Abstract
Climate change should be of special concern for the nephrologist, as the kidney has a critical role in protecting the host from dehydration, but it is also a favorite target of heat stress and dehydration. Here we discuss how rising temperatures and extreme heat events may affect the kidney. The most severe presentation of heat stress is heat stroke, which can result in severe electrolyte disturbance and both acute and chronic kidney disease (CKD). However, lesser levels of heat stress also have multiple effects, including exacerbating kidney disease and precipitating cardiovascular events in subjects with established kidney disease. Heat stress can also increase the risk for kidney stones, cause multiple electrolyte abnormalities and induce both acute and chronic kidney disease. Recently there have been multiple epidemics of CKD of uncertain etiology in various regions of the world, including Mesoamerica, Sri Lanka, India and Thailand. There is increasing evidence that climate change and heat stress may play a contributory role in these conditions, although other causes, including toxins, could also be involved. As climate change worsens, the nephrologist should prepare for an increase in diseases associated with heat stress and dehydration.
Keywords: CKD of unknown etiology, CKD of uncertain etiology, CKD of non-traditional cause, dehydration, global warming, heat stress, heat stroke, Mesoamerican nephropathy, nephrolithiasis, Sri Lankan nephropathy
INTRODUCTION
Climate change carries a significant threat to humanity. Increasing greenhouse gas emissions have raised ambient temperatures, triggered extreme weather events and caused sea level rise that can threaten food security and nutrition, encourage the spread of infectious diseases and displace populations, with major effects on human health [1, 2]. One of the major consequences of climate change is increasing temperatures, which has not only caused an increase in the mean temperature of 1.0°C in the last century, but is also responsible for up to 75% of heat extremes [3].
The kidney is on ‘center stage’ in climate change, having key roles in protecting against heat-associated morbidities, but also being one of the main organs injured by its wrath. Here we provide a brief discussion of the main heat-related illnesses that are expected to increase over the next decades and how they are expected to affect our specialty. Increasing temperatures are expected to not only increase the frequency of classic heat-associated diseases such as heat stroke, but may also exacerbate traditional kidney diseases and potentially lead to the emergence of new diseases.
HEAT STROKE: THE CLASSICAL HEAT-ASSOCIATED KIDNEY DISEASE
Heat stroke represents the most well-known heat-associated illness and presents with high fevers (core body temperature >40°C), confusion or coma, light headedness and headaches. It is a life-threatening condition that can be associated with seizures, shock, multiorgan failure and death. There are two major presentations (Figure 1) [4].
FIGURE 1.
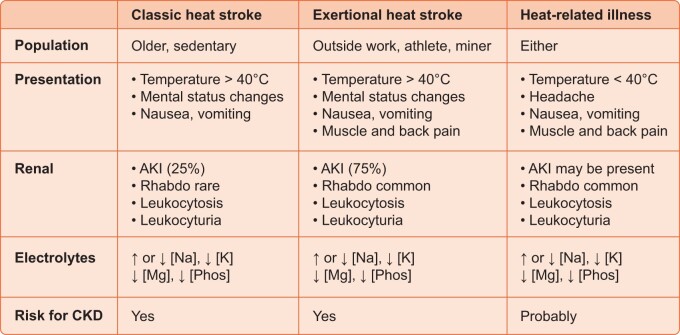
Renal manifestations of heat stroke and heat-associated illness. Heat stroke refers to a condition of high body core temperatures (>40°C) associated with mental status changes and can occur from simple heat exposure (classic or epidemic form) or from the combination of heat and exercise. The second form is more commonly associated with AKI and electrolyte abnormalities. In both cases, AKI may occur from either rhabdomyolysis (rhabdo) or from direct effects of heat. Both can be associated with electrolyte abnormalities and increased risk for CKD. Less severe heat-related illnesses in which body temperature does not reach 40°C are being increasingly recognized as also increasing the risk for AKI, electrolyte abnormalities and CKD.
Classic heat stroke tends to occur during heat waves and affects primarily older people who lack air conditioning and have limited access to water [5]. Epidemics of classic heat stroke have been associated with major heat waves, such as in Europe in 2003 [6], Chicago in 2005 [7] and India and Pakistan in 2015 [8, 9]. The other major presentation is exertional heat stroke, which typically involves individuals exercising or working in the heat, such as military recruits, athletes, agricultural workers and farmers, miners and factory workers [4]. Typically, exertional heat stroke is associated with much more sweating, and some subjects with classic heat stroke may have a history of minimal sweating. This is likely one reason electrolyte abnormalities tend to be more severe with exertional heat stroke [10]. Nevertheless, electrolyte abnormalities are common in both disorders. For example, ∼50% of subjects presenting with nonexertional heat stroke present with hyponatremia (32%) or hypernatremia (17%), with the latter being more commonly associated with obtundation and a higher mortality risk [11]. Hypokalemia is especially common in those not presenting with acute kidney injury (AKI) and total body potassium stores are usually low even if serum potassium is in the low ‘normal’ range [12]. Hypophosphatemia, hypocalcemia and hypomagnesemia may also occur [13–15]. Hyperuricemia is common. The urine is often concentrated, with leukocyturia, microhematuria and minimal proteinuria [16].
AKI also commonly complicates heat stroke and may be associated with septicemia (likely from heat-associated gut leak with endotoxemia or bacteremia) or may occur independent of infection [17]. Liver dysfunction or liver failure can also accompany AKI [18]. Approximately 75% of AKI is due to rhabdomyolysis, while 25% may relate to effects of high temperatures or dehydration, with the former being more common with exertional heat stroke [4, 10, 19]. Some individuals will need temporary dialysis [19]. Kidney biopsies, if performed, show not only acute tubular necrosis, but are also characterized by substantial interstitial inflammation resembling acute interstitial nephritis [16, 20]. While many recover their kidney function, over time there is a marked increased risk for chronic kidney disease (CKD), with biopsies showing chronic tubulointerstitial disease with glomerulosclerosis [20–22].
Pathogenesis
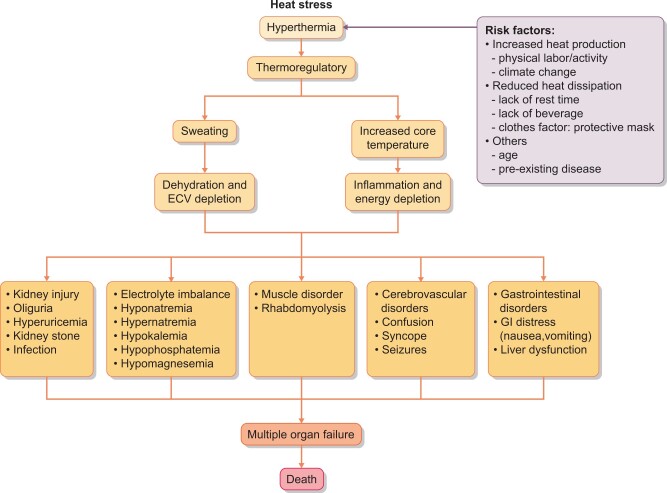
A primary goal of the body is to maintain body core temperature within a set range, and one of the main ways it does this is by sweating, which helps dissipate heat as it evaporates off the skin. Body heat increases not only from ambient and solar radiation, but also from ‘metabolic’ heat generated by body metabolism, which can increase markedly in the setting of exertion. The body can sweat as much as 10–12 L/day, which can lead to substantial loss of sodium and potassium [14]. This is associated with a relatively greater decrease in plasma and extracellular volume with some shift of water from the intracellular to extracellular spaces. The decrease in plasma volume stimulates vasopressin, catecholamines, cortisol and the renin–angiotensin–aldosterone system, and the urine will show a prerenal pattern with low urine sodium and paradoxical kaliuresis despite total body potassium deficiency.
These initial responses are all aimed at keeping body temperatures from rising. Indeed, subjects who work in hot conditions will undergo ‘heat acclimation’, which takes 3–14 days [23, 24]. This involves reducing their core temperature, increasing their sweating rate, expanding their plasma volume, increasing their cardiac output (by increasing stroke volume and lowering their heart rate) and reducing oxygen uptake and glycogen utilization in the muscle [23, 24]. This adaptation explains why subjects are most prone to heat stroke during the first week of working in an extremely hot environment.
A consequence of these adaptations is a high risk for both extracellular volume depletion (leading to hypotension) or total body water depletion (dehydration) if volume resuscitation is not maintained (Figure 2). Dehydration results in an increase in serum osmolality (Osm) that can stimulate both vasopressin secretion and activation of the aldose reductase pathway, and both can induce kidney injury if persistently stimulated [25, 26]. The decrease in intracellular potassium in the muscles prevents glycogen deposition and may be responsible for the rhabdomyolysis [12]. As compensation fails and temperatures rise, the primary effect is to stimulate inflammatory pathways, including heat shock proteins and cytokines [10, 17]. This is likely why inflammation is prominent in kidney biopsies.
FIGURE 2.
Pathogenesis of heat stroke. Heat stress leads to an increase in temperature. One of the basic defense systems is sweating, which can lead to dehydration and extracellular volume depletion. Increasing core temperatures also activate inflammatory pathways. The consequence is multiple organ dysfunction and increased risk for death.
OTHER HEAT-ASSOCIATED ILLNESSES
While heat stroke has a dramatic presentation with a body core temperature >40°C, there are also many other less severe presentations that can occur in the emergency room related to heat stress, including heat syncope, heat exhaustion, heat fatigue and heat cramps [21] (Figure 1). These milder presentations can also be associated with electrolyte abnormalities and AKI. For example, AKI is common with exercising in the heat and can be asymptomatic and associated with increased excretion of biomarkers of kidney damage or more severe with oliguric AKI from rhabdomyolysis [27, 28]. In fact, it is possible to induce markers of AKI with exercise in the heat, and the biomarkers tend to associate more with an inflammatory pattern than the one associated with dehydration. These milder illnesses can also be associated with long-term consequences. For example, a recent study from Taiwan provided evidence that individuals presenting with these conditions are also at increased risk for developing CKD later in life compared with age- and morbidity-matched controls who did not have any heat-associated illnesses [21].
Next, we will review some of the other manifestations of climate change on the kidney.
KIDNEY STONES AND CRYSTALLURIA
One of the major risk factors for kidney stones is dehydration, leading to concentration and acidification of the urine that increases the risk for uric acid nephrolithiasis [29]. Urinary concentration can also lead to an increased risk for supersaturation of calcium, with crystallization and stone formation [30]. The southern USA is famous for being the ‘Stone Belt’, due to its higher ambient temperatures and propensity for dehydration. Climate change is predicted to widen the Stone Belt and to markedly increase the risk for kidney stones in the future [31].
Hot climates may also stimulate the intake of sugary sodas, which are also a major risk factor for kidney stones [32]. The fructose in soft drinks also causes urinary concentration due to shifting plasma water into the cell, likely in association with glycogen production, and this, coupled with the stimulation of uric acid production and excretion, can further increase the risk for kidney stones [33]. Fructose also stimulates adenosine triphosphate (ATP) citrate lyase, which in the kidney governs urinary citrate concentrations, and we found that fructose administration could reduce urinary citrate levels in healthy volunteers [34].
In hot rural communities in Mesoamerica, Sri Lanka, India and Thailand, there is a condition in which subjects develop painful dysuria associated with the passing of sand-like material in the absence of urinary tract infection [35]. The passage of this sand-like or gravel-like material is thought to be due to crystalluria, likely from uric acid or calcium related to chronic dehydration associated with manual labor in the heat. Our group performed a study in which we evaluated sugarcane workers before and after their work shift during the sugarcane harvest. While we found that 15% of subjects tended to have urate crystalluria in postshift urine samples during the harvest [36], on one occasion we noted 100% to have urate crystalluria that also was at concentrations typically observed in tumor lysis syndrome (urine uric acid >100 mg/dL). It turned out that this latter analysis was done during a heat wave in which temperatures were the highest for that year.
EXACERBATION OF SUBJECTS WITH EXISTING CKD
Subjects with CKD are especially prone to heat-associated illnesses due to reduced thermoregulatory ability [37]. Indeed, the subjects most at risk for heat stroke include not only older subjects, but also those with diabetes, obesity and CKD. Recent studies suggest that subjects with obesity also tend to drink less water, to be hyperosmolar and to have elevated vasopressin (measured as copeptin) levels, so they are even more prone to volume depletion or hyperosmolality [38, 39]. Interestingly, they tend to have lower body temperatures, especially during the day, but their ability to dissipate heat is also less efficient [40]. Thus it is likely that the risk for AKI and electrolyte abnormalities from heat stress may be exacerbated in subjects with CKD, diabetes or metabolic syndrome [39]. Furthermore, dialysis patients are particularly sensitive to extreme heat events and have shown a relatively increased risk of hospital admission and mortality [41].
There is also an interesting but largely unstudied possibility that heat stress may increase the risk of developing obesity and diabetes. In addition to the linkage of heat stress with soft drink intake, there is increasing evidence that dehydration may increase endogenous fructose production, which can increase the risk for obesity, diabetes and both diabetic and nondiabetic kidney disease [42, 43]. Fructose metabolism has also been shown to drive vasopressin production, which can induce metabolic syndrome via activation of vasopressin 1b receptor [44]. Heat stress and dehydration, by stimulating fructose and vasopressin production, might be expected to increase the risk for both diabetes and obesity, as well as kidney injury associated with these diseases.
CKD OF UNKNOWN ETIOLOGY
In recent years, numerous epidemics of CKD have been identified primarily in hot, rural regions of the world. The best-described sites include Mesoamerican nephropathy along the Pacific Coast of Mesoamerica [45–47], CKD of unknown etiology in Tierra Blanca, Mexico [48], Sri Lankan nephropathy in northern Sri Lanka [49, 50] and Uddanam nephropathy in the Andhra Pradesh region of India [51, 52]. Other emerging sites include north central Thailand, Qatar and Egypt.
All of these epidemics have many similar characteristics. First, they are all occurring among people who are working outside under hot conditions, usually in agricultural communities. Most of those involved are young or middle-aged males (especially in Mesoamerica), who are often poor, with minimal if any medical insurance. In Sri Lanka, women working in the rice paddies account for nearly half of the cases. The occupations can vary, with working in the sugarcane fields being the most common in Mesoamerica and working in the rice paddies being most common in Sri Lanka. In India, there are a variety of occupations, including harvesting rice and cashews, while in Thailand it is usually either rice or sugarcane workers. In addition, kidney disease has also been reported in other nonagricultural jobs in these regions, including construction, fishing, gold mining and brick making [53]. While these diseases are common in individuals who have lived all of their lives in these regions, they also occurs among migrant workers. In Qatar, for example, most workers originate from Nepal [54].
The clinical presentation is similar in all of these epidemics. Usually the patient has elevated serum creatinine identified during a health screening [45, 55]. Diabetes is absent and blood pressure (BP) is normal or only slightly elevated (i.e. BP is usually <140/90 mm Hg). At this stage, the patient is often asymptomatic, although some may have a history of painful dysuria from crystalluria (see above). Laboratory tests often show hypokalemia, hyperuricemia, hypo- or hypernatremia and occasionally low serum phosphate or magnesium levels [56]. Urine usually shows low-grade (<1 g/day) or no proteinuria, often with occasional white cells and red cells [57, 58]. Some will have evidence of urate crystals in the urinary sediment [36]. Kidney biopsy, if performed, usually shows a type of chronic tubulointerstitial nephritis with a variable amount of fibrosis and inflammation. The glomeruli may show some wrinkling, as well as mesangial expansion with or without global glomerulosclerosis [56, 59, 60].
An acute presentation has also been reported in both Mesoamerica and Sri Lanka [61–63]. In this rarer presentation, patients will get sick while working and will present in the emergency room with fever (55%), nausea and vomiting (50%) and headaches (50%), often with muscle weakness and back pain. These patients may have anemia, leukocytosis, hypokalemia and hyperuricemia and often have a urinalysis that shows leukocyturia (98%) with or without hematuria and proteinuria. Biopsies in these patients show acute interstitial nephritis with tubular injury [61–63]. In Mesoamerica, those who present with AKI are from among the job categories with the heaviest workload.
The natural history of this mysterious CKD of unknown cause (CKDu) is one of progressive deterioration of renal function leading to uremia. Unfortunately, most of the individuals who develop these conditions are disadvantaged and lack adequate medical care, carry no insurance, are unable to receive adequate dialysis and end up dying of kidney failure.
Etiology
The similarities in epidemiology, clinical presentation, laboratory abnormalities, histologic findings and natural history have suggested that the CKDu from these endemic regions may have a common etiology. Today the leading hypothesis is that it represents a type of heat stress–related injury [17]. In particular, the concept is that these subjects are developing subtle injury to their kidneys each day while they are in the field that causes CKD over time, or they may have an occasional more severe AKI that has the same effect of progressing to CKD. Supporting evidence is that up to 30% of patients with AKI diagnosed during a sugarcane harvest will progress to CKD 6–12 months later [64]. Some patients with the more severe acute presentation also progress to CKD [65]. The reason these epidemics have emerged has also been attributed to climate change and an increase in heat waves [66].
There is substantial supporting evidence for this theory [67]. First, there is evidence, especially in Sri Lanka and Mesoamerica, that the epidemics are not simply due to better diagnosis and recognition, but rather represent a true increase in the prevalence of CKD since the 1970s and 1980s. For example, a study in the Guanacaste region of Costa Rica that was based on autopsy reports documented a dramatic increase in CKDu beginning in the 1970s [68]. Second, the sites where CKDu is occurring typically represent some of the hottest areas in the region [66, 69]. Third, all the occupations are associated with intense heat exposure and symptoms of dehydration are common [70, 71]. Some studies from Mesoamerica have also shown that the risk for developing CKD is greater if the workers are working in sugarcane fields at sea level where it is hotter, as opposed to sugarcane fields at higher altitudes where temperatures are cooler [57]. Fourth, there is evidence from cross-shift studies in Mesoamerica that individuals are often becoming mildly dehydrated and develop cross-shift evidence for acute reductions in kidney dysfunction and the development of hyperuricemia [72, 73]. Similar findings have been demonstrated for volunteers who exercise in the heat [27, 74]. Fifth, experimentally it has been possible to induce CKD in rats by repeated exposure to heat and dehydration that is histologically similar to that observed in these epidemics [25]. The degree of renal injury can be enhanced if the core temperature of the rats is increased by giving mitochondrial uncoupling agents [75]. Sixth, there is increasing evidence that measures to reduce heat stress, such as the implementation of better hydration, shade and rest, can reduce the frequency of individuals developing cross-shift AKI [76, 77]. Finally, the clinical and pathophysiological similarities between CKDu and the acute and chronic effects of heat stroke make a compelling case for a similar pathogenesis.
Pathogenesis
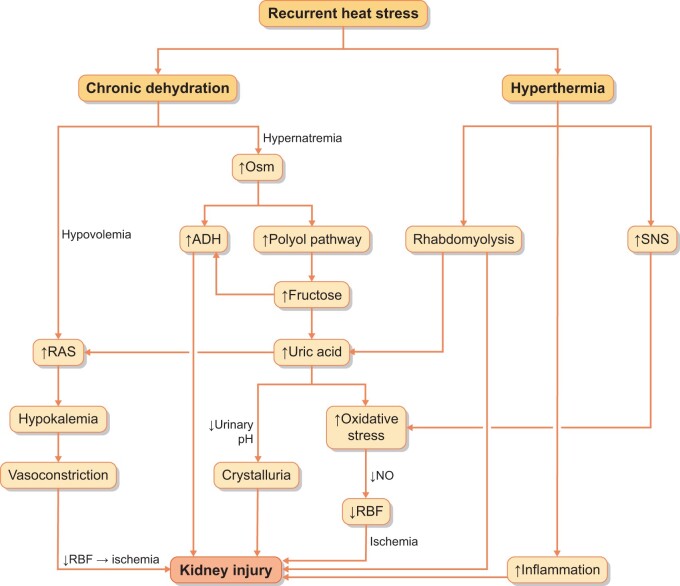
The pathogenesis of CKDu may involve both heat-related mechanisms and mechanisms associated with dehydration (Osm) (Figure 3). For example, recurrent hyperosmolality can induce activation of the polyol (aldose reductase) pathway in the kidney, leading to local fructose generation that can be metabolized in the proximal tubule to release oxidants, uric acid and inflammatory cytokines and chemotactic factors [25]. This injury can be amplified if the rehydration fluid has a high content of fructose (such as with sucrose or high fructose corn syrup) [78]. Similarly, recurrent dehydration also stimulates vasopressin production, and chronically elevated vasopressin levels can also induce both glomerular and tubular injury [79–81].
FIGURE 3.
Proposed pathogenesis of CKDu. Current thinking is that heat stress may lead to CKD via two major pathways. One mechanism results from excessive sweating, leading to dehydration, extracellular volume depletion and electrolyte abnormalities. The other major mechanism is from the effects of hyperthermia, stimulating inflammation, causing energy depletion and activating the sympathetic nervous system and renin–angiotensin system. RBF, renal blood flow; ADH, antidiuretic hormone or vasopressin.
Other potential mechanisms include a direct thermal pathway of injury that is linked with intracellular ATP depletion and stimulation of inflammation [82–85]. Other possible contributors could include the effects of chronic hypokalemia to cause vasoconstriction, the possibility of rhabdomyolysis as a potential contributor or the effects of hyperuricemia or uricosuria. Indeed, there is one experimental study that found that allopurinol can prevent both kidney and liver injury associated with recurrent heat stress and dehydration [86].
Limitations
While the evidence that heat stress and/or dehydration is involved in the pathogenesis of the epidemics of CKDu worldwide is strong, these studies do not exclude other potential contributing factors such as environmental toxins (agrochemicals and heavy metals in drinking water), infection diseases and working conditions. Dehydration stimulates the reabsorption of fluid in the proximal tubule and would be expected to amplify the uptake and toxicity of nephrotoxins. One toxin we have been concerned about is silica, which is present in sugarcane and rice husk ash and could be inhaled or ingested via contaminated drinking water. An ironic aspect is that the generation of this ash from burning the cane and rice husks increases black carbon and atmospheric biomass that can increase the greenhouse effect and contribute further to climate change.
In summary, climate change will, and likely is, having a very significant effect on our specialty. Climate change may be causing not only electrolyte disturbances and worsening existing kidney diseases, but it may also have a role in the appearance of new diseases that may dominate the future. Due to the increase in CKDu, medical expenses related to kidney disease treatment, including dialysis, will increase and more resources will be consumed, thus we need more physicians with an interest in investigating and developing new and effective therapies to treat diseases associated with heat stress and dehydration. Where is Sherlock Holmes when we need him? [67]
FUNDING
This study was supported by funds from the National Institutes of Health (DK125351) and the La Isla Network.
CONFLICT OF INTEREST STATEMENT
R.J.J. declares he has received honoraria from Danone and Horizon Pharma and also has equity in Colorado Research Partners and XORTX Therapeutics. M.A.L., C.R.R. and L.G.L. also have equity in Colorado Research Partners. All other authors declare no conflicts of interest. In addition, the University of Colorado has a Memorandum of Understanding with Pantaleon, Guatemala City, Guatemala to enable research in strict adherence to principles of scientific independence and integrity. The opinions expressed by the authors do not represent the position of the U.S. Department of Health and Human Services, the Centers for Disease Control and Prevention.
Contributor Information
Fumihiko Sasai, Division of Renal Diseases and Hypertension, University of Colorado Anschutz Medical Campus, Aurora, CO, USA.
Carlos Roncal-Jimenez, Division of Renal Diseases and Hypertension, University of Colorado Anschutz Medical Campus, Aurora, CO, USA.
Keegan Rogers, Department of Pharmaceutical Sciences, University of Colorado Anschutz Medical Campus, Aurora, CO, USA.
Yuka Sato, Department of Nephrology, Nagoya University Graduate School of Medicine, Nagoya, Japan.
Jared M Brown, Department of Pharmaceutical Sciences, University of Colorado Anschutz Medical Campus, Aurora, CO, USA.
Jason Glaser, La Isla Network, Léon, Nicaragua.
Gabriela Garcia, Division of Renal Diseases and Hypertension, University of Colorado Anschutz Medical Campus, Aurora, CO, USA.
Laura Gabriela Sanchez-Lozada, Laboratory of Renal Physiopathology, Instituto Nacional de Cardiologia, Ignacio Chavez, Mexico City.
Bernardo Rodriguez-Iturbe, Laboratory of Renal Physiopathology, Instituto Nacional de Cardiologia, Ignacio Chavez, Mexico City; Instituto Nacional de Cencias Médicas y Nutrición "Salvador Zubirán", Department of Nephrology, Mexico City, Mexico.
Jaime Butler Dawson, Center for Health, Work and Environment, Colorado School of Public Health, University of Colorado Anschutz Medical Campus, Aurora, CO, USA.
Cecilia Sorensen, Center for Health, Work and Environment, Colorado School of Public Health, University of Colorado Anschutz Medical Campus, Aurora, CO, USA.
Ana Andres Hernando, Division of Renal Diseases and Hypertension, University of Colorado Anschutz Medical Campus, Aurora, CO, USA.
Marvin Gonzalez-Quiroz, Research Centre on Health, Work and Environment (CISTA), National Autonomous University of Nicaragua, León, Nicaragua; Centre for Nephrology, University College London, London, UK.
Miguel Lanaspa, Division of Renal Diseases and Hypertension, University of Colorado Anschutz Medical Campus, Aurora, CO, USA.
Lee S Newman, Center for Health, Work and Environment, Colorado School of Public Health, University of Colorado Anschutz Medical Campus, Aurora, CO, USA.
Richard J Johnson, Division of Renal Diseases and Hypertension, University of Colorado Anschutz Medical Campus, Aurora, CO, USA.
REFERENCES
- 1. Lemery J, Knowlton K, Sorensen C.. Global Climate Change and Human Health: From Science to Practice. Hoboken, NJ: John Wiley & Sons, 2015 [Google Scholar]
- 2. Patz JA, Frumkin H, Holloway T. et al. Climate change: challenges and opportunities for global health. JAMA 2014; 312: 1565–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rahmstorf S, Coumou D.. Increase of extreme events in a warming world. Proc Natl Acad Sci USA 2011; 108: 17905–17909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hart GR, Anderson RJ, Crumpler CP. et al. Epidemic classical heat stroke: clinical characteristics and course of 28 patients. Medicine (Baltimore) 1982; 61: 189–197 [PubMed] [Google Scholar]
- 5. Hopp S, Dominici F, Bobb JF.. Medical diagnoses of heat wave-related hospital admissions in older adults. Prev Med 2018; 110: 81–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barriopedro D, Fischer EM, Luterbacher J. et al. The hot summer of 2010: redrawing the temperature record map of Europe. Science 2011; 332: 220–224 [DOI] [PubMed] [Google Scholar]
- 7. Hartz DA, Golden JS, Sister C. et al. Climate and heat-related emergencies in Chicago, Illinois (2003–2006). Int J Biometeorol 2012; 56: 71–83 [DOI] [PubMed] [Google Scholar]
- 8. Sarath Chandran MA, Subba Rao AVM, Sandeep VM. et al. Indian summer heat wave of 2015: a biometeorological analysis using half hourly automatic weather station data with special reference to Andhra Pradesh. Int J Biometeorol 2017; 61: 1063–1072 [DOI] [PubMed] [Google Scholar]
- 9. Saleem SG, Ansari T, Ali AS. et al. Risk factors for heat related deaths during the June 2015 heat wave in Karachi, Pakistan. J Ayub Med Coll Abbottabad 2017; 29: 320–324 [PubMed] [Google Scholar]
- 10. Leon LR, Bouchama A.. Heat stroke. Compr Physiol 2015; 5: 611–647 [DOI] [PubMed] [Google Scholar]
- 11. Hausfater P, Megarbane B, Fabricatore L. et al. Serum sodium abnormalities during nonexertional heatstroke: incidence and prognostic values. Am J Emerg Med 2012; 30: 741–748 [DOI] [PubMed] [Google Scholar]
- 12. Knochel JP. Potassium deficiency as the result of training in hot weather. In: Institute of Medicine (ed). Fluid Replacement and Heat Stress. Washington, DC: National Academy Press, 1993:117–126 [Google Scholar]
- 13. Knochel JP, Caskey JH.. The mechanism of hypophosphatemia in acute heat stroke. JAMA 1977; 238: 425–426 [PubMed] [Google Scholar]
- 14. Knochel JP, Beisel WR, Herndon EG Jr. et al. The renal, cardiovascular, hematologic and serum electrolyte abnormalities of heat stroke. Am J Med 1961; 30: 299–309 [DOI] [PubMed] [Google Scholar]
- 15. Satirapoj B, Kongthaworn S, Choovichian P. et al. Electrolyte disturbances and risk factors of acute kidney injury patients receiving dialysis in exertional heat stroke. BMC Nephrol 2016; 17: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kew MC, Abrahams C, Levin NW. et al. The effects of heatstroke on the function and structure of the kidney. Q J Med 1967; 36: 277–300 [PubMed] [Google Scholar]
- 17. Hansson E, Glaser J, Jakobsson K. et al. Pathophysiological mechanisms by which heat stress potentially induces kidney inflammation and chronic kidney disease in sugarcane workers. Nutrients 2020; 12: 1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kurowski J, Lin HC, Mohammad S. et al. Exertional heat stroke in a young athlete resulting in acute liver failure. J Pediatr Gastroenterol Nutr 2014; 63: e75–e76 [DOI] [PubMed] [Google Scholar]
- 19. Thongprayoon C, Qureshi F, Petnak T. et al. Impact of acute kidney injury on outcomes of hospitalizations for heat stroke in the United States. Diseases 2020; 8: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kew MC, Abrahams C, Seftel HC.. Chronic interstitial nephritis as a consequence of heatstroke. Q J Med 1970; 39: 189–199 [PubMed] [Google Scholar]
- 21. Wang JC, Chien WC, Chu P. et al. The association between heat stroke and subsequent cardiovascular diseases. PLoS One 2019; 14: e0211386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tseng MF, Chou CL, Chung CH. et al. Risk of chronic kidney disease in patients with heat injury: a nationwide longitudinal cohort study in Taiwan. PLoS One 2020; 15: e0235607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Benjamin CL, Sekiguchi Y, Struder JF. et al. Heat acclimation following heat acclimatization elicits additional physiological improvements in male endurance athletes. Int J Environ Res Public Health 2021; 18: 4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Saat M, Sirisinghe RG, Singh R. et al. Effects of short-term exercise in the heat on thermoregulation, blood parameters, sweat secretion and sweat composition of tropic-dwelling subjects. J Physiol Anthropol Appl Human Sci 2005; 24: 541–549 [DOI] [PubMed] [Google Scholar]
- 25. Roncal Jimenez CA, Ishimoto T, Lanaspa MA. et al. Fructokinase activity mediates dehydration-induced renal injury. Kidney Int 2014; 86: 294–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Garcia-Arroyo FE, Munoz-Jimenez I, Gonzaga G. et al. A role for both V1a and V2 receptors in renal heat stress injury amplified by rehydration with fructose. Int J Mol Sci 2019; 20: 5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Junglee NA, Di Felice U, Dolci A. et al. Exercising in a hot environment with muscle damage: effects on acute kidney injury biomarkers and kidney function. Am J Physiol Renal Physiol 2013; 305: F813–F820 [DOI] [PubMed] [Google Scholar]
- 28. Mansour SG, Verma G, Pata RW. et al. Kidney injury and repair biomarkers in marathon runners. Am J Kidney Dis 2017; 70: 252–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Borghi L, Meschi T, Amato F. et al. Hot occupation and nephrolithiasis. J Urol 1993; 150: 1757–1760 [DOI] [PubMed] [Google Scholar]
- 30. Borghi L, Meschi T, Amato F. et al. Urinary volume, water and recurrences in idiopathic calcium nephrolithiasis: a 5-year randomized prospective study. J Urol 1996; 155: 839–843 [PubMed] [Google Scholar]
- 31. Brikowski TH, Lotan Y, Pearle MS.. Climate-related increase in the prevalence of urolithiasis in the United States. Proc Natl Acad Sci USA 2008; 105: 9841–9846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ferraro PM, Taylor EN, Gambaro G. et al. Soda and other beverages and the risk of kidney stones. Clin J Am Soc Nephrol 2013; 8: 1389–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Johnson RJ, Stenvinkel P, Andrews P. et al. Fructose metabolism as a common evolutionary pathway of survival associated with climate change, food shortage and droughts. J Intern Med 2020; 287: 252–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Johnson RJ, Perez-Pozo SE, Lillo JL. et al. Fructose increases risk for kidney stones: potential role in metabolic syndrome and heat stress. BMC Nephrol 2018; 19: 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ramirez-Rubio O, Brooks DR, Amador JJ. et al. Chronic kidney disease in Nicaragua: a qualitative analysis of semi-structured interviews with physicians and pharmacists. BMC Public Health 2013; 13: 350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Roncal-Jimenez C, García-Trabanino R, Barregard L. et al. Heat stress nephropathy from exercise-induced uric acid crystalluria: a perspective on Mesoamerican nephropathy. Am J Kidney Dis 2016; 67: 20–30 [DOI] [PubMed] [Google Scholar]
- 37. Murota H. Sweating in systemic abnormalities: uremia and diabetes mellitus. Curr Probl Dermatol 2016; 51: 57–61 [DOI] [PubMed] [Google Scholar]
- 38. Stookey JD, Kavouras S, Suh H. et al. Underhydration is associated with obesity, chronic diseases, and death within 3 to 6 years in the U.S. population aged 51–70 years. Nutrients 2020; 12: 905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Velho G, Bouby N, Hadjadj S. et al. Plasma copeptin and renal outcomes in patients with type 2 diabetes and albuminuria. Diabetes Care 2013; 36: 3639–3645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Grimaldi D, Provini F, Pierangeli G. et al. Evidence of a diurnal thermogenic handicap in obesity. Chronobiol Int 2015; 32: 299–302 [DOI] [PubMed] [Google Scholar]
- 41. Remigio RV, Jiang C, Raimann J. et al. Association of extreme heat events with hospital admission or mortality among patients with end-stage renal disease. JAMA Netw Open 2019; 2: e198904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Song Z, Roncal-Jimenez CA, Lanaspa-Garcia MA. et al. Role of fructose and fructokinase in acute dehydration-induced vasopressin gene expression and secretion in mice. J Neurophysiol 2017; 117: 646–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lanaspa MA, Kuwabara M, Andres-Hernando A. et al. High salt intake causes leptin resistance and obesity in mice by stimulating endogenous fructose production and metabolism. Proc Natl Acad Sci USA 2018; 115: 3138–3143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Andres-Hernando A, Jensen TJ, Kuwabara M. et al. Vasopressin mediates fructose-induced metabolic syndrome by activating the V1b receptor. JCI Insight 2021; 6: e140848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wesseling C, Crowe J, Hogstedt C. et al. Mesoamerican Nephropathy: Report from the First International Research Workshop on MeN Heredia. San Jose, Costa Rica: SALTRA/IRET-UNA, 2013 [Google Scholar]
- 46. Garcia Trabanino R, Aguilar R, Silva CR. et al. Nefropatía terminal en pacientes de un hospital de referencia en El Salvador. Rev Panam Salud Publica 2002; 12: 202–206 [DOI] [PubMed] [Google Scholar]
- 47. Kupferman J, Amador JJ, Lynch KE. et al. Characterization of Mesoamerican nephropathy in a kidney failure hotspot in Nicaragua. Am J Kidney Dis 2016; 68: 716–725 [DOI] [PubMed] [Google Scholar]
- 48. Aguilar-Ramirez D, Rana-Custodio A, Villa A. et al. Decreased kidney function and agricultural work: a cross-sectional study in middle-aged adults from Tierra Blanca, Mexico. Nephrol Dial Transplant 2020; 36: 1030–1038 [DOI] [PubMed] [Google Scholar]
- 49. Wanigasuriya KP, Peiris-John RJ, Wickremasinghe R. et al. Chronic renal failure in North Central Province of Sri Lanka: an environmentally induced disease. Trans R Soc Trop Med Hyg 2007; 101: 1013–1017 [DOI] [PubMed] [Google Scholar]
- 50. Wijetunge S, Ratnatunga NV, Abeysekera TD. et al. Endemic chronic kidney disease of unknown etiology in Sri Lanka: correlation of pathology with clinical stages. Indian J Nephrol 2015; 25: 274–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tatapudi RR, Rentala S, Gullipalli P. et al. High prevalence of CKD of unknown etiology in Uddanam, India. Kidney Int Rep 2019; 4: 380–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Farag YMK, Karai Subramanian K, Singh VA. et al. Occupational risk factors for chronic kidney disease in Andhra Pradesh: ‘uddanam nephropathy’. Ren Fail 2020; 42: 1032–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gallo-Ruiz L, Sennett CM, Sanchez-Delgado M. et al. Prevalence and risk factors for CKD among brickmaking workers in La Paz Centro, Nicaragua. Am J Kidney Dis 2019; 74: 239–247 [DOI] [PubMed] [Google Scholar]
- 54. Pradhan B, Kjellstrom T, Atar D. et al. Heat stress impacts on cardiac mortality in Nepali migrant workers in Qatar. Cardiology 2019; 143: 37–48 [DOI] [PubMed] [Google Scholar]
- 55. Wimalawansa SJ. Escalating chronic kidney diseases of multi-factorial origin (CKD-mfo) in Sri Lanka: causes, solutions, and recommendations-update and responses. Environ Health Prev Med 2015; 20: 152–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wijkstrom J, Jayasumana C, Dassanayake R. et al. Morphological and clinical findings in Sri Lankan patients with chronic kidney disease of unknown cause (CKDu): similarities and differences with Mesoamerican nephropathy. PLoS One 2018; 13: e0193056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Torres C, Aragon A, Gonzalez M. et al. Decreased kidney function of unknown cause in Nicaragua: a community-based survey. Am J Kidney Dis 2010; 55: 485–496 [DOI] [PubMed] [Google Scholar]
- 58. O’Donnell JK, Tobey M, Weiner DE. et al. Prevalence of and risk factors for chronic kidney disease in rural Nicaragua. Nephrol Dial Transplant 2011; 26: 2798–2805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wijkstrom J, Gonzalez-Quiroz M, Hernandez M. et al. Renal morphology, clinical findings, and progression rate in Mesoamerican nephropathy. Am J Kidney Dis 2017; 69: 626–636 [DOI] [PubMed] [Google Scholar]
- 60. Anand S, Montez-Rath ME, Adasooriya D. et al. Prospective biopsy-based study of CKD of unknown etiology in Sri Lanka. Clin J Am Soc Nephrol 2019; 14: 224–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Fischer RSB, Mandayam S, Chavarria D. et al. Clinical evidence of acute Mesoamerican nephropathy. Am J Trop Med Hyg 2017; 97: 1247–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fischer RSB, Vangala C, Truong L. et al. Early detection of acute tubulointerstitial nephritis in the genesis of Mesoamerican nephropathy. Kidney Int 2018; 93: 681–690 [DOI] [PubMed] [Google Scholar]
- 63. Badurdeen Z, Nanayakkara N, Ratnatunga NV. et al. Chronic kidney disease of uncertain etiology in Sri Lanka is a possible sequel of interstitial nephritis!. Clin Nephrol 2016; 86: 106–109 [DOI] [PubMed] [Google Scholar]
- 64. Kupferman J, Ramirez-Rubio O, Amador JJ. et al. Acute kidney injury in sugarcane workers at risk for Mesoamerican nephropathy. Am J Kidney Dis 2018; 72: 475–482 [DOI] [PubMed] [Google Scholar]
- 65. Fischer RSB, Vangala C, Mandayam S. et al. Clinical markers to predict progression from acute to chronic kidney disease in Mesoamerican nephropathy. Kidney Int 2018; 94: 1205–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Glaser J, Lemery J, Rajagopalan B. et al. Climate change and the emergent epidemic of CKD from heat stress in rural communities: the case for heat stress nephropathy. Clin J Am Soc Nephrol 2016; 11: 1472–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Johnson RJ. Pro: heat stress as a potential etiology of Mesoamerican and Sri Lankan nephropathy: a late night consult with Sherlock Holmes. Nephrol Dial Transplant 2017; 32: 598–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wesseling C, van Wendel de Joode B, Crowe J. et al. Mesoamerican nephropathy: geographical distribution and time trends of chronic kidney disease mortality between 1970 and 2012 in Costa Rica. Occup Environ Med 2015; 72: 714–721 [DOI] [PubMed] [Google Scholar]
- 69. Hansson E, Mansourian A, Farnaghi M. et al. An ecological study of chronic kidney disease in five Mesoamerican countries: associations with crop and heat. BMC Public Health 2021; 21: 840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Crowe J, Wesseling C, Solano BR. et al. Heat exposure in sugarcane harvesters in Costa Rica. Am J Ind Med 2013; 56: 1157–1164 [DOI] [PubMed] [Google Scholar]
- 71. Siriwardhana EA, Perera PA, Sivakanesan R. et al. Dehydration and malaria augment the risk of developing chronic kidney disease in Sri Lanka. Indian J Nephrol 2015; 25: 146–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Garcia-Trabanino R, Jarquin E, Wesseling C. et al. Heat stress, dehydration, and kidney function in sugarcane cutters in El Salvador—a cross-shift study of workers at risk of Mesoamerican nephropathy. Environ Res 2015; 142: 746–755 [DOI] [PubMed] [Google Scholar]
- 73. Sorensen CJ, Butler-Dawson J, Dally M. et al. Risk factors and mechanisms underlying cross-shift decline in kidney function in Guatemalan sugarcane workers. J Occup Environ Med 2018; 61: 239–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Schlader ZJ, Chapman CL, Sarker S. et al. Firefighter work duration influences the extent of acute kidney injury. Med Sci Sports Exerc 2017; 49: 1745–1753 [DOI] [PubMed] [Google Scholar]
- 75. Sato Y, Roncal-Jimenez CA, Andres-Hernando A. et al. Increase of core temperature affected the progression of kidney injury by repeated heat stress exposure. Am J Physiol Renal Physiol 2019; 317: F1111–F1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Solis Zepeda GA. Impacto de las medidas preventivas para evitar el deterioro de la función renal por el Síndrome de Golpe por Calor en trabajadores agrícolas del Ingenio San Antonio del Occidente de Nicaragua, Ciclo Agrícola 2005–2006. PhD Thesis. Internal Medicine Department, León, Nicaragua: Universidad Nacional Autónoma de Nicaragua, 2007
- 77. Wegman DH, Apelqvist J, Bottai M. et al. Intervention to diminish dehydration and kidney damage among sugarcane workers. Scand J Work Environ Health 2018; 44: 16–24. [DOI] [PubMed] [Google Scholar]
- 78. Garcia-Arroyo FE, Cristobal M, Arellano-Buendia AS. et al. Rehydration with soft drink-like beverages exacerbates dehydration and worsens dehydration-associated renal injury. Am J Physiol Regul Integr Comp Physiol 2016; 311: R57–R65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bouby N, Bachmann S, Bichet D. et al. Effect of water intake on the progression of chronic renal failure in the 5/6 nephrectomized rat. Am J Physiol 1990; 258: F973–F979 [DOI] [PubMed] [Google Scholar]
- 80. Bankir L, Bouby N, Ritz E.. Vasopressin: a novel target for the prevention and retardation of kidney disease? Nat Rev Nephrol 2013; 9: 223–239 [DOI] [PubMed] [Google Scholar]
- 81. Garcia-Arroyo FE, Tapia E, Blas-Marron MG. et al. Vasopressin mediates the renal damage induced by limited fructose rehydration in recurrently dehydrated rats. Int J Biol Sci 2017; 13: 961–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Bouchama A, Hammami MM, Haq A. et al. Evidence for endothelial cell activation/injury in heatstroke. Crit Care Med 1996; 24: 1173–1178 [DOI] [PubMed] [Google Scholar]
- 83. Bouchama A, Bridey F, Hammami MM. et al. Activation of coagulation and fibrinolysis in heatstroke. Thromb Haemost 1996; 76: 909–915 [PubMed] [Google Scholar]
- 84. Zager RA. Hyperthermia: effects on renal ischemic/reperfusion injury in the rat. Lab Invest 1990; 63: 360–369 [PubMed] [Google Scholar]
- 85. Welc SS, Clanton TL, Dineen SM. et al. Heat stroke activates a stress-induced cytokine response in skeletal muscle. J Appl Physiol (1985) 2013; 115: 1126–1137 [DOI] [PubMed] [Google Scholar]
- 86. Roncal-Jimenez CA, Sato Y, Milagres T. et al. Experimental heat stress nephropathy and liver injury are improved by allopurinol. Am J Physiol Renal Physiol 2018; 315: F726–F733 [DOI] [PMC free article] [PubMed] [Google Scholar]