Abstract
Increased proliferation and survival of cells in small pulmonary arteries (PAs) drive pulmonary arterial hypertension (PAH). Because cell growth mediated by the mTOR-containing mTORC1 complex is inhibited by tuberous sclerosis complex 2 (TSC2), we investigated the role of this GTPase-activating protein in PAH pathology. TSC2 abundance was decreased in remodeled small PAs and PA vascular smooth muscle cells (PAVSMCs) from PAH patients or rodent pulmonary hypertension (PH) models, as well as PAVSMCs maintained on substrates that reproduced pathology-induced stiffness. Accordingly, mice with smooth muscle specific-reduction in TSC2 developed PH. At the molecular level, decreased TSC2 abundance led to stiffness-induced PAVSMC proliferation, increased abundance of the mechanosensitive transcriptional coactivators YAP/TAZ, and enhanced mTOR kinase activity. Moreover, extracellular matrix (ECM) produced by TSC2-deficient PAVSMCs stimulated the proliferation of non-diseased PA adventitial fibroblasts and PAVSMCs through fibronectin and its receptor, the α5β1 integrin. Reconstituting TSC2 in PAVSMCs from PAH patients through overexpression or treatment with the SIRT1 activator SRT2104 decreased YAP/TAZ abundance, mTOR activity, and ECM production, as well as inhibiting proliferation and inducing apoptosis. In two rodent models of PH, SRT2104 treatment restored TSC2 abundance, attenuated pulmonary vascular remodeling, and ameliorated PH. Thus, TSC2 in PAVSMCs integrates ECM composition and stiffness with pro-proliferative and survival signaling, and restoring TSC2 abundance could be an attractive therapeutic option to treat PH.
Introduction
Pulmonary arterial hypertension (PAH) is a progressive and rapidly fatal disease with a high mortality rate and no cures (1), and is a serious public health problem with increasing death and hospitalization rates (2-4). In PAH, vasoconstriction and remodeling of small pulmonary arteries (PAs) (5-10) leads to chronically increased PA pressure, right ventricular afterload, and eventually right ventricle failure and death (4). Available therapies fail to reverse established pulmonary vascular remodeling or prevent disease progression (5, 9), and development of novel anti-proliferative/anti-remodeling therapies is an unmet important need.
Increased proliferation and survival of resident pulmonary vascular cells, a key component of PA remodeling (8, 11, 12), are induced by the combination of soluble stimuli, such as excessive production of growth factors and pro-inflammatory mediators, and of physical factors, such as stiffening and remodeling of the extracellular matrix (ECM). As PAH progresses, PA vascular smooth muscle cells (PAVSMCs) undergo a switch to the secretory, proliferative, apoptosis-resistant phenotype, which is self-supported by constitutive inhibition of the growth suppressor HIPPO-large tumor suppressor 1 (LATS1) pathway and consequent up-regulation of its pro-proliferative downstream effectors, the transcriptional coactivators Yes-associated protein (YAP)/TAZ and the mechanistic target of rapamycin (mTOR) complex 1 (mTORC1) and mTORC2 (7-9, 12-15). The mechanisms coordinating growth-promoting signals with proliferative responses of resident PA cells in PAH are not completely understood, and clinical use of available anti-proliferative therapeutic strategies is challenging due to their side effects.
The growth suppressor tuberous sclerosis complex 2 (TSC2) (tuberin) inhibits mTORC1, cell growth, and proliferation (16). Deficiency or mutational inactivation of TSC2 is linked to proliferative diseases such as cancer, tuberous sclerosis and pulmonary lymphangioleiomyomatosis (LAM) (16-18). Several lines of evidence suggest that TSC2 may act as a coordinator of mTORC1 and HIPPO-YAP/TAZ pathways in PAH pulmonary vasculature. First, mTORC1 is activated in remodeled small PAs and contributes to human PAH PAVSMC proliferation, VSM remodeling and experimental PH in mice (7, 19, 20). Second, mTOR-induced accumulation of YAP is reported in TSC2-null cells (21). In addition, PH develops in mice with vascular smooth muscle (VSM)-specific knock-down of TSC1 (a binding partner of TSC2 that protects it from the degradation) (22). Lastly, a subset of patients with pulmonary LAM (caused by somatic mutations or loss of heterozygosity in TSC) develop disproportionate precapillary PH that cannot be fully explained by hypoxemia and is likely attributable to the loss of pulmonary vascular TSC function (23).
In the present study, we sought to determine the role of TSC2 in pulmonary vascular hyperproliferation in PAH. We found that TSC2 acted as a mechanosensor and mechanotransducer, and its deficiency in smooth muscle cells from small PAs permitted up-regulation of YAP-TAZ and mTOR; increased PAVSMC proliferation, survival, and remodeling; and promoted PH. We also mechanistically linked PAVSMC-specific TSC2 deficiency to excessive ECM production, ECM-dependent activation of YAP/TAZ and mTOR, and increased growth of PAVSMCs and PA adventitial fibroblasts (PAAFs). Lastly, we demonstrated that TSC2 restoration by SRT2104 was beneficial in inhibiting YAP/TAZ and mTOR, reversing pulmonary vascular remodeling, and reducing PH.
Results
TCS2 abundance is decreased in PAVSMCs from human PAH lungs
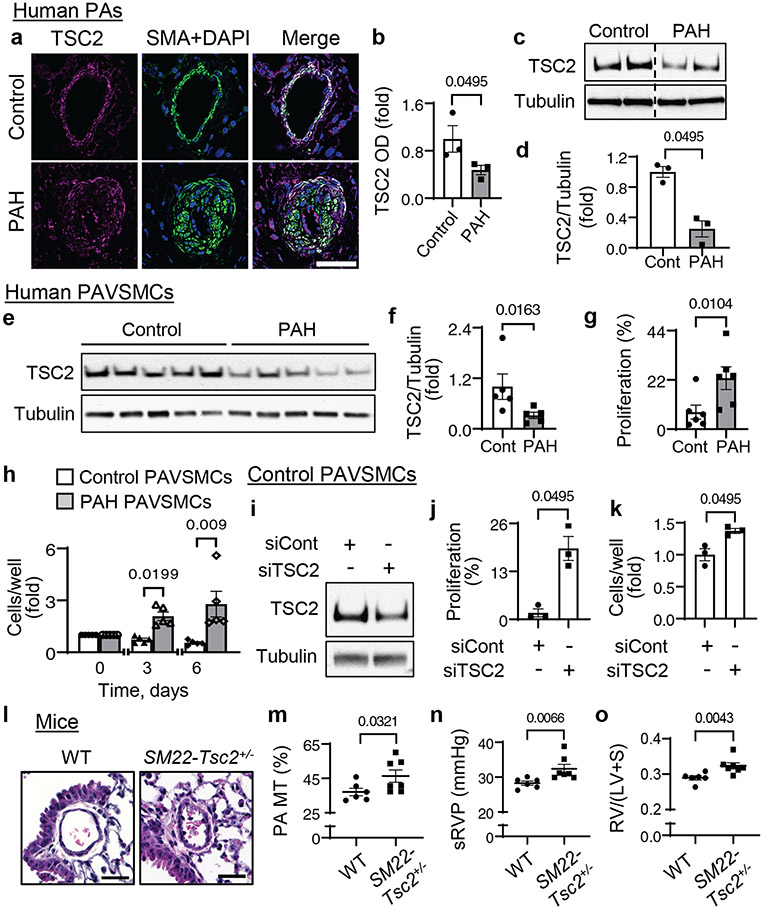
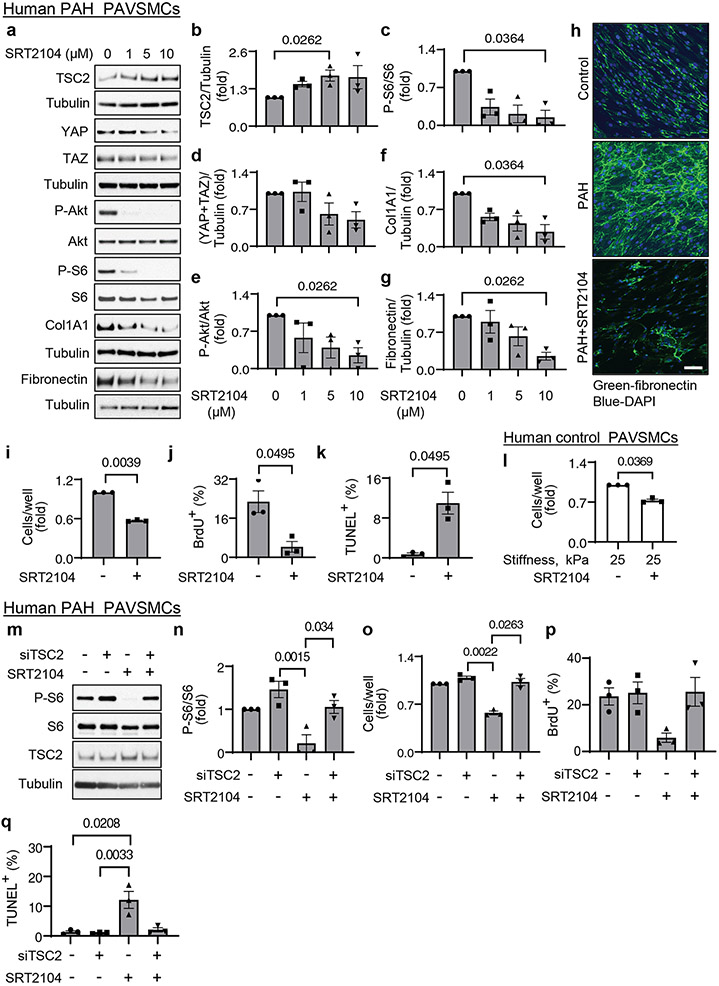
To determine the status of TSC2 in human PAH lungs, we performed immunohistochemical and immunoblot analysis of lung tissue specimens from subjects with PAH and from non-diseased (control) donors (table S1). We found that TSC2 protein levels were lower in smooth muscle α-actin (SMA)-positive areas and whole tissue lysates of small PAs from PAH lungs compared to those from control lungs (Fig. 1a-d). Supporting our observations, PAVSMCs from small (<1.5 mm outer diameter) human PAH PAs had significantly reduced TSC2 protein content (Fig. 1e, f) and significantly higher non-stimulated proliferation than those from non-diseased subjects (Fig. 1g, h). TSC2 abundance was not changed in human PAH PA endothelial cells (PAECs) or PAAFs (fig. S1a-d). Together, these data demonstrate that TSC2 is deficient in PAVSMCs in remodeled small PAs in human PAH lungs.
Figure 1. TSC2 deficiency results in increased PAVSMC proliferation, vascular remodeling and PH.
a, b. (a) Immunohistochemical analysis of human lung tissue sections to detect TSC2 (magenta), SMA (green) and DAPI (blue). Bar, 100 μm. Images are representative from 3 subjects/group, 6 PAs/subject. (b) Optical density (OD) measurement of TSC2 fluorescent signal in SMA-positive regions of human small PAs. Data are means±SE from 3 subjects/group, 6 PAs/subject, 12 areas/PA. PAH was normalized to control, which was set at 1. P value for PAH compared to control was determined by Mann Whitney U test. Images from different parts of the same gel are separated by dotted line.
c, d. Immunoblot analysis of small PAs. Data are means±SE from n=3 subjects/group. P value for PAH compared to control was determined by Mann Whitney U test.
e, f. Immunoblot analysis of human non-diseased (control) (white bars) and PAH (grey bars) PAVSMCs. Data are means±SE. PAH was normalized to control which was set to 1. N=5 subjects/group. P value for PAH compared to control was determined by Mann Whitney U test.
g. Proliferation of human control and PAH PAVSMCs as measured by Ki67 staining. Data represent % of Ki67-positive cells per total number of cells (as detected by DAPI) and are means±SE from n=6 subjects/group. P value for PAH compared to control was determined by Mann Whitney U test.
h. Equal quantity of cells was plated on each well of 6-well plate (day 0), cell counts were performed at days 3 and 6. Data are fold change to day 0. Data are means±SE from n=5 subjects/group. P values for PAH compared to control were determined by Mann Whitney U test.
i-k. Immunoblot (i), proliferation as measured by BrdU incorporation (j) or cell counting (k) performed on control human PAVSMCs transfected with siRNA directed against TSC2 (siTSC2) or control siRNA GLO (siCont) for 48 hours. Data are means±SE from n=3 subjects/group. P values for siTSC2 compared to siCont were determined by Mann Whitney U test.
l-o. Morphological and hemodynamic analysis of nine week old male SM22-Tsc2+/− and wild-type (WT) mice. Images (l) are representative of 6 mice/group, 12 PAs/mouse. Scale bar, 30 μm. Graphs should PA medial thickness (PA MT) (m), systolic RV pressure (sRVP) (n), and Fulton index (right ventricle (RV)/(left ventricle (LV) + septum) (o). Data are means±SE for n=6 WT mice and n=7 SM22-Tsc2+/− mice. P values for SM22-Tsc2+/− compared to WT by Mann Whitney U test.
Reduced TSC2 abundance enables PAVSMC hyper-proliferation, remodeling, and PH
To determine the functional outcome of decreased TSC2 content, we depleted TSC2 in human control PAVSMCs by siRNA, which significantly increased DNA synthesis (as assessed by BrdU incorporation) and cell proliferation (Fig. 1i-k). To test the in vivo consequences of TSC2 deficiency, we generated mice with SMC-specific Tsc2 knockout by crossing Tsc2flox/flox mice and Tagln (Sm22)-cre mice (13). Homozygous knockout of Tsc2 was embryonically lethal. Mice with heterozygous deletion of Tsc2 (Tsc2+/−) (fig. S2a, b) developed spontaneous mild experimental PH as early as nine weeks of age. Compared to the same-age wild-type controls (WT), nine week old male SM22-Tsc2+/− mice had significantly greater medial thickness of small (<150 μm) PAs (PA MT) (Fig. 1l,m), elevated systolic right ventricular (RV) pressure (sRVP) (Fig. 1n), and RV hypertrophy (as determined by the significant increase in the RV/(LV+S) ratio) (Fig. 1o). Four out of six female mice also developed mild experimental PH (sRVP≥30 mmHg) at nine weeks of age (fig. S3a). Exposing nine week old SM22-Tsc2+/− mice to hypoxia (10% O2, three weeks) resulted in significantly higher PA MT for male mice and RV hypertrophy for both male and female mice (fig. S3b-g) without altering sRVP compared to same-sex same-age controls (fig. S3h, i). Twenty-two week old male SM22-Tsc2+/− mice, but not their female counterparts, had significant pulmonary vascular remodeling compared to same-age controls and significantly greater RV hypertrophy than nine week old SM22-Tsc2+/− mice (fig. S4a-g), suggesting that the experimental PH phenotype continued to develop. Together, these data demonstrate that reduced TSC2 abundance results in increased PAVSMC proliferation, VSM remodeling, and spontaneous experimental PH.
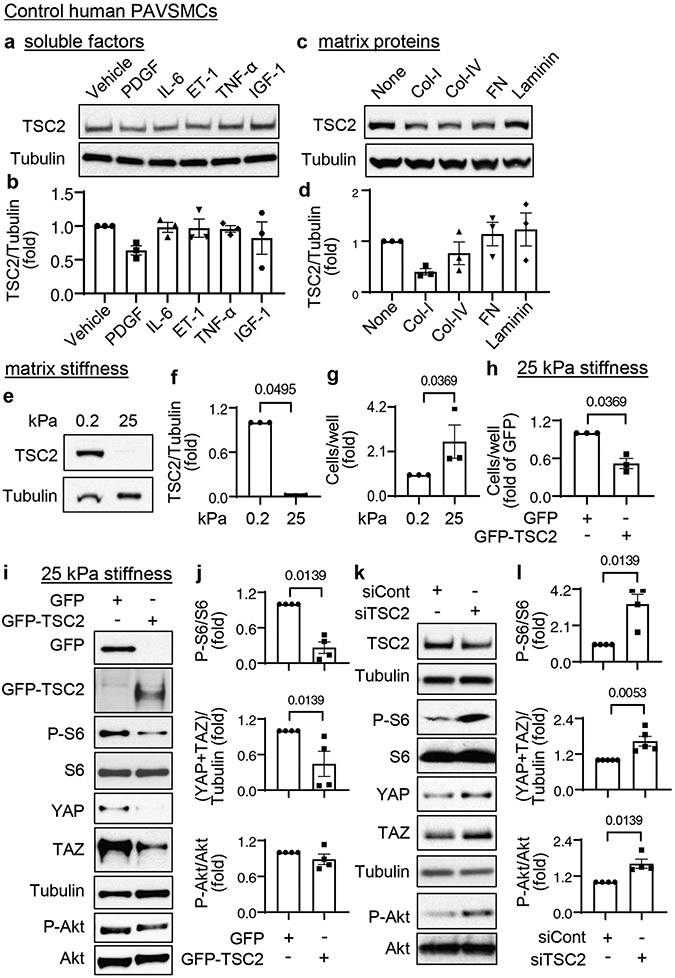
Decreased TSC2 content is induced by substrate stiffening and enables YAP/TAZ accumulation and PAVSMC proliferation
To identify pro-PAH stimuli that induce TSC2 deficiency in PAVSMCs, we exposed control human PAVSMCs to various soluble growth factors and pro-inflammatory mediators (platelet-derived growth factor-BB (PDGF-BB), insulin growth factor 1 (IGF-1), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and endothelin-1 (ET-1). We also maintained the cells on matrices of different composition (collagen 1, collagen IV, fibronectin, laminin) or stiffness (0.2 kPa, a stiffness seen under normal physiological conditions, and 25 kPa, a stiffness seen under pathological conditions) (8, 11, 13). We found that TSC2 protein levels showed a trend to decrease in PAVSMCs maintained on the collagen 1 matrices or treated with PDGF-BB (Fig. 2a-d), and that PDGF-dependent increases in the phosphorylation Akt and, in part, ribosomal protein S6 were attenuated in control PAVSMCs treated with the PDGF receptor inhibitor AG1295 (fig. S5 a-c). These data agree with previously published studies demonstrating that PDGF inhibits TSC2-mTORC1 signaling through Akt (24).
Figure 2. Decreased TSC2 content is induced by increased substrate stiffness and is required for YAP/TAZ accumulation, activation of Akt and mTOR, and increased PAVSMC proliferation.
a-d. Immunoblot analysis of control human PAVSMCs treated with the indicated soluble factors (a, b) or maintained on the indicated matrices (c,d) for 48 hours. Data are means±SE from n=3 subjects/group. Values were normalized to vehicle treatment or uncoated matrix, which were set at 1. P values were determined by Kruskal-Wallis test with Dunn’s pairwise comparison.
e, f. Immunoblot analysis of control human PAVSMCs maintained on softwell hydrogels with normal (0.2 kPa) or pathological (25 kPa) stiffness for 48 hours. Data are means±SE from n=3 subjects/group. 25 kPa was normalized to 0.2 kPa, which was set at 1. P value for 25 kPa compared to 0.2 kPa was determined by Mann Whitney U test.
g. Equal numbers of control human PAVSMCs were plated on hydrogels with 0.2 kPa or 25 kPa stiffness, and cell proliferation analysis was performed on day 4. Data are means±SE from n=3 subjects/group. 25 kPa was normalized to 0.2 kPa, which was set at 1. P value for 25 kPa compared to 0.2 kPa was determined by Mann Whitney U test.
h-j. Human control PAVSMCs were plated on softwell hydrogels with 25 kPa stiffness and transfected with mammalian vectors expressing GFP or GFP-tagged human TSC2 for 36 hours. Cell proliferation (h) and immunoblot analyses (i-j) were performed. Data are means±SE from n=3 (cell counts) and 4 (immunoblots) subjects/group. GFP-TSC2 was normalized to GFP, which was set at 1. P values for GFP-TSC2 compared to GFP were determined by Mann Whitney U test.
k, l. Human control PAVSMCs were transfected with siRNA directed against TSC2 (siTSC2) or control siRNA GLO (siCont) for 48 hours and were immunoblotted for the indicated proteins. Data are means±SE from n=4 or 5 subjects/group. siTSC2 was normalized to siCont, which was set at 1. P values for siTSC2 compared to siCont were determined by Mann Whitney U test.
Maintenance of PAVSMCs on stiff (25 kPa) (8, 12, 14, 15) matrices reduced TSC2 protein content and significantly increased cell proliferation compared to the cells seeded on soft (0.2 kPa) substrates (Fig. 2e-g). To determine whether TSC2 modulates stiffness-induced PAVSMC proliferation, we transfected control human PAVSMCs seeded on stiff (25 kPa) matrices with mammalian vectors expressing GFP-TSC2 or control GFP. GFP-TSC2-transfected PAVSMCs had ~twofold lower proliferation on stiff matrices than GFP-transfected cells (Fig. 2h), suggesting that TSC2 acts as a mechanosensor and mechanotransducer, and that decreased TSC2 abundance in PAVSMCs is induced by increased matrix stiffness and is required for stiffness-induced cell proliferation.
Stiffening of small PAs drives pulmonary vascular cell proliferation and remodeling in PAH through the transcriptional coactivators YAP/TAZ, which act partially by promoting pro-proliferative and survival signaling through the Akt-mTOR pathway (8, 12, 14). Re-expression of TSC2 in control PAVSMCs maintained on stiff matrices not only significantly decreased the phosphorylation of the ribosomal protein S6, a hallmark of mTORC1 activation, but also significantly reduced YAP/TAZ protein levels compared to those in GFP-expressing cells (Fig. 2i, j). Supporting our findings, siRNA-mediated depletion of TSC2 in control human PAVSMCs resulted in a significant increase in S6 and Akt phosphorylation and accumulation of YAP/TAZ compared to cells transfected with control siRNA (Fig. 2k, l). These data show that decreased TSC2 content in PAVSMCs is required for stiffness-induced PAVSMC proliferation and increases in YAP/TAZ abundance and mTOR activity.
PAVSMCs in PAH have an over-accumulation of active YAP in nuclei, leading to hyperproliferation and pulmonary vascular remodeling (8, 11, 14, 25). YAP could be phosphorylated at Ser127 by LATS1/2 and Akt, leading to its cytoplasmic retention and inhibition (26, 27). We observed no differences in the phosphorylation of Ser127 in YAP in human PAH PAVSMCs compared to controls (fig. S6a, b) and in SMA-positive areas of small PAs from SM22-Tsc2+/− mice compared to WT animals (fig. S6c, d). Treatment of human PAH PAVSMCs with the LATS1/2 kinase inhibitor LATS-IN-1 (28) significantly reduced the phosphorylation of Ser127 in YAP, an effect not observed with transfection of siRNA Akt1/2, which also did not affect LATS-IN-1-dependent inhibition of this phosphorylation event (fig. S7a, b). Together with published studies, these data suggest that LATS, rather than Akt, is responsible for the phosphorylation of Ser127 in YAP in human PAH PAVSMCs.
Akt1 is a key Akt isoform involved in pulmonary vascular remodeling (29), but cotransfection of siRNAs targeting TSC2 and Akt1 did not significantly reduce TSC2-dependent phosphorylation of S6K1 and S6 (fig. S8a, b), suggesting that TSC2 loss attenuates Akt-dependent mTORC1 activation. To confirm our observations, we used rat Eker leiomyoma tumor-3 (ELT3) cells, which carry a germline Tsc2 mutation, resulting in TSC2 loss and constitutive mTORC1 activation (fig. S8c) (30). Treatment of ELT3 cells with the Akt inhibitor VIII did not affect S6K1 and S6 phosphorylation (fig. S8d, e), demonstrating that TSC2 deficiency compromises Akt-dependent mTORC1 activation.
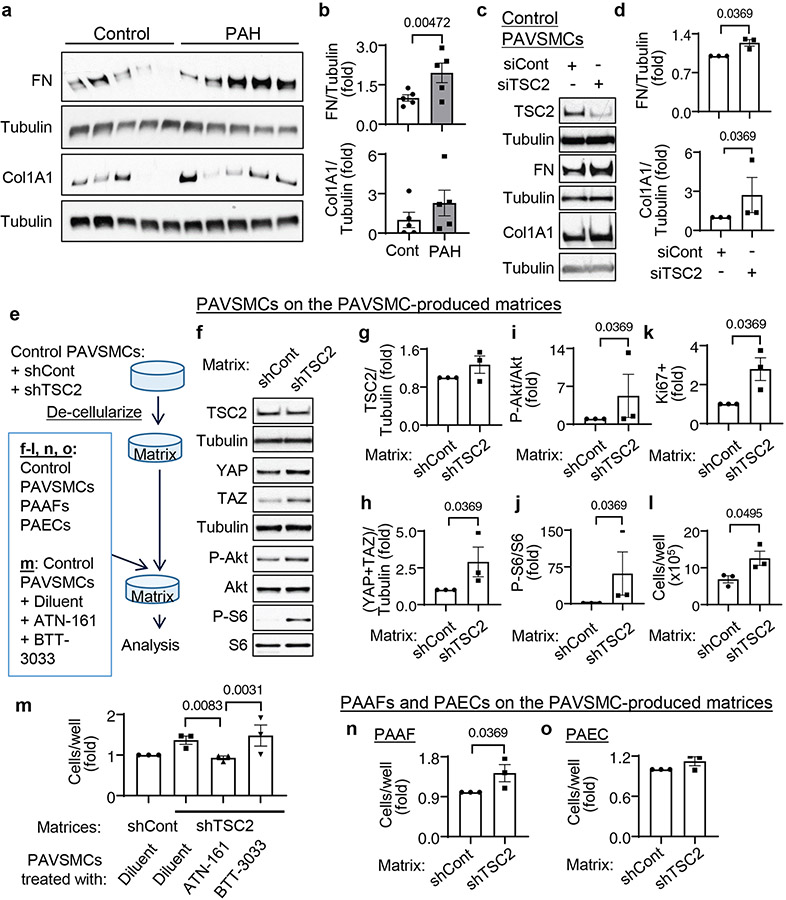
Decreased TSC2 content promotes increases in YAP/TAZ abundance, mTOR activity, and PAVSMC proliferation through ECM remodeling
Because YAP and TAZ facilitate resident pulmonary vascular cell proliferation in PAH through ECM remodeling (8, 11), we hypothesized that, in addition to canonical Rheb-dependent activation of mTORC1, reduced TSC2 abundance in PAH PAVSMCs may facilitate pro-proliferative signaling by modulating ECM production. In agreement with previous studies (8, 11), PAVSMCs from human PAH lungs had higher fibronectin and collagen 1A protein content compared to those from control lungs (Fig. 3a, b). Moreover, TSC2 knockdown significantly increased fibronectin and collagen 1A protein levels in control human PAVSMCs (Fig. 3c, d), suggesting that decreased TSC2 content facilitates production of the two key ECM proteins involved in PAH. Because excessive ECM production promotes stiffening of small muscular PAs (31), we next tested the effect of smooth muscle TSC2 loss on pulmonary vascular wall stiffness using atomic force microscopy (AFM) analysis. Mechanical characterization of small (< 200μm in diameter) PAs by AFM microindentation (32) revealed that nine week old male SM22-Tsc2+/− mice had significantly higher vascular wall stiffness compared to WT mice (fig. S9a, b). Together, these data demonstrate that a decrease in smooth muscle TSC2 elevates ECM production and induces pulmonary vascular wall stiffening.
Figure 3. Reduced TSC2 abundance in PAVSMCs promotes YAP/TAZ accumulation, mTOR activity, and proliferation of non-diseased pulmonary vascular cells through ECM remodeling.
a, b. Human control and PAH PAVSMCs (from 5 subjects/group) were immunoblotted for fibronectin (FN) and collagen 1A1 (Col1A1). Data are means±SE. PAH was normalized to Cont, which was set at 1. P values for PAH compared to Cont were determined by Mann Whitney U test.
c, d. Human control PAVSMCs were transfected with siRNA directed against TSC2 (siTSC2) or control siRNA GLO (siCont) and immunoblotted for FN and Col1A1. Data are means±SE from n=3 subjects/group. siTSC2 was normalized to siCont, which was set at 1. P values for siTSC2 compared to siCont were determined by Mann Whitney U test.
e-o. Pre-confluent control PAVSMCs were infected with adenoviruses encoding control shRNA (shCont) or shRNA directed against TSC2 (shTSC2) for six days (e). Cells were removed and an equal amount of non-diseased (control) untreated PAVSMCs (f-l), PAVSMCs treated with diluent, 10μM ATN161 (α5β1 integrin inhibitor), or 10μM BTT3033 (α2β1 integrin inhibitor) (m), control PAAFs (n), or control PAECs (o) were plated on the matrices. Four days after plating, immunoblot (f-j) and proliferation analyses as measured by Ki67 staining (k) or cell counting (l-o) were performed. Data are means±SE from n=3 subjects/group. P values for siTSC2 compared to siCont were determined by Mann Whitney U test (significance) (g-l, n, o) and by Kruskal-Wallis test with Dunn’s pairwise comparison (m).
Next, we evaluated whether reduced TSC2 abundance modulated pro-proliferative signaling in neighboring cells through the ECM. We used de-cellularized matrices from control human PAVSMCs infected with lentiviruses producing shTSC2 or control shRNA as substrates for the plating of control human PAVSMCs (Fig. 3e). We found that control PAVSMCs seeded on matrices produced by shTSC2-infected control PAVSMCs had significantly higher YAP/TAZ protein levels, increased phosphorylation of Ser473 in Akt and of S6, and elevated proliferation compared to cells seeded on matrices produced by shCont-infected PAVSMCs (Fig. 3f-l). To further confirm the role of ECM in the increase in YAP/TAZ abundance, Akt phosphorylation, mTOR activity, and cell proliferation in TSC2-deficient PAVSMCs, we maintained control PAVSMCs on cell-free matrices produced by shTSC2-infected PAVSMCs in the presence of ATN-161, a small peptide antagonist of α5β1 integrin, and BTT-3033, a selective inhibitor of α2β1 integrin, to block cell binding with fibronectin and collagen respectively (Fig. 3e). ATN-161, but not BTT3033, prevented matrix-induced PAVSMC proliferation (Fig. 3m), suggesting that decreased TSC2 abundance promotes PAVSMC proliferation by regulating extracellular fibronectin content. Supporting the relevance of these findings to human PAH, control PAVSMCs seeded at the de-cellularized ECM produced by human PAH PAVSMCs showed increases in the phosphorylation of Ser473 in Akt and of S6 and in YAP/TAZ protein content compared to cells maintained on matrices produced by non-diseased PAVSMCs (fig. S10a-f). Collectively, these data demonstrate that TSC2 deficiency promotes increases in YAP/TAZ abundance, mTOR activity, and PAVSMC proliferation through ECM remodeling.
In contrast to stiffness-induced TSC2 deficiency (Fig. 2e, f), control PAVSMCs seeded on the matrices produced by TSC2-deficient control or PAH PAVSMCs showed increased YAP/TAZ abundance and mTOR activity without a reduction in TSC2 protein levels (Fig. 3f-i, fig. S10a-f). These data support our observations that TSC2 acts upstream of YAP/TAZ and mTOR by regulating the ECM and suggest an ECM composition-independent mechanism(s) that maintains a self-sustaining suppression of TSC2 content in PAH PAVSMCs. We observed no significant differences in the protein levels of TSC2 binding partner TSC1 (17, 33) in SMA-positive areas of small PAs from SM22-Tsc2+/− and control mice as well as in human PAVSMCs from control and PAH subjects (fig. S11a-d). Because mTOR acts both upstream (through mTORC2) and downstream (through mTORC1) of TSC2 (17), and because the activity of both mTORC2 and mTORC1 is increased by YAP/TAZ in human PAH PAVSMCs (7, 8), we hypothesized that stiffness-independent TSC2 deficiency could be self-supported by a YAP/mTORC2 feedforward loop. Supporting our hypothesis, siRNA-mediated depletion of YAP and disruption of mTORC2 by siRNA-mediated depletion of Rictor led to TSC2 accumulation in human PAH PAVSMCs compared to cells transfected with control siRNA (figs. S12a, b, and S13a-c). Moreover, co-transfection with TSC2 siRNA reversed the Rictor siRNA-induced decrease in PAH PAVSMC proliferation (fig. S13d, e), suggesting that mTORC2-dependent reduction of TSC2 protein levels is required for PAH PAVSMC proliferation. However, treatment of human PAH PAVSMCs with either the mTOR kinase inhibitor PP242 (which inhibits mTOR in both mTORC1 and mTORC2) or the allosteric mTORC1 inhibitor rapamycin did not increase TSC2 protein levels or reduce YAP/TAZ accumulation (fig. S14a, b), suggesting that suppression of TSC2 abundance in PAH PAVSMCs is supported by YAP/mTORC2 independently of mTOR kinase activity.
ECM produced by TSC2-deficient PAVSMCs induces PAAF proliferation
Because changes in ECM composition affect the proliferative response of neighboring pulmonary vascular cells, we next tested whether the matrix produced by TSC2-deficient PAVSMCs modulates the proliferation of other resident pulmonary vascular cells. We seeded control human PAECs and PAAFs on matrices produced by control human PAVSMCs infected with adenovirus producing shTSC2 or control shRNA (Fig. 3e). We found that control PAAFs, but not PAECs, had significantly higher cellular proliferation when maintained on matrices produced by shTSC2-infected PAVSMCs compared to cells seeded on the matrices produced by shCont-infected PAVSMCs (Fig. 3n, o). These data show that TSC2 deficiency in PAVSMCs results in dysregulated ECM production, which promotes PAAF proliferation.
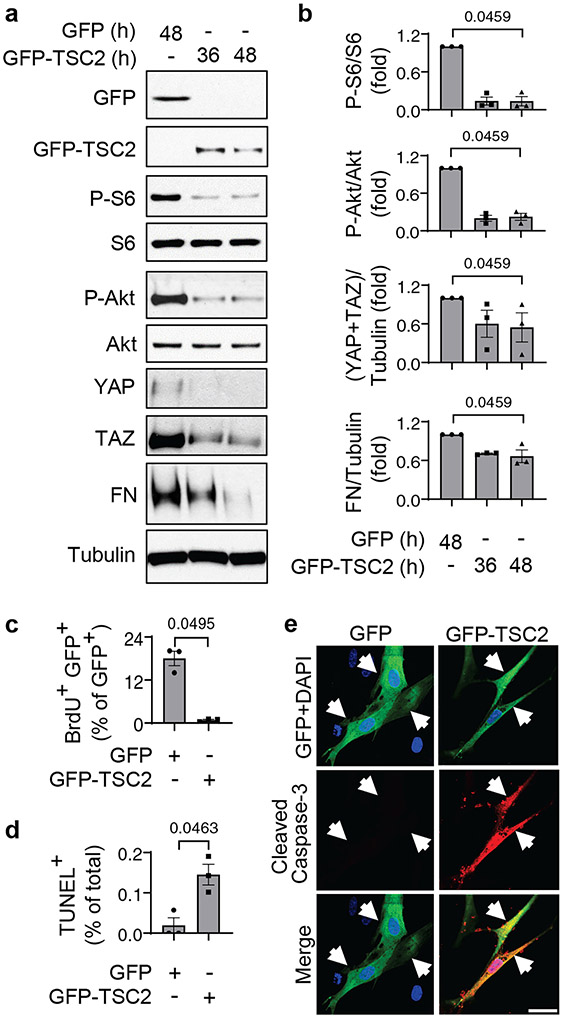
Restoration of TSC2 abundance suppresses proliferation and induces apoptosis in PAH PAVSMCs
We next sought to evaluate the potential benefits of restoring functional TSC2 in PAVSMCs from human PAH lungs. We found that TSC2 expression suppressed Akt and S6 phosphorylation, YAP/TAZ protein levels, and fibronectin and collagen 1A production compared to GFP-expressing cells (Fig. 4a, b, S15a). Moreover, expression of TSC2 suppressed PAH PAVSMC proliferation (Fig. 4c, fig. S15b) and induced the accumulation of cleaved caspase-3 and significant apoptosis (Fig. 4d, e), suggesting that restoration of TSC2 may be a possible therapeutic strategy to suppress both YAP/TAZ and mTOR pathways, halt excessive ECM production, inhibit proliferation, and induce apoptosis in PAH PAVSMCs.
Figure 4. TSC2 reconstitution reduces YAP/TAZ accumulation and fibronectin production, suppresses proliferation, and induces apoptosis in human PAH PAVSMCs.
a, b. Human PAH PAVSMCs were transfected with GFP or GFP-TSC2 for the indicated times and immunoblotted to detect the indicated proteins. Data are means±SE from 3 subjects/group. P values for GFP-TSC2 compared to GFP were determined by Kruskal-Wallis rank test with Dunn’s pairwise comparison.
c-e. Proliferation (as measured BrdU incorporation) (c), apoptosis (as measured by TUNEL staining) (d), and immunocytochemical analysis to detect cleaved caspase 3 (red), GFP (green), and DAPI (blue) (e). Data are means±SE from 3 subjects/group. A minimum of 12 transfected cells/subject and condition were analyzed. P values for GFP-TSC2 compared to GFP were determined by Mann Whitney U test. Scale bar, 50 μm. White arrows indicate transfected cells.
SRT2104 reverses the PAVSMC phenotype in PAH by restoring TSC2 abundance
Next, we evaluated potential pharmacological strategies to restore functional TSC2 in human PAH PAVSMCs. TSC2 is regulated by direct phosphorylation, which either prevents or promotes its degradation (17), and other types of posttranslational modification, such as deacetylation by SIRT1 (34). Positive and negative regulators of TSC2 involved in PAH are Akt and adenosine monophosphate-activated protein kinase (AMPK). Akt is activated whereas AMPK is inhibited in human PAH PAVSMCs, and either inhibition of Akt or activation of AMPK suppresses mTORC1 and PAH PAVSMC proliferation (8, 19, 29, 35-38). We found that pharmacological inhibition of Akt or activation of AMPK reduced S6 phosphorylation but did not elevate TSC2 protein content (fig. S16a, b). Together with the inability of mTORC1/2 inhibitor PP242 to restore TSC2 abundance in PAH PAVSMCs, our data suggest that mTOR, Akt and AMPK act downstream of or in parallel to TSC2 and targeting these signaling proteins cannot restore PAH-specific TSC2 deficiency.
Next, we tested the effect of the SIRT1 activator SRT2104. We found that treatment of human PAH PAVSMCs with SRT2104 significantly increased TSC2 protein levels and suppressed S6 phosphorylation (Fig. 5a-c), showing that SRT2104 restores functional TSC2. Moreover, SRT2104 significantly decreased the phosphorylation of Ser473 in Akt and the abundance of collagen 1A and fibronectin and showed a trend in reducing YAP/TAZ accumulation (Fig. 5a, d, e, f, g, h), and significantly inhibited proliferation and promoted apoptosis in PAH PAVSMCs (Fig. 5i-k). In addition, SRT2104 also reduced the proliferation of control PAVSMCs on stiff matrices (Fig. 5I), suggesting potential benefits of SRT2104 in targeting both self-sustaining and stiffness-induced PAVSMC proliferation in PAH. To test whether SRT2104 acted through TSC2, we targeted TSC2 by siRNA in SRT2104-treated cells. We found that siRNA-mediated knockdown of TSC2 prevented SRT2104-induced inhibition of S6 phosphorylation and of cell proliferation, and blocked SRT2104-induced apoptosis (Fig. 5m-q). Together, the data show that restoration of TSC2 abundance by SRT2104 inhibits proliferation and induces apoptosis in human PAH PAVSMC.
Figure 5. SRT2104 restores functional TSC2, inhibits proliferation, and induces apoptosis in human PAH PAVSMCs.
a-g. Human PAH PAVSMCs were treated with diluent (0) or the indicated concentrations of SRT2104 and immunoblotted for the indicated proteins. Data are means±SE from three experiments, each performed on the cells from a different subject. P values for SRT2104 treatment compared to diluent (0) were determined by Kruskal-Wallis rank test with Dunn’s pairwise comparison.
h. Immunocytochemical analysis to detect fibronectin produced by human non-diseased (control) and PAH PAVSMCs treated with diluent or 10 μM SRT2104 for 48 hr. Scale bar, 100 μm.
i-k. Human PAH PAVSMCs were treated with diluent (−) or 10 μM SRT2104 (+) for 48 hr. Proliferation assessed by cell counts (i) or BrdU incorporation (j) and apoptosis as determined by TUNEL staining (k) analyses were performed. Data are means±SE from three experiments, each performed on the cells from different subject. P values for SRT2104 treatment compared to diluent (0) were determined by Mann Whitney U test.
l. Equal numbers of human control PAVSMCs were seeded on hydrogels with 25 kPa stiffness, treated with diluent (−) or 10 μM SRT2104 (+) for 48hr, and counted. Data are means±SE from three experiments, each performed on the cells from a different subject. P value for SRT2104 treatment compared to diluent (0) was determined by Mann Whitney U test.
m-q. Immunoblot (m-n), proliferation (as measured by cell counting or BrdU incorporation) (o-p) and apoptosis (as measured by TUNEL staining) (q) analyses were performed on PAH PAVSMCs transfected with siContr or siTSC2 and treated with 10μM SRT2104 or diluent for 48hr. Data are means±SE from three experiments, each performed on the cells from a different subject. P values for SRT2104 and/or siTSC2 compared to siContr (Scr) and diluent were determined by Kruskal-Wallis rank test with Dunn’s pairwise comparison.
SRT2104 restores TSC2 in small PAs and reduces established PH in mice and rats
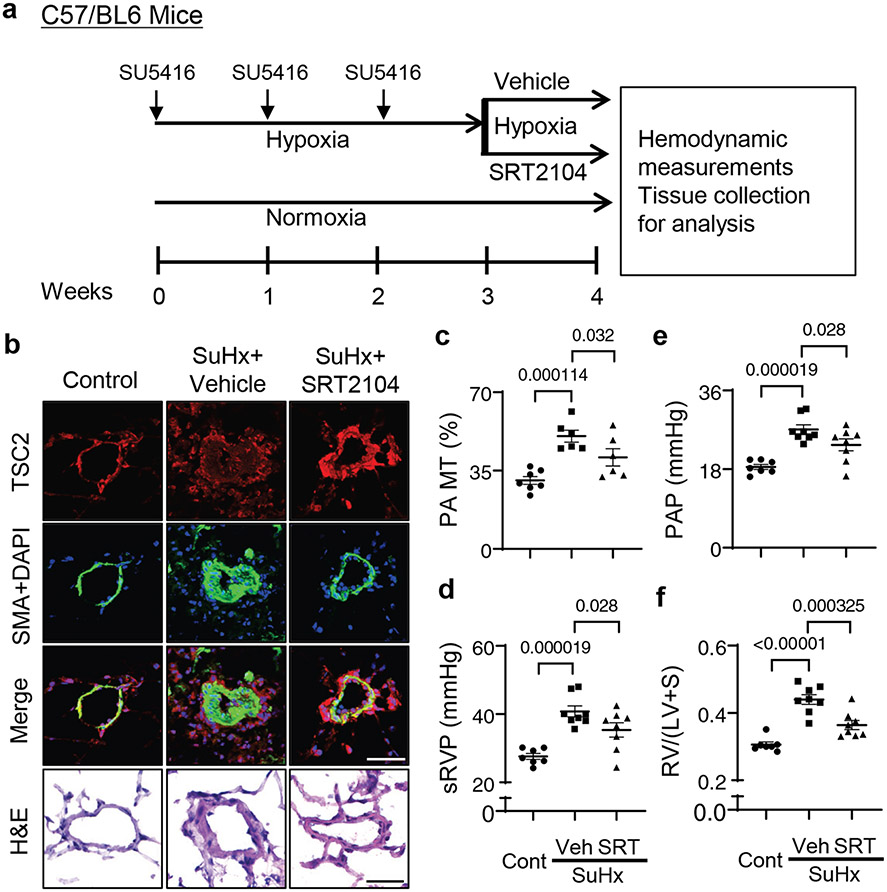
We examined whether short-term treatment of mice with SRT2104 after the establishment of SU5416/hypoxia (SuHx)-induced experimental PH led to TSC2 accumulation in small PAs (Fig. 6a). Similar to human PAH PAVSMCs, vehicle-treated mice from the SuHx group had reduced TSC2 protein levels in SMA-positive areas of small PAs (Fig. 6b), suggesting similar underlying mechanisms. As expected, these mice developed pulmonary vascular remodeling and experimental PH as evidenced by significantly higher PA medial thickness (PA MT), systolic right ventricular pressure (sRVP), PA pressure (PAP), and RV hypertrophy (Fig. 6b-f). The SRT2104-treated mice had higher TSC2 protein content in small PAs and significantly lower PA MT, sRVP, PAP, and RV hypertrophy compared to vehicle-treated SuHx mice (Fig. 6b-f). There were no significant differences between male and female mice. Systolic left ventricular pressure (sLVP), mean arterial pressure (MAP), and heart rate did not significantly differ between vehicle and SRT2014-treated groups (fig. S17a-c). These data show that short-term SRT2104 treatment increases Tsc2 abundance, attenuates VSM remodeling and PH, and improves RV hypertrophy in mice with SuHx-induced PH.
Figure 6. SRT2104 restores TSC2 in small PAs, attenuates PH, and reduces RV hypertrophy in mice.
a. Six to eight week old male and female mice were maintained under hypoxia for three weeks and received SU5416 injection at the beginning of every week. Starting at week 4, mice kept under hypoxia were randomly assigned to receive SRT2104 (SRT) or vehicle (Veh) for 5 days/week for one week, and hemodynamic and morphological analyses were performed. Controls were same-age and -sex mice kept under normoxia.
b. Immunohistochemical analysis to detect TSC2 (red), SMA (green), and DAPI (blue). Images are representative of 3 mice/group, 12 PAs/mice. Scale bar, 80 μm. For H&E staining, images are representative from 7 control mice and 6 SuHx+Vehicle or SuHx+SRT2104, 12 Pas/mouse. Scale bar, 30μm,
c. PA medial thickness (PA MT) was calculated from 12-24 Pas/mouse. Data are means±SE from n=7 control mice (3 male, 4 female), n=6 PH mice (3 male, 3 female), n=6 PH+SRT2104 mice (3 male, 3 female) groups. P values were determined by one-way ANOVA with a Fisher’s LSD post hoc test.
d-f. Systolic right ventricular pressure (sRVP) (d), pulmonary arterial pressure (PAP) (e), and Fulton index (RV/(LV + septum) weight ratio) (f) were calculated. Data are means±SE from n=7 control mice (3 male, 4 female), n=8 PH mice (4 male, 4 female), n=8 PH+SRT2104 mice (4 male, 4 female) groups. P values were determined by one-way ANOVA with a Fisher’s LSD post hoc test.
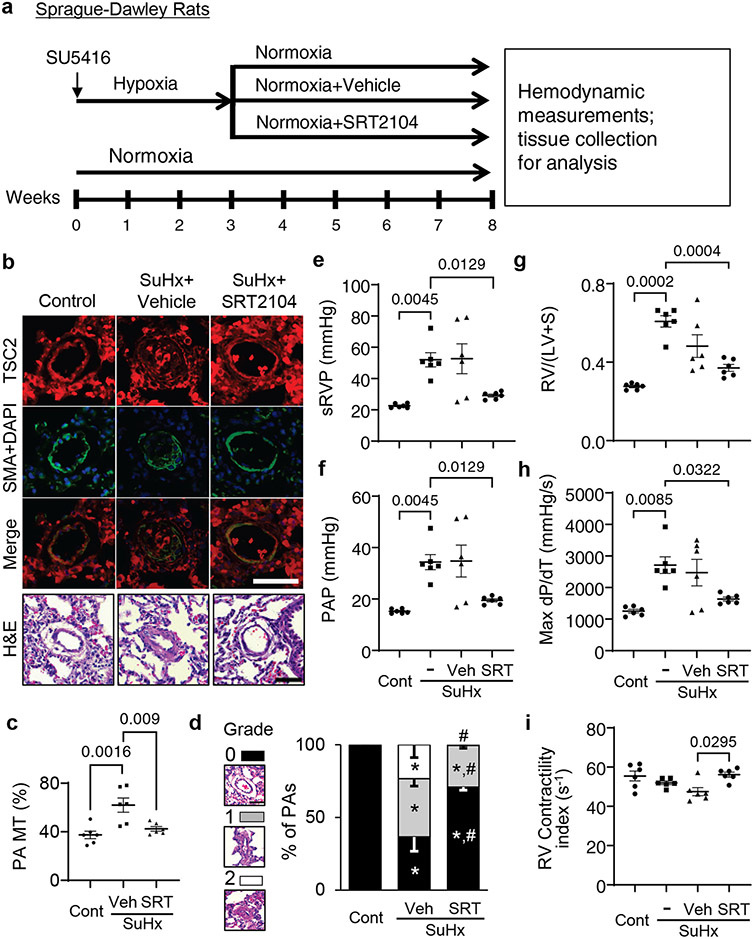
To evaluate the potential benefits of SRT2104 in severe experimental PH, we next tested the effects of long-term SRT2104 treatment using a rat SuHx model of experimental PH (Fig. 7a). Similar to human PAH PAVSMCs and the mouse PH model (Figs. 1a, 6b), vehicle-treated rats with SuHx-induced PH developed VSM-specific TSC2 deficiency in remodeled small PAs and robust pulmonary vascular remodeling, which were reversed by treatment with SRT2104 (Fig. 7b-d). SuHx-exposed rats developed experimental PH and RV hypertrophy compared to control rats (Fig. 7e-h). Treatment with SRT2104, but not vehicle, normalized sRVP and PAP and improved RV morphology and function (as shown by reversals of RV hypertrophy and normalized max dP/dT and the contractility index) (Fig. 7e-i). There were no significant differences in sLVP, MAP, and heart rate between SuHx+Vehicle and SuHx+SRT2104 groups (fig. S17d-f). Together, these data demonstrate that SRT2104 reverses established PH and improves RV morphology and function in a severe irreversible rat model of experimental PH.
Figure 7. SRT2104 restores TSC2 in small PAs, attenuates PH, and reduces RV hypertrophy in rats.
a. Male six to eight weeks old rats received one SU5416 injection and were maintained under hypoxia for three weeks. Starting week 4, rats were transferred to normoxia and were randomly assigned to receive SRT2104 (SRT) or vehicle (Veh) for five days/week for five weeks (days 21-56 of the experiment). Upon experiment termination, rats were subjected to hemodynamic and morphological analyses. Controls were same-age untreated male rats kept under normoxia for eight weeks or exposed to SU5416-hypoxia-normoxia without treatment.
b. Immunohistochemistry was performed for TSC2 (red), SMA (green) and DAPI (blue) and H&E analyses. Scale bar, 50 μm. Images are representative from 6 rats/group, 12 PAs/rat.
c-i: Percentage of fully (grade 2), partially (grade 1) and not occluded PAs (grade 0) was determined (c). Scale bar, 50 μm. PA MT (d), sRVP (e), PAP (f), Fulton index (g), RV contractility (Max(dP/dt)) (h), and RV contractility index (i) were measured on day 56. Data are means±SE from 6 rats/group. *p<0.05 compared to control, #p<0.05 compared to PH. P values were determined by one-way ANOVA with a Tukey’s post hoc test (c), one-way ANOVA with a Fisher’s LSD post hoc test (d), and Welch’s one-way ANOVA F test with Games-Howell post-hoc analysis (e-i).
Discussion
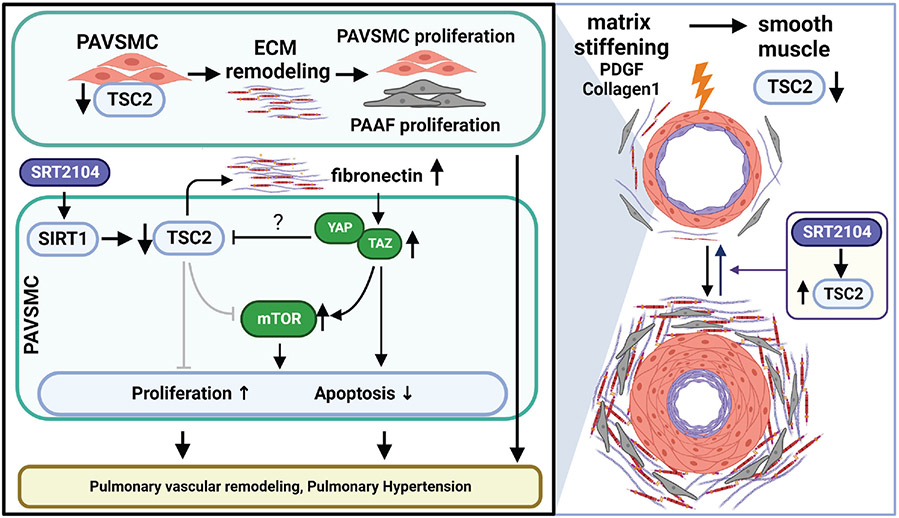
The present study provides evidence that reduced TSC2 abundance in the medial layer of small PAs is an important trigger of PH, and suggests that the restoration of functional TSC2 may be an attractive therapeutic strategy to reverse existing pulmonary vascular remodeling and PH. We found that TSC2 content was decreased in remodeled small PAs from subjects with PAH, two models of experimental PH, and early-passage PAVSMCs derived from small PAs of PAH patients. Reduced abundance or loss of TCS2 decreased PAVSMC hyper-proliferation, survival, pulmonary vascular remodeling, and development of spontaneous PH. Moreover, TSC2 in PAVSMCs acted as a mechanosensor and mechanotransducer and prevented the increases in YAP/TAZ abundance, mTOR activity, and cell proliferation induced by pathological stiffness. In addition, TSC2 controlled ECM composition, and reduced abundance or loss of TSC2 enhanced the production of abnormal ECM that resulted in increases in YAP/TAZ abundance, mTOR activity, and proliferation of PAVSMCs and PAAFs. Finally, pharmacological restoration of functional TSC2 by SRT2104 reversed molecular abnormalities, inhibited proliferation, induced apoptosis in human PAH PAVSMCs, and reduced established pulmonary vascular remodeling and PH in two rodent models of experimental PH (Fig. 8).
Figure 8. Schematic representation of the mechanism by which decreased TSC2 abundance in PAVSMCs promotes pulmonary vascular remodeling and PH.
Decreased TSC2 abundance in PAH PAVSMCs, caused predominantly by the ECM stiffening, results in excessive production of fibronectin, ECM remodeling, over-accumulation of YAP/TAZ, and activation of mTOR, leading to increased proliferation of PAVSMCs and PAAFs, reduced apoptosis of PAVSMCs, pulmonary vascular remodeling, and pulmonary hypertension. The SIRT1 activator SRT2104 restores functional TSC2, resolves the molecular and cellular abnormalities caused by decreased TSC2 protein content, and attenuates pulmonary vascular remodeling and pulmonary hypertension.
Increased proliferation and resistance to apoptosis of pulmonary vascular cells in small PAs underlie pulmonary vascular remodeling, a key component of PAH pathogenesis. Here, we showed that TSC2, a key inhibitor of mTORC1 and cell proliferation (39), was decreased in abundance in proliferative PAVSMCs, small remodeled PAs from human PAH lungs, and two rodent models of experimental PH. Our data indicated that reduced TSC2 content in PAVSMCs resulted in the unstimulated growth and proliferation of non-diseased human PAVSMCs, and SMC-specific Tsc2 heterozygosity in mice led to pulmonary vascular remodeling and early onset of spontaneous PH, indicating that even partial depletion of smooth muscle Tsc2 was sufficient to induce pulmonary vascular remodeling and PH.
A switch to the hyper-proliferative, apoptosis-resistant PAVSMC phenotype could be induced by various pro-PH factors, including exposure to soluble mitogens and changes in the ECM composition and stiffness (9, 40, 41). We found that decreased TSC2 content induced by pathological matrix stiffening was required for stiffness-induced proliferation of PAVSMCs, suggesting that TSC2 acts as a mechanosensor and a mechanotransducer. In addition to pathological stiffness, TSC2 abundance was reduced, although to a lesser extent, by its upstream inhibitor PDGF (39), suggesting the multi-factorial nature of TSC2 regulation. We found that TSC2 loss was required for the accumulation of the pro-proliferative/pro-survival mechanotransducers YAP/TAZ, which are implicated in the pathogenesis of PAH (8, 12, 42) and increased activity of the Akt-mTOR axis, suggesting that TSC2 integrates the mTOR and HIPPO networks, two major regulators of pulmonary vascular cell proliferation and survival in PAH (7-9, 43). Further, TSC2 regulates cellular metabolism, protein translation and autophagy, and the loss or mutational inactivation of TSC2 in pulmonary LAM activates mTORC1, RhoA, and CDK2 (44), pro-proliferative and vasoconstrictive molecules involved in PAH pathogenesis (45, 46). Although further studies are needed, TSC2 in PAH may integrate mechanobiological cues with PAVSMC contractility, cellular metabolism, proliferative signaling and cell cycle progression. If so, the reduced TSC2 abundance we observed in PAVSMCs from patients or disease models may be responsible for both vasoconstriction and pulmonary vascular remodeling, two major pathological features of PAH.
Our proposal of a central role of TSC2 in pulmonary vascular remodeling is further supported by our findings that TSC2 modulated ECM production by PAVSMCs. Reductions in TSC2 abundance in PAVSMCs led to over-production of two major ECM components, fibronectin and collagen 1 (8, 47) and formation of the abnormal ECM that induces PAH phenotypes. It is also important to note that the decellularized matrix produced by control PAVSMCs with shRNA-induced TSC2 depletion induced the proliferation of not only PAVSMCs, but also PAAFs, suggesting that TSC2 also modulates cell-cell communication in the pulmonary vasculature. Our findings agreed with evidence highlighting the importance of cell-matrix and cell-cell communications in PAH pathogenesis (47) and suggest that TSC2 may be an attractive molecular target to disrupt mechanotransduction signaling mediated by YAP and to inhibit ECM remodeling and the associated proliferation of pulmonary vascular cells in PAH.
In TSC2-deficient perivascular epithelioid tumors, YAP accumulation is induced by mTOR through impaired autophagosomal/lysosomal degradation (21). Our findings suggest a mechanism of TSC2-dependent YAP/TAZ and mTOR regulation through the ECM, specifically fibronectin and α5β1 integrin, and link TSC2 to the PAVSMC proliferative response. Supporting our in vitro findings, SM TSC2 deficiency in mice resulted in stiffening of small PAs, introducing a stiffness-dependent mechanism by which TSC2 regulates YAP in vivo. Stiffening of small PAs occurs as an early event preceding development of pulmonary vascular remodeling and PAH and worsens as the disease progresses (15). Although further studies are needed, it is possible that TSC2 deficiency increases PA wall stiffness through at least two mechanisms, excessive ECM production and activation of mTORC1-dependent PAVSMC proliferation and the resultant wall thickening due to VSMC remodeling. Further, our observations that YAP inhibited TSC2 in an ECM-independent manner provide evidence for negative intracellular TSC2-YAP crosstalk that supports unstimulated self-sustained proliferation and resistance to apoptosis of PAVSMCs in PAH.
Our study identified the SIRT1 activator SRT2104 as a potential approach to restore TSC2 abundance and reverse pulmonary vascular remodeling and PH. Reconstitution of TSC2 normalized PAH-specific molecular abnormalities and ECM production, reduced abnormal proliferation, and induced apoptosis in human PAH PAVSMCs. Similar effects were observed in human PAH PAVSMC treated with SRT2104. SIRT1 reduces vascular aging and atherosclerosis in systemic vasculature (48), and its activation improves systemic arterial vascular stiffness in smokers and subjects with type 2 diabetes. SIRT1 stabilizes TSC2 through lysine deacetylation that prevents its degradation (34). We showed that by restoring TSC2 abundance, SRT2104 reversed the PAH PAVSMC phenotype, reducing proliferation and inducing apoptosis. Orally administered SRT2104 restored smooth muscle TSC2 in small PAs in two animal models of SuHx-induced experimental PH, and even a short treatment of mice with established experimental PH led to reduced pulmonary vascular remodeling, sRVP, and RV hypertrophy, the central clinical features of this disease. Moreover, longer treatment of rats with established experimental PH reversed pulmonary vascular remodeling, sRVP, Max dP/dT, and RV hypertrophy, and improved the RV contractility index, making SRT2104 a promising drug for therapeutic intervention.
In agreement with our findings, a polyphenol resveratrol that acts at least partially by activating SIRT1 prevents monocrotaline-induced PH in rats (49). However, resveratrol is extensively metabolized in humans, resulting in low systemic bioavailability and limited potential for clinical utility (50). The rapamycin-based mTORC1 inhibitor ABI009 is now in a clinical trial for patients with severe PAH (ClinicalTrials.gov Identifier: NCT02587325) and shows promising interim results (51); however, intravenous delivery and reported side effects could make use of this drug challenging for PAH patients. SRT2104 has better bioavailability than ABI009, demonstrates benefits in pre-clinical models of age-related disorders (52), is well-tolerated in humans, has a favorable selectivity profile (53), and has already entered clinical trials for type 2 diabetes and psoriasis (54, 55), which makes it an attractive candidate for clinical trials for PAH patients.
We recognize that our study has several limitations. The first limitation is the small human sample size that arises from the nature of the studied disease. PAH is a rare disease, which limits the availability of human lung tissue specimens and early passage cells for mechanistic research of this type. However, the causal role of reduced TSC2 content in mediating PAVSMC hyper-proliferation and pulmonary vascular remodeling in PAH was supported by siRNA-mediated depletion in human PAH PAVSMCs and studies of transgenic mice with SM-specific Tsc2 deficiency. A second limitation was that not all our in vitro experiments used hydrogel matrices to model physiological or pathological stiffness. To separate stiffness-dependent TSC2 functions from their role in ECM remodeling and self-sustained proliferation and survival of PAH PAVSMCs, several data sets were collected from cells cultured on plastic. Although these data agreed with our human tissue-based and hydrogel-derived data, the different substrate may have affected the magnitude of the observed molecular changes. A third limitation was that only male rats were evaluated in the rat SuHx model of PH, because we did not observe differences between SRT2104-treated male and female SuHx mice. Finally, rodent models of PH cannot completely recapitulate human PAH. Moreover, SRT2104 was given to animals as a mono-treatment, and the combination of SRT2104 with currently used FDA-approved therapies was not evaluated. However, given our current findings in cells from PAH subjects and transgenic mice, further testing of targeting TSC2 to reverse PAH is worthy of further investigation.
Despite major progress in PAH treatment within the past decade, there are still no options to reverse advanced disease due to the multifactorial nature of this disease and to the molecular and metabolic reprogramming of pulmonary vascular cells supported by cell-cell and cell-matrix interactions. Our finding that TSC2 links mechanobiological cues, growth factors and ECM signals with hyper-proliferation of PAVSMCs and PAAFs offers a new target for therapeutic intervention. Our preclinical evidence shows that SRT2104, which is already in clinical trials for other diseases and has a favorable safety profile (56), has beneficial effects in human PAH PAVSMCs and two rodent models of PH, warranting further assessment in other pre-clinical models of PH and perhaps in clinical trials.
Materials and Methods
Human Tissues and Cell Culture
Human lung tissues from unused donor (control) and idiopathic PAH patients were provided by the University of Pittsburgh Medical Center Lung Transplant, Pulmonary Division Tissue Donation under protocols approved by the University of Pittsburgh institutional review board. Human primary distal PAVSMCs, primary pulmonary adventitial fibroblasts (PAAFs) and primary pulmonary arterial endothelial cells (PAECs) were provided by the Pulmonary Hypertension Breakthrough Initiative (PHBI) or by the University of Pittsburgh Vascular Medicine Institute (VMI) Cell Processing Core. Cell isolation, characterization and maintenance were performed under the rigorous and well-established study protocols adopted by the PHBI (7, 8). Briefly, pre-capillary PAs were dissected from the left lower lobe and adherent lung parenchymal tissue using microscissors and scalpel. The arteries were minced to 1mm2 blocks and placed on the tissue culture plates with a small drop of LONZA culture medium (LONZA) supplemented with the SmGM-2 or FGM-2 media kit for PAVSMC and PAAF isolation, respectively (7, 8). On the following day, a full volume of respective full growth culture medium was added to the plates, which were left undisturbed for three to five days. The medium was then subsequently changed every other day until the cells reached confluence. PAVSMCs were characterized using antibodies against three smooth muscle-specific markers (smooth muscle α-actin, smooth muscle myosin heavy chain, and SM22; Cell Signaling Technology) and cell morphology (7, 8). PAAFs were characterized using antibodies against vimentin and CD90, and negative staining for SM22, vWF, and cytokeratin (Cell Signaling Technology), to exclude contamination with smooth muscle, endothelial, and epithelial cells, respectively (57). Primary cells (3-8 passage) of the same passage from a minimum of three control and three PAH subjects were used for each experimental condition. Cells and tissues from de-identified human subjects were used (table S1). Cells were maintained at 37 °C in a humidified incubator with 5% CO2. For serum deprivation, cells were maintained for 24-48hr in basal PromoCell medium (PromoCell) supplemented with 0.1% bovine serum albumin (BSA) (Thermo Fisher Scientific, Waltham, MA). Softwell hydrogel-coated plates were purchased from Matrigen and used following the manufacturer’s protocol. Rat ELT3 cells (CRL-3371; RRID:CVCL_4616) were purchased from ATCC and maintained according to the manufacturer’s instructions. The cells were regularly tested for mycoplasma contamination.
Apoptosis
Apoptosis was measured with the In Situ Cell Death Detection Kit (Roche, Nutley, NJ) based on terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) technology according to the manufacturer’s protocol as previously described (7, 8, 58). Nuclei were detected by 4′,6-diamidino-2-phenylindole (DAPI) (Invitrogen). Images were captured by an All-in-One Fluorescence Microscope BZ-X810 (Keyence). Blind semi-automatic counts were performed. A minimum of 200 cells were counted per each condition in each experiment.
Cell proliferation
Cell proliferation was measured by DNA synthesis analysis (bromodeoxyuridine (BrdU) incorporation assay) or Ki67 detection with specific antibody (Cell Signaling Technology) as described previously (7, 8, 58, 59). For the BrdU incorporation assay, cells were serum-deprived for 48 hours, incubated with 10μM BrdU (Abcam) for 18 hours, fixed with 4% paraformaldehyde in PBS (Santa Cruz Biotechnology), permeabilized by 2M HCl, and stained with anti-BrdU antibody (BD Biosciences). Staining with DAPI and anti-GFP antibody (Cell Signaling Technology) was performed to detect nuclei and GFP, respectively. To detect Ki67, staining with anti-Ki67 antibody and DAPI was performed. Images were taken using an All-in-One Fluorescence Microscope BZ-X810 (Keyence). Blinded semi-automatic analysis was performed. Cell proliferation was calculated as the percentage of BrdU-positive or Ki67-positive cells per the total number of cells. A minimum of 200 cells were counted in each experiment per each condition. Cell proliferation was performed as described previously (7, 8, 58). Briefly, cells were treated with ATN-161 (MCE LLC), BTT-3033 (R&D Systems), SRT2104 (InvivoChem), or an appropriate diluent. Cells were trypsinized and resuspended in the same volume of basal medium supplemented with 0.1% BSA. Cell counts were performed using the Countess II FL Automated Cell Counter (Thermo Fisher Scientific).
Immunohistochemical, Immunocytochemical and Immunoblot Analyses
These analyses were performed as previously described (7, 8, 19, 60). Images were captured with an Olympus FV1000 Confocal Microscope (Olympus America Inc.) or the All-in-One Fluorescence Microscope BZ-X810 (Keyence). Immunoblot signals were captured with HyBlot CL® Autoradiography Films (Thomas Scientific), and fixed and developed by Medical Film Processor (Konica Minolta). Densitometry measurements for immunoblots and immunostainings were done with ImageJ (NIH). Antibodies for TSC2 (#4308; RRID: AB 10547134), cleaved caspase-3 (Asp175) (#9661; RRID:AB_2341188), GFP (#2956; RRID:AB_1196615), phospho-Ser235/236-S6 (#4856; RRID:AB_2181037), S6 (#2217; RRID:AB_331355), YAP/TAZ (#8418; RRID:AB_10950494), phopsho-Ser127YAP (#13008; RRID:AB_2650553), TSC1 (#4906; RRID:AB_2209790), RICTOR (#2114; RRID:AB_2179963), α/β-tubulin (#2148; RRID:AB_2288042), phospho-Ser473-Akt (#4060; RID:AB_2315049), Akt (#9272; RRID:AB_329827), and anti-rabbit IgG-HRP-linked antibody (#7074; RRID:AB_2099233) were purchased from Cell Signaling Technology. Anti-fibronectin antibody (ab2413; RRID:AB_2262874) was purchased from Abcam. Anti-collagen 1A1 antibody (AF6220; RRID:AB_10891543) was purchased from R&D Systems. Anti-actin, α-smooth muscle-FITC antibody (F3777; RRID:AB_476977) were purchased from Sigma-Aldrich. Alexa Fluor 488, 594 and 647 dyes, rabbit anti-sheep IgG-HRP-linked antibody (#61-8620; RRID:AB_2533942), and anti-TSC1 antibody (#PA5-18506; RRID:AB_10985558) were purchased from Thermo Fisher Scientific.
Transfection, Infection, and De-cellularization
These procedures were performed as described previously (7, 8, 61). The pEGFP plasmid (#6077-1) was obtained from Addgene. The pEGFP-TSC2 plasmid was previously generated (62, 63). siRNAs and shRNAs were purchased from Dharmacon (PerkinElmer) and Santa Cruz Biotechnology, respectively. siRNA transfection and shRNA infection were performed according to the manufacturers’ protocols. Effectene (Qiagen) was used to transfect cells according to the manufacturer’s protocol. To produce decellularized matrices, pre-confluent PAVSMCs were maintained on plastic plates for six days after which de-cellularization was performed.
Animals
All animal procedures were performed under the protocols approved by the Animal Care and Use Committees of the University of Pittsburgh and the University of California, Davis (protocols #17019864 and 21853, respectively). SM22-Tsc2+/− mice were generated by crossing female Tsc2f/f mice (Tsc2tm1.1Mjg/J, #027458) with male SM22-Cre mice (B6.Cg-Tg(Tagln-cre)1Her/J, #017491) (Jackson Laboratory) carrying mouse smooth muscle protein 22-alpha (SM22) promoter that induced Cre recombinase expression selectively in VSMCs (64, 65). Mice were genotyped prior to experiments. The genotyping of the DNA extracted from ears of transgenic mice by Extract-N-AMPK™ Tissue PCR Kit (Sigma) was performed following the manufacturer’s protocol. Polymerase chain reaction (PCR) samples were prepared using KAPA2G Fast PCR Kit (Sigma) following the manufacturer’s protocol. The SM22-Cre gene was detected using the primers 5’-GCAATTTCGGCTATACGTAACAGGG and 5’-GCAAGAACCTGATGGACATGTTCAG with the internal control primers 5’-CTAGGCCACAGAATTGAAAGATCT (oIMR7338) and 5’-GTAGGTGGAAATTCTAGCATCC (oIMR7339). Tsc2 gene was detected with the primers 5’-ACAATGGGAGGCACATTACC and 5’-AAGCAGCAGGTCTGCAGTG). Primers were purchased from Sigma. Control mice were the same age, sex, and background (C57BL/6J, Jackson Laboratories) housed under the same conditions (8, 66, 67). Experimental PH was induced by SU5416/hypoxia as described previously in (7, 8, 59).
Six to eight week old C57BL/6J mice were randomly assigned to experimental groups and exposed to hypoxia (10% O2) for up to 35 days. Subcutaneous injections of SU5416 (20 mg/kg) (Tocris) were performed at days 0, 7, and 14 of the experiment. At days 15-21 after experimental PH induction, SRT2104 (InvivoChem) suspended in warm corn oil (ACROS Organics) (100mg/kg/day, oral gavage, 200μL/mouse) or warm corn oil alone were administrated daily. Both male and female mice were used; controls were same-age same-sex mice maintained under normoxia.
Six to eight week old male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) received a single dose of SU5416 (sq 20 mg/kg) and were maintained for three weeks under hypoxia (10% O2) and for five weeks under normoxia. Then animals were randomly assigned to experimental groups. SRT2104 (InvivoChem) in warm corn oil (ACROS Organics) and vehicle (warm corn oil alone) were administrated starting at the beginning of week four of the experiment for five weeks (100mg/kg/day, oral gavage, five days/week). Negative controls were normoxia-maintained age-matched animals. Positive controls were untreated SU5416/hypoxia/normoxia exposed rats. The volume of the vehicle was equal for the volume of SRT2104 suspension (200μL/mouse or 3mL/rat). After blinded terminal hemodynamic analysis, animals were euthanized, and lung and heart tissues were collected for morphological analysis. Hearts were separated into RV and LV+Septum, and the Fulton index was calculated by the RV/(LV+Septum) weight ratio.
Blinded hemodynamic analysis was performed as previously described (8, 59, 67, 68). Briefly, animals were anesthetized by isoflurane (Minrad Inc.): 5% for induction, 2% during surgery, and 1% while performing PV loop measurements. Pressure-volume (PV) loop measurements were performed by PV catheters (Scisense, Inc.). The catheter attached to the data acquisition system (EMKA Instruments) was inserted into the RV and then into the LV. Data were acquired by the ADVantage PV System (Transonic Systems Inc.) and IOX2 software (EMKA Technologies Inc.). PAP was calculated as a sRVP×0.65+0.55 mmHg (59, 69). MAP was calculated as sLVP×0.65+0.55 mmHg (59, 69); RV contractility index was calculated as (Max dP/dT)/sRVP s−1 (59, 70).
Blinded morphological and immunohistological analyses were performed as described previously (7, 8, 19, 58, 59, 68, 71). Briefly, after being perfused by PBS through the PA, lungs were filled with 80% Tissue Plus OCT Compound (Fisher Scientific)/20% saline solution, snap frozen in dry ice and kept at −80°C or fixed in 4% paraformaldehyde solution in PBS and embedded in paraffin. After sectioning, lung tissue slides (5μm thickness) were stained by H&E or immunostained to detect TSC2, SMA, and nuclei (DAPI) (Invitrogen) as previously described (7, 8). Images for PA MT calculation were captured from blind-selected small PAs (25-100μm outer diameter from a minimum of six animals/group and a minimum of 12 PAs/animal) of H&E- or SMA-stained rodent’s lung tissues and calculated using the VMI Calculator (71). The entirety of H&E-stained rat lung tissue sections was captured (six rats/group, minimum of 29 PAs/rat) to count percentage of fully (grade 2), partially (grade 1), and non-occluded (grade 0) small PAs (25-50μm outer diameter) (8, 68, 71-73). Images were taken with an All-in-One Fluorescence Microscope BZ-X810.
AFM microscopy
Lungs were inflated with OCT (50 ml/kg body weight), frozen in dry ice–cooled 2-methyl butane and stored at −80°C. Lung parenchymal tissue strips 10μm in thickness were prepared using a cryostat (Leica CM1850), mounted on poly-L-lysine coated glass slides, stored at −20°C, and measured within 48 hours. Residual OCT was dissolved and rinsed away with PBS immediately before AFM measurements. PAs were identified by morphologic criteria using phase contrast microscopy. PAs (< 200μm in diameter) were mechanically characterized through microindentation using a JPK NanoWizard 4XP Bioscence (Bruker) atomic force microscope. Microindentation was performed using a sphere-tipped probe (Novascan) with a diameter of 5 μm and a nominal spring constant of approximately 60 pN/nm as described (15). The cantilever spring constant was further confirmed at each use by the thermal fluctuation method. This method correlated well with contact-based calibration. The entire AFM system was calibrated by following the manufacturer’s instructions before each measurement. Force-indentation profiles were acquired through force-scanning at an indentation force of 1-2nN with Z-length of 1.5 microns. Force curves were obtained over a 128 x 128 grid of 30-80 microns in size, depending on the size of the vessel. Young’s elastic modulus was calculated by fitting force-indentation data using a Hertz sphere model with JPK data processing software (Bruker). Height and Young’s modulus data were converted to 128x128 TIFF files and analyzed in image J to allow isolation of only those curves associated with vessel walls. 5-10 vessels were analyzed per animal.
Statistical Analysis
Statistical comparisons between two groups were performed by the Mann-Whitney U test and considered to be statistically different when the p-value was less than 0.05. Statistical comparisons among three or more groups were performed by the Kruskal-Wallis test with Dunn’s Pairwise Comparison (all data with sample size n<6/group and skewed data with sample size n≥6/group), analysis of variance (ANOVA) test with Fisher LSD or Tukey’s HSD posthoc comparison (all data with sample size n≥6/group that satisfied a test of normality assessed using the Kolmogorov-Smirnov test and had equal variances assessed using Bartlett’s test), or Welch’s one-way ANOVA F test with Games-Howell post-hoc analysis (all data with sample size n≥6/group that satisfied a test of normality but that failed the equal variances assumption test). Statistical analysis was performed using STATA (StataCorp), StatView (SAS Institute), and GraphPad Prism 9.2 (GraphPad Software) software. No animals were excluded from the analysis. To determine animal group sizes, Power calculations were performed using G*Power (Release 3.1.9.4) software.
Supplementary Material
Acknowledgements:
We thank Andrea Sebastiani and the Small Animal Hemodynamic Core at the University of Pittsburgh Heart, Lung, and Blood Vascular Medicine Institute (VMI) for the acquisition and analysis of animal hemodynamic data. We thank the Pulmonary Hypertension Breakthrough Initiative (PHBI) and the University of Pittsburgh VMI Cell processing Core for providing lung tissues and pulmonary vascular cells from patients with PAH and unused donor lungs. We thank John Sembrat from the University of Pittsburgh School of Medicine for his help with the acquisition of human tissues.
Funding:
This work is supported by NIH/NHLBI R01HL113178 (EAG), R01HL130261 (EAG), R01HL150638 (EAG), 5P01HL103455-05 (ALM, EAG), 1 U01HL145550 (MR), 5 P50AR060780 (MR), and American Heart Association Postdoctoral Fellowship 826806 (YS). The Pulmonary Hypertension Breakthrough Initiative is supported by NIH/NHLBI R24HL123767. Dr. Fredenburgh’s work related to pulmonary hypertension is supported by NIH/NHLBI and American Heart Association.
Footnotes
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper or the Supplementary Materials. The SM22-Tsc+/− mice are available from E.A.G. under a material transfer agreement from the Jackson Laboratories and from the University of California, Davis.
References and Notes:
- 1.Humbert M, Morrell NW, Archer SL, Stenmark KR, MacLean MR, Lang IM, Christman BW, Weir EK, Eickelberg O, Voelkel NF, Rabinovitch M. Cellular and molecular pathobiology of pulmonary arterial hypertension. Journal of the American College of Cardiology 2004; 43: S13–S24. [DOI] [PubMed] [Google Scholar]
- 2.Hyduk A, Croft JB, Ayala C, Zheng K, Zheng ZJ, Mensah GA. Pulmonary Hypertension Surveillance --- United States, 1980--2002. MMWR Surveillance Summaries 2005: 1–28. [PubMed] [Google Scholar]
- 3.George MG, Schieb LJ, Ayala C, Talwalkar A, Levant S. Pulmonary hypertension surveillance: United states, 2001 to 2010. Chest 2014; 146: 476–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frost AE, Badesch DB, Barst RJ, Benza RL, Elliott CG, Farber HW, Krichman A, Liou TG, Raskob GE, Wason P, Feldkircher K, Turner M, McGoon MD. The Changing Picture of Patients With Pulmonary Arterial Hypertension in the United States. Chest 2011; 139: 128–137. [DOI] [PubMed] [Google Scholar]
- 5.Guignabert C, Tu L, Le Hiress M, Ricard N, Sattler C, Seferian A, Huertas A, Humbert M, Montani D. Pathogenesis of pulmonary arterial hypertension: lessons from cancer. European Respiratory Review 2013; 22: 543–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morrell NW, Adnot S, Archer SL, Dupuis J, Jones PL, MacLean MR, McMurtry IF, Stenmark KR, Thistlethwaite PA, Weissmann N, Yuan JXJ, Weir EK. Cellular and Molecular Basis of Pulmonary Arterial Hypertension. Journal of the American College of Cardiology 2009; 54: S20–S31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goncharov DA, Kudryashova TV, Ziai H, Ihida-Stansbury K, DeLisser H, Krymskaya VP, Tuder RM, Kawut SM, Goncharova EA. Mammalian target of rapamycin complex 2 (mTORC2) coordinates pulmonary artery smooth muscle cell metabolism, proliferation, and survival in pulmonary arterial hypertension. Circulation 2014; 129: 864–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kudryashova TV, Goncharov DA, Pena A, Kelly N, Vanderpool R, Baust J, Kobir A, Shufesky W, Mora AL, Morelli AE, Zhao J, Ihida-Stansbury K, Chang B, DeLisser H, Tuder RM, Kawut SM, Silljé HHW, Shapiro S, Zhao Y, Goncharova EA. HIPPO–Integrin-linked Kinase Cross-Talk Controls Self-Sustaining Proliferation and Survival in Pulmonary Hypertension. American Journal of Respiratory and Critical Care Medicine 2016; 194: 866–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pullamsetti SS, Savai R, Seeger W, Goncharova EA. Translational Advances in the Field of Pulmonary Hypertension.From Cancer Biology to New Pulmonary Arterial Hypertension Therapeutics. Targeting Cell Growth and Proliferation Signaling Hubs. American Journal of Respiratory and Critical Care Medicine 2017; 195: 425–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Masri FA, Xu W, Comhair SAA, Asosingh K, Koo M, Vasanji A, Drazba J, Anand-Apte B, Erzurum SC. Hyperproliferative apoptosis-resistant endothelial cells in idiopathic pulmonary arterial hypertension. American Journal of Physiology - Lung Cellular and Molecular Physiology 2007; 293: L548–L554. [DOI] [PubMed] [Google Scholar]
- 11.Bertero T, Cottrill KA, Annis S, Bhat B, Gochuico BR, Osorio JC, Rosas I, Haley KJ, Corey KE, Chung RT, N CB, Chan SY A YAP/TAZ-miR-130/301 molecular circuit exerts systems-level control of fibrosis in a network of human diseases and physiologic conditions. Scientific Reports 2015; 5: 18277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bertero T, Oldham WM, Cottrill KA, Pisano S, Vanderpool RR, Yu Q, Zhao J, Tai Y, Tang Y, Zhang Y-Y, Rehman S, Sugahara M, Qi Z, Gorcsan JI, Vargas SO, Saggar R, Saggar R, Wallace WD, Ross DJ, Haley KJ, Waxman AB, Parikh VN, De Marco T, Hsue PY, Morris A, Simon MA, Norris KA, Gaggioli C, Loscalzo J, Fessel J, Chan SY. Vascular stiffness mechanoactivates YAP/TAZ-dependent glutaminolysis to drive pulmonary hypertension. The Journal of Clinical Investigation 2016; 126: 3313–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Savai R, Al-Tamari HM, Sedding D, Kojonazarov B, Muecke C, Teske R, Capecchi MR, Weissmann N, Grimminger F, Seeger W, Schermuly RT, Pullamsetti SS. Pro-proliferative and inflammatory signaling converge on FoxO1 transcription factor in pulmonary hypertension. Nature Medicine 2014; 20: 1289–1300. [DOI] [PubMed] [Google Scholar]
- 14.Bertero T, Cottrill KA, Lu Y, Haeger CM, Dieffenbach P, Annis S, Hale A, Bhat B, Kaimal V, Zhang Y-Y, Graham BB, Kumar R, Saggar R, Saggar R, Wallace WD, Ross DJ, Black SM, Fratz S, Fineman JR, Vargas SO, Haley KJ, Waxman AB, Chau BN, Fredenburgh LE, Chan SY. Matrix remodeling promotes pulmonary hypertension through feedback mechanoactivation of the YAP/TAZ-miR-130/301 circuit. Cell Reports 2015; 13: 1016–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu F, Haeger CM, Dieffenbach PB, Sicard D, Chrobak I, Coronata AMF, Velandia MMS, Vitali S, Colas RA, Norris PC, Marinković A, Liu X, Ma J, Rose CD, Lee S-J, Comhair SAA, Erzurum SC, McDonald JD, Serhan CN, Walsh SR, Tschumperlin DJ, Fredenburgh LE. Distal vessel stiffening is an early and pivotal mechanobiological regulator of vascular remodeling and pulmonary hypertension. JCI Insight 2016; 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krymskaya VP, Goncharova EA. PI3K/mTORC1 activation in hamartoma syndromes: therapeutic prospects. Cell Cycle 2009; 8: 403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang J, Manning BD. The TSC1–TSC2 complex: a molecular switchboard controlling cell growth. Biochemical Journal 2008; 412: 179–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huynh H, Hao H-X, Chan SL, Chen D, Ong R, Soo KC, Pochanard P, Yang D, Ruddy D, Liu M, Derti A, Balak MN, Palmer MR, Wang Y, Lee BH, Sellami D, Zhu AX, Schlegel R, Huang A. Loss of Tuberous Sclerosis Complex 2 (TSC2) Is Frequent in Hepatocellular Carcinoma and Predicts Response to mTORC1 Inhibitor Everolimus. Molecular Cancer Therapeutics 2015; 14: 1224–1235. [DOI] [PubMed] [Google Scholar]
- 19.Krymskaya VP, Snow J, Cesarone G, Khavin I, Goncharov DA, Lim PN, Veasey SC, Ihida-Stansbury K, Jones PL, Goncharova EA. mTOR is required for pulmonary arterial vascular smooth muscle cell proliferation under chronic hypoxia. The FASEB Journal 2011; 25: 1922–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Houssaini A, Abid S, Mouraret N, Wan F, Rideau D, Saker M, Marcos E, Tissot C-M, Dubois-Randé J-L, Amsellem V, Adnot S. Rapamycin Reverses Pulmonary Artery Smooth Muscle Cell Proliferation in Pulmonary Hypertension. American Journal of Respiratory Cell and Molecular Biology 2013; 48: 568–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang N, Zhang C, Dill P, Panasyuk G, Pion D, Koka V, Gallazzini M, Olson EN, Lam H, Henske EP, Dong Z, Apte U, Pallet N, Johnson RL, Terzi F, Kwiatkowski DJ, Scoazec JY, Martignoni G, Pende M. Regulation of YAP by mTOR and autophagy reveals a therapeutic target of tuberous sclerosis complex. Journal of Experimental Medicine 2014; 211: 2249–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Houssaini A, Abid S, Derumeaux G, Wan F, Parpaleix A, Rideau D, Marcos E, Kebe K, Czibik G, Sawaki D, Treins C, Dubois-Randé J-L, Li Z, Amsellem V, Lipskaia L, Pende M, Adnot S. Selective Tuberous Sclerosis Complex 1 Gene Deletion in Smooth Muscle Activates Mammalian Target of Rapamycin Signaling and Induces Pulmonary Hypertension. American Journal of Respiratory Cell and Molecular Biology 2016; 55: 352–367. [DOI] [PubMed] [Google Scholar]
- 23.Cottin V, Harari S, Humbert M, Mal H, Dorfmüller P, Jaïs X, Reynaud-Gaubert M, Prevot G, Lazor R, Taillé C, Lacronique J, Zeghmar S, Simonneau G, Cordier J-F, Cottin V, Zeghmar S, Cordier J-F, Harari S, Humbert M, Jaïs X, Simonneau G, Mal H, Taillé C, Dorfmüller P, Reynaud-Gaubert M, Prevot G, Lacronique J, Lazor R. Pulmonary hypertension in lymphangioleiomyomatosis: characteristics in 20 patients. European Respiratory Journal 2012; 40: 630–640. [DOI] [PubMed] [Google Scholar]
- 24.Mieulet V, Lamb RF. Tuberous sclerosis complex: linking cancer to metabolism. Trends in Molecular Medicine 2010; 16: 329–335. [DOI] [PubMed] [Google Scholar]
- 25.Bertero T, Oldham WM, Cottrill KA, Pisano S, Vanderpool RR, Yu Q, Zhao J, Tai Y, Tang Y, Zhang YY, Rehman S, Sugahara M, Qi Z, Gorcsan J 3rd, Vargas SO, Saggar R, Saggar R, Wallace WD, Ross DJ, Haley KJ, Waxman AB, Parikh VN, De Marco T, Hsue PY, Morris A, Simon MA, Norris KA, Gaggioli C, Loscalzo J, Fessel J, Chan SY Vascular stiffness mechanoactivates YAP/TAZ-dependent glutaminolysis to drive pulmonary hypertension. Journal of Clinical Investigations 2016; 126: 3313–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Basu S, Totty NF, Irwin MS, Sudol M, Downward J. Akt phosphorylates the Yes-associated protein, YAP, to induce interaction with 14-3-3 and attenuation of p73-mediated apoptosis. Molecular Cell 2003; 11: 11–23. [DOI] [PubMed] [Google Scholar]
- 27.Zheng Y, Pan D. The Hippo Signaling Pathway in Development and Disease. Developmental Cell 2019; 50: 264–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kastan N, Gnedeva K, Alisch T, Petelski AA, Huggins DJ, Chiaravalli J, Aharanov A, Shakked A, Tzahor E, Nagiel A, Segil N, Hudspeth AJ. Small-molecule inhibition of Lats kinases may promote Yap-dependent proliferation in postmitotic mammalian tissues. Nature Communications 2021; 12: 3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang H, Chen J, Fraidenburg DR, Song S, Sysol JR, Drennan AR, Offermanns S, Ye RD, Bonini MG, Minshall RD, Garcia JG, Machado RF, Makino A, Yuan JX. Deficiency of Akt1, but not Akt2, Attenuates the Development of Pulmonary Hypertension. American Journal of Physiology - Lung Cellular and Molecular Physiology 2014; 308(2): L208–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goncharova EA, Goncharov DA, Eszterhas A, Hunter DS, Glassberg MK, Yeung RS, Walker CL, Noonan D, Kwiatkowski DJ, Chou MM, Panettieri RA Jr, Krymskaya VP Tuberin regulates p70 S6 kinase activation and ribosomal protein S6 phosphorylation. A role for the TSC2 tumor suppressor gene in pulmonary lymphangioleiomyomatosis (LAM). Journal of Biological Chemistry 2002; 277: 30958–30967. [DOI] [PubMed] [Google Scholar]
- 31.Thenappan T, Chan SY, Weir EK. Role of extracellular matrix in the pathogenesis of pulmonary arterial hypertension. American Journal of Physiology - Heart and Circulatory Physiology 2018; 315: H1322–h1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu F, Haeger CM, Dieffenbach PB, Sicard D, Chrobak I, Coronata AM, Suárez Velandia MM, Vitali S, Colas RA, Norris PC, Marinković A, Liu X, Ma J, Rose CD, Lee SJ, Comhair SA, Erzurum SC, McDonald JD, Serhan CN, Walsh SR, Tschumperlin DJ, Fredenburgh LE. Distal vessel stiffening is an early and pivotal mechanobiological regulator of vascular remodeling and pulmonary hypertension. JCI Insight 2016; 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chong-Kopera H, Inoki K, Li Y, Zhu T, Garcia-Gonzalo FR, Rosa JL, Guan KL. TSC1 stabilizes TSC2 by inhibiting the interaction between TSC2 and the HERC1 ubiquitin ligase. Journal of Biological Chemistry 2006; 281: 8313–8316. [DOI] [PubMed] [Google Scholar]
- 34.García-Aguilar A, Guillén C, Nellist M, Bartolomé A, Benito M. TSC2 N-terminal lysine acetylation status affects to its stability modulating mTORC1 signaling and autophagy. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 2016; 1863: 2658–2667. [DOI] [PubMed] [Google Scholar]
- 35.Nagata D, Takeda R, Sata M, Satonaka H, Suzuki E, Nagano T, Hirata Y. AMP-Activated Protein Kinase Inhibits Angiotensin II-Stimulated Vascular Smooth Muscle Cell Proliferation. Circulation 2004; 110: 444–451. [DOI] [PubMed] [Google Scholar]
- 36.Rubin LJ, Magliola L, Feng X, Jones AW, Hale CC. Metabolic activation of AMP kinase in vascular smooth muscle. Journal of Applied Physiology 2005; 98: 296–306. [DOI] [PubMed] [Google Scholar]
- 37.Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nature Reviews Cancer 2009; 9: 563–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Szwed A, Kim E, Jacinto E. Regulation and metabolic functions of mTORC1 and mTORC2. Physiological Reviews 2021; 101: 1371–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goncharova EA, Krymskaya VP. Pulmonary lymphangioleiomyomatosis (LAM): Progress and current challenges. Journal of Cellular Biochemistry 2008; 103: 369–382. [DOI] [PubMed] [Google Scholar]
- 40.Stenmark KR, Mecham RP. Cellular and molecular mechanisms of pulmonary vascular remodeling. Annual Review of Physiology 1997; 59: 89–144. [DOI] [PubMed] [Google Scholar]
- 41.Sun W, Chan SY. Pulmonary Arterial Stiffness: An Early and Pervasive Driver of Pulmonary Arterial Hypertension. Frontiers in Medicine (Lausanne) 2018; 5: 204–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dieffenbach PB, Haeger CM, Coronata AMF, Choi KM, Varelas X, Tschumperlin DJ, Fredenburgh LE. Arterial stiffness induces remodeling phenotypes in pulmonary artery smooth muscle cells via YAP/TAZ-mediated repression of cyclooxygenase-2. Am J Physiol Lung Cell Mol Physiol 2017; 313: L628–l647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DeFelice A, Stewart D, Walker G, Truebel H, Stenmark K, Rabinovitch M, Fiszman M, Saulnier M, Morrell N, Schermuly R, Archer S, Evans S, Martin T. Anticipated classes of new medications and molecular targets for pulmonary arterial hypertension. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goncharova EA, Goncharov DA, Eszterhas A, Hunter DS, Glassberg MK, Yeung RS, Walker CL, Noonan D, Kwiatkowski DJ, Chou MM, Panettieri RA, Krymskaya VP. Tuberin regulates p70 S6 kinase activation and ribosomal protein S6 phosphorylation: a role for the TSC2 tumor suppressor gene in pulmonary lymphangioleiomyomatosis. The Journal of Biological Chemistry 2002; 277: 30958–30967. [DOI] [PubMed] [Google Scholar]
- 45.Weiss A, Neubauer MC, Yerabolu D, Kojonazarov B, Schlueter BC, Neubert L, Jonigk D, Baal N, Ruppert C, Dorfmuller P, Pullamsetti SS, Weissmann N, Ghofrani H-A, Grimminger F, Seeger W, Schermuly RT. Targeting cyclin-dependent kinases for the treatment of pulmonary arterial hypertension. Nature Communications 2019; 10: 2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barman SA, Zhu S, White RE. RhoA/Rho-kinase signaling: a therapeutic target in pulmonary hypertension. Vascular Health and Risk Management 2009; 5: 663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thenappan T, Chan SY, Weir EK. Role of extracellular matrix in the pathogenesis of pulmonary arterial hypertension. American Journal of Physiology - Heart and Circulatory Physiology 2018; 315: H1322–H1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bonkowski MS, Sinclair DA. Slowing ageing by design: the rise of NAD+ and sirtuin-activating compounds. Nature Reviews Molecular Cell Biology 2016; 17: 679–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Csiszar A, Labinskyy N, Olson S, Pinto JT, Gupte S, Wu JM, Hu F, Ballabh P, Podlutsky A, Losonczy G, de Cabo R, Mathew R, Wolin MS, Ungvari Z. Resveratrol Prevents Monocrotaline-Induced Pulmonary Hypertension in Rats. Hypertension 2009; 54: 668–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Knutson MD, Leeuwenburgh C. Resveratrol and novel potent activators of Sirt1: effects on aging and age-related diseases. Nutrition Reviews 2008; 66: 591–596. [DOI] [PubMed] [Google Scholar]
- 51.Simon M, Gomberg-Maitland M, Oudiz R, Machado R, Rischard F, Elinoff J, Grigorian B, Schmid A, Hou S, Desai N, Gladwin M. Patients with severe pulmonary arterial hypertension treated with ABI-009, nab-sirolimus, an mTOR inhibitor: interim results from a phase 1 clinical trial. Journal of the American College of Cardiology 2019; 73: 1927–1927. [Google Scholar]
- 52.Zhang X, Li X, Zhang J. Current Status and Future Perspectives of PI3K and mTOR Inhibitor as Anticancer Drugs in Breast Cancer. Current Cancer Drug Targets 2013; 13: 175–187. [DOI] [PubMed] [Google Scholar]
- 53.Hoffmann E, Wald J, Lavu S, Roberts J, Beaumont C, Haddad J, Elliott P, Westphal C, Jacobson E. Pharmacokinetics and tolerability of SRT2104, a first-in-class small molecule activator of SIRT1, after single and repeated oral administration in man. British Journal of Clinical Pharmacology 2013; 75: 186–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Venkatasubramanian S, Noh RM, Daga S, Langrish JP, Mills NL, Waterhouse BR, Hoffmann E, Jacobson EW, Lang NN, Frier BM, Newby DE. Effects of the small molecule SIRT1 activator, SRT2104 on arterial stiffness in otherwise healthy cigarette smokers and subjects with type 2 diabetes mellitus. Open Heart 2016; 3: e000402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baksi A, Kraydashenko O, Zalevkaya A, Stets R, Elliott P, Haddad J, Hoffmann E, Vlasuk GP, Jacobson EW. A phase II, randomized, placebo-controlled, double-blind, multi-dose study of SRT2104, a SIRT1 activator, in subjects with type 2 diabetes. British Journal of Clinical Pharmacology 2014; 78: 69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Krueger JG, Suárez-Fariñas M, Cueto I, Khacherian A, Matheson R, Parish LC, Leonardi C, Shortino D, Gupta A, Haddad J, Vlasuk GP, Jacobson EW. A Randomized, Placebo-Controlled Study of SRT2104, a SIRT1 Activator, in Patients with Moderate to Severe Psoriasis. PLOS ONE 2015; 10: e0142081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang D, Zhang H, Li M, Frid MG, Flockton AR, McKeon BA, Yeager ME, Fini MA, Morrell NW, Pullamsetti SS. MicroRNA-124 controls the proliferative, migratory, and inflammatory phenotype of pulmonary vascular fibroblasts. Circulation Research 2014; 114: 67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goncharova EA, Goncharov DA, Li H, Pimtong W, Lu S, Khavin I, Krymskaya VP. mTORC2 Is Required for Proliferation and Survival of TSC2-Null Cells. Molecular and Cellular Biology 2011; 31: 2484–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shen Y, Goncharov DA, Avolio T, Ray A, Okorie E, DeLisser H, Mora AL, Vanderpool R, Kudryashova TV, Goncharova EA. Differential effects of integrin-linked kinase inhibitor Cpd22 on severe pulmonary hypertension in male and female rats. Pulmonary Circulation 2020; 10: 2045894019898593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goncharova EA, Goncharov DA, Krymskaya VP. Assays for in vitro monitoring of human airway smooth muscle (ASM) and human pulmonary arterial vascular smooth muscle (VSM) cell migration. Nature Protocols 2007; 1: 2933–2939. [DOI] [PubMed] [Google Scholar]
- 61.Parmaksiz M, Elçin AE, Elçin YM. Decellularized Cell Culture ECMs Act as Cell Differentiation Inducers. Stem Cell Reviews and Reports 2020; 16: 569–584. [DOI] [PubMed] [Google Scholar]
- 62.Goncharova E, Goncharov D, Noonan D, Krymskaya VP. TSC2 modulates actin cytoskeleton and focal adhesion through TSC1-binding domain and the Rac1 GTPase. The Journal of Cell Biology 2004; 167: 1171–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Finlay GA, York B, Karas RH, Fanburg BL, Zhang H, Kwiatkowski DJ, Noonan DJ. Estrogen-induced smooth muscle cell growth is regulated by tuberin and associated with altered activation of platelet-derived growth factor receptor-beta and ERK-1/2. The Journal of Biological Chemistry 2004; 279: 23114–23122. [DOI] [PubMed] [Google Scholar]
- 64.Lesche R, Groszer M, Gao J, Wang Y, Messing A, Sun H, Liu X, Wu H. Cre/loxP-mediated inactivation of the murine Pten tumor suppressor gene. Genesis 2002; 32: 148–149. [DOI] [PubMed] [Google Scholar]
- 65.Schwenk F, Baron U, Rajewsky K. A cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucleic Acids Research 1995; 23: 5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Meloche J, Pflieger A, Vaillancourt M, Paulin R, Potus F, Zervopoulos S, Graydon C, Courboulin A, Breuils-Bonnet S, Tremblay È, Couture C, Michelakis ED, Provencher S, Bonnet S. Role for DNA Damage Signaling in Pulmonary Arterial Hypertension. Circulation 2014; 129: 786–797. [DOI] [PubMed] [Google Scholar]
- 67.Taraseviciene-Stewart L, Kasahara Y, Alger L, Hirth P, Mc Mahon G, Walrenberfer J, Voelkel NF, Tuder RM. Inhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. The FASEB Journal 2001; 15: 427–438. [DOI] [PubMed] [Google Scholar]
- 68.Pena A, Kobir A, Goncharov D, Goda A, Kudryashova TV. Pharmacological Inhibition of mTOR Kinase Reverses Right Ventricle Remodeling and Improves Right Ventricle Structure and Function in Rats. American Journal of Respiratory Cell and Molecular Biology 2017; 57: 615–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Syyed R, Reeves JT, Welsh D, Raeside D, Johnson MK, Peacock AJ. The relationship between the components of pulmonary artery pressure remains constant under all conditions in both health and disease. Chest 2008; 133: 633–639. [DOI] [PubMed] [Google Scholar]
- 70.MASON DT, Braunwald E, COVELL JW, SONNENBLICK EH, ROSS JR J. Assessment of cardiac contractility: the relation between the rate of pressure rise and ventricular pressure during isovolumic systole. Circulation 1971; 44: 47–58. [DOI] [PubMed] [Google Scholar]
- 71.Kelley EE, Baust J, Bonacci G, Golin-Bisello F, Devlin JE, St. Croix CM, Watkins SC, Gor S, Cantu-Medellin N, Weidert ER, Frisbee JC, Gladwin MT, Champion HC, Freeman BA, Khoo NKH, Fatty acid nitroalkenes ameliorate glucose intolerance and pulmonary hypertension in high-fat diet-induced obesity. Cardiovascular Research 2014; 101(3): 352–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Oka M, Homma N, Taraseviciene-Stewart L, Morris KG, Kraskauskas D, Burns N, Voelkel NF, McMurtry IF. Rho kinase-mediated vasoconstriction is important in severe occlusive pulmonary arterial hypertension in rats. Circulation Research 2007; 100: 923–929. [DOI] [PubMed] [Google Scholar]
- 73.Gairhe S, Joshi SR, Bastola MM, McLendon JM, Oka M, Fagan KA, McMurtry IF. Sphingosine-1-phosphate is involved in the occlusive arteriopathy of pulmonary arterial hypertension. Pulmonary Circulation 2016; 6: 369–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.