Abstract
Objective:
To summarize the evidence for the Pediatric Acute Lung Injury Consensus Conference-2 (PALICC-2) recommendations for assessment of outcomes among patients surviving pediatric acute respiratory distress syndrome (PARDS).
Data Sources:
MEDLINE (Ovid), Embase (Elsevier), and CINAHL Complete (EBSCOhost)
Study Selection:
We conducted a scoping review to identify studies evaluating outcomes following PARDS. We included studies of survivors of PARDS, acute respiratory failure with a high proportion of PARDS patients, or other critical illnesses if PARDS-specific outcomes could be extracted.
Data Extraction:
Title/abstract review, full text review, and data extraction using a standardized data collection form.
Data Synthesis:
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach was used to identify and summarize evidence and develop recommendations. Of 8,037 abstracts screened, we identified 20 articles for inclusion. Morbidity following PARDS was common and affected multiple domains of pulmonary and non-pulmonary function. There was insufficient evidence to generate any evidence-based recommendations. We generated eight good practice statements and five research statements. A panel of 52 experts discussed each proposed good practice statement and research statement, and the agreement rate was measured with an online voting process. Good practice statements describe the approach to clinical outcome assessment, assessment of pulmonary outcomes of children surviving PARDS, and assessment of non-pulmonary outcomes of children surviving PARDS including health-related quality of life and physical, neurocognitive, emotional, family, and social functioning. The five research statements relate to assessment of patient pre-illness status, use of post-discharge endpoints for clinical trials, the association between short-term and longer-term outcomes, the trajectory of recovery following PARDS, and practices to optimize follow-up.
Conclusions:
There is increasing evidence that children are at risk for impairments across a range of pulmonary and non-pulmonary health domains following hospitalization for PARDS. The results of this extensive scoping review and consensus conference involving experts in PARDS research, clinical care, and outcomes assessment provide guidance to clinicians and researchers on post-discharge follow-up to optimize the long-term health of patients surviving PARDS.
Keywords: Acute Lung Injury, Critical Care Outcomes, Follow-Up Studies, Intensive Care Units, Pediatric, Morbidity, Respiratory Distress Syndrome
There is increasing awareness that survivors of pediatric critical illness are at risk for ongoing morbidities across a range of health domains [1, 2]. Pediatric critical care clinicians, researchers, and families recommend assessment of child overall health, health-related quality of life (HRQL), physical, emotional, and cognitive function, pain, communication, and long-term survival following pediatric intensive care unit (PICU) discharge [3]. It is unknown whether these are also the most important domains to assess among survivors of pediatric acute respiratory distress syndrome (PARDS), who may in particular have additional pulmonary morbidity related to their acute lung injury. Pediatric Acute Lung Injury Consensuses Conference (PALICC) 2015 provided guidelines for outcome assessment following PARDS based on limited pediatric evidence [4]; we aimed to update these guidelines by reviewing new evidence published in the last 10 years. In this article for the Second Pediatric Acute Lung Injury Consensus Conference (PALICC-2), we address Key Question #9 as outlined in the accompanying methodology manuscript [5]: What are the morbidity and long-term outcomes of children with PARDS?
METHODS
Details of the literature search are outlined in the PALICC-2 methodology manuscript in this supplement [5]. We conducted a scoping review based on the PRISMA Extension for Scoping Reviews guidelines [6] to identify relevant studies related to outcomes of children surviving PARDS. We included studies that evaluated either pulmonary or non-pulmonary outcomes at the time of or following hospital discharge among survivors of PARDS, acute respiratory failure with a high proportion of PARDS patients, or other critical illnesses if PARDS-specific outcomes could be extracted. The complete search strategy can be found in the Supplemental Digital Content (Table S1). Details of title/abstract review, full text review, and data extraction, and generation of good practice statements and research statements are outlined in the PALICC-2 methodology manuscript [5].
RESULTS
Of 8,037 abstracts screened, 110 underwent full-text screening and 20 articles were included (Figure S1). From these articles, we developed four categories of recommendations/statements about: a) approach to clinical outcome assessment; b) pulmonary outcomes of patients who survive PARDS; c) non-pulmonary outcomes of patients who survive PARDS; and d) conducting PARDS outcomes research. Complete evidence-to-decision tables supporting the recommendations are provided as Tables S2–8.
Approach to Clinical Outcome Assessment
Post-PICU morbidities among children surviving PARDS and their families may go unrecognized if not intentionally evaluated. The optimal setting for and approach to post-discharge evaluation may differ based on the patient’s medical complexity and available resources, but for many patients may optimally occur in the patient’s existing medical home.
Good practice statement 9.1.1. In patients with PARDS, the patient’s primary care providers should be advised to screen for post-PICU morbidities within three months of discharge from the hospital.
(Ungraded good practice statement, 90% agreement)
Good practice statement 9.1.2. A stepwise approach to clinical evaluation of post-PICU morbidities should be used with initial screening by a primary care provider or electronic/telephonic screen.
(Ungraded good practice statement, 96% agreement)
Remarks: Full assessment, initial management, and serial re-evaluations of impairments should be made by a primary care provider if appropriate and referral to a specialist if deficits persist or are not in the scope of practice of the primary care provider. Location-specific resources should be considered, including availability of dedicated post-PICU follow-up clinics or remote consultation.
Justification
Opportunities for children to be evaluated in formal follow-up clinics remain limited [7]. While follow-up clinics have become increasingly common after adult and neonatal intensive care [8–10], most pediatric-specific clinics are focused on follow-up after pediatric neurocritical care [11–13]. Screening for morbidities following PARDS may alternatively (or even optimally) be performed by primary care providers after PICU discharge for patients without chronic respiratory support needs. For children requiring chronic respiratory support, subspecialty follow-up with pulmonologists or pediatricians with expertise in chronic respiratory care is advised. PICU providers familiar with the patient’s clinical course should advise primary care providers about the patient’s risk for adverse post-discharge outcomes and recommend screening. Primary care providers may elect to subsequently refer their patients to specialty providers including pulmonologists, rehabilitation specialists, physiotherapists, psychologists, or educators depending on the deficits identified.
Benefits
The initial months after PICU admission are resource-intensive for families and patients, and often include multiple healthcare visits, new equipment or medications, or outpatient therapy [14]. These months may represent a sensitive period of recovery for children who have acquired morbidities during their PICU stay, and provision of post-discharge care to optimize recovery is crucial. This resource-intensive period necessitates provision of care in a family-centered manner, which can be accomplished by incorporating post-PICU care into the patient’s medical home.
Harms and burdens
The burden of a one-time electronic or telephone communication from PICU providers to primary care clinicians is minimal but requires incorporation into standard PICU workflow. There is a time and potential financial burden on primary care clinicians to assume responsibility for screening.
Balance of effects
The burden associated with communication between the PICU provider and primary care clinician is minimal, while the risk of failure to communicate a patient’s risk for adverse outcomes and recommended screening for post-discharge impairments is high. The evaluation itself requires additional effort on the part of the clinician, family, and patient, but the associated risk is low while the potential for benefit if impairments are identified is high.
Implementation considerations
Post-PICU assessment and initial management of identified impairments by the primary care provider can minimize burden on patients, families, and specialists. Reserving subspecialty referrals for children with the highest risk of impairments or after impairments are identified may enhance efficiency and lessen the burden on families. The types of evaluation will differ based on local resources with varied settings presenting different opportunities to screen for and treat impairments congruent with resource availability.
Pulmonary Outcomes of Children Who Survive PARDS
While post-discharge pulmonary outcomes have been well-characterized in adult survivors of ARDS, studies of long-term pulmonary sequelae among survivors of PARDS are limited [15,16]. There is increasing evidence, however, that a subset of children surviving PARDS have ongoing pulmonary morbidities following discharge [17–20].
Good practice statement 9.2.1. Patients with PARDS should be screened for pulmonary function abnormalities within the first three months after hospital discharge.
(Ungraded good practice statement, 90% agreement)
Remarks: The screening should include a minimum of a respiratory symptom questionnaire, a respiratory exam, and pulse oximetry.
Good practice statement 9.2.2. Patients with PARDS who are of sufficient developmental age and capabilities should also be assessed by spirometry to screen for pulmonary function abnormalities within the first three months after discharge. (Ungraded good practice statement, 94% agreement)
Remarks: A follow-up assessment within the first year should be added if spirometry is abnormal.
Good practice statement 9.2.3. Patients should be referred to a specialist (pediatrician or pediatric pulmonologist) when deficits in pulmonary function are identified for further assessment, treatment, and long-term pulmonary follow-up.
(Ungraded good practice statement, 94% agreement)
Justification
The 2015 PALICC recommendations/statements were based on adult data and six small studies (n=2 to 11) reporting pulmonary function abnormalities in children following PARDS at variable time points after hospital discharge [4]. While still limited by small sample sizes, single-center cohorts, and loss to follow-up, four recent observational studies (n=29 to 47) provide additional evidence that children with PARDS are at risk for developing or having persistent respiratory symptoms and pulmonary function abnormalities post-discharge [17–20] (Table 1, Table S9). One study included children meeting the American European Consensus Conference (AECC) ARDS definition [20], while the other three included children meeting the PALICC definition of PARDS [17–19]. Abnormal respiratory symptoms including cough, breathing difficulty, and wheezing were reported on non-standardized questionnaires in approximately one-third of children with PARDS at three months post-discharge [17,18], with most symptoms resolving by 12 months [18]. A minority had an abnormal respiratory examination [18]. In one retrospective study conducted using insurance claims data, over 80% of the 47 children with severe PARDS were evaluated by a pulmonologist in the year following their PICU admission and 57.4% were treated with pulmonary medication(s) [19] (Figure 1).
Table 1:
Summary of evidence related to pulmonary outcomes following acute respiratory distress syndrome in children
| Ref (Year) | Centers | Population | Country | Outcome Domains | Timepoints | No.a | Results |
|---|---|---|---|---|---|---|---|
| 17 (2020) | Single center | General ARDS | Canada | Respiratory symptoms | 3 months | 38 | 36.8% any symptoms; 18.4% new symptoms |
| Pulmonary function | 3 months | 10 | 30% abnormal spirometry; 25% decreased inspiratory pressure | ||||
|
| |||||||
| 18 (2018) | Single center | General ARDS | India | Respiratory symptoms | 3 months 9–12 months |
29 27 |
37.9% any symptoms 0% any symptoms |
| Respiratory exam | 3 months 9–12 months |
29 27 |
3.4% abnormal exam 0% abnormal exam |
||||
| Pulmonary function | 3 months 9–12 months |
29 27 |
82.7% abnormal spirometry 18.5% abnormal spirometry |
||||
|
| |||||||
| 19 (2022) | Single center | Severe ARDS | US | Pulmonary care | 1 year | 47 | 80.9% ≥1 pulmonary outpatient visit; 57.4% pulmonary medication use |
|
| |||||||
| 20 (2017) | Single center | General ARDS | US | Pulmonary function | 11 monthsb | 17 | 35.3% abnormal spirometry |
ARDS, acute respiratory distress syndrome.
Number of patients evaluated at each follow-up timepoint
Mean time to follow-up
Figure 1.
Proportion of patients with impairments in pulmonary outcomes based on time of assessment and domain. Studies listed by population evaluated and reference number. ARDS, acute respiratory distress syndrome.
In addition to abnormal respiratory symptoms, 30–80% of children surviving PARDS had pulmonary function abnormalities on lung function testing at three months post-discharge [17,18,20], with 18.5% - 35% having persistent abnormalities at one year [18,20]. Abnormalities were mixed, including obstructive and restrictive disease, and some were not accompanied by respiratory symptoms. Spirometry, a technique that is often limited to cooperative school-aged or older children, was the most commonly used assessment method. We are unable to recommend other methods of pulmonary function testing as only a small number of patients underwent alternative methods including oscillometry [17], plethysmography [20], maximal inspiratory and expiratory pressure measurements [17], or infant pulmonary function testing [20].
Benefits
Post-discharge screening for respiratory symptoms, examination, and pulmonary function testing can identify at-risk children who may benefit from targeted management and follow-up, including reassessment within the first year post-discharge. These results should be interpreted considering the child’s pulmonary status prior to PARDS. While the initial assessment may be performed by a primary care provider, we recommend referral to a pediatric specialist if persistent respiratory symptoms or impairments in pulmonary function are identified. Additional testing (e.g., chest imaging, more extensive pulmonary function testing) may be recommended by the specialist to further evaluate the impairment and initiate targeted management, if appropriate.
Harms and burdens
A symptom questionnaire, clinical examination, and pulse oximetry are often performed in routine medical visits, and thus the associated burden is minimal. Although spirometry is a routine, non-invasive procedure that can be safely performed in age-appropriate, cooperative children (generally five years and older) [21], it can be time-consuming for patients and families and may necessitate traveling to another facility where the test is available. Additionally, in-depth assessments and referral to specialists may be a burden for families, generate additional anxiety, and increase costs to families and the health care system.
Balance of effects
Taken together, these findings suggest that children surviving PARDS are at risk of pulmonary morbidity. Failure to manage and follow these deficits, particularly when severe, may lead to long-term morbidity in affected children.
Implementation considerations
The proposed evaluations differ based on the resources available. Spirometry, particularly when performed in younger children, requires trained technicians, and may have limited availability in rural or low-resource settings [21]. Additionally, accessibility to specialist care may be limited, and the roles of various health providers may differ from one setting to another. The application of these good practice statements will need to be adapted to specific health care environments.
Non-Pulmonary Outcomes of Children Who Survive PARDS
Increasingly, pediatric critical care researchers have attempted to better understand the range of morbidities that children may experience following PICU hospitalization [2]. While studies of outcomes specific to PARDS survivors remain limited, in recent years there has been substantially more research into non-pulmonary outcomes following PARDS. Therefore, we have been able to augment the previous 2015 PALICC recommendations/statements regarding neurocognitive outcomes, which were based on the adult literature and only a single pediatric study demonstrating decline in Pediatric Overall Performance Category (POPC) and Pediatric Cerebral Performance Category (PCPC) at hospital discharge following acute lung injury [22].
Good practice statement 9.3.1. For patients who survive PARDS, health-related quality of life (HRQL), physical, neurocognitive, emotional, family, and social function should be evaluated within three months of hospital discharge.
(Ungraded good practice statement, 100% agreement)
Good practice statement 9.3.2. For infants and toddlers who survive PARDS, additional evaluation of HRQL, physical, neurocognitive, emotional, family, and social function should be performed prior to entering school, for example, at 4–6 years of age.
(Ungraded good practice statement, 90% agreement)
Good practice statement 9.3.3. Patients with identified abnormalities should be treated or referred for more in-depth assessment and treatment by appropriate subspecialists and educators.
(Ungraded good practice statement, 98% agreement)
Justification
Recent studies demonstrated impairments in HRQL [20,23–25], functional status [23–30], neurocognitive status [31,32], emotional health [24], and school participation [18,33] in the months and years after hospitalization with PARDS, as well as high rates of ongoing care needs after hospital discharge [28,29,34], post-discharge health resource use [14,17,19], late mortality [14,18,23,31,35,36], and work absences for parents [33] (Table 2, Table S9).
Table 2:
Summary of evidence related to non-pulmonary outcomes following acute respiratory distress syndrome in children
| Ref (Year) | Centers | Population | Country | Outcome Domains | Timepoints | No.a | Results |
|---|---|---|---|---|---|---|---|
| 14(2022) | Single center | Moderate-severe ARDS | US | Health resource use | 1 year | 241 | 15.8% high resource use |
| Late mortality | 1 year | 241 | 4.6% post-discharge mortality | ||||
|
| |||||||
| 17 (2020) | Single center | General ARDS | Canada | Health resource use | 3 months | 38 | 23.7% ED visit, 10.5% readmission, 18.4% specialist referral |
|
| |||||||
| 18 (2018) | Single center | General ARDS | India | Late mortality | 3 months 9–12 months |
29 27 |
0% post-discharge mortality 7.4% post-discharge mortality |
| Return to school | 3 months 9–12 months |
29 27 |
44.8% not in school 14.8% not in school |
||||
|
| |||||||
| 19 (2022) | Single center | Severe ARDS | US | Health resource use | 1 year | 47 | 66.0% ED visit; 25.5% readmission |
|
| |||||||
| 20 (2017) | Single center | General ARDS | US | HRQL | 11 monthsb | 23 | Health perception 46.5/100; general health 63/100; physical functioning 77/100; behavior 75/100 |
|
| |||||||
| 23 (2022) | Multicenter | General ARDS | US | HRQL | 28 days | 95 | 53% >10% decline from baseline |
| 3 months | 72 | 25% >10% decline from baseline | |||||
| 9 months | 61 | 31% >10% decline from baseline | |||||
| Functional status | 28 days | 95 | 48% FSS change ≥3 | ||||
| 3 months | 72 | 31% FSS change ≥3 | |||||
| 9 months | 61 | 23% FSS change ≥3 | |||||
|
| |||||||
| 24 (2018) | Multicenter | Acute respiratory failurec | US | Functional status | 6 months | 949 | 19% POPC decline; 11% PCPC decline |
| HRQL | 6 months | 838 | 27% impaired HRQL | ||||
| Emotional health | 6 months | 102 | 30.4% positive PTSD screen | ||||
|
| |||||||
| 25 (2019) | Multicenter | Moderate-severe ARDS | US | Functional status | 6 months | 689 | 22.4% decline in POPC |
| HRQL | 6 months | 448 | 21.0% impaired HRQL | ||||
|
| |||||||
| 26 (2021) | Multicenter | Acute respiratory failurec | US | Functional status | Discharge | 427 | 9.4% decline in POPC |
|
| |||||||
| 27 (2022) | Single center | ARDS requiring ECMO | US | Functional status | 3–4 yearsb | 59 | Mean POPC 2.3; mean PCPC 1.9 |
|
| |||||||
| 28 (2018) | Single center | General ARDS | US | Post-discharge care | Discharge | 274 | 24.4% to post-discharge care |
| Functional status | Discharge | 274 | 23.0% FSS change ≥3 | ||||
| Late mortality | 1 year 3 years |
274 259 |
5.5% post-discharge mortality 8.0% post-discharge mortality |
||||
|
| |||||||
| 29 (2020) | Single center | Trauma patients with ARDS | US | Post-discharge care | Discharge | 68 | 76.9% to post-discharge care |
| Functional status | Discharge | 68 | 77.1% abnormal FIM | ||||
|
| |||||||
| 30 (2021) | Single center | General ARDS | Singapore | Functional status | Discharge | 90 | 15.6% FSS change ≥3; 38.9% any change; 27.8% new feeding support; 11.1% new respiratory support |
|
| |||||||
| 31 (2017) | Single center | Immunocompromised patients with ARDS | Germany | Late mortality | 3 years | 15 | 40.0% post-discharge mortality |
| Neurologic outcome | 3 years | 15 | 26.7% learning difficulties; 6.7% cognitive impairment | ||||
|
| |||||||
| 32 (2022) | Multicenter | Moderate-severe ARDS | US | Neurocognitive status | 3–8 years | 74 | 18.9% IQ ≥15 points below sibling |
|
| |||||||
| 33 (2021) | Multicenter | Acute respiratory failurec | US | School absenteeism | 6 months | 399 | 69.9% missed school days; 38.3% chronic absenteeism |
| Work absenteeism | 6 months | 506 | 54.7% missed work days | ||||
|
| |||||||
| 34 (2019) | National database | Trauma patients with ARDS | US | Post-discharge care | Discharge | 2073 | 55.8% to post-discharge care |
| Tracheostomy | Discharge | 2073 | 18.4% discharged with tracheostomy | ||||
|
| |||||||
| 35 (2008) | Single center | ARDS requiring ECMO | UK | Late mortality | 1 year | 77 | 5.2% post-discharge mortality |
|
| |||||||
| 36 (2020) | Single center | ARDS requiring ECMO | France | Late mortality | 6 months | 111 | 3.0% post-discharge mortality |
|
| |||||||
| 39 (2020) | Single center | ARDS with NMB | India | Neuropathy/myopathy | 3 months | 23 | 0% neuropathy or myopathy |
ARDS, acute respiratory distress syndrome; ECMO, extracorporeal membrane oxygenation; ED, Emergency Department; FIM, Functional Independence Measure; FSS, Functional Status Scale; HRQL, health-related quality of life; IQ, intelligence quotient; NMB, neuromuscular blockade; PCPC, Pediatric Cerebral Performance Category; POPC, Pediatric Overall Performance Category; PTSD, post-traumatic stress disorder.
Number of patients evaluated at each follow-up timepoint
Mean time to follow-up
73% of cohort with moderate-severe ARDS.
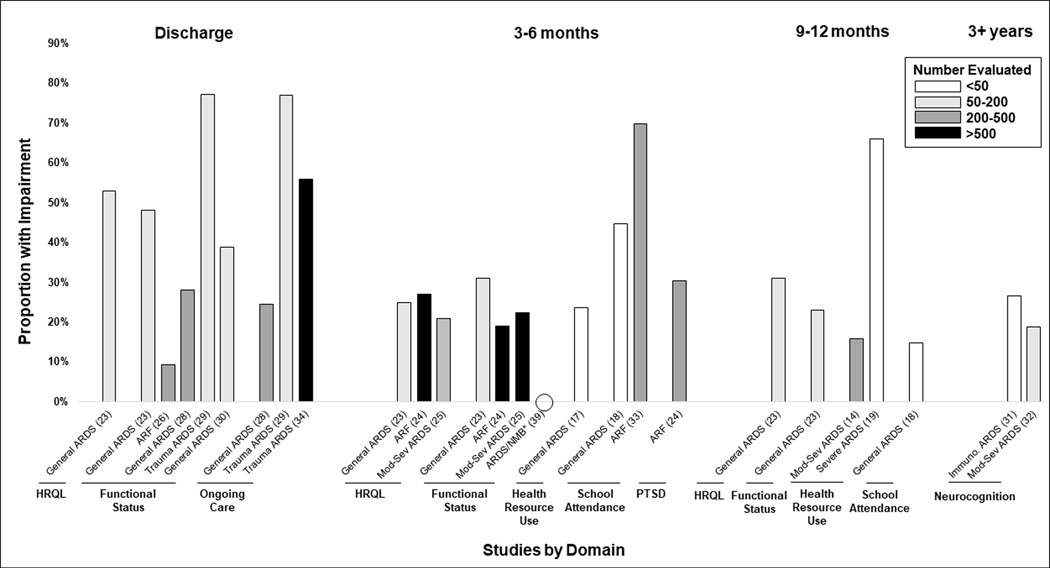
A small, single-center cohort study demonstrated that at a mean of 10.7 months post-admission, PARDS survivors had significantly worse HRQL than U.S. norms and children with asthma in the domains of general health perceptions and physical functioning; general health perception scores were particularly low at a mean of 46.5/100 [20]. In a larger multicenter study, 53% of PARDS survivors experienced a decline of >10% from their baseline HRQL at the earlier of 28 days post-admission or ICU discharge with 25% having persistent HRQL decline of >10% three months post-admission, a prevalence that rose to 31% by nine months post-admission [23]. The large multicenter Randomized Evaluation of Sedation Titration for Respiratory Failure (RESTORE) trial included HRQL assessment six months after ICU discharge in 838 survivors of acute respiratory failure, of whom 73% had moderate to severe PARDS; 27% of children and teens had impaired HRQL (at least one standard deviation below the population mean), with lower scores than healthy population norms across all HRQL domains. Scores for infants and toddlers were similarly lower than population norms for multiple HRQL domains [24]. Among the subset of participants who met criteria for moderate to severe PARDS and had normal pre-admission function, 21% of children and teens had impaired HRQL, and 22% of infants and toddlers had impaired growth and development [25] (Figure 2).
Figure 2.
Proportion of patients with impairments in non-pulmonary outcomes based on time of assessment and domain. Studies listed by population evaluated and reference number. ARDS, acute respiratory distress syndrome; ARF, acute respiratory failure; HRQL, health-related quality of life; Immuno, immunocompromised; NMB, neuromuscular blockade; PTSD, post-traumatic stress disorder. *Assessed neuropathy/myopathy.
In addition to the impaired physical functioning observed within domains of HRQL, multiple studies specifically evaluated functional status following PARDS. In a study of pediatric trauma patients with PARDS, 77.1% of were discharged with an abnormal Functional Independence Measure [37] score compared to 30.7% of trauma patients without PARDS [29], and 9.4% of these trauma patients with acute respiratory failure had a decline in POPC at discharge [26]. In two cohorts of general PICU patients with PARDS, 16–23% experienced new morbidity at hospital discharge based on increase in Functional Status Scale (FSS) [38] score by ≥3 points [28,30] with 39% experiencing any FSS increase, primarily manifested by 28% requiring new feeding support and 11% requiring new respiratory support [30]. Following discharge, 31% of PARDS patients had a new morbidity defined by FSS increase ≥3 points at three months post-admission relative to pre-illness baseline, and 23% had new morbidity at nine months [23]. Among patients with moderate to severe PARDS in the RESTORE trial, 22% experienced a decline in POPC and/or PCPC from baseline to six-month follow-up [25]. In a separate study, patients evaluated three to four years following PARDS requiring extracorporeal membrane oxygenation (ECMO) had ongoing impairments as demonstrated by mean POPC score of 2.3 and mean PCPC score of 1.9 [27]. In a study specifically evaluating neuromuscular outcomes following neuromuscular blockade for treatment of PARDS, neither myopathy nor neuropathy was identified at three-month follow-up [39].
Several studies report impairments in neurocognition, school participation, and emotional functioning following PARDS. In a small cohort of immunocompromised patients evaluated three years after admission with PARDS, 27% had learning difficulties and 6.7% had cognitive impairment [31], while among patients with moderate-severe PARDS in the RESTORE trial without a history of cognitive impairment, 19% had an estimated intelligence quotient at least one standard deviation below that of their sibling when measured three to eight years following their PARDS hospitalization [32]. A small cohort study in India found that only 55% of PARDS survivors had returned to school by three months, and only 85% had returned to school by nine to twelve months following hospitalization [18]. Among nearly 400 school-eligible patients in the RESTORE trial, 70% missed school in the six months post-admission, with 38% experiencing chronic absenteeism. Parents also struggled with absenteeism following their child’s PARDS admission, with 55% experiencing missed days of work during the six months post-admission [33]. Finally, 30% of patients in the RESTORE trial screened positive for post-traumatic stress disorder at six months after discharge [25].
Patients surviving PARDS also have high rates of ongoing care needs following hospital discharge, with 24% of general PICU patients with PARDS discharged to inpatient rehabilitation and 3.3% to chronic care facilities [28]. Among pediatric trauma patients with ARDS, nearly 50% required post-discharge care compared to 12–16% without PARDS [29,34], and 18.4% underwent tracheostomy placement [34]. In the three months following hospitalization, 18% of general PICU patients with PARDS were seen by a specialty care provider, 24% presented to an Emergency Department, and 11% were readmitted to the hospital [17]. Over the course of the first year, 66% of patients with severe PARDS presented to an Emergency Department and 26% were readmitted to the hospital [19]. A study using machine learning techniques applied to post-discharge insurance claims to identify phenotypes of outcomes after acute respiratory failure requiring at least three days of invasive ventilation identified that nearly one in five patients with moderate-to-severe PARDS demonstrated the high morbidity phenotype identified as having the highest health resource use in the year following hospitalization [14].
Post-discharge mortality varied by population and time point evaluated; among PARDS patients requiring ECMO who survived to hospital discharge, post-discharge mortality was 3.0% at six months [36] and 5.2% at one year [35], while among general PICU patients with PARDS, post-discharge mortality was 4.6–5.5% at one year [14,28] and 8.0% at three years [28]. Among 15 immunocompromised patients with PARDS, three-year post-discharge mortality was 40% [31] (Figure 3).
Figure 3.
Proportion of patients with post-discharge mortality based on time of assessment. Studies listed by population evaluated and reference number. ARDS, acute respiratory distress syndrome; ECMO, extracorporeal membrane oxygenation; Immuno, immunocompromised.
Benefits
These findings suggest that children surviving PARDS are at high risk for morbidities across a range of health domains. Early identification of impairments within three months following hospital discharge may facilitate treatment to optimize long-term outcomes. Given that impairments may not be as easily measured or recognized in young children due to inability to participate in evaluation, their developmental capacity, or inadequacy of standard measures to detect impairments in young children [40], re-evaluation prior to beginning school may identify previously undetected morbidities that could negatively impact development.
Harms and burdens
Screening for a wide range of morbidities may be time-consuming for patients, families, and clinicians, especially early after hospital discharge when families may be experiencing high rates of stress, anxiety, and healthcare encounters [41–44]. In-depth assessments and referrals to subspecialists may place an additional time and financial burden on patients and families. Interventions may also be costly and time-consuming.
Balance of effects
Identifying and treating deficits in HRQL, physical, neurocognitive, emotional, family, and social function among children surviving PARDS is important to optimize recovery. Identification of abnormalities in any of the domains recommended for screening warrants further evaluation to confirm and elucidate areas of impairments to develop appropriate treatment plans. The decision to provide more comprehensive assessments and/or treatment by a primary care provider versus a subspecialist will depend on the type and magnitude of the impairment, the experience and expertise of the primary care provider, and the availability of subspecialty providers. Failure to optimize recovery through identification and treatment of impairments may result in long-term harm to the patient or their family. Impairments could also progressively worsen over time if not treated. Shared decision-making among the provider, family, and patient, when appropriate, to discuss the risks and benefits of further assessment and treatment are likely to be valuable.
Implementation considerations
The type of evaluation will differ based on local resources. Some sites may have the capacity to conduct comprehensive in-person evaluations for patients through a post-PICU follow-up clinic, while other sites might conduct screening remotely via questionnaires, in primary care clinics, or through school-based assessments. We do not have evidence that non-pulmonary morbidities following PARDS are different than morbidities identified in other critically ill pediatric cohorts, and thus providers may refer to the pediatric critical care Core Outcome Set and Core Outcome Measures Set [2,3,45] for essential elements, domains, and instruments for screening, with acknowledgment that providers should consider where an instrument was validated and whether the validation is generalizable to their patient(s). Some locations have resources to provide additional in-depth assessments and availability of subspecialists to evaluate and treat post-discharge deficits among PARDS survivors. Others may have limited or no availability of subspecialty providers, and the responsibility for further assessment and treatment may fall to the primary care providers who may have variability in experience and resources to provide these services. By approaching the evaluation and treatment of post-PICU morbidities in a stepwise manner as recommended, the aspects of assessment and care that are most challenging to implement should be limited to the children at highest risk for ongoing deficits who have the greatest opportunity to benefit from treatment.
Conducting PARDS Outcomes Research
While the literature on outcomes among PARDS survivors is growing, our understanding of the associations between patient and clinical factors with poor long-term outcomes, and how post-discharge outcomes can be incorporated into clinical trials, remains limited.
Research statement 9.4.1. When feasible, pre-PICU baseline status for each outcome measure should be established or estimated when evaluation of post-PICU morbidity is anticipated.
(Ungraded research statement, 98% agreement)
Research statement 9.4.2. We cannot make a recommendation regarding the use of alternative post-discharge endpoints. Given declining mortality among children with PARDS, additional studies are needed to evaluate potential alternative post-discharge endpoints for clinical trials.
(Ungraded research statement, 96% agreement)
Remarks: Potential post-discharge endpoints to evaluate should include hospital and PICU readmissions (e.g., within 30 days of discharge), unplanned health resource use, HRQL, and physical, pulmonary, neurocognitive, emotional, family, and social function.
Research statement 9.4.3. Clinical studies should be designed to evaluate the association between short-term outcomes (e.g., new or progressive organ dysfunction) and longer-term post-discharge outcomes for patients with PARDS.
(Ungraded research statement, 100% agreement)
Research statement 9.4.4. Further studies are needed to determine factors that may affect the trajectory of recovery following PARDS.
(Ungraded research statement, 100% agreement)
Remarks: Additional research should include demographic characteristics, clinical factors, PICU exposures, social determinants of health, and access to care.
Research statement 9.4.5. Practices to optimize follow-up (e.g., incentives, multimodal evaluations) should be used to minimize bias due to differential loss-to-follow-up when conducting post-PICU outcomes research.
(Ungraded research statement, 96% agreement)
Justification
The heterogeneity of patient and hospitalization characteristics in children who develop PARDS limits our ability to clearly delineate the impact of severe lung injury and associated life-sustaining measures (e.g., immobility, sedation) likely to have negative side effects. To better delineate the impact of PARDS, it is imperative to understand the child’s functioning after resolution of PARDS relative to their pre-illness baseline, which is frequently omitted in pediatric critical illness research [2,46]. Additionally, understanding the long-term impact of lung injury and the associated critical care support on a PARDS survivor necessitates a breadth of outcome assessments including multiple health domains. The use of patient-centered endpoints is a central component of clinical outcomes research, and includes: quantifying the rate of subsequent hospital and PICU readmissions (e.g., within 30 days of discharge); recording unplanned health resource use; measuring pulmonary outcomes; and evaluating the domains of the PICU Core Outcome Set including overall health (inclusive of health-related quality of life) and physical, cognitive, emotional, and family functioning [3]. Additionally, the relationship between short- and long-term outcomes will need to be more clearly delineated to facilitate future targeting of key inpatient outcomes that may impact long-term outcomes. Lastly, outcomes research is frequently restricted to a limited population due to exclusion criteria that enhance study feasibility (e.g., language, need for internet access) at the expense of generalizability. High attrition after enrollment is also a substantial concern in many studies; loss-to-follow-up in the included prospective studies in this review ranged from 22–46% for survey data collection [23–25,32,33] and as high as 54% for in-person pulmonary evaluations [20]. There is also differential follow-up based on patient and family demographic factors; patients with follow-up data were more commonly of non-Hispanic White race [20,23,24] with higher parental level of education and greater household income than patients without follow-up data [24]. Future studies will need to facilitate participation by all patients, not only to ensure equity in access but also because results based on those who are currently followed-up may not be generalizable to those excluded or systematically lost to follow-up.
DISCUSSION
Literature characterizing outcomes of survivors of PARDS has grown substantially since PALICC 2015 [4]. We now have information about recovery after PICU admission for PARDS in single-center, multi-center, and population-based studies [14,17–20,23–36,39]. Unfortunately, it is increasingly clear that morbidity after hospital discharge from PARDS is common and affects all domains of function, HRQL, and patients’ families. The relationship of morbidity to illness severity is inconsistent among domains and populations studied. Thus, children with PARDS, regardless of severity, are at risk of new morbidity after the acute illness has resolved, and we advise screening for impairments in all patients surviving PARDS.
Many of the health domains identified in the literature as affecting PARDS survivors overlap with the domains recommended in the PICU Core Outcome Set [3] for survivors of pediatric critical illness. There were no studies, however, that directly compared outcomes among children surviving PARDS to children surviving other etiologies of critical illness. It thus remains unknown whether the prevalence and severity of impairments are similar for patients with and without PARDS. A domain of particular relevance to survivors of PARDS that was not identified as a core domain in the PICU Core Outcome Set is pulmonary morbidity. Pulmonary abnormalities have been identified in nearly one in three PARDS survivors and over half of PARDS survivors were prescribed pulmonary medications in the months following discharge [17–20]. While sample sizes for studies evaluating pulmonary function were small, the high prevalence of impairments across the range of PARDS severity suggest that all patients surviving PARDS may benefit from ongoing evaluation of their pulmonary function, including those without abnormal respiratory symptoms as some impairments may be subclinical.
To optimize their recovery, patients surviving PARDS warrant vigilant follow-up after discharge to identify and treat impairments in respiratory function as well as physical, emotional, cognitive, social, and family functioning. We generated eight good practice statements for the approach to clinical outcome assessment, assessment of pulmonary outcomes of children surviving PARDS, and assessment of non-pulmonary outcomes of children surviving PARDS including HRQL and physical, neurocognitive, emotional, family, and social functioning. There was no evidence for restricting follow-up evaluation only to patients with severe PARDS, and thus these good practice statements apply to all children surviving PARDS regardless of disease severity.
To minimize the burden to providers and families of conducting comprehensive post-discharge evaluations on all patients, we advise a stepwise approach to evaluation beginning with an initial screening followed by full assessment if deficits are identified on screening, with subsequent treatment and serial re-evaluations of impairments as appropriate. As most patients will not have access to dedicated PICU follow-up clinics, we suggest that follow-up care begin in the patient’s medical home with their primary care provider, with subsequent referral to subspecialty providers as needed. For this model to be effective, PICU providers must communicate with primary care providers of patients surviving PARDS to advise them of the post-discharge morbidities that the patient may face and suggest resources for screening, including the PICU Core Outcome Measures Set [45] that provides recommendations for screening measures and more in-depth assessment tools across the outcome domains recommended in the PICU Core Outcome Set [3]. More work is needed, in collaboration with primary care providers, to identify best practices for transitioning care of the PARDS patient from the PICU to the general hospital wards to the outpatient setting to ensure appropriate communication and adequate resources for ongoing evaluation. Additionally, further research is needed to determine how to optimize conducting post-discharge pulmonary evaluation, as we were unable to recommend specific screening tools for respiratory symptom screening based on the existing literature, and studies that have incorporated post-discharge spirometry assessments have been limited by small sample sizes and high rates of loss-to-follow-up.
We also generated five research statements reflecting the need to better delineate trajectories and mechanisms of impairment and recovery following PARDS, relationships between short- and long-term outcomes, and methods to optimize the study of post-discharge outcomes to ensure results are relevant to families from all backgrounds. Existing studies of PARDS outcomes have predominantly been conducted in North America and Western Europe, and multiple prospective studies have identified differential loss-to-follow-up based on patient and family demographic characteristics, with non-Hispanic White patients with higher socioeconomic status more commonly represented in follow-up cohorts.
This work has several limitations. While we employed an extensive search strategy, we may not have identified all relevant studies. Studies were primarily conducted in high-income countries and thus the statements we generated may not be relevant for patients in other geographic regions; however, we did develop these statements with experts from resource-limited settings to ensure broad applicability. No studies contained high quality evidence and thus we were only able to make ungraded good practice and research statements rather than recommendations for outcome assessment following PARDS.
CONCLUSION
Many children who survive PARDS suffer declines in physical, cognitive, and emotional function and diminished HRQL, and their families may also suffer. Their trajectory of recovery has not been well studied, but morbidities have been shown to persist for months or years after the episode of PARDS. The good practice statements developed through this extensive scoping review process and consensus conference involving experts in PARDS research, clinical care, and outcomes assessment provide guidance to clinicians caring for children surviving PARDS across the scope of pediatric care to identify and treat impairments to optimize recovery. The accompanying research statements address the limitations of existing PARDS outcomes research and aim to promote future research that advances our understanding of long-term outcomes among children surviving PARDS. This vulnerable population of children and their families warrant assiduous long-term care and additional rigorous research to inform efforts that will optimize their health and well-being.
Supplementary Material
ACKNOWLEDGEMENTS
The Second Pediatric Acute Lung Injury Consensus Conference (PALICC-2) group members are listed in Appendix 1 (see Supplemental Digital File).
Source of funding:
This work did not receive specific funding, but authors were supported by the following grants during manuscript preparation: NICHD K23HD100566 (Killien), NICHD K23HD096018 (Maddux), and salary support grant from the Fonds de recherche du Québec – Santé (Tse)
Footnotes
Work performed at: University of Washington, University of Colorado, and University of Montreal
Copyright Form Disclosure: Dr. Killien’s institution received funding from the National Institute of Child Health and Human Development (NICHD). Drs. Killien, Maddux, and Watson received support for article research from the National Institutes of Health (NIH). Dr. Maddux’s institution received funding from the NICHD (K23HD096018) and the Francis Family Foundation. Dr. Tse received funding from Fonds de recherche du Québec - Santé; he disclosed that he holds 3 active grants from the Canadian Institute of Health Research and 1 from the Quebec Respiratory Health Research Network. Dr. Watson’s institution received funding from the NIH.
REFERENCES
- 1.Watson RS, Choong K, Colville G, et al. Life after Critical Illness in Children-Toward an Understanding of Pediatric Post-intensive Care Syndrome. J Pediatr. 2018;198:16–24. [DOI] [PubMed] [Google Scholar]
- 2.Maddux AB, Pinto N, Fink EL, et al. Postdischarge Outcome Domains in Pediatric Critical Care and the Instruments Used to Evaluate Them: A Scoping Review. Crit Care Med. 2020;48(12):e1313–e1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fink EL, Maddux AB, Pinto N, et al. A Core Outcome Set for Pediatric Critical Care. Crit Care Med. 2020;48(12):1819–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quasney MW, Lopez-Fernandez YM, Santschi M, et al. The outcomes of children with pediatric acute respiratory distress syndrome: proceedings from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med. 2015;16(5 Suppl 1):S118–131. [DOI] [PubMed] [Google Scholar]
- 5.Iyer P, Khemani R, Emeriaud G, et al. Methodology of the Second Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med XXXX; X: X-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tricco AC, Lillie E, Zarin W, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med. 2018;169(7):467–473. [DOI] [PubMed] [Google Scholar]
- 7.Treble-Barna A, Beers SR, Houtrow AJ, et al. PICU-Based Rehabilitation and Outcomes Assessment: A Survey of Pediatric Critical Care Physicians. Pediatr Crit Care Med. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lasiter S, Oles SK, Mundell J, London S, Khan B. Critical Care Follow-up Clinics: A Scoping Review of Interventions and Outcomes. Clin Nurse Spec. 2016;30(4):227–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schofield-Robinson OJ, Lewis SR, Smith AF, McPeake J, Alderson P. Follow-up services for improving long-term outcomes in intensive care unit (ICU) survivors. Cochrane Database Syst Rev. 2018;11:Cd012701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuppala VS, Tabangin M, Haberman B, Steichen J, Yolton K. Current state of high-risk infant follow-up care in the United States: results of a national survey of academic follow-up programs. J Perinatol. 2012;32(4):293–298. [DOI] [PubMed] [Google Scholar]
- 11.Dodd JN, Hall TA, Guilliams K, et al. Optimizing Neurocritical Care Follow-Up Through the Integration of Neuropsychology. Pediatr Neurol. 2018;89:58–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wainwright MS, Grimason M, Goldstein J, et al. Building a pediatric neurocritical care program: a multidisciplinary approach to clinical practice and education from the intensive care unit to the outpatient clinic. Semin Pediatr Neurol. 2014;21(4):248–254. [DOI] [PubMed] [Google Scholar]
- 13.Williams CN, Kirby A, Piantino J. If You Build It, They Will Come: Initial Experience with a Multi-Disciplinary Pediatric Neurocritical Care Follow-Up Clinic. Children (Basel). 2017;4(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maddux AB, Mourani PM, Miller K, et al. Identifying Long-Term Morbidities and Health Trajectories After Prolonged Mechanical Ventilation in Children Using State All Payer Claims Data. Pediatr Crit Care Med. 2022;23(4):e189–e198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herridge MS. Fifty Years of Research in ARDS. Long-Term Follow-up after Acute Respiratory Distress Syndrome. Insights for Managing Medical Complexity after Critical Illness. Am J Respir Crit Care Med. 2017;196(11):1380–1384. [DOI] [PubMed] [Google Scholar]
- 16.Herridge MS, Tansey CM, Matté A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364(14):1293–1304. [DOI] [PubMed] [Google Scholar]
- 17.Boucher V, Mathy C, Lacroix J, Émériaud G, Jouvet P, Tse SM. Post-discharge respiratory outcomes of children with acute respiratory distress syndrome. Pediatr Pulmonol. 2020;55(2):468–473. [DOI] [PubMed] [Google Scholar]
- 18.Chakdour S, Vaidya PC, Angurana SK, Muralidharan J, Singh M, Singhi SC. Pulmonary Functions in Children Ventilated for Acute Hypoxemic Respiratory Failure. Pediatr Crit Care Med. 2018;19(9):e464–e471. [DOI] [PubMed] [Google Scholar]
- 19.Vo M, Miller K, Bennett TD, et al. Postdischarge health resource use in pediatric survivors of prolonged mechanical ventilation for acute respiratory illness. Pediatr Pulmonol. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ward SL, Turpin A, Spicer AC, Treadwell MJ, Church GD, Flori HR. Long-Term Pulmonary Function and Quality of Life in Children After Acute Respiratory Distress Syndrome: A Feasibility Investigation. Pediatr Crit Care Med. 2017;18(1):e48–e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Graham BL, Steenbruggen I, Miller MR, et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am J Respir Crit Care Med. 2019;200(8):e70–e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fiser DH. Assessing the outcome of pediatric intensive care. J Pediatr. 1992;121(1):68–74. [DOI] [PubMed] [Google Scholar]
- 23.Ames SG, Banks RK, Zinter MS, et al. Assessment of Patient Health-Related Quality of Life and Functional Outcomes in Pediatric Acute Respiratory Distress Syndrome. Pediatr Crit Care Med. 2022. [DOI] [PubMed] [Google Scholar]
- 24.Watson RS, Asaro LA, Hertzog JH, et al. Long-Term Outcomes after Protocolized Sedation versus Usual Care in Ventilated Pediatric Patients. Am J Respir Crit Care Med. 2018;197(11):1457–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watson RS, Asaro LA, Hutchins L, et al. Risk Factors for Functional Decline and Impaired Quality of Life after Pediatric Respiratory Failure. Am J Respir Crit Care Med. 2019;200(7):900–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carlton EF, Weeks HM, Dahmer MK, et al. Inflammatory Biomarkers Are Associated With a Decline in Functional Status at Discharge in Children With Acute Respiratory Failure: An Exploratory Analysis. Crit Care Explor. 2021;3(7):e0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farhat A, Li X, Huet B, Tweed J, Morriss MC, Raman L. Routine Neuroimaging: Understanding Brain Injury in Pediatric Extracorporeal Membrane Oxygenation. Crit Care Med. 2022;50(3):480–490. [DOI] [PubMed] [Google Scholar]
- 28.Keim G, Watson RS, Thomas NJ, Yehya N. New Morbidity and Discharge Disposition of Pediatric Acute Respiratory Distress Syndrome Survivors. Crit Care Med. 2018;46(11):1731–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Killien EY, Huijsmans RLN, Ticknor IL, et al. Acute Respiratory Distress Syndrome Following Pediatric Trauma: Application of Pediatric Acute Lung Injury Consensus Conference Criteria. Crit Care Med. 2020;48(1):e26–e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loh SW, Gan MY, Wong JJ, Ong C, Mok YH, Lee JH. High burden of acquired morbidity in survivors of pediatric acute respiratory distress syndrome. Pediatr Pulmonol. 2021;56(8):2769–2775. [DOI] [PubMed] [Google Scholar]
- 31.Fuchs H, Rossmann N, Schmid MB, et al. Permissive hypercapnia for severe acute respiratory distress syndrome in immunocompromised children: A single center experience. PLoS One. 2017;12(6):e0179974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watson RS, Beers SR, Asaro LA, et al. Association of Acute Respiratory Failure in Early Childhood With Long-term Neurocognitive Outcomes. Jama. 2022;327(9):836–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carlton EF, Donnelly JP, Prescott HC, et al. School and Work Absences After Critical Care Hospitalization for Pediatric Acute Respiratory Failure: A Secondary Analysis of a Cluster Randomized Trial. JAMA Netw Open. 2021;4(12):e2140732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Killien EY, Mills B, Watson RS, Vavilala MS, Rivara FP. Morbidity and Mortality Among Critically Injured Children With Acute Respiratory Distress Syndrome. Crit Care Med. 2019;47(2):e112–e119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pathan N, Ridout DA, Smith E, Goldman AP, Brown KL. Predictors of outcome for children requiring respiratory extra-corporeal life support: implications for inclusion and exclusion criteria. Intensive Care Med. 2008;34(12):2256–2263. [DOI] [PubMed] [Google Scholar]
- 36.Robert B, Guellec I, Jegard J, Jean S, Guilbert J, Soreze Y, Starck J, Piloquet JE, Leger PL, Rambaud J. Extracorporeal membrane oxygenation for immunocompromised children with acute respiratory distress syndrome: a French referral center cohort. Minerva Pediatr. 2020. Sep 22. [DOI] [PubMed] [Google Scholar]
- 37.Stineman MG, Shea JA, Jette A, et al. The Functional Independence Measure: tests of scaling assumptions, structure, and reliability across 20 diverse impairment categories. Arch Phys Med Rehabil. 1996;77(11):1101–1108. [DOI] [PubMed] [Google Scholar]
- 38.Pollack MM, Holubkov R, Glass P, et al. Functional Status Scale: new pediatric outcome measure. Pediatrics. 2009;124(1):e18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chandra S, Goel S, Dawra R. Early Neuromuscular Blockade in Children with Pediatric Acute Respiratory Distress Syndrome. J Pediatr Intensive Care. 2020;9(3):201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Killien EY, Farris RWD, Watson RS, Dervan LA, Zimmerman JJ. Health-Related Quality of Life Among Survivors of Pediatric Sepsis. Pediatr Crit Care Med. 2019;20(6):501–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Colville G, Pierce C. Patterns of post-traumatic stress symptoms in families after paediatric intensive care. Intensive Care Med. 2012;38(9):1523–1531. [DOI] [PubMed] [Google Scholar]
- 42.Murphy LK, Palermo TM, Meert KL, et al. Longitudinal Trajectories of Caregiver Distress and Family Functioning After Community-Acquired Pediatric Septic Shock. Pediatr Crit Care Med. 2020;21(9):787–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nelson LP, Gold JI. Posttraumatic stress disorder in children and their parents following admission to the pediatric intensive care unit: a review. Pediatr Crit Care Med. 2012;13(3):338–347. [DOI] [PubMed] [Google Scholar]
- 44.Woolf C, Muscara F, Anderson VA, McCarthy MC. Early Traumatic Stress Responses in Parents Following a Serious Illness in Their Child: A Systematic Review. J Clin Psychol Med Settings. 2016;23(1):53–66. [DOI] [PubMed] [Google Scholar]
- 45.Pinto NP, Maddux AB, Dervan LA, et al. ; POST-PICU Investigators of the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network and the Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network (CPCCRN); Core Outcome Measurement Set Expert Panel included the Scoping Review Steering Committee. A Core Outcome Measurement Set for Pediatric Critical Care. Pediatr Crit Care Med. 2022. Aug 29. [Google Scholar]
- 46.Killien EY, Watson RS, Zimmerman JJ. Inherent value of baseline measures when assessing the trajectory of health-related quality of life among children surviving critical illness. Intensive Care Med. 2018;44(11):1979–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.