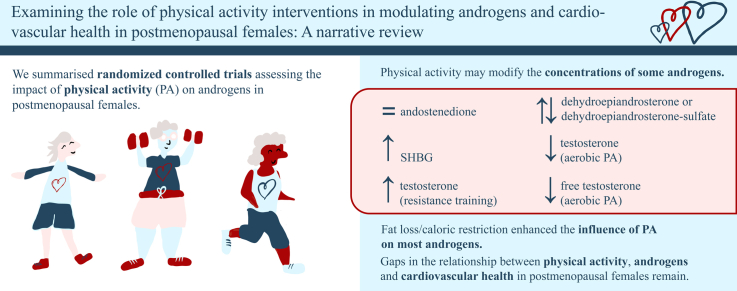
Abstract
A growing body of literature has examined the role of physical activity (PA) in modifying the effects of estrogen withdrawal on cardiovascular health in postmenopausal women, but the impact of PA on androgens is less clear. Changes in androgen concentrations following regular PA may improve cardiovascular health. This narrative review summarizes the literature assessing the impact of PA interventions on androgens in postmenopausal women. The association between changes in androgen concentrations and cardiovascular health following PA programs is also examined. Randomized controlled trials were included if they (i) implemented a PA program of any type and duration in postmenopausal women and (ii) measured changes in androgen concentrations. Following PA interventions, no changes in androstenedione, conflicting changes in dehydroepiandrosterone/dehydroepiandrosterone-sulfate, and increases in sex hormone–binding globulin concentrations were found. Total testosterone decreased following aerobic PA but increased after resistance training. Most aerobic PA interventions led to reductions in free testosterone. A combination of caloric restriction and/or fat loss enhanced the influence of PA on most androgens. Evidence exploring the relationship between changes in androgens and cardiovascular health indicators was scarce and inconsistent. PA has shown promise in modifying the concentrations of some androgens (free and total testosterone, sex hormone–binding globulin), and remains a well-known beneficial adjuvant option for postmenopausal women to manage their cardiovascular health. Fat loss influences the effect of PA on androgens, but the synergistic role of PA and androgens on cardiovascular health merits further examination. Many research gaps remain regarding the relationship between PA, androgens, and cardiovascular disease in postmenopausal women.
Graphical abstract
Résumé
Un nombre croissant de publications ont examiné le rôle de l’activité physique (AP) pour contrer les effets de la privation en œstrogènes sur la santé cardiovasculaire des femmes ménopausées, mais les effets de l’AP sur les androgènes sont moins évidents. Les variations des taux d’androgènes associées à l’AP régulière pourraient améliorer la santé cardiovasculaire. Cette revue narrative résume des articles ayant évalué les répercussions des interventions fondées sur l’AP sur les taux d’androgènes chez les femmes ménopausées. Le lien entre la santé cardiovasculaire et les variations des taux d’androgènes consécutives à des programmes d’AP a également été examiné. Des essais contrôlés randomisés étaient inclus s’ils comprenaient (i) la mise en œuvre d’un programme d’AP quel qu’en soit le type ou la durée chez des femmes ménopausées et (ii) la mesure des variations des taux d’androgènes. Aucune variation des taux d’androstènedione n’a été observée après des interventions fondées sur l’AP. Toutefois, des variations conflictuelles des taux de déhydroépiandrostérone et de sulfate de déhydroépiandrostérone et des hausses des taux de la globuline liant les hormones sexuelles ont été observés. Le taux de testostérone totale a diminué après l’AP en aérobie, mais a augmenté après l’entraînement contre résistance. La plupart des interventions fondées sur l’AP en aérobie ont entraîné des réductions du taux de testostérone libre. En association avec la restriction calorique et/ou une perte de graisse corporelle, l’AP exerce une influence accrue sur la plupart des androgènes. Les données probantes explorant le lien entre les variations des taux d’androgènes et les indicateurs de santé cardiovasculaire étaient rares et contradictoires. L’AP s’est révélée prometteuse pour ce qui est de modifier les taux de certains androgènes (testostérone libre et testostérone totale, globuline liant les hormones sexuelles); elle demeure une option adjuvante bénéfique bien connue pour aider les femmes ménopausées à prendre en charge leur santé cardiovasculaire. La perte de graisse corporelle influe sur les effets de l’AP sur les androgènes, mais le rôle synergique de l’AP et des androgènes sur la santé cardiovasculaire mérite un examen plus approfondi. De nombreuses lacunes subsistent quant à la recherche d’un lien entre l’AP, les androgènes et les maladies cardiovasculaires chez les femmes ménopausées.
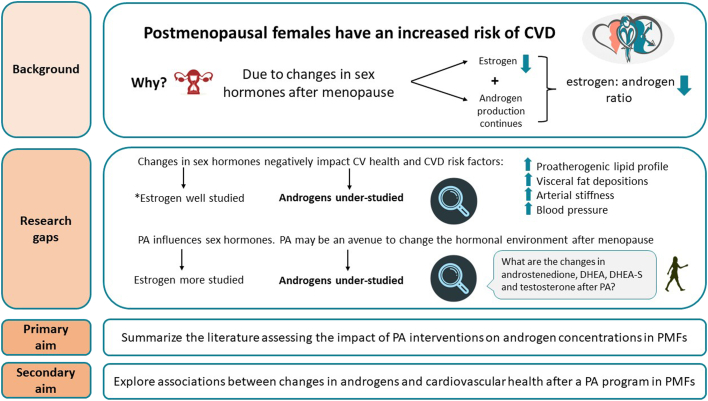
Cardiovascular disease (CVD) is the leading cause of premature death in women over 50 years of age in developed countries.1 During their reproductive years, women have a lower risk of CVD than men, but after menopause, this advantage disappears.2 Menopause is a female-specific risk factor for CVD, independent of natural aging.3 Menopause occurs at a median age of 51 years,2 and is characterized by a change in hormonal milieu resulting in the cessation of ovarian estrogen production, while the ovaries continue to synthesize and secrete testosterone (an androgen).4 In turn, the relative influence of androgens becomes more prominent after estrogen concentrations decrease post menopause.5
Alterations in the concentrations of sex hormones following menopause impact cardiovascular health.3,6 Previous research on the relationship between menopause and increased CVD risk has focused primarily on decreased estrogen concentrations.2 These outcomes include the evolution of a proatherogenic lipid profile and redistribution of fat to visceral depositions,3 development of subclinical vascular disease,4,7 and increased blood pressure8 (Fig. 1). However, the literature regarding the role of endogenous androgens on cardiovascular health in postmenopausal women is conflicting (see Androgens and Cardiovascular Health below). Many studies have attempted to characterize the relationship, but they have focused on sex-related differences in cardiovascular indicators9 or in women who have had androgenic therapy.10 Although menopausal hormone therapy (MHT) historically has been considered cardioprotective, it is no longer recommended for primary and secondary prevention against CVD.11 Similarly, in 2019, a Global Consensus Position Statement endorsed by ten regulatory societies declared here was insufficient data to recommend testosterone therapy for CVD prevention or treatment in postmenopausal women.12 In fact, the statement recommended against the use of oral testosterone therapy, as it has been associated with decreased high-density lipoprotein and increased low-density lipoprotein levels.12 Substantial gaps are present in the female-specific CVD research, as most CVD-related clinical trials were comprised of more than 85% male participants, and ∼66% of all CVD research has focused on male participants.13 These assumptions and gaps in key clinical knowledge warrant a narrative investigation of the influence of androgens on CVD in postmenopausal women.
Figure 1.
Rationale for the need to assess the impact of physical activity (PA) interventions on androgen concentrations and cardiovascular (CV) health in postmenopausal females (PMFs). CVD, cardiovascular disease; DHEA, dehydroepiandrosterone; DHEA-S, dehydroepiandrosterone-sulfate.
Physical activity (PA) is a safe, cost-effective strategy that has been shown to improve modifiable CVD risk factors associated with aging and postmenopausal status.1,14 For example, moderate-intensity aerobic PA has been shown to reduce cortisol-to-dehydroepiandrosterone (DHEA) ratios, fasting glucose, inflammation, and blood pressure.15 Increasing the number of minutes of aerobic PA has been associated with decreased adiposity in postmenopausal women.16 The increase in cardiorespiratory fitness (CRF) following regular PA also lowers the risk of CVD17 and attenuates the arterial stiffness that accompanies aging.14 For an in-depth review of the negative effects of menopause on CVD risk factors and the positive impact of PA on CVD in postmenopausal women, the reader is referred to El Khoudary et al.4 and Mendoza et al.,14 respectively.
Given the remarkable health benefits of PA, the World Health Organization and Canadian Society for Exercise Physiology recommend that adults (ages 18-64 years) and older adults (ages 64+ years) accumulate at least 150 to 300 minutes of moderate-intensity PA or 75 to 150 minutes of vigorous-intensity PA per week, and engage in muscle-strengthening activities on 2 or more days a week.18,19 However, women are often less physically active than men.20 Only 16.6% of women at least 40 years of age in Canada meet the PA recommendations, as measured with accelerometers.21 In a national cohort of previously inactive American adults over 60 years old, those who increased their PA frequency to 3 to 4 times per week significantly lowered their risk of CVD events, compared with those who remained inactive.22 Menopause may, thus, be a key period in which women should be encouraged to become physically active. If PA is an effective preventative therapy to reverse the negative cardiovascular consequences that follow altered hormone concentrations in postmenopausal women,15 the role of PA in modulating the specific effects of androgens on cardiovascular health must be considered.
Recent evidence suggests an interplay exists between fat loss, androgens, and PA in subpopulations of women, including those at increased risk of breast cancer and those with polycystic ovary syndrome (PCOS). Gonzalo-Encabo et al. investigated the role of PA on sex hormones and breast cancer risk in obese and overweight postmenopausal women and found that aerobic training, compared to aerobic training combined with resistance training, had a stronger impact on restoring sex hormone homeostasis (ie, decreasing estrogen and androgen concentrations [decreased DHEA, testosterone, and androstenedione concentrations] and increasing sex hormone–binding globulin {SHBG}concentration]).23 This effect was more pronounced when the training was combined with exercise-induced weight loss.23 Similarly, a systematic review including female participants of all ages with PCOS revealed that vigorous-intensity aerobic PA was associated with the largest improvements in insulin sensitivity, and resistance training lowered androgen concentrations more than aerobic, aquatic high-intensity interval training, or yoga PA modalities.24 However, a consensus on the influence of PA in altering androgen concentrations in a larger inclusive population of postmenopausal women has not been reached.25
Given the altered sex hormone profile that follows menopause and its subsequent influence on CVD risk, development of alternative preventative strategies that positively impact sex hormones and cardiovascular health is crucial. Changes in androgen concentrations following regular PA may improve cardiovascular health (Fig. 1). To address the limited literature surrounding androgens and CVD in postmenopausal women, calls for action have been made by experts in the field to investigate the sex-specific effects of androgens on CVD risk,26, 27, 28 and whether regular PA can modulate circulating androgen concentrations25 in postmenopausal women. The aims of this narrative review are as follows: (i) to provide a background in the production and function of androgens, alterations of androgens following menopause, and their potential impact on cardiovascular health; and (ii) to summarize the existing literature assessing the impact of PA interventions on androgen concentrations in postmenopausal women. As a secondary aim, we examine the association between changes in androgen concentrations and cardiovascular health indicators (eg, body mass and composition, blood pressure, CRF, blood biomarkers) following a PA program.
Androgens and Menopause
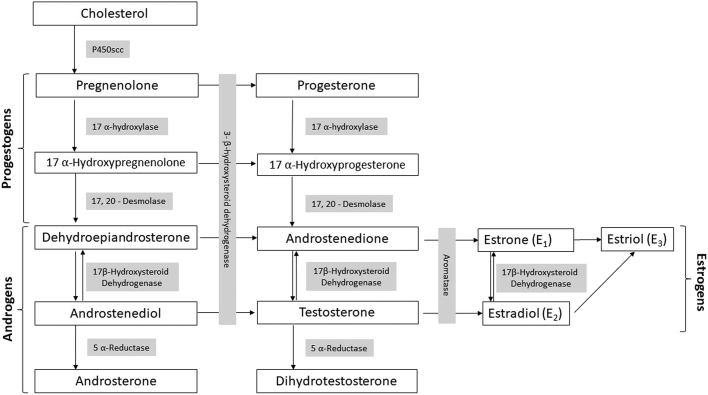
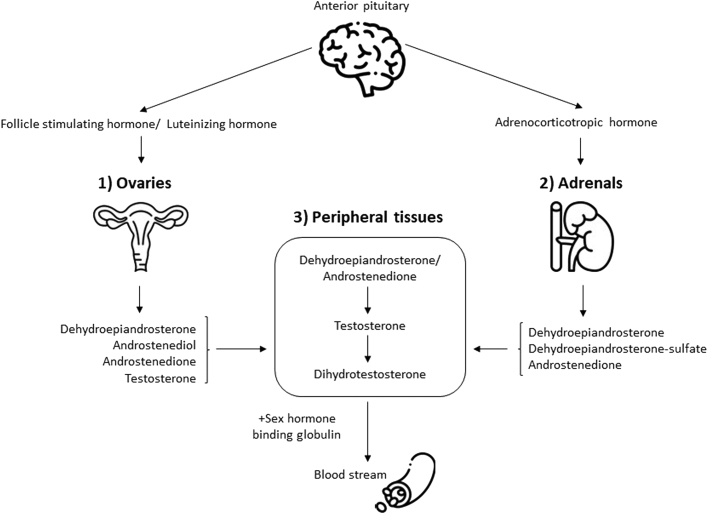
Androgens are essential steroid hormones with important roles in sexual function.26,29 They are one of 4 steroid hormone groups (including progesterone, estrogens, and corticoids) that originate from a common cholesterol precursor (Fig. 2). Sources of androgens are either direct secretion from endocrine glands (ie, the ovaries and adrenals) or indirect bioconversion of circulating androgenic prohormones into active hormones by enzymatic activity in peripheral tissues (Fig. 3). With the loss of ovarian follicular activity at menopause, the adrenal gland acts as the primary source of androgens: DHEA, DHEA-sulfate (DHEA-S), and androstenedione become major precursors for the extragonadal production of androgens in postmenopausal women.30
Figure 2.
Pathway of major ovarian and adrenal sex steroid synthesis. The syntheses of adrenal steroids aldosterone and cortisol have been omitted for the purposes of this review.
Figure 3.
A schematic representation of sources of androgen in postmenopausal women. 1) Ovaries; 2) adrenal glands; 3) peripheral tissues. Dehydroepiandrosterone is a major source of androgens in postmenopausal women and is converted into testosterone and dihydrotestosterone for action in target peripheral tissues. All androgens must be bound to sex hormone–binding globulin to enter the bloodstream. Brain, ovary, adrenal gland: Icons made by Freepik (https://www.flaticon.com/authors/freepik) from www.flaticon.com.
DHEA and androstenedione have little androgenic activity and are quickly converted into testosterone in peripheral tissues.31 Testosterone can then act directly on androgen receptors, be reduced to dihydrotestosterone by enzymatic action at target tissues (ie, ovaries, skin, and liver), or be aromatized to estradiol.12,28 Testosterone and dihydrotestosterone are considered the most potent source of androgens in terms of affinity for androgen receptors.28
Androgen physiology is highly dependent upon the binding of sex steroids to SHBG in circulating blood.32 Only unbound androgens bind to steroid receptors in peripheral tissues.28 SHBG has a high affinity for testosterone and dihydrotestosterone, but a low affinity for DHEA. SHBG does not bind to DHEA-S.28 A small proportion of testosterone (2%) remains unbound and biologically active, measured as “free” testosterone.32 “Total” testosterone refers to the amount of testosterone that is bound to SHBG plus the amount of unbound, free testosterone. A surrogate measure of free testosterone is the free androgen index (FAI), which is a ratio of total testosterone to SHBG and a validated marker of androgenicity in postmenopausal women.33 Therefore, androgen activity is dependent upon endocrine secretion, peripheral bioconversion, and SHBG concentrations.
Alterations in androgen concentrations after menopause
In postmenopausal women, circulating estrogen concentrations are lower when compared to premenopausal levels, contributing to a relative increase in androgenic concentrations.34 More specifically, androgen production decreases over the lifespan, including after menopause.34, 35, 36, 37 Estrogen production also decreases after menopause,3 but to a greater extent, allowing the relative influence of androgens to become more pronounced. DHEA concentrations decline by up to 60% prior to menopause and continue to decrease throughout the lifespan.38 Similarly, testosterone concentration decreases with reproductive aging,36 by up to 50% in women in their 40s compared to women in their 30s,37 and it plateaus after the age of 65 years.12 This change may reflect an age-related reduction rather than the influence of menopause. Minimal decreases in androstenedione concentrations have been observed after menopause.35 Finally, SHBG concentrations decline steadily throughout the menopausal transition, increasing the FAI.32,39 The cardiovascular changes that accompany the increased androgen concentrations following menopause are poorly understood.
Androgens and cardiovascular health
Testosterone and the risk of CVD
Conflicting results have been reported from longitudinal cohort studies, such that both high (ie, in n = 4600 pre- and postmenopausal women40; and n = 2634 postmenopausal41 women) and low total testosterone (in n = 2914 pre- and postmenopausal women42) concentrations have been associated with increased CVD risk. For instance, women with high (95th percentile) testosterone concentrations showed a 68% greater risk of ischemic heart disease, compared to those with lower (10th to 89th percentile) concentrations.40 A systematic review (n = 23 randomized controlled trials [RCTs] studying exogenous androgen administration) concluded that chronic states of hyperandrogenism (ie, high testosterone and low SHBG concentrations) contribute to increased CVD risk.43 Yet, low total testosterone concentration has been associated with an increased risk of cardiovascular events (ie, angina pectoris, myocardial infarction, percutaneous transluminal coronary angioplasty, coronary artery bypass surgery, stroke, transient ischemic attack; hazard ratio 0.68 [95% confidence interval {CI} 0.48-0.97]) after adjustment for body mass index (BMI) > 25 kg/m2, age, and smoking in postmenopausal women.42 Moreover, in a prospective population-based trial, postmenopausal women (n = 639) with the highest free testosterone ( ≥ 63 pg/mL) and lowest total testosterone ( ≤ 80 pg/mL) concentrations had the highest incidence of cardiovascular events, even after adjustment for PA levels, BMI, and other CVD risk factors.44 In summary, both high and low levels of total testosterone may have negative health consequences, and it remains unclear in the literature which association may be worse. Thus, the evidence suggests that an intermediate physiological range may benefit cardiovascular health, but the inconsistency of the findings limits our current understanding of these pathophysiological mechanisms.36,44
Finally, low SHBG concentration has been positively associated with an increased CVD risk, as reported in narrative28,36 and systematic reviews.43 Recent expert reviews have postulated that the development of CVD may be influenced more by low SHBG than by testosterone concentration,28,36 which may be particularly relevant in postmenopausal women who have a low serum SHBG concentration.32,39
Androgens and vascular disease progression
In postmenopausal women, higher androstenedione and free testosterone concentrations have been found to be significantly associated with reduced carotid artery intima-media thickness, a marker of atherosclerosis.44 Independent of CVD risk factors (ie, BMI, insulin resistance, systolic blood pressure, and lipids), higher serum total testosterone concentration and FAI corresponded to increased carotid artery intima-media thickness, and lower DHEA-S concentration was associated with arterial stiffness.45 Indeed, an increased incidence of atherosclerosis has been observed in women with PCOS who may have marked hyperandrogenism, even after adjusting for BMI.46 Therefore, although increased androgen levels appear to influence the development of atherosclerosis, the relative influence of each steroid independently has not been established.
Androgens and insulin resistance
Type 2 diabetes has been shown to increase the risk of coronary artery disease mortality more strongly in women than in men (ie, 3.5- vs 2-fold increase, respectively).47 A systematic review of prospective and cross-sectional studies by Ding et al. determined that, compared to controls, women with type 2 diabetes had a significantly higher total testosterone concentration (range: 449.6 to 605.2 ng/dL; mean difference, 6.1 ng/dL; 95% CI, 2.3 to 10.1; P < 0.001 for sex difference).48 Moreover, women with higher SHBG concentrations ( > 60 nmol/L) had an 80% lower risk of having type 2 diabetes (risk ratio, 0.20; 95% CI, 0.12 to 0.30), and men had a 52% lower risk.48 This review conversely found that high SHBG concentration led to low free testosterone concentration, suggesting that high SHBG concentration may protect against the adverse effects of free testosterone.48 SHBG concentration has been established as an independent marker for insulin resistance, and low SHBG concentration has been associated with the development of obesity, independent of estrogen and androgen concentrations. Insulin resistance can stimulate the ovarian production of androgens while also inhibiting SHBG, via increasing insulin-like growth factor (IGF-1).49,50 Finally, recent evidence has shown a positive correlation between left ventricular hypertrophy, increased FAI, and decreased SHBG concentration in postmenopausal hypertensive women, directly linking CVD with androgen concentrations outside of expected physiological ranges.51 Endogenous androgens may produce sex-specific modulation of glycemic control and onset of type 2 diabetes, influencing cardiovascular health largely through the effects of SHBG.
Androgens and body composition
Adipose tissue is a potent source of androgen metabolism through peripheral tissue bioconversion, and increased adiposity may heighten androgen concentrations in postmenopausal women with a higher BMI.25 Changes accompanying menopause include a redistribution of fat to the abdominal area.52 Increased abdominal circumference also has been independently associated with insulin resistance, increased low-density lipoprotein level, serum triglycerides, and blood pressure.6 Finding strategies to reduce amounts of adipose tissue, increase SHBG concentration, and improve insulin resistance may, therefore, alter androgen concentrations in postmenopausal women.
Methods
To summarize the existing literature, a search was created by a medical research librarian (S.V.) in collaboration with the project leads (C.E., J.R.). The search was created in MEDLINE (National Library of Medicine database) using a combination of key terms and index headings related to postmenopausal women, androgens, and PA and then translated to Cochrane Central Register of Controlled Trials, Embase, and CINAHL (Cumulative Index to Nursing and Allied Health Literature); Supplemental Tables S1-S4). The searches were conducted on August 10, 2020 and updated on December 17, 2021. When possible, French and English limits were applied. RCTs were included if they implemented a PA program of any type and duration in postmenopausal women and measured changes in androgen concentrations (ie, in androstenedione, DHEA, DHEA-S, total/free testosterone, and/or SHBG). Women of any health status were included if (i) they were > 40 years of age (as menopause before this age is considered premature ovarian insufficiency3), and > 12 months had passed since their last known period; (ii) they had no prior history of oophorectomy; and (iii) they did not take MHT. In studies with mixed populations (eg, both sexes, including women on MHT), only data from the postmenopausal women who were not taking MHT were extracted and included. Traditional cardiovascular health indicators (eg, BMI, body composition, CRF, blood pressure, blood biomarkers) reported at baseline and follow-up in at least 3 studies were extracted by the current authors.
Results
Study characteristics
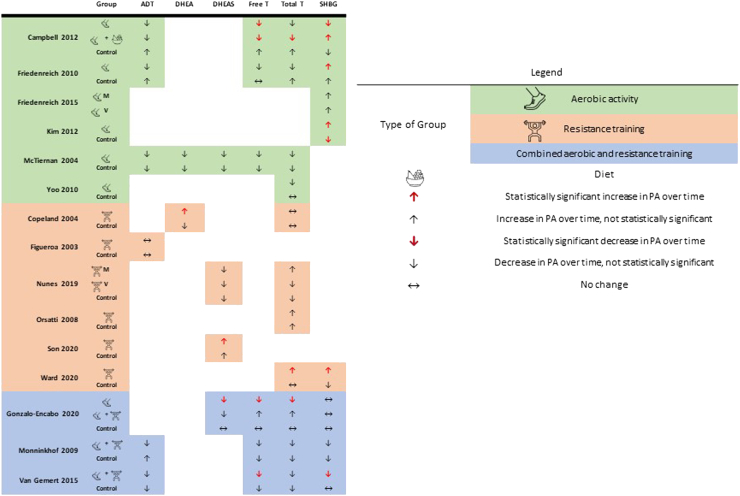
A total of 3253 articles were identified for this review; following the removal of duplicates (n = 1245), 2008 articles were screened by title and abstract. Full-text screening was conducted in 138 articles, and 15 RCTs were deemed eligible for inclusion (Supplemental Fig. S1). In 2 studies that included ineligible populations (ie, women on MHT; n = 3953, n = 1654), only data from postmenopausal women who were not taking MRT in the PA group were extracted. Characteristics of the included RCTs implementing a PA program and measuring androgen concentrations in postmenopausal women are presented in Table 1. Most women were sedentary53,65 and overweight or obese,53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 66,67 with a mean age between 53 and 70 years. Heterogeneity was high in the frequency (2-5 days per week), intensity (light, moderate, or vigorous), duration (30-75 minutes per session), and total length (12 weeks to 12 months) of the PA interventions. These interventions were categorized as aerobic PA,54, 55, 56,58,59,67 resistance training,53,61, 62, 63,65, 66 or combined aerobic and resistance training (Table 1).57,60,64 Figure 4 provides a visual representation of the changes in androgen concentrations following aerobic and/or resistance training.
Table 1.
Characteristics of RCTs implementing a physical activity program in postmenopausal women
| Study | Participant characteristics | Intervention characteristics | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Age (y), mean (SD) or range of groups | Inclusion criteria | Activity | Frequency, d/wk | Intensity | Duration, min | Length | Co-intervention/ control group | Hormone(s) studied | |
| Aerobic exercise | ||||||||||
| Campbell et al.54 (2012) USA | 439 from the “New” RCT | PA: 58 (5) Diet + PA: 58 (5) Diet: 58 (6) C: 57 (4) |
Healthy, BMI > 25, < 100 min/wk PA PMWs, aged 50-75 y | Supervised + at-home aerobic exercise (n = 117) | 5 (3 onsite, 2 at home) | Moderate- to-vigorous: 70%–85% HRMax or 4+ METS | ≥ 45 | 12 mo | Diet + PA (n = 117) Diet: caloric restriction (n = 118) C: MUA (n = 87) |
Androstenedione SHBG Testosterone (free, total) |
| Friedenreich et al.56 (2010) Canada | 320 | PA: 61 (5) C: 60 (5) |
Healthy, BMI 22-40 <120 min/wk PA, PMWs aged 50-74 y |
Supervised + at-home aerobic exercise (n = 160) | 5 (3 onsite, 2 at home) | 70%–80% HRR | ≥ 45 | 12 mo | C: MUA (n = 160) | Androstenedione SHBG Testosterone (free, total) |
| Friedenreich et al.55 (2015) Canada | 400 from the "BETA" trial | Moderate: 60 (5) High: 59 (5) |
Healthy, BMI 22-40, <120 min/wk PA, PMWs aged 50-74 y | Supervised + at-home aerobic exercise | 5 (3 onsite, 2 at home) | 60%–80% HRR |
Moderate group: 30 (n = 200) High group: 60 (n = 200) |
12 mo | No C | SHBG |
| Kim & Kim58 (2012) South Korea | 30 | Total: 55 (3) | Healthy, BF > 32%, < 40 min/wk PA, PMWs | Supervised aerobic dance (n = 15) | 3 | 55%–80% HRmax |
60 | 16 wk | C: MUA (n = 15) | SHBG |
| McTiernan et al.59 (2004) USA | 173 | PA: 61 (7) C: 61 (7) |
Healthy, BMI ≥ 24, < 60 min/wk PA, PMWs aged 50-79 y | Supervised + at-home aerobic exercise (n = 87) | 5 | 60%–75% HRmax |
45 | 12 mo | C: stretching exercises (1x/wk for 45 min) (n = 86) |
Androstenedione DHEA DHEA-S Testosterone (free, total) |
| Yoo et al.67 (2010) South Korea | 21 | PA: 70 (2) C: 71 (2) |
Healthy PMWs, aged > 65 y | Supervised walking with ankle weights (1 kg) (n = 11) | 3 | 60% HRR | 60 | 12 wk | C: MUA (n = 10) | Testosterone (total) |
| Resistance training | ||||||||||
| Copeland et al.66 (2004) Canada | 16 | PA: 53 (5) C: 54 (6) |
Healthy, sedentary PMWs aged > 50 y | Supervised resistance training (n = 8) | 3 | 10RM | NR | 12 wk | C: unsupervised flexibility exercises 3 d/wk (n = 8) | DHEA Testosterone (total) |
| Figueroa et al.53 (2003) USA | 74 | PA: 57 (1) C: 57 (1) |
Healthy, sedentary PMWs aged 40-65 y | Supervised resistance training (n = 24) | 3 | 70%–80 % 1RM |
60–75 | 12 mo | C: MUA (n = 28) | Androstenedione |
| Nunes et al.61 (2019) Brazil | 34 | Low: 64 (NR) High: 60 (NR) C: 59 (NR) |
Healthy, sedentary PMWs aged 49-79 y | Resistance training: Low-volume (3 sets) (n = 10); high-volume (6 sets) (n = 12) |
3 | 70% 1RM | Low-volume: ∼45 High-volume: ∼90 |
16 wk | C: stretching exercises 2x/wk (n = 12) | DHEA-S Testosterone (total) |
| Orsatti et al.62 (2008) Brazil | 43 | PA: 58 (8) C: 59 (6) |
Healthy, sedentary PMWs aged 45-70 y | Supervised resistance training (n = 22) | 3 | 60%–80% 1RM | 50–60 | 16 wk | C: MUA (n = 21) | Total testosterone |
| Son et al.63 (2020) South Korea | 20 | PA: 68 (1) C: 67 (1) |
Healthy, sedentary, Stage 1 HTN PMWs | Supervised resistance band training (n = 10) | 3 | Increased progressively: 40%–50% to 60%–80% 1RM | 60 | 12 wk | C: supervised sedentary activities (n = 10) | DHEA-S |
| Ward et al.65 (2020) Sweden | 55 | PA: 58 (5) C: 55 (5) |
Healthy, sedentary (< 75 min/wk MVPA) PMWs with vasomotor symptoms | Supervised and at-home resistance training (n = 26) | 3 | 8RM | NR | 15wk | C: remain sedentary (n = 29) | Testosterone SHBG |
| Combined aerobic and resistance training | ||||||||||
| Gonzalo-Encabo et al.57 (2020) Spain | 35 | Median Endurance: 56 Concurrent:58 C: 57 |
Healthy, BMI > 25, < 150 min/wk PA, PMWs aged 50-65 y | Supervised endurance (n = 10) or endurance + resistance (n = 13) training | 3 | Endurance: 55%–75% HRR Resistance: 65% 1RM |
Endurance: 60 of aerobic Concurrent: 20 aerobic + resistance exercises |
12 wk | C: MUA (n = 12) | DHEA-S Testosterone (free, total) SHBG |
| Monninkhof et al.60 (2009) The Netherlands | 189 from the "SHAPE" study | PA: 59 (5) C: 58 (4) |
Healthy, BMI > 22, < 120 min/wk PA, PMWs aged 50-69 y | Supervised or at-home aerobic + resistance training (n = 96) | Supervised: 2 At home: 1 |
MVPA | Supervised: 60 At home: 30 |
12 mo | C: MUA (n = 93) | Androstenedione SHBG Testosterone (free, total) |
| van Gemert et al.64 (2015) The Netherlands | 243 from the "SHAPE-2" trial | PA: 59 (4) Diet: 60 (5) C: 60 (5) |
Healthy, < 120 min/wk PA, BMI 25-35, PMWs aged 50-69 y | Supervised resistance + endurance training sessions, Nordic walking (n = 98) | 2 combined resistance + endurance training, 2 Nordic walking |
Target HRR increased progressively. Resistance: 1RM Endurance: 60%–90% HRR Nordic: 60%–65% HRR |
60 | 16 wk | Diet only: restriction to 3500 kcal/wk + nutrition education group sessions (5x total, 1 h) (n = 97) C: complete food diaries and usual activities (n = 48) |
Androstenedione SHBG Testosterone (free, total) |
BETA, Breast Cancer and Exercise Trial in Alberta; BF, body fat; BMI, body mass index; C, control; DHEA, dehydroepiandrosterone; DHEA-S, dehydroepiandrosterone sulphate; HRmax, heart rate maximum; HRR, heart rate reserve; HTN, hypertension; METS, metabolic equivalents of task; MUA, maintain usual activities; MVPA, moderate-to-vigorous physical activity; NR, not reported; PA, physical activity; PMW, postmenopausal women; RCT, randomized controlled trials; RM, repetition maximum; SD, standard deviation; SHAPE, Sex Hormones and Physical Exercise; SHBG, sex hormone–binding globulin.
Figure 4.
A visual representation of changes in androgen concentrations following aerobic and/or resistance training. ADT, androstenedione; DHEA, dehydroepiandrosterone; DHEAS, dehydroepiandrosterone-sulfate; M, moderate; PA, physical activity; SHBG, sex hormone–binding globulin; T, testosterone; V, vigorous.
Physical activity and androgen concentrations
Physical activity and androstenedione
Our findings demonstrate that studies implementing PA programs (n = 553,54,56,59,64) did not elicit significant changes in androstenedione concentrations in postmenopausal women (Table 2). Studies comparing PA of any intensity to control conditions (ie, maintenance of usual activities, diet, or stretching exercises) over 12 months showed no changes in androstenedione in postmenopausal women in response to different PA modalities (ie, aerobic exercise,54,56,59 resistance training,53 and combined aerobic and resistance training64). This evidence suggests that androstenedione concentrations are not influenced by PA interventions, regardless of their type, duration, or intensity, in postmenopausal women.
Table 2.
Changes in androgen hormone concentrations from baseline to follow-up after a physical activity intervention
|
Study |
Units: groups | Androstenedione | DHEA or DHEA-S | Testosterone (free) | Testosterone (total) | SHBG |
|---|---|---|---|---|---|---|
| Aerobic activity | ||||||
| Campbell et al.54 (2012) USA | Mean (95% CI); Δ% | pg/mL† | — | pg/mL | pg/mL† | nmol/L |
| PA | 502 (466–541) to 496 (456–540); –1.2% | — | 5.1 (4.7–5.5) to 4.9 (4.5–5.3); –4.5% ∗∗∗vs diet and PA ∗vs diet |
248 (230–267) to 236 (216 to 257); –4.9% | 39.1 (35.9–42.6) to 38.8 (35.6–42.4); –0.7% ∗∗∗vs diet ∗∗∗vs diet and PA |
|
| Diet | 511 (471–553) to 518 (477–562); +1.4% | — | 5.1 (4.7–5.6) to 4.6 (4.2–5.1); –10% ∗∗∗vs control ∗vs diet and PA |
239 (219–260) to 236 (216 to 258); –0.9% | 35.8 (33–38.8) to 43.8 (40.4–47.5); +22.4% ∗∗∗vs control |
|
| Diet + PA | 526 (491–564) to 508 (471–547); –3.5% | — | 5.3 (4.9–5.7) to 4.5 (4.1–4.8); –15.6% ∗∗∗vs control |
239 (221–258) to 225 (208 to 243); –5.9% ∗vs control |
34.1 (31.9–36.4) to 42.9 (40.2–45.6); +25.8% ∗∗∗ vs control |
|
| Control | 487 (439–540) to 494 (454–537); +1.5% | — | 4.9 (4.4–5.6) to 5.1 (4.6–5.7); +2.6% | 228 (202–257) to 232 (209–257); +1.8% | 34.7 (31.5–38.2) to 33.7 (30.3–37.5); –2.7% | |
| Friedenreich et al.56 (2010) Canada | Mean (95%CI) | pg/mL | — | pg/mL† | pg/mL† | nmol/L |
| PA | 578 (539–621) to 572 (537–610) | — | 3.5 (3.2–3.8) to 3.3 (3.1–3.6) | 239 (223–258) to 234 (217–253) | 40.3 (37.5 to 43.4) to 41.9 38.9 to 45.1 ∗∗∗vs control | |
| Control | 553 (514–595) to 577 (534–624) | — | 3.5 (3.2–3.8) to 3.5 (3.3–3.9) | 231 (213–251) to 237 (218–257) | 38.1 (35.7–40.8) to 38.4 (35.9–41.1) | |
| Friedenreich et al.55 (2015) Canada | Geometric mean (95% CI); Δ% | — | — | — | — | nmol/L |
| Moderate (150 min/wk PA) | — | — | — | — | 43.2 (40.6, 46.0) to 47.4 (44.6, 50.4); +9.6% | |
| High (300 min/wk PA) | — | — | — | — | 47.6 (44.7, 50.8) to 50.7 (47.7, 53.9); +6.4% | |
| Kim & Kim58 (2012) South Korea | Mean (SD) | — | — | — | — | nmol/L |
| PA | — | — | — | — | 43.3 (9.2) to 46.0 (10.0) ∗∗∗vs control ∗∗within group |
|
| Control | — | — | — | — | 44.2 (8.1) to 42.2 (8.8) ∗within group |
|
| McTiernan et al.59 (2004) USA | Geometric mean (95% Cl) | pg/mL | DHEA (ng/mL); DHEA-S (ug/dL) |
pg/mL | pg/mL | — |
| PA | 533 (494–575) to 480 (447–516) | 2.19 (1.93–2.49) to 1.93 (1.68–2.20); 53 (45.5–61.8) to 47.8 (41.2–55.5) |
4.6 (4.2–4.9) to 4.3 (3.9–4.7) | 211 (196–228) to 208 (190–227) | — | |
| Control | 585 (541–633) to 525 (489–564) | 2.46 (2.22–2.72) to 2.24 (2.03–2.47); 63.1 (54.8–72.7) to 47.8 (41.2–55.5) |
4.7 (4.3–5.2) to 4.6 (4.2–5.0) | 223 (204–243) to 218 (199–239) | — | |
| Yoo et al.67 (2010) South Korea | Mean (SD) | — | — | — | pg/mL | — |
| PA | — | — | — | 200 (100) to 100 (100) | — | |
| Control | — | — | — | 100 (100) to 100 (100) | — | |
| Resistance training | ||||||
| Copeland et al.66 (2004) Canada | Mean (SD) | — | DHEA (nmol/l) | — | pg/mL† | — |
| PA | — | 26 (13) to 35 (21) ∗within group |
— | 260 (115) to 260 (115) | — | |
| Control | — | 35 (15) to 34 (15) | — | 231 (173) to 231 (115) | — | |
| Figueroa et al.53 (2003) USA | Mean (SD) | NS | - | — | - | — |
| Nunes et al.61 (2019) Brazil | Mean (CI); Δ% | — | DHEA-S (ug/dL) | — | pg/mL† | — |
| Low (3 sets) | — | 35.7 (25.9–45.4) to 34.4 (25.4–43.3); +0.1% | — | 270 (110–420) to 290 (160–430) | — | |
| High (6 sets) | — | 69.2 (39.7–98.7) to 65.5 (38.5–92.6); -4.5% | — | 470 (320–620) to 350 (150–550) | — | |
| Control | — | 53.7 (35.5–71.9) to 51.1 (31.6–70.6); -6.7% | — | 440 (110–770) to 290 (160–430) | — | |
| Orsatti et al.62 (2008) Brazil | Mean (SD) | — | — | — | pg/mL† | — |
| PA | — | — | — | 231 (66) to 248 (82) | — | |
| Control | — | — | — | 269 (154) to 318 (21) | — | |
| Son et al.63 (2020) South Korea | Mean (SD) | — | DHEA-S (ug/dL) | — | — | — |
| PA | — | 81.6 (34.9) to 91.1 (40.2) ∗vs control ∗within group |
— | — | — | |
| Control | — | 81.0 (35) to 90.1 (35.6) | — | — | — | |
| Ward et al.65 (2020) Sweden | Median (IQR) | — | — | — | pg/mL† | nmol/L |
| PA | — | — | — | 700 (200) to 800 (200) ∗within group |
72.8 (40.0) to 84.5 (33.9) ∗vs control ∗within group |
|
| Control | — | — | — | 800 (300) to 800 (300) | 81.1 (42.5) to 76.8 (45.8) | |
| Combined aerobic and resistance training | ||||||
| Gonzalo-Encabo et al.57 (2020) Spain | Δ% | — | DHEA-S | — | — | — |
| Endurance (aerobic) | — | –13% ∗∗within group |
–41% ∗∗vs concurrent |
–40% ∗∗vs concurrent |
NS | |
| Concurrent (aerobic/resistance) | — | –7.5% | +21% | +25% | NS | |
| Control | NS | NS | NS | NS | ||
| Monninkhof et al.60 (2009) The Netherlands | Geometric mean; Δ% | pg/mL | — | pg/mL | pg/mL | nmol/L |
| PA | 1146 to 1097; –2.7% | — | 8.7 to 8.5; –2.9% | 528 to 508; –3.8% | 33.9 to 33.6; –0.7% | |
| Control | 1172 to 1199; +2.3% | — | 8.7 to 8.5; –1.8% | 535 to 548; –1.6% | 34.7 to 33.6; –3.3% | |
| van Gemert et al.64 (2015) The Netherlands | Geometric mean; Δ% | pg/mL | — | pg/mL | pg/mL | nmol/L |
| PA | 573 to 488; –14.7 | — | 2.4 to 2.0; –17.7% ∗vs diet ∗∗∗vs control |
186 to 172; –7.6% | 49 to 59; +19% ∗∗∗vs control |
|
| Diet | 562 to 537; –4.5% | — | 2.5 to 2.3; –11.2% | 197 to 189; –3.7% | 51 to 57; +13% ∗∗∗vs control |
|
| Control | 575 to 560; –2.6% | — | 2.7 to 2.6; –3.9% | 194 to 186; –4.1% | 44 to 44; –0.3% | |
Data were extracted as they were reported in the original article. In some cases, values were converted to the most frequently reported unit to facilitate comparisons between studies of the same hormone, not across hormones, as indicated by †. If available, percent change was reported.
CI, confidence interval; DHEA, dehydroepiandrosterone; DHEA-S, dehydroepiandrosterone-sulfate; IQR, interquartile range; NS, not significant; SD, standard deviation; SHBG, sex hormone–binding globulin.
P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001.
Physical activity and DHEA, DHEA-S
The effect of PA interventions on DHEA concentration (n = 2 studies59, 66) and DHEA-S concentration (n = 4 studies57,59,61,63) appears to be limited across the reviewed literature (Table 2). One study reported no change in DHEA concentration as a result of aerobic PA,59 whereas another showed significant increases in DHEA concentration following resistance training, compared to baseline.66 Notably, the sample size was smaller (n = 866 vs n = 8759) and the duration of intervention was shorter (n = 12 weeks66 vs n = 12 months59) in the latter investigation.
The changes in DHEA-S concentration following PA programs were variable. Nonsignificant changes in DHEA-S concentration were reported in various modalities and durations of PA. Following resistance training, (3 sessions of 60 minutes each per week at 80% 1 repetition maximum [RM]), DHEA-S concentration increased (+11.2%, P < 0.05) over 12 weeks.63 However, after 12 weeks of aerobic training (3 sessions of 60 minutes each per week at 75%, 1 RM), DHEA-S concentration decreased, compared to that in a combined aerobic and resistance training group (–13%, P < 0.01).57 DHEA-S concentration may be influenced by PA interventions; however, the most effective modality of PA and direction of change are unclear.
Physical activity and testosterone
Testosterone was the most widely studied androgen, reported as free (unbound to SHBG; n = 6 studies54,56,57,59,60,64) or total (free and bound to SHBG; n = 11 studies54,56,57,59, 60, 61, 62,63, 64, 65, 67) serum concentrations (Table 2). Most studies reported nonsignificant changes in total testosterone concentrations after PA interventions, compared to those in control conditions.56,58, 59, 60, 61,66,67 All of the interventions included a supervised component and ranged in duration from 12 weeks66,67 to 12 months.54,56 The studies that reported significant findings had variable results. Gonzalo-Encabo et al. reported that 12 weeks of aerobic exercise (55%-75% heart rate reserve [HRR]) reduced total testosterone (–40%, P < 0.05), whereas a combination of aerobic and resistance training (65% 1RM) increased total testosterone (+25%, P < 0.01).57 Ward et al. showed an increase in total testosterone over 15 weeks of a resistance training program.65 In an aerobic PA and diet group (ie, caloric restriction—participants ingested between 1200 and 2000 calories per day, based on baseline weight), significant reductions in testosterone were observed, compared to concentrations in controls.54 Resistance training may modestly increase and aerobic exercise may modestly decrease total testosterone, but factors such as diet may impact the strength of this association.
Unlike the findings for total testosterone concentration, all studies reported reductions in free testosterone concentrations among female exercisers.54,56,57,64 However, some findings became insignificant for when adjusting for changes in fat loss, implying that reductions in adiposity enhanced the effects of the PA intervention on free testosterone.59,60 Significant reductions in free testosterone were observed in women engaging in aerobic PA (–41%), but not in women engaging in combined aerobic and resistance training (+21%; between-group difference: P < 0.01).57 Findings from aerobic interventions over 12 months revealed reductions in free testosterone only in women who accumulated more than 225 min/wk of aerobic PA (70%-80% HRR), compared to less than 225 min/wk.56 Compared to the reduction following calorie-restriction alone, significantly greater decreases in free testosterone followed aerobic PA54 and a combination of aerobic and resistance PA (treatment effect ratio: 0.92, 95% CI 0.85 to 0.99).64 No studies that implemented a resistance program measured changes in free testosterone concentration. Aerobic PA may reduce free testosterone concentration more than diet alone, especially when performed for longer durations per week. Further, changes in body composition induced by regular PA may be an important mechanism responsible for changes in androgen concentrations.
Physical activity and SHBG
A total of 8 studies measured SHBG concentrations (Table 2).54, 55, 56, 57, 58,60,64,65 Postmenopausal women participating in aerobic PA for 30 to 60 minutes, 3 to 5 days per week demonstrated significant increases in SHBG, compared to the concentration in controls.58 Greater increases in SHBG were detected in the combined aerobic PA and diet group over 12 months, compared to those in the PA, diet, or control conditions alone (ie, PA + diet = +25.8%; PA = –0.7%; diet = +22.4%; control = –2.7%).54 Significant increases in SHBG were observed in those participating in combined aerobic and resistance PA, compared to the concentrations in controls (P < 0.001).64 Greater increases in SHBG were seen in women who exercised more than 225 min/wk, compared to concentrations in those who exercised less than 150 min/wk (P < 0.001).56 However, these findings were no longer significant (P = 0.29) when adjusting for body mass change over 12 months,56 and no significant increase in SHBG concentrations occurred in the aerobic PA group who exercised 300 min/wk, compared to the concentration change in those who exercised 150 min/wk.55 No changes were reported with resistance training over 1565 weeks, or in combined aerobic/resistance training over 12 weeks60 or 12 months.57 These findings suggest that aerobic PA increases SHBG concentrations, potentially to a greater extent than diet alone; however the dose of PA needed to effect change and its relationship with resistance training needs further clarification.
Follow-up trials
In a 30-month follow-up study to the Campbell et al. 2012 RCT,54 significantly greater increases in SHBG (+80.5%) were observed in women who were randomized to the 12-month aerobic PA and caloric restriction intervention, compared to increases in those in the control group (+80.5% vs +47.9%; P < 0.001).68 The original decrease in free testosterone in the aerobic PA and caloric-restriction group was lost at 30 months54; no significant changes in total or free testosterone concentration were observed in any group at 30 months.68 Unfortunately, this study did not report on the long-term PA levels of these women. In Friedenreich et al.’s 24-month follow up69 to the Breast Cancer and Exercise Trial in Alberta (BETA) trial,55 SHBG concentration significantly decreased (42.9 to 40.6 nmol/L) in the moderate aerobic PA group at follow-up,69 as compared to the original significant increase (42.9 to 46.7 nmol/L) observed at 12 months (P < 0.001).55 Changes in androgens may reverse with the cessation of PA. This finding emphasizes the importance of long-term regular PA in addition to fat loss achieved through caloric restriction for androgen maintenance.
Physical activity and CVD risk factors
CVD risk factors reported in the reviewed studies were scarce and inconsistent. The influence of anthropometric changes (ie, BMI, body mass, and body fat) on androgens following PA were the most frequently examined parameters.53, 66, 54, 55, 56, 57,60,62, 63, 64,66,67 Table 3 summarizes the changes in cardiovascular health indicators following the PA interventions.
Table 3.
Changes in cardiovascular disease risk factors data from baseline to follow-up after a physical activity (PA) intervention
| Study | Group | BMI, kg/m2 or body mass, kg | Body fat, % | Systolic BP (mmHg) | Cardiorespiratory fitness | †Fasting insulin (μU/mL) or IGF-1 (ng/mL) | Cortisol (nmol/L) |
|---|---|---|---|---|---|---|---|
| Aerobic activity | |||||||
| Campbell et al.54 (2012) USA | Insulin | ||||||
| PA | 83.7 (12.3) to 80.9 (12.2) kg; –3.3% ∗vs control ∗∗∗vs diet ∗∗∗vs PA + diet |
47.3 (4.1) to 45.5 (5.0); –3.8% ∗∗∗vs control ∗∗∗vs diet ∗∗∗vs PA + diet |
— | 1.9 (0.3) to 2.0 (0.4) L/min; +10.1% ∗∗∗vs control ∗∗∗vs diet |
10.9 (10.0–12.0) to 10.1 (9.1–11.0) μU/mL; -8.2% ∗∗vs diet ∗∗∗vs PA + diet |
— | |
| Diet | 84 (11.8) to 74.9 (12.3) kg; -10.8% ∗∗∗vs control |
47 (4.3) to 42.1 (6.4); –10.6% ∗∗∗vs control ∗∗∗vs PA + diet |
— | 1.9 (0.3) to 1.8 (0.3) L/min; –2.3% ∗∗∗vs PA + diet |
11 (9.8–12.2) to 8.1 (7.3 to 9) μU/mL; –26.1% ∗∗∗vs control |
— | |
| PA + diet | 82.5 (10.8) to 72.7 (10.9) kg; –11.9% ∗∗∗vs control |
47.4 (4.5) to 41.1 (7.0); –13.4% ∗∗∗vs control |
— | 1.9 (0.3) to 2.1 (0.4) L/min; +7.6% ∗∗∗vs control |
10.7 (9.7–11.8) to 7.9 (7.1-8.7) μU/mL; –26.5% ∗∗∗vs control |
— | |
| Control | 84.2 (12.5) to 83.7 (12.3) kg; –0.6% |
47.3 (4.4) to 47.2 (5.3); –0.5% | — | 1.9 (0.4) to 1.9 (0.3) L/min; –0.9% | 12.0 (10.8–13.3) to 11.6 (10.4–12.9) μU/mL; -3.7% | — | |
| Friedenreich et al.56 (2010) Canada | PA | Δ –2.3 (2.9–1.7) kg ∗∗∗vs control |
— | — | Δ +3.9 (2.8–4.9) mL/ kg/min ∗∗ vs control |
— | — |
| Control | Δ 0.5 (1.0–0.1) kg | — | — | Δ +0.7 (–0.2 to 1.6) mL/ kg/min | — | — | |
| Friedenreich et al.55 (2015) Canada | Moderate (150 min/wk PA) | Δ –1.9 (2.4–1.3) kg | Δ –1.1 (1.5–0.7) | — | Δ +4.0 (3.3–4.8) mL/ kg/min | — | — |
| High (300 min/wk PA) | Δ –2.6 (3.2–1.9) kg | Δ –2.0 (2.5–1.5) ∗∗vs moderate group |
— | Δ +5.0 (4.2–5.9) mL/ kg/min | — | — | |
| Kim & Kim58 (2012) South Korea | Insulin | ||||||
| PA | 25.0 (1.3) to 24.2(1.2) kg/m2 ∗∗∗vs control ∗∗within group |
36.0 (3.0) to 33.5 (3.3) ∗∗∗vs control ∗∗within group |
133 (5) to 125 (5) ∗∗∗ vs. control ∗∗∗within group |
— | 8.2 (1.0) 7.3(1.0) μU/mL ∗∗vs control ∗∗∗within group |
— | |
| Control | 25.1 (1.5) to 25.9(1.4) kg/m2 ∗∗within group | 36.6 (1.7) to 37.5 (2.4) ∗∗within group |
132 (4) to 134 (3) ∗∗within group |
— | 8.1 (1.2) to 8.4(1.2) μU/mL ∗∗∗within group |
— | |
| McTiernan et al.59 (2004) USA | — | — | — | — | — | — | — |
| Yoo et al.67 (2010) South Korea | PA | BMI 26.6 (2.9) to 26.3 (3.1) kg/m2 Body mass 63.9 (7.8) to 62.9 (8) kg |
— | 135 (8) to 130 (10) | — | — | — |
| Control | BMI 25.4 (3.0) to 25.1 (3.0) kg/m2 Body mass 59 (9.7) to 58.6 (10.1) kg |
— | 128 (14) to 125 (15) | — | — | — | |
| Resistance training | |||||||
| Copeland et al.66 (2004) Canada | IGF-1 | ||||||
| PA | 25.7 (2.7) to 25.6 (2.6) kg/m2 | 39.9 (2.6) to 40.4 (2.8) | — | — | 220 (175) to 203 (158) ng/mL ∗∗vs control |
401 (154) to 392 (120) nmol/L ∗∗within group |
|
| Control | 31.6 (7.6) to 31.6 (7.7) kg/m2 | 41.1 (5.5) to 40.9 (4.9) | — | — | 110 (33) to 109 (42) ng/mL | 406 (121) to 383 (102) nmol/L | |
| Figueroa et al.53 (2003) USA | PA | — | Δ –0.9% ∗within group |
— | — | NR | NR |
| Control | — | Δ +1.0% | — | — | — | — | |
| Nunes et al.61 (2019) Brazil | IGF-1 | ||||||
| Low (3 sets) | — | — | — | — | 114 (85–142) to 122 (81–163) ng/mL; +6.7% | 482 (328–639) to 630 (450–811) nmol/L; +73% | |
| High (6 sets) | — | — | — | — | 134 (111–157) to 142 (114–171) ng/mL; +7.3% | 518 (391–646) to 611 (442–779) nmol/L; +36.8% | |
| Control | — | — | — | — | 138 (104–172) to 135 (101–169) ng/mL; +4.1% | 451 (292–610) to 543 (263–823) nmol/L; +16.8% | |
| Orsatti et al.62 (2008) Brazil | IGF-1 | ||||||
| PA | 28.8 (4.5) to 29.4 (4.8) kg/m2 | 35.6 (8.1) to 34.9 (8.3) | — | — | 149 (71) to 205 (82) ng/mL ∗∗vs control ∗within group |
433 (124) to 447 (163) nmol/L | |
| Control | 27.6 (5.1) to 27.1 (5.1) kg/m2 | 32.6 (7.8) to 31.5 (7.8) | — | — | 129 (45) to 113 (53) ng/mL | 339 (127) to 356 (127) nmol/L | |
| Son et al.63 (2020) South Korea | IGF-1 | ||||||
| PA | 26.5 (1.0) to 26.0 (0.9) kg/m2 ∗vs control ∗within group |
35.6 (2.9) to 34.0 (3.0) ∗vs control ∗within group |
139 (3) to 136 (4) ∗vs control ∗within group |
— | 145 (19) to 151 (18) ng/mL ∗vs control ∗within group |
— | |
| Control | 26.9 (1.0) to 26.8 (1.1) kg/m2 | 35.8 (3.2) to 36.1 (2.9) | 138 (5) to 138 (4) | — | 144 (15) to 140 (15) ng/mL | — | |
| Ward et al.65 (2020) Sweden | PA | 28.1 (3.8) to 27.9 (3.9) kg/m2 | — | — | — | — | — |
| Control | 26.7 (3.6) to 26.8 (3.8) kg/m2 | — | — | — | — | — | |
| Combined aerobic and resistance training | |||||||
| Gonzalo-Encabo et al.57 (2020) Spain | Endurance (aerobic) | Δ –1.9 % ∗vs control |
— | — | Δ +13% ∗∗within group |
— | Δ –17.5% ∗within group |
| Concurrent (aerobic/ resistance) | Δ –1.4 % ∗vs control |
— | — | Δ +12% ∗∗∗within group |
— | NR | |
| Control | Δ –1.0 % | — | — | NR | — | NR | |
| Monninkhof et al.60 (2009) The Netherlands | PA | - | 39.8 to 38.9; –2.2% | — | — | — | — |
| Control | - | 40.9 to 40.9; 0% | — | — | — | — | |
| van Gemert et al.64 (2015) The Netherlands | PA | 29.0 to 27.0 kg/m2; –6.8% ∗∗∗vs control |
43.9 to 39.8; –9.3% ∗∗∗vs control ∗∗∗vs diet |
— | 1.8 to 1.9 L/min; +6.7% ∗∗vs control ∗∗∗vs diet |
— | — |
| Diet | 29.2 to 27.5; –6.1% ∗∗∗vs control |
44 to 41.5; –5.7% ∗∗∗vs control |
— | 1.7 to 1.7L/min; –2.5% | — | — | |
| Control | 29.3 to 29.4 kg/m2; +0.1% | 43.5 to 43.7; +0.5% | — | 1.8 to 1.7 L/min; –4.5% | — | — | |
Data are presented as mean (95% confidence interval or standard deviation) unless otherwise specified;
BMI, body mass index; BP, blood pressure; IGF-1, insulin-like growth factor-1; NR, not reported.
P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001.
Insulin was analyzed, although only 2 studies reported on it, owing to the well-established relationship between androgens and insulin.
Physical activity and body composition
Almost all aerobic PA54, 55, 56, 58 and combined aerobic and resistance57,64 programs showed significant decreases in BMI and/or body fat. Few resistance programs influenced BMI64 or body fat.53,63 The only aerobic program that did not yield significant decreases in BMI or body fat had the lowest exercise intensity (60% HRR).67
Inconsistencies were observed regarding the role of body composition in mediating the effects of PA on DHEA-S concentrations. Son et al. showed a moderate, negative correlation between the change in % body fat and DHEA-S concentrations (r = –0.4, P < 0.05) following a 12-week resistance-band PA program.63 Gonzalo-Encabo et al. found a significant increase in SHBG (+21%) and decrease in DHEA-S (–13%; P < 0.05) in women who lost more than 2 kg of fat mass in a combined aerobic and resistance program.57 Of note, among participants who lost more than 2% of body fat, those participating in a 12-month moderate-intensity aerobic PA program, compared to those in a control group engaging in stretching, experienced greater reductions in androstenedione (–17% vs –9%), DHEA (–20% vs –8%) and DHEA-S (–22% vs 3%), although this difference was not statistically significant.59 Future research should explore whether PA participation enhances reductions in androstenedione, DHEA, and DHEA-S concentrations independent of changes in body composition.59 No other studies reported on this relationship, so drawing conclusions is challenging.
Changes in testosterone concentrations following PA were more pronounced in participants who lost body fat or body mass.57,59,60 In women who lost more than 2% body fat, an aerobic PA program significantly reduced total testosterone at 3 months (PA: –10%; control: –1.6%; P < 0.005) and 12 months (PA: –8%; control: –3.6%; P < 0.02), compared to the concentration in controls (P < 0.001).60 Similarly, after 4 months of combined aerobic and resistance training, the reductions in total testosterone were significant in women who lost more than 2% body fat (PA: –12.9%; control: +0.2%; P = 0.005), but not in the whole sample of participants (PA: –3.8%; control: +2.4%; P = 0.14).60 These 2 studies had similar sample sizes (n = 87 and n = 96, respectively) and target intensity (moderate-to-vigorous PA), and both incorporated aerobic training into the intervention.59,60 Further, significant decreases in free testosterone concentration were detected in those participating in 12 months of aerobic exercise and who lost more than 2% of their body fat, compared to concentrations in controls.59,60
Decreases in body mass, body fat, and waist circumference were significantly associated with increases in SHBG following aerobic PA (P < 0.05).54,55,58 For instance, regardless of the minutes of aerobic PA per week (150 vs 250 min/wk), decreases in body mass were associated with increases in SHBG (r = –0.29, P < 0.0001).55 A greater decrease in free testosterone and a greater increase in SHBG (P < 0.001) were observed following aerobic PA and caloric restriction, compared to those following aerobic PA alone.54 These findings suggest that fat loss achieved through exercise strengthens the association of free testosterone, total testosterone, and SHBG concentrations with PA.
Physical activity and other cardiovascular health indicators
Other CVD health indicators examined included CRF, systolic blood pressure, fasting insulin, IGF-1, and cortisol. The included studies reported significant improvements in CRF following all types of PA interventions, compared to CRF in controls.54,56,57,64 Friedenreich et al. found that improvements in CRF were positively correlated with increases in SHBG (rs = 0.11, P < 0.04)56; no other significant associations between CRF and androgens were reported. Systolic blood pressure was rarely measured; this may be due to sampling bias inherent in the included RCTs, which enrolled predominantly healthy women. Small reductions of 3 to 8 mm Hg were reported in systolic blood pressure following several PA programs.58,63 Blood biomarkers, including fasting insulin, IGF-1, and cortisol, were measured in several studies, likely due to evidence showing their bidirectional relationship with androgens.70 A combination of PA and caloric restriction enhanced the reductions in fasting insulin (PA only: –8%; PA + diet: –27%).54 Kim and Kim showed that fasting insulin decreased following aerobic PA, yet it increased in the control group.58 Following PA, inconsistent findings were observed for IGF-1 and cortisol. PA interventions decreased,66 increased,62,63 or showed no significant changes in61 IGF-1. Similarly, 2 studies of different PA modalities reported decreases in cortisol (combined aerobic and resistance57 vs resistance only66), whereas 2 reported no changes.61,62 No studies directly examined the associations between insulin, IGF-1, or cortisol, and androgens.
Discussion
Summary of findings
This review explores the relationships among PA, androgen concentrations, and cardiovascular health indicators in postmenopausal women. The influence of PA on androgen concentration was varied. Trends across studies demonstrated no changes in androstenedione concentrations, and data is conflicting for DHEA and DHEA-S. The direction of change in total testosterone concentration appeared to depend on the type of PA implemented, with some reductions following aerobic PA programs and some increases following resistance training. Reductions in free testosterone followed most aerobic PA programs. Nearly all studies reported an increase in SHBG following a PA program, regardless of the type of PA. A combination of diet and/or fat loss enhanced the influence of PA interventions on DHEA-S, free and total testosterone, and SHBG concentrations; high-intensity aerobic PA interventions had the greatest influence on body composition.
Data on the association between cardiovascular health indicators and changes in androgen concentrations following PA interventions were sparsely reported and conflicting. Thus, we had only limited ability to discuss the potential mechanisms underpinning androgen concentrations and cardiovascular health indicators following PA in postmenopausal women. For all PA modalities, studies showed modest reductions in total body mass, fat mass, and BMI, increases in CRF, and inconsistent findings regarding IGF-1 and cortisol changes. Systolic blood pressure was rarely measured, and fasting insulin decreased in response to combined PA and diet interventions. The mechanisms that mediate the decrease in testosterone concentrations following aerobic training and the increase following resistance training should be further explored. Physiological changes related to increases in lean body mass and decreases in body fat may play a role.71
It is possible that the relationship between PA and androgens and PA and CVD exist independently of each other, but are both impacted by PA and weight loss. Women who are normal weight or underweight may have less opportunity for sex hormone improvements, as the degree of change may depend on the amount of fat mass lost. However, if the effects of fat loss from caloric restriction and PA are similar, exercise would be recommended as the preferred treatment, to avoid a decrease in muscle mass while simultaneously improving cardiovascular risk factors in postmenopausal women.
Strengths and limitations
Merits of our study included the use of evidence from RCTs, the highest quality of evidence, a robust search, and the inclusion of all androgens and traditional cardiovascular health indicators. This review also incorporated androgen concentrations adjusted for changes in BMI and/or fat loss, as appropriate, to investigate the complex relationship among adiposity, androgens, and SHBG. Limitations included a lack of homogeneity in reported androgen concentrations and cardiovascular health indicators in the previous literature, limiting a more comprehensive analysis. The relationship between changes in the ratio of estrogen to testosterone concentrations following PA was not reported in the included studies and was thus excluded from our review. We also did not report on cardiovascular health indicators that were not present in more than 3 studies (eg, waist circumference). Such additions may provide further insight into the association between androgens and cardiovascular health in postmenopausal women.
Future directions
The results of this review were heterogeneous with regard to sample sizes, androgens included, units of androgens used for analysis, and the frequency, duration, modality, and intensity of PA interventions. Most studies did not report similar cardiovascular health indicators or adjust for changes in adiposity, which are needed in future research. Specific suggestions for developing literature in this field include conducting RCTs with larger sample sizes of postmenopausal women to evaluate changes in androgens in participants with various BMI, PA levels, and differing health status. We recommend that future PA research include a comprehensive panel of all endogenous serum androgens and SHBG to standardize the evaluation and interpretation of the role of PA in modulating androgens and cardiovascular health. No “high” or “low” physiological androgen range has been defined in postmenopausal women,35 so future studies should aim to define this level to create a baseline value. Moreover, studies should explore changes in FAI, androstenediol, and dihydrotestosterone, as these were not reported in the included trials and thus could not be examined. RCTs comparing diverse PA modalities (eg, yoga, walking, endurance training, resistance training), intensities (eg, low, moderate, vigorous), and durations are necessary to delineate the most effective PA intervention in modulating the androgen environment. When exploring the relationship between androgens and PA, studies should investigate how cardiovascular health indicators impact this association, and adjust for changes in BMI and/or fat loss to explore the relative impact of adiposity and SHBG on resulting changes in androgen concentrations.
Conclusion
Our review has shown that PA interventions may alter some androgen concentrations (free and total testosterone, SHBG) in postmenopausal women. Fat loss influences the effect of PA on androgens, but the synergistic role of PA and androgens on cardiovascular health merits further examination. Evidence exploring the relationship between changes in androgens and cardiovascular health indicators was scarce and inconsistent. More research is needed to clarify the relationships among CVD, PA, and androgens in postmenopausal women. Future studies should focus on incorporating cardiovascular health indicators, standardizing the measurement of androgens to allow for direct comparison, including more diverse PA modalities, and accounting for the role of adiposity in androgen concentrations, PA, and cardiovascular health.
Acknowledgements
The authors acknowledge Laura Salisbury for the creation of the graphical abstract.
Funding Sources
S. V.-A. was supported by a University of Ottawa Heart Institute Research Scholarship. The other authors have no funding sources to declare.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: The research reported has adhered to the relevant ethical guidelines.
See page 69 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2022.10.008.
Supplementary Material
References
- 1.Garcia M., Mulvagh S.L., Merz C.N., Buring J.E., Manson J.E. Cardiovascular disease in women: clinical perspectives. Circ Res. 2016;118:1273–1293. doi: 10.1161/CIRCRESAHA.116.307547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mulvagh S.L., Mullen K.-A., Nerenberg K.A., et al. The Canadian women’s heart health alliance atlas on the epidemiology, diagnosis, and management of cardiovascular disease in women—Chapter 4: Sex- and gender-unique disparities: CVD across the lifespan of a woman. CJC Open. 2021;4:115–132. doi: 10.1016/j.cjco.2021.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis S.R., Lambrinoudaki I., Lumsden M., et al. Menopause. Nat Rev Dis Primers. 2015;1 doi: 10.1038/nrdp.2015.4. [DOI] [PubMed] [Google Scholar]
- 4.El Khoudary S.R., Aggarwal B., Beckie T.M., et al. Menopause transition and cardiovascular disease risk: implications for timing of early prevention: a scientific statement from the American Heart Association. Circulation. 2020;142:e506–e532. doi: 10.1161/CIR.0000000000000912. [DOI] [PubMed] [Google Scholar]
- 5.Labrie F. Intracrinology and menopause: the science describing the cell-specific intracellular formation of estrogens and androgens from DHEA and their strictly local action and inactivation in peripheral tissues. Menopause. 2019;26:220–224. doi: 10.1097/GME.0000000000001177. [DOI] [PubMed] [Google Scholar]
- 6.Vitale C., Fini M., Speziale G., Chierchia S. Gender differences in the cardiovascular effects of sex hormones. Fundam Clin Pharmacol. 2010;24:675–685. doi: 10.1111/j.1472-8206.2010.00817.x. [DOI] [PubMed] [Google Scholar]
- 7.Montalcini T., Gorgone G., Gazzaruso C., et al. Endogenous testosterone and endothelial function in postmenopausal women. Coron Artery Dis. 2007;18:9–13. doi: 10.1097/01.mca.0000236290.79306.d1. [DOI] [PubMed] [Google Scholar]
- 8.Son M.K., Lim N.-K., Lim J.-Y., et al. Difference in blood pressure between early and late menopausal transition was significant in healthy Korean women. BMC Women's Health. 2015;15:64. doi: 10.1186/s12905-015-0219-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vitale C., Mendelsohn M.E., Rosano G.M.C. Gender differences in the cardiovascular effect of sex hormones. Nat Rev Cardiol. 2009;6:532–542. doi: 10.1038/nrcardio.2009.105. [DOI] [PubMed] [Google Scholar]
- 10.Spoletini I., Vitale C., Pelliccia F., Fossati C., Rosano G.M. Androgens and cardiovascular disease in postmenopausal women: a systematic review. Climacteric. 2014;17:625–634. doi: 10.3109/13697137.2014.887669. [DOI] [PubMed] [Google Scholar]
- 11.Abramson B.L., Black D.R., Christakis M.K., Fortier M., Wolfman W. Guideline No. 422e: menopause and cardiovascular disease. J Obstet Gynaecol Can. 2021;43:1438–1443.e1431. doi: 10.1016/j.jogc.2021.09.010. [DOI] [PubMed] [Google Scholar]
- 12.Davis S.R., Baber R., Panay N., et al. Global consensus position statement on the use of testosterone therapy for women. J Clin Endocrinol Metab. 2019;104:4660–4666. doi: 10.1210/jc.2019-01603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heart and Stroke Foundation of Canada. Ms.Understood: heart and stroke 2018 heart report. Available at https://www.heartandstroke.ca/-/media/pdf-files/canada/2018-heart-month/hs_2018-heart-report_en.ashx. Accessed August 1, 2021.
- 14.Mendoza N., De Teresa C., Cano A., et al. Benefits of physical exercise in postmenopausal women. Maturitas. 2016;93:83–88. doi: 10.1016/j.maturitas.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 15.Bucciarelli V., Bianco F., Mucedola F., et al. Effect of adherence to physical exercise on cardiometabolic profile in postmenopausal women. Int J Environ Res Public Health. 2021;18:656. doi: 10.3390/ijerph18020656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalo-Encabo P., McNeil J., Pérez-López A., et al. Dose-response effects of aerobic exercise on adiposity markers in postmenopausal women: pooled analyses from two randomized controlled trials. Int J Obes. 2021;45:1298–1309. doi: 10.1038/s41366-021-00799-1. [DOI] [PubMed] [Google Scholar]
- 17.Haddock B.L., Hopp H.P., Mason J.J., Blix G., Blair S.N. Cardiorespiratory fitness and cardiovascular disease risk factors in postmenopausal women. Med Sci Sports Exerc. 1998;30:893–898. doi: 10.1097/00005768-199806000-00018. [DOI] [PubMed] [Google Scholar]
- 18.Bull F.C., Al-Ansari S.S., Biddle S., et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. 2020;54:1451. doi: 10.1136/bjsports-2020-102955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.CSEP Physiology. 24-hour movement guideline for adults (18-64 years and 65+ (years) 2021. https://csepguidelines.ca/guidelines/adults-18-64/. Accessed August 1, 2021.
- 20.Schiller J.S., Lucas J.W., Ward B.W., Peregoy J.A. Summary health statistics for U.S. adults: National Health Interview Survey, 2010. Vital Health Stat. 2012;10(252):1–207. [PubMed] [Google Scholar]
- 21.Clarke J., Colley R., Janssen I., Tremblay M.S. Accelerometer-measured moderate-to-vigorous physical activity of Canadian adults, 2007 to 2017. Health Rep. 2019;30:3–10. doi: 10.25318/82-003-x201900800001-eng. [DOI] [PubMed] [Google Scholar]
- 22.Kim K., Choi S., Hwang S.E., et al. Changes in exercise frequency and cardiovascular outcomes in older adults. Eur Heart J. 2020;41:1490–1499. doi: 10.1093/eurheartj/ehz768. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalo-Encabo P., Valadés D., De Cos A.I., García-Honduvilla N., Pérez-López A. Effects of exercise on circulating levels of sex hormones in overweight and obese postmenopausal women: a systematic review. Sci Sport. 2019;34:199–207. [Google Scholar]
- 24.Shele G., Genkil J., Speelman D. A systematic review of the effects of exercise on hormones in women with polycystic ovary syndrome. J Funct Morphol Kinesiol. 2020;5:35. doi: 10.3390/jfmk5020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Enea C., Boisseau N., Fargeas-Gluck M.A., Diaz V., Dugué B. Circulating androgens in women. Sports Med. 2011;41:1–15. doi: 10.2165/11536920-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 26.Davis S.R., Wahlin-Jacobsen S. Testosterone in women—the clinical significance. Lancet Diabetes Endocrinol. 2015;3:980–992. doi: 10.1016/S2213-8587(15)00284-3. [DOI] [PubMed] [Google Scholar]
- 27.Squiers G.T., McLellan M.A., Ilinykh A., et al. Cardiac cellularity is dependent upon biological sex and is regulated by gonadal hormones. Cardiovasc Res. 2020;117:2252–2262. doi: 10.1093/cvr/cvaa265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shifren J.L., Davis S.R. Androgens in postmenopausal women: a review. Menopause. 2017;24:970–979. doi: 10.1097/GME.0000000000000903. [DOI] [PubMed] [Google Scholar]
- 29.Brzozowska M., Lewiński A. Changes of androgens levels in menopausal women. Prz Menopauzalny. 2020;19:151–154. doi: 10.5114/pm.2020.101941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tulandi T., Gelfand M. CRC Press; London, England: 2005. Androgens and Reproductive Aging. [Google Scholar]
- 31.Gupta M.K., Chia S.-Y. In: Clinical Reproductive Medicine and Surgery: A Practical Guide. Falcone T., Hurd W.W., editors. Springer New York; New York: 2013. Ovarian hormones: structure, biosynthesis, function, mechanism of action, and laboratory diagnosis; pp. 1–30. [Google Scholar]
- 32.Hammond G.L. Plasma steroid-binding proteins: primary gatekeepers of steroid hormone action. J Endocrinol. 2016;230:R13–R25. doi: 10.1530/JOE-16-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Georgiopoulos G., Kontogiannis C., Lambrinoudaki I., Rizos D., Stamatelopoulos K. Free androgen index as a biomarker of increased cardiovascular risk in postmenopausal women. J Am Coll Cardiol. 2018;72:1986. doi: 10.1016/j.jacc.2018.07.082. [DOI] [PubMed] [Google Scholar]
- 34.Labrie F., Luu-The V., Labrie C., et al. Endocrine and intracrine sources of androgens in women: inhibition of breast cancer and other roles of androgens and their precursor dehydroepiandrosterone. Endocr Rev. 2003;24:152–182. doi: 10.1210/er.2001-0031. [DOI] [PubMed] [Google Scholar]
- 35.Davison S.L., Bell R., Donath S., Montalto J.G., Davis S.R. Androgen levels in adult females: changes with age, menopause, and oophorectomy. J Clin Endocrinol Metab. 2005;90:3847–3853. doi: 10.1210/jc.2005-0212. [DOI] [PubMed] [Google Scholar]
- 36.Wierman M.E., Arlt W., Basson R., et al. Androgen therapy in women: a reappraisal: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2014;99:3489–3510. doi: 10.1210/jc.2014-2260. [DOI] [PubMed] [Google Scholar]
- 37.Zumoff B., Strain G.W., Miller L.K., Rosner W. Twenty-four-hour mean plasma testosterone concentration declines with age in normal premenopausal women. J Clin Endocrinol Metab. 1995;80:1429–1430. doi: 10.1210/jcem.80.4.7714119. [DOI] [PubMed] [Google Scholar]
- 38.Labrie F., Bélanger A., Cusan L., Gomez J.L., Candas B. Marked decline in serum concentrations of adrenal C19 sex steroid precursors and conjugated androgen metabolites during aging. J Clin Endocrinol Metab. 1997;82:2396–2402. doi: 10.1210/jcem.82.8.4160. [DOI] [PubMed] [Google Scholar]
- 39.Sowers M.F.R., Zheng H., McConnell D., et al. Testosterone, sex hormone-binding globulin and free androgen index among adult women: chronological and ovarian aging. Hum Reprod. 2009;24:2276–2285. doi: 10.1093/humrep/dep209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benn M., Voss Sidsel S., Holmegard Haya N., et al. Extreme concentrations of endogenous sex hormones, ischemic heart disease, and death in women. Arterioscler Thromb Vasc Biol. 2015;35:471–477. doi: 10.1161/ATVBAHA.114.304821. [DOI] [PubMed] [Google Scholar]
- 41.Zhao D., Guallar E., Ouyang P., et al. Endogenous sex hormones and incident cardiovascular disease in post-menopausal women. J Am Coll Cardiol. 2018;71:2555–2566. doi: 10.1016/j.jacc.2018.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sievers C., Klotsche J., Pieper L., et al. Low testosterone levels predict all-cause mortality and cardiovascular events in women: a prospective cohort study in German primary care patients. Eur J Endocrinol. 2010;163:699–708. doi: 10.1530/EJE-10-0307. [DOI] [PubMed] [Google Scholar]
- 43.Laughlin G.A., Goodell V., Barrett-Connor E. Extremes of endogenous testosterone are associated with increased risk of incident coronary events in older women. J Clin Endocrinol Metab. 2010;95:740–747. doi: 10.1210/jc.2009-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manolakou P., Angelopoulou R., Bakoyiannis C., Bastounis E. The effects of endogenous and exogenous androgens on cardiovascular disease risk factors and progression. Reprod Biol Endocrinol. 2009;7:44. doi: 10.1186/1477-7827-7-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Creatsa M., Armeni E., Stamatelopoulos K., et al. Circulating androgen levels are associated with subclinical atherosclerosis and arterial stiffness in healthy recently menopausal women. Metabolism. 2012;61:193–201. doi: 10.1016/j.metabol.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 46.Talbott E.O., Zborowski J., Rager J., Stragand J.R. Is there an independent effect of polycystic ovary syndrome (PCOS) and menopause on the prevalence of subclinical atherosclerosis in middle aged women? Vasc Health Risk Manag. 2008;4:453–462. doi: 10.2147/vhrm.s1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huxley R., Barzi F., Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. BMJ. 2006;332:73–76. doi: 10.1136/bmj.38678.389583.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ding E.L., Song Y., Malik V.S., Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2006;295:1288–1299. doi: 10.1001/jama.295.11.1288. [DOI] [PubMed] [Google Scholar]
- 49.Davis S.R., Robinson P.J., Moufarege A., Bell R.J. The contribution of SHBG to the variation in HOMA-IR is not dependent on endogenous oestrogen or androgen levels in postmenopausal women. Clin Endocrinol. 2012;77:541–547. doi: 10.1111/j.1365-2265.2011.04301.x. [DOI] [PubMed] [Google Scholar]
- 50.Bianchi V.E., Bresciani E., Meanti R., et al. The role of androgens in women's health and wellbeing. Pharmacol Res. 2021;171 doi: 10.1016/j.phrs.2021.105758. [DOI] [PubMed] [Google Scholar]
- 51.Jianshu C., Qiongying W., Ying P., et al. Association of free androgen index and sex hormone–binding globulin and left ventricular hypertrophy in postmenopausal hypertensive women. J Clin Hypertens. 2021;23:1413–1419. doi: 10.1111/jch.14301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kostakis E.K., Gkioni L.N., Macut D., Mastorakos G. Androgens in menopausal women: not only polycystic ovary syndrome. Front Horm Res. 2019;53:135–161. doi: 10.1159/000494909. [DOI] [PubMed] [Google Scholar]
- 53.Figueroa A., Going S., Milliken L., et al. Effects of exercise training and hormone replacement therapy on lean and fat mass in postmenopausal women. J Gerontol. Ser A, Biol Sci Med Sci. 2003;58:266–270. doi: 10.1093/gerona/58.3.m266. [DOI] [PubMed] [Google Scholar]
- 54.Campbell K.L., Foster-Schubert K.E., Alfano C.M., et al. Reduced-calorie dietary weight loss, exercise, and sex hormones in postmenopausal women: randomized controlled trial. J Clin Oncol. 2012;30:2314–2326. doi: 10.1200/JCO.2011.37.9792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Friedenreich C.M., Neilson H.K., Wang Q., et al. Effects of exercise dose on endogenous estrogens in postmenopausal women: a randomized trial. Endocr Relat Cancer. 2015;22:863. doi: 10.1530/ERC-15-0243. [DOI] [PubMed] [Google Scholar]
- 56.Friedenreich C.M., Woolcott C.G., McTiernan A., et al. Alberta physical activity and breast cancer prevention trial: sex hormone changes in a year-long exercise intervention among postmenopausal women. J Clin Oncol. 2010;28:1458–1466. doi: 10.1200/JCO.2009.24.9557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gonzalo-Encabo P., Valadés D., García-Honduvilla N., et al. Exercise type and fat mass loss regulate breast cancer-related sex hormones in obese and overweight postmenopausal women. Eur J Appl Physiol. 2020;120:1277–1287. doi: 10.1007/s00421-020-04361-1. [DOI] [PubMed] [Google Scholar]
- 58.Kim J.W., Kim D.Y. Effects of aerobic exercise training on serum sex hormone binding globulin, body fat index, and metabolic syndrome factors in obese postmenopausal women. Metab Syndr Relat Disord. 2012;10:452–457. doi: 10.1089/met.2012.0036. [DOI] [PubMed] [Google Scholar]
- 59.McTiernan A., Tworoger S.S., Rajan K.B., et al. Effect of exercise on serum androgens in postmenopausal aomen: a 12-month randomized clinical trial. Cancer Epidem Biomar. 2004;13:1099. [PubMed] [Google Scholar]
- 60.Monninkhof E.M., Velthuis M.J., Peeters P.H., Twisk J.W., Schuit A.J. Effect of exercise on postmenopausal sex hormone levels and role of body fat: a randomized controlled trial. J Clin Oncol. 2009;27:4492–4499. doi: 10.1200/JCO.2008.19.7459. [DOI] [PubMed] [Google Scholar]
- 61.Nunes P.R.P., Barcelos L.C., Oliveira A.A., et al. Muscular strength adaptations and hormonal responses after two different multiple-set protocols of resistance training in postmenopausal women. J Strength Cond Res. 2019;33:1276–1285. doi: 10.1519/JSC.0000000000001788. [DOI] [PubMed] [Google Scholar]
- 62.Orsatti F.L., Nahas E.A.P., Maesta N., Nahas-Neto J., Burini R.C. Plasma hormones, muscle mass and strength in resistance-trained postmenopausal women. Maturitas. 2008;59:394–404. doi: 10.1016/j.maturitas.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 63.Son W.M., Pekas E.J., Park S.Y. Twelve weeks of resistance band exercise training improves age-associated hormonal decline, blood pressure, and body composition in postmenopausal women with stage 1 hypertension: a randomized clinical trial. Menopause. 2020;27:199–207. doi: 10.1097/GME.0000000000001444. [DOI] [PubMed] [Google Scholar]
- 64.van Gemert W.A., Schuit A.J., van der Palen J., et al. Effect of weight loss, with or without exercise, on body composition and sex hormones in postmenopausal women: the SHAPE-2 trial. Breast Cancer Res. 2015;17:120. doi: 10.1186/s13058-015-0633-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ward L.J., Nilsson S., Hammar M., et al. Resistance training decreases plasma levels of adipokines in postmenopausal women. Sci Rep. 2020;10 doi: 10.1038/s41598-020-76901-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Copeland J.L., Tremblay M.S. Effect of HRT on hormone responses to resistance exercise in post-menopausal women. Maturitas. 2004;48:360–371. doi: 10.1016/j.maturitas.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 67.Yoo E.J., Jun T.W., Hawkins S.A. The effects of a walking exercise program on fall-related fitness, bone metabolism, and fall-related psychological factors in elderly women. Res Sports Med. 2010;18:236–250. doi: 10.1080/15438627.2010.510098. [DOI] [PubMed] [Google Scholar]
- 68.Duggan C., Tapsoba J.D., Stanczyk F., et al. Long-term weight loss maintenance, sex steroid hormones, and sex hormone-binding globulin. Menopause. 2019;26:417–422. doi: 10.1097/GME.0000000000001250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Friedenreich C.M., Wang Q., Yasui Y., et al. Long-term effects of moderate versus high durations of aerobic exercise on biomarkers of breast cancer risk: follow-up to a randomized controlled trial. Cancer Epidemiol Biomarkers Prev. 2019;28:1725–1734. doi: 10.1158/1055-9965.EPI-19-0523. [DOI] [PubMed] [Google Scholar]
- 70.Baptiste C.G., Battista M.-C., Trottier A., Baillargeon J.-P. Insulin and hyperandrogenism in women with polycystic ovary syndrome. J Steroid Biochem. 2010;122:42–52. doi: 10.1016/j.jsbmb.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Marx J.O., Ratamess N.A., Nindl B.C., et al. Low-volume circuit versus high-volume periodized resistance training in women. Med Sci Sports Exerc. 2001;33:635–643. doi: 10.1097/00005768-200104000-00019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.