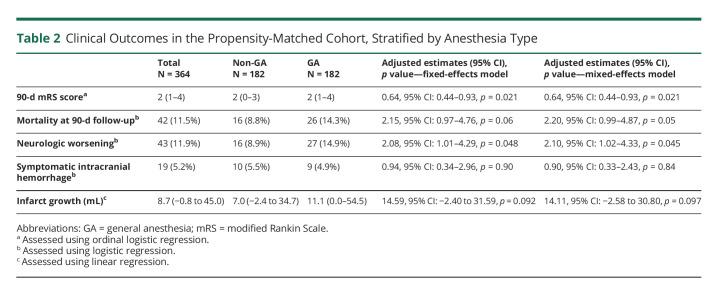
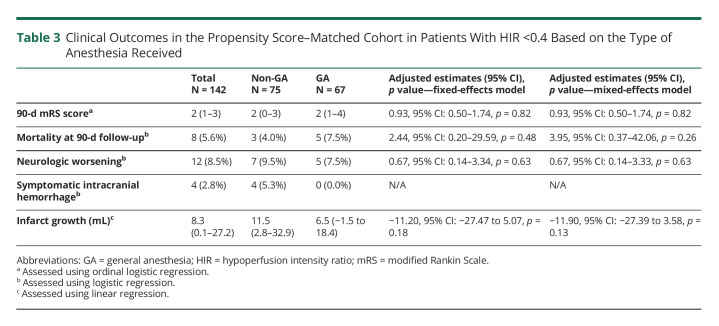
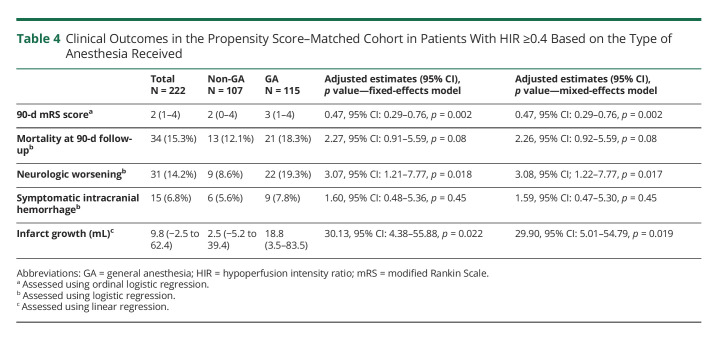
Amrou Sarraj
Amrou Sarraj, MD
1From the Case Western Reserve University (A.S.), Neurology; University Hospitals Cleveland Medical Center (A.S., D.P.), Neurology, OH; Stanford University (G.W.A., M.G.L.), Neurology, CA; The Royal Melbourne Hospital – University of Melbourne (P.J.M.), Radiology, Parkville, Victoria, Australia; University of Texas Rio Grande Valley - Valley Baptist Medical Center (A.E.H., W.G.T.), Harlingen; University of Kansas Medical Center (M.G.A., S.S., C.G.L., L.M., A.Q., L.R.), Neurology and Radiology; UTHealth McGovern Medical School (S.B., F.S.), Neurosurgery, Houston TX; The Royal Melbourne Hospitals (G.S., N.Y., L.C., G.A.D., S.M.D., B.C.V.C.), University of Melbourne, Neurology; The Walter and Eliza Hall Institute of Medical Research (N.Y.), Population Health and Immunity, Parkville, Victoria; Royal Adelaide Hospital (T.J.K.), Neurology, Adelaide, South Australia; Gold Coast University Hospital (D.G.S.), Neurology, Southport, Queensland, Australia; Christchurch Hospital (T.Y.W.), Neurology, Christchurch, Canterbury, New Zealand; Cleveland Clinic (M.S.H., G.T., M.A.A.), Cerebrovascular Unit, OH; University of Iowa Hospitals (S.O.G.), Neurosurgery; Baptist Health (A.N.A.), Lyerly Neurosurgery, Jacksonville, FL; Emory University (D.C.H., R.G.N.), Neurology, Atlanta, GA; Riverside Methodist Hospital (R.F.B., W.H., N.V.), Colombia, OH; Saint Louis University (R.C.E.), Neurology, MO; University of Tabuk (T.A.), Neurology, KSA; Baylor Scott & White Health (O.M.), Neurology, Dallas, TX; Greensboro | Cone Health (A.A.), Neurology, Greensboro, NC; Touro Infirmary and New Orleans East Hospital (S.M.-S.), Neurology, LA; UTHealth McGovern Medical School (C.W.S.), Diagnostic and Interventional Radiology, Houston, TX; WellStar Health System (R.G.), Neurology, Marietta, GA; and Memorial Hermann Hospital Texas Medical Center (J.C.G.), Neurology, Houston, TX.
1,✉,
Gregory W Albers
Gregory W Albers, MD
1From the Case Western Reserve University (A.S.), Neurology; University Hospitals Cleveland Medical Center (A.S., D.P.), Neurology, OH; Stanford University (G.W.A., M.G.L.), Neurology, CA; The Royal Melbourne Hospital – University of Melbourne (P.J.M.), Radiology, Parkville, Victoria, Australia; University of Texas Rio Grande Valley - Valley Baptist Medical Center (A.E.H., W.G.T.), Harlingen; University of Kansas Medical Center (M.G.A., S.S., C.G.L., L.M., A.Q., L.R.), Neurology and Radiology; UTHealth McGovern Medical School (S.B., F.S.), Neurosurgery, Houston TX; The Royal Melbourne Hospitals (G.S., N.Y., L.C., G.A.D., S.M.D., B.C.V.C.), University of Melbourne, Neurology; The Walter and Eliza Hall Institute of Medical Research (N.Y.), Population Health and Immunity, Parkville, Victoria; Royal Adelaide Hospital (T.J.K.), Neurology, Adelaide, South Australia; Gold Coast University Hospital (D.G.S.), Neurology, Southport, Queensland, Australia; Christchurch Hospital (T.Y.W.), Neurology, Christchurch, Canterbury, New Zealand; Cleveland Clinic (M.S.H., G.T., M.A.A.), Cerebrovascular Unit, OH; University of Iowa Hospitals (S.O.G.), Neurosurgery; Baptist Health (A.N.A.), Lyerly Neurosurgery, Jacksonville, FL; Emory University (D.C.H., R.G.N.), Neurology, Atlanta, GA; Riverside Methodist Hospital (R.F.B., W.H., N.V.), Colombia, OH; Saint Louis University (R.C.E.), Neurology, MO; University of Tabuk (T.A.), Neurology, KSA; Baylor Scott & White Health (O.M.), Neurology, Dallas, TX; Greensboro | Cone Health (A.A.), Neurology, Greensboro, NC; Touro Infirmary and New Orleans East Hospital (S.M.-S.), Neurology, LA; UTHealth McGovern Medical School (C.W.S.), Diagnostic and Interventional Radiology, Houston, TX; WellStar Health System (R.G.), Neurology, Marietta, GA; and Memorial Hermann Hospital Texas Medical Center (J.C.G.), Neurology, Houston, TX.
1,
Peter J Mitchell
Peter J Mitchell, MMed
1From the Case Western Reserve University (A.S.), Neurology; University Hospitals Cleveland Medical Center (A.S., D.P.), Neurology, OH; Stanford University (G.W.A., M.G.L.), Neurology, CA; The Royal Melbourne Hospital – University of Melbourne (P.J.M.), Radiology, Parkville, Victoria, Australia; University of Texas Rio Grande Valley - Valley Baptist Medical Center (A.E.H., W.G.T.), Harlingen; University of Kansas Medical Center (M.G.A., S.S., C.G.L., L.M., A.Q., L.R.), Neurology and Radiology; UTHealth McGovern Medical School (S.B., F.S.), Neurosurgery, Houston TX; The Royal Melbourne Hospitals (G.S., N.Y., L.C., G.A.D., S.M.D., B.C.V.C.), University of Melbourne, Neurology; The Walter and Eliza Hall Institute of Medical Research (N.Y.), Population Health and Immunity, Parkville, Victoria; Royal Adelaide Hospital (T.J.K.), Neurology, Adelaide, South Australia; Gold Coast University Hospital (D.G.S.), Neurology, Southport, Queensland, Australia; Christchurch Hospital (T.Y.W.), Neurology, Christchurch, Canterbury, New Zealand; Cleveland Clinic (M.S.H., G.T., M.A.A.), Cerebrovascular Unit, OH; University of Iowa Hospitals (S.O.G.), Neurosurgery; Baptist Health (A.N.A.), Lyerly Neurosurgery, Jacksonville, FL; Emory University (D.C.H., R.G.N.), Neurology, Atlanta, GA; Riverside Methodist Hospital (R.F.B., W.H., N.V.), Colombia, OH; Saint Louis University (R.C.E.), Neurology, MO; University of Tabuk (T.A.), Neurology, KSA; Baylor Scott & White Health (O.M.), Neurology, Dallas, TX; Greensboro | Cone Health (A.A.), Neurology, Greensboro, NC; Touro Infirmary and New Orleans East Hospital (S.M.-S.), Neurology, LA; UTHealth McGovern Medical School (C.W.S.), Diagnostic and Interventional Radiology, Houston, TX; WellStar Health System (R.G.), Neurology, Marietta, GA; and Memorial Hermann Hospital Texas Medical Center (J.C.G.), Neurology, Houston, TX.
1,
Ameer E Hassan
Ameer E Hassan, DO
1From the Case Western Reserve University (A.S.), Neurology; University Hospitals Cleveland Medical Center (A.S., D.P.), Neurology, OH; Stanford University (G.W.A., M.G.L.), Neurology, CA; The Royal Melbourne Hospital – University of Melbourne (P.J.M.), Radiology, Parkville, Victoria, Australia; University of Texas Rio Grande Valley - Valley Baptist Medical Center (A.E.H., W.G.T.), Harlingen; University of Kansas Medical Center (M.G.A., S.S., C.G.L., L.M., A.Q., L.R.), Neurology and Radiology; UTHealth McGovern Medical School (S.B., F.S.), Neurosurgery, Houston TX; The Royal Melbourne Hospitals (G.S., N.Y., L.C., G.A.D., S.M.D., B.C.V.C.), University of Melbourne, Neurology; The Walter and Eliza Hall Institute of Medical Research (N.Y.), Population Health and Immunity, Parkville, Victoria; Royal Adelaide Hospital (T.J.K.), Neurology, Adelaide, South Australia; Gold Coast University Hospital (D.G.S.), Neurology, Southport, Queensland, Australia; Christchurch Hospital (T.Y.W.), Neurology, Christchurch, Canterbury, New Zealand; Cleveland Clinic (M.S.H., G.T., M.A.A.), Cerebrovascular Unit, OH; University of Iowa Hospitals (S.O.G.), Neurosurgery; Baptist Health (A.N.A.), Lyerly Neurosurgery, Jacksonville, FL; Emory University (D.C.H., R.G.N.), Neurology, Atlanta, GA; Riverside Methodist Hospital (R.F.B., W.H., N.V.), Colombia, OH; Saint Louis University (R.C.E.), Neurology, MO; University of Tabuk (T.A.), Neurology, KSA; Baylor Scott & White Health (O.M.), Neurology, Dallas, TX; Greensboro | Cone Health (A.A.), Neurology, Greensboro, NC; Touro Infirmary and New Orleans East Hospital (S.M.-S.), Neurology, LA; UTHealth McGovern Medical School (C.W.S.), Diagnostic and Interventional Radiology, Houston, TX; WellStar Health System (R.G.), Neurology, Marietta, GA; and Memorial Hermann Hospital Texas Medical Center (J.C.G.), Neurology, Houston, TX.
1,
Michael G Abraham
Michael G Abraham, MD
1From the Case Western Reserve University (A.S.), Neurology; University Hospitals Cleveland Medical Center (A.S., D.P.), Neurology, OH; Stanford University (G.W.A., M.G.L.), Neurology, CA; The Royal Melbourne Hospital – University of Melbourne (P.J.M.), Radiology, Parkville, Victoria, Australia; University of Texas Rio Grande Valley - Valley Baptist Medical Center (A.E.H., W.G.T.), Harlingen; University of Kansas Medical Center (M.G.A., S.S., C.G.L., L.M., A.Q., L.R.), Neurology and Radiology; UTHealth McGovern Medical School (S.B., F.S.), Neurosurgery, Houston TX; The Royal Melbourne Hospitals (G.S., N.Y., L.C., G.A.D., S.M.D., B.C.V.C.), University of Melbourne, Neurology; The Walter and Eliza Hall Institute of Medical Research (N.Y.), Population Health and Immunity, Parkville, Victoria; Royal Adelaide Hospital (T.J.K.), Neurology, Adelaide, South Australia; Gold Coast University Hospital (D.G.S.), Neurology, Southport, Queensland, Australia; Christchurch Hospital (T.Y.W.), Neurology, Christchurch, Canterbury, New Zealand; Cleveland Clinic (M.S.H., G.T., M.A.A.), Cerebrovascular Unit, OH; University of Iowa Hospitals (S.O.G.), Neurosurgery; Baptist Health (A.N.A.), Lyerly Neurosurgery, Jacksonville, FL; Emory University (D.C.H., R.G.N.), Neurology, Atlanta, GA; Riverside Methodist Hospital (R.F.B., W.H., N.V.), Colombia, OH; Saint Louis University (R.C.E.), Neurology, MO; University of Tabuk (T.A.), Neurology, KSA; Baylor Scott & White Health (O.M.), Neurology, Dallas, TX; Greensboro | Cone Health (A.A.), Neurology, Greensboro, NC; Touro Infirmary and New Orleans East Hospital (S.M.-S.), Neurology, LA; UTHealth McGovern Medical School (C.W.S.), Diagnostic and Interventional Radiology, Houston, TX; WellStar Health System (R.G.), Neurology, Marietta, GA; and Memorial Hermann Hospital Texas Medical Center (J.C.G.), Neurology, Houston, TX.
1,
Spiros Blackburn
Spiros Blackburn, MD
1From the Case Western Reserve University (A.S.), Neurology; University Hospitals Cleveland Medical Center (A.S., D.P.), Neurology, OH; Stanford University (G.W.A., M.G.L.), Neurology, CA; The Royal Melbourne Hospital – University of Melbourne (P.J.M.), Radiology, Parkville, Victoria, Australia; University of Texas Rio Grande Valley - Valley Baptist Medical Center (A.E.H., W.G.T.), Harlingen; University of Kansas Medical Center (M.G.A., S.S., C.G.L., L.M., A.Q., L.R.), Neurology and Radiology; UTHealth McGovern Medical School (S.B., F.S.), Neurosurgery, Houston TX; The Royal Melbourne Hospitals (G.S., N.Y., L.C., G.A.D., S.M.D., B.C.V.C.), University of Melbourne, Neurology; The Walter and Eliza Hall Institute of Medical Research (N.Y.), Population Health and Immunity, Parkville, Victoria; Royal Adelaide Hospital (T.J.K.), Neurology, Adelaide, South Australia; Gold Coast University Hospital (D.G.S.), Neurology, Southport, Queensland, Australia; Christchurch Hospital (T.Y.W.), Neurology, Christchurch, Canterbury, New Zealand; Cleveland Clinic (M.S.H., G.T., M.A.A.), Cerebrovascular Unit, OH; University of Iowa Hospitals (S.O.G.), Neurosurgery; Baptist Health (A.N.A.), Lyerly Neurosurgery, Jacksonville, FL; Emory University (D.C.H., R.G.N.), Neurology, Atlanta, GA; Riverside Methodist Hospital (R.F.B., W.H., N.V.), Colombia, OH; Saint Louis University (R.C.E.), Neurology, MO; University of Tabuk (T.A.), Neurology, KSA; Baylor Scott & White Health (O.M.), Neurology, Dallas, TX; Greensboro | Cone Health (A.A.), Neurology, Greensboro, NC; Touro Infirmary and New Orleans East Hospital (S.M.-S.), Neurology, LA; UTHealth McGovern Medical School (C.W.S.), Diagnostic and Interventional Radiology, Houston, TX; WellStar Health System (R.G.), Neurology, Marietta, GA; and Memorial Hermann Hospital Texas Medical Center (J.C.G.), Neurology, Houston, TX.
1,
Gagan Sharma
Gagan Sharma, MS
1From the Case Western Reserve University (A.S.), Neurology; University Hospitals Cleveland Medical Center (A.S., D.P.), Neurology, OH; Stanford University (G.W.A., M.G.L.), Neurology, CA; The Royal Melbourne Hospital – University of Melbourne (P.J.M.), Radiology, Parkville, Victoria, Australia; University of Texas Rio Grande Valley - Valley Baptist Medical Center (A.E.H., W.G.T.), Harlingen; University of Kansas Medical Center (M.G.A., S.S., C.G.L., L.M., A.Q., L.R.), Neurology and Radiology; UTHealth McGovern Medical School (S.B., F.S.), Neurosurgery, Houston TX; The Royal Melbourne Hospitals (G.S., N.Y., L.C., G.A.D., S.M.D., B.C.V.C.), University of Melbourne, Neurology; The Walter and Eliza Hall Institute of Medical Research (N.Y.), Population Health and Immunity, Parkville, Victoria; Royal Adelaide Hospital (T.J.K.), Neurology, Adelaide, South Australia; Gold Coast University Hospital (D.G.S.), Neurology, Southport, Queensland, Australia; Christchurch Hospital (T.Y.W.), Neurology, Christchurch, Canterbury, New Zealand; Cleveland Clinic (M.S.H., G.T., M.A.A.), Cerebrovascular Unit, OH; University of Iowa Hospitals (S.O.G.), Neurosurgery; Baptist Health (A.N.A.), Lyerly Neurosurgery, Jacksonville, FL; Emory University (D.C.H., R.G.N.), Neurology, Atlanta, GA; Riverside Methodist Hospital (R.F.B., W.H., N.V.), Colombia, OH; Saint Louis University (R.C.E.), Neurology, MO; University of Tabuk (T.A.), Neurology, KSA; Baylor Scott & White Health (O.M.), Neurology, Dallas, TX; Greensboro | Cone Health (A.A.), Neurology, Greensboro, NC; Touro Infirmary and New Orleans East Hospital (S.M.-S.), Neurology, LA; UTHealth McGovern Medical School (C.W.S.), Diagnostic and Interventional Radiology, Houston, TX; WellStar Health System (R.G.), Neurology, Marietta, GA; and Memorial Hermann Hospital Texas Medical Center (J.C.G.), Neurology, Houston, TX.
1,
Nawaf Yassi
Nawaf Yassi, PhD
1From the Case Western Reserve University (A.S.), Neurology; University Hospitals Cleveland Medical Center (A.S., D.P.), Neurology, OH; Stanford University (G.W.A., M.G.L.), Neurology, CA; The Royal Melbourne Hospital – University of Melbourne (P.J.M.), Radiology, Parkville, Victoria, Australia; University of Texas Rio Grande Valley - Valley Baptist Medical Center (A.E.H., W.G.T.), Harlingen; University of Kansas Medical Center (M.G.A., S.S., C.G.L., L.M., A.Q., L.R.), Neurology and Radiology; UTHealth McGovern Medical School (S.B., F.S.), Neurosurgery, Houston TX; The Royal Melbourne Hospitals (G.S., N.Y., L.C., G.A.D., S.M.D., B.C.V.C.), University of Melbourne, Neurology; The Walter and Eliza Hall Institute of Medical Research (N.Y.), Population Health and Immunity, Parkville, Victoria; Royal Adelaide Hospital (T.J.K.), Neurology, Adelaide, South Australia; Gold Coast University Hospital (D.G.S.), Neurology, Southport, Queensland, Australia; Christchurch Hospital (T.Y.W.), Neurology, Christchurch, Canterbury, New Zealand; Cleveland Clinic (M.S.H., G.T., M.A.A.), Cerebrovascular Unit, OH; University of Iowa Hospitals (S.O.G.), Neurosurgery; Baptist Health (A.N.A.), Lyerly Neurosurgery, Jacksonville, FL; Emory University (D.C.H., R.G.N.), Neurology, Atlanta, GA; Riverside Methodist Hospital (R.F.B., W.H., N.V.), Colombia, OH; Saint Louis University (R.C.E.), Neurology, MO; University of Tabuk (T.A.), Neurology, KSA; Baylor Scott & White Health (O.M.), Neurology, Dallas, TX; Greensboro | Cone Health (A.A.), Neurology, Greensboro, NC; Touro Infirmary and New Orleans East Hospital (S.M.-S.), Neurology, LA; UTHealth McGovern Medical School (C.W.S.), Diagnostic and Interventional Radiology, Houston, TX; WellStar Health System (R.G.), Neurology, Marietta, GA; and Memorial Hermann Hospital Texas Medical Center (J.C.G.), Neurology, Houston, TX.
1,
Timothy J Kleinig
Timothy J Kleinig, PhD
1From the Case Western Reserve University (A.S.), Neurology; University Hospitals Cleveland Medical Center (A.S., D.P.), Neurology, OH; Stanford University (G.W.A., M.G.L.), Neurology, CA; The Royal Melbourne Hospital – University of Melbourne (P.J.M.), Radiology, Parkville, Victoria, Australia; University of Texas Rio Grande Valley - Valley Baptist Medical Center (A.E.H., W.G.T.), Harlingen; University of Kansas Medical Center (M.G.A., S.S., C.G.L., L.M., A.Q., L.R.), Neurology and Radiology; UTHealth McGovern Medical School (S.B., F.S.), Neurosurgery, Houston TX; The Royal Melbourne Hospitals (G.S., N.Y., L.C., G.A.D., S.M.D., B.C.V.C.), University of Melbourne, Neurology; The Walter and Eliza Hall Institute of Medical Research (N.Y.), Population Health and Immunity, Parkville, Victoria; Royal Adelaide Hospital (T.J.K.), Neurology, Adelaide, South Australia; Gold Coast University Hospital (D.G.S.), Neurology, Southport, Queensland, Australia; Christchurch Hospital (T.Y.W.), Neurology, Christchurch, Canterbury, New Zealand; Cleveland Clinic (M.S.H., G.T., M.A.A.), Cerebrovascular Unit, OH; University of Iowa Hospitals (S.O.G.), Neurosurgery; Baptist Health (A.N.A.), Lyerly Neurosurgery, Jacksonville, FL; Emory University (D.C.H., R.G.N.), Neurology, Atlanta, GA; Riverside Methodist Hospital (R.F.B., W.H., N.V.), Colombia, OH; Saint Louis University (R.C.E.), Neurology, MO; University of Tabuk (T.A.), Neurology, KSA; Baylor Scott & White Health (O.M.), Neurology, Dallas, TX; Greensboro | Cone Health (A.A.), Neurology, Greensboro, NC; Touro Infirmary and New Orleans East Hospital (S.M.-S.), Neurology, LA; UTHealth McGovern Medical School (C.W.S.), Diagnostic and Interventional Radiology, Houston, TX; WellStar Health System (R.G.), Neurology, Marietta, GA; and Memorial Hermann Hospital Texas Medical Center (J.C.G.), Neurology, Houston, TX.
1,
Darshan G Shah
Darshan G Shah, MBBS
1From the Case Western Reserve University (A.S.), Neurology; University Hospitals Cleveland Medical Center (A.S., D.P.), Neurology, OH; Stanford University (G.W.A., M.G.L.), Neurology, CA; The Royal Melbourne Hospital – University of Melbourne (P.J.M.), Radiology, Parkville, Victoria, Australia; University of Texas Rio Grande Valley - Valley Baptist Medical Center (A.E.H., W.G.T.), Harlingen; University of Kansas Medical Center (M.G.A., S.S., C.G.L., L.M., A.Q., L.R.), Neurology and Radiology; UTHealth McGovern Medical School (S.B., F.S.), Neurosurgery, Houston TX; The Royal Melbourne Hospitals (G.S., N.Y., L.C., G.A.D., S.M.D., B.C.V.C.), University of Melbourne, Neurology; The Walter and Eliza Hall Institute of Medical Research (N.Y.), Population Health and Immunity, Parkville, Victoria; Royal Adelaide Hospital (T.J.K.), Neurology, Adelaide, South Australia; Gold Coast University Hospital (D.G.S.), Neurology, Southport, Queensland, Australia; Christchurch Hospital (T.Y.W.), Neurology, Christchurch, Canterbury, New Zealand; Cleveland Clinic (M.S.H., G.T., M.A.A.), Cerebrovascular Unit, OH; University of Iowa Hospitals (S.O.G.), Neurosurgery; Baptist Health (A.N.A.), Lyerly Neurosurgery, Jacksonville, FL; Emory University (D.C.H., R.G.N.), Neurology, Atlanta, GA; Riverside Methodist Hospital (R.F.B., W.H., N.V.), Colombia, OH; Saint Louis University (R.C.E.), Neurology, MO; University of Tabuk (T.A.), Neurology, KSA; Baylor Scott & White Health (O.M.), Neurology, Dallas, TX; Greensboro | Cone Health (A.A.), Neurology, Greensboro, NC; Touro Infirmary and New Orleans East Hospital (S.M.-S.), Neurology, LA; UTHealth McGovern Medical School (C.W.S.), Diagnostic and Interventional Radiology, Houston, TX; WellStar Health System (R.G.), Neurology, Marietta, GA; and Memorial Hermann Hospital Texas Medical Center (J.C.G.), Neurology, Houston, TX.
1,
Teddy Y Wu
Teddy Y Wu, PhD
1From the Case Western Reserve University (A.S.), Neurology; University Hospitals Cleveland Medical Center (A.S., D.P.), Neurology, OH; Stanford University (G.W.A., M.G.L.), Neurology, CA; The Royal Melbourne Hospital – University of Melbourne (P.J.M.), Radiology, Parkville, Victoria, Australia; University of Texas Rio Grande Valley - Valley Baptist Medical Center (A.E.H., W.G.T.), Harlingen; University of Kansas Medical Center (M.G.A., S.S., C.G.L., L.M., A.Q., L.R.), Neurology and Radiology; UTHealth McGovern Medical School (S.B., F.S.), Neurosurgery, Houston TX; The Royal Melbourne Hospitals (G.S., N.Y., L.C., G.A.D., S.M.D., B.C.V.C.), University of Melbourne, Neurology; The Walter and Eliza Hall Institute of Medical Research (N.Y.), Population Health and Immunity, Parkville, Victoria; Royal Adelaide Hospital (T.J.K.), Neurology, Adelaide, South Australia; Gold Coast University Hospital (D.G.S.), Neurology, Southport, Queensland, Australia; Christchurch Hospital (T.Y.W.), Neurology, Christchurch, Canterbury, New Zealand; Cleveland Clinic (M.S.H., G.T., M.A.A.), Cerebrovascular Unit, OH; University of Iowa Hospitals (S.O.G.), Neurosurgery; Baptist Health (A.N.A.), Lyerly Neurosurgery, Jacksonville, FL; Emory University (D.C.H., R.G.N.), Neurology, Atlanta, GA; Riverside Methodist Hospital (R.F.B., W.H., N.V.), Colombia, OH; Saint Louis University (R.C.E.), Neurology, MO; University of Tabuk (T.A.), Neurology, KSA; Baylor Scott & White Health (O.M.), Neurology, Dallas, TX; Greensboro | Cone Health (A.A.), Neurology, Greensboro, NC; Touro Infirmary and New Orleans East Hospital (S.M.-S.), Neurology, LA; UTHealth McGovern Medical School (C.W.S.), Diagnostic and Interventional Radiology, Houston, TX; WellStar Health System (R.G.), Neurology, Marietta, GA; and Memorial Hermann Hospital Texas Medical Center (J.C.G.), Neurology, Houston, TX.
1,
Muhammad Shazam Hussain
Muhammad Shazam Hussain, MD
1From the Case Western Reserve University (A.S.), Neurology; University Hospitals Cleveland Medical Center (A.S., D.P.), Neurology, OH; Stanford University (G.W.A., M.G.L.), Neurology, CA; The Royal Melbourne Hospital – University of Melbourne (P.J.M.), Radiology, Parkville, Victoria, Australia; University of Texas Rio Grande Valley - Valley Baptist Medical Center (A.E.H., W.G.T.), Harlingen; University of Kansas Medical Center (M.G.A., S.S., C.G.L., L.M., A.Q., L.R.), Neurology and Radiology; UTHealth McGovern Medical School (S.B., F.S.), Neurosurgery, Houston TX; The Royal Melbourne Hospitals (G.S., N.Y., L.C., G.A.D., S.M.D., B.C.V.C.), University of Melbourne, Neurology; The Walter and Eliza Hall Institute of Medical Research (N.Y.), Population Health and Immunity, Parkville, Victoria; Royal Adelaide Hospital (T.J.K.), Neurology, Adelaide, South Australia; Gold Coast University Hospital (D.G.S.), Neurology, Southport, Queensland, Australia; Christchurch Hospital (T.Y.W.), Neurology, Christchurch, Canterbury, New Zealand; Cleveland Clinic (M.S.H., G.T., M.A.A.), Cerebrovascular Unit, OH; University of Iowa Hospitals (S.O.G.), Neurosurgery; Baptist Health (A.N.A.), Lyerly Neurosurgery, Jacksonville, FL; Emory University (D.C.H., R.G.N.), Neurology, Atlanta, GA; Riverside Methodist Hospital (R.F.B., W.H., N.V.), Colombia, OH; Saint Louis University (R.C.E.), Neurology, MO; University of Tabuk (T.A.), Neurology, KSA; Baylor Scott & White Health (O.M.), Neurology, Dallas, TX; Greensboro | Cone Health (A.A.), Neurology, Greensboro, NC; Touro Infirmary and New Orleans East Hospital (S.M.-S.), Neurology, LA; UTHealth McGovern Medical School (C.W.S.), Diagnostic and Interventional Radiology, Houston, TX; WellStar Health System (R.G.), Neurology, Marietta, GA; and Memorial Hermann Hospital Texas Medical Center (J.C.G.), Neurology, Houston, TX.
1,
Wondwoseen G Tekle
Wondwoseen G Tekle, MD
1From the Case Western Reserve University (A.S.), Neurology; University Hospitals Cleveland Medical Center (A.S., D.P.), Neurology, OH; Stanford University (G.W.A., M.G.L.), Neurology, CA; The Royal Melbourne Hospital – University of Melbourne (P.J.M.), Radiology, Parkville, Victoria, Australia; University of Texas Rio Grande Valley - Valley Baptist Medical Center (A.E.H., W.G.T.), Harlingen; University of Kansas Medical Center (M.G.A., S.S., C.G.L., L.M., A.Q., L.R.), Neurology and Radiology; UTHealth McGovern Medical School (S.B., F.S.), Neurosurgery, Houston TX; The Royal Melbourne Hospitals (G.S., N.Y., L.C., G.A.D., S.M.D., B.C.V.C.), University of Melbourne, Neurology; The Walter and Eliza Hall Institute of Medical Research (N.Y.), Population Health and Immunity, Parkville, Victoria; Royal Adelaide Hospital (T.J.K.), Neurology, Adelaide, South Australia; Gold Coast University Hospital (D.G.S.), Neurology, Southport, Queensland, Australia; Christchurch Hospital (T.Y.W.), Neurology, Christchurch, Canterbury, New Zealand; Cleveland Clinic (M.S.H., G.T., M.A.A.), Cerebrovascular Unit, OH; University of Iowa Hospitals (S.O.G.), Neurosurgery; Baptist Health (A.N.A.), Lyerly Neurosurgery, Jacksonville, FL; Emory University (D.C.H., R.G.N.), Neurology, Atlanta, GA; Riverside Methodist Hospital (R.F.B., W.H., N.V.), Colombia, OH; Saint Louis University (R.C.E.), Neurology, MO; University of Tabuk (T.A.), Neurology, KSA; Baylor Scott & White Health (O.M.), Neurology, Dallas, TX; Greensboro | Cone Health (A.A.), Neurology, Greensboro, NC; Touro Infirmary and New Orleans East Hospital (S.M.-S.), Neurology, LA; UTHealth McGovern Medical School (C.W.S.), Diagnostic and Interventional Radiology, Houston, TX; WellStar Health System (R.G.), Neurology, Marietta, GA; and Memorial Hermann Hospital Texas Medical Center (J.C.G.), Neurology, Houston, TX.
1,
Santiago Ortega Gutierrez
Santiago Ortega Gutierrez, MD, MS
1From the Case Western Reserve University (A.S.), Neurology; University Hospitals Cleveland Medical Center (A.S., D.P.), Neurology, OH; Stanford University (G.W.A., M.G.L.), Neurology, CA; The Royal Melbourne Hospital – University of Melbourne (P.J.M.), Radiology, Parkville, Victoria, Australia; University of Texas Rio Grande Valley - Valley Baptist Medical Center (A.E.H., W.G.T.), Harlingen; University of Kansas Medical Center (M.G.A., S.S., C.G.L., L.M., A.Q., L.R.), Neurology and Radiology; UTHealth McGovern Medical School (S.B., F.S.), Neurosurgery, Houston TX; The Royal Melbourne Hospitals (G.S., N.Y., L.C., G.A.D., S.M.D., B.C.V.C.), University of Melbourne, Neurology; The Walter and Eliza Hall Institute of Medical Research (N.Y.), Population Health and Immunity, Parkville, Victoria; Royal Adelaide Hospital (T.J.K.), Neurology, Adelaide, South Australia; Gold Coast University Hospital (D.G.S.), Neurology, Southport, Queensland, Australia; Christchurch Hospital (T.Y.W.), Neurology, Christchurch, Canterbury, New Zealand; Cleveland Clinic (M.S.H., G.T., M.A.A.), Cerebrovascular Unit, OH; University of Iowa Hospitals (S.O.G.), Neurosurgery; Baptist Health (A.N.A.), Lyerly Neurosurgery, Jacksonville, FL; Emory University (D.C.H., R.G.N.), Neurology, Atlanta, GA; Riverside Methodist Hospital (R.F.B., W.H., N.V.), Colombia, OH; Saint Louis University (R.C.E.), Neurology, MO; University of Tabuk (T.A.), Neurology, KSA; Baylor Scott & White Health (O.M.), Neurology, Dallas, TX; Greensboro | Cone Health (A.A.), Neurology, Greensboro, NC; Touro Infirmary and New Orleans East Hospital (S.M.-S.), Neurology, LA; UTHealth McGovern Medical School (C.W.S.), Diagnostic and Interventional Radiology, Houston, TX; WellStar Health System (R.G.), Neurology, Marietta, GA; and Memorial Hermann Hospital Texas Medical Center (J.C.G.), Neurology, Houston, TX.
1,
Amin Nima Aghaebrahim
Amin Nima Aghaebrahim, MD
1From the Case Western Reserve University (A.S.), Neurology; University Hospitals Cleveland Medical Center (A.S., D.P.), Neurology, OH; Stanford University (G.W.A., M.G.L.), Neurology, CA; The Royal Melbourne Hospital – University of Melbourne (P.J.M.), Radiology, Parkville, Victoria, Australia; University of Texas Rio Grande Valley - Valley Baptist Medical Center (A.E.H., W.G.T.), Harlingen; University of Kansas Medical Center (M.G.A., S.S., C.G.L., L.M., A.Q., L.R.), Neurology and Radiology; UTHealth McGovern Medical School (S.B., F.S.), Neurosurgery, Houston TX; The Royal Melbourne Hospitals (G.S., N.Y., L.C., G.A.D., S.M.D., B.C.V.C.), University of Melbourne, Neurology; The Walter and Eliza Hall Institute of Medical Research (N.Y.), Population Health and Immunity, Parkville, Victoria; Royal Adelaide Hospital (T.J.K.), Neurology, Adelaide, South Australia; Gold Coast University Hospital (D.G.S.), Neurology, Southport, Queensland, Australia; Christchurch Hospital (T.Y.W.), Neurology, Christchurch, Canterbury, New Zealand; Cleveland Clinic (M.S.H., G.T., M.A.A.), Cerebrovascular Unit, OH; University of Iowa Hospitals (S.O.G.), Neurosurgery; Baptist Health (A.N.A.), Lyerly Neurosurgery, Jacksonville, FL; Emory University (D.C.H., R.G.N.), Neurology, Atlanta, GA; Riverside Methodist Hospital (R.F.B., W.H., N.V.), Colombia, OH; Saint Louis University (R.C.E.), Neurology, MO; University of Tabuk (T.A.), Neurology, KSA; Baylor Scott & White Health (O.M.), Neurology, Dallas, TX; Greensboro | Cone Health (A.A.), Neurology, Greensboro, NC; Touro Infirmary and New Orleans East Hospital (S.M.-S.), Neurology, LA; UTHealth McGovern Medical School (C.W.S.), Diagnostic and Interventional Radiology, Houston, TX; WellStar Health System (R.G.), Neurology, Marietta, GA; and Memorial Hermann Hospital Texas Medical Center (J.C.G.), Neurology, Houston, TX.
1,
Diogo C Haussen
Diogo C Haussen, MD
1From the Case Western Reserve University (A.S.), Neurology; University Hospitals Cleveland Medical Center (A.S., D.P.), Neurology, OH; Stanford University (G.W.A., M.G.L.), Neurology, CA; The Royal Melbourne Hospital – University of Melbourne (P.J.M.), Radiology, Parkville, Victoria, Australia; University of Texas Rio Grande Valley - Valley Baptist Medical Center (A.E.H., W.G.T.), Harlingen; University of Kansas Medical Center (M.G.A., S.S., C.G.L., L.M., A.Q., L.R.), Neurology and Radiology; UTHealth McGovern Medical School (S.B., F.S.), Neurosurgery, Houston TX; The Royal Melbourne Hospitals (G.S., N.Y., L.C., G.A.D., S.M.D., B.C.V.C.), University of Melbourne, Neurology; The Walter and Eliza Hall Institute of Medical Research (N.Y.), Population Health and Immunity, Parkville, Victoria; Royal Adelaide Hospital (T.J.K.), Neurology, Adelaide, South Australia; Gold Coast University Hospital (D.G.S.), Neurology, Southport, Queensland, Australia; Christchurch Hospital (T.Y.W.), Neurology, Christchurch, Canterbury, New Zealand; Cleveland Clinic (M.S.H., G.T., M.A.A.), Cerebrovascular Unit, OH; University of Iowa Hospitals (S.O.G.), Neurosurgery; Baptist Health (A.N.A.), Lyerly Neurosurgery, Jacksonville, FL; Emory University (D.C.H., R.G.N.), Neurology, Atlanta, GA; Riverside Methodist Hospital (R.F.B., W.H., N.V.), Colombia, OH; Saint Louis University (R.C.E.), Neurology, MO; University of Tabuk (T.A.), Neurology, KSA; Baylor Scott & White Health (O.M.), Neurology, Dallas, TX; Greensboro | Cone Health (A.A.), Neurology, Greensboro, NC; Touro Infirmary and New Orleans East Hospital (S.M.-S.), Neurology, LA; UTHealth McGovern Medical School (C.W.S.), Diagnostic and Interventional Radiology, Houston, TX; WellStar Health System (R.G.), Neurology, Marietta, GA; and Memorial Hermann Hospital Texas Medical Center (J.C.G.), Neurology, Houston, TX.
1,
Gabor Toth
Gabor Toth, MD
1From the Case Western Reserve University (A.S.), Neurology; University Hospitals Cleveland Medical Center (A.S., D.P.), Neurology, OH; Stanford University (G.W.A., M.G.L.), Neurology, CA; The Royal Melbourne Hospital – University of Melbourne (P.J.M.), Radiology, Parkville, Victoria, Australia; University of Texas Rio Grande Valley - Valley Baptist Medical Center (A.E.H., W.G.T.), Harlingen; University of Kansas Medical Center (M.G.A., S.S., C.G.L., L.M., A.Q., L.R.), Neurology and Radiology; UTHealth McGovern Medical School (S.B., F.S.), Neurosurgery, Houston TX; The Royal Melbourne Hospitals (G.S., N.Y., L.C., G.A.D., S.M.D., B.C.V.C.), University of Melbourne, Neurology; The Walter and Eliza Hall Institute of Medical Research (N.Y.), Population Health and Immunity, Parkville, Victoria; Royal Adelaide Hospital (T.J.K.), Neurology, Adelaide, South Australia; Gold Coast University Hospital (D.G.S.), Neurology, Southport, Queensland, Australia; Christchurch Hospital (T.Y.W.), Neurology, Christchurch, Canterbury, New Zealand; Cleveland Clinic (M.S.H., G.T., M.A.A.), Cerebrovascular Unit, OH; University of Iowa Hospitals (S.O.G.), Neurosurgery; Baptist Health (A.N.A.), Lyerly Neurosurgery, Jacksonville, FL; Emory University (D.C.H., R.G.N.), Neurology, Atlanta, GA; Riverside Methodist Hospital (R.F.B., W.H., N.V.), Colombia, OH; Saint Louis University (R.C.E.), Neurology, MO; University of Tabuk (T.A.), Neurology, KSA; Baylor Scott & White Health (O.M.), Neurology, Dallas, TX; Greensboro | Cone Health (A.A.), Neurology, Greensboro, NC; Touro Infirmary and New Orleans East Hospital (S.M.-S.), Neurology, LA; UTHealth McGovern Medical School (C.W.S.), Diagnostic and Interventional Radiology, Houston, TX; WellStar Health System (R.G.), Neurology, Marietta, GA; and Memorial Hermann Hospital Texas Medical Center (J.C.G.), Neurology, Houston, TX.
1,
Deep Pujara
Deep Pujara, MBBS, MPH, MS
1From the Case Western Reserve University (A.S.), Neurology; University Hospitals Cleveland Medical Center (A.S., D.P.), Neurology, OH; Stanford University (G.W.A., M.G.L.), Neurology, CA; The Royal Melbourne Hospital – University of Melbourne (P.J.M.), Radiology, Parkville, Victoria, Australia; University of Texas Rio Grande Valley - Valley Baptist Medical Center (A.E.H., W.G.T.), Harlingen; University of Kansas Medical Center (M.G.A., S.S., C.G.L., L.M., A.Q., L.R.), Neurology and Radiology; UTHealth McGovern Medical School (S.B., F.S.), Neurosurgery, Houston TX; The Royal Melbourne Hospitals (G.S., N.Y., L.C., G.A.D., S.M.D., B.C.V.C.), University of Melbourne, Neurology; The Walter and Eliza Hall Institute of Medical Research (N.Y.), Population Health and Immunity, Parkville, Victoria; Royal Adelaide Hospital (T.J.K.), Neurology, Adelaide, South Australia; Gold Coast University Hospital (D.G.S.), Neurology, Southport, Queensland, Australia; Christchurch Hospital (T.Y.W.), Neurology, Christchurch, Canterbury, New Zealand; Cleveland Clinic (M.S.H., G.T., M.A.A.), Cerebrovascular Unit, OH; University of Iowa Hospitals (S.O.G.), Neurosurgery; Baptist Health (A.N.A.), Lyerly Neurosurgery, Jacksonville, FL; Emory University (D.C.H., R.G.N.), Neurology, Atlanta, GA; Riverside Methodist Hospital (R.F.B., W.H., N.V.), Colombia, OH; Saint Louis University (R.C.E.), Neurology, MO; University of Tabuk (T.A.), Neurology, KSA; Baylor Scott & White Health (O.M.), Neurology, Dallas, TX; Greensboro | Cone Health (A.A.), Neurology, Greensboro, NC; Touro Infirmary and New Orleans East Hospital (S.M.-S.), Neurology, LA; UTHealth McGovern Medical School (C.W.S.), Diagnostic and Interventional Radiology, Houston, TX; WellStar Health System (R.G.), Neurology, Marietta, GA; and Memorial Hermann Hospital Texas Medical Center (J.C.G.), Neurology, Houston, TX.
1,
Ronald F Budzik
Ronald F Budzik, MD
1From the Case Western Reserve University (A.S.), Neurology; University Hospitals Cleveland Medical Center (A.S., D.P.), Neurology, OH; Stanford University (G.W.A., M.G.L.), Neurology, CA; The Royal Melbourne Hospital – University of Melbourne (P.J.M.), Radiology, Parkville, Victoria, Australia; University of Texas Rio Grande Valley - Valley Baptist Medical Center (A.E.H., W.G.T.), Harlingen; University of Kansas Medical Center (M.G.A., S.S., C.G.L., L.M., A.Q., L.R.), Neurology and Radiology; UTHealth McGovern Medical School (S.B., F.S.), Neurosurgery, Houston TX; The Royal Melbourne Hospitals (G.S., N.Y., L.C., G.A.D., S.M.D., B.C.V.C.), University of Melbourne, Neurology; The Walter and Eliza Hall Institute of Medical Research (N.Y.), Population Health and Immunity, Parkville, Victoria; Royal Adelaide Hospital (T.J.K.), Neurology, Adelaide, South Australia; Gold Coast University Hospital (D.G.S.), Neurology, Southport, Queensland, Australia; Christchurch Hospital (T.Y.W.), Neurology, Christchurch, Canterbury, New Zealand; Cleveland Clinic (M.S.H., G.T., M.A.A.), Cerebrovascular Unit, OH; University of Iowa Hospitals (S.O.G.), Neurosurgery; Baptist Health (A.N.A.), Lyerly Neurosurgery, Jacksonville, FL; Emory University (D.C.H., R.G.N.), Neurology, Atlanta, GA; Riverside Methodist Hospital (R.F.B., W.H., N.V.), Colombia, OH; Saint Louis University (R.C.E.), Neurology, MO; University of Tabuk (T.A.), Neurology, KSA; Baylor Scott & White Health (O.M.), Neurology, Dallas, TX; Greensboro | Cone Health (A.A.), Neurology, Greensboro, NC; Touro Infirmary and New Orleans East Hospital (S.M.-S.), Neurology, LA; UTHealth McGovern Medical School (C.W.S.), Diagnostic and Interventional Radiology, Houston, TX; WellStar Health System (R.G.), Neurology, Marietta, GA; and Memorial Hermann Hospital Texas Medical Center (J.C.G.), Neurology, Houston, TX.
1,
William Hicks
William Hicks, MD
1From the Case Western Reserve University (A.S.), Neurology; University Hospitals Cleveland Medical Center (A.S., D.P.), Neurology, OH; Stanford University (G.W.A., M.G.L.), Neurology, CA; The Royal Melbourne Hospital – University of Melbourne (P.J.M.), Radiology, Parkville, Victoria, Australia; University of Texas Rio Grande Valley - Valley Baptist Medical Center (A.E.H., W.G.T.), Harlingen; University of Kansas Medical Center (M.G.A., S.S., C.G.L., L.M., A.Q., L.R.), Neurology and Radiology; UTHealth McGovern Medical School (S.B., F.S.), Neurosurgery, Houston TX; The Royal Melbourne Hospitals (G.S., N.Y., L.C., G.A.D., S.M.D., B.C.V.C.), University of Melbourne, Neurology; The Walter and Eliza Hall Institute of Medical Research (N.Y.), Population Health and Immunity, Parkville, Victoria; Royal Adelaide Hospital (T.J.K.), Neurology, Adelaide, South Australia; Gold Coast University Hospital (D.G.S.), Neurology, Southport, Queensland, Australia; Christchurch Hospital (T.Y.W.), Neurology, Christchurch, Canterbury, New Zealand; Cleveland Clinic (M.S.H., G.T., M.A.A.), Cerebrovascular Unit, OH; University of Iowa Hospitals (S.O.G.), Neurosurgery; Baptist Health (A.N.A.), Lyerly Neurosurgery, Jacksonville, FL; Emory University (D.C.H., R.G.N.), Neurology, Atlanta, GA; Riverside Methodist Hospital (R.F.B., W.H., N.V.), Colombia, OH; Saint Louis University (R.C.E.), Neurology, MO; University of Tabuk (T.A.), Neurology, KSA; Baylor Scott & White Health (O.M.), Neurology, Dallas, TX; Greensboro | Cone Health (A.A.), Neurology, Greensboro, NC; Touro Infirmary and New Orleans East Hospital (S.M.-S.), Neurology, LA; UTHealth McGovern Medical School (C.W.S.), Diagnostic and Interventional Radiology, Houston, TX; WellStar Health System (R.G.), Neurology, Marietta, GA; and Memorial Hermann Hospital Texas Medical Center (J.C.G.), Neurology, Houston, TX.
1,
Nirav Vora
Nirav Vora, MD
1From the Case Western Reserve University (A.S.), Neurology; University Hospitals Cleveland Medical Center (A.S., D.P.), Neurology, OH; Stanford University (G.W.A., M.G.L.), Neurology, CA; The Royal Melbourne Hospital – University of Melbourne (P.J.M.), Radiology, Parkville, Victoria, Australia; University of Texas Rio Grande Valley - Valley Baptist Medical Center (A.E.H., W.G.T.), Harlingen; University of Kansas Medical Center (M.G.A., S.S., C.G.L., L.M., A.Q., L.R.), Neurology and Radiology; UTHealth McGovern Medical School (S.B., F.S.), Neurosurgery, Houston TX; The Royal Melbourne Hospitals (G.S., N.Y., L.C., G.A.D., S.M.D., B.C.V.C.), University of Melbourne, Neurology; The Walter and Eliza Hall Institute of Medical Research (N.Y.), Population Health and Immunity, Parkville, Victoria; Royal Adelaide Hospital (T.J.K.), Neurology, Adelaide, South Australia; Gold Coast University Hospital (D.G.S.), Neurology, Southport, Queensland, Australia; Christchurch Hospital (T.Y.W.), Neurology, Christchurch, Canterbury, New Zealand; Cleveland Clinic (M.S.H., G.T., M.A.A.), Cerebrovascular Unit, OH; University of Iowa Hospitals (S.O.G.), Neurosurgery; Baptist Health (A.N.A.), Lyerly Neurosurgery, Jacksonville, FL; Emory University (D.C.H., R.G.N.), Neurology, Atlanta, GA; Riverside Methodist Hospital (R.F.B., W.H., N.V.), Colombia, OH; Saint Louis University (R.C.E.), Neurology, MO; University of Tabuk (T.A.), Neurology, KSA; Baylor Scott & White Health (O.M.), Neurology, Dallas, TX; Greensboro | Cone Health (A.A.), Neurology, Greensboro, NC; Touro Infirmary and New Orleans East Hospital (S.M.-S.), Neurology, LA; UTHealth McGovern Medical School (C.W.S.), Diagnostic and Interventional Radiology, Houston, TX; WellStar Health System (R.G.), Neurology, Marietta, GA; and Memorial Hermann Hospital Texas Medical Center (J.C.G.), Neurology, Houston, TX.
1,
Randall C Edgell
Randall C Edgell, MD
1From the Case Western Reserve University (A.S.), Neurology; University Hospitals Cleveland Medical Center (A.S., D.P.), Neurology, OH; Stanford University (G.W.A., M.G.L.), Neurology, CA; The Royal Melbourne Hospital – University of Melbourne (P.J.M.), Radiology, Parkville, Victoria, Australia; University of Texas Rio Grande Valley - Valley Baptist Medical Center (A.E.H., W.G.T.), Harlingen; University of Kansas Medical Center (M.G.A., S.S., C.G.L., L.M., A.Q., L.R.), Neurology and Radiology; UTHealth McGovern Medical School (S.B., F.S.), Neurosurgery, Houston TX; The Royal Melbourne Hospitals (G.S., N.Y., L.C., G.A.D., S.M.D., B.C.V.C.), University of Melbourne, Neurology; The Walter and Eliza Hall Institute of Medical Research (N.Y.), Population Health and Immunity, Parkville, Victoria; Royal Adelaide Hospital (T.J.K.), Neurology, Adelaide, South Australia; Gold Coast University Hospital (D.G.S.), Neurology, Southport, Queensland, Australia; Christchurch Hospital (T.Y.W.), Neurology, Christchurch, Canterbury, New Zealand; Cleveland Clinic (M.S.H., G.T., M.A.A.), Cerebrovascular Unit, OH; University of Iowa Hospitals (S.O.G.), Neurosurgery; Baptist Health (A.N.A.), Lyerly Neurosurgery, Jacksonville, FL; Emory University (D.C.H., R.G.N.), Neurology, Atlanta, GA; Riverside Methodist Hospital (R.F.B., W.H., N.V.), Colombia, OH; Saint Louis University (R.C.E.), Neurology, MO; University of Tabuk (T.A.), Neurology, KSA; Baylor Scott & White Health (O.M.), Neurology, Dallas, TX; Greensboro | Cone Health (A.A.), Neurology, Greensboro, NC; Touro Infirmary and New Orleans East Hospital (S.M.-S.), Neurology, LA; UTHealth McGovern Medical School (C.W.S.), Diagnostic and Interventional Radiology, Houston, TX; WellStar Health System (R.G.), Neurology, Marietta, GA; and Memorial Hermann Hospital Texas Medical Center (J.C.G.), Neurology, Houston, TX.
1,
Sabreena Slavin
Sabreena Slavin, MD
1From the Case Western Reserve University (A.S.), Neurology; University Hospitals Cleveland Medical Center (A.S., D.P.), Neurology, OH; Stanford University (G.W.A., M.G.L.), Neurology, CA; The Royal Melbourne Hospital – University of Melbourne (P.J.M.), Radiology, Parkville, Victoria, Australia; University of Texas Rio Grande Valley - Valley Baptist Medical Center (A.E.H., W.G.T.), Harlingen; University of Kansas Medical Center (M.G.A., S.S., C.G.L., L.M., A.Q., L.R.), Neurology and Radiology; UTHealth McGovern Medical School (S.B., F.S.), Neurosurgery, Houston TX; The Royal Melbourne Hospitals (G.S., N.Y., L.C., G.A.D., S.M.D., B.C.V.C.), University of Melbourne, Neurology; The Walter and Eliza Hall Institute of Medical Research (N.Y.), Population Health and Immunity, Parkville, Victoria; Royal Adelaide Hospital (T.J.K.), Neurology, Adelaide, South Australia; Gold Coast University Hospital (D.G.S.), Neurology, Southport, Queensland, Australia; Christchurch Hospital (T.Y.W.), Neurology, Christchurch, Canterbury, New Zealand; Cleveland Clinic (M.S.H., G.T., M.A.A.), Cerebrovascular Unit, OH; University of Iowa Hospitals (S.O.G.), Neurosurgery; Baptist Health (A.N.A.), Lyerly Neurosurgery, Jacksonville, FL; Emory University (D.C.H., R.G.N.), Neurology, Atlanta, GA; Riverside Methodist Hospital (R.F.B., W.H., N.V.), Colombia, OH; Saint Louis University (R.C.E.), Neurology, MO; University of Tabuk (T.A.), Neurology, KSA; Baylor Scott & White Health (O.M.), Neurology, Dallas, TX; Greensboro | Cone Health (A.A.), Neurology, Greensboro, NC; Touro Infirmary and New Orleans East Hospital (S.M.-S.), Neurology, LA; UTHealth McGovern Medical School (C.W.S.), Diagnostic and Interventional Radiology, Houston, TX; WellStar Health System (R.G.), Neurology, Marietta, GA; and Memorial Hermann Hospital Texas Medical Center (J.C.G.), Neurology, Houston, TX.
1,
Colleen G Lechtenberg
Colleen G Lechtenberg, MD
1From the Case Western Reserve University (A.S.), Neurology; University Hospitals Cleveland Medical Center (A.S., D.P.), Neurology, OH; Stanford University (G.W.A., M.G.L.), Neurology, CA; The Royal Melbourne Hospital – University of Melbourne (P.J.M.), Radiology, Parkville, Victoria, Australia; University of Texas Rio Grande Valley - Valley Baptist Medical Center (A.E.H., W.G.T.), Harlingen; University of Kansas Medical Center (M.G.A., S.S., C.G.L., L.M., A.Q., L.R.), Neurology and Radiology; UTHealth McGovern Medical School (S.B., F.S.), Neurosurgery, Houston TX; The Royal Melbourne Hospitals (G.S., N.Y., L.C., G.A.D., S.M.D., B.C.V.C.), University of Melbourne, Neurology; The Walter and Eliza Hall Institute of Medical Research (N.Y.), Population Health and Immunity, Parkville, Victoria; Royal Adelaide Hospital (T.J.K.), Neurology, Adelaide, South Australia; Gold Coast University Hospital (D.G.S.), Neurology, Southport, Queensland, Australia; Christchurch Hospital (T.Y.W.), Neurology, Christchurch, Canterbury, New Zealand; Cleveland Clinic (M.S.H., G.T., M.A.A.), Cerebrovascular Unit, OH; University of Iowa Hospitals (S.O.G.), Neurosurgery; Baptist Health (A.N.A.), Lyerly Neurosurgery, Jacksonville, FL; Emory University (D.C.H., R.G.N.), Neurology, Atlanta, GA; Riverside Methodist Hospital (R.F.B., W.H., N.V.), Colombia, OH; Saint Louis University (R.C.E.), Neurology, MO; University of Tabuk (T.A.), Neurology, KSA; Baylor Scott & White Health (O.M.), Neurology, Dallas, TX; Greensboro | Cone Health (A.A.), Neurology, Greensboro, NC; Touro Infirmary and New Orleans East Hospital (S.M.-S.), Neurology, LA; UTHealth McGovern Medical School (C.W.S.), Diagnostic and Interventional Radiology, Houston, TX; WellStar Health System (R.G.), Neurology, Marietta, GA; and Memorial Hermann Hospital Texas Medical Center (J.C.G.), Neurology, Houston, TX.
1,
Laith Maali
Laith Maali, MD
1From the Case Western Reserve University (A.S.), Neurology; University Hospitals Cleveland Medical Center (A.S., D.P.), Neurology, OH; Stanford University (G.W.A., M.G.L.), Neurology, CA; The Royal Melbourne Hospital – University of Melbourne (P.J.M.), Radiology, Parkville, Victoria, Australia; University of Texas Rio Grande Valley - Valley Baptist Medical Center (A.E.H., W.G.T.), Harlingen; University of Kansas Medical Center (M.G.A., S.S., C.G.L., L.M., A.Q., L.R.), Neurology and Radiology; UTHealth McGovern Medical School (S.B., F.S.), Neurosurgery, Houston TX; The Royal Melbourne Hospitals (G.S., N.Y., L.C., G.A.D., S.M.D., B.C.V.C.), University of Melbourne, Neurology; The Walter and Eliza Hall Institute of Medical Research (N.Y.), Population Health and Immunity, Parkville, Victoria; Royal Adelaide Hospital (T.J.K.), Neurology, Adelaide, South Australia; Gold Coast University Hospital (D.G.S.), Neurology, Southport, Queensland, Australia; Christchurch Hospital (T.Y.W.), Neurology, Christchurch, Canterbury, New Zealand; Cleveland Clinic (M.S.H., G.T., M.A.A.), Cerebrovascular Unit, OH; University of Iowa Hospitals (S.O.G.), Neurosurgery; Baptist Health (A.N.A.), Lyerly Neurosurgery, Jacksonville, FL; Emory University (D.C.H., R.G.N.), Neurology, Atlanta, GA; Riverside Methodist Hospital (R.F.B., W.H., N.V.), Colombia, OH; Saint Louis University (R.C.E.), Neurology, MO; University of Tabuk (T.A.), Neurology, KSA; Baylor Scott & White Health (O.M.), Neurology, Dallas, TX; Greensboro | Cone Health (A.A.), Neurology, Greensboro, NC; Touro Infirmary and New Orleans East Hospital (S.M.-S.), Neurology, LA; UTHealth McGovern Medical School (C.W.S.), Diagnostic and Interventional Radiology, Houston, TX; WellStar Health System (R.G.), Neurology, Marietta, GA; and Memorial Hermann Hospital Texas Medical Center (J.C.G.), Neurology, Houston, TX.
1,
Abid Qureshi
Abid Qureshi, MD
1From the Case Western Reserve University (A.S.), Neurology; University Hospitals Cleveland Medical Center (A.S., D.P.), Neurology, OH; Stanford University (G.W.A., M.G.L.), Neurology, CA; The Royal Melbourne Hospital – University of Melbourne (P.J.M.), Radiology, Parkville, Victoria, Australia; University of Texas Rio Grande Valley - Valley Baptist Medical Center (A.E.H., W.G.T.), Harlingen; University of Kansas Medical Center (M.G.A., S.S., C.G.L., L.M., A.Q., L.R.), Neurology and Radiology; UTHealth McGovern Medical School (S.B., F.S.), Neurosurgery, Houston TX; The Royal Melbourne Hospitals (G.S., N.Y., L.C., G.A.D., S.M.D., B.C.V.C.), University of Melbourne, Neurology; The Walter and Eliza Hall Institute of Medical Research (N.Y.), Population Health and Immunity, Parkville, Victoria; Royal Adelaide Hospital (T.J.K.), Neurology, Adelaide, South Australia; Gold Coast University Hospital (D.G.S.), Neurology, Southport, Queensland, Australia; Christchurch Hospital (T.Y.W.), Neurology, Christchurch, Canterbury, New Zealand; Cleveland Clinic (M.S.H., G.T., M.A.A.), Cerebrovascular Unit, OH; University of Iowa Hospitals (S.O.G.), Neurosurgery; Baptist Health (A.N.A.), Lyerly Neurosurgery, Jacksonville, FL; Emory University (D.C.H., R.G.N.), Neurology, Atlanta, GA; Riverside Methodist Hospital (R.F.B., W.H., N.V.), Colombia, OH; Saint Louis University (R.C.E.), Neurology, MO; University of Tabuk (T.A.), Neurology, KSA; Baylor Scott & White Health (O.M.), Neurology, Dallas, TX; Greensboro | Cone Health (A.A.), Neurology, Greensboro, NC; Touro Infirmary and New Orleans East Hospital (S.M.-S.), Neurology, LA; UTHealth McGovern Medical School (C.W.S.), Diagnostic and Interventional Radiology, Houston, TX; WellStar Health System (R.G.), Neurology, Marietta, GA; and Memorial Hermann Hospital Texas Medical Center (J.C.G.), Neurology, Houston, TX.
1,
Lee Rosterman
Lee Rosterman, MD
1From the Case Western Reserve University (A.S.), Neurology; University Hospitals Cleveland Medical Center (A.S., D.P.), Neurology, OH; Stanford University (G.W.A., M.G.L.), Neurology, CA; The Royal Melbourne Hospital – University of Melbourne (P.J.M.), Radiology, Parkville, Victoria, Australia; University of Texas Rio Grande Valley - Valley Baptist Medical Center (A.E.H., W.G.T.), Harlingen; University of Kansas Medical Center (M.G.A., S.S., C.G.L., L.M., A.Q., L.R.), Neurology and Radiology; UTHealth McGovern Medical School (S.B., F.S.), Neurosurgery, Houston TX; The Royal Melbourne Hospitals (G.S., N.Y., L.C., G.A.D., S.M.D., B.C.V.C.), University of Melbourne, Neurology; The Walter and Eliza Hall Institute of Medical Research (N.Y.), Population Health and Immunity, Parkville, Victoria; Royal Adelaide Hospital (T.J.K.), Neurology, Adelaide, South Australia; Gold Coast University Hospital (D.G.S.), Neurology, Southport, Queensland, Australia; Christchurch Hospital (T.Y.W.), Neurology, Christchurch, Canterbury, New Zealand; Cleveland Clinic (M.S.H., G.T., M.A.A.), Cerebrovascular Unit, OH; University of Iowa Hospitals (S.O.G.), Neurosurgery; Baptist Health (A.N.A.), Lyerly Neurosurgery, Jacksonville, FL; Emory University (D.C.H., R.G.N.), Neurology, Atlanta, GA; Riverside Methodist Hospital (R.F.B., W.H., N.V.), Colombia, OH; Saint Louis University (R.C.E.), Neurology, MO; University of Tabuk (T.A.), Neurology, KSA; Baylor Scott & White Health (O.M.), Neurology, Dallas, TX; Greensboro | Cone Health (A.A.), Neurology, Greensboro, NC; Touro Infirmary and New Orleans East Hospital (S.M.-S.), Neurology, LA; UTHealth McGovern Medical School (C.W.S.), Diagnostic and Interventional Radiology, Houston, TX; WellStar Health System (R.G.), Neurology, Marietta, GA; and Memorial Hermann Hospital Texas Medical Center (J.C.G.), Neurology, Houston, TX.
1,
Mohammad Ammar Abdulrazzak
Mohammad Ammar Abdulrazzak, MD
1From the Case Western Reserve University (A.S.), Neurology; University Hospitals Cleveland Medical Center (A.S., D.P.), Neurology, OH; Stanford University (G.W.A., M.G.L.), Neurology, CA; The Royal Melbourne Hospital – University of Melbourne (P.J.M.), Radiology, Parkville, Victoria, Australia; University of Texas Rio Grande Valley - Valley Baptist Medical Center (A.E.H., W.G.T.), Harlingen; University of Kansas Medical Center (M.G.A., S.S., C.G.L., L.M., A.Q., L.R.), Neurology and Radiology; UTHealth McGovern Medical School (S.B., F.S.), Neurosurgery, Houston TX; The Royal Melbourne Hospitals (G.S., N.Y., L.C., G.A.D., S.M.D., B.C.V.C.), University of Melbourne, Neurology; The Walter and Eliza Hall Institute of Medical Research (N.Y.), Population Health and Immunity, Parkville, Victoria; Royal Adelaide Hospital (T.J.K.), Neurology, Adelaide, South Australia; Gold Coast University Hospital (D.G.S.), Neurology, Southport, Queensland, Australia; Christchurch Hospital (T.Y.W.), Neurology, Christchurch, Canterbury, New Zealand; Cleveland Clinic (M.S.H., G.T., M.A.A.), Cerebrovascular Unit, OH; University of Iowa Hospitals (S.O.G.), Neurosurgery; Baptist Health (A.N.A.), Lyerly Neurosurgery, Jacksonville, FL; Emory University (D.C.H., R.G.N.), Neurology, Atlanta, GA; Riverside Methodist Hospital (R.F.B., W.H., N.V.), Colombia, OH; Saint Louis University (R.C.E.), Neurology, MO; University of Tabuk (T.A.), Neurology, KSA; Baylor Scott & White Health (O.M.), Neurology, Dallas, TX; Greensboro | Cone Health (A.A.), Neurology, Greensboro, NC; Touro Infirmary and New Orleans East Hospital (S.M.-S.), Neurology, LA; UTHealth McGovern Medical School (C.W.S.), Diagnostic and Interventional Radiology, Houston, TX; WellStar Health System (R.G.), Neurology, Marietta, GA; and Memorial Hermann Hospital Texas Medical Center (J.C.G.), Neurology, Houston, TX.
1,
Tareq AlMaghrabi
Tareq AlMaghrabi, MD
1From the Case Western Reserve University (A.S.), Neurology; University Hospitals Cleveland Medical Center (A.S., D.P.), Neurology, OH; Stanford University (G.W.A., M.G.L.), Neurology, CA; The Royal Melbourne Hospital – University of Melbourne (P.J.M.), Radiology, Parkville, Victoria, Australia; University of Texas Rio Grande Valley - Valley Baptist Medical Center (A.E.H., W.G.T.), Harlingen; University of Kansas Medical Center (M.G.A., S.S., C.G.L., L.M., A.Q., L.R.), Neurology and Radiology; UTHealth McGovern Medical School (S.B., F.S.), Neurosurgery, Houston TX; The Royal Melbourne Hospitals (G.S., N.Y., L.C., G.A.D., S.M.D., B.C.V.C.), University of Melbourne, Neurology; The Walter and Eliza Hall Institute of Medical Research (N.Y.), Population Health and Immunity, Parkville, Victoria; Royal Adelaide Hospital (T.J.K.), Neurology, Adelaide, South Australia; Gold Coast University Hospital (D.G.S.), Neurology, Southport, Queensland, Australia; Christchurch Hospital (T.Y.W.), Neurology, Christchurch, Canterbury, New Zealand; Cleveland Clinic (M.S.H., G.T., M.A.A.), Cerebrovascular Unit, OH; University of Iowa Hospitals (S.O.G.), Neurosurgery; Baptist Health (A.N.A.), Lyerly Neurosurgery, Jacksonville, FL; Emory University (D.C.H., R.G.N.), Neurology, Atlanta, GA; Riverside Methodist Hospital (R.F.B., W.H., N.V.), Colombia, OH; Saint Louis University (R.C.E.), Neurology, MO; University of Tabuk (T.A.), Neurology, KSA; Baylor Scott & White Health (O.M.), Neurology, Dallas, TX; Greensboro | Cone Health (A.A.), Neurology, Greensboro, NC; Touro Infirmary and New Orleans East Hospital (S.M.-S.), Neurology, LA; UTHealth McGovern Medical School (C.W.S.), Diagnostic and Interventional Radiology, Houston, TX; WellStar Health System (R.G.), Neurology, Marietta, GA; and Memorial Hermann Hospital Texas Medical Center (J.C.G.), Neurology, Houston, TX.
1,
Faris Shaker
Faris Shaker, MBChB
1From the Case Western Reserve University (A.S.), Neurology; University Hospitals Cleveland Medical Center (A.S., D.P.), Neurology, OH; Stanford University (G.W.A., M.G.L.), Neurology, CA; The Royal Melbourne Hospital – University of Melbourne (P.J.M.), Radiology, Parkville, Victoria, Australia; University of Texas Rio Grande Valley - Valley Baptist Medical Center (A.E.H., W.G.T.), Harlingen; University of Kansas Medical Center (M.G.A., S.S., C.G.L., L.M., A.Q., L.R.), Neurology and Radiology; UTHealth McGovern Medical School (S.B., F.S.), Neurosurgery, Houston TX; The Royal Melbourne Hospitals (G.S., N.Y., L.C., G.A.D., S.M.D., B.C.V.C.), University of Melbourne, Neurology; The Walter and Eliza Hall Institute of Medical Research (N.Y.), Population Health and Immunity, Parkville, Victoria; Royal Adelaide Hospital (T.J.K.), Neurology, Adelaide, South Australia; Gold Coast University Hospital (D.G.S.), Neurology, Southport, Queensland, Australia; Christchurch Hospital (T.Y.W.), Neurology, Christchurch, Canterbury, New Zealand; Cleveland Clinic (M.S.H., G.T., M.A.A.), Cerebrovascular Unit, OH; University of Iowa Hospitals (S.O.G.), Neurosurgery; Baptist Health (A.N.A.), Lyerly Neurosurgery, Jacksonville, FL; Emory University (D.C.H., R.G.N.), Neurology, Atlanta, GA; Riverside Methodist Hospital (R.F.B., W.H., N.V.), Colombia, OH; Saint Louis University (R.C.E.), Neurology, MO; University of Tabuk (T.A.), Neurology, KSA; Baylor Scott & White Health (O.M.), Neurology, Dallas, TX; Greensboro | Cone Health (A.A.), Neurology, Greensboro, NC; Touro Infirmary and New Orleans East Hospital (S.M.-S.), Neurology, LA; UTHealth McGovern Medical School (C.W.S.), Diagnostic and Interventional Radiology, Houston, TX; WellStar Health System (R.G.), Neurology, Marietta, GA; and Memorial Hermann Hospital Texas Medical Center (J.C.G.), Neurology, Houston, TX.
1,
Osman Mir
Osman Mir, MD
1From the Case Western Reserve University (A.S.), Neurology; University Hospitals Cleveland Medical Center (A.S., D.P.), Neurology, OH; Stanford University (G.W.A., M.G.L.), Neurology, CA; The Royal Melbourne Hospital – University of Melbourne (P.J.M.), Radiology, Parkville, Victoria, Australia; University of Texas Rio Grande Valley - Valley Baptist Medical Center (A.E.H., W.G.T.), Harlingen; University of Kansas Medical Center (M.G.A., S.S., C.G.L., L.M., A.Q., L.R.), Neurology and Radiology; UTHealth McGovern Medical School (S.B., F.S.), Neurosurgery, Houston TX; The Royal Melbourne Hospitals (G.S., N.Y., L.C., G.A.D., S.M.D., B.C.V.C.), University of Melbourne, Neurology; The Walter and Eliza Hall Institute of Medical Research (N.Y.), Population Health and Immunity, Parkville, Victoria; Royal Adelaide Hospital (T.J.K.), Neurology, Adelaide, South Australia; Gold Coast University Hospital (D.G.S.), Neurology, Southport, Queensland, Australia; Christchurch Hospital (T.Y.W.), Neurology, Christchurch, Canterbury, New Zealand; Cleveland Clinic (M.S.H., G.T., M.A.A.), Cerebrovascular Unit, OH; University of Iowa Hospitals (S.O.G.), Neurosurgery; Baptist Health (A.N.A.), Lyerly Neurosurgery, Jacksonville, FL; Emory University (D.C.H., R.G.N.), Neurology, Atlanta, GA; Riverside Methodist Hospital (R.F.B., W.H., N.V.), Colombia, OH; Saint Louis University (R.C.E.), Neurology, MO; University of Tabuk (T.A.), Neurology, KSA; Baylor Scott & White Health (O.M.), Neurology, Dallas, TX; Greensboro | Cone Health (A.A.), Neurology, Greensboro, NC; Touro Infirmary and New Orleans East Hospital (S.M.-S.), Neurology, LA; UTHealth McGovern Medical School (C.W.S.), Diagnostic and Interventional Radiology, Houston, TX; WellStar Health System (R.G.), Neurology, Marietta, GA; and Memorial Hermann Hospital Texas Medical Center (J.C.G.), Neurology, Houston, TX.
1,
Ashish Arora
Ashish Arora, MD
1From the Case Western Reserve University (A.S.), Neurology; University Hospitals Cleveland Medical Center (A.S., D.P.), Neurology, OH; Stanford University (G.W.A., M.G.L.), Neurology, CA; The Royal Melbourne Hospital – University of Melbourne (P.J.M.), Radiology, Parkville, Victoria, Australia; University of Texas Rio Grande Valley - Valley Baptist Medical Center (A.E.H., W.G.T.), Harlingen; University of Kansas Medical Center (M.G.A., S.S., C.G.L., L.M., A.Q., L.R.), Neurology and Radiology; UTHealth McGovern Medical School (S.B., F.S.), Neurosurgery, Houston TX; The Royal Melbourne Hospitals (G.S., N.Y., L.C., G.A.D., S.M.D., B.C.V.C.), University of Melbourne, Neurology; The Walter and Eliza Hall Institute of Medical Research (N.Y.), Population Health and Immunity, Parkville, Victoria; Royal Adelaide Hospital (T.J.K.), Neurology, Adelaide, South Australia; Gold Coast University Hospital (D.G.S.), Neurology, Southport, Queensland, Australia; Christchurch Hospital (T.Y.W.), Neurology, Christchurch, Canterbury, New Zealand; Cleveland Clinic (M.S.H., G.T., M.A.A.), Cerebrovascular Unit, OH; University of Iowa Hospitals (S.O.G.), Neurosurgery; Baptist Health (A.N.A.), Lyerly Neurosurgery, Jacksonville, FL; Emory University (D.C.H., R.G.N.), Neurology, Atlanta, GA; Riverside Methodist Hospital (R.F.B., W.H., N.V.), Colombia, OH; Saint Louis University (R.C.E.), Neurology, MO; University of Tabuk (T.A.), Neurology, KSA; Baylor Scott & White Health (O.M.), Neurology, Dallas, TX; Greensboro | Cone Health (A.A.), Neurology, Greensboro, NC; Touro Infirmary and New Orleans East Hospital (S.M.-S.), Neurology, LA; UTHealth McGovern Medical School (C.W.S.), Diagnostic and Interventional Radiology, Houston, TX; WellStar Health System (R.G.), Neurology, Marietta, GA; and Memorial Hermann Hospital Texas Medical Center (J.C.G.), Neurology, Houston, TX.
1,
Sheryl Martin-Schild
Sheryl Martin-Schild, MD
1From the Case Western Reserve University (A.S.), Neurology; University Hospitals Cleveland Medical Center (A.S., D.P.), Neurology, OH; Stanford University (G.W.A., M.G.L.), Neurology, CA; The Royal Melbourne Hospital – University of Melbourne (P.J.M.), Radiology, Parkville, Victoria, Australia; University of Texas Rio Grande Valley - Valley Baptist Medical Center (A.E.H., W.G.T.), Harlingen; University of Kansas Medical Center (M.G.A., S.S., C.G.L., L.M., A.Q., L.R.), Neurology and Radiology; UTHealth McGovern Medical School (S.B., F.S.), Neurosurgery, Houston TX; The Royal Melbourne Hospitals (G.S., N.Y., L.C., G.A.D., S.M.D., B.C.V.C.), University of Melbourne, Neurology; The Walter and Eliza Hall Institute of Medical Research (N.Y.), Population Health and Immunity, Parkville, Victoria; Royal Adelaide Hospital (T.J.K.), Neurology, Adelaide, South Australia; Gold Coast University Hospital (D.G.S.), Neurology, Southport, Queensland, Australia; Christchurch Hospital (T.Y.W.), Neurology, Christchurch, Canterbury, New Zealand; Cleveland Clinic (M.S.H., G.T., M.A.A.), Cerebrovascular Unit, OH; University of Iowa Hospitals (S.O.G.), Neurosurgery; Baptist Health (A.N.A.), Lyerly Neurosurgery, Jacksonville, FL; Emory University (D.C.H., R.G.N.), Neurology, Atlanta, GA; Riverside Methodist Hospital (R.F.B., W.H., N.V.), Colombia, OH; Saint Louis University (R.C.E.), Neurology, MO; University of Tabuk (T.A.), Neurology, KSA; Baylor Scott & White Health (O.M.), Neurology, Dallas, TX; Greensboro | Cone Health (A.A.), Neurology, Greensboro, NC; Touro Infirmary and New Orleans East Hospital (S.M.-S.), Neurology, LA; UTHealth McGovern Medical School (C.W.S.), Diagnostic and Interventional Radiology, Houston, TX; WellStar Health System (R.G.), Neurology, Marietta, GA; and Memorial Hermann Hospital Texas Medical Center (J.C.G.), Neurology, Houston, TX.
1,
Clark W Sitton
Clark W Sitton, MD
1From the Case Western Reserve University (A.S.), Neurology; University Hospitals Cleveland Medical Center (A.S., D.P.), Neurology, OH; Stanford University (G.W.A., M.G.L.), Neurology, CA; The Royal Melbourne Hospital – University of Melbourne (P.J.M.), Radiology, Parkville, Victoria, Australia; University of Texas Rio Grande Valley - Valley Baptist Medical Center (A.E.H., W.G.T.), Harlingen; University of Kansas Medical Center (M.G.A., S.S., C.G.L., L.M., A.Q., L.R.), Neurology and Radiology; UTHealth McGovern Medical School (S.B., F.S.), Neurosurgery, Houston TX; The Royal Melbourne Hospitals (G.S., N.Y., L.C., G.A.D., S.M.D., B.C.V.C.), University of Melbourne, Neurology; The Walter and Eliza Hall Institute of Medical Research (N.Y.), Population Health and Immunity, Parkville, Victoria; Royal Adelaide Hospital (T.J.K.), Neurology, Adelaide, South Australia; Gold Coast University Hospital (D.G.S.), Neurology, Southport, Queensland, Australia; Christchurch Hospital (T.Y.W.), Neurology, Christchurch, Canterbury, New Zealand; Cleveland Clinic (M.S.H., G.T., M.A.A.), Cerebrovascular Unit, OH; University of Iowa Hospitals (S.O.G.), Neurosurgery; Baptist Health (A.N.A.), Lyerly Neurosurgery, Jacksonville, FL; Emory University (D.C.H., R.G.N.), Neurology, Atlanta, GA; Riverside Methodist Hospital (R.F.B., W.H., N.V.), Colombia, OH; Saint Louis University (R.C.E.), Neurology, MO; University of Tabuk (T.A.), Neurology, KSA; Baylor Scott & White Health (O.M.), Neurology, Dallas, TX; Greensboro | Cone Health (A.A.), Neurology, Greensboro, NC; Touro Infirmary and New Orleans East Hospital (S.M.-S.), Neurology, LA; UTHealth McGovern Medical School (C.W.S.), Diagnostic and Interventional Radiology, Houston, TX; WellStar Health System (R.G.), Neurology, Marietta, GA; and Memorial Hermann Hospital Texas Medical Center (J.C.G.), Neurology, Houston, TX.
1,
Leonid Churilov
Leonid Churilov, PhD
1From the Case Western Reserve University (A.S.), Neurology; University Hospitals Cleveland Medical Center (A.S., D.P.), Neurology, OH; Stanford University (G.W.A., M.G.L.), Neurology, CA; The Royal Melbourne Hospital – University of Melbourne (P.J.M.), Radiology, Parkville, Victoria, Australia; University of Texas Rio Grande Valley - Valley Baptist Medical Center (A.E.H., W.G.T.), Harlingen; University of Kansas Medical Center (M.G.A., S.S., C.G.L., L.M., A.Q., L.R.), Neurology and Radiology; UTHealth McGovern Medical School (S.B., F.S.), Neurosurgery, Houston TX; The Royal Melbourne Hospitals (G.S., N.Y., L.C., G.A.D., S.M.D., B.C.V.C.), University of Melbourne, Neurology; The Walter and Eliza Hall Institute of Medical Research (N.Y.), Population Health and Immunity, Parkville, Victoria; Royal Adelaide Hospital (T.J.K.), Neurology, Adelaide, South Australia; Gold Coast University Hospital (D.G.S.), Neurology, Southport, Queensland, Australia; Christchurch Hospital (T.Y.W.), Neurology, Christchurch, Canterbury, New Zealand; Cleveland Clinic (M.S.H., G.T., M.A.A.), Cerebrovascular Unit, OH; University of Iowa Hospitals (S.O.G.), Neurosurgery; Baptist Health (A.N.A.), Lyerly Neurosurgery, Jacksonville, FL; Emory University (D.C.H., R.G.N.), Neurology, Atlanta, GA; Riverside Methodist Hospital (R.F.B., W.H., N.V.), Colombia, OH; Saint Louis University (R.C.E.), Neurology, MO; University of Tabuk (T.A.), Neurology, KSA; Baylor Scott & White Health (O.M.), Neurology, Dallas, TX; Greensboro | Cone Health (A.A.), Neurology, Greensboro, NC; Touro Infirmary and New Orleans East Hospital (S.M.-S.), Neurology, LA; UTHealth McGovern Medical School (C.W.S.), Diagnostic and Interventional Radiology, Houston, TX; WellStar Health System (R.G.), Neurology, Marietta, GA; and Memorial Hermann Hospital Texas Medical Center (J.C.G.), Neurology, Houston, TX.
1,
Rishi Gupta
Rishi Gupta, MD
1From the Case Western Reserve University (A.S.), Neurology; University Hospitals Cleveland Medical Center (A.S., D.P.), Neurology, OH; Stanford University (G.W.A., M.G.L.), Neurology, CA; The Royal Melbourne Hospital – University of Melbourne (P.J.M.), Radiology, Parkville, Victoria, Australia; University of Texas Rio Grande Valley - Valley Baptist Medical Center (A.E.H., W.G.T.), Harlingen; University of Kansas Medical Center (M.G.A., S.S., C.G.L., L.M., A.Q., L.R.), Neurology and Radiology; UTHealth McGovern Medical School (S.B., F.S.), Neurosurgery, Houston TX; The Royal Melbourne Hospitals (G.S., N.Y., L.C., G.A.D., S.M.D., B.C.V.C.), University of Melbourne, Neurology; The Walter and Eliza Hall Institute of Medical Research (N.Y.), Population Health and Immunity, Parkville, Victoria; Royal Adelaide Hospital (T.J.K.), Neurology, Adelaide, South Australia; Gold Coast University Hospital (D.G.S.), Neurology, Southport, Queensland, Australia; Christchurch Hospital (T.Y.W.), Neurology, Christchurch, Canterbury, New Zealand; Cleveland Clinic (M.S.H., G.T., M.A.A.), Cerebrovascular Unit, OH; University of Iowa Hospitals (S.O.G.), Neurosurgery; Baptist Health (A.N.A.), Lyerly Neurosurgery, Jacksonville, FL; Emory University (D.C.H., R.G.N.), Neurology, Atlanta, GA; Riverside Methodist Hospital (R.F.B., W.H., N.V.), Colombia, OH; Saint Louis University (R.C.E.), Neurology, MO; University of Tabuk (T.A.), Neurology, KSA; Baylor Scott & White Health (O.M.), Neurology, Dallas, TX; Greensboro | Cone Health (A.A.), Neurology, Greensboro, NC; Touro Infirmary and New Orleans East Hospital (S.M.-S.), Neurology, LA; UTHealth McGovern Medical School (C.W.S.), Diagnostic and Interventional Radiology, Houston, TX; WellStar Health System (R.G.), Neurology, Marietta, GA; and Memorial Hermann Hospital Texas Medical Center (J.C.G.), Neurology, Houston, TX.
1,
Maarten G Lansberg
Maarten G Lansberg, MD, PhD
1From the Case Western Reserve University (A.S.), Neurology; University Hospitals Cleveland Medical Center (A.S., D.P.), Neurology, OH; Stanford University (G.W.A., M.G.L.), Neurology, CA; The Royal Melbourne Hospital – University of Melbourne (P.J.M.), Radiology, Parkville, Victoria, Australia; University of Texas Rio Grande Valley - Valley Baptist Medical Center (A.E.H., W.G.T.), Harlingen; University of Kansas Medical Center (M.G.A., S.S., C.G.L., L.M., A.Q., L.R.), Neurology and Radiology; UTHealth McGovern Medical School (S.B., F.S.), Neurosurgery, Houston TX; The Royal Melbourne Hospitals (G.S., N.Y., L.C., G.A.D., S.M.D., B.C.V.C.), University of Melbourne, Neurology; The Walter and Eliza Hall Institute of Medical Research (N.Y.), Population Health and Immunity, Parkville, Victoria; Royal Adelaide Hospital (T.J.K.), Neurology, Adelaide, South Australia; Gold Coast University Hospital (D.G.S.), Neurology, Southport, Queensland, Australia; Christchurch Hospital (T.Y.W.), Neurology, Christchurch, Canterbury, New Zealand; Cleveland Clinic (M.S.H., G.T., M.A.A.), Cerebrovascular Unit, OH; University of Iowa Hospitals (S.O.G.), Neurosurgery; Baptist Health (A.N.A.), Lyerly Neurosurgery, Jacksonville, FL; Emory University (D.C.H., R.G.N.), Neurology, Atlanta, GA; Riverside Methodist Hospital (R.F.B., W.H., N.V.), Colombia, OH; Saint Louis University (R.C.E.), Neurology, MO; University of Tabuk (T.A.), Neurology, KSA; Baylor Scott & White Health (O.M.), Neurology, Dallas, TX; Greensboro | Cone Health (A.A.), Neurology, Greensboro, NC; Touro Infirmary and New Orleans East Hospital (S.M.-S.), Neurology, LA; UTHealth McGovern Medical School (C.W.S.), Diagnostic and Interventional Radiology, Houston, TX; WellStar Health System (R.G.), Neurology, Marietta, GA; and Memorial Hermann Hospital Texas Medical Center (J.C.G.), Neurology, Houston, TX.
1,
Raul G Nogueira
Raul G Nogueira, MD
1From the Case Western Reserve University (A.S.), Neurology; University Hospitals Cleveland Medical Center (A.S., D.P.), Neurology, OH; Stanford University (G.W.A., M.G.L.), Neurology, CA; The Royal Melbourne Hospital – University of Melbourne (P.J.M.), Radiology, Parkville, Victoria, Australia; University of Texas Rio Grande Valley - Valley Baptist Medical Center (A.E.H., W.G.T.), Harlingen; University of Kansas Medical Center (M.G.A., S.S., C.G.L., L.M., A.Q., L.R.), Neurology and Radiology; UTHealth McGovern Medical School (S.B., F.S.), Neurosurgery, Houston TX; The Royal Melbourne Hospitals (G.S., N.Y., L.C., G.A.D., S.M.D., B.C.V.C.), University of Melbourne, Neurology; The Walter and Eliza Hall Institute of Medical Research (N.Y.), Population Health and Immunity, Parkville, Victoria; Royal Adelaide Hospital (T.J.K.), Neurology, Adelaide, South Australia; Gold Coast University Hospital (D.G.S.), Neurology, Southport, Queensland, Australia; Christchurch Hospital (T.Y.W.), Neurology, Christchurch, Canterbury, New Zealand; Cleveland Clinic (M.S.H., G.T., M.A.A.), Cerebrovascular Unit, OH; University of Iowa Hospitals (S.O.G.), Neurosurgery; Baptist Health (A.N.A.), Lyerly Neurosurgery, Jacksonville, FL; Emory University (D.C.H., R.G.N.), Neurology, Atlanta, GA; Riverside Methodist Hospital (R.F.B., W.H., N.V.), Colombia, OH; Saint Louis University (R.C.E.), Neurology, MO; University of Tabuk (T.A.), Neurology, KSA; Baylor Scott & White Health (O.M.), Neurology, Dallas, TX; Greensboro | Cone Health (A.A.), Neurology, Greensboro, NC; Touro Infirmary and New Orleans East Hospital (S.M.-S.), Neurology, LA; UTHealth McGovern Medical School (C.W.S.), Diagnostic and Interventional Radiology, Houston, TX; WellStar Health System (R.G.), Neurology, Marietta, GA; and Memorial Hermann Hospital Texas Medical Center (J.C.G.), Neurology, Houston, TX.
1,
James C Grotta
James C Grotta, MD
1From the Case Western Reserve University (A.S.), Neurology; University Hospitals Cleveland Medical Center (A.S., D.P.), Neurology, OH; Stanford University (G.W.A., M.G.L.), Neurology, CA; The Royal Melbourne Hospital – University of Melbourne (P.J.M.), Radiology, Parkville, Victoria, Australia; University of Texas Rio Grande Valley - Valley Baptist Medical Center (A.E.H., W.G.T.), Harlingen; University of Kansas Medical Center (M.G.A., S.S., C.G.L., L.M., A.Q., L.R.), Neurology and Radiology; UTHealth McGovern Medical School (S.B., F.S.), Neurosurgery, Houston TX; The Royal Melbourne Hospitals (G.S., N.Y., L.C., G.A.D., S.M.D., B.C.V.C.), University of Melbourne, Neurology; The Walter and Eliza Hall Institute of Medical Research (N.Y.), Population Health and Immunity, Parkville, Victoria; Royal Adelaide Hospital (T.J.K.), Neurology, Adelaide, South Australia; Gold Coast University Hospital (D.G.S.), Neurology, Southport, Queensland, Australia; Christchurch Hospital (T.Y.W.), Neurology, Christchurch, Canterbury, New Zealand; Cleveland Clinic (M.S.H., G.T., M.A.A.), Cerebrovascular Unit, OH; University of Iowa Hospitals (S.O.G.), Neurosurgery; Baptist Health (A.N.A.), Lyerly Neurosurgery, Jacksonville, FL; Emory University (D.C.H., R.G.N.), Neurology, Atlanta, GA; Riverside Methodist Hospital (R.F.B., W.H., N.V.), Colombia, OH; Saint Louis University (R.C.E.), Neurology, MO; University of Tabuk (T.A.), Neurology, KSA; Baylor Scott & White Health (O.M.), Neurology, Dallas, TX; Greensboro | Cone Health (A.A.), Neurology, Greensboro, NC; Touro Infirmary and New Orleans East Hospital (S.M.-S.), Neurology, LA; UTHealth McGovern Medical School (C.W.S.), Diagnostic and Interventional Radiology, Houston, TX; WellStar Health System (R.G.), Neurology, Marietta, GA; and Memorial Hermann Hospital Texas Medical Center (J.C.G.), Neurology, Houston, TX.
1,
Geoffrey Alan Donnan
Geoffrey Alan Donnan, MD
1From the Case Western Reserve University (A.S.), Neurology; University Hospitals Cleveland Medical Center (A.S., D.P.), Neurology, OH; Stanford University (G.W.A., M.G.L.), Neurology, CA; The Royal Melbourne Hospital – University of Melbourne (P.J.M.), Radiology, Parkville, Victoria, Australia; University of Texas Rio Grande Valley - Valley Baptist Medical Center (A.E.H., W.G.T.), Harlingen; University of Kansas Medical Center (M.G.A., S.S., C.G.L., L.M., A.Q., L.R.), Neurology and Radiology; UTHealth McGovern Medical School (S.B., F.S.), Neurosurgery, Houston TX; The Royal Melbourne Hospitals (G.S., N.Y., L.C., G.A.D., S.M.D., B.C.V.C.), University of Melbourne, Neurology; The Walter and Eliza Hall Institute of Medical Research (N.Y.), Population Health and Immunity, Parkville, Victoria; Royal Adelaide Hospital (T.J.K.), Neurology, Adelaide, South Australia; Gold Coast University Hospital (D.G.S.), Neurology, Southport, Queensland, Australia; Christchurch Hospital (T.Y.W.), Neurology, Christchurch, Canterbury, New Zealand; Cleveland Clinic (M.S.H., G.T., M.A.A.), Cerebrovascular Unit, OH; University of Iowa Hospitals (S.O.G.), Neurosurgery; Baptist Health (A.N.A.), Lyerly Neurosurgery, Jacksonville, FL; Emory University (D.C.H., R.G.N.), Neurology, Atlanta, GA; Riverside Methodist Hospital (R.F.B., W.H., N.V.), Colombia, OH; Saint Louis University (R.C.E.), Neurology, MO; University of Tabuk (T.A.), Neurology, KSA; Baylor Scott & White Health (O.M.), Neurology, Dallas, TX; Greensboro | Cone Health (A.A.), Neurology, Greensboro, NC; Touro Infirmary and New Orleans East Hospital (S.M.-S.), Neurology, LA; UTHealth McGovern Medical School (C.W.S.), Diagnostic and Interventional Radiology, Houston, TX; WellStar Health System (R.G.), Neurology, Marietta, GA; and Memorial Hermann Hospital Texas Medical Center (J.C.G.), Neurology, Houston, TX.
1,
Stephen M Davis
Stephen M Davis, MD
1From the Case Western Reserve University (A.S.), Neurology; University Hospitals Cleveland Medical Center (A.S., D.P.), Neurology, OH; Stanford University (G.W.A., M.G.L.), Neurology, CA; The Royal Melbourne Hospital – University of Melbourne (P.J.M.), Radiology, Parkville, Victoria, Australia; University of Texas Rio Grande Valley - Valley Baptist Medical Center (A.E.H., W.G.T.), Harlingen; University of Kansas Medical Center (M.G.A., S.S., C.G.L., L.M., A.Q., L.R.), Neurology and Radiology; UTHealth McGovern Medical School (S.B., F.S.), Neurosurgery, Houston TX; The Royal Melbourne Hospitals (G.S., N.Y., L.C., G.A.D., S.M.D., B.C.V.C.), University of Melbourne, Neurology; The Walter and Eliza Hall Institute of Medical Research (N.Y.), Population Health and Immunity, Parkville, Victoria; Royal Adelaide Hospital (T.J.K.), Neurology, Adelaide, South Australia; Gold Coast University Hospital (D.G.S.), Neurology, Southport, Queensland, Australia; Christchurch Hospital (T.Y.W.), Neurology, Christchurch, Canterbury, New Zealand; Cleveland Clinic (M.S.H., G.T., M.A.A.), Cerebrovascular Unit, OH; University of Iowa Hospitals (S.O.G.), Neurosurgery; Baptist Health (A.N.A.), Lyerly Neurosurgery, Jacksonville, FL; Emory University (D.C.H., R.G.N.), Neurology, Atlanta, GA; Riverside Methodist Hospital (R.F.B., W.H., N.V.), Colombia, OH; Saint Louis University (R.C.E.), Neurology, MO; University of Tabuk (T.A.), Neurology, KSA; Baylor Scott & White Health (O.M.), Neurology, Dallas, TX; Greensboro | Cone Health (A.A.), Neurology, Greensboro, NC; Touro Infirmary and New Orleans East Hospital (S.M.-S.), Neurology, LA; UTHealth McGovern Medical School (C.W.S.), Diagnostic and Interventional Radiology, Houston, TX; WellStar Health System (R.G.), Neurology, Marietta, GA; and Memorial Hermann Hospital Texas Medical Center (J.C.G.), Neurology, Houston, TX.
1,
Bruce C V Campbell
Bruce C V Campbell, MBBS, PhD
1From the Case Western Reserve University (A.S.), Neurology; University Hospitals Cleveland Medical Center (A.S., D.P.), Neurology, OH; Stanford University (G.W.A., M.G.L.), Neurology, CA; The Royal Melbourne Hospital – University of Melbourne (P.J.M.), Radiology, Parkville, Victoria, Australia; University of Texas Rio Grande Valley - Valley Baptist Medical Center (A.E.H., W.G.T.), Harlingen; University of Kansas Medical Center (M.G.A., S.S., C.G.L., L.M., A.Q., L.R.), Neurology and Radiology; UTHealth McGovern Medical School (S.B., F.S.), Neurosurgery, Houston TX; The Royal Melbourne Hospitals (G.S., N.Y., L.C., G.A.D., S.M.D., B.C.V.C.), University of Melbourne, Neurology; The Walter and Eliza Hall Institute of Medical Research (N.Y.), Population Health and Immunity, Parkville, Victoria; Royal Adelaide Hospital (T.J.K.), Neurology, Adelaide, South Australia; Gold Coast University Hospital (D.G.S.), Neurology, Southport, Queensland, Australia; Christchurch Hospital (T.Y.W.), Neurology, Christchurch, Canterbury, New Zealand; Cleveland Clinic (M.S.H., G.T., M.A.A.), Cerebrovascular Unit, OH; University of Iowa Hospitals (S.O.G.), Neurosurgery; Baptist Health (A.N.A.), Lyerly Neurosurgery, Jacksonville, FL; Emory University (D.C.H., R.G.N.), Neurology, Atlanta, GA; Riverside Methodist Hospital (R.F.B., W.H., N.V.), Colombia, OH; Saint Louis University (R.C.E.), Neurology, MO; University of Tabuk (T.A.), Neurology, KSA; Baylor Scott & White Health (O.M.), Neurology, Dallas, TX; Greensboro | Cone Health (A.A.), Neurology, Greensboro, NC; Touro Infirmary and New Orleans East Hospital (S.M.-S.), Neurology, LA; UTHealth McGovern Medical School (C.W.S.), Diagnostic and Interventional Radiology, Houston, TX; WellStar Health System (R.G.), Neurology, Marietta, GA; and Memorial Hermann Hospital Texas Medical Center (J.C.G.), Neurology, Houston, TX.
1;
on behalf of the SELECT, EXTEND-IA, EXTEND-IA TNK, and EXTEND-IA TNK Part-II Investigators1