, Kinoshita N, et al. Rapidly progressive glomerulonephirits associated with PR3-ANCA positive subacute bacterial endocarditis. Intern Med. 2012;51:2587-2590. [DOI] [PubMed] [Google Scholar]
- 14. Khalighi MA, Nguyen S, Wiedeman JA, Palma Diaz MF. Bartonella endocarditis–associated glomerulonephritis: a case report and review of the literature. Am J Kidney Dis. 2014;63(6):1060-1065. [DOI] [PubMed] [Google Scholar]
- 15. Teoh LS, Hart HH, Soh MC, et al. Bartonella henselae aortic valve endocarditis mimicking systemic vasculitis. BMJ Case Rep. 2010;2010:bcr0420102945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Salvado C, Mekinian A, Rouvier P, Poignard P, Pham I, Fain O. Rapidly progressive crescentic glomerulonephritis and aneurism with antineutrophil cytoplasmic antibody: Bartonella henselae endocarditis. Presse Med. 2013;42(6, pt 1):1060-1061. [DOI] [PubMed] [Google Scholar]
- 17. Satake K, Ohsawa I, Kobayashi N, et al. Three cases of PR3-ANCA positive subacute endocarditis caused by attenuated bacteria (Propionibacterium, Gemella, and Bartonella) complicated with kidney injury. Mod Rheumatol. 2011;21(5):536-541. [DOI] [PubMed] [Google Scholar]
- 18. Yamada Y, Ohkusu K, Yanagihara M, et al. Prosthetic valve endocarditis caused by Bartonella quintana in a patient during immunosuppressive therapies for collagen vascular diseases. Diagn Microbiol Infect Dis. 2011;70(3):395-398. [DOI] [PubMed] [Google Scholar]
- 19. Turner JW, Pien BC, Ardoin SA, et al. A man with chest pain and glomerulonephritis. Lancet. 2005;365(9476):2062. [DOI] [PubMed] [Google Scholar]
- 20. Boils CL, Nasr SH, Walker PD, et al. Infective endocarditis–associated glomerulonephritis: a report of 37 cases [Abstract]. Mod Pathol. 2012;25:396A. [Google Scholar]
- 21. Beydon M, Rodriguez C, Karras A, et al. Bartonella and Coxiella infections presenting as systemic vasculitis: case series and review of literature. Rheumatology. 2021;61:2609-2618. [DOI] [PubMed] [Google Scholar]
- 22. Shah SH, Grahame-Clarke C, Ross CN. Touch not the cat bot a glove: ANCA-positive pauci-immune necrotizing glomerulonephritis secondary to Bartonella henselae. Clin Kidney J. 2014;7(2):179-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bookman I, Scholey JW, Jassal SV, Lajoie G, Herzenberg AM. Necrotizing glomerulonephritis caused by Bartonella henselae endocarditis. Am J Kidney Dis. 2004;43(2):e25-e30. [DOI] [PubMed] [Google Scholar]
- 24. van Tooren RM, van Leusen R, Bosch FH. Culture negative endocarditis combined with glomerulonephritis caused by Bartonella species in two immunocompetent adults. Neth J Med. 2001;59(5):218-224. [DOI] [PubMed] [Google Scholar]
- 25. Sexton DJ, Spelman D. Current best practices and guidelines: assessment and management of complications in infective endocarditis. Cardiol Clin. 2003;21(2):273-282. [DOI] [PubMed] [Google Scholar]
- 26. Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation. 2005;111(23):e394-e434. [DOI] [PubMed] [Google Scholar]
- 27. Ghosh GC, Sharma B, Katageri B, Bhardwaj M. ANCA positivity in a patient with infective endocarditis-associated glomerulonephritis: a diagnostic dilemma. Yale J Biol Med. 2014;87(3):373-377. [PMC free article] [PubMed] [Google Scholar]
- 28. Koya D, Shibuya K, Kikkawa R, Haneda M. Successful recovery of infective endocarditis-induced rapidly progressive glomerulonephritis by steroid therapy combined with antibiotics: a case report. BMC Nephrology. 2004;5(1):1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Le Moing V, Lacassin F, Delahousse M, et al. Use of corticosteroids in glomerulonephritis related to infective endocarditis: three cases and review. Clin Infect Dis. 1999;28(5):1057-1061. [DOI] [PubMed] [Google Scholar]