ABSTRACT
Doxorubicin (Dox) is an anthracycline antibiotic that treats a variety of malignancies. Unfortunately, its cardiotoxicity limits its therapeutic usefulness. Coenzyme Q10 (CoQ10) has effectively treated and prevented various cardiac diseases and toxicities. This study aimed to evaluate the possible antioxidative and anti-apoptotic cardioprotective effects of CoQ10 against doxorubicin-induced histopathological and molecular changes in cardiomyocytes. Twenty-eight adult Wistar rats were divided into positive control, negative control, Dox-treated group, and Dox+CoQ10-treated. On the 16th day after the start of treatment, the hearts of all rats were dissected, and the left ventricles were processed for histological evaluation; immunohistochemical staining with caspase-3 and inducible nitric oxide synthase (iNOS); ultrastructural examination of cardiomyocytes; molecular assessment of proapoptotic gene Bax and anti-apoptotic gene expression Bcl-2; and biochemical study of malondialdehyde (MDA). The Dox-treated group had disorganized cardiomyocytes with increased interstitial space, vacuolated cytoplasm, and multiple small-sized pyknotic nuclei. A significant increase in caspase-3 and iNOS immunoexpression was observed. Ultrastructurally, the mitochondria were large with abnormal shapes, vacuolated cytoplasm, multiple vacuoles and autophagosomes, collagen fibril accumulation, and multiple small hyperchromatic nuclei. The intercalated discs were disorganized with loss of desmosome junction. The cardiomyocytes also showed significantly increased MDA levels and upregulation of Bax/Bcl-2 gene expression ratio. Co-administration of CoQ10 resulted in significant improvement in the histopathological picture, with a significant decrease in caspase-3 and iNOS immunoexpression and downregulation of the Bax/Bcl-2 gene expression ratio. In conclusion, CoQ10 protects against Dox-induced cardiotoxicity through the regulation of proapoptotic and anti-apoptotic gene expression.
KEYWORDS: Doxorubicin, Coenzyme Q10, cardiomyocytes, Bax/Bcl-2
1. Introduction
Doxorubicin (Dox) is an anthracycline antibiotic and a highly effective chemotherapeutic drug. It is widely used to treat numerous adult and pediatric cancers, including breast cancer, Hodgkin’s disease, lymphoblastic leukemia, and sarcoma.1 However, human and animal studies have reported serious dose-dependent side effects.2,3 Cardiotoxicity (congestive heart failure and cardiomyopathy) is the most life-threatening health hazard that prominently constrains its clinical use, limits the appropriate treatment of cancer, and negatively impacts patient quality of life.4–6
Several mechanisms affect the pathogenesis of doxorubicin-induced cardiotoxicity, though the exact mechanism is not fully known. Cardiomyocytes have a lower capability for regeneration, lower antioxidant concentrations, greater dependence on oxidative substrate metabolism, and a greater volume of mitochondria. Therefore, they are more vulnerable to the long-term side effects of doxorubicin.7
Doxorubicin-induced cardiotoxicity is cumulative and permanent, but it can be of acute or chronic onset. It is the chief reason for mortality in doxorubicin-treated cases;8 therefore, studies have already been developed to reduce doxorubicin-induced cardiotoxicity through regulation of oxidative stress and apoptotic genes.9
Recent findings have proven that the use of antioxidants protects cardiomyocytes against doxorubicin-induced cardiotoxicity.1 Coenzyme Q10 (CoQ10), also known as ubiquinone, is a lipid-soluble benzoquinone that is internally produced in the smooth endoplasmic reticulum. In nature, it is found in CoQ10-rich food, such as fish, meat, broccoli, and cereals. It is also pharmacologically produced in the form of capsules and solutions.10 CoQ10 is an important component in mitochondrial energy production through participation in the mitochondrial respiratory chain and extra-mitochondrial electron transport. Therefore, CoQ10 is present in high concentrations in metabolically active organs, such as the liver, heart, kidney, and pancreas.11
CoQ10 plays a role in preventing and managing various heart diseases and toxicities, including hypertension, chronic heart failure, arrhythmias, and arteriosclerosis. It can also improve ischemic heart disease, valvular heart diseases, and drug-induced cardiomyopathy.12–14
CoQ10 has a potent antioxidant effect through free radical scavenging and inhibition of lipid peroxidation. Besides this, CoQ10 has been proven to be safe and well-tolerated by patients.15,16
Considering the beneficial role of doxorubicin as a chemotherapeutic drug and the restriction of its use due to cardiotoxicity, the present study aimed to evaluate the possible cardioprotective effect of CoQ10 against doxorubicin-induced histopathological and molecular changes in cardiomyocytes, with special consideration of the antioxidative and anti-apoptotic pathways.
2. Materials and methods
2.1. Animals
Twenty-eight adult Wistar rats (120–140 g in weight; 10 weeks old) were obtained from the Animal House, Faculty of Science, Mansoura University, Egypt. Rats were housed in clean, well-ventilated cages (two rats/cage). All cages were in a clean, climate-controlled room (temperature 22 ± 2°C, relative humidity 50–55%, with a 12:12 h light: dark cycle). Rats had free access to water and were fed ad libitum. They were allowed to acclimatize for one week before the start of the experiment. All experimental practices were performed according to the standards of the Institutional Animal Care and Use Committee and accepted by the Institutional Research Board (Ref R.21.07.1375), Faculty of Medicine, Mansoura University, Egypt.
2.2. Chemicals
Doxorubicin hydrochloride was obtained from Sigma Aldrich Chemical Company. It was dissolved in 0.9% sodium chloride. CoQ10 powder was bought from MEPACO Pharmaceutical Company, Egypt. It was dissolved in 1% carboxymethylcellulose.
Other reagents used and their sources are listed below:
•Anti-iNOS/rabbit polyclonal antibody [Product #PA3-030A] (Thermo Fisher Scientific, Waltham, USA)
•Anti-caspase 3/rabbit monoclonal antibody [EPR18297] (ab184787 Abcam, USA)
•TRIzol reagent (Zymo Research, Irvine, CA)
•SensiFAST cDNA Synthesis Kit (Bioline, Memphis, TN)
•HERA PLUS SYBR Green mix (Willowfort, Birmingham, UK)
•Thiobarbituric acid (Sigma Chemical Company, St-Louis, USA)
•Trichloroacetic acid (Sigma Aldrich, Ried str, USA)
2.3. Experimental design
The rats were randomly allocated into four equal groups (n = 7 per group). The required sample size was calculated using the IBMª SPSSª Sample Powerª version 3.0.1 (IBMª Corp., Armonk, NY, USA).
The control group (negative control): rats were given 1 ml of 1% carboxymethylcellulose per day by gastric tube for 7 days, followed by 1 ml of normal saline by intraperitoneal (IP) injection as a single dose on the 8th day, and another 7 days of 1% carboxymethylcellulose at 1 ml/day by gastric tube.
CoQ10-treated group (positive control): rats were given CoQ10 orally by gastric tube at a daily dose of 1 mg/kg/day for 7 days, followed by 1 ml of normal saline by IP injection as a single dose on the 8th day, and another 7 days of CoQ10 as described.
Dox-treated group: rats were given an initial 7 days of 1% carboxymethylcellulose at 1 ml/day by gastric tube, followed by doxorubicin at 12.5 mg/kg IP as a single dose on the 8th day, and another 7 days of 1% carboxymethylcellulose at 1 ml/day by gastric tube. The dose was chosen based on a previous study by Botelho et al.17
Dox+CoQ10-treated group: rats were given an initial 7 days of CoQ10 orally by gastric tube, then a single dose of doxorubicin IP on the 8th day of treatment, followed by another 7 days of oral CoQ10. Both CoQ10 and doxorubicin were given at the same dose as in the positive control group and the Dox-treated group.
2.4. Sampling and tissue processing
Twenty-four hours after the end of the experiment, all rats were euthanized by the intraperitoneal injection of sodium pentobarbital at 50 mg/kg. Each heart was dissected, and the left ventricle was divided into three parts. One part from each animal was fixed in 10% buffered formalin overnight for the preparation of paraffin blocks. A rotatory microtome was used to obtain 5-μm-thick sections to stain with hematoxylin and eosin (H&E) for histological evaluation and immunohistochemical staining with caspase-3 as an apoptotic marker and inducible nitric oxide synthase (iNOS) for detection of oxidative tissue stress.
A second part from each heart was processed for transmission electron microscopic examination.
The third and final part of each heart was immediately submerged in RNAlater solution (Qiagen, Germany) and stored at −80°C until used for real-time polymerase chain reaction (PCR) assessment of glyceraldehyde-3-phosphate dehydrogenase (GAPDH), Bax, and Bcl-2 gene expression level.
2.5. Immunohistochemical study
The streptavidin-biotin method was used: 5-µm-thick paraffin sections on positively charged glass slides were deparaffinized, hydrated, and embedded for 10 minutes in 3% hydrogen peroxide in phosphate buffer solution (PBS) for endogenous peroxidase blockage. Slides were put in 0.001 M citrate buffer (pH = 6) and boiled in a microwave for 5 minutes to unmask the antigenic sites. The slides were incubated with the primary rabbit polyclonal antibody at a dilution of 1:1000 for caspase-3 and1:200 for iNOS overnight at 4°C. After that, goat anti-rabbit IgG H&L (HRP) (ab97051) secondary antibody was used at 1:500 dilution, and sections were incubated in horseradish peroxidase-avidin-biotin complex (Vectastain Elite, Vector, CA) for 30 minutes at room temperature. Then, 3,3ʹ-diaminobenzidine in peroxide (DAB kit, Vector, CA) was added to the slides to visualize the reaction as a brown, insoluble product. Finally, 0.05% diaminobenzidine (DAB) (Dakopatts, Glostrup, Denmark) was used as a chromogen. The slides were counterstained with hematoxylin. Lastly, dehydration, clearing, and mounting were performed. Negative control slides were prepared the same way, with the replacement of the primary antibodies with PBS.
2.6. Ultrastructural study
Small-size samples (1 mm) were fixed in glutaraldehyde and osmic acid, dehydrated, and embedded in epoxy resin. Toluidine blue-stained semi-thin sections were prepared and examined. In addition, 80-nm-thick ultrathin sections were prepared and stained with uranyl acetate and lead citrate.18 The sections were examined and photographed by transmission electron microscope JEM-2100 (Gatan Inc., Tokyo, Japan) in the Electron Microscopy Unit, Faculty of Agriculture, Mansoura University, Egypt.
2.7. Morphometric study
Caspase-3 and iNOS immunohistochemically stained slides were examined by an Olympus microscope with an accompanying Olympus digital camera (E24- 10-megapixel, China). Three non-overlapping fields at x400 magnification were chosen per section (5 sections from 5 different rats/group). The images were examined on an Intel Core I3 computer via Video Test Morphology software (Saint Petersburg, Russia) with a specific, built-in routine for determining area percentage.
2.8. Molecular study
Total RNA was extracted according to the manufacturer’s instructions, using TRIzol reagent (Zymo Research, Irvine, CA). NanoDrop2000 was used to determine RNA concentration and purity (Thermo Fischer Scientific, Waltham, MA). A260/A280 and A260/A230 had ratios of >1.8 and >1.7, respectively. SensiFAST cDNA Synthesis Kit (Bioline, Memphis, TN) was used to prepare cDNA from each 1 g of RNA, as directed by the provider.
The HERA PLUS SYBR Green Mix was used to perform quantitative real-time polymerase chain reaction (PCR) (Willowfort, Birmingham, UK) analysis. Using an Applied Biosystems 7500 Real-Time PCR System, PCR reaction (20 μl) containing 10 μl SYBR Green Mix (2x), 1 μl of cDNA, 1 μl of each primer, and 7 μl nuclease-free H2O was performed (Applied Biosystems, Waltham, MA). The primers used for cDNA amplification are listed in Table 1.
Table 1.
Nucleotide sequences of primers
| Gene | Sequence |
|---|---|
| Bax | F5ʹ-ATGGAGCTGCAGAGGATGA-3ʹ R5ʹ-CCAGTTTGCTAGCAAAGTAG-3’ |
| Bcl2 | F5ʹ-GAGGATTGTGGCCTTCTTTG-3ʹ R5ʹ-AGGTACTCAGTCATCCACA-3’ |
| GAPDH | F5ʹ-TGCCACTCAGAAGACTGTGG-3ʹ R5ʹ-GGATGCAGGGATGATGTTCT-3’ |
The real-time PCR thermal conditions were as follows: initial cycle at 95°C for 10 minutes; then 40 cycles of denaturation at 95°C for 15 s each; annealing at 60°C (GAPDH), 55°C (Bax), or 58°C (Bcl-2) for 30 s; and extension at 72°C for 30 s. Relative expression of the target genes was calculated by (2− ΔΔCt) method.19 The internal standard was GAPDH.
2.9. Biochemical measurement of malondialdehyde (MDA)
Using mortar and pestle, about 25–50 mg of left ventricle tissue was homogenized with 1 ml of cold buffer (potassium phosphate, nM EDTA). The homogenate was centrifuged for 30 minutes at 9,000 × g, and the supernatant was utilized to assess the MDA levels. MDA interacts with thiobarbituric acid (TBA) to produce a red-colored, complex, thiobarbituric acid reactive substance with 532 nm peak absorbance.
In a centrifuge tube, 1 ml of 1% trichloroacetic acid and 1 ml of 0.6% TBA were added to 0.5 ml of tissue homogenate, and the mixture was heated for 45 minutes in a boiling water bath. After cooling, 70% TBA was added to the mixture, vortex mixed, and incubated at 37°C for 20 minutes, followed by 15 minutes of centrifugation at 2,000xg. Using a spectrophotometer, the absorbance reading of the supernatant portion was measured at 534 nm against reagent blank (Jenway, Genova model, serial no. 1722, UK). Results were expressed as nmol/mg tissue.20
2.10. Statistical analysis
The immunohistochemical, biochemical, and molecular results were statistically analyzed using Statistical Package for Social Sciences (SPSS) software version 15.0 (SPSS, Inc., Chicago, IL, USA). The data were expressed as mean value ± standard deviation. One way analysis of the variance was used to test the significance between three or more independent groups. Post-hoc Tukey was used to assess the significance between every two adjacent groups.
In all applied tests, the P-values associated with test statistics indicated the significance level at which the null-hypothesis (the hypothesis of no difference) was rejected, and it was set at 0.05 so that a P-values ≥ 0.05 are statistically non-significant, P-values < 0.05 are significant, and P-values < 0.01 are highly significant.
3. Results
3.1. H&E stain
H&E-stained sections from the control group and CoQ10-treated group revealed no histological differences. Both groups showed the usual histological architecture of the myocardium. The muscle fibers ran in different directions with a characteristic branching and anastomosing pattern. The muscle fibers had central oval vesicular nuclei and acidophilic faintly striated cytoplasm. A thin connective tissue layer, the endomysium, surrounded the cardiac myocytes and revealed the flat nuclei of fibroblasts and small blood capillaries (Figure 1a-b).
Figure 1.
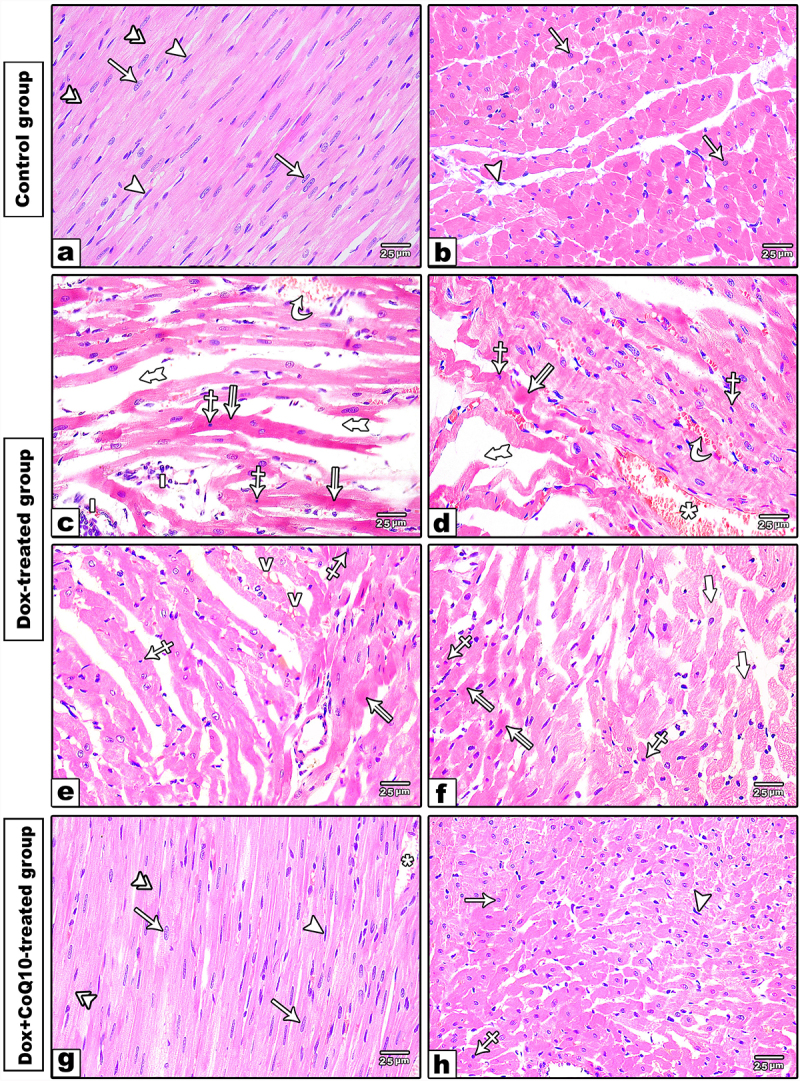
Photomicrographs of H &E-stained sections of in the left ventricle. (a-b) longitudinal and cross sections of the control group. The cardiac muscle fibers are branching and anastomosing with minimal interstitial spaces containing fibroblast cells with flat nuclei (arrowhead). The cardiomyocytes have acidophilic faintly striated cytoplasm (double arrowheads) and oval central vesicular nuclei (arrow). (c-f) Dox-treated group showing branched disorganized cardiac muscle fibers with wide interstitial spaces (thick tailed arrow) containing dilated congested blood capillaries (*) interstitial hemorrhage (curved arrow) and inflammatory cell infiltration (i). The sarcoplasm of cardiomyocytes showing pale stained (thick arrow) vacuolated (v) areas and others homogenous darkly acidophilic areas (split arrow). Some of cardiomyocytes having small sized darkly stained pyknotic nuclei (crossed arrow). (g-h) Dox+CoQ10-treated group revealing regular arrangement of cardiomyocytes with central vesicular nuclei (arrow) and acidophilic faintly straited cytoplasm (double arrowheads) apart from few small pyknotic nuclei (crossed arrow) and dilated blood capillaries (*). (H&E x 400)
The Dox-treated group revealed a disturbed histological organization of cardiac muscle with wide interstitial spaces between the muscle fibers, dilated congested capillaries, and areas of interstitial hemorrhage. Several cardiac myocytes had small-sized, darkly stained nuclei and vacuolated sarcoplasms; others had dark eosinophilic non-striated sarcoplasm (Figure 1c–f).
The Dox+CoQ10-treated group revealed a normal organization of cardiac muscle fibers, apart from slight increases in the intestinal space with dilated blood capillaries and few pyknotic nuclei were still observed (Figure 1g-h).
3.2. Immunohistochemical and morphometric results
3.2.1. Caspase-3 antibodies stain
Immunohistochemically stained sections of both the control and CoQ10-treated groups with anti-caspase-3 revealed few cells with positive immune reactions in the cytoplasm of cardiomyocytes (Figure 2a). The Dox-treated group showed a strong positive cytoplasmic reaction to anti-caspase-3 in most cardiac muscle fibers (Figure 2b). The Dox+CoQ10-treated group revealed few cells with positive immunoexpression (Figure 2c). Regarding the morphometric results, a highly significant increase in the area percentage occurred in the Dox-treated group compared with the control groups. A highly significant decrease in the Dox+CoQ10-treated group compared with the Dox-treated group was noted, along with a non-significant increase compared with the negative control group and significant increase compared with coQ10-treated group (Table 2).
Figure 2.

Photomicrographs of Caspase 3 immunostained sections (a-c) and iNOS immunostained sections (d-f). (a) Control group showing few cells with positive cytoplasmic caspase-3 immunoexpression (arrows). (b) DOX-treated group revealing strong positive reaction in the cytoplasm of most of cardiomyocytes (arrows). (c) Dox+CoQ10-treated group revealing mild positive caspase 3 immunoexpression (arrow). (d)The control group showing faint positive iNOS immunoexpression in the cytoplasm of few cardiomyocytes. (e) DOX-treated group showing a strong positive iNOS immunoexpression in the cytoplasm of most cardiomyocytes. (f) DOX+CoQ10-treated group showing mild positive iNOS immunoexpression (arrows). (a-c Anti-Caspase-3 immunostain × 400, d-f Anti-iNOS immunostain × 400)
Table 2.
Area percentage of caspase 3 in the studied groups
| Study groups |
Test of significance | ||||
|---|---|---|---|---|---|
| Control group (n = 15) | CO Q10 group (n = 15) | Doxorubicin treated group (n = 15) |
Doxorubicin treated group +CO Q10 group (n = 15) |
||
| Area percentage of caspase 3 | 0.76 ± 0.30 | 0.37 ± 0.22 | 6.10 ± 1.20 | 1.18 ± 0.51 | F = 84.89 P < .001** |
| P1 | 0.385 | < 0.001** | 0.346 | ||
| P2 | < 0.001** | 0.009* | |||
| P3 | < 0.001** | ||||
SD: standard deviation, F for ANOVA test
* statistically significant if P ≤ 0.05
** highly statistically significant result if P ≤ 0.001
P1: comparison in relation to control group
P2: comparison in relation to CO Q10 group
P3: comparison in relation to Doxorubicin treated group
3.2.2. iNOS antibodies stain
Anti-iNOS revealed faint positive immunoexpression in the cytoplasm of cardiac muscle fibers in the control groups (Figure 2d). The doxorubicin-treated group revealed a strong positive cytoplasmic immunoexpression in most cardiac myocytes (Figure 2e). On the one hand, the Dox+CoQ10-treated group showed relatively weak cytoplasmic iNOS immunoexpression (Figure 2f). Morphometric analysis revealed a highly significant increase in the area percentage of iNOS in the Dox-treated group compared with the control groups (p < .001). On the other hand, there was a highly significant decrease in the Dox+CoQ10-treated group compared with the Dox-treated group and a significant increase compared with the control groups (p = .015 & 0.007 respectively) (Table 3).
Table 3.
Area percentage of iNOS in the studied groups
| Study groups |
Test of significance | ||||
|---|---|---|---|---|---|
| Control group (n = 15) | CO Q10 group (n = 15) | Doxorubicin treated group (n = 15) |
Doxorubicin treated group +CO Q10 group (n = 15) |
||
| Area percentage of iNOS in the studied groups | 0.52 ± 0.18 | 0.49 ± 0.14 | 7.15 ± 0.47 | 0.83 ± 0.16 | F = 164.69 P < .001** |
| P1 | 0.994 | < 0.001** | 0.015* | ||
| P2 | < 0.001** | 0.007* | |||
| P3 | < 0.001** | ||||
SD: standard deviation, F for ANOVA test
* statistically significant if P ≤ 0.05
** highly statistically significant result if P ≤ 0.001
P1: comparison in relation to control group
P2: comparison in relation to CO Q10 group
P3: comparison in relation to Doxorubicin treated group
3.3. Electron microscopy results
Ultrastructural examination of the left ventricular walls of both the control and CoQ10-treated groups showed similar histological appearance. The sarcoplasm contained myofibrils which were parallel and regularly arranged. They were arranged in sarcomeres with alternating dark bands (A) intersected by H zones and light bands (I) intersected by Z lines. Longitudinally arranged rows of mitochondria with abundant tubular cristae appeared between the myofibrils. A characteristic step-like intercalated disk with transverse parts containing desmosomes and longitudinal parts contained gap junctions occurred. A centrally located oval euchromatic nucleus was visible (Figure 3a-c).
Figure 3.

Electron transmission photomicrographs of the left ventricular of the control groups (a-c). The myocyte showing regular arrangement of myofibrils within the cytoplasm with alternating light (i) bands intersected by Z line and dark bands intersected by H zone. The sarcomere is the distance between two adjacent Z lines. Mitochondria with characteristic tubular cristae are aligned in rows between the myofibrils. The nucleus (n) is oval and euchromatic. The cardiomyocyte is surrounded by sarcolemma (arrow). A step-like intercalated disc (crossed arrow) is seen connecting the two adjacent myocytes. Note, T tubule (t) below Z line. Dox. treated group (d-i) showing the nucleus (n) of cardiomyocyte is hyperchromatic with irregular and interrupted nuclear envelope (curved arrow). The cytoplasm showing irregular arrangement of myofibrils with areas of fragmented or lost myofibrils (*). Mitochondria are variable in size and shape (double arrowhead); some are electron dense (m) other with vacuolated cytoplasm (v). Disorganized and interrupted intercalated disc (crossed arrow). Abundant intracytoplasmic autophagosomes (zigzag arrow) and dilated T tubules (t) and sarcoplasmic reticulum (arrow) are seen. Collagen fibers (c) are accumulated. Dox+CoQ10-treated group (j-l), the cardiomyocyte showing euchromatic nucleus (N) with prominent nucleolus. The sarcomeres (s) reveal regular arrangement of myofibrils apart from small areas with myofibril lost (*). Normal shape and distribution of mitochondria (M) in rows between the myofibrils. Normal step-like intercalated disc (crossed arrow), T tubule (t) and sarcoplasmic reticulum (arrow) are seen
The Dox-treated group revealed cardiac myocytes with disorganized and fragmented myofibrils. Large-size mitochondria with abnormal shapes and swollen matrix with loss of cristae accumulated between the muscle fibers. Most nuclei were small-sized and hyperchromatic, with corrugated irregular outlines and areas of nuclear membrane discontinuity. Areas of the interrupted intercalated disc with loss of desmosomes were visible. Multiple vacuoles and autophagosomes were seen between muscle fibers (MF). Accumulation of collagen fibrils also appeared between cardiomyocytes. Some sections revealed areas of sarcoplasmic reticulum dilatation (Figure 3d-i).
Examination by transmission electron microscope of cardiomyocytes from the Dox+CoQ10-treated group revealed an ultrastructural appearance more or less similar to the control group (Figure 3j-l).
3.4. Biochemical and molecular results
The expression levels of Bax and Bcl-2 were measured using real-time quantitative PCR (RT-qPCR). Bax and Bcl-2 mRNA transcripts were significantly higher in both Dox- and Dox+CoQ10-treated groups compared to the control (p < .001). Also, the expression of Bax was significantly higher in the Dox group relative to the Dox+CoQ10-treated group (p < .001). In contrast, the relative expression of Bcl-2 was significantly lower in the Dox group compared with the Dox+CoQ10-treated group (p < .001) (Figure 4a-b)
Figure 4.

(a) BAX relative expression within the control and the experimental groups. (b) BCL2 relative expression within the control and the experimental groups. (c) BAX/BCL2 ratio. (d) MDA concentration
A: Significant in comparison to the control groupB: Significant in comparison to CO Q10 groupC: Significant in comparison to Doxorubicin treated group
The Bax/Bcl-2 gene expression ratio increased significantly in the Dox-treated group compared with both the control and CoQ10-treated groups. The Dox+CoQ10-treated group showed a significant decrease in comparison with the Dox-treated group (Figure 4c).
MDA concentration was significantly higher in the Dox- and Dox+CoQ10-treated groups than in the control (p < .001). Moreover, there was a significant increase in the Dox group compared to the Dox+CoQ10-treated group (p < .001) (Figure 4d).
4. Discussion
Although Dox has a potential anticancer effect, the clinical efficacy was limited, and a clinical restriction of its use occurred due to its cardiotoxic effect. There has been considerable effort to clarify the mechanisms of Dox-induced cardiotoxicity over the last few years.1 Meanwhile, the series of events that result in myocardial injury has remained elusive. In this study, we focus on oxidative stress and apoptotic pathways.
In the present work, acute cardiotoxicity was evident histologically in the form of cardiac muscle fiber disorganization, widened the interstitial spaces between these fibers, loss of myofilaments, and advanced atrophy of cardiomyocytes. These findings are consistent with previous research.21–23 Dox administration causes harmful effects on cardiomyocytes through the generation of free radicals and interference with DNA repair.24–27 Our results are in agreement since we revealed a significant elevation in MDA level in the Dox-treated group in comparison with the control group.
Oxidative stress with free radical formation is the most accepted theory for Dox-induced cardiotoxicity through lipid peroxidation.23,28 Evidence points toward doxorubicin-induced oxidative stress and liberation of reactive oxygen species (ROS) with reduction of endogenous antioxidants, multiple breaks in DNA double strands, and the consequent activation of the mitochondria-dependent apoptotic pathway.27–29
Regarding Dox treatment-induced myocardial congestion, areas of hemorrhage, and edema, Dox reportedly increased vascular endothelial growth factor that leads to vasodilatation and increased vascular permeability. Vacuolated sarcoplasm also occurred, which could be due to the destruction of cardiomyocyte plasma membranes by disruption of intracellular water and electrolyte distribution.30
Histological examination of Dox-treated H&E-stained sections revealed numerous cardiomyocytes with small, darkly stained pyknotic nuclei. Our result was confirmed by an electron microscopic study, which showed small-sized hyperchromatic nuclei. These findings were in line with Abdu et al.25 They observed nuclear pyknosis with loss of cardiomyocytes striations in the left ventricular of dox. treated rats. In addition, caspase-3 immunohistochemical staining revealed significant increases in the area percentage compared to the control group. Moreover, the RT-qPCR examination of Bax and Bcl-2 genes revealed a significant downregulation of the anti-apoptotic gene Bcl-2 and upregulation in proapoptotic gene Bax in the animals treated with Dox compared with the control group. A similar effect on Bax and Bcl-2 expression level was previously reported.31
Immunohistochemical examination of the Dox-treated group revealed a significant increase in iNOS immunoexpression. Our results were consistent with Wang et al.,32 who linked Dox-induced cardiotoxicity to elevated nitric oxide (NO) levels, oxidative/nitrosative stress, and myocardial inflammation. In addition, iNOS is usually used by cells as a pro-inflammatory cytokine.33 Moreover, Barakat et al. proved the cardioprotective role of iNOS inhibition in Dox-mediated cardiotoxicity.34
Cardiac NO development is aided by iNOS enzymes. NO is a primary regulator of vascular tone and plays an important role in cardiac function and the pathogenesis of cardiac disease. Although basal NO production controls cardiomyocyte contractility, blood flow, cell growth, and differentiation, excessive NO production is linked to cardiac disease.35 It played an essential role in initiating cardiac muscle death through apoptosis, remodeling, and vascularization. NO interacts with oxygen and produces peroxynitrites which cause DNA damage by activating Poly (ADP-ribose) polymerase, resulting in an energy imbalance and cell death.30,36
We used transmission electron microscopy to examine samples from the left ventricle for better evaluation of these lesions. Our results revealed areas of cardiomyocytes showing degenerative changes with loss of myofibrils and thick Z lines. This was previously explained by the interaction between Dox and actin myofilaments, which is the main component of the Z line.37,38 In addition to this, Dox caused actin polymerization.39
Mitochondrial ultrastructural changes were apparent in most of the sections in the Dox-treated group. The changes included large-sized mitochondria with abnormal shapes, mitochondrial vacuolation, and loss of cristae. Our results were in line with those of Wenceslau et al.40 They attributed the change to the direct effect of Dox on mitochondria through increased mitochondrial oxidative stress. On the other hand, Zhang et al.41 attributed Dox-induced mitochondrial damage, defective mitochondrial biogenesis, nuclear degeneration, and p53 activation to the interference of Dox with topoisomerase. In addition, an increased mitochondrial iron level was reported after Dox treatment. The hazardous effect of an increased mitochondrial iron level was confirmed by using dexrazoxane that exports mitochondrial iron.42,43
Notable ultrastructural deposition of collagen fibers between cardiomyocytes was visible. This could be considered cardiac remodeling for replacing damaged cardiac muscle fibers.44 Cardiac fibrosis could be due to the direct effect of Dox on cardiac fibroblasts: an attempt to enhanced collagen production and improve cell survival.45 In addition, Tanaka et al.46 proved that Dox-induced cardiac fibrosis occurs via the production of inflammatory cytokines by damaged mitochondria.
Multiple cytoplasmic vacuoles appeared in the Dox-treated group. These might be dilatations in the sarcoplasmic reticulum as described by Lushnikova et al.47 In addition, multiple autophagic vacuoles containing remnants of dead organelles were detected. Autophagy is a critical cellular mechanism that maintains cell and mitochondrial homeostasis. Changes in its dynamics can lead to abnormal mitochondrial function and cell death. Dox can alter the role of available lysosomes by interfering with their function. The autophagy mechanism results in the accumulation of autophagosomes, which results in excess mitochondrial damage via the accumulation of ROS.48
Intercalated discs play a vital role in the transmission of action potentials and Ca2+ during muscle contraction, where the Gap junction channels of the intercalated discs are responsible for direct cell-to-cell communication. Therefore, heart disease causes modification of gap junction organization.49 In the present study, we demonstrated ultrastructure changes in the intercalated disk and its gap junctions’ contents. TEM examination revealed substantially damaged intercalated discs. they appeared hazy, uneven, or interrupted. Our results are in accordance with previous studies.50,51 They attributed the contractility dysregulation in dox. induced heart failure to intercalated disc damage and uncoupling between T tubule and sarcoplasmic reticulum
There has recently been a boom in interest in CoQ10 research, both for experimental purposes and for its clinical use. CoQ10 has been found to protect against a variety of tissue damage in multiple clinical investigations, particularly heart disorders.52 In the present study, there was a significant decrease in MDA level in the Dox+CoQ10-treated group compared to the Dox-treated group. CoQ10 was proven to have a strong antioxidant activity that inhibits lipid peroxidation initiation and propagation.53
Immunohistochemical results revealed a significant decrease in area percentage of caspase-3 in the Dox+CoQ10-treated group compared to the Dox-treated group. The result was confirmed by RT-qPCR gene expression for Bax and Bcl-2, which revealed a significant increase in the anti-apoptotic gene Bcl-2 and downregulation in the proapoptotic gene Bax in the animals treated with Dox+CoQ10 compared with the Dox-treated group. This indicates the cardioprotective potential of CoQ10 against Dox-mediated cardiotoxicity by upregulation of Bcl-2 gene expression. The cardioprotective mechanism by the reduction in apoptotic cell death might be due to the antioxidant properties of CoQ10. These results are supported by those of El- Li et al. and Khadragy et al.,28,54 which demonstrated that CoQ10 treatment upregulated the expression of the anti-apoptotic gene Bcl-2 and downregulated the proapoptotic genes caspase-3 and Bax.
The preserved cell morphology detected by light microscopy and ultrastructural study further revealed CoQ10’s cardioprotective effect. A normal ultrastructure of mitochondria, with a notable decrease in their number and size in CoQ10 administration, was observed. This is consistent with research performed by Luo et al.55 where the addition of CoQ10 reduced the formation of ROS and superoxide anion in mitochondria. According to the findings, CoQ10 supplementation helps preserve mitochondrial activity during oxidative stress, resulting in reduced apoptotic cell death. Given the vital role of mitochondria in the heart, the histomorphometric preservation with CoQ10 administration, which reduced autophagosome formation, suggests a significant advantage in such treatment. Mitochondrial dysfunction is a key pathogenic pathway for Dox.17
This study has some limitations, as we relied on the transmission electron microscope in assessment of intercalated disc changes. Further study should be carried out to study the molecular changes in gap junction protein of intercalated disc in such cases. In addition to Echocardiographic examination of the rats to co-relate ultra-structural changes with the functional changes.
This study shows that treatment with coenzyme Q10 has a major cardioprotective effect in rats given Dox. Coenzyme Q10 reduces oxidative stress and regulates cell survival by preserving mitochondrial morphology and preventing apoptosis. Thus, avoiding oxidative stress-related disorders in the future may be as simple as preserving mitochondrial function.
Funding Statement
This research did not receive any specific grant
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Faridvand Y, Haddadi P, Vahedian V, Nozari S, Nejabati HR, Pezeshkian M, Afrasiabi A, Safaie N, Jodati A, Nouri M, et al. Human amnion membrane proteins prevent doxorubicin-induced oxidative stress injury and apoptosis in rat H9c2 cardiomyocytes. Cardiovasc Toxicol. 2020;20(4):1–14. doi: 10.1007/s12012-020-09564-8. [DOI] [PubMed] [Google Scholar]
- 2.Sandamali JAN, Hewawasam RP, Jayatilaka KAPW, Mudduwa LKB.. Cardioprotective potential of Murraya koenigii (L.) Spreng. Leaf extract against doxorubicin-induced cardiotoxicity in rats. Evid Based Complementary Altern Med: eCAM. 2020;2020:6023737. doi: 10.1155/2020/6023737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang B, Wan S, Peng X, Zhao M, Li S, Pu Y, He B.. Human serum albumin-based doxorubicin prodrug nanoparticles with tumor pH-responsive aggregation-enhanced retention and reduced cardiotoxicity. J Mater Chem B. 2020;8(17):3939–3948. doi: 10.1039/D0TB00327A. [DOI] [PubMed] [Google Scholar]
- 4.Kalyanaraman B. Teaching the basics of the mechanism of doxorubicin-induced cardiotoxicity: have we been barking up the wrong tree? Redox Biol. 2020;29(Jan):101394. doi: 10.1016/j.redox.2019.101394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bulten BF, Sollini M, Boni R, Massri K, de Geus-oei L-F, van Laarhoven HWM, Slart RHJA, Erba PA. Cardiac molecular pathways influenced by doxorubicin treatment in mice. Sci Rep. 2019;9(1):2514. doi: 10.1038/s41598-019-38986-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung WB, Youn HJ. Pathophysiology and preventive strategies of anthracycline-induced cardiotoxicity. Korean J Intern Med. 2016;31(4):625–633. doi: 10.3904/kjim.2016.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fang X, Wang H, Han D, Xie E, Yang X, Wei J, Gu S, Gao F, Zhu N, Yin X, et al. Ferroptosis as a target for protection against cardiomyopathy. Proc Natl Acad Sci U S A. 2019. Feb;116(7):2672–2680. doi: 10.1073/pnas.1821022116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang S, Liu X, Bawa-Khalfe T, Lu LS, Lyu YL, Liu LF, Yeh ET. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med. 2012;18(11):1639–1642. Epub 2012/10/30. doi: 10.1038/nm.2919. [DOI] [PubMed] [Google Scholar]
- 9.Zhao L, Zhang B. Doxorubicin induces cardiotoxicity through upregulation of death receptors mediated apoptosis in cardiomyocytes. Sci Rep. 2017;7:44735. doi: 10.1038/srep44735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holmberg MJ, Uber A, Stankovic N, Chen CO, Grossestreuer AV, Donnino MW, Andersen LW, Liu X, et al. Ubiquinol (reduced coenzyme Q10) and cellular oxygen consumption in patients undergoing coronary artery bypass grafting. J Intensive Care Med. 2020 Aug;35(8):797-804. doi: 10.1177/0885066618789114. Epub 2018 Jul 18. PMID: 30021499. [DOI] [PubMed] [Google Scholar]
- 11.Yang X, Zhang Y, Xu H, Luo X, Yu J, Liu J, Chang RC. Neuroprotection of coenzyme Q10 in neurodegenerative diseases. Curr Top Med Chem. 2016;16(8):858–866. doi: 10.2174/1568026615666150827095252. [DOI] [PubMed] [Google Scholar]
- 12.Kumar A, Kaur H, Devi P, Mohan V. Role of coenzyme Q10 (CoQ10) in cardiac disease, hypertension and meniere-like syndrome. Pharmacol Ther. 2009;124(3):259–268. PMID: 19638284 Google Scholar. doi: 10.1016/j.pharmthera.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Littarru GP, Tiano L, Belardinelli R, Watts GF. Coenzyme Q (10). BioFactors. 2011;37(5):366–373. PMID: 21674640 Google Scholar. doi: 10.1002/biof.154. [DOI] [PubMed] [Google Scholar]
- 14.Sattarinezhad E, Shafaroodi H, Sheikhnouri K, Mousavi Z, Moezi L. The effects of coenzyme Q10 on seizures in mice: the involvement of nitric oxide. Epilepsy Behav: E&B. 2014;37:36–42. doi: 10.1016/j.yebeh.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 15.Li X, Zhan J, Hou Y, Hou Y, Chen S, Luo D, Luan J, Wang L, Lin D. Coenzyme Q10 regulation of apoptosis and oxidative stress in H2O2 induced BMSC death by modulating the Nrf-2/NQO-1 signaling pathway and its application in a model of spinal cord injury. Oxid Med Cell Longev. 2019;2019:6493081. doi: 10.1155/2019/6493081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arafat E, Ghoneim F, Khalaf H, Elsamanoudy A. Anti-senescence role of coenzyme Q10 and 17 β-estradiol on submandibular gland of
- 17.Botelho AFM, Lempek MR, Branco SEMT, Nogueira MM, de Almeida ME, Costa AG, Freitas TG, Rocha MCRC, Moreira MVL, Barreto TO, et al. Coenzyme Q10 cardioprotective effects against doxorubicin-induced cardiotoxicity in Wistar rat. Cardiovasc Toxicol. 2020;20(3):222–234. doi: 10.1007/s12012-019-09547-4. [DOI] [PubMed] [Google Scholar]
- 18.Dykstra MJ, Reuss LE. Staining methods for semi thins and ultra-thins. Biological electron microscopy, theory, techniques and troubleshooting. 2nd. New York: Kluwer Academic Publishers/Plenum Press. 2003. 175–196. [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2- ΔΔCT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Halliwell B, Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br J Pharmacol. 2004;142(2):231–255. doi: 10.1038/sj.bjp.0705776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Green PS, Leeuwenburgh C. Mitochondrial dysfunction is an early indicator of doxorubicin-induced apoptosis. Biochim Biophys Acta. 2002;1588(1):94–101. doi: 10.1016/S0925-4439(02)00144-8. [DOI] [PubMed] [Google Scholar]
- 22.Gava FN, Zacché E, Ortiz EMG, Champion T, Bandarra MB, Vasconcelos RO, Barbosa JC, Camacho AA. Doxorubicin induced dilated cardiomyopathy in a rabbit model: an update. Res Vet Sci. 2013;94(1):115–121. doi: 10.1016/j.rvsc.2012.07.027. [DOI] [PubMed] [Google Scholar]
- 23.De Geest B, Mishra M. Doxorubicin-induced cardiomyopathy: tert gets to the heart of the matter. Mol Ther. 2021;29(4):1363–1365. doi: 10.1016/j.ymthe.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adwas AA, Elkhoely AA, Kabel AM, Abdelrahman MN, Eissa AA. Ameliorative potential of different doses of indol-3-carbinol on doxorubicin induced cardiotoxicity in mice. J Cancer Res Treat. 2016;2:26–31. [Google Scholar]
- 25.Abdu F, Ahmed F, Shaheen M, Mostafa S. A comparative study of the Ameliorative effect of doxorubicin with vitamin E versus liposomal doxorubicin on the left ventricular histological and immunohisochemical changes induced by doxorubicin in adult male albino rats. Egypt J Histol. 2019; doi: 10.21608/ejh.2019.7015.1061. [DOI] [Google Scholar]
- 26.Al-Malky HS, Al Harthi SE, Osman AMM. Major obstacles to doxorubicin therapy: cardiotoxicity and drug resistance. J Oncol Pharm Pract. 2020;26(2):434–444. doi: 10.1177/1078155219877931. [DOI] [PubMed] [Google Scholar]
- 27.Baniahmad B, Safaeian L, Vaseghi G, Rabbani M, Mohammadi B. Cardioprotective effect of vanillic acid against doxorubicin-induced cardiotoxicity in rat. Res Pharm Sci. 2020;15(1):87–96. doi: 10.4103/1735-5362.278718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li X, Zhan J, Hou Y, Chen S, Hou Y, Xiao Z, Luo D, Lin D. Coenzyme Q10 suppresses oxidative stress and apoptosis via activating the Nrf-2/NQO-1 and NF-?B signaling pathway after spinal cord injury in rats. Am J Transl Res. 2019;11:6544–6552. [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu H, Gao Y, Zhu S, Cui Q, Du J. Klotho improves cardiac function by suppressing reactive oxygen species (ROS) mediated apoptosis by modulating Mapks/Nrf2 signaling in doxorubicin-induced cardiotoxicity. Med Sci Monit. 2017;23:5283–5293. doi: 10.12659/MSM.907449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Attia GM, Elmansy RA, Algaidi SA. Silymarin decreases the expression of VEGF-A, iNOS and caspase-3 and preserves the ultrastructure of cardiac cells in doxorubicin induced cardiotoxicity in rats: a possible protective role. Int J Clin Exp Med. 2017;2:4158–4173. [Google Scholar]
- 31.Lu PP, Ma J, Liang XP, Guo CX, Yang YK, Yang KQ, Shen QM, Ma LH, Zhou XL. Xinfuli improves cardiac function, histopathological changes and attenuate cardiomyocyte apoptosis in rats with doxorubicin-induced cardiotoxicity. J Geriatr Cardiol: JGC. 2016;13(12):968–972. doi: 10.11909/j.1671-5411.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang J, Yao L, Wu X, Guo Q, Sun S, Li J, Shi G, Caldwell RB, Caldwell RW, Chen Y, et al. Protection against doxorubicin-induced cardiotoxicity through modulating iNOS/ARG 2 balance by electroacupuncture at PC6. Oxid Med Cell Longev. 2021;2021:6628957. doi: 10.1155/2021/6628957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abeles AM, Pillinger MH. Statins as antiinflammatory and immunomodulatory agents: a future in rheumatologic therapy? Arthritis Rheum. 2006;54(2):393–407. doi: 10.1002/art.21521. [DOI] [PubMed] [Google Scholar]
- 34.Barakat BM, Ahmed HI, Bahr HI, Elbahaie AM. Protective effect of boswellic acids against doxorubicin-induced hepatotoxicity: impact on Nrf2/HO-1 defense pathway. Oxid Med Cell Longev. 2018;2018:8296451. doi: 10.1155/2018/8296451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bahadır A, Kurucu N, Kadıoğlu M, Yenilme E. The role of nitric oxide in doxorubicin-induced cardiotoxicity: experimental study. Turk J Hematol. 2014;31(1):68–74. doi: 10.4274/Tjh.2013.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao Y, Zhou S, Xu Y, Sheng S, Qian SY, Huo X. Nitric oxide synthase inhibitors 1400W and L-NIO inhibit angiogenesis pathway of colorectal cancer. Nitric Oxide - Biol Chem. 2019;83:33–39. doi: 10.1016/j.niox.2018.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Balli E, Mete UO, Tuli A, Tap O, Kaya M. Effect of melatonin on the cardiotoxicity of doxorubicin. Histol Histopathol. 2004;19:1101–1108. [DOI] [PubMed] [Google Scholar]
- 38.Zare MFR, Rakhshan K, Aboutaleb N, Nikbakht F, Naderi N, Bakhshesh M, Azizi Y. Apigenin attenuates doxorubicin induced cardiotoxicity via reducing oxidative stress and apoptosis in male rats. Life Sci. 2019. Sep 1;232:116623. Epub 2019 Jul 4. PMID: 31279781. doi: 10.1016/j.lfs.2019.116623. [DOI] [PubMed] [Google Scholar]
- 39.Yassien R, Elsaid A. The possible protective role of melatonin on doxorubicin induced cardiomyopathy of adult male albino rats. Egypt J Histol. 2017;40(1):25–36. doi: 10.21608/EJH.2017.3694. [DOI] [Google Scholar]
- 40.Wenceslau CF, McCarthy CG, Szasz T, Spitler K, Goulopoulou S, Webb RC; Working Group on Damps in Cardiovascular Disease . Mitochondrial damage-associated molecular patterns and vascular function. Eur Heart J. 2014;35(18):1172–1177. doi: 10.1093/eurheartj/ehu047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang S, Liu X, Bawa-Khalfe T, Lu LS, Lyu YL, Liu LF, Yeh ET. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med. 2012;18:1639–1642. [DOI] [PubMed] [Google Scholar]
- 42.Tadokoro T, Ikeda M, Ide T, Deguchi H, Ikeda S, Okabe K, Ishikita A, Matsushima S, Koumura T, Yamada K-I, et al. Mitochondria-dependent ferroptosis plays a pivotal role in doxorubicin cardiotoxicity. JCI Insight. 2020;5(9). doi: 10.1172/jci.insight.132747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qin Y, Guo T, Wang Z, Zhao Y. The role of iron in doxorubicin-induced cardiotoxicity: recent advances and implication for drug delivery. J. Mater. Chem. B. 2021;9(24):4793–4803. doi: 10.1039/D1TB00551K. [DOI] [PubMed] [Google Scholar]
- 44.Zhang QL, Yang JJ, Zhang HS. Carvedilol (CAR) combined with carnosic acid (CAA) attenuates doxorubicin-induced cardiotoxicity by suppressing excessive oxidative stress, inflammation, apoptosis and autophagy. Biomed Pharmacother. 2019;109:71–83. doi: 10.1016/j.biopha.2018.07.037. [DOI] [PubMed] [Google Scholar]
- 45.Levick SP, Soto-Pantoja DR, Bi J, Hundley WG, Widiapradja A, Manteufel EJ, Bradshaw TW, Meléndez GC. Doxorubicin-induced myocardial fibrosis involves the Neurokinin-1 receptor and direct effects on cardiac fibroblasts. Heart Lung Circ. 2019. Oct;28(10):1598–1605. Epub 2018 Sep 2. PMID: 30205930 PMCID: PMC7901001. doi: 10.1016/j.hlc.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tanaka R, Umemura M, Narikawa M, Hikichi M, Osaw K, Fujita T, Yokoyama U, Ishigami T, Tamura K, Ishikawa Y, et al. Reactive fibrosis precedes doxorubicin-induced heart failure through sterile inflammation. ESC Heart Failure. 2020;7(2):588–603. doi: 10.1002/ehf2.12616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lushnikova EL, Nepomniashchich LM, Klinnikova MG, Molodykh OP. Ultrastructural manifestations of disturbances of cardiomyocyte regeneration after the doxorubicin treatment. Morfologiia. 2005;128:81–84. [PubMed] [Google Scholar]
- 48.Koleini N, Kardami E. Autophagy and mitophagy in the contexto of doxorubicin-induced cardiotoxicity. Oncotarget. 2017;8(28):46663–46680. doi: 10.18632/oncotarget.16944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Manring HR, Dorn LE, Ex-Willey A, Accornero F, Ackermann MA. At the heart of inter- and intracellular signaling: the intercalated disc. Biophys Rev. 2018;10:961–971. doi: 10.1007/s12551-018-0430-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Younis N,N, Salama A, Shaheen MA, Eissa RG. Pachymic acid attenuated doxorubicin-induced heart failure by suppressing miR-24 and preserving cardiac junctophilin-2 in rats. Int J Mol Sci. 2021;22(19):10710. doi: 10.3390/ijms221910710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li X, Xu G, Wei S, Zhang B, Yao H, Chen Y, Liu W, Wang B, Zhao J, Gao Y. Lingguizhugan decoction attenuates doxorubicin-induced heart failure in rats by improving TT-SR microstructural remodeling. BMC Complement Altern Med. 2019;19:1–11. doi: 10.1186/s12906-019-2771-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zozina VI, Covantev S, Goroshko OA, Krasnykh LM, Kukes VG. Coenzyme Q10 in cardiovascular and metabolic diseases: current state of the problem. Curr Cardiol Rev. 2018;14(3):164–174. doi: 10.2174/1573403X14666180416115428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khan NA, Abid M, Ahmad A, Abuzinadah MF, Basheikh M, Kishore K. Cardioprotective effect of coenzyme Q10 on apoptotic myocardial cell death by regulation of Bcl-2 gene expression. J Pharmacol Pharmacother. 2017;8(3):122–127. doi: 10.4103/jpp.JPP_47_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.El-khadragy M, Al-megrin W, A.s.n.a. M, D.m. E-H, Salem RE, Kassab, R.B. FH, Ahmed EAM, Abdel Moneim AE. Impact of Coenzyme Q10 administration on lead acetate induced testicular damage in rats. Oxid Med Cell Longev. 2020;2020:1–12. doi: 10.1155/2020/4981386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Luo K, Yu JH, Quan Y, Shin YJ, Lee KE, Kim HL, Ko EJ, Chung BH, Lim SW, Yang CW, et al. Therapeutic potential of coenzyme Q10 in mitochondrial dysfunction during tacrolimus-induced beta cell injury. Sci Rep. 2019;9(1):7995. doi: 10.1038/s41598-019-44475-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


