Abstract
In pregnancy-associated malaria, chemokines such as CXCL-4, CXCL-13, CXCL-16, and CCL-24 play critical roles in leucocyte trafficking to tissue sites in the infected placenta where inflammatory reactions are active. However, how plasma levels of these chemokines associate with Plasmodium falciparum placental malaria and pregnancy outcomes remains not well understood. The present study analyzed the plasma levels of CXCL-4, CXCL-13, CXCL-16, and CCL-24 chemokines in matched peripheral, placental and cord blood in relation with placental malaria (PM), and with submicroscopic parasitaemia. This was a retrospective case-control study (1:3 ratio) involving samples from 134 women (34 PM+ and 100 PM-) enrolled at delivery at the Marie Reine Health Center in Yaoundé, Cameroon between June 2013 and October 2018. Samples were collected just after delivery and used to diagnose microscopic and submicroscopic Plasmodium falciparum infections. Submicroscopic infections were detected by reverse transcription LAMP whereas chemokine levels were determined by Magnetic Luminex Screening Assay. Overall, PM was associated with increased plasma levels of CXCL-13 and CXCL-16 and low levels of CXCL-4 and CCL-24 in both peripheral and placental blood (0.0002 ≤ p ≤ 0.042). Similarly, CCL-24 levels in peripheral and placental blood samples were significantly lower in submicroscopically infected women compared to healthy controls (p = 0.04 and 0.02, respectively). Maternal hemoglobin levels increased with peripheral plasma levels of CXCL-4 (p = 0.005), CXCL-16 (p = 0.03), and CCL-24 (p = 0.002) while birth weight was lower for babies born from women with high levels of peripheral CXCL-13 (p = 0.0006) and low levels of cord CXCL-4 and CCL-24 (p = 0.02 and 0.08, respectively). Together the data suggest that low levels of CXCL-4 and CCL-24 coupled with high plasma levels of CXCL-13 and for a lesser extend CXCL-16 represent signatures of PM in the study population. These findings are relevant for understanding the immunopathogenesis of PM and developing new therapeutic or preventive strategies against severe PM outcomes.
Introduction
Plasmodium falciparum malaria remains a leading cause of mortality and morbidity in new-borns, particularly in sub-Saharan Africa where about 40 million women become pregnant each year [1]. In 2021, about 13.3 million women in sub-Saharan Africa were exposed to P. falciparum infection during pregnancy, resulting in 961 000 babies with low birth weights [1]. The main determinants of this include the sequestration of parasitized erythrocytes in the placental tissue causing placental malaria (PM) [2] and the subsequently strong inflammatory responses resulting from alteration of pro and anti-inflammatory cytokine/chemokine balance in the feto-maternal interface [3–6]. This condition might cause the intervillositis that alters efficient blood flow across the placenta, leading to poor pregnancy outcomes including fetal anemia, preterm delivery, and low birth weight [5–7]. Increasing evidence from human studies revealed the presence of host leucocytes at the site of parasite sequestration in the placental tissue [8]. These findings indicate that in addition to cytokines secretion, leucocytes might also contribute to the disease severity by migrating to placental tissue thereby exacerbating organ-specific inflammation.
Chemokines are best known for their ability to stimulate the migration of cells, mostly white blood cells [9]. As the first mobilizers of host response, chemokines play crucial role in the development and homeostasis of the immune system, and are involved in all protective immune and inflammatory responses [9]. The chemokine CXCL-4 also called Platelet Factor 4 (PF-4) is released by activated platelet α-granules [10]. This chemokine together with CXCL-13 and CXCL-16 have been shown to be involved in the implantation and embryonic development early during pregnancy [11, 12]. Moreover, data from previous reports suggest that Plasmodium-infected erythrocytes increase plasma levels of Th1 related chemokines including CXCL-13 and CXCL-16, leading to the formation of an ectopic germinal center which serves as a tertiary lymphoid organ in infected tissues [3, 11]; and such a Th1 immune response bias is generally associated with low birth weight [4, 13].
The diagnosis of PM infection remains a major challenge as 20–25% of PM infections are misdiagnosed in women from sub-Saharan Africa when using the microscopic examination of Giemsa-stained peripheral thick blood smears [14, 15], the WHO gold standard method. High sensitive methods such as nested-PCR, LAMP and RT-LAMP have been developed for the detection of low parasite density related infections including submicroscopic malaria infection [16, 17]. However, these techniques are very expensive, time consuming, require highly trained personnel, and cannot provide the information on the inflammatory status of the placental tissue. Therefore, the identification of molecular correlates of PM and submicroscopic infections in particular, as well as markers of protection remain invaluable.
Similar to microscopic parasitemia, submicroscopic malaria parasiteamia has been associated with maternal anemia, low birth weight [16] and alteration of the cytokine profile [18]. According to previous reports, submicroscopic Plasmodium falciparum infections during pregnancy are able to stimulate immune response mediators such as IFN-γ, TNF-α, and IP-10 altering their expression patterns [18, 19]. These evidences attracted substantial interest in identifying among chemokines useful biomarkers of P. falciparum PM. Most of the previous cytokines /chemokines identified as Plasmodium related markers are involved in different pathophysiological pathways, and thus, lack specificity to malaria infection. In addition, the majority of these cytokines/chemokines have not yet been analysed in relation with submicroscopic malaria parasitaemia, although high proportion of malaria infected pregnant women from sub-Saharan Africa are known to be asymptomatic, and bearing mostly submicroscopic parasitaemia [20, 21]. The present study therefore aimed to analyze the plasma levels of the pregnancy-associated chemokines CXCL-4, CXCL-13, CXCL-16, and CCL-24 in matched peripheral, placental, and cord blood, in relation with PM including submicroscopic parasitaemia and pregnancy outcomes, in women reporting for delivery in Yaoundé, Cameroon. This study might provide leads that should help to identify host molecules likely to be used as markers of PM or as correlates of protection.
Materials and methods
Ethics statement
Participation in the study was voluntary with written Informed Consent or Assent from each participant or their guardian prior to recruitment. The study protocol was reviewed and approved by the National Ethics Committee of Cameroon (Ethical Clearances N° 2013/02/ No 029/L/CNERSH/SP and N°2018/07/1067/CE/CNERSH/SP). Administrative Authorizations were obtained from the Ministry of Public Health of Cameroon (N° D30-392 AAR/MINSANTE/SG/DROS/ CRC/ CEA1) and from local health authorities. The study was performed following the guidelines and regulations of human clinical research as recommended by the Ministry of Public Health of Cameroon. Malaria rapid diagnostic test (RDT) was performed for each woman at the time of enrolment and positive results were reported to the physician for prescription of treatment according to the national guidelines.
Study site and population
Participant enrolment took place at the Marie Reine Health Center of Etoudi in Yaoundé, Cameroon. It was a retrospective case control study (1:3 ratio), involving an initial study undertaken between 2013 and 2018, where peripheral, placental and cord blood samples were collected from 134 women (34 placental malaria positive and 100 negative) enrolled at delivery. In addition, 134 pieces of placenta from the same women were collected immediately following delivery. Malaria transmission in this site is perennial, with 2 wet and 2 dry seasons. Entomological inoculation rates were estimated at 32 infectious bites per person per year in this peri-urban area of Yaoundé [22]. Information on the mother’s health, estimated length of pregnancy, parity, age, use of antimalarial drugs, HIV status, and baby weight were recorded in a standard questionnaire. Birth weights lower than 2.5 kg were considered as low this is the standard definition of low birthweight as per the World Health Organization’s guidelines [23]. Peripheral, placental and cord blood samples were aseptically collected in EDTA tubes. A portion of each sample was used to prepare smears for microscopic diagnostic of malaria infection and detection of submicroscopic malaria infection by RT-LAMP method. The remaining sample was centrifuged and the plasma collected and stored at -80°C for chemokine measurements. Placental tissues were also collected and used to prepare impression smears and for histology.
Diagnosis of placental malaria and determination of hemoglobin levels
For the microscopy-based diagnosis of malaria, maternal peripheral, placental and cord blood were used to prepare thick and thin blood smears while placenta tissue was used to prepare impression smears. Slides were stained using Giemsa and examined by two skilled microscopists for the presence of malaria parasites. P. falciparum infection was further established using the rapid diagnostic test, malaria Carestart™ HRP2 (Pf) (Access Bio Inc, NJ, USA). Placental tissue sections were fixed in buffered formalin, embedded, stained with haematoxylin-eosin, and examined for the presence of parasites and malaria pigments. A woman was considered placental P. falciparum positive if infected erythrocytes were detected in impression smears of placenta tissue, placental blood smear or in histological sections. The presence of malaria pigment in placental tissue was declared when detecting pigment within monocytes from placental impression smear and/or in intervillous spaces of placental histological sections. Acute placental P. falciparum infection was characterized by the presence of infected erythrocytes in impression smears of placenta tissue, placental blood smear or in histological sections with no malaria pigment in the placental tissue. Hemoglobin (Hb) levels in maternal blood and neonate cord blood were determined using a Coulter counter (URIT-3300, Europe). A woman was considered anemic if Hb < 11 g/dL, whereas new born was considered anemic if Hb < 12,5 g/dL.
Sample selection
In the present study, peripheral, placental and cord sera from 34 PM positive women (PM+) and 100 controls (PM-) were used. The 34 PM positive sera were selected based on the availability of the following informations: women’s age, gravidity and malarial status at delivery based on placental tissue impression smears. Then, peripheral, placental and cord sera from 100 PM-negative women of age 16 to 44 years and gravidity 1 to 9 matched to the selected PM+ women to avoid bias. That is, for each case (PM+), three controls of similar age or gravidity were enrolled for comparative analyses.
Diagnosis of submicroscopic malaria infection
The detection of P. falciparum submicroscopic malaria infection was performed using the RT-LAMP technique as previously described [17]. Briefly, the blood samples were diluted (1:50) in a lysis buffer comprising 10 mM Tris-buffered saline, pH 7.4, 0.2% Triton X-100, and 0.1% bovine serum albumin and kept at room temperature for 2 minutes. The lysate (2.5 uL) was immediately added to a reaction mix comprising 2 μl of pre-mixed primers forward (F3) and backward (B3) primers, forward-inner (FIP) and backward-inner (BIP) primers, and loop forward (LF) and loop backward (LB) primers), 15 μL of reconstituted enzyme (ISO-DR001, OptiGene, UK) and 5.5 μl of DEPC-treated water [24]. The RT-LAMP reaction was then performed at 68°C for 45 minutes using a Genie II real-time amplifier. An RT-LAMP inactivation/annealing step of 98–70°C with ramp at 0.1°C per minute was included, and the derived melting curves used to verify the reaction specificity. Samples were considered positive if resulting in an amplification peak with a characteristic annealing temperature of ~86.5°C [24]. Submicroscopic infection was characterized by a positive peripheral blood RT-LAMP result and a negative placental tissue impression smear result with absence of parasite in the placental histological sections.
Measurement of chemokines
Plasma levels of CXCL-4, CXCL-13, CXCL-16, and CCL-24 were measured by magnetic luminex screening assay, using Human Premixed Multi-Analyte Kit (R&D Systems, Inc. Minneapolis, MN, USA). Human, magnetic, premixed, microparticle cocktail of antibodies specific to CXCL-4, CXCL-13, CXCL-16, and CCL-24 was used to simultaneously screen maternal peripheral, placental and neonate cord plasma. The assay was carried out according to manufacturer’s instructions. Briefly, plates were incubated on the horizontal shaker with 50 μL/well of different diluted samples and concentration of standard chemokines provided alongside with 50 μL of human, magnetic, premixed, microparticle cocktail with antibodies specific for each chemokine. After washing using magnetic plate separator (Luminex, Austin, TX, USA, Cat# CN-0269-01), plates were incubated with 50 μL/well of human premixed biotin-antibodies cocktail specific for each chemokine. Antibody chemokine complexes were revealed using Streptavidin-PE. Plates were read using a Luminex MAGPix Analyzer (XMAP Technology, SN, USA) and results expressed as median fluorescence intensity (MFI). A standard curve was generated for each chemokine to convert MFI into corresponding chemokines relative concentrations.
Statistical analysis
The GraphPad Prism 6.0.1 software was used for statistical analyses. Results were reported as means with standard deviation or medians with interquartile ranges. Mann–Whitney rank sum test or Kruskal wallis test was used to evaluate inter-group differences in the levels of chemokines. Proportions were compared using Fisher’s exact test. P values <0.05 were considered statistically significant.
Results
Study population
The baseline characteristics of women involved in this study are summarized on the Table 1. Samples from 34 placental malaria positive (PM+) women (16 women with PM chronic infection, 18 women with PM acute infection) and 100 placental malaria negative (PM-) women (43 women with peripheral blood submicroscopic infection, 57 healthy controls) were used for the study. The mean age (25.4 years) and gravidity (2.2) of PM+ women were not significantly different from those of PM- women (26.3 years and 2.2, p = 0.43 and 0.96, respectively). As shown in Table 1, maternal hemoglobin levels in the present study were significantly lower in PM+ women (10.85 g/dL for PM chronic infection, 11.00 g/dL for PM acute infection) compared to PM-women (12.35 g/dL for PM- with peripheral blood submicroscopic infection, 12.60 dL for healthy controls) (p < 0.0001), and the prevalence of maternal anemia was higher in PM+ (50% for PM chronic infection, 50% for PM acute infection) than in PM- (19.0% for PM- with peripheral blood submicroscopic infection, 12.3% for healthy controls) (p = 0.0006). The mean birth weight of babies born from women with infected placenta (2,988 g and 3,122 g for PM chronic and PM acute infections respectively) was lower compared to that of babies born from PM- women (3,433 g and 3,239 g for PM-with peripheral blood submicroscopic infection and healthy controls respectively) (p = 0.006), although the percentage of baby with low birth weight was similar in PM+ (5.6%) and PM-women (5.0%). The pregnancy period as well as the preterm delivery prevalence were not significantly different across the different groups, and the identified low birth weight cases appear not related to premature delivery. The hemoglobin levels tended to be lower in neonates born from PM+ women (14.80 g/dL for neonate from PM chronic infection, 14.60 g/dL for neonate from PM acute infection) compared to those of neonate from PM-women (15.00 g/dL for PM- with peripheral blood submicroscopic infection, 15.30 dL for healthy controls), although not significant (p = 0.66). However, the prevalence of fetal anemia was higher in PM+ (20% for neonate from PM chronic infection, 22.2% for neonate from PM acute infection) than in PM- (9.3% for neonate from PM- with peripheral blood submicroscopic infection, 1.8% for neonate from healthy controls) (p = 0.02).
Table 1. Characteristics of the study population.
| Variables | PM chronic infection | PM acute infection | PM- with peripheral blood submicroscopic infection | Healthy control | p values |
|---|---|---|---|---|---|
| (n = 16) | (n = 18) | (n = 43) | (n = 57) | ||
| Age in years, mean ±SD | 24.4 ± 4.2 | 26.3 ± 5.4 | 27.2 ± 6.9 | 25.6 ± 5.1 | 0.31 |
| Parity, mean ±SD | 2.1± 1.1 | 2.2 ± 1.2 | 2.6 ± 1.5 | 1.9 ± 1.1 | 0.06 |
| Maternal hemoglobin levels in g/dL, median [25%-75% IQR] | 10.85 | 11.00 | 12.35 | 12.60 | P <0.0001 |
| [9.13, 11.20] | [9.96, 12.60] | [11.40, 13.13] | [11.60, 13.35] | ||
| Baby hemoglobin levels in g/dL, median [25%-75% IQR] | 14.80 | 14.60 | 15.00 | 15.30 | 0.66 |
| [12.80, 16.00] | [12.63, 16.03] | [13.80, 16.50] | [13.75, 16.40] | ||
| Percentage of maternal anemia (%) | 8/16(50,0) | 9/18 (50,0) | 8/42 (19,0) | 7/57 (12,3) | 0.0006 |
| Percentage of fetal anemia (%) | 3/15 (20.0) | 4/18 (22,2) | 4/43 (9,3) | 1/57 (1,8) | 0.02 |
| Baby birth weight, mean ± SD | 2,988 ± 406 | 3,122 ± 409 | 3,433 ± 473 | 3,239 ± 498 | 0.006 |
| Percentage of low birth weight (%) | 1/16(6.3) | 1/18 (5.6) | 1/43 (2.3) | 4/57 (7.0) | 0.76 |
| Gestational age (weeks), mean ± SD | 38 ± 2 | 39 ± 2 | 39 ± SD | 38 ± SD | 0.54 |
| Percentage of preterm delivery (%) | 1/16 (6.3) | 1/18 (5.6) | 4/43 (9.3) | 10/57 (17.5) | 0.36 |
| Peripheral microscopic parasitemia: trophozoites/μL, median [25%-75% IQR] | 26,645 | 1,691 | |||
| [1,006; 130,409] | [99.0; 20,445] | ||||
| Placental intervellous blood microscopic parasitemia: trophozoites/μL, median [25%-75% IQR] | 1917 | 209.5 | |||
| [29.8, 6205] | [0.0, 7613] | ||||
| Placental tissue microscopic parasitemia in %, median [25%-75% IQR] | 5.8 | 0.4 | |||
| [0.25, 18.1] | [0.09, 8.1] | ||||
| IPTp-SP usage (%) | 11/16 (68.8) | 15/18 (83.3) | 40/43 (93.0) | 51/57 (89.5) | 0.08 |
| ITNs usage (%) | 6/16 (37.5) | 14/18 (77.8) | 36/43 (83.7) | 43/56 (76.8) | 0.003 |
PM: Placenta Malaria; PM- with submicroscopic infection: infection characterised by the positive peripheral blood RT-LAMP result and a negative placental tissue impression smear result; Healthy controls: placental malaria negative women with submicroscopic uninfected peripheral blood.; IPTp-SP: Intermittent preventive treatment with sulphadoxine-pyrimethamine; ITNs: insecticide treated bed net; IQR: interquartile ranges, %: Percentage; Values in brackets represent percentages; SD: Standard deviation. Sample size for ITN is 99 as information about ITN usage was missing for one participant.
The percentage of chronic placental malaria infection (parasite and malaria pigment in the placental tissue) was 47% (16/34) among PM+ women and that of placentas with acute infection (parasite and no malaria pigment in placental tissue) was 53% (18/34). Moreover, the percentage of women who took at least 1 dose of SP as well as those who used ITN was significantly higher in PM-women: 91% (91/100) and 79.8% (79/99) respectively compared to PM+ women: 76.5% (26/34) and 58.8% (20/34) (p = 0.04 and 0.02 respectively).
Association of CXCL-4, CCL-24, CXCL-13 and CXCL-16 chemokine levels with PM
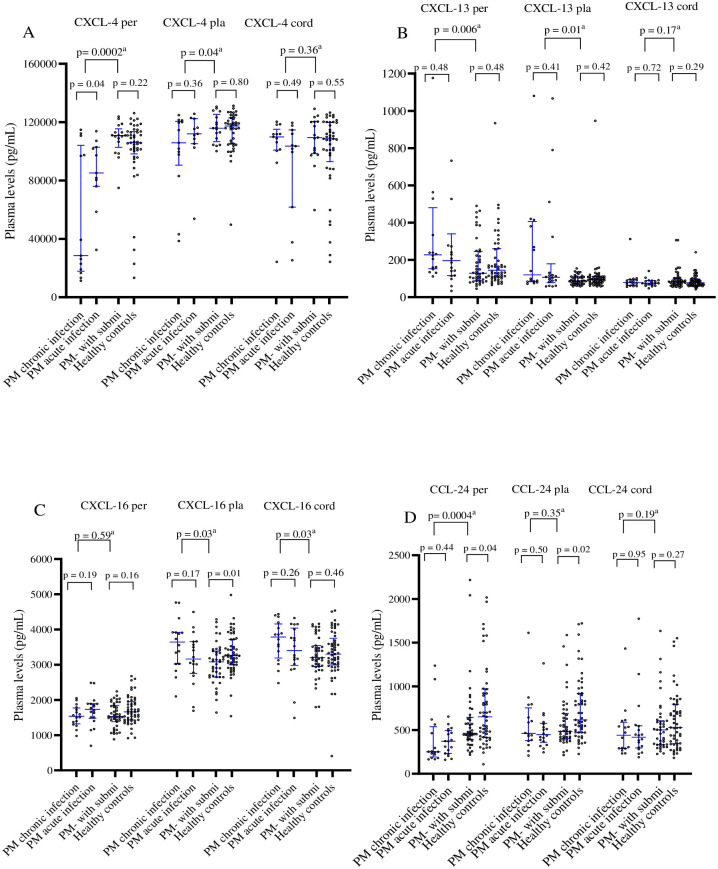
Plasma levels of CXCL-4, CXCL-13, CXCL-16 and CCL-24 chemokines in the peripheral, placental, and cord blood from PM+ women (PM with chronic and PM with acute infections) and PM- women (PM negative with submicroscopic peripheral blood infection) as well as healthy controls are presented in Fig 1. The levels of CXCL-4 in the peripheral and placenta bloods and CCL-24 in the peripheral blood were significantly lower in PM+ compared to PM- women (median levels in peripheral plasma: CXCL-4: 81, 071 vs. 110, 918 pg/mL, p = 0.0002; CCL-24: 299.9 vs. 462.8 pg/mL, p = 0.0004 and median levels in placental plasma: CXCL-4: 111, 503 vs. 115, 902 pg/mL, p = 0.04). The similar trends but not significant were observed for median level in the placental CCL-24: 457.2 vs. 482.6 pg/mL, (p = 0.35) and in cord plasma: CXCL-4: 107, 535 vs. 109, 430 pg/mL, p = 0.36; CCL-24: 426.1 vs.502.1 pg/mL, p = 0.19).
Fig 1. Plasma levels of CXCL-4, CXCL-13, CXCL-16, and CCL-24 chemokines in women at delivery in relation with placental malaria infection.
The levels of different chemokines in peripheral (Per), placental (Pla), and cord plasma were compared between different groups, using Man Whitney test. a: comparison between PM+ women and PM-with submicroscopic infection; PM- with submit (PM- with submicroscopic infection): Placental malaria negative women with positive peripheral blood RT-LAMP test; PM+ women: acute and chronic placental malaria positive women; Healthy controls: placental malaria negative women with negative peripheral blood RT-LAMP test.
Conversely, the levels of CXCL-13 were significantly higher in PM+ women compared to PM-women (median levels in the peripheral plasma: 215.2 vs.138.7 pg/mL, p = 0.006, median levels in the placental plasma: 106.6 vs. 86.85 pg/mL, p = 0.01). Concerning CXCL-16, the median levels in the placenta and cord plasma were also significantly higher in PM+ compared to PM- women (median levels in placental plasma: 3,395 vs 3,081 pg/ml, p = 0.03; median levels in cord plasma: 3, 635 vs 3,199 pg/ml, p = 0.03.
Association of CCL-24 chemokine levels with submicroscopic P. falciparum infection in women at delivery
The plasma levels of CXCL-4, CXCL-13, CXCL-16 and CCL-24 chemokines in the peripheral, placental, and cord blood of submicroscopic positive women (Placental malaria negative women with submicroscopic infection in peripheral blood) and healthy controls women (Placental malaria negative-women with submicroscopic uninfected peripheral blood) are also presented on Fig 1. The peripheral plasma levels of CXCL-4, CXCL-13 and CXCL-16 chemokines did not differ significantly between submicroscopic positive women and healthy controls women (median levels for CXCL-4: 110, 918 vs 105 892 pg/mL; p = 0.22; for CXCL-13: 128.3 vs 144.1 pg/mL p = 0.48, and for CXCL-16: 1,382 vs 1,433 pg/mL, p = 0.16). A similar trend was observed for CXCL-4 (median: 109,430 vs 92,948 pg/ml, p = 0.55), for CXCL-13 (median:82.91 vs 76.99 pg/ml, p = 0.29), for CXCL-16 (median: 3,199 vs 3,301 pg/ml, p = 0.46) and for CCL-24 (median: 482.6 vs 617.2 pg/ml, p = 0.27) in cord blood.
However, plasma levels of CCL-24 were significantly lower in PM- with submicroscopic positive women compared to the healthy controls (median levels in the peripheral plasma: CCL-24: 462. 8 vs 651. 8 pg/mL; p = 0.040, median levels in the placenta: 482.6 vs 617.2 pg/ml, p = 0.02). A similar trend was observed for placental levels of CXCL-16 (median: 3,081 vs 3,255 pg/ml, p = 0.01).
Association of CXCL-13 with gravidity in PM+ women
Among PM+ women, the levels of peripheral and placental CXCL-13 chemokine were higher in primigravidae [(N = 12) (272.4 and 323.7 pg/mL respectively)] compared to secundigravidae [(N = 10) (205.3 and 104.6 pg/mL respectively)] and multigravidae women [(N = 12) (158.9 and 89.82 pg/mL respectively)] (p = 0.02 and p = 0.03 respectively). However, the cord plasma levels of these chemokines as well as the levels of CXCL-4, CXCL-16 and CCL-24 in all the blood compartments did not differ significantly between primigravidae, secundigravidae and multigravid women (p> 0.11 for all) (S1 Table).
Differential association of chemokines with birth weight and maternal or foetal hemoglobin levels
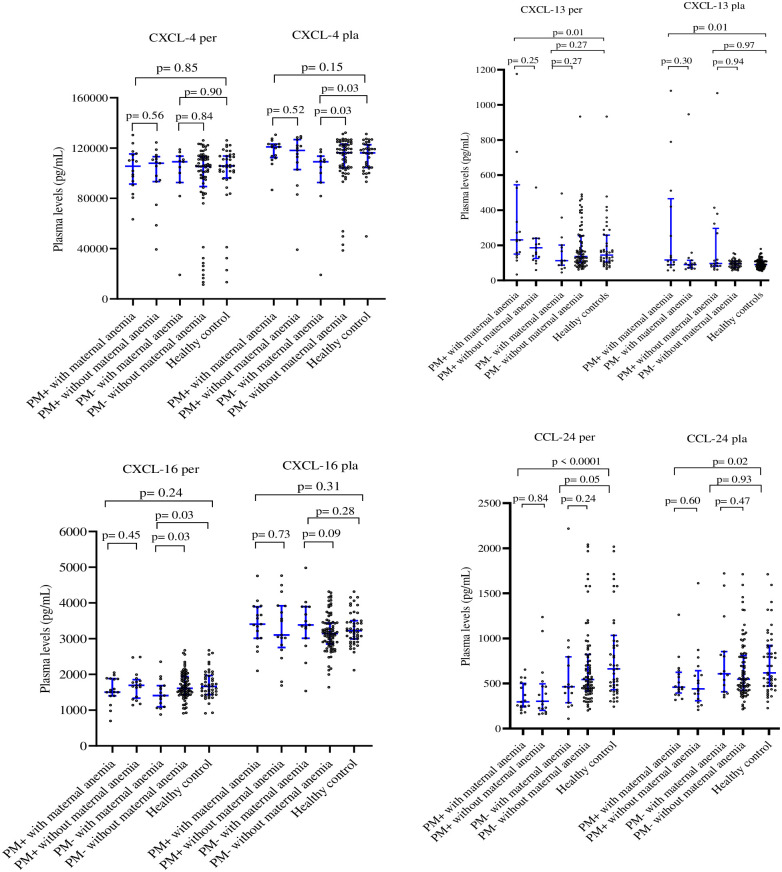
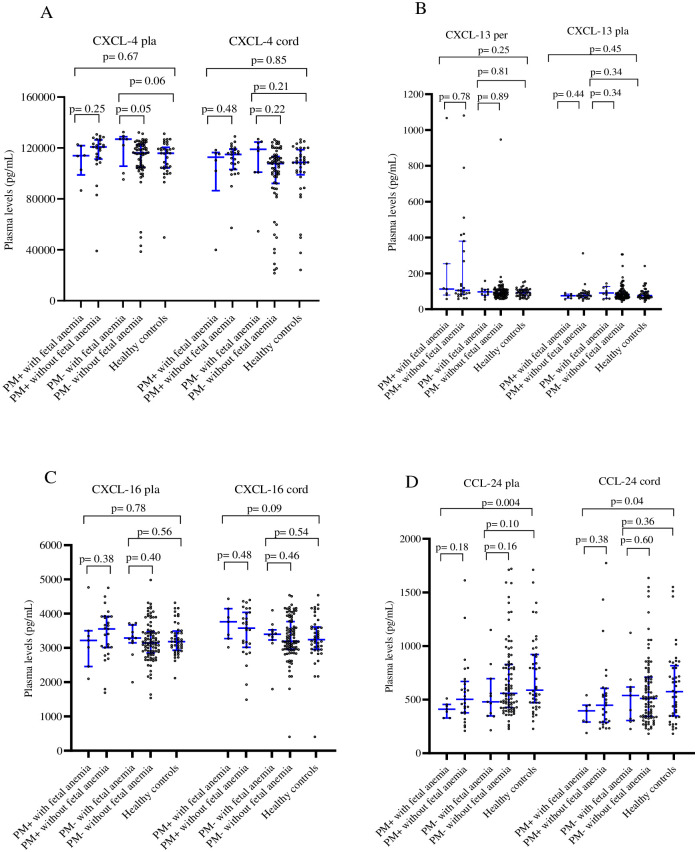
The data on the relationship between assayed chemokines and birth weight, maternal and neonate hemoglobin levels are presented on the Table 2. The birth weight increased with plasma level of placental and cord CXCL-4 chemokine (p = 0.07 and 0.02, respectively) but decreased with increasing peripheral plasma levels of CXCL-13 chemokine (p = 0.0006). Maternal hemoglobin levels were increased with levels of CXCL-4 and CCL-24 in the peripheral plasma (p = 0.005 and 0.002, respectively), and CCL-24 in the cord plasma (p = 0.008). Similarly, neonate hemoglobin levels were increased with levels of the CCL-24 chemokine in peripheral, placental and cord plasma (p = 0.022, 0.030 and 0.019 respectively), and with CXCL-4 in peripheral plasma (p = 0.003) (Table 2). As shown in Fig 2, only CXCL-13 and CCL-24 in both peripheral (p = 0.01 and <0.0001, respectively) and placental (p = 0.01 and p = 0.02, respectively) plasma were associated significantly with maternal anemia in PM+ women when compared to the healthy controls. Interestingly, the increase in CXCL-13 was accompanied by decrease in CCL-24, suggesting opposing roles for these two chemokines in PM-associated anemia. In contrast, Fig 3 shows that, only CCL-24 in both placental and cord plasma was associated significantly (p = 0.004 and 0.04, respectively) with fetal anemia in PM+ women.
Table 2. Plasma levels of chemokines in relation to birth weight, maternal hemoglobin and foetal haemoglobin levels.
| Variables | [CXCL-4 per] ≤ Q/4 | Q/4 < [CXCL-4 per] ≤ 3Q/4 | [CXCL-4 per] > 3Q/4 | p value |
| Birth weight | 3502 ± 441 | 3284 ± 562 | 3331 ± 544 | 0.99 |
| Maternal Hb | 11.80 [9.50; 12.70] | 12.75 [11.80; 13.40] | 12.70 [11.75; 13.70] | 0.005 |
| Cord Hb | 14.45 [12.95; 15.40] | 15.30 [14.58; 16.33] | 15.75 [14.30; 16.98] | 0.003 |
| [CXCL-4 pla] ≤ Q/4 | Q/4 < [CXCL-4 pla] ≤ 3Q/4 | [CXCL-4 pla] > 3Q/4 | p value | |
| Birth weight | 3213 ± 558 | 3242 ± 499 | 3495 ± 505 | 0.07 |
| Maternal Hb | 12.90 [11.20; 13.30] | 12.55 [11.58; 13.40] | 12.50 [11.55; 13.55] | 0.92 |
| Cord Hb | 15.10 [14.45; 16.50] | 15.05 [14.00; 16.30] | 15.50 [14.50; 16.40] | 0.67 |
| [CXCL-4 cord] ≤ Q/4 | Q/4 <[CXCL-4 cord] ≤ 3Q/4 | [CXCL-4 cord] > 3Q/4 | p value | |
| Birth weight | 3070 ± 465 | 3339 ± 580 | 3452 ± 387 | 0.02 |
| Maternal Hb | 12.60 [11.80; 13.23] | 12.50[11.20; 13.55] | 12.50 [11.60; 13.50] | 0.94 |
| Cord Hb | 14.95 [14.48; 16.23] | 15.20 [14.00; 16.78] | 15.05 [13.90; 16.38] | 0.91 |
| [CXCL-13 per] ≤ Q/4 | Q/4 < [CXCL-13 per] ≤ 3Q/4 | [CXCL-13 per] > 3Q/4 | p value | |
| Birth weight | 3523 ± 419 | 3257 ± 524 | 3194 ± 476 | 0.0006 |
| Maternal Hb | 12.10 [11.40; 12.60] | 12.40 [11.05; 13.30] | 12.00 [10.35; 13.05] | 0.69 |
| Cord Hb | 14.90 [13.90; 15.80] | 14.90 [13.75; 16.35] | 14.70 [12.80; 14.70] | 0.87 |
| [CXCL-13pla] ≤ Q/4 | Q/4 < [CXCL-13 pla] ≤ 3Q/4 | [CXCL-13 pla] > 3Q/4 | p value | |
| Birth weight | 3312 ± 468 | 3153 ± 441 | 3176 ± 497 | 0.62 |
| Maternal Hb | 11.90 [11.10; 12.53] | 12.65 [11.35; 13.30] | 11.70 [9.90; 12.70] | 0.007 |
| Cord Hb | 14.80 [13.75; 15.93] | 14.85 [13.73; 16.35] | 15.80 [13.10; 16.70] | 0.62 |
| [CXCL-13cord]≤ Q/4 | Q/4< [CXCL-13 cord] ≤ 3Q/4 | [CXCL-13 cord]> 3Q/4 | p value | |
| Birth weight | 3282± 368 | 3240 ± 526 | 3259 ± 519 | 0.92 |
| Maternal Hb | 11.90 [10.60; 12.80] | 11.90 [10.70; 13.20] | 12.35 [11.63; 13.28] | 0.14 |
| Cord Hb | 14.70 [13.48; 15.55] | 14.90 [13.70; 16.30] | 15.85 [14.03; 17.33] | 0.07 |
| [CXCL-16per] ≤ Q/4 | Q/4 < [CXCL-16per] ≤ 3Q/4 | [CXCL-16per] > 3Q/4 | p value | |
| Birth weight | 3145 ± 523 | 3262 ± 397 | 3350 ± 592 | 0.23 |
| Maternal Hb | 11.50 [9.8; 12.80] | 12.25 [11.20; 13.03] | 12.55 [11.55; 13.43] | 0.03 |
| Cord Hb | 14.90 [13.80; 16.35] | 14.80 [13.68; 16.05] | 15.75 [13.98; 16.53] | 0.52 |
| [CXCL-16 pla] ≤ Q/4 | Q/4 < [CXCL-16 pla] ≤ 3Q/4 | [CXCL-16 pla] > 3Q/4 | p value | |
| Birth weight | 3227 ± 479 | 3287 ± 475 | 3220 ± 525 | 0.28 |
| Maternal Hb | 12.50 [11.50; 13.20] | 12.10 [11.20; 13.20] | 11.60 [9.9; 12.90] | 0.19 |
| Cord Hb | 15.20 [13.75; 16.90] | 14.85 [13.70; 16.38] | 14.85 [13.83; 15.88] | 0.48 |
| [CXCL-16 cord] ≤ Q/4 | Q/4< [CXCL-16 cord] ≤ 3Q/4 | [CXCL-16 cord]> 3Q/4 | p value | |
| Birth weight | 3224 ± 431 | 3333 ± 513 | 3120 ± 460 | 0.11 |
| Maternal Hb | 12.60 [11.85; 13.20] | 12.10 [10.93; 13.28] | 11.55 [10.28; 12.68] | 0.05 |
| Cord Hb | 15.80 [14.23; 17.08] | 14.80 [12.80; 16.10] | 14.80 [13.83; 15.98] | 0.05 |
| [CCL-24 per] ≤ Q/4 | Q/4 < [CCL-24 per] ≤ 3Q/4 | [CCL-24 per] > 3Q/4 | p value | |
| Birth weight | 3157 ± 469 | 3310 ± 415 | 3247 ± 619 | 0.33 |
| Maternal Hb | 11.20 [9.92; 12.28] | 12.60 [11.40; 13.20] | 12.05 [11.53; 13.20] | 0.002 |
| Cord Hb | 14.10 [12.70; 15.35] | 15.30 [13.70; 16.60] | 16.00 [14.45; 16.50] | 0.022 |
| [CCL-24 pla] ≤ Q/4 | Q/4 < [CCL-24 pla] ≤ 3Q/4 | [CCL-24 pla] > 3Q/4 | p value | |
| Birth weight | 3386± 509 | 3194 ± 399 | 3252 ± 602 | 0.17 |
| Maternal Hb | 11.85 [10.88;12.85] | 12.10 [11.15; 12.93] | 12.60 [10.85; 13.35] | 0.45 |
| Cord Hb | 14.65 [12.73; 15.83] | 14.75 [13.68; 16.28] | 16.00 [14.50; 16.50] | 0.030 |
| [CCL-24 cord]≤ Q/4 | Q/4< [CCL-24 cord] ≤ 3Q/4 | [CCL-24 cord] > 3Q/4 | p value | |
| Birth weight | 3300 [2900; 3600] | 3300 [3100; 3500] | 2975 [2613; 3475] | 0.08 |
| Maternal Hb | 11.50 [9.55; 12.50] | 12.30 [11.20; 13.10] | 13.00 [11.30; 13.75] | 0.008 |
| Cord Hb | 14.40 [12.88; 15.65] | 14.90 [13.65; 16.25] | 16.10 [14.48; 16.55] | 0.019 |
Hb: hemoglobin level, Q/4: 25th percentiles, 3Q/4: 75th percentiles, Per: peripheral plasma, Pla: placental plasma. Birth weight was expressed as mean with standard deviation.
Fig 2. Plasma levels of CXCL-4, CXCL-13, CXCL-16, and CCL-24 chemokines in anemia positive-women and anemia negative-women at delivery.
The levels of different chemokines in peripheral (Per), placental (Pla) were compared between anemic and non-anemic women at delivery, using Mann Whitney test. PM-: Placental malaria negative-women; PM+: Placental malaria positive women; healthy controls: placental malaria negative-women with negative peripheral blood RT-LAMP test.
Fig 3. Plasma levels of CXCL-4, CXCL-13, CXCL-16, and CCL-24 chemokines in women at delivery in relation with fetal anemia.
The levels of different chemokines in placental (Pla) and cord plasma were compared between the fetal anemia positive-group and fetal anemia negative-women group, using Mann Whitney test. PM-: Placental malaria negative-women; PM+: Placental malaria positive-women; Healthy controls: placental malaria negative women with negative peripheral blood RT-LAMP test.
Discussion
The main findings of this study were: 1) the association of PM with enhanced levels of CXCL-13, CXCL-16 and low levels of CXCL-4 and CCL-24 in peripheral and placental plasma; 2) the association of submicroscopic P. falciparum parasitemia with decreased levels of CCL-24 in both peripheral and placental plasma. Moreover, maternal hemoglobin level in the present study increased with peripheral plasma levels of CXCL-4 while birth weight was lower for babies born from women with elevated levels of peripheral CXCL-13 but low plasma levels of cord CXCL-4 and CCL-24. These findings suggest that low levels of CXCL-4 and CCL-24 coupled with high plasma levels of CXCL-13 might represent the signature of PM and associated outcomes including maternal anemia and low birth weight. These findings have implications in the development of complementary diagnostic tools for PM diagnosis and prediction of placental malaria outcomes. Several studies have highlighted the involvement of cytokines and chemokines in the pathophysiology of the disease, but this study is the first to report the involvement of CXCL-4 and CCL-24 in PM. In a previous study, Wilson et al in 2011 [25] reported high expression of CXCL-4 in children with cerebral malaria, which contrast with the reduced levels of the chemokine found in PM+ women in the present study. This discrepancy might be explained by differences in the immuno-pathogenesis mechanisms of placental and cerebral malaria that include the differential expression of certain cytokines including CXCL4 and CCL24. According to previous studies, CXCL-4 binds to the erythrocyte Duffy antigen receptor, promoting lysis of the latter [26]. Moreover, this chemokine has been shown to exhibit both pro and anti-inflammatory effects as its level was significantly low in patients with acute hepatitis, and its absence was associated with severe liver damage [27]. CXCL-4 also has been shown to promote hematopoiesis by retaining progenitor cells [28], and this is consistent with the positive association observed in this study between plasma levels of CXCL-4 and maternal hemoglobin level.
Concerning CCL-24, pregnant women trophoblasts have been shown as one of main sources of this chemokine which binds to CCR3 receptors in decidual stromal cells [29]. In addition, the production of the progesterone, estrogen and human chorionic gonadotropin hormones is associated with upregulation of CCL-24 [29]. Thus, the low levels of CCL-24 found in this study in PM positive women could be due to the decrease in progesterone expression [30] and an alteration of the trophoblast [31] having been shown to be associated with PM. Placentas from women who were infected with P. falciparum during early pregnancy showed signs of damages such as reduced transport villi, increased syncytial knotting and increased placental lesions [32–34], while active infection at delivery was associated with reduced villous area and vascularity, increased basal membrane thickening, syncytial damage, increased syncytial knotting and fibrinoid necrosis [32, 35]. The enhanced plasma levels of CXCL-13 chemokine found in the present study in PM + women compared to PM-women is in agreement with previous studies [3, 4].
Submicroscopic parasitaemia is often associated with altered immune profile [36]. For the first time, we show in this study in relation with submicroscopic infection that the plasma concentration of CCL-24 in the peripheral and placental plasma was significantly higher in healthy controls compared to submicroscopic positive women. Only placental plasma concentration of CXCL-16 was higher in healthy controls. These results suggest that submicroscopic infection is also associated with decrease of peripheral and placental plasma concentration of CCL-24 and placental concentration of CXCL-16.
PM is known to cause maternal anemia, fetal anemia and low birth weight. Thus, the positive association observed between the levels of CXCL-4, CXCL-16, CCL-24 (in the peripheral, placental or cord blood depending on chemokine) and maternal or fetal Hb and between birth weight and CXCL-4 chemokine in the cord plasma, suggests the protective role of these chemokines against PM. In addition, the negative association observed between CXCL-13 levels in the peripheral plasma and birth weight as well as between the placental levels of this chemokine and the maternal hemoglobin level suggest its association with PM poor pregnancy outcomes.
The positive association found in the present study between CXCL-13 and decreasing birth weight is in line with previous findings by others [3, 4]. Indeed, CXCL-13 is produced by macrophage to activate the inflammatory T cells responses that might contribute to increased inflammatory cell infiltration and fibrinoid deposits and oxidative stress in the placenta [8, 37]. Increased oxidative insults may trigger apoptosis in human placenta [38, 39] and in mouse model of PM [40]. Collectively, the accumulation of fibrin in the intervellous spaces coupled with dysregulation of angiogenesis in the placenta can result in inadequate perfusion to placenta, causing necrosis [41] that may lead to growth restriction and low birth weight. Gravidity had no influence on the plasma levels of different assayed chemokines. The observed decrease in CXCL-13 in the peripheral blood with increasing gravidity among PM+ group can be explained on the basis of differences in parasitaemia that have been shown to be higher in paucigravid women compared to multigravid women.
Conclusion
These results suggest that conversely to high plasma levels of CXCL-13 and for a lesser extend CXCL-16, confirmed to be associated to P. falciparum PM, high plasma levels of CXCL-4 and CCL-24 chemokines might be associated with low risk of the disease in pregnant women. These findings are relevant for the identification of correlates of protection against PM and targets that can be used for the development of complementary diagnostic tools.
Supporting information
(DOCX)
(XLSX)
Acknowledgments
We are thankful to all the members and staff especially Mrs Philomina NYONGLEMA, Grace SAMA and Dr Livo F Esumu from the Immunology Laboratory of the Biotechnology Centre of the University of Yaoundé I, Cameroon for their technical assistance. Dr Sylvie KEMLEU, Mr Balotin FOGANG from the malaria Unit of the « Centre Pasteur du Cameroun » are also thanked for their assistance on RT-LAMP technique. We are very grateful to nurses at the antenatal service of the Marie Reine Health Centre of Etoudi, for assistance in specimen collection. Our sincere thanks to all the women at delivery who took part in this study making it possible.
Data Availability
All relevant data are within the paper and its Supporting information files.
Funding Statement
This work was supported by The World Academy of Sciences (TWAS) through Research Grant No: 12-081 RG/BIO/AF: AC_I---UNESCO FR: 3240271366), and the “Agence Universitaire de la Francophonie (AUF Région Afrique Centrale et des Grands Lacs” Through MATHINBIO; grants awarded to Rosette Megnekou.(RM). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization. World malaria report 2022, Geneva. World Health Organization, 2022; https://www.who.int/publications/i/item/9789240064898).
- 2.Pereira AM, Clausen MT, Pehrson C, Mao Y, Resende M, Daugaard M, et al. Placental Sequestration of Plasmodium falciparum Malaria Parasites Is Mediated by the Interaction Between VAR2CSA and Chondroitin Sulfate A on Syndecan-1. PLoS Pathog. 2016; 24;12(8):e1005831. doi: 10.1371/journal.ppat.1005831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muehlenbachs A, Fried M, Lachowitzer J, Mutabingwa TK, Duffy PE. Genome-Wide Expression Analysis of Placental Malaria Reveals Features of Lymphoid Neogenesis during Chronic Infection. J Immuno. 2007; l 179:557–565. doi: 10.4049/jimmunol.179.1.557 [DOI] [PubMed] [Google Scholar]
- 4.Dong S, Kurtis JD, Pond-Tor S, Kabyemela E, Duffy PE, Fried M. CXC Ligand 9 Response to Malaria during Pregnancy Is Associated with Low-Birth-Weight Deliveries. Infect Immun. 2012; 80:3034–3038. doi: 10.1128/IAI.00220-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Megnekou R, Djontu JC, Bigoga JD, Lissom A, Magagoum SH. Role of some biomarkers in placental malaria in women living in Yaoundé, Cameroon. Acta Trop. 2015a; 141:97–102. [DOI] [PubMed] [Google Scholar]
- 6.Djontu JC, Siewe Siewe S, Mpeke Edene YD, Nana BC, Chomga Foko EV, Bigoga JD, et al. Impact of placental Plasmodium falciparum malaria infection on the Cameroonian maternal and neonate’s plasma levels of some cytokines known to regulate T cells differentiation and function. Malar J. 2016; 15:561. doi: 10.1186/s12936-016-1611-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fried M, Kurtis JD, Swihart B, Pond-Tor S, Barry A, Sidibe Y, et al. Systemic Inflammatory Response to Malaria During Pregnancy Is Associated with Pregnancy Loss and Preterm Delivery. Clin Infect Dis. 2017; 65:1729–1735. doi: 10.1093/cid/cix623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suguitan AL Jr, Leke RG, Fouda G, Zhou A, Thuita L, Metenou S, et al. Changes in the levels of chemokines and cytokines in the placentas of women with Plasmodium falciparum malaria. J. Infect. Dis., 2003; 188(7):1074–1082. [DOI] [PubMed] [Google Scholar]
- 9.Hughes CE, Nibbs RJB. A guide to chemokines and their receptors. FEBS J. 2018; 285:2944–2971. doi: 10.1111/febs.14466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kasper B, and Petersen F. Molecular pathways of platelet factor 4/CXCL4 signaling. Eur J Cell Biol. 2011; 90 (7):521–526. doi: 10.1016/j.ejcb.2010.12.002 [DOI] [PubMed] [Google Scholar]
- 11.Nhan-Chang C-L, Romero R, Kusanovic JP, Gotsch F, Edwin SS, Erez O, et al. A role for CXCL13 (BCA-1) in pregnancy and intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med. 2008; 21:763–775. doi: 10.1080/14767050802244946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi J, Yang H, Fan D, Yang S, Qiu X, Wang Y, et al. The role of CXC chemokine ligand 16 in physiological and pathological pregnancies. Am J Reprod Immunol. 2020; 83. doi: 10.1111/aji.13223 [DOI] [PubMed] [Google Scholar]
- 13.Ayimba E, Hegewald J, Ségbéna AY, Gantin RG, Lechner CJ, Agosssou A, et al. Proinflammatory and regulatory cytokines and chemokines in infants with uncomplicated and severe Plasmodium falciparum malaria. Clin Exp Immunol. 2011; 166:218–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leke RF, Djokam RR, Mbu R, Leke RJ, Fogako J, Megnekou R, et al. Detection of the Plasmodium falciparum antigen histidine-rich protein 2 in blood of pregnant women: implications for diagnosing placental malaria. J Clin Microbiol. 1999, 37(9): 2992–2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Megnekou R, Djontu JC, Nana BC, Bigoga DJ, Fotso M, Fogang B, et al. Accuracy of One Step Malaria Rapid diagnostic tests in detecting Plasmodium falciparum placental malaria infection in women living in Yaounde Cameroon. Malar J. 2018; 17: 450 doi: 10.1186/s12936-018-2595-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samia AO, Noureldein NA, Hadeel E, Mutasim A, Hagir EI, Abdelrahim MA, et al. Impact of Submicroscopic Plasmodium falciparum Parasitaemia on Maternal Anaemia and Low Birth Weight in Blue Nile State, Sudan. Clin Microbiol Infect. 2019:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kemleu S, Guelig D, Eboumbou Moukoko C, Essangui E, Diesburg S, Mouliom A, et al. A Field-Tailored Reverse Transcription Loop-Mediated Isothermal Assay for High Sensitivity Detection of Plasmodium falciparum Infections. PLOS ONE. 2016; 11: e0165506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Álvarez-Larrotta C, Agudelo O, Duque Y, Gavina K, Yanow S, Maestre A, et al. Submicroscopic Plasmodium infection during pregnancy is associated with reduced antibody levels to tetanus toxoid. Clin Exp Immunol. 2018; 195:96–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ibitokou SA, Boström S, Brutus L, Tuikue Ndam N, Vianou B, Agbowaï C, et al. Erratum for Ibitokou et al., Submicroscopic Infections with Plasmodium falciparum during Pregnancy and Their Association with Circulating Cytokine, Chemokine, and Cellular Profiles. Clin Vaccine Immunol. 2014; 21:1468–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schantz-Dunn J, Nour NM. Malaria and pregnancy: a global health perspective. Rev Obstet Gynecol. 2009; 2:186–192. [PMC free article] [PubMed] [Google Scholar]
- 21.Massamba JE, Djontu JC, Vouvougui CJ, Gueye NCG, Kobawila C, Ntoumi F. Prevalence of Plasmodium falciparum Submicroscopic Infection and Pregnancy Outcomes in Congolese Women at Delivery. AmJ of Clin and Exp Med. 2022; 10 (1):15–22. [Google Scholar]
- 22.Ngom R, Siegmund A. Urban malaria in Africa: an environmental and socio-economic modelling approach for Yaoundé, Cameroon. Nat Hazards. 2010; 55:599–619. [Google Scholar]
- 23.World Health Organization. Nutrition Landscape information system (NLIS): Nutrition and nutrition–related health and development data. https://www.who.int/data/nutrition/nlis/info/low-birth-weight. Retrived 07 January 2023.
- 24.Kemleu Zebaze Temgoua SG, Ngando L, Nguekeng E, Fogang B, Mafo Kapen M, Fopa SI, et al. Diagnostic performance of a rapid whole blood-based RT-LAMP method for malaria diagnosis among apparently healthy blood donors and febrile neonates in Cameroon. PLOS ONE. 2021; 16: e0246205. doi: 10.1371/journal.pone.0246205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson NO, Jain V, Roberts CE, Lucchi N, Joel PK, Singh MP, et al. CXCL4 and CXCL10 Predict Risk of Fatal Cerebral Malaria. Dis Markers. 2011; 30:39–49. doi: 10.3233/DMA-2011-0763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McMorran BJ, Wieczorski L, Drysdale KE, Chan J-A, Huang HM, Smith C, et al. Platelet Factor 4 and Duffy Antigen Required for Platelet Killing of Plasmodium falciparum. Science. 2012; 338:1348–1351. [DOI] [PubMed] [Google Scholar]
- 27.Drescher HK, Brandt EF, Fischer P, Dreschers S, Schwendener RA, Kowalska MA, et al. Platelet Factor 4 Attenuates Experimental Acute Liver Injury in Mice. Front Physiol. 2019; 10:326. doi: 10.3389/fphys.2019.00326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonavita O, Poeta VM, Massara M, Mantovani A, Bonecchi R. Regulation of hematopoiesis by the chemokine system. Cytokine. 2018; 109: 76–80. doi: 10.1016/j.cyto.2018.01.021 [DOI] [PubMed] [Google Scholar]
- 29.Li H, Huang Y-H, Li M-Q, Meng Y-H, Chen X, Shao J, et al. Trophoblasts-derived chemokine CCL24 promotes the proliferation, growth and apoptosis of decidual stromal cells in human early pregnancy. Int J Clin Exp Pathol. 2013; 6:1028–1037. [PMC free article] [PubMed] [Google Scholar]
- 30.Megnekou R, Tenou S, Bigoga JD, Djontu JC, Medou FM, Lissom A. Placental malaria and modulation of immune and hormonal responses in Cameroonian women. Acta Trop. 2015c; 147:23–30. doi: 10.1016/j.actatropica.2015.04.001 [DOI] [PubMed] [Google Scholar]
- 31.Umbers AJ, Stanisic DI, Ome M, Wangnapi R, Hanieh S, Unger HW, et al. Does Malaria Affect Placental Development? Evidence from In Vitro Models. PLoS ONE. 2013; 8: e55269. doi: 10.1371/journal.pone.0055269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crocker IP, Tanner OM, Myers JE, Bulmer JN, Walraven G, Baker PN. Syncytiotrophoblast Degradation and the Pathophysiology of the Malaria-infected Placenta. Placenta. 2004; 25:273–282. doi: 10.1016/j.placenta.2003.09.010 [DOI] [PubMed] [Google Scholar]
- 33.Elphinstone RE, Weckman AM, McDonald CR, Tran V, Zhong K, Madanitsa M, et al. Early malaria infection, dysregulation of angiogenesis, metabolism and inflammation across pregnancy, and risk of preterm birth in Malawi: A cohort study. PLOS Med. 2019; 16: e1002914. doi: 10.1371/journal.pmed.1002914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moeller SL, Nyengaard JR, Larsen LG, Nielsen K, Bygbjerg IC, Msemo OA, et al. Malaria in Early Pregnancy and the Development of the Placental Vasculature. J Infect Dis. 2019; 220:1425–1434. doi: 10.1093/infdis/jiy735 [DOI] [PubMed] [Google Scholar]
- 35.Chaikitgosiyakul S, Rijken MJ, Muehlenbachs A, Lee SJ, Chaisri U, Viriyavejakul P, et al. A morphometric and histological study of placental malaria shows significant changes to villous architecture in both Plasmodium falciparum and Plasmodium vivax infection. Malar J. 2014; 13:4. doi: 10.1186/1475-2875-13-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agudelo OM, Aristizabal BH, Yanow SK, Arango E, Carmona-Fonseca J, Maestre A. Submicroscopic infection of placenta by Plasmodium produces Th1/Th2 cytokine imbalance, inflammation and hypoxia in women from north-west Colombia. Malar J. 2014; 13:122. doi: 10.1186/1475-2875-13-122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lucchi NW, Peterson DS, Moore JM. Immunologic activation of human syncytiotrophoblast by Plasmodium falciparum. Malar J. 2008; 7:42. doi: 10.1186/1475-2875-7-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Souza RM, Ataíde R, Dombrowski JG, Ippólito V, Aitken EH, Valle SN, et al. Placental Histopathological Changes Associated with Plasmodium vivax Infection during Pregnancy. PLoS Negl Trop Dis. 2013; 7: e2071. doi: 10.1371/journal.pntd.0002071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kawahara R, Rosa-Fernandes L, dos Santos AF, Bandeira CL, Dombrowski JG, Souza RM, et al. Integrated Proteomics Reveals Apoptosis-related Mechanisms Associated with Placental Malaria. Mol Cell Proteomics. 2019; 18:182–199. doi: 10.1074/mcp.RA118.000907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharma L, Kaur J, Shukla G. Role of Oxidative Stress and Apoptosis in the Placental Pathology of Plasmodium berghei Infected Mice. PLoS ONE. 2012; 7: e32694. doi: 10.1371/journal.pone.0032694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burton GJ, Jauniaux E. Pathophysiology of placental-derived fetal growth restriction. Am J Obstet Gynecol. 2018; 218: S745–S761. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting information files.