Abstract
Cell culture at liquid–liquid interfaces, for example, at the surface of oil microdroplets, is an attractive strategy to scale up adherent cell manufacturing while replacing the use of microplastics. Such a process requires the adhesion of cells at interfaces stabilized and reinforced by protein nanosheets displaying not only high elasticity but also presenting cell adhesive ligands able to bind integrin receptors. In this report, supercharged albumins are found to form strong elastic protein nanosheets when co-assembling with the co-surfactant pentafluorobenzoyl chloride (PFBC) and mediate extracellular matrix (ECM) protein adsorption and cell adhesion. The interfacial mechanical properties and elasticity of supercharged nanosheets are characterized by interfacial rheology, and behaviors are compared to those of native bovine serum albumin, human serum albumin, and α-lactalbumin. The impact of PFBC on such assembly is investigated. ECM protein adsorption to resulting supercharged nanosheets is then quantified via surface plasmon resonance and fluorescence microscopy, demonstrating that the dual role supercharged albumins are proposed to play as scaffold protein structuring liquid–liquid interfaces and substrates for the capture of ECM molecules. Finally, the adhesion and proliferation of primary human epidermal stem cells are investigated, at pinned droplets, as well as on bioemulsions stabilized by corresponding supercharged nanosheets. This study demonstrates the potential of supercharged proteins for the engineering of biointerfaces for stem cell manufacturing and draws structure–property relationships that will guide further engineering of associated systems.
Keywords: supercharged proteins, protein nanosheet, self-assembly, 2D nanomaterials, stem cells, liquid−liquid interface, emulsion
Introduction
Tissue culture plastics, glass, and other rigid substrates remain the main substrates on which adherent eukaryotic cells are routinely cultured and expanded. However, the use of such substrates constitutes an important hurdle to the scale up of cell manufacturing processes, and alternative microplastics used for implementation in 3D bioreactors1,2 pause increasing concerns in terms of contamination of cell products and to their processing. Liquid substrates, such as oil microdroplets, appear attractive alternatives for such 3D culture and manufacturing scale up.3 Indeed, it was recently proposed that the formation of mechanically strong protein or polymer nanosheets self-assembling at liquid–liquid interfaces and stabilizing oil microdroplets could provide a suitable local mechanical environment sustaining cell adhesion, while enabling readily processing of corresponding bioemulsions via centrifugation or potential filtration.4,5 In particular, the interfacial viscoelasticity of corresponding liquid–liquid systems was found to be regulating the ability of adherent stem cells to proliferate at corresponding liquid substrates.6 The mechanics of protein nanosheets and associated interfaces, in particular their elasticity, was found to be strongly impacted by co-assembly with co-surfactant molecules, in particular reactive co-surfactants forming covalent bonds with associated proteins. Although the ability of amphiphilic molecules and proteins, including globular proteins such as albumins, to stabilize liquid–liquid interfaces and act as tensioactive agents is well documented, the chemical and structural parameters regulating their interfacial viscoelasticity remain poorly understood.
Serum albumins are the most abundant proteins present in systemic circulation and play a significant role in the maintenance of osmotic pressure and pH of the blood.7,8 These albumins can bind a variety of substrates, including metal ions, fatty acids, and therapeutic molecules, and have found broad applications in biotechnologies.7−9 Owing to their hydrophobic core, serum albumins can bind water insoluble small negatively charged hydrophobic molecules which can regulate various interactions and functions such as the transportation of fatty acids in the blood.10 In addition, this inherent amphiphilicity has led to their wide application for the stabilization of emulsions, the formulation of food and healthcare products, and control of their rheological properties.11
Human serum albumin (HSA) displays a comparable molecular weight to that of bovine serum albumin (BSA), near 66 kDa,12 and high percentage of homology (76%).8 Both albumins display hydrophobic pockets allowing the binding of lipids and hydrophobic interfaces and 17 disulfide bridges controlling the overall shape of these predominantly α-helical proteins. In contrast, α-lactalbumin (αLA) is significantly smaller, with a molecular weight of 14.2 kDa, and displays a relative abundance of lysine, cysteine, tyrosine, and tryptophan residues.13,14 αLA participates in the binding of fatty acids or small molecules as for other albumins and contributes to lactose synthesis.13,15 Yuan et al. (2018) reported enhanced antioxidant properties of the αLA after ultrasound or enzymatic treatment, possibly due to the formation of the lactase cross-linked product with improved mechanical properties.15−17 This suggested that modification of albumins may result in the control of their physicochemical and mechanical properties.
Chemical modifications of proteins can lead to significant structural changes and can help in understanding the role of electrostatic interactions on their stability. For example, modifications such as succinylation and acetylation can expose, or block, reactive amino acids in a protein, affecting protein–protein interactions, normal unfolding, and aggregation.18−21 The extent to which the structure of the protein is affected by such external factors is often determined by the natural rigidity of the protein. Flexible proteins such as caseins are relatively resistant to significant conformational changes in comparison to BSA and whey proteins which often experience high degrees of denaturation upon acylation. In contrast, chemical modifications may enhance the flexibility of globular proteins and offer potential for further conjugation.19 In turn, increasing charge density at the surface of proteins, using polyelectrolyte block copolymers for example, can enhance their adsorption at liquid–liquid interfaces, as in the case of chloroform–water interfaces.22 Similarly, aggregation at the surface of nanoparticles can be promoted by such modifications and associated changes in surface charges,23 modulating colloidal stability and physicochemical properties. This implies a role for the charge density of proteins for the regulation of physicochemical and mechanical properties of resulting assemblies.
Supercharged proteins, naturally occurring, engineered or resulting from chemically modified native proteins, are attractive building blocks to design soft matter with emerging properties.24 Their engineering enabled the control of colloidal assembly,25 the formation of coacervates with RNA and other polyelectrolytes26 and the formation of nanostructured films.27 The architecture of supercharged proteins ranges from well-structured and folded, as in the case of β-barrel proteins such as engineered green fluorescent proteins, to disordered macromolecules such as histones, involved in the formation of coacervates and the structuring of DNA, to elastin-like proteins regulating the assembly of hydrogels and polyelectrolyte films with controlled mechanical properties. Hence, supercharged proteins appear as attractive candidates for the stabilization and structuring of liquid–liquid interfaces and the engineering of interfacial mechanics, while conferring high surface charge densities to resulting interfaces for subsequent extracellular matrix (ECM) protein adsorption.
In this report, the formation of supercharged protein nanosheets self-assembled at liquid–liquid interfaces is described. Supercharged BSA, generated via chemical modification, was assembled at the surface of the cytocompatible fluorinated oil Novec 7500, and the mechanical properties of resulting nanosheets were characterized via interfacial rheology and compared to that of other albumins. The impact of charge density, coupled to the formation of physical quadrupolar cross-links on interfacial shear moduli and viscoelasticity, is studied. In turn, the ability to adsorb ECM proteins at the surface of supercharged nanosheets is characterized, and the impact of the combined interfacial viscoelasticity and ECM adsorption on epidermal stem cell adhesion and proliferation is studied. Our results demonstrate the tuning of interfacial mechanics and ECM adsorption at liquid–liquid interfaces through supercharged nanosheet assembly and the potential of this platform for the culture of stem cells on bioemulsions.
Results
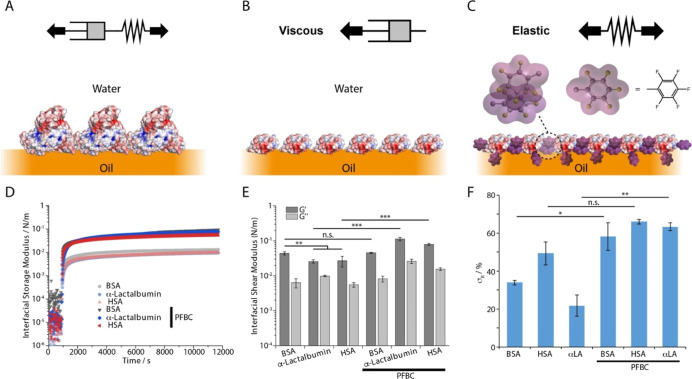
To explore structure–property relationships connecting albumin architecture, self-assembly, and mechanics of resulting nanosheets at liquid–liquid interfaces, we first investigated the impact of the molecular structure of different albumins (bovine serum albumin, BSA; human serum albumin, HSA; and α-lactalbumin, αLA) on the interfacial rheology of corresponding interfaces (Figure 1). In the absence of any co-surfactant, all three albumins adsorbed at corresponding interfaces rapidly (plateaus reached within 20 min), with comparable kinetics. At equilibrium, corresponding nanosheets displayed interfacial shear storage moduli in the range of 10–2–10–1 N/m, in agreement with literature reports for BSA4,6,28−30 (Figures 1D,E and S1A). The strong hydrophobic character of Novec 7500, compared to other oils investigated in the literature (e.g., alkanes and hydroxy-alkanes) may account for the relatively high moduli observed. Hence, BSA and other globular proteins were found to form softer nanosheets at oil interfaces, displaying more polar architectures.28 Compared to HSA and αLA, BSA formed stiffer nanosheets, displaying higher interfacial shear storage moduli (Figures 1E and S1A). In addition, BSA and HSA nanosheets displayed higher elasticities, determined from interfacial stress relaxation experiments, with stress retentions of 33.9 ± 1.3 and 49.4 ± 6.1%, respectively, compared to 21.8 ± 5.5% for αLA (Figures 1F and S2A). This was in agreement with the higher loss modulus, observed for αLA compared to BSA and HSA, and the stronger frequency dependence of the interfacial storage modulus measured for this protein (Figure S1A). However, relaxation constants associated with stress dissipation at corresponding interfaces remained relatively comparable (Figure S3A), implying similar dimensionalities for the protein networks assembled.
Figure 1.
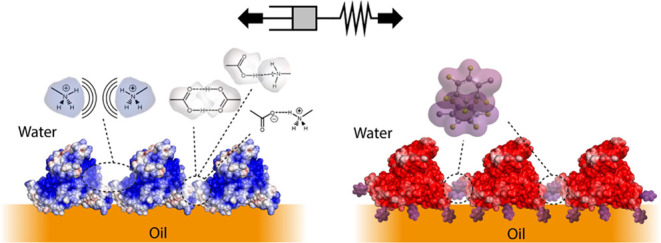
Formation and mechanical properties of albumin nanosheets at oil–water interfaces, in the presence of co-surfactant PFBC. (A) BSA/HSA form viscoelastic nanosheets at the oil–water interface. (B) αLA forms viscous nanosheets at the oil–water interface. (C) BSA, HSA, and αLA form more elastic nanosheets in the presence of PFBC. (D) Time sweeps of the evolution of interfacial storage moduli of Novec 7500/PBS interfaces during the assembly of native BSA, HSA, and αLA with and without co-surfactant PFBC (10 μg/mL in the oil phase). (E) Corresponding interfacial shear storage moduli extracted from frequency sweeps at an oscillating amplitude of 10–4 rad. (F) Residual elasticities σR (%) extracted from the fits of stress relaxation experiments at 0.5% strain. Note that protein conformations shown in (A–C) are only intended to illustrate the proposed structure of nanosheets and are not accurate representations of associated protein conformations.
The impact of the reactive co-surfactant pentafluoro benzoyl chloride (PFBC) was explored next. PFBC had previously been found to significantly impact the mechanics of protein nanosheets, in particular their elasticity.4−6 PFBC had a significant impact on the viscoelastic properties of nanosheets generated from the three proteins studied (Figures 1 and S1–S3). The interfacial storage moduli of nanosheets increased by almost 1 order of magnitude in the presence of PFBC (in the case of HSA and αLA, BSA displaying a more modest increase), as evidenced by frequency sweeps (Figures S1 and 1E). In addition, the elasticity of nanosheets formed in the presence of PFBC increased compared to nanosheets assembled in the absence of the co-surfactant (Figures 1F and S2), presumably reflecting the impact that reactive co-surfactants such as PFBC play on physical cross-linking of nanosheets.6 This was also in agreement with the decrease in frequency dependency of the interfacial storage moduli of corresponding nanosheets, especially in the case of αLA (Figure S1). Indeed, the increase in storage modulus and elastic stress retention σr in the presence of the co-surfactant PFBC was particularly pronounced in the case of αLA, switching from a relatively fluid, viscous interface, to a predominantly elastic interface (σr of 56.3 ± 2.1%). The comparable mechanical properties of BSA and HSA nanosheets may be anticipated from the similarity of their molecular weight (66 and 64 kDa, respectively), amino acid composition (76% homology), and isoelectric point (4.5 and 4.7, with ζ potentials of −20 and −21 mV, respectively; Figure S4).8,31−35 In contrast, αLA has a molecular weight of only 14 kDa and significantly different amino acid composition (36% homology with BSA, only 31% α-helix composition, Figure S4).36,37 Hence, we propose that the smaller size and more disordered structure of αLA result in more classic tensioactive properties, without the formation of protein networks at liquid–liquid interfaces (perhaps associated with reduced denaturation upon adsorption), resulting in more fluid interfaces with lower interfacial storage moduli, compared to BSA and HSA. However, in the presence of the co-surfactant PFBC, the abundance of functionalizable residues (e.g., lysine, serine, tyrosine, and threonine) at the surface of the three types of albumins tested (see Figure S4) underpinned the formation of physical cross-links and the establishment of a more interconnected protein network, associated with an increase in interfacial elasticity (Figures 1F, S2 and S3).
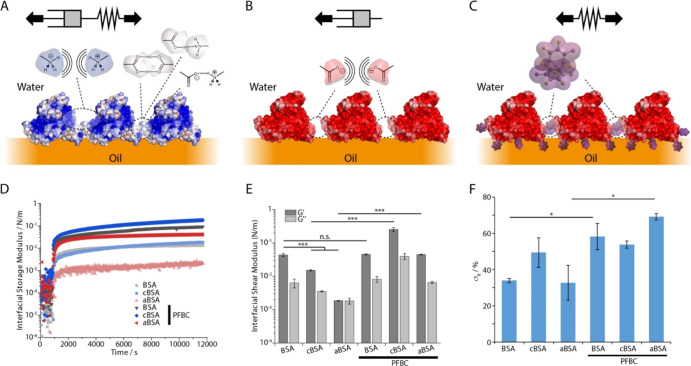
Therefore, the amino acid composition and conformation of globular proteins not only regulate their assembly and interfacial mechanics at liquid–liquid interfaces, as was previously reported,6,29,30,38 but also impact on the response of these proteins to co-assembly with surfactants such as PFBC. These observations raise the possibility of engineering protein nanosheets via the design of their amino acid composition and chemistry. To further demonstrate this concept, we studied the impact of functionalization of BSA with succinic anhydride [leading to a negatively supercharged protein, anionic BSA (aBSA), with a ζ-potential of −31.4 mV] and ethylene diamine residues [leading to a positively supercharged protein, cationic BSA (cBSA), with a ζ-potential of +13.9 mV]21 on self-assembly and interfacial mechanics (Figure 2). In the absence of the co-surfactant, the surface chemistry of BSA had a striking impact on the mechanical properties of corresponding nanosheets assembled at Novec 7500-water interfaces. Supercharged proteins resulted in softer nanosheets, presumably as a result of increasing repulsion between proteins assembled at corresponding interfaces (Figures 2D,E and S1B). This effect was particularly pronounced in the case of aBSA that had a higher charge density compared to cBSA. Indeed, the interfacial storage modulus increased only weakly upon exposure to aBSA and was comparable to the interfacial loss modulus extracted from measurements. At equilibrium, the residual elastic stress measured from stress relaxation experiments was particularly low in the case of aBSA (Figures 2F and S2B), and relaxation profiles were associated with reduced rate constants (Figure S3B). Interestingly, the conformation of aBSA was found to be comparable to that of BSA in solution, and comparable changes in protein conformation were observed upon assembly at liquid–liquid interfaces (in this case, Novec 7500/hexafluorobenzene mixture, to enable the contrast matching of both phases). In solution, BSA and aBSA displayed predominantly α-helical structures, with a positive peak at 194 nm, a negative peak at 209 nm, and shoulder near 220 nm (Figure S5A). Upon adsorption at liquid–liquid interfaces, the structure of both BSA and aBSA was found to significantly denature, with a loss of the low wavelength positive peak and a shift of the negative peak to 213 nm, with a loss of the associated shoulder.
Figure 2.
Formation and mechanical properties of supercharged albumin nanosheets at oil–water interfaces, in the presence of co-surfactant PFBC. (A) cBSA forms viscoelastic nanosheets at the oil–water interface. (B) aBSA forms viscous nanosheets at the oil–water interface. (C) In the presence of PFBC, supercharged albumins, including aBSA, form elastic nanosheets. (D) Time sweeps of the evolution of interfacial storage moduli of Novec 7500/PBS interfaces during the assembly of native BSA, cBSA, and aBSA, with and without co-surfactant PFBC (10 μg/mL in the oil phase). (E) Corresponding interfacial shear storage moduli extracted from frequency sweeps at an oscillating amplitude of 10–4 rad. (F) Residual elasticities σR (%) extracted from the fits of stress relaxation experiments at 0.5% strain. Note that protein conformations shown in (A–C) are only intended to illustrate the proposed structure of nanosheets and are not accurate representations of associated protein conformations.
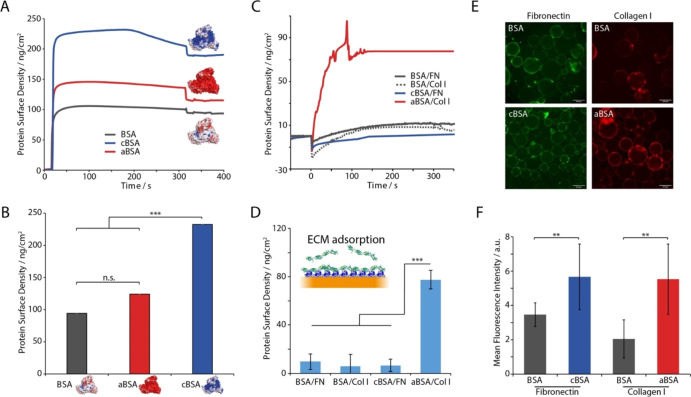
In contrast, cBSA formed slightly softer but more elastic interfaces (Figures 2E,F and S2B). We confirmed that protein densities adsorbed to fluorophilic model interfaces (monolayers of perfluorinated alkyl thiols) were comparable between BSA and aBSA and slightly higher for cBSA (Figure 3A). Therefore, the relatively strong surface potential associated with supercharged BSAs is proposed to result in enhanced repulsion between albumin molecules assembling at liquid–liquid interfaces, without substantial reduction in surface densities, resulting in softer interfaces, compared to native BSA nanosheets, in particular in the case of aBSA (Figure 2A–C). However, the occurrence of hydrogen bonding, and potentially solution aggregation, in the case of cBSA led to an increase in elasticity of corresponding interfacial networks. The interfacial storage moduli measured were in between those of BSA and aBSA nanosheets (Figure 2E) and, after an initial rapid increase, a gradual increase in modulus was observed. This may reflect that the surface charge density of cBSA, although more modest than that of aBSA, results in initial repulsion between surface-adsorbed macromolecules, but that with time further infiltration and interactions (perhaps between amine and carboxylic residues) result in physical cross-linking of associated nanosheets. Interestingly, comparable retention of cBSA conformation in solution and loss of structure upon adsorption to liquid–liquid interfaces were observed with this protein compared to BSA and aBSA (Figure S5). This suggests that conformational changes do not contribute significantly to variation in interfacial shear mechanics of associated supercharged protein nanosheets. The increased surface density of cBSA adsorbed at fluorophilic monolayers compared to BSA (Figure 3A), together with the increased elasticity of these interfaces, may also reflect the adsorption of small protein aggregates, rather than isolated proteins, that develop further interactions following adsorption. In agreement with such hypothesis, the frequency dependency of the interfacial storage modulus of cBSA nanosheets was moderate compared to that of aBSA nanosheets (Figure S1B).
Figure 3.
(A) Representative SPR traces of the adsorption of supercharged albumins and native BSA to perfluorodecanethiol monolayers modeling fluorinated oil interfaces. (B) Corresponding quantification of resulting protein surface densities. (C) SPR quantification of FN or collagen type I (Col I) adsorption at the surface of supercharged protein layers. (D) Corresponding protein surface densities. (E) Epifluorescence microscopy images of FN or Col I adsorption onto BSA, cBSA, and aBSA emulsions; green, FN; red, Col I. Scale bars, 200 μm. (F) Quantification (mean fluorescence intensity) of adsorbed FN or Col I on corresponding protein nanosheets. Error bars are s.e.m.; n = 3.
In the presence of the co-surfactant PFBC, supercharged proteins assembled into significantly stiffer nanosheets (Figure 2D,E). In particular, the interfacial storage modulus of cBSA and aBSA nanosheets was 17- and 24-fold higher, respectively, in the presence of PFBC, compared to only 5% increase for BSA. As for other albumins studied, the increase in hydrophobicity and associated physical cross-links enabled by PFBC moieties resulted in stiffening of nanosheets. In this respect, the higher storage moduli measured for cBSA nanosheets may reflect a combined impact of hydrophobic cross-links from strong quadrupole interactions between perfluorobenzene moieties39 and electrostatic cross-linking or hydrogen bonding associated with interactions between amines and carboxylic moieties of cBSA macromolecules. In contrast, the high surface charge of aBSA prevented the formation of the as-extensive hydrophobic cross-linked networks compared to BSA nanosheets.
These changes in interfacial modulus are also supported by the increase of the elasticity (quantified through σR) of all protein nanosheets in the presence of PFBC (Figure 2F), together with the increased relaxation times associated (Figure S3B), in particular in the case of supercharged nanosheets. In this respect, the particularly striking shift of aBSA nanosheets toward a highly elastic network behavior, despite a weaker interfacial shear modulus compared to cBSA (in the presence of PFBC) suggests that its higher surface charge density, and associated electrostatic repulsive forces may contribute to its stress relaxation. The increased elasticity evidenced in supercharged protein nanosheets formed in the presence of PFBC was also reflected in the reduction of the frequency dependency of their interfacial storage moduli (note the lack of decrease at high frequencies, see Figure S1B).
Overall, supercharged protein nanosheets stabilized by the co-surfactant PFBC display a combination of strong interfacial mechanical properties and high surface potential, ideal to promote the adsorption of ECM proteins such as fibronectin (FN) and collagen, to enable cell adhesion. To quantify ECM protein adsorption to supercharged protein nanosheets, we first studied the formation of protein assemblies at the surface of model perfluorinated monolayers, via surface plasmon resonance (SPR, Figure 3A,B). This constitutes an approximation, as the fluorinated self-assembled monolayer is distinct from the fluorinated oil investigated in this study, but allows direct quantification of adsorbed protein densities. Following protein injection, their adsorption rapidly increased, to reach a plateau near 90, 110, and 230 ng/cm2 for BSA, aBSA, and cBSA, respectively. Such rapid adsorption is in line with the adsorption reported for albumin at other hydrophobic interfaces and gold substrates and in agreement with the rapid increase in interfacial moduli observed via interfacial rheology.40−43 The higher rate at which protein adsorption was observed via SPR may be reflecting the fact that kinetics of evolution of interfacial mechanics not only depend on assembly at corresponding interfaces but also the formation of physical cross-links (via denaturation, hydrophobic bond formation, or electrostatic/hydrogen bonding) and a macroscale protein network. However, it is also likely that protein diffusion in the interfacial rheology trough is limiting the protein adsorption to liquid interfaces, as was reported in other systems.44 Finally, we also note that modeling adsorption in the presence of PFBC was not possible by SPR, as we cannot introduce PFBC through the fluorophilic monolayer. However, we note that neutron reflectometry data previously obtained for BSA did not indicate significant changes in protein density or nanosheet thickness, with and without PFBC.6 This is in good agreement with the relatively low level of tethering of PFBC moieties to BSA (only 11 PFBC per protein molecule4), unlikely to significantly change protein conformation, but providing physical cross-links between neighboring proteins.
Having examined the adsorption of supercharged albumins, we next quantified the secondary adsorption of ECM proteins to the resulting interfaces (Figure 3C,D). FN and collagen type I solutions [in phosphate buffered saline (PBS)] were injected on cBSA and aBSA interfaces (respectively), on the basis of their low and high isoelectric points (6.0 and 8–9, respectively).45,46 Differential adsorption of these two proteins to positively (for FN) and negatively charged interfaces [for collagen I (Col I)] has been previously reported.47 Adsorption levels were compared to those measured to native BSA interfaces. Col I adsorption was found to be enhanced on aBSA interfaces, supporting the hypothesis that enhanced charge density and associated electrostatic interactions, compared to native BSA, promote the adsorption of high pI proteins. In contrast, FN adsorption was moderate on both native BSA and cBSA, presumably as a result of the negative ζ-potential associated with native BSA, and the modest positive ζ-potential of cBSA compared to the negative potential achieved for aBSA.
To quantify protein adsorption at the surface of supercharged nanosheets assembled at liquid–liquid interfaces, we generated nanosheet-stabilized microdroplets and quantified FN and Col I adsorption via immunostaining and fluorescence microscopy (Figures 3E,F and S8). We note that all emulsions were stable, even after 7 days of incubation (Figure S6). Images of resulting droplets clearly demonstrated ECM protein adsorption at the surface of nanosheets and the enhancement of such adsorption at the surface of supercharged protein nanosheets, in agreement with SPR data. In particular, Col I was again found to adsorb strongly at the surface of aBSA, compared to native BSA, but FN adsorption was also found to be slightly promoted, compared to native BSA. Therefore, our data demonstrate that supercharged albumins not only allow the assembly of stiff, strong protein nanosheets at liquid–liquid interfaces but also enable the adsorption of ECM proteins relevant to regulate cell adhesion and stem cell expansion.
We note that direct adsorption of FN to hydrophobic interfaces, including fluorophilic liquids, is reported48 and was found to support the adhesion of mesenchymal stem cells. However, this phenomenon was found to depend significantly on the fluorinated oil type and only observed in the case of perfluorooctane. Indeed, upon direct adsorption of FN and collagen to Novec 7500, we observed an increase in interfacial shear moduli, although the difference between storage and loss moduli remained modest (Figure S7A). In addition, the resulting interfaces displayed negligible elasticities (from interfacial stress relaxation experiments, Figure S7B), suggesting that resulting FN and collagen interfaces did not form cross-linked networks. Finally, FN alone was not found to support the stabilization of emulsions (Figure S7C). Therefore, direct ECM protein adsorption appears as a limited strategy for the design of bioemulsions for cell culture.
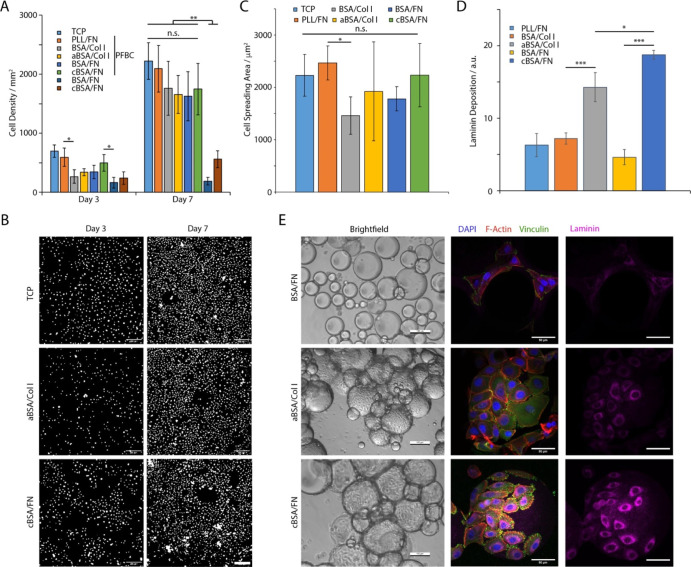
The adhesion and spreading of human primary keratinocytes (HPKs) are typically mediated by integrins and regulate their fate decision.49,50 Collagen (in particular type I and IV) is typically used to promote keratinocyte adhesion and selection, although FN is also often used, despite the lack of expression of associated integrin heterodimers in normal human interfollicular epidermis.51−54 To explore the ability to use microdroplets as microcarriers for the expansion of adherent stem cells, we first generated pinned droplets, stabilized by supercharged albumins, followed by adsorption of complementary ECM proteins (FN for cBSA and Col I for aBSA). HPKs were then cultured on the resulting droplets, enabling the quantification of cell densities at different time points (Figures 4, S9 and S10). As a comparison, we seeded cells on tissue culture polystyrene (TCP) and on poly(l-lysine) (PLL)-stabilized pinned droplets.5,6 After 3 days of culture, cell densities on PFBC/cBSA/FN-stabilized interfaces were comparable to those measured on TCP and PFBC/PLL/FN controls. In comparison, densities measured on supercharged nanosheets formed in the absence of PFBC, or on native BSA nanosheets, were significantly reduced compared to controls. Densities measured on PFBC/aBSA/COL were intermediate between those measured for native BSA nanosheets and the controls, despite the strong adhesion keratinocytes typically display for Col I-coated interfaces. After culture for 7 days, increased cell densities were observed for all conditions. Importantly, all PFBC reinforced nanosheets (displaying strong interfacial mechanical properties) enabled comparable cell densities to the controls to be achieved. In contrast, supercharged nanosheets generated in the absence of PFBC and displaying softer, more viscous behaviors were clearly unable to sustain rapid keratinocyte expansion (Figure 4A,B). This effect was particularly marked in the case of native nanosheets, in agreement with the combined weakness of corresponding interfaces and their lack of ability to mediate Col I adsorption.
Figure 4.
(A) HPK proliferation on interfaces conditioned with different supercharged albumins, functionalized with ECM proteins and assembled with or without co-surfactant PFBC. (B) Selected images of cells spreading at corresponding liquid–liquid interfaces after 3 and 7 days of culture at TCP, cBSA/FN, and aBSA/Col I with PFBC. Images are corresponding nuclear stainings. Scale bars are 200 μm. (C) Quantification of HPK spreading area (24 h after seeding) characterized on pinned droplets functionalized with corresponding nanosheets. (D) Quantification of laminin deposition at liquid–liquid interfaces. (E) Bright-field and confocal images of HPKs cultured for 7 days on emulsions stabilized by protein nanosheets (blue, DAPI; red, phalloidin; green, vinculin; and purple, laminin). Scale bars are 100 μm (bright-field) and 50 μm (confocal). Error bars are s.e.m.; n = 3.
In agreement with these observations, cell spreading on supercharged nanosheets, reinforced by PFBC and with ECM protein adsorption, was comparable to controls, whereas those spreading on nanosheets generated from native BSA displayed more rounded morphologies, 24 h after seeding (Figure 4C). Therefore, our data indicate that cell adhesion to liquid interfaces reinforced by supercharged protein nanosheets displaying strong mechanical properties and high ECM adsorption enable rapid keratinocyte adhesion, spreading and expansion, comparable to what is typically observed on tissue culture plastic or at cationic polymer nanosheets (based on PLL). Over prolonged culture times, only reinforced nanosheets promoted keratinocyte expansion on pinned droplets.
The ability to expand keratinocytes at the surface of bioemulsions stabilized by supercharged protein nanosheets was examined next (Figure 4D,E). Bioemulsions were generated by enabling native BSA and supercharged nanosheets to stabilize microdroplets, prior to the assembly of corresponding ECM proteins. Interfaces not reinforced by co-surfactants did not sustain keratinocyte proliferation (Figures 4E, S9 and S10), in agreement with results obtained on pinned droplets. Too few cells were observed on these systems to enable further characterization. Few keratinocytes could be observed at the surface of droplets stabilized by native BSA, reinforced with PFBC, irrespective of the ECM protein adsorbed (Figures 4E, S9–S12). Similarly, keratinocytes adhering to supercharged protein nanosheets, in the presence of PFBC, displayed mature focal adhesions, assembled a well-structured actin cytoskeleton, and deposited higher levels of laminin α1 compared to cell spreading at the surface of native BSA-stabilized droplets (Figures 4C,E and S13). It is possible that ECM protein adsorption, during initial conditioning or as keratinocytes proliferate and deposit laminin, contributes to strengthening the mechanics of protein nanosheets; however, this was not characterized further. The adsorption of serum proteins to albumin nanosheets4 (although not supercharged) was not found to impact the interfacial shear modulus of corresponding interfaces significantly, implying that such a process had a weak impact on the cross-linking of protein networks in the nanosheet. It is not clear whether remodeling and potential degradation of the protein nanosheet initially generated correlate with further ECM deposition.
Therefore, the more challenging adhesive environment associated with droplet curvature was met by the combination of high ECM protein adsorption and increased interfacial elasticity associated with supercharged protein nanosheets. As a proof-of-concept, we scaled up the culture of keratinocytes on cBSA/FN-stabilized emulsions, in conical flasks comparable to those used for the culture of E. coli and yeast (40 mL scale; orbital shaker used for agitation; see Figure S14A). Resulting cultures presented colonies adhering to the surface of droplets and comparable to those observed at a low scale (1 mL in multi-well plate), with comparable level of expression of the extra-cellular matrix protein laminin (Figure S14B,C).
Conclusions
Focal adhesion formation is typically regulated by the rigidity of the substrate on which cells are adhering.50,55−57 However, an increasing number of reports are suggesting that nanoscale mechanics, rather than bulk mechanical properties, regulate such processes.50,58,59 This concept is taken to its extreme in the phenomenon of cell adhesion to liquid substrates such as fluorophilic or silicone oils, providing that strong elastic polymer or protein nanosheets can be assembled at corresponding interfaces.4−6,60 Such a phenomenon had remained restricted to a relatively small number of proteins and polymers, and the translation of such systems to the culture of adherent cells on bioemulsions will require the engineering of more readily available macromolecules. Supercharged albumins appear as promising candidates for such applications, given their availability and amenability to engineering of chemical structures. Our data indicate that the combination of high interfacial modulus, elasticity, and ability to promote ECM adsorption of surfactant-reinforced supercharged protein nanosheets enhances cell attachment and focal adhesion formation, despite the absence of underlying rigid or elastic substrate.
The engineering of scaffold proteins that display suitable combination of amphiphilicity, in order to adsorb at the surface and stabilize oil droplets, and interfacial mechanics, to sustain cell-mediated contractile forces, remains challenging. Despite the wealth of data describing the stabilization of emulsions by a range of proteins and surfactants, in particular for application in the food industry or the formulation of healthcare products, the emphasis is most often placed on surface tension and dilatational mechanics. Parameters impacting interfacial viscoelasticity, independent on changes in surface areas, remain poorly understood and structure–property relationship enabling systematic design required. In this context, supercharged albumins were found not only to retain tensioactive properties suitable to stabilize microdroplets but also retained the ability to couple with reactive co-surfactants such as PFBC in order to provide physical cross-links required to achieve strong interfacial elastic properties. Therefore, supercharged albumin nanosheets offer a suitable combination of interfacial scaffolding properties and ability to promote fast ECM protein adsorption without the need for further coupling or reactivity.
The application of such protein engineering to the design of bioemulsions suitable for adherent cell culture remains in its infancy. A range of stem cells have been cultured at such interfaces, and the demonstration of long-term expansion of mesenchymal stem cells on such expansion paved the way toward the translation of these systems to bioreactors.3 A broad range of parameters remains to be investigated, such as the ability to sustain matrix remodeling or how the control of droplet size, stability, and curvature (which may affect cell adhesion, proliferation, and fate decision61) may also affect the quality of cells manufactured on resulting bioemulsions. However, these systems offer a unique opportunity to replace plastics and microplastics and revolutionize cell manufacturing processes.
Materials and Methods
Materials and Chemicals
Native BSA, aBSA, and cBSA were prepared and provided, as described in the literature.21 The fluorinated surfactant 2,3,4,5,6-perfluorobenzoyl chloride, PBS, trichloro (1H,1H,2H,2H-perfluorooctyl) silane (97%), the 1H,1H,2H,2H-perfluorodecanethiol (97%), and the nuclear staining agent 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) were purchased from Sigma-Aldrich Co. The fluorinated oil (Novec 7500) was obtained from ACOTA. The SPR-Au chips were obtained from Ssens.
Preparation of Emulsions
Emulsions were generated using 1 mL of fluorinated oil (Novec 7500, ACOTA) with or without fluorinated surfactant (2,3,4,5,6-pentafluorobenzoyl chloride, PFBC, final concentration of 0.01 mg/mL) and 2 mL of protein aqueous solution (1 mg/mL in PBS), added to a glass vial. The vial was shaken for 15 s and incubated for 1 h at room temperature. The upper liquid phase (aqueous) was aspirated and replaced with PBS 6 times.
Interfacial Shear Rheology Measurements
Interfacial shear rheology was selected for the evaluation of the shear mechanics of corresponding interfaces as it is highly sensitive (baselines typically in the range of 10–5–10–4 N/m), is not associated with changes in surface area and associated contribution of the surface tension, or sensitive to other physicochemical properties associated with probe–surface interactions.62 Interfacial rheological measurements were carried out on a Discovery Hydrid-Rheometer (DHR-3) from TA Instruments, using a Du Nouy ring geometry and a Delrin trough with a circular channel. The Du Nouy ring has a diamond-shaped cross section that improves positioning at the interface between two liquids to measure interfacial rheological properties while minimizing viscous drag from upper and sub-phases. The ring has a radius of 10 mm and is made of a platinum–iridium wire of 400 μm thickness. The Derlin trough was filled with 4 mL of fluorinated oil (with or without surfactant). Using axial force monitoring, the ring was positioned at the surface of the fluorinated oil and was then lowered by a further 200 μm to position the medial plane of the ring at the fluorinated phase interface. 4 mL of the PBS solution was then gently introduced to fully cover the fluorinated sub-phase. Time sweeps were performed at a frequency of 0.1 Hz and temperature of 25 °C, with a displacement of 1.0 × 10–3 rad (strain of 1%) to follow the self-assembly of the protein nanosheets at corresponding interfaces. In each case, the protein solution (1 mg/mL) was added after 15 min of incubation and continuous acquisition of interfacial rheology data for the naked liquid–liquid interface. Before and after each time sweep, a frequency sweep (with displacements of 1.0 × 10–3 rad) and amplitude sweeps (at a frequency of 0.1 Hz) were carried out to examine the frequency-dependent behavior of corresponding interfaces and to ensure that the selected displacement and frequency selected were within the linear viscoelastic region.
Surface Plasmon Resonance
SPR measurements were carried out on a BIACORE X from Biacore AB. SPR chips (SPR-Au 10 × 12 mm, Ssens) were plasma oxidized for 5 min and then incubated in 5 mM ethanolic solution of 1H,1H,2H,2H-perfluorodecanethiol, overnight at room temperature. This created a model fluorinated monolayer mimicking the fluoriphilic properties of Novec 7500. The chips were washed once with water, dried in an air stream, and kept dry at room temperature prior to mounting (within a few minutes). Thereafter, the sensor chip was mounted on a plastic support frame and placed in a Biacore protective cassette. The maintenance sensor chip cassette was first placed into the sensor chip port and docked onto the Integrated μ-Fluidic Cartridge (IFC) flow block, prior to priming the system with ethanol. The sample sensor chip cassette was then docked and primed once with PBS. Once the sensor chip primed, the signal was allowed to stabilize to a stable baseline, and the protein solution (1 mg/mL in PBS) was loaded into the IFC sample loop with a micropipette (volume of 50 μL). The sample and buffer flow rates were kept at 10 μL/min throughout. After the injection finished, washing of the surface was carried out in running buffer (PBS) for 10 min. Washing of the surface was allowed to continue for 10 min prior to injection of the second protein (collagen or FN at 10 μg/mL and 100 μg/mL in PBS, respectively; volume of 50 μL), at a flow rate of 10 μL/min. Buffer (PBS) was flown on the sensor chip for 10 min to wash off excess protein solution, and data were taken for a further 10 min.
Evaluation of Emulsion Stability
The emulsion samples were prepared, as described above, and stored at room temperature. The emulsion stability was monitored on the same day (day 0) or 7 days after the emulsion formation, using bright-field microscopy. A volume of 10 μL of emulsion was transferred to a 24-well plate, into 1 mL of PBS. Average microdroplet diameters were estimated from 100 droplets per condition.
Generation of Fluorinated Pinned Droplets for Cell Culture
Thin glass slides (25 × 60 mm, VWR) were washed with isopropanol and dried under nitrogen, prior to plasma oxidation for 10 min (Henniker Plasma HPT-200; air). Slides were then placed into an anhydrous ethanol solution (9.5 mL) containing trichloro-1H,1H,2H,2H-perfluorooctyl silane (97%, Sigma) (500 μL) for 1 h, at room temperature. The fluorinated glass slides were cut into chips (1 × 1 cm) and placed into a 24-well plate (for Hoechst staining), or the glass slides were kept at their original dimensions and embedded on sticky-slide eight-well plates (Ibidi), for imaging on a confocal microscope. After sterilization with 70% ethanol, the wells were washed (once) and then filled with 2 mL (or 600 μL for the sticky wells) of PBS (pH 7.4 for the different BSA types and pH 10.5 for PLL). 100 μL of fluorinated oil (or 10 μL for the sticky wells), with or without fluorinated surfactant (10 μg/mL) were added to the surface of the glass slide, forming a fluorinated pinned droplet. For samples prepared in 24-well plates, 30 μL of the oil phase was removed using a micropipette, to form a flatter oil droplet. For protein deposition, 10 μL of BSA solution (100 mg/mL) was added into the PBS phase contained in the well (final concentration of 1 mg/mL; volume used for Ibidi well was only 8 μL) and incubated for 1 h. After the incubation time, wells were washed six times with PBS (by dilution/aspiration, ensuring the oil surface did not become exposed to air). FN (10 μg/mL, final concentration) or collagen type I (100 μg/mL, final concentration) were added into the PBS solution and incubated for 1 h, followed by washing with PBS (four times) and with keratinocyte basal medium 2 (KBM2, twice).
Hoechst Staining
Cell proliferation was assessed via Hoechst staining, microscopy, and counting of nuclei. Cells were incubated in KBM2 containing 2 μL of Hoechst 33342 (5 mg/mL stock solution, Thermo Fisher Scientific) for 30 min before imaging by epifluorescence microscopy (see details below). The number of nuclei per image was determined manually and converted in cell densities per surface area.
Immuno-Fluorescence Staining and Antibodies
Samples (emulsions) were washed (dilution and aspiration, followed by addition of solutions) once with PBS and fixed with 4% paraformaldehyde (Sigma-Aldrich; 8% for samples in Ibidi well plates) for 10 min at room temperature. Thereafter, samples were washed three times with PBS and permeabilized with 0.2% Triton X-100 (Sigma-Aldrich; 0.4% for samples in Ibidi well plates) for 5 min at room temperature. After washing with PBS (three times), samples were blocked for 1 h in 10% fetal bovine serum (FBS). The blocking buffer was partly removed from the samples, not allowing them to be exposed to air, and the samples were incubated with primary antibodies at 4 °C overnight. Samples were washed six times with PBS and incubated for 1 h with the secondary antibodies (phalloidin, 1:500; DAPI, 1:1000; vinculin, 1:1000; laminin, 1:500) in blocking buffer (FBS 10% in PBS). After washing with PBS (six times), samples were transferred to Ibidi wells for imaging.
Immuno-Fluorescence Microscopy and Data Analysis
Fluorescence microscopy images were acquired with a Leica DMi8 fluorescence microscope. To determine the cell densities per mm2, cell counting was carried out by thresholding and watershedding nuclei images in Fiji ImageJ. In the case of cell clusters, for which this method did not allow the isolation of individual nuclei, cells were counted manually. To determine the cell adhesion areas, images (phalloidin stainings of the actin cytoskeleton) were analyzed by thresholding and watershedding. The area of cell clusters was removed when analyzing results. Confocal microscopy images were acquired with a Zeiss 710 confocal microscope.
Human Primary Keratinocyte Cell Line Culture and Seeding
HPKs were cultured in KBM2 (PromoCell). For proliferation assays, HPK cells were harvested with trypsin (0.25%) and versene solutions (Thermo Fisher Scientific, 0.2 g/L EDTA Na4 in PBS) at a ratio of 1/9. Cells were then resuspended with differentiation medium (FAD) prepared with DMEM/F12 (1:1) (1×) and DMEM (Thermo Fisher Scientific) at a ratio of 1:1, containing 1% l-glutamine (200 mM), 1% penicillin–streptomycin (5000 U/mL), 0.1% insulin, 0.1% hydrocortisone equivalent (HCE), and 10% of FBS (Labtech). HPK cells were centrifuged for 5 min at 1200 rpm, counted and resuspended in KBM2, at the desired density before seeding onto substrates. Cells were left to adhere and proliferate in an incubator (37 °C and 5% CO2) for different time points (at day 3 and day 7 of culture), prior to staining and imaging. For cell spreading assays, HPK cells were harvested and seeded onto fluorinated droplets at a density of 25,000 cells per well (13,000 cell/cm2). For passaging, cells were reseeded in a preconditioned T75 flask, with collagen type I (20 μL of collagen into 10 mL of PBS for 20 min), at a density of 250,000 cells per flask.
Statistical Analysis
Statistical analysis was carried out using OriginPro 9 through one-way ANOVA with Tukey test for posthoc analysis. Significance was determined by *P < 0.05, **P < 0.01, ***P < 0.001, and n.s., non-significant. A full summary of statistical analysis is provided in the Supporting Information.
Acknowledgments
The authors are grateful for experimental support and training from Dr Dexu Kong. Funding for this work from the European Research Council (ProLiCell, 772462; ProBioFac, 966740) and a Cyprus Scholarship (IKYK and 841C18) is gratefully acknowledged.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsami.2c20188.
Additional interfacial shear rheology data, proposed idealized protein structures, circular dichroism spectra, quantification of droplet size distributions, additional fluorescence and bright-field microscopy images of nanosheets and cells grown at liquid–liquid interfaces, and statistical analysis of data (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Tavassoli H.; Alhosseini S. N.; Tay A.; Chan P. P. Y.; Weng Oh S. K.; Warkiani M. E. Large-Scale Production of Stem Cells Utilizing Microcarriers: A Biomaterials Engineering Perspective from Academic Research to Commercialized Products. Biomaterials 2018, 181, 333–346. 10.1016/j.biomaterials.2018.07.016. [DOI] [PubMed] [Google Scholar]
- dos Santos F. F.; Andrade P. Z.; da Silva C. L.; Cabral J. M. S. Bioreactor Design for Clinical-Grade Expansion of Stem Cells. Biotechnol. J. 2013, 8, 644–654. 10.1002/biot.201200373. [DOI] [PubMed] [Google Scholar]
- Peng L.; Gautrot J. E. Long Term Expansion Profile of Mesenchymal Stromal Cells at Protein Nanosheet-Stabilised Bioemulsions for next Generation Cell Culture Microcarriers. Mater. Today Bio 2021, 12, 100159. 10.1016/j.mtbio.2021.100159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong D.; Megone W.; Nguyen K. D. Q.; Di Cio S.; Ramstedt M.; Gautrot J. E. Protein Nanosheet Mechanics Controls Cell Adhesion and Expansion on Low-Viscosity Liquids. Nano Lett. 2018, 18, 1946–1951. 10.1021/acs.nanolett.7b05339. [DOI] [PubMed] [Google Scholar]
- Kong D.; Peng L.; Di Cio S.; Novak P.; Gautrot J. E. Stem Cell Expansion and Fate Decision on Liquid Substrates Are Regulated by Self-Assembled Nanosheets. ACS Nano 2018, 12, 9206–9213. 10.1021/acsnano.8b03865. [DOI] [PubMed] [Google Scholar]
- Kong D.; Peng L.; Bosch-Fortea M.; Chrysanthou A.; Alexis C. V. J.-M.; Matellan C.; Zarbakhsh A.; Mastroianni G.; del Rio Hernandez A.; Gautrot J. E. Impact of the Multiscale Viscoelasticity of Quasi-2D Self-Assembled Protein Networks on Stem Cell Expansion at Liquid Interfaces. Biomaterials 2022, 284, 121494. 10.1016/j.biomaterials.2022.121494. [DOI] [PubMed] [Google Scholar]
- Carter D.; He X.; Munson S.; Twigg P.; Gernert K.; Broom M.; Miller T. Three-Dimensional Structure of Human Serum Albumin. Science 1989, 244, 1195–1198. 10.1126/science.2727704. [DOI] [PubMed] [Google Scholar]
- Huang B. X.; Kim H.-Y.; Dass C. Probing Three-Dimensional Structure of Bovine Serum Albumin by Chemical Cross-Linking and Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2004, 15, 1237–1247. 10.1016/j.jasms.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Fasano M.; Curry S.; Terreno E.; Galliano M.; Fanali G.; Narciso P.; Notari S.; Ascenzi P. The Extraordinary Ligand Binding Properties of Human Serum Albumin. IUBMB Life 2005, 57, 787–796. 10.1080/15216540500404093. [DOI] [PubMed] [Google Scholar]
- He X. M.; Carter D. C. Atomic Structure and Chemistry of Human Serum Albumin. Nature 1992, 358, 209–215. 10.1038/358209a0. [DOI] [PubMed] [Google Scholar]
- Damodaran S. Protein Stabilization of Emulsions and Foams. J. Food Sci. 2006, 70, R54–R66. 10.1111/j.1365-2621.2005.tb07150.x. [DOI] [Google Scholar]
- Babcock J. J.; Brancaleon L. Bovine Serum Albumin Oligomers in the E- and B-Forms at Low Protein Concentration and Ionic Strength. Int. J. Biol. Macromol. 2013, 53, 42–53. 10.1016/j.ijbiomac.2012.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layman D. K.; Lönnerdal B.; Fernstrom J. D. Applications for α-Lactalbumin in Human Nutrition. Nutr. Rev. 2018, 76, 444–460. 10.1093/nutrit/nuy004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakopović K. L.; Barukčić I.; Božanić R. Physiological Significance, Structure and Isolation of α-Lactalbumin. Mljekarstvo 2016, 66, 3–11. 10.15567/mljekarstvo.2016.0101. [DOI] [Google Scholar]
- Madureira A. R.; Pereira C. I.; Gomes A. M. P.; Pintado M. E.; Xavier Malcata F. Bovine Whey Proteins – Overview on Their Main Biological Properties. Food Res. Int. 2007, 40, 1197–1211. 10.1016/j.foodres.2007.07.005. [DOI] [Google Scholar]
- Yuan X.; Li X.; Zhang X.; Mu Z.; Gao Z.; Jiang L.; Jiang Z. Effect of Ultrasound on Structure and Functional Properties of Laccase-Catalyzed α-Lactalbumin. J. Food Eng. 2018, 223, 116–123. 10.1016/j.jfoodeng.2017.12.008. [DOI] [Google Scholar]
- Qayum A.; Hussain M.; Li M.; Li J.; Shi R.; Li T.; Anwar A.; Ahmed Z.; Hou J.; Jiang Z. Gelling, Microstructure and Water-Holding Properties of Alpha-Lactalbumin Emulsion Gel: Impact of Combined Ultrasound Pretreatment and Laccase Cross-Linking. Food Hydrocolloids 2021, 110, 106122. 10.1016/j.foodhyd.2020.106122. [DOI] [Google Scholar]
- Habeeb A. F. Quantitation of Conformational Changes on Chemical Modification of Proteins: Use of Succinylated Proteins as a Model. Arch. Biochem. Biophys. 1967, 121, 652–664. 10.1016/0003-9861(67)90050-1. [DOI] [PubMed] [Google Scholar]
- Lakkis J.; Villota R. Effect of Acylation on Substructural Properties of Proteins: A Study Using Fluorescence and Circular Dichroism. J. Agric. Food Chem. 1992, 40, 553–560. 10.1021/jf00016a005. [DOI] [Google Scholar]
- Vetri V.; Librizzi F.; Leone M.; Militello V. Thermal Aggregation of Bovine Serum Albumin at Different PH: Comparison with Human Serum Albumin. Eur. Biophys. J. 2007, 36, 717–725. 10.1007/s00249-007-0196-5. [DOI] [PubMed] [Google Scholar]
- Zhong W.; Wen M.; Xu J.; Wang H.; Tan L.-L.; Shang L. Simultaneous Regulation of Optical Properties and Cellular Behaviors of Gold Nanoclusters by Pre-Engineering the Biotemplates. Chem. Commun. 2020, 56, 11414–11417. 10.1039/D0CC04039H. [DOI] [PubMed] [Google Scholar]
- Jiménez-Ángeles F.; Kwon H.-K.; Sadman K.; Wu T.; Shull K. R.; Olvera de la Cruz M. Self-Assembly of Charge-Containing Copolymers at the Liquid–Liquid Interface. ACS Cent. Sci. 2019, 5, 688–699. 10.1021/acscentsci.9b00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamison J. A.; Bryant E. L.; Kadali S. B.; Wong M. S.; Colvin V. L.; Matthews K. S.; Calabretta M. K. Altering Protein Surface Charge with Chemical Modification Modulates Protein–Gold Nanoparticle Aggregation. J. Nanopart. Res. 2011, 13, 625–636. 10.1007/s11051-010-0057-5. [DOI] [Google Scholar]
- Ma C.; Malessa A.; Boersma A. J.; Liu K.; Herrmann A. Supercharged Proteins and Polypeptides. Adv. Mater. 2020, 32, 1905309. 10.1002/adma.201905309. [DOI] [PubMed] [Google Scholar]
- Korpi A.; Ma C.; Liu K.; Nonappa; Herrmann A.; Ikkala O.; Kostiainen M. A. Self-Assembly of Electrostatic Cocrystals from Supercharged Fusion Peptides and Protein Cages. ACS Macro Lett. 2018, 7, 318–323. 10.1021/acsmacrolett.8b00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- te Brinke E.; Groen J.; Herrmann A.; Heus H. A.; Rivas G.; Spruijt E.; Huck W. T. S. Dissipative Adaptation in Driven Self-Assembly Leading to Self-Dividing Fibrils. Nat. Nanotechnol. 2018, 13, 849–855. 10.1038/s41565-018-0192-1. [DOI] [PubMed] [Google Scholar]
- Liu K.; Pesce D.; Ma C.; Tuchband M.; Shuai M.; Chen D.; Su J.; Liu Q.; Gerasimov J. Y.; Kolbe A.; Zajaczkowski W.; Pisula W.; Müllen K.; Clark N. A.; Herrmann A. Solvent-Free Liquid Crystals and Liquids Based on Genetically Engineered Supercharged Polypeptides with High Elasticity. Adv. Mater. 2015, 27, 2459–2465. 10.1002/adma.201405182. [DOI] [PubMed] [Google Scholar]
- Bergfreund J.; Diener M.; Geue T.; Nussbaum N.; Kummer N.; Bertsch P.; Nyström G.; Fischer P. Globular Protein Assembly and Network Formation at Fluid Interfaces: Effect of Oil. Soft Matter 2021, 17, 1692–1700. 10.1039/D0SM01870H. [DOI] [PubMed] [Google Scholar]
- Bos A.; van Vliet T. Interfacial Rheological Properties of Adsorbed Protein Layers and Surfactants: A Review. Adv. Colloid Interface Sci. 2001, 91, 437–471. 10.1016/S0001-8686(00)00077-4. [DOI] [PubMed] [Google Scholar]
- Benjamins J.; Lyklema J.; Lucassen-Reynders E. H. Compression/Expansion Rheology of Oil/Water Interfaces with Adsorbed Proteins. Comparison with the Air/Water Surface. Langmuir 2006, 22, 6181–6188. 10.1021/la060441h. [DOI] [PubMed] [Google Scholar]
- Feng R.; Konishi Y.; Bell A. W. High Accuracy Molecular Weight Determination and Variation Characterization of Proteins Up To 80 Ku by Ionspray Mass Spectrometry. J. Am. Soc. Mass Spectrom. 1991, 2, 387–401. 10.1016/1044-0305(91)85005-Q. [DOI] [PubMed] [Google Scholar]
- Vlasova I. M.; Saletsky A. M. Study of the Denaturation of Human Serum Albumin by Sodium Dodecyl Sulfate Using the Intrinsic Fluorescence of Albumin. J. Appl. Spectrosc. 2009, 76, 536–541. 10.1007/s10812-009-9227-6. [DOI] [Google Scholar]
- Phan H. T. M.; Bartelt-Hunt S.; Rodenhausen K. B.; Schubert M.; Bartz J. C. Investigation of Bovine Serum Albumin (BSA) Attachment onto Self-Assembled Monolayers (SAMs) Using Combinatorial Quartz Crystal Microbalance with Dissipation (QCM-D) and Spectroscopic Ellipsometry (SE). PLoS One 2015, 10, e0141282 10.1371/journal.pone.0141282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R.; Wu Z.; Wangb Y.; Ding L.; Wang Y. Role of PH-Induced Structural Change in Protein Aggregation in Foam Fractionation of Bovine Serum Albumin. Biotechnol. Rep. 2016, 9, 46–52. 10.1016/j.btre.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell B. A.; Jachimska B.; Kralka I.; Mulheran P. A.; Chen Y. Human Serum Albumin Encapsulated Gold Nanoclusters: Effects of Cluster Synthesis on Natural Protein Characteristics. J. Mater. Chem. B 2016, 4, 6876–6882. 10.1039/C6TB01827K. [DOI] [PubMed] [Google Scholar]
- Permyakov E. A.; Berliner L. J. α-Lactalbumin: structure and function. FEBS Lett. 2000, 473, 269–274. 10.1016/S0014-5793(00)01546-5. [DOI] [PubMed] [Google Scholar]
- Spöttel J.; Brockelt J.; Falke S.; Rohn S. Characterization of Conjugates between α-Lactalbumin and Benzyl Isothiocyanate—Effects on Molecular Structure and Proteolytic Stability. Molecules 2021, 26, 6247. 10.3390/molecules26206247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergfreund J.; Bertsch P.; Kuster S.; Fischer P. Effect of Oil Hydrophobicity on the Adsorption and Rheology of β-Lactoglobulin at Oil-Water Interfaces. Langmuir 2018, 34, 4929–4936. 10.1021/acs.langmuir.8b00458. [DOI] [PubMed] [Google Scholar]
- Pace C. J.; Gao J. Exploring and Exploiting Polar−π Interactions with Fluorinated Aromatic Amino Acids. Acc. Chem. Res. 2013, 46, 907–915. 10.1021/ar300086n. [DOI] [PubMed] [Google Scholar]
- Dominguez-Medina S.; Blankenburg J.; Olson J.; Landes C. F.; Link S. Adsorption of a Protein Monolayer via Hydrophobic Interactions Prevents Nanoparticle Aggregation under Harsh Environmental Conditions. ACS Sustainable Chem. Eng. 2013, 1, 833–842. 10.1021/sc400042h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.; Herting G.; Odnevall Wallinder I.; Blomberg E. Adsorption of Bovine Serum Albumin on Silver Surfaces Enhances the Release of Silver at PH Neutral Conditions. Phys. Chem. Chem. Phys. 2015, 17, 18524–18534. 10.1039/C5CP02306H. [DOI] [PubMed] [Google Scholar]
- Lori J. A.; Hanawa T. Adsorption Characteristics of Albumin on Gold and Titanium Metals in Hanks’ Solution Using EQCM. Spectroscopy 2004, 18, 545–552. 10.1155/2004/834639. [DOI] [Google Scholar]
- Tsai D.-H.; DelRio F. W.; Keene A. M.; Tyner K. M.; MacCuspie R. I.; Cho T. J.; Zachariah M. R.; Hackley V. A. Adsorption and Conformation of Serum Albumin Protein on Gold Nanoparticles Investigated Using Dimensional Measurements and in Situ Spectroscopic Methods. Langmuir 2011, 27, 2464–2477. 10.1021/la104124d. [DOI] [PubMed] [Google Scholar]
- Baldursdottir S. G.; Fullerton M. S.; Nielsen S. H.; Jorgensen L. Adsorption of Proteins at the Oil/Water Interface—Observation of Protein Adsorption by Interfacial Shear Stress Measurements. Colloids Surf., B 2010, 79, 41–46. 10.1016/j.colsurfb.2010.03.020. [DOI] [PubMed] [Google Scholar]
- Highberger J. H. The Isoelectric Point of Collagen. J. Am. Chem. Soc. 1939, 61, 2302–2303. 10.1021/ja01878a010. [DOI] [Google Scholar]
- Hattori S.; Adachi E.; Ebihara T.; Shirai T.; Someki I.; Irie S. Alkali-Treated Collagen Retained the Triple Helical Conformation and the Ligand Activity for the Cell Adhesion via A2fil Integrin. J. Biochem. 1999, 125, 676. 10.1093/oxfordjournals.jbchem.a022336. [DOI] [PubMed] [Google Scholar]
- Tan K. Y.; Lin H.; Ramstedt M.; Watt F. M.; Huck W. T. S.; Gautrot J. E. Decoupling Geometrical and Chemical Cues Directing Epidermal Stem Cell Fate on Polymer Brush-Based Cell Micro-Patterns. Integr. Biol. 2013, 5, 899–910. 10.1039/c3ib40026c. [DOI] [PubMed] [Google Scholar]
- Jia X.; Minami K.; Uto K.; Chang A. C.; Hill J. P.; Nakanishi J.; Ariga K. Adaptive Liquid Interfacially Assembled Protein Nanosheets for Guiding Mesenchymal Stem Cell Fate. Adv. Mater. 2020, 32, 1905942. 10.1002/adma.201905942. [DOI] [PubMed] [Google Scholar]
- Connelly J. T.; Gautrot J. E.; Trappmann B.; Tan D. W.-M.; Donati G.; Huck W. T. S.; Watt F. M. Actin and Serum Response Factor Transduce Physical Cues from the Microenvironment to Regulate Epidermal Stem Cell Fate Decisions. Nat. Cell Biol. 2010, 12, 711–718. 10.1038/ncb2074. [DOI] [PubMed] [Google Scholar]
- Trappmann B.; Gautrot J. E.; Connelly J. T.; Strange D. G. T.; Li Y.; Oyen M. L.; Cohen Stuart M. A.; Boehm H.; Li B.; Vogel V.; Spatz J. P.; Watt F. M.; Huck W. T. S. Extracellular-Matrix Tethering Regulates Stem-Cell Fate. Nat. Mater. 2012, 11, 642–649. 10.1038/nmat3339. [DOI] [PubMed] [Google Scholar]
- Clark R. A. F.; Folkvord J. M.; Wertz R. L. Fibronectin, As Well As Other Extracellular Matrix Proteins, Mediate Human Keratinocyte Adherence. J. Invest. Dermatol. 1985, 84, 378–383. 10.1111/1523-1747.ep12265466. [DOI] [PubMed] [Google Scholar]
- O’Keefe E. J.; Payne R. E.; Russell N.; Woodley D. T. Spreading and Enhanced Motility of Human Keratinocytes on Fibronectin. J. Invest. Dermatol. 1985, 85, 125–130. 10.1111/1523-1747.ep12276531. [DOI] [PubMed] [Google Scholar]
- Takashima A.; Grinnell F. Human Keratinocyte Adhesion and Phagocytosis Promoted by Fibronectin. J. Invest. Dermatol. 1984, 83, 352–358. 10.1111/1523-1747.ep12264522. [DOI] [PubMed] [Google Scholar]
- Jones P. H.; Watt F. M. Separation of Human Epidermal Stem Cells from Transit Amplifying Cells on the Basis of Differences in Lntegrin Function and Expression. Cell 1993, 73, 713–724. 10.1016/0092-8674(93)90251-k. [DOI] [PubMed] [Google Scholar]
- Kanchanawong P.; Shtengel G.; Pasapera A. M.; Ramko E. B.; Davidson M. W.; Hess H. F.; Waterman C. M. Nanoscale Architecture of Integrin-Based Cell Adhesions. Nature 2010, 468, 580–584. 10.1038/nature09621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey P.; Hawkes W.; Hu J.; Megone W. V.; Gautrot J.; Anilkumar N.; Zhang M.; Hirvonen L.; Cox S.; Ehler E.; Hone J.; Sheetz M.; Iskratsch T. Cardiomyocytes Sense Matrix Rigidity through a Combination of Muscle and Non-Muscle Myosin Contractions. Dev. Cell 2018, 44, 326–336. 10.1016/j.devcel.2017.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M.; Gweon B.; Koh U.; Cho Y.; Shin D. W.; Noh M.; Shin J. H. Matrix Stiffness Induces Epithelial Mesenchymal Transition Phenotypes of Human Epidermal Keratinocytes on Collagen Coated Two Dimensional Cell Culture. Biomed. Eng. Lett. 2015, 5, 194–202. 10.1007/s13534-015-0202-2. [DOI] [Google Scholar]
- Bennett M.; Cantini M.; Reboud J.; Cooper J. M.; Roca-Cusachs P.; Salmeron-Sanchez M. Molecular Clutch Drives Cell Response to Surface Viscosity. Proc. Natl. Acad. Sci. 2018, 115, 1192–1197. 10.1073/pnas.1710653115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khetan S.; Guvendiren M.; Legant W. R.; Cohen D. M.; Chen C. S.; Burdick J. A. Degradation-Mediated Cellular Traction Directs Stem Cell Fate in Covalently Crosslinked Three-Dimensional Hydrogels. Nat. Mater. 2013, 12, 458–465. 10.1038/nmat3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong D.; Nguyen K. D. Q.; Megone W.; Peng L.; Gautrot J. E. The Culture of HaCaT Cells on Liquid Substrates Is Mediated by a Mechanically Strong Liquid–Liquid Interface. Faraday Discuss. 2017, 204, 367–381. 10.1039/C7FD00091J. [DOI] [PubMed] [Google Scholar]
- Pieuchot L.; Marteau J.; Guignandon A.; Dos Santos T.; Brigaud I.; Chauvy P.-F.; Cloatre T.; Ponche A.; Petithory T.; Rougerie P.; Vassaux M.; Milan J.-L.; Tusamda Wakhloo N.; Spangenberg A.; Bigerelle M.; Anselme K. Curvotaxis Directs Cell Migration through Cell-Scale Curvature Landscapes. Nat. Commun. 2018, 9, 3995. 10.1038/s41467-018-06494-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megone W.; Kong D.; Peng L.; Gautrot J. E. Extreme Reversal in Mechanical Anisotropy in Liquid-Liquid Interfaces Reinforced with Self-Assembled Protein Nanosheets. J. Colloid Interface Sci. 2021, 594, 650–657. 10.1016/j.jcis.2021.03.055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.