Abstract
In recent years, significant advancements have been made in the study of lymphatic vessels with the identification of their specific markers and the development of research tools that have accelerated our understanding of their role in tissue homeostasis and disease pathogenesis in many organs. Compared to other organs, the lymphatic system in the liver is understudied despite its obvious importance for hepatic physiology and pathophysiology. This review article presents basic knowledge of the hepatic lymphatic system and its role in a range of liver-related pathological conditions such as portal hypertension, ascites formation, malignant tumors, liver transplantation, congenital liver diseases, non-alcoholic fatty liver disease (NAFLD), and hepatic encephalopathy. The article concludes with a discussion on the modulation of lymphangiogenesis as a potential therapeutic strategy for liver diseases.
Keywords: Lymphangiogenesis, VEGFs, liver fibrosis, liver transplantation, portal hypertension
Introduction
The liver is recognized as the largest lymph producing organ, accounting for nearly 25 – 50% of lymph passing through the thoracic duct[1–3]. Their functional importance has been discussed mainly in regard to the regulation of lymph production, which is highly influenced by hemodynamic changes in the intrahepatic microcirculation. While lymphatic vessel numbers are known to increase (via lymphangiogenesis, new lymphatic vessel formation) in many liver diseases, the mechanism of lymphangiogenesis in the liver remains poorly understood[4, 5]. In addition to the maintenance of fluid homeostasis, hepatic lymphatic vessels are highly involved in the immune system and lipid metabolism by transporting various immune cells, antigens and lipids to lymph nodes. Despite these roles in key processes in the liver, hepatic lymphatic vessels have been understudied.
In the last decade, significant advancements have been made in the study of lymphatic vessels, including the identification of specific markers and the development of research tools, accelerating our understanding of their roles in tissue homeostasis and disease pathogenesis in many organs[6–8]. Lymphatic vessels exhibit a variety of immunoregulatory functions by expressing a wide range of chemokines and receptors. Thus, lymphatic vessels are more than conduits removing lymph, immune cells and cellular products from organs and tissues.
This review article aims to provide fundamental knowledge of the hepatic lymphatic system and its implications in liver diseases. We will discuss: 1) the fundamental knowledge of liver lymphatics, including structure, markers, lymph and lymphatic drainage; 2) lymphatics and the immune system; 3) mediators and signals leading to hepatic lymphangiogenesis; 4) lymphatics in liver diseases and their complications, including portal hypertension, ascites, malignant tumors, liver transplantation, congenital liver diseases, non-alcoholic fatty liver disease (NAFLD), and hepatic encephalopathy; 5) therapeutic potential and future directions in the study of liver lymphatics. For details of the biology of the hepatic lymphatic system, other review papers are referenced in Supplementary Table 1.
1. Hepatic lymphatic system
1–1. Structure of lymphatics (Figure 1)
Figure 1. Hepatic lymphatic system.
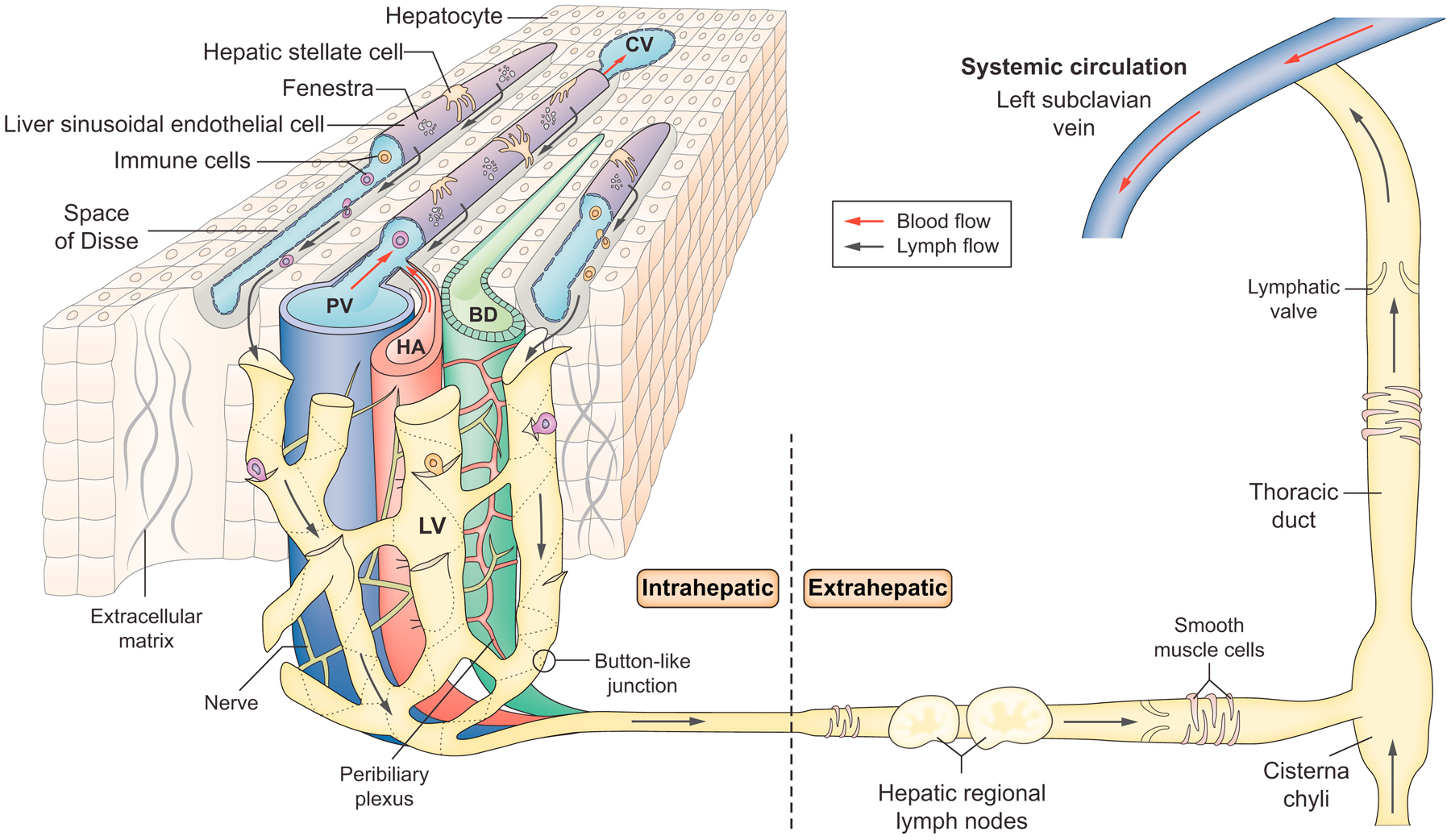
Lymph is thought to flow into lymphatic vessels located in three regions: portal, hepatic venous and sub-capsular areas. The illustration shows lymphatic vessels in the portal tract, the primary site of hepatic lymph drainage covering around 80% of the lymph produced by the liver. Because the hepatic sinusoids are highly permeable due to fenestrae, fluid in the hepatic sinusoids can flow through the channels traversing the limiting plate to the interstitial space of the portal tract. Endothelial cells of lymphatic capillaries have discontinuous, “button-like” junctions, which allow efficient entry of fluid, antigens and immune cells into lymphatic capillaries. Lymphatic capillaries in the portal tract coalesce into collecting lymphatic vessels surrounded by lymphatic muscle cells outside the liver. Lymphatic muscle cells covering collecting lymphatic vessels help to pump lymph into regional lymph nodes located in the hepatic hilum and then to the cisterna chyli located at the lower end of the thoracic duct. Lymph finally drains into the left subclavian vein via the thoracic duct and returns to the systemic blood circulation. LV: Lymphatic vessel, PV: Portal vein, HA: Hepatic artery, BD: Bile duct, CV: Central vein, ECM: Extracellular matrix protein, HSC: Hepatic stellate cell
A history of studies of the hepatic lymphatic system and how hepatic lymph reaches hepatic lymphatic vessels are described in an excellent review paper by Ohtani & Ohtani[5]. The lymphatic vascular system consists of lymphatic capillaries (also known as initial lymphatics) and collecting lymphatic vessels. Lymphatic capillaries consist of a single layer of lymphatic endothelial cells (LECs) with no coverage of “lymphatic muscle” cells. In contrast to lymphatic capillaries, collecting lymphatic vessels are covered with lymphatic muscle cells and located downstream of lymphatic capillaries. “Lymphatic vessels” are often defined as an intermediate structure between lymphatic capillaries and lymphatic collecting vessels. In this review article, however, we use the term “lymphatic vessels” to describe both lymphatic vessels and lymphatic capillaries, because lymphatic capillaries are most common in the liver. Endothelial cells of lymphatic capillaries have discontinuous, “button-like” junctions (buttons), which are strikingly different from continuous, “zipper-like” junctions (zippers) of collecting lymphatic vessels and blood vessels[9, 10]. The button-like junctions are thought to allow efficient entry of fluid, antigens and immune cells into lymphatic capillaries (Figure 2). In pathological conditions, it is reported that lymphatic capillaries lose this “button-like” structure with a change to the less permeable “zipper-like” structure[11, 12]. This change of junctional structure could impair transport of fluid and substances to lymphatic capillaries, thereby decreasing their clearance from tissues.
Figure 2. Button vs. Zipper-like structures.

The lymphatic vascular system includes lymphatic capillaries (also known as initial lymphatics) and collecting lymphatic vessels. Lymphatic capillaries consist of a single layer of lymphatic endothelial cells. Endothelial cells of lymphatic capillaries are attached by anchoring filaments to surrounding extracellular matrix proteins, which support their vessel structure. Endothelial cells of lymphatic capillaries have discontinuous, “button-like” junctions, which allow efficient entry of fluid, antigens and immune cells into lymphatic capillaries. In contrast, collecting lymphatic vessels have “zipper-like” junctions, similar to blood vessels. In pathological conditions, lymphatic capillaries lose this “button-like” structure with a change to the less permeable “zipper-like” structure, which results in impaired transport of fluid and substances to lymphatic capillaries, thereby decreasing their clearance from tissues. Created with BioRender.com.
1–2. Markers of lymphatics
Markers of LECs include vascular endothelial growth factor receptor 3 (VEGFR3)[13, 14], lymphatic vessel endothelial hyaluronan receptor 1 (Lyve1)[15, 16], prospero homeobox protein 1 (Prox1)[17] and podoplanin (also known as D2–40)[18]. However, these LEC markers are also expressed in other liver cells, which has made studies of the hepatic lymphatic system challenging[4, 19]. For example, VEGFR3 and Lyve1 are expressed in liver sinusoidal endothelial cells (LSECs), while Prox1 is expressed in hepatocytes. In the normal liver, the high localization of lymphatic capillaries in the portal tract allows these markers to be used for their identification. Because lymphatic capillaries are not covered by αSMA-positive smooth muscle cells, αSMA-labeling can differentiate lymphatics from blood vessels in the portal tract. In human liver specimens, podoplanin/D2–40 has frequently been used for identification of lymphatic vessels. However, identification of unique LEC proteins that are not expressed in other liver cells is indispensable for advancement of our understanding of lymphatic vessels in the liver.
The presence of LECs in the liver tends to be overlooked at least in part because they only account for a very small portion of the liver EC population in the normal liver, while liver cirrhosis increases the contribution of LECs to total ECs by 20-fold[20]. A recent study of the liver EC population using single-cell RNA sequencing (scRNA-seq) analysis identified several novel genes highly expressed in LECs, but with absent or very minimal expression in LSECs and arterial/venous ECs[20]. These potential liver LEC markers include Mmrn1, Rassf9, Tbx1 and interleukin 7 (IL7). Characterization of these genes may help to understand unique functions of LECs as opposed to LSECs.
1–3. Lymph and lymphatic drainage
Hepatic lymph (lymphatic fluid) consists of sinusoidal plasma filtered into the space of Disse[21], an interstitial space between LSECs and hepatocytes, through fenestrae of LSECs. Although detailed hepatic lymph contents have not yet been specified, these contents potentially include cellular byproducts discharged from hepatic cells in the space of Disse (Figure 1). Lymph is then thought to flow into lymphatic vessels located in three regions: portal, hepatic venous and sub-capsular areas[22]. Among them, lymphatic vessels in the portal tract are considered to be the primary site of hepatic lymph drainage, covering around 80% of the lymph produced by the liver[23]. Because the hepatic sinusoids are highly permeable and oncotic pressure along the sinusoids is negligible, fluid in the hepatic sinusoids can flow through the channels traversing the limiting plate to the interstitial space of the portal tract, following hydrostatic pressure gradients.
Lymphatic capillaries in the portal tract coalesce into collecting lymphatic vessels surrounded by lymphatic muscle cells outside the liver[4, 5, 24]. Lymphatic muscle cells covering collecting lymphatic vessels help to pump lymphatic fluid into regional lymph nodes known as hepatic or hilar lymph nodes located in the hepatic hilum. From these lymph nodes, lymphatic fluid flows to celiac lymph nodes through collecting lymphatic vessels and then drains to the cisterna chyli located at the lower end of the thoracic duct. Lymphatic capillaries running along the hepatic vein merge into 5–6 large lymphatic vessels, which traverse along with the inferior vena cava toward posterior mediastinal lymph nodes through the diaphragm. Lymphatic vessels along the hepatic capsule drain lymphatic fluid underneath the capsule of the convex surface of the liver to regional lymph nodes such as diaphragmatic lymph nodes in the thoracic region and then to mediastinal lymph nodes, similar to those along the hepatic vein.
While studies have suggested specific draining lymph nodes associated with lymphatic vessels in respective areas as mentioned above, the route of lymphatic drainage may be more complex and further analysis may be needed. Lymphatic vessels along the portal tract and the hepatic vein are called “the deep lymphatic system”, while those along the hepatic capsule are called “the superficial lymphatic system”[5]. A recent study[25] that examined drainage patterns of the deep lymphatic system in the mouse liver by lymphangiography showed that hepatic lymphatic fluid was preferentially drained into regional hilar lymph nodes when it came from the right or left lobe. However, hepatic lymphatic fluid from the median lobe was mainly drained to mediastinal lymph nodes rather than hilar lymph nodes. These observations may suggest that the hepatic lymphatic drainage system is organized in a lobe-specific manner in mice.
2. Lymphangiogenesis
2–1. Pro- and anti-lymphangiogenic factors in the liver
In adults, lymphatic vessels generally remain quiescent in normal conditions, and lymphangiogenesis occurs in pathological conditions such as tissue repair, inflammation and tumor-related conditions. Many cytokines and growth factors have been reported to promote or inhibit lymphangiogenesis in other organs. Among them, those cytokines and factors detected in the liver in physiological and pathophysiological conditions (thus not necessarily studied for lymphangiogenesis) are listed in Supplementary Table 2 as potential pro-and anti-lymphangiogenic factors in the liver. Cellular sources of these factors have not been fully identified in the liver.
2–2. VEGF-C/D and VEGFR3 signaling
Signals mediated by members of the VEGF and VEGFR families are known to play central roles in angiogenesis and lymphangiogenesis (Figure 3)[26–28]. VEGF-A binds to VEGFR1/Flt1 and VEGFR2/KDR[29, 30] and mediates angiogenesis, while VEGF-B and placental growth factor (PIGF) bind only to VEGFR1/Flt1[27, 31]. VEGF-C and D bind strongly to VEGFR3/Flt4, leading to lymphangiogenesis, while they also bind very weakly to VEGFR2/KDR[27, 32]. VEGF-C and D are initially synthesized as precursor forms that subsequently undergo proteolytic cleavage, removing their C and N-terminal propeptides for induction of lymphangiogenesis[27]. Currently, five different proteases are known to cleave VEGF-C, including plasmin, ADAMTS3 (A Distintegrin and Metalloprotease with Thrombospondin Motifs-3), prostate-specific antigen, cathepsin D and thrombin. All these proteases except for ADAMTS3 can also activate VEGF-D. The usual VEGF-C cleaving enzyme is ADAMTS3, which requires binding to its cofactor, CCBE1 (collagen and calcium binding EGF domains 1), for successful pro-VEGF-C activation[33, 34]. The inability of ADAMTS3-CCBE1 to activate VEGF-D[33] may suggest that biological events induced by VEGF-C and D are tissue or context dependent.
Figure 3. VEGFs and VEGFRs in angiogenesis and lymphangiogenesis.

VEGF (Vascular endothelial growth factor) is a potent mediator of both angiogenesis and lymphangiogenesis. All members of the VEGF family induce cellular responses by binding to specific VEGF receptors with tyrosine kinases, leading them to dimerize and activate through phosphorylation. VEGF-C and D bind strongly to VEGFR3/Flt4 and induce lymphangiogenesis, while they also bind very weakly to VEGFR2/KDR. VEGF-A binds to VEGFR1/Flt1 and VEGFR2/KDR and mediates angiogenesis. VEGF-B and placental growth factor (PIGF) bind only to VEGFR1/Flt1. Thick and thin arrows indicate strong and weak binding, respectively. Created with BioRender.com.
3. Lymphatics and the immune system
LECs express various chemokines and recruit immune cells. The most-studied chemokine in this regard is C-C motif chemokine ligand 21 (CCL21), a lymphoid homing chemokine[35]. CCL21 guides dendritic cells (DCs), which express its receptor C-C chemokine receptor type 7 (CCR7), and other CCR7-expressing immune cells such as T-cell subsets (naïve, memory and T regulatory T cells) and neutrophils to lymph nodes through lymphatic vessels[36–38]. LECs also express intracellular adhesion molecule 1 (ICAM1)[39] as well as C-X3-C motif chemokine ligand 1 (CX3CL1) to guide DCs to lymphatic vessels in inflamed skin[40]. Further, LEC-derived sphingosine-1-phosphate (S1P) has been identified as a critical lipid mediator molecule that interacts with S1P receptor 1 (S1P1) on T cells to promote their egression from lymph nodes[41] and spleen[42].
Besides T cell migration, LECs also regulate T cell function. Human and murine LECs express major histocompatibility complex (MHC) class I and class II molecules and may directly induce T cell tolerance, which prevents self-activation of T cells to innocuous proteins and self-antigens in normal conditions[43–46]. Further, multiple peripheral tissue antigens (PTAs) expressed in LECs are known to play an important role in inducing T cell tolerance and mediate deletion of self-reactive CD8+ T cells. LECs also secrete immunoregulatory factors, including TGF-β, indoleamine-2,3-dioxygenase (IDO) and nitric oxide, to suppress T cell activation[46, 47].
These observations of LEC’s immunomodulatory function from other organs could be applicable to hepatic LECs and help to understand the role of lymphatic vessels in liver functions. In chronic liver diseases, lymphatics play a role in immune cell trafficking. An increase in the number of CCL21-expressing LECs has been reported in the livers of patients with non-alcoholic steatohepatitis (NASH)[48]. Although the relationship between increased levels of CCL21 and hepatic lymphatics has not been investigated, other studies also reported an increase in hepatic CCL21 expression in primary biliary cholangitis (PBC), primary sclerosing cholangitis (PSC) and alcohol-associated liver disease (ALD)[49, 50]. Similarly, S1P signaling was shown as one of the key players in metabolic diseases and various liver pathologies including NAFLD, NASH and liver fibrosis[51]. The underlying mechanisms have been explored in regard to S1P’s role in hepatocytes and hepatic stellate cells in their regulation of hepatic glucose and lipid metabolism[52]. It is interesting to examine the role of S1P signaling in these diseases through its effect on hepatic lymphatics.
4. Lymphatics in liver diseases
4–1. Hepatic lymphangiogenesis
Lymphangiogenesis with increased and enlarged lymphatic vessels was reported in fibrotic/cirrhotic rat livers induced by carbon tetrachloride (CCl4)[53] as well as in patients with chronic viral hepatitis/cirrhosis[54]. In two rat models of portal hypertension (portacaval shunt and partial portal vein ligation), upregulation of VEGFR3 expression was observed. This observation leads us to speculate the occurrence of lymphangiogenesis in these models of portal hypertension as well[55]. In addition, liver specimens from patients with early-stage PBC showed an increase in the number and the luminal area of lymphatic vessels, indicating that lymphangiogenesis occurred even at the early-stage of PBC[56]. Further, microarray analysis revealed a 4-fold increase in VEGF-D expression in endothelial cells isolated from CCl4-induced cirrhotic rat livers compared with control rat livers[57], suggesting the role of VEGF-D in hepatic lymphangiogenesis observed in CCl4-induced cirrhotic rat livers.
4–2. Hepatic lymph production in liver cirrhosis with portal hypertension
Resistance to sinusoidal blood flow increases in cirrhotic livers because of their architectural deformations due to massive fibrosis. Consequently, hydrostatic pressure in the sinusoids is elevated, and plasma components filtrated through the sinusoids (which form lymph) increase[4, 58]. This mechanism is supported by observations in which lowering portal venous pressure by portocaval shunt surgery decreased the lymph flow rate in cirrhotic dogs[59] and patients[60, 61]. Transjugular intrahepatic portosystemic shunts (TIPSs) were also shown to have the same effect on thoracic duct lymph flow[62]. In cirrhosis, hepatic lymph production significantly increases in both humans[60] and rats[63, 64]. It was reported that patients with liver cirrhosis showed three to six times higher lymph flow rates in the thoracic duct than those patients without cirrhosis[60]. In this study, the thoracic duct was cannulated to measure the flow rate and compositions of lymph. For cirrhotic rats with portal hypertension, one study reported that hepatic lymph flow (measured by cannulating the hepatic lymph trunk) increased nearly 30-fold compared to control rats[63], while another study with cirrhotic rats estimated a 6-fold increase using a similar method of measurements[64]. This discrepancy in the magnitude of flow could be due to the timing of measurements or the severity of cirrhosis. In cirrhotic patients, the diameter of the thoracic duct was also dilated by two to four times, and the lymphatic flow rate of the thoracic duct increased by three to six times[60]. Increased collateral lymph flow in the mediastinum and esophagus results in bloody lymph in the thoracic duct due to communication opened between lymphatic vessels and veins[65].
4–3. Ascites formation
Ascites formation in association with cirrhosis is one of the most recognized clinical manifestations of disorders of the lymphatic system[58]. Currently, the most accepted theory of ascites formation is the “forward theory” (Figure 4)[66–68]. According to this theory, splanchnic arterial vasodilation caused by portal hypertension results in underfilling of the splanchnic arterial circulation or hypovolemia. In moderate stages, hypovolemia is compensated by renal retention of sodium and water[69]. However, severe portal hypertension and splanchnic arterial vasodilation make sodium and water retention persistent and lead to leaking fluid into the peritoneal cavity. In cirrhosis, decreased oncotic pressure caused by hypoalbuminemia also promotes fluid leakage into the peritoneal cavity[70]. As described above, increased intrahepatic vascular resistance due to liver cirrhosis results in elevated hydrostatic pressure and the consequent increase in hepatic lymph production as well.
Figure 4. Mechanisms of ascites formation in liver cirrhosis with portal hypertension.

Elevated hydrostatic pressure in the sinusoids due to liver cirrhosis causes an increased production of lymph. It is thought that ascitic fluid starts to accumulate when capsular or superficial lymphatics of the liver rupture and hepatic lymph with a high protein concentration leaks into the peritoneal cavity. This lymph leakage from the surface of the liver is known as the so-called ‘weeping liver’. When the total fluid flux into the peritoneal cavity exceeds the lymph draining capacity of the peritoneum, ascites forms. According to the “forward theory”, portal hypertension causes excessive arterial vasodilation in the splanchnic arterial circulation, leading to the underfilling of the arterial circulation or hypovolemia. Splanchnic arterial vasodilation makes sodium and water retention persistent and leads to leaking fluid into the peritoneal cavity and accumulation of ascites. Created with BioRender.com.
Levitt et al. looked into the local mechanism of ascites formation in the peritoneal cavity and developed a quantitative model[71]. Equations they formulated described the fluid flux based on hydrostatic pressure and colloid osmotic pressure, and were validated by actual parameters obtained from animal experiments and clinical data. This model demonstrates that an elevation of portal pressure itself does not cause appreciable ascites. Ascitic fluid starts to accumulate when capsular or superficial lymphatics of the liver rupture and hepatic lymph with a high protein concentration leaks into the peritoneal cavity. This ascitic fluid with high colloidal pressure causes transport of water from the mesentery to the peritoneal cavity, which results in further increases of ascitic fluid. The lymph leakage from the surface of the liver has been observed directly by laparoscopy and is known as the so-called ‘weeping liver’[72, 73], and no studies have yet quantified this lymph leakage. It is thought that ascites forms when the total fluid flux into the peritoneal cavity exceeds the lymph draining capacity of the peritoneum, which is approximately 50 ml/hr at most[74–77].
4–4. Malignant tumors
Lymphatic vessels play a pivotal role in the pathogenesis of malignant tumors as a pathway through which tumor cells spread[78, 79]. The incidence of lymph node metastasis differs among malignant tumors. In liver cancer, lymph node metastasis was observed in 5.1% of hepatocellular carcinoma (HCC) and 45.1% of intrahepatic cholangiocarcinoma (ICC)[80]. The prognosis of tumor-bearing patients with lymph node metastasis is worse than the cases without lymph node metastasis. In ICC, the lymphatic vessel density of surgically resected ICCs increased as the extent of malignancy worsened [81] and was correlated with higher incidences of lymphatic metastasis[82].
As mentioned above, CCL21 in LECs recruits CCR7-expressing DCs toward lymphatic vessels and facilitates their egression from the liver[83]. However, CCR7 is also expressed by a variety of malignant tumors, and the CCL21–CCR7 axis is considered as one of the causal factors for lymph node metastasis[84–89]. A positive correlation between expression levels of CCR7 and lymph node metastasis was reported in HCC patients[90].
In addition, many malignant tumors are known to secrete lymphangiogenic factors such as VEGF-C and VEGF-D and promote lymphangiogenesis in adjacent tissues, which helps tumor cells to metastasize to lymph nodes[91]. Many studies have demonstrated that tumor-associated macrophages play a vital role in lymphangiogenesis in malignant tumors by secreting VEGF-C and VEGF-D[92–95]. In ICC, VEGF-C expression was associated with a higher rate of lymph node metastasis and poorer prognosis[81, 96]. In HCC, VEGF-C expression was shown to correlate positively with the size of tumors and the number of extrahepatic metastasis and negatively with disease-free survival time[97]. Plasma VEGF-C levels in patients with liver transplantation for HCC treatment were negatively associated with both their disease-free survival rates and overall survival rates[98].
A recent study demonstrated that ICC-derived platelet-derived growth factor D (PDGF-D) recruited and activated cancer-associated fibroblasts (CAFs) in stromal components adjacent to ICC. Activated CAFs then secreted VEGF-A and VEGF-C, inducing lymphangiogenesis and thereby promoting lymph node metastasis[99]. The study also showed that a VEGFR3 antagonist reduced tumor-associated lymphangiogenesis in a xenograft model using SCID (severe combined immunodeficiency) mice transplanted with PDGF-D secreting human ICC cell lines. No selective agents that specifically suppress lymphangiogenesis have been approved for clinical use[100]. A phase I clinical trial of VEGF-C neutralizing antibody VGX-100 for adult patients with advanced or metastatic solid tumors (NCT01514123) was finished in 2014, but a phase II trial has not been opened yet[101, 102]. A phase I clinical trial of anti-VEGFR3 monoclonal antibody LY3022856/IMC-3C5 for patients with advanced solid tumors demonstrated a minimal anti-tumor effect[103].
Contrarily, interesting findings that VEGF-C/VEGFR3 driven lymphangiogenesis enhances an anti-tumor effect of immunotherapy have recently been reported in some tumors. One study performed inoculation of a melanoma cell line into VEGF-C overexpressing mice intradermally to evaluate a synergistic effect of a VEGFR3 blocking antibody on adaptive immunotherapy[104]. Contrary to expectations, blocking VEGFR3 suppressed the effect of immunotherapy and worsened the survival rate. It was demonstrated that VEGF-C overexpression facilitated intratumoral lymphangiogenesis, upregulated intratumoral CCL21 expression and promoted infiltration of CCR7-expressing naïve T cells into tumors. Conversely, VEGFR3 blocking inhibited intratumoral lymphangiogenesis, decreased intratumoral CCL21 expression and suppressed the number of intratumoral naïve T cells, which may have contributed to decreased sensitivity to immunotherapy. It was also shown that serum VEGF-C concentrations positively correlated with both T-cell activation after peptide vaccination and overall survival rates after a checkpoint blockade therapy in metastatic melanoma patients[104]. Another study using a mouse model of glioblastoma demonstrated that adeno-associated virus (AAV)-mediated VEGF-C gene transfer promoted meningeal lymphatic drainage and improved the survival rate[105]. Ligation of deep cervical lymph nodes, to which cerebrospinal fluid is drained through meningeal lymphatic vessels, as well as depletion of CD4- or CD8-positive T cells negated the beneficial effect of VEGF-C, indicating that the anti-tumor effect of VEGF-C was mediated by T cells that migrated to lymph nodes. It was also shown that VEGF-C gene transfer enhanced the effect of immunotherapy by anti-PD-L1 antibody. These findings indicate complex roles of tumor-associated lymphatic vessels, which act not only as a pathway for tumor dissemination, but also as an integral part of T-cell mediated anti-tumor immunity.
4–5. Liver transplantation
Graft rejection is one of the most serious concerns in solid organ transplantation. Alloimmunity (responses to non-self antigens from the same species) is established once alloantigens of the graft are drained into secondary lymphoid organs through lymphatic vessels and encounter T lymphocytes[106]. Therefore, the potential role of lymphangiogenesis in graft rejection has received considerable attention[4].
Post-transplant lymphangiogenesis in grafts was associated with acute cellular graft rejection in various organs (kidney[107], heart[108] and lung[109]) in humans. However, in a rat model of liver transplantation, increased post-transplant lymphangiogenesis in grafts was associated with long-term survival of recipients for more than 90 days. In addition, rats that had failed to graft by 11 days showed disappearance of lymphatic vessels from severely rejected areas. These observations may suggest that lymphangiogenesis plays a beneficial role in mitigation of inflammation at least in the early stage of liver transplantation[110]. This difference between the liver and other organs might be attributable in part to hepatic immune tolerance[111].
Hepatic immune tolerance was first reported in 1969, describing that liver allotransplantation did not lead to rejection of the second allograft transplanted from the same donor[112]. In liver transplantation, human leukocyte antigen (HLA) mismatch is not a problem[113], and immunosuppressant requirements are more mild compared with other organ transplantations[114]. While the mechanism of hepatic immune tolerance is not fully understood, unique phenotypes of liver resident cells may play a role. For example, it was reported that Kupffer cells suppressed T cell proliferation through secretion of nitric oxide in response to interferon-γ[115] and that LSECs negatively regulated activated T cells via expression of LSEC-specific lectin that recognizes these activated T cells[116]. In addition, different responses of hepatic DCs compared to other DCs may contribute to immune tolerant environments in the liver. One study demonstrated that hepatic DCs secreted anti-inflammatory cytokine IL10 upon toll-like receptor (TLR) 4 stimulation, while peripheral DCs secreted multiple proinflammatory cytokines[117]. This study also showed that CD4-positive T cells initially stimulated by hepatic DCs were less responsive to restimulation than those T cells pre-stimulated by blood DCs. Another study demonstrated that hepatic DCs secreted less inflammatory cytokine IL12 than spleen DCs upon activation by TLR9 ligand[118]. This study also showed that T cells stimulated by hepatic DCs secreted less IFN-γ than those stimulated by spleen DCs. Given that DCs present antigens to T cells in lymph nodes to establish alloimmunity in organ transplantation, hepatic lymphangiogenesis might contribute to immune tolerance in the liver by sending tolerant hepatic DCs to lymph nodes.
4–6. Congenital liver diseases
Lymphedema cholestasis syndrome (LCS) is an autosomal recessive syndrome characterized by primary lymphedema and cholestasis in the neonatal period with intermittent recurrences in childhood. A Norwegian type of LCS (a.k.a., Aagenaes syndrome) has the same haplotype on chromosome 15q and is classified as LCS1[119]. Out of 40 LCS1 patients studied, six patients developed severe cirrhosis with death or liver transplantation in infancy or early childhood, and three patients slowly developed progressive cirrhosis[120]. Recently, a mutation of CCBE1, a secreted protein essential for VEGF-C activation, was reported to be responsible for one LCS patient without the LCS1 mutation[121]. In addition, the CCBE1 mutation was identified in another patient who developed recurrent cholangitis at age 52 with a family history of primary lymphedema in lower limbs[122]. While the mechanism of cholestasis in LCS remains to be elucidated, these clinical findings may indicate that malfunctions of the hepatic lymphatic system are involved in the pathogenesis.
4–7. Non-alcoholic fatty liver disease (NAFLD)
Increased lymphatic vessel density was observed in areas of fibrosis and immune cell infiltration in patients with chronic liver diseases, including NASH[48]. In this study, scRNA-seq analysis of LECs from healthy controls and NASH patients revealed upregulation of genes related to IL13 signaling[48], which has been shown to regulate lymphangiogenesis negatively by inhibiting Prox1 expression as well as migration and proliferation of LECs[123, 124]. This study also showed in a preclinical model of NASH that oxidized low-density lipoprotein (oxLDL), which is known to be elevated in NASH livers[125, 126], could induce IL13 upregulation and reduce Prox1 transcript levels and LEC identity, suggesting an oxLDL-mediated mechanism of increased IL13 expression and LEC identity changes in NASH[48]. Later, this group recapitulated these findings in mice fed a high-fat, high cholesterol diet (another NASH model)[127]. They also showed that lymphatic transport activity was impaired in NASH mice, but it was rescued by administration of recombinant vascular endothelial growth factor C (rVEGF-C) concomitant with amelioration of hepatic inflammation. Drugs with pro-lymphangiogenic properties could be a new therapeutic strategy for NASH.
In recent years, a growing body of evidence has indicated that impairment of lipid transport by the lymphatic system (i.e., lacreals) could have systemic metabolic consequences[128–131]. Similarly, many studies have addressed the relationship between lymphatic dysfunction and obesity. For example, in heterozygous mice lacking Prox1 (Prox1+/–), lymphatic vascular defects and adult-onset obesity with elevated triglycerides were observed[131]. Lymphatic-specific Prox1 restoration rescued the adult-onset obesity phenotype in Prox1+/– mice, directly linking lymphatic dysfunction to the development of obesity. Obesity could also facilitate lymphatic dysfunction. Multiple mouse models of obesity demonstrated impaired lymphatic functions characterized by leaky lymphatics and reduced pumping capacities of collecting lymphatic vessels[128, 132–136]. Thus, it may be interesting to investigate NAFLD with a focus on impairment of lipid transfer due to lymphatic dysfunction.
4–8. Hepatic encephalopathy
Hepatic encephalopathy (HE) is a serious neurologic complication in patients with severe liver dysfunction resulting from acute liver failure or decompensated cirrhosis. In 2015, two groups of investigators re-discovered the presence of meningeal lymphatic vessels located underneath the skull[137, 138] and revealed that they are the major route for discharging waste materials and immune cells from the brain to lymph nodes in the neck called the deep cervical lymph nodes. Because of its critical functions for brain homeostasis, the meningeal lymphatic system has been receiving great attention as a potential therapeutic target for neurodegenerative and neuroinflammatory disorders.
A recent study using rats with 4-week bile duct ligation provided insight into the role of the meningeal lymphatic system in HE[139]. The study demonstrated that overexpression of VEGF-C via adeno-associated virus 8 (AAV8)-VEGF-C injection to the cisterna magna ameliorated HE, including motor dysfunction, by decreasing neuroinflammation and microglia activation through increased meningeal lymphangiogenesis and thereby enhanced meningeal drainage to the cervical lymph nodes. Manipulation of meningeal lymphangiogenesis could be a novel therapeutic strategy for HE.
5. Therapeutic potential and future directions
An increasing body of evidence supports an idea that modulation of lymphangiogenesis or lymphatic drainage is an effective therapeutic strategy for a wide range of pathological conditions[79, 140], including rheumatoid arthritis[141], psoriasis-like chronic dermatitis[142], inflammatory bowel disease[143], interstitial nephritis[144] and glioblastoma[105]. For liver diseases, several preclinical studies have shown that administration of VEGF-C or D ameliorates disease conditions by increasing lymphangiogenesis and lymphatic drainage. On the other hand, blocking lymphangiogenesis could also be an appropriate option for diseases such as cholangiocarcinoma where tumor-induced lymphangiogenesis may promote metastasis of malignant tumors.
An understanding of detailed molecular and cellular mechanisms of hepatic lymphangiogenesis is essential for its modulation. It is not adequately known which cells produce pro- or anti-lymphangiogenic factors and regulate hepatic lymphangiogenesis. Macrophages have been recognized for regulation of hepatic lymphangiogenesis by producing lymphangiogenic factors such as VEGF-C and D at the site of lymphatic vessel expansion[145]. Cholangiocarcinoma cells produce PDGF-D, which induces VEGF-C expression in myofibroblasts in tumor microenvironments, facilitating lymphangiogenesis and subsequent metastasis[99]. These observations may indicate that distinct mechanisms of lymphangiogenesis exist in different liver diseases.
While blocking VEGF-C is effective for decreasing lymphatic vessels and may prevent tumor metastasis, it will also reduce recruitment of T-cells for their tumor-killing activity[105]. Thus, identification of alternative lymphangiogenic pathways other than the VEGF-C/D/VEGFR3 axis, including additional pro- and anti-lymphangiogenic factors, will likely increase novel therapeutic options for cholangiocarcinoma in particular and for liver diseases in general[4]. A recent study showed thrombospondin (THBS)1, THBS2 and pigment epithelial-derived factor (PEDF) from ICC in intrahepatic tumor microenvironments promoted cancer-associated lymhangiogenesis, suggesting that THBS1, THBS2 and PEDF could be promising targets to reduce cancer-associated lymphangiogenesis and counteract invasiveness of ICC[146].
An origin of LECs for hepatic lymphangiogenesis in pathological conditions remains to be identified. A previous study reported a very small contribution of bone marrow-derived hematopoietic stem cells to LECs in the regenerating mouse liver[147]. Further studies with lineage tracing analysis are warranted to identify the origin of LECs in hepatic lymphangiogenesis.
Finally, it can be expected that hepatic lymph contains 80 to 90% of the proteins present in plasma[1, 3]. The content of the lymph, including self-peptides derived from intracellular, membrane-associated or matrix proteins, has been gaining attention as it may represent local conditions more accurately than blood[148, 149], suggesting the potential for containing more useful biomarkers. Moreover, lymph carries apoptotic cellular materials, cytokines, cell-derived microparticles and infectious agents, mediating communications between lymph generating organs (e.g., liver) and their associated draining lymph nodes, which is critical for host defense[149]. The relevance of small molecules and vesicles discharged via lymphatics to the etiology of liver diseases remains to be explored.
In conclusion, the lymphatic system in the liver remains underexplored. Given its pivotal homeostatic roles in the liver and other organs, investigations in this area will advance our knowledge of liver physiology and pathophysiology and lead to the development of effective therapies for liver diseases.
Supplementary Material
Key points.
The liver is recognized as the largest lymph producing organ.
Hepatic lymphatic vessels are involved in fluid homeostasis, the immune system and lipid metabolism by transporting various immune cells, antigens and lipids to lymph nodes.
While lymphatic vessel numbers are known to increase (via lymphangiogenesis) in many liver diseases, the mechanism of lymphangiogenesis in the liver remains poorly understood.
VEGF-C and D bind to VEGFR3/Flt4, leading to lymphangiogenesis, while VEGF-A binds to VEGFR1/Flt1 and VEGFR2/KDR, mediating angiogenesis.
Ascites formation in association with cirrhosis is one of the most recognized clinical manifestations of disorders of the lymphatic system.
While lymphatics help to reduce inflammation, they also serve as routes of cancer metastasis.
Modulation of lymphangiogenesis or lymphatic drainage could be an effective therapeutic strategy for a wide range of pathological conditions, including liver diseases.
Acknowledgements
We would like to thank Drs. Teruo Utsumi and Matthew McConnell (Yale University) for critical reading and editing.
Financial support
This study was supported by NIH grants (1R56DK121511, 1R01AA025342 and R01DK117597) to YI.
List of abbreviations
- NAFLD
non-alcoholic fatty liver disease
- LECs
lymphatic endothelial cells
- VEGFR3
vascular endothelial growth factor receptor 3
- Lyve1
lymphatic vessel endothelial hyaluronan receptor 1
- Prox1
prospero homeobox protein 1
- LSEC
liver sinusoidal endothelial cell
- ECs
endothelial cells
- IL7
interleukin 7
- PlGF
placental growth factor
- ADAMTS3
A Distintegrin and Metalloprotease with Thrombospondin Motifs-3
- CCBE1
collagen and calcium binding EGF domains 1
- DCs
dendritic cells
- CCL21
C-C motif chemokine ligand 21
- CCR7
C-C chemokine receptor type 7
- ICAM1
intracellular adhesion molecule 1
- CX3CL1
C-X3-C motif chemokine ligand 1
- S1P
sphingosine-1-phosphate
- S1P1
S1P receptor 1
- MHC
histocompatibility complex
- PTAs
peripheral tissue antigens
- IDO
indoleamine-2,3-dioxygenase
- NASH
non-alcoholic steatohepatitis
- PBC
primary biliary cholangitis
- PSC
primary sclerosing cholangitis
- ALD
alcohol-associated liver disease
- CCl4
carbon tetrachloride
- TIPS
Transjugular intrahepatic portosystemic shunt
- HCC
hepatocellular carcinoma
- ICC
intrahepatic cholangiocarcinoma
- AAV
adeno-associated virus
- PDGF-D
platelet-derived growth factor D
- CAFs
cancer-associated fibroblasts
- HLA
human leukocyte antigen
- TLR4
toll-like receptor 4
- LSC
lymphedema cholestasis syndrome
- HE
hepatic encephalopathy
- oxLDL
oxidized low-density lipoprotein
- PEDF
pigment epithelial-derived factor
- THBS1
thrombospondin 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have declared that no conflict of interest exists.
References
- [1].Morris B The hepatic and intestinal contributions to the thoracic duct lymph. Q J Exp Physiol Cogn Med Sci 1956;41:318–325. [DOI] [PubMed] [Google Scholar]
- [2].Cain JC, Grindlay JH, et al. Lymph from liver and thoracic duct; an experimental study. Surg Gynecol Obstet 1947;85:558–562. [PubMed] [Google Scholar]
- [3].Mobley WP, Kintner K, Witte CL, Witte MH. Contribution of the liver to thoracic duct lymph flow in a motionless subject. Lymphology 1989;22:81–84. [PubMed] [Google Scholar]
- [4].Tanaka M, Iwakiri Y. The Hepatic Lymphatic Vascular System: Structure, Function, Markers, and Lymphangiogenesis. Cell Mol Gastroenterol Hepatol 2016;2:733–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ohtani O, Ohtani Y. Lymph circulation in the liver. Anat Rec (Hoboken) 2008;291:643–652. [DOI] [PubMed] [Google Scholar]
- [6].Zheng W, Aspelund A, Alitalo K. Lymphangiogenic factors, mechanisms, and applications. J Clin Invest 2014;124:878–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tammela T, Alitalo K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell 2010;140:460–476. [DOI] [PubMed] [Google Scholar]
- [8].Norrmen C, Tammela T, Petrova TV, Alitalo K. Biological basis of therapeutic lymphangiogenesis. Circulation 2011;123:1335–1351. [DOI] [PubMed] [Google Scholar]
- [9].Baluk P, Fuxe J, Hashizume H, Romano T, Lashnits E, Butz S, et al. Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med 2007;204:2349–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Dejana E, Orsenigo F, Molendini C, Baluk P, McDonald DM. Organization and signaling of endothelial cell-to-cell junctions in various regions of the blood and lymphatic vascular trees. Cell Tissue Res 2009;335:17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Yao LC, Baluk P, Srinivasan RS, Oliver G, McDonald DM. Plasticity of button-like junctions in the endothelium of airway lymphatics in development and inflammation. Am J Pathol 2012;180:2561–2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cifarelli V, Eichmann A. The Intestinal Lymphatic System: Functions and Metabolic Implications. Cell Mol Gastroenterol Hepatol 2019;7:503–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, Kukk E, et al. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J 1996;15:290–298. [PMC free article] [PubMed] [Google Scholar]
- [14].Kukk E, Lymboussaki A, Taira S, Kaipainen A, Jeltsch M, Joukov V, et al. VEGF-C receptor binding and pattern of expression with VEGFR-3 suggests a role in lymphatic vascular development. Development 1996;122:3829–3837. [DOI] [PubMed] [Google Scholar]
- [15].Banerji S, Ni J, Wang SX, Clasper S, Su J, Tammi R, et al. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol 1999;144:789–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Prevo R, Banerji S, Ferguson DJ, Clasper S, Jackson DG. Mouse LYVE-1 is an endocytic receptor for hyaluronan in lymphatic endothelium. J Biol Chem 2001;276:19420–19430. [DOI] [PubMed] [Google Scholar]
- [17].Wigle JT, Harvey N, Detmar M, Lagutina I, Grosveld G, Gunn MD, et al. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J 2002;21:1505–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Breiteneder-Geleff S, Soleiman A, Kowalski H, Horvat R, Amann G, Kriehuber E, et al. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am J Pathol 1999;154:385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Tanaka M, Iwakiri Y. Lymphatics in the liver. Curr Opin Immunol 2018;53:137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Su T, Yang Y, Lai S, Jeong J, Jung Y, McConnell M, et al. Single-Cell Transcriptomics Reveals Zone-Specific Alterations of Liver Sinusoidal Endothelial Cells in Cirrhosis. Cell Mol Gastroenterol Hepatol 2021;11:1139–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Poonkhum R, Pisetpaisan K, Wang BJ, Anupunpisit V, Ohtani Y, Ohtani O. Origins and pathways of fluid entering sublobular lymphatic vessels in cat livers. Arch Histol Cytol 2003;66:317–326. [DOI] [PubMed] [Google Scholar]
- [22].Comparini L Lymph vessels of the liver in man. Microscopic morphology and histotopography. Angiologica 1969;6:262–274. [DOI] [PubMed] [Google Scholar]
- [23].Ritchie HD, Grindlay JH, Bollman JL. Flow of lymph from the canine liver. Am J Physiol 1959;196:105–109. [DOI] [PubMed] [Google Scholar]
- [24].Barbier L, Tay SS, McGuffog C, Triccas JA, McCaughan GW, Bowen DG, et al. Two lymph nodes draining the mouse liver are the preferential site of DC migration and T cell activation. J Hepatol 2012;57:352–358. [DOI] [PubMed] [Google Scholar]
- [25].Frenkel NC, Poghosyan S, Verheem A, Padera TP, Rinkes I, Kranenburg O, et al. Liver lymphatic drainage patterns follow segmental anatomy in a murine model. Sci Rep 2020;10:21808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 1989;246:1306–1309. [DOI] [PubMed] [Google Scholar]
- [27].Alitalo K, Carmeliet P. Molecular mechanisms of lymphangiogenesis in health and disease. Cancer Cell 2002;1:219–227. [DOI] [PubMed] [Google Scholar]
- [28].Ferrara N Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev 2004;25:581–611. [DOI] [PubMed] [Google Scholar]
- [29].de Vries C, Escobedo JA, Ueno H, Houck K, Ferrara N, Williams LT. The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science 1992;255:989–991. [DOI] [PubMed] [Google Scholar]
- [30].Terman BI, Dougher-Vermazen M, Carrion ME, Dimitrov D, Armellino DC, Gospodarowicz D, et al. Identification of the KDR tyrosine kinase as a receptor for vascular endothelial cell growth factor. Biochem Biophys Res Commun 1992;187:1579–1586. [DOI] [PubMed] [Google Scholar]
- [31].Olofsson B, Korpelainen E, Pepper MS, Mandriota SJ, Aase K, Kumar V, et al. Vascular endothelial growth factor B (VEGF-B) binds to VEGF receptor-1 and regulates plasminogen activator activity in endothelial cells. Proc Natl Acad Sci U S A 1998;95:11709–11714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Achen MG, Jeltsch M, Kukk E, Makinen T, Vitali A, Wilks AF, et al. Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4). Proc Natl Acad Sci U S A 1998;95:548–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bui HM, Enis D, Robciuc MR, Nurmi HJ, Cohen J, Chen M, et al. Proteolytic activation defines distinct lymphangiogenic mechanisms for VEGFC and VEGFD. J Clin Invest 2016;126:2167–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Le Guen L, Karpanen T, Schulte D, Harris NC, Koltowska K, Roukens G, et al. Ccbe1 regulates Vegfc-mediated induction of Vegfr3 signaling during embryonic lymphangiogenesis. Development 2014;141:1239–1249. [DOI] [PubMed] [Google Scholar]
- [35].Lo JC, Chin RK, Lee Y, Kang HS, Wang Y, Weinstock JV, et al. Differential regulation of CCL21 in lymphoid/nonlymphoid tissues for effectively attracting T cells to peripheral tissues. J Clin Invest 2003;112:1495–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Unsoeld H, Mueller K, Schleicher U, Bogdan C, Zwirner J, Voehringer D, et al. Abrogation of CCL21 chemokine function by transgenic over-expression impairs T cell immunity to local infections. International immunology 2007;19:1281–1289. [DOI] [PubMed] [Google Scholar]
- [37].Beauvillain C, Cunin P, Doni A, Scotet M, Jaillon S, Loiry ML, et al. CCR7 is involved in the migration of neutrophils to lymph nodes. Blood 2011;117:1196–1204. [DOI] [PubMed] [Google Scholar]
- [38].Saeki H, Moore AM, Brown MJ, Hwang ST. Cutting edge: secondary lymphoid-tissue chemokine (SLC) and CC chemokine receptor 7 (CCR7) participate in the emigration pathway of mature dendritic cells from the skin to regional lymph nodes. Journal of immunology (Baltimore, Md : 1950) 1999;162:2472–2475. [PubMed] [Google Scholar]
- [39].Podgrabinska S, Kamalu O, Mayer L, Shimaoka M, Snoeck H, Randolph GJ, et al. Inflamed lymphatic endothelium suppresses dendritic cell maturation and function via Mac-1/ICAM-1-dependent mechanism. Journal of immunology (Baltimore, Md : 1950) 2009;183:1767–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Johnson LA, Jackson DG. The chemokine CX3CL1 promotes trafficking of dendritic cells through inflamed lymphatics. Journal of cell science 2013;126:5259–5270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Schwab SR, Cyster JG. Finding a way out: lymphocyte egress from lymphoid organs. Nature immunology 2007;8:1295–1301. [DOI] [PubMed] [Google Scholar]
- [42].Lucaciu A, Kuhn H, Trautmann S, Ferreiros N, Steinmetz H, Pfeilschifter J, et al. A Sphingosine 1-Phosphate Gradient Is Linked to the Cerebral Recruitment of T Helper and Regulatory T Helper Cells during Acute Ischemic Stroke. Int J Mol Sci 2020;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Rouhani SJ, Eccles JD, Riccardi P, Peske JD, Tewalt EF, Cohen JN, et al. Roles of lymphatic endothelial cells expressing peripheral tissue antigens in CD4 T-cell tolerance induction. Nature communications 2015;6:6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Vokali E, Yu SS. Lymphatic endothelial cells prime naïve CD8(+) T cells into memory cells under steady-state conditions. 2020;11:538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Lund AW, Duraes FV, Hirosue S, Raghavan VR, Nembrini C, Thomas SN, et al. VEGF-C promotes immune tolerance in B16 melanomas and cross-presentation of tumor antigen by lymph node lymphatics. Cell Rep 2012;1:191–199. [DOI] [PubMed] [Google Scholar]
- [46].Malhotra D, Fletcher AL, Astarita J, Lukacs-Kornek V, Tayalia P, Gonzalez SF, et al. Transcriptional profiling of stroma from inflamed and resting lymph nodes defines immunological hallmarks. Nature immunology 2012;13:499–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Lukacs-Kornek V, Malhotra D, Fletcher AL, Acton SE, Elpek KG, Tayalia P, et al. Regulated release of nitric oxide by nonhematopoietic stroma controls expansion of the activated T cell pool in lymph nodes. Nature immunology 2011;12:1096–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Tamburini BAJ, Finlon JM, Gillen AE, Kriss MS, Riemondy KA, Fu R, et al. Chronic Liver Disease in Humans Causes Expansion and Differentiation of Liver Lymphatic Endothelial Cells. Frontiers in immunology 2019;10:1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Grant AJ, Goddard S, Ahmed-Choudhury J, Reynolds G, Jackson DG, Briskin M, et al. Hepatic expression of secondary lymphoid chemokine (CCL21) promotes the development of portal-associated lymphoid tissue in chronic inflammatory liver disease. Am J Pathol 2002;160:1445–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Borchers AT, Shimoda S, Bowlus C, Keen CL, Gershwin ME. Lymphocyte recruitment and homing to the liver in primary biliary cirrhosis and primary sclerosing cholangitis. Seminars in immunopathology 2009;31:309–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Kleuser B Divergent Role of Sphingosine 1-Phosphate in Liver Health and Disease. Int J Mol Sci 2018;19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Frej C, Linder A, Happonen KE, Taylor FB, Lupu F, Dahlbäck B. Sphingosine 1-phosphate and its carrier apolipoprotein M in human sepsis and in Escherichia coli sepsis in baboons. Journal of cellular and molecular medicine 2016;20:1170–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Vollmar B, Wolf B, Siegmund S, Katsen AD, Menger MD. Lymph vessel expansion and function in the development of hepatic fibrosis and cirrhosis. Am J Pathol 1997;151:169–175. [PMC free article] [PubMed] [Google Scholar]
- [54].Yamauchi Y, Michitaka K, Onji M. Morphometric analysis of lymphatic and blood vessels in human chronic viral liver diseases. Am J Pathol 1998;153:1131–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Guérin F, Wagner M, Liné A, Zappa M, Fasseu M, Paradis V, et al. Hepatic proliferation and angiogenesis markers are increased after portal deprivation in rats: a study of molecular, histological and radiological changes. PLoS One 2015;10:e0125493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Yamauchi Y, Ikeda R, Michitaka K, Hiasa Y, Horiike N, Onji M. Morphometric analysis of lymphatic vessels in primary biliary cirrhosis. Hepatol Res 2002;24:107. [DOI] [PubMed] [Google Scholar]
- [57].Tugues S, Morales-Ruiz M, Fernandez-Varo G, Ros J, Arteta D, Munoz-Luque J, et al. Microarray analysis of endothelial differentially expressed genes in liver of cirrhotic rats. Gastroenterology 2005;129:1686–1695. [DOI] [PubMed] [Google Scholar]
- [58].Chung C, Iwakiri Y. The lymphatic vascular system in liver diseases: its role in ascites formation. Clin Mol Hepatol 2013;19:99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Orloff MJ, Goodhead B, Windsor CW, Musicant ME, Annetts DL. Effect of portacaval shunts on lymph flow in the thoracic duct. Experiments with normal dogs and dogs with cirrhosis and ascites. American journal of surgery 1967;114:213–221. [DOI] [PubMed] [Google Scholar]
- [60].Dumont AE, Mulholland JH. Flow rate and composition of thoracic-duct lymph in patients with cirrhosis. N Engl J Med 1960;263:471–474. [DOI] [PubMed] [Google Scholar]
- [61].Witte MH, Dumont AE, Cole WR, Witte CL, Kintner K. Lymph circulation in hepatic cirrhosis: effect of portacaval shunt. Annals of internal medicine 1969;70:303–310. [DOI] [PubMed] [Google Scholar]
- [62].Vignaux O, Gouya H, Dousset B, Mazuir E, Buffet C, Calmus Y, et al. Refractory chylothorax in hepatic cirrhosis: successful treatment by transjugular intrahepatic portosystemic shunt. Journal of thoracic imaging 2002;17:233–236. [DOI] [PubMed] [Google Scholar]
- [63].Barrowman JA, Granger DN. Effects of experimental cirrhosis on splanchnic microvascular fluid and solute exchange in the rat. Gastroenterology 1984;87:165–172. [PubMed] [Google Scholar]
- [64].Nix JT, Flock EV, Bollman JL. Influence of cirrhosis on proteins of cisternal lymph. Am J Physiol 1951;164:117–118. [DOI] [PubMed] [Google Scholar]
- [65].Dumont AE, Witte CL, Witte MH, Cole WR. Origin of red blood cells in thoracic duct lymph in hepatic cirrhosis. Annals of surgery 1970;171:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].De Franchis R, Salerno F. Pathogenesis of ascites and predictors of resistance to therapy. J Gastroenterol Hepatol 2002;17 Suppl 3:S242–247. [DOI] [PubMed] [Google Scholar]
- [67].Arroyo V, Colmenero J. Ascites and hepatorenal syndrome in cirrhosis: pathophysiological basis of therapy and current management. J Hepatol 2003;38 Suppl 1:S69–89. [DOI] [PubMed] [Google Scholar]
- [68].Gordon FD. Ascites. Clin Liver Dis 2012;16:285–299. [DOI] [PubMed] [Google Scholar]
- [69].Schrier RW, Arroyo V, Bernardi M, Epstein M, Henriksen JH, Rodes J. Peripheral arterial vasodilation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology 1988;8:1151–1157. [DOI] [PubMed] [Google Scholar]
- [70].Mankin H, Lowell A. OSMOTIC FACTORS INFLUENCING THE FORMATION OF ASCITES IN PATIENTS WITH CIRRHOSIS OF THE LIVER. J Clin Invest 1948;27:145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Levitt DG, Levitt MD. Quantitative modeling of the physiology of ascites in portal hypertension. BMC Gastroenterol 2012;12:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Witte CL, Witte MH, Dumont AE, Frist J, Cole WR. Lymph protein in hepatic cirrhosis and experimental hepatic and portal venous hypertension. Annals of surgery 1968;168:567–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Dumont AE, Mulholland JH. Alterations in Thoracic Duct Lymph Flow in Hepatic Cirrhosis: Significance in Portal Hypertension. Annals of surgery 1962;156:668–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Leak LV, Rahil K. Permeability of the diaphragmatic mesothelium: the ultrastructural basis for “stomata”. The American journal of anatomy 1978;151:557–593. [DOI] [PubMed] [Google Scholar]
- [75].Henriksen JH, Lassen NA, Parving HH, Winkler K. Filtration as the main transport mechanism of protein exchange between plasma and the peritoneal cavity in hepatic cirrhosis. Scandinavian journal of clinical and laboratory investigation 1980;40:503–513. [DOI] [PubMed] [Google Scholar]
- [76].Henriksen JH, Siemssen O, Krintel JJ, Malchow-Møller A, Bendtsen F, Ring-Larsen H. Dynamics of albumin in plasma and ascitic fluid in patients with cirrhosis. J Hepatol 2001;34:53–60. [DOI] [PubMed] [Google Scholar]
- [77].Sanyal AJ, Bosch J, Blei A, Arroyo V. Portal hypertension and its complications. Gastroenterology 2008;134:1715–1728. [DOI] [PubMed] [Google Scholar]
- [78].Stacker SA, Williams SP, Karnezis T, Shayan R, Fox SB, Achen MG. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat Rev Cancer 2014;14:159–172. [DOI] [PubMed] [Google Scholar]
- [79].Oliver G, Kipnis J, Randolph GJ, Harvey NL. The Lymphatic Vasculature in the 21(st) Century: Novel Functional Roles in Homeostasis and Disease. Cell 2020;182:270–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Sun HC, Zhuang PY, Qin LX, Ye QH, Wang L, Ren N, et al. Incidence and prognostic values of lymph node metastasis in operable hepatocellular carcinoma and evaluation of routine complete lymphadenectomy. J Surg Oncol 2007;96:37–45. [DOI] [PubMed] [Google Scholar]
- [81].Aishima S, Nishihara Y, Iguchi T, Taguchi K, Taketomi A, Maehara Y, et al. Lymphatic spread is related to VEGF-C expression and D2–40-positive myofibroblasts in intrahepatic cholangiocarcinoma. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc 2008;21:256–264. [DOI] [PubMed] [Google Scholar]
- [82].Thelen A, Scholz A, Weichert W, Wiedenmann B, Neuhaus P, Gessner R, et al. Tumor-associated angiogenesis and lymphangiogenesis correlate with progression of intrahepatic cholangiocarcinoma. Am J Gastroenterol 2010;105:1123–1132. [DOI] [PubMed] [Google Scholar]
- [83].Förster R, Davalos-Misslitz AC, Rot A. CCR7 and its ligands: balancing immunity and tolerance. Nature reviews Immunology 2008;8:362–371. [DOI] [PubMed] [Google Scholar]
- [84].Günther K, Leier J, Henning G, Dimmler A, Weissbach R, Hohenberger W, et al. Prediction of lymph node metastasis in colorectal carcinoma by expressionof chemokine receptor CCR7. International journal of cancer 2005;116:726–733. [DOI] [PubMed] [Google Scholar]
- [85].Cabioglu N, Yazici MS, Arun B, Broglio KR, Hortobagyi GN, Price JE, et al. CCR7 and CXCR4 as novel biomarkers predicting axillary lymph node metastasis in T1 breast cancer. Clinical cancer research : an official journal of the American Association for Cancer Research 2005;11:5686–5693. [DOI] [PubMed] [Google Scholar]
- [86].Mashino K, Sadanaga N, Yamaguchi H, Tanaka F, Ohta M, Shibuta K, et al. Expression of chemokine receptor CCR7 is associated with lymph node metastasis of gastric carcinoma. Cancer research 2002;62:2937–2941. [PubMed] [Google Scholar]
- [87].Irino T, Takeuchi H, Matsuda S, Saikawa Y, Kawakubo H, Wada N, et al. CC-Chemokine receptor CCR7: a key molecule for lymph node metastasis in esophageal squamous cell carcinoma. BMC cancer 2014;14:291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Zhang S, Wang H, Xu Z, Bai Y, Xu L. Lymphatic Metastasis of NSCLC Involves Chemotaxis Effects of Lymphatic Endothelial Cells through the CCR7-CCL21 Axis Modulated by TNF-α. Genes 2020;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Pang MF, Georgoudaki AM, Lambut L, Johansson J, Tabor V, Hagikura K, et al. TGF-β1-induced EMT promotes targeted migration of breast cancer cells through the lymphatic system by the activation of CCR7/CCL21-mediated chemotaxis. Oncogene 2016;35:748–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Schimanski CC, Bahre R, Gockel I, Junginger T, Simiantonaki N, Biesterfeld S, et al. Chemokine receptor CCR7 enhances intrahepatic and lymphatic dissemination of human hepatocellular cancer. Oncology reports 2006;16:109–113. [PubMed] [Google Scholar]
- [91].Das S, Skobe M. Lymphatic vessel activation in cancer. Ann N Y Acad Sci 2008;1131:235–241. [DOI] [PubMed] [Google Scholar]
- [92].Schoppmann SF, Birner P, Stockl J, Kalt R, Ullrich R, Caucig C, et al. Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am J Pathol 2002;161:947–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Skobe M, Hamberg LM, Hawighorst T, Schirner M, Wolf GL, Alitalo K, et al. Concurrent induction of lymphangiogenesis, angiogenesis, and macrophage recruitment by vascular endothelial growth factor-C in melanoma. Am J Pathol 2001;159:893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Iwata C, Kano MR, Komuro A, Oka M, Kiyono K, Johansson E, et al. Inhibition of cyclooxygenase-2 suppresses lymph node metastasis via reduction of lymphangiogenesis. Cancer research 2007;67:10181–10189. [DOI] [PubMed] [Google Scholar]
- [95].Schoppmann SF, Fenzl A, Nagy K, Unger S, Bayer G, Geleff S, et al. VEGF-C expressing tumor-associated macrophages in lymph node positive breast cancer: impact on lymphangiogenesis and survival. Surgery 2006;139:839–846. [DOI] [PubMed] [Google Scholar]
- [96].Park BK, Paik YH, Park JY, Park KH, Bang S, Park SW, et al. The clinicopathologic significance of the expression of vascular endothelial growth factor-C in intrahepatic cholangiocarcinoma. American journal of clinical oncology 2006;29:138–142. [DOI] [PubMed] [Google Scholar]
- [97].Yamaguchi R, Yano H, Nakashima O, Akiba J, Nishida N, Kurogi M, et al. Expression of vascular endothelial growth factor-C in human hepatocellular carcinoma. J Gastroenterol Hepatol 2006;21:152–160. [DOI] [PubMed] [Google Scholar]
- [98].Duda DG, Dima SO, Cucu D, Sorop A, Klein S, Ancukiewicz M, et al. Potential Circulating Biomarkers of Recurrence after Hepatic Resection or Liver Transplantation in Hepatocellular Carcinoma Patients. Cancers 2020;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Cadamuro M, Brivio S, Mertens J, Vismara M, Moncsek A, Milani C, et al. Platelet-derived growth factor-D enables liver myofibroblasts to promote tumor lymphangiogenesis in cholangiocarcinoma. Journal of hepatology 2019;70:700–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Yamakawa M, Doh SJ, Santosa SM, Montana M, Qin EC, Kong H, et al. Potential lymphangiogenesis therapies: Learning from current antiangiogenesis therapies-A review. Medicinal research reviews 2018;38:1769–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Tampellini M, Sonetto C, Scagliotti GV. Novel anti-angiogenic therapeutic strategies in colorectal cancer. Expert Opin Investig Drugs 2016:1–14. [DOI] [PubMed] [Google Scholar]
- [102].Falchook GS, Goldman JW, Desai J, Leitch I, Hong DS, Subbiah V, et al. A first-in-human phase I study of VGX-100, a selective anti-VEGF-C antibody, alone and in combination with bevacizumab in patients with advanced solid tumors. Journal of Clinical Oncology 2014;32:2524–2524. [Google Scholar]
- [103].Saif MW, Knost JA, Chiorean EG, Kambhampati SR, Yu D, Pytowski B, et al. Phase 1 study of the anti-vascular endothelial growth factor receptor 3 monoclonal antibody LY3022856/IMC-3C5 in patients with advanced and refractory solid tumors and advanced colorectal cancer. Cancer chemotherapy and pharmacology 2016;78:815–824. [DOI] [PubMed] [Google Scholar]
- [104].Fankhauser M, Broggi MAS, Potin L, Bordry N, Jeanbart L, Lund AW, et al. Tumor lymphangiogenesis promotes T cell infiltration and potentiates immunotherapy in melanoma. Science translational medicine 2017;9. [DOI] [PubMed] [Google Scholar]
- [105].Song E, Mao T, Dong H, Boisserand LSB, Antila S, Bosenberg M, et al. VEGF-C-driven lymphatic drainage enables immunosurveillance of brain tumours. Nature 2020;577:689–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Liao S, von der Weid PY. Lymphatic system: an active pathway for immune protection. Semin Cell Dev Biol 2015;38:83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Kerjaschki D, Regele HM, Moosberger I, Nagy-Bojarski K, Watschinger B, Soleiman A, et al. Lymphatic neoangiogenesis in human kidney transplants is associated with immunologically active lymphocytic infiltrates. J Am Soc Nephrol 2004;15:603–612. [DOI] [PubMed] [Google Scholar]
- [108].Geissler HJ, Dashkevich A, Fischer UM, Fries JW, Kuhn-Regnier F, Addicks K, et al. First year changes of myocardial lymphatic endothelial markers in heart transplant recipients. Eur J Cardiothorac Surg 2006;29:767–771. [DOI] [PubMed] [Google Scholar]
- [109].Dashkevich A, Heilmann C, Kayser G, Germann M, Beyersdorf F, Passlick B, et al. Lymph angiogenesis after lung transplantation and relation to acute organ rejection in humans. Ann Thorac Surg 2010;90:406–411. [DOI] [PubMed] [Google Scholar]
- [110].Ishii E, Shimizu A, Kuwahara N, Arai T, Kataoka M, Wakamatsu K, et al. Lymphangiogenesis associated with acute cellular rejection in rat liver transplantation. Transplant Proc 2010;42:4282–4285. [DOI] [PubMed] [Google Scholar]
- [111].Dai H, Zheng Y, Thomson AW, Rogers NM. Transplant Tolerance Induction: Insights From the Liver. Frontiers in immunology 2020;11:1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Calne RY, Sells RA, Pena JR, Davis DR, Millard PR, Herbertson BM, et al. Induction of immunological tolerance by porcine liver allografts. Nature 1969;223:472–476. [DOI] [PubMed] [Google Scholar]
- [113].Lan X, Zhang MM, Pu CL, Guo CB, Kang Q, Li YC, et al. Impact of human leukocyte antigen mismatching on outcomes of liver transplantation: a meta-analysis. World journal of gastroenterology 2010;16:3457–3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Rodríguez-Perálvarez M, Germani G, Papastergiou V, Tsochatzis E, Thalassinos E, Luong TV, et al. Early tacrolimus exposure after liver transplantation: relationship with moderate/severe acute rejection and long-term outcome. Journal of hepatology 2013;58:262–270. [DOI] [PubMed] [Google Scholar]
- [115].Roland CR, Walp L, Stack RM, Flye MW. Outcome of Kupffer cell antigen presentation to a cloned murine Th1 lymphocyte depends on the inducibility of nitric oxide synthase by IFN-gamma. Journal of immunology (Baltimore, Md : 1950) 1994;153:5453–5464. [PubMed] [Google Scholar]
- [116].Tang L, Yang J, Liu W, Tang X, Chen J, Zhao D, et al. Liver sinusoidal endothelial cell lectin, LSECtin, negatively regulates hepatic T-cell immune response. Gastroenterology 2009;137:1498–1508.e1491– 1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Bamboat ZM, Stableford JA, Plitas G, Burt BM, Nguyen HM, Welles AP, et al. Human liver dendritic cells promote T cell hyporesponsiveness. Journal of immunology (Baltimore, Md : 1950) 2009;182:1901–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Abe M, Tokita D, Raimondi G, Thomson AW. Endotoxin modulates the capacity of CpG-activated liver myeloid DC to direct Th1-type responses. European journal of immunology 2006;36:2483–2493. [DOI] [PubMed] [Google Scholar]
- [119].Drivdal M, Slagsvold CE, Aagenaes O, Kase BF. Hereditary lymphedema, characteristics, and variations in 17 adult patients with lymphedema cholestasis syndrome 1/Aagenaes syndrome. Lymphatic research and biology 2014;12:251–257. [DOI] [PubMed] [Google Scholar]
- [120].Drivdal M, Trydal T, Hagve TA, Bergstad I, Aagenaes O. Prognosis, with evaluation of general biochemistry, of liver disease in lymphoedema cholestasis syndrome 1 (LCS1/Aagenaes syndrome). Scandinavian journal of gastroenterology 2006;41:465–471. [DOI] [PubMed] [Google Scholar]
- [121].Shah S, Conlin LK, Gomez L, Aagenaes O, Eiklid K, Knisely AS, et al. CCBE1 mutation in two siblings, one manifesting lymphedema-cholestasis syndrome, and the other, fetal hydrops. PloS one 2013;8:e75770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Viveiros A, Reiterer M, Schaefer B, Finkenstedt A, Schneeberger S, Schwaighofer H, et al. CCBE1 mutation causing sclerosing cholangitis: Expanding the spectrum of lymphedema-cholestasis syndrome. Hepatology (Baltimore, Md) 2017;66:286–288. [DOI] [PubMed] [Google Scholar]
- [123].Shin K, Kataru RP, Park HJ, Kwon BI, Kim TW, Hong YK, et al. TH2 cells and their cytokines regulate formation and function of lymphatic vessels. Nature communications 2015;6:6196. [DOI] [PubMed] [Google Scholar]
- [124].Savetsky IL, Ghanta S, Gardenier JC, Torrisi JS, Garcia Nores GD, Hespe GE, et al. Th2 cytokines inhibit lymphangiogenesis. PLoS One 2015;10:e0126908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Ampuero J, Ranchal I, Gallego-Durán R, Pareja MJ, Del Campo JA, Pastor-Ramírez H, et al. Oxidized low-density lipoprotein antibodies/high-density lipoprotein cholesterol ratio is linked to advanced non-alcoholic fatty liver disease lean patients. Journal of gastroenterology and hepatology 2016;31:1611–1618. [DOI] [PubMed] [Google Scholar]
- [126].McGettigan B, McMahan R, Orlicky D, Burchill M, Danhorn T, Francis P, et al. Dietary Lipids Differentially Shape Nonalcoholic Steatohepatitis Progression and the Transcriptome of Kupffer Cells and Infiltrating Macrophages. Hepatology (Baltimore, Md) 2019;70:67–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Burchill MA, Finlon JM, Goldberg AR, Gillen AE, Dahms PA, McMahan RH, et al. Oxidized Low-Density Lipoprotein Drives Dysfunction of the Liver Lymphatic System. Cell Mol Gastroenterol Hepatol 2021;11:573–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Blum KS, Karaman S, Proulx ST, Ochsenbein AM, Luciani P, Leroux JC, et al. Chronic high-fat diet impairs collecting lymphatic vessel function in mice. PLoS One 2014;9:e94713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Escobedo N, Proulx ST, Karaman S, Dillard ME, Johnson N, Detmar M, et al. Restoration of lymphatic function rescues obesity in Prox1-haploinsufficient mice. JCI Insight 2016;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Escobedo N, Oliver G. The Lymphatic Vasculature: Its Role in Adipose Metabolism and Obesity. Cell Metab 2017;26:598–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Harvey NL, Srinivasan RS, Dillard ME, Johnson NC, Witte MH, Boyd K, et al. Lymphatic vascular defects promoted by Prox1 haploinsufficiency cause adult-onset obesity. Nat Genet 2005;37:1072–1081. [DOI] [PubMed] [Google Scholar]
- [132].García Nores GD, Cuzzone DA, Albano NJ, Hespe GE, Kataru RP, Torrisi JS, et al. Obesity but not high-fat diet impairs lymphatic function. Int J Obes (Lond) 2016;40:1582–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Hespe GE, Kataru RP, Savetsky IL, García Nores GD, Torrisi JS, Nitti MD, et al. Exercise training improves obesity-related lymphatic dysfunction. The Journal of physiology 2016;594:4267–4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Nitti MD, Hespe GE, Kataru RP, García Nores GD, Savetsky IL, Torrisi JS, et al. Obesity-induced lymphatic dysfunction is reversible with weight loss. The Journal of physiology 2016;594:7073–7087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Savetsky IL, Torrisi JS, Cuzzone DA, Ghanta S, Albano NJ, Gardenier JC, et al. Obesity increases inflammation and impairs lymphatic function in a mouse model of lymphedema. American journal of physiology Heart and circulatory physiology 2014;307:H165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Torrisi JS, Hespe GE, Cuzzone DA, Savetsky IL, Nitti MD, Gardenier JC, et al. Inhibition of Inflammation and iNOS Improves Lymphatic Function in Obesity. Sci Rep 2016;6:19817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med 2015;212:991–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, et al. Structural and functional features of central nervous system lymphatic vessels. Nature 2015;523:337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Hsu SJ, Zhang C, Jeong J, Lee SI, McConnell M, Utsumi T, et al. Enhanced Meningeal Lymphatic Drainage Ameliorates Neuroinflammation and Hepatic Encephalopathy in Cirrhotic Rats. Gastroenterology 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Schwager S, Detmar M. Inflammation and Lymphatic Function. Front Immunol 2019;10:308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Shi JX, Liang QQ, Wang YJ, Mooney RA, Boyce BF, Xing L. Use of a whole-slide imaging system to assess the presence and alteration of lymphatic vessels in joint sections of arthritic mice. Biotech Histochem 2013;88:428–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Schwager S, Renner S, Hemmerle T, Karaman S, Proulx ST, Fetz R, et al. Antibody-mediated delivery of VEGF-C potently reduces chronic skin inflammation. JCI Insight 2018;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].D’Alessio S, Correale C, Tacconi C, Gandelli A, Pietrogrande G, Vetrano S, et al. VEGF-C-dependent stimulation of lymphatic function ameliorates experimental inflammatory bowel disease. J Clin Invest 2014;124:3863–3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Hasegawa S, Nakano T, Torisu K, Tsuchimoto A, Eriguchi M, Haruyama N, et al. Vascular endothelial growth factor-C ameliorates renal interstitial fibrosis through lymphangiogenesis in mouse unilateral ureteral obstruction. Lab Invest 2017;97:1439–1452. [DOI] [PubMed] [Google Scholar]
- [145].Nakamoto S, Ito Y, Nishizawa N, Goto T, Kojo K, Kumamoto Y, et al. Lymphangiogenesis and accumulation of reparative macrophages contribute to liver repair after hepatic ischemia-reperfusion injury. Angiogenesis 2020;23:395–410. [DOI] [PubMed] [Google Scholar]
- [146].Carpino G, Cardinale V, Di Giamberardino A, Overi D, Donsante S, Colasanti T, et al. Thrombospondin 1 and 2 along with PEDF inhibit angiogenesis and promote lymphangiogenesis in intrahepatic cholangiocarcinoma. J Hepatol 2021. [DOI] [PubMed] [Google Scholar]
- [147].Jiang S, Bailey AS, Goldman DC, Swain JR, Wong MH, Streeter PR, et al. Hematopoietic stem cells contribute to lymphatic endothelium. PLoS One 2008;3:e3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Clement CC, Cannizzo ES, Nastke MD, Sahu R, Olszewski W, Miller NE, et al. An expanded self-antigen peptidome is carried by the human lymph as compared to the plasma. PLoS One 2010;5:e9863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Clement CC, Rotzschke O, Santambrogio L. The lymph as a pool of self-antigens. Trends Immunol 2011;32:6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


