Abstract
New drug development is a race against the clock as soon as the first patents are filed, and understanding the potential timings from first-in-human studies to regulatory approval is crucial for strategic planning. Here, we use information gathered from US FDA review documents to provide insight into the timeframes of successful drug development programmes in the past decade. We define clinical development time as the number of days between the initiation of first-in-human clinical studies and regulatory marketing authorization, and we focus on the development of innovative drugs — those products that are being marketed for the first time that contain a new molecular entity or new active moiety (see Supplementary Box 1 for details of the dataset and analysis, and Supplementary Table 1 for the dataset). We also investigate how FDA regulatory programmes may affect this process.
Analysis of clinical development times
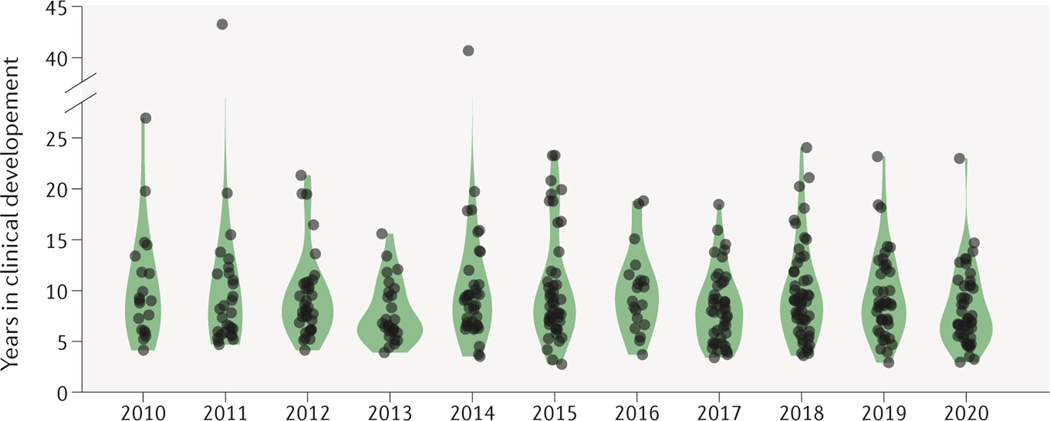
FDA-approved drugs from 2010–2020.
Between 2010 and 2020, 440 innovative drugs were approved for marketing by the FDA (Supplementary Table 1). Of these, clinical development start dates were not provided for 35 innovative drugs: the original submission date for the investigational new drug (IND) application was not provided for 10 drugs and for 25 drugs the initial clinical development occurred outside the United States and first-in-human trial dates were not available. The remaining 405 products were analysed for clinical development time and other product characteristics. Development times ranged from under 5 years to over 20 (Fig. 1).
Figure 1. Clinical development times for innovative drugs.
Development times for each year’s cohort of drugs has remained stable over the past decade; the median was 8.3 years. See Supplementary Box 1 for details of the dataset and analysis.
The two drugs with the shortest clinical development time were both small molecules: osimertinib (a non-small-cell lung cancer treatment) and elexacaftor (a cystic fibrosis treatment). Osimertinib entered clinical testing on 6 March 2013 and the marketed product Tagrisso received accelerated approval on 13 November 2015 (984 later). Tagrisso was granted full approval on 31 March 2017.
Elexacaftor’s IND opened on 12 December 2016 and the triple combination therapy including this drug, Trikafta, was approved on 21 October 2019 (1,043 days later). Even more impressive, this non-cancer, fixed-dose combination drug was approved without the benefit of accelerated approval. This was Vertex Pharmaceutical’s fourth approval for cystic fibrosis; earlier products had each spent over 2,000 days in clinical development, underscoring how experience and reduced regulatory uncertainty can accelerate product development.
The drugs with the shortest time between new drug application (NDA) submission and approval were blinatumomab (75 days) and remdesivir (76 days). While blinatumomab received accelerated approval on 3 December 2014, it was not granted full approval until 12 July 2017. The NDA for remdesivir for COVID-19 was submitted on 7 August 2020 and approval granted on 22 October 2020. Likewise, this programme also benefited from a prior emergency use authorization on 1 May 2020. The next fastest full approval was for cabazitaxel, for which the rolling NDA submission was completed on 31 March 2010 and was approved only 78 days later.
Trends for therapeutic class.
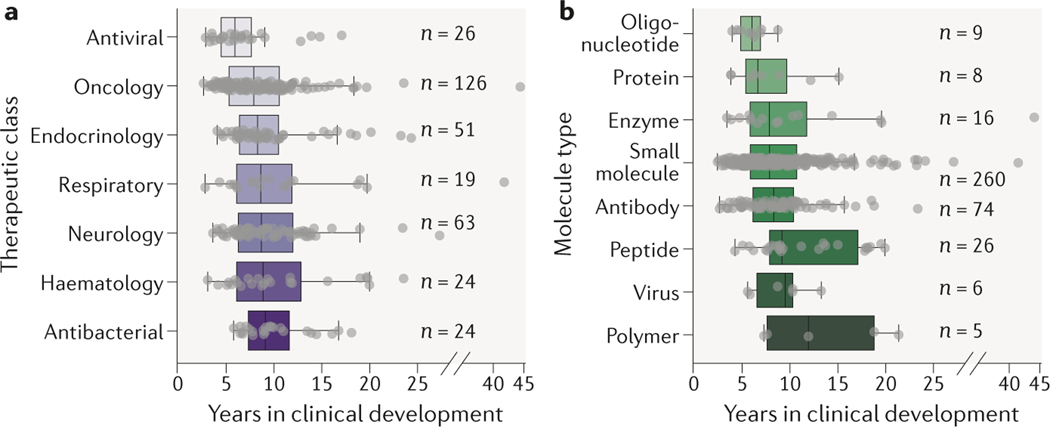
Clinical development times for recent innovative drugs grouped by therapeutic class reveal some interesting differences (Fig. 2a). Recent antiviral products (n = 26) had shorter development times than other product classes. Other differences were more modest, possibly reflecting differences in the amount of time and population size required to observe clinically meaningful benefit for different types of conditions. In general, there is significant variation in development times within each therapeutic class.
Figure 2. Clinical development times for innovative drugs as a function of therapeutic class and molecule type.
a. Therapeutic class; one-way ANOVA P = 0.04. b. Molecule type; one-way ANOVA P < 0.01. See Supplementary Box 1 for details of the analysis.
Trends for molecule type.
Despite regulatory risks associated with highly innovative technologies, recently approved small interfering RNA and antisense oligonucleotide products (n = 9) have had shorter development times than small molecules as a class (n = 260). In contrast, development times for approved gene therapy and viral products (n = 6) were comparable to small molecule products. While different molecule types can pose important manufacturing challenges, development times appear to be largely driven by the clinical context for the drug.
Impact of expedited programmes on clinical development times.
The FDA has a wide array of programmes to facilitate the development of certain medicines, such as those expected to help address unmet medical needs. To estimate how useful each programme is to clinical development, and control for multiple mechanisms being employed during a product’s development, we modelled clinical development time and, separately, review time between licensing application submission and approval, with programme and other covariates using multiple linear regressions.
Accelerated approval, breakthrough designation, orphan designation status and whether a product approval required more than one review cycle all significantly correlated with clinical development times (Table 1). And as expected, priority review status and whether a product approval required more than one review cycle significantly correlated with review times.
Table 1.
Regulatory factor impact
| Regulatory factor | Effect (years) | 95% CI |
|---|---|---|
| Accelerated approval | −3.0 | (−4.5, −1.5) |
| Breakthrough designation | −1.3 | (−2.6, 0.0) |
| Orphan designation | +1.5 | (+0.4, +2.6) |
| >1 Review cycle | +1.8 | (+0.4, +3.2) |
Effect size of US FDA regulatory factors on shortening (−) or increasing (+) clinical development times.
See Supplementary Box 1 for details of the dataset and analysis.
Controlling for other development factors, the clinical development time of a typical innovative drug is 9.1 years (95% confidence interval (CI) = 8.2–10.0 years). Drugs with the accelerated approval designation have a reduced clinical development time of 1,100 days (95% CI = 563–1637 days). Breakthrough designation was also associated with a reduction in clinical development times, although there is less certainty in the magnitude of this effect as compared to other regulatory programmes: 479 days (95% CI = 5–953 days).
In contrast, orphan designation is associated with an increase in clinical development times of 552 days (95% CI = 148–957 days). This suggests that despite the smaller trial sizes, such programmes may be hampered by issues such as challenges in identifying and recruiting patients, uncertainty in the natural history of the disease and a potential requirement for the development of novel clinical endpoints. It is noteworthy that in the United States, orphan-designated products are eligible to receive an additional two years of marketing exclusivity from the FDA, which offsets the additional costs associated with longer clinical development times.
Receipt of a complete response letter from the FDA and other failures to win approval within the first review cycle increased review times by 829 days (95% CI = 732–926 days) and likewise increased clinical development time by 643 days (95% CI = 132–1153 days).
Priority review status decreased review times by 103 days (19–187 days 95% CI), however, priority review status was not significantly correlated with overall clinical development time. Perhaps the analysis lacks sufficient power to detect this correlation.
Fast-track designation, whether the product ultimately includes a black box warning on the label and whether the product was a diagnostic imaging agent were not significantly correlated with development times.
Conclusions and outlook
Clinical development time, which can be calculated from publicly available information, provides a high-level metric for the speed of product development. Speed matters both to patients and drug developers. The FDA’s priority review voucher programme provides one estimate of its importance. The 120-day benefit of reducing review goal dates from 10 months to 6 months has recently had a value of US$100 million or more on the open market, providing an estimate of the cost of extra development time at nearly $1 million a day.
Clinical development times for innovative drugs remain stable over recent years. Most products spend the better part of a decade in clinical development, with others taking considerably longer. However, the FDA’s expedited development programmes clearly shorten the path from clinical trial entry to drug approval. And in the case of orphan products, extra product exclusivity offsets the additional time spent in development.
Efforts to improve clinical trial capacity and innovations in trial design, patient stratification and clinical biomarkers are also being pursued to accelerate drug development timelines. With the high number of orphan designations (approximately 40% of all new drugs), and potential for these to take longer in clinical development, strategic partnerships with rare disease foundations, patient advocacy groups and venture philanthropy models may prove to be critical for helping to improve development timelines. For example, partnerships that provide access to tissue banks, data, cell lines, biomarkers, patients and funding are now common for certain rare diseases. This has been highlighted as an important cornerstone leading to the success of the recent cystic fibrosis drugs, including the first disease-modifying treatment to be available to patients. Clinical development time will be a useful metric for measuring the impact of these strategies.
Supplementary Material
Disclaimer
The views and opinions presented here represent those of the authors and should not be considered to represent advice or guidance on behalf of the US Food and Drug Administration.
This study was supported in part by the Intramural Research Program of the NCATS, NIH.
Footnotes
Competing interests
D.G.B. and H.J.W. are employees of Jnana Therapeutics, which does not have a direct bearing on the subject of this paper. H.J.W is a shareholder in AstraZeneca. The other authors declare no competing interests.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.