ABSTRACT
Background
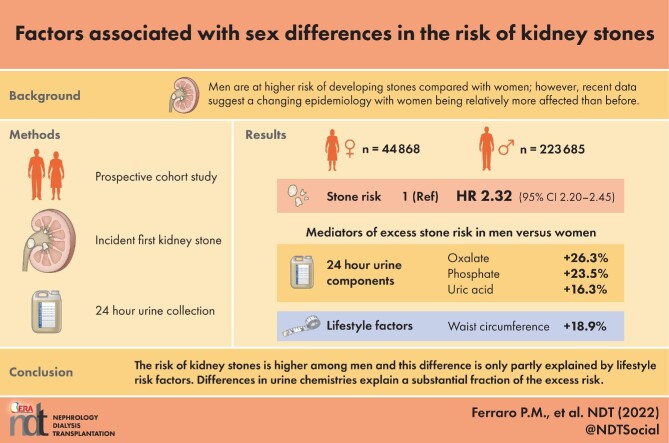
Men are at higher risk of developing stones compared with women; however, recent data suggest a changing epidemiology, with women being relatively more affected than before.
Methods
To estimate the proportion of excess risk among men, we analysed data from large cohorts (Health Professionals Follow-up Study and Nurses’ Health Study I and II). Kidney stone incidence rates were computed and hazard ratios (HRs) and 95% confidence intervals (CIs) generated with age-adjusted Cox proportional regression models. Mediation analysis estimated the excess risk for men explained by risk factors, including waist circumference, high blood pressure, diabetes, use of thiazides and dietary intake. The 24-h urine composition was also examined.
Results
The analysis included 268 553 participants, contributing 5 872 249 person-years of follow-up. A total of 10 302 incident stones were confirmed and the overall incidence rate was 271 and 159 per 100 000 person-years for men and women, respectively. The age-adjusted HR was 2.32 (95% CI 2.20, 2.45) and the risk of stones was consistently higher across categories of age (HRs ranging from 2.02 to 2.76) for men compared with women. The risk remained higher among men, but tended to decrease over time (48.1%), while it increased among women. Urine supersaturations for calcium oxalate and uric acid were higher among men, primarily because of higher oxalate (26.3%), uric acid (16.3%), phosphate (23.5%) and lower pH.
Conclusions
The risk of kidney stones is higher among men and this difference is only partly explained by lifestyle risk factors; differences in urine chemistries explain a substantial fraction of the excess risk.
Keywords: cohort studies, kidney stones, nephrolithiasis, risk factors, sex
Graphical Abstract
Graphical Abstract.
KEY LEARNING POINTS.
What is already known about this subject?
The higher prevalence of kidney stones among men compared with women has been previously reported, but there is limited evidence on why men are at higher risk.
What this study adds?
This study investigated three large cohorts with detailed and updated information on dietary habits and other conditions that might impact the risk of forming stones and found that men are overall at higher risk of stones compared with women.
Several factors, including differences in waist circumference, fluid intake and especially urine composition, explained a meaningful proportion of the excess risk among men.
What impact this may have on practice or policy?
Since lifestyle risk factors play a role in the excess risk of kidney stones among men, we can expect that vigorously tackling those factors would result in a reduction in the rate of stone formation.
INTRODUCTION
Kidney stone disease is common in the general population, with a prevalence higher than 10% in the most recent National Health and Nutrition Examination Survey [1]; it is also characterized by high recurrence rates [2]. A number of genetic [3] and environmental [4] factors are thought to play a role in its pathogenesis, including fluid intake, [5, 6] dietary calcium [7–9], animal protein [10] and adherence to a Dietary Approach to Stop Hypertension-style diet [11]. Men are more than twice as likely to be affected as women, although this gap seems to be decreasing [1]. The reasons for the sex difference and for the apparent change over time have not been thoroughly investigated. It is possible that some factors carry a differential risk in men compared with women, as suggested for example in different magnitudes of relative risks by sex for waist circumference [12] and intake of phytate [9], vitamin C [13] and vitamin D [14]. The aims of this study were to analyse data from three large, longitudinal cohorts to (i) compare incidence rates of kidney stones by sex overall and over categories of age and calendar time, (ii) define to what extent differences in incidence rates are explained by different risk factors and (iii) explore differences in 24-h urine composition relevant to kidney stones between men and women. The findings could provide insight into potential pathophysiologic differences in stone formation between men and women.
MATERIALS AND METHODS
Study cohorts
The Health Professionals Follow-up Study (HPFS) cohort was started in 1986 with the enrolment of 51 529 male health professionals (dentists, optometrists, osteopaths, pharmacists, podiatrists and veterinarians) aged 40–75 years; the Nurses’ Health Study (NHS) I cohort was started in 1976 with the enrolment of 121 700 female nurses aged 30–55 years; the NHS II cohort was started in 1989 with the enrolment of 116 429 female nurses aged 25–42 years. For all the cohorts, participants completed a detailed baseline questionnaire with information on lifestyle, medical history and medications. Questionnaires were subsequently mailed every 2 years to update information. These studies were approved by the Partners HealthCare Institutional Review Board and adhered to the principles of the declaration of Helsinki. The return of completed questionnaires was accepted by the institutional review board as implied informed consent.
Assessment of exposure
The primary exposure of interest was self-reported sex. In secondary analyses, we examined differences in sex-specific incidence rates by age and calendar time.
Assessment of outcome
The outcome of interest was time for a first, symptomatic kidney stone. Participants reporting an incident kidney stone were asked to complete a supplementary questionnaire with information about the date of occurrence and accompanying symptoms. A symptomatic kidney stone was defined as the presence of pain and/or haematuria. Self-reported diagnosis was found to be highly reliable by medical record review of a sample (confirmed in ≥95% who completed the supplementary questionnaire) [11]. In a subsample of the study population with stone composition reports, the stone type was predominantly calcium oxalate (>50%) in 86% of participants in the HPFS, 77% of participants in the NHS I and 79% of participants in the NHS II cohorts [11].
Assessment of covariates
Information about age, waist circumference, history of diabetes and thiazide use was obtained from the biennial questionnaires. Starting in 1986 (for HPFS and NHS I) and 1991 (for NHS II), participants completed a food frequency questionnaire providing information on the average use of ˃130 foods and ˃20 beverages during the previous year. Intake of individual nutrients was calculated from the frequency of consumption of foods and from data on the content of the relevant nutrients obtained from the US Department of Agriculture, except for oxalate intake, which was directly measured in foods using capillary electrophoresis [15]. The food frequency questionnaire has been sent every 4 years and also queries the use of multivitamins, as well as individual supplements. Information for nutrients obtained using the food frequency questionnaire has been demonstrated to be valid [16, 17].
Urine collections
Twenty-four-hour urine samples were collected in three cycles. In the first cycle, which spanned from 1994 to 1999, participants were eligible if they were aged ≤70 years (HPFS) or ≤65 years (NHS I) and had no history of cancer or cardiovascular disease. In the second cycle, which began in 2003, participants were eligible if they were aged ≤75 years and had no history of cancer (other than non-melanoma skin cancer). In the third cycle, which spanned 2010–11, NHS II participants with no history of hypertension were enrolled. Urine samples were analysed with the system provided by Mission Pharmacal (San Antonio, TX, USA) for the first two cycles and by Litholink (Labcorp, Chicago, IL, USA) for the third cycle. Participants with a history of kidney stones were oversampled in the first two cycles. Participants with possible over- or under-collections (defined as urinary creatinine excretion in the top or bottom 1% of the non-stone formers distribution) were removed from the analysis. For participants who provided more than one collection, the first sample was analysed. Supersaturation (SS) values were computed with the EQUIL-2 software.
Statistical analysis
The study design was prospective; information on variables of interest was collected before the incident kidney stone except for the 24-h urine collections. Time at risk started from the date of return of the 1986 (HPFS, NHS I) or 1991 (NHS II) questionnaire and participants were followed up until the development of a symptomatic kidney stone, an asymptomatic kidney stone, cancer, death or end of follow-up (2012 for HPFS and NHS I, 2015 for NHS II), whichever occurred first. Participants with a history of cancer (except nonmelanoma skin cancer) or a history of kidney stones at baseline were excluded from the study.
Cox proportional hazards regression models were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs). To explore how much of the exposure ‘effect’ (e.g. the excess risk of kidney stones among men) was explained by known risk factors for kidney stones, we implemented a mediation analysis by calculating the relative change in the coefficient for sex from a fully adjusted model to a model that did not include the given risk factor [18]. Models included age, waist circumference, history of high blood pressure, history of diabetes, use of thiazides, dietary intakes of animal protein, caffeine, fructose, potassium, sodium, oxalate and phytate, dietary and supplemental intakes of calcium, vitamin C and vitamin D and sugar-sweetened beverages, and total fluid intake. Percent changes of estimates were calculated using the non-exponentiated coefficients.
Linear regression models adjusted for age and kidney stone status were used for the analysis of urinary components. To determine the relative contribution of each urinary component to the excess risk of stones among men, we applied the mediation analysis approach described earlier by calculating the relative change in the coefficient for sex from a logistic regression model with kidney stone status as the dependent variable including the key lithogenic urinary components (calcium, oxalate, citrate, uric acid, magnesium, volume, pH) as well as age to a model that did not include the given urinary component.
A two-tailed P-value <0.05 was considered statistically significant. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
RESULTS
The analysis included 268 553 participants, contributing 5 872 249 person-years of follow-up, during which 10 302 incident stone events were confirmed. Baseline characteristics by sex are reported in Table 1 (baseline characteristics by cohort are reported in Supplementary data, Table S1). On average, men were older than women, had larger waist circumference, took less supplemental calcium and had higher intakes of animal protein, potassium, sodium, phytate and vitamins C and D.
Table 1.
Baseline characteristics of the study participants by sex
| Men (n = 44 868) | Women (n = 223 685) | |
|---|---|---|
| Age, yearsa | 54 (10) | 44 (10) |
| Waist circumference (cm) | 94 (9) | 79 (12) |
| Dietary calcium (mg/day) | 804 (300) | 805 (290) |
| Calcium supplement (mg/day) | 85 (244) | 241 (373) |
| Caffeine (mg/day) | 246 (255) | 263 (224) |
| Animal protein (g/day) | 67 (18) | 60 (16) |
| Fructose (g/day) | 25 (12) | 22 (10) |
| Potassium (mg/day) | 3360 (686) | 3006 (582) |
| Sodium (mg/day) | 3237 (1130) | 2495 (837) |
| Oxalate (mg/day) | 139 (129) | 146 (107) |
| Phytate (mg/day) | 925 (375) | 749 (258) |
| Vitamin C (mg/day) | 415 (472) | 303 (349) |
| Vitamin D (IU/day) | 383 (300) | 368 (258) |
| Sugar-sweetened beverages (servings/day) | 0.4 (0.7) | 0.3 (0.7) |
| Fluid intake (mL/day) | 1992 (825) | 2079 (791) |
| Diabetes, % | 1.8 | 2.6 |
| High blood pressure, % | 16.0 | 17.1 |
| Thiazide use, % | 5.9 | 6.9 |
Values are means (SD) for continuous variables, percentages for categorical variables and are standardized to the age distribution of the study population. aValue is not age-adjusted.
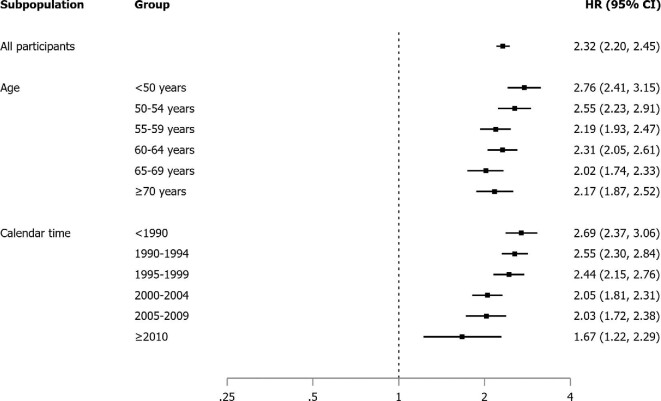
The association between sex and risk of stones is reported in Table 2. The overall incidence rates of kidney stones were 271 and 159 per 100 000 person-years for men and women, respectively. The age-adjusted HR for kidney stones in men compared with women was 2.32 (95% CI 2.20, 2.45).
Table 2.
Risk of incident kidney stones by sex overall and across categories of age
| Men | Women | |
|---|---|---|
| Overall | ||
| Cases | 2392 | 7910 |
| Person-years | 883 224 | 4 989 025 |
| Incidence ratea | 271 | 159 |
| HR (95% CI) | 2.32 (2.20, 2.45) | 1.00 (Ref.) |
| <50 years | ||
| Cases | 439 | 3291 |
| Person-years | 113 035 | 1 770 286 |
| Incidence ratea | 388 | 186 |
| HR (95% CI) | 2.76 (2.41, 3.15) | 1.00 (Ref.) |
| 50–54 years | ||
| Cases | 382 | 1314 |
| Person-years | 100 845 | 740 903 |
| Incidence ratea | 379 | 177 |
| HR (95% CI) | 2.55 (2.22, 2.91) | 1.00 (Ref.) |
| 55–59 years | ||
| Cases | 438 | 1305 |
| Person-years | 129 679 | 722 061 |
| Incidence ratea | 338 | 181 |
| HR (95% CI) | 2.18 (1.93, 2.47) | 1.00 (Ref.) |
| 60–64 years | ||
| Cases | 439 | 948 |
| Person-years | 148 886 | 656 760 |
| Incidence ratea | 295 | 144 |
| HR (95% CI) | 2.31 (2.04, 2.61) | 1.00 (Ref.) |
| 65–69 years | ||
| Cases | 325 | 595 |
| Person-years | 140 050 | 484 199 |
| Incidence ratea | 232 | 123 |
| HR (95% CI) | 2.02 (1.74, 2.33) | 1.00 (Ref.) |
| ≥70 years | ||
| Cases | 369 | 457 |
| Person-years | 250 730 | 614 816 |
| Incidence ratea | 147 | 74 |
| HR (95% CI) | 2.17 (1.87, 2.52) | 1.00 (Ref.) |
The estimates are age-adjusted as age is the time axis for the survival analysis. aNumber of events per 100 000 person-years
The associations between sex and incident kidney stones across categories of age and calendar time are reported in Figure 1, Tables 2 and 3, and Supplementary data, Table S2. The risk of stones was consistently higher across categories of age among men. Regarding calendar time, the risk remained higher among men, but tended to decrease over time, while it increased among women, resulting in a 48.1% decrease after 2009 compared with before 1990. All the previous analyses were repeated using cohort as the exposure of interest rather than sex (Supplementary data, Tables S3 and S4). Interestingly, while risk remained significantly higher among men (the HPFS cohort), women in the more recently started cohort (the NHS II) had a significantly higher risk compared with those in the less recently started cohort (the NHS I) across categories of age and calendar time. For instance, an NHS II participant in the age range 50–54 years had a 47% higher risk compared with an NHS I participant in the same age category, though from a different point in calendar time.
FIGURE 1:
Association between sex and incident kidney stones across categories of age and calendar time.
Table 3.
Risk of incident kidney stones by sex across categories of calendar time
| Men | Women | |
|---|---|---|
| <1990 | ||
| Cases | 498 | 520 |
| Person-years | 167 577 | 450 711 |
| Incidence ratea | 297 | 115 |
| HR (95% CI) | 2.69 (2.37, 3.06) | 1.00 (Ref.) |
| 1990–1994 | ||
| Cases | 691 | 1520 |
| Person-years | 233 742 | 1 055,81 |
| Incidence ratea | 296 | 144 |
| HR (95% CI) | 2.55 (2.30, 2.84) | 1.00 (Ref.) |
| 1995–1999 | ||
| Cases | 443 | 1707 |
| Person-years | 151 573 | 1 034 935 |
| Incidence ratea | 292 | 165 |
| HR (95% CI) | 2.44 (2.15, 2.76) | 1.00 (Ref.) |
| 2000–2004 | ||
| Cases | 462 | 1610 |
| Person-years | 184 830 | 957 218 |
| Incidence ratea | 250 | 168 |
| HR (95% CI) | 2.05 (1.81, 2.31) | 1.00 (Ref.) |
| 2005–2009 | ||
| Cases | 239 | 1421 |
| Person-years | 108 120 | 886 403 |
| Incidence ratea | 221 | 160 |
| HR (95% CI) | 2.02 (1.72, 2.38) | 1.00 (Ref.) |
| ≥2010 | ||
| Cases | 59 | 1132 |
| Person-years | 37 382 | 604 377 |
| Incidence ratea | 158 | 187 |
| HR (95% CI) | 1.67 (1.22, 2.29) | 1.00 (Ref.) |
The estimates are age-adjusted as age is the time axis for the survival analysis. aNumber of events per 100 000 person-years
Results of the mediation analysis, aimed at investigating what characteristics explained the higher risk of stones among men, are reported in Table 4 for those factors for which the percent mediated effect was statistically significant. Taken together, the non-urinary risk factors considered explained part of the excess risk among men and most of the mediation was due to differences in waist circumference (percent mediated effect 18.9%).
Table 4.
Non-urinary contributors to the difference in risk of incident kidney stones between men and women
| Contributor | Percent mediated effect (95% CI) |
|---|---|
| Waist circumference | 18.9 (15.6, 22.2) |
| Fluid intake | 5.7 (4.5, 6.9) |
| Sugar-sweetened beverages | 3.9 (2.9, 5.0) |
| Thiazides | 1.8 (1.0, 2.7) |
| Dietary oxalate | 0.7 (0.2, 1.2) |
| Dietary calcium | 0.7 (0, 1.4) |
This table shows the relative contribution of each non-urinary factor to the excess risk of incident kidney stones among men
Urine data were available for 6334 participants. Differences in 24-h urine composition between men and women are reported in Table 5. After adjustment for age and kidney stone status, men had significantly higher urinary excretion of potassium, oxalate, citrate, uric acid, sodium, magnesium and phosphate, and lower urinary volume and urine pH. Overall, the differences in urine composition resulted in significantly higher SS for calcium oxalate and uric acid among men. Interestingly, all SS values were higher among men compared with women in collections performed before 2000 as opposed to 2000 or after (differences between men and women pre- and post-2000 for SS CaOx: 2.15 versus 1.19; SS UA: 0.80 versus 0.61; SS CaP: 0.30 versus −0.06). After further adjustment for body weight, all the differences in urinary components remained statistically significant with the exception of citrate.
Table 5.
Twenty-four-hour urine components by sex
| Mean (SD) | ||||||
|---|---|---|---|---|---|---|
| Men (n = 1145) | Women (n = 5189) | Adjusted Difference (95% CI) | P-value | Adjusted Difference (95% CI)a | P-valuea | |
| Creatinine (g) | 1.65 (0.37) | 1.18 (0.25) | 0.56 (0.54, 0.58) | <0.001 | 0.47 (0.45, 0.48) | <0.001 |
| Potassium (mEq) | 76.4 (25.1) | 61.2 (21.1) | 16.1 (14.6, 17.6) | <0.001 | 14.5 (12.9, 16.0) | <0.001 |
| Calcium (mg) | 197 (106) | 201 (98) | 4.4 (−2.4, 11.2) | 0.21 | −1.8 (−8.8, 5.2) | 0.62 |
| Oxalate (mg) | 40.3 (13.0) | 29.7 (10.9) | 11.3 (10.5, 12.0) | <0.001 | 9.4 (8.6, 10.2) | <0.001 |
| Citrate (mg) | 696 (308) | 741 (306) | 29.1 (8.5, 49.8) | 0.006 | 2.5 (−18.8, 23.8) | 0.82 |
| Uric acid (mg) | 618 (229) | 517 (157) | 153 (142, 165) | <0.001 | 118 (106, 129) | <0.001 |
| Sodium (mEq) | 183 (70) | 142 (59) | 45.7 (41.5, 49.9) | <0.001 | 30.4 (26.3, 34.6) | <0.001 |
| Magnesium (mg) | 124 (44) | 104 (39) | 23.2 (20.5, 26.0) | <0.001 | 20.4 (17.5, 23.2) | <0.001 |
| Phosphate (mg) | 1067 (323) | 816 (262) | 301 (282, 319) | <0.001 | 237 (218, 255) | <0.001 |
| pH (U) | 5.86 (0.46) | 6.10 (0.51) | −0.19 (−0.23, −0.16) | <0.001 | −0.12 (−0.16, −0.09) | <0.001 |
| Volume (mL) | 1690 (650) | 1930 (810) | −143 (−196, −91) | <0.001 | −180 (−235, −126) | <0.001 |
| SS CaOx | 8.57 (5.08) | 6.26 (4.00) | 2.12 (1.85, 2.40) | <0.001 | 2.00 (1.71, 2.29) | <0.001 |
| SS CaP | 1.90 (1.63) | 1.57 (1.43) | 0.26 (0.17, 0.36) | <0.001 | 0.34 (0.24, 0.44) | <0.001 |
| SS UA | 2.09 (1.59) | 1.05 (1.16) | 0.94 (0.86, 1.03) | <0.001 | 0.76 (0.68, 0.85) | <0.001 |
Differences are reported with women as referent category and adjusted for age and kidney stone status. aFurther adjustment for body weight. CaOx, calcium oxalate; CaP, calcium phosphate; SD, standard deviation; SS, supersaturation; UA, uric acid.
Results of the mediation analysis on urine components are reported in Table 6. Taken together, urinary components explained a meaningful proportion of the excess risk among men. Urine volume, oxalate, pH and citrate were all significant contributors, with each factor being responsible for 6.8–28.0% of the higher risk of stones among men.
Table 6.
Urinary contributors to the difference in risk of kidney stones between men and women
| Contributor | Percent mediated effect (95% CI) |
|---|---|
| Volume | 26.0 (20.0, 32.3) |
| Oxalate | 17.0 (10.9, 23.0) |
| pH | 8.4 (4.0, 12.8) |
| Citrate | 6.8 (2.5, 11.1) |
This table shows the relative contribution of each urinary factor to the excess risk of incident kidney stones among men
DISCUSSION
In our study, we report several findings of interest. First, while it is well-known that kidney stone disease manifests more frequently among men compared with women, with almost double the risk of developing a first symptomatic kidney stone among men, the excess risk remained across the span of ages included in our study. Known lifestyle risk factors for kidney stone disease explained only a fraction of the observed excess risk in men, demonstrating that other factors such as genetic or hormonal causes play a role. Post-menopausal women have a higher risk of forming stones compared with pre-menopausal women, indirectly suggesting a role of hormonal status in the risk of kidney stones [19]. In animal models, testosterone promoted the activity of glycolate oxidase and increased urinary excretion of oxalate [20]; furthermore, testosterone replacement therapy in men with hypogonadism was associated with an increased risk of stone formation [21].
Although in our study men remained more affected than women across the time periods considered, this difference tended to attenuate over time, resulting in a ∼50% relative risk reduction from earlier to later time periods. This phenomenon could be explained by changes in dietary habits and/or body composition over time, as well as the ageing of the male cohort. Interestingly, when we repeated our set of analyses using cohort rather than sex as the exposure, we found that women enrolled in NHS II, the more recently established female cohort, had a significantly higher risk of stones compared with women enrolled in NHS I, even within strata defined by age. Considering that the design and methodology of the two cohorts are almost identical, this could be taken as indirect evidence that women are more exposed to lithogenic factors in recent years than in the past. Our data also show an apparent reduction of kidney stone rates over time among both men and women. This phenomenon is likely due to ageing of the study population.
Our finding of a higher risk of kidney stones among men was corroborated by our urine studies, showing significantly higher supersaturation values for calcium oxalate and uric acid, emphasizing the tendency for a more lithogenic urinary profile in men. Also consistent with our hypothesis of a shift towards a more lithogenic environment for women, we showed that sex differences for supersaturations of each crystal type, a measure of the likelihood of urine to become saturated with certain chemical species, tended to attenuate over time. Our analysis showed that differences in urinary composition explain a substantial fraction of the excess risk of kidney stones found in men. In particular, lower urine volume, pH and urinary excretion of citrate and higher levels of sodium and oxalate explained a substantial proportion of the excess risk. Unfortunately, it is not methodologically feasible to compute sum estimates of the percent mediated effect, due to the potential interplay between factors [22]. However, the individual estimates of percent mediated effect convey information on the weight of each parameter on the difference between men and women.
Although previous studies reported a difference in kidney stone disease by sex, they were mostly cross-sectional in design and thus focussed on prevalence rather than incidence: sex-specific estimates from longitudinal cohort studies are very rare [23]. Prevalence figures could be affected by other elements such as disease duration, which in turn might reflect patterns of treatment and access to care. Furthermore, previous studies used only self-reported information about kidney stones (without demonstration of the validity of the self-report), whereas in our study we confirmed the outcome of interest. We previously showed that lack of validation could have a significant impact on results [24]. Censoring of asymptomatic kidney stone events, as implemented in our study, further improved the robustness of our findings by reducing the risk of misclassifying the passage or discovery of a previously present kidney stone as a new stone event. Most importantly, no previous studies could rely on the combination of confirmed data on incident kidney stones, detailed and validated data on nutrient intakes repeated over time and extensive data on urine composition to explore the potential mechanisms underlying the epidemiology of kidney stones in men and women. A further strength of our study is the identical study design and procedures for information collection for the three cohorts analysed.
Our study also has limitations. First, participants were in large majority White, thus potentially limiting the generalizability of the findings to other races. We did not have information on younger age groups in men; however, since in our study we excluded those participants with a history of kidney stones at baseline, this limitation is unlikely to have influenced our estimates. Another potential limitation of our study is related to urine analysis being performed by different laboratories over time. Finally, we did not have information on stone composition for a significant number of participants, which would have been useful to further explore potential mechanisms of stone formation in men and women, but our previous work suggests that the majority are primarily calcium oxalate [11].
In conclusion, men have a higher risk of forming kidney stones compared with women. Such difference is explained by lifestyle and urinary risk factors, in particular a significantly more lithogenic urinary profile in men. Lifestyle risk factors for kidney stones could be changing over time, giving rise to trends toward increased incidence of kidney stones among women. Future studies should explore the mechanisms by which these factors result in a higher risk of stone formation in men.
Supplementary Material
ACKNOWLEDGEMENTS
Research grants from the NIH: DK094910, DK91417, DK118057, CA186107, CA176726 and CA167552. P.M.F. is a member of the European Reference Network for Rare Kidney Diseases (ERKNet)—Project ID No 739532.
Contributor Information
Pietro Manuel Ferraro, U.O.S. Terapia Conservativa della Malattia Renale Cronica, U.O.C. Nefrologia, Dipartimento di Scienze Mediche e Chirurgiche, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy; Dipartimento Universitario di Medicina e Chirurgia Traslazionale, Università Cattolica del Sacro Cuore, Rome, Italy.
Eric N Taylor, Renal Division and Channing Division of Network Medicine, Department of Medicine, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, USA; Section of Nephrology, VA Maine Healthcare System, Augusta, ME, USA.
Gary C Curhan, Renal Division and Channing Division of Network Medicine, Department of Medicine, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, USA.
CONFLICT OF INTEREST STATEMENT
P.M.F. received consultant fees and grant support from Allena Pharmaceuticals, Alnylam, AstraZeneca, BioHealth Italia and Vifor Fresenius, and royalties as an author for UpToDate. G.C.C. is an employee of OM1, Inc., has received consulting fees from Allena Pharmaceuticals and receives royalties as a Section Editor and author for UpToDate. The other authors have nothing to disclose. The results presented in this paper have not been published previously in whole or part, except in abstract format.
AUTHORS’ CONTRIBUTIONS
Funding acquisition was carried out by E.N.T. and G.C.C.; data curation was performed by E.N.T. and G.C.C.; conceptualization was by G.C.C. and P.M.F.; formal analysis was carried out by P.M.F.; visualization was by P.M.F.; writing of the original draft was done by P.M.F.; and writing—review and editing was done by E.N.T. and G.C.C.
REFERENCES
- 1. Chen Z, Prosperi M, Bird VY. Prevalence of kidney stones in the USA: the National Health and Nutrition Evaluation Survey. J Clin Urol 2019; 12: 296–302 [Google Scholar]
- 2. Ferraro PM, Curhan GC, D'Addessi Aet al. Risk of recurrence of idiopathic calcium kidney stones: analysis of data from the literature. J Nephrol 2017; 30: 227–233 [DOI] [PubMed] [Google Scholar]
- 3. Ferraro PM, D'Addessi A, Gambaro G. When to suspect a genetic disorder in a patient with renal stones, and why. Nephrol Dial Transplant 2013; 28: 811–820 [DOI] [PubMed] [Google Scholar]
- 4. Ferraro PM, Taylor EN, Gambaro Get al. Dietary and lifestyle risk factors associated with incident kidney stones in men and women. J Urol 2017; 198: 858–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Borghi L, Meschi T, Amato Fet al. Urinary volume, water and recurrences in idiopathic calcium nephrolithiasis: a 5-year randomized prospective study. J Urol 1996; 155: 839–843 [PubMed] [Google Scholar]
- 6. Ferraro PM, Taylor EN, Gambaro Get al. Soda and other beverages and the risk of kidney stones. Clin J Am Soc Nephrol 2013; 8: 1389–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Curhan GC, Willett WC, Rimm EBet al. A prospective study of dietary calcium and other nutrients and the risk of symptomatic kidney stones. N Engl J Med 1993; 328: 833–838 [DOI] [PubMed] [Google Scholar]
- 8. Curhan GC, Willett WC, Speizer FEet al. Comparison of dietary calcium with supplemental calcium and other nutrients as factors affecting the risk for kidney stones in women. Ann Intern Med 1997; 126: 497–504 [DOI] [PubMed] [Google Scholar]
- 9. Curhan GC, Willett WC, Knight ELet al. Dietary factors and the risk of incident kidney stones in younger women: Nurses’ Health Study II. Arch Intern Med 2004; 164: 885–891 [DOI] [PubMed] [Google Scholar]
- 10. Ferraro PM, Mandel EI, Curhan GCet al. Dietary protein and potassium, diet-dependent net acid load, and risk of incident kidney stones. Clin J Am Soc Nephrol 2016; 11: 1834–1844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Taylor EN, Fung TT, Curhan GC. DASH-style diet associates with reduced risk for kidney stones. J Am Soc Nephrol 2009; 20: 2253–2259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Taylor EN, Stampfer MJ, Curhan GC. Obesity, weight gain, and the risk of kidney stones. JAMA 2005; 293: 455–462 [DOI] [PubMed] [Google Scholar]
- 13. Ferraro PM, Curhan GC, Gambaro Get al. Total, dietary, and supplemental vitamin C intake and risk of incident kidney stones. Am J Kidney Dis 2016; 67: 400–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ferraro PM, Taylor EN, Gambaro Get al. Vitamin D intake and the risk of incident kidney stones. J Urol 2017; 197: 405–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Holmes RP, Kennedy M. Estimation of the oxalate content of foods and daily oxalate intake. Kidney Int 2000; 57: 1662–1667 [DOI] [PubMed] [Google Scholar]
- 16. Al-Shaar L, Yuan C, Rosner Bet al. Reproducibility and validity of a semi-quantitative food frequency questionnaire in men assessed by multiple methods. Am J Epidemiol 2021; 190: 1122–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yuan C, Spiegelman D, Rimm EBet al. Validity of a dietary questionnaire assessed by comparison with multiple weighed dietary records or 24-Hour recalls. Am J Epidemiol 2017; 185: 570–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lin DY, Fleming TR, De Gruttola V. Estimating the proportion of treatment effect explained by a surrogate marker. Stat Med 1997; 16: 1515–1527 [DOI] [PubMed] [Google Scholar]
- 19. Prochaska M, Taylor EN, Curhan G. Menopause and risk of kidney stones. J Urol 2018; 200: 823–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yoshihara H, Yamaguchi S, Yachiku S. Effect of sex hormones on oxalate-synthesizing enzymes in male and female rat livers. J Urol 1999; 161: 668–673 [PubMed] [Google Scholar]
- 21. McClintock TR, Valovska MTI, Kwon NKet al. Testosterone replacement therapy is associated with an increased risk of urolithiasis. World J Urol 2019; 37: 2737–2746 [DOI] [PubMed] [Google Scholar]
- 22. VanderWeele T, Vansteelandt S. Mediation analysis with multiple mediators. Epidemiol Method 2014; 2: 95–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kittanamongkolchai W, Vaughan LE, Enders FTet al. The changing incidence and presentation of urinary stones over 3 decades. Mayo Clin Proc 2018; 93: 291–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ferraro PM, Curhan GC, Sorensen MDet al. Physical activity, energy intake, and the risk of incident kidney stones. J Urol 2015; 193: 864–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.