PURPOSE
In SOLO1/GOG 3004 (ClinicalTrials.gov identifier: NCT01844986), maintenance therapy with the poly(ADP-ribose) polymerase inhibitor olaparib provided a sustained progression-free survival benefit in patients with newly diagnosed advanced ovarian cancer and a BRCA1 and/or BRCA2 (BRCA) mutation. We report overall survival (OS) after a 7-year follow-up, a clinically relevant time point and the longest follow-up for any poly(ADP-ribose) polymerase inhibitor in the first-line setting.
METHODS
This double-blind phase III trial randomly assigned patients with newly diagnosed advanced ovarian cancer and a BRCA mutation in clinical response to platinum-based chemotherapy to maintenance olaparib (n = 260) or placebo (n = 131) for up to 2 years. A prespecified descriptive analysis of OS, a secondary end point, was conducted after a 7-year follow-up.
RESULTS
The median duration of treatment was 24.6 months with olaparib and 13.9 months with placebo, and the median follow-up was 88.9 and 87.4 months, respectively. The hazard ratio for OS was 0.55 (95% CI, 0.40 to 0.76; P = .0004 [P < .0001 required to declare statistical significance]). At 7 years, 67.0% of olaparib patients versus 46.5% of placebo patients were alive, and 45.3% versus 20.6%, respectively, were alive and had not received a first subsequent treatment (Kaplan-Meier estimates). The incidence of myelodysplastic syndrome and acute myeloid leukemia remained low, and new primary malignancies remained balanced between treatment groups.
CONCLUSION
Results indicate a clinically meaningful, albeit not statistically significant according to prespecified criteria, improvement in OS with maintenance olaparib in patients with newly diagnosed advanced ovarian cancer and a BRCA mutation and support the use of maintenance olaparib to achieve long-term remission in this setting; the potential for cure may also be enhanced. No new safety signals were observed during long-term follow-up.
INTRODUCTION
The ultimate goal of treatment in women newly diagnosed with ovarian cancer is cure. However, disease is often advanced at the time of diagnosis and approximately 70% of patients who receive cytoreductive surgery followed by first-line platinum-based chemotherapy will relapse within 3 years,1 with a 10-year survival of 17% in patients with advanced epithelial ovarian cancer.2 Relapsed advanced ovarian cancer is typically incurable, highlighting the need for effective first-line treatments that delay relapse, prolong survival, and enhance the potential for cure.
CONTEXT
Key Objective
No overall survival (OS) benefit has yet been reported for poly(ADP-ribose) polymerase inhibitor maintenance therapy in women with newly diagnosed advanced ovarian cancer. The authors here report OS with maintenance olaparib in SOLO1 patients with newly diagnosed advanced BRCA-mutated ovarian cancer after a 7-year follow-up.
Knowledge Generated
Results indicate a clinically meaningful, albeit not statistically significant, improvement in OS with maintenance olaparib versus placebo, with two thirds of olaparib patients, versus fewer than half of placebo patients, alive at 7 years. No new safety signals were observed.
Relevance (G. Fleming)
-
The SOLO1 data support the use of maintenance olaparib therapy in patients with newly diagnosed advanced ovarian cancer and a BRCA mutation.*
*Relevance section written by JCO Associate Editor Gini Fleming, MD.
The poly(ADP-ribose) polymerase (PARP) inhibitor olaparib represents the new standard of care in the management of patients with newly diagnosed advanced ovarian cancer and a BRCA1 and/or BRCA2 (BRCA) mutation. In the pivotal SOLO1/GOG 3004 trial, maintenance olaparib provided a sustained progression-free survival (PFS) benefit beyond the end of treatment, which was capped at 2 years, in patients with newly diagnosed advanced ovarian cancer and a BRCA mutation.3,4 In the primary analysis (data cutoff [DCO]: May 17, 2018), maintenance olaparib provided a significant PFS benefit compared with placebo (hazard ratio [HR], 0.30; 95% CI, 0.23 to 0.41; P < .001).3 In an updated PFS analysis conducted after a 5-year follow-up (DCO: March 5, 2020), the median PFS was 56.0 months in the olaparib group compared with 13.8 months in the placebo group (HR, 0.33; 95% CI, 0.25 to 0.43).4 On the basis of Kaplan-Meier estimates, 48.3% versus 20.5% of patients, respectively, were progression-free at 5 years; overall survival (OS) data were immature.4
We report a descriptive analysis of OS after a 7-year follow-up in SOLO1. To our knowledge, this is the longest follow-up for any PARP inhibitor in newly diagnosed advanced ovarian cancer and the first report of long-term OS data for any PARP inhibitor in this setting. Seven years is considered a clinically relevant time point for survivorship, as modeling indicates that most ovarian cancer–related deaths occur within 7 years of diagnosis, with mortality approaching that of women in the general population after a 9-year follow-up.5
METHODS
Study Design and Patients
The design of the randomized, double-blind, placebo-controlled, international, phase III SOLO1/GOG 3004 study has been reported previously.3 In brief, eligible patients had newly diagnosed, histologically confirmed advanced (International Federation of Gynaecology and Obstetrics [FIGO] stage III or IV) high-grade serous or endometrioid ovarian, primary peritoneal, and/or fallopian tube cancer.
Patients were eligible for SOLO1 regardless of the timing of cytoreductive surgery or surgical outcome. Patients with FIGO stage III disease had undergone an attempt at optimal upfront or interval cytoreductive surgery, and those with FIGO stage IV disease had undergone a biopsy and/or upfront or interval cytoreductive surgery. Patients had a germline or somatic BRCA1 and/or BRCA2 mutation on local or central testing and were in clinical complete or partial response after first-line platinum-based chemotherapy (without bevacizumab). Patients who had received prior PARP inhibitor therapy or who had a history or myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML) were ineligible. Full eligibility criteria are given in the Data Supplement (online only).
The study Protocol (online only) was approved by the ethics committees at each participating site and performed in accordance with the Declaration of Helsinki, Good Clinical Practice guidelines, and the AstraZeneca policy on bioethics.6 All patients provided written informed consent.
Treatment
Patients were randomly assigned (2:1) to receive olaparib tablets (300 mg twice daily) or placebo within 8 weeks of receiving their last dose of chemotherapy. Random assignment was stratified according to clinical response (complete or partial) after platinum-based chemotherapy. Patients received treatment for up to 2 years or until investigator-assessed objective radiologic disease progression (according to modified RECIST, version 1.1), whichever occurred first, or treatment was stopped if other discontinuation criteria were met (Data Supplement). Patients with no evidence of disease at 2 years stopped receiving study treatment, but patients with evidence of disease at 2 years could continue to receive study treatment in a blinded manner if, in the opinion of the investigator, this was in the patient's best interest. Within the study, crossover between the treatment groups was not permitted. After study treatment discontinuation, patients could receive subsequent therapies at the investigators' discretion.
Outcomes
Secondary end points reported in this analysis are OS (defined as the time from random assignment to death because of any cause), time from random assignment to first subsequent therapy or death (TFST), time from random assignment to second subsequent therapy or death (TSST), time from random assignment to discontinuation of study treatment or death (TDT), and safety and tolerability. Adverse events (AEs) were monitored using the National Cancer Institute's Common Terminology Criteria for Adverse Events (version 4.0) throughout the treatment period and for 30 days after discontinuation of study treatment. In addition, patients were proactively followed for MDS/AML and new primary malignancies beyond the 30-day post-treatment safety follow-up period.
Investigator-assessed PFS, the primary end point,3,4 and additional end points3,4,7,8 have been reported previously.
Statistical Analysis
Efficacy was analyzed in all randomly assigned patients (full analysis set), and safety was analyzed in all patients who received at least one dose of randomized treatment.
This prespecified descriptive OS analysis was conducted 7 years after the last patient was randomly assigned (DCO: March 7, 2022). A final OS analysis is currently planned to be conducted at approximately 60% data maturity as prespecified in the study Protocol.3 OS was analyzed using a log-rank test stratified by response to first-line platinum-based chemotherapy, with HRs and 95% Cls estimated using a Cox proportional hazards model, including the stratification variable as a covariate. OS was not adjusted for subsequent PARP inhibitor therapy. A two-sided P value of < .0001 was required to declare statistical significance (Haybittle-Peto α = .0001). Kaplan-Meier methods were used to generate time-to-event curves, from which medians and survival proportions were calculated.
Analyses of TFST, TSST, and TDT were performed using a method similar to that used for the analysis of OS.
All statistical analyses were performed using SAS software version 9.4 (SAS Institute Inc, Cary, NC).
RESULTS
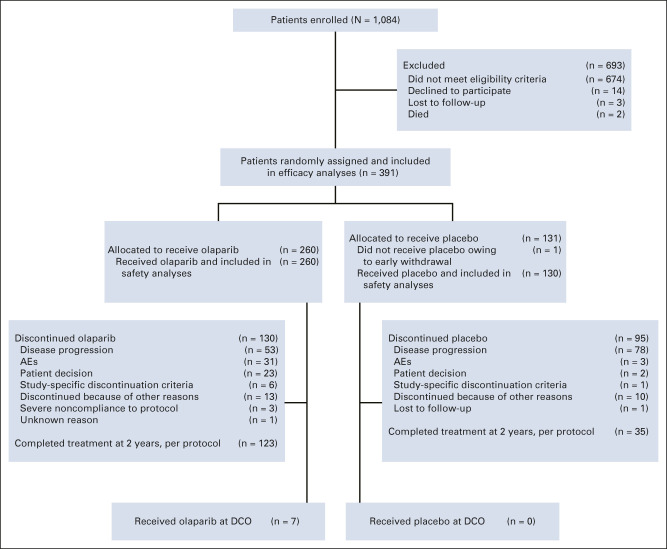
All 260 patients randomly assigned to olaparib and 130 of the 131 patients randomly assigned to placebo received study treatment (one patient assigned to placebo withdrew before receiving the intervention; Fig 1). Baseline characteristics were well balanced between the treatment groups (Data Supplement).
FIG 1.
CONSORT diagram of patients. AE, adverse event; DCO, data cutoff.
For this descriptive OS analysis, DCO (March 7, 2022) took place 7 years after the last patient was randomly assigned, with a median (interquartile range) duration of follow-up for OS of 88.9 (85.7-93.6) months in the olaparib group and 87.4 (84.3-91.7) months in the placebo group. The median (range) duration of treatment in the safety analysis set was 24.6 (0.0-97.5) months in the olaparib group, consistent with the 2-year treatment cap, and 13.9 (0.2-60.9) months in the placebo group. Study treatment was completed at 2 years, per the study Protocol, in 123 olaparib patients (47.3%) and 35 placebo patients (26.9%; Fig 1); 111 patients (42.7%) and 92 patients (70.8%), respectively, discontinued study treatment before 2 years, and 26 patients (10.0%) and three patients (2.3%), respectively, continued study treatment beyond 2 years. Seven of the 13 patients who were receiving olaparib at the primary DCO (May 17, 2018)3 were still receiving olaparib at the current DCO.
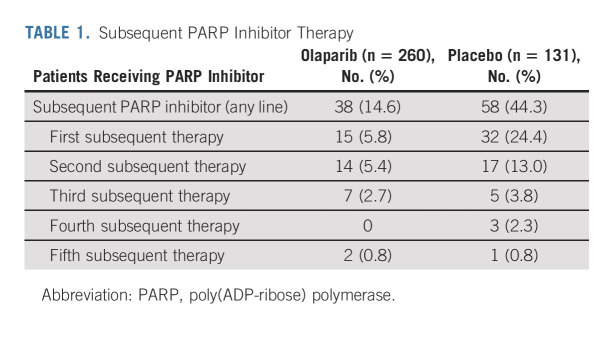
At DCO (March 7, 2022), 149 of 391 patients had died (data maturity 38.1%). The median OS was not reached (95% CI, not reached to not reached) in the olaparib group compared with 75.2 months (95% CI, 65.4 to not reached) in the placebo group, with an HR of 0.55 (95% CI, 0.40 to 0.76; P = .0004 [P < .0001 required to declare statistical significance]; Fig 2). This analysis was unadjusted for subsequent therapy, and the OS benefit was achieved despite 44.3% of patients in the placebo group having received a PARP inhibitor in a subsequent line of therapy (Table 1). Of the 122 olaparib patients and 97 placebo patients who received any subsequent therapy (Data Supplement), 31.1% and 59.8%, respectively, received a PARP inhibitor. On the basis of Kaplan-Meier estimates, 67.0% of olaparib patients versus 46.5% of placebo patients were alive 7 years after random assignment.
FIG 2.
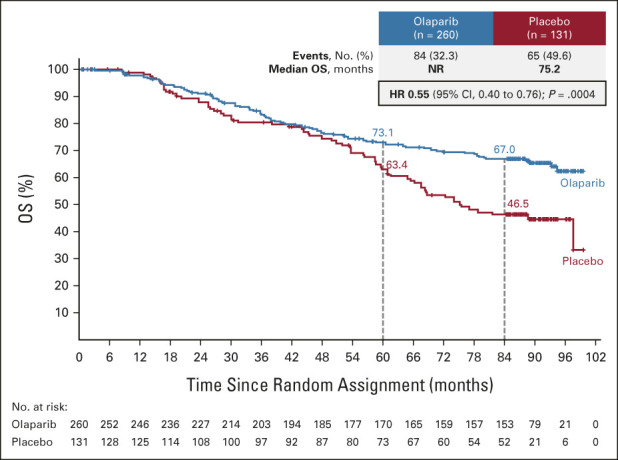
Kaplan-Meier estimates of OS. HR, hazard ratio; NR, not reached; OS, overall survival.
TABLE 1.
Subsequent PARP Inhibitor Therapy
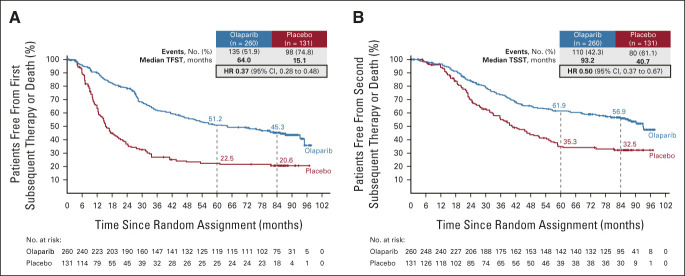
The median TFST (data maturity 59.6%) was 64.0 months (95% CI, 47.7 to 93.2) with olaparib compared with 15.1 months (95% CI, 12.7 to 20.5) with placebo, with an HR of 0.37 (95% CI, 0.28 to 0.48; Fig 3A). On the basis of Kaplan-Meier estimates, 45.3% of olaparib patients versus 20.6% of placebo patients were alive and had not received a first subsequent treatment after a 7-year follow-up. At the time of DCO, 122 (46.9%) patients in the olaparib group and 95 (72.5%) in the placebo group had received a first subsequent therapy (Data Supplement).
FIG 3.
Kaplan-Meier estimates of (A) TFST and (B) TSST. HR, hazard ratio; TFST, time to first subsequent therapy or death; TSST, time to second subsequent therapy or death.
The median TSST (data maturity 48.6%) was 93.2 months (95% CI, 84.2 to not reached) with olaparib compared with 40.7 months (95% CI, 32.9 to 54.4) with placebo, with an HR of 0.50 (95% CI, 0.37 to 0.67; Fig 3B). On the basis of Kaplan-Meier estimates, 56.9% of olaparib patients versus 32.5% of placebo patients were alive and had not received a second subsequent treatment after a 7-year follow-up. At the time of DCO, 68 (26.2%) patients in the olaparib group and 59 (45.0%) in the placebo group had received a second subsequent therapy (Data Supplement).
Consistent with the results reported previously,4 the median TDT (data maturity 98.2%) was 24.6 months (95% CI, 24.0 to 24.8) in the olaparib group compared with 13.8 months (95% CI, 11.2 to 16.4) in the placebo group, with an HR of 0.63 (95% CI, 0.51 to 0.78; Data Supplement).
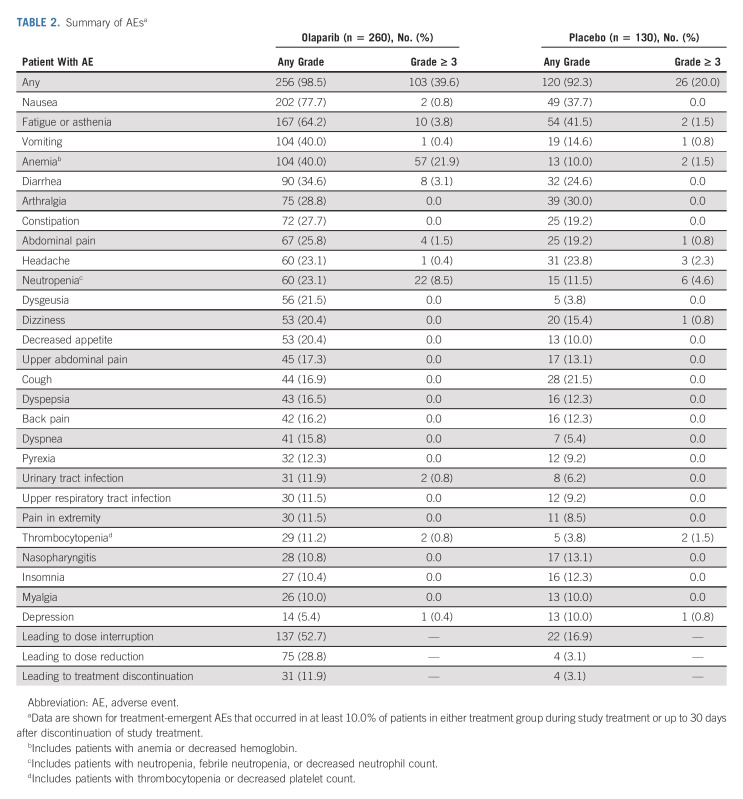
After a 7-year follow-up, the safety profile of maintenance olaparib was consistent with that reported at previous DCOs.3,4 The most common AEs of any grade reported in olaparib patients were nausea, fatigue/asthenia, vomiting, and anemia, and the most common grade ≥ 3 AE was anemia (Table 2). Serious AEs occurred in 21.2% of olaparib patients and 13.8% of placebo patients. The most commonly reported serious AEs were anemia (7.3% of olaparib patients v 0.0% of placebo patients) and neutropenia (1.5% v 0.0%).
TABLE 2.
Summary of AEsa
Data on MDS/AML and new primary malignancies were collected both during study treatment and after discontinuation of study treatment up to the time of DCO (March 7, 2022). Since the primary DCO (May 17, 2018), one (0.4%) new case of MDS has been reported in the olaparib group and one (0.8%) new case of acute myelomonocytic leukemia has been reported in the placebo group. In total, after a 7-year follow-up, four (1.5%) cases of MDS/AML were reported in the olaparib group and one (0.8%) case of MDS/AML was reported in the placebo group. In total, after a 7-year follow-up, new primary malignancies were reported in 14 (5.4%) olaparib patients (breast cancer [n = 10], lip and/or oral cavity cancer [n = 1], thyroid cancer [n = 1], pancreatic adenocarcinoma [n = 1], and gall bladder adenocarcinoma [n = 1]) and eight (6.2%) placebo patients (breast cancer [n = 5], lung adenocarcinoma [n = 1], squamous cell carcinoma of the tongue [n = 1], and chronic myeloid leukemia [n = 1]). Six (2.3%) new primary malignancies occurred in olaparib patients, and three (2.3%) occurred in placebo patients since the March 5, 2020, DCO.
AEs were usually managed by dose interruption or reduction, with few patients (11.9% of olaparib patients and 3.1% of placebo patients) requiring treatment discontinuation because of AEs (Table 2).
DISCUSSION
The median duration of follow-up of approximately 88 months reported in this descriptive SOLO1 analysis represents the longest follow-up for any PARP inhibitor in newly diagnosed advanced ovarian cancer. With an HR for OS of 0.55 (95% CI, 0.40 to 0.76) observed with maintenance olaparib (administered for ≤ 2 years in most patients) versus placebo and 67.0% of olaparib patients (v 46.5% of placebo patients) alive at 7 years, SOLO1 is the first study to indicate a clinically meaningful improvement in OS with PARP inhibitor maintenance therapy in the first-line setting.
Data maturity for OS was 38.1% in the current analysis, and the SOLO1 final OS analysis is currently planned to be conducted once data maturity reaches approximately 60%.3 Given that the event rate for OS is slower than that anticipated at the onset of the study and it may be many years before the threshold to conduct the final OS analysis is met, performing a descriptive OS analysis at 7 years, a clinically relevant time point, was important to help inform treatment decisions. The Haybittle-Peto α spending function required a P < .0001 to show statistical significance in the current descriptive analysis (administrative α spending), allowing the statistical power of the final OS analysis to be preserved. Although not reaching the threshold for statistical significance, we consider the OS benefit shown in this 7-year descriptive analysis to be clinically meaningful. Given the 5-year survival rate of 38.1% previously reported in patients with newly diagnosed advanced ovarian cancer and a BRCA mutation,9 the 5-year and 7-year OS rates of 73.1% and 67.0%, respectively, seen in SOLO1 patients receiving maintenance olaparib represent an important advance; it should be noted that OS rates in SOLO1 were calculated from the time of random assignment rather than from the time of diagnosis.
It is difficult to demonstrate improvements in OS in ovarian cancer trials because of the number and variety of uncontrolled postprogression treatment options including experimental agents.10,11 In this descriptive OS analysis, more than 40% of placebo patients (v 14.6% of olaparib patients) received subsequent therapy with a PARP inhibitor (and 59.8% of placebo patients v 31.1% of olaparib patients who received any subsequent therapy received a PARP inhibitor); this is likely to have affected the OS results, which were unadjusted for subsequent PARP inhibitor therapy. Subsequent treatment with a PARP inhibitor may also partly explain the relatively long median OS of 75.2 months observed in the placebo arm. This compares with a median OS of 58.3 months in patients, irrespective of biomarker status, who were in clinical complete response after first-line platinum-based chemotherapy and enrolled in the surveillance arm of the phase III GOG 0212 trial; < 20% of patients were alive and progression-free after a median follow-up in the overall patient population of 8.1 years.12 BRCA-mutated patients in the placebo arm of the phase III GOG 0218 trial had a median OS of 61.2 months13; it should be noted that compared with SOLO1, patients in GOG 0218 had a worse prognosis (patients with FIGO stage III disease and complete resection after cytoreductive surgery were excluded), and random assignment in GOG 0218 occurred before the start of chemotherapy. Advances in the management of relapsed ovarian cancer, including improvements in the sequencing of therapies and supportive care, might have also contributed to the median OS seen in placebo patients in SOLO1. OS results from other ongoing trials evaluating PARP inhibitor maintenance therapy in the newly diagnosed setting (eg, combination maintenance therapy with olaparib plus bevacizumab in PAOLA-1,14 maintenance niraparib in PRIMA,15 and maintenance rucaparib in ATHENA-MONO16) are awaited with interest.
The results of SOLO1 emphasize the importance of both testing for both germline and somatic BRCA mutations and providing PARP inhibitor maintenance therapy to all BRCA-mutated patients with advanced disease in the first-line setting, rather than delaying the introduction of PARP inhibitors until patients have experienced relapse. On the basis of the results of SOLO1, maintenance therapy with olaparib is capped at 2 years in the first-line setting although patients with evidence of disease at this time point can be treated beyond 2 years.17,18 This descriptive OS analysis (DCO March 7, 2022) confirms findings from earlier PFS analyses in SOLO1 (DCO May 17, 2018,3 and March 5, 20204) that the benefit of maintenance olaparib extends well beyond its 2-year treatment cap in patients with newly diagnosed advanced ovarian cancer and a BRCA mutation. Indeed, the SOLO1 OS data support the use of maintenance olaparib to achieve long-term remission in BRCA-mutated patients with newly diagnosed advanced ovarian cancer.
It is noteworthy that for a considerable proportion of olaparib patients, the SOLO1 OS data reflect disease-free survival. Although updated PFS data are not available, TFST was evaluated as a proxy for PFS.19 TFST data showed a substantial delay with maintenance olaparib versus placebo in the time between random assignment and the first subsequent treatment, with 45.3% of olaparib patients (v 20.6% of placebo patients) alive and still to receive a first subsequent therapy after a 7-year follow-up. These data suggest that maintenance olaparib might enhance the potential for cure although longer follow-up is needed for a more definitive evaluation of cure. Modeling data suggest that 10-year survival appears to be an appropriate surrogate of cure in this setting.5
TSST data are also consistent with the previously reported PFS benefit3,4 and indicate that the benefit of maintenance olaparib persists beyond the first subsequent therapy.19
After a 7-year follow-up, the safety profile of maintenance olaparib was consistent with that reported at earlier DCOs (May 17, 2018,3 and March 5, 20204), with no new safety signals detected. It is reassuring that the incidence of MDS/AML remained low and the incidence of new primary malignancies remained balanced between the treatment arms after 7 years of active follow-up for these events in SOLO1. Only one new case of MDS/AML has been reported in the olaparib arm since the primary DCO on May 17, 2018. The low risk of MDS/AML observed in SOLO1 is consistent with that reported in other PARP inhibitor maintenance therapy trials in the newly diagnosed setting.14-16 A higher incidence of MDS/AML has been observed in PARP inhibitor maintenance therapy trials in patients with relapsed ovarian cancer.20,21 The incidence of MDS/AML in the relapsed disease setting should be considered in the context of potential baseline risk factors for MDS/AML (eg, prior chemotherapy with DNA-damaging agents) and the long latency of these events. A contributing role for PARP inhibitors cannot be excluded, and long-term active surveillance for MDS/AML events after discontinuation of PARP inhibitor maintenance therapy is prudent.
In conclusion, after a 7-year follow-up, results indicate a clinically meaningful, albeit not statistically significant according to prespecified criteria, improvement in OS with maintenance olaparib versus placebo in patients with newly diagnosed advanced ovarian cancer and a BRCA mutation. These data support the use of maintenance olaparib to achieve long-term remission in this setting; the potential for cure may also be enhanced.
ACKNOWLEDGMENT
We thank all the women who participated in this study, their families, and the investigators. We thank Steve Keefe (Merck & Co Inc) for his contributions to this study. A complete list of collaborators from the SOLO1 investigators is provided in Appendix Table A1 (online only).
APPENDIX
TABLE A1.
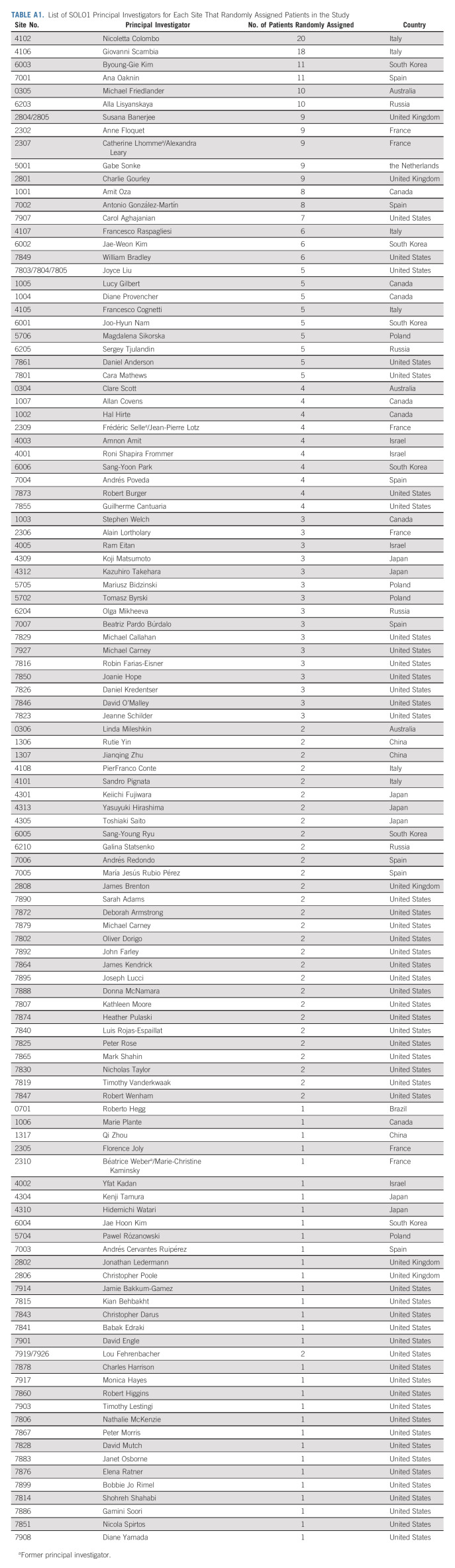
List of SOLO1 Principal Investigators for Each Site That Randomly Assigned Patients in the Study
Paul DiSilvestro
Consulting or Advisory Role: AstraZeneca, GlaxoSmithKline/Tesaro
Research Funding: Janssen Oncology (Inst), Tesaro (Inst), AstraZeneca (Inst), Genentech (Inst), AbbVie (Inst)
Susana Banerjee
Honoraria: AstraZeneca, GlaxoSmithKline, Clovis Oncology, Pfizer, Immunogen, MSD Oncology, Mersana, Roche, Takeda, Amgen
Consulting or Advisory Role: Amgen, GlaxoSmithKline, MSD Oncology, Mersana, AstraZeneca, Seattle Genetics, OncXerna Therapeutics, Shattuck Labs, Merck Serono, Immunogen
Research Funding: GlaxoSmithKline (Inst), AstraZeneca (Inst)
Nicoletta Colombo
Employment: Sarepta Therapeutics (I)
Honoraria: Roche/Genentech, AstraZeneca, Tesaro, GlaxoSmithKline, MSD Oncology, Clovis Oncology, Pfizer, Amgen, Immunogen, Novartis, Pfizer, Mersana, Eisai, Advaxis, Nuvation Bio
Consulting or Advisory Role: Roche/Genentech, AstraZeneca, Clovis Oncology, Pfizer, MSD Oncology, Tesaro, GlaxoSmithKline, Immunogen, Pfizer, Mersana, Eisai, Advaxis, Nuvation Bio
Giovanni Scambia
Consulting or Advisory Role: Clovis Oncology, AstraZeneca, PharmaMar, Roche, Tesaro
Speakers' Bureau: Clovis Oncology, MSD
Ana Oaknin
Consulting or Advisory Role: Roche, AstraZeneca, PharmaMar, Clovis Oncology, Tesaro, Immunogen, Genmab, Mersana, GSK, Deciphera, AGENUS, Corcept Therapeutics, Eisai, EMD Serono, F. Hoffmann-La Roche, Medison, Merck Sharp & Dohme, Novocure, prIME Oncology, Shattuck Labs, Sutro Biopharma, ITeos Therapeutics, Amgen
Research Funding: AbbVie (Inst), Ability Pharmaceuticals (Inst), Advaxis (Inst), Aeterna Zentaris (Inst), Aprea Therapeutics (Inst), Clovis Oncology Inc (Inst), Eisai (Inst), Roche (Inst), Regeneron (Inst), Agenus (Inst), AstraZeneca (Inst), BeiGene (Inst), Belgian Gynaecological Oncology Group (BGOG) (Inst), Bristol Myers Squibb International Corporation (BMS) (Inst), Corcept Therapeutics (Inst), Immunogen (Inst), Iovance Biotherapeutics (Inst), Lilly (Inst), Medimmune (Inst), Merck (Inst), Merck Sharp & Dohme (Inst), Mundipharma Research (Inst), /Inst), Seagen (Inst), Seattle Genetics (Inst), Sutro Biopharma (Inst), Tesaro (Inst), Verastem (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Clovis Oncology, PharmaMar, Roche
Michael Friedlander
Honoraria: AstraZeneca, MSD, Lilly, Takeda, Novartis, GlaxoSmithKline
Consulting or Advisory Role: AstraZeneca, MSD, AbbVie, Lilly, Takeda, Novartis, GlaxoSmithKline, Eisai, Incyclix
Speakers' Bureau: AstraZeneca, ACT Genomics, GlaxoSmithKline
Research Funding: BeiGene (Inst), AstraZeneca (Inst), Novartis (Inst)
Travel, Accommodations, Expenses: AstraZeneca
Alla Lisyanskaya
Honoraria: Incuron (Inst), MSD (Inst), AstraZeneca (Inst), Regeneron (Inst), Roche (Inst)
Research Funding: Incuron, Roche, AstraZeneca, Regeneron, MSD
Anne Floquet
Honoraria: GlaxoSmithKline
Travel, Accommodations, Expenses: GlaxoSmithKline, PharmaMar, AstraZeneca
Alexandra Leary
Honoraria: Medscape
Consulting or Advisory Role: Clovis Oncology (Inst), AstraZeneca (Inst), Tesaro (Inst), MSD (Inst), GlaxoSmithKline (Inst), Merck Serono (Inst), Zentalis, PEGASCY, Blueprint Medicines (Inst)
Research Funding: Inivata (Inst), Sanofi (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Tesaro
Gabe S. Sonke
Consulting or Advisory Role: Novartis (Inst), Seattle Genetics (Inst), Biovica (Inst)
Research Funding: Merck Sharp & Dohme (Inst), Agendia (Inst), AstraZeneca/Merck (Inst), Roche (Inst), Novartis (Inst)
Charlie Gourley
Honoraria: AstraZeneca, GlaxoSmithKline, MSD Oncology, Clovis Oncology, Takeda
Consulting or Advisory Role: AstraZeneca, Cor2Ed, GlaxoSmithKline, MSD Oncology, Clovis Oncology
Research Funding: AstraZeneca (Inst), GlaxoSmithKline (Inst), MSD Oncology (Inst), Novartis (Inst), BerGenBio (Inst), MedAnnex (Inst)
Patents, Royalties, Other Intellectual Property: One patent issued and four pending for a gene expression signature to predict cancer sensitivity to antiangiogenic therapy (Inst)
Expert Testimony: Amgen
Amit Oza
Uncompensated Relationships: Ozmosis Research
Antonio González-Martín
Consulting or Advisory Role: Roche, Tesaro/GSK, Clovis Oncology, AstraZeneca, MSD, Genmab, Immunogen, Oncoinvent, Pfizer/EMD Serono, Amgen, Mersana, SOTIO, Sutro Biopharma, MacroGenics, Novartis, Alkermes, Hedera Dx, Novocure, Seattle Genetics, Takeda
Speakers' Bureau: Roche, AstraZeneca, Tesaro/GSK, PharmaMar, Clovis Oncology, MSD Oncology
Research Funding: Roche (Inst), Tesaro/GSK (Inst)
Travel, Accommodations, Expenses: Roche, AstraZeneca, PharmaMar, Tesaro/GSK, MSD Oncology
Carol Aghajanian
Consulting or Advisory Role: Eisai, AstraZeneca/Merck, Roche/Genentech, Repare Therapeutics
Research Funding: Genentech/Roche (Inst), AbbVie (Inst), Clovis Oncology (Inst), AstraZeneca (Inst)
William Bradley
Consulting or Advisory Role: Celsion, Inovio Pharmaceuticals
Travel, Accommodations, Expenses: Inovio Pharmaceuticals, Clovis Oncology
Cara Mathews
Research Funding: AstraZeneca (Inst), Tesaro/GSK (Inst), Syros Pharmaceuticals (Inst), Astellas Pharma (Inst), Seattle Genetics (Inst), Deciphera (Inst), Moderna Therapeutics (Inst), Regeneron (Inst), Roche/Genentech (Inst), Pfizer (Inst), Laekna Therapeutics (Inst), EMD Serono (Inst), Merck (Inst)
Joyce Liu
Consulting or Advisory Role: Clovis Oncology, Genentech/Roche, GlaxoSmithKline, Regeneron, AstraZeneca, Eisai
Research Funding: Genentech/Roche (Inst), AstraZeneca (Inst), Boston Biomedical (Inst), Acetylon Pharmaceuticals (Inst), Bristol Myers Squibb (Inst), Agenus (Inst), CytomX Therapeutics (Inst), Regeneron (Inst), Tesaro (Inst), Clovis Oncology (Inst), Surface Oncology (Inst), 2× Oncology (Inst), Vigeo Therapeutics (Inst), Aravive (Inst), Arch Oncology (Inst), Zentalis (Inst)
Uncompensated Relationships: Merck
John McNamara
Employment: AstraZeneca
Stock and Other Ownership Interests: AstraZeneca
Consulting or Advisory Role: AstraZeneca
Travel, Accommodations, Expenses: AstraZeneca
Elizabeth S. Lowe
Employment: AstraZeneca
Stock and Other Ownership Interests: AstraZeneca
Mei-Lin Ah-See
Employment: AstraZeneca
Stock and Other Ownership Interests: AstraZeneca
Consulting or Advisory Role: AstraZeneca (I), Bard1 Bioscience (I)
Research Funding: GRAIL (I), Johnson and Johnson (I)
Patents, Royalties, Other Intellectual Property: Progression of precancerous lesion predictor (I)
Kathleen N. Moore
Leadership: GOG Partners, NRG Oncology (Inst)
Honoraria: Research To Practice, Prime Oncology, Physicians' Education Resource, Great Debates and Updates
Consulting or Advisory Role: Genentech/Roche, Immunogen, AstraZeneca, VBL Therapeutics, Merck, Aravive, Eisai, Mersana (Inst), Myriad Genetics, Alkermes (Inst), Blueprint Pharmaceuticals (Inst), GlaxoSmithKline/Tesaro (Inst), I-Mab (Inst), InxMed (Inst), Mereo BioPharma (Inst), OncXerna Therapeutics, Onconova Therapeutics, Mereo BioPharma, Novartis
Research Funding: PTC Therapeutics (Inst), Lilly (Inst), Merck (Inst), Tesaro (Inst), Genentech (Inst), Clovis Oncology (Inst), Lilly Foundation (Inst), Regeneron (Inst), Advaxis (Inst), Bristol Myers Squibb (Inst), Verastem (Inst), Novartis Pharmaceuticals UK Ltd (Inst), AstraZeneca (Inst), Agenus (Inst), Takeda (Inst), Forty Seven (Inst), Stem CentRx (Inst), Immunogen (Inst), Bayer (Inst), Novogen (Inst), AbbVie/Stemcentrx (Inst), Artios (Inst), Bolt Biotherapeutics (Inst), Amgen (Inst), Daiichi Sankyo/Lilly (Inst), Cyteir (Inst), Immunocore (Inst)
Patents, Royalties, Other Intellectual Property: UpToDate
Other Relationship: GOG Partners (Inst)
No other potential conflicts of interest were reported.
SUPPORT
Supported by AstraZeneca and this work is part of an alliance between AstraZeneca and Merck Sharp & Dohme LLC, a subsidiary of Merck & Co Inc, Rahway, NJ. Medical writing assistance was provided by Gillian Keating MBChB of Cence.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca's data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
AUTHOR CONTRIBUTIONS
Conception and design: Paul DiSilvestro, Giovanni Scambia, Gabe S. Sonke, Amit Oza, Elizabeth S. Lowe, Kathleen N. Moore
Administrative support: John McNamara
Provision of study materials or patients: Susana Banerjee, Nicoletta Colombo, Giovanni Scambia, Byoung-Gie Kim, Ana Oaknin, Michael Friedlander, Alla Lisyanskaya, Anne Floquet, Alexandra Leary, Gabe S. Sonke, Charlie Gourley, Amit Oza, Antonio González-Martín, Carol Aghajanian, William Bradley, Cara Mathews, Joyce Liu, Kathleen N. Moore
Collection and assembly of data: Susana Banerjee, Nicoletta Colombo, Giovanni Scambia, Byoung-Gie Kim, Ana Oaknin, Michael Friedlander, Alla Lisyanskaya, Anne Floquet, Alexandra Leary, Gabe S. Sonke, Charlie Gourley, Amit Oza, Antonio González-Martín, Carol Aghajanian, William Bradley, Cara Mathews, Joyce Liu, John McNamara, Elizabeth S. Lowe, Mei-Lin Ah-See, Kathleen N. Moore
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Overall Survival With Maintenance Olaparib at a 7-Year Follow-Up in Patients With Newly Diagnosed Advanced Ovarian Cancer and a BRCA Mutation: The SOLO1/GOG 3004 Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Paul DiSilvestro
Consulting or Advisory Role: AstraZeneca, GlaxoSmithKline/Tesaro
Research Funding: Janssen Oncology (Inst), Tesaro (Inst), AstraZeneca (Inst), Genentech (Inst), AbbVie (Inst)
Susana Banerjee
Honoraria: AstraZeneca, GlaxoSmithKline, Clovis Oncology, Pfizer, Immunogen, MSD Oncology, Mersana, Roche, Takeda, Amgen
Consulting or Advisory Role: Amgen, GlaxoSmithKline, MSD Oncology, Mersana, AstraZeneca, Seattle Genetics, OncXerna Therapeutics, Shattuck Labs, Merck Serono, Immunogen
Research Funding: GlaxoSmithKline (Inst), AstraZeneca (Inst)
Nicoletta Colombo
Employment: Sarepta Therapeutics (I)
Honoraria: Roche/Genentech, AstraZeneca, Tesaro, GlaxoSmithKline, MSD Oncology, Clovis Oncology, Pfizer, Amgen, Immunogen, Novartis, Pfizer, Mersana, Eisai, Advaxis, Nuvation Bio
Consulting or Advisory Role: Roche/Genentech, AstraZeneca, Clovis Oncology, Pfizer, MSD Oncology, Tesaro, GlaxoSmithKline, Immunogen, Pfizer, Mersana, Eisai, Advaxis, Nuvation Bio
Giovanni Scambia
Consulting or Advisory Role: Clovis Oncology, AstraZeneca, PharmaMar, Roche, Tesaro
Speakers' Bureau: Clovis Oncology, MSD
Ana Oaknin
Consulting or Advisory Role: Roche, AstraZeneca, PharmaMar, Clovis Oncology, Tesaro, Immunogen, Genmab, Mersana, GSK, Deciphera, AGENUS, Corcept Therapeutics, Eisai, EMD Serono, F. Hoffmann-La Roche, Medison, Merck Sharp & Dohme, Novocure, prIME Oncology, Shattuck Labs, Sutro Biopharma, ITeos Therapeutics, Amgen
Research Funding: AbbVie (Inst), Ability Pharmaceuticals (Inst), Advaxis (Inst), Aeterna Zentaris (Inst), Aprea Therapeutics (Inst), Clovis Oncology Inc (Inst), Eisai (Inst), Roche (Inst), Regeneron (Inst), Agenus (Inst), AstraZeneca (Inst), BeiGene (Inst), Belgian Gynaecological Oncology Group (BGOG) (Inst), Bristol Myers Squibb International Corporation (BMS) (Inst), Corcept Therapeutics (Inst), Immunogen (Inst), Iovance Biotherapeutics (Inst), Lilly (Inst), Medimmune (Inst), Merck (Inst), Merck Sharp & Dohme (Inst), Mundipharma Research (Inst), /Inst), Seagen (Inst), Seattle Genetics (Inst), Sutro Biopharma (Inst), Tesaro (Inst), Verastem (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Clovis Oncology, PharmaMar, Roche
Michael Friedlander
Honoraria: AstraZeneca, MSD, Lilly, Takeda, Novartis, GlaxoSmithKline
Consulting or Advisory Role: AstraZeneca, MSD, AbbVie, Lilly, Takeda, Novartis, GlaxoSmithKline, Eisai, Incyclix
Speakers' Bureau: AstraZeneca, ACT Genomics, GlaxoSmithKline
Research Funding: BeiGene (Inst), AstraZeneca (Inst), Novartis (Inst)
Travel, Accommodations, Expenses: AstraZeneca
Alla Lisyanskaya
Honoraria: Incuron (Inst), MSD (Inst), AstraZeneca (Inst), Regeneron (Inst), Roche (Inst)
Research Funding: Incuron, Roche, AstraZeneca, Regeneron, MSD
Anne Floquet
Honoraria: GlaxoSmithKline
Travel, Accommodations, Expenses: GlaxoSmithKline, PharmaMar, AstraZeneca
Alexandra Leary
Honoraria: Medscape
Consulting or Advisory Role: Clovis Oncology (Inst), AstraZeneca (Inst), Tesaro (Inst), MSD (Inst), GlaxoSmithKline (Inst), Merck Serono (Inst), Zentalis, PEGASCY, Blueprint Medicines (Inst)
Research Funding: Inivata (Inst), Sanofi (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Tesaro
Gabe S. Sonke
Consulting or Advisory Role: Novartis (Inst), Seattle Genetics (Inst), Biovica (Inst)
Research Funding: Merck Sharp & Dohme (Inst), Agendia (Inst), AstraZeneca/Merck (Inst), Roche (Inst), Novartis (Inst)
Charlie Gourley
Honoraria: AstraZeneca, GlaxoSmithKline, MSD Oncology, Clovis Oncology, Takeda
Consulting or Advisory Role: AstraZeneca, Cor2Ed, GlaxoSmithKline, MSD Oncology, Clovis Oncology
Research Funding: AstraZeneca (Inst), GlaxoSmithKline (Inst), MSD Oncology (Inst), Novartis (Inst), BerGenBio (Inst), MedAnnex (Inst)
Patents, Royalties, Other Intellectual Property: One patent issued and four pending for a gene expression signature to predict cancer sensitivity to antiangiogenic therapy (Inst)
Expert Testimony: Amgen
Amit Oza
Uncompensated Relationships: Ozmosis Research
Antonio González-Martín
Consulting or Advisory Role: Roche, Tesaro/GSK, Clovis Oncology, AstraZeneca, MSD, Genmab, Immunogen, Oncoinvent, Pfizer/EMD Serono, Amgen, Mersana, SOTIO, Sutro Biopharma, MacroGenics, Novartis, Alkermes, Hedera Dx, Novocure, Seattle Genetics, Takeda
Speakers' Bureau: Roche, AstraZeneca, Tesaro/GSK, PharmaMar, Clovis Oncology, MSD Oncology
Research Funding: Roche (Inst), Tesaro/GSK (Inst)
Travel, Accommodations, Expenses: Roche, AstraZeneca, PharmaMar, Tesaro/GSK, MSD Oncology
Carol Aghajanian
Consulting or Advisory Role: Eisai, AstraZeneca/Merck, Roche/Genentech, Repare Therapeutics
Research Funding: Genentech/Roche (Inst), AbbVie (Inst), Clovis Oncology (Inst), AstraZeneca (Inst)
William Bradley
Consulting or Advisory Role: Celsion, Inovio Pharmaceuticals
Travel, Accommodations, Expenses: Inovio Pharmaceuticals, Clovis Oncology
Cara Mathews
Research Funding: AstraZeneca (Inst), Tesaro/GSK (Inst), Syros Pharmaceuticals (Inst), Astellas Pharma (Inst), Seattle Genetics (Inst), Deciphera (Inst), Moderna Therapeutics (Inst), Regeneron (Inst), Roche/Genentech (Inst), Pfizer (Inst), Laekna Therapeutics (Inst), EMD Serono (Inst), Merck (Inst)
Joyce Liu
Consulting or Advisory Role: Clovis Oncology, Genentech/Roche, GlaxoSmithKline, Regeneron, AstraZeneca, Eisai
Research Funding: Genentech/Roche (Inst), AstraZeneca (Inst), Boston Biomedical (Inst), Acetylon Pharmaceuticals (Inst), Bristol Myers Squibb (Inst), Agenus (Inst), CytomX Therapeutics (Inst), Regeneron (Inst), Tesaro (Inst), Clovis Oncology (Inst), Surface Oncology (Inst), 2× Oncology (Inst), Vigeo Therapeutics (Inst), Aravive (Inst), Arch Oncology (Inst), Zentalis (Inst)
Uncompensated Relationships: Merck
John McNamara
Employment: AstraZeneca
Stock and Other Ownership Interests: AstraZeneca
Consulting or Advisory Role: AstraZeneca
Travel, Accommodations, Expenses: AstraZeneca
Elizabeth S. Lowe
Employment: AstraZeneca
Stock and Other Ownership Interests: AstraZeneca
Mei-Lin Ah-See
Employment: AstraZeneca
Stock and Other Ownership Interests: AstraZeneca
Consulting or Advisory Role: AstraZeneca (I), Bard1 Bioscience (I)
Research Funding: GRAIL (I), Johnson and Johnson (I)
Patents, Royalties, Other Intellectual Property: Progression of precancerous lesion predictor (I)
Kathleen N. Moore
Leadership: GOG Partners, NRG Oncology (Inst)
Honoraria: Research To Practice, Prime Oncology, Physicians' Education Resource, Great Debates and Updates
Consulting or Advisory Role: Genentech/Roche, Immunogen, AstraZeneca, VBL Therapeutics, Merck, Aravive, Eisai, Mersana (Inst), Myriad Genetics, Alkermes (Inst), Blueprint Pharmaceuticals (Inst), GlaxoSmithKline/Tesaro (Inst), I-Mab (Inst), InxMed (Inst), Mereo BioPharma (Inst), OncXerna Therapeutics, Onconova Therapeutics, Mereo BioPharma, Novartis
Research Funding: PTC Therapeutics (Inst), Lilly (Inst), Merck (Inst), Tesaro (Inst), Genentech (Inst), Clovis Oncology (Inst), Lilly Foundation (Inst), Regeneron (Inst), Advaxis (Inst), Bristol Myers Squibb (Inst), Verastem (Inst), Novartis Pharmaceuticals UK Ltd (Inst), AstraZeneca (Inst), Agenus (Inst), Takeda (Inst), Forty Seven (Inst), Stem CentRx (Inst), Immunogen (Inst), Bayer (Inst), Novogen (Inst), AbbVie/Stemcentrx (Inst), Artios (Inst), Bolt Biotherapeutics (Inst), Amgen (Inst), Daiichi Sankyo/Lilly (Inst), Cyteir (Inst), Immunocore (Inst)
Patents, Royalties, Other Intellectual Property: UpToDate
Other Relationship: GOG Partners (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Ledermann JA, Raja FA, Fotopoulou C, et al. : Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 24:vi24-vi32, 2013. (suppl 6) [DOI] [PubMed] [Google Scholar]
- 2.Cress RD, Chen YS, Morris CR, et al. : Characteristics of long-term survivors of epithelial ovarian cancer. Obstet Gynecol 126:491-497, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore K, Colombo N, Scambia G, et al. : Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 379:2495-2505, 2018 [DOI] [PubMed] [Google Scholar]
- 4.Banerjee S, Moore KN, Colombo N, et al. : Maintenance olaparib for patients with newly diagnosed advanced ovarian cancer and a BRCA mutation (SOLO1/GOG 3004): 5-year follow-up of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 22:1721-1731, 2021 [DOI] [PubMed] [Google Scholar]
- 5.Dood RL, Zhao Y, Armbruster SD, et al. : Defining survivorship trajectories across patients with solid tumors: An evidence-based approach. JAMA Oncol 4:1519-1526, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.AstraZeneca : AstraZeneca Code of Ethics. 2022. https://www.astrazeneca.com/sustainability/ethics-and-transparency.html [Google Scholar]
- 7.DiSilvestro P, Colombo N, Scambia G, et al. : Efficacy of maintenance olaparib for patients with newly diagnosed advanced ovarian cancer with a BRCA mutation: Subgroup analysis findings from the SOLO1 trial. J Clin Oncol 38:3528-3537, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedlander M, Moore KN, Colombo N, et al. : Patient-centred outcomes and effect of disease progression on health status in patients with newly diagnosed advanced ovarian cancer and a BRCA mutation receiving maintenance olaparib or placebo (SOLO1): A randomised, phase 3 trial. Lancet Oncol 22:632-642, 2021 [DOI] [PubMed] [Google Scholar]
- 9.Chetrit A, Hirsh-Yechezkel G, Ben-David Y, et al. : Effect of BRCA1/2 mutations on long-term survival of patients with invasive ovarian cancer: The national Israeli study of ovarian cancer. J Clin Oncol 26:20-25, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Colombo N, Sessa C, du Bois A, et al. : ESMO-ESGO consensus conference recommendations on ovarian cancer: Pathology and molecular biology, early and advanced stages, borderline tumours and recurrent diseasedagger. Ann Oncol 30:672-705, 2019 [DOI] [PubMed] [Google Scholar]
- 11.Wilson MK, Pujade-Lauraine E, Aoki D, et al. : Fifth ovarian cancer consensus conference of the Gynecologic Cancer InterGroup: Recurrent disease. Ann Oncol 28:727-732, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Copeland LJ, Brady MF, Burger RA, et al. : Phase III randomized trial of maintenance taxanes versus surveillance in women with advanced ovarian/tubal/peritoneal cancer: A Gynecologic Oncology Group 0212:NRG Oncology study. J Clin Oncol 40:4119-4128, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tewari KS, Burger RA, Enserro D, et al. : Final overall survival of a randomized trial of bevacizumab for primary treatment of ovarian cancer. J Clin Oncol 37:2317-2328, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ray-Coquard I, Pautier P, Pignata S, et al. : Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med 381:2416-2428, 2019 [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez-Martin A, Pothuri B, Vergote I, et al. : Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 381:2391-2402, 2019 [DOI] [PubMed] [Google Scholar]
- 16.Monk BJ, Parkinson C, Lim MC, et al. : A randomized, phase III trial to evaluate rucaparib monotherapy as maintenance treatment in patients with newly diagnosed ovarian cancer (ATHENA-MONO/GOG-3020/ENGOT-ov45). J Clin Oncol 40:3952-3964, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.AstraZeneca : LYNPARZA (Olaparib) Tablets, for Oral Use: Prescribing Information. 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/208558s023lbl.pdf [Google Scholar]
- 18.AstraZeneca : Lynparza Summary of Product Characteristics. 2022. https://www.ema.europa.eu/en/documents/product-information/lynparza-epar-product-information_en.pdf [Google Scholar]
- 19.Matulonis UA, Oza AM, Ho TW, et al. : Intermediate clinical endpoints: A bridge between progression-free survival and overall survival in ovarian cancer trials. Cancer 121:1737-1746, 2015 [DOI] [PubMed] [Google Scholar]
- 20.Poveda A, Floquet A, Ledermann JA, et al. : Olaparib tablets as maintenance therapy in patients with platinum-sensitive relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): A final analysis of a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 22:620-631, 2021 [DOI] [PubMed] [Google Scholar]
- 21.Matulonis U, Herrstedt J, Oza A, et al. : Long-term safety and secondary efficacy endpoints in the ENGOT-OV16/NOVA phase III trial of niraparib in recurrent ovarian cancer. Gynecol Oncol 162:S24-S25, 2021. (abstr) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca's data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.