Abstract
The first cells were plausibly bounded by membranes assembled from fatty acids with at least 8 carbons. Although the presence of fatty acids on the early Earth is widely assumed within the astrobiology community, there is no consensus regarding their origin and abundance. In this Review, we highlight three possible sources of fatty acids: (1) delivery by carbonaceous meteorites, (2) synthesis on metals delivered by impactors, and (3) electrochemical synthesis by spark discharges. We also discuss fatty acid synthesis by UV or particle irradiation, gas-phase ion–molecule reactions, and aqueous redox reactions. We compare estimates for the total mass of fatty acids supplied to Earth by each source during the Hadean eon after an extremely massive asteroid impact that would have reset Earth’s fatty acid inventory. We find that synthesis on iron-rich surfaces derived from the massive impactor in contact with an impact-generated reducing atmosphere could have contributed ∼102 times more total mass of fatty acids than subsequent delivery by either carbonaceous meteorites or electrochemical synthesis. Additionally, we estimate that a single carbonaceous meteorite would not deliver a high enough concentration of fatty acids (∼15 mM for decanoic acid) into an existing body of water on the Earth’s surface to spontaneously form membranes unless the fatty acids were further concentrated by another mechanism, such as subsequent evaporation of the water. Our estimates rely heavily on various assumptions, leading to significant uncertainties; nevertheless, these estimates provide rough order-of-magnitude comparisons of various sources of fatty acids on the early Earth. We also suggest specific experiments to improve future estimates. Our calculations support the view that fatty acids would have been available on the early Earth. Further investigation is needed to assess the mechanisms by which fatty acids could have been concentrated sufficiently to assemble into membranes during the origin of life.
Keywords: Fatty acid, membrane, prebiotic chemistry, origin of life, astrobiology
Introduction
Cells use bilayer membranes to separate themselves from their environment. In modern cells, these membranes are composed of phospholipids. During the origin of cells on Earth, more primitive membranes likely played a similar role,1 sequestering cellular building blocks2,3 and polymers.4 The hydrocarbons in modern phospholipids are tails of fatty acids connected by an ester linkage to the glycerol backbone. Fatty acids themselves can assemble into membranes. Fatty acids were likely more abundant than phospholipids on the early Earth, leading to a common hypothesis that the membranes of the first cells were composed of fatty acids.1
Fatty acids consist of a hydrocarbon tail and a carboxylic acid headgroup (Figure 1). Saturated fatty acids with eight or more carbons in a linear chain can assemble into membranes.5 Fatty acids with unsaturated6 or branched7 chains can also assemble into membranes, although in these cases it is unknown whether the minimum number of carbons for membrane assembly is greater or less than eight. Fatty acids with carboxylic acids at both ends of a carbon chain do not assemble into membranes on their own, although these dicarboxylic acids can incorporate into membranes when additional types of amphiphiles are present.8
Figure 1.
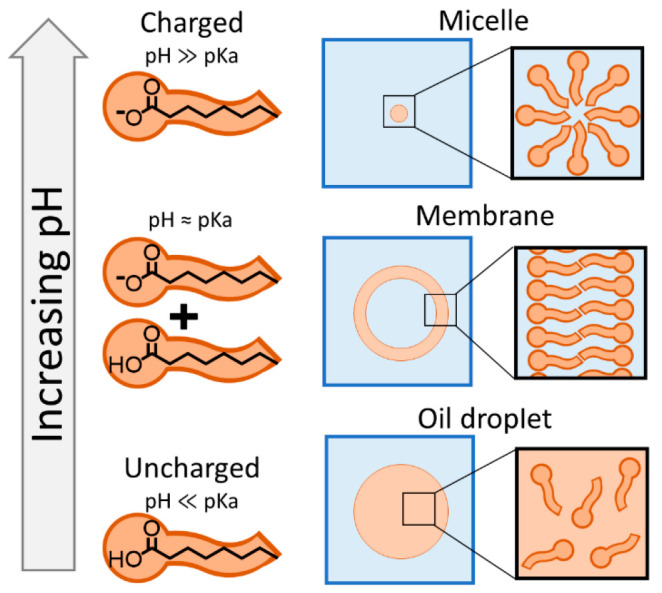
Fatty acid assembly depends on the pH of the surrounding solution. When the pH is below the effective pKa of the fatty acids in a bilayer, fatty acids form an oil that is immiscible with the surrounding aqueous solution (bottom). When the pH is near the effective pKa, fatty acids assemble into bilayers in a membrane (middle). Vesicles, spherical shells of these membranes, may have served as the membrane compartments for the first cells on Earth. When the pH is above the pKa of the fatty acids in a bilayer, the fatty acids assemble into micelles, which cannot encapsulate aqueous solutes (top).
To form membranes, the solution pH must be within about half a unit of the effective pKa of the fatty acid in a bilayer5 (where pKa = −log10 of the equilibrium constant, Ka, for the dissociation of the fatty acid into a proton and the negatively charged amphiphile). If the solution pH is high enough such that the vast majority of headgroups are charged or if the fatty acids have fewer than eight carbons, then fatty acids assemble into nanoscale micelles that cannot encapsulate aqueous solutes (Figure 1). On the other hand, if the solution pH is low enough that the vast majority of headgroups are uncharged, then fatty acids separate into an oil phase.9
Fatty acid membranes provide some advantages for early cell replication compared to modern phospholipid membranes.9 For example, fatty acid membranes are moderately permeable to salts and small organic molecules such as nucleotides, allowing internal replication of nucleic acids, which would have been critical for developing cells.10 The surface area of these vesicles increases when they incorporate additional fatty acids from the environment into the membrane.11,12 A growing vesicle can be supplied with fatty acids from micelles11,13 or from other vesicles.14 Vesicles that retain fatty acids grow while others shrink, which could have enabled competition between primitive cells for a limited supply of fatty acids.12 After acquiring excess membrane surface area, primitive cells could have divided when exposed to modest shear stress.13,15 Vesicles of phospholipids do not grow or divide as readily because aqueous solubility of a phospholipid with two hydrophobic tails is much lower than the solubility of a single-tailed fatty acid, so transfer through aqueous solution is slower.16,17
Here, we review how membrane-forming fatty acids could have been supplied on the early Earth. We identify three relatively well-characterized sources of abiotic fatty acids: delivery by meteorites, synthesis on the surface of metal catalysts, and synthesis by electrochemistry (Figure 2). There are also reports of fatty acid syntheses that fall outside these categories, but these have been less robustly investigated. We summarize the important details of the experiments that have been carried out and discuss whether similar reactions could have plausibly occurred on the early Earth. Finally, we estimate the total mass of fatty acids produced from each of the three relatively well-characterized sources during the Hadean eon when the first cells are hypothesized to have formed.18
Figure 2.
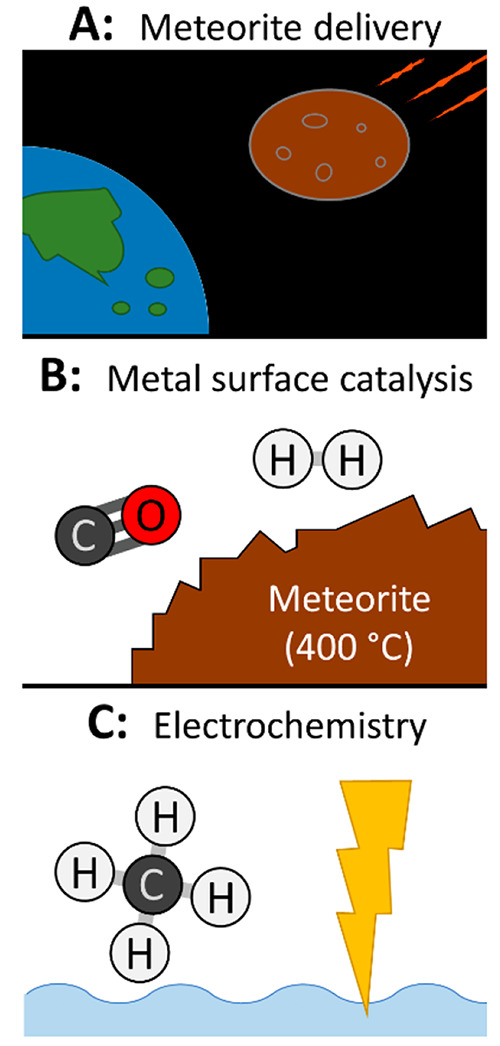
There are three well-characterized sources that could have provided fatty acids to the early Earth. (A) Carbonaceous meteorites can deliver fatty acids.19−22 (B) Metal surfaces can catalyze fatty acid synthesis. As one example, Nooner and Oro mixed filings of the Canyon Diablo meteorite (containing iron and nickel) with deuterium and carbon monoxide gases, and the mixture was heated to 400 °C to produce fatty acids.23 Similar experiments have used pure Fe, Ni, or Fe- and Ni-containing minerals as catalysts and a variety of carbon and hydrogen sources to synthesize fatty acids (Table 1). (C) Fatty acids can also be synthesized during electrical sparking (Table 2). As one example, Yuen et al. used an electric discharge to synthesize fatty acids from methane.24
Carbonaceous Meteorites Deliver Fatty Acids to Earth
Fatty acids have been detected in a variety of carbonaceous meteorites that have landed on Earth,19−22 and molecules extracted from at least one such meteorite assemble into membranes.25 At least 18 different carbonaceous meteorites have been analyzed, and both linear-chain and branched-chain fatty acids have been identified.26 Depending on the type of carbonaceous meteorite, the abundance of membrane-forming fatty acids can range from 1 ppb to 100 ppm by mass.26 Recent reviews provide detailed analyses of meteoritic fatty acids.26,27 Importantly, it remains unclear which types of reactions are responsible for synthesizing meteoritic fatty acids in space.26
Could fatty acids delivered by carbonaceous meteorites have dissolved into water and assembled into membranes? Meteorites can fragment in airbursts during passage through the atmosphere, allowing some fatty acids to remain intact.28,29 Meteorites with radii less than 100 m tend to fragment when differential pressure across the small body exceeds the material strength.28 The Chelyabinsk ordinary chondrite meteorite (radius ∼ 10 m) that fell in Russia in 2013 fragmented at an altitude above 25 km, and fragments were spread into an area 250–300 km2 around the trajectory.30 The largest fragment was ∼0.7 m mean diameter and fell into a lake. Fatty acids in carbonaceous chondrites would likely be similarly dispersed over a wide spatial area.
When meteorite fragments disperse into water on Earth’s surface, fatty acids can dissolve. Numerical models suggest that nucleobases leach out of 20 cm meteorite fragments and mix into the surrounding water within about three years.29 At moderately alkaline pH, fatty acids can be even more soluble because of their charged headgroup. However, in order for fatty acids to assemble into membranes, the fatty acids must accumulate in solution above the critical vesicle concentration (∼15 mM for decanoic acid3). If fatty acids are present at concentrations below the critical vesicle concentration, membrane assembly does not occur.
Here, we use the measured abundance of decanoic acid (a 10-carbon fatty acid) in carbonaceous meteorites26 to calculate the volume of a meteorite fragment that would be required to deliver enough decanoic acid into water so that the concentration of decanoic acid equals the critical vesicle concentration. Although it is not known precisely how meteorite size, initial velocity, or impact angle influences the fraction of fatty acids that survive impact, we note that a large portion of the meteorite’s initial mass (and thus a large portion of its fatty acids) may be destroyed by ablation and heating during travel through the atmosphere.31−33 Given that our estimates rely on the average mass fraction of decanoic acid that has been recovered from natural carbonaceous meteorites and that we do not know a priori the mass, velocity, and impact angle of each meteorite, we assume the measured fatty acid abundances represent the average survival over the entire population of possible impacts. Although this assumption introduces potential errors, a more precise calculation is beyond the scope of this study.
The volume of a meteorite fragment (Vmeteor, expressed in m3) that would be required to deliver enough decanoic acid to reach the critical vesicle concentration as a function of water volume (Vwater, expressed in m3) is given by eq 1:
| 1 |
where Ccvc is the critical vesicle concentration for decanoic acid (15 mol/m3 of water, equivalent to 15 mM), w is the molar mass of decanoic acid (0.1723 kg/mol), a is the dimensionless fraction of the meteorite’s mass that is decanoic acid (2 × 10–5 for CM2 type meteorites), and ρ is the meteorite density (2100 kg/m3 for CM type meteorites).
The result of our estimate is shown in Figure 3. To deliver enough decanoic acid to form membranes, the volume of the meteorite fragment would have to exceed the volume of the waterbody. If a meteorite fragment were to land in a large enough waterbody to submerge the fragment, the decanoic acid that subsequently dissolved in the water would be too dilute to form membranes. However, this does not rule out membrane formation. The concentration of fatty acids could increase during dry periods, accompanied by a net loss of water due to evaporation.34 Although we limited our calculation to decanoic acid because the critical vesicle concentration has been measured, a range of fatty acids from 8 to 12 carbons can be delivered simultaneously during a meteorite impact. The presence of these additional fatty acids could enable membrane formation at lower concentrations of decanoic acid.35,36 We do not consider that CM type meteorites can contain significant water (up to ∼9% by mass37). If all meteoritic fatty acids were to somehow dissolve in only this meteoritic water, the concentration of decanoic acid could exceed the critical vesicle concentration; this can be interpreted as an upper limit for the concentration of fatty acids delivered by a meteorite.
Figure 3.
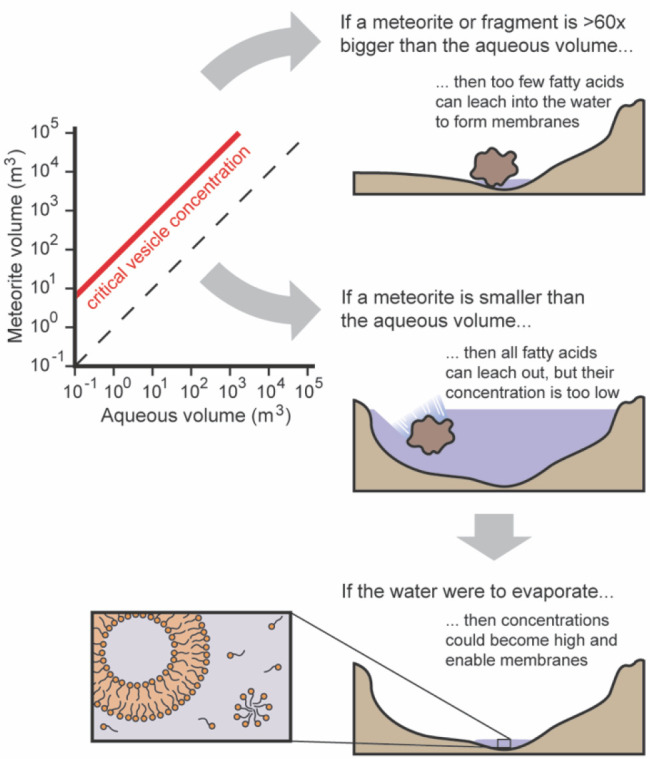
A single fragment of a carbonaceous meteorite cannot directly deliver enough decanoic acid to a body of water to form membranes. To exceed the critical vesicle concentration (∼15 mM),3 the volume of the meteorite (red line) would exceed the volume of the water. However, subsequent evaporation of the water could concentrate decanoic acid and enable membrane formation. A CM2 type meteorite is assumed because it contains the most decanoic acid on average (20 ppm by mass26). The density of CM-type meteorites is 2100 kg/m3.38 Only meteorites with radii less than 100 m (∼106 m3 for a spherical meteorite) can fragment and impact the Earth’s surface with low enough energy to preserve fatty acids.28,29 See eq 1 for details of the calculation.
To conclude our section on carbonaceous meteorites, we find that meteorite delivery was unlikely to directly yield high enough aqueous concentrations of fatty acids to form membranes on the early Earth. Additional processes would have been necessary to further concentrate fatty acids above the critical vesicle concentration, which we cannot rule out.
Catalysis of Fatty Acid Synthesis by Metal Surfaces
The most commonly reported abiotic synthesis of fatty acids involves catalytic metal surfaces. Within this class of syntheses, Fischer–Tropsch reactions are the most thoroughly investigated. Fischer–Tropsch reactions occur when H2 and either CO or CO2 adsorb onto a metal surface.39,40 Surfaces of solid iron or nickel are most commonly tested, although FeS, NiS, and Fe3O4 minerals have also been used to produce fatty acids.41−44 In most experiments, metal surfaces must be heated above 150 °C to produce fatty acids. Catalysts contain metals in their reduced form; the synthesis of membrane-forming fatty acids (at least 8 carbons) has not been demonstrated on oxidized metal surfaces.23,45−48 The synthesis of fatty acids seems to occur at the gas–solid interface (Figure 4). Even in experiments designed to eliminate gaseous headspace, reactions are suggested to proceed in gaseous bubbles within the aqueous solution.48 Whether or not an explicit gaseous headspace is present, the carbon-containing precursors are generally supplied as gases. However, for experimental convenience in hydrothermal experiments, both formic acid and oxalic acid have been used as aqueous starting materials for fatty acid synthesis because both compounds decompose into H2 and CO2 at temperatures above 150 °C.49
Figure 4.
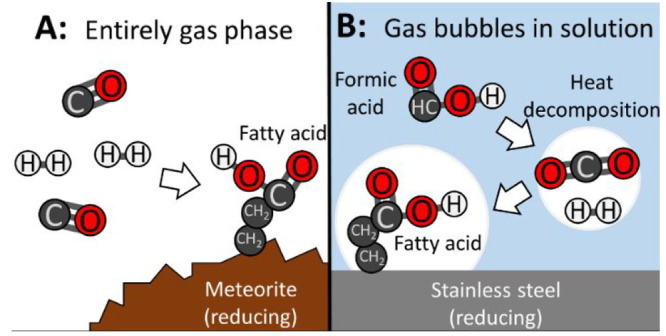
Fatty acid synthesis occurs at the interfaces between reducing metal surfaces and a gaseous headspace. (A) Nooner and Oro showed that deuterium and carbon monoxide gases react together on the surface of hot (400 °C) meteorite filings to produce membrane-forming fatty acids.23,50 When the meteorite filings were artificially oxidized, fatty acid synthesis was not observed.23 (B) In hydrothermal experiments, McCollum et al. report that the synthesis of membrane-forming fatty acids occurs within gaseous bubbles adsorbed onto oxidation-resistant stainless steel surfaces.49 When oxidized metal surfaces are present instead of stainless steel, only short-chain (<5 carbons) fatty acids are formed.23,45−48 In these hydrothermal experiments, aqueous formic acid or oxalic acid is used for experimental convenience as a source of H2 and CO2.
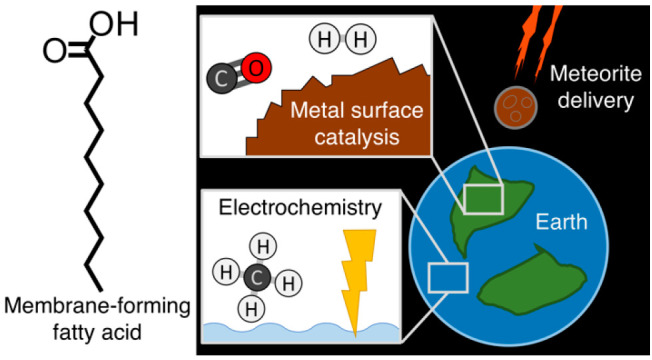
Metal-catalyzed reactions generally create a diverse mixture of product types, including hydrocarbons and fatty alcohols in addition to fatty acids.48,49,51−54 For each type of product, molecules with more carbons are less abundant.40 Many experiments have produced only short-chain carboxylic acids containing fewer than the 8 carbons required for membrane assembly5 (Figure 5). The carbon chain of a fatty acid could be elongated upon further reaction with a metal catalyst,40 although additional experiments are required to validate this hypothesis. Table 1 summarizes experiments in the literature that have produced fatty acids using metal catalysts.
Figure 5.
Length of fatty acids delivered by meteorites (labeled “Meteor.”) or produced in abiotic synthesis experiments. Fatty acids with at least eight carbons, indicated by the dashed red line, can assemble into membranes. All the fatty acids produced are saturated and unbranched (except in Scheidler et al. 2016, where experiments also produced unsaturated fatty acids). Note that detection of a fatty acid with a certain length may not have been attempted during every experiment.
Table 1. Summary of Experiments That Used Metal Surfaces to Catalyze the Synthesis of Fatty Acidsa.
| year and ref | explicit gas phase? | explicit aqueous phase? | H2 source | carbon source | solid surface | reaction conditions | number of carbons per fatty acid |
|---|---|---|---|---|---|---|---|
| 197650 | Yes | No | D2 (g) | CO (g) | Meteorite filings and K2CO3 | 50 h at 370 °C | 10–16 |
| 197923 | Yes | No | D2 (g) | CO (g) | Meteorite filings (containing iron and nickel) and carbonate salts | 48 h at 400 °C | 5–21 |
| 199741 | Yes | Yes | N/A | CO (g) and CH3SH (aq) | NiS–FeS | 1 week at 100 °C | 2 |
| 199949 | Yes | Yes | Formic acid or oxalic acid (aq) | Formic acid or oxalic acid (aq) | Stainless steel | >48 h at 175 °C | 6–22 |
| 200151 | No | Yes | Oxalic acid (aq) | Oxalic acid (aq) | Stainless steel | 48 h at 100 °C | 7–18 |
| 200363 | Yes | No | N/A | CH4 and CO2 | 5% Pt/alumina | 2 h with temperature increasing from 200 to 400 °C | 2 |
| 200464 | No | Yes | Formic acid (aq) | Nonane-thiol | Ni0b | 6 h at 250 °C and 200 MPa | 10–13 |
| 200452 | No | Yes | Oxalic acid (aq) | Oxalic acid (aq) | Stainless steel | 18 h at 300 °C | 8–20 |
| 200648 | No | Yes | Formic acid (aq) | Formic acid (aq) | Powdered iron | 86 h at 250 °C and 325 bar | 2–4 |
| 201065 | Yes | Yes | Water (l)c | CO2 (aq) | Iron nanoparticles | 25–200 h at fixed temp from 80 to 200 °C | 1–2 |
| 201666 | Yes | Yes | N/A | Formamide | Meteorite powder | 24 h at 140 °C | 16, 18 |
| 201642 | Yes | Yes | N/A | CO (g) and acetylene (g) | NiS | 1 week at 105 °C and 2.5 bar | Saturated: 3, 5 |
| Unsaturated: 3, 5, 7, 9 | |||||||
| 201853 | No | Yes | Oxalic acid (g) | Oxalic acid (g) | Stainless steel | >66 h at 175 °C | 7–26 |
| 201867 | Yes | Yes | N/A | CO2 (g) | Fe0, Ni0, Co0 | 16 h at fixed temp from 30 to 150 °C | 1–2 |
| 201954 | Yes | Yes | N/A | HCN, Na2CO3, formaldehyde | Macroporous Ni | Multistep reaction | 2–10 |
| 202043 | No | Yes | Water (l) | CO2 (aqueous) | NiS–FeS | 50 min at room temperature | 1 |
| 202044 | Yes | Yes | H2 (g) | CO2 (g) | Fe3S4, Fe3O4, Ni3Fe | 0–24 h at fixed temp from 60 to 100 °C | 1–2 |
Unless otherwise specified, fatty acids were saturated and unbranched. Note: The minimum and maximum length fatty acids may have been synthesized during separate experiments that were reported in the same reference.
Other minerals were tested as well, although reactions with Ni0 produced linear fatty acids with the greatest number of carbons.
H2 is generated when water oxidizes the Fe.
Metal-catalyzed reactions could have occurred on the early Earth after meteorite impacts, which delivered iron, nickel, and heat. Meteoritic metals could have been exposed to atmospheric H2, CO2, and CO, enabling surface-catalyzed synthesis of fatty acids. Extremely large impactors, about the size of the asteroid Vesta (∼1020 kg), could have transformed the Earth into a global Fischer–Tropsch reactor with surface temperatures >100 °C and high partial pressures of H2, CO2, and CO for thousands of years.55 Catalytic metal surfaces would be required to produce fatty acids, so future research in this area will be especially valuable if it constrains the location and quantity of reduced metals after such impacts.56 Although a large impact would have been catastrophic for any life that was already present on Earth, it could have potentially seeded Earth’s postimpact surface with fatty acids and other necessary biomolecules, enabling life to subsequently emerge.57
Ground-breaking experiments by Nooner and Oro modeled a postimpact scenario for fatty acid synthesis.23,50 It remains uncertain how the yield of fatty acids depends on experimental parameters such as partial pressure and temperature. Kinetic models for the Fischer–Tropsch process have been developed in industrial settings, which generally do not mimic plausible early Earth conditions, and the quantitative form of the models depends on the design of the reactor.58 Until experiments are conducted to understand how fatty acid production depends on reaction parameters more relevant to the early Earth, there will be substantial uncertainty in estimates of production of fatty acids by metal catalysts on the early Earth.
Another natural setting in which the metal catalyzed synthesis of fatty acids might occur is in a hydrothermal environment, where the conversion of CO2 and H2 into fatty acids is thermodynamically favorable.59 Fatty acids have been detected in natural hydrothermal systems; however, it is unclear what fraction of those fatty acids were produced by modern cells.60 Ultramafic rocks (relatively Fe- and Mg-rich and Si-poor) are common at some deep sea hydrothermal vents.61 Could metal-rich minerals within these rocks serve as catalysts for fatty acid synthesis? To address this question, we consider the oxidation state of the mineral surface. Ultramafic mineral surfaces become oxidized by reacting with seawater and generating H2 in serpentinization reactions.62 As noted above, to date, there have been no reports of the synthesis of membrane-forming fatty acids (at least 8 carbons) on oxidized metal surfaces.23,45−48 Laboratory experiments failed to produce fatty acids with more than 2 carbons when oxidized olivine (ultramafic mineral with general composition (Fe,Mg)SiO4) was the sole catalyst.45 In these experiments, the olivine was heated in water for 96 days to allow ample time for oxidation by serpentinization, and formic acid was included as an additional source of H2.45 It is unknown whether olivine surfaces could catalyze fatty acid synthesis before becoming oxidized during serpentinization.
Hydrothermal experiments with oxidation-resistant stainless steel surfaces, clearly not natural settings, do produce fatty acids from formic acid with up to 22 carbons.49 However, these experiments also permitted vapor-phase reactions, which may have enabled production of longer fatty acids regardless of the oxidation state of the surface. Thus, in natural hydrothermal settings, it remains unclear if catalytically active, reduced mineral surfaces could persist or be replenished quickly enough to catalyze fatty acid synthesis. Without a suitable catalyst, fatty acid synthesis would likely be slow or nonexistent in hydrothermal environments.62 In conclusion, additional experiments are necessary to determine whether fatty acids could be synthesized in natural hydrothermal settings.
Electrochemical Synthesis of Fatty Acids
Electrochemical reactions can generate diverse organic compounds, including amino acids,68,69 nucleobases,70 and fatty acids. For example, spark discharges between an electrode in the gaseous headspace and another electrode either in the same headspace71 or in solution24,72 can produce fatty acids. In the experiments summarized in Table 2, CH4 in the gaseous headspace served as the source of carbon in three of the four experiments generating linear fatty acids of varying lengths with only one experiment reporting chains long enough (at least 8 carbons) to form membranes.71 An additional electric discharge experiment produced carbon chains of 2–6 carbons with carboxylic acids on both ends.73
Table 2. Summary of Electrochemical Experiments That Have Synthesized Fatty Acidsa.
| year and ref | electric discharge description | carbon source | reaction conditions | number of carbons per fatty acid |
|---|---|---|---|---|
| 196772 | 1 electrode in solution, 1 electrode in headspace | CH4 (g) | 96 h of discharge | 2–5 |
| 198124 | 1 electrode in solution, 1 electrode in headspace | CH4 (g) | 24 h of discharge; pH = 8 | 2–7 |
| 201578 | Both electrodes in solution | CO2 (aq) | 150 h with electric potential cycling from −0.8 to 0.2 V | 1–2 |
| 202171 | Both electrodes in headspace | CH4 (g) | Discharge alternating on/off for 14 days, pH = 8.7, room temperature | 6, 9 |
All fatty acids were saturated and unbranched.
The goal of most electrochemical experiments is to simulate lightning strikes through a methane-rich atmosphere on the early Earth. However, attributes of natural lightning strikes are challenging to reproduce in the laboratory. Criado-Reyes et al. used a Tesla coil that generated a 3 × 104 V potential for 7 days at room temperature.71 In contrast, a natural lightning strike generates a potential of about 108 V for <1 s,74 and temperatures of the air surrounding a lightning strike can reach 30 000 °C.75 Unfortunately, experimental evidence is lacking to describe how fatty acid yields depend on the voltage, duration, or total energy dissipated by laboratory sparking. Chyba and Sagan assumed that electrochemical production of organic molecules on early Earth should depend on the total amount of electrical energy dissipated by lightning strikes and coronal discharges.76 Additional experiments are needed to validate this assumption.
Only slightly more is known about the role of the atmosphere and solid surfaces during electrochemical synthesis. Experiments by Schlesinger and Miller have shown that the yield of amino acids during sparking increases with the partial pressure of CH4;77 similar experiments with fatty acids are still needed. Borosilicate glass as a substrate has also been shown to increase the yields of electrochemical fatty acid synthesis.71 Although borosilicate does not occur naturally, silicates would have been ubiquitous on the early Earth because they are common rock-forming minerals. Additional experiments are necessary to better understand how the yields of electrochemical fatty acid production depend on the gaseous, solid, aqueous, and electrical environments. Nevertheless, the available experimental data suggest that fatty acid synthesis is possible under certain electrochemical conditions.
Alternative Types of Fatty Acid Synthesis
In addition to the three main sources reviewed above, there are references in the literature to four alternative types of fatty acid synthesis (Figure 6, Table 3). These include photochemical reactions,54,79 irradiation by massive particles,80,81 gas-phase ion–molecule reactions,82 and redox reactions in aqueous solution.83−85
Figure 6.
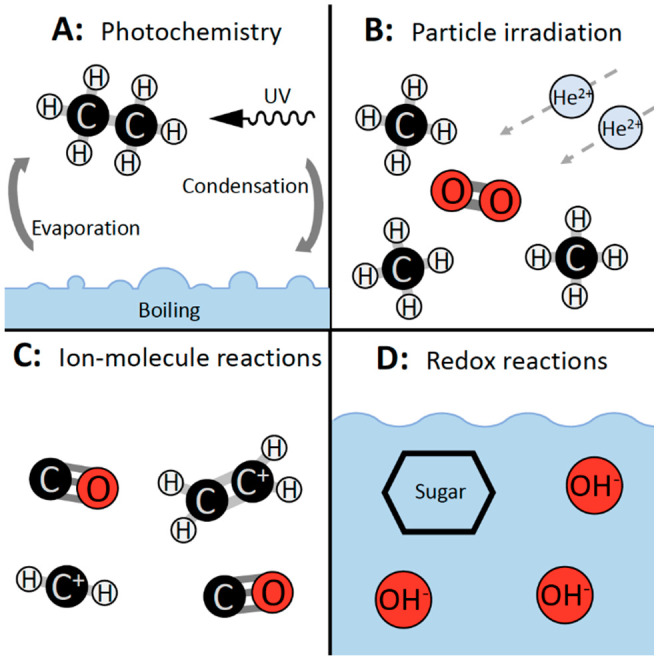
Alternative reaction types that have demonstrated fatty acid synthesis. (A) Groth and Weyssenhoff used UV photochemistry to convert ethane into fatty acids.79 (B) Kaiser et al. irradiated an ultracold (10 K) mixture of ∼99% CH4 and ∼1% O2 with 9 MeV alpha particles to produce fatty acids.80 (C) Blagojevic et al. report reactions between gas-phase ions (CH2+ and C2H4+) and CO to produce fatty acids.82 (D) Novotný et al. report the decomposition of monosaccharides into fatty acids under mild alkaline conditions (50 mM NaOH).84
Table 3. Summary of Experiments That Have Synthesized Fatty Acids by the Mechanisms Illustrated in Figure 6a.
| year and ref | reactants | reaction conditions | number of carbons per fatty acid |
|---|---|---|---|
| Photochemistry | |||
| 196079 | Ethane, NH3, H2O | 1 week of UV irradiation (185 and 254 nm) | 1–3 |
| 201954 | HCN, formaldehyde, Na2CO3 | Multistep reaction (254 nm UV irradiation and catalysis by Ni surface) | 2–10 |
| Irradiation by Massive Particles | |||
| 199580 | CH4, O2 | Irradiation with 9 MeV alpha particles at 10 K | 13, 15, 17 |
| 201081 | CO2, hydrocarbons (1–6 carbons) | Irradiation with 5 keV electrons at 10 K | undetermined |
| Gas-Phase Ion–Molecule Reactions | |||
| 200382 | CO and either CH2+ or C2H4+ | CH4 and C2H4 are ionized and reacted together in the presence of He and trace H2O at 0.35 Torr and room temperatureb | 2–3 |
| Redox Reactions in Solution | |||
| 196383 | HCN, NH3 | 90 °C for 18 h | 1 |
| 200085 | Fatty aldehydes (either 7 or 8 carbons) | 30% H2O2 solution at 90 °C for 2 h | 7 or 8 |
| 200884 | Glucose, fructose, arabinose, glyceraldehyde, or dihydroxyacetone | 50 mM NaOH for 1 h | 1–3 |
All fatty acids were saturated and unbranched.
The authors suggest that their synthesis models low temperature and low pressure environments.
There have been two reports of fatty acid synthesis by UV photochemistry. By irradiating a gaseous mixture of ethane and ammonia above boiling water (Figure 6A) at 185 and 254 nm, Groth and Weyssenhoff generated fatty acids 1–5 carbons long.79 Bonfio et al. used a multistep procedure to synthesize longer-chain fatty acids capable of membrane assembly.54 UV irradiation at 254 nm was used in one step, and catalysis by a nickel surface was used in a subsequent step. Photochemistry could provide a plausible explanation for the presence of fatty acids on the surface of the early Earth because UV radiation down to ∼200 nm could have penetrated prebiotic atmospheres.86 The experiments by Groth and Weyssenhoff did not generate fatty acids when methane was used instead of ethane;79 further investigation is needed to determine whether radiation from realistic early Earth solar spectra could have enabled photochemical conversion of methane into fatty acids. In contrast, Bonfio et al. used starting materials that are more plausibly prebiotic such as formaldehyde, HCN, NaH2PO4, Na2CO3, and NaSH,54 but these reagents impose a constraint on the geochemical scenario for the early Earth. In a separate experiment by Dworkin et al., membrane-forming amphiphiles were synthesized by irradiating ultracold ices composed of 100:50:1:1 H2O:CH3OH:NH3:CO with 121.6 and ∼160 nm UV photons, although the identity of these amphiphiles was not determined.87
The second type of “alternative” fatty acid synthesis is irradiation by particles. Experiments of this type were designed to simulate chemistry in the outer solar system, rather than the early Earth. By irradiating an ultracold (10 K) mixture of ∼99% CH4 and ∼1% O2 with 9 MeV alpha particles, Kaiser et al. produced linear fatty acids with 13, 15, or 17 carbons80 (Figure 6B). In a subsequent experiment by Kim and Kaiser, an ultracold (10 K) mixture of CO2 and hydrocarbons (1–6 carbons) was irradiated with 5 keV electrons. The presence of carboxylic acids was confirmed by Fourier-transform infrared spectroscopy, but specific fatty acids were not identified.81
A third alternative fatty acid synthesis is discussed by Blagojevic et al.82 In their experiments, reactions between gas-phase ions (CH2+ and C2H4+) and molecules (CO) resulted in carboxylic acids with 2 or 3 carbons (Figure 6C). Additional gas-phase ion–molecule reactions to produce formic acid (e.g., CH4 + O2+ → HCOOH2+ + H) have been suggested but not validated experimentally.88 These reactions were suggested to occur in the interstellar medium, and the relevance to early Earth conditions has not been explored.
The fourth group of lesser studied fatty acid syntheses are redox reactions. Three sets of redox reactions have been shown to produce fatty acids in solution. The first experiments from Sato et al. use hydrogen peroxide to oxidize fatty aldehydes (7–8 carbons) to produce fatty acids with the same carbon-chain length.85 It is unlikely that sufficient hydrogen peroxide would have been present on the early Earth.89 In the second set of experiments, Novotný et al. report the decomposition of monosaccharides (3–6 carbons) into linear carboxylic acids (1–3 carbons) under mild alkaline conditions (50 mM NaOH, Figure 6D).84 Diverse monosaccharides are obtained in low yield during the formose reaction,90 and alkaline lakes on early Earth might have been sites for decomposition into short-chain fatty acids.91,92 Finally, Lowe et al. reported production of formic acid and potentially other carboxylic acids by heating a mixture of ammonia and hydrogen cyanide to 90 °C.83 Hydrogen cyanide is considered a prebiotic reagent that can be sequestered and concentrated as ferrocyanide within early Earth environments.91,93
There are numerous additional pathways for the synthesis of short-chain (1–3 carbons) linear fatty acids,94 but because membrane assembly requires fatty acids with at least 8 carbons, we have generally omitted them. We are unaware of any abiotic mechanisms by which carbon chains of existing fatty acids are elongated. Modern cells synthesize fatty acids from acetyl-CoA using the sophisticated enzyme complex fatty acid synthetase.95 Because the intermediate compounds that are produced during this process are unstable, it is believed that fatty acid synthesis via these reactions would have occurred at a negligible rate on the early Earth before the emergence of enzymes.96 Finally, thermodynamic calculations suggest that fatty acids can be synthesized from polyaromatic hydrocarbons,97 but to our knowledge, this synthesis has never been demonstrated experimentally.
Critical Analysis of Analytical Techniques
Many of the reactions discussed above produce a wide variety of fatty acids and other products. Uniquely identifying products can be challenging, and determining the concentration of each product is even more difficult. In Table 4, we summarize the reported concentrations of fatty acids from each experiment and comment on potential limitations of the analyses. In general, the papers in Table 4 convincingly identify fatty acids of various lengths but do not provide substantial evidence for the fatty acid concentrations that they report. Moreover, many papers report only relative concentrations of fatty acids (e.g., X% of all products by mass or moles) instead of absolute concentrations (e.g., X moles or X grams), so it is difficult to compare product yields between experiment types. An ideal strategy for the characterization and absolute quantitation of fatty acids was employed by Yuen et al., which involved chromatography and tandem mass spectrometry with isotope-labeled internal standards to eliminate matrix effects.24 Additional synthesis experiments that determine the absolute concentration of fatty acids would be valuable.
Table 4. Summary of Analytical Techniques in Each Reporta.
| year and ref | carbons per fatty acid | yield information | analytical techniques | identification of products | quantitation of product concentrations |
|---|---|---|---|---|---|
| Method 1: Metal Surface Catalysis | |||||
| 197650 | 10–16 | None | GC-MS | Unambiguous identification by comparing fragmentation spectra from (derivatized) products with fragmentation spectra from authentic standards | N/A |
| 197923 | 5–21 | ≤0.08% yield of normal fatty acids (by mass, relative to initial CO) | GC-MS | Unclear how products were identified. Comparisons with authentic standards were not shown. | No data were shown to validate the FID procedure for quantifying product concentrations. |
| GC-FID | |||||
| 199741 | 2 | ≤40% yield (by moles, relative to initial CH3SH) | GC-MS | Unclear how products were identified. Comparisons with authentic standards were not shown. | No data were shown to validate the GC-MS procedure for quantifying product concentrations. |
| 199949 | 6–22 | ≤20.8% of all products are fatty acids (by moles) | GC-MS | Unambiguous identification of select products by comparing fragmentation spectra and retention times with authentic standards. | No data were shown to validate the procedure for quantifying product concentrations. |
| GC-FID | |||||
| 200151 | 7–18 | ≤20% yield of fatty acids (by mass, relative to total mass of reaction extract) | GC-MS | Unambiguous identification by comparing fragmentation spectra and retention times with authentic standards. | No data were shown to validate the GC-MS procedure for quantifying product concentrations. |
| 200363 | 2 | None | Diffuse reflectance infrared Fourier transform spectroscopy | Using diffuse reflectance infrared Fourier transform spectroscopy, experimental spectra were compared to authentic standards. | N/A |
| 200464 | 10–13 | C10: 53.7% yield | GC-MS | Unambiguous identification by comparing fragmentation spectra with authentic standards. | Calibration curves were described in the text to validate the procedure for quantifying product concentrations. Pentadecane was used as an internal standard for all compounds. |
| 200452 | 8–20 | 2.8% of all products are fatty acids (unclear whether by mass or moles) | GC-MS | Unambiguous identification by comparing fragmentation spectra and retention times with authentic standards. | No data were shown to validate the MS procedure for quantifying product concentrations. |
| 200648 | 2–4 | C2: 1% yield | GC-MS | Unclear how products were identified. Comparisons with authentic standards were not shown. | No data were shown to validate the FID procedure for quantifying product concentrations. |
| C3: 0.06% yield | GC-FID | ||||
| C4: <0.02% yield | |||||
| (by moles, relative to initial formic acid) | |||||
| 201065 | 1–2 | C2: 9.0 mM | GC-MS | Unambiguous identification by comparing fragmentation spectra with authentic standards. | Calibration curves were shown to validate the procedure for quantifying product concentrations. Internal standards were not used. |
| C3: 3.5 mM | |||||
| 201666 | 16, 18 | C16: 0.011% yield | GC-MS | Unambiguous identification by comparing fragmentation spectra with authentic standards and literature references. | No data were shown to validate the MS procedure for quantifying product concentrations. Betulinic acid was mentioned as the internal standard for all products. |
| C18: 0.02% yield | |||||
| (by moles, relative to initial NH2CHO) | |||||
| 201642 | Saturated: 3, 5 | C3: 7.1 mM | GC-MS | For most products, unambiguous identification by comparing fragmentation spectra with authentic standards. For select products, identification was inferred on the basis of mass spectra and relative retention times. | Calibration curves were mentioned (but not shown) to validate the procedure for quantifying product concentrations. For some products, calibration curves were made with authentic standards. For other products, related compounds were used for calibration. Internal standards were not used. |
| C5: 0.3 mM | |||||
| C3 unsaturated: 3.9 mM | |||||
| Unsaturated: 3, 5, 7, 9 | |||||
| C5 unsaturated: 7.9 mM | |||||
| C7 unsaturated: 0.44 mM | |||||
| C9 unsaturated: 0.011 mM | |||||
| 201853 | 7–26 | 7.8% of all products are fatty acids (unclear whether by mass or moles) | GC-MS | Unambiguous identification by comparing fragmentation spectra with authentic standards. | No data were shown to validate the GC-MS procedure for quantifying product concentrations. N-Eicosane-d42 was mentioned as the internal standard for all experiments. |
| 201867 | 1–2 | Formic acid: ≤0.21% yield | GC-MS | Unambiguous identification by comparing GC-MS fragmentation spectra and retention times with authentic standards. In addition, H NMR spectra were compared with authentic standards. | H NMR calibration curves were shown to validate the procedure for quantifying product concentrations. Authentic standards were used, and DSS-Na was used as an internal standard. |
| Acetic acid: ≤0.053% yield | 1H NMR | ||||
| (by moles, relative to initial CO2) | |||||
| 201954 | 2–10 | 36% of all 8-carbon products are fatty acids (by moles) | 1H NMR | Unambiguous identification by comparing H NMR spectra with authentic standards. | Data from H NMR spectra were used to determine the relative abundance of fatty acids. Internal standards were not discussed. |
| 202043 | 1 | C1: 1.5 μM | 1H NMR | Unambiguous identification by comparing H NMR spectra with authentic standards. | H NMR spectra with an internal standard (acetone) were used to quantitate formate concentration. To validate the quantitation, additional formate was spiked in and a corresponding increase in H NMR signal was observed. |
| 13C-NMR | |||||
| 202044 | 1–2 | C1: ∼20% yield | LC-MS | Unambiguous identification by comparing H NMR spectra with authentic standards. | H NMR calibration curves were mentioned (not shown) to validate the procedure for quantifying product concentrations. Authentic standards were used, and DSS-Na was used as an internal standard. |
| C2: ∼0.4% yield | |||||
| (by moles, relative to CO2) | LC-UV | ||||
| 1H NMR | |||||
| Method 2: Electrochemistry | |||||
| 196772 | 2–5 | C2: 1.2% yield | GC-MS | Unambiguous identification by comparing fragmentation spectra and retention times with authentic standards. | No data were shown to validate the MS procedure for quantifying product concentrations. |
| C3: 0.86% yield | |||||
| C4: 0.088% yield | |||||
| C5: 0.066% yield | |||||
| (by moles, relative to initial CH4) | |||||
| 198124 | 2–7 | C2: 0.10% yield | GC-MS | Unambiguous identification by comparing fragmentation spectra and retention times with authentic standards. | Calibration curves were shown to validate the procedure for quantifying product concentrations. Singly deuterated internal standards were used for each product. |
| C3: 0.68% yield | |||||
| C4: 0.014% yield | |||||
| C5: 0.0050% yield | |||||
| C6: 0.00073% yield | |||||
| C7:0.00025% yield | |||||
| (by moles, relative to initial CH4) | |||||
| 201578 | 1–2 | C1: ∼1 μmoles | 1H NMR | Unambiguous identification by comparing H NMR spectra with authentic standards. | H NMR calibration curves were shown (for formic acid) to validate the procedure for quantifying product concentrations. Authentic standards were used and Me4Si was used as an internal standard. |
| C2: ∼0.5 μmoles | |||||
| 202171 | 6, 9 | C6: ≤0.0078% yield | GC-MS | Unambiguous identification by comparing fragmentation spectra and retention times with authentic standards. | No data were shown to validate the GC-MS procedure for quantifying product concentrations. |
| C9: ≤0.016% yield | |||||
| (by mass, relative to total mass of reaction extract) | |||||
| Method 3: Photochemistry | |||||
| 196079 | 1–3 | C1: 82 μmoles | Silica gel chromatography | Identification of products by comparison of retention times with authentic standards. | Silica gel chromatography was used to separate C1, C2, and C3 products. Each putatively pure product was titered with NaOH to find the equivalence point. |
| C2: 234 μmoles | |||||
| Acid–base titration | |||||
| C3: 15 μmoles | |||||
| 201954 | 2–10 | 36% of all 8-carbon products are fatty acids (by moles) | 1H NMR | Unambiguous identification by comparing H NMR spectra with authentic standards. | Data from H NMR spectra were shown and used to determine the relative abundance of fatty acids. Internal standards were not mentioned. |
| Method 4: Irradiation by Massive Particles | |||||
| 199580 | 13, 15, 17 | C13: maximum 18.6 picograms | GC-MS | Unclear how products were identified. Comparison with authentic standards were not mentioned. | No data were shown to validate the MS procedure for quantifying product concentrations. |
| C15: maximum 83.9 picograms | |||||
| C17: maximum 57.0 picograms | |||||
| 201081 | Undetermined | 1016 molecules/cm2 | FTIR | Regions of the FTIR spectra were considered diagnostic for carboxylic acids: | The intensity of the FTIR spectra at 1720 cm–1 was used to provide an upper limit on carboxylic acid concentration. |
| ν(O–H) stretching = 3500–2500 cm–1. | |||||
| ν(C=O) = 1720 cm–1 | |||||
| ν(C–O) = 1282 cm–1 | |||||
| Method 5: Gas-Phase Ion–Molecule Reactions | |||||
| 200382 | 2–3 | No data were shown | GC-MS | Unambiguous identification by comparing fragmentation spectra with authentic standards. | N/A |
| Method 6: Redox Reactions in Solution | |||||
| 196383 | 1 | No quantitative data were shown | Paper chromat-ography | The presence of formic acid was inferred on the basis of the comparison of paper chromatography mobility to an authentic standard, and on the basis of a reaction with ammoniacal silver nitrate. | N/A |
| 200085 | 7 or 8 | C7: 73–85% yield | GC-MS | Unambiguous identification by comparing fragmentation spectra with authentic standards. | No data were shown to validate the GC-MS procedure for quantifying product concentrations. |
| C8: 65% yield | |||||
| (unclear whether by mass or moles) | |||||
| 200884 | 1–3 | C1: 7–20% yield | GC-MS | Unambiguous identification by comparing fragmentation spectra with publicly available data and by comparing retention times with authentic standards. | Calibration curves were mentioned (but not shown) to validate the procedure for quantifying product concentrations. An internal standard (heptadecane) was used. |
| C2: 0.7–12% yield | |||||
| C3: <0.01–0.2% yield | |||||
| (by moles) | |||||
Unless otherwise specified, fatty acids are saturated and unbranched.
Estimating the Contribution of Each Source to the Early Earth Fatty Acid Inventory
To gauge the relative importance of each fatty acid source for the origin of cells, we go beyond purely reviewing the literature with a goal of estimating how much fatty acid could be supplied to the early Earth from three sources: delivery by carbonaceous chondrites, catalysis by metal surfaces, and electrochemistry. Many relevant parameters for these estimates lack experimental constraints, so assumptions are needed. Our first assumption is that cells formed during the latter part of the Hadean eon; the full eon was ∼4.6 to 4.0 billion years ago (Gya).18 During the early Hadean, Earth was potentially hit by impactors that were large enough to sterilize the planet’s surface and reset the fatty acid inventory, so life must have originated after the last such event.98 The median age of estimates for the last ocean-vaporizing impact is ∼4.3 Gya,99 and we assume that life had originated by 4.0 Gya.18 In the subsections below, we construct back-of-the-envelope estimates for the total mass of fatty acids supplied to Earth during this interval. Our estimates are based on empirical data from the literature, and we indicate when existing models from the literature are applied. We articulate our assumptions and suggest experiments that will help to refine these estimates. Our estimates cannot distinguish fatty acids that form membranes (more than 8 carbons) from those that do not (less than 8 carbons) because the empirical data that one of our estimates relies on does not do so.
Meteorite Delivery
We estimate the total mass of 2–12 carbon fatty acids delivered to Earth by carbonaceous meteorites, Ms, using the following equation:
| 2 |
In eq 2, fL is the dimensionless fraction of a meteorite’s mass that comprises fatty acids of length L, where the length denotes the number of carbons in the fatty acid chain. Values for fL depend on the type of carbonaceous meteorite, and average values can range from 10–9 for 10-carbon fatty acids to 10–3 for 2-carbon fatty acids.26 Parameter M is the time-integrated total mass of carbonaceous meteorites with radii of 1–100 m that would impact Earth from 4.3 Gya to 4.0. To estimate M, we integrate the following equation for the mass flux (kg/year) at time t (years) in the past from 4.3 to 4.0 Gya:
| 3 |
This equation is adapted from Chyba and Sagan.100 In eq 3, ṁ is the mass flux (mass/year) of the total mass of carbonaceous meteorites with mass from mmin to mmax that would impact Earth per year; C is the frequency of carbonaceous meteorites relative to all types of meteorites (∼4%) observed in the meteorite fall record;101 τ is the decay constant of 144 million years for the impactor population; mmin is taken as the mass of a meteorite with a 1 m radius; mmax is taken as the mass of a meteorite with a 100 m radius; q is 1 kg0.54/year. We assume that all meteorites are spherical with a uniform density (2.1 g/cm3 for CM-type meteorites38 or 3.2 g/cm3 for C2-type meteorites102), so we can calculate the mass of a meteorite in kilograms from its radius in meters. Meteorites with radii less than 100 m can fragment into pieces that impact the Earth’s surface with low enough energy that fatty acids are preserved.28,29 Using eq 3, we estimate that ∼1015 kg of carbonaceous meteorites with radii between 1 and 100 m would impact Earth from 4.3 to 4.0 Gya.
Assuming that all carbonaceous meteorites are either CM1-type or C2-type, we use eqs 2 and 3 to calculate bounds for the total mass of fatty acids with a length of 2–12 carbons that could have been delivered to Earth from 4.3 to 4.0 Gya. We find that 1010–1013 kg of fatty acids with a length of 2–12 carbons could have been delivered to Earth from 4.3 to 4.0 Gya by carbonaceous meteorites. Our estimate varies over 3 orders of magnitude because the abundance of fatty acids on each type of meteorite (fL) varies considerably between different meteorite types. We consider only carbonaceous chondrites because they are the most well-characterized type of meteorite that can deliver fatty acids. We do not quantify additional uncertainties in the meteorite flux, nor do we quantify the influence of meteorite size, impact velocity, and impact angle on the fraction of fatty acids that survive impact.103
Catalysis by Metal Surfaces after Vesta-Sized Impact
Next, we consider fatty acid synthesis on metal surfaces in the wake of a large Vesta-sized (∼1020 kg) asteroid impact on the early Earth. Such an impact would deliver iron to Earth’s surface, which could both act as a catalyst for fatty acid synthesis and generate a H2-rich atmosphere by reactions with steam from the vaporized ocean,57 as in eq 4:
| 4 |
An H2-rich atmosphere appears to be required for fatty acid synthesis by metal catalysts (Table 1). Such a massive asteroid impact would generate a high enough surface temperature to destroy most organic molecules on Earth; then, as the Earth cooled, the synthesis of fatty acids could occur in the H2-rich atmosphere. We consider only the last impactor that would reset Earth’s fatty acid inventory, which most likely hit Earth between 4.4 and 4.1 Gya.99 Significantly smaller meteorite impacts would not generate high partial pressures of H2 in the atmosphere because H2 escapes to space on time scales of ∼106–107 years. Additionally, small impactors would not generate global surface temperatures above 200 °C, which we assume is the minimum temperature required for fatty acid synthesis. Therefore, smaller impactors would not produce many fatty acids, and we do not consider their contribution here.
To estimate the total mass of fatty acids synthesized by metal catalysts after such an impact, Mc, we assume that the rate of fatty acid synthesis depends linearly on gas pressures according to the following equation:
| 5 |
Here, kc is an empirical rate constant for fatty acid synthesis, calculated from the results of experiments at 400 °C by Nooner and Oro.23 They provide the only measurement to date of the absolute concentration of fatty acids produced on a metal catalyst in the absence of an aqueous phase. The value of kc is 7.4 × 10–6 kilograms of 6–18 carbon fatty acids (excluding 12-carbon fatty acids, for which data are not available) per bar of H2, per bar of CO, per hour of reaction time, per kilogram of catalytic surface available.23 Only the summed mass of all fatty acids is reported by Nooner and Oro;23 there is no information given about the number of moles of individual fatty acids. Here, mcat is the mass of available metal catalyst, in kilograms. PH2 and PCO are the atmospheric partial pressures of hydrogen and carbon monoxide, respectively, in bar; both are functions of time, tc. The product is integrated over time from t400 to t200. t400 is the number of hours after the impact until the surface temperature reaches 400 °C, and t200 is the number of hours after the impact until the surface temperature cools to 200 °C. We assume that fatty acid synthesis occurs at a constant rate kc when the temperature is within this range and that the reaction does not occur when the temperature is outside this range.
We assume that 33% of the asteroid’s mass is iron and that 7% of this iron (i.e., 2.3% of the total asteroid mass) remains available on Earth’s surface to catalyze fatty acid synthesis (giving mcat = 2.3 × 1018 kg) based on a linear extrapolation of the impact simulation performed by Citron and Stewart.56 The assumption of ∼33% iron mass follows Zahnle et al.,55 which is the total iron in high iron enstatite (EH-type) meteorites104 and also the fraction of Earth’s mass in its iron core.105 Bodies with enstatite composition are candidates for impactors that hit the Earth after the Moon-forming impact and Earth’s core formation, although the contribution of carbonaceous versus enstatite compositions is debated.106−108 In EH enstatites, which are highly reduced, most of the iron is metallic with a smaller fraction of iron sulfide.109 In a postimpact vapor plume, the iron is vaporized into atoms, which condensation sequences show condenses to form metallic iron with subsequent cooling.110
We assume that another fraction of the asteroid’s iron (between 1% and 90%) is used to generate H2 from excess H2O according to eq 4. We adapted a thermochemical model previously developed by Zahnle et al. to compute PH2, PCO, and temperature as a function of time after the impact.55 Depending on the fraction of the asteroid’s iron that reacts with water to produce H2 gas, we find that PH2 ranges from 10–3 to 10–1 bar and PCO ranges from 10–3 to 10–7 bar. We calculate that the Earth’s postimpact temperature would be between 200 and 400 °C for ∼1000 years, and we assume that fatty acid synthesis occurs at a constant rate during this time. Equation 5 estimates that between 1011 and 1015 kg of fatty acids with a length 6–18 of carbons (excluding 12 carbons, for which data are not available) would be synthesized using metal catalysts in the wake of a Vesta-size impactor.
Our estimate for the total mass of fatty acids synthesized by metal catalysts is uncertain because many factors in our analysis are poorly constrained. First, it is not known whether reduced metals would remain exposed to the atmosphere on Earth’s surface after an extremely massive impact.56 Additionally, it is unclear how a H2O steam atmosphere would influence the rate of fatty acid synthesis; steam can affect the yield of Fischer–Tropsch reactions in different ways, depending on the type of catalyst and the type of reaction product.111 Even without steam in the atmosphere, it is uncertain whether the rate of fatty acid synthesis depends linearly on gas partial pressures. Kinetic models for the Fischer–Tropsch process have been developed in industrial settings, which generally do not mimic plausible early Earth conditions; functions for each partial pressure depend on the engineered design of the catalyst.58 Additionally, the effect of temperature on the final product distribution also appears to depend on the catalyst design.51,112 Separate experiments indicate that the yield of fatty acids depends linearly on the amount of catalyst surface area that is available.64 In general, further experiments are necessary to constrain these reaction parameters and the precise functional form for fatty acid production in plausible conditions on the early Earth.
Electrochemical Synthesis
We also construct an estimate for the total mass of fatty acids that could be synthesized by electrochemistry from 4.3 to 4.0 Gya,
| 6 |
where R is an empirical rate constant for the synthesis of 2–7 carbon fatty acids, calculated from electrochemical experiments by Yuen et al.24 The value of R is 1.1 × 10–17 kilograms of 2–7 carbon fatty acids per bar of CH4, per joule of electrical energy dissipated by sparking, per hour of reaction time.24 Although there are no electrochemical experiments that provide absolute concentrations for membrane-forming (longer than 8 carbons) fatty acids, Yuen et al.24 provide absolute concentrations for the largest number of fatty acids. PCH4 is the partial pressure of methane on the early Earth, which we estimate to be between 10–15 to 10–1 bar after the last Vesta-sized (∼1020 kg) asteroid impact. This value depends on the initial preimpact abundance of atmospheric CO2, the fraction of the impactor’s iron that becomes oxidized (1–100%), and the importance of methane-forming catalysts, which may reduce the quench temperature of methane, thereby increasing its abundance.55B is the amount of electrical energy dissipated by lightning and corona discharges on the Hadean Earth during a year. We assume that B is 1.5 × 1018 joules per year, which is the electrical energy dissipated per year on the modern Earth.76tE is the reaction time in years. We assume tE is 105 years, which is an estimate for the lifetime of a methane-rich atmosphere after a Vesta-sized (∼1020 kg) asteroid impact.55
By applying these assumptions, we calculate that 10–4 to 1010 kg of fatty acids with a length of 2–7 carbons could be synthesized on Earth from 4.3 to 4.0 Gya by electrochemical reactions. Although separate experiments have shown that electrochemical production of membrane forming fatty acids with more than 8 carbons is possible,71 membrane-forming fatty acids were not detected in the experiments by Yuen et al.,24 so our estimate is not directly informative about membrane formation on early Earth. More data are needed about the absolute concentration of membrane-forming fatty acids produced during electrochemical experiments.
There are uncertainties in our electrochemistry estimate. First, there is insufficient information available to estimate the total energy dissipated by the Tesla coil during the experiments by Yuen et al.24 If we assume that their Tesla coil used 30 000 V potential71 and 15 A, we can estimate that ∼4 × 1010 joules of energy were dissipated during their 24 h experiment. Even if we had perfect information about the Tesla coil voltage and current, it might not be straightforward to estimate the amount of energy that is usable for chemical synthesis.113 Furthermore, we assumed that the yield of fatty acids depends only linearly on the amount of available electrical energy.76 Further experiments are necessary to validate this assumption. Experiments by Schlesinger and Miller suggest that the yield of amino acids during sparking does increase linearly with increasing CH4 partial pressure (below ∼ 0.06 bar),77 but further experiments are needed to validate this assumption for fatty acids.
Summary of Estimates
We have compared the total mass of fatty acids produced by 3 different sources during the Hadean eon, following the last extremely massive impactor that would have reset Earth’s fatty acid inventory. We estimate that 1011 to 1015 kg of 6–18 carbon fatty acids could have been synthesized by metal catalysts derived from the massive impactor. The total mass of fatty acids that could have been delivered by carbonaceous meteorites is 1010 to 1013 kg of 2–12 carbon fatty acids. The yield of 2–7 carbon fatty acids from electrochemical processes is potentially smaller, between 10–4 and 1010 kg. Consequently, an integrated supply of fatty acids to the Earth’s surface from all sources (dominated by metal surface production) between ∼10–4 and ∼100 kg/m2 is possible, given the Earth’s surface area of 5.1 × 1014 m2.
Ultimately, the local concentration of fatty acids determines whether or not membranes form, so the possible sources should be evaluated by this criterion. Although meteorites could have delivered a significant mass of fatty acids across the Earth’s surface, the aqueous concentration of fatty acids in a single waterbody would not have been high enough to form membranes without evaporating a significant volume of water (Figure 3). In contrast, a local stockpile of fatty acids could have been produced on atmosphere-exposed metal surfaces after an extremely massive impact, and subsequent dissolution into water could have allowed membrane formation. Although little is known about the electrochemical synthesis rate for membrane-forming fatty acids, repeated lightning strikes into the same small waterbody seem unlikely, so it is unclear whether a high enough local concentration of membrane-forming fatty acids could have formed via electrochemistry. Fatty acids in aqueous solution can be degraded via photochemistry,114 so fatty acids that are slowly synthesized by electrochemistry may not have attained high enough concentrations to form membranes, whereas a large stockpile of fatty acids dissolving off metal surfaces may have been less sensitive to photochemical degradation.
Although the estimates above have many uncertainties, they are valuable as a first attempt to quantitatively compare fatty acid sources on the early Earth. We hope that future experiments can further constrain these estimates.
Alternative Amphiphiles
In addition to fatty acids, alternative types of amphiphiles may have been synthesized on the early Earth,115−117 and these amphiphiles might have incorporated into the membranes of the earliest cells. For example, alcohols are commonly produced along with fatty acids in many experiments,48,49,51−54 and long-chain fatty alcohols are known to stabilize fatty acid membranes.5,118 An excess of long-chain fatty alcohols form oil droplets, and the oil may disrupt membranes. In addition, phase-separated coacervates could have served as another type of prebiotic compartment,119,120 and fatty acid membranes may have even assembled around such coacervate compartments.121
Conclusions
Fatty acids can assemble into membranes and could have formed the boundaries for the first cells. Our Review highlights multiple potential sources of fatty acids on the early Earth. The three most well-characterized sources are meteorite delivery, synthesis on metal surfaces, and synthesis by electrochemistry. Other reactions involving photochemistry, irradiation by massive particles, ion–molecule reactions, and diverse redox reactions in aqueous solution may have also produced fatty acids in natural environments. To refine quantitative estimates for the relative importance of each fatty acid source, more detailed constraints are needed. We highlight the following questions to help any future experiments have the widest possible impact:
How do the yields of fatty acids from metal-catalyzed reactions depend on the temperature and the partial pressures of gases such as H2, CO2, and H2O? Data would help to constrain estimates for fatty acid production following ocean-vaporizing impacts.
Can membrane-forming fatty acids (>8 carbons) form in hydrothermal experiments with nonoxidized, ultramafic minerals as the sole catalyst? Data would elucidate the potential for fatty acid production at hydrothermal vents.
How do the yields of fatty acids depend on the voltage, duration, or total energy dissipated during electrical sparking? Data would help to constrain estimates for fatty acid production during lightning strikes.
What are the absolute concentrations of fatty acids in these types of experiments?
In summary, our analysis suggests that fatty acids could have been available on the early Earth. We have not assessed whether those fatty acids would have been sufficiently concentrated to assemble into membranes except in the limited case of small meteorite fragments delivered into aqueous environments. For fatty acids supplied via alternative sources, further data are required to assess the potential for membrane formation. By investigating possible sources of fatty acids on the early Earth, we hope to constrain the environmental setting for the origin of cells.
Acknowledgments
We thank Milomir Suvira for helpful discussions of electrochemical sparking, Martin Sadilek for helpful discussions of analytical methods, Kevin Zahnle for helpful comments on all portions of the manuscript, and Ben K. D. Pearce for sharing raw data on meteoritic fatty acid abundances that he and his colleagues compiled.26 This work was supported in part by grant NNX17AK86G (Exobiology) from NASA to S.L.K., by a grant (MCB 1925731) from the NSF to S.L.K., and by a grant (511570FY20, DCC) from the Simons Foundation to D.C.C. Z.R.C. was funded by an NSF fellowship (NSF GRFP DGE 1762114).
Author Contributions
Z.R.C. conducted the literature review. Z.R.C., Z.R.T., and N.W. calculated fatty acid abundances. Z.R.C., Z.R.T., N.W., R.A.B, S.L.K, and D.C.C. wrote the paper.
The authors declare no competing financial interest.
References
- Deamer D.; Dworkin J. P.; Sandford S. A.; Bernstein M. P.; Allamandola L. J. The First Cell Membranes. Astrobiology 2002, 2 (4), 371–381. 10.1089/153110702762470482. [DOI] [PubMed] [Google Scholar]
- Black R. A.; Blosser M. C.; Stottrup B. L.; Tavakley R.; Deamer D. W.; Keller S. L. Nucleobases Bind to and Stabilize Aggregates of a Prebiotic Amphiphile, Providing a Viable Mechanism for the Emergence of Protocells. Proc. Natl. Acad. Sci. USA 2013, 110 (33), 13272. 10.1073/pnas.1300963110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornell C. E.; Black R. A.; Xue M.; Litz H. E.; Ramsay A.; Gordon M.; Mileant A.; Cohen Z. R.; Williams J. A.; Lee K. K.; Drobny G. P.; Keller S. L. Prebiotic Amino Acids Bind to and Stabilize Prebiotic Fatty Acid Membranes. Proc. Natl. Acad. Sci. USA 2019, 116 (35), 17239–17244. 10.1073/pnas.1900275116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue M.; Black R. A.; Cohen Z. R.; Roehrich A.; Drobny G. P.; Keller S. L. Binding of Dipeptides to Fatty Acid Membranes Explains Their Colocalization in Protocells but Does Not Select for Them Relative to Unjoined Amino Acids. J. Phys. Chem. B 2021, 125 (29), 7933–7939. 10.1021/acs.jpcb.1c01485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apel C. L.; Deamer D. W.; Mautner M. N. Self-Assembled Vesicles of Monocarboxylic Acids and Alcohols: Conditions for Stability and for the Encapsulation of Biopolymers. Biochimica et Biophysica Acta (BBA) - Biomembranes 2002, 1559 (1), 1–9. 10.1016/S0005-2736(01)00400-X. [DOI] [PubMed] [Google Scholar]
- Cistola D. P.; Hamilton J. A.; Jackson D.; Small D. M. Ionization and Phase Behavior of Fatty Acids in Water: Application of the Gibbs Phase Rule. Biochemistry 1988, 27 (6), 1881–1888. 10.1021/bi00406a013. [DOI] [PubMed] [Google Scholar]
- Jordan S. F.; Rammu H.; Zheludev I. N.; Hartley A. M.; Maréchal A.; Lane N. Promotion of Protocell Self-Assembly from Mixed Amphiphiles at the Origin of Life. Nat. Ecol Evol 2019, 3, 1705. 10.1038/s41559-019-1015-y. [DOI] [PubMed] [Google Scholar]
- Caschera F.; de la Serna J. B.; Löffler P. M. G.; Rasmussen T. E.; Hanczyc M. M.; Bagatolli L. A.; Monnard P.-A. Stable Vesicles Composed of Monocarboxylic or Dicarboxylic Fatty Acids and Trimethylammonium Amphiphiles. Langmuir 2011, 27 (23), 14078–14090. 10.1021/la203057b. [DOI] [PubMed] [Google Scholar]
- Chen I. A.; Walde P. From Self-Assembled Vesicles to Protocells. Cold Spring Harb Perspect Biol. 2010, 2 (7), a002170. 10.1101/cshperspect.a002170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansy S. S.; Schrum J. P.; Krishnamurthy M.; Tobé S.; Treco D. A.; Szostak J. W. Template-Directed Synthesis of a Genetic Polymer in a Model Protocell. Nature 2008, 454 (7200), 122–125. 10.1038/nature07018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen I. A.; Szostak J. W. A Kinetic Study of the Growth of Fatty Acid Vesicles. Biophys. J. 2004, 87 (2), 988–998. 10.1529/biophysj.104.039875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen I. A.; Roberts R. W.; Szostak J. W. The Emergence of Competition Between Model Protocells. Science 2004, 305 (5689), 1474–1476. 10.1126/science.1100757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu T. F.; Szostak J. W. Coupled Growth and Division of Model Protocell Membranes. J. Am. Chem. Soc. 2009, 131 (15), 5705–5713. 10.1021/ja900919c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toparlak Ö. D.; Wang A.; Mansy S. S. Population-Level Membrane Diversity Triggers Growth and Division of Protocells. JACS Au 2021, 1 (5), 560–568. 10.1021/jacsau.0c00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanczyc M. M.; Fujikawa S. M.; Szostak J. W. Experimental Models of Primitive Cellular Compartments: Encapsulation, Growth, and Division. Science 2003, 302 (5645), 618–622. 10.1126/science.1089904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coreta-Gomes F. M.; Vaz W. L. C.; Moreno M. J. Effect of Acyl Chain Length on the Rate of Phospholipid Flip-Flop and Intermembrane Transfer. J. Membr. Biol. 2018, 251 (3), 431–442. 10.1007/s00232-017-0009-4. [DOI] [PubMed] [Google Scholar]
- Budin I.; Szostak J. W. Physical Effects Underlying the Transition from Primitive to Modern Cell Membranes. Proc. Natl. Acad. Sci. USA 2011, 108 (13), 5249–5254. 10.1073/pnas.1100498108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell E. A.; Boehnke P.; Harrison T. M.; Mao W. L. Potentially Biogenic Carbon Preserved in a 4.1 Billion-Year-Old Zircon. Proc. Natl. Acad. Sci. USA 2015, 112 (47), 14518. 10.1073/pnas.1517557112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawless J. G.; Yuen G. U. Quantification of Monocarboxylic Acids in the Murchison Carbonaceous Meteorite. Nature 1979, 282 (5737), 396–398. 10.1038/282396a0. [DOI] [Google Scholar]
- Naraoka H.; Shimoyama A.; Harada K. Molecular Distribution of Monocarboxylic Acids in Asuka Carbonaceous Chondrites from Antarctica. Orig Life Evol Biosph 1999, 29 (2), 187–201. 10.1023/A:1006547127028. [DOI] [PubMed] [Google Scholar]
- Aponte J. C.; Alexandre M. R.; Wang Y.; Brearley A. J.; Alexander C. M. O.; Huang Y. Effects of Secondary Alteration on the Composition of Free and IOM-Derived Monocarboxylic Acids in Carbonaceous Chondrites. Geochim. Cosmochim. Acta 2011, 75 (9), 2309–2323. 10.1016/j.gca.2011.01.040. [DOI] [Google Scholar]
- Hilts R. W.; Herd C. D. K.; Simkus D. N.; Slater G. F. Soluble Organic Compounds in the Tagish Lake Meteorite. Meteoritics & Planetary Science 2014, 49 (4), 526–549. 10.1111/maps.12272. [DOI] [Google Scholar]
- Nooner D. W.; Oro J.. Synthesis of Fatty Acids by a Closed System Fischer–Tropsch Process. In Hydrocarbon Synthesis from Carbon Monoxide and Hydrogen; Advances in Chemistry; American Chemical Society, 1979; Vol. 178, pp 159–171. [Google Scholar]
- Yuen G. U.; Lawless J. G.; Edelson E. H. Quantification of Monocarboxylic Acids from a Spark Discharge Synthesis. J. Mol. Evol 1981, 17 (1), 43–47. 10.1007/BF01792423. [DOI] [Google Scholar]
- Deamer D. W. Boundary Structures Are Formed by Organic Components of the Murchison Carbonaceous Chondrite. Nature 1985, 317 (6040), 792–794. 10.1038/317792a0. [DOI] [Google Scholar]
- Lai J. C.-Y.; Pearce B. K. D.; Pudritz R. E.; Lee D. Meteoritic Abundances of Fatty Acids and Potential Reaction Pathways in Planetesimals. Icarus 2019, 319, 685–700. 10.1016/j.icarus.2018.09.028. [DOI] [Google Scholar]
- Sephton M. A. Organic Compounds in Carbonaceous Meteorites. Nat. Prod. Rep. 2002, 19 (3), 292–311. 10.1039/b103775g. [DOI] [PubMed] [Google Scholar]
- Chyba C. F.; Thomas P. J.; Brookshaw L.; Sagan C. Cometary Delivery of Organic Molecules to the Early Earth. Science 1990, 249 (4967), 366–373. 10.1126/science.11538074. [DOI] [PubMed] [Google Scholar]
- Pearce B. K. D.; Pudritz R. E.; Semenov D. A.; Henning T. K. Origin of the RNA World: The Fate of Nucleobases in Warm Little Ponds. Proc. Natl. Acad. Sci. USA 2017, 114 (43), 11327–11332. 10.1073/pnas.1710339114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamozdra S. N.; Kocherov A. V.. Underwater Excavations and Welcoming the Big Meteorite. In Chelyabinsk Superbolide; Gorkavyi N., Dudorov A., Taskaev S., Eds.; Springer Praxis Books; Springer International Publishing: Cham, 2019; pp 122–147. [Google Scholar]
- Popova O. P.; Jenniskens P.; Emel’yanenko V.; Kartashova A.; Biryukov E.; Khaibrakhmanov S.; Shuvalov V.; Rybnov Y.; Dudorov A.; Grokhovsky V. I.; Badyukov D. D.; Yin Q.-Z.; Gural P. S.; Albers J.; Granvik M.; Evers L. G.; Kuiper J.; Kharlamov V.; Solovyov A.; Rusakov Y. S.; Korotkiy S.; Serdyuk I.; Korochantsev A. V.; Larionov M. Yu.; Glazachev D.; Mayer A. E.; Gisler G.; Gladkovsky S. V.; Wimpenny J.; Sanborn M. E.; Yamakawa A.; Verosub K. L.; Rowland D. J.; Roeske S.; Botto N. W.; Friedrich J. M.; Zolensky M. E.; Le L.; Ross D.; Ziegler K.; Nakamura T.; Ahn I.; Lee J. I.; Zhou Q.; Li X.-H.; Li Q.-L.; Liu Y.; Tang G.-Q.; Hiroi T.; Sears D.; Weinstein I. A.; Vokhmintsev A. S.; Ishchenko A. V.; Schmitt-Kopplin P.; Hertkorn N.; Nagao K.; Haba M. K.; Komatsu M.; Mikouchi T.; Chelyabinsk Airburst, Damage Assessment, Meteorite Recovery, and Characterization. Science 2013, 342 (6162), 1069–1073. 10.1126/science.1242642. [DOI] [PubMed] [Google Scholar]
- Artemieva N. A.; Shuvalov V. V. From Tunguska to Chelyabinsk via Jupiter. Annual Review of Earth and Planetary Sciences 2016, 44 (1), 37–56. 10.1146/annurev-earth-060115-012218. [DOI] [Google Scholar]
- Mehta C.; Perez A.; Thompson G.; Pasek M. A. Caveats to Exogenous Organic Delivery from Ablation, Dilution, and Thermal Degradation. Life 2018, 8 (2), 13. 10.3390/life8020013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark B. C.; Kolb V. M. Comet Pond II: Synergistic Intersection of Concentrated Extraterrestrial Materials and Planetary Environments to Form Procreative Darwinian Ponds. Life 2018, 8 (2), 12. 10.3390/life8020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cape J. L.; Monnard P.-A.; Boncella J. M. Prebiotically Relevant Mixed Fatty Acid Vesicles Support Anionic Solute Encapsulation and Photochemically Catalyzed Trans-Membrane Charge Transport. Chem. Sci. 2011, 2 (4), 661–671. 10.1039/c0sc00575d. [DOI] [Google Scholar]
- Budin I.; Prywes N.; Zhang N.; Szostak J. W. Chain-Length Heterogeneity Allows for the Assembly of Fatty Acid Vesicles in Dilute Solutions. Biophys. J. 2014, 107 (7), 1582–1590. 10.1016/j.bpj.2014.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trigo-Rodríguez J. M.; Rimola A.; Tanbakouei S.; Soto V. C.; Lee M. Accretion of Water in Carbonaceous Chondrites: Current Evidence and Implications for the Delivery of Water to Early Earth. Space Sci. Rev. 2019, 215 (1), 18. 10.1007/s11214-019-0583-0. [DOI] [Google Scholar]
- Britt D. T.; Consolmagno G. J. S. J. Stony Meteorite Porosities and Densities: A Review of the Data through 2001. Meteoritics & Planetary Science 2003, 38 (8), 1161–1180. 10.1111/j.1945-5100.2003.tb00305.x. [DOI] [Google Scholar]
- de Klerk A.Fischer–Tropsch Synthesis. In Fischer–Tropsch Refining; John Wiley & Sons, Ltd.: Hoboken, NJ; pp 73–103. [Google Scholar]
- Schulz H.; Beck K.; Erich E.. Mechanism of the Fischer–Tropsch Process. In Studies in Surface Science and Catalysis; Bibby D. M., Chang C. D., Howe R. F., Yurchak S., Eds.; Methane Conversion; Elsevier, 1988; Vol. 36, pp 457–471. [Google Scholar]
- Huber C.; Wächtershäuser G. Activated Acetic Acid by Carbon Fixation on (Fe,Ni)S Under Primordial Conditions. Science 1997, 276 (5310), 245–247. 10.1126/science.276.5310.245. [DOI] [PubMed] [Google Scholar]
- Scheidler C.; Sobotta J.; Eisenreich W.; Wächtershäuser G.; Huber C. Unsaturated C 3,5,7,9 -Monocarboxylic Acids by Aqueous, One-Pot Carbon Fixation: Possible Relevance for the Origin of Life. Sci. Rep. 2016, 6 (1), 1–7. 10.1038/srep27595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson R.; de Graaf R.; Strandoo Rodin M.; Ohno A.; Lane N.; McGlynn S. E.; Yamada Y. M. A.; Nakamura R.; Barge L. M.; Braun D.; Sojo V. CO2 Reduction Driven by a PH Gradient. Proc. Natl. Acad. Sci. USA 2020, 117 (37), 22873–22879. 10.1073/pnas.2002659117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preiner M.; Igarashi K.; Muchowska K. B.; Yu M.; Varma S. J.; Kleinermanns K.; Nobu M. K.; Kamagata Y.; Tüysüz H.; Moran J.; Martin W. F. A Hydrogen-Dependent Geochemical Analogue of Primordial Carbon and Energy Metabolism. Nat. Ecol Evol 2020, 4 (4), 534–542. 10.1038/s41559-020-1125-6. [DOI] [PubMed] [Google Scholar]
- McCollom T. M.; Seewald J. S. A Reassessment of the Potential for Reduction of Dissolved CO2 to Hydrocarbons during Serpentinization of Olivine. Geochim. Cosmochim. Acta 2001, 65 (21), 3769–3778. 10.1016/S0016-7037(01)00655-X. [DOI] [Google Scholar]
- McCollom T. M.; Seewald J. S. Experimental Constraints on the Hydrothermal Reactivity of Organic Acids and Acid Anions: I. Formic Acid and Formate. Geochim. Cosmochim. Acta 2003, 67 (19), 3625–3644. 10.1016/S0016-7037(03)00136-4. [DOI] [Google Scholar]
- McCollom T. M.; Seewald J. S. Experimental Study of the Hydrothermal Reactivity of Organic Acids and Acid Anions: II. Acetic Acid, Acetate, and Valeric Acid. Geochim. Cosmochim. Acta 2003, 67 (19), 3645–3664. 10.1016/S0016-7037(03)00135-2. [DOI] [Google Scholar]
- McCollom T. M.; Seewald J. S. Carbon Isotope Composition of Organic Compounds Produced by Abiotic Synthesis under Hydrothermal Conditions. Earth and Planetary Science Letters 2006, 243 (1), 74–84. 10.1016/j.epsl.2006.01.027. [DOI] [Google Scholar]
- McCollom T. M.; Ritter G.; Simoneit B. R. T. Lipid Synthesis Under Hydrothermal Conditions by Fischer- Tropsch-Type Reactions. Orig Life Evol Biosph 1999, 29 (2), 153–166. 10.1023/A:1006592502746. [DOI] [PubMed] [Google Scholar]
- Nooner D. W.; Gibert J. M.; Gelpi E.; Oró J. Closed System Fischer–Tropsch Synthesis over Meteoritic Iron, Iron Ore and Nickel-Iron Alloy. Geochim. Cosmochim. Acta 1976, 40 (8), 915–924. 10.1016/0016-7037(76)90140-X. [DOI] [Google Scholar]
- Rushdi A. I.; Simoneit B. R. T. Lipid Formation by Aqueous Fischer–Tropsch-Type Synthesis over a Temperature Range of 100 to 400 °C. Orig Life Evol Biosph 2001, 31 (1), 103–118. 10.1023/A:1006702503954. [DOI] [PubMed] [Google Scholar]
- Rushdi A. I.; Simoneit B. R. T. Condensation Reactions and Formation of Amides, Esters, and Nitriles Under Hydrothermal Conditions. Astrobiology 2004, 4 (2), 211–224. 10.1089/153110704323175151. [DOI] [PubMed] [Google Scholar]
- Mißbach H.; Schmidt B. C.; Duda J.-P.; Lünsdorf N. K.; Goetz W.; Thiel V. Assessing the Diversity of Lipids Formed via Fischer–Tropsch-Type Reactions. Org. Geochem. 2018, 119, 110–121. 10.1016/j.orggeochem.2018.02.012. [DOI] [Google Scholar]
- Bonfio C.; Caumes C.; Duffy C. D.; Patel B. H.; Percivalle C.; Tsanakopoulou M.; Sutherland J. D. Length-Selective Synthesis of Acylglycerol-Phosphates through Energy-Dissipative Cycling. J. Am. Chem. Soc. 2019, 141 (9), 3934–3939. 10.1021/jacs.8b12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahnle K. J.; Lupu R.; Catling D. C.; Wogan N. Creation and Evolution of Impact-Generated Reduced Atmospheres of Early Earth. Planet. Sci. J. 2020, 1 (1), 11. 10.3847/PSJ/ab7e2c. [DOI] [Google Scholar]
- Citron R. I.; Stewart S. T.. Large Impacts onto the Early Earth: Planetary Sterilization and Iron Delivery. arXiv 2022, arXiv:2201.09349; https://arxiv.org/abs/2201.09349. [Google Scholar]
- Benner S. A.; Bell E. A.; Biondi E.; Brasser R.; Carell T.; Kim H.-J.; Mojzsis S. J.; Omran A.; Pasek M. A.; Trail D. When Did Life Likely Emerge on Earth in an RNA-First Process?. ChemSystemsChem. 2020, 2 (2), e1900035. 10.1002/syst.202000009. [DOI] [Google Scholar]
- Zhou L.-P.; Hao X.; Gao J.-H.; Yang Y.; Wu B.-S.; Xu J.; Xu Y.-Y.; Li Y.-W. Studies and Discriminations of the Kinetic Models for the Iron-Based Fischer–Tropsch Catalytic Reaction in a Recycle Slurry Reactor. Energy Fuels 2011, 25 (1), 52–59. 10.1021/ef101270u. [DOI] [Google Scholar]
- Shock E.; Canovas P. The Potential for Abiotic Organic Synthesis and Biosynthesis at Seafloor Hydrothermal Systems. Geofluids 2011, 10 (1–2), 161–192. 10.1002/9781444394900.ch12. [DOI] [Google Scholar]
- Konn C.; Charlou J. L.; Donval J. P.; Holm N. G.; Dehairs F.; Bouillon S. Hydrocarbons and Oxidized Organic Compounds in Hydrothermal Fluids from Rainbow and Lost City Ultramafic-Hosted Vents. Chem. Geol. 2009, 258 (3), 299–314. 10.1016/j.chemgeo.2008.10.034. [DOI] [Google Scholar]
- Bach W.; Banerjee N. R.; Dick H. J. B.; Baker E. T. Discovery of Ancient and Active Hydrothermal Systems along the Ultra-Slow Spreading Southwest Indian Ridge 10°–16°E. Geochemistry, Geophysics, Geosystems 2002, 3 (7), 1–14. 10.1029/2001GC000279. [DOI] [Google Scholar]
- McCollom T. M.; Seewald J. S. Abiotic Synthesis of Organic Compounds in Deep-Sea Hydrothermal Environments. Chem. Rev. 2007, 107 (2), 382–401. 10.1021/cr0503660. [DOI] [PubMed] [Google Scholar]
- Wilcox E. M.; Roberts G. W.; Spivey J. J. Direct Catalytic Formation of Acetic Acid from CO2 and Methane. Catal. Today 2003, 88 (1), 83–90. 10.1016/j.cattod.2003.08.007. [DOI] [Google Scholar]
- Cody G. D.; Boctor N. Z.; Brandes J. A.; Filley T. R.; Hazen R. M.; Yoder H. S. Assaying the Catalytic Potential of Transition Metal Sulfides for Abiotic Carbon Fixation 1 1Associate Editor: P. A. O’Day. Geochim. Cosmochim. Acta 2004, 68 (10), 2185–2196. 10.1016/j.gca.2003.11.020. [DOI] [Google Scholar]
- He C.; Tian G.; Liu Z.; Feng S. A Mild Hydrothermal Route to Fix Carbon Dioxide to Simple Carboxylic Acids. Org. Lett. 2010, 12 (4), 649–651. 10.1021/ol9025414. [DOI] [PubMed] [Google Scholar]
- Rotelli L.; Trigo-Rodríguez J. M.; Moyano-Cambero C. E.; Carota E.; Botta L.; Di Mauro E.; Saladino R. The Key Role of Meteorites in the Formation of Relevant Prebiotic Molecules in a Formamide/Water Environment. Sci. Rep 2016, 6 (1), 38888. 10.1038/srep38888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma S. J.; Muchowska K. B.; Chatelain P.; Moran J. Native Iron Reduces CO2 to Intermediates and End-Products of the Acetyl-CoA Pathway. Nat. Ecol Evol 2018, 2 (6), 1019–1024. 10.1038/s41559-018-0542-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. L. A Production of Amino Acids Under Possible Primitive Earth Conditions. Science 1953, 117 (3046), 528–529. 10.1126/science.117.3046.528. [DOI] [PubMed] [Google Scholar]
- Johnson A. P.; Cleaves H. J.; Dworkin J. P.; Glavin D. P.; Lazcano A.; Bada J. L. The Miller Volcanic Spark Discharge Experiment. Science 2008, 322 (5900), 404–404. 10.1126/science.1161527. [DOI] [PubMed] [Google Scholar]
- Ferus M.; Pietrucci F.; Saitta A. M.; Knížek A.; Kubelík P.; Ivanek O.; Shestivska V.; Civiš S. Formation of Nucleobases in a Miller–Urey Reducing Atmosphere. Proc. Natl. Acad. Sci. USA 2017, 114 (17), 4306–4311. 10.1073/pnas.1700010114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criado-Reyes J.; Bizzarri B. M.; García-Ruiz J. M.; Saladino R.; Di Mauro E. The Role of Borosilicate Glass in Miller–Urey Experiment. Sci. Rep 2021, 11 (1), 21009. 10.1038/s41598-021-00235-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen W. V.; Ponnamperuma C. A Possible Prebiotic Synthesis of Monocarboxylic Acids. Biosystems 1967, 1 (1), 24–28. 10.1016/0303-2647(67)90017-2. [DOI] [PubMed] [Google Scholar]
- Zeitman B.; Chang S.; Lawless J. G. Dicarboxylic Acids from Electric Discharge. Nature 1974, 251 (5470), 42–43. 10.1038/251042a0. [DOI] [Google Scholar]
- Williams E. R. The Electrification of Thunderstorms. SCIENTIFIC AMERICAN 1988, 7, 13. 10.1109/44.16811. [DOI] [Google Scholar]
- Orville R. E. A High-Speed Time-Resolved Spectroscopic Study of the Lightning Return Stroke: Part II. A Quantitative Analysis. Journal of the Atmospheric Sciences 1968, 25 (5), 839–851. . [DOI] [Google Scholar]
- Chyba C.; Sagan C. Electrical Energy Sources for Organic Synthesis on the Early Earth. Origins Life Evol Biosphere 1991, 21 (1), 3–17. 10.1007/BF01809509. [DOI] [PubMed] [Google Scholar]
- Schlesinger G.; Miller S. L. Prebiotic Synthesis in Atmospheres Containing CH4, CO, and CO2. J. Mol. Evol 1983, 19 (5), 376–382. 10.1007/BF02101642. [DOI] [PubMed] [Google Scholar]
- Roldan A.; Hollingsworth N.; Roffey A.; Islam H.-U.; Goodall J. B. M.; Catlow C. R. A.; Darr J. A.; Bras W.; Sankar G.; Holt K. B.; Hogarth G.; de Leeuw N. H. Bio-Inspired CO2 Conversion by Iron Sulfide Catalysts under Sustainable Conditions. Chem. Commun. 2015, 51 (35), 7501–7504. 10.1039/C5CC02078F. [DOI] [PubMed] [Google Scholar]
- Groth W. E.; Weyssenhoff H. v. Photochemical Formation of Organic Compounds from Mixtures of Simple Gases. Planetary and Space Science 1960, 2 (2), 79–85. 10.1016/0032-0633(60)90001-5. [DOI] [Google Scholar]
- Kaiser R. I.; Gabrysch A.; Roessler K. Cosmic Ray Simulator: A Versatile Apparatus for Quantitative Studies on the Interaction of Cosmic Rays with Frozen Solids by on Line and in Situ Quadrupole Mass Spectrometry and Fourier Transform Infrared Spectroscopy. Rev. Sci. Instrum. 1995, 66 (4), 3058–3066. 10.1063/1.1145529. [DOI] [Google Scholar]
- Kim Y. S.; Kaiser R. I. Abiotic Formation of Carboxylic Acids (RCOOH) in Interstellar and Solar System Model Ices. ApJ. 2010, 725 (1), 1002–1010. 10.1088/0004-637X/725/1/1002. [DOI] [Google Scholar]
- Blagojevic V.; Petrie S.; Bohme D. K. Gas-Phase Syntheses for Interstellar Carboxylic and Amino Acids. Mon. Not. R. Astron. Soc. 2003, 339 (1), L7–L11. 10.1046/j.1365-8711.2003.06351.x. [DOI] [Google Scholar]
- Lowe C. U.; Rees M. W.; Markham R. Synthesis of Complex Organic Compounds from Simple Precursors: Formation of Amino-Acids, Amino-Acid Polymers, Fatty Acids and Purines from Ammonium Cyanide. Nature 1963, 199 (4890), 219–222. 10.1038/199219a0. [DOI] [PubMed] [Google Scholar]
- Novotný O.; Cejpek K.; Velíšek J. Formation of Carboxylic Acids during Degradation of Monosaccharides.. Czech J. Food Sci. 2008, 26 (2), 113–131. 10.17221/2465-CJFS. [DOI] [Google Scholar]
- Sato K.; Hyodo M.; Takagi J.; Aoki M.; Noyori R. Hydrogen Peroxide Oxidation of Aldehydes to Carboxylic Acids: An Organic Solvent-, Halide- and Metal-Free Procedure. Tetrahedron Lett. 2000, 41 (9), 1439–1442. 10.1016/S0040-4039(99)02310-2. [DOI] [Google Scholar]
- Ranjan S.; Sasselov D. D. Constraints on the Early Terrestrial Surface UV Environment Relevant to Prebiotic Chemistry. Astrobiology 2017, 17 (3), 169–204. 10.1089/ast.2016.1519. [DOI] [PubMed] [Google Scholar]
- Dworkin J. P.; Deamer D. W.; Sandford S. A.; Allamandola L. J. Self-Assembling Amphiphilic Molecules: Synthesis in Simulated Interstellar/Precometary Ices. Proc. Natl. Acad. Sci. USA 2001, 98 (3), 815–819. 10.1073/pnas.98.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenfreund P.; d’Hendecourt L.; Charnley S.; Ruiterkamp R. Energetic and Thermal Processing of Interstellar Ices. Journal of Geophysical Research: Planets 2001, 106 (E12), 33291–33301. 10.1029/2000JE001349. [DOI] [Google Scholar]
- Pecoits E.; Smith M. L.; Catling D. C.; Philippot P.; Kappler A.; Konhauser K. O. Atmospheric Hydrogen Peroxide and Eoarchean Iron Formations. Geobiology 2015, 13 (1), 1–14. 10.1111/gbi.12116. [DOI] [PubMed] [Google Scholar]
- Omran A.; Menor-Salvan C.; Springsteen G.; Pasek M. The Messy Alkaline Formose Reaction and Its Link to Metabolism. Life 2020, 10 (8), 125. 10.3390/life10080125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toner J. D.; Catling D. C. Alkaline Lake Settings for Concentrated Prebiotic Cyanide and the Origin of Life. Geochim. Cosmochim. Acta 2019, 260, 124–132. 10.1016/j.gca.2019.06.031. [DOI] [Google Scholar]
- Toner J. D.; Catling D. C. A Carbonate-Rich Lake Solution to the Phosphate Problem of the Origin of Life. Proc. Natl. Acad. Sci. USA 2020, 117 (2), 883–888. 10.1073/pnas.1916109117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasselov D. D.; Grotzinger J. P.; Sutherland J. D. The Origin of Life as a Planetary Phenomenon. Sci. Adv. 2020, 6 (6), eaax3419. 10.1126/sciadv.aax3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda N.; Kusano S.; Yasui M.; Pujado P.; Wilcher S. Recent Advances in Processes and Catalysts for the Production of Acetic Acid. Appl. Catal. A: General 2001, 221 (1), 253–265. 10.1016/S0926-860X(01)00800-6. [DOI] [Google Scholar]
- Berg J. M.; Tymoczko J. L.; Stryer L.. Biochemistry, 5th ed.; W. H. Freeman: New York, NY, 2002. [Google Scholar]
- Weber A. L. Origin of Fatty Acid Synthesis: Thermodynamics and Kinetics of Reaction Pathways. J. Mol. Evol 1991, 32 (2), 93–100. 10.1007/BF02515381. [DOI] [PubMed] [Google Scholar]
- Shock E. L.; Schulte M. D. Amino-Acid Synthesis in Carbonaceous Meteorites by Aqueous Alteration of Polycyclic Aromatic Hydrocarbons. Nature 1990, 343 (6260), 728–731. 10.1038/343728a0. [DOI] [PubMed] [Google Scholar]
- Sleep N. H.; Zahnle K. J.; Kasting J. F.; Morowitz H. J. Annihilation of Ecosystems by Large Asteroid Impacts on the Early Earth. Nature 1989, 342 (6246), 139–142. 10.1038/342139a0. [DOI] [PubMed] [Google Scholar]
- Marchi S.; Bottke W. F.; Elkins-Tanton L. T.; Bierhaus M.; Wuennemann K.; Morbidelli A.; Kring D. A. Widespread Mixing and Burial of Earth’s Hadean Crust by Asteroid Impacts. Nature 2014, 511 (7511), 578–582. 10.1038/nature13539. [DOI] [PubMed] [Google Scholar]
- Chyba C.; Sagan C. Endogenous Production, Exogenous Delivery and Impact-Shock Synthesis of Organic Molecules: An Inventory for the Origins of Life. Nature 1992, 355 (6356), 125–132. 10.1038/355125a0. [DOI] [PubMed] [Google Scholar]
- Bevan A. W. R.; Bland P. A.; Jull A. J. T.. Meteorite Flux on the Nullarbor Region, Australia. In Meteorites: Flux with Time and Impact Effects; Geological Society: London, Special Publications, 1998; Vol. 140, pp 59–73. [Google Scholar]
- Macke R. J.; Consolmagno G. J.; Britt D. T. Density, Porosity, and Magnetic Susceptibility of Carbonaceous Chondrites. Meteoritics & Planetary Science 2011, 46 (12), 1842–1862. 10.1111/j.1945-5100.2011.01298.x. [DOI] [Google Scholar]
- Pierazzo E.; Chyba C. F.. Impact Delivery of Prebiotic Organic Matter to Planetary Surfaces. In Comets and the Origin and Evolution of Life; Thomas P. J., Hicks R. D., Chyba C. F., McKay C. P., Eds.; Advances in Astrobiology and Biogeophysics; Springer: Berlin, Heidelberg, 2006; pp 137–168. [Google Scholar]
- Keil K. Mineralogical and Chemical Relationships among Enstatite Chondrites. J. Geophys. Res. 1968, 73 (22), 6945–6976. 10.1029/JB073i022p06945. [DOI] [Google Scholar]
- McDonough W. F. Compositional Model for the Earth’s Core. Treatise on Geochemistry 2003, 2, 547. 10.1016/B0-08-043751-6/02015-6. [DOI] [Google Scholar]
- Dauphas N. The Isotopic Nature of the Earth’s Accreting Material through Time. Nature 2017, 541 (7638), 521–524. 10.1038/nature20830. [DOI] [PubMed] [Google Scholar]
- Bermingham K. R.; Worsham E. A.; Walker R. J. New Insights into Mo and Ru Isotope Variation in the Nebula and Terrestrial Planet Accretionary Genetics. Earth Planet Sci. Lett. 2018, 487, 221–229. 10.1016/j.epsl.2018.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellmann J. L.; Hopp T.; Burkhardt C.; Becker H.; Fischer-Gödde M.; Kleine T. Tellurium Isotope Cosmochemistry: Implications for Volatile Fractionation in Chondrite Parent Bodies and Origin of the Late Veneer. Geochim. Cosmochim. Acta 2021, 309, 313–328. 10.1016/j.gca.2021.06.038. [DOI] [Google Scholar]
- Macke R. J.; Consolmagno G. J.; Britt D. T.; Hutson M. L. Enstatite Chondrite Density, Magnetic Susceptibility, and Porosity. Meteoritics & Planetary Science 2010, 45 (9), 1513–1526. 10.1111/j.1945-5100.2010.01129.x. [DOI] [Google Scholar]
- Lewis J. S.; Prinn R. G.. Planets and Their Atmospheres: Origin and Evolution; International Geophysics Series; Academic Press Inc.: Orlando, FL, 1984; Vol. 33. [Google Scholar]
- Wang Z.; Mai K.; Kumar N.; Elder T.; Groom L. H.; Spivey J. J. Effect of Steam During Fischer–Tropsch Synthesis Using Biomass-Derived Syngas. Catal. Lett. 2017, 147 (1), 62–70. 10.1007/s10562-016-1881-8. [DOI] [Google Scholar]
- Pendyala V. R. R.; Shafer W. D.; Jacobs G.; Davis B. H. Fischer–Tropsch Synthesis: Effect of Reaction Temperature for Aqueous-Phase Synthesis Over a Platinum Promoted Co/Alumina Catalyst. Catal. Lett. 2014, 144 (6), 1088–1095. 10.1007/s10562-014-1247-z. [DOI] [Google Scholar]
- Navarro-González R.; Romero A.; Honda Y. Power Measurements of Spark Discharge Experiments. Orig Life Evol Biosph 1998, 28 (2), 131–153. 10.1023/A:1006577104654. [DOI] [PubMed] [Google Scholar]
- Chiu R.; Tinel L.; Gonzalez L.; Ciuraru R.; Bernard F.; George C.; Volkamer R. UV Photochemistry of Carboxylic Acids at the Air-Sea Boundary: A Relevant Source of Glyoxal and Other Oxygenated VOC in the Marine Atmosphere. Geophys. Res. Lett. 2017, 44 (2), 1079–1087. 10.1002/2016GL071240. [DOI] [Google Scholar]
- Simoneit B. R. T.; Rushdi A. I.; Deamer D. W. Abiotic Formation of Acylglycerols under Simulated Hydrothermal Conditions and Self-Assembly Properties of Such Lipid Products. Adv. Space Res. 2007, 40 (11), 1649–1656. 10.1016/j.asr.2007.07.034. [DOI] [Google Scholar]
- Rapf R. J.; Perkins R. J.; Dooley M. R.; Kroll J. A.; Carpenter B. K.; Vaida V. Environmental Processing of Lipids Driven by Aqueous Photochemistry of α-Keto Acids. ACS Cent. Sci. 2018, 4 (5), 624–630. 10.1021/acscentsci.8b00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibard C.; Bhowmik S.; Karki M.; Kim E.-K.; Krishnamurthy R. Phosphorylation, Oligomerization and Self-Assembly in Water under Potential Prebiotic Conditions. Nat. Chem. 2018, 10 (2), 212–217. 10.1038/nchem.2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S.; Dagar S.; Verma A.; Rajamani S. Compositional Heterogeneity Confers Selective Advantage to Model Protocellular Membranes during the Origins of Cellular Life. Sci. Rep 2020, 10 (1), 4483. 10.1038/s41598-020-61372-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia T. Z.; Chandru K.; Hongo Y.; Afrin R.; Usui T.; Myojo K.; Cleaves H. J. Membraneless Polyester Microdroplets as Primordial Compartments at the Origins of Life. Proc. Natl. Acad. Sci. USA 2019, 116 (32), 15830–15835. 10.1073/pnas.1902336116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fares H. M.; Marras A. E.; Ting J. M.; Tirrell M. V.; Keating C. D. Impact of Wet-Dry Cycling on the Phase Behavior and Compartmentalization Properties of Complex Coacervates. Nat. Commun. 2020, 11 (1), 5423. 10.1038/s41467-020-19184-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dora Tang T-Y.; Rohaida Che Hak C.; Thompson A. J.; Kuimova M. K.; Williams D. S.; Perriman A. W.; Mann S. Fatty Acid Membrane Assembly on Coacervate Microdroplets as a Step towards a Hybrid Protocell Model. Nat. Chem. 2014, 6 (6), 527–533. 10.1038/nchem.1921. [DOI] [PubMed] [Google Scholar]