Abstract
Morphine appears to be the most active metabolite of heroin, therefore, the effects of morphine are important in understanding the ramifications of heroin abuse. Opioid physical dependence (withdrawal response) may have very long-lasting effects on the motivation for reward, including the incubation of cue-induced drug-seeking behavior. However, the exact mechanisms of morphine withdrawal (MW) are not clear yet, and its treatment remains elusive. Periaqueductal gray (PAG) is one of the important sites in the pathogenesis of MW. Here, we used recombinant herpes simplex virus (HSV) vectors that encode the sod2 gene expressing manganese superoxide dismutase (MnSOD) to evaluate its therapeutic potential in MW. Microinjection of HSV vectors expressing MnSOD into the PAG reduced the MW syndrome. MnSOD vectors suppressed the upregulated mitochondrial superoxide, and endoplasmic reticulum (ER) stress markers (glucose related protein 78 (GRP78) and activating transcription factor 6 (ATF6α) in the PAG induced by MW. Immunostaining showed that mitochondrial superoxide, GRP78, and ATF6α were colocalized with neuronal nuclei (NeuN, a neuronal-specific marker), suggesting that they are located in the neurons in the PAG. These results suggest that overexpression of MnSOD by HSV vectors may relieve opioid dependence. This study may provide a novel therapeutic approach to morphine physical withdrawal response.
Keywords: morphine withdrawal, PAG, ROS, ER stress, MnSOD, gene therapy
Introduction
Heroin addiction may lead to very serious medical problems. Morphine appears to be the most active metabolite of heroin, therefore, the effects of morphine are important in understanding the ramifications of heroin abuse 1. Chronic opioids induces a variety of changes in the nervous system resulting in dependence2, 3. Opioid dependence is characterized by the withdrawal reaction (physical dependence) and/or the presence of a “drug-craving” component (psychological dependence) 4. The withdrawal phenomena may occur when continuous administration of the opiate drug is stopped suddenly, or may be precipitated by administration of an opioid antagonist5–7. This withdrawal state can produce dramatic and long-lasting motivational disorders and reward-seeking relevant to drug dependence8, 9 and poses a problem for both patients with opioid analgesia and opioid abusers. However, the exact mechanisms of morphine withdrawal (MW) are elusive, and its treatment is still not achieved.
An oxidative stress condition occurs in cells as a result of an imbalance between the generation and the detoxification of reactive oxygen species (ROS)10. Superoxide dismutase (SOD) scavenger enzymes are the major ROS detoxifying enzymes protecting the cells from potential damage induced by ROS 10, 11. The SOD scavenger enzymes convert superoxide radicals into H2O2 and molecular oxygen. In mammalian cells, the SOD family of enzymes consist of (i) the copper/zinc-containing superoxide dismutase (Cu/ZnSOD, SOD1) localized to the cytosol and in the intermembrane space of mitochondria, (ii) manganese superoxide dismutase (MnSOD, SOD2) located in the mitochondrial matrix, and (iii) extracellular superoxide dismutase (ECSOD, SOD3) found in the extracellular space (see Review 10). The steady-state level of superoxide is inversely proportional to the activity of MnSOD that appears to be a central player in the redox biology of cells and tissues 12. Oxidative stress is involved in the development of morphine physical dependence. Heroin decreased the total antioxidant capacity of serum and the antioxidative enzymes activities in brains 3. Pretreatment with antioxidants could inhibit oxidative stress, and alleviate opioid withdrawal in animals 13–15. Therefore, strategies for blocking oxidative stress may be useful in the development of a new therapy for opiate abuse.
Gene transfer offers the possibility to produce and release bioactive macromolecules in a local site in vivo. Among the several gene transfer vectors that are available, herpes simplex virus (HSV) vectors are particularly well suited for delivery of genes to the nervous system as HSV vectors readily transduce and persist within neurons, and can express therapeutic genes for the treatment of neuropathies and pain 16–18. A role of the periaqueductal gray (PAG) in the pathogenesis of opioid withdrawal has been suggested by both functional and biochemical studies 19, 20. In this report, we investigated the effect of non-replacing HSV vector-mediated expression of human MnSOD in the ventrolateral PAG (vlPAG) on naloxone-precipitated MW in rats. Scavenging ROS with antioxidants significantly diminishes induction of the endoplasmic reticulum (ER) stress response21, 22Mol Biol Cell. 2006 Feb; 17(2):770–8 Mol Biol Cell. 2006 Feb; 17(2):770–8. We also investigated the effect of MnSOD mediated by the HSV vector on ER stress markers in MW.
Results
HSV vector QHMnSOD expresses MnSOD in the PAG
Figure 1A showed the schematic diagrams of the QHMnSOD and Q0ZHG control HSV replication defective vectors. Our previous studies have shown that HSV vector encoding enhanced green fluorescent protein (EGFP) injected into the PAG induced GFP expression, and GFP was colocalized with neuronal nuclei (NeuN, a specific neuronal marker) immunostaining 19, suggesting that the HSV vector infected neurons. To determine the expression of MnSOD, in naïve rats 7 days after microinjection of vector QHMnSOD expressing MnSOD into the ventrolateral PAG (vlPAG), the vlPAG was harvested and MnSOD was measured by western blots analysis. Microinjection of QHMnSOD significantly increased the expression of MnSOD in the vlPAG compared to control vector (Figure 1B). There was no significant difference in the MnSOD expression in the PAG between rats that received microinjection of PBS or control vector Q0ZHG (Figure 1C).
Figure 1.
Schematic diagrams of QHMnSOD and QOZHG control HSV replication defective vectors and the expression of MnSOD in vivo. (A) The QHMnSOD and QOZHG vectors contain either an HCMV IE promoter driven the human MnSOD or E.coli lacZ gene cassette within the UL41 gene locus of an ICP4/ICP27 deletion virus that expresses both ICP22 and ICP47 as early genes (βICP22, βICP47) and an HCMV IE promoter GFP reporter cassette in the ICP27 locus. (B) HSV vector mediated expression of MnSOD. In naïve rats, 7 days after vector injection into the PAG, the PAG was collected and MnSOD was measured using western blots analysis. QHMnSOD microinjection significantly induced the expression of MnSOD in the PAG, * P < 0.05, two-tailed t test, n = 4–5. (C) The effect of microinjection of PBS or QOZHG into the vlPAG on the expression of MnSOD.
Microinjection of HSV vectors expressing MnSOD into the PAG reduced MW scores
Oxidative stress may be involved in the withdrawal process of opioids 15. Antioxidant has been shown to decrease abstinence signs of MW in animals23. In this study, we determined whether overexpression of MnSOD mediated by HSV vectors reduced MW behavioral signs. Rats received HSV vector microinjection into the PAG 1 week before MW or the sham procedure. In sham animals, HSV vector QHMnSOD encoding sod2 gene or control vector Q0ZHG was microinjected into the PAG, and intraperitoneal (IP) injection of saline (3 ml, twice a day, 8AM and 8PM, IP) was administered for 5 days. At day 5, rats received saline (1 ml, IP) 1 hour after the last chronic saline injection. There was no significant difference in the MW scores in sham rats treated with Q0ZHG compared to QHMnSOD rats (Figure 2A–F).
Figure 2.
The effect of QHMnSOD microinjected into the vlPAG on naloxone-precipitated morphine withdrawal. QHMnSOD significantly lowered the scores of teeth chatter (A), penile erection (B), rhinorrhea (C), abnormal posture (D), wet-dog shakes (E), and the global withdrawal score (F), **P < 0.01, ***P < 0.001, n =5–7, one-way ANOVA with post hoc PLSD test. (G) The time course of morphine withdrawal. Two-way ANOVA repeated measure showed that the global scores in the QHMnSOD+MW group was significant lower than that in Q0ZHG+MW rats (F(15,100) interaction=5.54, P < 0.0001); F(5,100) time=10.43, P < 0.0001; F(3,100) treatment=32.24, P < 0.0001). There was a significant increase in MW behavioral global scores in the Q0ZHG+MW rat compared to that in rats with Q0ZHG+sham at time points of 5 (***P < 0.001), 10 (***P < 0.001), 15 (***P < 0.001), 20 (*P < 0.05), and 25 (***P < 0.001) min (G), Bonferroni posttests. MW behavioral global scores in the QHMnSOD+MW group were significantly lower than that in Q0ZHG+MW group at time points of 5 (###P < 0.001), 15 (#P <0.05), and 25 (##P < 0.01) min (G), Bonferroni posttests.
In MW rats, at 1 week after HSV vector injection, rats received chronic morphine for 5 days; at day 5, one hour after the last injection of morphine, animals received naloxone (4mg/kg, IP). There was a significant increase in MW behavioral scores of teeth chat (Figure 2A), penile erection (Figure 2B), rhinorrhea (Figure 2C), abnormal posture (Figure 2D), wet-dog shakes (Figure 2E), and global scores (Figure 2F) in rats with Q0ZHG+MW compared to Q0ZHG+sham rats. MW behavioral scores of teeth chat, penile erection, rhinorrhea, abnormal posture, wet-dog shakes, and global scores in the QHMnSOD+MW group, were significantly lower than that in the Q0ZHG+MW group (Figure 2A–F). For the comparison of the time course of MW behavioral response, 2-way ANOVA showed the global scores in the QHMnSOD+MW group was significant lower than that in Q0ZHG+MW (F(15,100) interaction=5.54, P < 0.0001); F(5,100) time=10.43, P < 0.0001; F(3,100) treatment=32.24, P < 0.0001). Bonferroni post test showed that there was a significant increase in MW behavioral global scores in the Q0ZHG+MW rats compared to the Q0ZHG+sham group at time points of 5 (P < 0.001), 10 (P < 0.001), 15 (P < 0.001), 20 (P < 0.05), and 25 (P < 0.001) min (Figure 2G), and that MW global scores in the QHMnSOD+MW group were significantly lower than that in the Q0ZHG+MW group at time points of 5 (P < 0.001), 15 (P <0.05), and 25 min (P < 0.01) (Figure 2G).
We also investigated the effect of chronic morphine alone or naloxone on the MW behavioral response. Rats received chronic saline (IP) for 5 days plus saline (IP) at day 5, chronic saline for 5 days plus naloxone at day 5, or chronic morphine for 5 days plus saline at day 5. After the last saline or naloxone treatment, MW behavioral signs were observed for 30 min. We found there was no significant difference in the global scores in the 3 groups (Supplementary Figure S1).
QHMnSOD complemented the loss of MnSOD activity in the PAG induced by MW
We examined the MnSOD activity in sham groups treated with Q0ZHG, MW with Q0ZHG or MW with QHMnSOD. There was a decrease in MnSOD activity in the vlPAG of rats with Q0ZHG+MW compared to Q0ZHG+sham, P < 0.05, one-way ANOVA with posthoc PLSD test; MnSOD activity in the QHMnSOD+MW rats was significantly higher than that in the Q0ZHG+MW group, P < 0.05, one-way ANOVA with posthoc PLSD test, n=4–6 (Figure 3A).
Figure 3.

The activity of MnSOD mediated by HSV vectors in the PAG during morphine withdrawal. (A) Group of Q0ZHG with MW showed the lowered MnSOD activity in the vlPAG compared to sham, * P < 0.05, one-way ANOVA with posthoc PLSD test, n =5–6. The MnSOD activity in the QHMnSOD rats with MW was higher than that in Q0ZHG with MW, * P < 0.05 vs. Q0ZHG+MW, one-way ANOVA with posthoc PLSD test, n =4–5. Double-label immunostaining showed MnSOD immunoreactivity mainly colocalized with NeuN (B-D). MnSOD was not colocalized with GFAP (E-G) or Iba1 (H-J) immunostaining, arrows denote the cells in which the signal colocalizes, scale bar, 50 μm.
To determine the cellular localization of MnSOD, double-label immunostaining was carried out in rats with MW. MnSOD was mainly colocalized with NeuN (a specific neuronal marker, Figure 3B–D), but not glial fibrillary acidic protein (GFAP, a marker of astrocytes) (Figure 3E–G) or ionized calcium-binding adapter molecule 1 (Iba1, a marker of microglia) (Figure 3H–J) immunostaining, indicating that MnSOD is expressed in neurons, but not glia.
QHMnSOD decreased mitochondrial superoxide in the PAG
ROS is produced by multiple mechanisms, and mainly originates from mitochondria with superoxide (O2•−) being the main form of ROS. We, and others have used MitoSox Red (a mitochondrial superoxide indicator) to label mitochondrial superoxide (mtO2•−) in neurons in animal tissue 24–27. To determine whether mtO2•− played a role in the PAG in MW, we injected MitoSox into the PAG at 75 min prior to perfusion. MitoSox positive image was detected using a fluorescent microscope with rhodamine filter and the number of MitoSox positive neurons in the PAG were counted. The representative MitoSox positive images in the group of Q0ZHG+sham, Q0ZHG+MW, or QHMnSOD+MW rats are shown in Figure 4A, B, and C, respectively. We observed that there was no significant difference in the MitoSox positive cell number between Q0ZHG+sham and QHMnSOD+sham groups, P > 0.05, one-way ANOVA with posthoc PLSD test, n=6 (Figure 4D). We observed an increase in the number of MitoSox positive cells in the vlPAG in the Q0ZHG+MW rats compared to the Q0ZHG+sham group, P < 0.05, one-way ANOVA with posthoc PLSD test. The number of MitoSox positive cells in the QHMnSOD+MW rats was significantly lower than that in the Q0ZHG+MW group, P < 0.05, one-way ANOVA with posthoc PLSD test, n=6 (Figure 4D). MitoSox image profile was mainly co-localized with NeuN immunostaining in the PAG (Figure 4E–G), but not with glial markers (GFAP or Iba1, data not shown) suggesting that MitoSox is in the neurons, but not glia in the PAG.
Figure 4.
The effect of QHMnSOD mediated by HSV vectors on mitochondrial superoxide in the PAG in naloxone-precipitated morphine withdrawal. Representative images of MitoSox positive cells in Q0ZHG+sham (A), Q0ZHG+MW (B) and QHMnSOD+MW (C) rats. (D) In sham groups microinjected with Q0ZHG or QHMnSOD, there was no significant difference in the number of MitoSox cells in the PAG, P > 0.05, one-way ANOVA with posthoc PLSD test, n=6. The number of MitoSox cells in Q0ZHG+MW rats is higher than that in Q0ZHG+sham rats, *P <0.05, one-way ANOVA with posthoc PLSD test, n=6. The number of MitoSox positive cells in QHMnSOD+MW rats was lower than that in Q0ZHG+MW rats, *P < 0.05, one-way ANOVA with posthoc PLSD test, n=6. MitoSox image profile co-localized with NeuN neuronal marker immunostaining in the PAG (E-G), but not with glial markers (GFAP or Iba1, data not shown).
QHMnSOD reduced GRP78 in the PAG of rats with MW
ROS regulates numerous cellular pathways22. ER stress is considered one of the mechanisms contributing to ROS-mediated cell damage 28. ER stress-induced unfolded protein response (UPR) is known to produce ROS leading to apoptosis 29. In ER stress situations UPR is activated and results in reduced global protein synthesis and increased production of proteins, such as chaperones required for proper folding at the ER. The main UPR-upregulated protein is the 78 kDa glucose-regulated protein (GRP78, also called binding immunoglobulin protein (BiP)). Elevated level of GRP78 was observed during ER stress 30. Mutant GRP78 deficient mice showed decreased chronic morphine tolerance 31. We observed the GRP78 expression in the PAG in MW, and found that there was no significant difference in the expression of GRP78 between Q0ZHG+sham and QHMnSOD+sham injected rats, P > 0.05, n=6 (Figure 5A). There was an increase in the expression of GRP78 in the PAG of rats injected with Q0ZHG+MW compared to Q0ZHG+sham, P < 0.001, one-way ANOVA with posthoc PLSD test, n=5. The expression of GRP78 in the QHMnSOD+MW rats was significantly lower than that detected in the Q0ZHG+MW rats, P < 0.001, one-way ANOVA with posthoc PLSD test, n=4–5 (Figure 5B). Double immunostaining showed that GRP78 positive immunoreactivity (ir) was co-localized with NeuN-ir (neuronal marker, Figure 5C–E), but neither OX42-ir (microglia marker, Figure 5F–H) nor GFAP-ir (astrocytes marker, Figure 5I–K), suggesting that GRP78 is located in the neurons in the PAG, but not glia.
Figure 5.

The effect of MnSOD mediated by HSV vectors on GRP78 in the PAG in naloxone-precipitated morphine withdrawal. (A) In sham groups treated with Q0ZHG or QHMnSOD, there was no significant difference in the expression of GRP78 in the PAG, P > 0.05, t test, n=6. (B) The expression of GRP78 in Q0ZHG+MW rats was higher than that in Q0ZHG+sham rats, *** P < 0.001, one-way ANOVA with posthoc PLSD test, n=5. The expression of GRP78 in QHMnSOD+MW rats was lower than that in Q0ZHG+MW rats, ***P < 0.001, one-way ANOVA with posthoc PLSD test, n=4–5. Double immunostaining showed that GRP78 positive immunoreactivity (ir) was co-localized with NeuN-ir (neuronal marker, C-E), but neither OX42-ir (microglia marker, F-H) nor GFAP-ir (astrocytes marker, I-K). Arrow showed the co-localized cells, scale bar, 50μm.
QHMnSOD decreased ATF6α in the PAG of rats with MW
In mammalian cells UPR can be mediated by activation of different stress transducers which sense the level of unfolded proteins in the ER lumen and pass the signal to the cytoplasm and nucleus32. Activating transcription factor-6 alpha (ATF6α) is one of the major membrane-associated sensors of the UPR. Upon ER stress, GRP78 binds preferentially to misfolded proteins, releasing ATF6α, which then translocate to the Golgi where ATF6α constitutively expressed as a 90-kDa protein (p90ATF6α) is directly converted to a 50-kDa soluble protein (p50ATF6); 50-kDa ATF6α plays a role in the ER stress downstream pathway33, 34. Thus, we determined the expression of 50-kDa ATF6α in the PAG of rats with MW. In shams rats with HSV vector alone, there was no significant difference in the expression of ATF6α between Q0ZHG+sham and QHMnSOD+sham rats, P > 0.05, n=6 (Figure 6A). However, we observed an increase in the expression of ATF6α in rats with Q0ZHG+MW compared to Q0ZHG+sham, P < 0.001, one-way ANOVA with posthoc PLSD test. ATF6α expression in the QHMnSOD+MW group was significantly lower than that in the Q0ZHG+MW rats, P < 0.01, one-way ANOVA with posthoc PLSD test, n=4–5 (Figure 6B). Double immunostaining showed that ATF6α-ir was co-localized with NeuN-ir (neuronal marker, Figure 6C–E), but neither OX42-ir (microglia marker, Figure 6F–H) nor GFAP-ir (astrocytes marker, Figure 6I–K), suggesting that ATF6α is located in the neurons in the PAG, but not glia.
Figure 6.
The effect of MnSOD mediated by HSV vectors on ATF6α in the PAG in naloxone-precipitated morphine withdrawal. (A) In sham groups injected with Q0ZHG or QHMnSOD, there was no significant difference in the expression of ATF6α in the PAG, P > 0.05, two-tailed t test, n=6. (B) There was a significant increase in the expression of ATF6α in Q0ZHG+MW rats compared to that in Q0ZHG+sham rats, *** P < 0.001, one-way ANOVA with posthoc PLSD test, n=5. The expression of ATF6α in QHMnSOD+MW rats was lower than that in Q0ZHG+MW rats, **P < 0.01, one-way ANOVA with posthoc PLSD test, n=4–5. Double immunostaining showed that ATF6α-ir was co-localized with NeuN-ir (neuronal marker, C-E), but neither OX42 (microglia marker, F-H) nor GFAP (astrocytes marker, I-K). Arrows show the co-localized cells, scale bar, 50 μm.
Determination of cellular co-localization of MnSOD, GRP78, and ATF6α in the vlPAG of rats with MW
To determine the cellular co-localization of MnSOD, GRP78, and ATF6α in the PAG of rats with MW, double-label immunostaining was carried out. MnSOD immunostaining was colocalized with either GRP78 (Figure 7A–C) or ATF6α (Figure 7D–F), and that GRP78 immunostaining was colocalized with ATF6α (Figure 7G–I), suggesting the activity of MnSOD may play a role in the GRP78 and ATF6α-positive neurons in reducing ROS.
Figure 7.
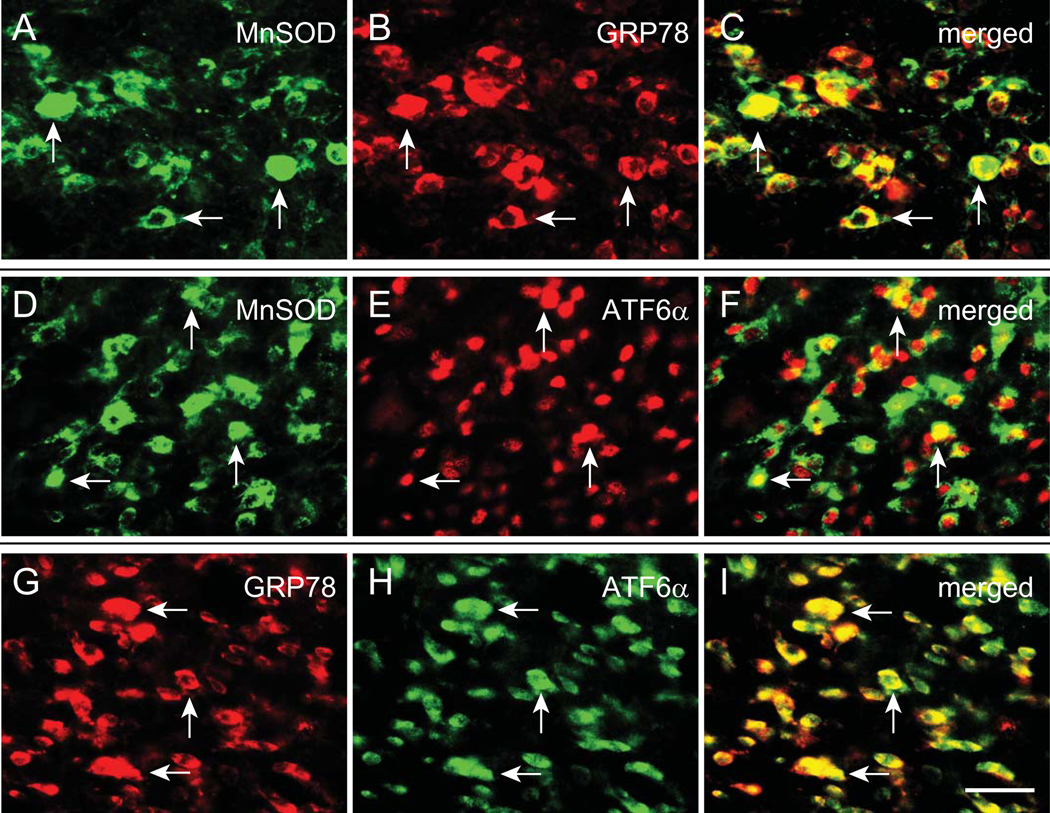
Co-localization of MnSOD, GRP78, and ATF6α in the PAG. To determine the cellular co-localization of MnSOD, GRP78, and ATF6α in the PAG with MW, double-label immunostaining was carried out. MnSOD immunostaining was colocalized with either GRP78 (A-C) or ATF6α (D-F), and that GRP78 immunostaining was colocalized with ATF6α (G-I). Arrows indicate double-labeled cells. Scale bar, 50 μm.
Discussion
Morphine appears to be the most active metabolite of heroin, therefore, the effects of morphine are important in understanding the ramifications of heroin abuse 1. Opioid dependence continues to be a significant public health concern, and can occur due to prescription opioid use, recreational opioid use, or as a result of opioid use for the treatment of drug addiction. Current treatment of opioid dependence is still not adequately addressed in the clinic. The PAG is one of important sites in the pathogenesis of opioid withdrawal 19, 20. Functional and biochemical studies suggest the PAG is involved in MW 35–37. In the current studies, we found that microinjection of HSV vector expressing human MnSOD into the vlPAG reduced the MW syndrome, and suppressed the upregulated mitochondrial superoxide and ER stress markers in the vlPAG.
The withdrawal phenomena may occur when continuous administration of the opiate drug is stopped suddenly, or may be precipitated by administration of an opioid antagonist5–7. Naloxone precipitating chronic morphine has been used for more than 40 years 38. Naloxone induces withdrawal in an individual who is already opiate dependent. Naloxone does this by knocking the opiate off of opioid receptors, hence depriving the brain of the opiate signal, leading to quick (acute) withdrawal response (personal communication, Dr. Eric Nestler, Mount Sinai School of Medicine, NY). The detailed mechanisms of naloxone precipitation in MW are still not very clear.
The magnitude of morphine withdrawal behavior is dependent on the species and the experimental procedure 38. Early studies of MW show that guinea pigs treated with a slowly released morphine suspension (300 mg/kg) exhibited a quantifiable withdrawal syndrome after naloxone injection (0.01–1 mg/kg s.c.) 38. In rats, naloxone (0.0005–5.0 mg/kg) dose-dependently induced jumping, weight loss and autonomic signs, however, the incidence of wet shakes didn’t correlate with naloxone dose 39. Guinea-pigs showed jumping, hyperactivity and wet dog shakes, the intensity of which was directly related to the dose of naloxone during opioid (subcutaneous morphine pellets) withdrawal precipitated by naloxone (0.1–10 mg/kg, IP) 40. In morphine-stabilized opiate-dependent human volunteers, naloxone (0.25–1.0 mg/kg, intravenously) dose-dependently in combination with buprenorphine increased opiate withdrawal signs and symptoms 41. In the central nucleus of the rat central amygdala, c-Fos immunoreactivity was increased by naloxone (0.2 to 1.0 mg/kg) in a dose-dependent manner in rats pretreated with morphine 42.
Recently many studies use higher dose of morphine for the precipitation of withdrawal response. For example, in mice with physical dependence the incidence of jumping was precipitated by naloxone (5 mg/kg, i.p.) 2 h after challenge dose of morphine on the sixth day 43. In adult mice with morphine physical dependence, withdrawal jumping was precipitated by a dose of subcutaneous naloxone (50 mg/kg)44. In a chronic morphine dependence test, mice were injected with morphine (30 mg/kg, twice daily for 3 days, s.c.). On Day 4, 2 h after treatment with morphine, mice were injected with naloxone (4 mg/kg, IP) to precipitate the withdrawal syndrome 45. In the current studies, we also used naloxone (4 mg/kg) for MW precipitation. Further investigation is needed to determine the dose-effect of naloxone in the model.
Mitochondria consume nearly 85% of a cell’s oxygen to support oxidative phosphorylation for the synthesis of ATP. ROS can be generated by multiple mechanisms, and mainly originates from mitochondria. Accumulation of ROS can result in oxidative stress, impairment of cell function, and necrosis or apoptosis 22. ROS is also a signaling molecule that regulates various cellular pathways22. Superoxide (O2•−) is the main ROS component produced by xanthine oxidase, the mitochondrial respiratory chain, and nitric oxide enzymes. The primary ROS made in mitochondria is O2•−, which is converted to H2O2, either by spontaneous dismutation or by the actions of the mitochondrial MnSOD.
Chronic morphine exposure induces some pathological consequences including neurotoxicity and neuronal dysfunction, oxidative stress and apoptosis 46. Morphine and its analogue derivatives alter oxidative metabolism, and studies have shown that morphine causes peroxide generation in vitro and in vivo. Opiates such as heroin and morphine are able to induce the formation of ROS in cultured cells 47, 48. Heroin-dependent mice show the decrease in total antioxidant capacity in serum and antioxidant enzymes such as superoxide dismutase in brain, but also exhibit the oxidative DNA damage; exogenous antioxidants alleviate withdrawal syndrome and restrain oxidative stress 15. Pretreatment with an exogenous antioxidant alleviates opioid withdrawal syndrome and inhibits oxidative stress in mice 13, 14. Systemic alpha-lipoic acid, a powerful antioxidant decreases abstinence signs of MW in animals23, suggesting ROS may participate in the process of MW. It appears that different tissues may respond to morphine diversely and are distinctly susceptible to oxidative stress and subsequent oxidative damage of biomolecules 46. Oxidative stress pathways in the PAG are associated with chronic morphine exposure 49. To our knowledge we are the first to report that MW increased mitochondrial superoxide and reduced the activity of MnSOD in the vlPAG.
GRP78 is one of the most abundant ER chaperones that play a central role in ER function. Under conditions of homeostasis and equilibrium, abundant GRP78 chaperone complex is in contact with the three major membrane associated sensors of the unfolded protein response: ATF6α, PKR-like ER-associated kinase, and inositol-requiring kinase-1 alpha 34, 50. The persistent accumulation of misfolded proteins causes ER stress leading to cellular apoptosis and is involved in numerous human disorders, such as neurodegenerative diseases 51, 52. Mutant GRP78 mice show reduced chronic morphine tolerance, suggesting that GRP78 may play an important role in the development of morphine tolerance31. In the present studies, we found that MW increased GRP78 levels in the PAG, and that HSV vector-MnSOD expression reduced GRP78, supporting the relationship of ER stress and mitochondrial oxidative stress.
ATF6α is one of the major membrane-associated sensors of the UPR. Upon ER stress, GRP78 binds preferentially to misfolded proteins, releasing ATF6α, which then translocate to the Golgi where ATF6α the 90-kDa protein is directly converted to a 50-kDa soluble protein, leading to induced transcription in the nucleus 33, 34. Our current results showed that MW increased ATF6α, and that MnSOD mediated by HSV reduced the increased levels of ATF6α, suggesting that ATF6α is the downstream factor of MnSOD in the PAG of MW.
It is possible that an interaction of ROS and ER stress exists. Prolonged accumulation of misfolded or unfolded proteins caused by cellular stress, including oxidative stress, induces ER stress, which in turn activates UPR 53. ER stressor calcimycin present in human lens epithelial cell lines induces UPR and ROS leading to apoptosis 29. ER stress is considered one of the mechanisms contributing to ROS-mediated cell apoptosis 28. Evidence suggests that ER stress can be either a cause, or a result of increased ROS generation54, 55. In the present study, we found that MW increased ROS and ER stress markers in the PAG, and that HSV vector-mediated MnSOD reduced ER stress, which supports the interaction of ROS and ER stress. However, the detailed interaction of oxidative stress and ER stress in the PAG during MW remains unclear. We will examine their relationship in a future study.
The effect of systemic delivery of therapeutic agents to the CNS is limited by the blood-brain barrier 18. Gene transfer to supply gene products that permanently restore function might be an attractive alternative to standard pharmacological approaches that can produce systemic side effects 18. Direct gene transfer to the brain in vivo offers advantages for these experimental applications to reduce the off-target effects of systemic drugs; the most effective gene delivery vehicles in the CNS in vivo are recombinant virus vectors 56. HSV is particularly well suited for delivery of targeted genes to the nervous system (naturally neurotropic viral vectors) 57–60. However, prolonged transgene expression will be required for the treatment of diseases of the CNS using HSV vectors. We, and others have used HSV vectors in a rodent model of Parkinson disease 16, 61. Anti-inflammatory cytokines mediated by the HSV into the PAG reduced MW 19. HSV vectors possess a large transgene capacity displaying the potential for effective gene targeting and sustained transgene expression. These vectors can, however, display limited toxicity and inflammation stemming from ‘leaky’ expression of viral genes and reaction to the vector coat in pre-immune animals18, 62. Despite these limitations, HSV vectors have proven to be highly effective gene delivery tools for treating nervous system disease17–19.
In summary, our current findings suggest that oxidative stress in the PAG plays an important role in naloxone-precipitated withdrawal from chronic morphine. Inhibition of oxidative stress in the PAG may suppress MW through reducing ER stress markers. This study might provide a novel therapeutic approach to the response of morphine physical withdrawal.
Materials and Methods
Construction of the HSV vectors expressing MnSOD
The HSV vector expressing the human sod2 gene was generated as described previously 63. Briefly, the HSV control vector Q0ZHG contains deletions of the essential immediate early genes ICP4 and ICP27, while the immediate early genes ICP22 and ICP47 genes are expressed as early ß-genes only when the virus is propagated in complementing cells in culture. The control virus Q0ZHG contains an HCMV promoter-EGFP cassette in the ICP27 locus and an ICP0 IE promoter-lacZ cassette inserted into the UL41 locus. The MnSOD vector was engineered from the control vector by homologous recombination with a plasmid that contains the HCMV promoter driving the human MnSOD gene that is flanked by UL41 sequences that allows for the homologous recombination of MnSOD into the UL41 locus of Q0ZHG, thereby replacing the ICP0p-lacZ expression cassette. The schematic diagrams of HSV replication defective vectors are detailed within Figure 1A.
Microinjection HSV vectors into vlPAG
Male Sprague-Dawley rats (body weight 230–250 g) were housed one to two per cage approximately 1 week prior to the beginning of the study, with free access to food and water and maintained on a 12h:12h, light: dark schedule at 21 °C and 60% humidity. All housing conditions and experimental procedures were approved by the University of Miami Institutional Animal Care and Use Committee (IACUC). Rats were prepared for intracranial HSV vectors administration by placing the rats anesthetized with isoflurane into a stereotaxic headholder (David Kopf Instruments, Tujunga, CA). The skull was exposed, and a needle with microsyringe (33-gauge) was directed bilaterally toward the vlPAG (AP −8.3 mm using bregma as 0, ML ±0.75 mm, DV −4.5 mm from the base of the dura). Rats were randomly divided to receive a bilateral vlPAG injection of HSV vector QHMnSOD expressing MnSOD or control vector Q0ZHG (1 μl containing 109 pfu/ml) with a micropump (0.5 μl/min). After microinjection, the injector was left in situ for an additional 2 min before it was removed to prevent vector from migrating up the needle tract.
Animal and evaluation of chronic morphine physical dependence
Morphine withdrawal was induced as described previously64. Rats were given escalating doses of morphine for a period of 5 days as follows: day 1, 10 mg/kg (8AM, IP) and 15 mg/kg (8PM); day 2, 20 and 25 mg/kg; day 3, 30 and 35 mg/kg; day 4, 40 and 45 mg/kg. On day 5, animals received a morning injection of 50 mg/kg, and 1 h later, naloxone (4 mg/kg, IP) was administered to precipitate a morphine withdrawal response. Immediately after naloxone, animals were placed individually in test chambers consisting of boxes (50×35×45 cm), and withdrawal signs were evaluated over the course of a 30 min period. Two types of signs were measured during abstinence, as described previously 65, 66. Episodes of wet-dog shakes and jumps were counted (i.e. recorded quantitatively); teeth chatter (vacuous chewing), diarrhea, rhinorrhea, ptosis, lacrimation, escaping, penile erection, and abnormal posture were evaluated over 5 min periods with one point being given for the presence of each sign during each period. The body weight of each rat was recorded before the injection of naloxone and then again at 60 min after naloxone. A global withdrawal score was calculated for each rat by assigning a weighting factor to the various physical signs of withdrawal 67. Morphine injection and behavior counting were carried out by a blinded operator. Animals showing neurological deficits or 85 % loss of body weight after microinjection were excluded. Randomization was used to determine samples/animals to experimental groups.
Western blot analyses
Under deep anesthesia the brains were harvested. A tissue block including a segment at the level of the vlPAG was cut on an ice cold glass plate 19. The vlPAG from the tissue block was harvested by taking punches with a 14 gauge puncture needle as described previously 68. The punched tissue was homogenized with 100 μl of ice-cold lysis buffer (150 mM sodium chloride, 1.0% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris, pH 8.0) containing protease inhibitors (Sigma, St Louis, MO) and phosphatase inhibitor cocktail 2 and 3 (Sigma, St Louis, MO). Tissue homogenates were sonicated and then centrifuged at 15,000x g for 20 min at 4 °C. The supernatant was collected and assayed for protein content using the BCA assay method (Pierce, Rockford, IL, USA) and stored at −80 °C until further use. Total protein (20 μg) was electrophoresed on an 8% or 15% SDS-PAGE gel, then transferred to a PVDF membrane, and blocked with 1x Rapid block solution (Amresco, Fountain Parkway Solon, OH) at room temperature for one hour. The primary antibodies (rabbit anti-MnSOD, 1:2000, cata.# 06–984, Millipore, Billerica, MA; mouse anti-GRP78, 1:2000, cata.# 610978, BD Transduction Laboratories, San Jose, CA; rabbit anti-ATF6α, 1:1000, cata.# sc-22799, Santa Cruz biotechnology, Dallas, TX; mouse monoclonal anti-β-actin, 1:8000, cata.# A5441, Sigma, St Louis, MO), were incubated overnight at 4 °C in fresh blocking buffer. The membrane was washed three times for 15 minutes. The membranes were incubated with complementary secondary antibodies, (1:4000, horseradish peroxidase conjugated IgG antibody, cat.# sc-2004, sc-2005, Santa Cruz biotechnology, Dallas, TX) for 1 h at room temperature. The membranes were washed in washing buffer for 10 minutes three times and the bands detected were then revealed using Super Signal west dura extended duration substrate (Thermo, Rockford, IL, USA). For densitometric analyses, blots were quantified with Quantity One analysis software (Bio-Rad, Hercules, CA). The membranes were stripped and re-probed with mouse anti-β-actin IgG (Sigma Aldrich, Saint Louis, Missouri) as a loading control. The results were expressed as the ratio to β-actin immunoreactivity.
Mitochondrial superoxide image in the PAG
MitoSox Red (a mitochondrial superoxide indicator, Invitrogen, Grand Island, NY) was dissolved in a 1:1 mixture of dimethylsulfoxide and saline to a final concentration of 33 μM as described previously 69, 70. MitoSox (1 μl) was microinjected into the PAG. Approximately 75 minutes after injection, rats were perfused intracardially with 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer, and the brains were removed, postfixed in the same solution overnight, and cryoprotected with 30% sucrose in phosphate-buffered solution for 2 days. The 25-μm sections were examined under a fluorescent microscope with a rhodamine filter. The number of MitoSox-positive cellular profiles with distinctive nuclei (dark oval-shaped space surrounded by red granules) were counted blindly from the pictures as described previously 69.
MnSOD activity assay
vlPAG samples were collected, homogenized, and sonicated in 50mM sodium phosphate buffer (pH 7.4) containing protease inhibitor cocktail. The homogenates were centrifuged at 12,500 x g for 20 minutes at 4 °C. Supernatant was collected, and protein concentration was measured by DC protein assay (Biorad, CA, USA). SOD activity was measured using SOD assay kit (Cat# 19160, Sigma, MO, USA). Samples were run in duplicates as well as standards. Twenty microliter of sample, 200 μl of WST-1 solution, and 20 μl of enzyme solution were added, and then absorbance was read at 450-nm from time 0 to 9 minutes (1 minute intervals) using an Epoch microplate reader (BioTek, Winooska, VT). Bovine Cu/ZnSOD (Sigma, St Louis, MO) was used as standard, and SOD1 activity was inhibited by adding 2 mM NaCN into the samples 30 minutes prior to assay69. MnSOD activity was calculated as units of activity/mg of total protein and shown as 100% of sham.
Immunohistochemistry
For immunoreactivity in the PAG, rats were perfused intracardially with 4% PFA in 0.1 M phosphate buffer, and the brains were removed, postfixed in the same solution overnight, and cryoprotected with 30% sucrose in phosphate-buffered solution for 3 days. To examine the co-localization of MnSOD, GRP78 or ATF6α with NeuN, GFAP, or OX42, cryostat sections (25 μm thickness) were incubated overnight at 4 °C with primary antibodies, rabbit anti-MnSOD (Cata.#06–984, 1:300, Millipore, Temecula, CA), rabbit anti-GRP78 (cata. # SAB4501452, 1:5000 Sigma, Louis, MO), mouse anti-ATF6α (cata. # SC-22799, 1:100, Santa Cruz Biotechnology, Dallas, TX), mouse anti-NeuN (cata.# MAB377, 1:500, Millipore, Temecula, CA), rabbit anti-NeuN (cata # ABN78, 1:2000, Millipore, Temecula, CA), rabbit anti-GFAP (cata.# Z0334, 1:3000, Dako, Midland, ON Canada), mouse anti-GFAP (cata. # G3893, 1: 3000, Sigma, St Louis, MO), mouse anti-OX-42 (cata.#, CBL1512, 1:100, Millipore, Temecula, CA), and rabbit anti-Iba1 (cata. # 019–19741, 1:2000, Wako, Richmond, VA) followed by fluorescent IgG with Alexa Fluor 488 or Alexa Fluor 594 (1:1000, Invitrogen Life Technologies) for 2 h at room temperature. Fluorescence images were captured by a fluorescent microscopy (Fluorescent M Leica/Micro CDMI 6000B)24.
Drugs and data analysis
Morphine sulfate was purchased from West-Ward Pharmaceuticals, Eatontown, NJ. Naloxone hydrochloride was obtained from Sigma St. Louis, MO. Naloxone was injected intraperitoneally in a volume of 1 ml/kg of body weight. All drugs were dissolved in physiologic (0.9%) saline.
The sample size estimate was based on our previous studies19, 24. The statistical significance of the differences was determined by one-way ANOVA followed by a posthoc PLSD test (IBM SPSS21, Armonk, NY), or two-tailed t test. The difference between the time-course curves of the withdrawal behavior was determined using 2-way ANOVA repeated measure with Bonferroni posttests (GraphPad Prism 5). P-values of less than 0.05 were considered to be statistically significant.
Supplementary Material
ACKNOWLEDGMENTS
This work was partially supported by grants from the NIH DA020078 (PI: S.H.), NS066792 (PI: S.H.), DA34749 (PI: S.H. and Co-I, J.C.G.). We thank the support from the Department (Chair Dr. Lubarsky) of Anesthesiology, University of Miami, FL.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest. None of the authors have received compensation for professional services or anticipate receiving such compensation in the near future.
References
- 1.Steele AD, Henderson EE, Rogers TJ. Mu-opioid modulation of HIV-1 coreceptor expression and HIV-1 replication. Virology 2003; 309(1): 99–107. [DOI] [PubMed] [Google Scholar]
- 2.Hasanein P, Teimuri Far M, Emamjomeh A. Salvia officinalis L. attenuates morphine analgesic tolerance and dependence in rats: possible analgesic and sedative mechanisms. Am. J. Drug Alcohol Abuse 2015; 41(5): 405–13. [DOI] [PubMed] [Google Scholar]
- 3.Cooper ZD, Johnson KW, Pavlicova M, Glass A, Vosburg SK, Sullivan MA et al. The effects of ibudilast, a glial activation inhibitor, on opioid withdrawal symptoms in opioid-dependent volunteers. Addict. Biol 2015; 21(4): 895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor DA, Fleming WW. Unifying perspectives of the mechanisms underlying the development of tolerance and physical dependence to opioids. J. Pharmacol. Exp. Ther 2001; 297(1): 11–8. [PubMed] [Google Scholar]
- 5.Cami J, Farre M. Drug addiction. N. Engl. J. Med 2003; 349(10): 975–86. [DOI] [PubMed] [Google Scholar]
- 6.Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science 1997; 278(5335): 52–8. [DOI] [PubMed] [Google Scholar]
- 7.Fishbain DA, Rosomoff HL, Rosomoff RS. Drug abuse, dependence, and addiction in chronic pain patients. Clin. J. Pain 1992; 8(2): 77–85. [DOI] [PubMed] [Google Scholar]
- 8.Rouibi K, Contarino A. Increased motivation to eat in opiate-withdrawn mice. Psychopharmacology (Berl.) 2012; 221(4): 675–84. [DOI] [PubMed] [Google Scholar]
- 9.Anderson EM, Neubert JK, Caudle RM. Long-term changes in reward-seeking following morphine withdrawal are associated with altered N-methyl-D-aspartate receptor 1 splice variants in the amygdala. Neuroscience 2012; 223: 45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Candas D, Li JJ. MnSOD in oxidative stress response-potential regulation via mitochondrial protein influx. Antioxid. Redox Signal 2014; 20(10): 1599–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fridovich I. Superoxide radical and superoxide dismutases. Annu. Rev. Biochem 1995; 64: 97–112. [DOI] [PubMed] [Google Scholar]
- 12.Buettner GR. Superoxide dismutase in redox biology: the roles of superoxide and hydrogen peroxide. Anticancer Agents Med. Chem 2011; 11(4): 341–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan J, Zhang Q, Zhang Y, Ouyang Z, Zheng Q, Zheng R. Oxidative stress in heroin administered mice and natural antioxidants protection. Life Sci. 2005; 77(2): 183–93. [DOI] [PubMed] [Google Scholar]
- 14.Cemek M, Buyukokuroglu ME, Hazman O, Konuk M, Bulut S, Birdane YO. The roles of melatonin and vitamin E plus selenium in prevention of oxidative stress induced by naloxone-precipitated withdrawal in heroin-addicted rats. Biol. Trace Elem. Res 2011; 142(1): 55–66. [DOI] [PubMed] [Google Scholar]
- 15.Xu B, Wang Z, Li G, Li B, Lin H, Zheng R et al. Heroin-administered mice involved in oxidative stress and exogenous antioxidant-alleviated withdrawal syndrome. Basic Clin. Pharmacol. Toxicol 2006; 99(2): 153–61. [DOI] [PubMed] [Google Scholar]
- 16.Puskovic V, Wolfe D, Wechuck J, Krisky D, Collins J, Glorioso JC et al. HSV-mediated delivery of erythropoietin restores dopaminergic function in MPTP-treated mice. Mol. Ther 2006; 14(5): 710–5. [DOI] [PubMed] [Google Scholar]
- 17.Guedon JM, Wu S, Zheng X, Churchill CC, Glorioso JC, Liu CH et al. Current gene therapy using viral vectors for chronic pain. Molecular pain 2015; 11: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simonato M, Bennett J, Boulis NM, Castro MG, Fink DJ, Goins WF et al. Progress in gene therapy for neurological disorders. Nat. Rev. Neurol 2013; 9(5): 277–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hao S, Liu S, Zheng X, Zheng W, Ouyang H, Mata M et al. The role of TNFalpha in the periaqueductal gray during naloxone-precipitated morphine withdrawal in rats. Neuropsychopharmacology 2011; 36(3): 664–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hutchinson MR, Lewis SS, Coats BD, Skyba DA, Crysdale NY, Berkelhammer DL et al. Reduction of opioid withdrawal and potentiation of acute opioid analgesia by systemic AV411 (ibudilast). Brain. Behav. Immun 2009; 23(2): 240–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borradaile NM, Buhman KK, Listenberger LL, Magee CJ, Morimoto ET, Ory DS et al. A critical role for eukaryotic elongation factor 1A-1 in lipotoxic cell death. Mol. Biol. Cell 2006; 17(2): 770–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brookheart RT, Michel CI, Listenberger LL, Ory DS, Schaffer JE. The non-coding RNA gadd7 is a regulator of lipid-induced oxidative and endoplasmic reticulum stress. J. Biol. Chem 2009; 284(12): 7446–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pinelli A, Cighetti G, Trivulzio S. Plasma malondialdehyde levels and opiate withdrawal signs observed in rats treated with morphine plus naloxone: effects of alpha-lipoic acid administration. Fundam. Clin. Pharmacol 2008; 22(4): 439–45. [DOI] [PubMed] [Google Scholar]
- 24.Iida T, Yi H, Liu S, Huang W, Kanda H, Lubarsky DA et al. Spinal CPEB-mtROS-CBP signaling pathway contributes to perineural HIV gp120 with ddC-related neuropathic pain in rats. Exp. Neurol 2016; 281: 17–27. [DOI] [PubMed] [Google Scholar]
- 25.Kanda H, Liu S, Iida T, Yi H, Huang W, Levitt R et al. Inhibition of mitochondrial fission protein reduced mechanical allodynia and suppressed spinal mitochondrial superoxide induced by perineural HIV gp120 in rats. Anesth. Analg 2016; 122(1): 264–272. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz ES, Lee I, Chung K, Chung JM. Oxidative stress in the spinal cord is an important contributor in capsaicin-induced mechanical secondary hyperalgesia in mice. Pain 2008; 138(3): 514–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim HY, Lee KY, Lu Y, Wang J, Cui L, Kim SJ et al. Mitochondrial Ca(2+) uptake is essential for synaptic plasticity in pain. J. Neurosci 2011; 31(36): 12982–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu ZW, Zhu HT, Chen KL, Dong X, Wei J, Qiu C et al. Protein kinase RNA-like endoplasmic reticulum kinase (PERK) signaling pathway plays a major role in reactive oxygen species (ROS)-mediated endoplasmic reticulum stress-induced apoptosis in diabetic cardiomyopathy. Cardiovasc. Diabetol 2013; 12: 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang HZ, Yang LM. Activation of the unfolded protein response in aged human lenses. Mol Med Rep 2015; 12(1): 389–93. [DOI] [PubMed] [Google Scholar]
- 30.Kania E, Pajak B, Orzechowski A. Calcium homeostasis and ER stress in control of autophagy in cancer cells. BioMed research international 2015; 2015: 352794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dobashi T, Tanabe S, Jin H, Mimura N, Yamamoto T, Nishino T et al. BiP, an endoplasmic reticulum chaperone, modulates the development of morphine antinociceptive tolerance. J. Cell. Mol. Med 2010; 14(12): 2816–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ogata M, Hino S, Saito A, Morikawa K, Kondo S, Kanemoto S et al. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol. Cell. Biol 2006; 26(24): 9220–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haze K, Yoshida H, Yanagi H, Yura T, Mori K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol. Biol. Cell 1999; 10(11): 3787–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maurel M, Chevet E. Endoplasmic reticulum stress signaling: the microRNA connection. Am. J. Physiol. Cell Physiol 2013; 304(12): C1117–26. [DOI] [PubMed] [Google Scholar]
- 35.Laschka E, Teschemacher H, Mehraein P, Herz A. Sites of action of morphine involved in the development of physical dependence in rats. II. Morphine withdrawal precipitated by application of morphine antagonists into restricted parts of the ventricular system and by microinjection into various brain areas. Psychopharmacologia 1976; 46(2): 141–7. [DOI] [PubMed] [Google Scholar]
- 36.Maldonado R, Stinus L, Gold LH, Koob GF. Role of different brain structures in the expression of the physical morphine withdrawal syndrome. J. Pharmacol. Exp. Ther 1992; 261(2): 669–77. [PubMed] [Google Scholar]
- 37.Bozarth MA. Physical dependence produced by central morphine infusions: an anatomical mapping study. Neurosci. Biobehav. Rev 1994; 18(3): 373–83. [DOI] [PubMed] [Google Scholar]
- 38.Frederickson RC, Hewes CR, Aiken JW. Correlation between the in vivo and an in vitro expression of opiate withdrawal precipitated by naloxone: their antagonism by l-(−)-delta9-tetrahydrocannabinol. J. Pharmacol. Exp. Ther 1976; 199(2): 375–84. [PubMed] [Google Scholar]
- 39.Geary WA 2nd, Wooten GF. Dose effects of naloxone on fixed morphine dependence: simultaneous behavioral and 2-deoxyglucose study in the rat. Brain Res. 1985; 332(1): 69–78. [DOI] [PubMed] [Google Scholar]
- 40.Antonelli T, Beani L, Bianchi C, Rando S, Simonato M, Tanganelli S. Cortical acetylcholine release is increased and gamma-aminobutyric acid outflow is reduced during morphine withdrawal. Br. J. Pharmacol 1986; 89(4): 853–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mendelson J, Jones RT, Welm S, Baggott M, Fernandez I, Melby AK et al. Buprenorphine and naloxone combinations: the effects of three dose ratios in morphine-stabilized, opiate-dependent volunteers. Psychopharmacology (Berl.) 1999; 141(1): 37–46. [DOI] [PubMed] [Google Scholar]
- 42.Jin C, Araki H, Nagata M, Shimosaka R, Shibata K, Suemaru K et al. Expression of c-Fos in the rat central amygdala accompanies the acquisition but not expression of conditioned place aversion induced by withdrawal from acute morphine dependence. Behav. Brain Res 2005; 161(1): 107–12. [DOI] [PubMed] [Google Scholar]
- 43.Mansouri MT, Khodayar MJ, Tabatabaee A, Ghorbanzadeh B, Naghizadeh B. Modulation of morphine antinociceptive tolerance and physical dependence by co-administration of simvastatin. Pharmacol. Biochem. Behav 2015; 137: 38–43. [DOI] [PubMed] [Google Scholar]
- 44.Wu SZ, Chen KT, Chen JY, Sung KC, Wang JJ, Liu KS et al. Phenothiazine-type antipsychotics may attenuate naloxone-precipitated withdrawal jumping in morphine-dependent mice. Acta anaesthesiologica Taiwanica : official journal of the Taiwan Society of Anesthesiologists 2012; 50(4): 167–71. [DOI] [PubMed] [Google Scholar]
- 45.Yang L, Zhu CJ, Cao JL, Zeng YM. Inhibition of the spinal phosphoinositide 3-kinase exacerbates morphine withdrawal response. Neurosci. Lett 2006; 404(1–2): 237–41. [DOI] [PubMed] [Google Scholar]
- 46.Skrabalova J, Drastichova Z, Novotny J. Morphine as a Potential Oxidative Stress-Causing Agent. Mini-reviews in organic chemistry 2013; 10(4): 367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oliveira MT, Rego AC, Morgadinho MT, Macedo TR, Oliveira CR. Toxic effects of opioid and stimulant drugs on undifferentiated PC12 cells. Ann. N. Y. Acad. Sci 2002; 965: 487–96. [DOI] [PubMed] [Google Scholar]
- 48.Sharp BM, Keane WF, Suh HJ, Gekker G, Tsukayama D, Peterson PK. Opioid peptides rapidly stimulate superoxide production by human polymorphonuclear leukocytes and macrophages. Endocrinology 1985; 117(2): 793–5. [DOI] [PubMed] [Google Scholar]
- 49.Bajic D, Berde CB, Commons KG. Periaqueductal gray neuroplasticity following chronic morphine varies with age: role of oxidative stress. Neuroscience 2012; 226: 165–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang M, Kaufman RJ. The impact of the endoplasmic reticulum protein-folding environment on cancer development. Nat. Rev. Cancer 2014; 14(9): 581–97. [DOI] [PubMed] [Google Scholar]
- 51.Katayama T, Imaizumi K, Sato N, Miyoshi K, Kudo T, Hitomi J et al. Presenilin-1 mutations downregulate the signalling pathway of the unfolded-protein response. Nat. Cell Biol 1999; 1(8): 479–85. [DOI] [PubMed] [Google Scholar]
- 52.Imai Y, Soda M, Inoue H, Hattori N, Mizuno Y, Takahashi R. An unfolded putative transmembrane polypeptide, which can lead to endoplasmic reticulum stress, is a substrate of Parkin. Cell 2001; 105(7): 891–902. [DOI] [PubMed] [Google Scholar]
- 53.Kim B, Kim HS, Jung EJ, Lee JY, B KT, Lim JM et al. Curcumin induces ER stress-mediated apoptosis through selective generation of reactive oxygen species in cervical cancer cells. Mol. Carcinog 2016; 55(5): 918–28. [DOI] [PubMed] [Google Scholar]
- 54.Rigoulet M, Yoboue ED, Devin A. Mitochondrial ROS generation and its regulation: mechanisms involved in H(2)O(2) signaling. Antioxid. Redox Signal 2011; 14(3): 459–68. [DOI] [PubMed] [Google Scholar]
- 55.Huang J, Lam GY, Brumell JH. Autophagy signaling through reactive oxygen species. Antioxid. Redox Signal 2011; 14(11): 2215–31. [DOI] [PubMed] [Google Scholar]
- 56.Cannon JR, Sew T, Montero L, Burton EA, Greenamyre JT. Pseudotype-dependent lentiviral transduction of astrocytes or neurons in the rat substantia nigra. Exp. Neurol 2011; 228(1): 41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wilson SP, Yeomans DC, Bender MA, Lu Y, Goins WF, Glorioso JC. Antihyperalgesic effects of infection with a preproenkephalin-encoding herpes virus. Proc. Natl. Acad. Sci. U. S. A 1999; 96(6): 3211–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Westlund KN. Gene therapy for pancreatitis pain. Gene Ther. 2009; 16(4): 483–92. [DOI] [PubMed] [Google Scholar]
- 59.Fink DJ, DeLuca NA, Goins WF, Glorioso JC. Gene transfer to neurons using herpes simplex virus-based vectors. Annu. Rev. Neurosci 1996; 19: 265–87. [DOI] [PubMed] [Google Scholar]
- 60.Yeomans DC, Wilson SP. Herpes virus-based recombinant herpes vectors: gene therapy for pain and molecular tool for pain science. Gene Ther. 2009; 16(4): 502–8. [DOI] [PubMed] [Google Scholar]
- 61.Puskovic V, Wolfe D, Goss J, Huang S, Mata M, Glorioso JC et al. Prolonged biologically active transgene expression driven by HSV LAP2 in brain in vivo. Mol. Ther 2004; 10(1): 67–75. [DOI] [PubMed] [Google Scholar]
- 62.Nayak S, Herzog RW. Progress and prospects: immune responses to viral vectors. Gene Ther. 2010; 17(3): 295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guo HL, Wolfe D, Epperly MW, Huang S, Liu K, Glorioso JC et al. Gene transfer of human manganese superoxide dismutase protects small intestinal villi from radiation injury. J. Gastrointest. Surg 2003; 7(2): 229–35; discussion 235–6. [DOI] [PubMed] [Google Scholar]
- 64.Trang T, Sutak M, Quirion R, Jhamandas K. Spinal administration of lipoxygenase inhibitors suppresses behavioural and neurochemical manifestations of naloxone-precipitated opioid withdrawal. Br. J. Pharmacol 2003; 140(2): 295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hao S, Hu J, Fink DJ. Transgene-mediated enkephalin expression attenuates signs of naloxone-precipitated morphine withdrawal in rats with neuropathic pain. Behav. Brain Res 2009; 197(1): 84–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Valverde O, Noble F, Beslot F, Dauge V, Fournie-Zaluski MC, Roques BP. Delta9-tetrahydrocannabinol releases and facilitates the effects of endogenous enkephalins: reduction in morphine withdrawal syndrome without change in rewarding effect. Eur. J. Neurosci 2001; 13(9): 1816–24. [DOI] [PubMed] [Google Scholar]
- 67.Gellert VF, Holtzman SG. Development and maintenance of morphine tolerance and dependence in the rat by scheduled access to morphine drinking solutions. J. Pharmacol. Exp. Ther 1978; 205(3): 536–46. [PubMed] [Google Scholar]
- 68.Guo W, Wang H, Watanabe M, Shimizu K, Zou S, LaGraize SC et al. Glial-cytokine-neuronal interactions underlying the mechanisms of persistent pain. J. Neurosci 2007; 27(22): 6006–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schwartz ES, Kim HY, Wang J, Lee I, Klann E, Chung JM et al. Persistent pain is dependent on spinal mitochondrial antioxidant levels. J. Neurosci 2009; 29(1): 159–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kanao M, Kanda H, Huang W, Liu S, Yi H, Candiotti KA et al. Gene Transfer of Glutamic Acid Decarboxylase 67 by Herpes Simplex Virus Vectors Suppresses Neuropathic Pain Induced by Human Immunodeficiency Virus gp120 Combined with ddC in Rats. Anesth. Analg 2015; 120(6): 1394–404. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






