Abstract
Background
The management of atrial fibrillation and flutter (AF) patients undergoing percutaneous coronary intervention (PCI) has evolved rapidly in the past decade. We determine whether the publication of the 2016 Canadian Cardiovascular Society AF guidelines were associated with a shift in practice patterns.
Methods
Using Quebec provincial administrative database information for the period from 2010-2017, a retrospective cohort of patients with inpatient or outpatient coding for AF, who subsequently underwent PCI with placement of a coronary stent, was created and analyzed for the antithrombotic regimen received in the following year. Prescribing behavior was compared among 3 time periods (2010-2011, 2012-2015, 2016-2017), and use of antithrombotics was compared to guideline-predicted therapy using the χ2 test. Predictors of oral anticoagulation (OAC) prescription were identified using adjusted logistic regression.
Results
A total of 3740 AF patients undergoing PCI were included. The proportion of OAC prescription increased over time (2010-2011 = 51.4%; 2012-2015 = 54.3%; 2016-2017 = 56.6%; P = 0.13), with a significant increase in direct OAC prescription (P < 0.01). A substantial treatment gap in OAC prescription persisted after publication of the 2016 guidelines (56.6% observed vs 89.7% predicted; P < 0.01). Previous stroke, CHADS2 score, Charlson Comorbidity Index ≥ 4, and prior use of direct OAC or warfarin were predictors of being exposed to OAC claims; previous major bleeding, and low-dose acetylsalicylic acid or P2Y12 inhibitor use were predictors of not being exposed to OACs.
Conclusion
Expert guidance contributed to an increase in OAC prescription following PCI, but up to 2017, substantial further changes in practice patterns would have been required to achieve the recommended rates of OAC prescription.
Résumé
Contexte
La prise en charge des patients qui sont atteints de fibrillation auriculaire (FA) ou de flutter auriculaire et qui subissent une intervention coronarienne percutanée (ICP) a évolué rapidement au cours de la dernière décennie. Nous avons voulu déterminer si la publication des lignes directrices sur la fibrillation auriculaire de la Société canadienne de cardiologie en 2016 s’était traduite par un changement de pratiques.
Méthodologie
À partir de renseignements recueillis dans la base de données administratives du Québec en ciblant la période allant de 2010 à 2017, nous avons créé une cohorte rétrospective de patients qui, selon le code diagnostique, avaient été hospitalisés ou reçus en consultation externe pour cause de FA ou de flutter auriculaire et qui avaient par la suite subi une ICP avec mise en place d'une endoprothèse coronaire. La cohorte a été l’objet d’une analyse visant à caractériser le traitement antithrombotique administré au cours de l’année suivant l’opération, et le comportement des prescripteurs a été comparé sur trois périodes (2010-2011, 2012-2015, 2016-2017). En outre, le recours aux antithrombotiques a été comparé au traitement prévu suivant les lignes directrices au moyen du test χ2. Les facteurs prédictifs de prescription d’anticoagulants oraux (ACO) ont été cernés par régression logistique corrigée.
Résultats
Au total, 3 740 patients atteints de FA ou de flutter auriculaire et ayant subi une ICP ont été inclus dans la cohorte. La proportion d’ordonnances d’ACO a augmenté au fil du temps (2010-2011 = 51,4 %, 2012-2015 = 54,3 %, 2016-2017 = 56,6 %; P = 0,13), et la prescription d’ACO directs a connu une augmentation significative (P < 0,01). Un écart important sur le plan thérapeutique en matière de prescription d’ACO a persisté après la publication des lignes directrices en 2016 (proportion observée de 56,6 % vs proportion prévue de 89,7 %; P < 0,01). Les antécédents d’AVC, le score CHADS2, un indice de comorbidité de Charlson ≥ 4 et les antécédents de traitement par des ACO directs ou la warfarine étaient des facteurs prédictifs d’exposition aux ACO; les antécédents de saignement majeur et la prise à faible dose d’acide acétylsalicylique ou d’un inhibiteur de P2Y12 étaient des facteurs prédictifs de non-exposition aux ACO.
Conclusion
Les avis des spécialistes ont contribué à une augmentation de la prescription d’ACO après une ICP. Toutefois, jusqu’en 2017, d’autres changements de pratique substantiels auraient été nécessaires pour atteindre les taux recommandés d’utilisation des ACO.
Contemporary antithrombotic management of patients with either atrial fibrillation/flutter (AF) or coronary artery disease is well established in clinical guidelines.1, 2, 3, 4 Up to 30% of patients with AF also have coronary artery disease,5 and the optimal management of AF patients requiring percutaneous coronary intervention (PCI) has, up until recently, been ill defined. Although oral anticoagulation (OAC) is preferred for the prevention of stroke and systemic embolism for most AF patients who are age 65 years or older or have a Congestive Heart Failure, Hypertension, Age ≥ 75, Diabetes, and Prior Stroke/Transient Ischemic Attack (doubled) (CHADS2) score ≥ 1 (strong recommendation; high-quality evidence),3 dual antiplatelet therapy is the standard of care after PCI in the absence of AF.6,7 However, combining these 2 recommendations in patients with AF who require PCI (so-called triple antithrombotic therapy [TATT]) increases the bleeding risk significantly.8
Recently, an international multicenter analysis demonstrated that the availability of newer antiplatelet and anticoagulant agents in the absence of guidance was associated with increased practice variability in the antithrombotic management of AF patients post-PCI.9 However, the Canadian Cardiovascular Society (CCS) and European Society of Cardiology (ESC) published recommendations in 2016 to help clinicians balance bleeding and thrombotic risks in these patients.1,3 The landmark Open-label, Randomized, Controlled, Multicenter Study Exploring Two Treatment Strategies of Rivaroxaban and One of Oral Vitamin K Antagonist in Patients With Atrial Fibrillation Who Undergo Percutaneous Coronary Intervention (PIONEER AF-PCI) study10 was also published in 2016, followed by the Randomized Evaluation of Dual Antithrombotic Therapy with Dabigatran versus Triple Therapy with Warfarin in Patients with Nonvalvular Atrial Fibrillation Undergoing Percutaneous Coronary Intervention(RE-DUAL PCI) study11 in 2017, both of which supported the use of dual pathway (OAC + P2Y12-inhibitor) antithrombotic regimens using direct oral anticoagulants (DOACs) to reduce bleeding risk in AF patients who underwent PCI. We therefore sought to determine whether these publications were associated with significant changes in OAC prescription using province-wide Quebec healthcare claims databases.
Methods
We conducted a retrospective cohort analysis using Quebec healthcare claims databases in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.12 The study protocol was consistent with the ethical guidelines of the 1975 Declaration of Helsinki. Ethics approval of the project was obtained from the University of Montreal Ethics Committee.
Data sources
Administrative databases (hospital discharges from Med-Echo; medical services; and public drug plan) administered by the Régie de l’Assurance Maladie du Quebec (RAMQ) were linked using encrypted health insurance numbers and were used to derive the study cohort.13, 14, 15, 16 The RAMQ covers all Quebec residents for the cost of physician visits, hospitalizations, and procedures, and it covers 94% of Quebec citizens aged ≥65 years on the drug insurance plan.15
Population
We identified patients aged ≥ 18 years with 1 inpatient or 2 outpatient diagnostic coding for AF within a 2-year period from January 1, 2010, to December 31, 2017, using International Classification of Diseases (ICD)-9 codes (427.3, 427.31, or 427.32) or the ICD-10 code (I48).17,18 AF patient data from the RAMQ database are available until December 31, 2017. The follow-up of the antithrombotic regimen can be conducted up to a year after the diagnosis depending on the year of entry into the cohort. The first instance of AF coding was used to determine eligibility. ICD-9 diagnostic codes for AF have a median positive predictive value of 89%.19 The cohort was then restricted to patients who subsequently underwent coronary stenting before December 31, 2017, using the procedural code 20521 in RAMQ databases.20 The date of the PCI was defined as the date of cohort entry. Patients who were hospitalized for ≥ 14 days following the PCI procedure were excluded, as were patients who resided in long-term care facilities, which typically provide medications to patients. Patients were required to have been enrolled in the provincial drug insurance plan for a minimum of 12 months prior to cohort entry. We then excluded any patient who underwent PCI for an acute coronary syndrome (ACS) indication and with coding for any non-AF or non-PCI condition or procedure that might have impacted the choice of antithrombotic regimen at discharge (Supplemental Table S1).
The total study cohort was subsequently divided into 3 time periods of interest, as follows: (i) subcohort 2010-2011 represents a “historic” period before DOACs were commercially available; (ii) subcohort 2012-2015 corresponds to a “pre-guidelines” period once DOACs and newer P2Y12 inhibitors were commercially available, but prior to publication of the 2016 CCS AF guidelines; and (iii) subcohort 2016-2017 represents a period in which guidance from the CCS AF guidelines and early landmark studies was emerging.
Patient demographics and clinical characteristics
Demographic data were extracted at cohort entry, whereas comorbidities were determined using inpatient and outpatient ICD-9 and ICD-10 diagnoses occurring in the 3 years preceding cohort entry.18,21,22 Using this information, we calculated the CHADS2 score (Supplemental Table S2), a modified Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly (> 65 Years), Drugs/Alcohol Concomitantly (HAS-BLED) score (Supplemental Table S3), and the Charlson Comorbidity Index for each patient.23 Estimated glomerular filtration rate (eGFR) was estimated with an algorithm based on diagnosis code, drug use, and nephrologist visits from administrative databases that was shown to be valid when compared with medical chart reviews in older adults.22 The algorithm used for eGFR definition had a positive predictive value ranging from 94.5% to 97.7%.22
Outcomes
The primary outcome of interest was the antithrombotic regimen (antiplatelet and anticoagulant) claimed after the entry into the cohort, which was assessed at the following 4 time points: (i) at 1 month; (ii) at 3 months; (iii) at 6 months; and (iv) at 12 months. In Quebec, most medications are dispensed for 30 days at a time. Consequently, medication exposure was measured within the 14 days preceding and the 14 days following each time point of follow-up.
Statistical analysis
Data are presented for the total cohort and the 3 subcohorts. Continuous data are expressed as mean with standard deviation, whereas categorical data are expressed as count and percentage. Comparisons among the 3 subcohorts were made using the Kruskal-Wallis test for continuous data, and the χ2 test for categorical data.
The primary analysis consisted of an evaluation of the difference in prescription patterns across subcohorts using the χ2 test. Secondarily, we used the χ2 test to perform an evaluation of the differences between antithrombotic prescription patterns in subcohort 2012-2015 and subcohort 2016-2017, compared with the patterns that would have been expected with perfect adherence to the 2016 CCS AF guidelines. Individual guideline-expected treatment was determined by assessing the indication for anticoagulation according to the CHADS2 score and the patient’s age. Patients aged ≥ 65 years or with a CHADS2 score ≥ 1 were expected to receive an OAC prescription, whereas patients aged < 65 years and with a CHADS2 score < 1 were not expected to receive an OAC prescription 1 month after cohort entry.
To better take into consideration the time necessary for guideline assimilation into clinical practice, a subanalysis of the difference in prescription patterns entry was performed as a sensitivity analysis using the χ2 test across the following subcohorts: (i) subcohort January 1, 2010 to December 31, 2011 represents a “historic” period before the commercial availability of DOACs; (ii) subcohort January 1, 2012 to August 31, 2017 corresponds to a “pre- and early guidelines” period once DOACs and newer P2Y12 inhibitors were commercially available, but prior to publication of the 2016 CCS AF guidelines; and (iii) subcohort September 1, 2017 to December 31, 2017 represents a period in which guidance from CCS AF guidelines was most likely assimilated into clinical practice. To consider deaths during the follow-up period, incident rates (per 100 person-years) of antithrombotic therapy (acetylsalicylic acid (ASA), P2Y12 inhibitor. and OAC) during the year following cohort entry were also provided.
Determinants of OAC prescription 1 month following PCI were identified using multivariable logistic regression analysis. Covariates were included if they were judged to be potential confounders, based on a combination of expert opinion and results of univariate analyses (P < 0.05). The variables included in the final model were the following: age ≥ 65 years; female sex; CHADS2 score ≥ 3; HAS-BLED score ≥ 3; Charlson Comorbidity Index ≥ 4; previous stroke; prior major bleeding; chronic renal failure (eGFR ≤ 30 mL/min); peripheral artery disease; liver disease; DOAC use within the 2 weeks prior to cohort entry; as well as warfarin use, low-dose ASA use, and P2Y12 inhibitor use within the 2 weeks. Crude and adjusted odds ratios (ORs) with 95% confidence intervals (CIs) are reported.
All analyses were performed using SAS 9.4 statistical software (SAS Institute, Cary, NC). A 2-tailed P-value < 0.05 was considered statistically significant, without correction for multiple analyses.
Results
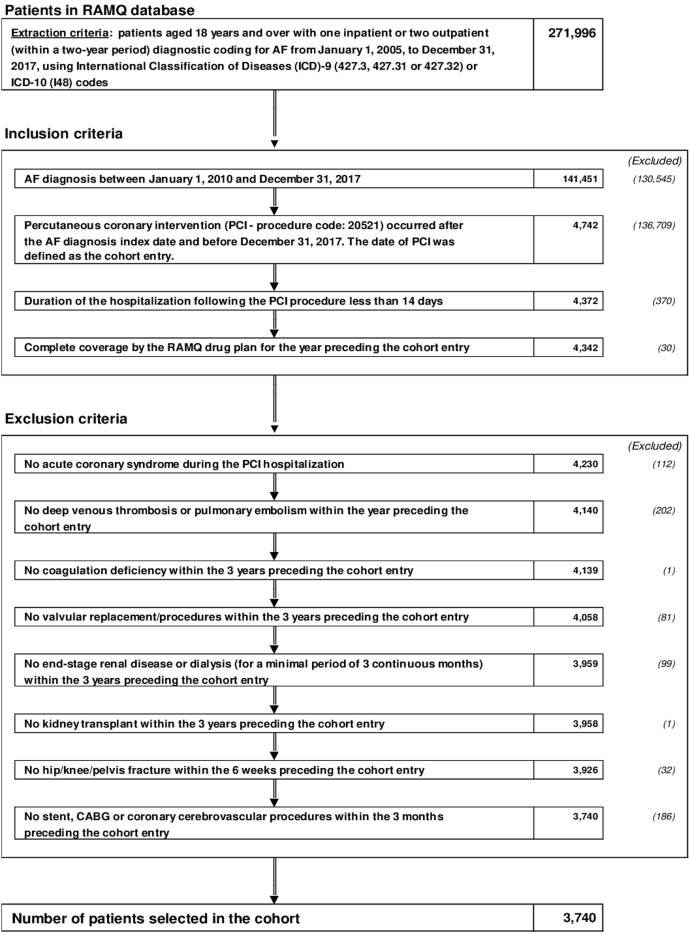
A total of 3740 patients with AF undergoing PCI were included in the cohort (Fig. 1). A total of 88% of AF patients were hospitalized during their index PCI. The mean duration of hospitalization was 6.2 ± 5.9 days, and 75.6% of AF patients were hospitalized for < 4 days. Baseline and demographic characteristics for the entire cohort as well as the 3 subcohorts are detailed in Table 1. Medication received within the 2 weeks prior to cohort entry are available in Supplemental Table S4.
Figure 1.
Flow chart of study design and patient cohort. AF, atrial fibrillation; CABG, coronary artery bypass graft surgery; ICD (9-10), International Classification of Diseases, 9th or 10th revision; PCI, percutaneous coronary intervention; RAMQ, Régie de l’assurance maladie du Québec.
Table 1.
Baseline and demographic characteristics at cohort entry
| Characteristic | Total cohort (n = 3740) | Subcohort 2010–2011 (n = 474) | Subcohort 2012–2015 (n = 1914) | Subcohort 2016–2017 (n = 1352) | P∗ |
|---|---|---|---|---|---|
| Age, y | 75.3 ± 8.8 75.8 (69.7–81.9) |
74.0 ± 9.7 75.0 (67.8–81.2) |
75.1 ± 8.7 75.5 (69.5–81.5) |
76.1 ± 8.7 76.4 (70.5–82.8) |
< 0.01 |
| Male | 2458 (65.7) | 307 (64.8) | 1264 (66.0) | 887 (65.6) | 0.87 |
| CHADS2 score | 3.7 ± 1.5 4.0 (3.0–5.0) |
3.6 ± 1.5 4.0 (3.0–5.0) |
3.8 ± 1.5 4.0 (3.0–5.0) |
3.8 ± 1.5 4.0 (3.0–5.0) |
0.03 |
| CHADS2 score categories | |||||
| 0–1 | 229 (6.1) | 41 (8.7) | 115 (6.0) | 73 (5.4) | 0.10 |
| 2–3 | 1410 (37.7) | 187 (39.4) | 704 (36.8) | 519 (38.4) | |
| 4 | 991 (26.5) | 125 (26.4) | 517 (27.0) | 349 (25.8) | |
| ≥ 5 | 1110 (29.7) | 121 (25.5) | 578 (30.2) | 411 (30.4) | |
| HAS-BLED score | 3.0 ± 1.3 3.0 (2.0–4.0) |
2.8 ± 1.2 3.0 (2.0–3.0) |
3.0 ± 1.3 3.0 (2.0–4.0) |
3.0 ± 1.3 3.0 (2.0–4.0) |
< 0.01 |
| HAS-BLED score ≥ 3 | 2427 (64.9) | 276 (58.2) | 1283 (67.0) | 868 (64.2) | < 0.01 |
| Charlson Comorbidity Index | 5.2 ± 3.5 5.0 (3.0–7.0) |
4.6 ± 3.2 4.0 (2.0–6.0) |
5.3 ± 3.4 5.0 (3.0–7.0) |
5.3 ± 3.6 5.0 (3.0–7.0) |
< 0.01 |
| Comorbidities within the 3 years prior to cohort entry | |||||
| Hypertension | 3237 (86.6) | 396 (83.5) | 1673 (87.4) | 1168 (86.4) | 0.09 |
| Coronary artery disease | 3697 (98.9) | 471 (99.4) | 1900 (99.3) | 1326 (98.1) | < 0.01 |
| Acute myocardial infarction | 2300 (61.5) | 285 (60.1) | 1233 (64.4) | 782 (57.8) | < 0.01 |
| Chronic heart failure | 1795 (48.0) | 205 (43.3) | 943 (49.3) | 647 (47.9) | 0.06 |
| Valvular heart disease | 916 (24.5) | 106 (22.4) | 477 (24.9) | 333 (24.6) | 0.50 |
| Stroke | 449 (12.3) | 53 (11.2) | 234 (12.2) | 172 (12.7) | 0.68 |
| Cardiomyopathy | 397 (10.6) | 49 (10.3) | 201 (10.5) | 147 (10.9) | 0.92 |
| Other cardiac dysrhythmias | 995 (26.6) | 107 (22.6) | 541 (28.3) | 347 (25.7) | 0.03 |
| Peripheral arterial disease | 1173 (31.4) | 141 (29.8) | 610 (31.9) | 422 (31.2) | 0.66 |
| Dyslipidemia | 2829 (75.6) | 342 (72.2) | 1451 (75.8) | 1036 (76.6) | 0.14 |
| Diabetes | 1686 (45.1) | 201 (42.4) | 882 (46.1) | 603 (44.6) | 0.32 |
| Major bleeding | 1265 (33.8) | 129 (27.2) | 660 (34.5) | 476 (35.2) | < 0.01 |
| Chronic renal failure (eGFR ≤ 30 mL/min) | 239 (6.4) | 29 (6.1) | 125 (6.5) | 85 (6.3) | 0.93 |
| Acute renal failure | 921 (24.6) | 73 (15.4) | 495 (25.9) | 353 (26.1) | < 0.01 |
| Liver disease | 100 (2.7) | 9 (1.9) | 55 (2.9) | 36 (2.7) | 0.50 |
| Chronic obstructive pulmonary disease | 1394 (37.3) | 155 (32.7) | 715 (37.4) | 524 (38.8) | 0.06 |
| Systemic embolism | 95 (2.5) | 7 (1.5) | 47 (2.5) | 41 (3.0) | 0.17 |
| Helicobacter pylori infection | 30 (0.8) | 5 (1.1) | 15 (0.8) | 10 (0.7) | 0.80 |
| Depression | 331 (8.9) | 37 (7.8) | 180 (9.4) | 114 (8.4) | 0.44 |
| Hypothyroidism | 761 (20.4) | 84 (17.7) | 389 (20.3) | 288 (21.3) | 0.25 |
| Neurologic disorder | 785 (21.0) | 78 (16.5) | 417 (21.8) | 290 (21.5) | 0.03 |
| Malignant cancer | 895 (23.9) | 88 (18.6) | 451 (23.6) | 356 (26.3) | < 0.01 |
| Medical procedures within the 3 years prior to cohort entry | |||||
| Coronary artery bypass grafting | 176 (4.7) | 18 (3.8) | 92 (4.8) | 66 (4.9) | 0.60 |
| Implantable cardiac devices | 18 (0.5) | 8 (1.7) | 8 (0.4) | 2 (0.2) | < 0.01 |
| Medical services within the year prior to cohort entry | |||||
| Specialty visits | 4.2 ± 6.1 | 3.8 ± 5.4 | 4.3 ± 6.5 | 4.3 ± 5.7 | 0.18 |
| Family physician visits | 3.2 ± 7.9 | 4.4 ± 7.7 | 3.3 ± 9.0 | 2.7 ± 6.1 | < 0.01 |
| Hospital services within the year prior to cohort entry | |||||
| Emergency visits | 6.0 ± 6.0 | 4.9 ± 4.7 | 6.2 ± 6.0 | 6.2 ± 6.4 | < 0.01 |
| All-cause hospital admissions | 3.1 ± 2.2 | 3.0 ± 2.0 | 3.2 ± 2.3 | 3.1 ± 2.3 | < 0.01 |
Values are mean ± standard deviation, median (interquartile range), or n (%), unless otherwise indicated.
ASA, acetylsalicylic acid; CHADS2, Congestive Heart Failure, Hypertension, Age ≥ 75, Diabetes, and Prior Stroke/Transient Ischemic Attack (doubled); eGFR, estimated glomerular filtration rate; HAS-BLED, Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly (> 65 Years), Drugs/Alcohol Concomitantly.
Significance applies to the difference among the 3 subcohorts.
Post-PCI antithrombotic treatment
Antithrombotic therapy during the first year after cohort entry for the total cohort and the subcohorts is presented in Table 2. Among patients receiving OAC and antiplatelet therapy at 1 month, the first prescriber was a cardiologist in 44.8% and 25.2% of patients, respectively. Over time, the proportion of patients receiving newer, more potent P2Y12 inhibitors (prasugrel or ticagrelor) increased at the expense of a decrease in clopidogrel prescription in the first month after PCI (P < 0.05 for all). The proportion of patients receiving ASA during the first year after cohort entry decreased significantly (P < 0.01 for all). More patients made OAC prescription claims over time (P < 0.01, except for OAC at 1 month), driven by a significant increase in DOAC prescription (P < 0.01 for all) and despite a concomitant decrease in warfarin prescription (P < 0.01 for all). Dual pathway therapy (DOAC + single antiplatelet) increased, whereas TATT prescription remained stable within 3 months after the index PCI, and then decreased. Accordingly, dual antiplatelet therapy decreased significantly during the first year after cohort entry. Results from the sensitivity analysis were similar to those of the primary analysis (Supplemental Table S5). Incident rates of antithrombotic therapy during the year following cohort entry are available in Supplemental Table S6. The incident OAC rate in the study cohort was 57.6 per 100 person-years (2012-2011 = 47.2; 2012-2015 = 57.8; 2016-20117 = 60.9 per 100 person-years).
Table 2.
Medication and antithrombotic therapy (ATT) during the year following cohort entry
| Medication/therapy |
Total cohort (n = 3740) |
Subcohort 2010–2011 (n = 474) |
Subcohort 2012–2015 (n = 1914) |
Subcohort 2016–2017 (n = 1352) |
P∗ |
|---|---|---|---|---|---|
| ATT at 1 month following the cohort entry | (n = 3552) | (n = 451) | (n = 1812) | (n = 1289) | |
| Low-dose ASA | 3141 (88.4) | 414 (91.8) | 1613 (89.0) | 1114 (86.4) | < 0.01 |
| P2Y12 inhibitor | |||||
| Ticagrelor | 332 (9.4) | 1 (0.2) | 171 (9.4) | 160 (12.4) | < 0.01 |
| Clopidogrel | 3040 (85.6) | 426 (94.5) | 1540 (85.0) | 1074 (83.3) | < 0.01 |
| Prasugrel | 52 (1.5) | 5 (1.1) | 37 (2.0) | 10 (0.8) | 0.01 |
| Oral anticoagulant | |||||
| Warfarin | 1118 (31.5) | 223 (49.5) | 676 (37.3) | 219 (17.0) | < 0.01 |
| DOAC | 865 (24.4) | 12 (2.7) | 327 (18.1) | 526 (40.8) | < 0.01 |
| Warfarin and/or DOAC | 1945 (54.8) | 232 (51.4) | 983 (54.3) | 730 (56.6) | 0.13 |
| Combination therapy | 0.02† | ||||
| DAPT | 1407 (39.6) | 193 (42.8) | 725 (40.0) | 489 (37.9) | |
| Dual pathway‡ | 232 (6.5) | 15 (3.3) | 111 (6.1) | 106 (8.2) | |
| TATT§ | 1652 (46.5) | 209 (46.3) | 843 (46.5) | 600 (46.7) |
| ATT at 3 months following the cohort entry§ | (n = 3477) | (n = 442) | (n = 1770) | (n = 1265) | |
|---|---|---|---|---|---|
| Low-dose ASA | 2753 (792) | 394 (89.1) | 1465 (82.8) | 894 (70.7) | < 0.01 |
| P2Y12 inhibitor | |||||
| Ticagrelor | 269 (7.7) | 1 (0.2) | 143 (8.1) | 125 (9.9) | < 0.01 |
| Clopidogrel | 2478 (71.3) | 314 (71.0) | 1170 (66.1) | 994 (78.6) | < 0.01 |
| Prasugrel | 47 (1.4) | 6 (1.4) | 32 (1.8) | 9 (0.7) | 0.04 |
| Oral anticoagulant | |||||
| Warfarin | 1037 (29.8) | 206 (46.6) | 624 (35.3) | 207 (16.4) | < 0.01 |
| DOAC | 934 (26.9) | 16 (3.6) | 371 (21.0) | 547 (43.2) | < 0.01 |
| Warfarin and/or DOAC | 1935 (55.7) | 222 (50.2) | 973 (55.0) | 740 (58.5) | < 0.01 |
| Combination therapy | < 0.01† | ||||
| DAPT | 1284 (36.9) | 186 (42.1) | 657 (37.1) | 441 (34.9) | |
| Dual-pathway‡ | 478 (13.8) | 17 (3.9) | 173 (9.8) | 288 (22.8) | |
| TATT§ | 929 (26.7) | 109 (24.7) | 458 (25.9) | 362 (28.6) |
| ATT at 6 months following the cohort entry§ | (n = 3373) | (n = 431) | (n = 1710) | (n = 1232) | |
|---|---|---|---|---|---|
| Low-dose ASA | 2422 (71.8) | 375 (87.0) | 1340 (78.4) | 707 (57.4) | < 0.01 |
| P2Y12 inhibitor | |||||
| Ticagrelor | 225 (6.7) | 0 (0.0) | 116 (6.8) | 109 (8.9) | < 0.01 |
| Clopidogrel | 2119 (62.8) | 284 (65.9) | 982 (57.4) | 853 (69.2) | < 0.01 |
| Prasugrel | 42 (1.3) | 4 (0.9) | 29 (1.7) | 9 (0.7) | 0.06 |
| Oral anticoagulant | |||||
| Warfarin | 917 (27.2) | 194 (45.0) | 549 (32.1) | 174 (14.1) | < 0.01 |
| DOAC | 971 (28.8) | 20 (4.6) | 396 (23.2) | 555 (45.1) | < 0.01 |
| Warfarin and/or DOAC | 1868 (55.4) | 214 (49.7) | 931 (54.4) | 723 (58.7) | < 0.01 |
| Combination therapy | < 0.01† | ||||
| DAPT | 1178 (34.9) | 180 (41.8) | 605 (35.4) | 393 (31.9) | |
| Dual-pathway‡ | 631 (18.7) | 19 (4.4) | 198 (11.6) | 414 (33.6) | |
| TATT§ | 462 (13.7) | 79 (18.3) | 260 (15.2) | 123 (10.0) |
| ATT at 12 months following the cohort entry | (n = 3219) | (n = 411) | (n = 1639) | (n = 1169) | |
|---|---|---|---|---|---|
| Low-dose ASA | 2178 (67.7) | 340 (82.7) | 1213 (74.0) | 625 (53.5) | < 0.01 |
| P2Y12 inhibitor | |||||
| Ticagrelor | 180 (5.6) | 0 (0.0) | 87 (5.3) | 93 (8.0) | < 0.01 |
| Clopidogrel | 1668 (51.8) | 248 (60.3) | 742 (45.3) | 678 (58.0) | < 0.01 |
| Prasugrel | 35 (1.1) | 3 (0.7) | 24 (1.5) | 8 (0.7) | 0.13 |
| Oral anticoagulant | |||||
| Warfarin | 766 (23.8) | 158 (38.4) | 484 (29.5) | 124 (10.6) | < 0.01 |
| DOAC | 1036 (32.2) | 33 (8.0) | 441 (26.9) | 562 (48.1) | < 0.01 |
| Warfarin and/or DOAC | 1787 (55.5) | 188 (45.7) | 914 (55.8) | 685 (58.6) | < 0.01 |
| Combination therapy | < 0.01† | ||||
| DAPT | 982 (30.5) | 164 (39.9) | 488 (29.8) | 330 (28.2) | |
| Dual-pathway‡ | 536 (16.7) | 18 (4.4) | 173 (10.6) | 345 (29.5) | |
| TATT§ | 251 (7.8) | 53 (12.9) | 137 (8.4) | 61 (5.2) |
Values are n (%), unless otherwise indicated.
ASA, acetylsalicylic acid; ATT, antithrombotic therapy; DAPT, dual antiplatelet therapy; DOAC, direct oral anticoagulant; TATT, triple antithrombotic therapy.
Medication at cohort entry was evaluated within the first 30 days following the discharge of the percutaneous coronary intervention (PCI) hospitalization, or within the first 30 days following the PCI diagnosis for patients not hospitalized for PCI. Medication exposure was measured within the 14 days preceding and the 14 days following the time of follow-up (1 month, 6 months, 12 months).
Significance applies to the difference among the 3 subcohorts.
P-value for the association between the 3 categories of combination therapy (mutually exclusive) and the 3 categories of subcohort.
Dual pathway: P2Y12 inhibitor + oral anticoagulant.
TATT: DAPT + oral anticoagulant.
DOAC choice and dosage 1 month after cohort entry are presented in Supplemental Table S7. Prescription of full- and reduced-dose DOACs increased over time for all, except for full-dose dabigatran. Reduced-dose DOACs were prescribed more frequently than full-dose DOACs as part of dual pathway and TATT regimens, except for full-dose apixaban as part of a dual-pathway regimen. Rivaroxaban and apixaban were the DOACs prescribed most frequently in these cohorts.
Guideline adherence
Observed and guideline-expected proportions and type of OAC are presented in Table 3. The observed proportion of OAC was significantly below the 2016 CCS guideline-expected proportion in both the early (54.3% vs 88.4%; P < 0.01) and later (56.6% vs 89.7%; P < 0.01) periods.
Table 3.
Observed anticoagulation at 1 month following cohort entry vs guideline-expected proportions of oral anticoagulation
| Anticoagulation, n (%) |
P | ||
|---|---|---|---|
| Observed | Expected dispensation according to 2016 CCS AF guidelines | ||
| Pre-guidelines period, 2012–2015 (n = 1812)∗ | 983 (54.3) | 1601 (88.4) | < 0.01 |
| Post-guidelines period, 2016–2017 (n = 1289)† | 730 (56.6) | 1186 (89.7) | < 0.01 |
AF, atrial fibrillation; CCS, Canadian Cardiovascular Society.
Among the 1914 patients in subcohort 2012–2015, a total of 1812 had available data concerning the medication exposure at 1 month following cohort entry.
Among the 1352 patients in subcohort 2016–2017, a total of 1289 had available data concerning the medication exposure at 1 month following cohort entry.
Determinants of OAC prescription
Determinants of OAC prescription are presented in Table 4. Significant determinants of OAC prescription 1 month following cohort entry in the adjusted model were a CHADS2 score ≥ 3 (OR 1.67; 95% CI 1.33-2.09), a Charlson Comorbidity Index ≥ 4 (OR 1.28; 95% CI 1.08-1.52), a previous stroke (OR 1.33; 95% CI 1.05-1.68), DOAC use within the 2 weeks prior to cohort entry (OR 7.79; 95% CI 5.94-10.23), and warfarin use (OR 6.18; 95% CI 4.52-8.45). Conversely, prior major bleeding (OR 0.81; 95% CI 0.68-0.96), low-dose ASA use (OR 0.49; 95% CI 0.41-0.58), and P2Y12 inhibitor use (OR 0.54; 95% CI 0.42-0.70) were determinants of being less likely to be exposed to an OAC. Again, the determinants of warfarin claims were similar to those for OAC prescription, with the exception of a Charlson Comorbidity Index ≥ 4, previous stroke, previous major bleeding, and DOAC use within the 2 weeks prior to cohort entry, the latter being a predictor of being less likely to be exposed to warfarin (OR 0.71; 95% CI 0.59-0.89). The only significant predictor of being exposed to DOAC is a prior use within the 2 weeks prior to cohort entry (OR 9.04; 95% CI 7.29-11.21). Having a prior exposure to low-dose ASA (OR 0.60; 95% CI 0.48-0.75) and warfarin (OR 0.37; 95% CI 0.24-0.57) within the 2 weeks prior to cohort entry, as well as having chronic renal failure (eGFR ≤ 30 mL/min; OR 0.51; 95% CI 0.33-0.79) were determinants of being less likely to be exposed to DOACs.
Table 4.
Determinants of oral anticoagulation prescribed within the month following cohort entry
| Determinants | OAC |
Warfarin |
DOACs |
|||
|---|---|---|---|---|---|---|
| Crude | Adjusted | Crude | Adjusted | Crude | Adjusted | |
| Age, y, ≥ 65 vs < 65 | 1.72 (1.40–2.12) | 1.29 (0.99–1.67) | 1.59 (1.25–2.01) | 1.18 (0.88–1.57) | 1.27 (0.99–1.62) | 1.18 (0.87–1.62) |
| Female vs male | 1.08 (0.94–1.25) | 0.91 (0.78–1.08) | 1.11 (0.95–1.29) | 0.98 (0.83–1.16) | 0.98 (0.83–1.15) | 0.88 (0.73–1.07) |
| CHADS2 score ≥ 3 vs < 3 | 1.74 (1.48–2.04) | 1.67 (1.33–2.09) | 1.84 (1.52–2.22) | 1.60 (1.25–2.05) | 1.07 (0.89–1.29) | 1.12 (0.86–1.46) |
| HAS-BLED score ≥ 3 vs < 3 | 0.91 (0.79–1.05) | 0.85 (0.70–1.03) | 1.15 (0.99–1.34) | 1.00 (0.82–1.23) | 0.75 (0.64–0.87) | 0.84 (0.67–1.04) |
| Charlson Comorbidity Index ≥ 4 vs < 4 | 1.33 (1.16–1.53) | 1.28 (1.08–1.52) | 1.40 (1.20–1.62) | 1.18 (0.99–1.41) | 1.02 (0.87–1.20) | 1.17 (0.96–1.43) |
| Previous stroke (yes vs no) | 1.43 (1.16–1.75) | 1.33 (1.05–1.68) | 1.39 (1.12–1.71) | 1.32 (1.05–1.66) | 1.09 (0.87–1.38) | 1.04 (0.80–1.37) |
| Prior major bleeding (yes vs no) | 0.87 (0.76–0.99) | 0.81 (0.68–0.96) | 1.03 (0.89–1.20) | 0.88 (0.74–1.06) | 0.80 (0.68–0.95) | 0.87 (0.71–1.07) |
| Chronic renal failure (eGFR ≤ 30 mL/min) vs ≥ 30 | 0.93 (0.71–1.23) | 0.96 (0.71–1.29) | 1.53 (1.16–2.02) | 1.35 (0.99–1.84) | 0.43 (0.29–0.64) | 0.51 (0.33–0.79) |
| Peripheral artery disease (yes vs no) | 1.03 (0.89–1.18) | 0.90 (0.76–1.06) | 1.11 (0.95–1.29) | 0.99 (0.84–1.18) | 0.90 (0.76–1.06) | 0.84 (0.69–1.03) |
| Liver disease (yes vs no) | 0.63 (0.41–0.95) | 0.65 (0.41–1.03) | 0.68 (0.42–1.10) | 0.66 (0.39–1.09) | 0.80 (0.48–1.34) | 0.94 (0.54–1.63) |
| Medication use within the 2weeks prior to cohort entry | ||||||
| DOAC | 6.27 (4.82–8.15) | 7.79 (5.94–10.23) | 0.60 (0.48–0.75) | 0.71 (0.56–0.89) | 9.22 (7.50–11.35) | 9.04 (7.29–11.21) |
| Warfarin | 4.42 (3.27–5.97) | 6.18 (4.52–8.45) | 8.19 (6.26–10.71) | 8.14 (6.17–10.75) | 0.24 (0.16–0.36) | 0.37 (0.24–0.57) |
| Baseline ASA use at cohort entry (excluding antiplatelet) | 0.54 (0.46–0.62) | 0.49 (0.41–0.58) | 0.68 (0.58–0.80) | 0.61 (0.51–0.74) | 0.61 (0.51–0.74) | 0.60 (0.48–0.75) |
| Baseline P2Y12 inhibitor use | 0.44 (0.35–0.55) | 0.54 (0.42–0.70) | 0.56 (0.43–0.73) | 0.62 (0.46–0.83) | 0.58 (0.43–0.77) | 0.72 (0.52–1.00) |
Values are odds ratio (95% confidence interval).
ASA, acetylsalicylic acid; CHADS2, Congestive Heart Failure, Hypertension, Age ≥ 75, Diabetes, and Prior Stroke/Transient Ischemic Attack (doubled); DOAC, direct oral anticoagulant; eGFR, estimated glomerular filtration rate; HAS-BLED, Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly (> 65 Years), Drugs/Alcohol Concomitantly; OAC, oral anticoagulation.
Discussion
This retrospective cohort analysis reveals several findings pertinent to both clinical practice and the design of professional educational initiatives. Significant changes in baseline medication over time were observed. Despite a decline in warfarin prescription, OAC prescription increased, both at cohort entry and within the first year following PCI, owing to a substantial uptake of DOAC therapy, associated with an increase in both TATT and dual-pathway antithrombotic regimens. However, in spite of these significant shifts in clinical practice, the overall proportion of OAC prescription remains below the proportions expected with perfect guideline adherence, up to 2017. Lastly, we identified important clinical determinants of both OAC and DOAC prescription at discharge.
The observed increase in OAC prescription is in line with the recommendation from both the 2016 CCS AF guidelines and landmark studies3,10,11 of TATT for 3 to 6 months in patients with a CHADS2 score ≥ 1 who undergo PCI for an ACS, placing greater weight on reduction of thromboembolic events and comparatively less weight on risk of major bleeding.3 A course of TATT of up to 6 months for patients with a CHADS2 score ≥ 1 in the setting of an ACS or elective PCI with a high thrombotic risk is suggested in a recent update of the CCS antiplatelet guidelines.24,25 The emergence of a dual-pathway regimen, in the period up to 2017, represents an integration of randomized trial data, from PIONEER AF-PCI (rivaroxaban) and RE-DUAL PCI (dabigatran), that showed that such a regimen could reduce bleeding risk without a signal for an increase in ischemic events.10,11 Although dual-pathway therapy was recommended only in AF patients who undergo an elective PCI, in the 2016 CCS AF guidelines,3 a broader shift to dual-pathway antithrombotic management is advocated in the 2018 updates of the CCS antiplatelet and AF guidelines.24,25 The subsequently published Apixaban Versus Warfarin in Patients with AF and ACS or PCI (AUGUSTUS) (apixaban) and Edoxaban Treatment Versus Vitamin K Antagonist in Patients With Atrial Fibrillation Undergoing Percutaneous Coronary Intervention (ENTRUST-AF-PCI) trials reinforced the safety advantage of dual-pathway over triple therapy,26,27 and similar results were found in recent retrospective studies of AF patients undergoing PCI in Asia and Europe.28,29
An international multicentre analysis, including AF patients undergoing PCI from 2010 to 2015, showed that the availability of newer antiplatelet and anticoagulant agents increased practice variability in the antithrombotic management of AF patients post-PCI. As with the present analysis using administrative data, it revealed that a major change in clinical practice would be necessary to achieve a high degree of agreement with AF guidelines.9 A recent analysis of an Alberta administrative database showed that, after the publication of the 2016 CCS AF guidelines and the landmark PIONEER-AF-PCI and RE-DUAL PCI trials, more patients were anticoagulated, and the choice of agent favoured DOACs over warfarin.30 However, almost half of the post-guideline cohort did not receive an OAC prescription.30 This treatment gap has also been reported in large observational studies of AF patients without PCI. Introductions of DOACs combined with professional guidance and early landmark trials reduced, but did not eliminate, OAC underuse.30, 31, 32 Although clinically appropriate reasons for this discrepancy may not have been captured in observational studies, clinicians might still place a greater weight on reduction of stent thrombosis/restenosis and comparatively lesser weight on the risk of stroke early after the index PCI. Nevertheless, the short period of observation after publication of landmark trials is not sufficient to explain this treatment gap, given that our sensitivity analysis, examining the antithrombotic regimen received after PCI, longer after the publication of the 2016 AF CCS Guidelines, led to similar results. As in our analysis, female sex and concomitant use of ASA and other antiplatelets have repeatedly been identified as determinants of OAC nonprescription, and high CHADS2 or Congestive Heart Failure, Hypertension, Age (≥ 75 Years) (doubled), Diabetes Mellitus, Stroke (doubled), Vascular Disease, Age (65-74) Years, Sex Category (Female) (CHA2DS2-VASc) scores have been identified as determinants of OAC prescription in the AF population.31, 32, 33 As for agent choice, an eGFR ≥ 30 mL/min also has been identified as a predictor of being prescribed a DOAC instead of warfarin among AF patients.33
Certain limitations of the present analysis must be acknowledged. First, this retrospective observational analysis relied on administrative data that depend on complete and accurate recording of diagnoses, as well as procedure and drug codes. Reassuringly, however, diagnostic, procedural, and drug codes have been well validated in this dataset,13, 14, 15, 16,19 but a risk of ascertainment bias remains. Second, the use of over-the-counter medications (eg, ASA) may lead to inadequate assessment of the antithrombotic regimen therapy received within the first year after PCI. However, the probability of inadequate ASA claims assessment is very low, since more than 95% of older patients are using ASA claims instead of over-the-counter use. Third, clinically appropriate reasons for the discrepancy between the overall proportion of OAC prescription and the expected proportion under perfect guideline adherence might have not been captured in our analysis (eg, short-duration, transient AF). Forth, the antithrombotic regimen therapy received within the first 14 days after PCI was not assessed, as it may be imprecise (eg, some patients who were prescribed P2Y12 inhibitors prior to their PCI might have been able to wait before filling their new prescription). Fifth, we did not have the exact eGFR values. However, the algorithm used to estimate eGFR has been validated by chart review.22 Sixth, the transferability is limited to AF patients who were hospitalized for more than 14 days, and patients with an ACS, since they were excluded from the analysis. Lastly, AF patient data from the RAMQ database were available until the 31st of December 2017; therefore, we were unable to assess antithrombotic regimen prescription patterns in the AF population undergoing PCI after this date.
Conclusion
The overall proportion of patients taking an OAC remained significantly lower than expected according to current guidelines at the time. Understanding impediments to OAC prescription in this patient population is critical to the planning of educational initiatives. Prior major bleeding and the use of antiplatelet therapy at cohort entry were determinants of non-OAC prescription 1 month after PCI in this cohort.
Acknowledgments
Funding Sources
No financial support was received for this publication. B.J.P. is supported by a Fonds de recherche du Québec-Santé career award (267436). The other authors have no funding sources to declare.
Disclosures
B.J.P. has served as a consultant for and/or received research funding from Bayer Canada, Boerhinger-Ingelhiem Canada, BMS-Pfizer Canada, and Servier Canada. The other authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: The study protocol was consistent with the ethical guidelines of the 1975 Declaration of Helsinki. Ethics approval of the project was obtained from the University of Montreal Ethics Committee.
See page 22 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2022.10.004.
Supplementary Material
References
- 1.Kirchhof P., Benussi S., Kotecha D., et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur J Cardiothorac Surg. 2016;50:e1–88. doi: 10.1093/ejcts/ezw313. [DOI] [PubMed] [Google Scholar]
- 2.Montalescot G., Sechtem U., Achenbach S., et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the Management of Stable Coronary Artery Disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949–3003. doi: 10.1093/eurheartj/eht296. [DOI] [PubMed] [Google Scholar]
- 3.Macle L., Cairns J., Leblanc K., et al. 2016 focused update of the Canadian Cardiovascular Society guidelines for the management of atrial fibrillation. Can J Cardiol. 2016;32:1170–1185. doi: 10.1016/j.cjca.2016.07.591. [DOI] [PubMed] [Google Scholar]
- 4.Mancini G.B.J., Gosselin G., Chow B., et al. Canadian Cardiovascular Society guidelines for the diagnosis and management of stable ischemic heart disease. Can J Cardiol. 2014;30:837–849. doi: 10.1016/j.cjca.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 5.Budhraja V. The net clinical benefit of warfarin anticoagulation in atrial fibrillation. Ann Intern Med. 2010;152:265. doi: 10.7326/0003-4819-152-4-201002160-00017. [DOI] [PubMed] [Google Scholar]
- 6.Bell A.D., Roussin A., Cartier R., et al. The use of antiplatelet therapy in the outpatient setting: Canadian Cardiovascular Society guidelines. Can J Cardiol. 2011;27:S1–59. doi: 10.1016/j.cjca.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 7.Tanguay J.-F., Bell A.D., Ackman M.L., et al. Focused 2012 update of the Canadian Cardiovascular Society guidelines for the use of antiplatelet therapy. Can J Cardiol. 2013;29:1334–1345. doi: 10.1016/j.cjca.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Lamberts M., Olesen J.B., Ruwald M.H., et al. Bleeding after initiation of multiple antithrombotic drugs, including triple therapy, in atrial fibrillation patients following myocardial infarction and coronary intervention. Circulation. 2012;126:1185. doi: 10.1161/CIRCULATIONAHA.112.114967. [DOI] [PubMed] [Google Scholar]
- 9.Potter B.J., Andò G., Cimmino G., et al. Time trends in antithrombotic management of patients with atrial fibrillation treated with coronary stents: results from TALENT-AF (The internAtionaL stENT – Atrial Fibrillation study) multicenter registry. Clin Cardiol. 2018;41:470–475. doi: 10.1002/clc.22898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibson C.M., Mehran R., Bode C., et al. Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N Engl J Med. 2016;375:2423–2434. doi: 10.1056/NEJMoa1611594. [DOI] [PubMed] [Google Scholar]
- 11.Cannon C.P., Bhatt D.L., Oldgren J., et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med. 2017;377:1513–1524. doi: 10.1056/NEJMoa1708454. [DOI] [PubMed] [Google Scholar]
- 12.von Elm E., Altman D.G., Egger M., et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12:1495–1499. doi: 10.1016/j.ijsu.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 13.Tamblyn R., Lavoie G., Petrella L., Monette J. The use of prescription claims databases in pharmacoepidemiological research: the accuracy and comprehensiveness of the prescription claims database in Quebec. J Clin Epidemiol. 1995;48:999–1009. doi: 10.1016/0895-4356(94)00234-h. [DOI] [PubMed] [Google Scholar]
- 14.Eguale T., Winslade N., Hanley J.A., Buckeridge D.L., Tamblyn R. Enhancing pharmacosurveillance with systematic collection of treatment indication in electronic prescribing: a validation study in Canada. Drug Saf. 2010;33:559–567. doi: 10.2165/11534580-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 15.Wilchesky M., Tamblyn R.M., Huang A. Validation of diagnostic codes within medical services claims. J Clin Epidemiol. 2004;57:131–141. doi: 10.1016/S0895-4356(03)00246-4. [DOI] [PubMed] [Google Scholar]
- 16.Tamblyn R., Reid T., Mayo N., McLeod P., Churchill-Smith M. Using medical services claims to assess injuries in the elderly: sensitivity of diagnostic and procedure codes for injury ascertainment. J Clin Epidemiol. 2000;53:183–194. doi: 10.1016/s0895-4356(99)00136-5. [DOI] [PubMed] [Google Scholar]
- 17.Humphries K.H., Jackevicius C., Gong Y., et al. Population rates of hospitalization for atrial fibrillation/flutter in Canada. Can J Cardiol. 2004;20:869–876. [PubMed] [Google Scholar]
- 18.Perreault S., Shahabi P., Cote R., et al. Rationale, design, and preliminary results of the Quebec Warfarin Cohort Study. Clin Cardiol. 2018;41:576–585. doi: 10.1002/clc.22948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jensen P.N., Johnson K., Floyd J., et al. A systematic review of validated methods for identifying atrial fibrillation using administrative data. Pharmacoepidemiol Drug Saf. 2012;21(suppl 1):S141–S147. doi: 10.1002/pds.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Québec Régie de l'assurance maladie Manuel des médecins spécialistes: rénumération à l’acte. https://www.ramq.gouv.qc.ca/fr/professionnels/medecins-specialistes/manuels/Pages/remuneration-acte.aspx Available at: [in French]
- 21.C.L.L. Blais, D. Hamel, K. Brown, et al., Évaluation des soins et surveillance des maladies cardiovasculaires de santé publique du Québec et de L’institut national d’excellence en santé et services sociaux, Gouvernement du Québec, Institut national de santé publique , Institut national d’excellence en santé et des services sociaux, 2012, pp. 1–9. Available at: https://www.inspq.qc.ca/publications/1558. Accessed December 12, 2022.
- 22.Roy L., Zappitelli M., White-Guay B., et al. Agreement between administrative database and medical chart review for the prediction of chronic kidney disease G category. Can J Kidney Health Dis. 2020;7 doi: 10.1177/2054358120959908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D'Hoore W., Bouckaert A., Tilquin C. Practical considerations on the use of the Charlson Comorbidity Index with administrative data bases. J Clin Epidemiol. 1996;49:1429–1433. doi: 10.1016/s0895-4356(96)00271-5. [DOI] [PubMed] [Google Scholar]
- 24.Andrade J.G., Verma A., Mitchell L.B., et al. 2018 focused update of the Canadian Cardiovascular Society guidelines for the management of atrial fibrillation. Can J Cardiol. 2018;34:1371–1392. doi: 10.1016/j.cjca.2018.08.026. [DOI] [PubMed] [Google Scholar]
- 25.Mehta S.R., Bainey K.R., Cantor W.J., et al. 2018 Canadian Cardiovascular Society/Canadian Association of Interventional Cardiology focused update of the guidelines for the use of antiplatelet therapy. Can J Cardiol. 2018;34:214–233. doi: 10.1016/j.cjca.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 26.Lopes R.D., Heizer G., Aronson R., et al. Antithrombotic therapy after acute coronary syndrome or PCI in atrial fibrillation. N Engl J Med. 2019;380:1509–1524. doi: 10.1056/NEJMoa1817083. [DOI] [PubMed] [Google Scholar]
- 27.Vranckx P., Valgimigli M., Eckardt L., et al. Edoxaban-based versus vitamin K antagonist-based antithrombotic regimen after successful coronary stenting in patients with atrial fibrillation (ENTRUST-AF PCI): a randomised, open-label, phase 3b trial. Lancet. 2019;394:1335–1343. doi: 10.1016/S0140-6736(19)31872-0. [DOI] [PubMed] [Google Scholar]
- 28.Lane D.A., Dagres N., Dan G.A., et al. Antithrombotic treatment in patients with atrial fibrillation and acute coronary syndromes: results of the European Heart Rhythm Association survey. Europace. 2019;21:1116–1125. doi: 10.1093/europace/euz033. [DOI] [PubMed] [Google Scholar]
- 29.Park J., Choi E.K., Han K.D., et al. Temporal trends in prevalence and antithrombotic treatment among Asians with atrial fibrillation undergoing percutaneous coronary intervention: a nationwide Korean population-based study. PLoS One. 2019;14 doi: 10.1371/journal.pone.0209593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eberhardt T.E., Bungard T.J., Graham M.M., et al. Effect of new evidence on antithrombotic therapies in atrial fibrillation patients who undergo percutaneous coronary intervention in Alberta, Canada. CJC Open. 2021;4:378–382. doi: 10.1016/j.cjco.2021.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lubitz S.A., Khurshid S., Weng L.-C., et al. Predictors of oral anticoagulant non-prescription in patients with atrial fibrillation and elevated stroke risk. Am Heart J. 2018;200:24–31. doi: 10.1016/j.ahj.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marzec L.N., Wang J., Shah N.D., et al. Influence of direct oral anticoagulants on rates of oral anticoagulation for atrial fibrillation. J Am Coll Cardiol. 2017;69:2475–2484. doi: 10.1016/j.jacc.2017.03.540. [DOI] [PubMed] [Google Scholar]
- 33.Brais C., Larochelle J., Turgeon M.H., et al. Predictors of direct oral anticoagulants utilization for thromboembolism prevention in atrial fibrillation. J Pharm Pharm Sci. 2017;20:8–14. doi: 10.18433/J30W4F. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.