Abstract
Background
Patients with follicular lymphoma (FL) can have high response rates to early lines of treatment. However, among FL patients relapsed/refractory (r/r) after ≥2 prior lines of therapy (LOT), remission tends to be shorter and there is limited treatment guidance. This study sought to evaluate the clinical outcomes for r/r FL after ≥2 prior LOT identified through systematic literature review.
Methods
Eligible studies included comparative or non-comparative interventional or observational studies of systemic therapies among adults with FL r/r after ≥2 prior LOT published prior to 31st May 2021. Prior LOT must have included an anti-CD20 monoclonal antibody and an alkylating agent, in combination or separately. Overall response rate (ORR) and complete response (CR) were estimated using inverse-variance weighting with Freeman-Tukey double-arcsine transformations. Kaplan-Meier (KM) curves for progression-free survival (PFS) and overall survival (OS) estimated by reconstructing digitized curves using the Guyot algorithm, and survival analyses were conducted, stratified by ≥2 prior LOT and ≥ 3 prior LOT groups (as defined in the source material). Restricting the analyses to the observational cohorts was investigated as a sensitivity analysis.
Results
The analysis-set included 20 studies published between 2014 and 2021. Studies were primarily US and/or European based, with the few exceptions using treatments approved in US/Europe. The estimated ORR was 58.47% (95% confidence interval [CI]: 51.13–65.62) and proportion of patients with CR was 19.63% (95% CI: 15.02–24.68). The median OS among those ≥2 prior LOT was 56.57 months (95% CI: 47.8–68.78) and median PFS was 9.78 months (95% CI: 9.01–10.63). The 24-month OS decreased from 66.50% in the ≥2 prior LOT group to 59.51% in the ≥3 prior LOT group, with a similar trend in PFS at 24-month (28.42% vs 24.13%).
Conclusions
This study found that few r/r FL patients with ≥2 prior LOT achieve CR, and despite some benefit, approximately 1/3 of treated patients die within 24 months. The shorter median PFS with increasing prior LOT suggest treatment durability is suboptimal in later LOT. These findings indicate that patients are underserved by treatments currently available in the US and Europe.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-023-10546-6.
Keywords: Relapsed/refractory follicular lymphoma, Clinical outcomes, Systematic literature review, Meta-analysis
Background
Non-Hodgkin lymphoma (NHL) is the eleventh most commonly diagnosed malignancy in the world and accounts for the eleventh highest cancer-related mortality [1]. It is estimated that in 2020 there were 544,352 cases of NHL diagnosed globally, and more than 259,793 deaths among patients afflicted by this malignancy [1]. The primary risk factor for NHL is older age, with greater than half of patients being diagnosed at age 65 or older [2]. In the coming decades the generational aging in many areas of the world is likely to lead to a subsequent increase in global NHL cases.
NHL can be broadly categorized into aggressive and indolent NHL (iNHL) based on rate of progression [3]. iNHL is typically a slow growing cancer that is often asymptomatic and discovered incidentally. Approximately one-third of malignant lymphomas are iNHL [4], which are further subdivided by histology, with follicular lymphoma (FL) and marginal zone lymphoma (MZL) being the most commonly diagnosed histologies. Notably, despite its relatively high incidence and prevalence, FL is generally considered to be incurable with standard front-line therapies [5].
The introduction of front-line chemoimmunotherapy, employing an alkylator and anti-CD20 monoclonal antibody combination, such as R-CHOP (rituximab, cyclophosphamide, doxorubicin hydrochloride, vincristine and prednisone), has led to a nearly 100% overall response rate among first-line FL patients [6]. Approximately 20% of FL patients are expected to experience disease relapse within 2 years of treatment [2], and the disease tends to become increasingly refractory to treatment with successive each line of therapy [7]. Among relapsing patients, remission tends to also become shorter with additional line of therapy [8].
There are numerous treatments that have recently come to market or are currently under study. Anti-CD19 chimeric antigen receptor T cells (CAR-T) have shown promise in patients with B-cell cancers [9]. Following the approval of CAR-T for adult patients with r/r diffuse large B-cell lymphoma (DLBCL) and primary mediastinal large B-cell lymphoma (PMBCL) after 2 or more lines of systemic therapy, CAR-T was recently approved for r/r FL [10]. Clinical trials are ongoing for several CAR-T therapies in r/r FL [11, 12]. Other novel therapies that have been investigated in r/r FL [13, 14] include the anti-CD20/CD3 bispecific antibody odronextamab, and the PI3K inhibitor idelalisib. Many of the recent and ongoing trials are non-comparative in nature, so better understanding the treatment landscape for r/r FL patients would help to contextualize their results.
Critically, despite the advent of newer therapies being added to the r/r iNHL armamentarium, there is a need for data on the impact of currently available agents on long-term prognosis for patients with r/r iNHL. The current study therefore utilized a comprehensive methodological approach to evaluate and summarize the clinical outcomes of currently available agents through a systematic literature review (SLR) and meta-analysis of treatments available for therapy in Europe and the US for r/r FL patients having been failed by ≥2 prior lines of therapy.
Methods
Systematic literature review
A comprehensive systematic search of the literature was conducted on 31 March 2021 using the following databases on the Ovid platform: Medical Literature Analysis and Retrieval System Online (MEDLINE), Excerpta Medica database (EMBASE), and Cochrane Central Register of Controlled Trials (Additional file: Tables S1, S2, S3). Searches were conducted in accordance with recommendations from the Cochrane Collaboration, National Institute for Health and Care Excellence (NICE) guidance, Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (IQWiG in Germany). Manual searches were also undertaken of relevant conference proceedings over the previous 2 years, as well as international clinical trial databases, to identify additional eligible studies.
Eligible studies for the SLR were among adults (aged ≥18 years) with r/r iNHL after failure of two or more lines of therapy. For the purpose of this study, the analysis-set was further reduced to r/r FL patients as discussed in further detail below. Randomized control trials, non-randomized trials, observational studies and registries were all eligible study designs. Eligible interventions were any approved for treatment in the US or Europe, best supportive care or placebo. Here too, the SLR scope was broad, including genetic therapies and therapies approved for other iNHL indications (e.g., ibrutinib is approved for marginal zone lymphoma and other iNHL, but not for FL). The full study eligibility criteria, defined in terms of the population, interventions, comparisons, outcomes, and study design (PICOS), are outlined in Additional file 1: Table S4.
Two reviewers, working independently, reviewed all abstracts and proceedings identified in the searches according to the selection criteria, with the exception of outcome criteria which were adjudicated during full-text screening. Eligible studies then underwent full-text screening by the same two reviewers, and full-text studies that met the inclusion criteria were identified for data extraction. Any disagreement between the two reviewers was adjudicated and resolved by a third reviewer. This process is detailed in the PRISMA [15] flow diagram (Fig. 1).
Fig. 1.
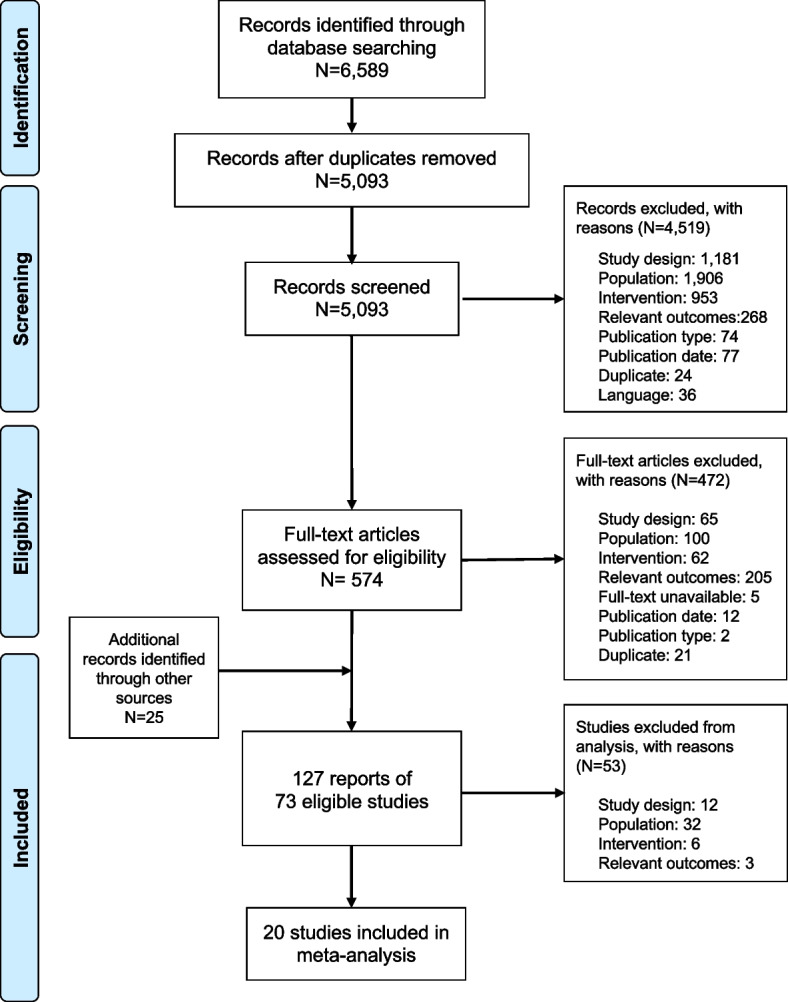
PRISMA flow diagram
Data on study characteristics, interventions, patient characteristics, and outcomes for the final list of included studies was extracted by the two independent reviewers. Since direct access to study data was not available for time-to-event outcomes, survival curves were digitally extracted using the DigitizeIt software. These were then used to generate pseudo-individual patient-level data by applying the Guyot algorithm with numbers at risk tables [16]. Time-to-event data from the reconstructed survival curves were extracted by one reviewer and then independently verified by the second reviewer.
Given the mixed study designs eligible for the evidence base (i.e., the eligibility of both randomized and non-randomized studies), the quality assessment for the evidence base was performed using the Downs and Black checklist [17]. This study quality tool is well established and lends itself to all eligible study designs, which allowed for a single assessment tool to be used for all studies.
Study selection for inclusion in the analysis set was conducted in two steps. First, a feasibility-assessment-set was identified by reducing patients to the scope of the project at hand. Studies including small lymphocytic lymphoma, lymphoplasmacytic lymphoma, MZL only or transformed FL/MZL were removed, unless subgroups excluding these patients were available. Studies restricted to Grade 1 and 2 FL were also excluded from analyses. One study explicitly included Grade 3b patients [18], which, after further review, a judgment was made that the few Grade 3b patients included in the trial would have negligible impact on the outcomes of interest, and thus this study was included in the analysis set. Studies examining CAR-T therapy were also removed as CAR-T did not represent an available treatment modality at the time of analysis. Second, studies were further restricted following the results of the feasibility assessment. The analysis set was restricted to sample sizes of at least 20 patients because a few studies reporting on FL as a subgroup had very small sample sizes (often below 5) that led to high levels of heterogeneity.
Statistical analyses
A frequentist meta-analysis approach was used for the ORR, CR, PFS and OS outcomes and a Bayesian approach was used in meta-analysis of the digitized Kaplan-Meier curve data for the time-to-event outcomes. Treatments identified from studies that met the inclusion criteria were simplified for the purpose of analysis into the following categories: standard of care (SoC), PI3k-δ inhibitors, Lenalidomide + Rituximab, Bortezomib + Rituximab, Obinutuzumab + Benda, 90Y + Anti-CD20 combination, Autologous stem cell transplant (SCT), and Allogeneic SCT. The evidence base included data from three studies [7, 19, 20] that included a heterogenous sampling of both treatments and patient populations. These were considered to be representative of typical care and thus were dubbed to be representative cohorts. The most common treatments were anti-CD20 monoclonal antibodies, with or without chemotherapy [21, 22], and PI3k-δ inhibitors [23–26].
All meta-analyses using single summary statistics of proportions were based on dichotomous outcomes: ORR and CR. For the analysis of each of these outcomes, inverse-variance meta-analyses were used. The Freeman-Tukey double arcsin transform was used throughout to ensure stability in the extreme proportion values (near 1 or 0). Our review of the data revealed multiple instances of observed proportions of 1, so this was deemed necessary. The analyses were stratified by the treatment categories outlined above. Both fixed- and random-effects were used within the strata, but random-effects were not used between them. The results from each stratum were combined using a weighted mean with relative sample size as the weight. Weights were designed to sum up to 1 to ensure an unbiased estimate. Heterogeneity within strata was assessed using the I2 statistic.
Meta-analyses for the digitized Kaplan-Meier survival curves, for both OS and PFS, were analyzed in both the frequentist and Bayesian framework. Bayesian analyses used non-informative prior distributions and were based on methods for network meta-analyses of survival data using a multidimensional treatment effect as an alternative to the synthesis of the constant hazard ratios, as developed be Ouwens et al. [27] and Jansen [28]. Namely, the hazard functions of the interventions in a trial were modeled using known parametric survival functions or fractional polynomials. Given the non-comparative nature of this evidence base, a simple version of the model introduced by Jansen was used for the meta-analyses of OS and PFS [28, 29].
Of note, patients included in the representative cohorts were followed from one line to the next and as a result, observations were not fully independent for OS and PFS. In addition, restricting analyses to include only patients in their third line of treatment was deemed more detrimental than having repeated measures among some patients, and thus no such restrictions were implemented. Where permitted by the evidence, analyses also included those patients receiving a fourth line or more of treatment.
For Bayesian analyses, the deviance information criterion (DIC) was used to compare the goodness-of-fit of competing survival models [30]. A difference in DIC of approximately 5 points was considered meaningful and, in the case of survival models, the hazard functions were visually inspected for over-fitting [16]. The parameters of the different models were estimated using a Markov Chain Monte Carlo method implemented in the JAGS software package. A first series of 20,000 iterations from the JAGS sampler were discarded as ‘burn-in’, and the inferences were based on an additional 40,000 iterations using two chains. For all analyses, model convergence was assessed through trace plots, density plots and Gelman-Rubin-Brooks (shrink factor) plots [31].
The patient population in the primary analyses were restricted to patients with FL receiving therapies other than transplant because: a) this treatment modality represents a very different intervention to those being studied; b) the SCT study populations tended to be significantly younger and healthier; and c) these studies appeared to be overrepresented in the evidence base. Furthermore, as these studies only reported on patients who survived through to SCT, these studies were at risk of immortal time bias. The primary model also excluded off-label treatments for FL, as these were considered atypical. A second model included only study cohorts that were representative of care. Two supplemental models included a) off-label treatments, and b) only SCT studies. The viability of each model depended upon data availability (Additional File 1: Table S5).
Results
From the 6589 citations identified in the database and 25 through conference proceedings searches, a total of 126 publications describing 72 unique studies were eligible for inclusion in the SLR iNHL evidence base. The analysis-set excluded studies for the following reasons: 32 on the basis of population [MZL (3 studies), CLL/SLL/LPL/Transformed FL or Grade 3b FL (9 studies), FL grade 1 and 2 only (2 studies) and older studies guaranteed to have no prior anti-CD20 (18 studies)], 3 for outcomes, 6 for intervention and 12 for study design, including small sample sizes. The complete flow diagram leading to the selection of the SLR evidence base is presented in Fig. 1.
Of the 20 studies included in the analysis-set, 9 were single-arm clinical trials [21, 24, 32–38], 9 were retrospective cohort studies [7, 19, 22, 23, 25, 39–42] and 2 were prospective cohort studies [20, 43]. Two of the single arm trials were Phase I dose escalation studies, whilst the rest were Phase II non-comparative trials. Studies were conducted in a variety of countries, with nearly half being conducted in the US and the majority conducted in the US and/or Europe. Further study characteristics, including location, are presented in Table 1. We assessed the risk of patient overlap between the cohort studies, and concluded some overlap was possible, but due to the different geographies, treatment regimens, treatment centers and dates of patient inclusion, this overlap was minimal and not of concern. The quality assessment of the included studies, performed using the Downs and Black checklist [17], rated 13 studies as fair and 7 studies as poor (Additional file 1: Table S6). However, studies of poor quality tended to be non-comparative, for which a considerable number of items on the check-list are non-applicable (i.e., it would be reasonable to qualify these as fair quality). The majority of the studies reported response criteria used (Additional File 1: Table S7), with the 2007 IWG revised guidelines being the most frequently used [44]. However four studies used the 1999 IWG criteria, [45] and UNITY-NHL used Lugano classification [46].
Table 1.
Study characteristics of included studies
| Study | Location | Year | Treatment | Study design | N | Median follow up (months) | Follow-up range |
|---|---|---|---|---|---|---|---|
| Andorsky 2019 [23] | US | 2019 | Idelalisib | Retrospective cohort study | 54 | 18.6 | 0.6–49.5 |
| Assouline 2020 [32] | US, Australia, South Korea, Canada, Europe | 2020 | Mosunetuzumab | Phase I trial | 62 | 14.4 | – |
| Batlevi 2020 [7] | US | 2020 | Representative cohort | Retrospective cohort study | 299 | 87.6 | 2.4–200.4 |
| CHRONOS 1 part B [33] | Europe, Asia, US, Australia, New Zealand | 2017 | Copanlisib | Phase II trial | 104 | 6.69 | 0.23–24.01 |
| DAWN [34] | Europe, Asia, US, South America, Australia | 2018 | Ibrutinib | Phase II trial | 110 | 27.7 | 1.1–37.1 |
| DELTA [24] | Europe, US | 2014 | Idelalisib | Phase II trial | 72 | 33.9 | 1.2–81.4 |
| ELM-1 [21] | Europe, US, Canada, Australia, Asia | 2020 | Odronextamab | Phase I trial | 28 | 6.8 | 1.0–22.1 |
| Evens 2013 [43] | US | 2013 | Allo-SCT | Prospective cohort study | 184 | 48 | N/R |
| EZH [35] | Europe, US, Canada, Australia, Taiwan | 2018 | Tazemetostat | Phase II trial | 99 | N/R | N/R |
| Fuji 2020 [19] | Japan | 2020 | Representative cohort | Retrospective cohort study | 41 | 89.64 | 28.56–134.40 |
| Ito 2013 [39] | Japan | 2013 | Allo-SCT | Retrospective cohort study | 30 | 21.4 | 0.4–165.4 |
| Khouri 2008 [36] | US | 2008 | Allo-SCT | Phase II trial | 47 | 107 | 72–142 |
| Laport 2016 [37] | US | 2016 | Allo-SCT, Auto SCT | Phase II trial | 62 | 47 | 30–73 |
| Link 2019 [20] | US | 2019 | Representative cohort | Prospective cohort study | 438 | 96 | 0.24–124.8 |
| Lunning 2016 [40] | US | 2016 | Auto-SCT | Retrospective cohort study | 44 | 60 | 21.8–96 |
| Muntanola 2020 [22] | Spain | 2020 | R-ESHAP | Retrospective cohort study | 28 | N/R | N/R |
| Robert 2019 [25] | France | 2019 | Idelalisib | Retrospective cohort study | 24 | 23 | 20–24 |
| Sesques 2020 [41] | France | 2020 | Auto-SCT | Retrospective cohort study | 61 | 105.6 | 27.6–291.6 |
| UNITY-NHL [38] | Europe, US, Australia, Korea | 2021 | Umbralisib | Phase II trial | 117 | 27.5 | 20.9–37.1 |
| Vose 2008 [42] | US | 2008 | Auto-SCT | Retrospective cohort study | 108 | 72 | 12–192 |
Response outcomes
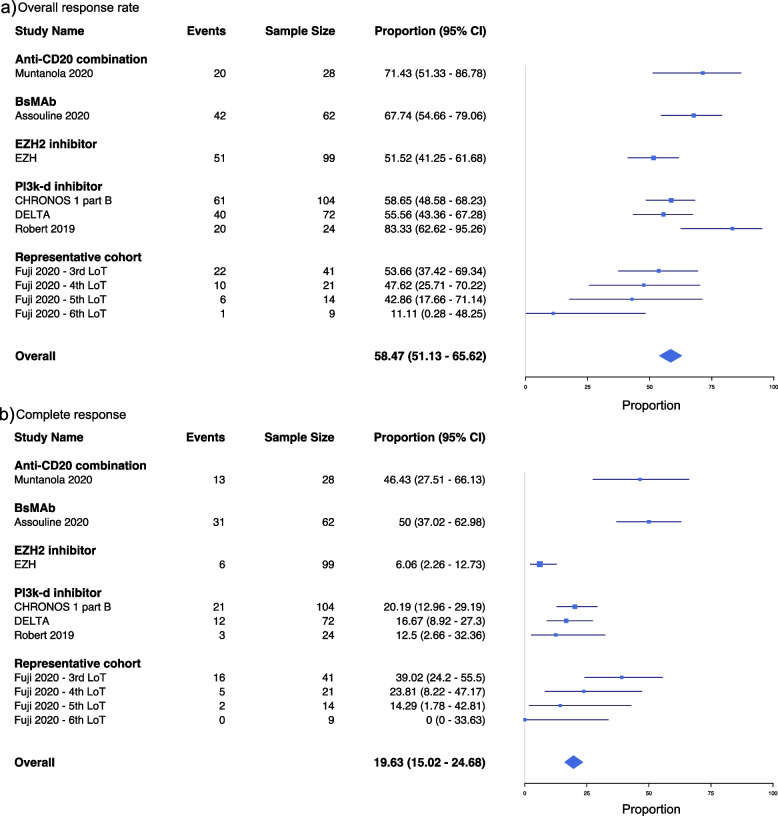
The meta-analysis revealed an overall ORR of 58.47% (CI: 51.13–65.62%) and an overall CR of 19.63% (CI: 15.02–24.68%) (Fig. 2). As can be observed, there was notable heterogeneity between studies. In the supplementary model (Additional File 1: Table S8, Fig. S1), the inclusion of off-label treatments found similar results as the primary analyses, with an ORR of 52.40% (CI: 46.37–58.39%), CR of 17.46% (CI: 13.59–21.70%). Off-label treatments included ibrutinib, which is only approved for other iNHL indications by both the EMA and FDA, odronextamab, which is not yet approved globally, and umbralisib, which is aimed at MZL but indicated in the US for 4 L+ patients only (EMA has granted a waiver to all mature B cell malignancies).
Fig. 2.
Meta-analysis of response outcomes. BsMAb, bispecific monoclonal antibody; CI, confidence interval; EZH2. Enhancer of zeste homolog 2; PI3k-d Phosphoinositide 3-kinase delta
Time-to-event outcomes
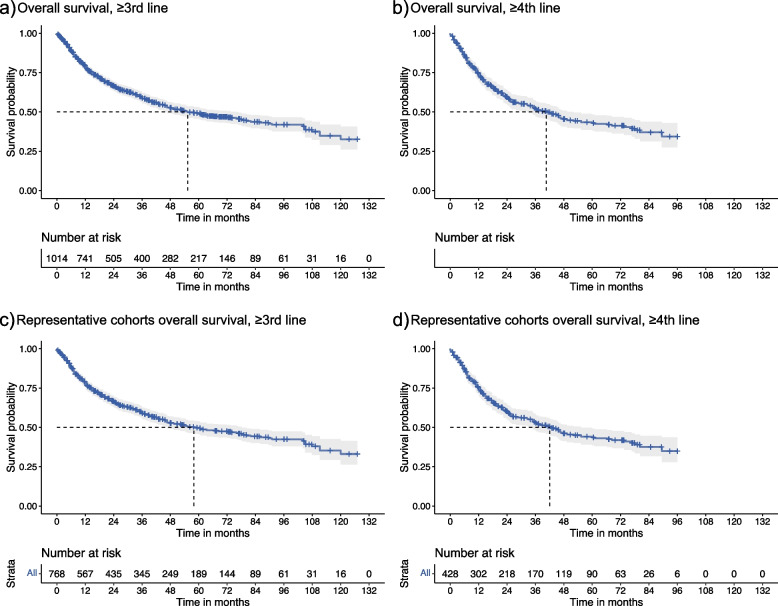
The Bayesian and frequentist analyses provided similar results with regard to clinical outcomes, with results for each approach presented in Table 2 and Table 3, respectively. The selected fractional polynomial parameters for each model are shown in Table S9. Summary KM curves are also presented (Figs. 3 and 4) for OS and PFS for those in the 3rd or greater LOT, 4th or greater LOT, and subsequent representative cohorts. With regard to OS, the main analyses and representative cohorts were similar in magnitude. A notable decrease in the median OS was evident among those in the 4th or greater LOT as compared to the 3rd or greater LOT (39.89 months vs. 56.57 months), suggesting that the data from the 3rd or greater LOT group may be attenuated by the inclusion of the latter group. A similar pattern was observed in the representative cohorts being treated in these later LOTs. Supplementary analyses of patients undergoing SCT showed a significantly higher OS, with a median OS of 93.9 months (CI: 81.8–107.96) in the 3rd or greater LOT (Additional File 1: Table S10, Fig. S2, S3).
Table 2.
Median OS and PFS using pseudo IPD from Kaplan-Meier curves
| Population | Frequentist analyses | Bayesian fractional polynomial meta-analyses | ||
|---|---|---|---|---|
| Median OS Months (95% CI) | Median PFS Months (95% CI) | Median OS Months (95% CI) | Median PFS Months (95% CI) | |
| ≥3rd line | 55.34 (46.60, 76.00) | 10.09 (9.25, 11.10) | 56.57 (47.8–68.78) | 9.78 (9.01–10.63) |
| ≥4th line | 40.63 (32.58, 52.12) | 8.41 (7.47, 9.48) | 39.89 (31.79–51.94) | 8.11 (7.3–9.04) |
| Representative cohorts ≥3rd line | 57.94 (46.60, 78.41) | 9.99(8.62, 11.10) | 58.67 (48.56–72.47) | 9.43 (8.57–10.4) |
| Representative cohorts ≥4th line | 42.02 (34.80, 55.34) | 8.33 (7.11, 9.56) | 41.63 (32.93–55.12) | 7.9 (7.02–8.93) |
*Frequentist results and median as estimated using Bayesian fractional polynomials
Table 3.
OS and PFS at 18 months and 24 months using frequentist meta-analysis
| Population | OS at 18 m %(95% CI) | PFS at 18 m % (95% CI) | OS at 24 m % (95% CI) | PFS at 24 m (95% CI) |
|---|---|---|---|---|
| ≥3rd line | 71.49 (68.68, 74.42) | 35.37 (33.15, 37.75) | 66.50 (63.54, 69.60) | 28.26 (26.15, 30.55) |
| ≥4th line | 65.07 (60.84, 69.59) | 31.36 (28.30, 34.75) | 59.51 (55.12, 64.24) | 24.13 (21.29, 27.35) |
| Representative cohorts ≥3rd line | 70.92 (67.71, 74.29) | 35.52 (33.16, 38.05) | 66.45 (63.08, 69.99) | 28.42 (26.18, 30.85) |
| Representative cohorts ≥4th line | 65.07 (60.84, 69.59) | 31.36 (28.30, 34.75) | 59.51 (55.12, 64.24) | 24.13 (21.29, 27.35) |
Fig. 3.
Summary KM curves for overall survival. Dotted line shows median, shaded area = 95% CI
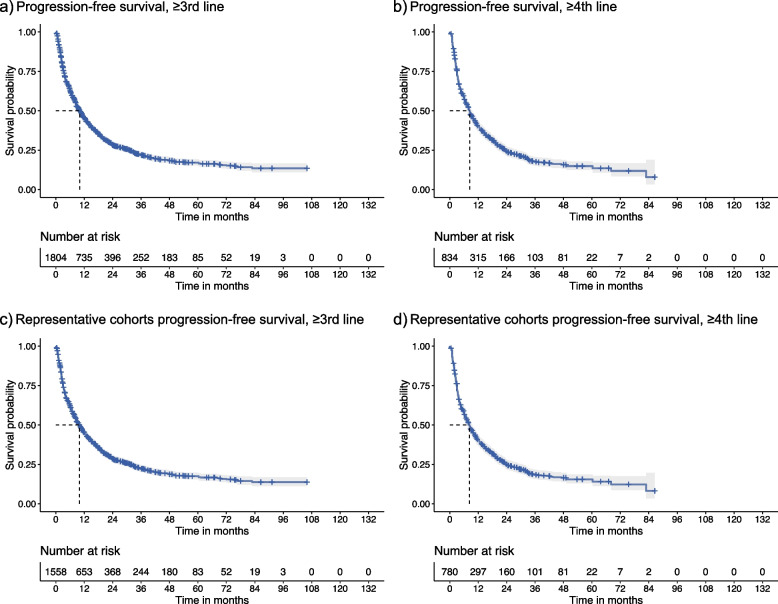
Fig. 4.
Summary KM curves for progression-free survival. Dotted line shows median, shaded area = 95% CI
A similar pattern of results was observed with regard to PFS, with a median PFS of 9.78 (CI: 9.01–10.63) months among those receiving their 3rd or greater LOT as compared 8.11 (CI: 7.3–9.04) months among the 4 or greater LOT group. Observations in the representative cohorts (9.43 months vs. 7.9 months) suggested a similar pattern of attenuation in the 3rd or greater LOT results. In the supplementary analyses, the inclusion of off-label treatments did not have a marked effect on the median PFS (9.86 (CI: 9.16–10.67) months), whereas those patients undergoing SCT were reported to have longer median PFS of 38.58 (CI: 31.37–47.94) months.
The 24-month OS decreased from 66.50% (CI: 63.54–69.60) in the ≥2 prior LOT group to 59.51% (CI: 55.12–64.24) in the ≥3 prior LOT group, with a similar trend in PFS at 24-month (28.26% vs 24.13%). Once again, a similar pattern of results was observed in the representative cohorts, with a reduction in OS from 66.45 to 59.51% and PFS from 28.42 to 24.13%.
Discussion
The purpose of this study was to determine the therapeutic effects of treatments available in Europe and the US for r/r FL patients having been failed by ≥2 prior lines of therapy. Our SLR identified multiple studies including large representative cohorts. Results of the analyses point to a number of unmet needs in this population. The overall response rate was low (57%), despite the inclusion of more studies of recent-to-market treatments (e.g., PIK3-δ and EZH2 inhibitors) that are less commonly used in real-world settings. The median progression-free survival time was also low (median: 9 months), indicating an unmet need. The median survival time was high (59 months), which reflects the indolent nature of the disease. This study provides important context for the results of clinical trials and future studies in r/r FL.
The search methodology we employed was comprehensive and identified studies that were geographically diverse and featured a mix of retrospective cohort studies and non-randomized single-arm clinical trials; thus, the poor clinical outcomes identified in this study emphasize the significant unmet need among this patient population being treated with existing therapeutic agents. This important insight into the limited efficacy of therapies currently available in the treatment of r/r FL can also be used as a point of comparison for ongoing clinical trials of CAR T-cell therapies in this disease space. For example, Jacobsen and colleagues reported a 95% ORR and an 81% CR among FL patients in the ZUMA-5 trial [47] and Fowler and colleagues reported an 85% ORR and a 69% CR among FL patients in the ELARA trial [48]. Whereas there exist several potential differences in the population examined in this review and those enrolled in the ZUMA-5 and ELARA trials, the response rates reported in these clinical trials were notably higher than those found in the SLR reported here. These population differences may explain the differences in overall survival. Jacobson and colleagues reported a > 80% survival at 24 months compared to the 57% noted in this review; although the median OS in this trial has not been reached and thus conclusions regarding OS must be tempered.
The natural disease course of iNHL, with its relapsing and refractory nature and limited treatment options, particularly in later lines of therapy, can exert significant burden on patients and their families. The uncertainty associated with long-term prognosis, ongoing treatment regimens and their toxicities, and frequent interactions with the medical establishment, can all lead to diminished quality of life and poorer psychosocial outcomes [49, 50]. Given the limited treatment efficacy observed in this study, and the prolonged disease course associated with iNHL, it may be prudent for healthcare providers to engage in shared decision making with patients and select treatment regimens that strike a balance between minimizing tumor burden and toxicity while also maximizing quality of life [51, 52].
The current study possesses both strengths and limitations that should be noted. Among the strengths is the robustness of the survival analysis, where sophisticated methods were used to maximize the inclusion of information available in the literature. Through digitization of survival curves, pseudo individual patient data were obtained which allowed for estimation of the entire survival curve all at once, rather than only at specific time points.
In terms of limitations, firstly the sample population was non-representative. Importantly, the goal of this study was to characterize a patient population and not to estimate a comparative treatment effect, and thus measures were taken to create a sample that is reflective of the general population. Despite these efforts, such a condition was not met by our evidence base, with the most notable difference being that concerning treatments received. Generally, there was an over-representation of modern treatments (e.g., SCTs and PI3K-δ inhibitors) and a subsequent under-representation of anti-CD20 and/or chemotherapies that remain common (e.g., R-CHOP). This non-representativeness was further seen in response outcomes, where PI3K-δ inhibitors were heavily represented as only one representative cohort study reported response outcomes. In these analyses response outcomes may be biased towards these more recently approved treatments. The exclusion of SCT studies was necessary due to the immortal time bias introduced and the lack of intention-to-treat analyses in a setting where many patients do not meet criteria to receive treatment. Nonetheless, it is important to note that the representative cohort analyses did include SCT patients, and thus were included in the main analysis. Also, SCT studies reported high survival rates, suggesting SCT is an effective treatment.
A second important limitation pertains to the representative cohort studies that were included in the analyses. A series of three recent studies [7, 19, 20] were the primary sources of insight here. Notably, the results of these studies were presented by line of treatment such that some patients provided data at multiple points. Given the aggregate nature of the data, it was impossible to disaggregate the data to adjust for the repeated measures among patients. In an ideal situation, patients progressing from 3rd line to 4th line would be censored for time-to-event analyses at the time of switch. While this study could have restricted the analyses to a specific line only, it wouldn’t have allowed for inference on the target population, namely 3 L+ r/r FL patients. The potential bias due to repeated measures was deemed less detrimental than the removal of later lines altogether. The issue of repeated measures was reduced for PFS relative to OS because the events were unlikely to be shared across lines of therapy. Typically progression leads to a subsequent change of line of therapy.
Finally, response assessment differed both within and between studies. For the representative cohort studies, response assessment criteria were not reported. For the studies that did report criteria, the 1999 IWG-NHL criteria [45], the 2007 IWG-NHL criteria [44] and the Lugano classification [46] were all used, dependent upon when patients received the index treatment, and what imaging was available. The imaging modality used for response assessment may lead to differences in CR rates, with CT based assessment resulting in lower CR than PET-CT based assessment. This potential bias should be considered when interpreting the CR results.
In conclusion, this comprehensive systematic literature review and meta-analysis further emphasize the significant unmet need among those patients diagnosed with r/r FL patients being failed by ≥2 prior lines of therapy. The low to moderate rates of CR and ORR, as well as the short median time to progression, highlight the need for novel treatment options to be developed and approved among this patient population.
Supplementary Information
Additional file 1: Table S1. Embase search strategy. Table S2. Cochrane Central Register of Controlled Trials search strategy. Table S3. MEDLINE search strategy. Table S4. Study selection criteria to identify trials for the systematic literature review. Table S5. Studies included in each meta-analysis. Table S6. Study quality assessment results. Table S7. Response criteria used for each study included in the meta-analysis. Table S8. Meta-analysis of response outcomes, separated by treatment category, a. Main analysis, b. With inclusion of off-label treatments. Table S9. Model selection across the Bayesian analyses. Table S10. Time-to-event meta-analysis results for supplemental models. Fig. S1. Meta-analysis of response outcomes, including off-label treatments, A) Overall response rate, B) Complete response. Fig. S2. Pooled KM curves for supplemental model including off-label treatments, A) PFS 3rd line plus, B) OS 3rd line plus, Fig. S3. Pooled KM curves for supplemental model including only SCT studies, A) PFS 3rd line plus, B) OS 3rd line plus.
Acknowledgements
We would like to thank the numerous individuals who participated in various parts of the study identification and data collecting throughout this study.
Authors’ contributions
Study concept and design: AP, JTS, AS, SK, and BK. Acquisition, analysis, or interpretation of data: SK, BG, ELO, and AP. Drafting of the manuscript: SK, BK, EHLO, GB, and AP. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: SK and EHLO. Administrative, technical, or material support: BK, AW, GB, JTS, and AP. Study supervision: JTS and AP. All authors take responsibility for the integrity of the data, the accuracy of the data analysis, and the final decision to submit for publication. The author(s) read and approved the final manuscript.
Funding
This project was funded by Kite, a Gilead company.
Availability of data and materials
The data analyzed in this study were obtained from publicly available sources. The following sources were used: Medical Literature Analysis and Retrieval System Online (MEDLINE), Excerpta Medica database (EMBASE), and Cochrane Central Register of Controlled Trials.
Declarations
Ethics approval and consent to participate
As all data used in this study were publicly available, no ethics approval was required. All methods were carried out in accordance with the relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
SK: Employment or leadership position: RainCity Analytics; Research funding: RainCity Analytics has received funds from for-profit healthcare companies for research. BK: Consulting: Abbvie, Acerta, Astra Zeneca, ADCT, Celgene, Genentech, Juno, Kite, Morphosys, BeiGene, Pharmacyclics, Janssen, Genmab, Incyte, MEI; Research Funding: Genentech, ADCT, Acerta, Celgene, BeiGene. AW: Employment or leadership position - Kite, A Gilead company; Stock ownership - Kite, A Gilead company. BG: Employment or leadership position: IQVIA. EHLO: Employment or leadership position: RainCity Analytics. AS: Employment or leadership position: Atara Biotherapeutics, Kite Pharma; Stock ownership: Atara Biotherapeutics, Gilead. GB: Employment or leadership position - Kite, A Gilead company; Stock ownership - Kite, A Gilead company. JTS: Employment or leadership position: Kite, A Gilead Company, Stock ownership: Gilead Sciences. AP: Employment or leadership position - Kite, A Gilead company; Stock ownership - Kite, A Gilead company.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Steve Kanters, Email: skanters@raincity-analytics.com.
Graeme Ball, Email: graeme.ball@gilead.com.
Brad Kahl, Email: bkahl@wustl.edu.
Adriana Wiesinger, Email: adriana.wiesinger@gilead.com.
Eve H. Limbrick-Oldfield, Email: elimbrickoldfield@raincity-analytics.com
Akshay Sudhindra, Email: asudhindra@kitepharma.com.
Julia Thornton Snider, Email: jsnider2@kitepharma.com.
Anik R. Patel, Email: apatel23@kitepharma.com
References
- 1.International Agency for Research on Cancer. Non-Hodgkin Lymphoma. 2020. https://gco.iarc.fr/today/data/factsheets/cancers/34-Non-hodgkin-lymphoma-fact-sheet.pdf
- 2.Casulo C, Byrtek M, Dawson KL, et al. Early Relapse of Follicular Lymphoma After Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone Defines Patients at High Risk for Death: An Analysis From the National LymphoCare Study. J Clin Oncol. 2015;33(23):2516–2522. doi: 10.1200/jco.2014.59.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leukemia and Lymphoma Society. NHL subtypes https://www.lls.org/lymphoma/non-hodgkin-lymphoma/diagnosis/nhl-subtypes
- 4.Gribben JG, How I. treat indolent lymphoma. Blood. 2007;109(11):4617–4626. doi: 10.1182/blood-2006-10-041863. [DOI] [PubMed] [Google Scholar]
- 5.Wang TP, Scott JH, Barta SK. The evolving role of targeted biological agents in the management of indolent B-cell lymphomas. Ther Adv Hematol. 2017;8(12):329–344. doi: 10.1177/2040620717738740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flinn IW, van der Jagt R, Kahl BS, et al. Randomized trial of bendamustine-rituximab or R-CHOP/R-CVP in first-line treatment of indolent NHL or MCL: the BRIGHT study. Blood. 2014;123(19):2944–2952. doi: 10.1182/blood-2013-11-531327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Batlevi CL, Sha F, Alperovich A, et al. Follicular lymphoma in the modern era: survival, treatment outcomes, and identification of high-risk subgroups. Blood. Cancer J. 2020;10(7):74. doi: 10.1038/s41408-020-00340-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Institute for Health and Care Excellence. Single Technology Appraisal - Idelalisib for treating follicular lymphoma refractory to 2 treatments [ID1379]. https://www.nice.org.uk/guidance/ta604/evidence/appraisal-consultation-committee-papers-pdf-6906724813
- 9.Kochenderfer JN, Dudley ME, Kassim SH, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015;33(6):540–549. doi: 10.1200/jco.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Food and Drug Administration. FDA grants accelerated approval to axicabtagene ciloleucel for relapsed or refractory follicular lymphoma. 2021. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-axicabtagene-ciloleucel-relapsed-or-refractory-follicular-lymphoma
- 11.Jacobson C, Chavez JC, Sehgal AR, et al. Primary analysis of Zuma-5: a phase 2 study of Axicabtagene Ciloleucel (Axi-Cel) in patients with relapsed/refractory (R/R) indolent non-Hodgkin lymphoma (iNHL) Blood. 2020;136(Supplement 1):40–41. doi: 10.1182/blood-2020-136834. [DOI] [Google Scholar]
- 12.Schuster SJ, Dickinson MJ, Dreyling MH, et al. Efficacy and safety of tisagenlecleucel (Tisa-cel) in adult patients (Pts) with relapsed/refractory follicular lymphoma (r/r FL): primary analysis of the phase 2 Elara trial. J Clin Oncol. 2021;39(15_suppl):7508–7508. 10.1200/JCO.2021.39.15_suppl.7508.
- 13.Apostolidis J, Mokhtar N, Al Omari R, Darweesh M, Al HH. Follicular lymphoma: update on management and emerging therapies at the dawn of the new decade. Hematol Oncol. 2020;38(3):213–222. doi: 10.1002/hon.2711. [DOI] [PubMed] [Google Scholar]
- 14.Luminari S, Trotman J, Federico M. Advances in treatment of follicular lymphoma. Cancer J May/Jun. 2020;26(3):231–240. doi: 10.1097/ppo.0000000000000444. [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 16.Guyot P, Ades AE, Beasley M, Lueza B, Pignon JP, Welton NJ. Extrapolation of survival curves from Cancer trials using external information. Med Decis Mak. 2017;37(4):353–366. doi: 10.1177/0272989x16670604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health Jun 1998;52(6):377–384. 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed]
- 18.Morschhauser F, Tilly H, Chaidos A, et al. Tazemetostat for patients with relapsed or refractory follicular lymphoma: an open-label, single-arm, multicentre, phase 2 trial. Lancet Oncol. 2020;21(11):1433–1442. doi: 10.1016/s1470-2045(20)30441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuji S, Tada Y, Nozaki K, et al. A multi-center retrospective analysis of patients with relapsed/refractory follicular lymphoma after third-line chemotherapy. Ann Hematol. 2020;99(9):2133–2139. doi: 10.1007/s00277-020-04126-y. [DOI] [PubMed] [Google Scholar]
- 20.Link BK, Day BM, Zhou X, et al. Second-line and subsequent therapy and outcomes for follicular lymphoma in the United States: data from the observational national LymphoCare study. Br J Haematol. 2019;184(4):660–663. doi: 10.1111/bjh.15149. [DOI] [PubMed] [Google Scholar]
- 21.Bannerji R, Allan JN, Arnason JE. Odronextamab (REGN1979), a human CD20 x CD3 bispecific antibody, induces durable, complete responses in patients with highly refractory b-cell non-Hodgkin lymphoma, including patients refractory to CAR T therapy. Blood. 2020;136(SUPPL 1):42–43. doi: 10.1182/blood-2020-136659. [DOI] [Google Scholar]
- 22.Muntañola A, Baumann T, Caballero AC, et al. Results of R-ESHAP as salvage therapy in refractory/relapsed follicular lymphoma: a real-world experience on behalf of GELCAB group. Ann Hematol. 2020;99(7):1627–1634. doi: 10.1007/s00277-020-04101-7. [DOI] [PubMed] [Google Scholar]
- 23.Andorsky D, Chan RJ, Clark J, Ruzicka B, Robert NJ, Awan FT. Treatment patterns and outcomes of patients with relapsed or refractory follicular lymphoma treated with Idelalisib in a community oncology setting. Blood. 2019;134(Supplement_1):2810. doi: 10.1182/blood-2019-124013. [DOI] [Google Scholar]
- 24.Gopal AK, Kahl BS, de Vos S, et al. PI3Kδ inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med. 2014;370(11):1008–1018. doi: 10.1056/NEJMoa1314583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robert P, Rossi C, Aucagne R, et al. Somatic mutation profile analyzed by next-generation sequencing in relapsed/refractory follicular lymphoma treated with Idelalisib. Blood. 2019;134(Supplement_1):1503. doi: 10.1182/blood-2019-126459. [DOI] [Google Scholar]
- 26.Fowler NH, Samaniego F, Jurczak W, et al. Umbralisib, a dual PI3Kdelta/CK1epsilon inhibitor in patients with relapsed or refractory indolent lymphoma. J Clin Oncol. 2021;39(15):1609–1618. doi: 10.1200/JCO.20.03433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ouwens MJNM, Philips Z, Jansen JP. Network meta-analysis of parametric survival curves. Res Synth Methods. 2011;1(3–4):258–271. doi: 10.1002/jrsm.25. [DOI] [PubMed] [Google Scholar]
- 28.Jansen JP. Network meta-analysis of survival data with fractional polynomials. BMC Med Res Methodol. 2011;11:61. doi: 10.1186/1471-2288-11-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jansen JP, Cope S. Meta-regression models to address heterogeneity and inconsistency in network meta-analysis of survival outcomes. BMC Med Res Methodol. 2012;12(1):152. doi: 10.1186/1471-2288-12-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dempster AP. The direct use of likelihood for significance testing. Stat Comput. 1997;7(4):247–252. doi: 10.1023/A:1018598421607. [DOI] [Google Scholar]
- 31.Brooks SP, Gelman A. General methods for monitoring convergence of iterative simulations. J Comput Graph Stat. 1998;7(4):434–455. doi: 10.1080/10618600.1998.10474787. [DOI] [Google Scholar]
- 32.Assouline SE, Seog Kim W, Sehn LH, Schuster SJ, Cheah CY, Nastoupil LJ, Shadman M, Yoon SS, Matasar MJ, Diefenbach C, Gregory GP, Bartlett NL, Wei MC, Doral MY, Yin S, Negricea R, Li CC, Penuel EM, Buddle LE. Mosunetuzumab Shows Promising Efficacy in Patients with Multiply Relapsed Follicular Lymphoma: Updated Clinical Experience from a Phase I Dose-Escalation Trial. Blood. 2020;136:42–44. doi: 10.1182/blood-2020-135839. [DOI] [Google Scholar]
- 33.Dreyling M, Santoro A, Mollica L, et al. Phosphatidylinositol 3-kinase inhibition by Copanlisib in relapsed or refractory indolent lymphoma. J Clin Oncol. 2017;35(35):3898–3905. doi: 10.1200/JCO.2017.75.4648. [DOI] [PubMed] [Google Scholar]
- 34.Gopal AK, Schuster SJ, Fowler NH, et al. Ibrutinib as treatment for patients with relapsed/refractory follicular lymphoma: results from the open-label, multicenter, phase II DAWN study. J Clin Oncol. 2018;36(23):2405–2412. doi: 10.1200/JCO.2017.76.8853. [DOI] [PubMed] [Google Scholar]
- 35.Morschhauser F, Tilly H, Chaidos A, et al. Interim update from a phase 2 multicenter study of tazemetostat, an EZH2 inhibitor, in patients with relapsed or refractory follicular lymphoma (FL) Hematol Oncol. 2019;37:154–156. doi: 10.1002/hon.111_2629. [DOI] [Google Scholar]
- 36.Khouri IF, McLaughlin P, Saliba RM, et al. Eight-year experience with allogeneic stem cell transplantation for relapsed follicular lymphoma after nonmyeloablative conditioning with fludarabine, cyclophosphamide, and rituximab. Blood. 2008;111(12):5530–5536. doi: 10.1182/blood-2008-01-136242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laport GG, Wu J, Logan B, et al. Reduced-intensity conditioning with Fludarabine, cyclophosphamide, and high-dose rituximab for allogeneic hematopoietic cell transplantation for follicular lymphoma: a phase two multicenter trial from the blood and marrow transplant clinical trials network. Biol Blood Marrow Transplant. 2016;22(8):1440–1448. doi: 10.1016/j.bbmt.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fowler NH, Samaniego F, Jurczak W, et al. Umbralisib, a dual PI3Kδ/CK1ε inhibitor in patients with relapsed or refractory indolent lymphoma. J Clin Oncol. 2021;39(15):1609–1618. doi: 10.1200/jco.20.03433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ito Y, Miyamoto T, Kamimura T, et al. Clinical outcomes of allogeneic stem cell transplantation for relapsed or refractory follicular lymphoma: a retrospective analysis by the Fukuoka blood and marrow transplantation group. Int J Hematol. 2013;98(4):463–471. doi: 10.1007/s12185-013-1430-9. [DOI] [PubMed] [Google Scholar]
- 40.Lunning MA, Migliacci JC, Hilden P, et al. The potential benefit of allogeneic over autologous transplantation in patients with very early relapsed and refractory follicular lymphoma with prior remission duration of ≤12 months. Br J Haematol. 2016;173(2):260–264. doi: 10.1111/bjh.13947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sesques P, Bourcier J, Golfier C, et al. Clinical characteristics and outcomes of relapsed follicular lymphoma after autologous stem cell transplantation in the rituximab era. Hematol Oncol. 2020;38(2):137–145. doi: 10.1002/hon.2713. [DOI] [PubMed] [Google Scholar]
- 42.Vose JM, Bierman PJ, Loberiza FR, et al. Long-term outcomes of autologous stem cell transplantation for follicular non-Hodgkin lymphoma: effect of histological grade and follicular international prognostic index. Biol Blood Marrow Transplant. 2008;14(1):36–42. doi: 10.1016/j.bbmt.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 43.Evens AM, Vanderplas A, LaCasce AS, et al. Stem cell transplantation for follicular lymphoma relapsed/refractory after prior rituximab: a comprehensive analysis from the NCCN lymphoma outcomes project. Cancer. 2013;119(20):3662–3671. doi: 10.1002/cncr.28243. [DOI] [PubMed] [Google Scholar]
- 44.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 45.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 17(4):1244. 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed]
- 46.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059–3067. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jacobson CA, Chavez JC, Sehgal AR, et al. Axicabtagene ciloleucel in relapsed or refractory indolent non-Hodgkin lymphoma (ZUMA-5): a single-arm, multicentre, phase 2 trial. The Lancet Oncology. 23(1):91–103. [DOI] [PubMed]
- 48.Fowler NH, Dickinson M, Dreyling M, et al. Tisagenlecleucel in adult relapsed or refractory follicular lymphoma: the phase 2 ELARA trial. Nat Med. 2021;28(2):325–332. doi: 10.1038/s41591-021-01622-0. [DOI] [PubMed] [Google Scholar]
- 49.Oerlemans S, Issa DE, van den Broek EC, et al. Impact of therapy and disease-related symptoms on health-related quality of life in patients with follicular lymphoma: results of the population-based PHAROS-registry. Eur J Haematol. 2014;93(3):229–238. doi: 10.1111/ejh.12335. [DOI] [PubMed] [Google Scholar]
- 50.Pettengell R, Donatti C, Hoskin P, et al. The impact of follicular lymphoma on health-related quality of life. Ann Oncol Mar 2008;19(3):570–576. 10.1093/annonc/mdm543. [DOI] [PubMed]
- 51.Matasar MJ, Luminari S, Barr PM, et al. Follicular lymphoma: recent and emerging therapies, treatment strategies, and remaining unmet needs. Oncologist. 2019;24(11):e1236–e1250. doi: 10.1634/theoncologist.2019-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pagel JM, Burke JM, Leslie LA. Refining the management of relapsed or refractory follicular lymphoma. Clin Adv Hematol Oncol. 2020;18(12):1–24. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Embase search strategy. Table S2. Cochrane Central Register of Controlled Trials search strategy. Table S3. MEDLINE search strategy. Table S4. Study selection criteria to identify trials for the systematic literature review. Table S5. Studies included in each meta-analysis. Table S6. Study quality assessment results. Table S7. Response criteria used for each study included in the meta-analysis. Table S8. Meta-analysis of response outcomes, separated by treatment category, a. Main analysis, b. With inclusion of off-label treatments. Table S9. Model selection across the Bayesian analyses. Table S10. Time-to-event meta-analysis results for supplemental models. Fig. S1. Meta-analysis of response outcomes, including off-label treatments, A) Overall response rate, B) Complete response. Fig. S2. Pooled KM curves for supplemental model including off-label treatments, A) PFS 3rd line plus, B) OS 3rd line plus, Fig. S3. Pooled KM curves for supplemental model including only SCT studies, A) PFS 3rd line plus, B) OS 3rd line plus.
Data Availability Statement
The data analyzed in this study were obtained from publicly available sources. The following sources were used: Medical Literature Analysis and Retrieval System Online (MEDLINE), Excerpta Medica database (EMBASE), and Cochrane Central Register of Controlled Trials.