Abstract
Exercise is the main treatment for patients with metabolic-associated fatty liver disease (MAFLD); however, it may be difficult for some patients to adhere to or tolerate an exercise regime. Thus, finding a treatment alternative to exercise is of particular importance. The authors have previously demonstrated that the high expression of microRNA (miRNA/miR)-212 promotes lipogenesis in vitro. The present study aimed to explore the therapeutic potential, as well as the mechanisms of action of miR-212 in MAFLD. The expression of miR-212-3p, but not that of miR-212-5p, was found to be significantly elevated in MAFLD and to be decreased by exercise. Compared with exercise treatment, the inhibition of miR-212-3p expression in a mouse model fed a high-fat diet exerted beneficial effects on MAFLD similar to those of exercise. Conversely, the overexpression of miR-212-3p abolished the ameliorative effects of exercise on MAFLD. Fibroblast growth factor 21 (FGF21) and chromodomain helicase DNA binding protein 1 (CHD1) were identified as target genes of miR-212-3p in lipid metabolism using bioinformatics analysis. Mechanistically, the inhibition of miR-212-3p mimicked the effects of exercise on lipid metabolism by regulating FGF21, but not CHD1. The exercise-related transcription factor, early growth response 1 (EGR1), was identified upstream of miR-212-3p through promoter motif analysis. EGR1 overexpression inhibited miR-212-3p expression. The overexpression of miR-212-3p abolished the effects of exercise on lipid metabolism by exogenously attenuating the transcriptional repression of EGR1. Moreover, the overexpression of miR-212-3p abolished the regulatory effects of EGR1 on FGF21. On the whole, the present study demonstrates that miR-212-3p plays a key role in the effects of exercise on MAFLD. The findings presented herein suggest a potential therapeutic effect of targeting miR-212-3p in MAFLD.
Keywords: metabolic-associated fatty liver disease, microRNA-212-3p, fibroblast growth factor 21, early growth response 1, chromodomain helicase DNA binding protein 1, lipogenesis
Introduction
The incidence rate of metabolic-associated fatty liver disease (MAFLD) has rapidly increased, and it has become a major health concern, posting a threat to numerous lives (1-3). The ectopic accumulation of liver fat aggravates the metabolic burden, and when accompanied by multiple factors, such as lipotoxic substances, the inflammatory response and oxidative stress, MAFLD can progress from steatosis to cirrhosis, and in some cases, even hepatocellular carcinoma. Exercise is often used as the main treatment modality for MAFLD, which can effectively improve intrahepatic lipid accumulation (4-6). Previous research and a randomized controlled trials (RCT) on the effects of aerobic exercise training doses on liver fat accumulation have revealed that even a minimal amount of physical activity (150-180 min of moderate-intensity exercise per week) can reduce liver fat in the short-term (7,8). However, the majority of patients who were included in the RCT went through the diet and exercise program without success (8). As with all clinical trials, some patients cannot tolerate or adhere to the prescribed exercise program due to their physical condition or lifestyle habits. Thus, exploring an effective alternative therapy to simulate exercise may provide a feasible solution.
MicroRNAs (miRNAs/miRs) are evolutionarily conserved, small (~21 nucleotides) non-coding RNAs (9). miRNA precursors (pre-miRNAs) are stem-loop structures which are exported to the cytoplasm by the karyopherin, exportin 5 (Exp5) transporter. In the cytoplasm, pre-miRNAs are processed by the Dicer enzyme to produce mature miRNAs of ~22 nucleotides in length (9). In a previous study by the authors, miR-212 was identified to be closely related to lipid metabolism (10). It has also been shown to be involved in a variety of pathophysiological processes, including cell proliferation (11), angiogenesis (12), intestinal permeability (13) and blood glucose metabolism (14). Furthermore, its dysregulation is strongly related to metabolic diseases, such as hypertension, cardiac hypertrophy (15) and alcoholic liver disease (13). Mature miR-212 consists mainly of miR-212-3p and miR-212-5p, both of which are closely related to lipid metabolism. Leucine deprivation suppresses triglyceride (TG) accumulation in the liver, which usually causes elevated levels of miR-212-5p (16). A high-fat diet (HFD) accelerates liver lipid accumulation, which usually leads to elevated levels of miR-212-3p (10). In addition, exercise improves hepatic lipid accumulation, which decreases miR-212-3p levels in patients with MAFLD. The study of miR-212 may contribute to the development of potential exercise replacement therapies for MAFLD in the future. The therapeutic potential of miR-212 (an alternative treatment which can mimic the effects of exercise), as well as the detailed functional mechanisms of MAFLD warrant further investigation.
The present study explored three functional roles of miR-212-3p in MAFLD; i.e., the therapeutic effects of targeting miR-212-3p, its association with exercise and the mechanisms underlying its functions. The data confirmed that the inhibition of miR-212-3p exerted beneficial effects on MAFLD similar to those of exercise by targeting fibroblast growth factor 21 (FGF21), rather than chromodomain helicase DNA binding protein 1 (CHD1). In addition, the regulatory effects of exercise on miR-212-3p were investigated. Exercise played a protective role in MAFLD by activating early growth response 1 (EGR1), which transcriptionally suppressed miR-212-3p and increased FGF21 expression. The overexpression of miR-212-3p abolished the regulatory effects of EGR1 on FGF21, validating a regulatory association among EGR1, miR-212-3p and FGF21. miR-212-3p was a key molecule in the effects of exercise on MAFLD. These findings suggest a potential therapeutic effect of targeting miR-212-3p on MAFLD.
Materials and methods
Animals experiments and grouping
A total of 30 male C57BL/6 mice (aged 8 weeks; weighing 20-23 g; mean ± standard error, 21.7±0.9 g) were purchased from the Shanghai SLAC Laboratory Animal Co., Ltd. The mice were maintained on a 12-h light/dark cycle at 25°C, a humidity of 50-60% and lighting with ad libitum access to food and water. The animal study was approved by the Animal Ethics Committee of Tongji University, Shanghai, China (approval no. TJHBLAC-2 019-024). All procedures performed in experiments involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted. The mice were randomly divided into five groups as follows: i) The chow group (n=6), in which mice were fed standard chow for 16 weeks, and administered tail vein injections of adeno-associated virus (AAV) serotype 8 gene vectors [1×1011 viral genomes in 100 µl saline; Hanheng Biotechnology (Shanghai) Co., Ltd.] in the 4th week; ii) the HFD group (n=6), in which mice were fed a HFD for 16 weeks, and received a tail vein injection of AAV serotype 8 gene vectors [1×1011 viral genomes in 100 µl saline; Hanheng Biotechnology (Shanghai) Co., Ltd.] in the 4th week; iii) the HFD and SP group (n=6), in which mice were fed a HFD for 16 weeks, and received a tail vein injection of recombinant AAV serotype 8 gene vectors carrying miR-212-3P sponge [1×1011 viral genomes in 100 µl saline; Hanheng Biotechnology (Shanghai) Co., Ltd.] in the 4th week; iv) the HE group (n=6), in which mice were fed a HFD, subjected to daily exercise on a running machine (10 m/min, 60 min/day, 16 weeks) and received a tail vein injection of recombinant AAV serotype 8 gene vectors [1×1011 viral genomes in 100 µl saline; Hanheng Biotechnology (Shanghai) Co., Ltd.] in the 4th week; and v) the HE and OE group (n=6), in which mice were fed a HFD, subjected to daily exercise on a running machine (10 m/min, 60 min/day, 16 weeks) and received a tail vein injection of recombinant AAV serotype 8 gene vectors carrying miR-212-3P [1×1011 viral genomes in 100 µl saline; Hanheng Biotechnology (Shanghai) Co., Ltd.] in the 4th week. The standard chow diet (cat. no. LAD3001M; 14.1% kcal protein, 75.9% carbohydrate and 10.0% fat; 3.6 kcal/g) and HFD (cat. no. TP23400; 14.1% kcal protein, 25.9% carbohydrate and 60.0% fat; 5.0 kcal/g) were obtained from Trophic Animal Feed High-tech Co. Ltd.
For the glucose tolerance test (GTT), the mice were fasted overnight for 16 h with free access to water and injected intraperitoneally (i.p.) with 2 g/kg glucose. For the insulin tolerance test (ITT), the mice were fasted 4 h prior to being i.p. injected with human insulin (0.75 U/kg; Eli Lilly and Company). The fasting blood glucose levels were detected after the mice were fasted for 6 h.
The criteria for humane endpoints in this experiment were as follows: Markedly reduced food or water intake, waddling, dyspnea, ruffled fur or self-mutilation, inability to stand and unresponsiveness to external stimuli. Any animals reaching these endpoints were to be euthanized with 1% pentobarbital sodium (150 mg/kg, i.p., P3761, MilliporeSigma). All animals were observed daily for behavior and food or water intake to assess their health status. No abnormal signs indicating the humane endpoint of the experiment were observed in any of the mice during the experiment.
At the end of the experiment (16 weeks), a total of 30 male C57BL/6 mice were euthanized by an overdose of inhalant anesthetic (isoflurane; concentration: 5%; exposure time, 5 min), followed by ensuring the mouse was unconscious by testing the retraction of the foot pedal and the reflexes of the eyelids, and finally exsanguination under anesthesia to ensure death. When the mice exhibited no respiration, no heartbeat and no response to any external stimuli, they were considered dead. Samples required for testing were collected.
Microarray data and human samples
Microarray data (GSE65978) were obtained from the Gene Expression Omnibus (GEO) database, as previously described (10). Non-coding RNA expression information was measured for liver tissue samples from 4 patients with MAFLD and 4 patients with non-fatty liver at Tongji Hospital, Tongji University. The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN) criteria (17) were used to assess liver histology. Combined with the clinical history, the Alcohol Use Disorders Identification Test (AUDIT) questionnaire (18) was used to exclude the interference of a history of alcohol consumption. The present study was approved by the Ethics Committee of Tongji Hospital (approval no. K-KYSB-2020-139) and was performed in accordance with the Declaration of Helsinki. Written informed consent was obtained from the patients or their guardians.
Measurement of serum and liver metabolites
Serum was separated at 5,000 × g for 15 min at room temperature and stored at −80°C. Alanine transaminase (ALT), aspartate transaminase (AST) and TG levels were measured using routine clinical chemical assays (cat. nos. C009-2-1, C010-2-1 and A110-1-1, respectively; Nanjing Jiancheng Bioengineering Institute). The process was carried out according to the manufacturer's protocols. Serum FGF21 and insulin levels were measured using a mouse FGF21 ELISA kit and a mouse insulin ELISA kit (JM-03104M1 and JM-02862M1, respectively; Jiangsu Jingmei Biotechnology, Co., Ltd.), according to the manufacturer's protocols.
Histopathological analysis and immunohistochemistry
The liver tissue samples were fixed in 4% paraformaldehyde for 24 h at temperature. For the preparation of paraffin-embedded tissues, the tissues were routinely dehydrated at room temperature using an increasing ethanol gradient followed by xylene. The tissues were then embedded in paraffin. The non-alcoholic fatty liver disease (NAFLD) activity score (NAS) was calculated by determining steatosis, inflammation and ballooning. For the preparation of frozen tissues, tissues were routinely dehydrated at room temperature using an increasing sucrose followed by optimal cutting temperature compound embedding, and preservation at −80°C. Liver sections (5-µm-thick) were respectively stained with hematoxylin and eosin (H&E) dye (cat. no. G1120; Beijing Solarbio Science & Technology Co., Ltd.) and Oil Red O dye (cat. no. D027-1-1; Nanjing Jiancheng Bioengineering Institute) to observe hepatic lipid droplets. For H&E staining, the tissue sections were stained with hematoxylin solution for 10 min at room temperature and washed in running water for 5 min, followed by the addition of differentiation solution for 10 sec at room temperature. The tissue sections were subsequently stained with eosin solution for 10 sec at room temperature, dehydrated in alcohol (75, 85, 95 and 100%; 2-3 sec each at room temperature), and rinsed in 100% alcohol for 1 min at room temperature. Finally, the tissue sections were cleared with xylene and sealed with neutral balsam. For Oil Red O staining, frozen sections were dried at room temperature for 15-20 min, followed by incubation in 100% isopropanol for 5 min. Subsequently, 0.5% Oil Red O solution was incubated for 8 min at 60°C, distilled water was cleaned for 5 min. Hematoxylin was stained for 10 min and washed with distilled water for 5 min. Finally, the sections were sealed with glycerol gelatin. The 'analyze particles' function (size-pixel2 from 0.1 to infinity; circularity from 0.1 to 1) of Image J software (Version: 1.52a; National Institutes of Health) was used for lipid droplet quantification. The sections were scanned using a Pannoramic Whole Slide Scanner (Pannoramic Desk; 3DHISTECH Ltd.) and viewed using CaseViewer 2.2 (3DHISTECH Ltd.).
For immunohistochemical staining, the sections were immersed in 0.01 M sodium citrate (100°C, 20 min) for antigen retrieval. Endogenous peroxidase activity was quenched by incubation with 3% H2O2 for 10 min at room temperature. The sections were then incubated with 10% bovine serum albumin for 1 h at room temperature, and then incubated with anti-FGF21 antibody (1:200, cat. no. ab171941, Abcam) at 4°C overnight. A goat anti-rabbit IgG H&L (HRP) secondary antibody (1:1,000, cat. no. ab97051, Abcam) specific to the primary antibody was selected and allowed to react at room temperature for 60 min. DAB (MilliporeSigma) color reaction solution was used for slice color development. The sections were scanned using a Pannoramic Whole Slide Scanner (Pannoramic Desk; 3DHISTECH Ltd.) and viewed using CaseViewer 2.2 (3DHISTECH Ltd.).
Cell transfection and Nile Red staining
The liver cancer cell line (HepG2) was purchased from The Cell Bank of Type Culture Collection of The Chinese Academy of Sciences (serial no. SCSP-510). The liver cancer cell line (HepG2) used in the study was authenticated by STR profiling. The liver cancer cell line (HepG2) was treated with 1 mM long-chain free fatty acid (FFA; oleate, palmitate at a molar ration of 2:1; MilliporeSigma) in 1% bovine serum albumin for 24 h to induce lipogenesis in vitro. The liver cancer cells (HepG2) were plated in six-well plates in antibiotic-free medium for 24 h prior to transfection and transfected at 70% confluency for 48 h using Lipofectamine 2000® reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions. The miR-212-3p mimics and inhibitor purchased from Guangzhou Ruibo Co., Ltd. The sequence of siRNA duplex targeting EGR1 was referenced from the literature (19) and synthesized by Sangon Biotech (Shanghai) Co., Ltd. EGR1 overexpression plasmid with the pcDNA3.1 vector were purchased from Changsha Youzhe Co., Ltd. For the transfection experiments, the HepG2 cells were transfected with miR-212-3p mimics/control (50 nM), inhibitors/control (100 nM), FGF21-siRNA/control (75 nM), CHD1-siRNA/control (75 nM), EGR1-siRNA/control (75 nM) and EGR1 overexpression plasmids/blank vector (1.25 µg/ml) according to the experimental purpose. The efficiency of transfection was evaluated using reverse transcription-quantitative (RT-qPCR).
For Nile Red staining, the cells were washed three times with phosphate-buffered saline (PBS) and then fixed with 3.7% paraformaldehyde for 1 h at room temperature and washed thrice again with PBS. To evaluate intracellular lipogenesis, lipid droplets were stained with Nile Red dye (0.1 µmol/ml; CAS no. 7385-67-3; Sigma-Aldrich; Merck KGaA) for 15 min in the dark at room temperature, and cell nuclei were stained with DAPI (CAS no. KGA215-10; Jiangsu Keygen Biotech Corp., Ltd) for 5 min in the dark at room temperature. Finally, the cells were washed three times with PBS to clean the dye solution. The results were analyzed using a Leica fluorescence microscope (Leica DM6B; Leica Microsystems, Inc.). The whole staining process was conducted at room temperature avoiding direct light. Each experiment was performed in triplicate. The siRNA and negative control sequences used in the experiments are presented in Table SI.
Luciferase reporter assay
The 293T cell line was purchased from The Cell Bank of Type Culture Collection of The Chinese Academy of Sciences (Serial: SCSP-502). To test for promoter activity, the 293T cells were seeded at 5×104 per well in 24-well plates and co-transfected with pGL3-miR-promoter/pGL3-basic (containing the −1,500 to +500 bp promoter sequence of miR-212) (1 µg/ml), pRL-TK (1 µg/ml) (control for fluorescence) and EGR1-siRNA/control (75 nM) or pcDNA3.1-EGR1/pcDNA3.1-basic (1 µg/ml) using Lipofectamine 2000® reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to manufacturer's instructions. At 48 h post-transfection, the luciferase activities were determined using a Dual Luciferase Reporter Assay kit (DL101, Nanjing Vazyme Biotech Co., Ltd), and normalized to Renilla luciferase activity, respectively. Each experiment was performed in triplicate.
Western blot analysis
The liver tissues and cells were lysed using RIPA lysis buffer (Beyotime Institute of Biotechnology) containing 1% phenylmethanesulfonyl fluoride. A total of 30 µg protein was loaded onto 10 and 12.5% SDS-polyacrylamide gels, and transferred onto PVDF membranes. The membranes were incubated overnight at 4°C with primary antibodies (as indicated below) followed by an incubation with the horseradish peroxidase-conjugated goat anti-rabbit IgG secondary antibody (1:10,000; cat. no. A0208) and goat anti-mouse IgG (1:10,000; cat. no. A0216) (both from Beyotime Institute of Biotechnology) at room temperature for 1 h. The protein bands were visualized using ECL Moon from Beyotime Institute of Biotechnology. The blots were scanned using an Odyssey Two-color Infrared Laser Imaging System (LI-COR Biosciences). The primary antibodies used were as follows: Anti-EGR1 (1:1,000; cat. no. 55117-1-AP, ProteinTech Group, Inc.), anti-CHD1 (1:1,000; cat. no. 4351S, Cell Signaling Technology, Inc.), anti-FGF21 (1:1,000; cat. no. ab171941, Abcam), anti-ERK1/2 (1:1,000; cat. no. ab184699, Abcam), anti-phosphorylated (p-)ERK1/2 (1:1,000; cat. no. 4377s, Cell Signaling Technology, Inc.), anti-fatty acid synthase (FASN; 1:5,000; cat. no. ab128870, Abcam), anti-carnitine palmitoyltransferase (CPT)1α (1:1,000; cat. no. 15184-1-AP, ProteinTech Group, Inc.) and anti-β-actin (1:5,000; cat. no. MA1-140, Thermo Fisher Scientific, Inc.). The protein bands were visualized using BeyoECL Plus (Beyotime Institute of Biotechnology). QuantityOne v4.6.6 software (Bio Rad Laboratories, Inc.) was used for the analysis and for the ratio of the gray value of the target protein band to the gray.
RNA isolation and RT-qPCR
Total RNA was isolated from the cells and tissues using TRIzol reagent (Sigma-Aldrich; Merck KGaA). For mRNA detection, RNA was reverse transcribed into cDNA using PrimeScript™ RT Master Mix (cat. no. RR036B; Takara Bio, Inc.). The temperature protocol was as follows: 37°C for 15 min, 85°C for 5 sec. qPCR was carried out using TB Green® Premix Ex Taq™ (cat. no. RR042B; Takara Bio, Inc.) with the ABI Q5 system. The full thermocycling conditions for qPCR are as follows: Initial denaturation at 95°C for 30 sec, followed by 40 cycles of 95°C for 5 sec and 60°C for 30 sec. For miRNA analysis, miRNA was reverse transcribed using the miRNA 1st Strand cDNA Synthesis kit (cat. no. MR101-01; Nanjing Vazyme Biotech Co., Ltd), and amplified with miRNA universal SYBR qPCR Master Mix (cat. no. MQ101-02; Nanjing Vazyme Biotech Co., Ltd). The temperature protocol for cDNA synthesis were as follows: 25°C for 5 min, 50°C for 15 min, 85°C for 5 min. The full thermocycling conditions for qPCR are as follows: Initial denaturation at 95°C for 5 min, followed by 40 cycles of 95°C for 10 sec and 60°C for 30 sec. β-actin was used as an internal control for each coding genes of interests. U6 was used as an internal control for each non-coding genes of interests. The primers used for RT-qPCR are presented in Table SI.
Bioinformatics analysis
For the determination of target genes, the TargetScan (20-25) (http://www.targetscan.org/vert_80/) and miRwalk (26) (http://mirwalk.umm.uni-heidelberg.de/) online website software tools were used that query possible downstream gene sets by inputing miR-212-3p in the search bar of the website. Subsequently, enrichment analysis was carried out using Gene Ontology (GO) to identify biological processes and pathways. Specifically, terms with a P-value <0.01, a minimum count of 3, and an enrichment factor >1.5 (the enrichment factor is the ratio between the observed counts and the counts expected by chance) were collected and grouped into clusters based on their membership similarities. Finally, experimentally confirmed literatures were examined as the final screening (10,27). For transcription factor prediction, the UCSC online website (http://genome.ucsc.edu/) (28) was queried for promoter region sequences of miR-212-3p. Subsequently, the sequence was input into Jasper (https://jaspar.genereg.net/) (29) and regRNA 2.0 (http://regrna2.mbc.nctu.edu.tw.) (30) online software to obtain predicted transcription factors. MGI mammalian phenotype (http://www.informatics.jax.org/) was used for functional screening (31).
Statistical analysis
GraphPad Prism software (Version: 7.00; GraphPad Software, Inc.) and SPSS 20.0 (IBM Corp.) were used for statistical analysis. The Metascape online website (http://metascape.org/gp/index.html#/main/step1) was used for functional enrichment analysis (32). The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as (fasting glucose level x fasting insulin level)/22.4. An unpaired Student's t-test was used for comparisons between two groups. One-way analysis of variance (ANOVA) was used for comparisons of three or more groups, and following ANOVA, Dunnett's test or Tukey's test were used as post hoc tests according to the analytical purpose. A Mann-Whitney U test was used to evaluate non-parametric data. All data are expressed as the mean ± standard error of the mean (SEM), or median (25-75th percentile). P<0.05 was considered to indicate a statistically significant difference.
Results
miR-212-3p, but not miR-212-5p is involved in lipid metabolism in MAFLD
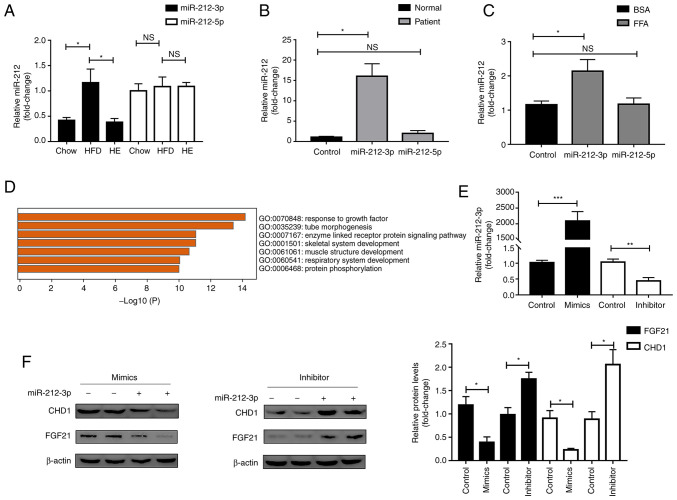
The authors have previously demonstrated that a high expression of miR-212 promotes lipogenesis in vitro (10). In the present study, it was found that among miR-212, both miR-212-3p and miR-212-5p were stably expressed in vivo, as determined using miRBase database. The association between miR-212-3p and miR-212-5p in lipid metabolism warrants clarification. Firstly, following the chow diet, the HFD and HE models, the microarray data and RT-qPCR revealed that miR-212-3p expression was elevated in the HFD model and decreased with exercise intervention. However, miR-212-5p expression was not consistent with miR-212-3p; this did not increase with the HFD (Fig. 1A and Table SII). Secondly, miR-212-3p and miR-212-5p were detected in human samples from patients with MAFLD. The expression of miR-212-3p, but not that of miR-212-5p, was significantly elevated in liver tissues from patients with MAFLD compared with normal livers (Fig. 1B). Finally, the expression of miR-212-3p, but not that of miR-212-5p, was significantly upregulated in the FFA-stimulated cell model (Fig. 1C).
Figure 1.
miR-212-3p, but not miR-212-5p is involved in MAFLD. (A) miR-212-3p, but not miR-212-5p expression, was elevated in HFD and decreased by exercise (n=6). (B) miR-212-3p, but not miR-212-5p expression, was elevated in patients with MAFLD (n=4). (C) miR-212-3p, but not miR-212-5p expression, was elevated in the FFA-treated cell model (n=3). (D) Functional enrichment analysis of the target gene. (E) miR-212-3p was overexpressed/knocked down by miR-212-3p mimics/inhibitor, respectively. (F) miR-212-3p negatively regulated FGF21/CHD1 at the protein level under FFA treatment. The data are presented as the mean ± SEM. *P<0.05, **P<0.01 and ***P<0.001. NS, not significant; MAFLD, metabolic-associated fatty liver disease; HFD, high-fat diet; FFA, free fatty acid; FGF21, fibroblast growth factor 21; CHD1, chromodomain helicase DNA binding protein 1; HE, high-fat diet and exercise.
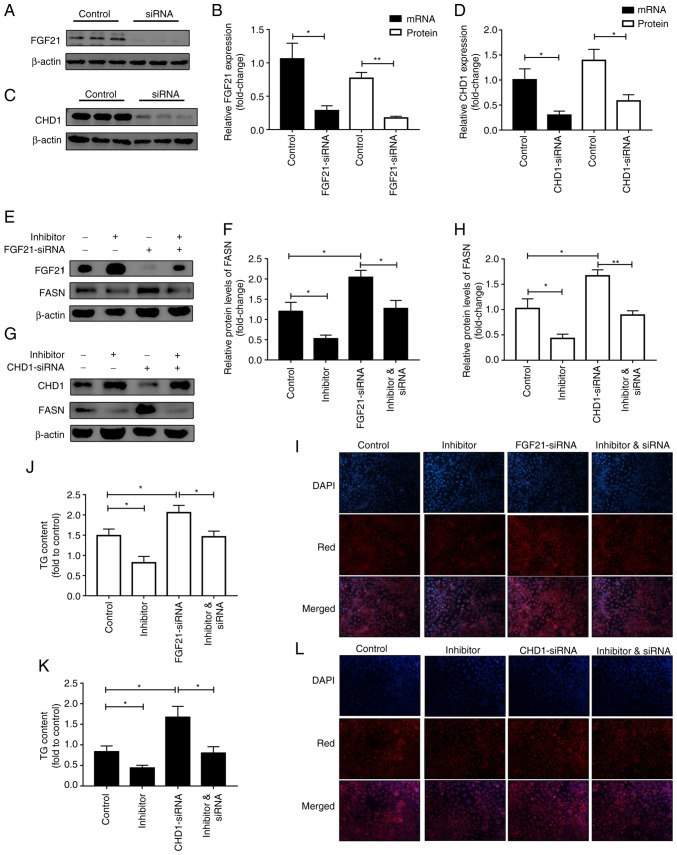
Subsequently, the target genes (CHD1 and FGF21) of miR-212-3p involved in lipid metabolism were selected using TargetScan (20-25), miRwalk (26) and experimental validation (10,27), combined with functional enrichment analysis (Fig. 1D), in which FGF21 was one of the molecules previously identified and reported by the authors (10). On this basis, miR-212-3p mimics/inhibitor were separately transfected for further validation (Fig. 1E). The overexpression of miR-212-3p suppressed the protein levels, but not the mRNA levels of CHD1 and FGF21. Conversely, the inhibition of miR-212-3p significantly upregulated the protein levels, but not the mRNA levels of CHD1 and FGF21 (Figs. 1F and S1). Furthermore, a rescue experiment was performed to further verify whether the lipogenic effect of miR-212-3p was realized by regulating FGF21/CHD1. FGF21/CHD1 was knocked down using siRNA (Fig. 2A-D). Subsequently, following co-transfection with miR-212-3p inhibitor and FGF21-siRNA, the decrease in FASN expression induced by transfection with miR-212-3p inhibitor alone was abolished by FGF21-siRNA (Fig. 2E and F). Moreover, Nile Red staining and TG content assay also revealed that the silencing of FGF21 significantly attenuated the suppressive effects of miR-212 inhibitor on lipogenesis (Fig. 2I and J). The same experiments were also performed for miR-212-3p and CHD1 (Fig. 2G, H, K and L). The data indicated that the effect of miR-212-3p on lipogenesis was achieved by negatively regulating FGF21/CHD1. FGF-21/CHD1 is a target gene of miR-212-3p in lipogenesis.
Figure 2.
miR-212-3p negatively regulates FGF21/CHD1 to promote lipogenesis. (A and B) FGF21 was knocked down using FGF21-siRNA. (C and D) CHD1 was knocked down using CHD1-siRNA. (E and F) The decrease in FASN expression caused by transfection with miR-212-3p inhibitor alone was blocked by FGF21-siRNA. (G and H) The decrease in FASN expression caused by transfection with miR-212-3p inhibitor alone was blocked by CHD1-siRNA. (I-L) The knockdown of FGF21/CHD1 abolished the inhibitory effects of miR-212-3p inhibitor on lipogenesis, as evidenced by Nile Red staining and TG content detection. The data are presented as the mean ± SEM. *P<0.05, **P<0.01. FGF21, fibroblast growth factor 21; CHD1, chromodomain helicase DNA binding protein 1; FASN, fatty acid synthase; TG, triglyceride.
Inhibition of miR-212-3p expression mimics exercise to improve lipid accumulation induced by a HFD
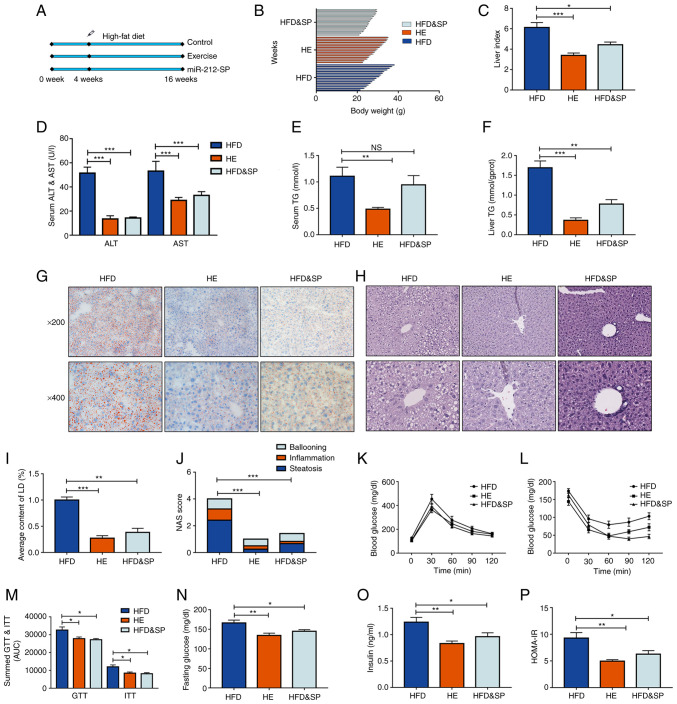
miR-212-3p was significantly elevated in MAFLD and could be decreased through exercise. On this basis, a HFD and HE model was constructed to explore the therapeutic potential of targeting miR-212-3p. The workflow is illustrated in Fig. 3A. Exercise therapy was used as a positive control. After 16 weeks on a HFD, the mice were obese. The increased body weight and liver index were reduced by inhibiting miR-212-3p as effectively as exercise therapy (Fig. 3B and C). The long-term feeding of the HFD led to an elevation in the levels of ALT and AST, which were reduced through the inhibition of miR-212-3p (Fig. 3D). The liver and serum TG contents both exhibited similar downward trends following the inhibition of miR-212-3p and exercise therapy (Fig. 3E and F). Intrahepatic fat accumulation was determined using H&E and Oil Red O staining; the inhibition of miR-212-3p exerted a similar ameliorative effect to that of exercise, as assessed using the NAS score and lipid droplet quantification (Fig. 3G-J). The GTT and ITT were performed to examine glucose metabolism homeostasis. Similar to exercise therapy, both the GTT and ITT revealed substantially improved insulin sensitivity and glucose tolerance in the HFD and SP group (Fig. 3K-M). The fasting blood glucose and insulin levels of the mice in each group were detected to construct a HOMA-IR. The results revealed that the inhibition of miR-212-3p ameliorated the impairment of insulin signaling in mice fed the HFD, similar to that of exercise therapy (Fig. 3N-P). These results indicated that the inhibition of miR-212-3p exerted ameliorative effects on MAFLD similar to those of exercise.
Figure 3.
Inhibition of miR-212-3p expression mimics the effects of exercise to improve lipid accumulation induced by a HFD. (A) The experimental protocol for the animals. (B-D) The body weight, liver index, and serum ALT and AST levels were increased in the HFD group, while they were decreased in the HFD and SP, and HE group. (E and F) The liver TG content and serum TG content exhibited a similar decrease following the inhibition of miR-212-3p compared with exercise treatment. (G-J) Hematoxylin and eosin and Oil Red O staining confirmed the ameliorative effect of miR-212-3p inhibition on lipid accumulation. (K-M) Similar to exercise treatment, the inhibition of miR-212-3p improved glucose homeostasis through GTT and ITT. (N-P) Similar to exercise therapy, the inhibition of miR-212-3p improved insulin resistance through fasting blood glucose, serum insulin and the calculated HOMA-IR. The data are presented as the mean ± SEM. *P<0.05, **P<0.01 and ***P<0.001. NS, not significant; HFD, high-fat diet; ALT, alanine transaminase; AST, aspartate transaminase; SP, serotype (mice that received the tail vein injection of recombinant adeno-associated virus serotype 8 gene vectors); TG, triglyceride; LD, lipid droplets; NAS, NAFLD activity score; GTT, glucose tolerance test; ITT, insulin tolerance test; HOMA-IR, homeostasis model assessment of insulin resistance; HE, high-fat diet and exercise.
miR-212-3p plays a key role in the effects of exercise on MAFLD
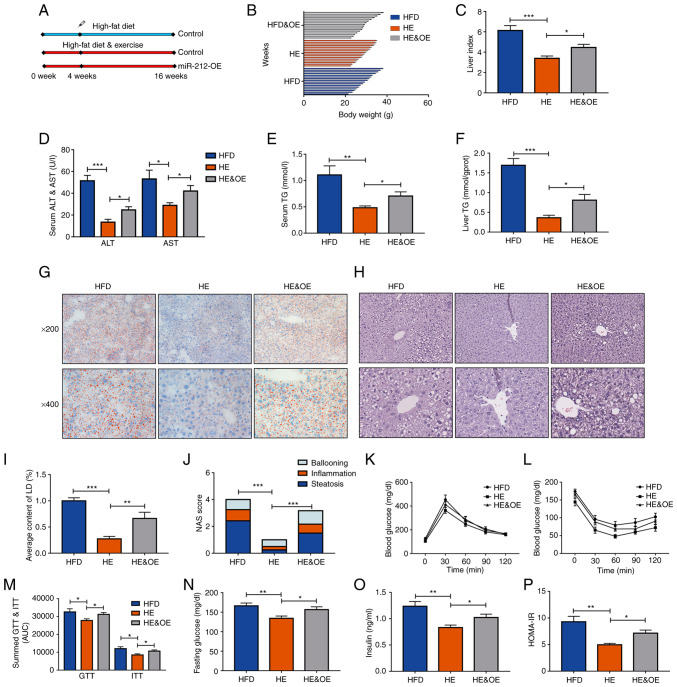
The aforementioned results suggested that the inhibition of miR-212-3p reduced lipid accumulation as effectively as exercise therapy. Of note, exercise decreased miR-212-3p expression in the HFD group. However, whether miR-212-3p plays a key role in the effects of exercise on MAFLD remained to be determined. To further explore this, hepatic miR-212-3p was overexpressed in the HE group through AAV to explore its function. The HFD group was used as a negative control to verify the effects of exercise. The workflow is presented in Fig. 4A. Body weight, liver index, serum ALT and AST all increased as a result of the high expression of miR-212-3p in the HE and OE group, compared with the HE group (Fig. 4B-D). With the overexpression of miR-212-3p, hepatic TG and serum TG levels also significantly increased even with the intervention of exercise (Fig. 4E and F). Pathological evidence collected through the consideration of the NAS score and lipid droplet quantification further indicated that the overexpression of miR-212-3p promoted hepatic fatty infiltration similar to that of the HFD group (Fig. 4G-J). The GTT and ITT assays indicated that the overexpression of miR-212-3p induced a dysregulation of glucose homeostasis compared to that of the HE group. The corresponding curve was significantly higher in the HE and OE group (Fig. 4K-M). Moreover, the HOMA-IR also revealed that the overexpression of miR-212-3p abolished the ameliorative effects of long-term regular exercise on insulin resistance (Fig. 4N-P). These results indicated that the overexpression of miR-212-3p abolished the therapeutic effects of exercise on MAFLD.
Figure 4.
The overexpression of miR-212-3p blocks the beneficial effects of exercise on MAFLD. (A) The experimental procedures on animals. (B-D) The body weight, liver index, and serum ALT and AST levels were decreased in the HE group, while they were increased in the HE and OE, and HFD group. (E and F) The liver and serum TG content exhibited similar increase following the overexpression of miR-212-3p compared with the HFD group. (G-J) Pathological evidence by NAS score and lipid droplet quantification suggested that the overexpression of miR-212-3p promoted hepatic fatty infiltration similar to the HFD group. (K-M) The high expressed miR-212-3p resulted in dysregulation of glucose homeostasis, as determined by GTT and ITT. (N-P) Overexpression of miR-212-3p abolished the ameliorative effects of long-term regular exercise in insulin resistance. The data are presented as the mean ± SEM. *P<0.05, **P<0.01 and ***P<0.001. HFD, high-fat diet; ALT, alanine transaminase; AST, aspartate transaminase; OE, overexpression; TG, triglyceride; LD, lipid droplets; NAS, NAFLD activity score; GTT, glucose tolerance test; ITT, insulin tolerance test; HOMA-IR, homeostasis model assessment of insulin resistance; HE, high-fat diet and exercise.
The function of miR-212-3p is associated with regulating hepatic FGF21
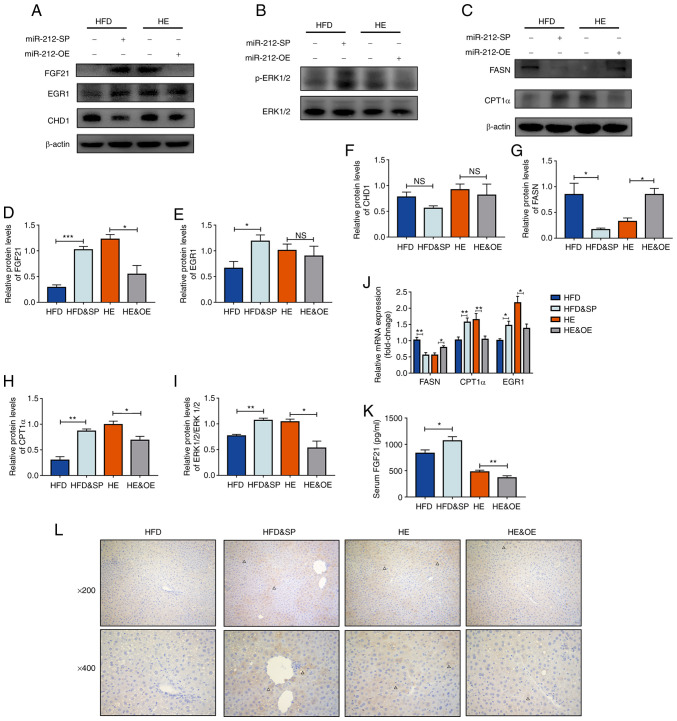
The inhibition of miR-212-3p exerted beneficial effects on MAFLD similar to those of exercise therapy. Conversely, the overexpression of miR-212 abolished the ameliorative effects of exercise on MAFLD. The present study then further explored the mechanisms of miR-212-3p in these processes. Consistent with the in vitro experiments, FGF21 was a target gene of miR-212-3p in vivo (Fig. 5A and D). However, CHD1 was also detected and it was not regulated by miR-212-3p in both the HFD and HE models (Fig. 5A and F). These results suggested that miR-212-3p played a role in the beneficial effects of exercise on MAFLD by regulating FGF21 rather than CHD1 in vivo. Subsequently, the activation state of the FGF21 pathway was detected to further verify the association between miR-212-3p and FGF21. Compared with the HFD group, the expression of p-ERK1/2 and EGR1 significantly increased following the inhibition of miR-212-3p (Fig. 5A, B, E, I and J). Conversely, the expression of p-ERK1/2 markedly decreased following the overexpression of miR-212-3p (Fig. 5A and B). In addition, the overexpression of miR-212-3p enhanced lipogenic activity, as shown by the upregulation of FASN mRNA and protein levels (Fig. 5C, G and J). The inhibition of miR-212-3p enhanced lipolytic activity, as shown by the increase in CPT1α mRNA and protein levels (Fig. 5C, H and J). Finally, ELISA, immunohistochemistry and luciferase reporter assay were used to re-validate the association between miR-212-3p and FGF21. The results confirmed that the functions of miR-212-3p were associated with the negative regulation of hepatic FGF21 (Figs. 5K and L; and S2).
Figure 5.
The function of miR-212-3p is associated with the regulation of hepatic FGF21. (A and B) miR-212-3p mimics the effects of exercise on MAFLD by regulating the FGF21 pathway, but not CHD1. (C) The inhibition of miR-212-3p suppresses lipogenesis, whereas the overexpression of miR-212-3p promotes lipogenesis. (D-I) Protein quantification of FGF21, EGR1, CHD1, p-ERK1/2, FASN and CPT1α. (J) Relative mRNA levels of FASN, CPT1α and EGR1. (K) Serum ELISA levels of FGF21. (L) Immunohistochemistry of FGF21. The positive areas are marked by triangles. The data are presented as the mean ± SEM. *P<0.05, **P<0.01 and ***P<0.001. NS, not significant; FGF21, fibroblast growth factor 21; CHD1, chromodomain helicase DNA binding protein 1; EGR1, early growth response 1; FASN, fatty acid synthase; CPT1α, carnitine palmitoyltransferase 1α; HFD, high-fat diet; HE, high-fat diet and exercise; SP, serotype (mice that received the tail vein injection of recombinant adeno-associated virus serotype 8 gene vectors).
Exercise improves lipid accumulation by inhibiting miR-212-3p via activating EGR1
The inhibition of miR-212-3p mimicked the therapeutic effects of exercise on MAFLD, while the overexpression of miR-212-3p abolished the beneficial effects of exercise on MAFLD. miR-212-3p appeared to play a key role in the beneficial effects of exercise on MAFLD. To confirm the link between exercise and miR-212-3p, a transcription factor motif analysis of the miR-212-3p promoter region was performed. Sequences of genes in the upstream region of the miR-212-encoding gene in human and mouse genomes were accessed through the online genome browser UCSC (28) (Fig. 6A). A total of 18 transcription factors that could bind to the sequences in this region were also predicted online using Jasper and regRNA 2.0 software (29,30). Four transcription factors were found to have simultaneous upstream region binding sites for miR-212 in both humans and mice (Table SIII). Subsequently, the MGI mammalian phenotype was used to analyze the functions and pathways of the aforementioned 18 transcription factors and found that EGR1 may be simultaneously involved in regulating body weight, abnormal injury response and liver inflammation (Fig. 6B). Of note, EGR1 is an exercise-related transcription factor; its expression can increase 11.4-fold following exercise (33). Notably, EGR1 was also found to be a downstream target gene of FGF21.
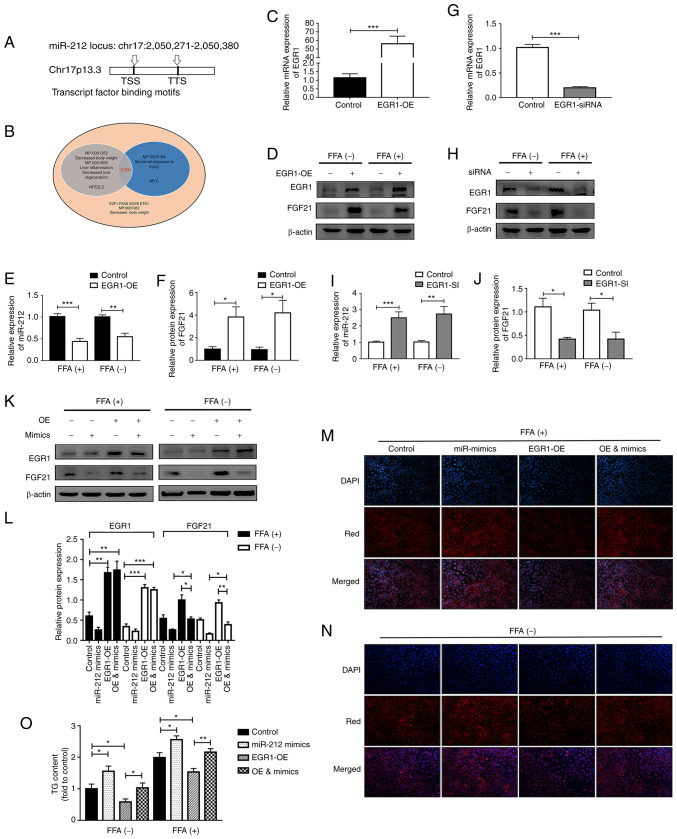
Figure 6.
Exercise improves lipid accumulation by inhibiting miR-212-3p via activating EGR1. (A) Schematic diagram of miR-212-3p promoter predictions. (B) Mammalian phenotype analysis. (C-F) miR-212-3p expression decreased and FGF21 expression increased upon EGR1 overexpression. (G-J) miR-212-3p expression increased and FGF21 expression decreased upon EGR1 overexpression. (K, L) The corresponding elevation of FGF21 by the overexpression of EGR1 was blocked at the protein level by co-transfection of miR-212-3p mimics. (M-O) The overexpression of miR-212-3p abolished the inhibitory effect of EGR1-OE in lipogenesis as evidenced using Nile Red staining and TG content detection. Data are from three independent experiments. The data are presented as the mean ± SEM. *P<0.05, **P<0.01 and ***P<0.001. EGR1, early growth response 1; FGF21, fibroblast growth factor 21; OE, overexpression; SI, siRNA; FFA, free fatty acid.
Gain and loss of function assays for EGR1 were performed separately. In the case of EGR1 overexpression, the expression of miR-212-3p was suppressed and that of FGF21 significantly increased, both with or without FFA treatment (Fig. 6C-F). Conversely, the knockdown of EGR1 significantly increased miR-212-3p expression, whereas it decreased FGF21 expression (Fig. 6G-J). Subsequently, rescue experiments were performed to clarify whether EGR1 regulates FGF21 via miR-212-3p. The corresponding increase in FGF21 expression when EGR1 was overexpressed was abolished by co-transfection with miR-212-3p mimics (Fig. 6K and L). As detected using Nile Red staining and by examining the TG content, EGR1 overexpression induced a notable decrease in the lipid content, while this effect was blocked through the high expression of miR-212-3p, regardless of FFA treatment (Fig. 6M-O).
Finally, luciferase reporter assays were conducted to further illustrate the direct mechanisms involved in the interaction between EGR1 and miR-212. 293T cells were transiently transfected with modified pGL3 vectors containing the 2 kb promoter segment of miR-212 (containing the -1,500 to +500 bp promoter sequence of miR-212) (Fig. S3A). Firefly luciferase expression was used to assess the activity of the transcriptional promoter. The pRL-TK vector expressing Renilla luciferase was co-transfected to control for transfection efficiency. To further analyze the regulatory effects of EGR1 on pri-miR-212 transcription, EGR1 protein levels were altered in 293T cells using siRNA and an overexpression plasmid. Compared to the control group, the overexpression of EGR1 decreased the luciferase activity of the miR-212 promoter by >50% (Fig. S3B). Conversely, the knockdown of EGR1 increased the transcriptional activity (Fig. S3C). It was thus concluded that the overexpression of miR-212-3p abolished the effects of exercise on MAFLD by exogenously relieving the transcriptional repression of EGR1.
Discussion
Exercise is known to be beneficial for MAFLD treatment (34). During the course of studying the effects of exercise in the treatment of MAFLD, a previous study by the authors demonstrated that miR-212-3p was a key factor in delivering the beneficial effects of exercise on MAFLD (10). An HFD promotes liver lipid accumulation, which causes elevated levels of miR-212-3p. Moreover, exercise improves hepatic lipid accumulation, which decreases miR-212-3p expression in patients with MAFLD. Despite the strong association between miR-212-3p, exercise and MAFLD, existing data on the gain of function, loss of function and rescue experiments lack clarity on whether miR-212-3p is critical for exercise to protect against MAFLD (10). Therefore, the present study focused on the role of miR-212-3p in the progression of MAFLD.
Mature miRNAs are derived from the shearing of precursors, whereby miR-212-3p and miR-212-5p are stably expressed. Studies have reported that a leucine-deficient diet can lead to an increase in the expression of miR-212-5p, which in turn decreases lipogenesis by targeting FASN and stearoyl-CoA desaturase 1 (16). Therefore, the present study first examined whether miR-212-5p also plays a role using a mouse model of HFD-induced MAFLD. In the HFD and exercise mouse model, miR-212-3p, but not miR-212-5p, was regulated by the high-fat environment and exercise intervention. However, whether the elevation of miR-212-3p expressions was responsible for the HFD-induced intrahepatic lipid accumulation in MAFLD also needed to be determined. Pathological and serological indicators revealed that the inhibition of miR-212-3p effectively inhibited intrahepatic lipid accumulation. Exercise inhibited the high expression of miR-212-3p in MAFLD, while the exogenous high expression of miR-212-3p effectively blocked the lipid-lowering effects of exercise. Based on the aforementioned results, it can be suggested that miR-212-3p is a key factor involved in the effectiveness of exercise. After confirming that miR-212-3p was critical for the protective effects of exercise against MAFLD, the present study further examined the mechanisms through which the effects of miR-212-3p were achieved. For this purpose, two potential targets were systematically screened out through target prediction, functional enrichment and literature curation. Furthermore, it was confirmed that miR-212-3p exerted its effects on lipogenesis through the regulation of CHD1 and FGF21 in vitro. However, under a more complex environment in vivo, only FGF21 was regulated by miR-212-3p. Therefore, these results suggested that the utility of miR-212-3p in lipogenesis was achieved through the regulation of FGF21 both in vitro and in vivo.
To elucidate the association between exercise and miR-212-3p, a transcription factor motif prediction analysis was performed along with mammalian phenotyping to gain further insight into the mechanisms (35). EGR1 is a member of the immediate early genes family and plays a critical role in regulating cell growth and proliferation (36). It has been shown that EGR1 is involved in the inhibitory effects of leptin on PPARγ, suggesting that EGR1 may inhibit lipid accumulation (37). Of note, EGR1 responds to exercise in the early stages, that is, exercise can directly and rapidly alter the expression level of EGR1 (33). An increase of as much as 11.4-fold in EGR1 expression can be observed following exercise. EGR1 expression is increased after an organism is stimulated with the recombinant protein FGF21, and EGR1 has been found to be the downstream target gene of the FGF21 signaling pathway (38,39). Taken together, these findings may explain why the overexpression of miR-212-3p in the exercise model did not cause an increase in EGR1 expression (Fig. 5A and E). The association between EGR1, miR-212-3p and FGF21 is crucial for delivering the beneficial effects of exercise on MAFLD. Through a luciferase reporter gene, gain of function, loss of function and rescue experiments, the present study successfully confirmed that EGR1 can increase FGF21 expression through the transcriptional inhibition of miR-212-3p. FGF21 is a myokine which can improve the metabolic regulation of systemic organs when produced by skeletal muscle in response to exercise (15). A previous study demonstrated that mice overexpressing FGF21 are were to resist HFD-induced obesity (40). Another study demonstrated that the injection of FGF21 recombinant protein was able to attenuate obesity-induced hyper-TG levels and liver damage (41). An increasing number of studies have suggested that FGF21 is a potent anti-MAFLD molecule (42,43). In the present study, in the exercise model, it was observed that the overexpression of miR-212-3p blocked the increase in the expression of FGF21 by EGR1, and significantly increased lipid accumulation. At the same time, the FGF21 pathway, which was activated through exercise, was also blocked by miR-212-3p. Furthermore, when miR-212-3p expression was inhibited, the regulation of FGF21 by EGR1 was restored. The data also indicated that the FGF21 pathway was once again activated and lipid accumulation was reduced.
Finally, the present study still has some limitations. It was observed that targeting miR-212-3p significantly improved lipid deposition and prevented NAFLD. However, a greater number of patients need to be treated when they already have NAFLD. In this case, it remains to be determined whether miR-212-3p would be equally effective. In addition, the regulatory association between EGR1, miR-212-3p and FGF21 was supported by in vitro experiments; however, whether the in vivo environment would still effective warrants to be further exploration.
In conclusion, the inhibition of miR-212-3p exerts effects on MAFLD similar to those of exercise. Gene therapy targeting miR-212-3p may thus mimic the ameliorative effects of exercise on MAFLD and may provide novel strategies for the comprehensive treatment of patients with MAFLD, particularly those who cannot adhere to or tolerate exercise programs.
Supplementary Data
Acknowledgments
Not applicable.
Funding Statement
The present study was supported by a grant from the National Natural Science Foundation of China (grant no. 81873567).
Availability of data and materials
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
BS and YZ were involved in data curation and analyses. MZ was involved in the study supervision and data validation. RL and WY were involved in the conceptualization of the study, in the formal analysis and in funding acquisition. BS and YZ confirm the authenticity of all the raw data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
For the use of patient samples, the present study was approved by the Ethics Committee of Tongji Hospital (approval no. K-KYSB-2020-139) and was performed in accordance with the Declaration of Helsinki. Written informed consent was obtained from the patients or their guardians. The animal experiments were approved by the Animal Ethics Committee of Tongji University (approval no. TJHBLAC-2019-024).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Simon TG, Roelstraete B, Khalili H, Hagström H, Ludvigsson JF. Mortality in biopsy-confirmed nonalcoholic fatty liver disease: Results from a nationwide cohort. Gut. 2021;70:1375–1382. doi: 10.1136/gutjnl-2020-322786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cotter TG, Rinella M. Nonalcoholic fatty liver disease 2020: The state of the disease. Gastroenterology. 2020;158:1851–11864. doi: 10.1053/j.gastro.2020.01.052. [DOI] [PubMed] [Google Scholar]
- 3.Younossi ZM. Patient-reported outcomes and the economic effects of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: The value proposition. Hepatology. 2018;68:2405–2412. doi: 10.1002/hep.30125. [DOI] [PubMed] [Google Scholar]
- 4.Rohilla S, Awasthi A, Kaur S, Puria R. Evolutionary conservation of long non-coding RNAs in non-alcoholic fatty liver disease. Life Sci. 2021;264:118560. doi: 10.1016/j.lfs.2020.118560. [DOI] [PubMed] [Google Scholar]
- 5.El-Agroudy NN, Kurzbach A, Rodionov RN, O'Sullivan J, Roden M, Birkenfeld AL, Pesta DH. Are lifestyle therapies effective for NAFLD treatment? Trends Endocrinol Metab. 2019;30:701–709. doi: 10.1016/j.tem.2019.07.013. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi H, Kotani K, Tanaka K, Egucih Y, Anzai K. Therapeutic approaches to nonalcoholic fatty liver disease: Exercise intervention and related mechanisms. Front Endocrinol (Lausanne) 2018;9:588. doi: 10.3389/fendo.2018.00588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keating SE, Hackett DA, Parker HM, O'Connor HT, Gerofi JA, Sainsbury A, Baker MK, Chuter VH, Caterson ID, George J, Johnson NA. Effect of aerobic exercise training dose on liver fat and visceral adiposity. J Hepatol. 2015;63:174–182. doi: 10.1016/j.jhep.2015.02.022. [DOI] [PubMed] [Google Scholar]
- 8.Glass O, Filozof C, Noureddin M, Berner-Hansen M, Schabel E, Omokaro SO, Schattenberg JM, Barradas K, Miller V, Francque S, et al. Standardisation of diet and exercise in clinical trials of NAFLD-NASH: Recommendations from the liver forum. J Hepatol. 2020;73:680–693. doi: 10.1016/j.jhep.2020.04.030. [DOI] [PubMed] [Google Scholar]
- 9.Fabian MR, Sonenberg N. The mechanics of miRNA-mediated gene silencing: A look under the hood of miRISC. Nat Struct Mol Biol. 2012;19:586–593. doi: 10.1038/nsmb.2296. [DOI] [PubMed] [Google Scholar]
- 10.Xiao J, Bei Y, Liu J, Dimitrova-Shumkovska J, Kuang D, Zhou Q, Li J, Yang Y, Xiang Y, Wang F, et al. MiR-212 downregulation contributes to the protective effect of exercise against non-alcoholic fatty liver via targeting FGF-21. J Cell Mol Med. 2016;20:204–216. doi: 10.1111/jcmm.12733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang X, Zeng J, Wang L, Fang M, Wang Q, Zhao M, Xu X, Liu Z, Li W, Liu S, et al. Histone demethylase retinoblastoma binding protein 2 is overexpressed in hepatocellular carcinoma and negatively regulated by hsa-miR-212. PLoS One. 2013;8:e69784. doi: 10.1371/journal.pone.0069784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumarswamy R, Volkmann I, Beermann J, Napp LC, Jabs O, Bhayadia R, Melk A, Ucar A, Chowdhury K, Lorenzen JM, et al. Vascular importance of the miR-212/132 cluster. Eur Heart J. 2014;35:3224–3231. doi: 10.1093/eurheartj/ehu344. [DOI] [PubMed] [Google Scholar]
- 13.Tang Y, Banan A, Forsyth CB, Fields JZ, Lau CK, Zhang LJ, Keshavarzian A. Effect of alcohol on miR-212 expression in intestinal epithelial cells and its potential role in alcoholic liver disease. Alcohol Clin Exp Res. 2008;32:355–364. doi: 10.1111/j.1530-0277.2007.00584.x. [DOI] [PubMed] [Google Scholar]
- 14.Mollet IG, Malm HA, Wendt A, Orho-Melander M, Eliasson L. Integrator of stress responses calmodulin binding transcription activator 1 (Camta1) regulates miR-212/miR-132 expression and insulin secretion. J Biol Chem. 2016;291:18440–18452. doi: 10.1074/jbc.M116.716860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ucar A, Gupta SK, Fiedler J, Erikci E, Kardasinski M, Batkai S, Dangwal S, Kumarswamy R, Bang C, Holzmann A, et al. The miRNA-212/132 family regulates both cardiac hypertrophy and cardiomyocyte autophagy. Nat Commun. 2012;3:1078. doi: 10.1038/ncomms2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo Y, Yu J, Wang C, Li K, Liu B, Du Y, Xiao F, Chen S, Guo F. miR-212-5p suppresses lipid accumulation by targeting FAS and SCD1. J Mol Endocrinol. 2017;59:205–217. doi: 10.1530/JME-16-0179. [DOI] [PubMed] [Google Scholar]
- 17.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 18.Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption-II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 19.Park SE, Lee SW, Hossain MA, Kim MY, Kim MN, Ahn EY, Park YC, Suh H, Kim GY, Choi YH, Kim ND. A chenodeoxycholic derivative, HS-1200, induces apoptosis and cell cycle modulation via Egr-1 gene expression control on human hepatoma cells. Cancer Lett. 2008;270:77–86. doi: 10.1016/j.canlet.2008.04.038. [DOI] [PubMed] [Google Scholar]
- 20.McGeary SE, Lin KS, Shi CY, Pham TM, Bisaria N, Kelley GM, Bartel DP. The biochemical basis of microRNA targeting efficacy. Science. 2019;366:eaav1741. doi: 10.1126/science.aav1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agarwal V, Bell GW, Nam JW, Bartel DP. Predicting effective microRNA target sites in mammalian mRNAs. ELife. 2015;4:e05005. doi: 10.7554/eLife.05005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia DM, Baek D, Shin C, Bell GW, Grimson A, Bartel DP. Weak seed-pairing stability and high target-site abundance decrease the proficiency of lsy-6 and other microRNAs. Nat Struct Mol Biol. 2011;18:1139–1146. doi: 10.1038/nsmb.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grimson A, Farh KK, Johnston WK, Garrett-Engele P, Lim LP, Bartel DP. MicroRNA targeting specificity in mammals: Determinants beyond seed pairing. Mol Cell. 2007;27:91–105. doi: 10.1016/j.molcel.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 26.Sticht C, De La Torre C, Parveen A, Gretz N. MiRWalk: An online resource for prediction of microRNA binding sites. PLoS One. 2018;13:e0206239. doi: 10.1371/journal.pone.0206239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Green CD, Huang Y, Dou X, Yang L, Liu Y, Han JDJ. Impact of dietary interventions on noncoding RNA networks and mRNAs encoding chromatin-related factors. Cell Rep. 2017;18:2957–2968. doi: 10.1016/j.celrep.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez JN, Zweig AS, Speir ML, Schmelter D, Rosenbloom KR, Raney BJ, Powell CC, Nassar LR, Maulding ND, Lee CM, et al. The UCSC genome browser database: 2021 update. Nucleic Acids Res. 2021;49:D1046–D1057. doi: 10.1093/nar/gkaa1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fornes O, Castro-Mondragon JA, Khan A, van der Lee R, Zhang X, Richmond PA, Modi BP, Correard S, Gheorghe M, Baranašić D, et al. JASPAR 2020: Update of the open-access database of transcription factor binding profiles. Nucleic Acids Res. 2020;48:D87–D92. doi: 10.1093/nar/gkz1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang TH, Huang HY, Hsu JB, Weng SL, Horng JT, Huang HD. An enhanced computational platform for investigating the roles of regulatory RNA and for identifying functional RNA motifs. BMC Bioinformatics. 2013;14(Suppl 2):S4. doi: 10.1186/1471-2105-14-S2-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith CL, Eppig JT. The mammalian phenotype ontology: Enabling robust annotation and comparative analysis. Wiley Interdiscip Rev Syst Biol Med. 2009;1:390–399. doi: 10.1002/wsbm.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou Y, Zhou B, Pache L, Chang M, Khodabakhshi AH, Tanaseichuk O, Benner C, Chanda SK. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun. 2019;10:1523. doi: 10.1038/s41467-019-09234-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McLean CS, Mielke C, Cordova JM, Langlais PR, Bowen B, Miranda D, Coletta DK, Mandarino LJ. Gene and microRNA expression responses to exercise; relationship with insulin sensitivity. PLoS One. 2015;10:e0127089. doi: 10.1371/journal.pone.0127089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Orci LA, Gariani K, Oldani G, Delaune V, Morel P, Toso C. Exercise-based interventions for nonalcoholic fatty liver disease: A meta-analysis and meta-regression. Clin Gastroenterol Hepatol. 2016;14:1398–1411. doi: 10.1016/j.cgh.2016.04.036. [DOI] [PubMed] [Google Scholar]
- 35.Hayamizu TF, Baldock RA, Ringwald M. Mouse anatomy ontologies: Enhancements and tools for exploring and integrating biomedical data. Mamm Genome. 2015;26:422–430. doi: 10.1007/s00335-015-9584-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han MS, Perry RJ, Camporez JP, Scherer PE, Shulman GI, Gao G, Davis RJ. A feed-forward regulatory loop in adipose tissue promotes signaling by the hepatokine FGF21. Genes Dev. 2021;35:133–146. doi: 10.1101/gad.344556.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou Y, Jia X, Zhou M, Liu J. Egr-1 is involved in the inhibitory effect of leptin on PPARgamma expression in hepatic stellate cell in vitro. Life Sci. 2009;84:544–551. doi: 10.1016/j.lfs.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 38.Li J, Xu C, Liu Y, Li Y, Du S, Zhang R, Sun Y, Zhang R, Wang Y, Xue H, et al. Fibroblast growth factor 21 inhibited ischemic arrhythmias via targeting miR-143/EGR1 axis. Basic Res Cardiol. 2020;115:9. doi: 10.1007/s00395-019-0768-4. [DOI] [PubMed] [Google Scholar]
- 39.Adams AC, Yang C, Coskun T, Cheng CC, Gimeno RE, Luo Y, Kharitonenkov A. The breadth of FGF21's metabolic actions are governed by FGFR1 in adipose tissue. Mol Metab. 2012;2:31–37. doi: 10.1016/j.molmet.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Samms RJ, Murphy M, Fowler MJ, Cooper S, Emmerson P, Coskun T, Adams AC, Kharitonenkov A, Ebling FJP, Tsintzas K. Dual effects of fibroblast growth factor 21 on hepatic energy metabolism. J Endocrinol. 2015;227:37–47. doi: 10.1530/JOE-15-0334. [DOI] [PubMed] [Google Scholar]
- 41.Wang WF, Li SM, Ren GP, Zheng W, Lu YJ, Yu YH, Xu WJ, Li TH, Zhou LH, Liu Y, Li DS. Recombinant murine fibroblast growth factor 21 ameliorates obesity-related inflammation in monosodium glutamate-induced obesity rats. Endocrine. 2015;49:119–129. doi: 10.1007/s12020-014-0433-5. [DOI] [PubMed] [Google Scholar]
- 42.Maratos-Flier E. Fatty liver and FGF21 physiology. Exp Cell Res. 2017;360:2–5. doi: 10.1016/j.yexcr.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 43.Byun S, Seok S, Kim YC, Zhang Y, Yau P, Iwamori N, Xu HE, Ma J, Kemper B, Kemper JK. Fasting-induced FGF21 signaling activates hepatic autophagy and lipid degradation via JMJD3 histone demethylase. Nat Commun. 2020;11:807. doi: 10.1038/s41467-020-14384-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.