Abstract
Islet transplantation has proven to be an effective treatment for type 1 diabetes (T1D) yet is hampered by the shortage of available tissue. Recently, two reports from a Viacyte multicenter clinical trial demonstrate the feasibility, safety, and potential efficacy of transplanting macro-encapsulated human stem cell-derived pancreatic endoderm cells into patients with T1D, highlighting the promise of a stem cell-based therapeutic approach.
Allogenic cadaveric islet transplantation using non-steroidal immune suppression can provide a practical cure for patients suffering from autoimmune diabetes (Shapiro and Lakey, 2000). Islet transplant recipients commonly achieve independence from exogenous insulin injections, which limits life-threatening hypoglycemic episodes and leads to improved quality of life (Barton et al., 2012). However, widespread implementation of islet transplantation has been severely hampered by limited access to islet tissue. While many different approaches have been explored to address this tissue shortage, in recent years the differentiation of human pluripotent stem cells (hPSCs) has emerged as one of the most promising options. Coordinated research efforts from many different laboratories have led to stepwise differentiation protocols that result in the generation of human stem cell-derived pancreatic endoderm cells (PECs) and, with somewhat less efficiency, glucose responsive insulin-producing cells (D’Amour et al., 2006; Kroon et al., 2008; Pagliuca et al., 2014; Rezania et al., 2014; Russ et al., 2015). Both of these in vitro differentiated pancreatic populations have shown promising outcomes when transplanted into preclinical animal models; PECs differentiate into functional beta cells over several months and produce high levels of circulating human insulin that can reverse diabetes, whereas transplanted insulin-producing beta-like cells can exhibit function at earlier time points post-transplantation but yield reduced insulin levels compared to beta cells derived from PECs in vivo (Pagliuca et al., 2014; Rezania et al., 2014; Russ et al., 2015). Although each approach presents relatively balanced experimental advantages and disadvantages, from a translational perspective, PEC generation requires a shorter time under Good Manufacturing Practices (GMP) conditions, which considerably reduces the cost associated with production for broad clinical implementation.
Based on the lessons learned from stem cell-derived therapies in preclinical models, two exciting papers by Shapiro et al. (Shapiro et al., 2021) and Ramzy et al. (Ramzy et al., 2021) describe preliminary results from the first year of an ongoing multicenter phase I/II clinical trial by Viacyte. The study was designed to test non-immunoprotective macrocapsulation “VC-02” devices that were loaded with stem cell-derived PECs and could be vascularized by host blood vessels following transplant. The loaded VC-02 devices were transplanted subcutaneously into patients with type 1 diabetes (T1D) in combination with immune suppression agents, with the goal of assessing the safety and efficacy of the approach. The reported outcomes from two cohorts of patients (15 and 17 individuals, respectively) at independent trial sites provide the first evidence to support the feasibility of stem cell-based cell replacement therapy for individuals with diabetes. Importantly, the procedure was found to be relatively safe, with only a small percentage of all adverse events potentially attributed to VC-02 devices; the majority of adverse events were predominantly related to surgical complications or systemic immune suppression. Furthermore, transplanted PECs did not develop into teratomas, providing additional relevant safety data. Although many of the trial participants withdrew from the trial prematurely, ~35% of the individuals from each site showed variable response to the treatment, and several patients developed meal responsive insulin secretion that increased over time, providing evidence that PECs were able to mature into functional beta-like cells following transplantation. In some patients, insulin requirements were reduced when compared to pre-transplant levels indicating therapeutic efficacy; however, none of the patients achieved insulin independence, and the reduced insulin requirements did not appear to correlate with C-peptide values, a surrogate read out for insulin secretion. In general, circulating C-peptide levels were extremely low, so to reach the therapeutic goal of insulin independence, circulating C-peptide levels would ideally need to be increased by ~100-fold, which may not be feasible with the current state of the technology.
In addition to reporting clinical outcomes, both studies demonstrated that, similar to the preclinical animal data, transplanted PECs are able to survive, differentiate, and mature within the transplanted VC-02 devices. Both papers also described histological analysis of grafts explanted at different time points post-transplantation to assess the presence of differentiated endocrine cells. Many of the insulin-producing cells present in the graft expressed functional beta cell markers, indicating that they were able to achieve a mature phenotype. However, the predominant cell type within the endocrine component of the graft were glucagon-expressing alpha cells, indicating there was favorable differentiation of PECs into alpha cells or a survival/growth advantage of alpha cells over time. Interestingly, analysis of explanted VC-02 grafts also revealed that a considerable proportion (>50%) of cells within the lumen of the devices originated from the host. Host-derived blood vessels appeared to have readily integrated into the devices via the open ports, concentrating primarily near the endocrine cell clusters and likely facilitating improved function by providing sufficient blood flow. Immune cell populations were only rarely detected, but a large population of myofibroblasts, cells commonly associated with wound healing, were present. The presence of myofibroblasts was inversely correlated with endocrine cell numbers, suggesting that preventing myofibroblast accumulation at early stages of engraftment may have the potential to increase endocrine cell mass within the VC-02 device. Although neither study directly assessed ischemia-induced cell death within the grafts immediately post-transplantation, the degree of cell loss upon graft retrieval suggests that, similar to transplanted cadaveric islets, sustained viability of the implanted cells remains an unresolved challenge.
The findings of these two clinical trials highlight the promise of stem cell-derived therapies for individuals with T1D but have also identified many of the remaining challenges that will need to be overcome to reverse diabetes (Figure 1). Although the encapsulated PECs were able to differentiate into functional beta cells, the process took several months, and these cells represented only a minor population within the graft. Attempts to directly differentiate stem cells into beta cells in vitro face similar challenges; therefore, additional studies aimed at deciphering the precise molecular mechanisms underlying the directed differentiation of functional beta cells are necessary to enhance the efficacy of stem cell-based cell therapy. Ultimately, the use of in vitro differentiated beta-like cells would provide distinct advantages over the in vivo approach by 1) enabling the loading of an increased percentage of functional insulin-producing cells into the graft and 2) providing a more mature cell product that should function immediately upon transplantation. However, as mentioned above, a caveat associated with the in vitro differentiation approach is the need for substantially longer-term culturing of cells under GMP conditions, which incurs greater expense when scaling up production. Recently, the company Vertex has initiated a phase I/II clinical trial using non-encapsulated stem cell-derived beta-like cells that are infused through the portal vein into the liver of patients with T1D in conjunction with systemic immune suppression (NCT04786262). A press release describing promising efficacy results from a single patient 90 days post transplantation has received considerable media attention; however, results from this trial cannot be fully evaluated until more details are shared through peer-reviewed scientific publication. Regardless, comparison of these two trials will provide the opportunity for an unbiased assessment of the relative influence of stem cell-derived PECs versus beta-like cells and the transplantation site.
Figure 1. Current and potential stem cell-based therapeutic strategies for T1D treatment.
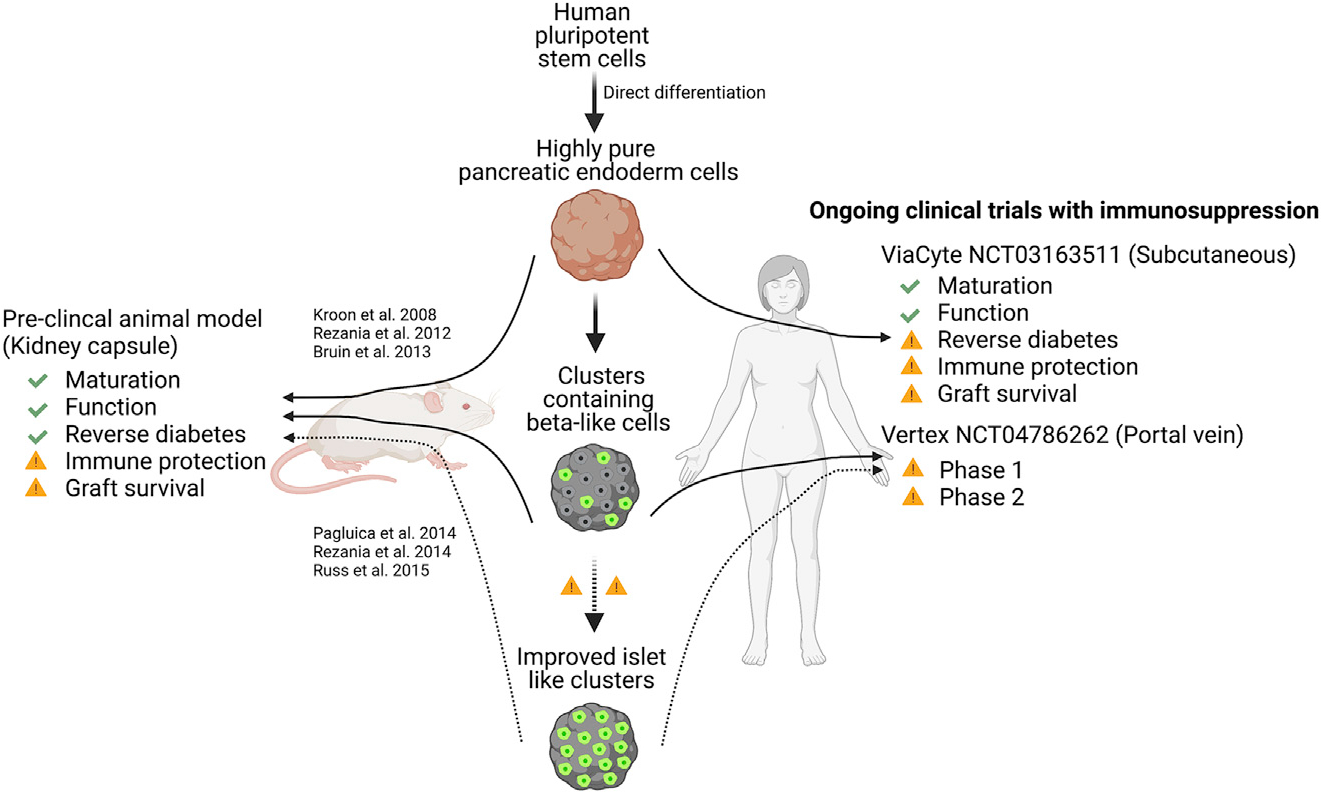
Transplantation of pancreatic endoderm cells (PECs) and stem cell-derived beta-like cells into preclinical animal models has demonstrated the ability to successfully reverse diabetes, albeit with caveats associated with each approach. Recently, results from a multi-center clinical trial (NCT03163511) provided preliminary results showing the feasibility of using encapsulated stem cell-derived PECs as replacement therapy for patients suffering from T1D. A combined phase 1/2 clinical trial (NCT04786262) is also ongoing and awaits dissemination of peer-reviewed results.
Another challenge for the stem cell-derived islet cell transplantation field is the reliance on systemic immunosuppression to protect against allogeneic immune responses and potentially recurring autoimmunity that may negatively impact long term graft survival and function. One appealing approach would be to provide localized immune suppression via functionalized biomaterials and/or engineered immune cell populations. Alternatively, the use of genome editing technologies to generate immune-privileged hPSCs and their derivative cells has received considerable attention. Protection from xenogenic, allogenic, and diabetogenic immune destruction has been successfully achieved in preclinical in vitro and in vivo studies (Castro-Gutierrez et al., 2021; Yoshihara et al., 2020). These strategies have focused on expressing high levels of immune suppressive proteins, such as PD-L1, on the cell surface and/or removing surface proteins critical for antigen presentation, such as human leukocyte antigen (HLA) molecules. Viacyte, in collaboration with CRISPR Therapeutics, has recently announced the initiation of a first-in-human trial using genome edited hypoimmune PECs delivered to adult patients in a non-immune protective macroencaspulation device.
In summary, despite the challenges that remain, these preliminary studies transplanting human stem cell-derived PECs into patients with diabetes provide promising evidence for the feasibility, safety, and efficacy of using stem cell-based therapies to treat this condition. With the continued ongoing collaborative efforts of the field, we anticipate exciting advancements of this approach in the near future.
ACKNOWLEDGMENTS
We thank members of the Sussel lab for providing critical feedback of the manuscript. The H.A.R. lab is supported by the NIH (DK120444, AI140044, and P30-DK116073), a New Investigator Award from the NIDDK-supported Human Islets Research Network (HIRN, RRID:SCR_014393 and UC24 DK1041162), a Culshaw Junior Investigator Award in Diabetes, the Juvenile Diabetes Research Foundation (JDRF 2-SRA-2019–781-S-B), and the Children’s Diabetes Foundation. A.H.S. is supported by NIH T32 GM136444. The L.S. lab is supported by NIH R01 DK082590, DK111405, DK125360, DK118155, U01 DK127505, and P30 DK116073.
Footnotes
DECLARATION OF INTERESTS
H.A.R. is an SAB member at Sigilon Therapeutics and Prellis Biologics and consultant to Eli Lilly and Minutia. H.A.R. has filed patent applications in the research space.
REFERENCES
- Barton FB, Rickels MR, Alejandro R, Hering BJ, Wease S, Naziruddin B, Oberholzer J, Odorico JS, Garfinkel MR, Levy M, et al. (2012). Improvement in outcomes of clinical islet transplantation: 1999–2010. Diabetes Care 35, 1436–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Gutierrez R, Alkanani A, Mathews CE, Michels A, and Russ HA (2021). Protecting Stem Cell Derived Pancreatic Beta-Like Cells From Diabetogenic T Cell Recognition. Front. Endocrinol. (Lausanne) 12, 707881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, and Baetge EE (2006). Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat. Biotechnol. 24, 1392–1401. [DOI] [PubMed] [Google Scholar]
- Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, Young H, Richardson M, Smart NG, Cunningham J, et al. (2008). Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat. Biotechnol. 26, 443–452. [DOI] [PubMed] [Google Scholar]
- Pagliuca FW, Millman JR, Gürtler M, Segel M, Van Dervort A, Ryu JH, Peterson QP, Greiner D, and Melton DA (2014). Generation of functional human pancreatic β cells in vitro. Cell 159, 428–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramzy A, Thompson DM, Ward-Hartstonge KA, Ivison S, Cook L, Garcia RV, Loyal J, Kim PTW, Warnock GL, Levings MK, et al. (2021). Implanted pluripotent stem-cell-derived pancreatic endoderm cells secrete glucose-responsive C-peptide in patients with type 1 diabetes. Cell Stem Cell 28, 2047–2061.e2045. [DOI] [PubMed] [Google Scholar]
- Rezania A, Bruin JE, Arora P, Rubin A, Batushansky I, Asadi A, O’Dwyer S, Quiskamp N, Mojibian M, Albrecht T, et al. (2014). Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat. Biotechnol. 32, 1121–1133. [DOI] [PubMed] [Google Scholar]
- Russ HA, Parent AV, Ringler JJ, Hennings TG, Nair GG, Shveygert M, Guo T, Puri S, Haataja L, Cirulli V, et al. (2015). Controlled induction of human pancreatic progenitors produces functional beta-like cells in vitro. EMBO J. 34, 1759–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro AM, and Lakey JR (2000). Future trends in islet cell transplantation. Diabetes Technol. Ther. 2, 449–452. [DOI] [PubMed] [Google Scholar]
- Shapiro AMJ, Thompson D, Donner TW, Bellin MD, Hsueh W, Pettus J, Wilensky J, Daniels M, Wang RM, Brandon EP, et al. (2021). Insulin expression and C-peptide in type 1 diabetes subjects implanted with stem cell-derived pancreatic endoderm cells in an encapsulation device. Cell Reports Medicine 2, 100466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara E, O’Connor C, Gasser E, Wei Z, Oh TG, Tseng TW, Wang D, Cayabyab F, Dai Y, Yu RT, et al. (2020). Immune-evasive human islet-like organoids ameliorate diabetes. Nature 586, 606–611. [DOI] [PMC free article] [PubMed] [Google Scholar]


