Abstract
In this study we utilized immortalized morphologically and functionally distinct epithelial cell lines from normal human endocervix, ectocervix, and vagina to characterize gonococcal epithelial interactions pertinent to the lower female genital tract. Piliated, but not nonpiliated, N. gonorrhoeae strain F62 variants actively invaded these epithelial cell lines, as demonstrated by an antibiotic protection assay and confocal microscopy. Invasion of these cells by green fluorescent protein-expressing gonococci was characterized by colocalization of gonococci with F actin, which were initially detected 30 min postinfection. In all three cell lines, upregulation of interleukin 8 (IL-8) and IL-6, intercellular adhesion molecule 1 (CD54), and the nonspecific cross-reacting antigen (CD66c) were detected 4 h after infection with piliated and nonpiliated gonococci. Furthermore, stimulation of all three cell lines with gonococcal whole-cell lysates resulted in a similar upregulation of IL-6 and IL-8, confirming that bacterial uptake is not essential for this response. Increased levels of IL-1 were first detected 8 h after infection with gonococci, suggesting that the earlier IL-8 and IL-6 responses were not mediated through the IL-1 signaling pathway. The IL-1 response was limited to cultures infected with piliated gonococci and was more vigorous in the endocervical epithelial cells. The ability of gonococci to stimulate distinct proinflammatory host responses in these morphologically and functionally different compartments of the lower female genital tract may contribute directly to the inflammatory signs and symptoms characteristic of disease caused by N. gonorrhoeae.
Cervicovaginal epithelial cells are increasingly recognized as active moderators of both innate and acquired immune functions in the genital tract via the production of a variety of proinflammatory and anti-inflammatory mediators. Increased levels of the cytokines interleukin 1 (IL-1), tumor necrosis factor alpha (TNF-α), and IL-6 and the chemokines RANTES, MIP-1α, and MIP-1β in cervicovaginal secretions have been associated with human immunodeficiency virus type 1 infection and bacterial vaginosis; however, little is known about the role of locally produced proinflammatory mediators in the pathogenesis of bacterial vaginitis and cervicitis (10). We recently developed three epithelial cell lines from normal human vagina, ectocervix, and endocervix, immortalized by expression of the E6 and E7 genes of human papillomavirus type 16 (8). The morphological and immunocytochemical characteristics of the immortalized lines closely resembled those of their tissues of origin and primary cultures, and all three differed significantly from the HeLa cervical adenocarcinoma cell line, the most commonly used cell line derived from the human female lower genital tract mucosa. The three cell lines constitutively produced distinctive arrays of cytokines (IL-1, IL-6, IL-7, macrophage colony-stimulating factor, and transforming growth factor β) and chemokines (IL-8 and RANTES), with the endocervical epithelial cells being more active in cytokine secretion than the ectocervical and the vaginal epithelial cells (9). These results suggested that the endocervical, ectocervical, and vaginal compartments of the lower female genital tract not only are represented by different epithelial cell types and mucosal tissue architecture (simple versus stratified squamous epithelium) but also demonstrate differences in their immunobiological functions. The different cytokine patterns of the three epithelial cell types may be related to the fact that under healthy conditions the endocervical cells differentiate in a sterile environment, while the ectocervical and vaginal epithelial cells are constantly exposed to the normal vaginal microbiota and may have developed cytokine-orchestrated mechanisms for downregulation of inflammatory responses to bacterial components.
Based on the unique characteristics of these cell lines, we postulated that they could be developed as models to study invasion by sexually transmitted pathogens such as Neisseria gonorrhoeae. N. gonorrhoeae is the etiologic agent of gonorrhea, which is typically manifested as a mucosal infection of the male urethra and the lower genital tract of women. Among several different sexually transmitted diseases, gonorrhea has the highest probability of transmission per sexual contact (2). More than 90% of men with urethral gonorrhea will develop symptoms within 5 days of infection; however, fewer than 50% of women with genital gonorrhea will do so (22). Women with asymptomatic infections are at higher risk than men of developing secondary complications such as pelvic inflammatory disease and disseminated gonococcal infection (23). Although N. gonorrhoeae causes extensive morbidity, little is known about the immune defense mechanisms in the different compartments of the human genital tract. One recent report has demonstrated a rapid increase of local production and systemic levels of the chemokine IL-8 and the proinflammatory cytokine IL-6 in men with experimental urethral gonococcal infection (29). Similar investigations on acute gonococcal infections are lacking in women. Hedges et al. (13) have reported an increase of levels of IL-6 in plasma in association with natural uncomplicated gonococcal infection in women, which was potentiated by coinfections with other sexually transmitted pathogens. However, detailed studies describing molecular mechanisms of gonococcal host epithelial interactions in the lower female genital tract have not been reported to date. The ability to examine the consequences of gonococcal infection of cervical and vaginal epithelial cells would aid our understanding of the gender differences in gonococcal disease as well as the preferences for primary and secondary infection sites.
In this study we demonstrate that normal human vaginal, ectocervical, and endocervical epithelial cells are capable of mounting a proinflammatory response to infection with N. gonorrhoeae. Furthermore, both piliated and nonpiliated gonococci induced a marked upregulation of chemokines (IL-8), cytokines (IL-6), and intercellular adhesion molecule 1 (ICAM-1) (CD54 and CD66), which was detectable in all three cell lines 4 h following infection and was independent of bacterial uptake and IL-1 release. Although the three epithelial cell lineages could not be distinguished by these early proinflammatory responses, a later increase of IL-1, a powerful amplifier of proinflammatory events, was limited to the endocervical epithelial cells and the more invasive piliated gonococcal variant. These findings suggest that distinct temporal and spatial host inflammatory responses may operate in the different compartments of the lower female genital tract.
MATERIALS AND METHODS
Bacterial strains.
Piliated and nonpiliated variants of N. gonorrhoeae strain F62 were used in this study (5). Gonococci were routinely maintained in a 5% CO2 incubator at 37°C on GC medium base (Difco Laboratories, Detroit, Mich.) with 1% Kellogg's supplement. The piliated (FP+) and nonpiliated phenotypes were confirmed using a dissecting microscope prior to their use in the study. At the time of epithelial inoculation, the nonpiliated variant appeared to be morphologically opacity (Opa) negative while the piliated variant appeared to be Opa positive when examined under a dissecting microscope.
Epithelial cell cultures.
The generation and basic characteristics of the three immortalized human epithelial cell lines, endocervical (End/E6E7), ectocervical (Ect1/E6E7), and vaginal (Vk2/E6E7) cells, used in this study have been described previously (8, 9). Briefly, the cell lines were maintained in keratinocyte serum-free medium (KSFM) (Gibco BRL Life Technologies, Gaithersburg, Md.) supplemented with 50 μg of bovine pituitary extract per ml, 0.1 ng of epidermal growth factor per ml, and CaCl2 to a final concentration of 0.4 mM. Penicillin and streptomycin at concentrations of 100 U/ml and 100 mg/ml, respectively, were used when necessary. The cell lines repeatedly tested negative for mycoplasma contamination by enzyme-linked immunosorbent assay (Boehringer-Mannheim GmbH, Mannheim, Germany).
Adherence and invasion assays.
Adherence and invasion of the gonococcal strains were assayed by a standard method described previously with slight modifications (32). The target cell lines were seeded in six-well tissue culture plates (Becton Dickinson and Company, Franklin Lakes, N.J.) at 2.5 × 105 epithelial cells/well and allowed to grow in antibiotic-free KSFM to complete confluence (106 cells/well). Preliminary experiments using 10:1, 100:1, and 1,000:1 multiplicities of infection (MOI) were conducted to determine the optimal bacterial-to-epithelial cell ratio in our infection model. These pilot investigations demonstrated a saturation of adhesion of gonococci to the endocervical epithelial cells at an MOI of 10:1, with the highest invasion frequency observed at an MOI of 100:1. Therefore, for all subsequent infection experiments described in this study we utilized an MOI of 100:1. A bacterial cell suspension of approximately 5 × 107 CFU/ml of KSFM was standardized using an optical density at 600 nm of ∼0.025 to 0.030. A 2.0-ml volume of this suspension was added to each epithelial layer, giving an MOI of 100:1, and the bacterial-epithelial cocultures were maintained at 37°C in a 5% CO2 incubator. Adherence was monitored after 4 h, and the invasion assays were carried out for 4 and 8 h.
To assay for adherent bacteria, the monolayers were gently washed three times with 1 ml of Dulbecco's phosphate buffered saline (D-PBS), pH 7.0, without Ca2+ or Mg2+ ions (Gibco BRL Life Technologies) with a sterile transfer pipette. Excess D-PBS was aspirated followed by the addition of 1 ml of D-PBS supplemented with 0.5 mM EDTA to each well. The monolayer from each well was scraped off with a sterile tissue culture scraper and collected into a sterile 1.5-ml Eppendorf tube, vortexed vigorously for 1 min, diluted, and plated on GC agar (Difco) to determine the number of viable bacteria (CFU/106 epithelial cells).
To assay for bacterial invasion at the desired time points, the monolayers were washed three times as described above but with D-PBS containing Ca2+ and Mg2+. After the third wash, 2 ml of KSFM containing gentamicin (20 μg/ml) was added to each well, and the plates were incubated for 1 h at 37°C in a 5% CO2 incubator. At the end of the gentamicin treatment the supernatants were aspirated, and the monolayers were washed three times and scraped off as described above for the adherence assay. Each adherence and/or invasion experiment was run in duplicate and repeated at least three times.
Construction of GFP fusion in N. gonorrhoeae.
A green fluorescent protein (GFP)-expressing gonococcal strain was constructed as follows. The tac promoter was first cloned into the plasmid pFPV25, which contains a promoterless GFP gene; this construct was then used as the template to amplify the tacgfp fusion, and the resulting PCR product was ligated into the gonococcal vector pLES94 (31). The fusion plasmid (pLES95) was then transformed into Escherichia coli, and transformants were selected on Luria-Bertani agar supplemented with ampicillin (100 μg/ml). Following confirmation of the tacgfp insertion in pLES94, the resulting plasmid construct (pLES95) was used to transform N. gonorrhoeae, and the presence of the tacgfp fusions in gonococcal transformants was confirmed by PCR. The expression of GFP in gonococcal transformants was confirmed by fluorescence-activated cell sorting.
Confocal microscopy.
The human endocervical (End/E6E7) epithelial cell line was used to assess adherence to and invasion of piliated N. gonorrhoeae F62 expressing GFP, using a laser scanning confocal microscope. Invasion assays were conducted as described above, but a sterile coverslip was placed into each six-well tissue culture plate prior to seeding of epithelial cells. Epithelial cells were allowed to reach 90 to 100% confluence. N. gonorrhoeae F62 expressing GFP (MOI of 100) was added to the epithelial cells and incubated as described above for 30 min, 1 h, and 4 h. F-actin staining of the infected monolayer using the fluorescent phallotoxin Alexa-Fluor 568 (Molecular Probes, Eugene, Oreg.) was performed according to the manufacturer's protocol. Briefly, at the end of the desired infection period, the coverslips containing the monolayers were washed three times with prewarmed PBS, pH 7.0. The target monolayers were then fixed with 3.7% formaldehyde solution in PBS for 10 minutes at room temperature. Fixed monolayers were washed three times with 0.1% Triton X-100 in PBS for 5 min and incubated with Alexa-Fluor (1 to 2 U/200 ml in PBS containing 1% bovine serum albumin) for 20 min at room temperature. Finally, the coverslips were washed three times with PBS, air dried, mounted in Cytoseal 60 mounting medium (VWR Scientific Products, Willard, Ohio), and stored in the dark at 4°C. Reading for Alexa-Fluor and GFP was performed at 568 and 485 nm, respectively. To confirm that N. gonorrhoeae expressing GFP adhered to and invaded the epithelial cells at levels similar to that observed for the wild-type strain, we examined adherence and invasion using the standard antibiotic protection assay as described above.
Detection of soluble immunobiological mediators.
Culture supernatants were collected from quadruplicate endocervical cultures incubated with N. gonorrhoeae expressing GFP for 1, 4, and 8 h or from endocervical, ectocervical, and vaginal cultures incubated with piliated and nonpiliated N. gonorrhoeae F62 for 4, 8, and 24 h. Parallel cultures without added bacteria were used as appropriate controls. Each culture supernatant was aspirated using a sterile 3-ml syringe, filtered with a 0.45-μm low-protein-binding filter (Millipore, Bedford, Mass.), aliquoted in 500-μl volumes, and stored immediately at −20°C. Supernatants were also collected from triplicate epithelial cultures stimulated with N. gonorrhoeae lysates. Lysates were obtained by incubation of 108/cells of piliated or nonpiliated N. gonorrhoeae strain F62 variants/ml in distilled H2O for 30 min at 37°C followed by centrifugation at 10,000 × g for 10 min. The lysates were aliquoted and stored at −20°C. An equivalent of 5 × 106 gonococci per well (lysates diluted 20×) were incubated with epithelial monolayers in six-well culture plates for 24 h. Controls in each experiment included monolayers with no bacteria added (negative control) or monolayers stimulated with 20 ng of recombinant human TNF-α (R&D Systems, Minneapolis, Minn.) per ml. Concentrations of cytokines, chemokines, and soluble ICAM-1 (sICAM-1) were determined by commercial enzyme-linked immunosorbent assay kits (R&D Systems and Endogen, Cambridge, Mass.) using a Dynatech MRX microplate reader and Revelation software (Dynex Technologies, Chantilly, Va.). At least two independent experiments per cell line were conducted.
Immunocytochemistry.
Expression of the membrane cofactor protein (CD46), members of the carcinoembryonic family of proteins (CD66), and ICAM-1 (CD54) by epithelial cells in vivo was examined in frozen methanol-fixed tissue sections from normal human vagina, ectocervix, and endocervix by two different immunohistochemical methods. Vaginal and cervical tissue specimens were obtained from discarded surgical material in agreement with human subject protection policies. The expression of the same adhesion molecules was examined in the immortalized cervical and vaginal epithelial cell lines under baseline (uninfected) conditions as well as following TNF-α stimulation or gonococcal infection, performed as described above for cytokine expression. Anti-human CD46 antibody (mouse monoclonal MAS657) was purchased from Accurate Chemical and Scientific Corporation, Westbury, N.Y. Fluorescein isothiocyanate (FITC)-conjugated pan-CD66 antibody (mouse monoclonal F7112) recognizing an epitope common to four members of the CD66 family (CD66a, -b, -c, and -e) was purchased from Dako, Carpinteria, Calif. The anti-human CD66 antibody (mouse monoclonal By114), selective for the 90-kDa nonspecific cross-reactive antigen (CD66c), was purchased from BioGenex, San Ramon, Calif. An antibody cocktail against human ICAM-1 (mouse monoclonals R6.5, R6.1, CA7, and CA8) was a courtesy of Robert Rothlein from Boehringer Ingelheim Pharmaceuticals, Ridgefield, Conn.
For the immunostaining experiments, the epithelial cell lines were grown to confluence in Falcon eight-chamber tissue culture glass slides (Becton Dickinson Labware). Immunohistochemical analysis for CD46, CD66c, and ICAM-1 was performed as previously described in detail (8) using a streptavidin-biotin-alkaline phosphatase kit (StrAviGen; BioGenex) and the fast red substrate system K699 (Dako). A direct immunofluorescent assay using the FITC-conjugated pan-CD66 antibody was performed as follows. Briefly, slides were incubated for 1 h at 37°C with blocking solution of 10% albumin in PBS and for 1 h at 4°C in the dark with the FITC-labeled antibody and then washed three times in PBS and sealed with Vectashield mounting medium with propidium iodide (Vector Laboratories, Burlingame, Calif.). An epifluorescent microscope was used to read slides.
Statistical analysis.
The results from the antibiotic protection assay and the cytokine experiments were analyzed by one-way analysis of variance using Instat version 3.0 (Graphpad, San Diego, Calif.). A P value of <0.05 was considered significant.
RESULTS
N. gonorrhoeae strain F62 invades both cervical and vaginal epithelial cells.
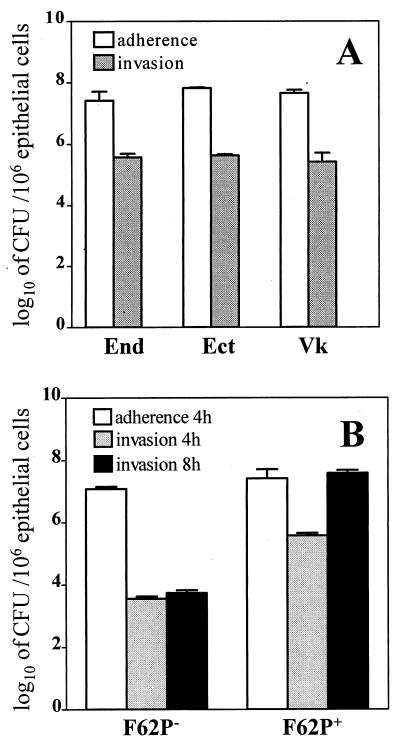
Results from a standard antibiotic protection assay demonstrated that the immortalized endocervical, ectocervical, and vaginal epithelial cells supported the attachment and the intracellular uptake of piliated N. gonorrhoeae F62 (Fig. 1A). We did not detect statistically significant differences among the three epithelial cell lines with regard to their ability to attach and internalize gonococci. Piliated N. gonorrhoeae F62 invaded the endocervical epithelial cells at 5.57 ± 0.24 log10 CFU per 106 epithelial cells, as determined at 4 h postinfection. Likewise, this strain was found to invade the ectocervical and vaginal epithelial cells at 5.63 ± 0.11 and 5.42 ± 0.58 log10 CFU, respectively, as detected 4 h postinfection. As shown in Fig. 1B, nonpiliated N. gonorrhoeae F62 variants attached to the endocervical epithelial cells with an efficiency similar to that of the piliated variants (7.08 ± 0.15 and 7.41 + 0.59 log10 CFU/106 epithelial cells, respectively, P > 0.05), suggesting that pili were not absolutely required for attachment to these cells. However, despite their similar adherence abilities, the nonpiliated and piliated F62 variants demonstrated markedly different invasion capacities. Nonpiliated N. gonorrhoeae F62 variants were poor invaders of the endocervical epithelial cells, as indicated by the significantly lower numbers of internalized microorganisms observed (2-log difference at 4 h and 4-log difference at 8 h between piliated and nonpiliated gonococci; P < 0.05) (Fig. 1B). Moreover, the numbers of intracellular piliated gonococci measured by the antibiotic protection assay increased by 2 logs over 8 h, while the intracellular nonpiliated gonococci did not increase significantly within the 8-h period postinfection. Similar adhesion and invasion patterns were obtained when ectocervical and vaginal epithelial cells were challenged with nonpiliated F62 gonococci (data not shown).
FIG. 1.
Interactions of N. gonorrhoeae with human cervical and vaginal epithelial cells. (A) Adherence of the N. gonorrhoeae F62 piliated variant to and invasion of the endocervical (End), ectocervical (Ect), and vaginal (Vk) immortalized epithelial cell lines 4 h postinfection. (B) Adherence and invasion of nonpiliated (F62P−) and piliated (F62P+) N. gonorrhoeae F62 following 4 and 8 h infection of endocervical epithelial cells. Values are means plus standard deviations of logarithmically transformed determinations obtained from three independent experiments.
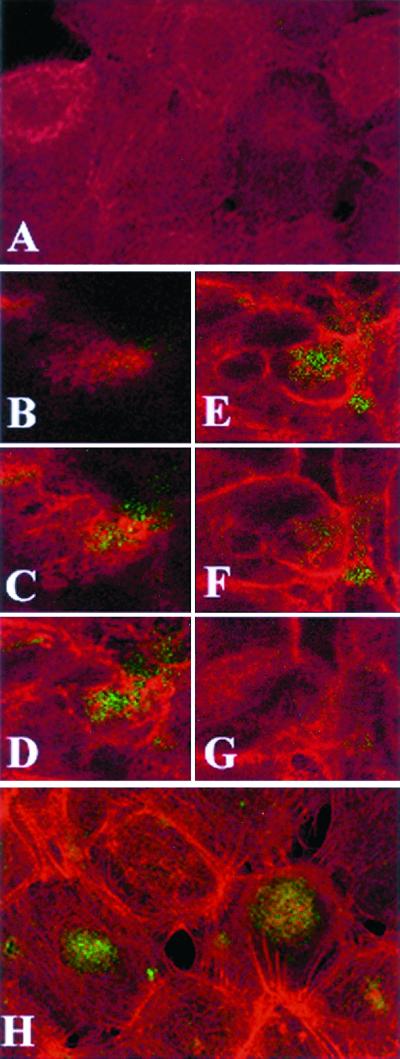
Internalization of piliated N. gonorrhoeae by the endocervical epithelial cells was confirmed by laser scanning confocal microscopy analysis using N. gonorrhoeae expressing GFP (Fig. 2). While no F-actin polymerization was seen in uninfected cultures (Fig. 2A), infected cultures demonstrated polymerization of F-actin around the epithelial cell surface, suggesting active internalization of N. gonorrhoeae following a 30-min infection period (Fig. 2B to G). This was intensified following 4 h (Fig. 2H) postinfection. Large clusters of gonococci contained foci that coincided with F actin and typically appeared to be unevenly distributed within subpopulations of epithelial cells. This may reflect distinct differentiation states previously characterized within confluent epithelial monolayers using specific markers of epithelial cell differentiation (8).
FIG. 2.
Confocal microscopy analysis of endocervical epithelial cell monolayers infected with GFP-expressing N. gonorrhoeae. F actin was visualized using red fluorescent Alexa-Fluor. Images were obtained by combining a Z series of 15 optical sections taken at 2-μm intervals. The slides were consecutively read for red and green fluorescence using 485- and 568-nm filters, respectively. (A) Control uninfected culture. Diffuse red staining indicates lack of F-actin polymerization. (B to G) Single apical-basal confocal slices taken in consecutive order (1, 4, 6, 8, 10, and 12 optical sections) from a Z series 30 min postinfection. The Alexa-Fluor labeling (C to G) demonstrates increased cortical polymerization of F actin in most epithelial cells. The GFP Alexa-Fluor overlap (yellow color [C to F]) indicates colocalization of F actin with gonococci. (H) Composite image of a 15-section Z series 4 h postinfection. GFP-expressing gonococci appear in large round clusters within the infected epithelial cells (typically seen between the sixth and tenth optical sections). Polymerized actin bundles extend toward the middle of infected epithelial cells.
N. gonorrhoeae infection of cervical and vaginal epithelial cells induces upregulation of proinflammatory mediators.
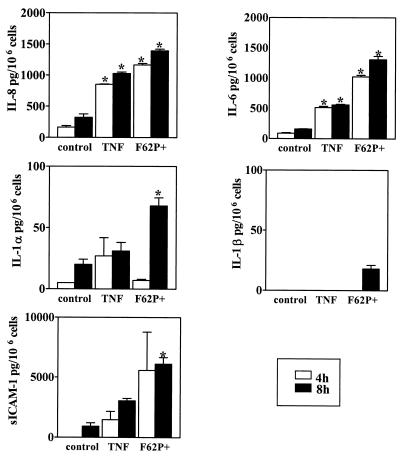
As demonstrated in Fig. 3, infection with piliated N. gonorrhoeae induced a distinct temporal pattern of proinflammatory response by the endocervical epithelial cells. At 4 and 8 h post infection, we detected significantly elevated concentrations of IL-8 and IL-6 in supernatants obtained from infected compared to uninfected cell cultures (Fig. 3). In contrast, IL-1α, IL-1β, and sICAM-1 levels were initially increased at 8 h postinfection (Fig. 3). TNF-α was not detectable at these time points (data not shown).
FIG. 3.
Levels of IL-8, IL-6, IL-1β, IL-1α, and sICAM-1 following infection of endocervical cells with N. gonorrhoeae. Culture supernatants were collected 4 and 8 h after incubation under the following conditions: uninfected nonstimulated (control), uninfected stimulated with TNF-α, and infected with the N. gonorrhoeae F62 piliated variant (F62P+) expressing GFP. Values are means plus standard deviations of quadruplicate determinations and are representative of two independent experiments. ∗, P < 0.05 versus nonstimulated control.
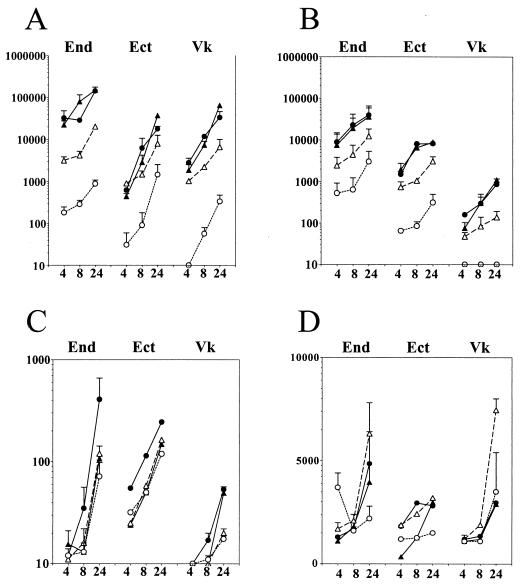
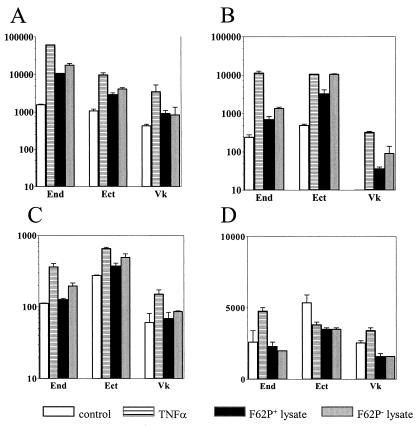
In another set of experiments, we compared the levels of these proinflammatory mediators in supernatants obtained from endocervical, ectocervical, and vaginal epithelial cells infected with piliated and nonpiliated cultures of N. gonorrhoeae F62 strain (Fig. 4). Although as previously reported (8) the endocervical cell line showed higher baseline production of IL-8 and IL-6 than the ectocervical and vaginal epithelial cell lines, all three cell lines demonstrated a significant upregulation of both mediators within a 24-h infection period, compared to uninfected nonstimulated or TNF-α-stimulated cultures (P < 0.05) (Fig. 4A and B). The magnitude of the IL-8 and IL-6 upregulation was similar for the three cell lines (approximately a 2-log increase over the control untreated cultures 8 and 24 h following infection). As observed in the previous set of experiments, the endocervical cells demonstrated a delayed release of IL-1α compared to IL-8 and IL-6 following infection with piliated N. gonorrhoeae F62 (Fig. 4C), while the ectocervical and vaginal cells failed to show any significant increase of IL-1α in response to infection. Moreover, all three cell lines failed to show any significant increase of IL-1α and sICAM-1 in response to nonpiliated F62 (Fig. 4C and D). On the other hand, there was no statistically significant difference between the IL-8 and IL-6 upregulation by the piliated and nonpiliated F62 variants, suggesting that bacterial uptake was not essential for upregulation of these two mediators.
FIG. 4.
Levels of IL-8 (A), IL-6 (B), IL-1α (C), and sICAM-1 (D) in supernatants collected from endocervical (End), ectocervical (Ect), and vaginal (Vk) epithelial cell cultures during a 24-h time course of infection with N. gonorrhoeae. Data (picograms per 106 epithelial cells) are means plus standard deviations of duplicate determinations obtained in two independent experiments for each cell line. Closed symbol represent levels of mediators in cultures infected with piliated (circles) and nonpiliated (triangles) N. gonorrhoeae strain F62. Controls included uninfected nonstimulated (open circles) and TNF-α-stimulated cultures (open triangles) examined at the same time points.
To further test this hypothesis, we examined the cytokine production in epithelial cell cultures incubated for 24 h with whole-cell lysates obtained from N. gonorrhoeae strain F62 piliated and nonpiliated variants. A significant increase in both IL-8 and IL-6 expression was detected in culture supernatants following stimulation with these lysates, which was comparable to that observed after TNF-α stimulation (Fig. 5A and B). In contrast, IL-1α and sICAM-1 concentrations were not altered significantly by incubation of the three cell lines with the gonococcal lysates (Fig. 5C and D). Taken together, these results indicated that both piliated and nonpiliated gonococci can stimulate IL-1α-independent IL-8 and IL-6 upregulation in endocervical, ectocervical, and vaginal epithelial cells while the late IL-1α response is restricted to the endocervical cells infected with piliated gonococci.
FIG. 5.
Levels of IL-8 (A), IL-6 (B), IL-1α (C), and sICAM-1 (D) in 24-h supernatants from endocervical (End), ectocervical (Ect), and vaginal (Vk) epithelial cell cultures exposed to whole-cell lysates obtained from N. gonorrhoeae F62 piliated (F62P+) and nonpiliated (F62P−) variants. Controls include uninfected unstimulated cultures (control) and TNF-α-stimulated cultures (TNFα) examined at the same time point (24 h). Values (picograms per 106 epithelial cells) are means plus standard deviations of triplicate determinations for each cell line and represent two independent experiments.
N. gonorrhoeae infection of endocervical, ectocervical, and vaginal epithelial cells induces expression of adhesion molecules.
The intimate attachment and invasion of human cells by N. gonorrhoeae appears to be mediated by diverse mechanisms in the different cell types that have been examined (19). While CD46 is regarded as the primary host receptor for neisserial pilus in both phagocytic and nonphagocytic cell types (18), the neisserial Opa proteins utilize various receptor mechanisms depending on the host cell type, including the carcinoembryonic receptors (CD66) in phagocytes or the proteoglycan receptors in cancer cells (19, 26, 28). On the other hand, clustering of ICAM-1 at the site of gonococcal entry in cancer cell lines has been reported (16, 25, 27). However, the expression of these adhesion molecules by epithelial cells in the lower genital tract has not been systemically studied, and their role in the pathogenesis of gonococcal infection of normal human cervical and vaginal epithelial cells remains obscure. Thus, we next examined the expression of CD46, CD66, and ICAM-1 in vaginal and cervical epithelial cells before and after gonococcal infection. We also examined the expression of these adhesion molecules in normal vaginal and cervical tissues collected from six women without history of gonococcal infection.
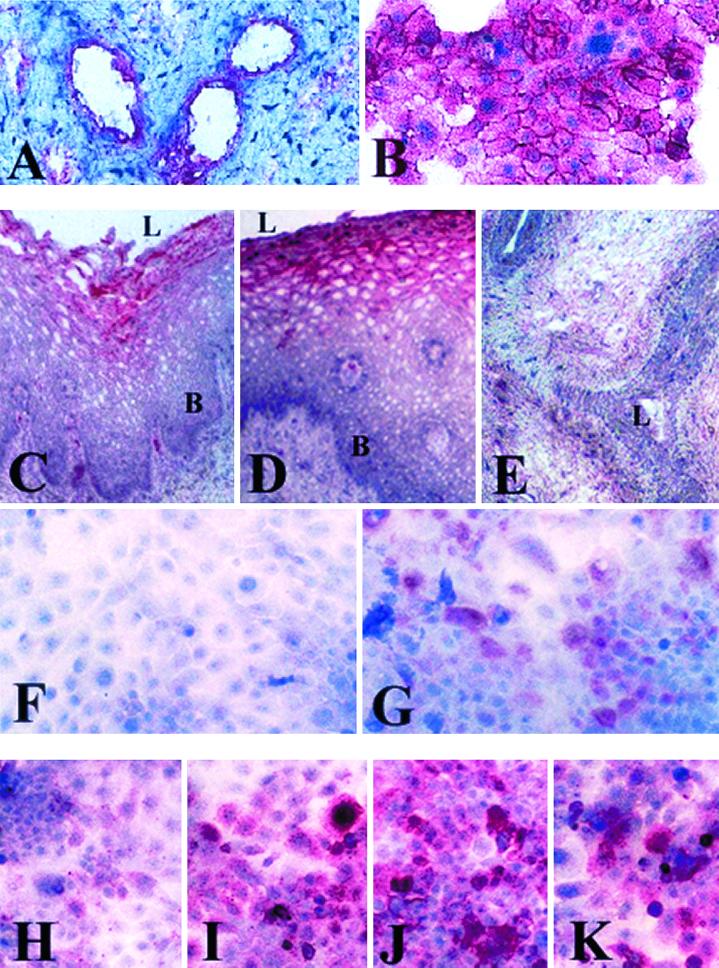
CD46 was constitutively expressed by epithelial cells in endocervical, ectocervical, and vaginal tissue specimens collected from women without history of gonococcal infection (Fig. 6A and data not shown). Similarly, the endocervical, ectocervical, and vaginal cell lines demonstrated strong and uniform constitutive CD46 expression, as demonstrated for the endocervical cells in Fig. 6B, which was unchanged following infection with gonococci for 4 to 24 h (data not shown).
FIG. 6.
Immunocytochemical analysis of adhesion molecule expression by cervicovaginal epithelial cells in vitro and in vivo. Positive cells appear red. (A and B) Constitutive expression of CD46 in endocervical tissue (A) and endocervical cell culture (B). (C to G) Expression of CD66 in vaginal tissue (C), ectocervical tissue (D), endocervical tissue (E), uninfected endocervical cell culture (F), and endocervical cell culture after 8 h of infection with the N. gonorrhoeae F62 piliated variant (G). (H to K) Expression of ICAM-1 in endocervical cell cultures with no infection (H), following 8 h of TNF-α stimulation (I), following 8 h of infection with N. gonorrhoeae piliated F62 (J), or following 8 h of infection with N. gonorrhoeae nonpiliated F62 (K). L. lumenal epithelial surface: B. basal epithelial layers. Magnification, ×125 (A, C to E, and H to K) and ×250 (B, F, and G).
Others have shown ubiquitous CD66 expression in human endocervical and ectocervical epithelial tissue (7), which was confirmed by our investigations using the same pan-CD66 (CD66a, -b, -c, and -e) antibody (data not shown). However, when we used a CD66c-specific antibody, we found differences in tissue distribution of this epitope. As demonstrated in Fig. 6C to F, the expression of CD66c was abundant in the luminal suprabasal layers of the stratified vaginal and ectocervical epithelia in vivo and not detectable within the basal and parabasal proliferative layers of these epithelia. However, in contrast to the panepitope, it was not detectable in the endocervical epithelium in vivo (Fig. 6E) or any of the uninfected epithelial cell lines in vitro (Fig. 6F). Interestingly, a considerable number of CD66c-positive cells were detected in all three epithelial cell lines following infection with both piliated and nonpiliated gonococci (Fig. 6G and data not shown).
ICAM-1 was expressed by a subpopulation of cells in the vaginal and cervical epithelial cultures under nonstimulated or uninfected conditions (Fig. 6H). This adhesion molecule was markedly upregulated in the three immortalized epithelial cell lines by both piliated and nonpiliated F62 gonococci at 4, 8, and 24 h after infection (Fig. 6J and K).
DISCUSSION
Our study is the first to systematically compare the interactions of N. gonorrhoeae with epithelial cells originating from three morphologically and functionally distinct compartments of the lower female genital tract, the endocervix, ectocervix, and vagina. The use of immortalized epithelial cell lines which closely resemble the epithelial differentiation patterns of normal human vaginal and cervical tissues enabled us to establish a reproducible in vitro model that is anatomically relevant. Our results demonstrated marked IL-8, IL-6, and ICAM-1 upregulation by the cervical and vaginal epithelial cells in response to infection with N. gonorrhoeae F62 piliated and nonpiliated cultures. These proinflammatory molecules are under the transcriptional control by the nuclear factor κB (NF-κB) and can be induced in vivo as well as in vitro in many cell types by IL-1 or TNF-α or by direct contact with pathogenic bacteria or bacterial products, such as endotoxin or lipopolysaccharide (14). Our results suggested that the gonococcus-triggered IL-8 and IL-6 upregulation was IL-1α and TNF-α independent within the first 4 h of infection. Likewise, IL-1α-independent IL-6 and or IL-8 upregulation has been demonstrated in gastroenteric, urinary, and respiratory epithelial cell models of mucosal infections with gram-negative bacteria (1, 4, 11, 17, 33). An IL-1-independent IL-8 and IL-6 local mucosal production has been reported following experimental gonococcal inoculation of the male urethra (29). Furthermore, evidence that the major proinflammatory transcription factor NF-κB can be activated in infected cervical cancer cell lines prior to gonococcal uptake and IL-1 release (27) supports our findings of invasion-independent and IL-1α-independent proinflammatory response to gonococcal infection. In contrast, the release of intracellular IL-1 by invaded and lysed host cells appears to be essential for cytokine and chemokine upregulation by chlamydial infection of endocervical epithelial cells (30), suggesting pathogen-specific host inflammatory responses in the lower female genital tract.
An important conclusion of our experiments with whole gonococcal lysates is that the IL-8 and IL-6 response by cervical and vaginal epithelial cells was not restricted to the interactions with viable gonococci. Similarly, a recent study has shown that viable N. gonorrhoeae is not essential for proinflammatory response by innate immune cells, since mature human macrophages generate an array of cytokines and chemokines in response to purified gonococcal surface antigens (24). Also, the comparison between the two N. gonorrhoeae F62 variants in our study showed that IL-6 and IL-8 responses did not correlate with the different magnitudes of gonococcal internalization by the epithelial cells. Moreover, our findings as well as other published reports suggest that the proinflammatory cytokine response to gram-negative bacteria by epithelial cells in the lower female genital tract and other nonsterile mucosal compartments is independent of bacterial lipopolysaccharide (6, 1, 33; G. A. Jarvis, J. Li, and K. Swanson, Keystone Symposia: Interfaces Between Innate and Adaptive Immunity, abstr. 119, 2000). Taken together, these findings suggest that gonococcal components can stimulate proinflammatory responses, which are independent of either gonococcal metabolic activity (viability) or entry into the host cells.
The ability of gonococci to stimulate the expression of IL-8, IL-6, and ICAM-1 by the different epithelial compartments of the lower female genital tract, observed in this study, may contribute directly to the inflammatory infiltrate characteristic of disease caused by N. gonorrhoeae (20). While IL-8 is a powerful chemoattractant and activator of neutrophils (21) and IL-6 is responsible for neutrophil priming to chemotactic factors (23), ICAM-1 can facilitate transepithelial migration and retention of leukocytes at the bacterial aggression site (11, 15, 35, 37). The shedding of ICAM-1 may, on the other hand, represent a mechanism for limiting the inflammatory events. Soluble ICAM-1 appears to attract neutrophils (36) and can compete with membrane-bound ICAM-1 for neutrophil integrins, thus impeding their firm adherence to the infection site (12). Similarly, the expression of CD66c by the superficial layers of the vaginal and ectocervical epithelium may be a specific host epithelial mechanism for downregulation of inflammatory events in the stratified epithelia by eliminating adherent N. gonorrhoeae through the rapid shedding of the luminal layers in vivo. A similar mechanism to control the bacterial colonization of the gastrointestinal mucosa has been proposed based on in vitro E. coli epithelial interactions (34). In contrast, the expression of CD66c by the endocervical epithelial cells upon infection can facilitate the gonococcal entry into the simple columnar epithelium of the endocervix, which is the primary site for gonococcal infection in the lower female genital tract (38).
Our finding that endocervical epithelial cells can release higher levels of IL-1α in response to gonococcal infection than ectocervical and vaginal cells reveals an additional molecular mechanism for amplification of the proinflammatory responses which may contribute to the development of cervicitis, the most common clinical characteristic of the disease caused by N. gonorrhoeae in women (38). Moreover, other in vitro models have demonstrated that although macrophages upregulated a myriad of proinflammatory molecules following challenge with purified gonococcal antigens, they did not respond by significantly increased IL-1 production (24), suggesting that the endocervical epithelial cells may be the primary source of IL-1 during advanced stages of gonococcal disease. Furthermore, increased levels of mucosally derived IL-1 have been associated with the development of symptoms in experimental urethral gonorrhea (29), which suggests that the deficiency of vaginal IL-1 response to gonococcal infection may underlie the prevalence of asymptomatic genital gonorrhea in women.
In summary, the endocervical, ectocervical, and vaginal epithelial cell lines used in this study can support adhesion and invasion of N. gonorrhoeae and initiate vigorous proinflammatory events in response to gonococcal infection. These immortalized cervical and vaginal epithelial cell lines can be further used as monolayers or as polarized or stratified cultures to supplement tissue explant and primary cell in vitro models to identify host and pathogen features participating in the molecular and cellular events of N. gonorrhoeae infection.
ACKNOWLEDGMENTS
We thank Deborah Anderson, Director of the Fearing Research Laboratory at Brigham and Women's Hospital, Harvard Medical School, Boston, Mass. for providing lab resources for the development of our infection model; Alison Quayle, from the Fearing Research Laboratory, for providing frozen blocks of tissue for our immunohistochemistry assays; and Virginia Clarke for the N. gonorrhoeae F62 strain. We also acknowledge Lee Wetzler for critical review of the manuscript.
This work was supported by the Connors Seed Grant for Gender Biology Research, Brigham and Women's Hospital, a Rockefeller Foundation Microbicides Basic Science Network Grant, grant PO1AI4596701 from the National Institutes of Health, Bethesda, Md. (R.N.F.), and Public Health Service grant U19AI38515 (C.A.G.).
REFERENCES
- 1.Agace W, Hedges S, Andersson U, Andersson J, Ceska M, Svanborg C. Selective cytokine production by epithelial cells following exposure to Escherchia coli. Infect Immun. 1993;61:602–609. doi: 10.1128/iai.61.2.602-609.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson R M. Transmission dynamics of sexually transmitted infections. In: Holmes K K, Sparling P F, Mardh P A, Lemon S M, Satm W E, Piot P, Wasserheit J N, editors. Sexually transmitted diseases. New York, N.Y: McGraw-Hill; 1999. pp. 25–37. [Google Scholar]
- 3.Christodoulides M, Everson J S, Liu B L, Lambden P R, Watt P J, Thomas E J, Heckels J E. Interaction of primary human endometrial cells with Neisseria gonorrhoeae expressing green fluorescent protein. Mol Microbiol. 2000;35:32–43. doi: 10.1046/j.1365-2958.2000.01694.x. [DOI] [PubMed] [Google Scholar]
- 4.Crowe S E, Alvarez L, Dytoc M, Hunt R H, Miller M, Sherman P, Patel J, Jin Y, Ernst P B. Expression of interleukin 8 and CD54 by human gastric epithelium after Helicobacter pylori infection in vitro. Gastroenterology. 1995;108:65–74. doi: 10.1016/0016-5085(95)90009-8. [DOI] [PubMed] [Google Scholar]
- 5.Desai P J, Nzeribi R, Genco C A. Binding and accumulation of hemin in Neisseria gonorrhoeae. Infect Immun. 1995;63:4634–4641. doi: 10.1128/iai.63.12.4634-4641.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eckman L, Kagnoff M F, Fierer J. Epithelial cells secrete the chemokine interleukin-8 in response to bacterial entry. Infect Immun. 1993;61:4569–4574. doi: 10.1128/iai.61.11.4569-4574.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edwards J L, Shao J Q, Ault K A, Apicella M A. Neisseria gonorrhoeae elicits membrane ruffling and cytoskeletal rearrangements upon infection of primary human endocervical and ectocervical cells. Infect Immun. 2000;68:5354–5363. doi: 10.1128/iai.68.9.5354-5363.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fichorova R N, Rheinwald J G, Anderson D J. Generation of papillomavirus-immortalized cell lines from normal human ectocervical, endocervical, and vaginal epithelium that maintain expression of tissue-specific differentiation proteins. Biol Reprod. 1997;57:847–855. doi: 10.1095/biolreprod57.4.847. [DOI] [PubMed] [Google Scholar]
- 9.Fichorova R N, Anderson D J. Differential expression of immunobiological mediators by immortalized human cervical and vaginal epithelial cells. Biol Reprod. 1999;60:508–514. doi: 10.1095/biolreprod60.2.508. [DOI] [PubMed] [Google Scholar]
- 10.Fichorova R N, Anderson D J. Cytokines in the cervical vaginal environment. In: Hill J A, editor. Cytokines in reproduction. New York, N.Y: Wiley-Liss; 2000. pp. 79–91. [Google Scholar]
- 11.Frick A G, Joseph T J, Pang L, Rabe A M, St. Geme III J W, Look D C. Haemophilus influenzae stimulates ICAM-1 expression on respiratory epithelial cells. J Immunol. 2000;164:4185–4196. doi: 10.4049/jimmunol.164.8.4185. [DOI] [PubMed] [Google Scholar]
- 12.Gearing A J H, Newman W. Circulating adhesion molecules in disease. Immunol Today. 1993;14:506–514. doi: 10.1016/0167-5699(93)90267-O. [DOI] [PubMed] [Google Scholar]
- 13.Hedges S R, Sibley D A, Mayo M S, Hook III E W, Russel M W. Cytokine and antibody responses in women infected with Neisseria gonorrhoeae: effects of concomitant infections. J Infect Dis. 1998;178:742–751. doi: 10.1086/515372. [DOI] [PubMed] [Google Scholar]
- 14.Holtmann H, Winzen R, Holland P, Eickemeier S, Hofmann E, Wallach D, Malinin N L, Cooper J A, Resch K, Kracht M. Induction of interleukin-8 synthesis integrates effects on transcription and mRNA degradation from at least three different cytokine- or stress-activated signal transduction pathways. Mol Cell Biol. 1999;19:6742–6753. doi: 10.1128/mcb.19.10.6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang G T-J, Eckman L, Savidge T C, Kagnoff M F. Infection of human intestinal epithelial cells with invasive bacteria upregulates apical intracellular adhesion molecule-1 (ICAM-1) expression and neutrophil adhesion. J Clin Invest. 1996;98:572–583. doi: 10.1172/JCI118825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jarvis G A, Li J, Swanson K. Invasion of human mucosal epithelial cells by Neisseria gonorrhoeae upregulates expression of intercellular adhesion molecule 1 (ICAM-1) Infect Immun. 1999;67:1149–1156. doi: 10.1128/iai.67.3.1149-1156.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jung H C, Eckman L, Yang S-K, Panja A, Fierer J, Morzycka-Wroblewska E, Kagnoff M F. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Invest. 1995;95:55–65. doi: 10.1172/JCI117676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kallstromi H, Liszewski M K, Atkinson J P, Jonsson A-B. Membrane cofactor protein (MCP or CD46) is a cellular pilus receptor for pathogenic Neisseria. Mol Microbiol. 1997;25:639–647. doi: 10.1046/j.1365-2958.1997.4841857.x. [DOI] [PubMed] [Google Scholar]
- 19.Kerr J R. Cell adhesion molecules in the pathogenesis of and host defense against microbial infections. Mol Pathol. 1999;52:220–230. doi: 10.1136/mp.52.4.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.King G, James J F, Swanson J. Studies on gonococcus infection. XI. Comparison of in vivo and in vitro association of Neisseria gonorrhoeae with human neutrophils. J Infect Dis. 1978;137:38–42. doi: 10.1093/infdis/137.1.38. [DOI] [PubMed] [Google Scholar]
- 21.Kunkel S L, Lukacs N W, Chensue S W, Strieter R M. Chemokines and the inflammatory response. In: Remick D G, Friedland J S, editors. Cytokines in health and disease. 2nd ed. New York, N.Y: Marcel Dekker, Inc; 1997. pp. 121–131. [Google Scholar]
- 22.Lin J S, Donegan S P, Heeren T C, Hunberg M, Flaherty E E, Haivany R, Su X H, Dean D, Neutrau W J, Knapp J S, Rice R S, Morse S A, Rice P A. Transmission of Chlamydia trachomatis and Neisseria gonorrhoeae among men with urethritis and their female sex partners. J Infect Dis. 1998;78:1707–1712. doi: 10.1086/314485. [DOI] [PubMed] [Google Scholar]
- 23.Linder H, Engberg I, van Kooten C, de Man P, Svanborg-Edén C. Effects of anti-inflammatory agents on mucosal inflammation induced by infection with gram-negative bacteria. Infect Immun. 1990;58:2056–2060. doi: 10.1128/iai.58.7.2056-2060.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Makepeace B L, Watt P J, Heckels J E, Christodoulides M. Interactions of Neisseria gonorrhoeae with mature human macrophage opacity proteins influence production of proinflammatory cytokines. Infect Immun. 2001;69:1909–1913. doi: 10.1128/IAI.69.3.1909-1913.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Merz A J, Enns C A, So M. Type IV pili of pathogenic Neisseria elicit cortical plaque formation in epithelial cells. Mol Med. 1999;32:1316–1332. doi: 10.1046/j.1365-2958.1999.01459.x. [DOI] [PubMed] [Google Scholar]
- 26.Muenzner P, Dehio C, Fujiwara T, Achtman M, Meyer T F, Gray-Owen S D. Carcinoembryonic antigen family receptor specificity of Neisseria meningitidis Opa variants influences adherence to and invasion of proinflammatory cytokine-activated endothelial cells. Infect Immun. 2000;68:3601–3607. doi: 10.1128/iai.68.6.3601-3607.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naumann M, Wessler S, Bartsch C, Wieland B, Meyer T F. Neisseria gonorrhoeae epithelial cell interaction leads to the activation of the transcription factors nuclear factor κB and activator protein 1 and the induction of inflammatory cytokines. J Exp Med. 1997;186:247–258. doi: 10.1084/jem.186.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naumann M, Rudel T, Meyer T F. Host cell interactions and signaling with Neisseria gonorrhoeae. Curr Opin Microbiol. 1999;2:62–70. doi: 10.1016/s1369-5274(99)80011-3. [DOI] [PubMed] [Google Scholar]
- 29.Ramsey K, Schneider H, Cross A S, Boslego J W, Hoover D L, Staley T L, Kuschner R A, Deal C D. Inflammatory cytokines produced in response to experimental human gonorrhea. J Infect Dis. 1995;172:186–191. doi: 10.1093/infdis/172.1.186. [DOI] [PubMed] [Google Scholar]
- 30.Rasmussen S J, Eckman L, Quayle A J, Shen L, Zhang Y-X, Anderson D J, Flere J, Stephens R S, Kagnoff M F. Secretion of proinflammatory cytokines by epithelial cells in response to Chlamydia infection suggests a central role for epithelial cells in chlamydial pathogenesis. J Clin Invest. 1997;99:77–87. doi: 10.1172/JCI119136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silver L E, Clark V L. Construction of a translational lacZ fusion to study gene regulation in Neisseria gonorrhoeae. Gene. 1995;166:101–104. doi: 10.1016/0378-1119(95)00605-6. [DOI] [PubMed] [Google Scholar]
- 32.Spence J M, Chen J C-R, Clark V L. Contact inducible gonococcal invasion of Hec1B cells: proposed role of the lutropin receptor. Infect Immun. 1997;65:3736–3742. doi: 10.1128/iai.65.9.3736-3742.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Svanborg C, Agace W, Hedges S, Linder H, Svensson M. Bacterial adherence and epithelial cell cytokine production. Zentbl Bakteriol. 1993;278:359–364. [PubMed] [Google Scholar]
- 34.Thompson J A, Grunert F, Zimmermann W. Carcinoembryonic antigen gene family: molecular biology and clinical perspective. J Clin Lab Anal. 1991;5:344–366. doi: 10.1002/jcla.1860050510. [DOI] [PubMed] [Google Scholar]
- 35.Tonetti M S. Molecular factors associated with compartmentalization of gingival immune responses and transepithelial neutrophil migration. J Periodontal Res. 1997;32:104–109. doi: 10.1111/j.1600-0765.1997.tb01389.x. [DOI] [PubMed] [Google Scholar]
- 36.Vainer B, Nielsen O H. Chemotactic properties of ICAM-1 and PECAM-1 on neutrophil granulocytes in ulcerative colitis: effects of prednisolone and metasalazine. Aliment Pharmacol Ther. 2000;14:1023–1031. doi: 10.1046/j.1365-2036.2000.00797.x. [DOI] [PubMed] [Google Scholar]
- 37.Verdrengh M, Springer T A, Gutierrez-Ramos J C, Tarkowski A. Role of intercellular adhesion molecule 1 in pathogenesis of staphylococcal arthritis and in host defense against staphylococcal bacteremia. Infect Immun. 1996;64:2804–2807. doi: 10.1128/iai.64.7.2804-2807.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zenilman J M, Deal C D. Gonorrhea: epidemiology, control and prevention. In: Stanberry L R, Bernstein D I, editors. Sexually transmitted diseases. Vaccines, prevention and control. San Diego, Calif: Academic Press; 2000. pp. 369–385. [Google Scholar]