Abstract
Exposure to metals may contribute to the development of metabolic syndrome (MetS); however, evidence from midlife women who are at greater risk of cardiometabolic disease is limited. We assessed the associations of 15 urinary metal concentrations with incident MetS in a prospective cohort of midlife women in the United States. The study population included 947 White, Black, Chinese and Japanese women, aged 45–56 years, free of MetS at baseline (1999–2000), who participated in the Study of Women’s Health Across the Nation Multi-Pollutant Study. Fifteen metals were detected in almost all participants urine samples using inductively coupled plasma mass spectrometry at the baseline. Incident MetS was identified annually through 2017 as having at least three of the following five components: high blood pressure, impaired fasting glucose, abdominal obesity, high triglycerides, and poor high-density lipoprotein cholesterol. We used the Cox proportional hazards models to investigate the associations between individual metals and MetS incidence. The adjusted hazard ratios (HR) (95% CI) for MetS in associations with each doubling of urinary metal concentration were 1.14 (1.08, 1.23) for arsenic, 1.14 (1.01, 1.29) for cobalt, and 1.20 (1.06, 1.37) for zinc. We further evaluated the associations between metal mixtures and MetS using the elastic net penalized Cox model and summarized the results into the environmental risk score (ERS). Arsenic, barium, cobalt, copper, nickel, antimony, thallium, and zinc had positive weights, and cadmium, cesium, mercury, molybdenum, lead, and tin had negative weights in the construction of the ERS. The adjusted HR of MetS comparing 75th vs. 25th percentiles of the ERS was 1.45 (1.13, 1.87). These findings support the view that that arsenic, cobalt, zinc, as well as metal mixtures, might influence the risks of incident MetS in midlife women.
Keywords: metals, arsenic, cobalt, zinc, metabolic syndrome, women
1. Introduction
Cardiometabolic disorders including cardiovascular disease and type 2 diabetes are major public health issues, accounting for around 30% of deaths in the United States (U.S.) (Heron, 2019). Metabolic syndrome (MetS), a collection of interconnected cardiometabolic risk factors that includes high blood pressure, impaired fasting glucose, abdominal obesity, and dyslipidemia, is frequently utilized in clinical practice as a predictor of diabetes, cardiovascular disease, and mortality (Alberti et al., 2009). Midlife women had a substantially higher risk of MetS than women in earlier life stages (Beltrán-Sánchez et al., 2013), contributing to more pronounced health effects, including cardiovascular disease and all-cause mortality (Lin et al., 2010). A greater knowledge of the risk factors of MetS is critical for preventing its development in midlife women and promoting health in later life. Increased caloric consumption and lack of physical activity have been recognized as major factors to the MetS (Grundy, 2016). Environmental exposures, including metals, may also have a potential role in development of cardiometabolic disorders, according to growing research (Planchart et al., 2018; Wang et al., 2020a).
Metals and metalloids (for convenience, referred to collectively as metals) are broadly distributed in the environment with common sources of smoking, food, drinking water, consumer goods, and air (Tchounwou et al., 2012; Wang et al., 2019a). Biological evidence suggests that some metals may impact MetS. For example, arsenic, cadmium, mercury, and lead are all oxidative stress inducers, and their accumulation in various tissues has been shown to lead to elevated blood pressure and lipid peroxidation (Han et al., 2003; Jomova and Valko, 2011; Lu et al., 2011; Perry et al., 1979; Preuss et al., 1994; Wakita, 1987; Yang et al., 2007). Certain metals may also act as endocrine disruptors. For example, arsenic has been found to disrupt insulin function through decreasing insulin-stimulated glucose absorption in adipocytes and skeletal muscle cells and modifying gene expression of a range of glucose homeostasis-related factors (Walton et al., 2004). In animal and in vitro studies, manganese and zinc have been shown to increase testosterone and estradiol (Denier et al., 2009; Lee et al., 2006), which in turn may increase the risk of metabolic disorders (Ding et al., 2007). In rats, exposure to copper was observed to impair hepatic and renal functions and lipid metabolism and to disrupt thyroid hormones, all of which are linked to metabolic disorders (Su et al., 2017). Toxicological evidence on other metals is scant. However, limited evidence indicates the potential metabolic toxicity of these metals: barium (ATSDR, 2007), cobalt (ATSDR, 2004), molybdenum (ATSDR, 2017a), nickel (ATSDR, 2005a), antimony (ATSDR, 2017b), tin (ATSDR, 2005b), and thallium (ATSDR, 1992) may impair liver function and lead to metabolic disorders. Findings on metals and MetS from population-based studies are relatively limited and mixed (Ayoub et al., 2021; Bulka et al., 2019; Guo et al., 2019; L. Liu et al., 2022; Lo et al., 2021; Ma et al., 2020; Moon, 2014; Park and Oh, 2021; Tinkov et al., 2017), though evidence from epidemiologic studies of individual components of MetS suggests a contributing role of metals (Alissa and Ferns, 2011; Buhari et al., 2020; Wang et al., 2020b; X. Wang et al., 2018). Moreover, even though people are routinely exposed to metal mixtures (Wang et al., 2019a), the majority of studies have concentrated on single metals, possibly attributed to the data unavailability and statistical challenges posed by the complex correlations between mixture components (Park et al., 2017; Wang et al., 2019b; X. Wang et al., 2018). Finally, women become more susceptible to cardiometabolic disorders in midlife due to a shift in sex hormone profiles (Polotsky and Polotsky, 2010; Stuenkel, 2017). Menopause is also linked with an increased burden of oxidative stress caused by decreased estrogen levels (Sánchez-Rodríguez et al., 2012). Given this increased susceptibility, exposure to metals could be a risk factor of MetS, especially for women in midlife. Nonetheless, to our knowledge, no study has been conducted on the impact of metals on the development of MetS in midlife women.
Given the inconsistent findings and the paucity of studies on some metals and their metabolic toxicity, we conducted exploratory analysis examining the associations of 15 urinary metal concentrations with the MetS incidence and its components in a prospective cohort of midlife women representing multiple racial/ethnic groups, using the data from the Study of Women’s Health Across the Nation (SWAN). Additionally, we developed an environmental risk score (ERS) (Park et al., 2017, 2014; Wang et al., 2020b, 2019b; X. Wang et al., 2018) to assess the relationship between metal mixtures and MetS.
2. Material and methods
2.1. Study population
We utilized data from SWAN, which is a longitudinal, multi-site, multi-racial/ethnic, community-based cohort of 3,302 midlife women designed to investigate physiological and psychosocial changes during the menopausal transition. During 1996 and 1997, 3,302 women were recruited from seven study sites across the United States. This study included White women from all study sites and women from one of the following racial/ethnic groups, including, Black women from Boston, MA, Pittsburgh, PA, southeast Michigan, MI, and Chicago, IL; Hispanic women from Newark, NJ; Chinese women from Oakland, CA; and Japanese women from Los Angeles, CA (Sowers et al., 2000). Eligibility criteria were being aged 42–52 years, having an intact uterus and at least one ovary, having had at least one menstrual period in the past three months, having not taken hormone therapy in the past three months, and not being pregnant or lactating. SWAN has approximately annual or biannual follow-up visits. The institutional review board at each participating site approved the study protocol, and all participants provided written, signed informed consent.
Beginning in 2016, the SWAN Multi-Pollutant Study (MPS) was initiated to undergo measurements of environmental pollutants among 1,400 women with available SWAN repository samples from the five SWAN sites including Michigan, MI, Boston, MA, Oakland, CA, Los Angeles, CA, and Pittsburgh, PA (Ding et al., 2020; Park et al., 2019; Wang et al., 2019a). Metal concentrations were assessed in repository urine samples collected at SWAN visit 03 (1999–2000, the MPS baseline). Of these 1,400 participants, we excluded 366 women with prevalent MetS at the MPS baseline, and 87 women who had missing information on key covariates, yielding a final analytic sample of 947 women with 8,283 observations followed from 1999 to 2017. A flow chart of the current study is shown in Figure 1.
Figure 1.
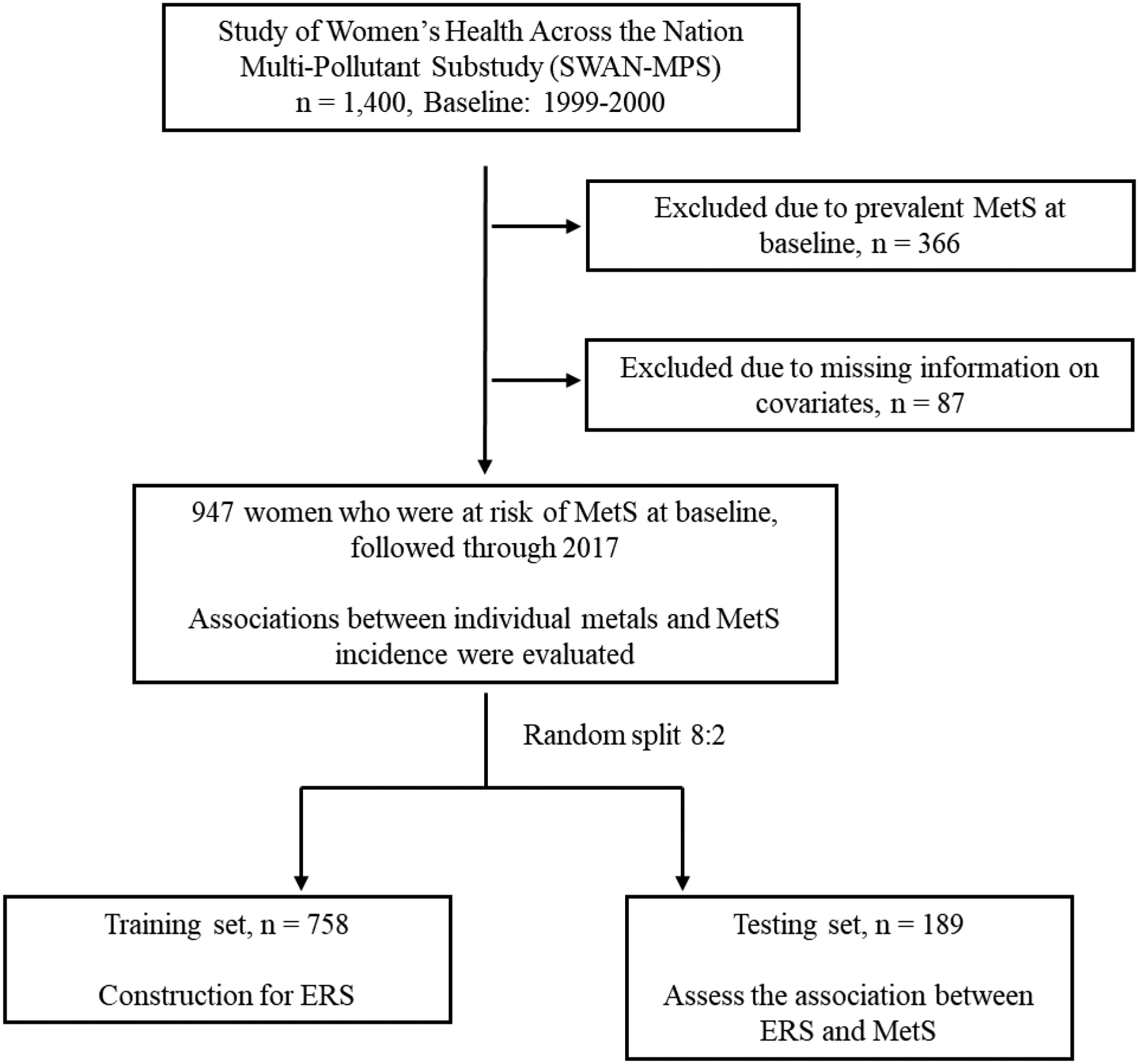
Flow chart of the study design. MetS: metabolic syndrome; ERS: Environment Risk Score.
2.2. Metabolic syndrome
Blood pressure and waist circumference were measured using standardized protocols by trained and certified personnel. Serum samples were collected to measure fasting glucose and lipid levels. Incident MetS and its components were determined at SWAN visit 04, 05, 06, 07, 09, 12, 13, and 15 using the National Cholesterol Education Program Adult Treatment Panel III criteria for MetS for women (Grundy et al., 2005). Women who had at least 3 of the following 5 components were identified as having MetS: (1) high blood pressure defined as systolic blood pressure ≥ 130 mmHg, or diastolic blood pressure ≥ 85 mmHg, or current use of antihypertensive medication; (2) impaired fasting glucose ascertained by fasting glucose ≥ 100 mg/dL or current use of antidiabetic medication; (3) abdominal obesity defined as waist circumference ≥ 88 cm for White and Black women and ≥ 80 cm for Chinese and Japanese women; (4) high triglyceride defined as serum triglyceride ≥ 150 mg/dL; and (5) low high-density lipoprotein cholesterol (HDL) defined as serum HDL < 50 mg/dL.
2.3. Urinary metals
Concentrations of a panel of 15 metals, including arsenic, barium, cadmium, cobalt, cesium, copper, mercury, manganese, molybdenum, nickel, lead, antimony, tin, thallium, and zinc, were measured in morning spontaneously voided urine samples using high-resolution inductively-coupled plasma mass spectrometry (Thermo Scientific iCAP RQ, Waltham, MA) at the SWAN-MPS baseline. The measurements followed the CDC method 3018.3 (CDC, 2012), with modifications for the expanded metals panel, performed at the Applied Research Center of NSF International (Ann Arbor, Michigan) (Wang et al., 2019a). Concentrations below the limits of detection (LODs) were substituted with the LODs divided by the square root of 2 (Lubin et al., 2004). Urinary creatinine was measured using the Cobas Mira analyzer (Horiba ABX, Montpellier, France) as a marker of urine dilution.
2.4. Other covariates
Adjustment covariates included were race/ethnicity, study site, education, smoking status, alcohol drinking, physical activity score, total energy intake, menopausal status, body mass index (BMI), and urinary creatinine (log-transformed). Self-reported race/ethnicity (defined as White, Black, Chinese, or Japanese), and education (categorized as high school or less, some college, or college degree or higher) were assessed through a self-administered questionnaire at the MPS baseline. At each study visit, smoking status (never smoked, former smokers, or current smoking), alcohol drinking (<1 drink/month, >1 drink/month and ≤1/week, and >1 drink/week), physical activity, and menopausal status (pre-menopausal, post-menopausal, and unknown due to hormone therapy use) were obtained from standardized interviews. Physical activity was measured using a modified version of the Kaiser Physical Activity Survey (Sternfeld et al., 2000). The total score ranged from 3 to 15 was calculated, indicating the activity levels during the previous 12 months in 3 distinct domains: active living (1–5), household/caregiving (1–5), and sports/exercise (1–5). A total score of 3 indicated least, and 15 indicated most physically active. Total energy intake, and zinc and Vitamin B12 intake from diet and supplements were assessed using a detailed semi-quantitative food frequency questionnaire (FFQ) adopted from the Block FFQ (Block et al., 1986). BMI was calculated as weight in kilograms divided by the square of height in meters. We used a Directed Acyclic Graph to show the hypothesized relations between metals, confounders, and MetS (Figure S1) (Baik and Shin, 2008; Cena et al., 2011; He et al., 2014; Scuteri et al., 2008; Wang et al., 2019a).
2.5. Statistical analysis
We used Cox proportional hazards models to estimate the hazard ratio (HR) and 95% confidence interval (CI) for incident MetS associated with each metal. We used age as the time scale, and participants contributed survival time from the SWAN-MPS baseline to the date of the first MetS event for incident cases and participants without incident MetS were right-censored at the date of the last study visit. Metal concentrations were modeled as continuous variables in the Cox models. Given the right-skewed distributions of metal concentrations, logarithmic transformations with base two were applied to all metal concentrations. Effect estimates were thus interpreted as HR of MetS per doubling of each urinary metal concentration. To capture the potential non-linear associations, metal concentrations were also categorized into quartiles and HRs were calculated comparing the second, third, and fourth quartiles to the first quartile (the reference group). A linear trend of the association across the quartiles was tested by including metal quartiles as a continuous variable. All the models were adjusted for race/ethnicity, study site, and urinary creatinine (log-transformed), smoking (time-varying), alcohol drinking (time-varying), physical activity score (time-varying), total energy intake (time-varying), and menopausal status (time-varying), and BMI at baseline. Time-varying BMI was not included in the analysis because of its possible role as an intermediate variable (X. Wang et al., 2018). For the association between urinary zinc and MetS, we adjusted for total zinc intake from food and supplements in all three models. Dietary zinc intake has been associated with lower risk of diabetes and MetS (Sun et al., 2009; Y. Wang et al., 2018). Therefore, urinary zinc adjusted for dietary zinc intake and zinc supplements could better capture the excessive renal clearance and excretion of zinc independent of beneficial zinc intake from diet. For the association between urinary cobalt and MetS, we also adjusted for dietary and supplemental Vitamin B12 intake since the cobalt is an important metal constitute of vitamin B12 and vitamin B12 intake has been associated with a lower risk of MetS (Li et al., 2018). For other essential elements, such as copper, we did not adjust for dietary intake due to a lack of data. We also examined associations of metals with the incidence of each of the five MetS components. Given the relatively large number of associations examined for MetS components, we addressed multiple comparisons at a false discovery rate (FDR) of 0.05 using the Benjamini–Hochberg Method (Benjamini and Hochberg, 1995).
We developed an ERS an integrative score of health risk associated with multiple environmental exposures to summarize the associations between metal mixtures and MetS (Park et al., 2017; Wang et al., 2019b; X. Wang et al., 2018). We randomly split the study population into the training set to construct the MetS-related ERS of metal mixtures and the testing set to evaluate its association with MetS while avoid overfitting. We tried multiple split ratios and found that a ratio of 8:2 (N=758 for training set and N=189 for testing set) was the most accurate split with the least prediction errors in the testing set. In the training set, we first used the elastic net (ENET) penalized Cox regression (Yang and Zou, 2013), a machine learning algorithm designed for analyzing high-dimensional data in survival analyses, to identify metals associated with incident MetS while accounting for the potential multicollinearity due to the complex correlations between metals. This ENET penalized Cox model included all 15 metals (log2-transformed) as independent variables and coefficients of “unimportant” metals were shrunk to zero in the model fitting process. The covariates from the Cox model in the individual metal analyses were adjusted in the ENET model. The regularization parameters (λ and α) were ascertained through a grid search based on minimal 10-fold cross-validation errors. The R package ‘glmnet’ was used to implement the ENET penalized Cox model (Friedman et al., 2010). ERS was then computed as a weighted sum of non-zero metal predictors estimated from ENET penalized Cox model by
where (j = 1, … , p) is the log-transformed concentration of the j-th metal and is the beta coefficient (weight) of the j-th metal. In the testing set, we fitted the ERS in the Cox model and report the adjusted HR of MetS comparing the 75th vs. the 25th percentile of the ERS. All metals were fitted as continuous variables. It is not statistically efficient to incorporate quartiles of all metal concentrations in the ENET model if all the metals are treated as categorical variables, and it is possible that the ENET only select one but not all quartiles of specific metals that the ERS cannot be calculated.
We recognized that the associations between metals and MetS might be influenced by the selective participation into the SWAN-MPS. To account for this potential bias, we calculated weights to participation into the SWAN-MPS using probability weighting (IPW) to create a pseudo population representing the women who were at risk of developing incident MetS at the time of metal measurements in the original SWAN cohort. Details illustrating the construction of IPW are presented in our previous study (Wang et al., 2020a).
To test the robustness of our results, we performed following sensitivity analyses. First, we used covariate-adjusted creatinine standardization instead of adjusting for urinary creatinine concentration as a covariate in regression models for adjusting urine dilution (O’Brien et al., 2016). Briefly, we first fitted the linear regression with log-transformed urinary creatinine as dependent variables and all covariates included in the primary analysis as independent variables and predicted each participant’s creatinine concentration based on the regression results. The predicted creatinine concentrations (after back transformed) are therefore independent of the covariates and capture only variations due to urine dilution. We then calculated the covariates-adjusted creatinine standardized metal concentrations by dividing the urinary metal concentrations by the ratio of the measured to the predicted urinary creatinine concentration. Second, we additionally adjusted for seafood and rice intake in analyses for arsenic, cadmium, and mercury as we have identified these dietary components as important determinants in a previous study (Wang et al., 2019a). Finally, we examined the effect modification by race/ethnicity and menopausal status at baseline by incorporating the interaction terms between metals and modifiers in the Cox models. All analyses were conducted using R, version 4.0.3 (www.R-project.org).
3. Results
Among 947 women free of MetS at the SWAN-MPS baseline, 173 developed incident cases with the median follow-up of 15.7 years. Participants who developed MetS tended to be Black and from Michigan. They tended to have a higher BMI but less education and poorer individual components of MetS (Table 1).
Table 1.
Characteristics of participants in the Study of Women’s Health Across the Nation Multi-Pollutant Substudy at baseline.
| Non-MetS (n= 774) | Incident MetS (n= 173) | |
|---|---|---|
| Age (years)a | 49.4 (47.3, 51.3) | 49.5 (47.4, 51.7) |
| Race/ethnicity | ||
| White | 415 (53.6) | 75 (43.4) |
| Black | 123 (15.9) | 41 (23.7) |
| Chinese | 108 (14.0) | 23 (13.3) |
| Japanese | 128 (16.5) | 34 (20.0) |
| Study site | ||
| Michigan | 100 (12.9) | 39 (22.5) |
| Boston | 136 (17.6) | 18 (10.4) |
| Oakland | 181 (23.4) | 37 (21.4) |
| Los Angeles | 235 (30.4) | 50 (28.9) |
| Pittsburgh | 122 (15.8) | 29 (16.8) |
| Body mass index (kg/m2) | 23.5 (21.2, 26.6) | 27.6 (25.0, 32.2) |
| Education | ||
| High school or less | 113 (14.6) | 43 (24.9) |
| Some college | 228 (29.5) | 64 (37.0) |
| College or higher | 433 (55.9) | 66 (38.1) |
| Smoking status | ||
| Never | 509 (65.8) | 103 (59.5) |
| Former | 197 (25.5) | 52 (30.1) |
| Current | 68 (8.8) | 18 (10.4) |
| Alcohol drinking | ||
| ≤1 drink/month | 361 (46.6) | 96 (55.5) |
| >1 drink/month and ≤1/week | 186 (24.0) | 43 (24.9) |
| >1 drink/week | 227 (29.3) | 34 (19.7) |
| Physical activity score | 8.2 (7.0, 9.3) | 7.5 (6.2, 8.6) |
| Menopausal status | ||
| Pre-menopausal | 549 (70.9) | 126 (72.8) |
| Post-menopausal | 113 (14.6) | 15 (8.7) |
| Unknownb | 112 (14.5) | 32 (18.5) |
| Systolic blood pressure (mmHg) | 107 (99, 116) | 117 (105, 124) |
| Diastolic blood pressure (mmHg) | 70 (65, 77) | 75 (69, 80) |
| Fasting glucose (mg/dL) | 84.0 (79.4, 88.5) | 87.6 (83.0, 93.1) |
| Waist circumference (cm) | 75.8 (70.4, 83.3) | 86.2 (79.6, 93.9) |
| HDL cholesterol (mg/dL) | 66 (57, 76) | 56 (50, 64) |
| Triglyceride (mg/dL) | 84 (65, 109) | 111 (79, 142) |
| Total energy intake (kCal) | 1657 (1332, 2070) | 1604 (1236, 2318) |
| Total zinc intake (mg/day) | 10.6 (7.4, 19.9) | 11.6 (7.5, 20.4) |
| Total Vitamin B12 intake (μg/day) | 2.4 (1.7, 3.4) | 2.4 (1.5, 3.6) |
| Dietary seafood intake (times/week) | 1.5 (0.8, 2.5) | 1.6 (0.8, 2.8) |
| Dietary rice intake (times/week) | 2.0 (1.0, 5.5) | 2.0 (1.0, 5.5) |
Note: MetS, metabolic syndrome.
Data are median (interquartile range) or n (%).
Menopausal status unknown due to hormone therapy or hysterectomy.
Table 2 presents the distribution of urinary metal concentrations. The percentage of participants with detectable metal concentrations ranged from 78.1% to 100%, while most metals had detection rates greater than 90%. Participants who developed incident MetS were more likely to have higher arsenic and zinc concentrations.
Table 2.
Detection rates and concentrations of urinary metals by incident metabolic syndrome.
| Metals | LOD | Percent >LOD | Median concentration (IQR), μg/L | |
|---|---|---|---|---|
| Non-MetS (n= 774) | Incident MetS (n= 173) | |||
| Arsenic | 0.3 | 100 | 13.85 (6.59, 38.66) | 14.41 (7.02, 41.53) |
| Barium | 0.1 | 99.6 | 1.76 (0.98, 2.95) | 1.73 (0.92, 3.06) |
| Cadmium | 0.06 | 94.0 | 0.46 (0.23, 0.79) | 0.47 (0.23, 0.82) |
| Cobalt | 0.05 | 99.2 | 0.63 (0.37, 0.91) | 0.61 (0.42, 1.20) |
| Cesium | 0.01 | 100 | 4.69 (3.13, 7.27) | 4.92 (2.98, 7.23) |
| Copper | 2.5 | 96.8 | 9.29 (6.14, 13.28) | 10.14 (6.40, 12.80) |
| Mercury | 0.05 | 100 | 1.31 (0.71, 2.52) | 1.12 (0.61, 2.18) |
| Manganese | 0.08 | 99.7 | 0.90 (0.61, 1.45) | 0.89 (0.59, 1.34) |
| Molybdenum | 0.3 | 100 | 45.13 (24.35, 73.68) | 45.79 (24.86, 70.58) |
| Nickel | 0.8 | 96.2 | 3.77 (2.30, 5.92) | 3.76 (2.22, 6.21) |
| Lead | 0.1 | 97.5 | 0.80 (0.50, 1.28) | 0.85 (0.46, 1.29) |
| Antimony | 0.04 | 78.1 | 0.07 (0.04, 0.13) | 0.09 (0.05, 0.14) |
| Tin | 0.1 | 96.6 | 0.95 (0.49, 1.79) | 0.87 (0.52, 1.80) |
| Thallium | 0.02 | 92.0 | 0.14 (0.08, 0.22) | 0.14 (0.08, 0.24) |
| Zinc | 2 | 100 | 292 (159, 479) | 332 (188, 541) |
Note: LOD: limit of detection; IQR: interquartile range; MetS: metabolic syndrome.
Table 3 summarizes the associations between urinary metal concentrations and the incidence of MetS. After adjustment for race/ethnicity, study site, and urinary creatinine (log-transformed), education, smoking status, alcohol drinking, physical activity score, total energy intake, menopausal status, BMI at baseline, and dietary intake of zinc or Vitamin B12, comparing the highest to the lowest quartiles, the HR for MetS was 1.72 (95% CI: 1.20, 2.46) for arsenic (P for trend=0.002), 1.85 (95% CI: 1.25, 2.74) for cobalt (P for trend=0.02), and 1.66 (95% CI: 1.08, 2.58) for zinc. The associations of arsenic, cobalt, and zinc with MetS were log linear. The HR for MetS associated with each doubling of urinary metal concentration was 1.14 (95% CI: 1.08, 1.23) for arsenic, 1.14 (95% CI: 1.01, 1.29) for cobalt, and 1.20 (95% CI: 1.06, 1.37) for zinc, when they were fit as continuous variables (log2-transformed).
Table 3.
Hazard ratios (HR) (95% confidence intervals, 95% CI) for incident metabolic syndrome in relation to urinary metal concentrations.
| Metals | Quartile of metal concentrations | P for trend | Per doublinga | P | |||
|---|---|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | ||||
| Arsenic | |||||||
| Range, μg/L | 0.73, 7.37 | 7.40, 15.55 | 15.65, 44.94 | 45.02, 2983.79 | |||
| HR (95% CI)b | Ref | 1.32 (0.96, 1.82) | 1.78 (1.23, 2.58) | 1.72 (1.20, 2.46) | 0.002 | 1.14 (1.07, 1.23) | 0.0001 |
| Barium | |||||||
| Range, μg/L | <LOD, 0.98 | 0.98, 1.75 | 1.76, 2.93 | 2.94, 51.03 | |||
| HR (95% CI) | Ref | 0.66 (0.48, 0.91) | 0.71 (0.51, 0.99) | 0.82 (0.59, 1.15) | 0.96 | 1.04 (0.94, 1.14) | 0.45 |
| Cadmium | |||||||
| Range, μg/L | <LOD, 0.22 | 0.23, 0.44 | 0.44, 0.77 | 0.77, 23.97 | |||
| HR (95% CI) | Ref | 0.95 (0.67, 1.35) | 0.93 (0.63, 1.38) | 0.98 (0.65, 1.49) | 0.99 | 0.95 (0.85, 1.06) | 0.35 |
| Cobalt | |||||||
| Range, μg/L | <LOD, 0.38 | 0.38, 0.63 | 0.63, 0.95 | 0.95, 8.32 | |||
| HR (95% CI) | Ref | 1.47 (1.02, 2.11) | 0.89 (0.59, 1.34) | 1.85 (1.25, 2.74) | 0.02 | 1.14 (1.01, 1.29) | 0.03 |
| Cesium | |||||||
| Range, μg/L | 0.37, 3.12 | 3.13, 4.78 | 4.81, 7.35 | 7.36, 104.43 | |||
| HR (95% CI) | Ref | 0.75 (0.53, 1.06) | 1.04 (0.71, 1.52) | 1.03 (0.65, 1.63) | 0.58 | 1.04 (0.86, 1.25) | 0.22 |
| Copper | |||||||
| Range, μg/L | <LOD, 5.96 | 5.97, 9.17 | 9.18, 13.22 | 13.22, 1889.40 | |||
| HR (95% CI) | Ref | 1.03 (0.71, 1.50) | 1.13 (0.76, 1.69) | 0.75 (0.47, 1.20) | 0.22 | 1.00 (0.87, 1.15) | 0.99 |
| Mercury | |||||||
| Range, μg/L | 0.07, 0.70 | 0.70, 1.30 | 1.30, 2.47 | 2.48, 32.37 | |||
| HR (95% CI) | Ref | 1.01 (0.74, 1.37) | 1.06 (0.77, 1.47) | 0.65 (0.44, 0.94) | 0.06 | 0.91 (0.82, 1.01) | 0.06 |
| Manganese | |||||||
| Range, μg/L | <LOD, 0.58 | 0.59, 0.87 | 0.87, 1.43 | 1.43, 41.97 | |||
| HR (95% CI) | Ref | 0.93 (0.66, 1.30) | 0.76 (0.54, 1.09) | 0.77 (0.53, 1.13) | 0.11 | 1.01 (0.89, 1.15) | 0.87 |
| Molybdenum | |||||||
| Range, μg/L | 2.48, 24.60 | 24.65, 45.95 | 46.00, 74.80 | 74.82, 694.53 | |||
| HR (95% CI) | Ref | 1.30 (0.92, 1.82) | 1.35 (0.95, 1.91) | 1.13 (0.76, 1.68) | 0.55 | 1.10 (0.97, 1.24) | 0.14 |
| Nickel | |||||||
| Range, μg/L | <LOD, 2.35 | 2.35, 3.79 | 3.80, 5.94 | 5.94, 73.60 | |||
| HR (95% CI) | Ref | 1.02 (0.72, 1.44) | 0.77 (0.53, 1.12) | 1.01 (0.69, 1.48) | 0.77 | 1.01 (0.89, 1.16) | 0.84 |
| Lead | |||||||
| Range, μg/L | <LOD, 0.47 | 0.47, 0.78 | 0.78, 1.25 | 1.25, 43.59 | |||
| HR (95% CI) | Ref | 0.57 (0.40, 0.81) | 0.78 (0.55, 1.12) | 0.75 (0.51, 1.12) | 0.57 | 0.90 (0.79, 1.02) | 0.09 |
| Antimony | |||||||
| Range, μg/L | <LOD, 0.04 | 0.04, 0.07 | 0.07, 0.12 | 0.12, 1.38 | |||
| HR (95% CI) | Ref | 1.35 (0.94, 1.94) | 1.58 (1.09, 2.31) | 1.24 (0.84, 1.84) | 0.32 | 1.04 (0.92, 1.17) | 0.55 |
| Tin | |||||||
| Range, μg/L | <LOD, 0.48 | 0.48, 0.90 | 0.90, 1.73 | 1.74, 106.82 | |||
| HR (95% CI) | Ref | 1.53 (1.10, 2.21) | 1.01 (0.70, 1.46) | 1.03 (0.71, 1.48) | 0.44 | 0.96 (0.88, 1.04) | 0.28 |
| Thallium | |||||||
| Range, μg/L | <LOD, 0.08 | 0.08, 0.14 | 0.14, 0.22 | 0.22, 15.73 | |||
| HR (95% CI) | Ref | 1.41 (1.01, 1.95) | 0.96 (0.66, 1.39) | 1.52 (1.05, 2.21) | 0.12 | 1.03 (0.93, 1.14) | 0.55 |
| Zinc | |||||||
| Range, μg/L | 7.01, 157.38 | 157.44, 277.26 | 277.71, 464.33 | 466.99, 2295.46 | |||
| HR (95% CI) | Ref | 1.37 (0.94, 1.98) | 1.04 (0.69, 1.56) | 1.66 (1.08, 2.58) | 0.05 | 1.20 (1.06, 1.37) | 0.006 |
Note: LOD: limit of detection.
Results based on when log2-transformed metal concentrations were fitted.
All models were adjusted for race/ethnicity, study sites, urinary creatinine (log-transformed), education, smoking status, alcohol drinking, physical activity score, total energy intake, menopausal status, and body mass index at baseline. Zinc intake from diet and supplements was additionally adjusted for zinc model. Vitamin B12 intake from diet and supplements was additionally adjusted for cobalt model.
Associations between metals and the incidence of MetS components are presented in Table 4. After full adjustment for confounders, arsenic was associated with higher incidences of high blood pressure and impaired fasting glucose but a lower incidence of high triglyceride. Cobalt was associated with higher incidences of high blood pressure, impaired fasting glucose, and abdominal obesity. Zinc was associated with higher incidences of high blood pressure, impaired fasting glucose, abdominal obesity, and high triglyceride. For other metals where the association with MetS were not observed, we found that barium was associated with higher incidences of impaired fasting glucose and abdominal obesity, cadmium was associated with high blood pressure, copper and lead were associated with a higher incidence of abdominal obesity, molybdenum was associated with a lower incidence of abdominal obesity, and nickel was associated with higher incidences of high blood pressure and impaired fasting glucose, after adjusting for multiple comparison with FDRs < 5%.
Table 4.
Hazard ratios (HR) (95% confidence intervals, 95% CI) for incident metabolic syndrome components for a doubling increase in urinary metal concentrations.
| Metals | High blood pressure | FDR | Impaired fasting glucose | FDR | Abdominal obesity | FDR | High triglyceride | FDR | Low HDL | FDR |
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | ||||||
| Arsenic | 1.06 (1.01, 1.12) | 0.03 | 1.16 (1.07, 1.26) | 0.01 | 0.96 (0.92, 1.01) | 0.23 | 0.90 (0.83, 0.96) | 0.02 | 0.93 (0.86, 1.00) | 0.15 |
| Barium | 1.07 (1.00, 1.13) | 0.08 | 1.19 (1.06, 1.33) | 0.02 | 1.10 (1.04, 1.17) | 0.01 | 0.93 (0.86, 1.01) | 0.33 | 0.99 (0.91, 1.07) | 0.92 |
| Cadmium | 1.09 (1.02, 1.16) | 0.03 | 1.16 (1.02, 1.31) | 0.05 | 1.05 (0.99, 1.12) | 0.22 | 0.96 (0.88, 1.05) | 0.59 | 1.02 (0.94, 1.12) | 0.92 |
| Cobalt | 1.12 (1.03, 1.21) | 0.02 | 1.25 (1.07, 1.46) | 0.02 | 1.12 (1.04, 1.22) | 0.008 | 0.94 (0.85, 1.05) | 0.58 | 0.88 (0.79, 0.97) | 0.08 |
| Cesium | 1.11 (0.98, 1.25) | 0.12 | 1.16 (0.94, 1.43) | 0.21 | 1.02 (0.90, 1.15) | 0.81 | 0.92 (0.79, 1.08) | 0.58 | 0.87 (0.74, 1.03) | 0.33 |
| Copper | 1.08 (0.98, 1.19) | 0.12 | 1.16 (0.99, 1.36) | 0.11 | 1.24 (1.14, 1.34) | 0.001 | 0.95 (0.81, 1.10) | 0.59 | 0.82 (0.70, 0.96) | 0.08 |
| Mercury | 0.95 (0.89, 1.01) | 0.13 | 1.09 (0.96, 1.24) | 0.20 | 0.88 (0.83, 0.94) | 0.001 | 1.01 (0.93, 1.11) | 0.88 | 0.97 (0.89, 1.06) | 0.92 |
| Manganese | 0.96 (0.89, 1.04) | 0.29 | 1.14 (1.00, 1.30) | 0.08 | 1.03 (0.95, 1.10) | 0.57 | 0.85 (0.76, 0.94) | 0.02 | 0.97 (0.87, 1.07) | 0.92 |
| Molybdenum | 0.96 (0.88, 1.04) | 0.29 | 1.17 (1.01, 1.36) | 0.08 | 0.90 (0.83, 0.98) | 0.03 | 1.05 (0.94, 1.17) | 0.59 | 1.01 (0.90, 1.13) | 0.92 |
| Nickel | 1.14 (1.04, 1.24) | 0.02 | 1.26 (1.07, 1.49) | 0.02 | 1.07 (0.98, 1.16) | 0.22 | 0.99 (0.87, 1.12) | 0.88 | 0.99 (0.88, 1.12) | 0.92 |
| Lead | 1.08 (1.00, 1.17) | 0.09 | 1.15 (1.00, 1.33) | 0.08 | 1.14 (1.06, 1.24) | 0.004 | 0.94 (0.84, 1.04) | 0.55 | 1.12 (1.00, 1.24) | 0.15 |
| Antimony | 0.93 (0.86, 1.01) | 0.11 | 1.08 (0.93, 1.25) | 0.32 | 1.06 (0.99, 1.14) | 0.19 | 1.04 (0.94, 1.16) | 0.59 | 1.02 (0.92, 1.13) | 0.92 |
| Tin | 1.03 (0.98, 1.08) | 0.29 | 1.08 (0.98, 1.19) | 0.15 | 0.97 (0.93, 1.02) | 0.33 | 1.00 (0.93, 1.07) | 0.93 | 1.00 (0.94, 1.07) | 0.92 |
| Thallium | 1.05 (0.99, 1.12) | 0.12 | 1.00 (0.88, 1.14) | 0.99 | 1.01 (0.95, 1.07) | 0.81 | 0.93 (0.85, 1.01) | 0.30 | 0.99 (0.92, 1.08) | 0.92 |
| Zinc | 1.15 (1.05, 1.25) | 0.02 | 1.58 (1.34, 1.87) | 0.002 | 1.15 (1.06, 1.25) | 0.004 | 1.28 (1.14, 1.44) | 0.002 | 1.08 (0.96, 1.21) | 0.55 |
Note: all models were constructed by Cox proportional hazards model. FDR: false discovery rate. All models were adjusted for race/ethnicity, study sites, urinary creatinine (log-transformed), education, smoking status, alcohol drinking, physical activity score, total energy intake, menopausal status, and body mass index at baseline. Zinc intake from diet and supplements was additionally adjusted for zinc model. Vitamin B12 intake from diet and supplements was additionally adjusted for cobalt model.
We further examined the associations between metal mixtures and MetS using the ERS approach. In the training set, 14 metals were selected in the ENET penalized Cox model associated with MetS incidence, with eight (arsenic, barium, cobalt, copper, nickel, antimony, thallium, and zinc) showing positive and six (cadmium, cesium, mercury, molybdenum, lead, and tin) showing negative beta coefficients, representing log-transformed hazard ratio of MetS for a two-fold increase in metal concentrations (Table 5). The beta coefficient of manganese was shrunk to zero. The ERS was then constructed using these beta coefficients as weights, with higher ERS indicating a combination of higher concentrations of metals with positive weights and lower concentrations of metals with negative weights. Meanwhile, individuals with a greater ERS had a higher risk of MetS than those with a lower ERS in the training set. The distributions of the ERS are similar in the training and testing sets (Figure S2). In the testing set, an increase in the ERS from 25th percentile (0.25) to 75th percentile (0.44) was associated with a higher incidence of MetS with an adjusted HR of 1.45 (95% CI: 1.13, 1.87).
Table 5.
Selected non-zero beta coefficients of metals for incidence of metabolic syndrome in elastic-net (ENET) penalized Cox modela.
| Selected non-zero metal predictorsb | β for log2-transformed metal concentrationsc |
|---|---|
| Arsenic | 0.038 |
| Barium | 0.006 |
| Cadmium | −0.037 |
| Cesium | −0.024 |
| Cobalt | 0.033 |
| Copper | 0.053 |
| Mercury | −0.091 |
| Manganese | 0d |
| Molybdenum | −0.028 |
| Nickel | 0.008 |
| Lead | −0.026 |
| Antimony | 0.032 |
| Tin | −0.017 |
| Thallium | 0.028 |
| Zinc | 0.051 |
Model was adjusted for race/ethnicity, study sites, and urinary creatinine (log-transformed), education, smoking status, alcohol drinking, physical activity score, total energy intake, menopausal status, body mass index (baseline), and dietary intake of zinc and Vitamin B12.
Logarithmic transformations with base 2 were applied to all urinary metal concentrations.
Beta coefficients of selected predictors were used as weights in the following construction of the environmental risk score.
Beta coefficient was shrunk to zeros.
Similar findings were found in sensitivity analyses when covariate-adjusted creatinine standardization was used (Table S1). Additional adjustment for seafood and rice intake did not alter the results for arsenic, cadmium, and mercury (Table S2). When examining effect modifications by race/ethnicity, we found a stronger positive association between manganese and MetS in Asian (Chinese and Japanese) women than White and Black women (Table S3). A stronger association between nickel and MetS was observed in White and Asian women compared to Black women. We also found significant effect modification of the association between manganese and MetS that a stronger association was observed in post-menopausal women (Table S4). Stronger associations between most other metals and MetS incidence were also observed in post-menopausal women than pre-menopausal women, though the interactions were not statistically significant, possibly due to reduced statistical power in accordance with the smaller number of post-menopausal women at baseline.
4. Discussion
In this 18-year multi-site, prospective cohort study of 947 midlife women with diverse racial/ethnic groups, higher urinary arsenic, cobalt, and zinc concentrations were significantly associated with elevated MetS incidence. These associations persisted after controlling for demographic, socioeconomic, lifestyle factors, menopausal status, BMI, and dietary factors. Using the ENET penalized Cox model and integrating the associations into the ERS, we again found a significant association between the ERS and the risk of MetS after controlling for overfitting. These findings suggest that metals, including arsenic, cobalt, and zinc, as well as metal mixtures, may play a role in the development of MetS in a cohort of midlife women with similar metal concentrations as women of the same age range in the U.S. general population (Wang et al., 2019a).
More than one-third of women in the U.S. have MetS (Hirode and Wong, 2020). The risk of developing MetS increases substantially across the menopausal transition (Beltrán-Sánchez et al., 2013), and MetS becomes a more pronounced predictor of cardiovascular disease in postmenopausal women (Lin et al., 2010). A better knowledge of the risk factors for the MetS is of substantial public health importance as effective preventive efforts can be undertaken. This is the first prospective study, to the best of our knowledge, that assessed the associations of a panel of 15 metals with the incidence of MetS in midlife women. The mixture analysis in our study (ENET penalized Cox model) was conducted to account for potential confounding from co-exposure to other metals in the mixture. For example, copper was not associated with incident MetS in the single metal Cox model; however, it showed one of the strongest associations in the ENET penalized Cox model, suggesting that there may be confounding by co-exposure to other metals in either direction. Finally, we summarized the joint effects of metal mixtures into the ERS, and our findings indicate that people with higher ERS as a weighted combination of multiple metal concentrations may be at higher risk of MetS.
We observed that urinary arsenic was positively associated with an elevated incidence of MetS, high blood pressure, and impaired fasting glucose. Arsenic is pervasive in the environment, and inorganic arsenic is a toxicant that people can be exposed through drinking water and foods such as cereal and rice (Wang et al., 2019a). Urinary arsenic was associated with higher MetS prevalence in two highly exposed Taiwanese populations and the U.S. general population (Bulka et al., 2019; Chen et al., 2012; Wang et al., 2007). Epidemiologic studies have also found associations of arsenic with high blood pressure (Abhyankar et al., 2012; Jiang et al., 2015), impaired fasting glucose (Spratlen et al., 2018), and type 2 diabetes (Grau-Perez et al., 2017; Navas-Acien et al., 2008; Wang et al., 2014, 2020a), which are consistent with the results observed in the current study. Arsenic induces the generation of reactive oxygen species, leading to oxidative stress and related inflammation, endothelial dysfunction, and renal dysfunction, and the development of cardiovascular disorders such as hypertension (Abhyankar et al., 2012; Chen et al., 2011). Arsenic is also related to a higher risk of insulin resistance through disrupting insulin-stimulated glucose uptake in peripheral tissues (Mohammed Abdul et al., 2015; Walton et al., 2004). In this study, we also observed an inverse association between arsenic and the incidence of high triglyceride levels. In contrast to our finding, a positive association between arsenic exposure and triglyceride was shown in Mexican Adults (Mendez et al., 2016), while a null association was reported in American Indian adults and adults in the U.S. general population (Bulka et al., 2019; Spratlen et al., 2018).
Cobalt is a metal component of Vitamin B12 (cyanocobalamin), a vital nutrient for human health. Despite the beneficial role of cyanocobalamin, other cobalt compounds have been described as environmental toxicants, and people can be exposed to cobalt through ambient air and drinking water (Leyssens et al., 2017). In the current study, cobalt was associated with higher incidence of MetS, high blood pressure, impaired fasting glucose, and abdominal obesity, and these associations persisted after adjusting for dietary and supplement intake of Vitamin B12. Existing evidence on the impact of cobalt exposure on MetS is extremely limited: a null association between urinary cobalt and MetS prevalence was recently reported in a large community-based cross-sectional study in China (Ma et al., 2020). The cardiovascular effects of cobalt, in contrast, have been more extensively examined in occupational settings. Epidemiologic studies in highly occupationally exposed populations have found associations of cobalt exposure with cardiovascular endpoints, including altered diastole, reduced left ventricular systolic function, left ventricular and atrial hypertrophy, reversible electrocardiographic changes, arrhythmias, and hypertension (D’Adda et al., 1994; Horowitz et al., 1988; Leyssens et al., 2017; Linna et al., 2004; Machado et al., 2012; Oldenburg et al., 2009). Cobalt appears to exert toxic effects through the inhibition of cellular respiration due to interruption of the mitochondrial function (Leyssens et al., 2017). Our findings, leveraging a community-based prospective cohort design, suggest cobalt exposure may also increase the risk of MetS among midlife women from the U.S. general population (Wang et al., 2019a). Finally, we observed a positive association between cobalt and abdominal obesity in the fully adjusted model. In contrast, two cross-sectional studies in the U.S. found an inverse association of cobalt with waist circumference and BMI (Niehoff et al., 2020; X. Wang et al., 2018). We found a null association in models without BMI adjustment in the current analysis. Given the positive correlation between BMI at baseline and waist circumference at follow-up (β=1.93, 95% CI: 1.87, 1.99 for BMI at baseline in the linear mixed regression with time-varying waist circumference as the outcome), it is possible that the association observed could be positively biased due to the overadjustment.
Our analyses also revealed that urinary zinc was associated with an elevated risk of MetS and its components, including high blood pressure, impaired fasting glucose, abdominal obesity, and high triglyceride. Zinc is an essential element that people need on a daily basis to be healthy and prevent disease (Jansen et al., 2009). Zinc is excreted in the urine and feces (Roohani et al., 2013). After adjusting for dietary and supplemental zinc intake, we observed a positive association between urinary zinc and MetS, demonstrating that women who had higher urinary zinc excretion might be at a greater risk of MetS independent of zinc intake. Mechanistic evidence suggests that zinc can protect against oxidative stress by inhibiting lipid peroxidation and inflammatory cytokines expression (Goel et al., 2005; Hennig et al., 2001, 1999; Mansour and Mossa, 2009). Increased urinary zinc excretion was also linked with zinc loss in β-cells, affecting insulin synthesis, storage, and secretion. Urinary zinc excretion has previously been associated with a higher incidence of type 2 diabetes, accelerated increase in insulin resistance, and decrease in β-cell function over time in SWAN (Wang et al., 2020a, 2020b). In contrast, some observational studies have demonstrated a positive association of serum zinc with adverse cardiometabolic outcomes, including MetS, hypertension, and dyslipidemia (Bulka et al., 2019; Ghasemi et al., 2014; Kunutsor and Laukkanen, 2016), while the underlying biological mechanisms are still unclear. Future prospective studies with the quantification of zinc in multiple biological matrices are needed to confirm these findings, and further mechanistic studies are needed to unravel the underlying biological pathways. Finally, we need to acknowledge that hyperglycemia may inhibit the active transport of zinc into renal cells, resulting in a zinc loss through urine (Chausmer, 1998). It is possible that the observed association between urinary zinc and MetS is a result of increased urinary zinc excretion among women with relatively high glucose levels at baseline. However, the prospective cohort design of our study, and the fact that women with prevalent MetS were excluded in the survival analyses, reduce the likelihood that our finding occurred as a result of reverse causation.
Our data provided evidence for associations between other metals and MetS and its components. A significant positive association between manganese and MetS was observed in Asian women. Race/ethnicity was not found as a determinant for urinary manganese concentration in SWAN (Wang et al., 2019a). In a most recent study of the U.S. general population, no association was observed between urinary manganese and MetS prevalence (Lo et al., 2021). Cadmium was positively associated with high blood pressure. Existing evidence regarding cadmium and high blood pressure is mixed. Positive associations between urinary cadmium and blood pressure have been reported in U.S. adults (Tellez-Plaza et al., 2008) and in a large cohort study of American Indian adults (Franceschini et al., 2017). By contrast, no association was found in other studies (Mordukhovich et al., 2012; Park and Oh, 2021; Staessen et al., 2000). We observed positive associations of barium with impaired fasting glucose and abdominal obesity, which is in line with findings from two large cross-sectional studies in China that barium was associated with higher prevalence of impaired fasting glucose and high waist circumference (Feng et al., 2015; Zhang et al., 2020). We found positive associations of nickel with high blood pressure and impaired fasting glucose, and MetS in White and Asian women, who showed higher concentrations compared to Black women in SWAN (Wang et al., 2019a). One cross-sectional study conducted in China showed that urinary nickel was associated with higher diabetes prevalence, higher fasting glucose, HbA1c, and elevated insulin resistance (Liu et al., 2015). By contrast, an inverse association between urinary nickel and diastolic blood pressure was reported in U.S. adults without hypertension in a most recent study (Y. Liu et al., 2022). Molybdenum was inversely associated with abdominal obesity in our study, which is supported by an inverse association between molybdenum and waist circumference in the U.S. adults (X. Wang et al., 2018). In our latest SWAN study, favorable relationships between molybdenum and adipokine profiles were observed (Wang et al., 2021b). Copper was positively associated with abdominal obesity. Though no identified literature has examined the association between urinary copper and waist circumference, a recent meta-analysis suggested a possible link between serum copper and obesity risk (Gu et al., 2020). Given the limited and conflicting data now exist, further research is required to confirm these findings in the future. Finally, the null association between one specific metal and MetS does not necessarily mean null associations between this metal and MetS components. For example, cadmium was not associated with MetS but was significantly associated with high blood pressure.
Strengths of the present study include the large sample size, diverse racial/ethnic groups, and longitudinal design with up to 18 years of follow-up of SWAN. A list of 15 metals was also measured, enabling us to examine a reasonably large number of associations between MetS of multiple individual metals, as well as metal mixtures. We note, however, that all metal concentrations were determined in urine, which may not capture all metal forms and exposure sources. Furthermore, total arsenic concentrations in urine samples were measured, but not for arsenic metabolites. Based upon the evidence that arsenic metabolites may impact metabolic outcomes (Grau-Perez et al., 2017; Spratlen et al., 2018), further measures of arsenic metabolites will give a clearer understanding of arsenic body burden and related health problems in the future. Additionally, metals examined in this study have varying half-lives. Urinary metals having short half-lives, such as arsenic, primarily represent recent exposures. In comparison, metals such as cadmium have half-lives ranging from years to decades. Future studies should incorporate repeated measures of metal concentrations to better elucidate the associations with MetS, given that metal exposures over a more extended time span are expected to impact MetS risk. Furthermore, although we applied IPW to address the potential for selective participation into the SWAN-MPS, we cannot rule out potential selection bias due to the initial selection process of the parent SWAN design. Estimates of the metal-MetS associations may be underestimated because women who might be more susceptible to metabolic effects of metals were more likely to excluded at the enrollment. Arsenic, for example, has been associated to an earlier age at natural menopause (Wang et al., 2021a), which is a risk factor for MetS (Janssen et al., 2008). Finally, we utilized cross-validation to reduce the prediction errors of the ENET penalized Cox model, and the association between ERS and MetS was examined in the testing set. Nevertheless, these results may have limited generalizability to populations with distinct characteristics due to a lack of external validation datasets. While further prospective studies are required to confirm the results of individual metals and the ERS, our findings from a broad, multi-racial/ethnic population indicate that exposure to metals and their mixtures may influence the risk of MetS.
5. Conclusion
Our findings found that urinary arsenic, cobalt, and zinc concentrations were associated with the incidence of MetS in midlife women. Using the ENET penalized Cox model and integrating the associations into the ERS with positive weights of arsenic, barium, cobalt, copper, nickel, antimony, thallium, and zinc, and negative weights of cadmium, cesium, mercury, molybdenum, lead, and tin, we again found a significant association between the ERS and the risk of MetS. These findings provide evidence that metals may be an underappreciated contributing factor to MetS, especially during the sensitive period of midlife for women. More prospective studies are needed to confirm these findings, and mechanistic studies are encouraged to investigate the underlying biological mechanisms.
Supplementary Material
Acknowledgements
The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants U01NR004061; U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495, and U19AG063720). The study was supported by the SWAN Repository (U01AG017719).
This study was also supported by grants from the National Institute of Environmental Health Sciences (NIEHS) R01-ES026578, R01-ES026964 and P30-ES017885, and by the Center for Disease Control and Prevention (CDC)/National Institute for Occupational Safety and Health (NIOSH) grant T42-OH008455.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
Clinical Centers: University of Michigan, Ann Arbor - Carrie Karvonen-Gutierrez, PI 2021 - present, Siobán Harlow, PI 2011 - 2021, MaryFran Sowers, PI 1994-2011; Massachusetts General Hospital, Boston, MA - Joel Finkelstein, PI 1999 - present; Robert Neer, PI 1994 - 1999; Rush University, Rush University Medical Center, Chicago, IL - Howard Kravitz, PI 2009 - present; Lynda Powell, PI 1994 - 2009; University of California, Davis/Kaiser - Ellen Gold, PI; University of California, Los Angeles - Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY - Carol Derby, PI 2011 - present, Rachel Wildman, PI 2010 - 2011; Nanette Santoro, PI 2004 - 2010; University of Medicine and Dentistry - New Jersey Medical School, Newark - Gerson Weiss, PI 1994 - 2004; and the University of Pittsburgh, Pittsburgh, PA - Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD - Chhanda Dutta 2016- present; Winifred Rossi 2012-2016; Sherry Sherman 1994 - 2012; Marcia Ory 1994 - 2001; National Institute of Nursing Research, Bethesda, MD - Program Officers.
Central Laboratory: University of Michigan, Ann Arbor - Daniel McConnell (Central Ligand Assay Satellite Services).
SWAN Repository: University of Michigan, Ann Arbor - Siobán Harlow 2013 - Present; Dan McConnell 2011 - 2013; MaryFran Sowers 2000 - 2011.
Coordinating Center: University of Pittsburgh, Pittsburgh, PA - Maria Mori Brooks, PI 2012 - present; Kim Sutton-Tyrrell, PI 2001 - 2012; New England Research Institutes, Watertown, MA - Sonja McKinlay, PI 1995 - 2001.
Steering Committee: Susan Johnson, Current Chair
Chris Gallagher, Former Chair
We thank the study staff at each site and all the women who participated in SWAN.
Abbreviations:
- BMI
body mass index
- CVD
cardiovascular disease
- ENET
elastic net
- ERS
environmental risk score
- FFQ
food frequency questionnaire
- IPW
inverse probability weighting
- MetS
metabolic syndrome
- SWAN
Study of Women’s Health Across the Nation
- SWAN-MPS
Study of Women’s Health Across the Nation Multi-Pollutant Substudy
- T2DM
type 2 diabetes mellitus.
Footnotes
Conflict of interest
The authors declare they have no actual or potential competing interest.
References
- Abhyankar LN, Jones MR, Guallar E, Navas-Acien A, 2012. Arsenic exposure and hypertension: a systematic review. Environ. Health Perspect 120, 494–500. 10.1289/ehp.1103988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberti KGMM, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WPT, Loria CM, Smith SC, 2009. Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; National heart, lung, and blood institute; American heart association; World heart federation; International atherosclerosis society; And international association for the study of obesity. Circulation 120, 1640–1645. 10.1161/CIRCULATIONAHA.109.192644 [DOI] [PubMed] [Google Scholar]
- Alissa EM, Ferns GA, 2011. Heavy metal poisoning and cardiovascular disease. J. Toxicol 2011. 10.1155/2011/870125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATSDR, 2017a. Toxicological profile for Molybdenum. [PubMed]
- ATSDR, 2017b. Toxicological profile for antimony.
- ATSDR, 2007. Toxicological profile for Barium.
- ATSDR, 2005a. Toxicological profile for Nickel.
- ATSDR, 2005b. Toxicological profile for Tin.
- ATSDR, 2004. Toxicological profile for cobalt.
- ATSDR, 1992. Toxicological profile for Thallium. [PubMed]
- Ayoub N, Mantash H, Dhaini HR, Mourad A, Hneino M, Daher Z, 2021. Serum Cadmium Levels and Risk of Metabolic Syndrome: A Cross-Sectional Study. Biol. Trace Elem. Res 199, 3625–3633. 10.1007/S12011-020-02502-3/TABLES/2 [DOI] [PubMed] [Google Scholar]
- Baik I, Shin C, 2008. Prospective study of alcohol consumption and metabolic syndrome. Am. J. Clin. Nutr 87, 1455–1463. 10.1093/AJCN/87.5.1455 [DOI] [PubMed] [Google Scholar]
- Beltrán-Sánchez H, Harhay MO, Harhay MM, McElligott S, 2013. Prevalence and Trends of Metabolic Syndrome in the Adult U.S. Population, 1999–2010. J. Am. Coll. Cardiol 62, 697–703. 10.1016/j.jacc.2013.05.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y, 1995. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 57, 289–300. 10.2307/2346101 [DOI] [Google Scholar]
- Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L, 1986. A data-based approach to diet questionnaire design and testing. Am. J. Epidemiol 124, 453–69. [DOI] [PubMed] [Google Scholar]
- Buhari O, Dayyab FM, Igbinoba O, Atanda A, Medhane F, Faillace RT, 2020. The association between heavy metal and serum cholesterol levels in the US population: National Health and Nutrition Examination Survey 2009–2012. Hum. Exp. Toxicol 39, 355–364. 10.1177/0960327119889654 [DOI] [PubMed] [Google Scholar]
- Bulka CM, Persky VW, Daviglus ML, Durazo-Arvizu RA, Argos M, 2019. Multiple metal exposures and metabolic syndrome: A cross-sectional analysis of the National Health and Nutrition Examination Survey 2011–2014. Environ. Res 168, 397–405. 10.1016/j.envres.2018.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC, 2012. Laboratory Procedure Manual, Multi-Element in urine. NHANES 2011–2012. [Google Scholar]
- Cena H, Fonte ML, Turconi G, 2011. Relationship between smoking and metabolic syndrome. Nutr. Rev 69, 745–753. 10.1111/J.1753-4887.2011.00446.X [DOI] [PubMed] [Google Scholar]
- Chausmer AB, 1998. Zinc, Insulin and Diabetes. J. Am. Coll. Nutr 17, 109–115. 10.1080/07315724.1998.10718735 [DOI] [PubMed] [Google Scholar]
- Chen JW, Chen HY, Li WF, Liou SH, Chen CJ, Wu JH, Wang SL, 2011. The association between total urinary arsenic concentration and renal dysfunction in a community-based population from central Taiwan. Chemosphere 84, 17–24. 10.1016/j.chemosphere.2011.02.091 [DOI] [PubMed] [Google Scholar]
- Chen JW, Wang SL, Wang YH, Sun CW, Huang YL, Chen CJ, Li WF, 2012. Arsenic methylation, GSTO1 polymorphisms, and metabolic syndrome in an arseniasis endemic area of southwestern Taiwan. Chemosphere 88, 432–438. 10.1016/j.chemosphere.2012.02.059 [DOI] [PubMed] [Google Scholar]
- D’Adda F, Borleri D, Migliori M, Mosconi G, Medolago G, Virotta G, Colombo F, Seghizzi P, 1994. Cardiac function study in hard metal workers. Sci. Total Environ 150, 179–186. 10.1016/0048-9697(94)90148-1 [DOI] [PubMed] [Google Scholar]
- Denier X, Hill EM, Rotchell J, Minier C, 2009. Estrogenic activity of cadmium, copper and zinc in the yeast estrogen screen. Toxicol. Vitr 23, 569–573. 10.1016/J.TIV.2009.01.006 [DOI] [PubMed] [Google Scholar]
- Ding EL, Song Y, Manson JE, Rifai N, Buring JE, Liu S, 2007. Plasma sex steroid hormones and risk of developing type 2 diabetes in women: A prospective study. Diabetologia 50, 2076–2084. 10.1007/S00125-007-0785-Y/TABLES/5 [DOI] [PubMed] [Google Scholar]
- Ding N, Harlow SD, Batterman S, Mukherjee B, Park SK, 2020. Longitudinal trends in perfluoroalkyl and polyfluoroalkyl substances among multiethnic midlife women from 1999 to 2011: The Study of Women′s Health Across the Nation. Environ. Int 135, 105381. 10.1016/j.envint.2019.105381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W, Cui X, Liu B, Liu C, Xiao Y, Lu W, Guo H, He M, Zhang X, Yuan J, Chen W, Wu T, 2015. Association of Urinary Metal Profiles with Altered Glucose Levels and Diabetes Risk: A Population-Based Study in China. PLoS One 10, e0123742. 10.1371/journal.pone.0123742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschini N, Fry RC, Balakrishnan P, Navas-Acien A, Oliver-Williams C, Howard AG, Cole SA, Haack K, Lange EM, Howard BV, Best LG, Francesconi KA, Goessler W, Umans JG, Tellez-Plaza M, 2017. Cadmium body burden and increased blood pressure in middle-aged American Indians: The Strong Heart Study. J. Hum. Hypertens 31, 225–230. 10.1038/jhh.2016.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J, Hastie T, Tibshirani R, 2010. Regularization paths for generalized linear models via coordinate descent. J. Stat. Softw 33, 1–22. [PMC free article] [PubMed] [Google Scholar]
- Ghasemi A, Zahediasl S, Hosseini-Esfahani F, Azizi F, 2014. Gender differences in the relationship between serum zinc concentration and metabolic syndrome. Ann. Hum. Biol 41, 436–442. 10.3109/03014460.2013.870228 [DOI] [PubMed] [Google Scholar]
- Goel A, Dani V, Dhawan DK, 2005. Protective effects of zinc on lipid peroxidation, antioxidant enzymes and hepatic histoarchitecture in chlorpyrifos-induced toxicity. Chem. Biol. Interact 156, 131–140. 10.1016/j.cbi.2005.08.004 [DOI] [PubMed] [Google Scholar]
- Grau-Perez M, Kuo C-C, Gribble MO, Balakrishnan P, Jones Spratlen M, Vaidya D, Francesconi KA, Goessler W, Guallar E, Silbergeld EK, Umans JG, Best LG, Lee ET, Howard BV, Cole SA, Navas-Acien A, 2017. Association of Low-Moderate Arsenic Exposure and Arsenic Metabolism with Incident Diabetes and Insulin Resistance in the Strong Heart Family Study. Environ. Health Perspect 125, 127004. 10.1289/EHP2566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy SM, 2016. Metabolic syndrome update. Trends Cardiovasc. Med 26, 364–373. 10.1016/j.tcm.2015.10.004 [DOI] [PubMed] [Google Scholar]
- Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Spertus JA, Costa F, 2005. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation 112, 2735–2752. 10.1161/CIRCULATIONAHA.105.169404 [DOI] [PubMed] [Google Scholar]
- Gu K, Li X, Xiang W, Jiang X, 2020. The Relationship Between Serum Copper and Overweight/Obesity: a Meta-analysis. Biol. Trace Elem. Res 194, 336–347. 10.1007/S12011-019-01803-6/FIGURES/10 [DOI] [PubMed] [Google Scholar]
- Guo X, Yang Q, Zhang W, Chen Y, Ren J, Gao A, 2019. Associations of blood levels of trace elements and heavy metals with metabolic syndrome in Chinese male adults with microRNA as mediators involved. Environ. Pollut 248, 66–73. 10.1016/j.envpol.2019.02.015 [DOI] [PubMed] [Google Scholar]
- Han JC, Park SY, Hah BG, Choi GH, Kim YK, Kwon TH, Kim EK, Lachaal M, Jung CY, Lee W, 2003. Cadmium induces impaired glucose tolerance in rat by down-regulating GLUT4 expression in adipocytes. Arch. Biochem. Biophys 413, 213–20. [DOI] [PubMed] [Google Scholar]
- He D, Xi B, Xue J, Huai P, Zhang M, Li J, 2014. Association between leisure time physical activity and metabolic syndrome: A meta-analysis of prospective cohort studies. Endocrine 46, 231–240. 10.1007/S12020-013-0110-0/TABLES/2 [DOI] [PubMed] [Google Scholar]
- Hennig B, Meerarani P, Toborek M, McClain CJ, 1999. Antioxidant-Like Properties of Zinc in Activated Endothelial Cells. J. Am. Coll. Nutr 18, 152–158. 10.1080/07315724.1999.10718843 [DOI] [PubMed] [Google Scholar]
- Hennig B, Toborek M, Hennig B, Toborek M, McClain CJ, McClain CJ, 2001. High-Energy Diets, Fatty Acids and Endothelial Cell Function: Implications for Atherosclerosis. J. Am. Coll. Nutr 20, 97–105. 10.1080/07315724.2001.10719021 [DOI] [PubMed] [Google Scholar]
- Heron M, 2019. National Vital Statistics Reports Deaths- Leading Causes for 2017. [PubMed]
- Hirode G, Wong RJ, 2020. Trends in the Prevalence of Metabolic Syndrome in the United States, 2011–2016. JAMA - J. Am. Med. Assoc 323, 2526–2528. 10.1001/jama.2020.4501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz SF, Fischbein A, Matza D, Rizzo JN, Stern A, Machac J, Solomon SJ, 1988. Evaluation of right and left ventricular function in hard metal workers. Br. J. Ind. Med 45, 742–746. 10.1136/oem.45.11.742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen J, Karges W, Rink L, 2009. Zinc and diabetes — clinical links and molecular mechanisms. J. Nutr. Biochem 20, 399–417. 10.1016/J.JNUTBIO.2009.01.009 [DOI] [PubMed] [Google Scholar]
- Janssen I, Powell LH, Crawford S, Lasley B, Sutton-Tyrrell K, 2008. Menopause and the Metabolic Syndrome: The Study of Women’s Health Across the Nation. Arch. Intern. Med 168, 1568–1575. 10.1001/ARCHINTE.168.14.1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Liu M, Parvez F, Wang B, Wu F, Eunus M, Bangalore S, Newman JD, Ahmed A, Islam T, Rakibuz-Zaman M, Hasan R, Sarwar G, Levy D, Slavkovich V, Argos M, Bryan MS, Farzan SF, Hayes RB, Graziano JH, Ahsan H, Chen Y, 2015. Association between Arsenic Exposure from Drinking Water and Longitudinal Change in Blood Pressure among HEALS Cohort Participants. Environ. Health Perspect 123, 806–812. 10.1289/EHP.1409004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jomova K, Valko M, 2011. Advances in metal-induced oxidative stress and human disease. Toxicology 283, 65–87. 10.1016/j.tox.2011.03.001 [DOI] [PubMed] [Google Scholar]
- Kunutsor SK, Laukkanen JA, 2016. Serum zinc concentrations and incident hypertension. J. Hypertens 34, 1055–1061. 10.1097/HJH.0000000000000923 [DOI] [PubMed] [Google Scholar]
- Lee B, Pine M, Johnson L, Rettori V, Hiney JK, Dees W. Les, 2006. Manganese acts centrally to activate reproductive hormone secretion and pubertal development in male rats. Reprod. Toxicol 22, 580–585. 10.1016/J.REPROTOX.2006.03.011 [DOI] [PubMed] [Google Scholar]
- Leyssens L, Vinck B, Van Der Straeten C, Wuyts F, Maes L, 2017. Cobalt toxicity in humans—A review of the potential sources and systemic health effects. Toxicology 387, 43–56. 10.1016/j.tox.2017.05.015 [DOI] [PubMed] [Google Scholar]
- Li Z, Gueant-Rodriguez RM, Quilliot D, Sirveaux MA, Meyre D, Gueant JL, Brunaud L, 2018. Folate and vitamin B12 status is associated with insulin resistance and metabolic syndrome in morbid obesity. Clin. Nutr 37, 1700–1706. 10.1016/J.CLNU.2017.07.008 [DOI] [PubMed] [Google Scholar]
- Lin J-W, Caffrey JL, Chang M-H, Lin Y-S, 2010. Sex, Menopause, Metabolic Syndrome, and All-Cause and Cause-Specific Mortality—Cohort Analysis from the Third National Health and Nutrition Examination Survey. J. Clin. Endocrinol. Metab 95, 4258–4267. 10.1210/jc.2010-0332 [DOI] [PubMed] [Google Scholar]
- Linna A, Oksa P, Groundstroem K, Halkosaari M, Palmroos P, Huikko S, Uitti J, 2004. Exposure to cobalt in the production of cobalt and cobalt compounds and its effect on the heart. Occup. Environ. Med 61, 877–885. 10.1136/oem.2003.009605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Sun L, Pan A, Zhu M, Li Z, Wang Z, Liu X, Ye X, Li H, Zheng H, Ong CN, Yin H, Lin X, Chen Y, 2015. Nickel exposure is associated with the prevalence of type 2 diabetes in Chinese adults. Int. J. Epidemiol 44, 240–248. 10.1093/ije/dyu200 [DOI] [PubMed] [Google Scholar]
- Liu L, Li X, Wu M, Yu M, Wang L, Hu L, Li Y, Song L, Wang Y, Mei S, 2022. Individual and joint effects of metal exposure on metabolic syndrome among Chinese adults. Chemosphere 287, 132295. 10.1016/J.CHEMOSPHERE.2021.132295 [DOI] [PubMed] [Google Scholar]
- Liu Y, Wu M, Xu B, Kang L, 2022. Association between the urinary nickel and the diastolic blood pressure in general population. Chemosphere 286, 131900. 10.1016/J.CHEMOSPHERE.2021.131900 [DOI] [PubMed] [Google Scholar]
- Lo K, Yang JL, Chen CL, Liu L, Huang YQ, Feng YQ, Yang AM, 2021. Associations between blood and urinary manganese with metabolic syndrome and its components: Cross-sectional analysis of National Health and Nutrition Examination Survey 2011–2016. Sci. Total Environ 780, 146527. 10.1016/J.SCITOTENV.2021.146527 [DOI] [PubMed] [Google Scholar]
- Lu T-H, Su C-C, Chen Y-W, Yang C-Y, Wu C-C, Hung D-Z, Chen C-H, Cheng P-W, Liu S-H, Huang C-F, 2011. Arsenic induces pancreatic β-cell apoptosis via the oxidative stress-regulated mitochondria-dependent and endoplasmic reticulum stress-triggered signaling pathways. Toxicol. Lett 201, 15–26. 10.1016/j.toxlet.2010.11.019 [DOI] [PubMed] [Google Scholar]
- Lubin JH, Colt JS, Camann D, Davis S, Cerhan JR, Severson RK, Bernstein L, Hartge P, 2004. Epidemiologic Evaluation of Measurement Data in the Presence of Detection Limits. Environ. Health Perspect 112, 1691. 10.1289/EHP.7199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Zhou Y, Wang D, Guo Y, Wang B, Xu Y, Chen W, 2020. Associations between essential metals exposure and metabolic syndrome (MetS): Exploring the mediating role of systemic inflammation in a general Chinese population. Environ. Int 140, 105802. 10.1016/j.envint.2020.105802 [DOI] [PubMed] [Google Scholar]
- Machado C, Appelbe A, Wood R, 2012. Arthroprosthetic Cobaltism and Cardiomyopathy. Hear. Lung Circ. 21, 759–760. 10.1016/j.hlc.2012.03.013 [DOI] [PubMed] [Google Scholar]
- Mansour SA, Mossa ATH, 2009. Lipid peroxidation and oxidative stress in rat erythrocytes induced by chlorpyrifos and the protective effect of zinc. Pestic. Biochem. Physiol 93, 34–39. 10.1016/j.pestbp.2008.09.004 [DOI] [Google Scholar]
- Mendez MA, González-Horta C, Sánchez-Ramírez B, Ballinas-Casarrubias L, Cerón RH, Morales DV, Terrazas FAB, Ishida MC, Gutiérrez-Torres DS, Saunders RJ, Drobná Z, Fry RC, Buse JB, Loomis D, García-Vargas GG, Del Razo LM, Stýblo M, 2016. Chronic Exposure to Arsenic and Markers of Cardiometabolic Risk: A Cross-Sectional Study in Chihuahua, Mexico. Environ. Health Perspect 124, 104–111. 10.1289/ehp.1408742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed Abdul KS, Jayasinghe SS, Chandana EPS, Jayasumana C, De Silva PMCS, 2015. Arsenic and human health effects: A review. Environ. Toxicol. Pharmacol 40, 828–846. 10.1016/j.etap.2015.09.016 [DOI] [PubMed] [Google Scholar]
- Moon S-S, 2014. Additive effect of heavy metals on metabolic syndrome in the Korean population: the Korea National Health and Nutrition Examination Survey (KNHANES) 2009–2010. Endocrine 46, 263–271. 10.1007/s12020-013-0061-5 [DOI] [PubMed] [Google Scholar]
- Mordukhovich I, Wright RO, Hu H, Amarasiriwardena C, Baccarelli A, Litonjua A, Sparrow D, Vokonas P, Schwartz J, 2012. Associations of toenail arsenic, cadmium, mercury, manganese, and lead with blood pressure in the Normative aging study. Environ. Health Perspect 120, 98–104. 10.1289/EHP.1002805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navas-Acien A, Silbergeld EK, Pastor-Barriuso R, Guallar E, 2008. Arsenic Exposure and Prevalence of Type 2 Diabetes in US Adults. JAMA 300, 814. 10.1001/jama.300.7.814 [DOI] [PubMed] [Google Scholar]
- Niehoff NM, Keil AP, O’Brien KM, Jackson BP, Karagas MR, Weinberg CR, White AJ, 2020. Metals and trace elements in relation to body mass index in a prospective study of US women. Environ. Res 184, 109396. 10.1016/j.envres.2020.109396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien KM, Upson K, Cook NR, Weinberg CR, 2016. Environmental Chemicals in Urine and Blood: Improving Methods for Creatinine and Lipid Adjustment. Environ. Health Perspect 124, 220–7. 10.1289/ehp.1509693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldenburg M, Wegner R, Baur X, 2009. Severe Cobalt Intoxication Due to Prosthesis Wear in Repeated Total Hip Arthroplasty. J. Arthroplasty 24, 825.e15–825.e20. 10.1016/j.arth.2008.07.017 [DOI] [PubMed] [Google Scholar]
- Park SK, Peng Q, Ding N, Mukherjee B, Harlow SD, 2019. Determinants of per- and polyfluoroalkyl substances (PFAS) in midlife women: Evidence of racial/ethnic and geographic differences in PFAS exposure. Environ. Res 175, 186–199. 10.1016/j.envres.2019.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SK, Tao Y, Meeker JD, Harlow SD, Mukherjee B, 2014. Environmental Risk Score as a New Tool to Examine Multi-Pollutants in Epidemiologic Research: An Example from the NHANES Study Using Serum Lipid Levels. PLoS One 9, e98632. 10.1371/journal.pone.0098632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SK, Zhao Z, Mukherjee B, 2017. Construction of environmental risk score beyond standard linear models using machine learning methods: application to metal mixtures, oxidative stress and cardiovascular disease in NHANES. Environ. Heal 16, 102. 10.1186/s12940-017-0310-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YJ, Oh CU, 2021. Association of lead, mercury, and cadmium with metabolic syndrome of young adults in South Korea: The Korea National Health and Nutrition Examination Survey (KNHANES) 2016. Public Health Nurs. 38, 232–238. 10.1111/PHN.12855 [DOI] [PubMed] [Google Scholar]
- Perry HM, Erlanger M, Perry EF, 1979. Increase in the systolic pressure of rats chronically fed cadmium. Environ. Health Perspect 28, 251–260. 10.1289/ehp.7928251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planchart A, Green A, Hoyo C, Mattingly CJ, 2018. Heavy Metal Exposure and Metabolic Syndrome: Evidence from Human and Model System Studies. Curr. Environ. Heal. reports 5, 110–124. 10.1007/s40572-018-0182-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polotsky H, Polotsky A, 2010. Metabolic Implications of Menopause. Semin. Reprod. Med 28, 426–434. 10.1055/s-0030-1262902 [DOI] [PubMed] [Google Scholar]
- Preuss HG, Jiang G, Jones JW, Macarthy PO, Andrews PM, Gondal JA, 1994. Early lead challenge and subsequent hypertension in sprague-dawley rats. J. Am. Coll. Nutr 13, 578–583. 10.1080/07315724.1994.10718451 [DOI] [PubMed] [Google Scholar]
- Roohani N, Hurrell R, Kelishadi R, Schulin R, 2013. Zinc and its importance for human health: An integrative review. J. Res. Med. Sci 18, 144–157. 10.1016/j.foodpol.2013.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Rodríguez MA, Zacarías-Flores M, Arronte-Rosales A, Correa-Muñoz E, Mendoza-Núñez VM, 2012. Menopause as risk factor for oxidative stress. Menopause J. North Am. Menopause Soc 19, 361–367. 10.1097/gme.0b013e318229977d [DOI] [PubMed] [Google Scholar]
- Scuteri A, Vuga M, Najjar SS, Mehta V, Everson-Rose SA, Sutton-Tyrrell K, Matthews K, Lakatta EG, 2008. Education eclipses ethnicity in predicting the development of the metabolic syndrome in different ethnic groups in midlife: the Study of Women’s Health Across the Nation (SWAN). Diabet. Med 25, 1390–1399. 10.1111/J.1464-5491.2008.02596.X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowers MF, Crawford SL, Sternfeld B, Morganstein D, Gold EB, Greendale GA, Evans D, Neer R, Matthews K, Sherman S, Lo A, Weiss G, Kelsey J, 2000. SWAN: a multi-center, multi-ethnic, community-based cohort study of women and the menopausal transition, in: Lobo RA, Kelsey J, Marcus R (Eds.), Menopause : Biology and Pathobiology. Academic Press, pp. 175–188. [Google Scholar]
- Spratlen MJ, Grau-Perez M, Best LG, Yracheta J, Lazo M, Vaidya D, Balakrishnan P, Gamble MV, Francesconi KA, Goessler W, Cole SA, Umans JG, Howard BV, Navas-Acien A, 2018. The Association of Arsenic Exposure and Arsenic Metabolism With the Metabolic Syndrome and Its Individual Components: Prospective Evidence From the Strong Heart Family Study. Am. J. Epidemiol 187, 1598–1612. 10.1093/aje/kwy048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staessen JA, Kuznetsova T, Roels HA, Emelianov D, Fagard R, 2000. Exposure to cadmium and conventional and ambulatory blood pressures in a prospective population study. Am. J. Hypertens 13, 146–156. 10.1016/S0895-7061(99)00187-9 [DOI] [PubMed] [Google Scholar]
- Sternfeld B, Cauley J, Harlow S, Liu G, Lee M, 2000. Assessment of Physical Activity with a Single Global Question in a Large, Multiethnic Sample of Midlife Women. Am. J. Epidemiol 152, 678–687. 10.1093/aje/152.7.678 [DOI] [PubMed] [Google Scholar]
- Stuenkel CA, 2017. Menopause, hormone therapy and diabetes. Climacteric 20, 11–21. 10.1080/13697137.2016.1267723 [DOI] [PubMed] [Google Scholar]
- Su H, Li Z, Kenston SSF, Shi H, Wang Y, Song X, Gu Y, Barber T, Aldinger J, Zou B, Ding M, Zhao J, Lin X, 2017. Joint Toxicity of Different Heavy Metal Mixtures after a Short-Term Oral Repeated-Administration in Rats. Int. J. Environ. Res. Public Heal. 2017, Vol. 14, Page 1164 14, 1164. 10.3390/IJERPH14101164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Van Dam RM, Willett WC, Hu FB, 2009. Prospective study of zinc intake and risk of type 2 diabetes in women. Diabetes Care 32, 629–634. 10.2337/dc08-1913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ, 2012. Heavy metal toxicity and the environment. Mol. Clin. Environ. Toxicol 101, 133–64. 10.1007/978-3-7643-8340-4_6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellez-Plaza M, Navas-Acien A, Crainiceanu CM, Guallar E, 2008. Cadmium Exposure and Hypertension in the 1999–2004 National Health and Nutrition Examination Survey (NHANES). Environ. Health Perspect 116, 51–56. 10.1289/ehp.10764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinkov AA, Filippini T, Ajsuvakova OP, Aaseth J, Gluhcheva YG, Ivanova JM, Bjørklund G, Skalnaya MG, Gatiatulina ER, Popova EV, Nemereshina ON, Vinceti M, Skalny AV, 2017. The role of cadmium in obesity and diabetes. Sci. Total Environ 601–602, 741–755. 10.1016/J.SCITOTENV.2017.05.224 [DOI] [PubMed] [Google Scholar]
- Wakita Y, 1987. Hypertension induced by methyl mercury in rats. Toxicol. Appl. Pharmacol 89, 144–147. 10.1016/0041-008X(87)90185-2 [DOI] [PubMed] [Google Scholar]
- Walton FS, Harmon AW, Paul DS, Drobná Z, Patel YM, Styblo M, 2004. Inhibition of insulin-dependent glucose uptake by trivalent arsenicals: possible mechanism of arsenic-induced diabetes. Toxicol. Appl. Pharmacol 198, 424–433. 10.1016/J.TAAP.2003.10.026 [DOI] [PubMed] [Google Scholar]
- Wang SL, Chang FH, Liou SH, Wang HJ, Li WF, Hsieh DPH, 2007. Inorganic arsenic exposure and its relation to metabolic syndrome in an industrial area of Taiwan. Environ. Int 33, 805–811. 10.1016/j.envint.2007.03.004 [DOI] [PubMed] [Google Scholar]
- Wang W, Xie Z, Lin Y, Zhang D, 2014. Association of inorganic arsenic exposure with type 2 diabetes mellitus: a meta-analysis. J Epidemiol Community Heal. 68, 176–184. [DOI] [PubMed] [Google Scholar]
- Wang X, Ding N, Harlow SD, Randolph JF, Mukherjee B, Gold EB, Park SK, 2021a. Urinary metals and metal mixtures and timing of natural menopause in midlife women: The Study of Women’s Health Across the Nation. Environ. Int 157, 106781. 10.1016/J.ENVINT.2021.106781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Karvonen-Gutierrez CA, Herman WH, Mukherjee B, Harlow SD, Park SK, 2020a. Urinary metals and incident diabetes in midlife women: Study of Women’s Health Across the Nation (SWAN). BMJ Open Diabetes Res. Care 8, e001233. 10.1136/bmjdrc-2020-001233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Karvonen-Gutierrez CA, Mukherjee B, Herman WH, Park SK, 2021b. Urinary metals and adipokines in midlife women: The Study of Women’s Health Across the nation (SWAN). Environ. Res 196, 110426. 10.1016/J.ENVRES.2020.110426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Mukherjee B, Batterman S, Harlow SD, Park SK, 2019a. Urinary metals and metal mixtures in midlife women: The Study of Women’s Health Across the Nation (SWAN). Int. J. Hyg. Environ. Health 222, 778–789. 10.1016/J.IJHEH.2019.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Mukherjee B, Karvonen-Gutierrez CA, Herman WH, Batterman S, Harlow SD, Park SK, 2020b. Urinary metal mixtures and longitudinal changes in glucose homeostasis: The Study of Women’s Health Across the Nation (SWAN). Environ. Int 145, 106109. 10.1016/j.envint.2020.106109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Mukherjee B, Park SK, 2019b. Does Information on Blood Heavy Metals Improve Cardiovascular Mortality Prediction? J. Am. Heart Assoc 8, e013571. 10.1161/JAHA.119.013571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Mukherjee B, Park SK, 2018. Associations of cumulative exposure to heavy metal mixtures with obesity and its comorbidities among U.S. adults in NHANES 2003–2014. Environ. Int 121, 683–694. 10.1016/j.envint.2018.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Jia XF, Zhang B, Wang ZH, Zhang JG, Huang FF, Su C, Ouyang YF, Zhao J, Du WW, Li L, Jiang HR, Zhang J, Wang HJ, 2018. Dietary Zinc Intake and Its Association with Metabolic Syndrome Indicators among Chinese Adults: An Analysis of the China Nutritional Transition Cohort Survey 2015. Nutr. 2018, Vol. 10, Page 572 10, 572. 10.3390/NU10050572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HT, Chou HJ, Han BC, Huang SY, 2007. Lifelong inorganic arsenic compounds consumption affected blood pressure in rats. Food Chem. Toxicol 45, 2479–2487. 10.1016/j.fct.2007.05.024 [DOI] [PubMed] [Google Scholar]
- Yang Y, Zou H, 2013. A cocktail algorithm for solving the elastic net penalized Cox’s regression in high dimensions. Stat. Interface 6, 167–173. 10.4310/SII.2013.v6.n2.a1 [DOI] [Google Scholar]
- Zhang W, Du J, Li H, Yang Y, Cai C, Gao Q, Xing Y, Shao B, Li G, 2020. Multiple-element exposure and metabolic syndrome in Chinese adults: A case-control study based on the Beijing population health cohort. Environ. Int 143, 105959. 10.1016/J.ENVINT.2020.105959 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


