Abstract
Melanins are implicated in the pathogenesis of several human diseases, including some microbial infections. In this study, we analyzed whether the conidia and the yeasts of the thermally dimorphic fungal pathogen Paracoccidioides brasiliensis produce melanin or melanin-like compounds in vitro and during infection. Growth of P. brasiliensis mycelia on water agar alone produced pigmented conidia, and growth of yeasts in minimal medium with l-3,4-dihydroxyphenylalanine (l-DOPA) produced pigmented cells. Digestion of the pigmented conidia and yeasts with proteolytic enzymes, denaturant, and hot concentrated acid yielded dark particles that were the same size and shape as their propagules. Immunofluorescence analysis demonstrated reactivity of a melanin-binding monoclonal antibody (MAb) with the pigmented conidia, yeasts, and particles. Electron spin resonance spectroscopy identified the yeast-derived particles produced in vitro when P. brasiliensis was grown in l-DOPA medium as a melanin-like compound. Nonreducing polyacrylamide gel electrophoresis of cytoplasmic yeast extract revealed a protein that catalyzed melanin synthesis from l-DOPA. The melanin binding MAb reacted with yeast cells in tissue from mice infected with P. brasiliensis. Finally digestion of infected tissue liberated particles reactive to the melanin binding MAb that had the typical morphology of P. brasiliensis yeasts. These data strongly suggest that P. brasiliensis propagules, both conidia and yeast cells, can produce melanin or melanin-like compounds in vitro and in vivo. Based on what is known about the function of melanin in the virulence of other fungi, this pigment may play a role in the pathogenesis of paracoccidioidomycosis.
Paracoccidioides brasiliensis is the causative agent of paracoccidioidomycosis, one of the most important systemic mycoses in Central and South America (30). The disease initially involves the lungs, with subsequent dissemination to other organs; secondary lesions may occur in the mucous membranes, the skin, lymph nodes, and the adrenal glands. Two forms of disease are recognized: the more common chronic form (adult type), and the rare acute or subacute form (juvenile type) (2, 30). The organism is presumed to exist in the environment in the mycelial phase, where it produces airborne conidia. In experimental models, conidia are infectious; when inhaled into the lungs, they transform into the yeast phase and disseminate to other organs (20). This pattern of infection is consistent with clinical observations (30). Little is known of the pathogenic processes that underpin this sequence of events or of the mechanisms by which the organism survives in the environment.
Melanins are multifunctional polymers found in diverse species that include representatives of all biological kingdoms (13). Typically, they are dark brown or black pigments of high molecular weight formed by the oxidative polymerization of phenolic and/or indolic compounds (26, 45). In fungi, melanins have been implicated in the virulence of plant pathogens (19, 25). With regard to human fungal pathogens, most attention has focused on the melanization of Cryptococcus neoformans. In this encapsulated yeast, melanization is catalyzed by a laccase when cells are grown in the presence of exogenous dihydroxyphenolic compounds (14, 15, 46). In vitro studies have shown that melanized C. neoformans cells are less susceptible to UV light-induced damage (41), macrophage-mediated phagocytosis (1, 43), oxidant-mediated damage (44), antimicrobial peptides (4), heavy metal toxicity (9), and antifungal drugs such as amphotericin B (42) than nonmelanized cells. These results suggest that melanins play a role in protection against environmental insults, host defense mechanisms, and antimicrobial therapies. Both classical genetic and gene disruption studies have demonstrated that wild-type melanin-producing (Mel+) C. neoformans cells are more virulent than their corresponding albino (Mel−) mutants (17, 18, 31, 36). There is now strong evidence that melanization in C. neoformans occurs in vivo, since monoclonal antibodies (MAbs) to melanin label yeasts in tissue (24, 34, 35), melanin particles can be isolated from infected tissue, yeast cells in tissue darken progressively with time of infection and undergo cell wall changes consistent with melanin deposition (6), and infected animals produce an antibody response against melanin (21, 23). C. neoformans cells isolated from pigeon feces (a major environmental source) have also recently been demonstrated to express the pigment (22), suggesting that the infectious propagule is probably melanized at the point of inhalation.
No previous substantive efforts have been made to detect melanization in P. brasiliensis. However, P. brasiliensis mycelial cultures, which are typically white, sometimes produce a brown pigment, and conidia are darkly colored after collection from water-agar medium (A. Restrepo, unpublished data). Accordingly, given the potential role of melanin in protection in the environment and in virulence, we investigated whether the conidia and yeasts of P. brasiliensis synthesize melanin or melanin-like compounds. We used recently developed techniques and a melanin isolation protocol (24, 35) to determine whether the conidial and yeast forms of P. brasiliensis melanize in vitro and during infection. The results demonstrate the presence of melanin or melanin-like pigments in conidia and yeast of P. brasiliensis.
MATERIALS AND METHODS
Fungal strains.
P. brasiliensis strains 60855 and 32069 isolated from Colombian patients were obtained from the American Type Culture Collection (Manassas, Va.).
Growth of P. brasiliensis mycelia and production of conidia.
P. brasiliensis isolate ATCC 60855, previously known to sporulate on special media, was used for the production of conidia (29). The techniques used to grow the mycelial form and to collect and dislodge conidia have been reported elsewhere (29). Briefly, the stock mycelial culture was grown in a liquid, chemically defined medium (28) for 10 to 15 days at 18°C with continuous shaking at 150 rpm. The mycelial masses were homogenized, and portions were used to inoculate agar plates (10 g of Bacto Agar [Difco, Detroit, Mich.] per liter of distilled water), which were then incubated at 18°C for 2 to 3 months. All cultures were performed in the dark to prevent photopolymerization. Fungal growth was scraped off in phosphate-buffered saline (PBS; 0.1 M, pH 7.4) containing 0.85% Tween 20, and conidia were dislodged by agitation with glass beads. The suspension was filtered through a syringe packed with sterile glass wool (8 μm; Pyrex fiberglass; Corning Glass Works, Coming, N.Y.) and then concentrated by centrifugation. The conidia were washed in PBS and counted with a hemocytometer. The viability of the conidia was assessed via ethidium bromide fluorescein diacetate as described elsewhere (3).
Growth of P. brasiliensis yeast with or without l-DOPA.
P. brasiliensis isolates ATCC 60855 and 32069 were transformed from the mycelilim to the yeast form as described previously (7), and a cytoplasmic yeast extract (CYE) from both isolates was produced as described elsewhere (11). Yeast cells of isolate ATCC 60855 were also grown either on a solid chemically defined medium (28) supplemented with 1.0 mM l-3,4-dihydroxyphenylalanine (l-DOPA) for a total of 10 days at 37°C or in a defined liquid minimal medium (15.0 mM glucose, 10.0 mM MgSO4, 29.4 mM KH2PO4, 13.0 mM glycine, 3.0 M vitamin B1 [pH 5.5]) with or without 1.0 mM l-DOPA (Sigma Chemical Co., St Louis, Mo.) for 15 days at 37°C in a rotary shaker at 150 rpm. All cultures were performed in the dark to prevent photopolymerization. Cells were collected either by scraping or by centrifugation at 3,000 rpm for 30 min, autoclaved, washed with PBS, and stored at 4°C until use. Wild-type (Mel+) C. neoformans JEC21 and its albino mutant (Mel−) C. neoformans HMC6 were used as positive and negative controls, respectively. These C. neoformans strains have been described elsewhere (34). In addition, a C. albicans clinical isolate (ER 2841) was also grown on media with and without l-DOPA under the conditions described above.
Isolation and purification of conidia and yeast melanin particles, scanning electron microscopy, and ESR-spectroscopy.
Melanin particles were isolated from conidia and yeasts by a modification of a methodology described previously (34). Briefly, conidia and yeast cells were collected by centrifugation at 3,000 rpm for 30 min, autoclaved, washed with PBS, and suspended in 1.0 M sorbitol–0.1 M sodium citrate (pH 5.5). Cell wall-lysing enzymes (from Trichoderma harzianum [Sigma]) were added at a concentration of 10 mg/ml, and the suspensions were incubated overnight at 30°C to generate protoplasts. The protoplasts were collected by centrifugation, washed with PBS, and incubated in 4.0 M guanidine thiocyanate (denaturant) overnight at room temperature. Cell debris was collected by centrifugation, washed three times with PBS, and treated with proteinase K (1.0 mg/ml; Roche Laboratories; Indianapolis, Ind.), made up in reaction buffer (10.0 mM Tris, 1.0 mM CaCl2, 0.5% sodium dodecyl sulfate [SDS] [pH 7.8]), overnight at 37°C. The resultant materials were washed three times with PBS and then boiled in 6.0 M HCl for 1 h. The materials remaining after acid digestion were collected by centrifugation, washed extensively with PBS, and dialyzed against distilled water for 10 days. Scanning electron microscopy of melanin particles from both conidia and yeast of P. brasiliensis 60855 was then performed as described elsewhere (44). ESR spectroscopy analyses were performed only on the melanin from yeast grown in l-DOPA media as described previously (44) except that a Gunn diode replaced the klystron as microwave source.
To investigate whether fungal chitin interfered with the melanin isolation procedure, chitin flakes (Sigma) were subjected to the denaturant, enzymes, and boiling acid. In addition, calcofluor white (Sigma) was used to label conidia and yeasts before and after the above treatment.
Experimental infection of mice with P. brasiliensis conidia.
Isogenic BALB/c male mice (4 to 6 weeks old and weighing 18 to 20 g) were obtained from the breeding colony of the Corporación para Investigaciones Biológicas (Medellín, Colombia) and used for all experiments. Mice were supplied with sterilized commercial food pellets, sterilized bedding, and fresh acidified water. Eleven mice were inoculated intranasally with 3 × 106 viable conidia of P. brasiliensis suspended in 60 μl of PBS (experimental group) and six mice were inoculated with PBS (control group) as described elsewhere (8). Mice were sacrificed at 12 weeks postinoculation, and the lungs, spleen, and liver were removed and either embedded in paraffin wax or kept frozen at −70°C.
MAbs.
MAb 6D2 (μκ) was generated against C. neoformans-derived DOPA-melanin and binds other types of melanins (35). The MAb does not bind C. albicans, Saccharomyces cerevisiae yeast cells, or a laccase-deficient mutant of C. neoformans (35). MAb 5C11 (μκ) to mycobacterial lipoarabinomannan (10) was used as an isotype-matched negative control. The MAbs were purified from concentrated cell culture supernatants by ultralinked mannan-binding protein chromatography (Pierce, Rockford, Ill.) according to the manufacturer's instructions, and their concentrations were determined by enzyme-linked immunosorbent assay relative to purified murine polyclonal immunoglobulin M (IgM; 1.0 mg/ml; ICN Biomedicals, Aurora, Ohio). The MAbs were suspended in PBS with 0.02% azide at 1.0 mg/ml. Antibody solutions were kept at −20°C until use in immunofluorescence (IF) analyses.
IF analysis.
Approximately 106 P. brasiliensis conidia or yeasts (and particles derived from the same number of cells via denaturation and enzyme and acid treatment) were paraffin embedded, and 4-μm sections were cut. Tissue sections from infected mice were also cut, together with negative control biopsy material from two human infections of C. albicans. Paraffin sections were deparaffinized in xylene, rehydrated in an ethanol series, treated with 20 μg of proteinase K per ml for 1 h at room temperature, and then heated in 10 mM citric acid in a microwave oven for 5 min. Sections were incubated with SuperBlock (Pierce) blocking buffer in PBS for 4 h and then incubated with the relevant MAb (10 μg/ml) for 2 h at 37°C. After washing with PBS, sections were incubated with a 1:100 dilution of fluorescein isothiocyanate-conjugated anti-mouse IgM (Southern Biotechnologies Associates, Inc.) for 1.5 h at 37°C. The sections were washed with PBS to eliminate unbound antibody and then mounted using 50% glycerol–50% PBS–0.1 M N-propyl gallate solution. An Olympus AX70 microscope (Olympus America Inc., Melville, N.Y.) was used to examine sections at a magnification of ×250. Negative controls consisted of sections incubated with fluorescein-conjugated anti-mouse IgM antibody only and sections incubated with MAb 5C11 in place of 6D2.
SDS-PAGE analysis of laccase activity.
The laccase activity of CYE was determined as described elsewhere (44). Briefly, CYE protein concentrations were determined via the Coomasie blue method (27). Commercially prepared laccase (from Rhus vernificera) was obtained from Sigma (activity, 50 U per mg of solid). R. vernificera laccase (200 μg) and 150 and 300 μg of CYE from P. brasiliensis isolates ATCC 32069 and ATCC 60855, respectively, were separated by 10% polyacrylamide gel electrophoresis (PAGE) run at 18 mA overnight under nondenatring conditions. As controls, samples of each of the above were treated with 10 μl of a 1 M solution of potassium cyanide (KCN), an irreversible inhibitor of laccase enzyme activity, before loading onto the gel. In addition, a cytoplasmic extract from a C. albicans isolate (ER 2841) was also tested. Gels were then incubated with 1 mM l-DOPA in 0.1 M citric acid–0.2 M Na2HPO4 (pH 6.0) buffer for 6 to 8 h.
Extraction of melanin particles from infected tissues.
Frozen and paraffin-embedded tissue (lung and spleen) from P. brasiliensis-infected mice were used. Fresh tissues spiked with melanized or nonmelanized organisms were also used as controls; in addition, mice were intranasally inoculated with heat-killed nonmelanized yeast and sacrificed after 4 days, and the lungs were then removed for processing. The paraffin-embedded tissues were initially dewaxed in xylene. All tissues were then treated in succession with cell wall-lysing enzymes (from T. harzianum [Sigma]), at a concentration of 10 mg/ml, 4 M guanidine thiocyanate, proteinase K, and boiling 6 M HCl (24) (as described earlier). After washing, residual material was processed for immunofluorescence development with MAbs and for scanning electron microscopy as described above (34).
RESULTS
Melanization of P. brasiliensis conidia and yeast cells.
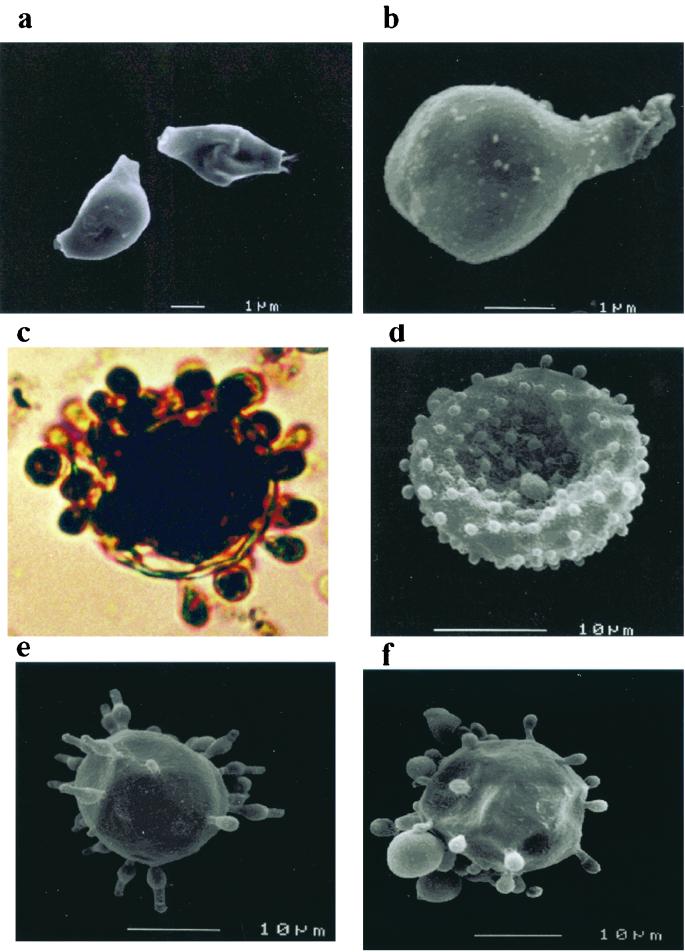
Mycelia grown on water agar produced conidia, which when dislodged and collected by centrifugation gave rise to a dark pellet. Treatment of the pellet with proteolytic and glycolytic enzymes, denaturant, and hot concentrated acid left a black residue. This consisted of dark particles that retained the size and shape of the conidia, as determined by scanning electron microscopy (Fig. 1a and b). Some of the pigmented particles were still attached to the remnants of mycelial elements. P. brasiliensis yeasts grown on solid agar medium supplemented with l-DOPA became dark after 8 days, and microscopic examination revealed that about 10% of the yeasts were visibly darker than other cells, with a dark brown internal pigment present (data not shown). After a period of 5 to 7 days, P. brasiliensis yeasts grown on liquid minimal medium supplemented with l-DOPA began to produce black cultures, which when examined with the light microscope were seen to consist of darkly pigmented yeast cells (approximately 30% of them) (Fig. 1c and d). The pigment was localized within the cytoplasm as well as in the cell wall. For positive and negative controls, Mel+ C. neoformans JEC21 and the Mel− mutant HMC6, respectively, were used. Pigmentation was observed with strain JEC21 but not with the Mel− strain (data not shown). The C. albicans isolate tested did not show pigmentation when grown on media with l-DOPA (data not shown). No pigmentation of P. brasiliensis yeasts was observed when cells were grown without l-DOPA. Pigmented yeast cells treated with proteolytic and glycolytic enzymes, denaturant, and hot concentrated acid yielded a dark residue when collected by centrifugation. Yeast cells grown in the absence of l-DOPA treated with the melanin isolation protocol were completely solubilized. Chitin was also solubilized by this procedure. Calcofluor white labeled the walls of conidia and yeasts but did not stain the residual particles left after treatment with denaturant and acid (data not shown). Scanning electron microscopy of the debris from pigmented cells revealed individual particles of approximately the same size and shape as intact P. brasiliensis yeasts (Fig. le and f). These particles are presumed to be melanin “ghosts” of melanized cells analogous to similar structures that have been isolated from melanized C. neoformans.
FIG. 1.
Scanning electron micrographs of P. brasiliensis conidia and photomicrographs and scanning electron micrographs of yeast cells (ATCC 60855) before and after treatment with proteinases, guanadinium isothiocyanate, and hydrochloric acid. (a and b) Conidia before and after treatment respectively, (c and d) light photomicrograph and scanning electron micrograph, respectively, of P. brasiliensis (ATCC 60855) yeasts grown on l-DOPA (without treatment); (e and f) yeast cells grown on minimal media supplemented with l-DOPA before and after treatment, respectively.
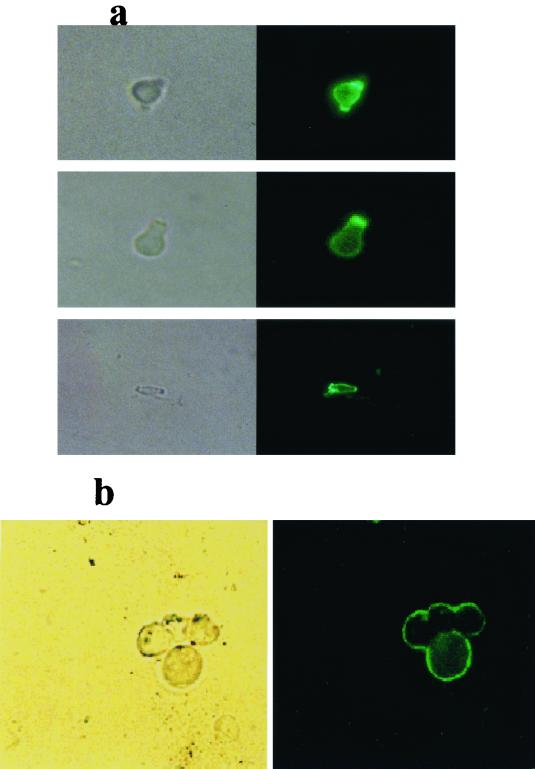
IF analyses demonstrated reactivity of the melanin-binding MAb 6D2 with conidia and yeast cells embedded in paraffin wax and with melanin particles produced from the latter after denaturation, enzyme treatment, and boiling with acid. Examples are provided of the MAb staining the intact conidial cell wall and the outer extremities of yeast particles grown on l-DOPA (Fig. 2a and b, respectively). Negative control MAb 5C11 did not react with conidia or yeasts or with the particles derived from them (data not shown). Yeasts grown in the absence of l-DOPA did not react with MAb 6D2 (data not shown) and did not produce any particles after denaturation, enzyme treatment and boiling with acid.
FIG. 2.
Immunofluorescent reactivity of MAb 6D2 (raised against melanin from C. neoformans) to P. brasiliensis (ATCC 60855) to intact conidia grown in vitro (a) and to melanin particles remaining after denaturation, enzyme, and acid treatment of yeasts produced in vitro (b), grown with l-DOPA.
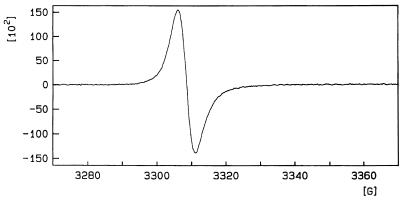
ESR spectroscopy.
ESR spectroscopy of particles collected from P. brasiliensis yeasts grown in culture with l-DOPA produced a strong and reproducible signal (Fig. 3). This spectrum demonstrates the presence of a stable free-radical population identical to that which defines a pigment as melanin (5). In addition, the spectrum was almost identical to that previously identified for C. neoformans melanin (44). Unfortunately, material sufficient for ESR analysis could not be collected from conidia, as they are much more difficult to harvest in the quantities necessary for this measurement.
FIG. 3.
ESR spectroscopy of melanin particles collected from P. brasiliensis yeasts (ATCC 60855).
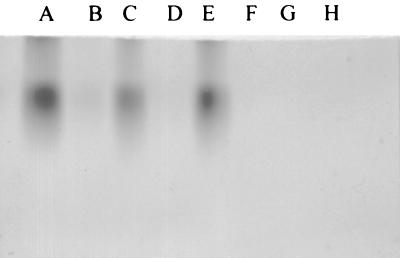
Laccase activity in CYE.
To determine whether P. brasiliensis had laccase activity, CYE from P. brasiliensis 60855 and 32069 were subjected to SDS-PAGE in a nondenaturing gel and then incubated with l-DOPA for 2 h. This resulted in the formation of dark bands consistent with polymerized DOPA-melanin (Fig. 4). The same results were observed with the commercially available R. vernificera laccase. Treatment of the P. brasiliensis CYE and R. vernificera laccase with KCN abolished the enzymatic activity. A C. albicans extract did not produce a band of polymerized DOPA-melanin.
FIG. 4.
Nonreducing SDS-polyacrylamide gel of CYE of P. brasiliensis developed with l-DOPA. Synthesis of a black pigment consistent with melanin occurs in situ on the gel. Tracks: A, commercial laccase (40-U equivalent); B, as for A but treated with KCN; C, 150 μg of CYE of P. brasiliensis isolate 32069; D, as for C but treated with KCN; E, 300 μg of CYE of P. brasiliensis isolate 60855; F, as for E but treated with KCN; G, 300 μg of CYE of C. albicans isolate; H, as for G but treated with KCN.
Immunohistochemical analysis of infected tissue.
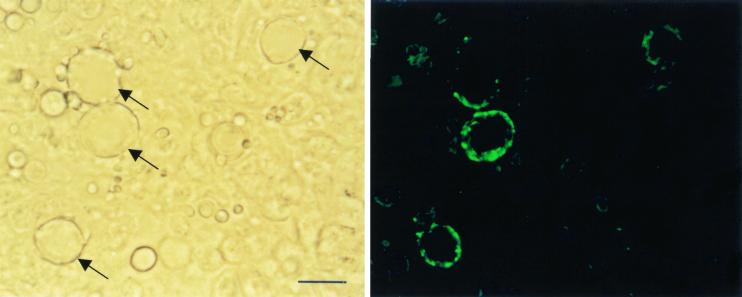
MAb 6D2 demonstrated reactivity to yeast cells in paraffin wax-embedded tissue from mice infected with P. brasiliensis (Fig. 5). Reactivity was confined to the exterior of cells, which presumably is the cell wall area. Not all cells were reactive. This may represent cell-to-cell heterogeneity in melanin production and/or differences in the plane of focus at which the photographs were taken. No reactivity was observed with the negative control MAb 5C11 (data not shown) or when goat anti-mouse IgG-fluorescein conjugate was incubated directly on sections in the absence of MAb 6D2 (data not shown). C. albicans yeast cells in human biopsy material were unreactive with MAb 6D2 (data not shown).
FIG. 5.
Reactivity of MAb 6D2 to yeast cells in paraffin wax-embedded tissue from mice infected with P. brasiliensis. Bar represents 40 μm. Reactive yeast cells are arrowed.
Extraction of melanin particles from infected mouse tissue.
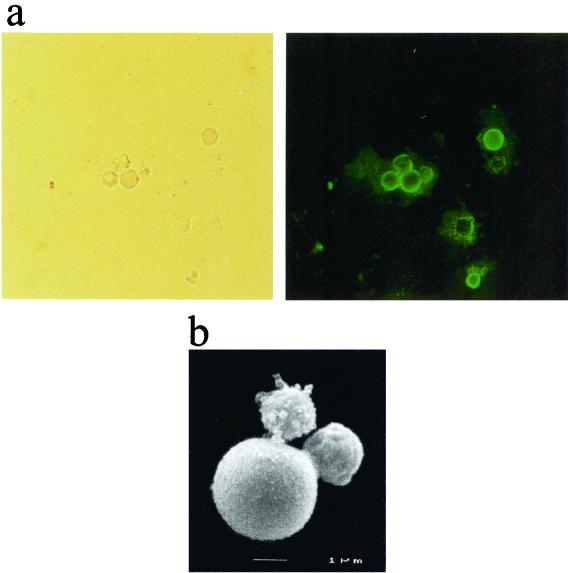
Treatment of lung and spleen tissue from three mice with denaturant, enzymes, and hot concentrated acid yielded a small amount of dark residue which when observed microscopically contained particles which were the same size and shape as yeast cells (Fig. 6a). These particles were reactive by immunofluorescence with the antimelanin MAb, whereas particles incubated with the fluorescein conjugate only or with the control antibody (and conjugate) did not show any reactivity (data not shown). Scanning electron microscopy demonstrated that the particles had the typical morphology of P. brasiliensis yeasts (Fig. 6b). Dark particles were also observed following treatment of fresh tissue spiked with in vitro-melanized yeast cells (data not shown). Fresh tissues spiked with nonmelanized yeast cells or processed tissues from mice intranasally inoculated with heat-killed nonmelanized yeast cells were completely solubilized.
FIG. 6.
Extraction of melanin particles from P. brasiliensis-infected mouse tissue. (a) Light microscope appearance of particles and their immunofluorescent reactivity with MAb 6D2 raised against melanin; (b) scanning electron micrograph of extracted particle.
DISCUSSION
P. brasiliensis mycelial form has been observed to produce pigments under certain conditions (30), but the chemical nature of these pigments is unknown. We have applied techniques recently developed for the study of melanization of C. neoformans (23, 24, 35) to investigate the nature of the P. brasiliensis pigment. The following lines of evidence suggest that the pigment(s) produced by P. brasiliensis in vitro and in vivo is a type of melanin: (i) recovery of pigmented particles after chemical and enzymatic treatment of conidia and yeasts grown in vitro (on water agar in the former case and in the presence of l-DOPA in the latter), (ii) reactivity of a melanin-binding MAb to a cell wall component of P. brasiliensis conidia and yeast grown in vitro, (iii) detection of an ESR profile consistent with that of melanin from the extracted yeast pigment for cells grown in the presence of l-DOPA, (iv) detection of laccase activity in protein extracts of P. brasiliensis, (v) reactivity of a melanin-binding MAb with the cell wall of P. brasiliensis yeast in infected tissue, and (vi) recovery of melanin-like particles from infected mouse tissue after chemical and enzymatic treatment (these particles were reactive with antibody to melanin and had yeast like morphology).
In this study, we noted that yeast cells grown in cultures with l-DOPA became black and that the pigment was a melanin, as indicated by resistance to acid hydrolysis, ESR spectra, and reactivity with a MAb to melanin. A laccase-like activity was detected in nondenaturing protein gels, consistent with the presence of such an enzyme in P. brasiliensis. Although the occurrence of melanization in vitro and the presence of a laccase-like activity in protein extracts provide strong suggestive evidence for enzymatic synthesis of melanin in yeast cells, we cannot completely rule out l-DOPA autopolymerization on cell walls in this situation. Conclusive evidence for catalytic synthesis of melanin from l-DOPA in vitro must await the more detailed characterization of the putative laccase and the generation of mutants deficient in this enzyme. In contrast, the evidence for in vitro conidial melanization is significantly stronger. P. brasiliensis conidia become pigmented when suspended in water, indicating a capacity to synthesize melanin-like pigment in the absence of l-DOPA. This indicates that P. brasiliensis has the enzymatic machinery to synthesize melanin. Recently dihydroxynaphthalene melanin precursors have been detected in conidia from the pulmonary pathogen Aspergillus fumigatus (39, 40), and it appears that the production of this type of melanin is associated with virulence. A similar association is likely in Sporothrix schenckii (32). It is possible that DHN melanin is the principal or sole type produced in P. brasiliensis conidia, although further investigation of the relevant biosynthetic pathways is required to confirm this possibility.
P. brasiliensis does not stain with the Masson-Fontana stain, an observation which led to the assumption that it does not melanize (38). However, this stain is not very sensitive, and it is not specific for melanin (5), with both nonmelanized and melanized C. neoformans yeasts staining positive (16). The positive staining observed with the melanin-binding MAb in infected tissue and the recovery of yeast-like particles from infected tissue and their reactivity to the melanin-binding MAb are observations consistent with in vivo melanization. We considered the possibility that the particles recovered from tissue were composed of chitin, but this is extremely unlikely since chitin is solubilized by the extraction procedure and the particles do not stain with calcofluor. Although these findings are strongly suggestive for the occurrence of P. brasiliensis yeast cell melanization, a definitive conclusion will require studies with mutants as was done with C. neoformans (34). Furthermore, we note that although the recovery of acid-resistant particles from tissue that stain with MAb to melanin suggests the synthesis of melanin in the cell of P. brasiliensis during infection, the chemical nature of this polymer is unknown and there is no evidence that it would necessarily be DOPA-melanin.
Melanin has been implicated in virulence for several fungal pathogens. At this time we have no data to suggest that melanin deposition plays a role in the virulence of P. brasiliensis. However, at the very least, conidial melanization is likely to protect this stage in the life cycle from various environmental insults, such as UV radiation (41) and extremes of temperature (33). Given the fact that conidia presumably have a dual role as infectious propagules and as agents of environmental dissemination, it is possible that the extra protection provided by melanization would constitute an important attribute. The ability of conidia to produce melanin-like compounds in the absence of substrates such as l-DOPA differentiates the melanization process seen in this phase of the development of P. brasiliensis from that seen in C. neoformans, which relies on the presence of exogenous substrate to drive the process (12).
As is the case with conidial melanization, we have no evidence as to whether the deposition of melanin-like pigments in yeasts plays any role in virulence; this too is clearly a rich area for further research. There is now strong evidence suggesting that melanization serves a protective role for C. neoformans (36, 43, 44), Exophiala dermatitidis (37), and S. schenckii (32). Consequently, melanin could be expected to perform a similar protective role in P. brasiliensis.
In summary, our results indicate that (i) P. brasiliensis conidia are melanized; (ii) melanin pigment can form on P. brasiliensis yeast forms when grown in vitro; and (iii) particles similar to the C. neoformans melanin ghosts can be isolated from P. brasiliensis-infected tissues. These results suggest a need for additional studies of the potential for this fungus to synthesize melanin-like pigments in vitro and in vivo. If this is the case, then melanization in P. brasiliensis would be a potential target for therapeutic intervention. Experimental animal studies in which melanin production or expression are blocked will contribute to a better understanding of the in vivo functions of melanin in P. brasiliensis.
ACKNOWLEDGMENTS
We thank the UNESCO/ASM travel award (1999) for sponsoring Beatriz L. Gómez during her time in A. Casadevall's laboratory. Joshua Nosanchuk is supported by NIH grant K08-AI01489; Arturo Casadevall is supported by NIH grants R01-AI33774, AI13342, and HL59842 and a Burroughs Wellcome Scholar award in Experimental Therapeutics. Philip Aisen is supported by grant 5 RO1 DK15056. Angela Restrepo and Luz E. Cano are supported by the CIB and Colciencias in Colombia, and Soraya Diez is supported by the CIB and an ORS (UK Government) award.
Andrew J. Hamilton and Arturo Casadevall share senior authorship on the manuscript.
REFERENCES
- 1.Blasi E, Barluzzi R, Marzolla R, Tancini B, Saleppico S, Pulti M, Pitzurra L, Bistoni F. Role of nitric oxide and melanogenesis in the accomplishment of anticryptococcal activity by the BV-2 microglial cell line. J Neuroimmunol. 1995;58:111–116. doi: 10.1016/0165-5728(95)00016-u. [DOI] [PubMed] [Google Scholar]
- 2.Brummer E, Castañeda E, Restrepo A. Paracoccidioidomycosis: an update. Clin Microbiol Rev. 1993;6:89–117. doi: 10.1128/cmr.6.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calich V L, Purchio A, Paula C R. A new fluorescent viability test for fungal cells. Mypopathology. 1978;66:175–177. doi: 10.1007/BF00683967. [DOI] [PubMed] [Google Scholar]
- 4.Doering T L, Nosanchuk J D, Roberts W K, Casadevall A. Melanin as a potential cryptococcal defense against microbicidal peptides. Med Mycol. 1999;37:175–181. [PubMed] [Google Scholar]
- 5.Enochs W S, Nilges M J, Swartz H M. A standardized test for the identification and characterization of melanin using electron paramagnetic (EPR) spectroscopy. Pigm Cell Res. 1993;6:91–99. doi: 10.1111/j.1600-0749.1993.tb00587.x. [DOI] [PubMed] [Google Scholar]
- 6.Feldmesser, M., Y. Kress, and A. Casadevall. Dynamic changes in the morphology of Cryptococcus neoformans during infection. Microbiology, in press. [DOI] [PubMed]
- 7.Figueroa J, Hamilton A J, Bartholomew M A, Fenelon L E, Hay R J. Preparation of species specific murine monoclonal antibodies against the yeast phase of Paracoccidioides brasiliensis. J Clin Microbiol. 1990;28:1766–1769. doi: 10.1128/jcm.28.8.1766-1769.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franco L, Najvar L, Gómez B L, Restrepo S, Graybill J R, Restrepo A. Experimental pulmonary fibrosis induced by Paracoccidioides brasiliensis conidia: measurement of local host responses. Am J Trop Med Hyg. 1998;58:424–430. doi: 10.4269/ajtmh.1998.58.424. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Rivera, J., and A. Casadevall. Melanization of Cryptococcus neoformans reduces its susceptibility to the antimicrobial effects of silver nitrate. Med. Mycol., in press. [DOI] [PubMed]
- 10.Glatman-Freedman A, Martin J M, Rifka P F, Bloom B R, Casadevall A. Monoclonal antibodies to surface antigens of Mycobacterium tuberculosis and their use in a modified enzyme-linked immunosorbent spot assay for detection of mycobacteria. J Clin Microbiol. 1996;34:2795–2802. doi: 10.1128/jcm.34.11.2795-2802.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gómez B L, Figueroa J I, Hamilton A J, Ortiz B, Robledo M A, Hay R J, Restrepo A. Use of monoclonal antibodies in diagnosis of paracoccidioidomycosis: new strategies for detection of circulating antigens. J Clin Microbiol. 1997;35:3278–3283. doi: 10.1128/jcm.35.12.3278-3283.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamilton A J, Holdom M. Antioxidant system in the pathogenic fungi of man and their role in virulence. Med Mycol. 1999;37:375–389. doi: 10.1046/j.1365-280x.1999.00208.x. [DOI] [PubMed] [Google Scholar]
- 13.Hill H Z. The function of melanin or six blind people examine an elephant. Bioassays. 1992;14:49–56. doi: 10.1002/bies.950140111. [DOI] [PubMed] [Google Scholar]
- 14.Ikeda R, Jacobson E S. Heterogeneity of phenol oxidases in Cryptococcus neoformans. Infect Immun. 1992;60:3552–3555. doi: 10.1128/iai.60.9.3552-3555.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ikeda R, Shinoda T, Morita T, Jacobson E S. Characterization of a phenol oxidase from Cryptococcus neoformans var. neoformans. Microbiol Immunol. 1993;37:759–764. doi: 10.1111/j.1348-0421.1993.tb01702.x. [DOI] [PubMed] [Google Scholar]
- 16.Kwon-Chung K J, Hill W B, Bennett J E. New, special stain for histopathological diagnosis of cryptococcosis. J Clin Microbiol. 1981;13:383–387. doi: 10.1128/jcm.13.2.383-387.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwon-Chung K J, Polachek I, Popkin T J. Melanin-lacking mutants of Cryptococcus neoformans and their virulence in mice. J Bacteriol. 1982;150:1414–1421. doi: 10.1128/jb.150.3.1414-1421.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwon-Chung K J, Rhodes J C. Encapsulation and melanin formation as indicators of virulence in Cryptococcus neoformans. Infect Immun. 1986;51:218–223. doi: 10.1128/iai.51.1.218-223.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lundqvist T, Rice J, Hodge C N, Basarab G S, Rice J, Pierce J. Crystal structure of scytalone dehydratase—the disease determinant of the rice pathogen Magnaporthe grisea. Structure. 1996;15:937–944. doi: 10.1016/s0969-2126(94)00095-6. [DOI] [PubMed] [Google Scholar]
- 20.McEwen J G, Bedoya V, Patiño M, Salazar M E, Restrepo A. Experimental murine paracoccidioidomycosis induced by inhalation of conidia. J Med Vet Mycol. 1987;25:165–175. doi: 10.1080/02681218780000231. [DOI] [PubMed] [Google Scholar]
- 21.Nosanchuk J D, Rosas A L, Casadevall A. The antibody response to fungal melanin in mice. J Immunol. 1998;160:6026–6031. [PubMed] [Google Scholar]
- 22.Nosanchuk J D, Rudolph J, Rosas A L, Casadevall A. Evidence that Cryptococcus neoformans is melanized in pigeon excreta: implications for pathogenesis. Infect Immun. 1999;67:5477–5479. doi: 10.1128/iai.67.10.5477-5479.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nosanchuk J D, Valadon P, Feldmesser M, Casadevall A. Melanization of Cryptococcus neoformans in murine infection. Infect Immun. 1999;19:745–750. doi: 10.1128/mcb.19.1.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nosanchuk J D, Rosas A L, Lee S-H, Casadevall A. Melanization of Cryptococcus neoformans in infected human brain tissue. Lancet. 2000;355:2049–2050. doi: 10.1016/S0140-6736(00)02356-4. [DOI] [PubMed] [Google Scholar]
- 25.Perpetua N S, Kubo Y, Yasuda N, Takano Y, Furusawa I. Cloning and characterization of a melanin biosynthetic THR1 reductase gene essential for appressorial penetration of Colletotrichum lagenarium. Mol Plant Microbe Interact. 1996;9:323–329. doi: 10.1094/mpmi-9-0323. [DOI] [PubMed] [Google Scholar]
- 26.Polak A. Melanin as a virulence factor in pathogenic fungi. Mycoses. 1989;33:215–224. doi: 10.1111/myc.1990.33.5.215. [DOI] [PubMed] [Google Scholar]
- 27.Read S M, Northcote D H. Minimization of variation in the response to different proteins of the Coomassie blue G dye-binding assay for protein. Anal Biochem. 1981;116:53–64. doi: 10.1016/0003-2697(81)90321-3. [DOI] [PubMed] [Google Scholar]
- 28.Restrepo A, Jimenez B E. Growth of Paracoccidioides brasiliensis yeast phase in a chemically defined culture medium. J Clin Microbiol. 1980;12:279–281. doi: 10.1128/jcm.12.2.279-281.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Restrepo A, Salazar M E, Cano L E, Patiño M M. A technique to collect and dislodge conidia produced by Paracoccidioides brasiliensis mycelial form. J Med Vet Mycol. 1986;24:247–250. [PubMed] [Google Scholar]
- 30.Restrepo A. Paracoccidioides brasiliensis. In: Mandell G L, Bennett J E, Dolin R, editors. Principles and practice of infectious diseases. 5th ed. Philadelphia, Pa: Churchill Livingstone; 2000. pp. 2768–2772. [Google Scholar]
- 31.Rhodes J C, Polacheck I, Kwon-Chung K J. Phenoloxidase activity and virulence in isogenic strains of Cryptococcus neoformans. Infect Immun. 1982;36:1175–1184. doi: 10.1128/iai.36.3.1175-1184.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Romero-Martinez R, Wheeler M, Guerrero-Plata A, Rico G, Torres-Guerrero H. Biosynthesis and functions of melanin in Sporothrix schenckii. Infect Immun. 2000;68:3696–3703. doi: 10.1128/iai.68.6.3696-3703.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosas A L, Casadevall A. Melanization affects susceptibility of Cryptococcus neoformans to heat and cold. FEMS Microbiol Lett. 1997;153:265–272. doi: 10.1111/j.1574-6968.1997.tb12584.x. [DOI] [PubMed] [Google Scholar]
- 34.Rosas A L, Nosanchuk J D, Feldmesser M, Cox G M, McDade H C, Casadevall A. Synthesis of polymerized melanin by Cryptococcus neoformans in infected rodents. Infect Immun. 2000;68:2845–2853. doi: 10.1128/iai.68.5.2845-2853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosas A L, Nosanchuk J D, Gómez B L, Edens W A, Henson J M, Casadevall A. Isolation and serological analysis of fungal melanins. J Immunol Methods. 2000;244:69–80. doi: 10.1016/s0022-1759(00)00255-6. [DOI] [PubMed] [Google Scholar]
- 36.Salas S D, Bennett J E, Kwon-Chung K J, Perfect J R, Williamson P R. Effect of the laccase gene CNLAC1, on virulence of Cryptococcus neoformans. J Exp Med. 1996;184:377–386. doi: 10.1084/jem.184.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schnitzler N, Peltroche-Llacahuanga H, Bestier N, Zundorf J, Haase G. Effect of melanin and carotenoids of Exophiala (Wangiella) dermatitidis on phagocytosis, oxidative burst, and killing by human neutrophils. Infect Immun. 1999;67:94–101. doi: 10.1128/iai.67.1.94-101.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taborda V B A, Taborda P R O, McGinnis M R. Constitutive melanin in the cell wall of the etiologic agent of Lobo's disease. Rev Inst Med Trop São Paulo. 1999;41:9–12. doi: 10.1590/s0036-46651999000100003. [DOI] [PubMed] [Google Scholar]
- 39.Tsai H F, Washburn R G, Chang Y C, Kwon-Chung K J. Aspergillus fumigatus arp1 modulates conidial pigmentation and complement deposition. Mol Microbiol. 1997;26:175–183. doi: 10.1046/j.1365-2958.1997.5681921.x. [DOI] [PubMed] [Google Scholar]
- 40.Tsai H F, Chang Y C, Washburn R G, Wheeler M H, Kwon-Chung K J. The developmentally regulated alb1 gene of Aspergillus fumigatus: its role in modulation of conidial morphology and virulence. J Bacteriol. 1998;180:3031–3038. doi: 10.1128/jb.180.12.3031-3038.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y, Casadevall A. Decreased susceptibility of melanized Cryptococcus neoformans to UV light. Appl Environ Microbiol. 1994;60:3864–3866. doi: 10.1128/aem.60.10.3864-3866.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Y, Casadevall A. Growth of Cryptococcus neoformans in presence of l-dopa decreases its susceptibility to amphotericin B. Antimicrob Agents Chemother. 1994;38:2648–2650. doi: 10.1128/aac.38.11.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y, Casadevall A. Susceptibility of melanized and nonmelanized Cryptococcus neoformans to nitrogen- and oxygen-derived oxidants. Infect Immun. 1995;62:3004–3007. doi: 10.1128/iai.62.7.3004-3007.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y, Aisen P, Casadevall A. Cryptococcus neoformans melanin and virulence: mechanism of action. Infect Immun. 1995;63:3131–3136. doi: 10.1128/iai.63.8.3131-3136.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wheeler M H, Bell A A. Melanins and their importance in pathogenic fungi. Curr Top Med Mycol. 1988;2:338–387. doi: 10.1007/978-1-4612-3730-3_10. [DOI] [PubMed] [Google Scholar]
- 46.Williamson P R. Biochemical and molecular characterization of the diphenol oxidase of Cryptococcus neoformans: identification as a laccase. J Bacteriol. 1994;176:656–664. doi: 10.1128/jb.176.3.656-664.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]