Abstract
Gestational diabetes mellitus and hypertensive disorders in pregnancy are adverse pregnancy outcomes (APOs) that affect 15% of pregnancies in the United States. These APOs have long-term health implications, with greater risks of future cardiovascular and chronic disease later in life. In this manuscript, we review the importance of timely postpartum follow-up and transition to primary care after APOs for future disease prevention. We also discuss interventions to improve postpartum follow-up and long-term health after an APO. In recognizing racial and ethnic disparities in APOs and chronic disease, we review important considerations of these interventions through a health equity lens.
Keywords: Gestational diabetes mellitus, hypertensive disorders in pregnancy, chronic disease, cardiovascular disease, postpartum interventions
1. Adverse pregnancy outcomes and long-term health
Gestational diabetes mellitus (GDM) and hypertensive disorders in pregnancy (HDP) affect an estimated 15% of pregnancies in the United States (US).1,2 GDM is defined as glucose intolerance developed during pregnancy; HDP include preeclampsia, gestational hypertension, and eclampsia. Individuals with GDM have an increased risk of HDP,1,3,4 and these adverse pregnancy outcomes (APO) have overlapping long-term risks. Specifically, individuals with HDP have a 2-fold increased risk of developing cardiovascular disease (CVD) or type 2 diabetes (T2D), with even greater risk for those with more severe manifestations of HDP;2,5,6 risk of T2D is 7-fold higher among those with a history GDM compared to those without.1,6–8 Although the term APO captures these conditions as well as others, including preterm birth and small-for-gestational-age neonatal status,9 GDM and HDP are major sources of morbidity during pregnancy and beyond. Thus, we are limiting our discussion of APOs to GDM and HDP.
Racial and ethnic disparities in APOs are well-established. Over half of pregnant people diagnosed with GDM identify as underrepresented racial or ethnic minorities. Non-Hispanic Black pregnant individuals bear a disproportionate burden of HDP, and the prevalence among Hispanic pregnant individuals increased at a steeper rate than all other ethnic groups between 2007–2018.10–13 These disparities are expected to further widen, mirroring national rises in obesity, sedentary lifestyle, and poor nutrition.14 The economic, environmental, political, and social conditions in which individuals are born, live, and interact with are recognized as social determinants of health. Minoritized groups more commonly experience multilevel structural disadvantages that contribute to health disparities, such as exposure to racism, food and housing insecurity, language barriers, low health literacy, and limited access to quality healthcare.15–18 Therefore, in discussing these disparities, we recognize racial and ethnic disparities as differences in health outcomes by the categorization of individuals based on socially constructed definitions that have no genetic basis.19 Addressing such disparities requires a focus on the promotion of health equity, defined as the absence of health disparities when every person has the opportunity to “attain his or her full health potential.”20
With rising rates and widening disparities of GDM and HDP in the US, the prevention of APOs and their sequelae are national priorities. Despite the acute issues related to these conditions typically resolving in the early postpartum period, the notion of “pregnancy as a window into future health” has been coined to illustrate that the development of APOs may be the first clinical signal of an increased future risk of hypertension, T2D, and CVD,21–23 all of which affect minoritized individuals at disproportionate rates.24,25 1–3 To achieve health equity in the postpartum period and beyond for the birthing individual, interventions addressing these disparities are of critical importance.
Thus, we aim to 1) review the importance of timely postpartum follow-up and transition to primary care after APOs, and 2) discuss interventions to improve postpartum follow-up and long-term health. In recognizing that interventions can reduce disparities only if they include those for whom health inequities are greatest, our goal is to discuss interventions through a health equity lens.
2. Barriers to postpartum and long-term health care after a pregnancy complicated by APOs
The American College of Obstetricians and Gynecologists (ACOG), American Academy of Family Physicians, American Diabetes Association, and other professional societies have affirmed that postpartum care is an important healthcare episode to optimize long-term health.26–29 Postpartum care is especially critical after APOs, as additional testing is recommended beyond routine follow-up.1,2,30 Short-term care testing includes the 2-hour 75-gram oral glucose tolerance test (OGTT) after GDM and blood pressure monitoring after HDP. Administration of these tests is vital to prevent short-term morbidity (e.g., stroke) and secure appropriate long-term follow-up for the prevention of chronic diseases.
Despite its importance, attendance at postpartum visits varies from 5.7% to 95.4% completion.26,31 Rates are especially low among individuals of minoritized racial and ethnic groups or those with limited resources,32,33 despite the disproportionate risk of T2D and CVD.34–36 Additionally, individuals with suboptimal prenatal care utilization are less likely to receive postpartum blood pressure screening,37 suggesting that those with decreased access to prenatal care are less likely to remain engaged in the healthcare system, compounding existing disparities.
Barriers to receiving postpartum care are well documented, with contributing factors at the clinician, patient, and societal levels.32,38–41 In fact, one study from 2015 found that both internal medicine and obstetric and gynecologic clinicians failed to consistently identify and provide appropriate follow-up for patients who experienced APOs.42 At the patient level, barriers include lack of access to quality healthcare, limited childcare, and low health literacy. Low health literacy is associated with inadequate health service utilization,43–45 which in this population could limit understanding of chronic disease risk or result in confusion regarding instructions.38,46 Additionally, some postpartum individuals may understand their elevated risk for chronic disease but feel anxious about future complications and potential diagnoses.45 On a societal level, structural racism - defined as public policies, institutional practices, cultural representations, and other norms that work in various, often reinforcing ways to perpetuate racial group inequity - contributes to and exacerbates existing disparities in postpartum health.19,47 Interventions that aim to increase postpartum follow-up must improve clinician education, ease patient-level barriers, and recognize the role of social determinants.
Further, the transition from obstetrics to primary care after an APO requires care coordination for counseling, monitoring, and treatment to optimize short- and long-term cardiometabolic health. Only half of individuals successfully transition from obstetric care to primary care within the first year postpartum, with lower rates of primary care engagement among individuals of minoritized racial and ethnic groups compared to non-Hispanic White postpartum individuals.48 This gap in care amplifies racial and ethnic disparities in chronic illness,48 and represents a missed opportunity for prevention. In recognition of this gap, ACOG and the American Heart Association released a joint statement to promote risk identification and reduction of CVD by encouraging collaborations bridging obstetrics and cardiology.49
Barriers to this transition are compounded by the fragmentation between obstetric health care and other subspecialties.50 Among those who do transition, some will continue to see their obstetrician-gynecologist for well-woman care, but many will transition to a general internist or family medicine clinician. Advocates for care coordination have called for a “warm hand-off” between the obstetrician and new clinician to facilitate this transition, which can be done via electronic health records (EHR), written or verbal communication, or postpartum transition clinics.51,52 Fragmentation, combined with the structural barriers to quality healthcare that minoritized individuals are more likely to experience, perpetuates racial and ethnic disparities in long-term health.50,51 Additionally, structural and interpersonal racism, unequal treatment in the healthcare system, and lack of culturally-tailored health services are among a few of the reasons that individuals of color avoid seeking care,53–55 which is particularly detrimental after an APO. Experiences of racism impact pregnancy outcomes and chronic illness.54,56,57 Due to these complex, multifactorial inequities, it is imperative to address disparities on multiple levels, which are outlined by the socioecological model in Figure 1.
Figure 1.
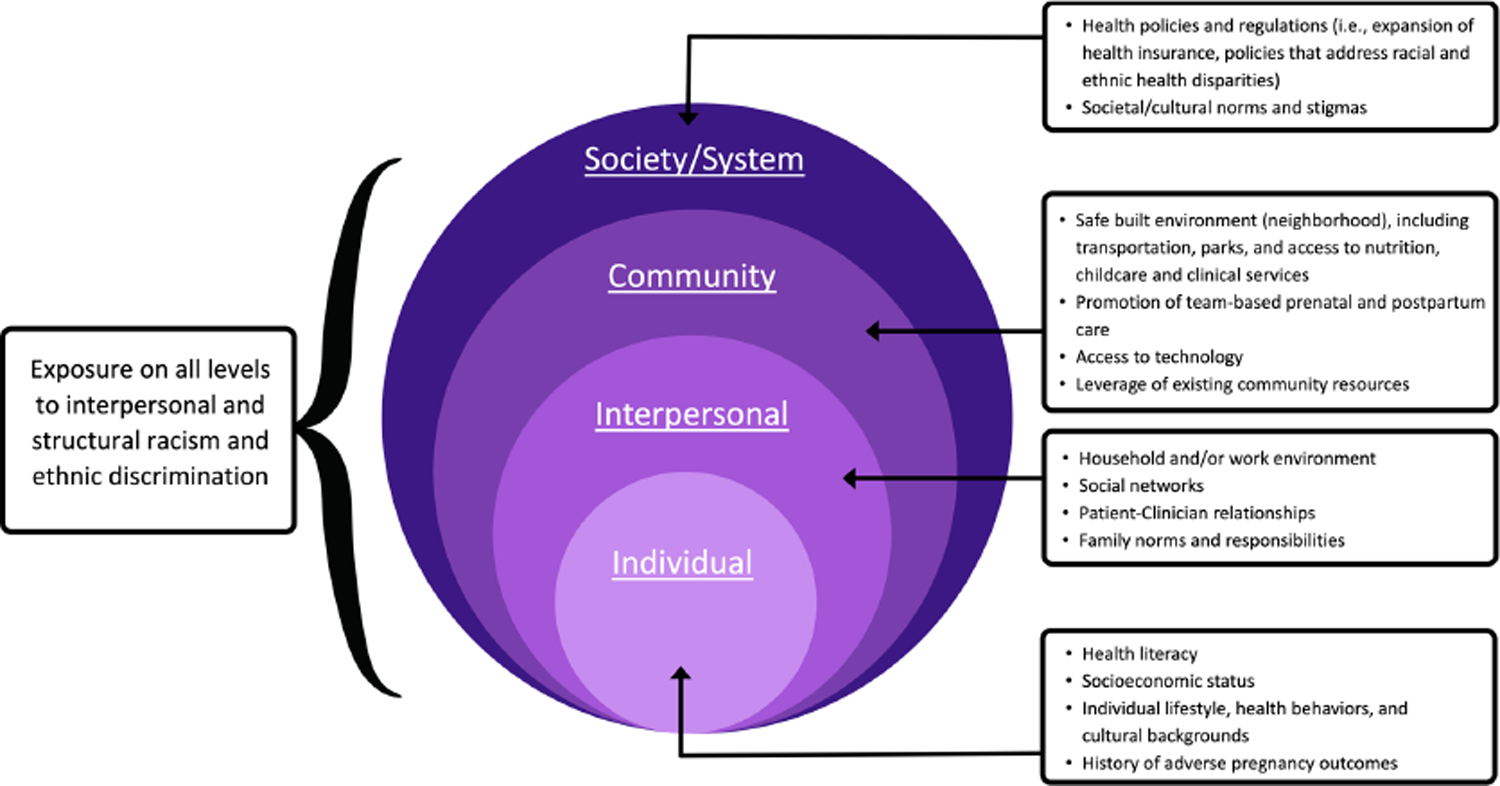
Considerations for developing an intervention from a health equity perspective for postpartum individuals with APOs, guided by the socioecological model.
Although we emphasize the importance of bridging the postpartum to primary care gap, the role of primary care is not limited to one year after birth. In fact, establishment of care can improve preconception health, if future pregnancy is desired; individuals who have a preconception primary care visit are 3-fold more likely to receive optimal monitoring and care in the immediate postpartum period and 3 years after giving birth.58 Improved transition of care may facilitate access and utilization of preconception care and monitoring, thus promoting lifelong individual and family wellness.
3. Interventions to improve postpartum monitoring, screening, and follow-up after an APO
Multiple interventions aimed to improve postpartum follow-up after APOs have attempted to address health disparities, including technology and healthcare clinic-based interventions. These interventions are outlined in Table 1. This is not an exhaustive review, but instead a discussion of interventions with preliminary success which are most applicable to the US healthcare system. In recognizing the importance of health policy initiatives on expanding access to healthcare, we have summarized selected policies in Table 2. Although a full discussion of these policies is beyond the scope of this manuscript, health policies aimed at improving postpartum health must exist in tandem with the individual and system-level interventions we describe below.
Table 1.
Characteristics of postpartum interventions to improve monitoring, screening, and follow-up among postpartum individuals after an adverse pregnancy outcome.
| Category | Type of intervention | Definition | Postpartum population | Description of example interventions | Summary of results |
|---|---|---|---|---|---|
| Technology-based interventions | Virtual reminders | Text or EHR reminders for postpartum screening and follow-up testing; can be on the patient or clinician level | GDM40,60–65 and HDP66 | ||
| mHealth monitoring | Mobile health technologies for remote patient monitoring | HDP66,70–73 | |||
| mHealth education and support | Mobile health technologies for patient education and support | GDM78 and HDP80 |
|
||
| Healthcare system-based | Medical home model | Joint postpartum visits and 2-month well infant visits | GDM81 |
|
|
| Patient navigation | Barrier-focused, patient-centered intervention that offers support related to general postpartum health and T2D prevention | General population and GDM82,86 |
|
||
| Postpartum transition clinics | Specialized outpatient care clinics for individuals who had complicated pregnancies | HDP87,89 |
|
|
EHR, electronic health record; GDM, gestational diabetes mellitus; HDP, hypertensive disorders of pregnancy; SMS, short message services; OGTT, oral glucose tolerance test; mHealth, mobile health; T2D, type 2 diabetes.
Table 2.
Brief overview of selected US health policy initiatives to improve access to health care in the postpartum period.
| Overarching aim | Name | Policy description and potential impact |
|---|---|---|
| Medicaid expansion and extending Medicaid coverage to one-year postpartum | Affordable Care Act 2014 Medicaid Expansion | State-dependent Medicaid expansion for adults at or below 138 percent of the federal poverty level and extended insurance eligibility preconception and 60 days postpartum; several studies have found that ACA Medicaid expansion increased postpartum Medicaid enrollment100 |
| American Rescue Plan Act of 2021 | COVID-19 relief package facilitating the extension of pregnancy-related Medicaid coverage from 60 days to 1 year postpartum;101 supports continuity of insurance coverage postpartum | |
| Addressing racial disparities in maternal health | Preventing Maternal Death Acts of 2018 | Authorizes $12 million a year in funds for 5 years for states to establish and financially support maternal mortality review committees; facilitates investigation of maternal deaths via review and the collection of data to study maternal mortality causes and avenues for prevention102 |
| Prematurity Research Expansion and Education for Mothers who deliver Infants Early (PREEMIE) Reauthorization Act of 2018 | Aims to address birth outcome disparities among Black individuals; helps provide funding for federal services and research on preterm neonates and their families to close existing racial equity gaps in obstetric and postpartum health103 | |
| Black Maternal Health Momnibus Act of 2021 | Aims to address racial and ethnic disparities in maternal health outcomes and mortality with 12 standalone bills with goals such as improving data collection, investing in digital healthcare, and funding community-based initiatives104,105 | |
| State-specific policy initiatives for advancing maternal and child health | An Act Improving the Quality of Health Care and Reducing Costs through Increased Transparency, Efficiency, and Innovation 2012 (Massachusetts) | Aims to control healthcare costs via the adoption of novel delivery system and payment models, investments in preventive care programs, and implementation of assessment measures to ensure the quality of health care services106 |
| California Pregnancy Associated Mortality Review (CA-PAMR) | Funded by the California Department of Public Health and Maternal, Child and Adolescent Health Title V Maternal and Child Health Block Grant, the review committee that uses a mixed-methods approach for the investigation of maternal deaths in order to inform future prevention strategies and policies; promotes improved accuracy for identifying the cause of pregnancy-related deaths associated with racial disparities and further insight into cardiovascular disease as one of the primary causes of pregnancy-related mortality107 |
COVID-19, coronavirus disease 2019.
Technology-based interventions
Technology provides a promising avenue for public health interventions to both optimize the nearly universal use of smartphones and allow for healthcare information to be readily accessible.59 Examples include virtual reminders and mobile health (mHealth) applications.
Virtual reminders, via phone calls or EHR patient portals, may improve patient awareness regarding the OGTT, blood pressure surveillance, and appointments.40,60–66 Postpartum individuals have competing demands for their attention. As a result, virtual reminders are appealing in terms of low cost, preliminary success, and ease of implementation. However, these reminders may be less successful among those experiencing high perceived barriers; for example, individuals may be aware of the importance of the OGTT after GDM but may not have the time or resources to attend a 2-hour appointment. A review article by Nielsen et al. identified that postpartum individuals of high socioeconomic status found it easier to overcome barriers to OGTT completion which subsequently made it easier to prioritize the test, suggesting that reminders alone may not improve outcomes among those who are disproportionately affected by APOs.67
Further, there are racial and ethnic disparities in EHR portal use during pregnancy,68 thus, systems must ensure that patients are universally introduced, offered, and taught how to navigate EHR portals. Despite initial success in trials of virtual reminders for OGTTs, the majority of data are among highly educated White patients, thereby limiting our knowledge of the efficacy among groups who are most likely to experience APOs.
Technology-based reminders provide an opportunity to improve postpartum test ordering and completion. Vesco et al. incorporated a checkbox into the EHR to remind clinicians to order a fasting plasma glucose test at the postpartum visit for patients who experienced GDM.60 Education on the importance of the test was administered via regional department and local clinic meetings by a maternal-fetal medicine clinician. Using technology to remind and educate clinicians of the importance of postpartum testing after GDM may enhance patient-clinician discussions regarding the importance of such testing, subsequently improving rates of OGTT completion. Data on clinician technology-based reminders for postpartum blood pressure screening are limited.
Another technology-based intervention is mHealth, which refers to the use of mobile devices or Bluetooth technology in public health practice. mHealth may be particularly helpful among those with limited access to healthcare, as it enables health monitoring or education to occur virtually, overall reducing logistical barriers.69 Postpartum remote blood pressure monitoring via Bluetooth systems linked to clinics for individuals with HDP have high retention rates and show improvements in postpartum visit attendance.66,70–73 Many individuals report being satisfied with addressing health concerns at home rather than the clinic, which may be particularly burdensome among those with limited transportation or childcare. Heart Safe Motherhood, a postpartum text message-based at-home blood pressure surveillance program, demonstrated a 50% reduction in racial differences in compliance with blood pressure measurement attainment.74 These findings were similar when implemented at another institution,72 indicating that high adherence in blood pressure monitoring may be achieved with the combination of text message reminders and Bluetooth-enabled home blood pressure cuffs.
Nevertheless, Rhoads et al. identified that non-users of a remote blood pressure monitoring system reported barriers to access, including limited number of minutes on their cellular phones and disruptions in internet connectivity in rural communities.73 These barriers have been well documented among non-pregnant populations,75 subsequently contributing to the digital divide in which the benefits of technology bias towards White, high socioeconomic status, and highly educated individuals. Additionally, affordability of devices in clinical settings outside of research or philanthropy may be limited. If patients are expected to buy devices themselves, this adds a financial burden, highlighting the importance of the role of payors or institutions to support technology-based monitoring devices.76
Few postpartum mHealth education and support interventions after an APO exist. SweetMama, a smartphone app for individuals with GDM, extends up to 6-weeks postpartum with appointment reminders and resources such as recipes, educational videos, and links to local and federal resources to improve health literacy and self-efficacy among this population.77,78 This app was specifically designed according to feedback from qualitative interviews among the target patient population.77,79 In order to be culturally appropriate for the target audience, the acceptability, efficiency, and effectiveness of the app are designed in accordance with patient preferences. Similarly, Social Ties to Encourage Physical activity among Postpartum Mothers is designed specifically for postpartum individuals with HDP;80 participants are enrolled in a mobile, team-based gamification intervention with points, levels, and small prizes to encourage daily step count goals. Further study is required to determine whether mHealth interventions can improve postpartum health and reduce disparities on a larger scale.
Despite disparities in use, technology-based interventions have the potential to reach a wide audience due to the growing number of individuals with access to a smartphone in the US. To narrow the racial and socioeconomic gap in use of mHealth, interventions must account for barriers specifically identified by marginalized communities, ensure universal offer by health systems and clinicians, and include culturally-tailored features based on user preferences.
Healthcare system-based interventions for postpartum follow-up
Healthcare system-based interventions may improve rates of postpartum follow-up after an APO. These interventions target health disparities by specifically addressing multilevel barriers. For instance, the medical home model can allow the postpartum visit and infant’s 2-month check-up to be scheduled at the same time and location.30,81 This addresses a logistical burden (e.g., one drive to the doctor’s office instead of two) and changes the current system of postpartum care in recognition of parental tendency to prioritize the neonate over themselves; however, there are limited data on this application after an APO, with only one study reporting null results.81 This intervention may be particularly successful at clinics that are family medicine-focused or multispecialty health systems. Feasibility and coordination may be difficult at clinics with fragmentation between obstetricians and pediatricians or for patients who receive primary care in the internal medicine environment where pediatric care is often separate. Nevertheless, this is a promising intervention, particularly considering the length of the OGTT, as postpartum individuals with GDM commonly cite time as a major barrier to completing the test.
Originally implemented for oncology, patient navigation is a barrier-focused, patient-centered intervention employing trained personnel to identify patient-level barriers and facilitate complete and timely access to health services.82,83 It has been adapted to postpartum care demonstrating preliminary success with improvements in postpartum visit attendance, receipt of contraception and depression screening, and high perceived utility among stakeholders.82,84,85 The principles of patient navigation as adapted to individuals with GDM are currently under investigation in an ongoing feasibility trial by our team designed to promote connectivity to primary care, enhance patient awareness of T2D risk after GDM, and engage patients in diabetes prevention activities.86
Postpartum transition clinics, initially piloted in Canada, have aimed to improve postpartum follow-up, cardiovascular screening, and patient education among individuals with a history of HDP.87 Importantly, Canada has universal healthcare coverage which accounts for the reimbursement system that postpartum transition clinics are funded upon, whereas the payment environment in the US healthcare system limits equitable access for those living in states with short-term loss of Medicaid after giving birth.88 Nevertheless, data at a single academic institution in the US are promising, including among those with public insurance with high rates of insurance approvals.89 Future adaptations of multidisciplinary transition clinics may be led by various types of providers, including primary care clinicians, endocrinologists, cardiologists, dietitians, or behavioral lifestyle coaches. If postpartum transition clinics are to be implemented in the US, it is vital to ensure universal healthcare, along with continued advocacy for expanded postpartum insurance coverage (Table 2).
4. Interventions to improve long-term health and transition to primary care after an APO
There are a number of evidence-based interventions to improve long-term health after an APO. Existing interventions can be grouped into lifestyle and healthcare system-based interventions (Table 3).
Table 3.
Characteristics of interventions to improve primary care transition and long-term health after an adverse pregnancy outcome.
| Category | Type of intervention | Definition | Postpartum Population | Description of example interventions | Summary of results |
|---|---|---|---|---|---|
| Lifestyle interventions | Diabetes Prevention Program (DPP) | Adaptation of the DPP, a lifestyle modification intervention to prevent future T2D; can be web-based, in-person, or in print | GDM95–98 |
|
|
| Lifestyle intervention | Web-based intervention with modules on healthy eating and physical activity; personalized coaching with a dietician | HDP92 |
|
||
| Health care system-based interventions | Patient navigation | Barrier-focused, episode-specific, patient-centered intervention that offers support related to T2D prevention | GDM86 |
|
|
| Postpartum transition clinics | Specialized outpatient care clinics for individuals who had complicated pregnancies | HDP89
|
|
|
|
| Primary care transition task forces | Collaboration between obstetricians, diabetic nurses, and primary care clinicians | GDM, HDP93 |
|
|
T2D, type 2 diabetes; GDM, gestational diabetes mellitus; HDP, hypertensive disorders of pregnancy
Lifestyle interventions
Lifestyle interventions are designed to prevent chronic disease by promoting a healthy diet, exercise, and weight loss. Perhaps the most widely known is the Diabetes Prevention Program (DPP), a national program created by the Centers for Disease Control in 2010 following a core curriculum with lifestyle coaching to prevent T2D. The original study required one risk factor for enrollment, including history of GDM.90 The DPP was designed to include an ethnically diverse population, and indeed, 45% of the participants were from racial and ethnic minority groups. The DPP partnered with community stakeholders, “case managers,” who identified as the same racial or ethnic group as the participant to tailor the intervention to be culturally adaptive. To address social determinants of health such as language or health literacy, the modules were available in Spanish and English and the pace of the program components (i.e., speed that new information was introduced) could be modified.
The DPP has been adapted for postpartum individuals with GDM via in-person, web-based, and phone call platforms. Despite preliminary success in improving postpartum weight loss, the extent to which original efforts to individualize the intervention have been maintained is unclear. Non-profit organizations, such as Chicago CARES to Prevent Diabetes,91 have attempted to increase the visibility of community-based DPP programs, yet the success of these programs is unknown. Further, there are often not enough DPP spots available for patients, so scalability remains an issue. To ensure that the original DPP principles are maintained throughout adaptations, policy must fund preventive health - rather than management of current chronic illness - in a culturally adaptive manner to ensure relevancy and usefulness to those seeking lifestyle change.
Another lifestyle intervention example is the Heart Health 4 Moms program, which is a virtual lifestyle intervention to reduce cardiovascular risk after a pregnancy complicated by preeclampsia.92 This intervention includes personalized lifestyle coaching, online community forums, and resources such as videos of exercises that can be done with infants, whose presence may compound barriers to physical activity. Although this intervention demonstrated considerable success across the US, many participants were White with high levels of education, thus limiting the generalizability to minoritized racial and ethnic individuals who bear the largest burden of APOs.
Healthcare system-based interventions to promote long-term health
Many healthcare system-based interventions that occur in the early postpartum period, described previously, are also relevant for long-term health and connection to primary care. For instance, a mutual goal of patient navigation and postpartum transition clinics is to connect postpartum individuals to primary care.86,89 In one study, patient navigators directly connect patients to a primary care office and provide the clinician with a high-level summary of the patient’s immediate and long-term clinical needs via the EHR.86 Postpartum transition clinics operate in a similar fashion.89 However, a unique advantage of patient navigation is that it can be successfully implemented without requiring clinicians to perform duties beyond routine clinical care, thereby improving clinic efficiency.82 Additionally, the patient navigator routinely checks in with the patient regarding individual barriers to care and clinical goals such as postpartum weight loss, thus helping patients achieve their goals and provide resources, such as nutritionist referrals, local DPP programs, or lifestyle medicine resources.83
In 2018, the Bridging the Gap program was developed at an urban, academic medical center; postpartum individuals with APOs were routed through a referral pathway to a general internal medicine practice via a warm handoff between the obstetrician and primary care clinician.93 This program was well received and demonstrated pilot success in connecting patients to care, yet disparities in receipt of primary care persisted, suggesting that more intensive strategies to identify and address social determinants of health are needed in concordance with institutional referral pathways. The bundling of multiple strategies - such as variations of navigation along with referral pathways - may be the optimal next step to reduce inequities for patients at greatest risk.
5. Conclusions and future directions
Racial and ethnic disparities in APOs are well documented, yet evaluation of interventions to optimize care after birth and eliminate these disparities are limited. In this review, we center on promising interventions that clinicians may be interested in implementing. Making significant strides in postpartum and long-term health after APOs requires consideration of the unique needs of marginalized populations and subsequently developing targeted interventions. We have outlined key recommendations (Box) to consider when looking to implement an intervention in one’s community. Additionally, it is essential that health care policies, such as insurance coverage, paid parental leave, and funding for maternal health research, remain focused on the importance of this life stage and the unique needs of populations at risk for adverse long-term health outcomes after APOs. To achieve health equity, continued research must recognize and prioritize addressing racial and ethnic disparities in postpartum and long-term health after an APO.
Box. Key recommendations for the development of an equity-focused intervention targeted to improve postpartum follow-up, care coordination, and long-term health after an adverse pregnancy outcome.
| Key Questions | Recommendations |
| How can community perspectives remain forefront in intervention development? | Engage stakeholders (e.g., patients, physicians, social workers) in the development, implementation, and evaluation of the intervention. Consider community leadership and/or community advisory boards.19 |
| Is the intervention designed to be adaptable and flexible? | Consider the importance of constant adaptation through continuous evaluation. Changing social, medical, financial, and structural contexts may alter needs. |
| How can interventions be extend beyond pilot and research phases? | Develop interventions with a focus on long-term sustainability, funding, leadership, and infrastructure. Implementation characteristics such as sustainability features must be a component of initial planning. |
| Is the intervention improving health outcomes or reducing health disparities? | Improving health and reducing health disparities are not synonymous. Remember that not all health promotion interventions reduce inequities if the intervention does not consider those with disproportionate burden of disease risk. |
FUNDING:
This project was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development R01 HD098178 and the National Institute of Diabetes and Digestive and Kidney Diseases R34 DK125958. Comments and views of the authors do not necessarily represent views of the National Institutes of Health.
Footnotes
DISCLOSURES: The authors report no conflict of interest.
REFERENCES
- 1.American College of Obstetricians and Gynecologists. Practice Bulletin No. 190: Gestational Diabetes Mellitus. Obstet Gynecol 2018;131(2):e49–64. DOI: 10.1097/AOG.0000000000002501. [DOI] [PubMed] [Google Scholar]
- 2.Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin, Number 222. Obstet Gynecol 2020;135(6):e237–e260. (In eng). DOI: 10.1097/aog.0000000000003891. [DOI] [PubMed] [Google Scholar]
- 3.Yogev Y, Xenakis EM, Langer O. The association between preeclampsia and the severity of gestational diabetes: the impact of glycemic control. Am J Obstet Gynecol 2004;191(5):1655–60. (In eng). DOI: 10.1016/j.ajog.2004.03.074. [DOI] [PubMed] [Google Scholar]
- 4.Zhou TS DL, Xiang H, Yoriko N, Hoirun H, Gang PXS, Xiayun S, Qi L. Prevalence and Trends in Gestational Diabetes Mellitus among Women in the United States, 2006–2016. Am Diabetes Assoc; 2018. [Google Scholar]
- 5.Heida KY, Bots ML, de Groot CJ, et al. Cardiovascular risk management after reproductive and pregnancy-related disorders: A Dutch multidisciplinary evidence-based guideline. Eur J Prev Cardiol 2016;23(17):1863–1879. (In eng). DOI: 10.1177/2047487316659573. [DOI] [PubMed] [Google Scholar]
- 6.Haas DM, Parker CB, Marsh DJ, et al. Association of Adverse Pregnancy Outcomes With Hypertension 2 to 7 Years Postpartum. J Am Heart Assoc 2019;8(19):e013092. (In eng). DOI: 10.1161/jaha.119.013092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.England LJ, Dietz PM, Njoroge T, et al. Preventing type 2 diabetes: public health implications for women with a history of gestational diabetes mellitus. Am J Obstet Gynecol 2009;200(4):365.e1–8. (In eng). DOI: 10.1016/j.ajog.2008.06.031. [DOI] [PubMed] [Google Scholar]
- 8.Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care 2002;25(10):1862–8. (In eng). DOI: 10.2337/diacare.25.10.1862. [DOI] [PubMed] [Google Scholar]
- 9.Mehta P, Minissian M, Merz C. Adverse pregnancy outcomes and cardiovascular risk factor management. Semin Perinatol 2015;39(4):268–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bornstein E, Eliner Y, Chervenak FA, Grünebaum A. Racial Disparity in Pregnancy Risks and Complications in the US: Temporal Changes during 2007–2018. J Clin Med 2020;9(5) (In eng). DOI: 10.3390/jcm9051414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grobman WA, Bailit JL, Rice MM, et al. Racial and ethnic disparities in maternal morbidity and obstetric care. Obstet Gynecol 2015;125(6):1460–7. (In eng). DOI: 10.1097/aog.0000000000000735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esakoff TF, Caughey AB, Block-Kurbisch I, Inturrisi M, Cheng YW. Perinatal outcomes in patients with gestational diabetes mellitus by race/ethnicity. J Maternal Fetal Neonatal Med 2011;24(3):422–6. [DOI] [PubMed] [Google Scholar]
- 13.Berggren EK, Boggess KA, Funk MJ, Stuebe AM. Racial disparities in perinatal outcomes among women with gestational diabetes mellitus. Journal of Women’s Health 2012;21(5):521–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petersen R, Pan L, Blanck HM. Racial and Ethnic Disparities in Adult Obesity in the United States: CDC’s Tracking to Inform State and Local Action. Prev Chronic Dis 2019;16:E46. (In eng). DOI: 10.5888/pcd16.180579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang E, Glazer KB, Howell EA, Janevic TM. Social Determinants of Pregnancy-Related Mortality and Morbidity in the United States: A Systematic Review. Obstet Gynecol 2020;135(4):896–915. (In eng). DOI: 10.1097/aog.0000000000003762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American College of Obstetricians and Gynecologists. Importance of Social Determinants of Health and Cultural Awareness in the Delivery of Reproductive Health Care (Committee Opinion No. 729). Obstet Gynecol 2018;131(1):e43–e48. (In eng). DOI: 10.1097/aog.0000000000002459. [DOI] [PubMed] [Google Scholar]
- 17.Crear-Perry J, Correa-de-Araujo R, Lewis Johnson T, McLemore MR, Neilson E, Wallace M. Social and Structural Determinants of Health Inequities in Maternal Health. J Womens Health (Larchmt) 2021;30(2):230–235. (In eng). DOI: 10.1089/jwh.2020.8882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DiTosto JD, Holder K, Soyemi E, Beestrum M, Yee LM. Housing instability and adverse perinatal outcomes: a systematic review. Am J Obstet Gynecol MFM 2021;3(6):100477. (In eng). DOI: 10.1016/j.ajogmf.2021.100477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wheeler SM, Bryant AS, Bonney EA, Howell EA. Society for Maternal-Fetal Medicine Special Statement: Race in maternal-fetal medicine research- Dispelling myths and taking an accurate, antiracist approach. Am J Obstet Gynecol 2021. (In eng). DOI: 10.1016/j.ajog.2021.11.023. [DOI] [PubMed] [Google Scholar]
- 20.(NCCDPHP) NCfCDPaHP. Health Equity. 3/11/2020 (https://www.cdc.gov/chronicdisease/healthequity/index.htm).
- 21.Smith GN, Saade G, for the Society for Maternal-Fetal Medicine for the SMFM Health Policy Committee. SMFM White Paper: Pregnancy as a Window to Future Health:. (www.smfm.org/publications/141-smfm-white-paper-pregnancy-as-a-window-to-future-health).
- 22.Cain MA, Salemi JL, Tanner JP, Kirby RS, Salihu HM, Louis JM. Pregnancy as a window to future health: maternal placental syndromes and short-term cardiovascular outcomes. American Journal of Obstetrics and Gynecology 2016;215:484.e1–14. [DOI] [PubMed] [Google Scholar]
- 23.Saade G. Pregnancy as a window to future health. Obstet Gynecol 2009;114(5):958–60. [DOI] [PubMed] [Google Scholar]
- 24.Graham W, Smith P, Kamal A, Fitzmaurice A, Smith N, Hamilton N. Randomised controlled trial comparing effectiveness of touch screen system with leaflet for providing women with information on prenatal tests. British Medical Journal 2000;320(7228):155–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Javed Z, Haisum Maqsood M, Yahya T, et al. Race, Racism, and Cardiovascular Health: Applying a Social Determinants of Health Framework to Racial/Ethnic Disparities in Cardiovascular Disease. Circ Cardiovasc Qual Outcomes 2022;15(1):e007917. (In eng). DOI: 10.1161/circoutcomes.121.007917. [DOI] [PubMed] [Google Scholar]
- 26.American College of Obstetricians and Gynecologists. Committee Opinion No. 736: Optimizing Postpartum Care. Obstet Gynecol 2018;131(5):e140–50. [DOI] [PubMed] [Google Scholar]
- 27.Paladine HL, Blenning CE, Strangas Y. Postpartum Care: An Approach to the Fourth Trimester. Am Fam Physician 2019;100(8):485–491. (In eng). [PubMed] [Google Scholar]
- 28.Maxwell YL. Making space for preconception counseling in primary care. ACP Internist. [Google Scholar]
- 29.Mehta LS, Sharma G, Creanga AA, et al. Call to Action: Maternal Health and Saving Mothers: A Policy Statement From the American Heart Association. Circulation 2021;144(15):e251–e269. (In eng). DOI: 10.1161/cir.0000000000001000. [DOI] [PubMed] [Google Scholar]
- 30.Martinez NG, Niznik CM, Yee LM. Optimizing postpartum care for the patient with gestational diabetes mellitus. Am J Obstet Gynecol 2017;217(3):314–321. (In eng). DOI: 10.1016/j.ajog.2017.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilcox A, Levi E, Garrett J. Predictors of non-attendance to the postpartum follow-up visit. Matern Child Health J 2016;20(Suppl 1):22–7. [DOI] [PubMed] [Google Scholar]
- 32.Battarbee AN, Yee LM. Barriers to Postpartum Follow-Up and Glucose Tolerance Testing in Women with Gestational Diabetes Mellitus. Am J Perinatol 2018;35(4):354–360. (In eng). DOI: 10.1055/s-0037-1607284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qafiti F, Kaur S, Bahado-Singh R. Development of a Clinical Risk Assessment Tool for 6-Week Postpartum Visit Nonadherence. Am J Perinatol 2018;35(7):688–694. (In eng). DOI: 10.1055/s-0037-1612640. [DOI] [PubMed] [Google Scholar]
- 34.Cameron NA, Everitt I, Seegmiller LE, Yee LM, Grobman WA, Khan SS. Trends in the Incidence of New-Onset Hypertensive Disorders of Pregnancy Among Rural and Urban Areas in the United States, 2007 to 2019. J Am Heart Assoc 2022;11(2):e023791. (In eng). DOI: 10.1161/jaha.121.023791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang MC, Freaney PM, Perak AM, et al. Trends in prepregnancy cardiovascular health in the United States, 2011–2019. Am J Prev Cardiol 2021;7:100229. (In eng). DOI: 10.1016/j.ajpc.2021.100229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodríguez JE, Campbell KM. Racial and Ethnic Disparities in Prevalence and Care of Patients With Type 2 Diabetes. Clin Diabetes 2017;35(1):66–70. DOI: 10.2337/cd15-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Campbell A, Stanhope KK, Platner M, Joseph NT, Jamieson DJ, Boulet SL. Demographic and Clinical Predictors of Postpartum Blood Pressure Screening Attendance. J Womens Health (Larchmt) 2021. (In eng). DOI: 10.1089/jwh.2021.0161. [DOI] [PubMed] [Google Scholar]
- 38.Bennett W, Ennen C, Carrese J, et al. Barriers to and facilitators of postpartum follow-up care in women with recent gestational diabetes mellitus: a qualitative study. J Womens Health (Larchmt) 2011;20(2):239–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DiBari JN, Yu SM, Chao SM, Lu MC. Use of postpartum care: Predictors and barriers. J Pregnancy 2014;2014:530769. (In eng). DOI: 10.1155/2014/530769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ko JY, Dietz PM, Conrey EJ, et al. Strategies associated with higher postpartum glucose tolerance screening rates for gestational diabetes mellitus patients. J Womens Health (Larchmt) 2013;22(8):681–6. DOI: 10.1089/jwh.2012.4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Young B, Hacker MR, Rana S. Physicians’ knowledge of future vascular disease in women with preeclampsia. Hypertens Pregnancy 2012;31(1):50–8. (In eng). DOI: 10.3109/10641955.2010.544955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilkins-Haug L, Celi A, Thomas A, Frolkis J, Seely EW. Recognition by Women’s Health Care Providers of Long-Term Cardiovascular Disease Risk After Preeclampsia. Obstet Gynecol 2015;125(6):1287–1292. (In eng). DOI: 10.1097/aog.0000000000000856. [DOI] [PubMed] [Google Scholar]
- 43.American College of Obstetricians and Gynecologists. Committee Opinion 585: Health literacy. Obstetrics and Gynecology 2014;123:380–3. [DOI] [PubMed] [Google Scholar]
- 44.American College of Obstetricans and Gynecologists. Committee Opinion No. 676: Health Literacy to Promote Quality of Care. Obstet Gynecol 2016;128(4):e183–6. (In eng). DOI: 10.1097/aog.0000000000001714. [DOI] [PubMed] [Google Scholar]
- 45.Yee LM, Niznik CM, Simon MA. Examining the Role of Health Literacy in Optimizing the Care of Pregnant Women with Diabetes. Am J Perinatol 2016;33(13):1242–1249. (In eng). DOI: 10.1055/s-0036-1584540. [DOI] [PubMed] [Google Scholar]
- 46.Stasenko M, Liddell J, Cheng Y, Sparks T, Killion M, Caughey AB. Patient counseling increases postpartum follow-up in women with gestational diabetes mellitus. Am J Obstet Gynecol 2011;204(6):522.e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chambers BD, Arabia SE, Arega HA, et al. Exposures to structural racism and racial discrimination among pregnant and early post-partum Black women living in Oakland, California. Stress Health 2020;36(2):213–219. (In eng). DOI: 10.1002/smi.2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shankar M, Chan CS, Frayne SM, Panelli DM, Phibbs CS, Shaw JG. Postpartum Transition of Care: Racial/Ethnic Gaps in Veterans’ Re-Engagement in VA Primary Care after Pregnancy. Womens Health Issues 2021;31(6):603–609. (In eng). DOI: 10.1016/j.whi.2021.06.003. [DOI] [PubMed] [Google Scholar]
- 49.Brown HL, Warner JJ, Gianos E, et al. Promoting Risk Identification and Reduction of Cardiovascular Disease in Women Through Collaboration With Obstetricians and Gynecologists: A Presidential Advisory From the American Heart Association and the American College of Obstetricians and Gynecologists. Circulation 2018;137(24):e843–e852. (In eng). DOI: 10.1161/cir.0000000000000582. [DOI] [PubMed] [Google Scholar]
- 50.McCloskey L, Bernstein J, The Bridging The Chasm C, et al. Bridging the Chasm between Pregnancy and Health over the Life Course: A National Agenda for Research and Action. Womens Health Issues 2021;31(3):204–218. (In eng). DOI: 10.1016/j.whi.2021.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yee LM, Miller EC, Greenland P. Mitigating the Long-term Health Risks of Adverse Pregnancy Outcomes. JAMA 2022. DOI: 10.1001/jama.2021.23870. [DOI] [PubMed] [Google Scholar]
- 52.Ogunwole SM, Chen X, Mitta S, et al. Interconception Care for Primary Care Providers: Consensus Recommendations on Preconception and Postpartum Management of Reproductive-Age Patients With Medical Comorbidities. Mayo Clin Proc Innov Qual Outcomes 2021;5(5):872–890. (In eng). DOI: 10.1016/j.mayocpiqo.2021.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Timmins CL. The impact of language barriers on the health care of Latinos in the United States: a review of the literature and guidelines for practice. J Midwifery Womens Health 2002;47(2):80–96. (In eng). DOI: 10.1016/s1526-9523(02)00218-0. [DOI] [PubMed] [Google Scholar]
- 54.Bailey ZD, Feldman JM, Bassett MT. How Structural Racism Works - Racist Policies as a Root Cause of U.S. Racial Health Inequities. N Engl J Med 2021;384(8):768–773. (In eng). DOI: 10.1056/NEJMms2025396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Collins JW Jr., David RJ, Symons R, Handler A, Wall SN, Dwyer L. Low-income African-American mothers’ perception of exposure to racial discrimination and infant birth weight. Epidemiology 2000;11(3):337–9. (In eng). DOI: 10.1097/00001648-200005000-00019. [DOI] [PubMed] [Google Scholar]
- 56.Wallace M, Crear-Perry J, Richardson L, Tarver M, Theall K. Separate and unequal: Structural racism and infant mortality in the US. Health Place 2017;45:140–144. (In eng). DOI: 10.1016/j.healthplace.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 57.Bailey ZD, Krieger N, Agénor M, Graves J, Linos N, Bassett MT. Structural racism and health inequities in the USA: evidence and interventions. Lancet 2017;389(10077):1453–1463. (In eng). DOI: 10.1016/s0140-6736(17)30569-x. [DOI] [PubMed] [Google Scholar]
- 58.McCloskey L, Quinn E, Ameli O, et al. Interrupting the Pathway from Gestational Diabetes Mellitus to Type 2 Diabetes: The Role of Primary Care. Womens Health Issues 2019;29(6):480–488. (In eng). DOI: 10.1016/j.whi.2019.08.003. [DOI] [PubMed] [Google Scholar]
- 59.Center PR. Mobile Fact Sheet. April 7, 2021. (https://www.pewresearch.org/internet/fact-sheet/mobile/).
- 60.Vesco KK, Dietz PM, Bulkley J, et al. A system-based intervention to improve postpartum diabetes screening among women with gestational diabetes. Am J Obstet Gynecol 2012;207(4):283.e1–6. [DOI] [PubMed] [Google Scholar]
- 61.Zera C, Bates D, Stuebe A, Ecker J, Seely E. Diabetes screening reminder for women with prior gestational diabetes: A randomized controlled trial. Obstet Gynecol 2015;126(1):109–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Soffer MD, Factor SH, Rosenman A, Levy C, Stone J. Improving postpartum glucose monitoring in women with gestational diabetes. Journal of Maternal-Fetal & Neonatal Medicine 2017;30(24):3014–3019. (In eng). DOI: 10.1080/14767058.2016.1271411. [DOI] [PubMed] [Google Scholar]
- 63.Heatley E, Middleton P, Hague W, Crowther C. The DIAMIND study: postpartum SMS reminders to women who have had gestational diabetes mellitus to test for type 2 diabetes: a randomised controlled trial - study protocol. BMC Pregnancy Childbirth 2013;13:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Van Ryswyk E, Middleton P, Hague W, Crowther C. Postpartum SMSM reminders to women who have experienced gestational diaebtes to test for Type 2 diabetes: the DIAMIND randomized trial. Diabet Med 2015;32(10):1368–76. [DOI] [PubMed] [Google Scholar]
- 65.Lawrence JM, Black MH, Hsu JW, Chen W, Sacks DA. Prevalence and timing of postpartum glucose testing and sustained glucose dysregulation after gestational diabetes mellitus. Diabetes Care 2010;33(3):569–76. (In eng). DOI: 10.2337/dc09-2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hirshberg A, Downes K, Srinivas S. Comparing standard office-based follow-up with text-based remote monitoring in the management of postpartum hypertension: a randomised clinical trial. BMJ Qual Saf 2018;27(11):871–877. (In eng). DOI: 10.1136/bmjqs-2018-007837. [DOI] [PubMed] [Google Scholar]
- 67.Nielsen KK, Kapur A, Damm P, de Courten M, Bygbjerg IC. From screening to postpartum follow-up: the determinants and barriers for gestational diabetes (GDM) services, a systematic review. BMC Pregnancy Childbirth 2014;14(41):1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ukoha EP, Feinglass J, Yee LM. Disparities in Electronic Patient Portal Use in Prenatal Care: Retrospective Cohort Study. J Med Internet Res 2019;21(9):e14445. (In eng). DOI: 10.2196/14445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kern-Goldberger A, Hirshberg A. Reducing Disparities Using Telehealth Approaches for Postdelivery Preeclampsia Care. Clin Obstet Gynecol 2021;64(2):375–383. (In eng). DOI: 10.1097/grf.0000000000000605. [DOI] [PubMed] [Google Scholar]
- 70.Hoppe KK, Williams M, Thomas N, et al. Telehealth with remote blood pressure monitoring for postpartum hypertension: A prospective single-cohort feasibility study. Pregnancy Hypertens 2019;15:171–176. (In eng). DOI: 10.1016/j.preghy.2018.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hauspurg A, Lemon LS, Quinn BA, et al. A Postpartum Remote Hypertension Monitoring Protocol Implemented at the Hospital Level. Obstet Gynecol 2019;134(4):685–691. (In eng). DOI: 10.1097/aog.0000000000003479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Triebwasser JE, Janssen MK, Hirshberg A, Srinivas SK. Successful implementation of text-based blood pressure monitoring for postpartum hypertension. Pregnancy Hypertens 2020;22:156–159. (In eng). DOI: 10.1016/j.preghy.2020.09.001. [DOI] [PubMed] [Google Scholar]
- 73.Rhoads SJ, Serrano CI, Lynch CE, et al. Exploring Implementation of m-Health Monitoring in Postpartum Women with Hypertension. Telemed J E Health 2017;23(10):833–841. (In eng). DOI: 10.1089/tmj.2016.0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hirshberg A, Sammel MD, Srinivas SK. Text message remote monitoring reduced racial disparities in postpartum blood pressure ascertainment. Am J Obstet Gynecol 2019;221(3):283–285. (In eng). DOI: 10.1016/j.ajog.2019.05.011. [DOI] [PubMed] [Google Scholar]
- 75.Liu P, Astudillo K, Velez D, Kelley L, Cobbs-Lomax D, Spatz ES. Use of Mobile Health Applications in Low-Income Populations: A Prospective Study of Facilitators and Barriers. Circ Cardiovasc Qual Outcomes 2020;13(9):e007031. (In eng). DOI: 10.1161/circoutcomes.120.007031. [DOI] [PubMed] [Google Scholar]
- 76.Ukoha EP, Davis K, Yinger M, et al. Ensuring Equitable Implementation of Telemedicine in Perinatal Care. Obstet Gynecol 2021;137(3):487–492. (In eng). DOI: 10.1097/aog.0000000000004276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yee LM JJ, Leziak K, Niznik CM, Saber R, Yeh C, Simon MA. SweetMama: Usability testing of a novel mobile application for diabetes education and support during pregnancy. Society for Maternal-Fetal Medicine 40th Annual Meeting. Grapevine, TX2020. [Google Scholar]
- 78.United States National Library of Medicine ClinicalTrials.gov. SweetMama: Testing of a novel technology for diabetes education and support to pregnant women (NCT03240874). (https://clinicaltrials.gov/ct2/show/NCT03240874?term=sweetmama&draw=2&rank=1).
- 79.Leziak K, Birch E, Jackson J, Strohbach A, Niznik C, Yee LM. Identifying Mobile Health Technology Experiences and Preferences of Low-Income Pregnant Women with Diabetes. J Diabetes Sci Technol 2021;15(5):1018–1026. (In eng). DOI: 10.1177/1932296821993175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lewey J, Murphy S, Zhang D, et al. Effectiveness of a Text-Based Gamification Intervention to Improve Physical Activity Among Postpartum Individuals With Hypertensive Disorders of Pregnancy: A Randomized Clinical Trial. JAMA Cardiol 2022. (In eng). DOI: 10.1001/jamacardio.2022.0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Soffer MD, Rekawek P, Pan S, Overbey J, Stone J. Improving Postpartum Attendance among Women with Gestational Diabetes Using the Medical Home Model of Care. Am J Perinatol 2021. (In eng). DOI: 10.1055/s-0041-1727216. [DOI] [PubMed] [Google Scholar]
- 82.Yee LM, Martinez NG, Nguyen AT, Hajjar N, Chen MJ, Simon MA. Using a Patient Navigator to Improve Postpartum Care in an Urban Women’s Health Clinic. Obstet Gynecol 2017;129(5):925–933. (In eng). DOI: 10.1097/aog.0000000000001977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yee LM, Williams B, Green HM, et al. Bridging the postpartum gap: best practices for training of obstetrical patient navigators. Am J Obstet Gynecol 2021;225(2):138–152. (In eng). DOI: 10.1016/j.ajog.2021.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ruderman RS, Dahl EC, Williams BR, et al. Obstetric Provider Perspectives on Postpartum Patient Navigation for Low-Income Patients. Health Educ Behav 2021:10901981211043117. (In eng). DOI: 10.1177/10901981211043117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hu F, Strohbach A, Martinez NG, Simon MA, Yee LM. Patient and Provider Perceptions of a Patient Navigation Program to Improve Postpartum Care Among Publicly Insured Women. Am J Perinatol 2019. (In eng). DOI: 10.1055/s-0039-1696671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.United States National Library of Medicine ClinicalTrials.gov. SWEET: Postpartum Navigation After GDM: NCT04583839. (https://clinicaltrials.gov/ct2/show/NCT04583839?term=lynn+yee&draw=2&rank=2).
- 87.Cusimano MC, Pudwell J, Roddy M, Cho C-KJ, Smith GN. The maternal health clinic: an initiative for cardiovascular risk identification in women with pregnancy-related complications. American Journal of Obstetrics and Gynecology 2014;210(5):438.e1–438.e9. DOI: 10.1016/j.ajog.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 88.Daw JR, Hatfield LA, Swartz K, Sommers BD. Women In The United States Experience High Rates Of Coverage ‘Churn’ In Months Before And After Childbirth. Health Aff (Millwood) 2017;36(4):598–606. (In eng). DOI: 10.1377/hlthaff.2016.1241. [DOI] [PubMed] [Google Scholar]
- 89.Celi AC, Seely EW, Wang P, Thomas AM, Wilkins-Haug LE. Caring for Women After Hypertensive Pregnancies and Beyond: Implementation and Integration of a Postpartum Transition Clinic. Matern Child Health J 2019;23(11):1459–1466. (In eng). DOI: 10.1007/s10995-019-02768-7. [DOI] [PubMed] [Google Scholar]
- 90.The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care 2002;25(12):2165–71. (In eng). DOI: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chicago CARES to Prevent Diabetes. (https://chicagocaresdpp.org/).
- 92.Rich-Edwards JW, Stuart JJ, Skurnik G, et al. Randomized Trial to Reduce Cardiovascular Risk in Women with Recent Preeclampsia. J Womens Health (Larchmt) 2019;28(11):1493–1504. (In eng). DOI: 10.1089/jwh.2018.7523. [DOI] [PubMed] [Google Scholar]
- 93.Natalie A Cameron HB, Niznik Charlotte M., Michon Ruth, Donelan Emily, Yee Lynn M., Dolan Brigid. Bridging the Gap: A pilot program to improve postpartum care for birthing individuals with cardiometabolic complications of pregnancy. Society of General Internal Medicine 2022 Annual Meeting. Orlando, FL2022. [Google Scholar]
- 94.Hirshberg A, Bittle M, VanDerTuyn M, et al. Rapid-cycle innovation testing of text-based monitoring for management of postpartum hypertension. J Clin Outcomes Manag 2017;24(2):77–85. [Google Scholar]
- 95.Nicklas JM, Zera CA, England LJ, et al. A Web-Based Lifestyle Intervention for Women With Recent Gestational Diabetes Mellitus: A Randomized Controlled Trial. Obstet Gynecol 2014;124(3):563–570. DOI: 10.1097/aog.0000000000000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ferrara A, Hedderson MM, Brown SD, et al. The Comparative Effectiveness of Diabetes Prevention Strategies to Reduce Postpartum Weight Retention in Women With Gestational Diabetes Mellitus: The Gestational Diabetes’ Effects on Moms (GEM) Cluster Randomized Controlled Trial. Diabetes Care 2016;39(1):65–74. (In eng). DOI: 10.2337/dc15-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ferrara A, Hedderson MM, Albright CL, et al. A pregnancy and postpartum lifestyle intervention in women with gestational diabetes mellitus reduces diabetes risk factors: a feasibility randomized control trial. Diabetes Care 2011;34(7):1519–25. (In eng). DOI: 10.2337/dc10-2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nicholson WK, Beckham AJ, Hatley K, et al. The Gestational Diabetes Management System (GooDMomS): Development, feasibility and lessons learned from a patient-informed, web-based pregnancy and postpartum lifestyle intervention. BMC Pregnancy and Childbirth 2016;16(277):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nicklas J, Zera C, England L, et al. A web-based lifestyle intervention for women with recent gestational diabetes mellitus. Obstet Gynecol 2014;124(3):563–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bellerose M, Collin L, Daw JR. The ACA Medicaid Expansion And Perinatal Insurance, Health Care Use, And Health Outcomes: A Systematic Review. Health Aff (Millwood) 2022;41(1):60–68. (In eng). DOI: 10.1377/hlthaff.2021.01150. [DOI] [PubMed] [Google Scholar]
- 101.Gordon SH, Hoagland A, Admon LK, Daw JR. Extended Postpartum Medicaid Eligibility Is Associated With Improved Continuity Of Coverage In The Postpartum Year. Health Aff (Millwood) 2022;41(1):69–78. (In eng). DOI: 10.1377/hlthaff.2021.00730. [DOI] [PubMed] [Google Scholar]
- 102.Blog HA. “Beyond The Preventing Maternal Deaths Act: Implementation And Further Policy Change”. February 4, 2019.
- 103.Sutherland R. The State of the Field: Legislation Addressing Disparities in Birth Outcomes and Maternal Mortality among Black Mothers and Infants.
- 104.Ferranti EP, Jones EJ, Bush S, et al. A Call to Action: Cardiovascular-Related Maternal Mortality: Inequities in Black, Indigenous, and Persons of Color. J Cardiovasc Nurs 2021;36(4):310–311. DOI: 10.1097/jcn.0000000000000823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Alma Adams LU. About the Black Maternal Health Momnibus Act of 2021.
- 106.Glynn A, MacKenzie R, Fitzgerald T. Taming Healthcare Costs: Promise and Pitfalls for Women’s Health. J Womens Health (Larchmt) 2016;25(2):110–6. (In eng). DOI: 10.1089/jwh.2015.5295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mitchell C, Lawton E, Morton C, McCain C, Holtby S, Main E. California Pregnancy-Associated Mortality Review: mixed methods approach for improved case identification, cause of death analyses and translation of findings. Matern Child Health J 2014;18(3):518–26. (In eng). DOI: 10.1007/s10995-013-1267-0. [DOI] [PubMed] [Google Scholar]


