PURPOSE
BRAF V600 mutations occur in many childhood cancers, including approximately 20% of low-grade gliomas (LGGs). Here, we describe a phase I/II study establishing pediatric dosing and pharmacokinetics of trametinib with or without dabrafenib, as well as efficacy and safety in a disease-specific cohort with BRAF V600–mutant LGG; other cohorts will be reported elsewhere.
METHODS
This is a four-part, phase I/II study (ClinicalTrials.gov identifier: NCT02124772) in patients age < 18 years with relapsed/refractory malignancies: trametinib monotherapy dose finding (part A) and disease-specific expansion (part B), and dabrafenib + trametinib dose finding (part C) and disease-specific expansion (part D). The primary objective assessed in all patients in parts A and C was to determine pediatric dosing on the basis of steady-state pharmacokinetics. Disease-specific efficacy and safety (across parts A-D) were secondary objectives.
RESULTS
Overall, 139 patients received trametinib (n = 91) or dabrafenib + trametinib (n = 48). Trametinib dose-limiting toxicities in > 1 patient (part A) included mucosal inflammation (n = 3) and hyponatremia (n = 2). There were no dose-limiting toxicities with combination therapy (part C). The recommended phase II dose of trametinib, with or without dabrafenib, was 0.032 mg/kg once daily for patients age < 6 years and 0.025 mg/kg once daily for patients age ≥ 6 years; dabrafenib dosing in the combination was as previously identified for monotherapy. In 49 patients with BRAF V600–mutant glioma (LGG, n = 47) across all four study parts, independently assessed objective response rates were 15% (95% CI, 1.9 to 45.4) for monotherapy (n = 13) and 25% (95% CI, 12.1 to 42.2) for combination (n = 36). Adverse event–related treatment discontinuations were more common with monotherapy (54% v 22%).
CONCLUSION
The trial design provided efficient evaluation of pediatric dosing, safety, and efficacy of single-agent and combination targeted therapy. Age-based and weight-based dosing of trametinib with or without dabrafenib achieved target concentrations with manageable safety and demonstrated clinical efficacy and tolerability in BRAF V600–mutant LGG.
INTRODUCTION
Genetic alterations leading to constitutive mitogen-activated protein kinase pathway activation have been identified in many pediatric malignancies, suggesting that molecularly targeted therapies against these alterations may offer improved outcomes over current options. For example, BRAF V600 mutation occurs in approximately 20% of pediatric low-grade gliomas (LGGs) and is particularly common in pleomorphic xanthoastrocytoma and ganglioglioma.1-3 BRAF V600–mutant pediatric LGG is associated with poor response to standard-of-care chemotherapy and risk of transformation to secondary high-grade glioma (HGG).2,4 These patients also have poor progression-free survival (PFS) and overall survival rates compared with patients with BRAF V600 wild-type disease.2,5
CONTEXT
Key Objective
Genetic alterations driving aberrant mitogen-activated protein kinase pathway signaling are found in many pediatric malignancies, supporting a potential role for BRAF- and/or MEK inhibitor–targeted therapies. This phase I/II trial in children with relapsed/refractory malignancies encompassed dose finding for the MEK inhibitor trametinib with or without the BRAF inhibitor dabrafenib, plus efficacy and safety analyses in disease-specific cohorts.
Knowledge Generated
Recommended phase II doses for trametinib with or without dabrafenib in pediatric patients were identified, with exposures similar to those established in adults. Trametinib with or without dabrafenib also demonstrated clinical efficacy and manageable toxicity in a disease-specific cohort with BRAF V600–mutant low-grade glioma.
Relevance (S. Bhatia)
-
This study supports the need to conduct molecular profiling in children with cancer to inform targeted therapy.*
*Relevance section written by JCO Associate Editor Smita Bhatia, MD, MPH.
Case studies and early-phase clinical trials of BRAF inhibitors such as dabrafenib and vemurafenib have demonstrated clinical activity and manageable safety in BRAF V600–mutant pediatric malignancies such as LGG.6-15 In adults, it is well established that combination of BRAF + MEK inhibitors yields improved outcomes, mitigates the emergence of resistance, and reduces incidence of skin-related toxicities compared with BRAF inhibitor monotherapy in BRAF V600–mutant melanoma and non–small-cell lung cancer.16-19 The dabrafenib + trametinib combination, in particular, is approved for the treatment of BRAF V600–mutant solid tumors in patients age ≥ 6 years20,21 and has demonstrated clinical activity in adult BRAF V600–mutant gliomas.22 However, results from pediatric clinical trials of BRAF + MEK inhibitor combination have not yet been reported in the literature.
With pediatric dosing, efficacy, and safety of dabrafenib monotherapy established in a previous phase I/II trial,6,7 we describe here a phase I/II study designed to efficiently evaluate the same for trametinib monotherapy and dabrafenib + trametinib combination in children with relapsed/refractory malignancies, using a four-part trial design including dose-escalation and disease-specific expansion cohorts. As this is the initial report of the first trial of trametinib with or without dabrafenib in pediatric patients, dose finding and recommended phase II doses (RP2Ds) determined in all patients (regardless of diagnosis) in the dose-escalation parts are presented. Efficacy and safety in the cohort of patients with BRAF V600–mutant LGG enrolled across all study parts are also presented; other pediatric disease-specific cohorts with distinct management considerations will be reported elsewhere.
METHODS
Clinical Study Design
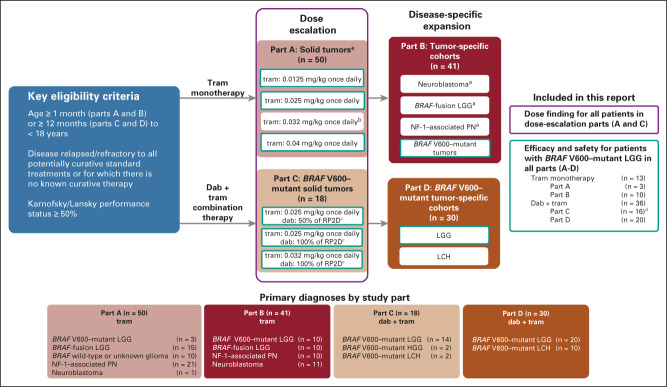
This phase I/II, multicenter, open-label study (An Open-Label, Dose-Escalation, Phase I/II Study to Investigate the Safety, Pharmacokinetics, Pharmacodynamics and Clinical Activity of the MEK Inhibitor Trametinib in Children and Adolescents Subjects With Cancer or Plexiform Neurofibromas and Trametinib in Combination With Dabrafenib in Children and Adolescents With Cancers Harboring V600 Mutations; ClinicalTrials.gov identifier: NCT02124772) had four parts (Fig 1): trametinib monotherapy dose escalation (followed by age-specific extension; part A) and disease-specific expansion (part B), and dabrafenib + trametinib dose escalation (followed by age-specific extension; part C) and disease-specific expansion (part D).
FIG 1.
Study design. aCohort not restricted to patients with BRAF V600–mutant disease. bIntermediate dose added on the basis of steady state pharmacokinetics and the tolerability of 0.025 mg/kg and 0.04 mg/kg once-daily doses. cPreviously determined RP2D for dabrafenib monotherapy7: age < 12 years, 5.25 mg/kg; age ≥ 12 years, 4.5 mg/kg, divided into two equal doses daily. dTwo patients with BRAF V600–mutant HGG were enrolled in part C and are included in this predominantly LGG cohort as a diagnosis of HGG did not define any other disease-specific cohort in the trial. dab, dabrafenib; HGG, high-grade glioma; LCH, Langerhans cell histiocytosis; LGG, low-grade glioma; NF-1, neurofibromatosis type 1; PN, plexiform neurofibroma; RP2D, recommended phase II dose; tram, trametinib.
The initial trametinib monotherapy dose level (0.0125 mg/kg once daily, tablet or oral solution) was 50% of the adult RP2D (2 mg/day). At first, three dose levels (0.0125, 0.025, and 0.04 mg/kg once daily) were evaluated; another dose level (0.032 mg/kg once daily) was subsequently added on the basis of evolving pharmacokinetic (PK) observations. On the basis of the monotherapy results, two of these (0.025 mg/kg once daily and 0.032 mg/kg once daily) were selected for evaluation in combination with dabrafenib. In this combination, the starting dose of dabrafenib was 50% of the previously established pediatric RP2D7 (age < 12 years, 5.25 mg/kg; age ≥ 12 years, 4.5 mg/kg, capsule or oral suspension divided into two equal doses daily); both 50% RP2D and 100% RP2D of dabrafenib were evaluated in combination with trametinib. Treatment was administered until unacceptable toxicity, progressive disease or lack of clinical benefit, or a patient/physician decision to discontinue. At study completion for all patients (December 29, 2020), those who were deriving clinical benefit per investigator opinion were eligible to continue in a rollover study (ClinicalTrials.gov identifier: NCT03975829) for long-term follow-up.
The trial was performed in compliance with Good Clinical Practice and conducted according to the principles of the Declaration of Helsinki. The study Protocol (online only) was approved by an independent ethics committee or review board at each institution and by medical authorities, and written informed consent was obtained from each patient and/or parent/legal representative according to local laws.
Study Objectives
The primary objective was to determine the RP2D of trametinib in pediatric patients, which would achieve an exposure level similar to that observed in adults. Secondary objectives included determination of the RP2D of dabrafenib + trametinib in pediatric patients, characterization of PK, safety and tolerability, and efficacy of trametinib with or without dabrafenib.
Patient Selection
Eligible patients had relapsed/refractory malignancies (exhausting any potentially curative treatments including surgery, radiation, chemotherapy, or combination thereof), had a Karnofsky/Lansky performance status of ≥ 50%, and were age ≥ 1 month (parts A and B) or ≥ 12 months (parts C and D) to < 18 years. Part A enrolled patients with solid tumors (ie, BRAF V600 mutation was not required), and part B included expansion cohorts for neuroblastoma, BRAF-fusion LGG, neurofibromatosis type 1 (NF-1)–associated plexiform neurofibroma (PN), and BRAF V600–mutant tumors. In parts C and D, patients were required to have BRAF V600–mutant disease; disease-specific expansion cohorts in part D included LGG and Langerhans cell histiocytosis (Fig 1). Specific eligibility criteria for each part are given in detail in the Data Supplement (online only).
Assessments
Adverse events (AEs), defined by the National Cancer Institute Common Terminology Criteria for Adverse Events v4.03, were assessed at each visit; additional safety assessments are described in the Data Supplement. For trametinib with or without dabrafenib dose finding (parts A and C), dose-limiting toxicities (DLTs) were defined as potentially related to study treatment per the investigator, occurred during the first 28 days of treatment, and met specific criteria outlined in the Data Supplement. PK samples were obtained on days 15 and 22 (part A); day 15 (part B); or days 1, 15, and 22 (parts C and D). Dose escalation and RP2D determination were based on DLTs and PK criteria outlined in the Data Supplement.
For the BRAF V600–mutant LGG cohort, radiographic disease assessments were performed at baseline, every 8 weeks × 3, and then every 12 weeks and evaluated by independent radiology review and investigators using Response Assessment in Neuro-Oncology (RANO) criteria23,24; RANO 2017 criteria use T2 FLAIR magnetic resonance imaging sequences, were considered more relevant for pediatric LGG, and are presented here.
Statistical Analysis
DLTs were evaluated in all patients in parts A and C who received ≥ 75% of planned doses (or < 75% if dose reduction was due to related toxicity). Target exposure was based on adult PK and efficacy studies: average steady state concentration (Cavg) trametinib, approximately 10 ng/mL; dabrafenib, approximately 300 ng/mL. PK parameters were calculated using noncompartmental methods, and AEs and PK parameters were summarized descriptively.
Efficacy and safety were assessed in all patients who received ≥ 1 dose of study treatment; the response-evaluable population had ≥ 1 postdose efficacy assessment or progression before the first on-treatment assessment. For patients with BRAF V600–mutant LGG, the cohort included individuals with BRAF V600–mutant glioma diagnoses from all four parts of the study. The protocol specified a ≥ 10-patient expansion for trametinib monotherapy and ≥ 20-patient expansion for dabrafenib + trametinib for initial efficacy exploration in a small sample to inform future studies. Objective response rates (complete + partial [PR] responses) per RANO criteria are summarized with corresponding 95% CIs. For PFS, survival functions were estimated using the Kaplan-Meier method; median estimates with 95% CI are presented.
RESULTS
Study Overview
Between January 15, 2015, and December 29, 2020, 139 patients were enrolled (Fig 1). In parts A and B, a total of 91 patients received trametinib monotherapy, including those with BRAF V600–mutant LGG (n = 13), BRAF-fusion LGG (n = 25), BRAF wild-type or unknown glioma (n = 10), neuroblastoma (n = 12), or NF-1–associated PN (n = 31). Median durations of exposure were 24 and 19 months in parts A and B, respectively, and 20 patients (22%) entered the rollover study (see the Methods section). In parts C and D, a total of 48 patients received dabrafenib + trametinib, including those with BRAF V600–mutant LGG (n = 34), HGG (n = 2), or LCH (n = 12). Median durations of exposure were 21 and 24 months in parts C and D, respectively, and 31 patients (65%) entered the rollover study. Demographics, disposition, and exposure are further summarized in the Data Supplement.
All Patients in Dose-Finding Parts (A and C): DLTs and RP2Ds of Trametinib With or Without Dabrafenib
PK parameters for 133 patients receiving trametinib with or without dabrafenib (Data Supplement) determined that trametinib doses above 0.025 mg/kg once daily achieved mean steady state exposures at or above the target. Both dabrafenib doses tested in combination with trametinib exceeded the target exposures. Additional PK results are given in the Data Supplement.
In part A (trametinib monotherapy), 8 of 48 DLT-evaluable patients (17%) experienced a total of 10 DLTs (Data Supplement) at the doses of 0.025 (n = 3) and 0.04 (n = 7) mg/kg once daily, including mucosal inflammation (n = 3) and hyponatremia (n = 2) in > 1 patient. Most (7 of 10) were grade 3; there were one grade 2 DLT (intolerable mucosal inflammation) at 0.025 mg/kg once daily and two grade 4 DLTs (hyponatremia and hypotension) at 0.04 mg/kg once daily. Although the dose of 0.04 mg/kg once daily was not well tolerated, it was estimated on the basis of PK data that approximately 23% of patients age < 6 years would not achieve target trametinib exposure at the dose of 0.025 mg/kg once daily. Therefore, an intermediate dose (0.032 mg/kg once daily) was explored and found to be tolerable, with no DLTs. Thus, RP2Ds selected for trametinib were 0.032 mg/kg once daily for patients age < 6 years and 0.025 mg/kg once daily for patients age ≥ 6 years.
No DLTs were seen in part C (dabrafenib + trametinib), and no maximum tolerated dose was reached. Ocular, cardiac, and skin-related AEs of potential interest were primarily grade 1/2 and not dose limiting with trametinib with or without dabrafenib (Data Supplement). Thus, RP2Ds selected for use in combination were the same as those for each monotherapy: trametinib as determined above, plus 100% of the previously determined RP2D of dabrafenib (5.25 mg/kg for patients age < 12 years; 4.5 mg/kg for patients age ≥ 12 years, divided into two equal doses daily).
BRAF V600–Mutant LGG (Parts A-D): Efficacy and Safety of Trametinib With or Without Dabrafenib
Forty-nine patients with BRAF V600–mutant glioma were treated across the four parts of this study; 47 had LGG, whereas the remaining two (both in part C) had HGG but were included in this analysis as that diagnosis did not define a separate expansion cohort (Data Supplement). A total of 13 patients with BRAF V600–mutant LGG received trametinib monotherapy: three in part A and 10 in part B; 36 patients with BRAF V600–mutant glioma received dabrafenib + trametinib: 16 in part C and 20 in part D. Additional demographics, exposure, and disposition for this disease-specific cohort are given in Table 1.
TABLE 1.
Demographics, Prior Treatment, Disposition, and Exposure in Patients With BRAF V600–Mutant LGGa
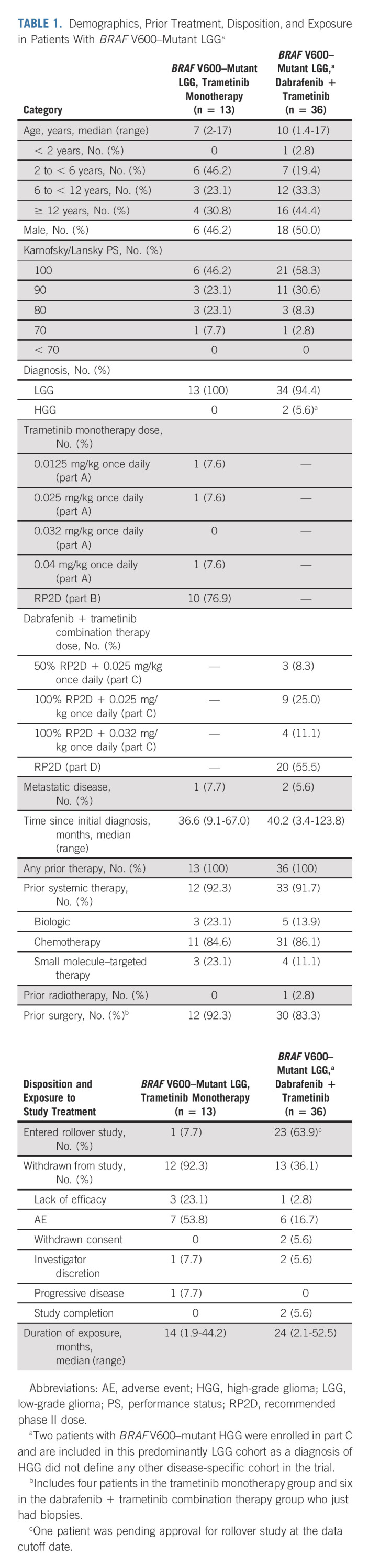
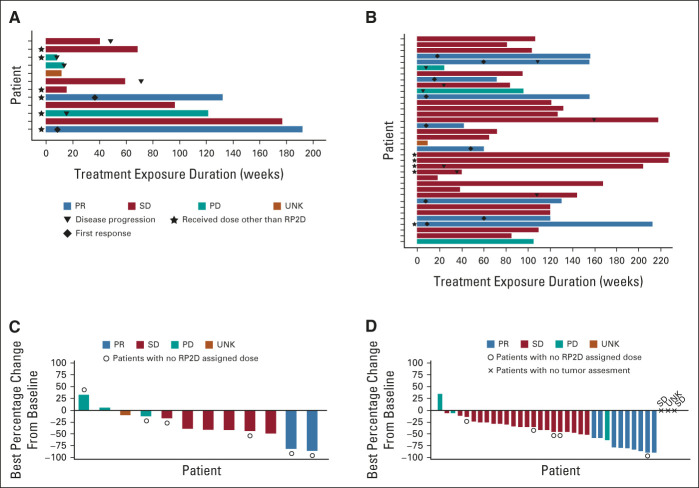
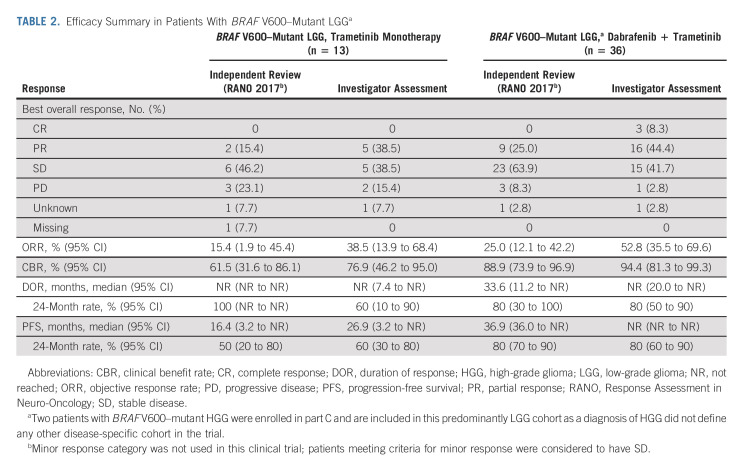
The best percentage change from baseline in the sum of the products of biperpendicular diameters in measurable lesions per independent review in patients with BRAF V600–mutant LGG is shown in Figure 2, and their sum over time is shown in the Data Supplement; the majority of patients had a reduction in tumor cross-sectional area. Two of 13 patients with BRAF V600–mutant LGG treated with trametinib monotherapy (15%; 95% CI, 1.9 to 45.4) had objective PRs by independent review, and six (46%) had stable disease ≥ 12 weeks from the first dose of therapy. The median duration of response (DOR) was not reached (NR), and both responses were ongoing at data cutoff. The estimated 24-month DOR rate was 100%. For patients with BRAF V600–mutant glioma treated with dabrafenib + trametinib, 9 of 36 (25%; 95% CI, 12.1 to 42.2) had objective PRs by independent review and 23 (64%) had stable disease. The median DOR was 33.6 months (95% CI, 11.2 to NR), with seven responses ongoing at data cutoff. The estimated 24-month DOR rate was 80% (95% CI, 30 to 100; Table 2). The median independently assessed PFS was 16.4 months (95% CI, 3.2 to NR) in the trametinib monotherapy group (n = 13) and 36.9 months (95% CI, 36.0 to NR) in the combination therapy group (n = 36; Table 2 and Fig 3). Efficacy per investigator assessment is summarized in Table 2 and the Data Supplement.
FIG 2.
Duration of exposure to study treatment and best percentage change from baseline in measurable lesions by independent review in patients with BRAF V600–mutant LGGa. (A) Duration of exposure to trametinib monotherapy (n = 13; one best overall response missing) or (B) dabrafenib + trametinib combination therapy (n = 36) in patients with BRAF V600–mutant LGG; best overall response assessed by independent review (RANO 2017) is shown. Best percentage change from baseline in the sum of the products of biperpendicular diameters in measurable lesions assessed by independent review (RANO 2017) in patients with BRAF V600–mutant LGG receiving (C) trametinib monotherapy (n = 13; one best overall response missing) or (D) dabrafenib + trametinib combination therapy (n = 36). aTwo patients with BRAF V600–mutant HGG were enrolled in part C and are included in this predominantly LGG cohort as a diagnosis of HGG did not define any other disease-specific cohort in the trial. HGG, high-grade glioma; LGG, low-grade glioma; PD, progressive disease; PR, partial response; RANO, Response Assessment in Neuro-Oncology; RP2D, recommended phase III dose; SD, stable disease; UNK, unknown.
TABLE 2.
Efficacy Summary in Patients With BRAF V600–Mutant LGGa
FIG 3.
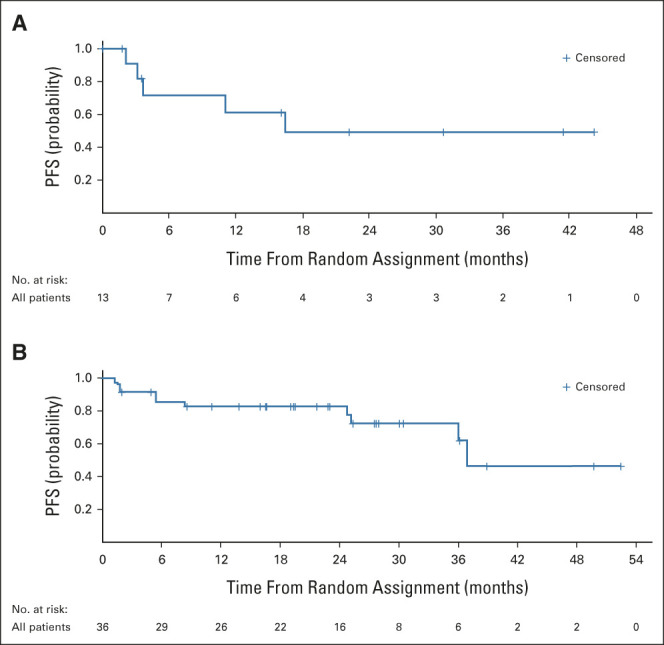
PFS by independent review in patients with BRAF V600–mutant LGGa. Kaplan-Meier plots showing PFS in patients with BRAF V600–mutant LGG treated with (A) trametinib monotherapy (n = 13) or (B) dabrafenib + trametinib combination therapy (n = 36), assessed by independent review (RANO 2017). aTwo patients with BRAF V600–mutant HGG were enrolled in part C and are included in this predominantly LGG cohort as a diagnosis of HGG did not define any other disease-specific cohort in the trial. HGG, high-grade glioma; LGG, low-grade glioma; PFS, progression-free survival; RANO, Response Assessment in Neuro-Oncology.
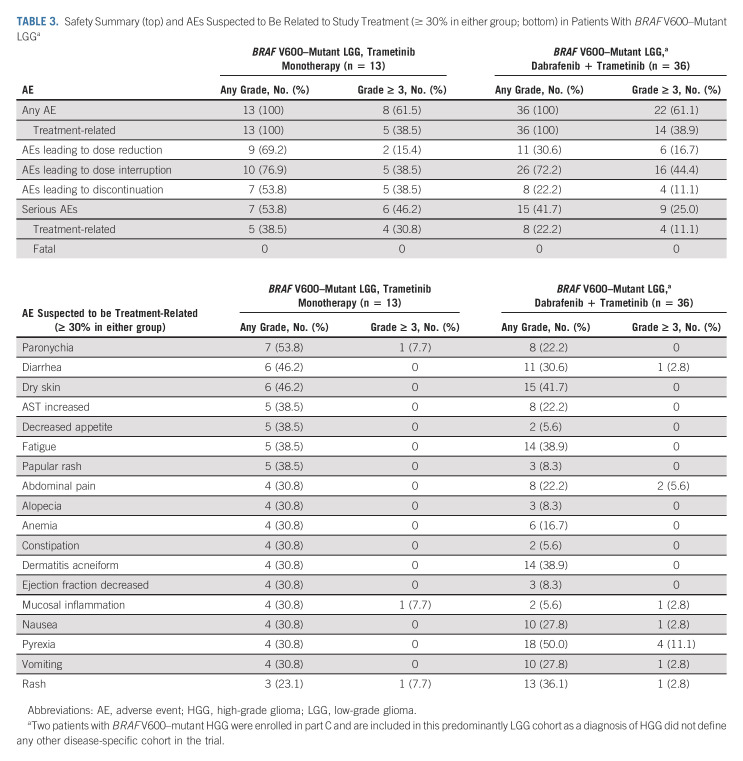
All patients with BRAF V600–mutant glioma experienced ≥ 1 AE, either all-cause (Data Supplement) or suspected to be treatment-related (Table 3). In the trametinib monotherapy group (n = 13), the most frequent treatment-related AEs (TRAEs) were paronychia (n = 7; 54%), diarrhea, and dry skin (n = 6 each; 46%). AEs led to dose reduction, interruption, or discontinuation of trametinib monotherapy in 9 (69%), 10 (77%), and 7 (54%) patients with BRAF V600–mutant LGG, respectively; only rash led to discontinuation in > 1 patient (n = 2). In the combination therapy group (n = 36), the most frequent TRAEs were pyrexia (n = 18; 50%) and dry skin (n = 15; 42%). AEs led to dose reduction, interruption, or discontinuation of dabrafenib + trametinib in 11 (31%), 26 (72%), and 8 (22%) patients with BRAF V600–mutant glioma, respectively; only decreased ejection fraction led to discontinuation in > 1 patient (n = 2). There were no on-treatment deaths with trametinib with or without dabrafenib in patients with BRAF V600–mutant LGG.
TABLE 3.
Safety Summary (top) and AEs Suspected to Be Related to Study Treatment (≥ 30% in either group; bottom) in Patients With BRAF V600–Mutant LGGa
DISCUSSION
To our knowledge, this phase I/II clinical trial is the first to evaluate trametinib with or without dabrafenib in pediatric patients with relapsed/refractory malignancies. The four-part design enabled efficient dose finding for both trametinib monotherapy and combination therapy guided by PK analysis to model similar exposures established in adults treated at the approved doses.25,26 Incorporation of PK as a driver of dose decisions enabled patients to reach therapeutic levels of exposure without dosing to excess or finding a maximum tolerable dose. On the basis of PK and DLTs, the recommended age-based and weight-based pediatric dosing of trametinib was established to be 0.032 mg/kg once daily for patients age < 6 years and 0.025 mg/kg once daily for patients age ≥ 6 years. The RP2Ds for combination therapy were the same as those established for each monotherapy (pediatric dosing for dabrafenib monotherapy had been previously determined7), which were well tolerated with no clear evidence of drug-drug interactions.
The study also enabled preliminary investigation of the pediatric efficacy and safety of trametinib with or without dabrafenib through the inclusion of disease-specific expansion cohorts. Results presented here demonstrated that, consistent with the well-established profile of these agents in adult BRAF V600–mutant solid tumors,17,19,27 trametinib with or without dabrafenib exhibits clinical efficacy with manageable toxicity in BRAF V600–mutant pediatric LGG. Pyrexia was the most commonly reported TRAE with combination therapy in BRAF V600–mutant LGG, consistent with observations in adult patients,28,29 but led to discontinuation in only one patient (3%). Trametinib monotherapy appeared to be less well tolerated in BRAF V600–mutant LGG, with 54% of patients discontinuing treatment for the primary reason of toxicity compared with 22% treated with combination therapy. In these patients, paronychia was the most frequently reported TRAE, leading to discontinuation in one patient (8%). Electrolyte disturbances, including hypo- and hypernatremia, were observed in two patients with hypothalamic/suprasellar tumors. Monitoring of sodium levels should be continued in patients with a history of electrolyte disturbances, including patients with pre-existing diabetes insipidus.30
Durable and rapid responses were seen in many patients with BRAF V600–mutant LGG receiving trametinib with or without dabrafenib, with the majority of responses achieved within 2 months and ongoing at 24 months. Efficacy appeared to be greater with dabrafenib + trametinib, with response rates of 25% with combination therapy and 15% with trametinib monotherapy. Although few progression events were reported before study completion, median PFS with combination therapy was also longer than with trametinib monotherapy, at 36.9 and 16.4 months, respectively. Based in part on these results, dabrafenib + trametinib received tumor-agnostic approval from the US Food and Drug Administration for the treatment of patients age ≥ 6 years with unresectable/metastatic BRAF V600E–mutant solid tumors who have progressed after prior treatment and have no satisfactory alternative.20,21 Moreover, recently presented results from a phase II randomized trial comparing dabrafenib + trametinib with standard-of-care chemotherapy in previously untreated BRAF V600–mutant pediatric LGG (ClinicalTrials.gov identifier: NCT02684058) further support BRAF + MEK inhibitor targeted therapy as an emerging standard of care in BRAF V600–mutant pediatric LGG.31
Indirect comparison of trametinib monotherapy and combination therapy within our trial suggests that BRAF + MEK inhibitor combination may offer improved efficacy and safety over MEK inhibitor monotherapy in BRAF V600–mutant pediatric LGG. However, the relative benefit of combination therapy versus BRAF inhibitor monotherapy in these patients is less clear.32 In adult BRAF V600–mutant solid tumors such as melanoma, combination therapy is used almost exclusively, on the basis of head-to-head comparative evidence from large phase III trials.16-18 Such direct evidence is not available for adults with BRAF V600–mutant gliomas although cross-trial comparison of BRAF inhibitor monotherapy with combination therapy favors the latter.22,33 However, in pediatric phase I/II studies of dabrafenib (n = 32) or vemurafenib (n = 19) monotherapy, response rates comparable with those observed with combination therapy in the present study were achieved in BRAF V600–mutant LGG.6,15 This is consistent with a retrospective analysis of pediatric patients with BRAF V600–mutant LGG treated with BRAF inhibitors or chemotherapy in which the objective response rates were reported to be 42% versus 10%, respectively.14 These results illustrate the number of potentially confounding factors inherent in cross-trial comparison, including the heterogeneity of patients with LGG and different versions of RANO response criteria applied. As emergence of resistance may be a key differentiator, additional PFS analyses from the ongoing rollover study (ClinicalTrials.gov identifier: NCT03975829) may further elucidate the relative merits of BRAF inhibitor monotherapy versus combination therapy in this patient population and provide insight into the optimal treatment duration for targeted therapy in this setting. However, lack of head-to-head comparison and small trial sizes are limitations.
The present trial included several cohorts with non–BRAF V600–mutant diagnoses, including BRAF-fusion LGG and NF-1–associated PN. Evidence suggests that these malignancies have management considerations distinct from those for BRAF V600–mutant disease and benefit from MEK inhibition only34,35; as such, they will be reported separately in the future. Phase II data have been reported for the MEK inhibitor selumetinib in a molecularly mixed pediatric LGG population, with a response rate of 36% in the cohort of patients with BRAF aberrations (n = 25), the majority of which were KIAA1549:BRAF fusions.36 Thus, the present trial adds to a growing body of evidence supporting the utility of molecularly targeted therapy in pediatric malignancies and the importance of conducting molecular profiling in this population.
ACKNOWLEDGMENT
The authors thank the patients who participated in the trial and their families. The authors also thank the physicians, nurses, research coordinators, and other staff at each site who assisted with the study. We also thank Karen Wright, Kenneth Cohen, and Lindsay Kilburn for enrollment of patients onto the trial and Neha Pakhle (Novartis Pharmaceuticals Corporation) for statistical analysis, guidance, and critical review of the report. Editorial assistance was provided by Amy Ghiretti, PhD; Allison Lytle, PhD; and Laura Hilditch, PhD (Articulate Science LLC), and was funded by Novartis Pharmaceuticals Corporation.
Eric Bouffet
Consulting or Advisory Role: Novartis
Research Funding: Roche (Inst)
Birgit Geoerger
Consulting or Advisory Role: Boehringer Ingelheim, AZD, Novartis
Christopher Moertel
Stock and Other Ownership Interests: OX2 Therapeutics
Patents, Royalties, Other Intellectual Property: Patents related to intellectual property held by OX2 Therapeutics and the University of Minnesota
James A. Whitlock
Consulting or Advisory Role: Jazz Pharmaceuticals
Research Funding: Novartis (Inst), Daiichi Sankyo (Inst), Syndax (Inst)
Isabelle Aerts
Consulting or Advisory Role: AstraZeneca
Travel, Accommodations, Expenses: Jazz Pharmaceuticals
Darren Hargrave
Honoraria: AstraZeneca/MedImmune, Bayer, Alexion Pharmaceuticals
Consulting or Advisory Role: AstraZeneca, Roche/Genentech, Novartis, Bayer, Boehringer Ingelheim
Speakers' Bureau: Alexion Pharmaceuticals
Research Funding: AstraZeneca
Expert Testimony: AstraZeneca
Travel, Accommodations, Expenses: Boehringer Ingelheim, Novartis, Roche/Genentech, Alexion Pharmaceuticals
Other Relationship: Celgene, Novartis, Bristol Myers Squibb, Epizyme, AbbVie, AstraZeneca/MedImmune, Day One Therapeutics
Lisa Osterloh
Employment: Novartis
Eugene Tan
Employment: Novartis
Stock and Other Ownership Interests: Novartis
Jeea Choi
Employment: Novartis
Mark Russo
Employment: Novartis
Stock and Other Ownership Interests: Novartis
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at the 2020 ASCO Annual Meeting, virtual, May 29-31, 2020 (abstr 10506).
SUPPORT
Supported by Novartis Pharmaceuticals Corporation. The study was designed by the academic authors in conjunction with representatives of the sponsor. Data were collected by the sponsor and analyzed and interpreted in collaboration with the authors. The sponsor was involved in the writing of the report. All authors had full access to all the data in the study and accept responsibility to submit for publication.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
Novartis is committed to sharing with qualified external researchers, access to patient-level data, and supporting clinical documents from eligible studies. Requests are reviewed and approved by an independent review panel on the basis of scientific merit. All data provided are anonymized to respect the privacy of patients who have participated in the trial in line with applicable laws and regulations. This trial data availability is according to the criteria and process described on ClinicalStudyDataRequest.com.
AUTHOR CONTRIBUTIONS
Conception and design: Eric Bouffet, Birgit Geoerger, James A. Whitlock, Darren Hargrave, Mark Russo, Elizabeth Fox
Administrative support: Eugene Tan
Provision of study materials or patients: Birgit Geoerger, Christopher Moertel, Isabelle Aerts, Elizabeth Fox
Collection and assembly of data: Eric Bouffet, Birgit Geoerger, Christopher Moertel, Isabelle Aerts, Darren Hargrave, Mark Russo, Elizabeth Fox
Data analysis and interpretation: Eric Bouffet, Birgit Geoerger, Christopher Moertel, Darren Hargrave, Lisa Osterloh, Eugene Tan, Jeea Choi, Mark Russo, Elizabeth Fox
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Efficacy and Safety of Trametinib Monotherapy or in Combination with Dabrafenib in Pediatric BRAF V600–Mutant Low-Grade Glioma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Eric Bouffet
Consulting or Advisory Role: Novartis
Research Funding: Roche (Inst)
Birgit Geoerger
Consulting or Advisory Role: Boehringer Ingelheim, AZD, Novartis
Christopher Moertel
Stock and Other Ownership Interests: OX2 Therapeutics
Patents, Royalties, Other Intellectual Property: Patents related to intellectual property held by OX2 Therapeutics and the University of Minnesota
James A. Whitlock
Consulting or Advisory Role: Jazz Pharmaceuticals
Research Funding: Novartis (Inst), Daiichi Sankyo (Inst), Syndax (Inst)
Isabelle Aerts
Consulting or Advisory Role: AstraZeneca
Travel, Accommodations, Expenses: Jazz Pharmaceuticals
Darren Hargrave
Honoraria: AstraZeneca/MedImmune, Bayer, Alexion Pharmaceuticals
Consulting or Advisory Role: AstraZeneca, Roche/Genentech, Novartis, Bayer, Boehringer Ingelheim
Speakers' Bureau: Alexion Pharmaceuticals
Research Funding: AstraZeneca
Expert Testimony: AstraZeneca
Travel, Accommodations, Expenses: Boehringer Ingelheim, Novartis, Roche/Genentech, Alexion Pharmaceuticals
Other Relationship: Celgene, Novartis, Bristol Myers Squibb, Epizyme, AbbVie, AstraZeneca/MedImmune, Day One Therapeutics
Lisa Osterloh
Employment: Novartis
Eugene Tan
Employment: Novartis
Stock and Other Ownership Interests: Novartis
Jeea Choi
Employment: Novartis
Mark Russo
Employment: Novartis
Stock and Other Ownership Interests: Novartis
No other potential conflicts of interest were reported.
REFERENCES
- 1.Tan JY, Wijesinghe IVS, Alfarizal Kamarudin MN, et al. : Paediatric gliomas: BRAF and histone H3 as biomarkers, therapy and perspective of liquid biopsies. Cancers (Basel) 13:607, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lassaletta A, Zapotocky M, Mistry M, et al. : Therapeutic and prognostic implications of BRAF V600E in pediatric low-grade gliomas. J Clin Oncol 35:2934-2941, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Penman CL, Faulkner C, Lowis SP, et al. : Current understanding of BRAF alterations in diagnosis, prognosis, and therapeutic targeting in pediatric low-grade gliomas. Front Oncol 5:54, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mistry M, Zhukova N, Merico D, et al. : BRAF mutation and CDKN2A deletion define a clinically distinct subgroup of childhood secondary high-grade glioma. J Clin Oncol 33:1015-1022, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryall S, Zapotocky M, Fukuoka K, et al. : Integrated molecular and clinical analysis of 1,000 pediatric low-grade gliomas. Cancer Cell 37:569-583 e5, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hargrave DR, Bouffet E, Tabori U, et al. : Efficacy and safety of dabrafenib in pediatric patients with BRAF V600 mutation-positive relapsed or refractory low-grade glioma: Results from a phase I/IIa study. Clin Cancer Res 25:7303-7311, 2019 [DOI] [PubMed] [Google Scholar]
- 7.Kieran MW, Geoerger B, Dunkel IJ, et al. : A phase I and pharmacokinetic study of oral dabrafenib in children and adolescent patients with recurrent or refractory BRAF V600 mutation-positive solid tumors. Clin Cancer Res 25:7294-7302, 2019 [DOI] [PubMed] [Google Scholar]
- 8.Hargrave DR, Moreno L, Broniscer A, et al. : Dabrafenib in pediatric patients with BRAF V600–positive high-grade glioma. J Clin Oncol 36:10505, 2018 [Google Scholar]
- 9.Whitlock JA, Broniscer A, Ambati SR, et al. : Dabrafenib in pediatric patients with BRAF V600–positive Langerhans cell histiocytosis. Hemasphere 2:1-1113, 2018. (suppl 1; abstr PF627) [Google Scholar]
- 10.del Bufalo F, Carai A, Figa-Talamanca L, et al. : Response of recurrent BRAFV600E mutated ganglioglioma to vemurafenib as single agent. J Transl Med 12:356, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rush S, Foreman N, Liu A: Brainstem ganglioglioma successfully treated with vemurafenib. J Clin Oncol 31:e159-e160, 2013 [DOI] [PubMed] [Google Scholar]
- 12.Upadhyaya SA, Robinson GW, Harreld JH, et al. : Marked functional recovery and imaging response of refractory optic pathway glioma to BRAFV600E inhibitor therapy: A report of two cases. Childs Nerv Syst 34:605-610, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lassaletta A, Guerreiro Stucklin A, Ramaswamy V, et al. : Profound clinical and radiological response to BRAF inhibition in a 2-month-old diencephalic child with hypothalamic/chiasmatic glioma. Pediatr Blood Cancer 63:2038-2041, 2016 [DOI] [PubMed] [Google Scholar]
- 14.Nobre L, Zapotocky M, Ramaswamy V, et al. : Outcomes of BRAF V600E pediatric gliomas treated with targeted BRAF inhibition. JCO Precis Oncol 4:561-571, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nicolaides T, Nazemi KJ, Crawford J, et al. : Phase I study of vemurafenib in children with recurrent or progressive BRAF(V600E) mutant brain tumors: Pacific Pediatric Neuro-Oncology Consortium study (PNOC-002). Oncotarget 11:1942-1952, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ascierto PA, Dreno B, Larkin J, et al. : 5-year outcomes with cobimetinib plus vemurafenib in BRAFV600 mutation-positive advanced melanoma: Extended follow-up of the coBRIM study. Clin Cancer Res 27:5225-5235, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robert C, Grob JJ, Stroyakovskiy D, et al. : Five-year outcomes with dabrafenib plus trametinib in metastatic melanoma. N Engl J Med 381:626-636, 2019 [DOI] [PubMed] [Google Scholar]
- 18.Ascierto PA, Dummer R, Gogas HJ, et al. : Update on tolerability and overall survival in COLUMBUS: Landmark analysis of a randomised phase 3 trial of encorafenib plus binimetinib vs vemurafenib or encorafenib in patients with BRAF V600-mutant melanoma. Eur J Cancer 126:33-44, 2020 [DOI] [PubMed] [Google Scholar]
- 19.Planchard D, Besse B, Groen HJM, et al. : Dabrafenib plus trametinib in patients with previously treated BRAF(V600E)-mutant metastatic non-small cell lung cancer: An open-label, multicentre phase 2 trial. Lancet Oncol 17:984-993, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MEKINIST (trametinib) [package insert]. East Hanover, NJ: Novartis Pharmaceutical Corporation; 2022 [Google Scholar]
- 21.TAFINLAR (dabrafenib) [package insert]. East Hanover, NJ: Novartis Pharmaceutical Corporation; 2022 [Google Scholar]
- 22.Wen PY, Stein A, van den Bent M, et al. : Dabrafenib plus trametinib in patients with BRAF(V600E)-mutant low-grade and high-grade glioma (ROAR): A multicentre, open-label, single-arm, phase 2, basket trial. Lancet Oncol 23:53-64, 2022 [DOI] [PubMed] [Google Scholar]
- 23.Wen PY, Chang SM, Van den Bent MJ, et al. : Response assessment in neuro-oncology clinical trials. J Clin Oncol 35:2439-2449, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wen PY, Macdonald DR, Reardon DA, et al. : Updated response assessment criteria for high-grade gliomas: Response assessment in neuro-oncology working group. J Clin Oncol 28:1963-1972, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Falchook GS, Long GV, Kurzrock R, et al. : Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: A phase 1 dose-escalation trial. Lancet 379:1893-1901, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Infante JR, Fecher LA, Falchook GS, et al. : Safety, pharmacokinetic, pharmacodynamic, and efficacy data for the oral MEK inhibitor trametinib: A phase 1 dose-escalation trial. Lancet Oncol 13:773-781, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Robert C, Flaherty K, Nathan P, et al. : Five-year outcomes from a phase 3 METRIC study in patients with BRAF V600 E/K-mutant advanced or metastatic melanoma. Eur J Cancer 109:61-69, 2019 [DOI] [PubMed] [Google Scholar]
- 28.Long GV, Hauschild A, Santinami M, et al. : Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N Engl J Med 377:1813-1823, 2017 [DOI] [PubMed] [Google Scholar]
- 29.Long GV, Stroyakovskiy D, Gogas H, et al. : Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: A multicentre, double-blind, phase 3 randomised controlled trial. Lancet 386:444-451, 2015 [DOI] [PubMed] [Google Scholar]
- 30.Egan G, Hamilton J, McKeown T, et al. : Trametinib toxicities in patients with low-grade gliomas and diabetes insipidus: Related findings? J Pediatr Hematol Oncol 42:e248-e250, 2020 [DOI] [PubMed] [Google Scholar]
- 31.Bouffet E, Hansford JR, Garre ML, et al. : Primary analysis of a phase II trial of dabrafenib + trametinib in BRAD V600-mutant pediatric low-grade glioma. J Clin Oncol 40, 2022. (suppl 17; abstr LBA2002) [Google Scholar]
- 32.Marks AM, Bindra RS, DiLuna ML, et al. : Response to the BRAF/MEK inhibitors dabrafenib/trametinib in an adolescent with a BRAF V600E mutated anaplastic ganglioglioma intolerant to vemurafenib. Pediatr Blood Cancer 65:e26969, 2018 [DOI] [PubMed] [Google Scholar]
- 33.Kaley T, Touat M, Subbiah V, et al. : BRAF inhibition in BRAF(V600)-mutant gliomas: Results from the VE-BASKET study. J Clin Oncol 36:3477-3484, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schreck KC, Grossman SA, Pratilas CA: BRAF mutations and the utility of RAF and MEK inhibitors in primary brain tumors. Cancers (Basel) 11:1262, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harder A: MEK inhibitors—Novel targeted therapies of neurofibromatosis associated benign and malignant lesions. Biomark Res 9:26, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fangusaro J, Onar-Thomas A, Young Poussaint T, et al. : Selumetinib in paediatric patients with BRAF-aberrant or neurofibromatosis type 1-associated recurrent, refractory, or progressive low-grade glioma: A multicentre, phase 2 trial. Lancet Oncol 20:1011-1022, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Novartis is committed to sharing with qualified external researchers, access to patient-level data, and supporting clinical documents from eligible studies. Requests are reviewed and approved by an independent review panel on the basis of scientific merit. All data provided are anonymized to respect the privacy of patients who have participated in the trial in line with applicable laws and regulations. This trial data availability is according to the criteria and process described on ClinicalStudyDataRequest.com.