Abstract
Systems vaccinology has defined molecular signatures and mechanisms of immunity to vaccination. However, comparative analysis of immunity to different vaccines is lacking. We integrated transcriptional data of over 3,000 samples, from 820 adults across 28 studies of 13 vaccines and analyzed vaccination-induced signatures of antibody responses. Most vaccines induced signatures of innate immunity and plasmablasts at Days 1 and 7 respectively post-vaccination. However, the yellow fever vaccine induced an early transient signature of T and B cell activation at Day 1, followed by delayed antiviral/interferon and plasmablast signatures that peaked at Days 7 and 14–21, respectively. Thus, there was no evidence for a “universal signature” that predicted antibody response to all vaccines. However, accounting for the asynchronous nature of responses we defined a time-adjusted signature that predicted antibody responses across vaccines. These results provide a transcriptional atlas of immunity to vaccination and define a common, time-adjusted signature of antibody responses.
Introduction
Systems vaccinology employs high-throughput-omics measurements together with systems-based analysis approaches to better understand immune responses to vaccination1, 2. The recent growth of this field, which began with initial studies of yellow fever3, 4 and seasonal influenza5, 6 vaccines, has rapidly expanded to include studies profiling responses to a range of vaccines and vaccine platforms, including those targeting diverse pathogens and age groups7–20. These studies have led to important discoveries such as the role for the nutrient sensor general control nonderepressible 2 (GCN2) in enhancing antigen presentation during responses to yellow fever vaccination21, as well as the impact of the gut microbiota in promoting antibody responses to inactivated influenza vaccination22, 23. However, outside of a few studies8–10, thus far the vast majority have examined immune responses to a single vaccine, hindering the ability to contextualize the findings and understand how differences in vaccine formulations can impact immunogenicity.
Another important outcome of such studies has been the identification of early transcriptional signatures predictive of immune response quality such as subsequent antibody3, 5, 18 or antigen-specific T cell3, 7, 14 responses. These findings may enable more rapid and personalized evaluation of vaccine efficacy and development of improved next-generation vaccines. Yet again, a current limitation is that the identified predictive signatures thus far have been described in the context of responses to a single vaccine, and the extent to which predictors of immune response quality are conserved across vaccines is unclear24, 25. We previously sought to address the question of whether there was a ‘universal signature’ that could be used to predict antibody responses to any vaccine by analyzing the transcriptional response to 5 different human vaccines. Our analysis revealed distinct transcriptional signatures of antibody responses to different classes of vaccines, and provided key insights into primary viral, protein recall and anti-polysaccharide responses8, yet failed to identify a universal signature of vaccination.
Here we leverage Immune Signatures Data Resource26, a curated database of publicly available datasets containing transcriptional and immune response profiling of peripheral blood following vaccination in humans, to perform a comparative analysis of transcriptional responses from 820 healthy young adults across 13 different vaccines. We find that while a common transcriptional program is shared across many vaccines, there is considerable heterogeneity especially in the kinetics of immune responses. In particular, the live attenuated yellow fever vaccine induces a unique transcriptional response, with a surprisingly early upregulation of B and T cell modules within a day of vaccination, and a delayed induction of innate responses, including antiviral and interferon signaling, peaking at 10–14 days following vaccination. Furthermore, in an analysis of predictive signatures of antibody responses across vaccines, adjusting for time of peak expression enabled a gene module associated with plasma cells and immunoglobulins to consistently predict antibody responses across vaccines, demonstrating the importance of accounting for immune response kinetics in the development of universal predictors of response quality. Together, these findings highlight the spectrum of immune responses across vaccines and serve as a basis for future studies to understand the mechanisms underlying variation in immune responses across vaccines and inform future vaccine development.
Results
An integrated database of transcriptional responses to vaccination
As part of an effort to enable comparative studies and benchmarking of human vaccine responses, we curated a database of transcriptomic responses of 820 healthy adults (18–50 years old) across 13 different vaccines from previously published datasets. These datasets were compiled into ImmPort, an NIH-funded repository for immunological data27, and uploaded to ImmuneSpace (http://www.ImmuneSpace.org) for centralized QC and processing (Figure 1A). This combined database, named the Immune Signatures Data Resource26, includes responses to a broad range of vaccines, including live viruses (e.g. yellow fever, smallpox and influenza vaccines), recombinant viral vectors (e.g. Ebola and HIV vaccines), inactivated viruses (e.g. seasonal influenza vaccine), glycoconjugate vaccines (e.g. pneumococcal and meningococcal vaccines) (Table 1 and Extended Data Table 1). It also contains samples spanning multiple response timepoints, ranging from a few hours to more than 3 weeks post-vaccination (Figure 1B). Included participants in our initial analysis were restricted to 18–50 years old, and there were similar age and sex distributions across vaccines (Figure 1C). For analysis, all post-vaccination samples were normalized by pairwise fold change calculation with their matched pre-vaccination samples. Principal variance component analysis (PVCA)28 revealed that demographic features such as age and sex had relatively small contributions to variation in responses. In contrast, the post-vaccination timepoint of the sample explained 15% of the variance in the data, suggesting that there are shared kinetics of immune responses across vaccines (Figure 1D).
Figure 1. An integrated database of transcriptional responses to vaccination.

A) Workflow for collection, curation, and standardization of datasets in the Immune Signatures Data Resource. B) Histogram of the number of samples included per vaccine at each timepoint. Day 0 represents Day of vaccination. C) Age distribution of participants in the Immune Signatures Data Resource by vaccine. Shape of points denotes the subject’s inferred sex based on Y chromosome-specific gene expression. For participants with missing age data, ages were estimated using RAPToR43. Center line, median; box limits, upper and lower quartiles; whiskers, 1.5x interquartile range. Ebola (RVV): n=13, Hepatitis A/B (IN/RP): n=26, HIV (RVV): n=10, Influenza (IN): n=496, Influenza (LA): n=28, Malaria (RP): n=44, Meningococcus (CJ): n=19, Meningococcus (PS): n=14, Pneumococcus (PS): n=12, Smallpox (LA): n=8, Tuberculosis (RVV): n=12, Varicella Zoster (LA): n=31, Yellow Fever (LA): n=107. D) Bar plot representing the proportion of variance in post-vaccination transcriptional responses that can be attributed to clinical (age, sex, ethnicity) and experimental variables (time after vaccination, vaccine) via Principal Component Variance Analysis. The residual represents the proportion of the variance that could not be explained by any of the included variables.
Table 1.
Table of included vaccines.
| Vaccine | Pathogen | Vaccine Type | Adjuvant/Vector |
|---|---|---|---|
|
| |||
| rVSV-ZEBOV | Ebola | Recombinant Viral Vector (RVV) | Vesicular stomatitis virus (VSV) |
| Hepatitis A/B vaccine* | Hepatitis A/B | Inactivated/Recombinant Protein (IN/RP) | None |
| MRKAd5/HIV | HIV | Recombinant Viral Vector (RVV) | Adenovirus (AdV) |
| Influenza vaccine | Influenza | Inactivated (IN) | None |
| Influenza vaccine live | Influenza | Live virus (LV) | Live attenuated influenza virus (LAIV) |
| RTS,S | Malaria | Recombinant protein (RP) | AS01/AS02 |
| MCV4 | Meningococcus | Conjugate (CJ) | None |
| MPSV4 | Meningococcus | Polysaccharide (PS) | None |
| PPSV-23 | Pneumococcus | Polysaccharide (PS) | None |
| Smallpox vaccine | Smallpox | Live virus (LV) | Vaccinia virus |
| MVA85A | Tuberculosis | Recombinant Viral Vector (RVV) | Vaccinia virus |
| Zoster vaccine live | Varicella Zoster | Live virus (LV) | Varicella zoster virus (VZV) |
| Yellow fever vaccine | Yellow Fever | Live virus (LV) | Yellow fever 17D (YF17D) |
Participants receiving Hepatitis A/B vaccine also received tetanus/diptheria and cholera vaccines at the same time.
Common and unique transcriptional responses across vaccines
To examine the overlap in responses across vaccines, we identified differentially expressed genes post-vaccination relative to the pre-vaccination baseline as well as differential expression of blood transcriptional modules (BTMs), a set of gene modules developed through large-scale network integration of publicly available human blood transcriptomes10. There was much less overlap at a gene level (Extended Data Figure 1A) than at a module level (Extended Data Figure 1B), where a majority of differentially expressed modules were shared across 4 or more vaccines. Based on temporal expression patterns, the most commonly induced modules clustered into four groups (Figure 2A). Cluster 1 (indicated by the blue vertical bar to the left of the heatmap), upregulated at days 1 and 3 post-vaccination, represented BTMs related to innate responses and included modules associated with Toll-like receptor (TLR) and inflammatory signaling, antigen presentation, and monocyte signatures. Cluster 2 (yellow vertical bar to the left) contained multiple natural killer (NK) cell modules and was significantly downregulated on Day 1 (Extended Data Figure 1C). Finally, Clusters 3 (pink bar) and 4 (green bar) generally peaked in activity on Day 7 and reflected plasma cell and cell cycle signatures, respectively, corresponding with expansion of antibody-producing plasmablasts. The “innate” Cluster 1 was most prominently induced in vaccines containing a live viral vector (Ebola, HIV), or an adjuvant (malaria) (Figure 2B). Meanwhile, the plasma cell signature in Cluster 3 was strongly increased in the polysaccharide pneumococcal vaccine and the conjugate meningococcal vaccine (Figure 2C).
Figure 2. Common and unique transcriptional responses across different vaccines.
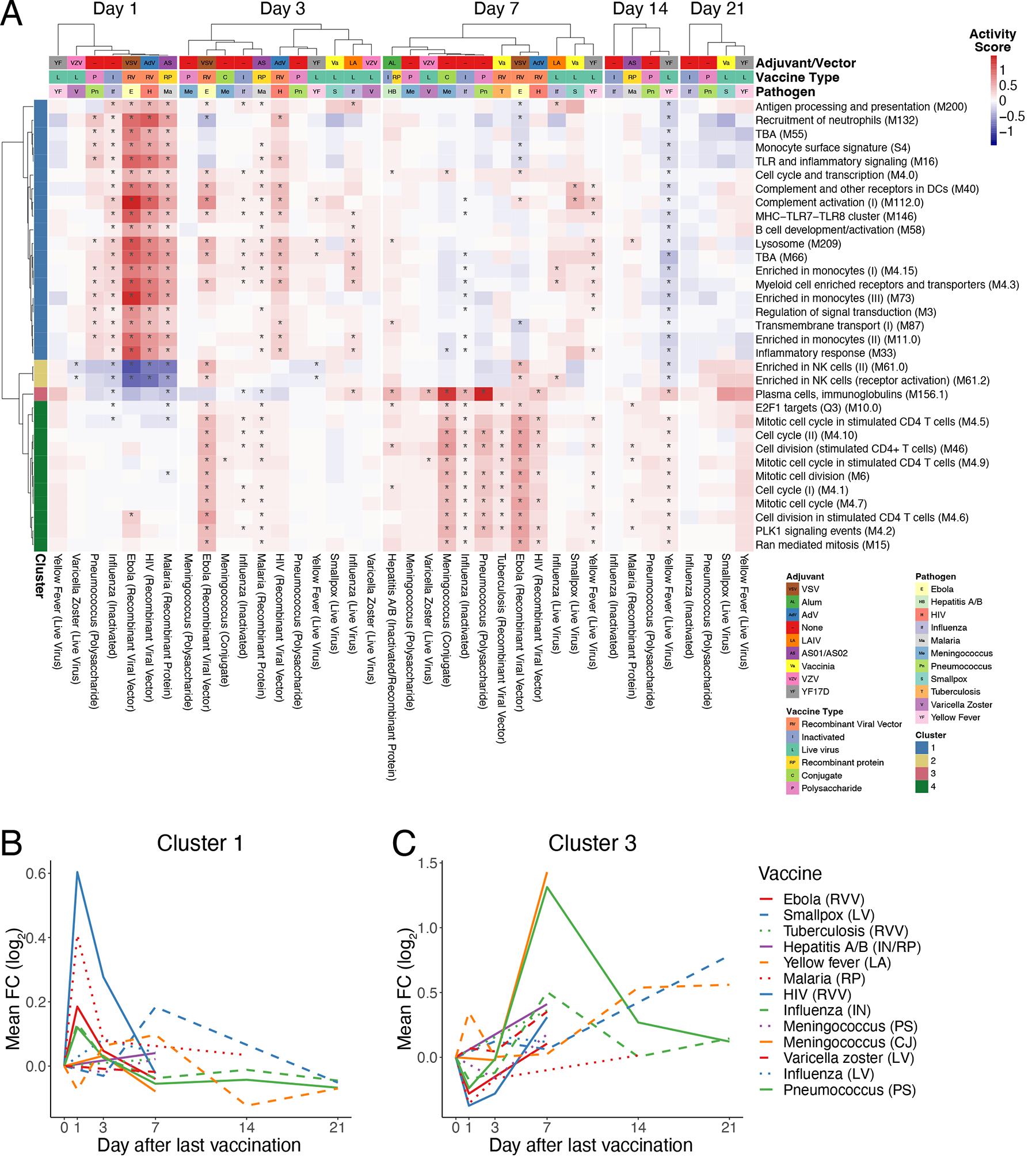
A) Heatmap of common differentially expressed modules (regulated in 7 or more vaccines) over time (*FDR<0.05). Color represents the QuSAGE activity score. Clustering on columns was performed separately for Days 1, 3, 7, 14, and 21 post-vaccination. TBA – To be annotated. B) Kinetics of the mean FC of cluster 1 modules across vaccines C) Kinetics of the mean FC of cluster 3 modules across vaccines.
We next analyzed how differentially expressed modules were shared across vaccine responses (Figure 3). In agreement with the prior analysis of common responses (Figure 2), the response to most vaccines on Days 1 (Figure 3A) and 7 (Figure 3C) reflected innate and plasma cell/cell cycle responses, respectively, while the Day 3 response (Figure 3B) appeared as an intermediate between these states, with both innate and cell cycle signatures present. However, such responses were not universally shared across all of the vaccines. In particular, the early innate and antiviral responses common to most vaccines on Day 1 were not observed in the varicella zoster (VZV) and yellow fever vaccine responses. While these signatures appeared at later timepoints (Days 3 and 7) in yellow fever vaccine responses, they were not observed at all following VZV. Additionally, the Day 7 cell cycle signature was not observed following smallpox, VZV, and polysaccharide meningococcal vaccines. Notably, this signature was observed in the case of the meningococcal conjugate vaccine, where the bacterial polysaccharides have been conjugated to a diphtheria toxoid protein to induce memory and T helper cell responses29. Since diphtheria toxoid protein is used in other vaccines such as the Haemophilus influenza type B (Hib) vaccine30, the cell cycle signature observed at day 7 likely reflects the plasmablast response of the recall response to diphtheria toxoid, consistent with our previous study10.
Figure 3. Overlap in transcriptional responses across vaccines.
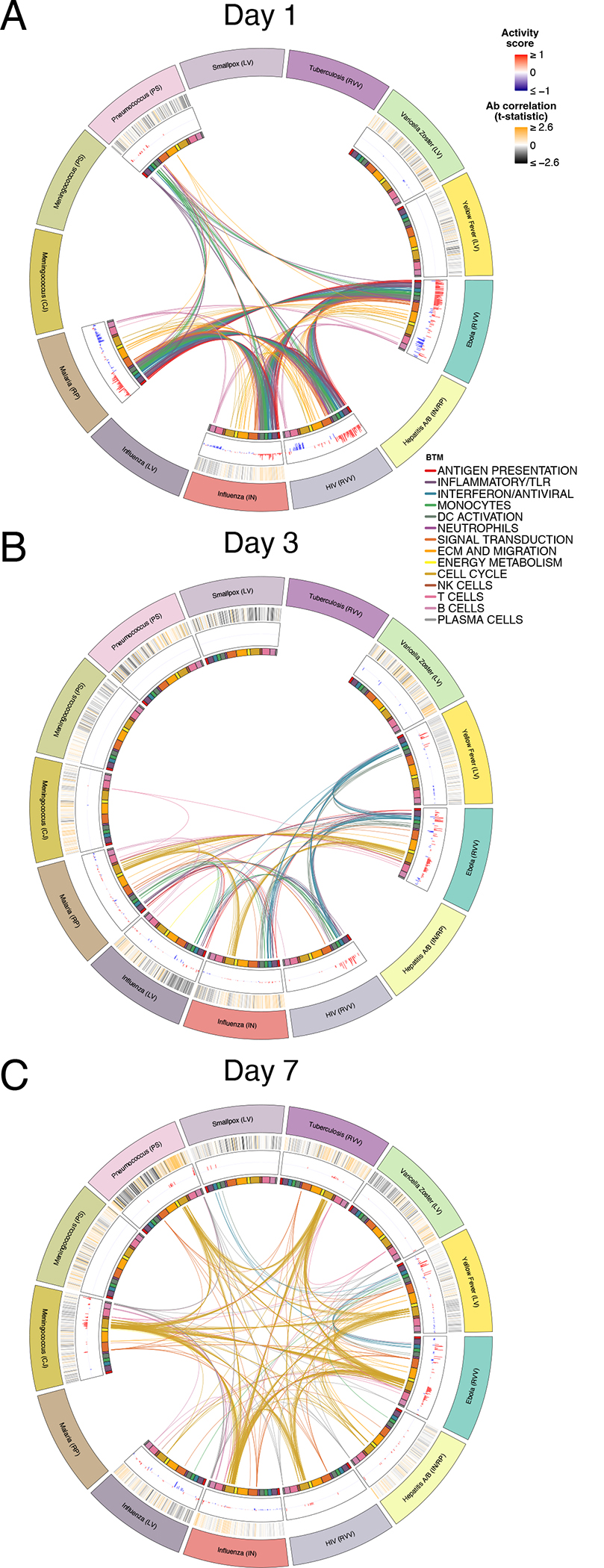
A-C) Circos plots of the overlap in differentially expressed modules (FDR<0.05) across vaccines on Days (A) 1, (B) 3, and (C) 7. Each segment of the circle represents one vaccine, and each point in a segment represents a single module. Heatmaps in the outermost ring represent correlation with Day 28 antibody responses, and bars in middle ring represent the activity score of differentially expressed modules. Lines connect modules with a significant positive score shared between vaccines. Inner ring boxes and line colors represent the functional groups of the modules.
Early adaptive and delayed innate signatures of yellow fever vaccine
At the gene level, responses were highly correlated across most vaccines on Day 1 post-vaccination (Figure 4A) but became more divergent at later timepoints (Extended Data Figures 2A–B). On Day 1, Ebola, inactivated influenza, HIV, and malaria vaccines exhibited the strongest similarity (Figure 4A, Extended Data Figure 2C). However, the yellow fever vaccine YF-17D induced a very distinct response that had little or even negative correlation with responses to all other vaccines, including other live viral vaccines such as VZV, HIV, and Ebola (Figure 4A, Extended Data Figure 2D). The innate pathways that were upregulated in other vaccine responses were, in fact, downregulated in response to yellow fever vaccine on Day 1 (Figure 4B). Instead, YF-17D induced early expression of multiple B and T cell modules. Analysis using the xCell deconvolution algorithm31 detected an increase in estimated frequencies of total B cells (Extended Data Figure 2E), suggesting that this signature may reflect an induction of adaptive cells into the periphery.
Figure 4. Early adaptive and delayed innate transcriptional signatures of yellow fever vaccine.
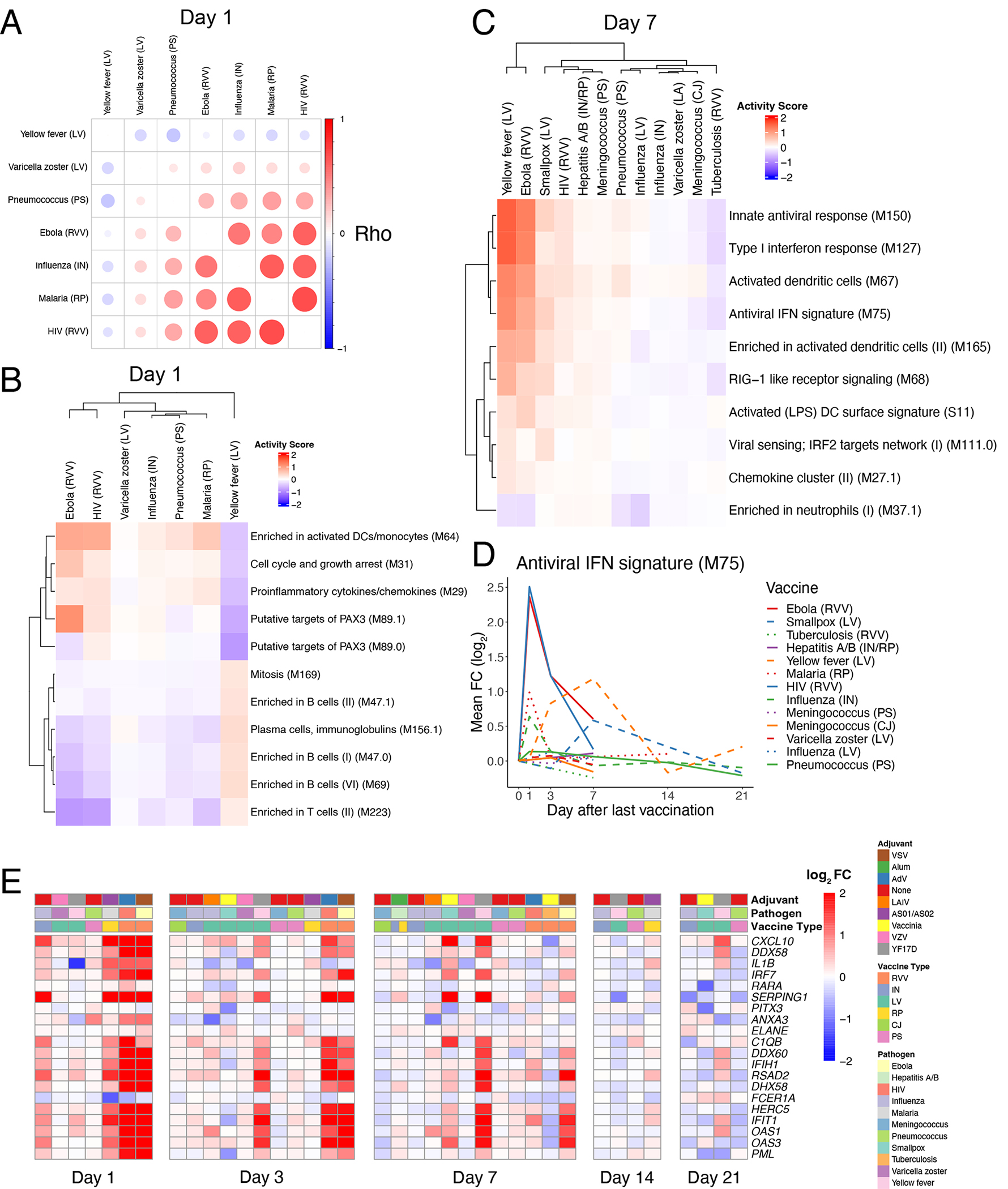
A) Correlation matrix of pairwise Spearman correlations of Day 1 gene-level fold changes between vaccines. B) Heatmap of Day 1 activity scores of modules differentially expressed in response to YF vaccination (QuSAGE FDR<0.2). C) Heatmap of Day 7 activity scores of modules differentially expressed in response to YF vaccination (QuSAGE FDR<0.05, activity score >0.2). D) Kinetics of the mean FC of module M75 across vaccines. E) Heatmap of the post-vaccination FC of genes in module M75.
Another surprising feature of the yellow fever vaccine response was the relatively late expression of antiviral and interferon pathways, whose expression starts to be observed on Day 3 and peaks on Day 7 (Figure 4C–D). While these modules were also upregulated at this timepoint in Ebola vaccine responses, their expression waned rapidly following a robust early induction at Day 1. Some of the genes in these pathways that were strongly upregulated on Day 1 in response to most vaccines, such as CXCL10 and OAS1, were upregulated as late as 21 days post-vaccination with YF-17D (Figure 4E). Importantly, both the early adaptive and delayed innate responses were consistent across multiple studies from diverse geographical locations (Extended Data Figure 2F–G). Together, these results highlight the unique kinetics of transcriptional responses to yellow fever vaccine relative to other vaccines.
Impact of pre-existing immunity on transcriptional responses to vaccination
One possible contributor to the unique kinetics of responses to the yellow fever vaccine is that of the studies included in the Data Resource, all participants were naïve to yellow fever and the vaccine was inducing a primary response. This is in contrast to other vaccines such as influenza and varicella zoster that elicit a recall response in the adult populations studied. However, it is difficult to evaluate the impact of pre-existing immunity and compare primary versus recall responses across vaccines because of intrinsic differences in the nature of these vaccines as well as other factors such as the vaccine platform and type of pathogen that also affect the nature and kinetics of immune response and confound such an analysis.
To address this, we therefore performed a vaccine-specific analysis using all inactivated influenza studies. The inactivated influenza vaccine was ideal for this analysis as there are a large number of such studies in the Data Resource, and there is substantial heterogeneity in the amount of pre-existing immunity and prior exposure to influenza across the population. We used pre-vaccination antibody levels as a marker of pre-existing immunity and defined ‘high’ and ‘low’ baseline antibody participants as the top and bottom 30% of participants in each study based on baseline geometric mean antibody titer. Increased levels of pre-existing antibodies resulted in diminished induction of interferon (Extended Data Figure 3A,C) and plasma cell signatures (Extended Data Figure 3B,D) on Days 1 and 7, respectively, but did not appear to impact the kinetics of these responses (Extended Data Figure 3E–F). One possible explanation for this effect is that increased amounts of pre-existing antibodies may bind antigen from the vaccine, thereby reducing the amount available to be processed and presented by innate cells and lowering the vaccine ‘take’.
Time-adjusted transcriptional predictors of antibody responses
A key goal of systems vaccinology is to identify early signatures predictive of subsequent protection from infection. Antibody titers have been established as a reliable correlate of protection against many pathogens32 and previous studies have identified transcriptional signatures predictive of antibody responses to several vaccines, including inactivated influenza6, 11, 18, 33, 34, yellow fever3, and hepatitis B12. However, these signatures have thus far been developed for single vaccines, and it remains to be seen whether a ‘universal signature’ exists that can predict antibody responses across vaccines. Our curated data resource is uniquely suited to address this question, as 10 of the encompassed vaccines had at least one dataset with antibody titer measurements pre- and ~1 month post-vaccination (Extended Data Figure 4A). As there was substantial variability in antibody responses across vaccines, we defined ‘high’ and ‘low’ responders on a per dataset basis as the top and bottom 30% of participants according to antibody titer fold changes. We then used an elastic-net machine learning algorithm to develop classifiers capable of distinguishing between high and low responders based on early transcriptional signatures (see Methods section for further details).
As an initial approach, we wanted to examine whether a model trained using responses to a single vaccine could reliably predict responses to other vaccines. We therefore first built models using all 15 inactivated influenza vaccine datasets (the vaccine for which there was the largest number of samples) in a leave-one-study-out training/testing configuration. As validation that our model could predict responses within the same vaccine, classifiers trained using Day 7 fold-change expression data were able to predict high versus low antibody response in the left-out influenza dataset, with areas under the ROC curve (AUCs), a measure of classification performance taking into account both true positive and false positive rates, ranging between 0.55–0.9 (Figure 5A). The modules in these classifiers were primarily associated with cell cycle and plasma cell modules (Extended Data Figure 4B). These results are consistent with prior work showing that classifiers built using similar pathways are predictive of antibody responses to influenza vaccination across multiple seasons18. However, when we examined their performance in other vaccines, they were not reliably predictive (Extended Data Figure 4C). Moreover, the expression of modules associated with antibody response to inactivated influenza vaccination at Day 7 was not generally correlated with antibody responses across vaccines (Figure 5B).
Figure 5. Time-adjusted transcriptional predictors of antibody responses.
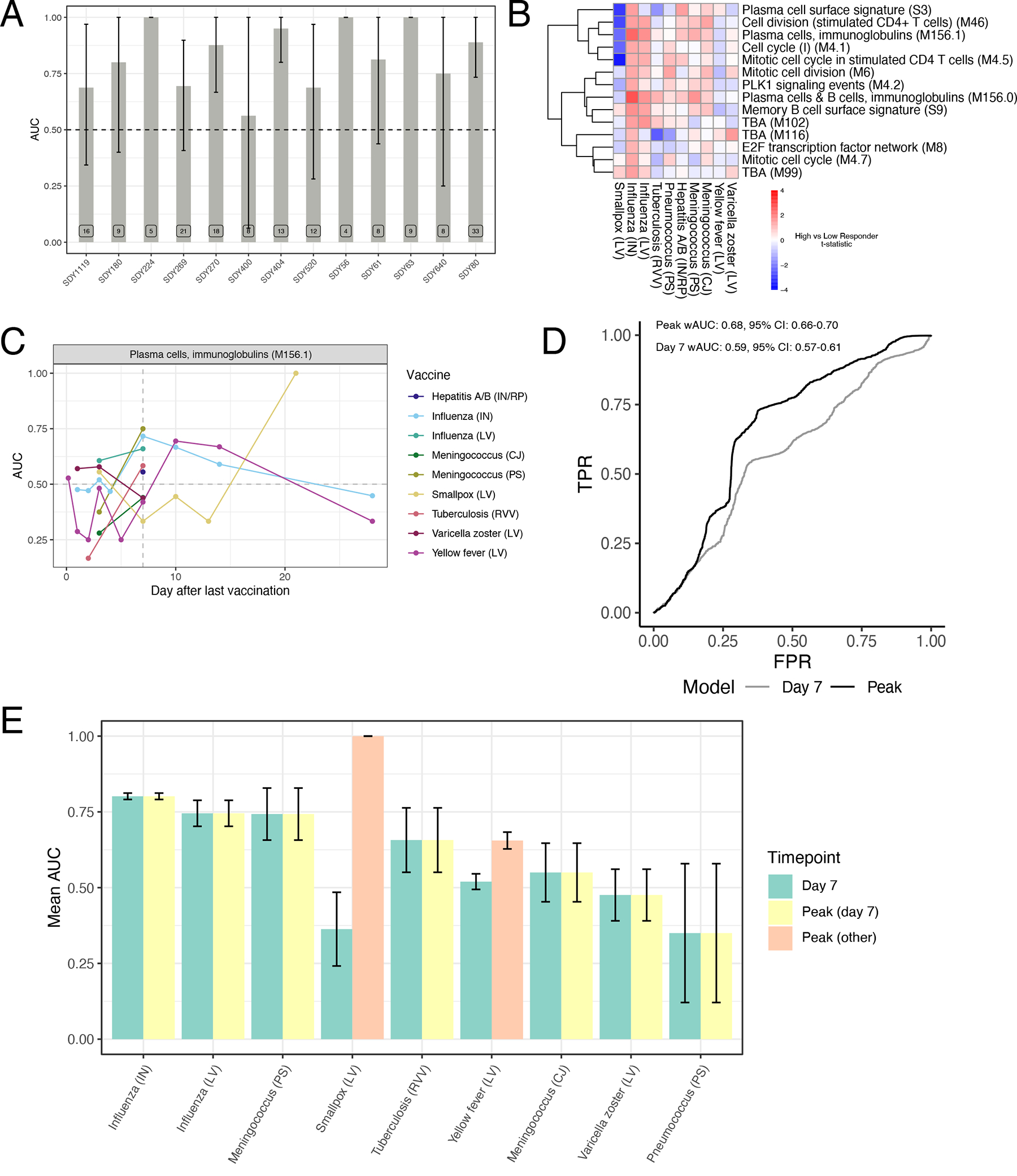
A) Area under the ROC curve (AUC) barplot of antibody response prediction performance per dataset for the elastic net classifier trained on inactivated influenza datasets only. Data are presented as mean values +/− 95% confidence interval. n=2000 bootstrap replicates. B) Heatmap of high versus low antibody responder difference across vaccines of modules differentially expressed (FDR<0.05) between high and low antibody responders to inactivated influenza vaccination. C) Kinetics of the predictive power of M156.1 across vaccines. For each vaccine/timepoint combination, the AUC is computed based on difference in the geometric mean of the fold changes of the genes in the M156.1 between high and low responders (see Methods for details). D) Weighted ROC curves for a logistic regression classifier using M156.1 expression either at Day 7 in all vaccines (Day 7) or at the vaccine-specific peak expression timepoint (Peak). E) Per vaccine AUC barplot for a logistic regression classifier using M156.1 expression either at Day 7 in all vaccines (yellow) or at the vaccine-specific peak expression timepoint (green – peak at Day 7, pink – peak at other timepoints). Data are presented as mean values +/− 95% confidence interval. n=2000 bootstrap replicates.
We then asked whether training across multiple vaccines would improve the universality of the identified signatures. Neither a leave-one-vaccine-out approach, nor a 10-fold cross-validation approach combining all datasets, were able to identify signatures on Day 3 or Day 7 post-vaccination that could accurately discriminate high versus low responders across all vaccines (Extended Data Figure 4D–E). However, analysis of the predictive power of specific modules over time, such as M156.1, one of the plasma cell modules associated with response in influenza vaccination on Day 7, revealed that this module was predictive of response across many vaccines but at different timepoints (Figure 5C). While many vaccines saw a strong association between M156.1 on Day 7 and subsequent antibody response, in certain vaccines such as yellow fever and smallpox, expression of the module was not associated with response until much later, at Days 10–14 and 21, respectively, consistent with the delayed kinetics of this BTM with these vaccines (Figure 2).
These results suggest that differential kinetics of immune responses across vaccines pose a confounding variable in the identification of universal predictive signatures of response at a single timepoint, but that using vaccine-specific timepoints dictated by the particular kinetics of immune responses for identification of predictive biomarkers of vaccine responses may improve the universality of such signatures. To test this hypothesis in the context of the plasma cell signature, we identified the timepoint at which expression of the plasma cell module M156.1 peaked in response to each vaccine (Figure 2C). We then trained a logistic regression classifier with M156.1 expression as an input in a 10-fold cross-validation approach using fold-change data at the peak M156.1 expression timepoint for each vaccine. Indeed, using M156.1 peak expression timepoints improved the overall performance of the classifier compared with using a single timepoint (Day 7) for all of the vaccines (Figure 5D). This improvement was driven by increases in response prediction among vaccines in which the plasma cell signature peaked at timepoints other than Day 7, such as the yellow fever and smallpox vaccines (Figure 5E). Thus, expression of the plasma cell module M156.1 acts as a time-variable universal signature of antibody responses to vaccination.
Impact of aging on transcriptional responses to vaccination
The impairment of vaccine efficacy with age is a major challenge for vaccine development and public health. Although declining vaccine efficacy can broadly be attributed to effects of immunosenescence such as loss and dysfunction of naïve T cells35, diminished class-switch capability of B cells36, and decreased TLR function among innate cells37, 38, the molecular mechanisms responsible for impaired vaccine responses among older adults are not yet fully understood. While most of the curated datasets in the HIPC resource contained only young adult participants, some studies, including those of inactivated influenza18, varicella zoster13, and hepatitis B12 vaccines, profiled responses of both young (≤50) and older (≥60) vaccinees. As expected, post-vaccination antibody responses were diminished in older compared to younger participants across all three vaccines (Figure 6A).
Figure 6. Impact of aging on transcriptional responses to vaccination.
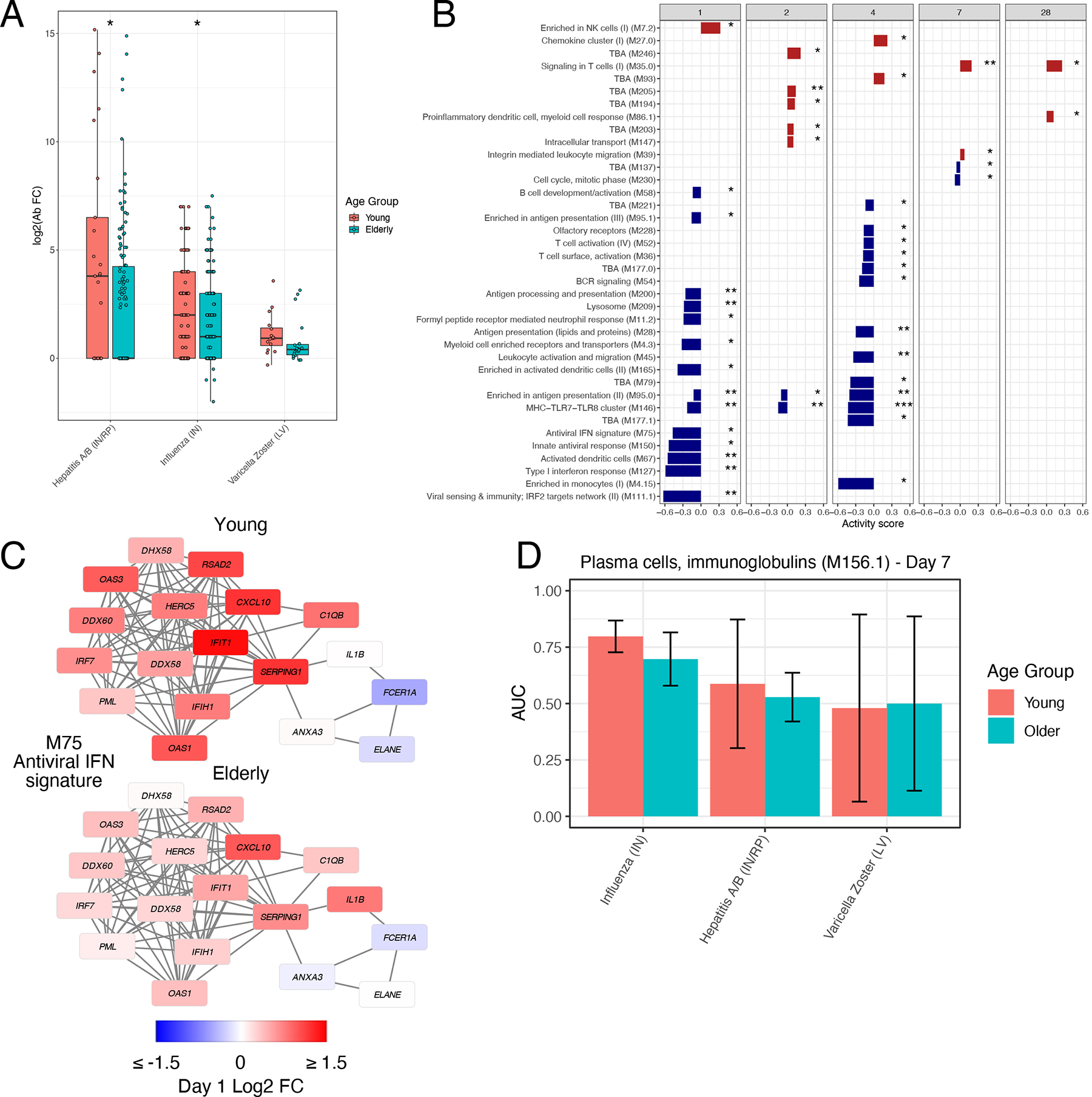
A) Boxplots of Day 30 antibody responses to vaccination in young (≤50) and older (≥60) participants across vaccines. Center line, median; box limits, upper and lower quartiles; whiskers, 1.5x interquartile range. Hepatitis A/B (IN/RP) − Young: n=25, Hepatitis A/B (IN/RP) − Older: n=135, Influenza (IN) − Young: n=123, Influenza (IN) − Older: n=175, Varicella Zoster (LA) − Young: n=16, Varicella Zoster (LA) − Older: n=19. B) Modules differentially expressed between young and older participants in response to inactivated influenza vaccination (QuSAGE FDR<0.05). C) Network plot of module M75 on Day 1 following inactivated influenza vaccination in young and older participants. Each edge represents a co-expression relationship, as described in Li et al.10; colors represent the Day 1 log2 FC. D) Barplot of the Day 7 AUC of module M156.1 across vaccines in young and older participants. Data are presented as mean values +/− 95% confidence interval. n=2000 bootstrap replicates.
We sought to examine for the effect of aging on immune responses across vaccines by comparing BTM activity scores of the most commonly induced BTMs (Figure 2A) between young and older participants across all three vaccines at each timepoint. Broadly, transcriptional responses to the three vaccines were similar between the two age groups (Extended Data Figure 5A). However, there were significant age-associated differences in several pathways in response to inactivated influenza vaccination, including decreased expression of interferon and other innate immune modules in older compared to young participants early post-vaccination (Figure 6B–C), consistent with prior findings18. Despite these differences, the power of the plasma cell signature to predict the antibody response was similar in both young and older individuals (Figure 6D). These results suggest conservation in the pathways responsible for successful antibody production post-vaccination, consistent with prior findings for influenza vaccination20.
Discussion
The high degree of homology in the vaccine-induced signatures induced demonstrates that diverse vaccines that differ widely in target pathogens and composition stimulate conserved immunological networks. Despite this homology, there was still substantial heterogeneity in both the magnitude and kinetics of the induced responses across vaccines. The most distinct in this regard were responses to the yellow fever vaccine YF-17D, which displayed several unique features: (1) a delayed innate and antiviral response which did not peak until Days 3–7 post-vaccination (Figure 4D), (2) an early upregulation of B and T cell signatures at Day 1 (Figure 4B, Extended Data Figure 2E) not observed in other vaccines until much later, and (3) a delay in cell cycle and plasma cell signatures typically associated with the expansion of antigen-specific antibody-secreting cells (Figures 2A–B).
The observed temporal differences in the plasmablast signature likely reflects differences in the kinetics of the plasmablast response. This in turn will depend on a number of factors, such as immune memory caused by prior exposure to the vaccine or pathogen, the persistence and distribution of the vaccine in the body, as well as the nature of the innate signals triggered by the vaccine. Vaccines such as YF-17D that induce a primary immune response in individuals who have not previously been exposed to the vaccine or virus, will mount a delayed plasmablast response. Furthermore, since YF-17D consists of a live virus that causes an acute viral infection during which viral loads in the serum can be detected for a week or longer, the systemic and sustained presence of the virus, may result in a more prolonged and robust plasmablast response. Finally, the triggering of multiple TLRs and innate receptors by YF-17D3, 39 may result in potent activation of the innate immune system that results in a prolonged antibody response. Indeed, consistent with this notion, our previous work has shown that synthetic nanoparticles containing antigens plus ligands that signal through TLR4 and TLR7 induces synergistic increases in antigen-specific, neutralizing antibodies compared to immunization with nanoparticles containing antigens plus a single TLR ligand40.
Of note, yellow fever and other flaviviruses have a specific capability to inhibit interferon signaling via multiple mechanisms, including suppression of JAK-STAT signaling41, which could potentially cause the observed delay in interferon responses following YF-17D vaccination. Interestingly, the Vaccinia virus also has several mechanisms for inhibition of interferon responses, including prevention of IRF-3 and NFκB activation and dephosphorylation of STAT1/242. Although early response data was not available, the smallpox vaccine containing Vaccinia also induced some degree of delayed interferon response following vaccination (Figure 4D).
While YF-17D demonstrated delayed induction of interferon signatures, induction of B and T cell signatures at Day 1 was much earlier than typically observed with other vaccines. This timing is most likely too early to represent an antigen-specific response but could reflect non-specific activation or recruitment of naïve cells into the circulation. Alternatively, these signatures could be a result of increased relative proportions of adaptive cells in the blood due to extravasation of innate cells into tissues at the site of injection. Further investigation at a cellular level is required to address these hypotheses and elucidate the mechanisms by which YF-17D exerts such unique early effects on the adaptive immune system.
Finally, our analysis of predictive signatures of antibody responses (Figure 5) indicates that vaccine response kinetics play an important role in determining such signatures. Here we have illustrated this principal for a single plasma cell transcriptional module, however future analyses may enable detection of additional and more accurate signatures. We have previously proposed the concept of a ‘vaccine chip’ that could measure defined biomarkers and be used to predict protective immune responses across vaccines25. We proposed that this chip would be designed to measure expression of a select set of genes or modules, subsets of which would predict a particular type of functional or protective immune response (e.g., neutralizing antibody titers, effector CD8+ T cell responses, frequency of polyfunctional T cells, T helper 1 (Th1) versus Th2 response bias, etc)25. The results of the present study pave the way for the development of a simple PCR assay (a “vaccine chip”) that can be used to monitor plasmablast signatures in vaccinees. Indeed, PCR based assays are practical and widely deployable globally, as witnessed during the COVID pandemic. Thus, they are likely to be of greater practical value than a FACS based assay, involving multi color FACS analysis to define plasmablast signatures, as such an assay may be too complex to set up in the field. The concept of time-adjusted signatures based on vaccine-specific response kinetics will be useful in the development of future signatures. In practice, small phase I/II trials could be used to define response kinetics and enable the successful application of a ‘vaccine chip’ to predict immune responses in subsequent trials.
Due to the considerable costs needed to perform a clinical trial of sufficient size, such vaccine studies are rarely performed with more than one vaccine. Here, we have demonstrated that meta-analysis of vaccine trials can provide valuable insights into the common and unique aspects of immune responses across vaccines. These findings complement those of a companion manuscript by Fourati et al. describing a set of pre-vaccination transcriptional immune states within the same set of participants that influence responsiveness to vaccination. Combined with the Immune Signatures Data Resource26, these computational approaches and repositories will enable future research into the mechanisms of vaccine-induced immunity to inform development of improved adjuvants and vaccines.
Methods
Gene expression preprocessing
An extensive description of the preprocessing of microarray and RNA-Sequencing (RNA-Seq) datasets included in the Immune Signatures Data Resource can be found in the associated manuscript26. The dataset includes 2,949 samples from published studies and 228 samples not included in previously published studies. All these samples were assembled into a single resource. Briefly, raw probe intensity data for Affymetrix studies were background corrected and summarized using the RMA algorithm44. For studies using the Illumina array platform, background corrected raw probe intensities were used. For RNA-Seq studies, count data was voom-transformed45 to mimic the distribution of microarray expression intensities. Expression data within each study was quantile normalized and log-transformed separately for each study.
Batch correction
An extensive description of the across studies normalization used to correct for batch effects can be found in the Immune Signatures Data Resource manuscript26. Briefly, a linear model was fit using the pre-vaccination normalized gene expression as a dependent variable and platform, study, and blood sample type (i.e., whole blood or PBMC) as independent variables. The estimated effect of the platform, study and sample type was then subtracted from the entire gene expression (pre- and post-vaccination) to obtain batch corrected gene expression.
Identification of differentially expressed genes
To determine differentially expressed genes, p values were first computed within each study using two-sided paired student’s t-tests. Next, Stouffer’s method was used to combine p values across studies via the sumz function in the metap R package46, with weighting according to the square root of the study sample size. Finally, combined p values were then adjusted for multiple testing using the Benjamini-Hochberg procedure. Similarly, average gene fold changes for each vaccine at each timepoint were computed by averaging across studies while using weighting equal to the study sample size.
Gene set enrichment analysis
The enrichment analysis of BTMs was performed in two steps. First, for every study and time point, enrichment was calculated using QuSAGE47, providing as contrast “Day X – Day 0” where X is the current time point, and also a “pairVector” containing the subject identifiers so that a paired analysis would be performed. Second, to integrate the results from multiple studies of the same vaccine, we performed a meta-analysis for every vaccine + timepoint combination, using the “combinePDFs” function of QuSAGE.
Gene and module sharing analysis
The sharing number of a gene/module is computed as the maximum number of vaccines it is significantly differentially expressed (FDR<0.05) in, irrespective of time point. For modules, the p-values were calculated using QuSAGE47 (see Gene set enrichment analysis). A null distribution for sharing was generated by performing 10,000 permutations of gene/module labels within each vaccine + timepoint group.
Antibody titer measurements and identification of high and low responders
Depending on the study, antibody titers were measured by neutralization assays, hemagglutination inhibition assay (HAI), or Immunoglobulin G (IgG) levels measured by ELISA1. Since some vaccines include multiple strains of viral antigens, the fold change in the antibody response metric was defined as the maximum fold change (MFC) of any strain in the vaccine at day 28 (+/− 7 days) compared to pre-vaccination. To minimize the difference in antibody response between studies (e.g., due to different vaccines or different techniques used for antibody concentration assessment), the high and low responders were identified for each study separately by selecting the participants with MFC equal or above the 70th percentile as high responders and participants with MFC equal or below the 30th percentile as low responders.
Identification of predictive signatures of antibody responses
Four training/testing setups employed for identification of predictive signatures of antibody responses: 1) inactivated influenza datasets only, leave-one-study-out 2) training on all inactivated influenza datasets, testing on other vaccines 3) leave-one-vaccine-out (all datasets combined) 4) 10-fold cross validation (all datasets combined). All models were trained using elastic-net logistic regression using the ‘caret’ and ‘glmnet’ R packages. BTM enrichment scores were calculated for each sample using the single-sample Gene Set Enrichment Analysis (ssGSEA) function and used as input features to the models, filtering for modules with a standard deviation > 75% quantile of the standard deviation. Models were fit using either Day 3 fold-change or Day 7 fold-change of ssGSEA score separately. Tuning parameters and performance metrics were estimated using 10-fold cross-validation. Confidence intervals were estimated using the ‘ci.auc’ function from the pROC R package.
When developing predictive models for the timepoint adjustment approach, logistic regression models were trained using ssGSEA score fold-change for module M156.1, either at Day 7 or at the timepoint of peak expression in a given vaccine. AUC confidence intervals were estimated using linear-mixed effects models fitted with 100 Monte-Carlo resamples. When computing AUCs across multiple vaccines, a weighted AUC was computed using sample size as the weights. For the analysis of temporal change in the predictive capability of M156.1 (Figure 4C), a weighted mean AUC (based on number of samples in each study) was computed using the calculateROC function of MetaIntegrator R package based on the geometric mean of gene fold changes in the M156.1 module.
Statistics & Reproducibility
No statistical method was used to predetermine sample size. ArrayQualityMetrics R package was used for quality control of all microarray data. Outlier detection was based on the following statistics: i) Mean absolute difference of M-values (log-ratios) of each pair of arrays, ii) the Kolmogorov-Smirnov statistic Ka between each array’s signal intensity distribution and the distribution of the pooled data and, iii) the Hoeffding’s statistic Da on the joint distribution of A (average) and M values for each array. Samples that failed all three quality control statistics were removed from further analysis. As this study only involved reanalysis of public datasets, no statistical method was used to predetermine sample size, experiments were not randomized, and investigators were not blinded to allocation during experiments and outcome assessment.
Extended Data
Extended Data Figure 1. Overlap in differentially expressed genes/modules and kinetics of common module clusters.
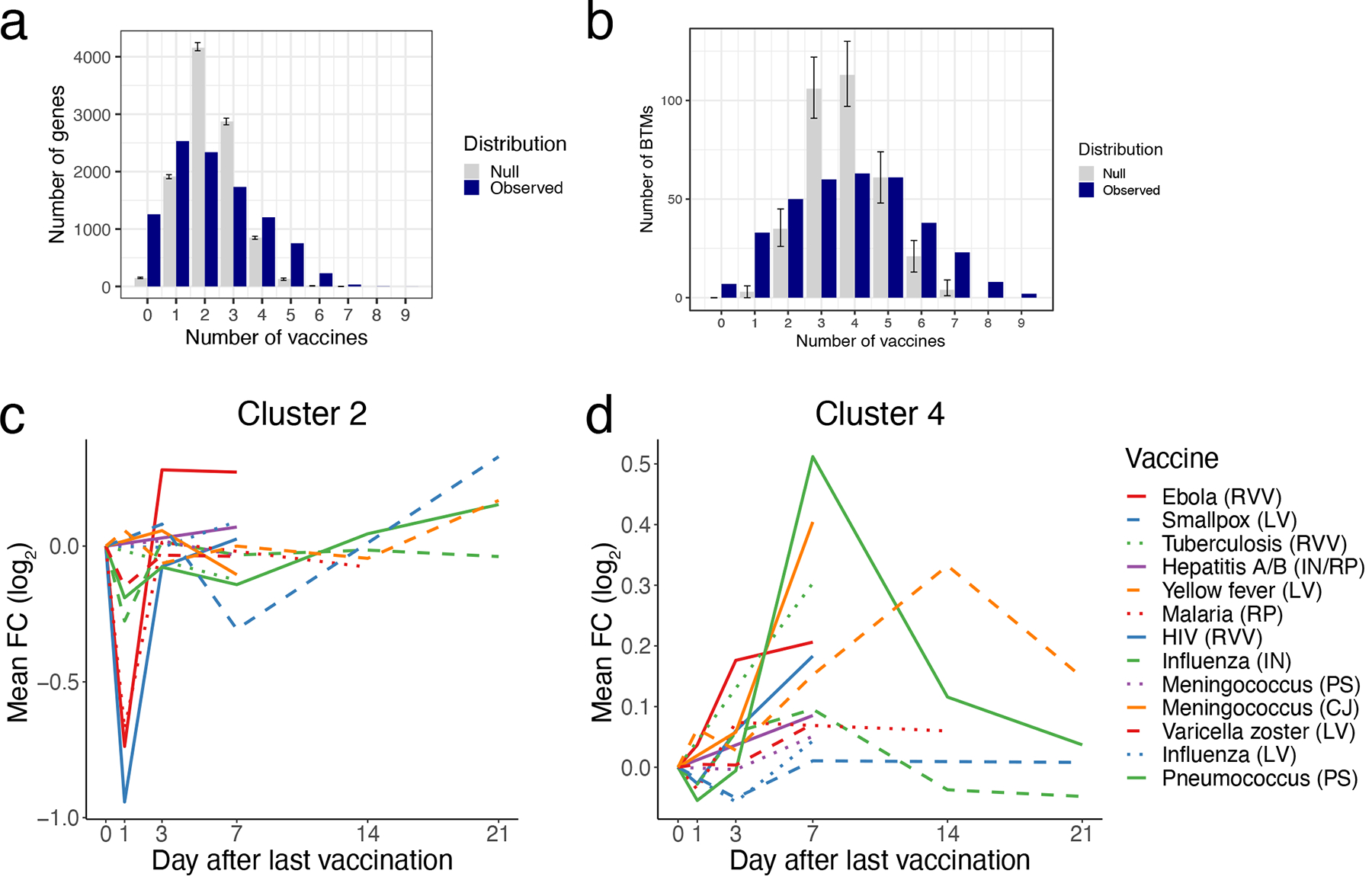
A-B) Histograms of overlap in DEGs (A) or differentially expressed modules (B) between vaccines. A gene/module is shared with another vaccine if it is significantly (FDR < 0.05) regulated in the same direction, irrespective of time point. Blue bars, number of genesets shared (y-axis) between the same number of vaccines (x-axis). Grey bars represent the null distribution generated by n=10,000 permutations of gene/module labels within vaccine + timepoint groups. Data are presented as mean values +/− 95% confidence interval. C) Kinetics of the mean FC of cluster 2 BTMs across vaccines. D) Kinetics of the mean FC of cluster 4 modules across vaccines.
Extended Data Figure 2. Gene-level correlations between vaccines and estimated cell frequencies.
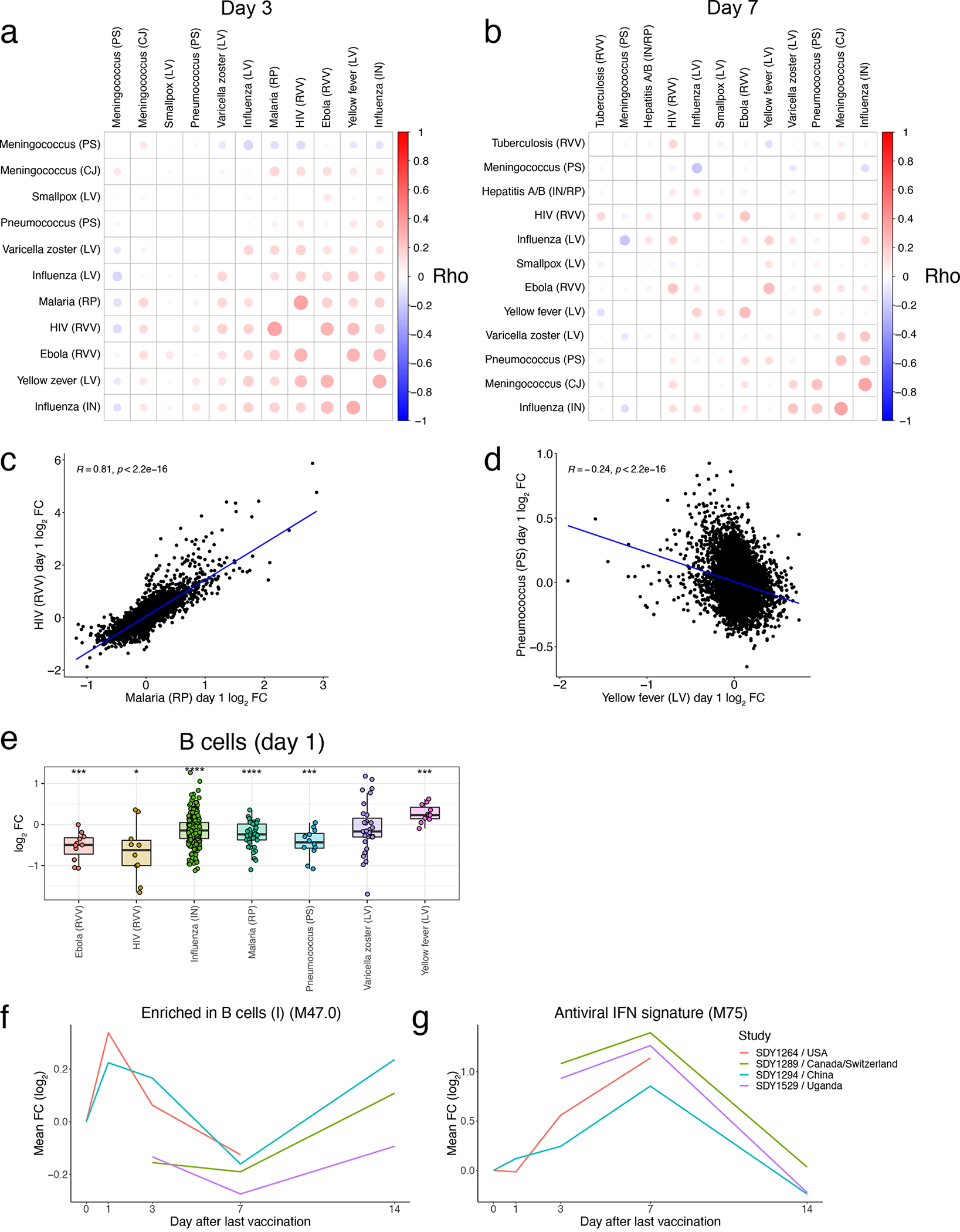
A) Correlation matrix of pairwise Spearman correlations of Day 3 gene-level fold changes between vaccines. B) Correlation matrix of pairwise Spearman correlations of Day 7 gene-level fold changes between vaccines. C) Scatterplot of Day 1 gene FCs between HIV and Malaria vaccines. D) Scatterplot of Day 1 gene FCs between Yellow Fever and Pneumococcus vaccines. E) Boxplot of Day 1 FC in xCell31 estimated B cell frequencies across vaccines. Center line, median; box limits, upper and lower quartiles; whiskers, 1.5x interquartile range. Ebola (RVV): n=11, HIV (RVV): n=10, Influenza (IN): n=298, Malaria (RP): n=42, Pneumococcus (PS): n=12, Varicella Zoster (LA): n=31, Yellow Fever (LA): n=11. F-G) Kinetics of the mean FC of modules (F) M47.0 and (G) M75 across YF vaccine studies. In C-D, correlation coefficient and p value determined via Pearson correlation. In E, statistical differences were determined via two-sided paired Student’s t-tests within each study and integrating p values across studies within each vaccine using Stouffer’s method (see Methods for further details). *p < 0.05, **p < 0.01, ***p < 0.001, **** p < 0.0001.
Extended Data Figure 3. Impact of pre-existing antibody levels on transcriptional responses to influenza vaccination.

A) Differentially expressed modules at Day 1 (FDR <0.05, t-test between mean fold changes) between participants with high and low baseline antibody titers (negative score indicates increased expression in the low baseline antibody group). Differentially expressed modules at Day 7 between high and low baseline antibody groups. C-D) Boxplots of (C) IFN signature module M75 expression at Day 1 and (D) plasma cell module M156.1 expression at Day 7 between high and low baseline antibody groups at Day 1. Center line, median; box limits, upper and lower quartiles; whiskers, 1.5x interquartile range. Day 1: SDY1276_D: High – n=35, Low – n=31; SDY1276_V: High – n=31, Low – n=31; SDY180: High – n=4, Low – n=4; SDY56: High – n=4, Low – n=7; SDY80: High – n=14, Low – n=14. Day 7: SDY1119: High – n=6, Low – n=6; SDY180: High – n=4, Low – n=4; SDY270: High – n=10, Low – n=9; SDY56: High – n=4, Low – n=7; SDY61: High – n=3, Low – n=3; SDY63: High – n=3, Low – n=4; SDY640: High – n=6, Low – n=4; SDY80: High – n=13, Low – n=13. E-F) Line graphs of (E) M75 and (F) M156.1 expression across time in high and low baseline antibody groups. Error bars represent standard error of the mean. Day 1: High – n=88, Low – n=87; Day 3: High – n=83, Low – n=88; Day 7: High – n=49, Low – n=50; Day 14: High – n=64, Low – n=66; In C-F, statistical differences were determined via two-sided unpaired Student’s t-tests. *p < 0.05, **p < 0.01, ***p < 0.001.
Extended Data Figure 4. Antibody response prediction across vaccines.
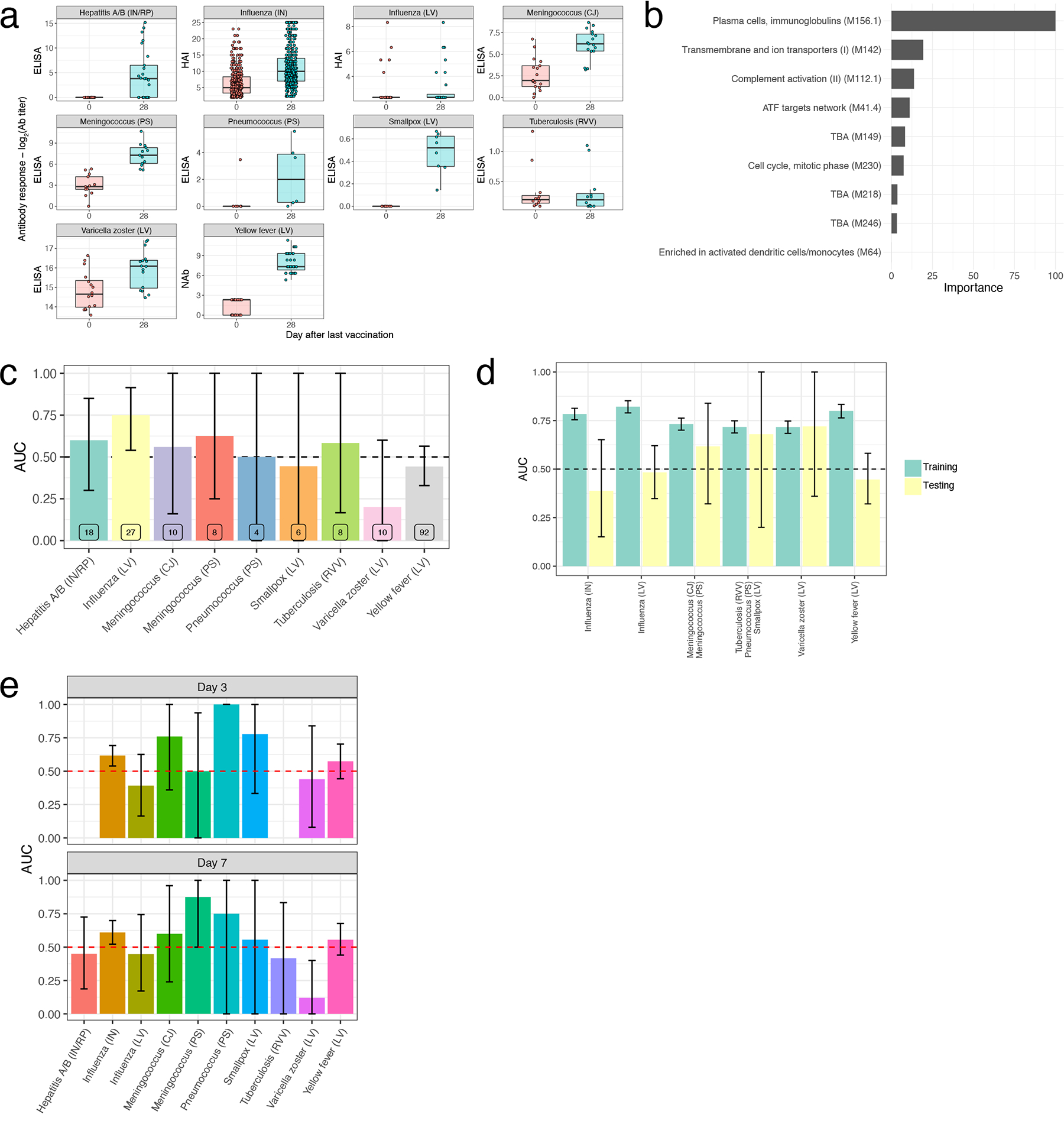
A) Boxplots of Day 30 antibody responses to vaccination across vaccines. Center line, median; box limits, upper and lower quartiles; whiskers, 1.5x interquartile range. Hepatitis A/B (IN/RP): n=25, Influenza (IN): n=412, Influenza (LA): n=28, Meningococcus (CJ): n=17, Meningococcus (PS): n=13, Pneumococcus (PS): n=6, Smallpox (LA): n=8, Tuberculosis (RVV): n=12, Varicella Zoster (LA): n=16, Yellow Fever (LA): n=35. B) Barplot of feature importance for the GLM classifier trained on inactivated influenza datasets only. C) AUC barplot of antibody response prediction performance across vaccines for the GLM classifier trained on inactivated influenza datasets only. Data are presented as mean values +/− 95% confidence interval. n=2000 bootstrap replicates. D) AUC barplot of antibody response prediction performance of the leave-one-vaccine-out GLM classifier. Data are presented as mean values +/− 95% confidence interval. n=2000 bootstrap replicates. E) AUC barplot of antibody response prediction performance of the 10-fold cross-validation GLM classifier. Data are presented as mean values +/− 95% confidence interval. n=2000 bootstrap replicates.
Extended Data Figure 5. Comparison of common transcriptional responses between age groups.
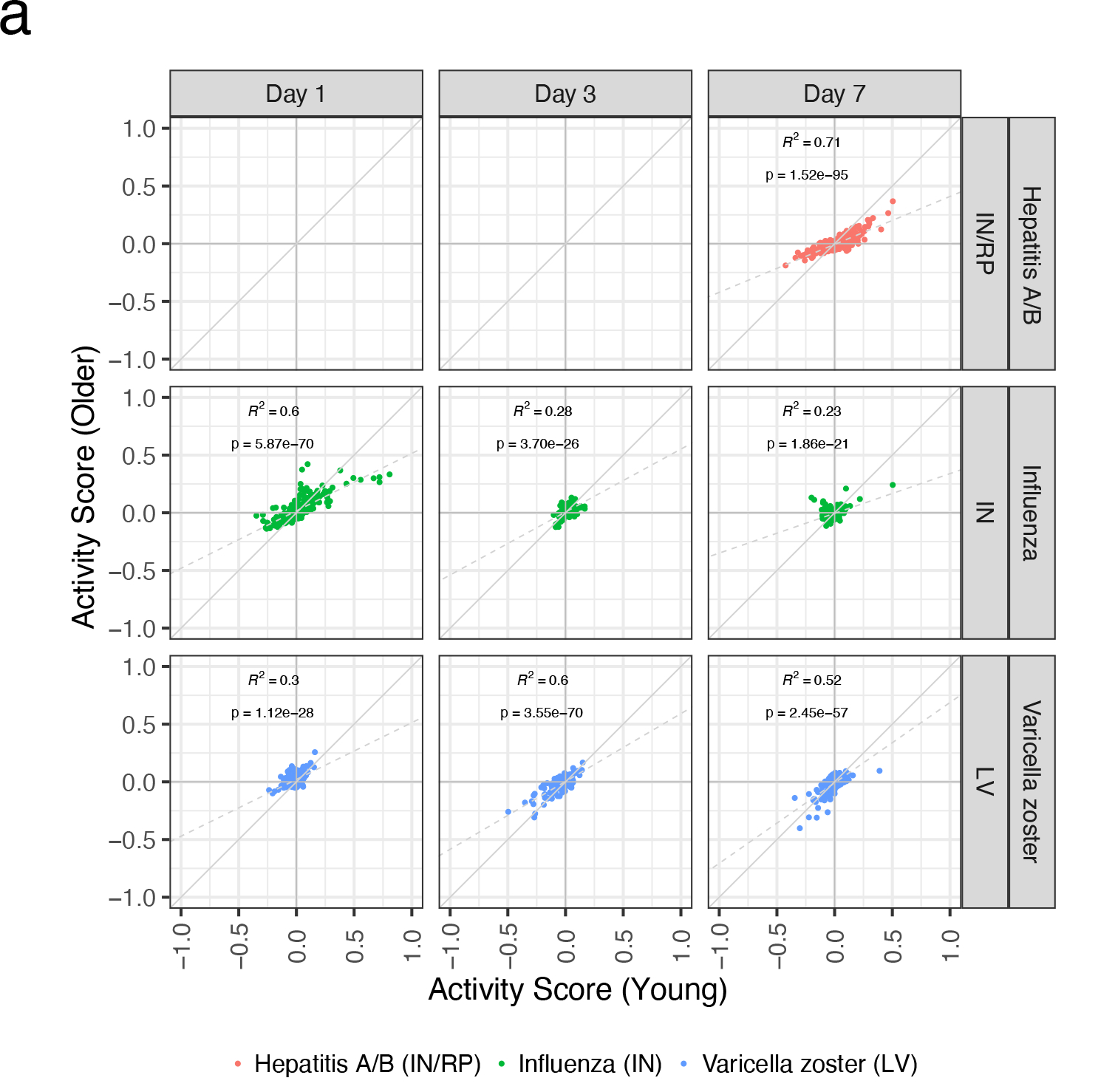
A) Scatterplots of module activity scores in each vaccine among young (x-axis) and older participants (y-axis) of the most commonly expressed modules (Figure 2A) on days 1–7. Correlation coefficient and p value determined via Pearson correlation.
Extended Data Table 1.
Summary of vaccine datasets in the Immune Signatures Data Resource.
| Vaccine | ImmPort Accession | Pathogen | Vaccine Type | Adjuvant/Vector | Transcriptomics Sample Type | Antibody Measurement # of samples | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| rVSV-ZEBOV | SDY1373 | Ebola | Recombinant Viral Vector | VSV | Whole blood | None | 46 |
| Hepatitis A/B vaccine* | SDY1328 | Hepatitis A/B | Inactivated/Recombinant Protein | Alum | Whole blood | ELISA | 51 |
| MRKAd5/HIV | SDY1291 | HIV | Recombinant Viral Vector | AdV | PBMC | None | 50 |
| Influenza vaccine | SDY1119** | Influenza | Inactivated | None | PBMC | HAI | 67 |
| Influenza vaccine | SDY1276 | Influenza | Inactivated | None | Whole blood | HAI | 828 |
| Influenza vaccine | SDY180 | Influenza | Inactivated | None | Whole blood | HAI/NAb | 102 |
| Influenza vaccine | SDY212 | Influenza | Inactivated | None | Whole blood | HAI | 29 |
| Influenza vaccine | SDY224 | Influenza | Inactivated | None | PBMC | HAI | 55 |
| Influenza vaccine | SDY269 | Influenza | Inactivated | None | PBMC | HAI | 80 |
| Influenza vaccine | SDY270 | Influenza | Inactivated | None | PBMC | HAI | 83 |
| Influenza vaccine | SDY400 | Influenza | Inactivated | None | PBMC | HAI | 60 |
| Influenza vaccine | SDY404 | Influenza | Inactivated | None | PBMC | HAI | 64 |
| Influenza vaccine | SDY520 | Influenza | Inactivated | None | Whole blood | HAI | 51 |
| Influenza vaccine | SDY56 | Influenza | Inactivated | None | PBMC | HAI | 96 |
| Influenza vaccine | SDY61 | Influenza | Inactivated | None | PBMC | HAI | 27 |
| Influenza vaccine | SDY63 | Influenza | Inactivated | None | PBMC | HAI | 42 |
| Influenza vaccine | SDY640 | Influenza | Inactivated | None | Whole blood | HAI | 44 |
| Influenza vaccine | SDY80 | Influenza | Inactivated | None | PBMC | NAb | 256 |
| Influenza vaccine live | SDY269 | Influenza | Live Virus | LAIV | PBMC | HAI | 83 |
| RTS,S | SDY1293 | Malaria | Recombinant protein | AS01/AS02 | PBMC | None | 165 |
| MCV4 | SDY1260 | Meningococcus | Conjugate | None | PBMC | ELISA | 51 |
| MCV4 | SDY1325 | Meningococcus | Conjugate | None | Whole blood | SBA | 4 |
| MPSV4 | SDY1260 | Meningococcus | Polysaccharide | None | PBMC | ELISA | 39 |
| MPSV4 | SDY1325 | Meningococcus | Polysaccharide | None | Whole blood | SBA | 2 |
| PPSV-23 | SDY180 | Pneumococcus | Polysaccharide | None | Whole blood | NAb | 155 |
| Smallpox vaccine | SDY1370 | Smallpox | Live Virus | Vaccinia | PBMC | ELISA | 48 |
| MVA85A | SDY1364 | Tuberculosis | Recombinant Viral Vector | Vaccinia | PBMC | ELISA | 36 |
| Zoster vaccine live | SDY984 | Varicella Zoster | Live Virus | VZV | PBMC | ELISA | 124 |
| Yellow fever vaccine | SDY1264 | Yellow Fever | Live Virus | YF17D | PBMC | NAb | 87 |
| Yellow fever vaccine | SDY1289 | Yellow Fever | Live Virus | YF17D | Whole blood | NAb | 117 |
| Yellow fever vaccine | SDY1294 | Yellow Fever | Live Virus | YF17D | PBMC | NAb | 109 |
| Yellow fever vaccine | SDY1529 | Yellow Fever | Live Virus | YF17D | Whole blood | NAb | 180 |
Participants receiving Hepatitis A/B vaccine also received tetanus/diptheria and cholera vaccines at the same time.
4 participants in SDY1119 had type II diabetes.
VSV – vesicular stomatitis virus, AdV – adenovirus, LAIV – live attenuated influenza vaccine, VZV – varicella zoster virus, ELISA – enzyme-linked immunosorbent assay, HAI - hemagglutination inhibition assay, NAb – neutralizing antibody, SBA – serum bactericidal assay
Acknowledgements
This research was performed as a project of the Human Immunology Project Consortium (HIPC) and supported by the National Institute of Allergy and Infectious Diseases. This work was supported in part by NIH grants U19AI118608, U19AI128949, U19AI090023, U19AI118626, U19AI089992, U19AI128914, U19AI128910, U19AI118610, U19AI128913, and the Intramural Program of NIAID and NIH institutes supporting the Trans-NIH Center for Human Immunology (CHI). OL is supported in part by the Department of Pediatrics at Boston Children’s Hospital. Work in Bali Pulendran’s lab is supported in part by National Institutes of Health (R37 DK057665; R01 AI048638; U19 AI057266; U19 AI167903), Bill and Melinda Gates Foundation, Open Philanthropy, and the Violetta L. Horton and Soffer Endowments to B.P.
Footnotes
Competing Interests
OL is a named inventor on patents held by Boston Children’s Hospital regarding human in vitro systems modeling vaccine action and vaccine adjuvants. BP serves on the External Immunology Network of GSK, and on the scientific advisory board of Medicago, CircBio, Sanofi, EdJen and Boehringer-Ingelheim. SHK receives consulting fees from Northrop Grumman and Peraton. TH owns stock in GSK and Pfizer, Inc. The remaining authors declare no competing interests.
Code availability
R code used to generate the figures presented in the paper can be found at (www.immunespace.org/is2.url).
Human Immunology Project Consortium
Deckhut-Augustine A.17, Gottardo R.9,15,16, Haddad EK.18, Hafler DA.3; Harris E.19, Farber D.20, Kleinstein SH.3; Levy O.10,11,14; McElrath J.9, Montgomery RR.3, Peters B.21, Pulendran B.13, Rahman A.22, Reed EF.23, Rouphael N.5, Sarwal MM.8, Sékaly RP.5, Fernandez-Sesma A.22, Sette A.21, Stuart K.24, Togias A.14, Tsang JS.6
17NIAID, NIH, Bethesda, MD, USA
18Department of Medicine, Division of Infectious Diseases and HIV Medicine, Drexel University, Philadelphia, PA, USA
19Division of Infectious Disease and Vaccinology, School of Public Health, University of California, Berkeley, Berkeley, CA, USA.
20Department of Microbiology and Immunology, Columbia University Irving Medical Center, New York, NY, USA
21La Jolla Institute for Immunology, La Jolla, CA, USA
22Department of Microbiology, Icahn School of Medicine at Mount Sinai, New York, NY, USA
23Department of Pathology, University of California, Los Angeles, California, USA
24Center for Global Infectious Disease Research, Seattle Children’s Research Institute, Seattle, WA, USA
Data availability
All data used in this study are available from ImmuneSpace (www.immunespace.org/is2.url).
References
- 1.Hagan T, Nakaya HI, Subramaniam S, Pulendran B. Systems vaccinology: Enabling rational vaccine design with systems biological approaches. Vaccine. 2015;33(40):5294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pulendran B Systems vaccinology: probing humanity’s diverse immune systems with vaccines. Proc Natl Acad Sci U S A. 2014;111(34):12300–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Querec TD, Akondy RS, Lee EK, Cao W, Nakaya HI, Teuwen D, Pirani A, Gernert K, Deng J, Marzolf B, Kennedy K, Wu H, Bennouna S, Oluoch H, Miller J, Vencio RZ, Mulligan M, Aderem A, Ahmed R, Pulendran B. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat Immunol. 2009;10(1):116–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaucher D, Therrien R, Kettaf N, Angermann BR, Boucher G, Filali-Mouhim A, Moser JM, Mehta RS, Drake DR 3rd, Castro E, Akondy R, Rinfret A, Yassine-Diab B, Said EA, Chouikh Y, Cameron MJ, Clum R, Kelvin D, Somogyi R, Greller LD, Balderas RS, Wilkinson P, Pantaleo G, Tartaglia J, Haddad EK, Sekaly RP. Yellow fever vaccine induces integrated multilineage and polyfunctional immune responses. J Exp Med. 2008;205(13):3119–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakaya HI, Wrammert J, Lee EK, Racioppi L, Marie-Kunze S, Haining WN, Means AR, Kasturi SP, Khan N, Li GM, McCausland M, Kanchan V, Kokko KE, Li S, Elbein R, Mehta AK, Aderem A, Subbarao K, Ahmed R, Pulendran B. Systems biology of vaccination for seasonal influenza in humans. Nat Immunol. 2011;12(8):786–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bucasas KL, Franco LM, Shaw CA, Bray MS, Wells JM, Nino D, Arden N, Quarles JM, Couch RB, Belmont JW. Early patterns of gene expression correlate with the humoral immune response to influenza vaccination in humans. J Infect Dis. 2011;203(7):921–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zak DE, Andersen-Nissen E, Peterson ER, Sato A, Hamilton MK, Borgerding J, Krishnamurty AT, Chang JT, Adams DJ, Hensley TR, Salter AI, Morgan CA, Duerr AC, De Rosa SC, Aderem A, McElrath MJ. Merck Ad5/HIV induces broad innate immune activation that predicts CD8(+) T-cell responses but is attenuated by preexisting Ad5 immunity. Proc Natl Acad Sci U S A. 2012;109(50):E3503–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Obermoser G, Presnell S, Domico K, Xu H, Wang Y, Anguiano E, Thompson-Snipes L, Ranganathan R, Zeitner B, Bjork A, Anderson D, Speake C, Ruchaud E, Skinner J, Alsina L, Sharma M, Dutartre H, Cepika A, Israelsson E, Nguyen P, Nguyen QA, Harrod AC, Zurawski SM, Pascual V, Ueno H, Nepom GT, Quinn C, Blankenship D, Palucka K, Banchereau J, Chaussabel D. Systems scale interactive exploration reveals quantitative and qualitative differences in response to influenza and pneumococcal vaccines. Immunity. 2013;38(4):831–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banchereau R, Baldwin N, Cepika AM, Athale S, Xue Y, Yu CI, Metang P, Cheruku A, Berthier I, Gayet I, Wang Y, Ohouo M, Snipes L, Xu H, Obermoser G, Blankenship D, Oh S, Ramilo O, Chaussabel D, Banchereau J, Palucka K, Pascual V. Transcriptional specialization of human dendritic cell subsets in response to microbial vaccines. Nat Commun. 2014;5:5283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li S, Rouphael N, Duraisingham S, Romero-Steiner S, Presnell S, Davis C, Schmidt DS, Johnson SE, Milton A, Rajam G, Kasturi S, Carlone GM, Quinn C, Chaussabel D, Palucka AK, Mulligan MJ, Ahmed R, Stephens DS, Nakaya HI, Pulendran B. Molecular signatures of antibody responses derived from a systems biology study of five human vaccines. Nat Immunol. 2014;15(2):195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsang JS, Schwartzberg PL, Kotliarov Y, Biancotto A, Xie Z, Germain RN, Wang E, Olnes MJ, Narayanan M, Golding H, Moir S, Dickler HB, Perl S, Cheung F, Baylor HC, Consortium CHI. Global analyses of human immune variation reveal baseline predictors of postvaccination responses. Cell. 2014;157(2):499–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fourati S, Cristescu R, Loboda A, Talla A, Filali A, Railkar R, Schaeffer AK, Favre D, Gagnon D, Peretz Y, Wang IM, Beals CR, Casimiro DR, Carayannopoulos LN, Sekaly RP. Pre-vaccination inflammation and B-cell signalling predict age-related hyporesponse to hepatitis B vaccination. Nat Commun. 2016;7:10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li S, Sullivan NL, Rouphael N, Yu T, Banton S, Maddur MS, McCausland M, Chiu C, Canniff J, Dubey S, Liu K, Tran V, Hagan T, Duraisingham S, Wieland A, Mehta AK, Whitaker JA, Subramaniam S, Jones DP, Sette A, Vora K, Weinberg A, Mulligan MJ, Nakaya HI, Levin M, Ahmed R, Pulendran B. Metabolic Phenotypes of Response to Vaccination in Humans. Cell. 2017;169(5):862–77 e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kazmin D, Nakaya HI, Lee EK, Johnson MJ, van der Most R, van den Berg RA, Ballou WR, Jongert E, Wille-Reece U, Ockenhouse C, Aderem A, Zak DE, Sadoff J, Hendriks J, Wrammert J, Ahmed R, Pulendran B. Systems analysis of protective immune responses to RTS,S malaria vaccination in humans. Proc Natl Acad Sci U S A. 2017;114(9):2425–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rechtien A, Richert L, Lorenzo H, Martrus G, Hejblum B, Dahlke C, Kasonta R, Zinser M, Stubbe H, Matschl U, Lohse A, Krahling V, Eickmann M, Becker S, Consortium V, Thiebaut R, Altfeld M, Addo MM. Systems Vaccinology Identifies an Early Innate Immune Signature as a Correlate of Antibody Responses to the Ebola Vaccine rVSV-ZEBOV. Cell Rep. 2017;20(9):2251–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kotliarov Y, Sparks R, Martins AJ, Mule MP, Lu Y, Goswami M, Kardava L, Banchereau R, Pascual V, Biancotto A, Chen J, Schwartzberg PL, Bansal N, Liu CC, Cheung F, Moir S, Tsang JS. Broad immune activation underlies shared set point signatures for vaccine responsiveness in healthy individuals and disease activity in patients with lupus. Nat Med. 2020;26(4):618–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arunachalam PS, Scott MKD, Hagan T, Li C, Feng Y, Wimmers F, Grigoryan L, Trisal M, Edara VV, Lai L, Chang SE, Feng A, Dhingra S, Shah M, Lee AS, Chinthrajah S, Sindher SB, Mallajosyula V, Gao F, Sigal N, Kowli S, Gupta S, Pellegrini K, Tharp G, Maysel-Auslender S, Hamilton S, Aoued H, Hrusovsky K, Roskey M, Bosinger SE, Maecker HT, Boyd SD, Davis MM, Utz PJ, Suthar MS, Khatri P, Nadeau KC, Pulendran B. Systems vaccinology of the BNT162b2 mRNA vaccine in humans. Nature. 2021;596(7872):410–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakaya HI, Hagan T, Duraisingham SS, Lee EK, Kwissa M, Rouphael N, Frasca D, Gersten M, Mehta AK, Gaujoux R, Li GM, Gupta S, Ahmed R, Mulligan MJ, Shen-Orr S, Blomberg BB, Subramaniam S, Pulendran B. Systems Analysis of Immunity to Influenza Vaccination across Multiple Years and in Diverse Populations Reveals Shared Molecular Signatures. Immunity. 2015;43(6):1186–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thakar J, Mohanty S, West AP, Joshi SR, Ueda I, Wilson J, Meng H, Blevins TP, Tsang S, Trentalange M, Siconolfi B, Park K, Gill TM, Belshe RB, Kaech SM, Shadel GS, Kleinstein SH, Shaw AC. Aging-dependent alterations in gene expression and a mitochondrial signature of responsiveness to human influenza vaccination. Aging (Albany NY). 2015;7(1):38–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Avey S, Mohanty S, Chawla DG, Meng H, Bandaranayake T, Ueda I, Zapata HJ, Park K, Blevins TP, Tsang S, Belshe RB, Kaech SM, Shaw AC, Kleinstein SH. Seasonal Variability and Shared Molecular Signatures of Inactivated Influenza Vaccination in Young and Older Adults. J Immunol. 2020;204(6):1661–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ravindran R, Khan N, Nakaya HI, Li S, Loebbermann J, Maddur MS, Park Y, Jones DP, Chappert P, Davoust J, Weiss DS, Virgin HW, Ron D, Pulendran B. Vaccine activation of the nutrient sensor GCN2 in dendritic cells enhances antigen presentation. Science. 2014;343(6168):313–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hagan T, Cortese M, Rouphael N, Boudreau C, Linde C, Maddur MS, Das J, Wang H, Guthmiller J, Zheng NY, Huang M, Uphadhyay AA, Gardinassi L, Petitdemange C, McCullough MP, Johnson SJ, Gill K, Cervasi B, Zou J, Bretin A, Hahn M, Gewirtz AT, Bosinger SE, Wilson PC, Li S, Alter G, Khurana S, Golding H, Pulendran B. Antibiotics-Driven Gut Microbiome Perturbation Alters Immunity to Vaccines in Humans. Cell. 2019;178(6):1313–28 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oh JZ, Ravindran R, Chassaing B, Carvalho FA, Maddur MS, Bower M, Hakimpour P, Gill KP, Nakaya HI, Yarovinsky F, Sartor RB, Gewirtz AT, Pulendran B. TLR5-mediated sensing of gut microbiota is necessary for antibody responses to seasonal influenza vaccination. Immunity. 2014;41(3):478–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pulendran B Learning immunology from the yellow fever vaccine: innate immunity to systems vaccinology. Nat Rev Immunol. 2009;9(10):741–7. [DOI] [PubMed] [Google Scholar]
- 25.Pulendran B, Li S, Nakaya HI. Systems vaccinology. Immunity. 2010;33(4):516–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diray-Arce J, Miller HER, Henrich E, Gerritsen B, Mulè MP, Fourati S, Gygi J, Hagan T, Tomalin L, Rychkov D, Kazmin D, Chawla DG, Meng H, Dunn P, Campbell J, Consortium THIP, Sarwal M, Tsang JS, Levy O, Pulendran B, Sekaly R, Floratos A, Gottardo R, Kleinstein SH, Suárez-Fariñas M. The Immune Signatures Data Resource: A compendium of systems vaccinology datasets. bioRxiv. 2021:2021.11.05.465336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhattacharya S, Dunn P, Thomas CG, Smith B, Schaefer H, Chen J, Hu Z, Zalocusky KA, Shankar RD, Shen-Orr SS, Thomson E, Wiser J, Butte AJ. ImmPort, toward repurposing of open access immunological assay data for translational and clinical research. Sci Data. 2018;5:180015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scherer A Batch effects and noise in microarray experiments : sources and solutions. Chichester, U.K.: J. Wiley; 2009. xx, 252 p. p. [Google Scholar]
- 29.Gasparini R, Panatto D. Meningococcal glycoconjugate vaccines. Hum Vaccin. 2011;7(2):170–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Granoff DM, Rathore MH, Holmes SJ, Granoff PD, Lucas AH. Effect of immunity to the carrier protein on antibody responses to Haemophilus influenzae type b conjugate vaccines. Vaccine. 1993;11 Suppl 1:S46–51. [DOI] [PubMed] [Google Scholar]
- 31.Aran D, Hu Z, Butte AJ. xCell: digitally portraying the tissue cellular heterogeneity landscape. Genome Biol. 2017;18(1):220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Plotkin SA. Correlates of protection induced by vaccination. Clin Vaccine Immunol. 2010;17(7):1055–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Furman D, Jojic V, Kidd B, Shen-Orr S, Price J, Jarrell J, Tse T, Huang H, Lund P, Maecker HT, Utz PJ, Dekker CL, Koller D, Davis MM. Apoptosis and other immune biomarkers predict influenza vaccine responsiveness. Mol Syst Biol. 2013;9:659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sobolev O, Binda E, O’Farrell S, Lorenc A, Pradines J, Huang Y, Duffner J, Schulz R, Cason J, Zambon M, Malim MH, Peakman M, Cope A, Capila I, Kaundinya GV, Hayday AC. Adjuvanted influenza-H1N1 vaccination reveals lymphoid signatures of age-dependent early responses and of clinical adverse events. Nat Immunol. 2016;17(2):204–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moro-Garcia MA, Alonso-Arias R, Lopez-Larrea C. When Aging Reaches CD4+ T-Cells: Phenotypic and Functional Changes. Front Immunol. 2013;4:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frasca D, Blomberg BB. Effects of aging on B cell function. Curr Opin Immunol. 2009;21(4):425–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Panda A, Qian F, Mohanty S, van Duin D, Newman FK, Zhang L, Chen S, Towle V, Belshe RB, Fikrig E, Allore HG, Montgomery RR, Shaw AC. Age-associated decrease in TLR function in primary human dendritic cells predicts influenza vaccine response. J Immunol. 2010;184(5):2518–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kollmann TR, Levy O, Montgomery RR, Goriely S. Innate immune function by Toll-like receptors: distinct responses in newborns and the elderly. Immunity. 2012;37(5):771–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Querec T, Bennouna S, Alkan S, Laouar Y, Gorden K, Flavell R, Akira S, Ahmed R, Pulendran B. Yellow fever vaccine YF-17D activates multiple dendritic cell subsets via TLR2, 7, 8, and 9 to stimulate polyvalent immunity. J Exp Med. 2006;203(2):413–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kasturi SP, Skountzou I, Albrecht RA, Koutsonanos D, Hua T, Nakaya HI, Ravindran R, Stewart S, Alam M, Kwissa M, Villinger F, Murthy N, Steel J, Jacob J, Hogan RJ, Garcia-Sastre A, Compans R, Pulendran B. Programming the magnitude and persistence of antibody responses with innate immunity. Nature. 2011;470(7335):543–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thurmond S, Wang B, Song J, Hai R. Suppression of Type I Interferon Signaling by Flavivirus NS5. Viruses. 2018;10(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith GL, Benfield CTO, Maluquer de Motes C, Mazzon M, Ember SWJ, Ferguson BJ, Sumner RP. Vaccinia virus immune evasion: mechanisms, virulence and immunogenicity. J Gen Virol. 2013;94(Pt 11):2367–92. [DOI] [PubMed] [Google Scholar]
- 43.Bulteau R, Francesconi M. Real age prediction from the transcriptome with RAPToR. Nat Methods. 2022;19(8):969–75. [DOI] [PubMed] [Google Scholar]
Methods References:
- 44.Irizarry RA et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4, 249–264 (2003). [DOI] [PubMed] [Google Scholar]
- 45.Law CW, Chen Y, Shi W & Smyth GK voom: Precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol 15, R29 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dewey M metap: meta-analysis of significance values. R package version 1.8; 2022. [Google Scholar]
- 47.Yaari G, Bolen CR, Thakar J & Kleinstein SH Quantitative set analysis for gene expression: a method to quantify gene set differential expression including gene-gene correlations. Nucleic Acids Res 41, e170 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used in this study are available from ImmuneSpace (www.immunespace.org/is2.url).


