Abstract
The antigen-specific T-cell unresponsiveness seen in lymphatic filariasis is mediated, in part, by diminished antigen-presenting cell function and is most specific for microfilariae (MF), the parasite stage found in large numbers in the peripheral circulation. We investigated the effect of MF antigen (MFAg) on dendritic cells (DC) in both their differentiation process from monocyte precursors and also after they have developed into DC. When MFAg was added to cultures of monocytes during their differentiation process to immature DC, the production of interleukin 12 (IL-12) p40, p70 protein, and IL-10 was significantly (P < 0.03) inhibited in response to Staphylococcus aureus Cowan (SAC) and SAC-gamma interferon (IFN-γ) (60% to 80% inhibition). IL-10 was also inhibited (P = 0.04) in response to CD40 ligand–IFN-γ. Moreover, MFAg inhibited the mRNA expression of IL-12 p40 and IL-10 as assessed by RNA protection assays. This effect was antigen specific, as another parasite antigen (soluble Toxoplasma gondii antigen) did not inhibit the production of these cytokines. This effect was also not a result of diminished cell viability nor of an alteration in surface expression of most costimulatory surface molecules, including major histocompatibility complex class I and class II. In contrast to exposure throughout the differentiation process, MFAg added to immature DC had no effect on DC cytokine expression. Although MF-differentiated DC were capable of inducing an allogeneic mixed lymphocyte reaction, they did so to a significantly lesser degree than DC without antigen exposure. These data collectively suggest that once DC are differentiated from their precursor cells, they become resistant to changes by MFAg.
Among the various outcomes associated with infection with the lymphatic-dwelling filariae (lymphedema, adenolymphangitis, elephantiasis, and tropical pulmonary eosinophilia), the most immunologically intriguing is a subclinical condition associated with both high levels of circulating microfilariae (MF) (or parasite antigen) and the inability to proliferate or produce gamma interferon (IFN-γ) in response to parasite antigen (24). This lack of cellular responsiveness has been shown to be primarily directed at the parasite stage found in the blood circulation (MF) (21), a stage that represents (at the quantitative level) the major repository of parasite antigen. Whether the antigen-specific cellular hyporesponsiveness is a cause or a result of the heavy intravascular parasite burden remains to be determined.
The regulatory controls on the T-cell responses in these patently infected individuals affect type 1 responses preferentially, with interleukin 10 (IL-10) and transforming growth factor beta being the cytokines most often implicated in mediating this type 1 downregulation (15, 38). Indeed, IL-10 production is not only elevated in asymptomatic (or subclinical) MF individuals but is also preferentially induced by the MF stage of the parasite (22, 23). While many factors may be invoked for modulating the immune response to parasite antigen in these asymptomatic individuals, including cross-regulatory cytokines (15, 38), in utero sensitization to parasite antigen (48, 55), the antigenic composition of the parasites themselves (migration inhibition factor [33] or transforming growth factor beta homologs [8]), and underlying genetic factors, the deviation of the immune system away from a type 1 response suggests that early priming events may play a critical role in determining the nature of the secondary (and long-lasting) response (48). While the type of antigen-presenting cells (APC) (5) and the antigen dose used at priming (2) have each been implicated as major determining factors in the differentiation process from naïve to “mature” memory cells, it is the cytokine milieu, most notably the IL-12–IL-4 balance at the time of priming, that may be the most important factor (3, 30, 32, 39, 40, 42, 56, 58).
IL-12 stands at the interface between the innate and adaptive immune responses and has been shown to play a major role in initiating type 1 responses (43). With its ability to stimulate IFN-γ and to modulate IL-4 production, it is a critical cytokine in most responses to microbial pathogens, the exception being helminth parasites (31). Indeed, helminths most typically induce an immune response characterized by high levels of serum immunoglobulin E (IgE) and peripheral blood eosinophilia with concomitant increase in frequencies of IL-4- and IL-5-producing T cells (20).
The cells that clearly initiate the early immune response to parasitic worms (just as with other pathogens) are the dendritic cells (DC), a major source of IL-12 and IL-10. Immature DC have a propensity to take up antigen in the periphery, mature, and migrate into secondary lymphoid organs, where they prime antigen-specific T cells (4). In the case of lymphatic-dwelling filariae, MF must travel from the afferent lymphatics to the peripheral circulation. In so doing, these MF must encounter DC at different stages of their maturation and may influence the function of these cells by altering their function and/or their maturation process.
Thus, the objective of our study is to investigate the role of these MF antigens (MFAg) on the differentiation process of DC from CD14+ precursor monocytes as well as their effect on immature DC. We demonstrate that the exposure of DC to MFAg at the beginning and during their differentiation from monocytes inhibits the production of IL-12 and IL-10 following activation with Staphylococcus aureus Cowan (SAC) and IFN-γ (SAC–IFN-γ) and CD40 ligand (CD40L) and IFN-γ (CD40L–IFN-γ) at both the protein and mRNA levels. Furthermore, exposure to this antigen reduces the capacity of DC to stimulate an allogeneic mixed lymphocyte reaction (MLR).
MATERIALS AND METHODS
MFAg preparation.
Soluble MFAg was made from ∼108 live Brugia malayi MF (provided by John McCall, University of Georgia, Athens, Ga.) as described previously (48). Briefly, MF were collected by peritoneal lavage of infected jirds and were separated from peritoneal cells by Ficoll diatrizoate density centrifugation. The MF were then washed repeatedly in RPMI medium with antibiotics and cultured overnight at 37°C in 5% CO2. Worms were harvested the following day, washed with phosphate-buffered saline (PBS), and frozen at −20°C. The frozen MF were pulverized, sonicated, and extracted in PBS at 37°C for 4 h and than at 4°C overnight. Following centrifugation at 20,000 × g for 30 min, the supernatant was passed through a 0.45-μm-pore-size filter and stored in aliquots at −70°C. The antigen was tested for endotoxin (QCL-1000 kit; BioWhittaker) and found to be endotoxin free.
In vitro generation of DC.
CD14+ peripheral blood-derived monocytes were isolated from leukopacks from healthy donors by counterflow centrifugal elutriation (7). Monocytes were cryopreserved at 5 × 107/vial and were thawed for culture in six-well tissue culture plates at 2 × 106 to 3 × 106/ml (no. 3596; Costar) in complete RPMI 1640 (BioWhittaker) supplemented with 20 mM glutamine (BioWhittaker), 10% heat-inactivated fetal calf serum (Harlan Bioproducts for Science), 100 IU of penicillin/ml, and 100 μg of streptomycin (Biofluids, Inc.)/ml. Recombinant human IL-4 and recombinant human granulocyte-macrophage colony-stimulating factor (PeproTech) were added to the culture at 50 ng/ml on days 1, 4, and 7 of culture. When the effect of MFAg was studied on the differentiation process of DC, the antigen was added on the same days as cytokines (days 1, 4, and 7) at a final concentration of 50 μg/ml. Cells were harvested at day 10 of culture with versene-EDTA (Biofluids, Inc.), washed twice with PBS (without Ca2+-Mg2+), and used for flow cytometry analysis or other functional studies. DC harvested at day 10 were repeatedly shown to be CD1a+, HLA-DR+, CD86+, CD40+, CD3−, CD14−/lo, CD19−, and CD56− by flow cytometry (FACSCalibur; Becton Dickinson).
When the effect of MFAg was studied during the later stages of DC differentiation, the antigen was added at the day of harvest (day 10).
Isolation of T cells.
Blood was obtained from normal volunteer blood donors at the National Institutes of Health by apheresis, and lymphocytes were isolated using elutriation. They were washed twice with PBS and frozen in aliquots. When needed, the cells were thawed and washed. Resting CD4+ T cells were subsequently obtained by negative selection as described (12), using a cocktail of monoclonal antibody (MAb) and rigorous immunomagnetic negative selection with BioMag beads (Polysciences, Inc.) bound to goat anti-mouse IgG (heavy plus light chains). The purity of the isolated cells was shown by flow cytometry to be greater than 97%. The selected CD4+ T cells were free of monocytes based both on flow cytometry and on the criterion that there is no proliferative response to optimal concentrations (1/200 dilution) of phytohemagglutinin (M form; Gibco-BRL).
In vitro activation of DC.
On day 10 of culture, DC were harvested and cultured at 0.5 × 106/ml in a 48-well tissue culture plate in media alone or activated with SAC (10 μg/ml), SAC–IFN-γ (1 ng/ml), soluble CD40L (2 μg/ml), or CD40L–IFN-γ. Supernatants were collected at 16 or 48 h. For RNase protection assays, RNA was prepared 16 h after activation.
Flow cytometry.
Staining of cells with antibody was carried out according to standard protocols. Propidium iodide (Sigma Chemical Co.) was used to exclude nonviable cells from the analysis. DC (0.2 × 106 to 0.5 × 106) were harvested and washed with fluorescence-activated cell sorter media (Hanks balanced salt solution) without phenol red and without Ca2+-Mg2+ (BioWhittaker) containing 0.2% human serum albumin (Sigma) and 0.2% sodium azide (Sigma). Cells were incubated with human gamma globulin (Sigma) at 10 mg/ml for 10 min at 4°C to inhibit subsequent binding of MAb to FcR. Then cells were incubated with specific MAb conjugated with fluorescein isothiocyanate (FITC) or phycoerythrin (PE) at saturating concentrations for 30 min at 4°C, washed twice with fluorescence-activated cell sorter media, and analyzed using a FACSCalibur (Becton Dickinson) and CellQuest software. All antibodies used were mouse anti-human MAb and consisted of the following: CD1a-FITC (clone BB-5; Biosource); CD11a-FITC (clone MEM25; Caltag); CD11b-FITC (clone CR3; Caltag); CD11c-PE (clone 3.9; Caltag); CD14 (clone B-A8-FITC; Biosource); CD18 (clone CLB-LFA-1/1; Caltag); CD40-FITC (clone 5C3; PharMingen); CD54 (intercellular adhesion molecule 1 [ICAM-1]) (clone MEM111; Caltag); CD58-FITC (clone IC3; PharMingen); CD80 (B7-1)-PE (clone L307.4; Becton Dickinson); CD86 (B7-2)-FITC (clone 2331; PharMingen); CD83-PE (clone HB15e, PharMingen); HLA-A,B, C-FITC (clone G46-2.6; PharMingen); HLA-DR-FITC (clone L243; PharMingen). For isotype controls, FITC-mouse IgG1 (clone MOPC-21), PE-mouse IgG1 (clone MOPC-21), and FITC-mouse IgG2b (clone 27-35) (all from PharMingen) were used.
RNase protection assay.
RNA was prepared using RNAqueous (Ambion). RNA populations were analyzed using a multiprobe RNase protection assay. Defined riboprobes for human cytokines were purchased from PharMingen. Assays were performed as described previously (11). Radioactivity contained in bands on dried polyacrylamide gels was quantified using a Storm PhosphorImager (Molecular Dynamics). The net counts per minute (cpm) for a given band was calculated by the following formula:
 |
and was expressed as a percentage of the housekeeping gene transcript glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Immunofluorescent staining of DC.
DC or MF-differentiated DC were harvested on day 10 of culture. Cells were washed with PBS and were cytospun. Cells were fixed in acetone-methanol (1:1) at −20°C. They were stained with polyclonal rabbit anti-MF antibody or control rabbit sera for 1 h. After washing, the secondary antibody FITC-conjugated AffiniPure F(ab′)2 fragment goat anti-rabbit IgG heavy plus light chains was used to detect rabbit anti-MF antibody. Immunofluorescence microscopy was used to detect the uptake of MFAg.
MLR.
Purified CD4+ T cells (50,000) were cultured in 96-well U-bottom microplates with 5,000 or 10,000 DC. Thymidine incorporation was measured on day 7 after a 24-h pulse with [3H]thymidine solution (5 mCi/ml, 2 mCi/mmol specific activity; New England Nuclear). Incorporation of radioactive label was measured using liquid scintillation spectroscopy. Results are expressed as the arithmetic mean counts per minute of triplicate cultures.
Cytokine assays.
All cytokines were detected in culture supernatants using a cytokine-specific enzyme-linked immunosorbent assay. For IL-12 p70, paired antibodies (R&D Systems) were used; for IL-12 p40 and IL-10, PharMingen paired antibodies were used. Assays were performed according to the manufacturer's guidelines. The lower limits of detection for the assays were as follows: for IL-12 p70, 33 pg/ml; for IL-12 p40, 78 pg/ml; and for IL-10, 39 pg/ml. A PGE2 immunoassay was performed using an R&D Systems kit (catalog no. DE0100).
Statistical analysis.
The nonparametric Wilcoxon signed rank test was used to examine the significant effects of culture conditions on cytokine secretion. All statistics were performed with StatView 5 (SAS Institute).
RESULTS
MFAg uptake by monocyte-derived DC.
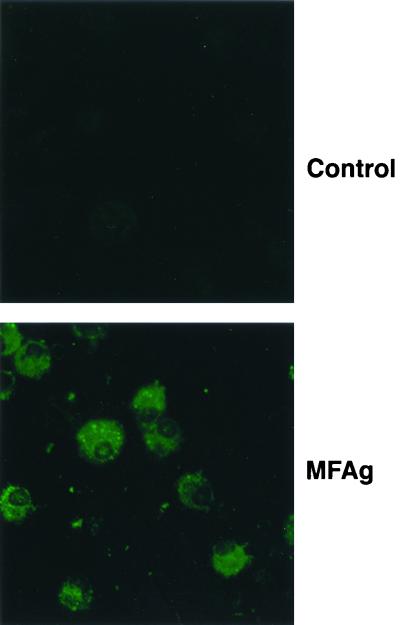
To demonstrate that MFAg could be taken up by DC, immature DC were generated by culturing CD14+ elutriated monocytes for 10 days in the presence of granulocyte-macrophage colony-stimulating factor and IL-4. MFAg was added to the culture at days 1, 4, and 7 during the process of differentiation at a final concentration of 50 μg/ml. At day 10 of culture, DC were harvested, placed on glass slides using a cytospin, and stained with polyclonal rabbit anti-MF antibody. As shown by immunofluorescence staining for Fig. 1, immature DC are capable of taking up MFAg. This finding was confirmed separately by demonstrating that DC differentiated for 10 days in the absence of MFAg were also capable of taking up MFAg (data not shown). Moreover, this antigen did not interfere with cell recovery, as the viability and number of DC (analyzed by flow cytometry studies using propidium iodide staining; data not shown) were similar for unstimulated and antigen-differentiated DC.
FIG. 1.
MFAg uptake by DC. Photomicrographs of DC cultured with MFAg and stained with control rabbit antibody (top) or monospecific polyclonal rabbit anti-MF antibody (bottom).
MFAg does not significantly alter the expression of cell surface molecules.
To investigate whether MFAg uptake by DC would result in changes in the expression of cell surface molecules, viable DC differentiated in the presence or absence of MFAg were analyzed by flow cytometry. As expected, monocyte-derived DC were CD14lo and had upregulated their expression of CD1a, showing a phenotype typical of immature DC. There were no significant differences in the expression of CD11a, CD11b, CD18, and CD58 between DC and MF-differentiated DC. MF-differentiated DC showed a 1.6-fold increase (of the mean fluorescent intensity) in the surface expression of CD40, a 1.5- to 2.6-fold increase (in some donors) in CD54, an almost 2.0-fold increase in the expression of CD80, and an almost 2-fold increase in major histocompatibility complex class I (MHC-I) expression compared with normal DC (Table 1).
TABLE 1.
The influence of MFAg on the expression of cell surface molecules on DCa
| Cell type | Testing results for:
|
|||
|---|---|---|---|---|
| DC
|
DC with MF
|
|||
| Geometric mean | Range | Geometric mean | Range | |
| CD1a | 30.4 | (17.0–54.4) | 43.3 | (26.5–70.8) |
| CD14 | 21.2 | (16.0–25.1) | 21.6 | (11.3–49.9) |
| CD11a | 28.0 | (17.5–42.0) | 27.0 | (19.2–33.5) |
| CD11b | 126.5 | (61.5–325.5) | 152.1 | (92.1–339.5) |
| CD18 | 57.6 | (41.3–91.8) | 61.5 | (49.2–89.2) |
| CD40 | 39.0 | (21.5–84.8) | 58.2 | (25.4–141.5) |
| CD54 | 124.2 | (99.3–193.0) | 196.1 | (98.8–248.6) |
| CD58 | 61.3 | (47.0–97.2) | 75.7 | (59.1–106.4) |
| CD80 | 34.9 | (18.7–77.6) | 68.2 | (43.9–146.6) |
| CD86 | 25.0 | (15.7–40.1) | 28.6 | (17.9–60.2) |
| MHC-I | 189.4 | (87.6–339.7) | 316.1 | (91.1–595.0) |
| MHC-II | 133.6 | (64.2–316.3) | 168.2 | (51.1–370.8) |
DC generated in vitro with or without MF were harvested at day 10. Flow cytometry analysis was performed using MAbs to the cell surface markers. Results shown are the geometric mean of the mean fluorescent intensity and, in parentheses, the range of mean fluorescent intensity of three independent experiments.
MF-differentiated DC produce less IL-12 p40, IL-12 p70, and IL-10.
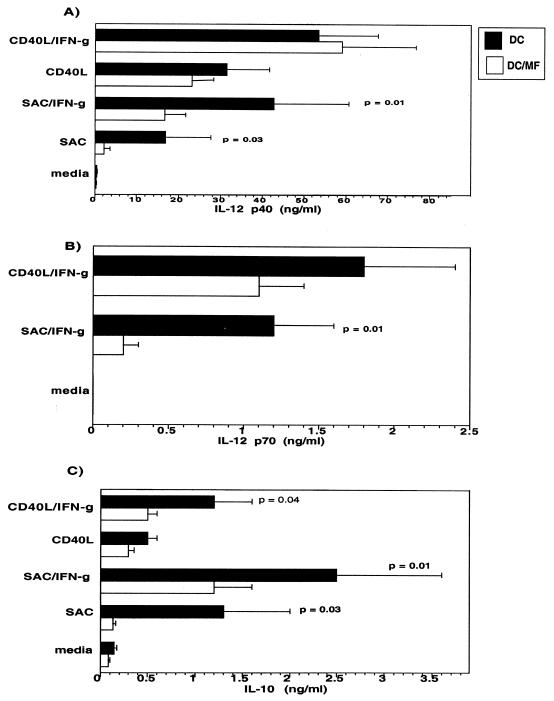
IL-10 has been implicated in mediating the antigen-specific unresponsiveness in filarial infections that, in turn, might negatively modulate IL-12 through preventing Th1 development. Therefore, the expression of IL-10 and IL-12 in response to MFAg becomes critical in understanding this aspect of the host-parasite interaction. Thus, we examined the effect of MFAg on the production of IL-10 and IL-12 by culturing the MFAg-differentiated DC (or those differentiated in media alone) for an additional 2 days in media alone or with SAC, SAC–IFN-γ, soluble CD40L, or CD40L–IFN-γ (Fig. 2). The data indicate that MF-treated DC produce significantly less IL-12 p40 following stimulation with either SAC (P = 0.03) or SAC–IFN-γ (P = 0.01) (Fig. 2A). This downregulation was less profound when DC were stimulated with CD40L or CD40L–IFN-γ (Fig. 2A). DC produced IL-12 p70, the biologically active form of IL-12, in greatest quantities when IFN-γ was used in combination with either SAC or CD40L. MF-differentiated DC produced significantly less IL-12p70 with SAC–IFN-γ (P = 0.01) than did the unexposed DC (Fig. 2B). Although IL-12 p70 inhibition was not shown to be statistically different in MF-differentiated DC following CD40L–IFN-γ activation, the trend was shown to be similar. Of interest, the level of IL-10 was also shown to be significantly inhibited in MF-differentiated DC compared with that in normal DC. This inhibition was observed under all activation conditions, including CD40L–IFN-γ cultures (P = 0.04) (Fig. 2C). This experiment was repeated in six to eight DC donors. Although there was donor-to-donor variability in the amount of cytokines produced, each individual donor's DC responded similarly.
FIG. 2.
MF-differentiated DC produce less IL-12 p40, IL-12 p70, and IL-10. DC generated without (black bars) or with (white bars) MF were cultured in media alone or activated with SAC, SAC–IFN-γ, CD40L, or CD40L–IFN-γ and assessed for IL-12 p40 (A), IL-12 p70 (B), and IL-10 (C) production. Results shown are the mean + standard error of six to eight independent experiments.
MFAg does not alter cytokine production of immature DC.
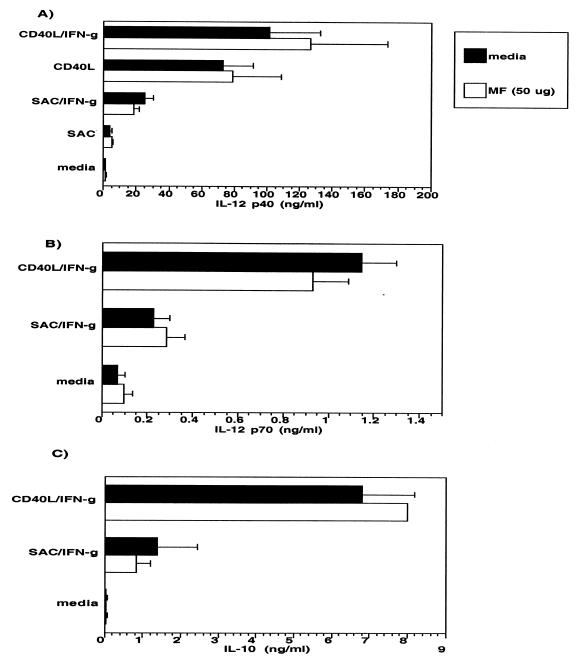
To determine the effect of MFAg on differentiated DC (immature DC), MFAg was added to the culture of immature DC at day 10, which was then compared with that of normal, unexposed DC (Fig. 3). The data indicated that MFAg added to DC at day 10 had no effect on the production of IL-12 p40, IL-12 p70, or IL-10 either in unactivated cultures (media) or following activation with SAC–IFN-γ or CD40L–IFN-γ (Fig. 3A to C). Furthermore, MFAg was incapable of altering expression of the many cell surface markers tested (data not shown), suggesting that MFAg exerts its effect primarily during the differentiation process of DC from monocytes. Of interest, this antigen, if added on day 5 or later, had little or no effect on the functional capacity of the DC, suggesting that MFAg is active only during the earliest stages of differentiation.
FIG. 3.
MFAg has no effect on the production of IL-10 or IL-12 by immature DC. DC were generated in the absence of MFAg and cultured without (black bars) or with (white bars) 50 μg of MFAg/ml. The production of IL-12 p40 (A), IL-12 p70 (B), and IL-10 (C) was assessed in media alone or following activation with SAC–IFN-γ or CD40L–IFN-γ. Results shown are the mean + standard error of four independent experiments.
Cytokine downregulation by MFAg is parasite specific.
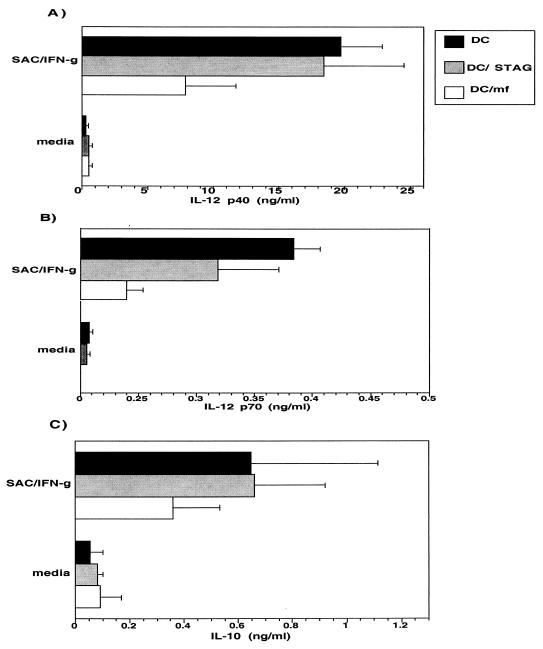
To demonstrate that the downregulation of IL-10 and IL-12 is truly antigen specific, an unrelated but parasite-derived antigen, soluble extract of the intracellular parasite Toxoplasma gondii (STAG), was added to the culture of monocytes from the beginning and during the differentiation (days 1, 4, and 7) at the antigen concentration of 50 μg/ml that was used for MFAg. Our data indicate that, unlike MFAg, STAG did not inhibit the production of IL-10 or IL-12 (p40 or p70) when added at the beginning or during their process of differentiation from monocytes (Fig. 4A to C). Although the findings are preliminary, when we compared the effect of MFAg with that of the antigens from different stages of the brugian parasite (e.g., the infective-stage L3 antigen), only IL-12 production was downregulated, while IL-10 was increased (data not shown). These data need to be confirmed in more donors but suggest that stages of the parasite other than MF may also affect DC differentiation.
FIG. 4.
IL-10 and IL-12 inhibition in DC is MF specific. DC generated in the absence of antigen (black bars) or in the presence of STAG (gray bars) or MF (white bars) were cultured in media alone or with SAC–IFN-γ. The production of IL-12 p40 (A), IL-12 p70 (B), and IL-10 (C) was assessed in these cultures. Results shown are the mean + standard error of four independent experiments.
Downregulation of IL-10 and IL-12 p40 in MF-differentiated DC is at the mRNA level.
We investigated the effect of MFAg on the expression of IL-10, IL-12 p40, and IL-12 p35 at the RNA level in DC using an RNase protection assay (Fig. 5). As shown, there is a major decrease in the level of IL-12 p40 and a slight downregulation in the level of IL-10 in MF-differentiated DC compared with that in normal DC when either SAC–IFN-γ or CD40L–IFN-γ was used to stimulate these cells. When each band was calculated as a percentage of the housekeeping gene GAPDH, there was a threefold reduction in the expression of IL-12 p40 in MF-differentiated DC compared with that in normal DC. These data correlate not only with the protein results detected by enzyme-linked immunosorbent assay after 48 h (Fig. 2) but also with those obtained at the time that the RNA was prepared (data not shown). These data indicate that downregulation of IL-10 and IL-12 p40 in MF-differentiated DC is at the level of their mRNA. The expression of other cytokines, such as IL-1α, IL-1Rα, and IL-6, seemed to be downregulated as well. There was also a reduction in the expression of IL-1β and a slight decrease in the level of IFN-γ following CD40L–IFN-γ activation. In contrast, MFAg added to the DC after differentiation was unable to alter RNA expression of IL-12 p40 upon SAC–IFN-γ or CD40L–IFN-γ activation (Fig. 6), a finding that parallels the protein data (demonstrated in Fig. 3).
FIG. 5.
Inhibition of IL-10 and IL-12 p40 in MF-differentiated DC is at the level of mRNA. DC were generated without (A) or with (B) MFAg and cultured in media alone or activated with SAC–IFN-γ or CD40L–IFN-γ for 16 h. After 16 h, RNA was prepared and analyzed by a multiprobe RNase protection assay. Results represent one of three independent experiments.
FIG. 6.
MFAg does not alter the expression of IL-10 or IL-12 p40 RNA in immature DC. DC were generated in the absence of MFAg and cultured without (A) or with (B) MFAg in media alone, SAC–IFN-γ, or CD40L–IFN-γ for 16 h. After 16 h, RNA was prepared and analyzed by a multiprobe RNase protection assay. Results represent one of two independent experiments.
MF-differentiated DC have a reduced capacity to induce an allogeneic MLR.
To assess the functional capacity of MFAg-differentiated DC, their ability to activate CD4+ T cells in an allogeneic MLR was compared with that of normal DC (Fig. 7) by using several different T-cell donors. As the DC-to-T-cell ratio increased, there was a decrease in the proliferation of T cells with both DC and MF-differentiated DC. Moreover, MF-differentiated DC had a diminished capacity to induce an allogeneic MLR compared with that of normal DC at both DC-to-T-cell ratios (Fig. 7), with the difference being more profound at lower DC-to-T-cell ratios (1:5). As shown, there was a 40 to 60% inhibition in proliferation at a 1:5 DC-to-T-cell ratio and approximately a 20 to 40% inhibition at a 1:10 DC-to-T-cell ratio. These data suggest that exposure to MFAg during the differentiation process interferes with an ability to induce allogeneic MLR. Again, DC harvested at day 10 of culture are resistant to this inhibition by MFAg (data not shown).
FIG. 7.
MF-differentiated DC have diminished capacity to induce allogeneic CD4+ T cells in MLR. DC generated without (black bars) or with (white bars) MFAg were used to stimulate allogeneic CD4+ T cells in MLR. Experiments 1, 2, 3, and 4 are from four different T-cell donors and two different DC donors. Results shown are the mean + standard error of counts per minute of triplicate cultures.
DISCUSSION
Through their route of travel from the lymphatics to the peripheral blood, MF may encounter many cell types at various stages of development and differentiation. The present study demonstrates that antigen derived from MF is being taken up by DC early in their developmental process and, as a consequence, there is not only a significant decrease in their ability to produce IL-10 and IL-12 p40 and p70 but also an inability to present antigen optimally to T cells, as was demonstrated in MLR studies (Fig. 7); however, upon differentiation of DC to immature DC, MFAg has no significant effect on cytokine production or on induction of allogeneic MLR.
Antigen-specific T-cell proliferative hyporesponsiveness and the lack of IFN-γ production are the characteristics of immunologic findings in patients with patent (MF+ or circulating antigen-positive) filarial infection. Among the myriad possible mechanisms for this lack of response is alteration in APC function. Indeed, this concept has found support in animal models used in the study of filarial infection in which parasite antigen has been shown to allow macrophages to be “alternatively” activated, an activation process that appears to inhibit T-cell proliferation by a contact-dependent mechanism (19). Because DC (or their precursors) are literally bathed in MFAg, the question of whether these antigens alter the function of DC and/or affect their development from CD14+ monocyte precursors becomes important. We thus examined the effect of MFAg on both the process of DC differentiation from monocytes and its influence on DC that have already differentiated; we have shown that the primary influence of MFAg is on the DC maturation process per se.
How antigens derived from a large extracellular parasite are taken up by APC of the host and how these antigens are processed have not been fully elucidated. Most likely, it is the excretory-secretory products that are being picked up and processed by APC. Not only have our data demonstrated antigen uptake by DC (e.g., Fig. 1), but a phosphorylcholine-containing excretory-secretory antigen of a related rodent filarial nematode (Acanthocheilonema viteae) has also been shown to alter the maturation process of murine bone marrow-derived DC (57). Moreover, these antigens do not appear to influence DC maturation by a loss of viability as assessed by propidium iodine staining (the present study) or by inducing apoptosis (57).
The intensity of T-cell signaling by DC not only determines the potential to initiate T-cell responses but can affect the balance of Th1-Th2 subset differentiation. For example, increasing the concentration of peptide administered in vivo is known to promote Th1 responses preferentially (2). This process may not occur during a normal immune response after parasite infection, however, as low-dose chronic antigenic stimulation is the rule. Under conditions of low antigen density, costimulation through CD28-B7 may be essential for stimulation of the primary T-cell response (17). Moreover, signals from specific costimulatory molecules in conjunction with signals from the T-cell receptor have been shown to alter both the duration and amplitude of the signaling pathways, leading to a specific type of immune response (1, 41, 44, 51). Therefore, an increase in the expression of a particular costimulatory molecule may result in (i) an increased avidity of antigen-specific T-cell–DC conjugates and (ii) a Th1 phenotype.
Additionally, it has been shown that other costimulatory molecules (such as ICAM-1) may influence T-cell subset development by inhibiting the production of cytokines such as IL-10 (37). To investigate whether MFAg alters the Th1 response by lowering the expression of a costimulatory molecule, we looked at the expression of these molecules on MF-differentiated DC and compared it with that of normal DC. MFAg did not result in decreased expression of any of the costimulatory molecules that we tested (Table 1). The presence of this antigen did result in a slight increase in expression of CD40, CD80, CD86, CD54 (ICAM-1), MHC-I, and MHC-II (Table 1); although statistically not significant, the trend was similar in all donors tested. This increase in cell surface expression may merely suggest that DC were undergoing maturation (36). Similar increases of expression of HLA-DR, CD86, and CD40 on human monocyte-derived DC have been shown with infection with intracellular parasites such as Leishmania major (25) and by a soluble leishmanial antigen, LeIF (34). In addition, there are several reports indicating that a number of microbial products (reference 29 and references therein), such as lipopolysaccharide (LPS), bacterial DNA with CpG motifs, mannans, and glycans, induce expression of the costimulatory molecules CD80 and CD86 on APC.
Many factors have been shown to influence the differentiation and development of DC from their precursor cells. These factors include a wide range of stimuli, such as corticosteroids (26, 53), IFN-α and IFN-β (28), and parasitic infectious agents, such as Leishmania. It is well known that bacterial components such as LPS, carbohydrate polymers (6, 46), peptidoglycans (54), and insoluble cell walls from gram-positive bacteria (SAC) are able to induce cytokine responses in monocytes, macrophages, and DC. DC produce IL-12 in response to SAC and LPS (10, 13, 47, 52). Because SAC alone (and in combination with IFN-γ) can stimulate IL-12 and IL-10, we investigated whether MFAg could interfere with this process. Our study demonstrated that if MFAg comes in contact with monocytes at the beginning and during the process of their differentiation to DC, the production of cytokines such as IL-10 and IL-12 by DC is diminished upon stimulation with SAC or SAC–IFN-γ. If, however, this antigen encounters immature DC that have already been differentiated and are further along in their maturation process, MFAg appear to have no effect on the production of these cytokines. This effect on DC differentiation is less likely to be the result of the LPS or LPS-like molecules from the andosymbiotic bacterium Wolbachia in B. malayi, as exposure of monocyte-derived DC to LPS was shown to not only enhance T-cell stimulatory capacity in MLR (9) but also to secrete IL-6 and IL-12 (27). This is clearly different from the case for MFAg-differentiated DC, which have a diminished production of IL-12 and a concurrent diminished capacity to induce an allogeneic CD4+ MLR. Because it has been shown that PGE2 may be released by MF of B. malayi (18), it is possible that the effect of MFAg on DC differentiation could be, in part, due to PGE2 contained in the MFAg: however, DC generated in the presence of elevated levels of PGE2 have been previously shown to be deficient in IL-12 p70 production and, in contrast to the present study, shown to produce high levels of IL-10 (13). In addition, the concentration at which the MFAg affect DC differentiation (50 μg/ml) was measured to contain approximately 8.5 × 10−10 M PGE2 (data not shown), a concentration only slightly lower than that seen to be active on DC IL-12 p70 production (13).
IL-12 production can also be induced by CD40 ligation (10, 13, 16). While CD40 triggering alone is sufficient to induce production of the p40 subunit of IL-12, induction of biologically active IL-12 p70 requires an additional signal that may be provided by IFN-γ (45). The combination of IL-12 and IFN-γ represents an endogenous pathway of IL-12 induction that operates during the interaction of CD40-bearing DC with CD40L-expressing Th cells (10, 45). Using CD40L or CD40L–IFN-γ to activate DC, we demonstrated that there is a general decrease in the production of IL-12 p70. This inhibition is less profound for IL-12 p40 protein. At the mRNA level, however, there is a significant reduction in the expression of IL-12 p40 in MF-differentiated DC following activation with either SAC–IFN-γ or CD40L–IFN-γ.
Differences in IL-12 production by DC during the course of their differentiation in response to bacterial stimuli and CD40 ligation have been previously reported (14). The reduced IL-12-producing capacity of mature DC has been reported to be mainly from their impaired responsiveness to IFN-γ, which correlated with reduced surface expression of IFN-γR (CD119) by mature DC (14). In addition, investigators have shown that while immature DC produced IL-12 and IL-6 after stimulation with SAC, mature DC became unresponsive. Our data suggest that the decreased levels of IL-12 may be a result of further maturation of these DC following MFAg differentiation. Although these cells are still not fully mature (they do not express CD83; data not shown), they appear to become less responsive to activation by SAC–IFN-γ or CD40L–IFN-γ than are DC unexposed to MF. For this decrease in IL-12 and IL-10 to be observed, the cells have to be exposed to MFAg repeatedly. If these cells are exposed to MF only at the monocyte level or at an early stage and not throughout the culture, this downregulation of cytokines could not be seen (data not shown). This has in vivo relevance, in that maturing DC are consistently exposed to MFAg in the blood of MF patients.
It has recently been reported that a particular subset of DC is capable of inducing Th2 responses. For example, DC derived from the lymphoid lineage (35) or DC generated in the presence of PGE2 are capable of inducing a type 2 T-cell response (13). In fact, a phosphorylcholine-containing glycoprotein, ES-62, secreted by the filarial nematode A. viteae has also been shown to induce maturation of the so-called DC2 (57). This ES-62-exposed DC produced significantly less IL-12 but not IL-10 than did LPS-generated DC following CD4+ T-cell interaction. This is somewhat different from the results shown in the present study, which indicated a general inhibition in cytokine production associated with a reduced capacity to induce allogeneic CD4+ T cells in MLR. These differences may be attributed to both the source of antigen and the nature of the host cells. In the studies done by Whelan et al. (57), bone marrow-derived DC from mice were exposed to a specific protein (ES-62), whereas the present study utilizes monocyte-derived DC and has as its antigen a crude parasite extract that contains many filaria-derived antigens. Therefore, it is likely that human DC present very different epitopes based on the differences in the antigens that they encounter. Of note, both MFAg-exposed and -unexposed DC were capable of inducing activation and differentiation of naïve Th cells in an anti-CD3-dependent manner (data not shown); preliminary results indicate that there were no significant differences in the cytokines produced by autologous, activated T cells in response to MF-differentiated DC. One possible explanation for the lack of differences in proliferation using anti-CD3 could be the strength of the first signal. Anti-CD3 is clearly a strong first signal, which may override the inhibitory signals that might be sent by MF-differentiated DC. Furthermore, preliminary results indicated that allogeneic CD4+ T cells in the presence of MF-differentiated DC or normal DC produced high but similar levels of IFN-γ, pointing toward the potency of the anti-CD3 signal (data not shown). Moreover, there were no significant MF-induced differences in IL-5 or IL-10 production by these T cells; however, because the precursor frequency of naïve T cells to MFAg is extremely low (range, 1/87,000 to 1/200,000) (48), it is almost impossible to compare the potency of MF-differentiated DC with the normal potency in an autologous, antigen-driven system.
Although the underlying mechanisms may differ, it is clear that parasites as diverse as unicellular protozoa (Leishmania, plasmodia [49], and trypanosomes [50]) and multicellular helminths may influence the development and differentiation of DC. Whether they do so in a manner that alters the nature of the effector T-cell responses remains to be elucidated.
ACKNOWLEDGMENTS
We thank Elaine K. Thomas (Immunex Corporation, Seattle, Wash.) for providing soluble CD40L and Alan Sher and Sara Hieny for providing STAG. We also thank Brenda Rae Marshall for editorial help.
REFERENCES
- 1.Bluestone J A. New perspectives of CD28–B7-mediated T cell costimulation. Immunity. 1995;2:555–559. doi: 10.1016/1074-7613(95)90000-4. [DOI] [PubMed] [Google Scholar]
- 2.Constant S, Pfeiffer A, Woodard T, Pasqualini T, Bottomly K. Extent of T cell receptor ligation can determine the functional differentiation of naive CD4+ T cells. J Exp Med. 1995;182:1591–1596. doi: 10.1084/jem.182.5.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Demeure C E, Wu C Y, Shu U, Schneider P V, Heusser C, Yssel H, Delespesse G. In vitro maturation of human neonatal CD4+ T lymphocytes. II. Cytokines present at priming modulate the development of lymphokine production. J Immunol. 1994;152:4775–4782. [PubMed] [Google Scholar]
- 4.Dieu M C, Vanbervliet B, Vicari A, Bridon J M, Oldham E, Yahia A, Briere F, Zlotnik A, Lebecque S, Caux C. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J Exp Med. 1998;188:373–386. doi: 10.1084/jem.188.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duncan D D, Swain S L. Role of antigen-presenting cells in the polarized development of helper T cell subsets: evidence for differential cytokine production by Th0 cells in response to antigen presentation by B cells and macrophages. Eur J Immunol. 1994;24:2506–2514. doi: 10.1002/eji.1830241037. [DOI] [PubMed] [Google Scholar]
- 6.Espevik T, Ottelei M, Skjek-Braek G, Ryan L, Wright S D, Sundan A. The involvement of CD14 in stimulation of cytokine production by uronic acid polymers. Eur J Immunol. 1993;23:255–261. doi: 10.1002/eji.1830230140. [DOI] [PubMed] [Google Scholar]
- 7.Gerrard T L, Jurgenson C H, Fauci A S. Differential effect of monoclonal anti-DR antibody on monocytes in antigen- and mitogen-stimulated responses: mechanism of inhibition and relationship to interleukin 1 secretion. Cell Immunol. 1983;82:394–402. doi: 10.1016/0008-8749(83)90172-7. [DOI] [PubMed] [Google Scholar]
- 8.Gomez-Escobar N, Gregory W F, Maizels R M. Identification of tgh-2, a filarial nematode homolog of Caenorhabditis elegans daf-7 and human transforming growth factor β, expressed in microfilarial and adult stages of Brugia malayi. Infect Immun. 2000;68:6402–6410. doi: 10.1128/iai.68.11.6402-6410.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hertz C J, Kiertscher S M, Godowski P J, Bouis D A, Norgard M V, Roth M D, Modlin R L. Microbial lipopeptides stimulate dendritic cell maturation via toll-like receptor 2. J Immunol. 2001;166:2444–2450. doi: 10.4049/jimmunol.166.4.2444. [DOI] [PubMed] [Google Scholar]
- 10.Hilkens C M U, Kalinski P, de Boer M, Kapsenberg M L. Human dendritic cells require exogenous interleukin-12-inducing factors to direct the development of naive T-helper cells toward the Th1 phenotype. Blood. 1997;90:1920–1926. [PubMed] [Google Scholar]
- 11.Hodge J W, Sabzevari H, Yafal A G, Gritz L, Lorenz M G, Schlom J. A triad of costimulatory molecules synergize to amplify T-cell activation. Cancer Res. 1999;59:5800–5807. [PubMed] [Google Scholar]
- 12.Horgan K J, Shaw S. Immunomagnetic purification of T cell populations. In: Coligan J E, Kruisbeek A M, Margulies D H, Shevach E M, Strober W, editors. Current protocols in immunology. New York, N.Y: Wiley Interscience; 1991. p. 7.4.1-7.4.5. [Google Scholar]
- 13.Kalinski P, Hilkens C M U, Snijders A, Snijdewint F G M, Kapsenberg M L. IL-12-deficient dendritic cells, generated in the presence of prostoglandin E2, promote type 2 cytokine production in maturing human naive T helper cells. J Immunol. 1997;159:28–35. [PubMed] [Google Scholar]
- 14.Kalinski P, Schuitemaker J H, Hilkens C M, Wierenga E A, Kapsenberg M L. Final maturation of dendritic cells is associated with impaired responsiveness to IFN-γ and to bacterial IL-12 inducers: decreased ability of mature dendritic cells to produce IL-12 during the interaction with Th cells. J Immunol. 1999;162:3231–3236. [PubMed] [Google Scholar]
- 15.King C L, Mahanty S, Kumaraswami V, Abrams J S, Regunathan J, Jayaraman K, Otteson E A, Nutman T B. Cytokine control of parasite-specific anergy in human lymphatic filariasis. Preferential induction of a regulatory T helper Type 2 lymphocyte subset. J Clin Investig. 1993;92:1667–1673. doi: 10.1172/JCI116752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koch F, Stanzl U, Jennewein P, Janke K, Heufler C, Kampgen E, Romani N, Schuler G. High level IL-12 production by murine dendritic cells: upregulation via MHC class II and CD40 molecules and downregulation by IL-4 and IL-10. J Exp Med. 1996;184:741–746. doi: 10.1084/jem.184.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lenschow D J, Walunas T L, Bluestone J A. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 18.Liu L X, Buhlmann J E, Weller P F. Release of prostaglandin E2 by microfilariae of Wuchereria bancrofti and Brugia malayi. Am J Trop Med Hyg. 1992;46:520–523. doi: 10.4269/ajtmh.1992.46.520. [DOI] [PubMed] [Google Scholar]
- 19.Loke P, MacDonald A S, Robb A, Maizels R M, Allen J E. Alternatively activated macrophages induced by nematode infection inhibit proliferation via cell-to-cell contact. Eur J Immunol. 2000;30:2669–2678. doi: 10.1002/1521-4141(200009)30:9<2669::AID-IMMU2669>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 20.Mahanty S, King C L, Kumaraswami V, Regunathan J, Maya A, Jayaraman K, Abrams J S, Ottesen E A, Nutman T B. IL-4- and IL-5-secreting lymphocyte populations are preferentially stimulated by parasite-derived antigens in human tissue invasive nematode infections. J Immunol. 1993;151:3704–3711. [PubMed] [Google Scholar]
- 21.Mahanty S, Luke H E, Kumaraswami V, Narayanan P R, Vijayshekaran V, Nutman T B. Stage-specific induction of cytokines regulates the immune response in lymphatic filariasis. Exp Parasitol. 1996;84:282–290. doi: 10.1006/expr.1996.0114. [DOI] [PubMed] [Google Scholar]
- 22.Mahanty S, Mollis S N, Ravichandran M, Abrams J S, Kumaraswami V, Jayaraman K, Ottesen E A, Nutman T B. High levels of spontaneous and parasite antigen-driven interleukin-10 production are associated with antigen-specific hyporesponsiveness in human lymphatic filariasis. J Infect Dis. 1996;173:769–773. doi: 10.1093/infdis/173.3.769. [DOI] [PubMed] [Google Scholar]
- 23.Mahanty S, Nutman T B. Immunoregulation in human lymphatic filariasis: the role of interleukin 10. Parasite Immunol. 1995;17:385–392. doi: 10.1111/j.1365-3024.1995.tb00906.x. [DOI] [PubMed] [Google Scholar]
- 24.Maizels R M, Allen J E, Yazdanbakhsh M. Immunology of lymphatic filariasis: current controversies. In: Nutman T B, editor. dsfaLymphatic filariasis. London, United Kingdom: Imperial College Press; 2000. pp. 217–243. [Google Scholar]
- 25.Marovich M A, McDowell M A, Thomas E K, Nutman T B. IL-12p70 production by Leishmania major-harboring human dendritic cells is a CD40/CD40 ligand-dependent process. J Immunol. 2000;164:5858–5865. doi: 10.4049/jimmunol.164.11.5858. [DOI] [PubMed] [Google Scholar]
- 26.Matyszak M K, Citterio S, Rescigno M, Ricciardi-Castagnoli P. Differential effects of corticosteroids during different stages of dendritic cell maturation. Eur J Immunol. 2000;30:1233–1242. doi: 10.1002/(SICI)1521-4141(200004)30:4<1233::AID-IMMU1233>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 27.McRae B L, Beilfuss B A, van Seventer G A. Interferon-β differentially regulates CD40-induced cytokine secretion by human dendritic cells. J Immunol. 2000;164:23–28. doi: 10.4049/jimmunol.164.1.23. [DOI] [PubMed] [Google Scholar]
- 28.McRae B L, Nagai T, Semnani R T, van Seventer J M, van Seventer G A. Interferon-α and -β inhibit the in vitro differentiation of immunocompetent human dendritic cells from CD14+ precursors. Blood. 2000;96:210–217. [PubMed] [Google Scholar]
- 29.Medzhitov R, Janeway C A. Innate immunity: impact on the adaptive immune response. Curr Opin Immunol. 1997;9:4–9. doi: 10.1016/s0952-7915(97)80152-5. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura T, Kamogawa K, Bottomly K, Flavell R A. Polarization of IL-4 and IFN-γ producing CD4+ T cells following activation of naive CD4+ T cells. J Immunol. 1997;158:1085–1094. [PubMed] [Google Scholar]
- 31.Nutman T B. Immune responses to helminth parasites. In: Rich R R, Fleisher T A, Schwartz B D, Shearer W T, Strober W, editors. Clinical immunology: principles and practice. St. Louis, Mo: Mosby Year-Book; 1996. pp. 561–570. [Google Scholar]
- 32.Openshaw P, Murphy E E, Hosken N A, Maino V, Davis K, Murphy K M, O'Garra A. Heterogeneity of intracellular cytokine synthesis at the single-cell level in polarized T helper 1 and T helper 2 population. J Exp Med. 1995;182:1357–1367. doi: 10.1084/jem.182.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pastrana D V, Raghavan N, Fitzgerald P, Eisinger S W, Metz C, Bucala R, Schleimer R P, Bickel C, Scott A L. Filarial nematode parasites secrete a homologue of the human cytokine macrophage migration inhibitory factor. Infect Immun. 1998;66:5955–5963. doi: 10.1128/iai.66.12.5955-5963.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Probst P, Skeiky Y A W, Steeves M, Gervassi A, Grabstein K H, Reed S G. A Leishmania protein that modulates interleukin (IL)-12, IL-10 and tumor necrosis factor-α production and expression of B7–1 in human monocyte-derived antigen-presenting cells. Eur J Immunol. 1997;27:2634–2642. doi: 10.1002/eji.1830271024. [DOI] [PubMed] [Google Scholar]
- 35.Rissoan M-C, Soumelis V, Kadowaki N, Grouard G, Briere F, Malefyt R, Liu Y-J. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999;283:1183–1186. doi: 10.1126/science.283.5405.1183. [DOI] [PubMed] [Google Scholar]
- 36.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salomon B, Bluestone J A. LFA-1 interaction with ICAM-1 and ICAM-2 regulates Th2 cytokine production. J Immunol. 1998;161:5138–5142. [PubMed] [Google Scholar]
- 38.Sartono E, Kruize Y C M, Partono F, Kurniawan-Atmadja A, Maizels R M, Yazdanbakhsh M. Specific T cell unresponsiveness in human filariasis: diversity in underlying mechanisms. Parasite Immunol. 1995;17:587–594. doi: 10.1111/j.1365-3024.1995.tb01002.x. [DOI] [PubMed] [Google Scholar]
- 39.Schmitt E, Hoehn P, Germann T, Rude E. Differential effects of interleukin 12 on the development of naive mouse CD4+ T cells. Eur J Immunol. 1994;24:343–347. doi: 10.1002/eji.1830240211. [DOI] [PubMed] [Google Scholar]
- 40.Schmitt E, Hoehn P, Huels S, Goedert S, Palm N, Rude E, Germann T. T helper type 1 development of naive CD4+ T cells requires the coordinate action of interleukin 12 and interferon-γ and is inhibited by transforming growth factor-β. Eur J Immunol. 1994;24:793–798. doi: 10.1002/eji.1830240403. [DOI] [PubMed] [Google Scholar]
- 41.Schweitzer A N, Borriello F, Wong R C, Abbas A K, Sharpe A H. Role of costimulators in T cell differentiation: studies using antigen-presenting cells lacking expression of CD80 or CD86. J Immunol. 1997;158:2713–2722. [PubMed] [Google Scholar]
- 42.Seder R A. High-dose IL-2 and IL-15 enhance the in vitro priming of naive CD4+ T cells for IFN-γ but have differential effects on priming for IL-4. J Immunol. 1996;156:2413–2422. [PubMed] [Google Scholar]
- 43.Seder R A, Gazzinelli R T, Sher A, Paul W E. Interleukin 12 acts directly on CD4+ T cells to enhance priming for interferon γ production and diminishes interleukin 4 inhibition of such priming. Proc Natl Acad Sci USA. 1993;90:10188–10192. doi: 10.1073/pnas.90.21.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Semnani R T, Nutman T B, Hochman P, Shaw S, van Seventer G A. Costimulation by purified intercellular adhesion molecule 1 and lymphocyte function-associated antigen 3 induces distinct proliferation, cytokine and cell surface antigen profiles in human “naive” and “memory” CD4+ T cells. J Exp Med. 1994;180:2125–2135. doi: 10.1084/jem.180.6.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Snijders A, Kalinski P, Hilkens C M, Kapsenberg M L. High level IL-12 production by human dendritic cells requires two signals. Int Immunol. 1998;10:1593–1598. doi: 10.1093/intimm/10.11.1593. [DOI] [PubMed] [Google Scholar]
- 46.Soell M, Lett E, Holveck F, Scholler M, Wachsmann D, Klein J P. Activation of human monocytes by streptococcal rhamnose glucose polymers is mediated by CD14 antigen, and mannan binding protein inhibits TNF-α release. J Immunol. 1995;154:851–860. [PubMed] [Google Scholar]
- 47.Sousa C R E, Hieny S, Scharton-Kersten T, Jankovic D, Charest H, Germani R N, Sher A. In vivo microbial stimulation induces rapid CD40 ligand-independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas. J Exp Med. 1997;186:1819–1829. doi: 10.1084/jem.186.11.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steel C, Nutman T B. Helminth antigens selectively differentiate unsensitized CD45RA+ CD4+ human T cells in vitro. J Immunol. 1998;160:351–360. [PubMed] [Google Scholar]
- 49.Urban B C, Ferguson D J, Pain A, Willcox N, Plebanski M, Austyn J M, Roberts D J. Plasmodium falciparum-infected erythrocytes modulate the maturation of dendritic cells. Nature. 1999;400:73–77. doi: 10.1038/21900. [DOI] [PubMed] [Google Scholar]
- 50.Van Overtvelt L, Vanderheyde N, Verhasselt V, Ismaili J, De Vos L, Goldman M, Willems F, Vray B. Trypanosoma cruzi infects human dendritic cells and prevents their maturation: inhibition of cytokines, HLA-DR, and costimulatory molecules. Infect Immun. 1999;67:4033–4040. doi: 10.1128/iai.67.8.4033-4040.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Seventer G A, Semnani R T, Palmer E M, McRae B L, van Seventer J M. Integrins and T helper cell activation. Transplant Proc. 1998;30:4270–4274. doi: 10.1016/s0041-1345(98)01410-9. [DOI] [PubMed] [Google Scholar]
- 52.Verhasselt V, Buelens C, Willems F, De Groote D, Haeffiner-Cavaillon N, Goldman M. Bacterial lipopolysaccharide stimulates the production of cytokines and the expression of costimulatory molecules by human peripheral blood dendritic cells: evidence for a soluble CD14-dependent pathway. J Immunol. 1997;158:2919–2925. [PubMed] [Google Scholar]
- 53.Vieira P L, Kalinski P, Wierenga E A, Kapsenberg M L, de Jong E C. Glucocorticoids inhibit bioactive IL-12 p70 production by in vitro-generated human dendritic cells without affecting their T cell stimulatory potential. J Immunol. 1998;161:5245–5251. [PubMed] [Google Scholar]
- 54.Weidemann B, Brade H, Rietschel E T, Dziarski R, Bazil V, Kusumoto S, Flad H D, Ulmer A. Soluble peptidoglycan-induced monokine production can be blocked by anti-CD14 monoclonal antibodies and by lipid A partial structures. J Clin Investig. 1994;62:4709–4715. doi: 10.1128/iai.62.11.4709-4715.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weil G J, Hussain R, Kumaraswami V, Tripathy S P, Phillips K S, Ottesen E A. Prenatal allergic sensitization to helminth antigens in offspring of parasite-infected mothers. J Clin Investig. 1983;71:1124–1129. doi: 10.1172/JCI110862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wenner C A, Guler M L, Macatonia S E, O'Garra A, Murphy K M. Roles of IFN-γ in IL-12-induced T helper cell-1 development. J Immunol. 1996;156:1442–1447. [PubMed] [Google Scholar]
- 57.Whelan M, Harnett M M, Houston K M, Patel V, Harnett W, Rigley K P. A filarial nematode-secreted product signals dendritic cells to acquire a phenotype that drives development of Th2 cells. J Immunol. 2000;164:6453–6460. doi: 10.4049/jimmunol.164.12.6453. [DOI] [PubMed] [Google Scholar]
- 58.Yoshimoto K, Swain S L, Bradley L M. Enhanced development of Th2-like primary CD4 effectors in response to sustained exposure to limited rIL-4 in vivo. J Immunol. 1996;156:3267–3274. [PubMed] [Google Scholar]