Abstract
The molecular complexes underlying planar cell polarity (PCP) were first identified in Drosophila through analysis of mutant phenotypes in the adult cuticle and the orientation of associated polarized protrusions such as wing hairs and sensory bristles. The same molecules are conserved in vertebrates and are required for the localization of polarized protrusions such as primary or sensory cilia and the orientation of hair follicles. Not only is PCP signaling required to align cellular structures across a tissue, it is also required to coordinate movement during embryonic development and adult homeostasis. PCP signaling allows cells to interpret positional cues within a tissue to move in the appropriate direction and to coordinate this movement with their neighbors. In this review we outline the molecular basis of the core Wnt-Frizzled/PCP pathway, and describe how this signaling orchestrates collective motility in Drosophila and vertebrates. Here we cover the paradigms of ommatidial rotation and border cell migration in Drosophila, and convergent extension in vertebrates. The downstream cell biological processes that underlie polarized motility include cytoskeletal reorganization, and adherens junctional and extracellular matrix remodeling. We discuss the contributions of these processes in the respective cell motility contexts. Finally, we address examples of individual cell motility guided by PCP factors during nervous system development and in cancer disease contexts.
1. Overview of planar cell polarity (PCP) signaling
Cells in tissues often require directional features for proper cellular and organ function. For example, epithelial cells are uniformly polarized along the apical-basal axis, enabling their vectorial functions like protein secretion into a lumen. Most epithelia are further polarized within the planar axis, which is referred to as planar cell polarity (PCP). PCP provides cells and tissues with positional information allowing them to generate polarized structures oriented with respect to tissue axes, to embed specialized cells (e.g., sensory cells) with a specific orientation or to move in a directed fashion (Adler, 2002; Butler & Wallingford, 2017; Davey & Moens, 2017; Devenport, 2016; Goodrich & Strutt, 2011; Humphries & Mlodzik, 2018; Peng & Axelrod, 2012; Seifert & Mlodzik, 2007).
PCP has been best studied and characterized in Drosophila, where it is evident in all adult cuticular structures and the compound eye. In wings, for example, cellular hairs are oriented in the proximo-distal axis and this uniform pattern is disrupted in PCP mutants (Fig. 1A). Similarly, in eyes, the regular arrangement of ommatidia with respect to anterior-posterior (AP) and dorsal-ventral (DV) axes is altered in PCP mutants (Fig. 2) (Adler, 2002; Goodrich & Strutt, 2011; Humphries & Mlodzik, 2018; Peng & Axelrod, 2012; Seifert & Mlodzik, 2007). Based on these phenotypes, a core group of evolutionarily conserved PCP genes have been identified, which is referred to as “the core Frizzled (Fz)/PCP factors.” The core Fz/PCP module includes the transmembrane proteins Fz, Flamingo (Fmi, a.k.a. Starry night/Stan), and Van Gogh (Vang; a.k.a. Strabismus/Stbm) and the cytoplasmic factors Dishevelled (Dsh), Prickle (Pk), and Diego (Dgo) (Adler, 2012; Goodrich & Strutt, 2011; Humphries & Mlodzik, 2018; Peng & Axelrod, 2012; Seifert & Mlodzik, 2007; Wu & Mlodzik, 2009). The core Fz/PCP factors interact with each other and localize asymmetrically to generate cellular polarization. This polarity information is then transmitted to downstream effectors to elicit tissue-specific responses (Figs. 1 and 2) (Adler, 2002; Goodrich & Strutt, 2011; Harrison, Shao, Strutt, & Strutt, 2020; Humphries & Mlodzik, 2018; Peng & Axelrod, 2012; Seifert & Mlodzik, 2007; Wu & Mlodzik, 2009).
Fig. 1.
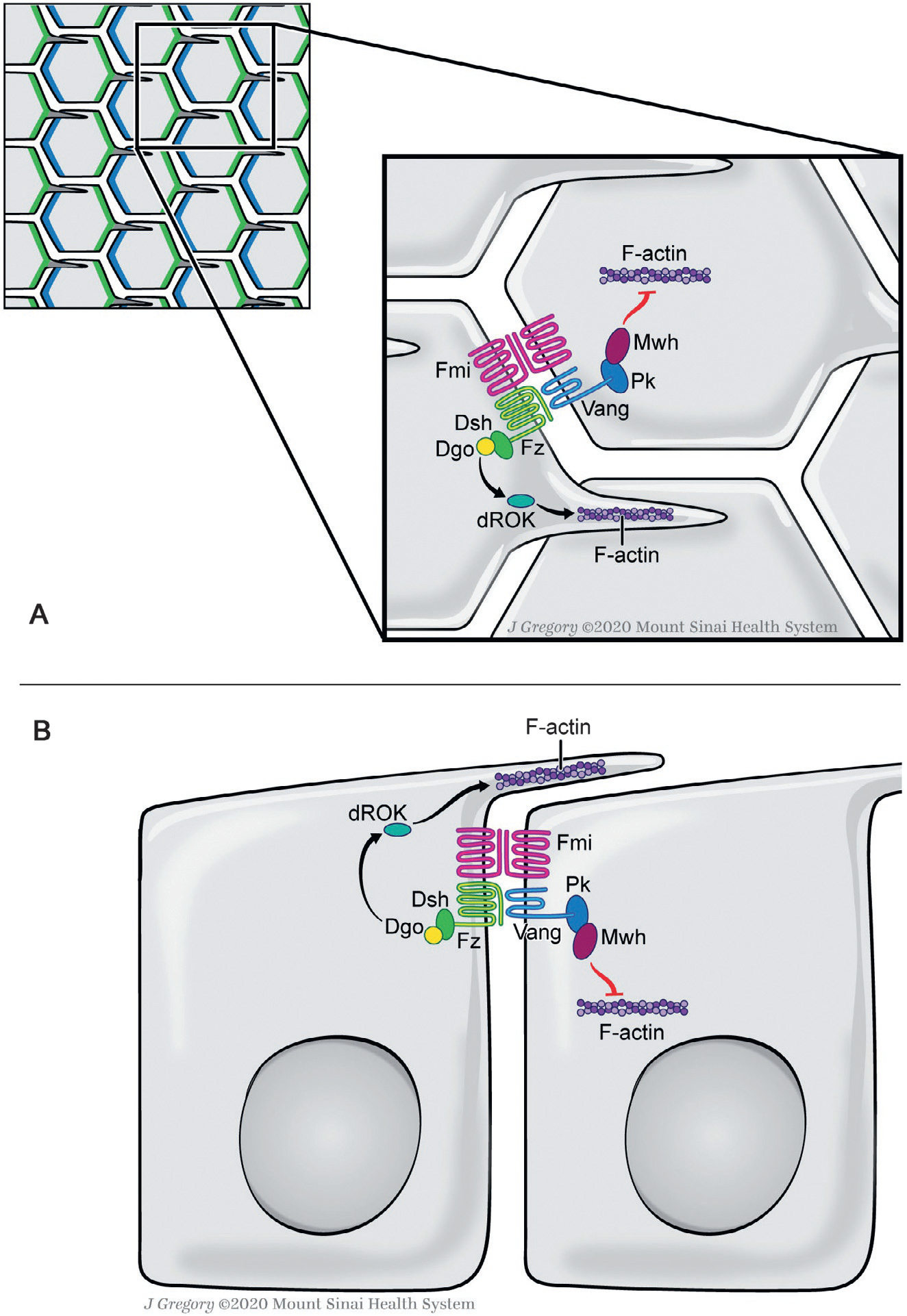
Planar cell polarity in the Drosophila wing epithelium. Schematic X-Y (top) view in (A) and lateral (Z-section) view in (B) of core PCP complexes is shown. Note that the core PCP complexes are located at/near the adherens junctions in the subapical domain of the cells. In wing epithelia, one of the simplest PCP paradigms, asymmetric distribution of the core PCP factors starts to emerge at late larval stages and is most obvious in pupal stages (shown in upper left panel schematic, Fz-Dsh-Dgo (blue) and Vang-Pk complexes (green), both stabilized by interactions with Fmi, asymmetrically localize in distal and proximal apical membranes, respectively (Adler, 2012; Goodrich & Strutt, 2011; Humphries & Mlodzik, 2018; Jenny, 2010; Peng & Axelrod, 2012; Seifert & Mlodzik, 2007; Wu & Mlodzik, 2009). The PCP complexes then trigger the polarization of the cytoskeleton through downstream regulators to ensure single spike actin hair formation via the Fz/Dsh/Dgo complex at the distal vertex of each cell (Gault, Olguin, Weber, & Mlodzik, 2012; Strutt & Warrington, 2008; Winter et al., 2001; Yan et al., 2008). Proximal is left. See main text for further details.
Fig. 2.
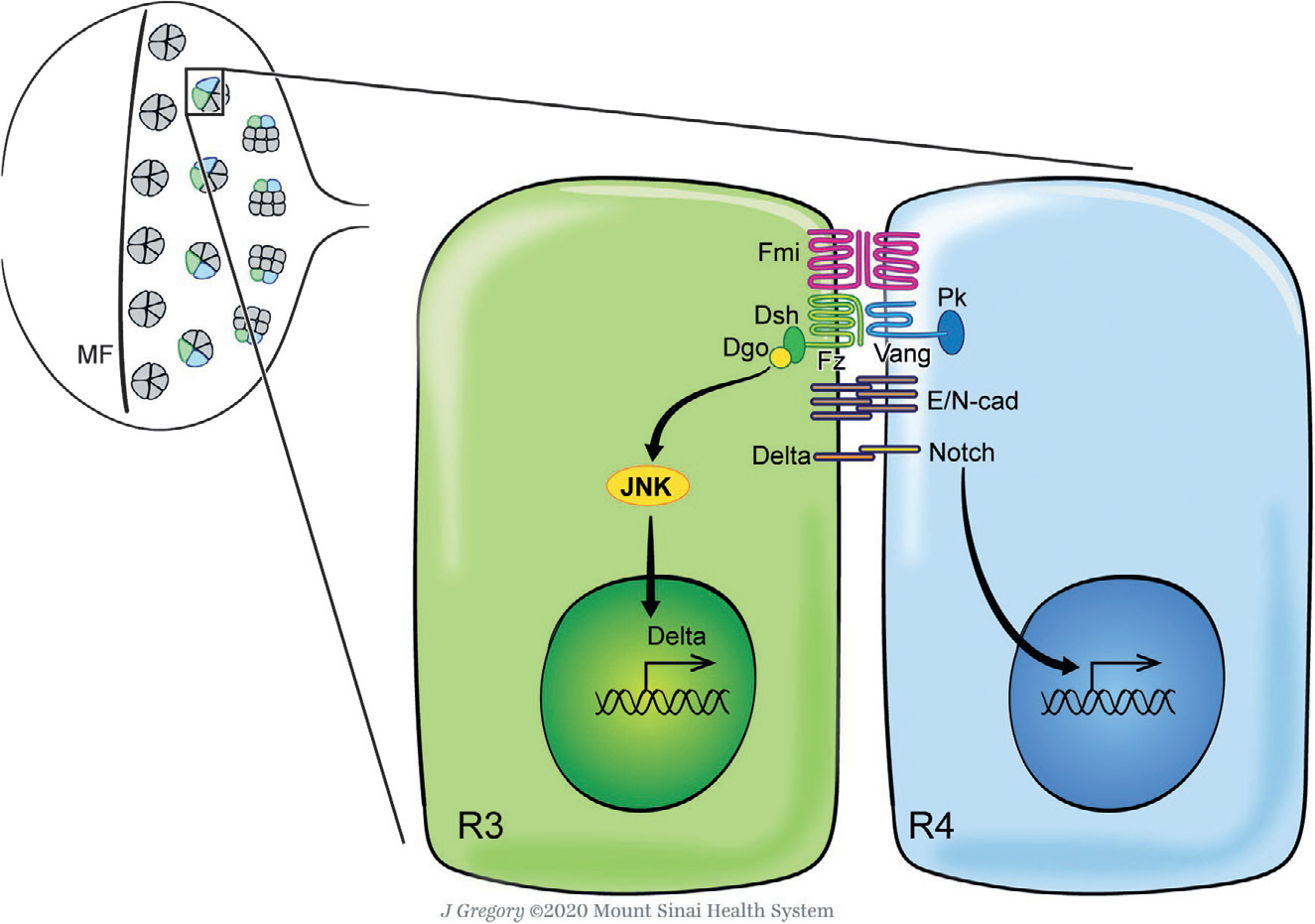
Planar cell polarity signaling and ommatidial rotation in the Drosophila eye. The Drosophila eye develops from an epithelial imaginal disc during larval stages, which is initially composed of identical pluripotent precursor cells ahead of the morphogenetic furrow (MF) (upper left panel). As the MF sweeps across the disc from posterior to anterior, preclusters of differentiating cells start to emerge which will then develop into mature ommatidia (Cagan & Ready, 1989; Roignant & Treisman, 2009; Tomlinson & Ready, 1987). During differentiation and maturation, these clusters rotate 90° in opposite directions in the dorsal and ventral halves of the eye to establish the final mirror-symmetric pattern of ommatidia across the D/V midline ( Jenny, 2010; Mlodzik, 1999). Posterior to the MF, Fz/PCP signaling mainly takes place between the photoreceptors R3 (green) and R4 (blue), inducing R4 fate via Notch signaling activation (Cooper & Bray, 1999; Das, Jenny, Klein, Eaton, & Mlodzik, 2004; Das, Reynolds-Kenneally, & Mlodzik, 2002; del Alamo & Mlodzik, 2006; Fanto & Mlodzik, 1999; Tomlinson & Struhl, 1999; Wolff & Rubin, 1998; Wu, Klein, & Mlodzik, 2004). The core PCP factors become asymmetrically localized in the subapical membrane region of the R3/R4 pair cell boundary, leading to differential downstream nuclear and/or cellular responses in each cell mediated by signaling (JNK, Delta, Notch) and simultaneously adhesion (E- and N-cadherin) molecules which are thought to mediate the cell motility process of ommatidial rotation (Cooper & Bray, 1999; Das et al., 2002, 2004; del Alamo & Mlodzik, 2006; Fanto & Mlodzik, 1999; Mirkovic et al., 2011; Mirkovic & Mlodzik, 2006; Tomlinson & Struhl, 1999; Wolff & Rubin, 1998; Wu et al., 2004). Dorsal is up, anterior is left. See main text for details.
A second (independent) set of PCP factors identified in Drosophila is centered around the proto-cadherins Fat and Dachsous (Ds) which heterophilically interact with each other across cell membranes and have their own set of effectors to generate polarization (Lawrence & Casal, 2018; Matis & Axelrod, 2013; Thomas & Strutt, 2012). Although recent studies suggest that this module also has a role in vertebrate PCP, the mechanism of Fat/Ds signaling and their conservation, and whether they directly or indirectly relate to the core Fz/PCP factors or act upstream or in parallel to them remain largely elusive (Lawrence & Casal, 2018; Matis & Axelrod, 2013; Strutt & Strutt, 2021).
Importantly, and as further outlined below, the core Wnt-Fz/PCP factors regulate a set of cellular responses and read-outs that, like the molecular PCP signaling cassette, appear conserved across animal species (Butler & Wallingford, 2017; Davey & Moens, 2017; Devenport, 2016; Humphries & Mlodzik, 2018; Klein & Mlodzik, 2005; Seifert & Mlodzik, 2007). The two most obvious and common read-outs include (i) polarization of cytoskeletal elements and (ii) regulation of directed cell motility (see also review by Davey & Moens, 2017). While these two read-outs are in many ways linked, they can be separated in certain contexts, as not all PCP associated read-outs cause cell movement or rearrangement of cells relative to each other. While the latter read-out is the focus of this review (see below), the former—affecting cytoskeletal organization—is also linked to the asymmetric, subcellular localization of cilia and centrioles (reviewed in Carvajal-Gonzalez, Mulero-Navarro, & Mlodzik, 2016) and the anchoring of centrosomes required for the orientation of the mitotic spindle (reviewed in Segalen & Bellaiche, 2009). Here, we focus on Wnt/Fz-PCP signaling regulated cell motility in its many aspects including, besides cell migration during development and disease per se, cellular rearrangements within a field of cells and neuronal pathfinding.
1.1. PCP in Drosophila
Studies in Drosophila, mainly in wings and eyes but also in other tissues like the abdomen, have provided fundamental mechanistic insights into how PCP core factors interact and how PCP is established in individual cells and across tissues (Adler, 2012; Goodrich & Strutt, 2011; Humphries & Mlodzik, 2018; Jenny, 2010; Peng & Axelrod, 2012; Seifert & Mlodzik, 2007; Wu & Mlodzik, 2009). In wings, arguably the best understood PCP system, Fz/PCP signaling leads to asymmetric distribution of core PCP factors from late larval/early pupal stages. Fz-Dsh-Dgo and Vang-Pk complexes become asymmetrically enriched in distal and proximal membranes respectively, as they antagonize each other within the same cell and stabilize each other across cell membranes, to enforce and maintain an initial polarization bias set-up by localized Wnt-expression (Fig. 1) (Adler, 2012; Seifert & Mlodzik, 2007; Wu & Mlodzik, 2009; Wu, Roman, Carvajal-Gonzalez, & Mlodzik, 2013). The well-characterized read-out to this polarized signaling complexes in wing cells is the formation of a single distally pointing actin spike, called trichome, downstream of the Fz-Dsh complex (Fig. 1). In this context, multiple wing hair (Mwh) and Rho-associated kinase (dROK) are two effectors that are employed downstream of the Vang-Pk and Fz-Dsh complexes, respectively, ensuring that a single distal actin hair is generated. Mwh, an anti-Formin, is recruited to the proximal edge of the cells to locally inhibit actin hair formation (Strutt & Warrington, 2008; Yan et al., 2008), whereas Rho and dROK and their associated trafficking acts in the distal vertex to restrict hair formation to a single actin spike (Fig. 1) (Gault et al., 2012; Winter et al., 2001). Thus, polarity is established in the wing through local cytoskeletal rearrangements mediated by the Fz/PCP cassette.
In the Drosophila eye, Fz/PCP signaling first instructs R3/R4 photoreceptor cell fate specification, which is followed by directed rotation of ommatidial clusters (Fig. 2) ( Jenny, 2010; Mlodzik, 1999). An initial bias in Fz/PCP activity between the R3/R4 precursors specifies the cell that has higher Fz-Dsh/PCP activity as R3 and induces its neighbor to adopt the R4 fate. This specification depends on JNK-mediated transcriptional responses created by Fz-Dsh/PCP signaling that culminate in differential upregulation of Delta (Dl) and neuralized in R3 (Cooper & Bray, 1999; del Alamo & Mlodzik, 2006; Fanto & Mlodzik, 1999; Tomlinson & Struhl, 1999) which act together to activate Notch signaling in R4 (within the R3/R4 pair), and Notch-signaling specifying the R4 fate (reviewed in Jenny, 2010; Mlodzik, 1999). During this process, the core PCP factors become asymmetrically localized in R3/R4 cells and thus also guide the subsequent directed and coordinated movement of ommatidial clusters via cytoskeletal and junctional rearrangements (Fig. 2) ( Jenny, 2010; Seifert & Mlodzik, 2007). Hence, core Fz/PCP signaling triggers distinct cellular responses in wings and eyes by employing tissue-specific downstream effectors, including the regulation of a directed cell movement process in the eye ( Jenny, 2010; Klein & Mlodzik, 2005, see dedicated chapter on ommatidial rotation below).
1.2. PCP in vertebrates
Data from many vertebrate models reveal that the molecular mechanism(s) and function of core Wnt-Fz/PCP signaling is conserved across species, and that the core PCP factor cassette is involved in polarity establishment in many (if not all) vertebrate tissues (Butler & Wallingford, 2017; Davey & Moens, 2017; Devenport, 2016; Humphries & Mlodzik, 2018; Seifert & Mlodzik, 2007). Similar to PCP establishment in the Drosophila wing epithelium (or the Drosophila cuticle in general), the mammalian epidermis is planar polarized with hair follicles aligned along the A/P axis and this pattern is disrupted in Fzd6 mutant mice (Guo, Hawkins, & Nathans, 2004). In this context, Vangl2, Celsr1, and Fzd6 are asymmetrically localized along the A/P axis during hair follicle development (Devenport & Fuchs, 2008), mirroring their polarized localization features from Drosophila. In the mammalian inner ear, PCP signaling orients and aligns the sensory hair cells to uniformly polarize the bundles of actin-based stereocilia, a pattern that is randomized in Fzd3/6, Vangl2 and Celsr1 mutants (Curtin et al., 2003; Montcouquiol et al., 2006; Wang et al., 2006). Fzd3 and Vangl2 are enriched at opposite cell membranes, reiterating the asymmetric distribution of the core PCP factors (Montcouquiol et al., 2006). There are many other examples of planar polarized tissues in vertebrates and these prominently include ciliated cells, ranging from multi-ciliated cells of the airway epithelia and kidney tubules (Brzoska et al., 2016; Vladar, Bayly, Sangoram, Scott, & Axelrod, 2012) to cells displaying a polarized localization of the primary cilium, which is critical for their function (Wallingford, 2010).
In addition to polarizing tissues and embedded organ features, PCP has been implicated in multiple morphogenetic processes that require directed cell motility in vertebrates, and associated asymmetric localization of PCP proteins has also been documented in motile cells despite technical challenges (reviewed in Butler & Wallingford, 2017; Davey & Moens, 2017; Devenport, 2016). For example, core Fz/PCP signaling coordinates convergent extension (CE) movements that take place during vertebrate gastrulation and neurulation. Accordingly, PCP mutant embryos show defects in elongation of the body axis and neural tube closure due to the failure and/or misregulation of CE cellular movements and intercalation (Butler & Wallingford, 2017; Davey & Moens, 2017; Devenport, 2016). Similarly, human patient derived mutations in Vangl genes associated with neural tube closure defects have been shown to affect core PCP signaling (Humphries, Narang, & Mlodzik, 2020). Overall, in the past 15 years, significant progress has been made in dissecting cellular asymmetries of core PCP factors in the context of CE processes during gastrulation and neurulation and how these might coordinate cellular convergence and extension movements in general (Ciruna, Jenny, Lee, Mlodzik, & Schier, 2006; Nishimura, Honda, & Takeichi, 2012; Roszko, Sepich, Jessen, Chandrasekhar, & Solnica-Krezel, 2015; Williams, Yen, Lu, & Sutherland, 2014; Yin, Kiskowski, Pouille, Farge, & Solnica-Krezel, 2008; reviewed in Butler & Wallingford, 2017; Davey & Moens, 2017; Devenport, 2016). Other PCP-mediated collective motility processes in vertebrates include the migration of facial branchiomotor neurons and growth cone guidance, where perturbing PCP signaling activity causes abnormalities in coordinated cell movements (reviewed in Butler & Wallingford, 2017; Davey & Moens, 2017; Devenport, 2016). Furthermore, mutations in core Fz-PCP factors and associated signaling has been implicated in cancer metastasis (e.g., reviewed in Humphries & Mlodzik, 2018), highlighting the importance of understanding how PCP regulates cell motility in development for addressing related mechanisms in disease contexts. Several of these specific processes will be discussed in this review.
2. PCP and cell motility in Drosophila
2.1. Ommatidial rotation (OR)
During Drosophila eye development, Fz/PCP signaling regulates, besides general ommatidial patterning via cell fate specification ( Jenny, 2010; Mlodzik, 1999), the directed movement of the ommatidial preclusters. The eye consists of ~800 regularly arranged ommatidia, with each consisting of 8 photoreceptor neurons arranged into an invariant trapezoidal pattern and 12 accessory (cone, pigment, and bristle) cells. During larval stages, the eye develops from an epithelial imaginal disc, which is initially composed of identical pluripotent precursor cells (Fig. 2). As a wave of cell proliferation and differentiation (called morphogenetic furrow, MF) travels across the disc from posterior to anterior, it leaves in its wake preclusters of differentiating cells that will mature into ommatidia (Cagan & Ready, 1989; Roignant & Treisman, 2009; Tomlinson & Ready, 1987). During differentiation and maturation, these clusters rotate 90° in opposite directions in the dorsal and ventral halves of the eye to establish the mirror-symmetric pattern of adult ommatidia across the D/V midline (Fig. 2) (Jenny, 2010; Mlodzik, 1999). As such, posterior to the MF, Fz/PCP signaling instructs not only R3/R4 cell fate specification, but also coordinates the direction and degree of rotation. The rotation of ommatidial clusters has served as a key model to study PCP regulated cell motility.
Loss-of-function (LOF) mutations in core PCP genes randomize R3/R4 specification and thus chiral ommatidial arrangements or even eliminate chirality, giving rise to R3-R3 or R4-R4 symmetrical ommatidia (Jenny, 2010; Mlodzik, 1999). At the same time, they also cause a randomization of both direction and degree of rotation, suggesting that PCP is critical for both directing and executing rotation and coordinating the process with cell fate induction (Das et al., 2002; Jenny, Darken, Wilson, & Mlodzik, 2003; Rawls & Wolff, 2003; Wolff & Rubin, 1998; Wu et al., 2004). Similarly, interference with PCP signaling and establishment via overexpression of the core components causes phenotypes similar to LOF defects, affecting both cell fate specification and rotation of ommatidia (Das et al., 2002; Fanto & Mlodzik, 1999; Jenny, Reynolds-Kenneally, Das, Burnett, & Mlodzik, 2005; Rawls & Wolff, 2003; Wolff, Guinto, & Rawls, 2007). However, ommatidial rotation (OR) can be genetically uncoupled from cell fate specification, as several genes have been reported to affect OR without interfering with R3/R4 cell fate choice. Genes that affect rotation in a specific manner include argos (aos, an inhibitory ligand for EGF receptor) (Choi & Benzer, 1994; Gaengel & Mlodzik, 2003; Strutt & Strutt, 2003), nemo (nmo, a serine-threonine kinase of the MAPK superfamily) (Choi & Benzer, 1994; Fiehler & Wolff, 2008; Mirkovic et al., 2011; Mirkovic & Mlodzik, 2006), shotgun (shg, DE-cadherin, a cell adhesion molecule) (Mirkovic & Mlodzik, 2006), zipper (zip, a myosin/motor protein) (Fiehler & Wolff, 2007), scabrous (sca, a secreted fibrinogen-related protein) (Chou & Chien, 2002), and integrins and extracellular matrix (ECM) components (Thuveson et al., 2019). Involvement of these genes in OR suggests that receptor tyrosine kinase (RTK) signaling, cell-cell and cell-matrix interactions, and cytoskeletal dynamics are critical to regulate this cell motility process. These OR-“specific” factors act downstream (or in parallel) to core PCP signaling and translate oriented core PCP factor input to the directional movement of the cell clusters.
Recently, mechanistic insight into how core PCP proteins may regulate OR and cell motility in general is starting to emerge. Nmo, the founding member of the Nlk superfamily of MAP kinases, was the first rotation-specific gene to be identified (Choi & Benzer, 1994). nmo null mutants are characterized by severe underrotation of otherwise largely normal ommatidial preclusters (Fiehler & Wolff, 2008; Mirkovic et al., 2011). Although nmo is required in all photoreceptors and interommatidial cells for OR to proceed normally, it is enriched at the R4 side of R3/R4 cell border through a physical interaction with the Vang-Pk complex. Nmo phosphorylates β-catenin/E-cadherin complexes whereby it may regulate the dynamics (or adhesive properties) of the E-cad complexes. As nmo genetically interacts with Vang and pk, and β-cat (arm)/E-cad (shg) in the OR context, its involvement as an effector linking core PCP factors and cell adhesion molecules is functionally supported (Mirkovic et al., 2011). Cadherin-dependent cell adhesion has been implicated in OR and mutations in both Drosophila E-cad and N-cad genes have been shown to cause misrotation (Mirkovic & Mlodzik, 2006). Based on their mutant and overexpression phenotypes, they have opposing effects on rotation: DE-cad promotes rotation, whereas DN-cad restricts it. Strikingly, the localization patterns of two cadherins in rotating preclusters are complementary to each other: DE-cad becomes enriched at the membranes between all precluster cells except the R3/R4 border, whereas DN-cad is upregulated in the R3/R4 pair and enriched at their border. E-cad is also detected and required at all adherens junctions in eye imaginal discs and notably at the borders between precluster cells and inter-ommatidial cells (Mirkovic & Mlodzik, 2006). Classically, cadherins may serve a role to hold the cells of the precluster together; nevertheless, normal organization of ommatidia with (under) rotation defects in hypomorphic shg mutants indicates that cadherin-based junctional remodeling is critically required for the OR process (Mirkovic & Mlodzik, 2006). In other words, cell adhesion dynamics must be tightly controlled in ommatidial clusters to achieve the correct rate of rotation, and local asymmetries generated by core PCP molecules, via Nmo or other regulators, may translate into polarized remodeling of adherens junctions, both providing a directional input—with Nmo being enriched in R4 via core PCP factors (Mirkovic et al., 2011)—and also coordinate the OR movement process through its general function in all eye disc cells (Fiehler & Wolff, 2008; Mirkovic et al., 2011; Munoz-Soriano, Ruiz, Perez-Alonso, Mlodzik, & Paricio, 2013).
Cytoskeletal dynamics must also be regulated for proper cell motility during OR. Rotation-specific phenotypes in mutants of dRhoA and dROK (Strutt, Weber, & Mlodzik, 1997; Winter et al., 2001), which are critical regulators of actin dynamics, support the idea that cytoskeletal rearrangements contribute to the OR process. RhoA genetically interacts with fz and dsh suggesting that core PCP signaling provides input into cytoskeletal reorganization (Strutt & Strutt, 2003). Similarly, perturbations in the activity of zip, encoding Drosophila Myosin II heavy chain, also cause ommatidial misrotation (Fiehler & Wolff, 2007). Zip acts downstream of RhoA and dROK in many contexts, and thus a similar mode of signaling may link core PCP factors to Zip regulation during OR (Verdier, Guang Chao, & Settleman, 2006). Importantly, Zip localizes to cell junctions between rotating (ommatidial precluster) and non-rotating (interommatidial) cells, a pattern that is maintained as new cells join the rotating (pre)cluster (Fiehler & Wolff, 2007). In this context, Zip may regulate rotational forces and/or it may affect the remodeling of adherens junctions at cell boundaries, enabling the rotating cluster to slide along the non-rotating cells. These data collectively suggest that Zip (Myosin II) may be involved in spatially and temporally distinct domains of rotation, integrating parallel upstream inputs into cytoskeletal reorganization.
Core Fz/PCP signaling cooperates with other signaling pathways during OR. A mutant allele of aos, encoding an inhibitory ligand for Drosophila EGF-Receptor (EGFR), was among the first OR specific mutations identified (Choi & Benzer, 1994). In addition, aos was isolated in a genetic screen for loci that interact with fz (Strutt & Strutt, 2003). Accordingly, aos LOF mutants, as well as hypomorphic EGFR alleles, show rotation defects without affecting ommatidial chirality (Brown & Freeman, 2003; Choi & Benzer, 1994; Gaengel & Mlodzik, 2003; Strutt & Strutt, 2003). Although mechanistic details of the involvement of EGFR during OR remain unknown, several lines of data point to an interaction between EGFR signaling and the regulators of cell adhesion and cytoskeleton. Firstly, the expression pattern of Fmi in R3/R4 cells is altered in aos mutants suggesting that EGFR signaling affects the establishment of polarity during R3/R4 specification, at least in part by controlling the adhesive properties of the cluster (Fmi is an atypical cadherin) (Gaengel & Mlodzik, 2003). Rotation defects that arise from reduced EGFR activity are enhanced in fmi and shg mutants, further linking EGFR signaling to cadherin-based adhesion (Gaengel & Mlodzik, 2003). As junctional dynamics must be regulated throughout rotation, EGFR signaling may feed into cellular adhesion at multiple stages of rotation. Indeed, perturbations of EGFR signaling at larval stages of rotation can lead to misorientation of ommatidia at later pupal stages, potentially by altering the adhesive properties of the rotating clusters (Brown & Freeman, 2003). While the effect of EGFR on rotation is mediated by Ras GTPase activity, expression of various Ras constructs that activate specific subsets of Ras effectors revealed that besides Ras/Raf/MAPK signaling, a Raf/MAPK-independent pathway downstream of Ras is employed during OR: Canoe (Cno/AF-6), a Raf/MAPK-independent Ras effector postulated to link cytoskeleton to cellular junctions acts downstream of EGFR/Ras signaling in the OR setting (Gaengel & Mlodzik, 2003). Consistent with this hypothesis, cno mutants show rotation-specific phenotypes and cno genetically enhances OR phenotypes associated with the EGFR-ligand regulator Star (Gaengel & Mlodzik, 2003). Taken together, these data suggest that EGFR signaling modulates junctional and cytoskeletal dynamics in parallel to core PCP signaling at multiple stages of ommatidial rotation.
In addition to the above discussed cell-cell adhesion features, cell-ECM interactions are also required for eye morphogenesis (Thuveson et al., 2019). All photoreceptor cells express Myospheroid, the Drosophila homolog of β1 Integrin, which localizes to the outside membrane of the photoreceptors within each cluster, forming a cup-like shape inside each cluster, where it interacts with the ECM (Thuveson et al., 2019). Mutations in any of the integrin-ECM associated components lead to rotation defects, with clusters rotating initially too quickly and later asynchronously (Thuveson et al., 2019). Thus, it is worth noting that the basolateral integrin-ECM mediated adhesion acts in the opposite manner to Nmo in regulating OR. Furthermore, an additional gene that could be associated with ECM function(s), scabrous (sca), which encodes a fibrinogen-related secreted protein (Mlodzik, Baker, & Rubin, 1990), has also been postulated to inhibit rotation, as sca mutants are characterized by overrotation of ommatidia (Chou & Chien, 2002), similar to integrin and ECM mutants. Sca is suggested to be transported in vesicles from the furrow to rows 6–8 posterior to it to slow down the OR process (Chou & Chien, 2002). This model is supported by observations that in “furrow stop” mutants ommatidia often overrotate and ectopic expression of Sca in these mutants can rescue this phenotype. Interestingly, sca associated overrotation phenotypes require nmo activity, as sca and nmo double mutants display nmo underrotation pattern, whereas underrotation phenotype associated with Sca overexpression is enhanced in nmo heterozygosity and suppressed by Nmo overexpression (Chou & Chien, 2002). It has been suggested that Sca may change the properties of the ECM, possibly by creating a “barrier” to the rotating cluster to slow it down. However, as Sca has also been suggested to modulate Notch signaling, or even act as a Notch ligand (Baker, Mlodzik, & Rubin, 1990; Mlodzik et al., 1990; Powell, Wesley, Spencer, & Cagan, 2001), other functional models are possible, either through Notch signaling or the interaction between Notch and EGFR signaling via the Notch-dependent expression of Aos (Koca, Housden, Gault, Bray, & Mlodzik, 2019).
Taken together, ommatidial rotation is a well-studied PCP cell motility process and (at least at the genetic level) it helped significantly to identify and define the role of many genes that are involved in PCP-regulated cell motility in general, covering distinct branches of the cellular machinery ranging from signaling to adhesion and to cytoskeletal regulation. Work on OR also underlines how multiple signaling pathways and local cellular processes are interlinked in achieving precision in a highly regulated cell motility process.
2.2. Border cell migration
A second paradigm of collective cell motility in Drosophila is border cell migration (BCM) during oogenesis (Fig. 3). The early egg chamber consists of 16 germline cells—an oocyte and 15 nurse cells—that are enveloped by an epithelial layer of (somatic) follicle cells (Wu et al., 2008). At anterior and posterior edges of this follicular epithelium emerges a pair of differentiated cells, referred to as polar cells (Montell, 2003; Montell et al., 2012; Wu et al., 2008). As the egg chamber matures, follicle cells undergo rearrangements during which anterior polar cells signal to their neighbors—so called border cells—and induce their epithelial-to-mesenchymal transition and delamination (Wu et al., 2008). These border cells then migrate posteriorly, toward the anterior border of the oocyte, carrying along the polar cells (Fig. 3) (Montell, 2003; Montell et al., 2012).
Fig. 3.
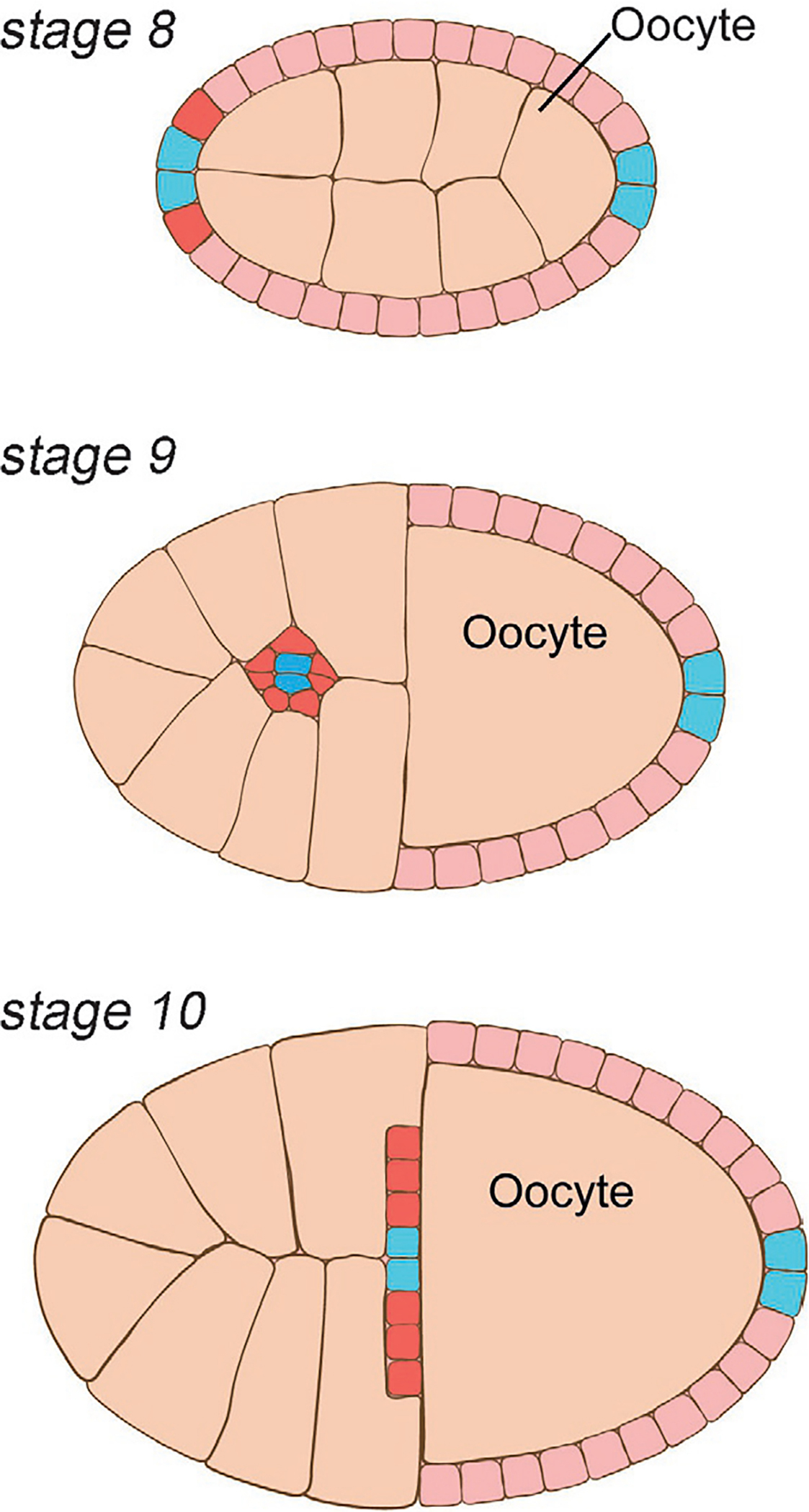
Schematic of border cell migration in the Drosophila egg chamber. The early egg chamber is composed of 16 germline cells—an oocyte and 15 nurse cells—that are surrounded by an epithelial monolayer of somatic follicle cells (Wu, Tanwar, & Raftery, 2008). As cell divisions in the follicular epithelium continue, a pair of cells at the anterior and posterior ends of the follicular epithelium differentiates into so called “polar cells” (stage 8, blue) (Montell, 2003; Montell, Yoon, & Starz-Gaiano, 2012; Wu et al., 2008). Anterior polar cells start to recruit a cluster from their neighboring epithelial cells, referred to as border cells (stage 8, red), through intercellular signaling events (Wu et al., 2008). While the follicular epithelium undergoes further cellular rearrangements during egg chamber development, the polar-border cell cluster migrates posteriorly, toward the anterior border of the oocyte (stages 9 and 10) (Montell, 2003; Montell et al., 2012). Fz/PCP signaling has been implicated in border cell migration. Anterior is left. See main text for details.
Efficient BCM requires the activity of core Fz/PCP signaling. Mutations in fz, Vang, dsh and pk cause significant delays in migration (Bastock & Strutt, 2007). Knockdown of fz and Vang specifically in border cells or in polar cells delays migration, suggesting that PCP is required in both cell types for their normal motility. This was further confirmed by the analysis of genetically mosaic clusters, where only polar or only border cells lacked the core PCP genes fz or Vang. Importantly, in mosaic clusters with only one polar cell retaining fz activity, the polar cell that was Fz+ was always positioned toward the leading edge, suggesting that Fz/PCP activity in a polar cell promotes their migratory behavior although the inverse pattern was not observed for Vang mutants (Bastock & Strutt, 2007). In addition, Fz and Vang localize to adherens junctions between polar and border cells, consistent with the idea that they mediate the “communication” between polar and border cells (Bastock & Strutt, 2007).
Very similar to ommatidial rotation, the core Fz/PCP cassette seems to cooperate with RTK signaling to promote BCM and the migration process entails junctional and cytoskeletal remodeling in the cells of the cluster. EGFR and PVR signaling pathways act redundantly to guide the process, as blocking the activity of both receptors, EGFR and PVR, severely inhibits migration (Duchek & Rorth, 2001; Duchek, Somogyi, Jekely, Beccari, & Rorth, 2001). In fact, individual border cells show differential RTK activity due to a gradient of the respective ligands emanating from the oocyte. This generates distinct cellular responses between the front and the back of the cluster, enabling the border cell cluster to move forward (Bianco et al., 2007). In particular, Rac activity was shown to be asymmetric in border cell clusters with highest levels in cells at the front, a pattern that was lost upon elimination of RTK signaling (Cai et al., 2014; Wang, He, Wu, Hahn, & Montell, 2010). This pattern was accompanied by a difference in the protrusive behavior of the front and the rear cells such that the protrusion number and speed was higher in the front, whereas loss of Rac activity or RTK signaling abolished this asymmetric feature. Border cells communicate with each other to generate and maintain this polarized behavior and DE-cad is an essential element of this communication (Cai et al., 2014; Wang et al., 2010). Cells of the border cell cluster are held together by increased levels of E-cad on the inner cell membranes within the cluster (Niewiadomska, Godt, & Tepass, 1999), which resembles the increased E-cad levels within the photoreceptor cluster during the OR process (Mirkovic & Mlodzik, 2006). Knockdown of E-cad in border cells, or in nurse cells, causes migration to ectopic positions, although the border cells retain their cluster formation and some motility, suggesting that E-cad levels sustain directed motility (Niewiadomska et al., 1999), which is again very similar to E-cad requirements during OR (Mirkovic & Mlodzik, 2006). A detailed analysis of E-cad engagement in border cells revealed that E-cad tension levels also show an RTK signaling- and Rac-dependent gradient in the border cells, with higher tension in the front of the cluster and this gradient is required for polarized Rac activity and protrusive behavior in border cells (Cai et al., 2014), suggesting that E-cad acts in a positive feedback loop with Rac downstream of RTK signaling to reinforce asymmetric cell behavior in border cells during BCM (Cai et al., 2014).
How Fz/PCP cooperates with RTK signaling in border cell migration remains unclear. Notably, elimination of PCP signaling delays BCM but does not block it, unlike RTK signaling (Bastock & Strutt, 2007). Whether there are Wnt gradients in the oocyte at the stage of BCM has not been reported. However, considering the involvement of Rac and E-cad downstream of PCP signaling in Drosophila OR, the involvement of Fz/PCP signaling may provide additional input into these molecules to make BCM more efficient. In line with this notion, Fz/PCP signaling was shown to regulate the protrusive activity of border cells during migration, and removal of fz, Vang, or dsh activity in border cells leads to a loss of RhoA-mediated protrusions (Bastock & Strutt, 2007). Furthermore, knockdown of fz altered distribution of RhoA in border cells and suppressed “no-protrusion phenotypes” associated with RhoA overactivation. This suggests that Fz/PCP signaling affects the actin cytoskeleton by positively regulating RhoA activity (Bastock & Strutt, 2007). On the other hand, Rac-mediated asymmetric protrusive activity in border cells was lost upon inhibition of JNK signaling (Wang et al., 2010), which is often activated downstream of Fz/PCP signaling in the Drosophila eye (Boutros, Paricio, Strutt, & Mlodzik, 1998; Strutt et al., 1997), consistent with a cross-talk between Fz/PCP and RTK signaling pathways in the BCM context.
It is worth noting here that in both these cell migration contexts in Drosophila, ommatidial rotation and border cell migration, the core Fz/PCP pathway and RTK signaling cooperate and largely act on the same downstream effectors, both regarding the cytoskeletal regulation as well as cell-adhesion mediated by cadherins, in particular, E-cad.
3. PCP regulated cell motility processes in vertebrates
3.1. Convergent extension cellular movements and the core PCP factors
Convergent Extension (abbreviated here as CE) is a key morphogenetic phenomenon that shapes the body plan during embryogenesis (Keller et al., 2000; Tada & Heisenberg, 2012; Yin, Ciruna, & Solnica-Krezel, 2009). CE was the first morphogenetic process in vertebrates linked to Wnt/Fz-PCP signaling. Cellular movements during CE are best characterized during vertebrate gastrulation, where mesendodermal cells move dorsally toward the midline of the gastrulating embryo and intercalate between their neighbors along the A/P-axis (Fig. 4A) (Keller et al., 2000; Tada & Heisenberg, 2012; Yin et al., 2009). These cells extend bipolar protrusions at medial and lateral ends that make stable contacts with neighboring cells and generate traction, which allows them to elongate and intercalate (Keller et al., 2000; Tada & Heisenberg, 2012; Yin et al., 2009). These coordinated cellular movements and rearrangements narrow the body along the medio-lateral axis and elongate it along the anterior-posterior (A/P) axis. Similarly, during neurulation, the neuroepithelium thickens apico-basally via CE rearrangements of neural plate cells, with apical wedging elevating the neural folds which then fuse dorsally and continue CE rearrangements toward the midline to close the neural tube (Fig. 4B) (Keller et al., 1992, 2000; Nikolopoulou et al., 2017; Tada & Heisenberg, 2012). Notably, cells of the neuroepithelium show monopolar protrusive activity with lamellipodia orienting medially, which might suggest that there are differences in the mechanisms regulating CE during gastrulation and neurulation.
Fig. 4.
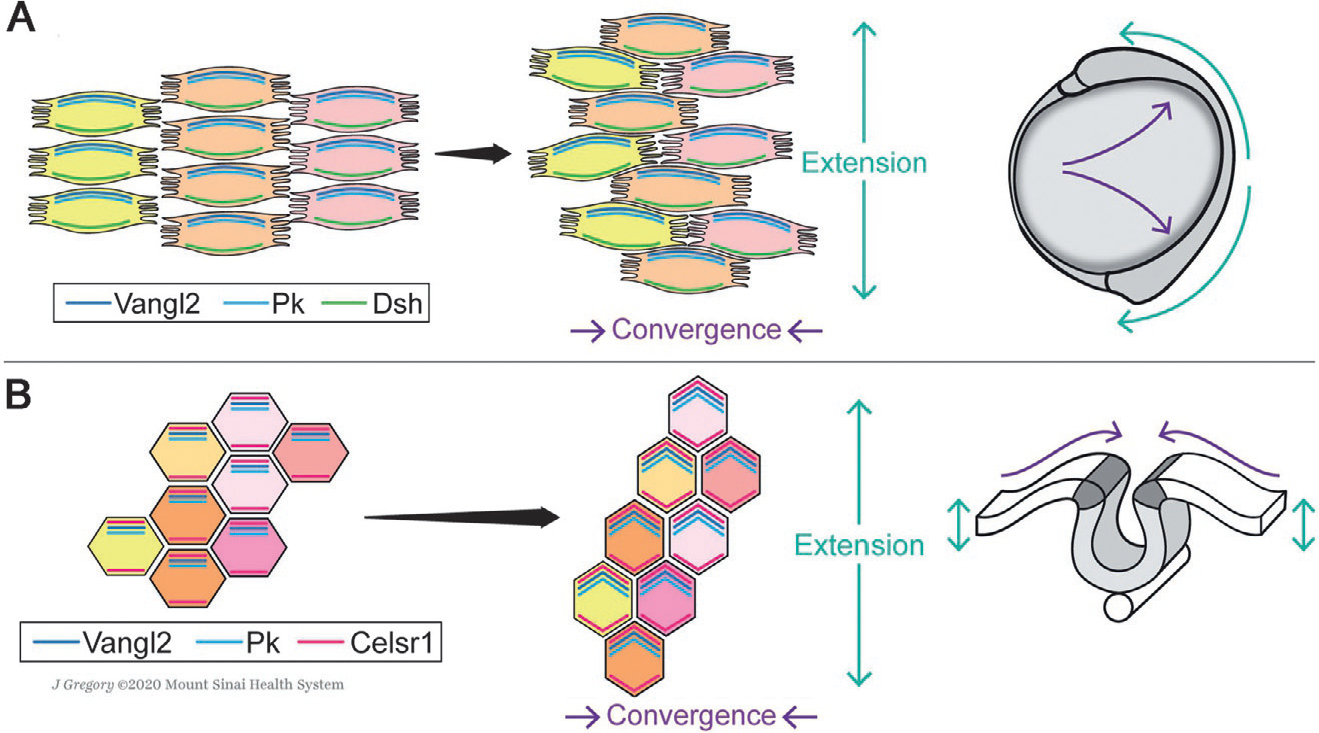
Schematic of planar cell polarity core factor localization during CE movements of gastrulation and neurulation. (A) During vertebrate gastrulation, mesendodermal cells move dorsally toward the midline of the embryo and intercalate between their neighbors along the A/P-axis (Keller et al., 2000; Tada & Heisenberg, 2012; Yin et al., 2009), narrowing the body along the medio-lateral (M/L) axis and elongate it along the anterior-posterior (A/P) axis. Bipolar protrusions emanating from mesendodermal cells at medial and lateral edges facilitate this process by allowing for stable contacts with the neighboring cells and generating traction (Keller et al., 2000; Tada & Heisenberg, 2012; Yin et al., 2009). The core PCP factors localize asymmetrically in the converging cells, as shown here schematically for Vangl2/Pk (note that there are multiple Pk factors) and Dsh (Dvl in mammals), and mediate their protrusive behavior (Roszko et al., 2015; Yin et al., 2008). (B) During neurulation, the neural folds that emerge from apical thickening of the neuroepithelium unite dorsally and close the neural tube via CE rearrangements toward the midline (Keller et al., 2000; Keller, Shih, & Sater, 1992; Nikolopoulou, Galea, Rolo, Greene, & Copp, 2017; Tada & Heisenberg, 2012). In this context, cell-cell intercalations were reported to be driven by polarized actomyosin contractions within the neuroepithelium, regulated by asymmetric core PCP factor localization. Vangl2, Pk and Celsr1/Fmi bridges the cells in the A/P axis, signaling in the apical domain (Butler & Wallingford, 2018; Ciruna et al., 2006; McGreevy, Vijayraghavan, Davidson, & Hildebrand, 2015; Nishimura et al., 2012; Ossipova, Kim, & Sokol, 2015). Anterior is up in all images. See main text for details.
Around the time the Wnt-Fz/PCP pathway was defined as a signaling pathway in Drosophila (Boutros et al., 1998; Strutt et al., 1997), several groups reported that an equivalent non-canonical Wnt-dependent Fz/PCP signaling system is critical for CE movements in vertebrates. The first implications of non-canonical Wnt-signaling in gastrulation movements came from studies in Xenopus frogs where perturbations in Wnt5a, Fz8 and Dvl2 disrupted gastrulation processes and axis elongation without causing phenotypes associated with disrupting Wnt/β-catenin (canonical) signaling, and subsequent studies confirmed this during CE in neurulation (Deardorff, Tan, Conrad, & Klein, 1998; Moon et al., 1993; Sokol, 1996). In Xenopus, interference with Wnt11 function caused CE defects marked by shortening of the A/P axis with normal cell specification; and Wnt11-dependent inhibition of elongation in animal pole explants was rescued by expression of a truncated form of Dsh impaired in canonical Wnt-signaling capability (Tada & Smith, 2000). Importantly, a non-canonical Wnt-PCP signaling requirement in CE was confirmed genetically, when zebrafish screens identified wnt11/silberblick mutants, which caused a shortening of axial mesoderm and central nervous system (CNS) and their broadening due to the impairment of convergence and cell intercalations, which was again rescued by expression of a Dsh construct that lacked canonical signaling activity (Heisenberg et al., 2000). It was later shown that in Xenopus embryos, targeted (over)expression of mutant Xdsh that disrupted Wnt-PCP signaling specifically caused a lack of elongation of the A/P-axis and neural epithelium, and a failure in neural tube closure (Wallingford & Harland, 2002). Consistently, targeted overexpression of Wnt5a and XFz-8 phenocopied Xdsh overexpression phenotypes, confirming that a Wnt-Fz/PCP-pathway, related to the Fz/PCP signaling casette in Drosophila, is at work regulating CE in vertebrates (Wallingford & Harland, 2001, 2002).
Genetic screens in zebrafish and subsequent functional studies on core PCP gene homologs in Xenopus and zebrafish confirmed and refined the involvement of a conserved Wnt-Fz/PCP signaling cassette in vertebrate CE movements assembling the vertebrate PCP factors into largely the same molecular pathway as in Drosophila (e.g., reviewed in Montero & Heisenberg, 2004; Roszko, Sawada, & Solnica-Krezel, 2009; Tada, Concha, & Heisenberg, 2002; Veeman, Axelrod, & Moon, 2003; Wallingford, Fraser, & Harland, 2002). For example, both gain-of-function and LOF of Vangl2/Stbm, Fz7, and Pk in Xenopus and zebrafish severely perturbed CE processes often marked by shortening of the notochord and neural plate, without affecting cell fate specification (Carreira-Barbosa et al., 2003; Ciruna et al., 2006; Darken et al., 2002; Djiane, Riou, Umbhauer, Boucaut, & Shi, 2000; Goto & Keller, 2002; Takeuchi et al., 2003; Veeman, Slusarski, Kaykas, Louie, & Moon, 2003; Yin et al., 2008). In zebrafish, shortening of the A/P-axis in trilobite (tri: the zebrafish Vangl2 gene) mutants was shown to be caused by the inability of cells to elongate in the mediolateral axis, which resulted in the impairment of dorsal cell migration and subsequent CE-type intercalation ( Jessen et al., 2002; Roszko et al., 2015). In this context, Tri/Vangl2 is required in both the elongating cells and the neighboring tissue, suggesting a cell autonomous requirement with a non-cell autonomous effect, similar to its function in Drosophila ( Jessen et al., 2002). In Xenopus, Stbm/Vangl-associated neural tube closure defects was also reported to be due to lack of neural CE and cell intercalation movements (Park & Moon, 2002). Here, engrafted single cells mutant for Stbm/Vangl function were able to intercalate between their wild type neighbors, supporting a non-cell autonomous requirement during CE. Besides the highly conserved core PCP genes, several additional regulators of Wnt-Fz/PCP-signaling have also been identified in vertebrates based on their reported CE phenotypes (for other CE focused reviews see (Butler & Wallingford, 2017; Huebner & Wallingford, 2018; Keller et al., 2000; Montero & Heisenberg, 2004; Roszko et al., 2009; Tada & Heisenberg, 2012; Veeman, Axelrod, & Moon, 2003; Wallingford, 2012). Taken together, these studies are consistent with Wnt-Fz/PCP signaling acting critically in the context of CE processes, both during gastrulation and neurulation, to provide cells with directional information that guides their migration and intercalation behavior. However, it should be noted that interactions among the PCP genes are more complicated than in Drosophila, as there are several gene family members for each Drosophila gene, and additional vertebrate specific PCP regulators exist.
3.2. Downstream effectors of PCP in CE movements
As in Drosophila eye PCP establishment (Boutros et al., 1998; Strutt et al., 1997), the JNK pathway is employed downstream of Fz/PCP signaling during CE processes. Hyperactivation or depletion of JNK interferes with gastrulation movements. For example, Wnt5a can activate JNK in cultured cells and CE phenotype induced by Wnt5a overexpression in Xenopus can be rescued by expression of a dominant negative JNK (Yamanaka et al., 2002). Similarly, Wnt11/Fz7 signaling can activate JNK during Xenopus gastrulation through Rac GTPase activity (Habas, Dawid, & He, 2003; Habas, Kato, & He, 2001). Although Vangl/Stbm and Pk can also activate JNK in Xenopus and synergize with Dsh to elevate JNK activity, the JNK activation read-out here might be more complicated (Habas et al., 2001, 2003). However, it remains unclear how JNK activation regulates CE processes. Although nuclear responses are likely involved via JNK activation, its upstream activators of the Rho family of GTPases (acting as effectors of Fz-Dsh signaling (Boutros et al., 1998; Strutt et al., 1997)) appear as global regulators of CE movements and, in addition to their nuclear effects via JNK, they regulate multiple other cellular processes (Habas et al., 2001, 2003) as discussed below.
Coordination of multiple signaling inputs and cellular processes is essential during CE regulation, including protrusive activity, actomyosin contractility, cell-cell adhesion, and cell-matrix interactions (Butler & Wallingford, 2017; Huebner & Wallingford, 2018; Keller et al., 2000; Montero & Heisenberg, 2004; Roszko et al., 2009; Skoglund & Keller, 2010; Tada & Heisenberg, 2012; Veeman, Axelrod, & Moon, 2003; Wallingford, 2012). Besides the conserved requirement of Rho-family GTPases and JNK activation downstream of the Wnt-Fz/PCP pathway, FGF signaling has also been linked to CE defects (Nutt, Dingwell, Holt, & Amaya, 2001). Thus, a scenario of Fz/PCP and RTK signaling cooperation, similar to what has been observed in Drosophila ommatidial rotation and border cell migration, is likely in play during CE regulation.
3.2.1. Cytoskeletal rearrangements
As in Drosophila OR and border cell migration, Wnt-Fz/PCP signaling mediates cytoskeletal rearrangements during CE processes. A key aspect of CE regulation is polarized protrusive cellular activity, which has often been linked to PCP signaling (Butler & Wallingford, 2017; Huebner & Wallingford, 2018; Keller et al., 2000; Montero & Heisenberg, 2004; Roszko et al., 2009; Tada & Heisenberg, 2012; Veeman, Axelrod, & Moon, 2003; Wallingford, 2012). It was initially discovered that expression of Dsh isoforms that interfere with Wnt-Fz/PCP signaling causes alterations in the protrusive behavior of cells (Wallingford et al., 2000). Lamellipodia that extend from mediolateral ends of cells during CE fail to do so upon expression of PCP-interfering Dsh isoforms. Such loss of polarity was accompanied by a decrease in length to width ratio of the respective cells and a loss of alignment in the mediolateral axis during CE (Wallingford et al., 2000). Many studies later confirmed protrusive activity as an important effector read-out of PCP signaling that enables cells to elongate, migrate, and intercalate while being tightly linked to multiple other local cellular processes that need to be coordinated during CE movements, as discussed below.
An elegant study using the chick neural tube linked Fz/PCP signaling to polarized actomyosin activity during CE (Nishimura et al., 2012). During neural plate bending, active myosin and F-actin were enriched at mediolateral edges of neural-plate cells and time-lapse images revealed that contractions of these cells occur in mediolateral directions, which helps them intercalate and elongate (Nishimura et al., 2012). This cellular behavior was dependent on Rho kinase (ROCK) activation and lost upon ROCK inhibition. Importantly, Celsr1/Fmi was enriched at mediolateral edges of these cells along with PDZ-RhoGEF, and upon knockdown of Celsr1/Fmi or expression of a dominant negative Dsh, the polarized pattern of PDZ-RhoGEF and actomyosin cables was lost. PDZ-RhoGEF physically interacts with Dsh via the adapter protein Daam1 (Habas et al., 2001, 2003), suggesting that the Rho regulator may be recruited to mediolateral edges through Dsh (Nishimura et al., 2012). Consistently, during Xenopus neural tube closure, PCP signaling was reported to be required for polarized actomyosin contractility (Butler & Wallingford, 2018). In this context, Vangl2 and Pk2 localization displayed a significant bias to anterior cell junctions, while being dynamically enriched in shrinking junctions in temporal correlation with actomyosin contractions (Butler & Wallingford, 2018; Ossipova et al., 2015). Taken together, Fz/PCP signaling provides input into the regulators of cytoskeletal dynamics, namely actin machinery and actomyosin network, to confer polarized protrusive and contractile activity required to drive convergence and extension (Fig. 4).
3.2.2. Regulation of junctional adhesion
Intercellular interactions through cell adhesion molecules are crucial for CE movements and they appear to act in the context of Fz/PCP signaling. Classical cadherins in vertebrates, C-cad, N-cad and E-cad, are all highly expressed in mesoderm cells during gastrula stages. Overexpression of wild type or dominant-negative C-cad was shown to perturb gastrulation movements in Xenopus. Remarkably, the adhesive activity of C-cad decreased during activin-induced elongation of animal cap explants, whereas co-treatment with C-cad activating antibodies inhibited this elongation (Zhong, Brieher, & Gumbiner, 1999). Accordingly, cdh1 zygotic mutant zebrafish embryos exhibit mild defects in convergence and extension with wider somites at the segmentation stage (Shimizu et al., 2005). N-cad LOF in zebrafish impairs the extension of posterior axial mesodermal cells, thereby shortening the A/P axis (Harrington, Hong, Fasanmi, & Brewster, 2007). N-cad was further shown to be involved in neural CE processes in zebrafish: in N-cad-depleted embryos, lateral/dorsal neural keel cells failed to form stable protrusions, elongate and radially intercalate (Hong & Brewster, 2006). In addition to classical cadherins, paraxial protocadherin was shown to have role in vertebrate gastrulation. Overexpression of XPAPC or its depletion by morpholinos in Keller explants inhibited constriction but not elongation in the involuting marginal zone (IMZ) (Unterseher et al., 2004). Although XPAPC morpholinos do not severely affect the general morphology of Xenopus embryos, a closer examination with mesodermal markers revealed that the axial and paraxial tissue was broadened due to its lack of constriction. Consistently, XPAPC depletion randomized the polarization axis of mesodermal cells in explants and slowed down their dorsal migration. Nevertheless, PAPC could have a signaling activity in this context, as JNK activation was decreased upon XPAPC depletion and this was rescued by expression of a constitutively active RhoA, which was also deactivated upon PAPC depletion (Unterseher et al., 2004). Cell adhesion molecules have often been linked to the Fz/PCP pathway. Wnt5a/Fz7/Ror2 signaling, morpholinos of which phenocopy the PAPC like CE defects, was shown to regulate PAPC expression in a JNK-dependent manner (Schambony & Wedlich, 2007; Unterseher et al., 2004). Wnt11/Fz7 signaling, on the other hand, stabilizes PAPC on the membrane by blocking its clathrin- and dynamin-1-mediated internalization (Kraft, Berger, Wallkamm, Steinbeisser, & Wedlich, 2012). Strikingly, Fz7 binds C-cad and PAPC on the membrane, where PAPC abundance inhibits C-cad clustering, pointing to an interplay between different adhesion molecules (Kraft et al., 2012). Consistently, during Xenopus CE movements, C-cad clustering, which is essential for successful CE movements, was further shown to be dependent on the Fz/PCP pathway (Huebner et al., 2021).
During gastrulation in zebrafish, there are many modes of cell movements that contribute to convergence and extension of tissue and elongation of the A/P axis in each of the germ layers (see Williams & Solnica-Krezel, 2020, for a comprehensive review). The genetic and imaging tools available in zebrafish have allowed for a greater understanding of the localization and contribution of PCP components during these cell rearrangement processes. During gastrulation, cells in the endoderm undergo CE movements, similarly to the mesodermal cells as described above. Recent high resolution imaging studies of endodermal CE have shown that components of the Wnt-Fz/PCP pathway (including gpc4/knypek, a heparan-sulfate proteoglycan that influences non-canonical Wnt signaling), are required for polarization of cell shape and behavior (Balaraju, Hu, Rodriguez, Murry, & Lin, 2021). GFP-Vangl2 is localized to the anterior of these cells, which intercalate in a mediolateral direction. In addition to the polarized protrusions discussed above, junctional remodeling is also required for directed migration. The level of adhesive contacts during CE must be tightly balanced to maintain tissue integrity and yet allow cells to change position within the tissue to alter tissue dimensions. gpc4-mutant embryos show elevated levels of Cdh2 (N-cadherin) at the membrane, which through increased adhesive contacts counteracts the remodeling of adhesive junctions as cells try to exchange neighbors during intercalation. Gpc4 was shown to regulate the Rab5c-dependent endocytosis of Cdh2 to control the level of Cdh2 at the membrane, and thus neighbor adhesion.
The link between Wnt-Fz/PCP and Rab5c-dependent cadherin trafficking is strengthened by experiments investigating the directed migration of prechordal plate progenitors toward the animal pole following cellular internalization (Ulrich et al., 2005) (see also review Williams & Solnica-Krezel, 2020, for comprehensive outline of this process). Wnt11/Slb is required to coordinate prechordal plate progenitor movement toward the animal pole. In wnt11/slb mutant mesendodermal cells, cohesion between cells is reduced and this is accompanied by changes in E-cad membrane localization. Through genetic interactions and biophysical methods, it was determined that Wnt11 acts via Rab-5c- and dynamin1-dependent endocytosis of E-cad to regulate mesendodermal cell adhesion.
At earlier stages of development, during blastoderm spreading, termed “doming,” Wnt-Fz/PCP signaling is also required to spatially regulate adhesion to increase cell movement (Petridou, Grigolon, Salbreux, Hannezo, & Heisenberg, 2019). During doming, the cells in the blastoderm begin to spread over the yolk, and this cohesive movement of cells represents a decrease in tissue viscosity. Prior to doming, cells are tightly packed together and resemble a solid, whereas at the onset of doming cell-cell adhesion is reduced and cells are able to flow around the yolk, akin to the movement of molecules in a fluid. This is termed tissue fluidization. E-cad contacts are destabilized, allowing for increased neighbor exchange and cell movements, and these processes are reduced in wnt11/slb mutants (Petridou et al., 2019). Interestingly, a similar process of tissue fluidization is evident during posterior elongation, in which mesodermal precursors migrate and progressively differentiate into mature mesodermal cells that are then incorporated into the presomitic mesoderm (Mongera et al., 2018). This process is also dependent upon N-cad regulation, and it will be interesting to see whether this regulation is also dependent upon the Wnt/PCP-regulated endocytosis.
A link between convergent-extension movements and cadherin trafficking is also conserved in Drosophila polarized cell rearrangements in the early embryo. A/P axis elongation occurs during a process known as germband extension (reviewed in Pare & Zallen, 2020). This process involves polarized cellular intercalations that are impacted by multiple signals, with receptors of the Toll superfamily being the predominant drivers of this process during Drosophila germband extension. Interestingly, the core PCP factors are not required in the Drosophila embryo for such intercalations; it is likely that the fast speed of the cell intercalation process and the abundance of maternal product of some of the core PCP factors contributes to their lack of involvement. Imaging and biophysical methods of a related process, tracheal tube morphogenesis during later Drosophila embryogenesis, determined that at junctions along the A/P borders of cells, PCP components are enriched and, through recruitment of RhoGEF2, there is a corresponding decrease in E-cadherin levels, which would allow for polarized junctional remodeling (Warrington, Strutt, & Strutt, 2013). Here also E-cadherin levels are controlled by polarized regulation of clathrin- and dynamin-dependent endocytosis (Levayer & Lecuit, 2012). This relationship extends to intercalations in the pupal wing (Classen, Anderson, Marois, & Eaton, 2005; Warrington et al., 2013). During pupal wing development, cell packing increases through junctional remodeling to produce a hexagonal array of cells (depicted in Fig. 1). PCP proteins are required for this process and regulate Rab11- and dynamin-dependent E-cad recycling (Classen et al., 2005). Fmi was also shown to localize E-cad-containing exocyst vesicles, further supporting a role for PCP complexes in regulating E-cad junctional dynamics to allow for neighbor exchange and tissue remodeling (Classen et al., 2005).
Together these data highlight both the importance and complexity of differential regulation of adhesion molecules during CE processes and the requirement of Wnt-Fz/PCP signaling in regulating complex adhesive behavior. PCP-dependent fine-tuning of adhesive behavior is likely to share common regulators between Drosophila and vertebrates. For example, a vertebrate homolog of Drosophila Nmo, Nlk1, was shown to affect Wnt11 signaling and CE movements during Xenopus gastrulation (Thorpe & Moon, 2004). Moreover, Nlk1 and PAPC genetically and physically interact, and reciprocally stabilize each other during Wnt11 signaling and Xenopus CE processes (Kumar, Ciprianidis, Theiss, Steinbeisser, & Kaufmann, 2017). Despite the existence of Nmo, as a conserved downstream Wnt-Fz/PCP effector, the regulation of adhesive behavior by the core PCP factors is likely to be more complex in vertebrates than in Drosophila.
3.2.3. ECM remodeling and signaling
Wnt-Fz/PCP signaling has also been linked to the remodeling of ECM during CE processes in vertebrates, possibly a similar manner to Drosophila ommatidial rotation (Thuveson et al., 2019). Cell-matrix interactions through integrin and its ligand fibronectin (FN) are critical for CE gastrulation processes. Xenopus embryos that lack FN fibrils showed a shorter and broader A/P axis (Marsden & DeSimone, 2003). For example, neural and mesodermal cells of Keller explants injected with FN morpholinos or anti-integrin function-blocking antibodies failed to polarize their protrusions, elongate, and intercalate, whereas this behavior was rescued when the explants were cultured on fibronectin (Davidson, Marsden, Keller, & Desimone, 2006). Fibronectin organization has been linked to PCP signaling. Essentially, the assembly of fibronectin fibrils starts at the onset of gastrulation and is observed only along the surface of the mesoderm. This restricted or “polarized” pattern is critical for proper CE movements. Overexpression of Vangl2/Stbm, Pk or Fz in Xenopus embryos disrupts this localized assembly of fibronectin fibrils along the surface of the mesoderm (Goto, Davidson, Asashima, & Keller, 2005). PCP signaling appears to have a dual role in this context: While it provides the cues to polarize the ECM, it is further required for ECM-dependent polarization of protrusions, as evident by experiments when culturing Xenopus notochord explants on fibronectin was not sufficient to rescue the protrusion, elongation, and intercalation defects caused by the PCP factor overexpression (Goto et al., 2005). Similarly to these results, fibrillar FN matrix failed to form on the surface of the dorsal marginal zone (DMZ) upon dominant negative Wnt11 expression in animal caps and this effect was rescued by expression of a Dsh isoform that only activates Wnt/PCP signaling, or by activation of Rho and Rac GTPases (Dzamba, Jakab, Marsden, Schwartz, & DeSimone, 2009). As these rescue experiments required integrin activity, these data implicated Wnt-Fz/PCP signaling in the (re)organization of ECM through integrin signaling. However, core PCP factors might affect fibronectin organization through various mechanisms. For example, loss of Vangl2 and Pk1a function in zebrafish causes a reduction in fibronectin levels as a result of increased matrix metalloproteinase activity during gastrulation, and here Vangl2 was shown to promote MMP endocytosis in vitro by antagonizing focal adhesion kinase (FAK) activity (Williams et al., 2012). Conversely, glypican4 and fz7a/7b zebrafish mutants have increased fibronectin assembly albeit normal matrix metalloproteinase activity (Dohn, Mundell, Sawyer, Dunlap, & Jessen, 2013), and surprisingly this effect was due to high accumulation of N-cad on cell membranes, as it was rescued by N-cad knockdown.
Taken together, these studies all emphasize the significance of Wnt-Fz/PCP signaling in regulating cellular behavior at multiple layers during mesendodermal and neural CE processes, ranging from polarized cytoskeletal protrusions to cell-cell adhesion and to the communication between cells and the ECM (Butler & Wallingford, 2017; Huebner & Wallingford, 2018; Keller et al., 2000; Montero & Heisenberg, 2004; Roszko et al., 2009; Skoglund & Keller, 2010; Tada et al., 2002; Tada & Heisenberg, 2012; Veeman, Axelrod, & Moon, 2003; Wallingford, 2012). A handful of pathways have been shown to be critical in CE processes that likely co-operate with Wnt-Fz/PCP signaling. For example, similarly to ommatidial rotation and border cell migration in Drosophila, RTK signaling pathways appear important contributors to CE regulation. A negative regulator of FGF signaling in Xenopus, XSprouty or XSpry, causes the shortening of the A/P-axis by impairing the CE processes when overexpressed (Nutt et al., 2001). Unlike dominant negative FGFR-expressing embryos, which show severe developmental defects, mesoderm induction was normal in XSpry overexpressing embryos. Xspry overexpression halted FGF-dependent Ca2 + efflux but did not affect MAPK activity in oocytes, suggesting that a Ras/MAPK-independent pathway downstream of FGF signaling may modulate CE movements, reminiscent of the Ras/MAPK-independent involvement of EGFR signaling during ommatidial rotation (Gaengel & Mlodzik, 2003). Moreover, as XSpry was shown to inhibit Fz/PCP signaling, by decreasing Dsh recruitment to the membrane in embryos, crosstalk between the FGF and PCP signaling pathways is likely (Wang et al., 2008). As XSpry also genetically and physically interacts with PAPC during Xenopus gastrulation (Wang et al., 2008), it may be feeding into multiple effector pathways regulating CE processes. In summary, taking data from Drosophila PCP processes and vertebrate gastrulation together, Wnt-Fz/PCP and RTK signaling are likely to co-operate in many such contexts, orchestrating the local cellular readouts necessary for cell motility and intercalation throughout animal development. Although there are significant differences between mammalian embryogenesis, which occurs in utero, and the examples outlined above, Wnt-Fz/PCP signaling is still required for directed cell movements during such processes (Williams et al., 2014) including neural tube closure (reviewed in Wang, Marco, Capra, & Kibar, 2019) and cardiac outflow tract morphogenesis (Sinha, Wang, Evans, Wynshaw-Boris, & Wang, 2012).
3.3. Facial branchiomotor neuron migration
An interesting migratory process is tangential migration of facial branchiomotor neurons (FBMNs) in the vertebrate hindbrain, which has been shown to require Wnt-Fz/PCP signaling (Fig. 5). FBMNs are a group of cranial branchiomotor neurons that are born in rhombomere 4 (r4) and the cell bodies undergo a posterior migration to r6 and r7, where they form the facial motor nucleus whilst their axons remain in r4 and then exit to innervate those muscles derived from the second branchial arch (Chandrasekhar, 2004).
Fig. 5.
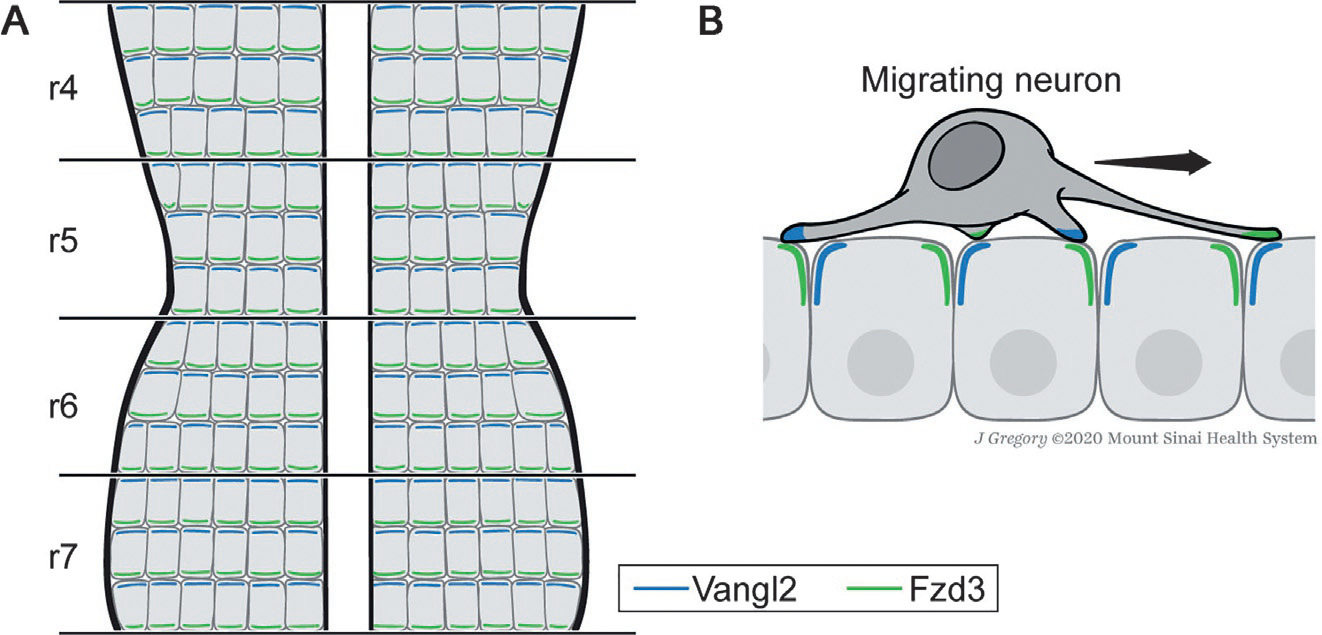
Model of PCP-mediated FBMN migration in the vertebrate hindbrain. The vertebrate hindbrain is segmented into developmental units called rhombomeres during embryonic development. FBMNs are born in rhombomere 4 (r4) and tangentially migrate to the more posterior r7 (Chandrasekhar, 2004). In zebrafish, rhombomeres have been shown to be planar polarized with Fzd3 and Vangl2 being asymmetrically enriched in anterior and posterior (sub)apical membranes, respectively (A) (Davey, Mathewson, & Moens, 2016). Migrating FBMNs enrich filopodial protrusions over the neuroepithelium in the direction of migration. Vangl2 becomes transiently enriched at the tips of filopodia in FBMNs preceding retraction, suggesting that transient PCP-mediated signaling events between FBMNs and the polarized neuroepithelium may promote FBMN migration (B) (Davey et al., 2016). Anterior is up. See text for details.
Forward genetic screens in zebrafish revealed that core PCP components are required for FBMN migration. LOF alleles of tri/Vangl2, Pk1a/Pk1b, Fz3a, Celsr2 and scribble1 in zebrafish cause an impairment in caudal migration of FBMNs (Bingham, Higashijima, Okamoto, & Chandrasekhar, 2002; Carreira-Barbosa et al., 2003; Jessen et al., 2002; Rohrschneider, Elsen, & Prince, 2007; Wada et al., 2005; Wada, Tanaka, Nakayama, Iwasaki, & Okamoto, 2006). Chimeric analyses showed that the transmembrane core PCP factors Vangl2, Fzd3a and Celsr2 have a cell autonomous role in the migrating neurons as well as a non-autonomous role in the cellular environment of the migration process (Davey et al., 2016; Jessen et al., 2002; Wada et al., 2006), whereas the cytoplasmic Pk1b has a mostly cell autonomous involvement (Rohrschneider et al., 2007). Consistently, FBMNs that express a truncated form of Dvl that is dominant negative for the non-canonical pathway largely fail to migrate in a wild-type environment and expression of an equivalent Dvl construct in the migratory environment blocks the migration of wild type FBMNs (Davey et al., 2016). These data suggest that the communication between FBMNs and the migratory environment through core Fz/PCP signaling drives the migration of FBMNs. Nevertheless, in this context, interference with the established PCP Wnt-ligands, silberblick/Wnt11, pipetail/Wnt5a and the glypican knypek, does not impair FBMN migration in zebrafish (although they are critical for CE movements, see above) (Bingham et al., 2002; Jessen et al., 2002). These data suggest that there might be different extracellular upstream regulators of PCP-dependent FBMN migration in zebrafish, as compared to CE processes, or that there is functional redundancy among the zebrafish Wnts in this context. Nonetheless, the core Fz/PCP-dependence in FBMN migration is conserved in vertebrates, and in mice Wnt involvement has been suggested (Glasco et al., 2016; Qu et al., 2010; Vivancos et al., 2009). Further research in vertebrates will be required to better understand the exact nature of the Fz/PCP signaling involvement during this process.
During migration, FBMNs generate filopodial protrusions which have often been associated with neuronal cell migration and this protrusive activity appears to be regulated by Fz/PCP signaling (Fig. 5). As FBMNs migrate posteriorly through r5 and r6, they enrich their filopodia in the direction of migration (Davey et al., 2016). Strikingly, FBMNs fail to polarize their protrusive activity in tri/vangl2 mutants. Chimeric analyses showed that Vangl2 and Fzd3a have opposing cell autonomous and non-autonomous functions in regulating protrusive activity. In FBMNs, Vangl2 destabilizes filopodia whereas Fzd3a stabilizes them. Conversely, in the migratory environment, Vangl2 acts to stabilize FBMN protrusions while Fzd3a has a destabilizing role (Davey et al., 2016). These findings are reminiscent of the antagonism between Fz and Vang in regulating actin polymerization during hair formation in the Drosophila wing epithelium (Adler, 2012; Klein & Mlodzik, 2005). Strikingly, Vangl2 is transiently enriched at the tips of filopodia in FBMNs preceding retraction, and the migratory environment or the “substrate” on which the neurons migrate display polarized localization of Vangl2 and Fzd3a to opposite sides of each cell, along the A/P axis, suggesting that transient interactions between FBMNs and the “substrate” neuroepithelium through these two core PCP factors (and associated signaling) may drive the migration of FBMNs (Fig. 5) (Davey et al., 2016). It has been proposed that cell-autonomous functions of Fzd3a and Vangl2 are enabled in filopodia, as they contact polarized Vangl2 and Fzd3a domains of neuroepithelial cells respectively to promote migration. In this context, the cell-autonomous activities of the Fz/PCP factors are likely to involve not only cytoskeletal remodeling (as seen by the formation or retraction of filopodia), but also cellular adhesion (Davey et al., 2016). Additional studies will be required to elucidate potential other interactors of PCP signaling including cell adhesion factors to promote FBMN migration.
3.4. Wnt/Fz-PCP signaling regulated axonal pathfinding
During nervous system development and patterning, axonal pathfinding can be considered a specialized cell movement process, through which the growth cone of the axon moves along and across tissues and guideposts to reach its target location. While the cell body stays “local,” the growth cone “migrates” through tissues, guided by activating and inhibitory cues. Wnt signals have been identified as a set of such conserved growth cone guiding cues (reviewed in Dickson, 2005; Zou, 2006). In this context, Wnt/Fz-PCP signaling has been established as a major player, with Wnts working as guidance cues and the core Fz/PCP module mediating the cellular responses to these cues to direct the growth cone (reviewed in Goodrich, 2008; Zou, 2020). In particular, graded expression of Wnt molecules along the A/P axis of the developing central nervous system guides axons along this axis (reviewed in Hollis 2nd & Zou, 2012) and this feature of Wnt molecules is evolutionarily conserved. Wnt family members have been initially demonstrated to affect axonal pathfinding along the A/P axis in Drosophila (Yoshikawa, McKinnon, Kokel, & Thomas, 2003), C. elegans (Hilliard & Bargmann, 2006; Pan et al., 2006), and mouse (Lyuksyutova et al., 2003) and subsequently shown to do so in other vertebrates as well (reviewed in Zou, 2020). Moreover, injury to the vertebrate spinal cord has been shown to cause reactivation of expression of several Wnt members that are thought to regulate the associated de novo growth cone guidance and migration (reviewed in Hollis 2nd & Zou, 2012).
The impact of Wnts on axonal pathfinding in several parts of the nervous system has been firmly linked to the core Fz/PCP module both in Drosophila (Gombos et al., 2015; Mrkusich, Flanagan, & Whitington, 2011; Shimizu, Sato, & Tabata, 2011; Yuan et al., 2016) and in vertebrates (Fenstermaker et al., 2010; Hua, Smallwood, & Nathans, 2013; Onishi et al., 2013; Shafer, Onishi, Lo, Colakoglu, & Zou, 2011). The mechanistic understanding of how Wnts regulate growth cone extension and guidance via the Wnt/Fz-PCP pathway is, however, less well developed. Studies in mouse suggested that within the growth cone, there are separated domains of either Vangl2 or Fzd3 signaling units, and the protrusive behavior depends on complex interactions between these factors and the Dvl isoforms, which control Fzd3 internalization and the activation of the JNK signaling (Fig. 6A) (reviewed in Onishi, Hollis, & Zou, 2014; Zou, 2020). During growth cone migration, the core PCP factors are thought to be cooperating mainly with N-cad based adhesion, as compared to major E-cad involvement in many of the processes discussed above. As growth cone based “migration” shares the same principles with other migratory processes, regarding the involvement of adhesion junctions and polarity proteins, both with respect to PCP as well as apical-basal polarity factors, the growth cone has been coined a “half adherens junction” (reviewed in Zou, 2020).
Fig. 6.
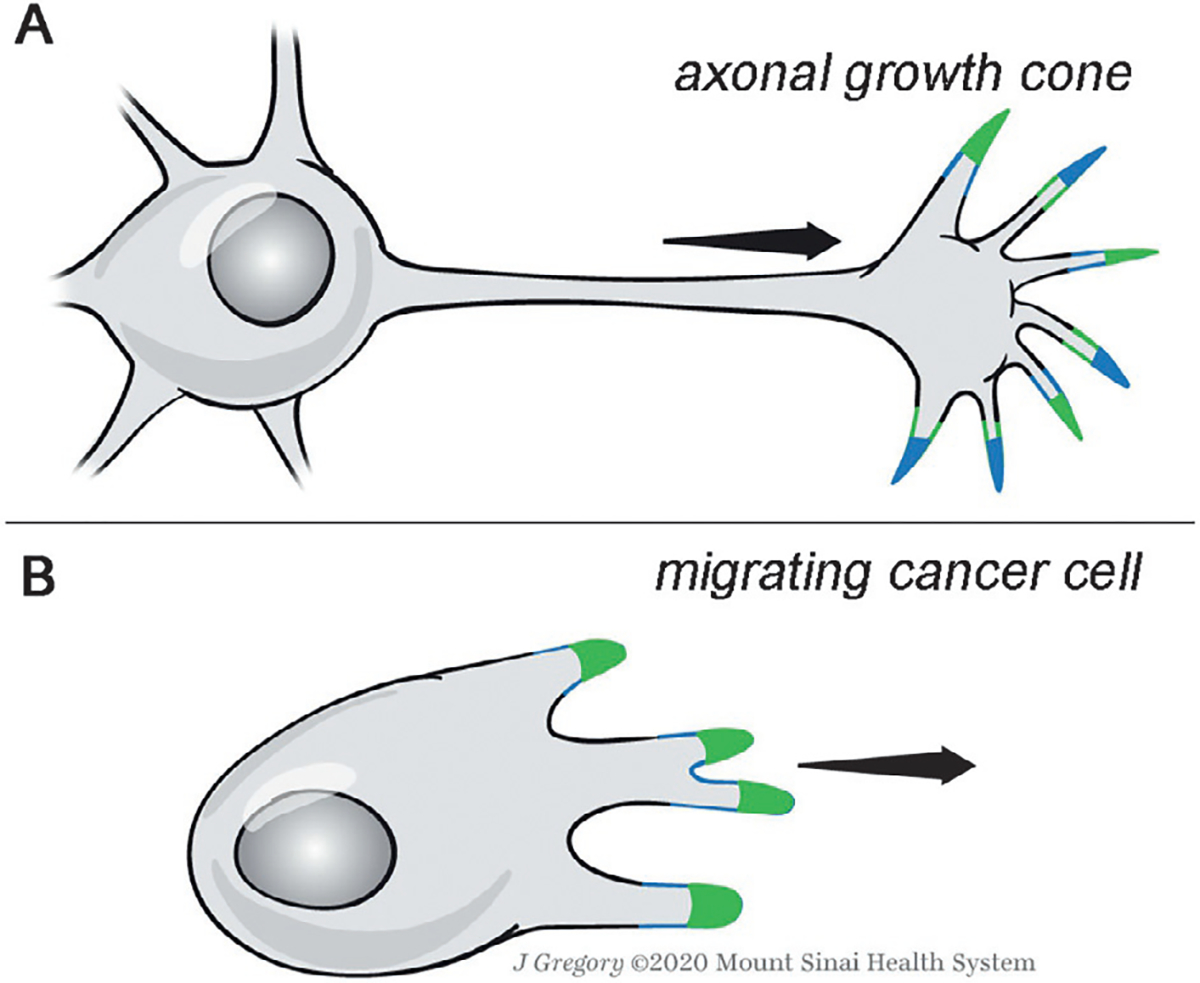
Schematics of axonal growth cone migration (A) and cancer cell migration (B) as mediated by core PCP factors. (A) Model for the growth cone migration of commissural axons as guided by Wnt signaling via the core Fz/PCP factors. Vangl2 complexes (green) and Fzd3-Dvl complexes (blue) are subcellularly asymmetrically localized in the filopodial tips of the growth cone protrusions, mediating a regulated Fzd3/Dvl endocytosis and associated effects on actin protrusions. Note that in the absence of Wnts these core PCP complex localization domains become randomized and the formation/retraction of protrusions is also randomized, causing stalling of the axonal extension (Dos-Santos Carvalho et al., 2020). (B) Schematic of migrating cancer cell with localized core PCP complex domains that also lead to organized Fzd/Dvl endocytosis and protrusion formation. It is not yet clearly defined how Wnt signals influence cancer cell migration regarding paracrine and autocrine signaling in general. See main text for details and references.
While the mechanistic aspects of core PCP regulated growth cone guidance are very difficult to study in vivo in the mouse central nervous system, a model of action has recently received strong support through an elegant set of experiments with cochlear mouse neurons, linking Vangl2 complex function in axonal growth cones to N-cad dynamics and actin flow (Dos-Santos Carvalho et al., 2020). Here, the authors demonstrate that Vangl2 activity restricts neuronal outgrowth by controlling N-cad dynamics and increasing retrograde actin flow in spikes of the growth cone. Vangl2 is thought to positively regulate N-cad diffusion and mobility at the membrane, likely via affecting the mechanical coupling between N-cad and actin filaments. This type of regulatory interactions between the core PCP factors and cell adhesion machinery is again similar to the cadherin based regulations in the context of ommatidial rotation, border cell migration, and convergence extension movements (see chapters above). Interestingly, the effects of Vangl2 (or PCP complexes in general) on N-cad activity might be reciprocal as N-cad substrates also affect Vangl2 localization and distribution, and by extension also the core PCP complexes in general (Dos-Santos Carvalho et al., 2020).
Although not linked to neuronal and axonal cell motility directly, it is worth noting in this context that the core PCP complexes have also been linked to synapse formation and stability. For example, in the developing glutamatergic neurons of mice, Vangl2 and Celsr3/Fmi have been shown to have opposing functions, with Vangl2 inhibiting synapse formation (Thakar et al., 2017). Here the Celsr3/Fzd3 complexes sit on the bouton side of the synapse, while Celsr3/Vangl2 complexes are located on the spine. As the synapse formation heavily relies on regulated cell adhesive behavior, it again highlights a strong regulatory link between the core PCP complexes and cell adhesion regulation in general and in this specialized context.
The molecular scenario employed by the core PCP module during growth cone migration and guidance is likely complicated with (at least) the receptor tyrosine kinase (RTK) family member Ryk/Derailed also playing an important role as a Wnt receptor (Liu et al., 2005; Yoshikawa et al., 2003). Whether and how it influences the core PCP module biology remain unclear, and even if Ryk/Derailed would act in parallel to the core PCP module factors, it is likely to function through some of the downstream effectors of the Fz and Vang complexes. For additional insight and references, please see a comprehensive recent review of Wnt-PCP signaling regulation of axonal growth cone guidance and migration (Zou, 2020).
3.5. Wnt/PCP signaling and cancer cell dissemination and migration
The molecular core PCP factor cassette has a broad applicability during many developmental processes, including cell migration (see above). It is thus not a surprise that defective PCP signaling plays a causative role in many disorders ranging from ciliopathies to neural tube closure and organ defects (Butler & Wallingford, 2017; Simons & Mlodzik, 2008). Recent analyses also highlight a critical role for Wnt-Fz/PCP signaling in cancer and cancer cell dissemination or migration. While the canonical Wnt/β-catenin signaling pathway has long been causatively linked to cancer initiation and progression, the function of Wnt/PCP signaling in cancer has been underappreciated. However, recent work has revealed a strong correlation between core PCP component upregulation, which also includes several Wnt ligands, and unfavorable prognosis in several different cancer types (Daulat & Borg, 2017). Nonetheless, the situation is more complex as PCP factors can also act as tumor suppressors (Daulat & Borg, 2017). It is thus apparent that the contribution of Wnt/PCP signaling to cancer progression can differ significantly depending on the type of cancer and, importantly, the stage of the disease.
Along with the function of Wnt/PCP signaling in axonal growth guidance and developmental cell migration in general, an important role for PCP in cancer is linked to cell dissemination (for example, Daulat & Borg, 2017). In particular, PCP features have been analyzed in detail in mouse models of breast cancer, where fibroblast-derived exosomes promote autocrine Wnt11/PCP signaling and cause invasive cellular behavior (Luga et al., 2012). Importantly, migrating breast cancer cells displayed mutually exclusive localization of the core PCP complexes, with Vangl and Fzd anchored complexes being separated into adjacent domains (see schematic in Fig. 6B), which is somewhat reminiscent of the developmental asymmetric localization of the PCP factors during growth cone guidance (Fig. 6A). Here again, the Fzd-Dvl complex induces actin-based protrusions and the Vangl-Pk complex antagonizes this process (Luga et al., 2012). Along these lines, a pathway termed “lateral signaling” has been defined in breast cancer cells, which builds upon the principles of the spatially separated, but locally antagonistic functions of the core PCP complexes, with again Fzd-Dvl complexes promoting protrusive activity (Zhang et al., 2016). Furthermore, Pk1 can form a complex with Arhgap21/23 to downregulate RhoA activity, providing a mechanistic insight into how the Vangl/Pk complexes antagonize protrusive activities (Zhang et al., 2016). Taken together with the general functions of the molecular PCP cassette, this builds a model whereby the antagonistic behavior of the two PCP complexes establishes a spatially regulated activation of RhoA, modulating actomyosin activity and focal adhesion to promote efficient cell migration (Zhang et al., 2016). While the interaction of Pk1 with Arhgap21/23 was detected by a mass spectrometry analysis in breast cancer cells (and not yet pursued further), it is very likely that a similar link could be detected in other PCP regulated cell migration and growth cone guidance scenarios (see above). The protrusion promoting effects of the Fzd-Dvl complexes, with focus on Fzd6, are further discussed elsewhere (Corda & Sala, 2017). It is highly probable that multiple signaling pathways, both downstream of PCP and RTK signaling for example, converge in cancer cells to impact actin dynamics and promote cancer cell dissemination.
4. Concluding remarks
In this chapter, we have outlined the molecular details of the Wnt-Fz/PCP complexes and how they lead to the polarized activity of downstream effectors in processes that are direct cell migration or related morphological process. The outcome of core Wnt/Fz-PCP activity on motility depends upon the cell type, but in each case covered here, PCP signaling allows a cell to move in a directed manner relative to its position within a whole tissue. In general, PCP signaling can affect (i) the cytoskeleton through actomyosin regulation, which is required for force generation; (ii) junctional remodeling through cadherin trafficking, which is required for detaching from old neighbors and attaching to new ones; and (iii) modification of the ECM substrate upon which the cell is migrating. Each of these processes is vital for a cell to undergo directed movement. Core PCP signaling does not function in isolation, and in many instances cells integrate PCP signaling with input from other pathways, particularly RTKs, to ensure motility is appropriately regulated within the respective cellular environment.
PCP information is propagated across a tissue and can be used to coordinate the orientation of all cells within a field; this coordination is required for the CE movements that occur in each of the germ layers during gastrulation. In addition to responding to global cues such as A/P or D/V axes, PCP signaling can also allow groups of cells to respond to more localized cues, such as ommatidial clusters during rotation and border cells during migration to the oocyte. Even on an individual cell level, particularly in the nervous system, PCP complexes can direct the growth and movement of individual cells. Much of the work uncovering the molecular basis of PCP and its impact on motility has come from model organisms such as Drosophila, zebrafish, and Xenopus, however these processes are highly conserved and are at play during most if not all stages of mammalian development.
The impact of dysregulated PCP signaling can be seen in the many congenital syndromes that accompany mutations in core PCP genes, and many such disease links are now being discovered and studied. Similarly, a contribution of core Wnt/Fz-PCP signaling to cancer cell dissemination and migration has been established, but many questions remain unanswered in the disease contexts. Despite a growing knowledge base, much remains to be discovered and further research into specific functions and facets of PCP signaling will improve our understanding of both development and disease. As discussed in this chapter, the core PCP pathway has been mainly associated with the asymmetric localization of the core components with relevance in contexts of both cell polarity and cell migration during development and disease. There is still much to learn about the temporal organization of core complexes and their links to downstream effectors. With the advent of innovative live-imaging tools and biophysical methods to complement genetic and molecular studies, we look forward to further advances in the understanding of PCP-regulated motility at a molecular and cellular level. There are many exciting discoveries still to come in the core Wnt/PCP field and its impact on cell motility.
Acknowledgments
We are grateful to all Mlodzik lab members for their helpful discussions and support. We wish to thank Jill Gregory at the Academic Medical Illustration Department at the Icahn School of Medicine at Mount Sinai for excellent assistance with figure preparation. Related research in the Mlodzik laboratory is supported by National Institutes of Health grants from NIGMS and NEI.
References
- Adler PN (2002). Planar signaling and morphogenesis in Drosophila. Developmental Cell, 2, 525–535. [DOI] [PubMed] [Google Scholar]
- Adler PN (2012). The frizzled/stan pathway and planar cell polarity in the Drosophila wing. Current Topics in Developmental Biology, 101, 1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker NE, Mlodzik M, & Rubin GM (1990). Spacing differentiation in the developing Drosophila eye: A fibrinogen-related lateral inhibitor encoded by scabrous. Science, 250, 1370–1377. [DOI] [PubMed] [Google Scholar]
- Balaraju AK, Hu B, Rodriguez JJ, Murry M, & Lin F (2021). Glypican 4 regulates planar cell polarity of endoderm cells by controlling the localization of Cadherin 2. Development, 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastock R, & Strutt D (2007). The planar polarity pathway promotes coordinated cell migration during Drosophila oogenesis. Development, 134, 3055–3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco A, Poukkula M, Cliffe A, Mathieu J, Luque CM, Fulga TA, et al. (2007). Two distinct modes of guidance signalling during collective migration of border cells. Nature, 448, 362–365. [DOI] [PubMed] [Google Scholar]
- Bingham S, Higashijima S, Okamoto H, & Chandrasekhar A (2002). The Zebrafish trilobite gene is essential for tangential migration of branchiomotor neurons. Developmental Biology, 242, 149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutros M, Paricio N, Strutt DI, & Mlodzik M (1998). Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell, 94, 109–118. [DOI] [PubMed] [Google Scholar]
- Brown KE, & Freeman M (2003). Egfr signalling defines a protective function for ommatidial orientation in the Drosophila eye. Development, 130, 5401–5412. [DOI] [PubMed] [Google Scholar]
- Brzoska HL, d’Esposito AM, Kolatsi-Joannou M, Patel V, Igarashi P, Lei Y, et al. (2016). Planar cell polarity genes Celsr1 and Vangl2 are necessary for kidney growth, differentiation, and rostrocaudal patterning. Kidney International, 90, 1274–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MT, & Wallingford JB (2017). Planar cell polarity in development and disease. Nature Reviews. Molecular Cell Biology, 18, 375–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MT, & Wallingford JB (2018). Spatial and temporal analysis of PCP protein dynamics during neural tube closure. eLife, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagan RL, & Ready DF (1989). The emergence of order in the Drosophila pupal retina. Developmental Biology, 136, 346–362. [DOI] [PubMed] [Google Scholar]
- Cai D, Chen SC, Prasad M, He L, Wang X, Choesmel-Cadamuro V, et al. (2014). Mechanical feedback through E-cadherin promotes direction sensing during collective cell migration. Cell, 157, 1146–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreira-Barbosa F, Concha ML, Takeuchi M, Ueno N, Wilson SW, & Tada M (2003). Prickle 1 regulates cell movements during gastrulation and neuronal migration in zebrafish. Development, 130, 4037–4046. [DOI] [PubMed] [Google Scholar]
- Carvajal-Gonzalez JM, Mulero-Navarro S, & Mlodzik M (2016). Centriole positioning in epithelial cells and its intimate relationship with planar cell polarity. BioEssays, 38, 1234–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekhar A (2004). Turning heads: Development of vertebrate branchiomotor neurons. Developmental Dynamics, 229, 143–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KW, & Benzer S (1994). Rotation of photoreceptor clusters in the developing Drosophila eye requires the nemo gene. Cell, 78, 125–136. [DOI] [PubMed] [Google Scholar]
- Chou YH, & Chien CT (2002). Scabrous controls ommatidial rotation in the Drosophila compound eye. Developmental Cell, 3, 839–850. [DOI] [PubMed] [Google Scholar]
- Ciruna B, Jenny A, Lee D, Mlodzik M, & Schier AF (2006). Planar cell polarity signalling couples cell division and morphogenesis during neurulation. Nature, 439, 220–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Classen AK, Anderson KI, Marois E, & Eaton S (2005). Hexagonal packing of Drosophila wing epithelial cells by the planar cell polarity pathway. Developmental Cell, 9, 805–817. [DOI] [PubMed] [Google Scholar]
- Cooper MT, & Bray SJ (1999). Frizzled regulation of Notch signalling polarizes cell fate in the Drosophila eye. Nature, 397, 526–530. [DOI] [PubMed] [Google Scholar]
- Corda G, & Sala A (2017). Non-canonical WNT/PCP signalling in cancer: Fzd6 takes centre stage. Oncogensis, 6, e364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin JA, Quint E, Tsipouri V, Arkell RM, Cattanach B, Copp AJ, et al. (2003). Mutation of Celsr1 disrupts planar polarity of inner ear hair cells and causes severe neural tube defects in the mouse. Current Biology, 13, 1129–1133. [DOI] [PubMed] [Google Scholar]
- Darken RS, Scola AM, Rakeman AS, Das G, Mlodzik M, & Wilson PA (2002). The planar polarity gene strabismus regulates convergent extension movements in Xenopus. The EMBO Journal, 21, 976–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das G, Jenny A, Klein TJ, Eaton S, & Mlodzik M (2004). Diego interacts with Prickle and Strabismus/Van Gogh to localize planar cell polarity complexes. Development, 131, 4467–4476. [DOI] [PubMed] [Google Scholar]
- Das G, Reynolds-Kenneally J, & Mlodzik M (2002). The atypical cadherin Flamingo links Frizzled and Notch signaling in planar polarity establishment in the Drosophila eye. Developmental Cell, 2, 655–666. [DOI] [PubMed] [Google Scholar]
- Daulat AM, & Borg JP (2017). Wnt/planar cell polarity signaling: New opportunities for cancer treatment. Trends in Cancer, 3, 113–125. [DOI] [PubMed] [Google Scholar]
- Davey CF, Mathewson AW, & Moens CB (2016). PCP signaling between migrating neurons and their planar-polarized neuroepithelial environment controls filopodial dynamics and directional migration. PLoS Genetics, 12, e1005934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey CF, & Moens CB (2017). Planar cell polarity in moving cells: Think globally, act locally. Development, 144, 187–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson LA, Marsden M, Keller R, & Desimone DW (2006). Integrin alpha5beta1 and fibronectin regulate polarized cell protrusions required for Xenopus convergence and extension. Current Biology, 16, 833–844. [DOI] [PubMed] [Google Scholar]
- Deardorff MA, Tan C, Conrad LJ, & Klein PS (1998). Frizzled-8 is expressed in the Spemann organizer and plays a role in early morphogenesis. Development, 125, 2687–2700. [DOI] [PubMed] [Google Scholar]
- del Alamo D, & Mlodzik M (2006). Frizzled/PCP-dependent asymmetric neuralized expression determines R3/R4 fates in the Drosophila eye. Developmental Cell, 11, 887–894. [DOI] [PubMed] [Google Scholar]
- Devenport D (2016). Tissue morphodynamics: Translating planar polarity cues into polarized cell behaviors. Seminars in Cell & Developmental Biology, 55, 99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devenport D, & Fuchs E (2008). Planar polarization in embryonic epidermis orchestrates global asymmetric morphogenesis of hair follicles. Nature Cell Biology, 10, 1257–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson BJ (2005). Wnts send axons up and down the spinal cord. Nature Neuroscience, 8, 1130–1132. [DOI] [PubMed] [Google Scholar]
- Djiane A, Riou J, Umbhauer M, Boucaut J, & Shi D (2000). Role of frizzled 7 in the regulation of convergent extension movements during gastrulation in Xenopus laevis. Development, 127, 3091–3100. [DOI] [PubMed] [Google Scholar]
- Dohn MR, Mundell NA, Sawyer LM, Dunlap JA, & Jessen JR (2013). Planar cell polarity proteins differentially regulate extracellular matrix organization and assembly during zebrafish gastrulation. Developmental Biology, 383, 39–51. [DOI] [PubMed] [Google Scholar]
- Dos-Santos Carvalho S, Moreau MM, Hien YE, Garcia M, Aubailly N, Henderson DJ, et al. (2020). Vangl2 acts at the interface between actin and N-cadherin to modulate mammalian neuronal outgrowth. eLife, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchek P, & Rorth P (2001). Guidance of cell migration by EGF receptor signaling during Drosophila oogenesis. Science, 291, 131–133. [DOI] [PubMed] [Google Scholar]
- Duchek P, Somogyi K, Jekely G, Beccari S, & Rorth P (2001). Guidance of cell migration by the Drosophila PDGF/VEGF receptor. Cell, 107, 17–26. [DOI] [PubMed] [Google Scholar]
- Dzamba BJ, Jakab KR, Marsden M, Schwartz MA, & DeSimone DW (2009). Cadherin adhesion, tissue tension, and noncanonical Wnt signaling regulate fibronectin matrix organization. Developmental Cell, 16, 421–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanto M, & Mlodzik M (1999). Asymmetric Notch activation specifies photoreceptors R3 and R4 and planar polarity in the Drosophila eye. Nature, 397, 523–526. [DOI] [PubMed] [Google Scholar]
- Fenstermaker AG, Prasad AA, Bechara A, Adolfs Y, Tissir F, Goffinet A, et al. (2010). Wnt/planar cell polarity signaling controls the anterior-posterior organization of monoaminergic axons in the brainstem. The Journal of Neuroscience, 30, 16053–16064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiehler RW, & Wolff T (2007). Drosophila Myosin II, Zipper, is essential for ommatidial rotation. Developmental Biology, 310, 348–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiehler RW, & Wolff T (2008). Nemo is required in a subset of photoreceptors to regulate the speed of ommatidial rotation. Developmental Biology, 313, 533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaengel K, & Mlodzik M (2003). Egfr signaling regulates ommatidial rotation and cell motility in the Drosophila eye via MAPK/Pnt signaling and the Ras effector Canoe/AF6. Development, 130, 5413–5423. [DOI] [PubMed] [Google Scholar]
- Gault WJ, Olguin P, Weber U, & Mlodzik M (2012). Drosophila CK1-gamma, gilgamesh, controls PCP-mediated morphogenesis through regulation of vesicle trafficking. The Journal of Cell Biology, 196, 605–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasco DM, Pike W, Qu Y, Reustle L, Misra K, Di Bonito M, et al. (2016). The atypical cadherin Celsr1 functions non-cell autonomously to block rostral migration of facial branchiomotor neurons in mice. Developmental Biology, 417, 40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gombos R, Migh E, Antal O, Mukherjee A, Jenny A, & Mihaly J (2015). The formin DAAM functions as molecular effector of the planar cell polarity pathway during axonal development in Drosophila. The Journal of Neuroscience, 35, 10154–10167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich LV (2008). The plane facts of PCP in the CNS. Neuron, 60, 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich LV, & Strutt D (2011). Principles of planar polarity in animal development. Development, 138, 1877–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto T, Davidson L, Asashima M, & Keller R (2005). Planar cell polarity genes regulate polarized extracellular matrix deposition during frog gastrulation. Current Biology, 15, 787–793. [DOI] [PubMed] [Google Scholar]
- Goto T, & Keller R (2002). The planar cell polarity gene strabismus regulates convergence and extension and neural fold closure in Xenopus. Developmental Biology, 247, 165–181. [DOI] [PubMed] [Google Scholar]
- Guo N, Hawkins C, & Nathans J (2004). Frizzled6 controls hair patterning in mice. Proceedings of the National Academy of Sciences of the United States of America, 101, 9277–9281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas R, Dawid IB, & He X (2003). Coactivation of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate gastrulation. Genes & Development, 17, 295–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas R, Kato Y, & He X (2001). Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell, 107, 843–854. [DOI] [PubMed] [Google Scholar]
- Harrington MJ, Hong E, Fasanmi O, & Brewster R (2007). Cadherin-mediated adhesion regulates posterior body formation. BMC Developmental Biology, 7, 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison C, Shao H, Strutt H, & Strutt D (2020). Molecular mechanisms mediating asymmetric subcellular localisation of the core planar polarity pathway proteins. Biochemical Society Transactions, 48, 1297–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisenberg CP, Tada M, Rauch GJ, Saude L, Concha ML, Geisler R, et al. (2000). Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature, 405, 76–81. [DOI] [PubMed] [Google Scholar]
- Hilliard MA, & Bargmann CI (2006). Wnt signals and frizzled activity orient anterior-posterior axon outgrowth in C. elegans. Developmental Cell, 10, 379–390. [DOI] [PubMed] [Google Scholar]
- Hollis ER 2nd, & Zou Y (2012). Expression of the Wnt signaling system in central nervous system axon guidance and regeneration. Frontiers in Molecular Neuroscience, 5, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong E, & Brewster R (2006). N-cadherin is required for the polarized cell behaviors that drive neurulation in the zebrafish. Development, 133, 3895–3905. [DOI] [PubMed] [Google Scholar]
- Hua ZL, Smallwood PM, & Nathans J (2013). Frizzled3 controls axonal development in distinct populations of cranial and spinal motor neurons. eLife, 2, e01482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebner RJ, Malmi-Kakkada AN, Sarikaya S, Weng S, Thirumalai D, & Wallingford JB (2021). Mechanical heterogeneity along single cell-cell junctions is driven by lateral clustering of cadherins during vertebrate axis elongation. eLife, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebner RJ, & Wallingford JB (2018). Coming to consensus: A unifying model emerges for convergent extension. Developmental Cell, 46, 389–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries AC, & Mlodzik M (2018). From instruction to output: Wnt/PCP signaling in development and cancer. Current Opinion in Cell Biology, 51, 110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries AC, Narang S, & Mlodzik M (2020). Mutations associated with human neural tube defects display disrupted planar cell polarity in Drosophila. eLife, 9, e535322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenny A (2010). Planar cell polarity signaling in the Drosophila eye. Current Topics in Developmental Biology, 93, 189–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenny A, Darken RS, Wilson PA, & Mlodzik M (2003). Prickle and Strabismus form a functional complex to generate a correct axis during planar cell polarity signaling. The EMBO Journal, 22, 4409–4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenny A, Reynolds-Kenneally J, Das G, Burnett M, & Mlodzik M (2005). Diego and Prickle regulate Frizzled planar cell polarity signalling by competing for Dishevelled binding. Nature Cell Biology, 7, 691–697. [DOI] [PubMed] [Google Scholar]
- Jessen JR, Topczewski J, Bingham S, Sepich DS, Marlow F, Chandrasekhar A, et al. (2002). Zebrafish trilobite identifies new roles for Strabismus in gastrulation and neuronal movements. Nature Cell Biology, 4, 610–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R, Davidson L, Edlund A, Elul T, Ezin M, Shook D, et al. (2000). Mechanisms of convergence and extension by cell intercalation. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 355, 897–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R, Shih J, & Sater A (1992). The cellular basis of the convergence and extension of the Xenopus neural plate. Developmental Dynamics, 193, 199–217. [DOI] [PubMed] [Google Scholar]
- Klein TJ, & Mlodzik M (2005). Planar cell polarization: An emerging model points in the right direction. Annual Review of Cell and Developmental Biology, 21, 155–176. [DOI] [PubMed] [Google Scholar]
- Koca Y, Housden BE, Gault WJ, Bray SJ, & Mlodzik M (2019). Notch signaling coordinates ommatidial rotation in the Drosophila eye via transcriptional regulation of the EGF-receptor ligand Argos. Scientific Reports, 9, 18628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft B, Berger CD, Wallkamm V, Steinbeisser H, & Wedlich D (2012). Wnt-11 and Fz7 reduce cell adhesion in convergent extension by sequestration of PAPC and C-cadherin. The Journal of Cell Biology, 198, 695–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Ciprianidis A, Theiss S, Steinbeisser H, & Kaufmann LT (2017). Nemo-like kinase 1 (Nlk1) and paraxial protocadherin (PAPC) cooperatively control Xenopus gastrulation through regulation of Wnt/planar cell polarity (PCP) signaling. Differentiation, 93, 27–38. [DOI] [PubMed] [Google Scholar]
- Lawrence PA, & Casal J (2018). Planar cell polarity: Two genetic systems use one mechanism to read gradients. Development, 145. [DOI] [PubMed] [Google Scholar]
- Levayer R, & Lecuit T (2012). Biomechanical regulation of contractility: Spatial control and dynamics. Trends in Cell Biology, 22, 61–81. [DOI] [PubMed] [Google Scholar]
- Liu Y, Shi J, Lu CC, Wang ZB, Lyuksyutova AI, Song XJ, et al. (2005). Ryk-mediated Wnt repulsion regulates posterior-directed growth of corticospinal tract. Nature Neuroscience, 8, 1151–1159. [DOI] [PubMed] [Google Scholar]
- Luga V, Zhang L, Viloria-Petit AM, Ogunjimi AA, Inanlou MR, Chiu E, et al. (2012). Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell, 151, 1542–1556. [DOI] [PubMed] [Google Scholar]
- Lyuksyutova AI, Lu CC, Milanesio N, King LA, Guo N, Wang Y, et al. (2003). Anterior-posterior guidance of commissural axons by Wnt-frizzled signaling. Science, 302, 1984–1988. [DOI] [PubMed] [Google Scholar]
- Marsden M, & DeSimone DW (2003). Integrin-ECM interactions regulate cadherin-dependent cell adhesion and are required for convergent extension in Xenopus. Current Biology, 13, 1182–1191. [DOI] [PubMed] [Google Scholar]
- Matis M, & Axelrod JD (2013). Regulation of PCP by the fat signaling pathway. Genes & Development, 27, 2207–2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGreevy EM, Vijayraghavan D, Davidson LA, & Hildebrand JD (2015). Shroom3 functions downstream of planar cell polarity to regulate myosin II distribution and cellular organization during neural tube closure. Biology Open, 4, 186–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirkovic I, Gault WJ, Rahnama M, Jenny A, Gaengel K, Bessette D, et al. (2011). Nemo kinase phosphorylates beta-catenin to promote ommatidial rotation and connects core PCP factors to E-cadherin-beta-catenin. Nature Structural & Molecular Biology, 18, 665–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirkovic I, & Mlodzik M (2006). Cooperative activities of drosophila DE-cadherin and DN-cadherin regulate the cell motility process of ommatidial rotation. Development, 133, 3283–3293. [DOI] [PubMed] [Google Scholar]
- Mlodzik M (1999). Planar polarity in the Drosophila eye: A multifaceted view of signaling specificity and cross-talk. The EMBO Journal, 18, 6873–6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlodzik M, Baker NE, & Rubin GM (1990). Isolation and expression of scabrous, a gene regulating neurogenesis in Drosophila. Genes & Development, 4, 1848–1861. [DOI] [PubMed] [Google Scholar]
- Mongera A, Rowghanian P, Gustafson HJ, Shelton E, Kealhofer DA, Carn EK, et al. (2018). A fluid-to-solid jamming transition underlies vertebrate body axis elongation. Nature, 561, 401–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montcouquiol M, Sans N, Huss D, Kach J, Dickman JD, Forge A, et al. (2006). Asymmetric localization of Vangl2 and Fz3 indicate novel mechanisms for planar cell polarity in mammals. The Journal of Neuroscience, 26, 5265–5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell DJ (2003). Border-cell migration: The race is on. Nature Reviews. Molecular Cell Biology, 4, 13–24. [DOI] [PubMed] [Google Scholar]
- Montell DJ, Yoon WH, & Starz-Gaiano M (2012). Group choreography: Mechanisms orchestrating the collective movement of border cells. Nature Reviews. Molecular Cell Biology, 13, 631–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero JA, & Heisenberg CP (2004). Gastrulation dynamics: Cells move into focus. Trends in Cell Biology, 14, 620–627. [DOI] [PubMed] [Google Scholar]
- Moon RT, Campbell RM, Christian JL, McGrew LL, Shih J, & Fraser S (1993). Xwnt-5A: A maternal Wnt that affects morphogenetic movements after overexpression in embryos of Xenopus laevis. Development, 119, 97–111. [DOI] [PubMed] [Google Scholar]
- Mrkusich EM, Flanagan DJ, & Whitington PM (2011). The core planar cell polarity gene prickle interacts with flamingo to promote sensory axon advance in the Drosophila embryo. Developmental Biology, 358, 224–230. [DOI] [PubMed] [Google Scholar]
- Munoz-Soriano V, Ruiz C, Perez-Alonso M, Mlodzik M, & Paricio N (2013). Nemo regulates cell dynamics and represses the expression of miple, a midkine/pleiotrophin cytokine, during ommatidial rotation. Developmental Biology, 377, 113–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niewiadomska P, Godt D, & Tepass U (1999). DE-Cadherin is required for intercellular motility during Drosophila oogenesis. The Journal of Cell Biology, 144, 533–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolopoulou E, Galea GL, Rolo A, Greene ND, & Copp AJ (2017). Neural tube closure: Cellular, molecular and biomechanical mechanisms. Development, 144, 552–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T, Honda H, & Takeichi M (2012). Planar cell polarity links axes of spatial dynamics in neural-tube closure. Cell, 149, 1084–1097. [DOI] [PubMed] [Google Scholar]
- Nutt SL, Dingwell KS, Holt CE, & Amaya E (2001). Xenopus Sprouty2 inhibits FGF-mediated gastrulation movements but does not affect mesoderm induction and patterning. Genes & Development, 15, 1152–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi K, Hollis E, & Zou Y (2014). Axon guidance and injury-lessons from Wnts and Wnt signaling. Current Opinion in Neurobiology, 27, 232–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi K, Shafer B, Lo C, Tissir F, Goffinet AM, & Zou Y (2013). Antagonistic functions of Dishevelleds regulate Frizzled3 endocytosis via filopodia tips in Wnt-mediated growth cone guidance. The Journal of Neuroscience, 33, 19071–19085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossipova O, Kim K, & Sokol SY (2015). Planar polarization of Vangl2 in the vertebrate neural plate is controlled by Wnt and Myosin II signaling. Biology Open, 4, 722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan CL, Howell JE, Clark SG, Hilliard M, Cordes S, Bargmann CI, et al. (2006). Multiple Wnts and frizzled receptors regulate anteriorly directed cell and growth cone migrations in Caenorhabditis elegans. Developmental Cell, 10, 367–377. [DOI] [PubMed] [Google Scholar]
- Pare AC, & Zallen JA (2020). Cellular, molecular, and biophysical control of epithelial cell intercalation. Current Topics in Developmental Biology, 136, 167–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M, & Moon RT (2002). The planar cell-polarity gene stbm regulates cell behaviour and cell fate in vertebrate embryos. Nature Cell Biology, 4, 20–25. [DOI] [PubMed] [Google Scholar]
- Peng Y, & Axelrod JD (2012). Asymmetric protein localization in planar cell polarity: Mechanisms, puzzles, and challenges. Current Topics in Developmental Biology, 101, 33–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petridou NI, Grigolon S, Salbreux G, Hannezo E, & Heisenberg CP (2019). Fluidization-mediated tissue spreading by mitotic cell rounding and non-canonical Wnt signalling. Nature Cell Biology, 21, 169–178. [DOI] [PubMed] [Google Scholar]
- Powell PA, Wesley C, Spencer S, & Cagan RL (2001). Scabrous complexes with Notch to mediate boundary formation. Nature, 409, 626–630. [DOI] [PubMed] [Google Scholar]
- Qu Y, Glasco DM, Zhou L, Sawant A, Ravni A, Fritzsch B, et al. (2010). Atypical cadherins Celsr1–3 differentially regulate migration of facial branchiomotor neurons in mice. The Journal of Neuroscience, 30, 9392–9401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls AS, & Wolff T (2003). Strabismus requires Flamingo and Prickle function to regulate tissue polarity in the Drosophila eye. Development, 130, 1877–1887. [DOI] [PubMed] [Google Scholar]
- Rohrschneider MR, Elsen GE, & Prince VE (2007). Zebrafish Hoxb1a regulates multiple downstream genes including prickle1b. Developmental Biology, 309, 358–372. [DOI] [PubMed] [Google Scholar]
- Roignant JY, & Treisman JE (2009). Pattern formation in the Drosophila eye disc. The International Journal of Developmental Biology, 53, 795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roszko I, Sawada A, & Solnica-Krezel L (2009). Regulation of convergence and extension movements during vertebrate gastrulation by the Wnt/PCP pathway. Seminars in Cell & Developmental Biology, 20, 986–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roszko I, Sepich DS, Jessen JR, Chandrasekhar A, & Solnica-Krezel L (2015). A dynamic intracellular distribution of Vangl2 accompanies cell polarization during zebrafish gastrulation. Development, 142, 2508–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schambony A, & Wedlich D (2007). Wnt-5A/Ror2 regulate expression of XPAPC through an alternative noncanonical signaling pathway. Developmental Cell, 12, 779–792. [DOI] [PubMed] [Google Scholar]
- Segalen M, & Bellaiche Y (2009). Cell division orientation and planar cell polarity pathways. Seminars in Cell & Developmental Biology, 20, 972–977. [DOI] [PubMed] [Google Scholar]
- Seifert JR, & Mlodzik M (2007). Frizzled/PCP signalling: A conserved mechanism regulating cell polarity and directed motility. Nature Reviews, 8, 126–138. [DOI] [PubMed] [Google Scholar]
- Shafer B, Onishi K, Lo C, Colakoglu G, & Zou Y (2011). Vangl2 promotes Wnt/planar cell polarity-like signaling by antagonizing Dvl1-mediated feedback inhibition in growth cone guidance. Developmental Cell, 20, 177–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu K, Sato M, & Tabata T (2011). The Wnt5/planar cell polarity pathway regulates axonal development of the Drosophila mushroom body neuron. The Journal of Neuroscience, 31, 4944–4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Yabe T, Muraoka O, Yonemura S, Aramaki S, Hatta K, et al. (2005). E-cadherin is required for gastrulation cell movements in zebrafish. Mechanisms of Development, 122, 747–763. [DOI] [PubMed] [Google Scholar]
- Simons M, & Mlodzik M (2008). Planar cell polarity signaling: From fly development to human disease. Annual Review of Genetics, 42, 517–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha T, Wang B, Evans S, Wynshaw-Boris A, & Wang J (2012). Disheveled mediated planar cell polarity signaling is required in the second heart field lineage for outflow tract morphogenesis. Developmental Biology, 370, 135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoglund P, & Keller R (2010). Integration of planar cell polarity and ECM signaling in elongation of the vertebrate body plan. Current Opinion in Cell Biology, 22, 589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol SY (1996). Analysis of Dishevelled signalling pathways during Xenopus development. Current Biology, 6, 1456–1467. [DOI] [PubMed] [Google Scholar]
- Strutt H, & Strutt D (2003). EGF signaling and ommatidial rotation in the Drosophila eye. Current Biology, 13, 1451–1457. [DOI] [PubMed] [Google Scholar]
- Strutt H, & Strutt D (2021). How do the Fat-Dachsous and core planar polarity pathways act together and independently to coordinate polarized cell behaviours? Open Biology, 11, 200356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutt D, & Warrington SJ (2008). Planar polarity genes in the Drosophila wing regulate the localisation of the FH3-domain protein Multiple Wing Hairs to control the site of hair production. Development, 135, 3103–3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutt DI, Weber U, & Mlodzik M (1997). The role of RhoA in tissue polarity and Frizzled signalling. Nature, 387, 292–295. [DOI] [PubMed] [Google Scholar]
- Tada M, Concha ML, & Heisenberg CP (2002). Non-canonical Wnt signalling and regulation of gastrulation movements. Seminars in Cell & Developmental Biology, 13, 251–260. [DOI] [PubMed] [Google Scholar]
- Tada M, & Heisenberg CP (2012). Convergent extension: Using collective cell migration and cell intercalation to shape embryos. Development, 139, 3897–3904. [DOI] [PubMed] [Google Scholar]
- Tada M, & Smith JC (2000). Xwnt11 is a target of Xenopus Brachyury: Regulation of gastrulation movements via Dishevelled, but not through the canonical Wnt pathway. Development, 127, 2227–2238. [DOI] [PubMed] [Google Scholar]
- Takeuchi M, Nakabayashi J, Sakaguchi T, Yamamoto TS, Takahashi H, Takeda H, et al. (2003). The prickle-related gene in vertebrates is essential for gastrulation cell movements. Current Biology, 13, 674–679. [DOI] [PubMed] [Google Scholar]
- Thakar S, Wang L, Yu T, Ye M, Onishi K, Scott J, et al. (2017). Evidence for opposing roles of Celsr3 and Vangl2 in glutamatergic synapse formation. Proceedings of the National Academy of Sciences of the United States of America, 114, E610–E618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C, & Strutt D (2012). The roles of the cadherins Fat and Dachsous in planar polarity specification in Drosophila. Developmental Dynamics, 241, 27–39. [DOI] [PubMed] [Google Scholar]
- Thorpe CJ, & Moon RT (2004). nemo-like kinase is an essential co-activator of Wnt signaling during early zebrafish development. Development, 131, 2899–2909. [DOI] [PubMed] [Google Scholar]
- Thuveson M, Gaengel K, Collu GM, Chin ML, Singh J, & Mlodzik M (2019). Integrins are required for synchronous ommatidial rotation in the Drosophila eye linking planar cell polarity signalling to the extracellular matrix. Open Biology, 9, 190148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson A, & Ready DF (1987). Neuronal differentiation in Drosophila ommatidium. Developmental Biology, 120, 366–376. [DOI] [PubMed] [Google Scholar]
- Tomlinson A, & Struhl G (1999). Decoding vectorial information from a gradient: Sequential roles of the receptors Frizzled and Notch in establishing planar polarity in the Drosophila eye. Development, 126, 5725–5738. [DOI] [PubMed] [Google Scholar]
- Ulrich F, Krieg M, Schotz EM, Link V, Castanon I, Schnabel V, et al. (2005). Wnt11 functions in gastrulation by controlling cell cohesion through Rab5c and E-cadherin. Developmental Cell, 9, 555–564. [DOI] [PubMed] [Google Scholar]
- Unterseher F, Hefele JA, Giehl K, De Robertis EM, Wedlich D, & Schambony A (2004). Paraxial protocadherin coordinates cell polarity during convergent extension via Rho A and JNK. The EMBO Journal, 23, 3259–3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeman MT, Axelrod JD, & Moon RT (2003). A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Developmental Cell, 5, 367–377. [DOI] [PubMed] [Google Scholar]
- Veeman MT, Slusarski DC, Kaykas A, Louie SH, & Moon RT (2003). Zebrafish Prickle, a modulator of noncanonical Wnt/Fz signaling, regulates gastrulation movements. Current Biology, 13, 680–685. [DOI] [PubMed] [Google Scholar]
- Verdier V, Guang Chao C, & Settleman J (2006). Rho-kinase regulates tissue morphogenesis via non-muscle myosin and LIM-kinase during Drosophila development. BMC Developmental Biology, 6, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivancos V, Chen P, Spassky N, Qian D, Dabdoub A, Kelley M, et al. (2009). Wnt activity guides facial branchiomotor neuron migration, and involves the PCP pathway and JNK and ROCK kinases. Neural Development, 4, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vladar EK, Bayly RD, Sangoram AM, Scott MP, & Axelrod JD (2012). Microtubules enable the planar cell polarity of airway cilia. Current Biology, 22, 2203–2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada H, Iwasaki M, Sato T, Masai I, Nishiwaki Y, Tanaka H, et al. (2005). Dual roles of zygotic and maternal Scribble1 in neural migration and convergent extension movements in zebrafish embryos. Development, 132, 2273–2285. [DOI] [PubMed] [Google Scholar]
- Wada H, Tanaka H, Nakayama S, Iwasaki M, & Okamoto H (2006). Frizzled3a and Celsr2 function in the neuroepithelium to regulate migration of facial motor neurons in the developing zebrafish hindbrain. Development, 133, 4749–4759. [DOI] [PubMed] [Google Scholar]
- Wallingford JB (2010). Planar cell polarity signaling, cilia and polarized ciliary beating. Current Opinion in Cell Biology, 22, 597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallingford JB (2012). Planar cell polarity and the developmental control of cell behavior in vertebrate embryos. Annual Review of Cell and Developmental Biology, 28, 627–653. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Fraser SE, & Harland RM (2002). Convergent extension: The molecular control of polarized cell movement during embryonic development. Developmental Cell, 2, 695–706. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, & Harland RM (2001). Xenopus Dishevelled signaling regulates both neural and mesodermal convergent extension: Parallel forces elongating the body axis. Development, 128, 2581–2592. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, & Harland RM (2002). Neural tube closure requires Dishevelled-dependent convergent extension of the midline. Development, 129, 5815–5825. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Rowning BA, Vogeli KM, Rothbacher U, Fraser SE, & Harland RM (2000). Dishevelled controls cell polarity during Xenopus gastrulation. Nature, 405, 81–85. [DOI] [PubMed] [Google Scholar]
- Wang J, Hamblet NS, Mark S, Dickinson ME, Brinkman BC, Segil N, et al. (2006). Dishevelled genes mediate a conserved mammalian PCP pathway to regulate convergent extension during neurulation. Development, 133, 1767–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, He L, Wu YI, Hahn KM, & Montell DJ (2010). Light-mediated activation reveals a key role for Rac in collective guidance of cell movement in vivo. Nature Cell Biology, 12, 591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Janicki P, Koster I, Berger CD, Wenzl C, Grosshans J, et al. (2008). Xenopus paraxial protocadherin regulates morphogenesis by antagonizing Sprouty. Genes & Development, 22, 878–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Marco P, Capra V, & Kibar Z (2019). Update on the role of the non-canonical Wnt/planar cell polarity pathway in neural tube defects. Cell, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington SJ, Strutt H, & Strutt D (2013). The Frizzled-dependent planar polarity pathway locally promotes E-cadherin turnover via recruitment of RhoGEF2. Development, 140, 1045–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BB, Cantrell VA, Mundell NA, Bennett AC, Quick RE, & Jessen JR (2012). VANGL2 regulates membrane trafficking of MMP14 to control cell polarity and migration. Journal of Cell Science, 125, 2141–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MLK, & Solnica-Krezel L (2020). Cellular and molecular mechanisms of convergence and extension in zebrafish. Current Topics in Developmental Biology, 136, 377–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M, Yen W, Lu X, & Sutherland A (2014). Distinct apical and basolateral mechanisms drive planar cell polarity-dependent convergent extension of the mouse neural plate. Developmental Cell, 29, 34–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter CG, Wang B, Ballew A, Royou A, Karess R, Axelrod JD, et al. (2001). Drosophila Rho-associated kinase (Drok) links Frizzled-mediated planar cell polarity signaling to the actin cytoskeleton. Cell, 105, 81–91. [DOI] [PubMed] [Google Scholar]
- Wolff T, Guinto JB, & Rawls AS (2007). Screen for genetic modifiers of stbm reveals that photoreceptor fate and rotation can be genetically uncoupled in the Drosophila eye. PLoS One, 2, e453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff T, & Rubin GM (1998). Strabismus, a novel gene that regulates tissue polarity and cell fate decisions in Drosophila. Development, 125, 1149–1159. [DOI] [PubMed] [Google Scholar]
- Wu J, Klein TJ, & Mlodzik M (2004). Subcellular localization of frizzled receptors, mediated by their cytoplasmic tails, regulates signaling pathway specificity. PLoS Biology, 2, E158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, & Mlodzik M (2009). A quest for the mechanism regulating global planar cell polarity of tissues. Trends in Cell Biology, 19, 295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Roman AC, Carvajal-Gonzalez JM, & Mlodzik M (2013). Wg and Wnt4 provide long-range directional input to planar cell polarity orientation in Drosophila. Nature Cell Biology, 15, 1045–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Tanwar PS, & Raftery LA (2008). Drosophila follicle cells: Morphogenesis in an eggshell. Seminars in Cell & Developmental Biology, 19, 271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka H, Moriguchi T, Masuyama N, Kusakabe M, Hanafusa H, Takada R, et al. (2002). JNK functions in the non-canonical Wnt pathway to regulate convergent extension movements in vertebrates. EMBO Reports, 3, 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Huen D, Morely T, Johnson G, Gubb D, Roote J, et al. (2008). The multiple-wing-hairs gene encodes a novel GBD-FH3 domain-containing protein that functions both prior to and after wing hair initiation. Genetics, 180, 219–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin C, Ciruna B, & Solnica-Krezel L (2009). Convergence and extension movements during vertebrate gastrulation. Current Topics in Developmental Biology, 89, 163–192. [DOI] [PubMed] [Google Scholar]
- Yin C, Kiskowski M, Pouille PA, Farge E, & Solnica-Krezel L (2008). Cooperation of polarized cell intercalations drives convergence and extension of presomitic mesoderm during zebrafish gastrulation. The Journal of Cell Biology, 180, 221–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa S, McKinnon RD, Kokel M, & Thomas JB (2003). Wnt-mediated axon guidance via the Drosophila Derailed receptor. Nature, 422, 583–588. [DOI] [PubMed] [Google Scholar]
- Yuan L, Hu S, Okray Z, Ren X, De Geest N, Claeys A, et al. (2016). The Drosophila neurogenin Tap functionally interacts with the Wnt-PCP pathway to regulate neuronal extension and guidance. Development, 143, 2760–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Luga V, Armitage SK, Musiol M, Won A, Yip CM, et al. (2016). A lateral signalling pathway coordinates shape volatility during cell migration. Nature Communications, 7, 11714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y, Brieher WM, & Gumbiner BM (1999). Analysis of C-cadherin regulation during tissue morphogenesis with an activating antibody. The Journal of Cell Biology, 144, 351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y (2006). Navigating the anterior-posterior axis with Wnts. Neuron, 49, 787–789. [DOI] [PubMed] [Google Scholar]
- Zou Y (2020). Breaking symmetry—Cell polarity signaling pathways in growth cone guidance and synapse formation. Current Opinion in Neurobiology, 63, 77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]


