PURPOSE
Brexucabtagene autoleucel (KTE-X19) autologous anti-CD19 chimeric antigen receptor (CAR) T-cell therapy is approved for the treatment of relapsed/refractory mantle cell lymphoma (MCL). Outcomes after a 3-year follow-up in the pivotal ZUMA-2 study of KTE-X19 in relapsed/refractory MCL are reported, including for subgroups by prior therapy (bendamustine and type of Bruton tyrosine kinase inhibitor [BTKi]) or high-risk characteristics.
METHODS
Patients with relapsed/refractory MCL (one to five prior therapies, including prior BTKi exposure) received a single infusion of KTE-X19 (2 × 106 CAR T cells/kg).
RESULTS
After a median follow-up of 35.6 months, the objective response rate among all 68 treated patients was 91% (95% CI, 81.8 to 96.7) with 68% complete responses (95% CI, 55.2 to 78.5); medians for duration of response, progression-free survival, and overall survival were 28.2 months (95% CI, 13.5 to 47.1), 25.8 months (95% CI, 9.6 to 47.6), and 46.6 months (95% CI, 24.9 to not estimable), respectively. Post hoc analyses showed that objective response rates and ongoing response rates were consistent among prespecified subgroups by prior BTKi exposure or high-risk characteristics. In an exploratory analysis, patients with prior bendamustine benefited from KTE-X19, but showed a trend toward attenuated T-cell functionality, with more impact of bendamustine given within 6 versus 12 months of leukapheresis. Late-onset toxicities were infrequent; only 3% of treatment-emergent adverse events of interest in ZUMA-2 occurred during this longer follow-up period. Translational assessments revealed associations with long-term benefits of KTE-X19 including high-peak CAR T-cell expansion in responders and the predictive value of minimal residual disease for relapse.
CONCLUSION
These data, representing the longest follow-up of CAR T-cell therapy in patients with MCL to date, suggest that KTE-X19 induced durable long-term responses with manageable safety in patients with relapsed/refractory MCL and may also benefit those with high-risk characteristics.
INTRODUCTION
Despite recent therapeutic advances in mantle cell lymphoma (MCL), most treatments provide limited duration responses, indicating high unmet need for novel therapies.1-7 In patients who discontinue the Bruton tyrosine kinase inhibitor (BTKi) ibrutinib because of progressive disease or intolerance, reports indicate that the median overall survival (OS) ranges from 2.5 to 14.2 months.8-12 MCL prognosis depends on MCL risk factors, with important high-risk factors including blastoid variant,13,14 high Ki-67 proliferation index (PI),15 tumor protein p53 gene (TP53) mutation16 or high P53 expression,17 and disease progression within 24 months after initial diagnosis (POD24).18,19 Patients with these characteristics have limited treatment options and poor outcomes, with a median OS of 6.6 months to 4 years after initial therapy7,18-20 In addition, treatments in previous lines may affect outcomes with subsequent therapies; for example, bendamustine-containing treatments may be associated with reduced T-cell number and function, potentially affecting cellular therapies.21
CONTEXT
Key Objective
Relapsed/refractory mantle cell lymphoma (MCL) remains an area of high unmet need despite availability of novel therapies like Bruton tyrosine kinase inhibitors (BTKis). Survival times are particularly low for patients who discontinue BTKis. This study examined long-term outcomes after a single infusion of the chimeric antigen receptor T-cell therapy brexucabtagene autoleucel (KTE-X19) in patients with relapsed/refractory MCL with prior BTKi exposure, including those with high-risk characteristics. These 3-year follow-up data comprise the longest follow-up for chimeric antigen receptor T-cell therapy in patients with MCL to date.
Knowledge Generated
Our findings demonstrate durable responses and sustained survival with a manageable safety profile for KTE-X19 in patients with relapsed/refractory MCL. These findings were also seen in patients within prespecified subgroups, such as high-risk characteristics or prior therapies.
Relevance
Durable outcomes suggest that the use of KTE-X19 in earlier lines of treatment may be beneficial for patients with relapsed/refractory MCL, including those with high-risk characteristics.
ZUMA-2 (ClinicalTrials.gov identifier: NCT02601313) is a pivotal, single-arm, multicenter, phase II trial of the autologous anti-CD19 chimeric antigen receptor (CAR) T-cell therapy brexucabtagene autoleucel (KTE-X19) in patients with heavily pretreated MCL that was relapsed/refractory to one to five prior therapies, including a BTKi (ibrutinib or acalabrutinib).22 With a median follow-up of 12.3 months, the primary efficacy analysis demonstrated a 93% objective response rate (ORR) by an independent radiologic review committee (IRRC), including a 67% complete response (CR) rate.22 On the basis of these results, KTE-X19 was approved in the United States and Europe for the treatment of adults with relapsed/refractory MCL.10,23
Here, long-term efficacy, safety, and pharmacology of KTE-X19 are reported in patients with relapsed/refractory MCL with a median follow-up of 35.6 months in ZUMA-2. In addition, post hoc analyses of outcomes and the pharmacologic and pharmacodynamic profile of KTE-X19 in subgroups by prior bendamustine, prior BTKi exposure type, and high-risk disease characteristics are reported, accompanied by product attribute characterization.
METHODS
Patients and Study Design
Detailed study procedures for the pivotal, multicenter, single-arm ZUMA-2 study (ClinicalTrials.gov identifier: NCT02601313) have been reported (Data Supplement, online only).22 Briefly, patients (≥ 18 years) had histologically confirmed MCL that was relapsed/refractory to one to five prior regimens. Patients must have received prior anthracycline-containing or bendamustine-containing chemotherapy, an anti-CD20 monoclonal antibody, and BTKi therapy (ibrutinib or acalabrutinib). The trial was conducted in accordance with the principles of the Declaration of Helsinki, the Protocol (online only) was approved by each site's institutional review board, and all patients provided written informed consent.
After leukapheresis and before conditioning therapy, patients with high disease burden could receive bridging therapy with steroids or BTKi at investigators' discretion.22 Conditioning chemotherapy (fludarabine 30 mg/m2 once a day; cyclophosphamide 500 mg/m2 once a day) was administered intravenously on days –5, –4, and –3. On day 0, KTE-X19 was given as a single intravenous infusion (target dose: 2 × 106 CAR T cells/kg).
End Points and Assessments
The primary end point was ORR (partial response [PR] or CR) as assessed by IRRC (Lugano classification).22,24 Secondary end points included duration of response (DOR), progression-free survival (PFS), OS, adverse event (AE) incidence, blood CAR T-cell levels, and serum cytokine levels. Minimal residual disease (MRD) was assessed as an exploratory end point using next-generation sequencing (sensitivity: 1 in 105 cells), as previously described (Data Supplement).22 Post hoc assessment of subgroups was performed on the basis of prognostic features, including MCL morphology (classical, blastoid, or pleomorphic), Ki-67 PI (< 30%, ≥ 30%, < 50%, and ≥ 50%), TP53 mutation (present or not [next-generation sequencing]), POD24 status (yes/with or no/without), MRD status at month 6 (positive v negative), and prior BTKi exposure (ibrutinib, acalabrutinib, or both). An exploratory analysis examined the impact of timing of prior bendamustine exposure.
Statistical Analysis
End points are reported in all patients treated with any dose of CAR T cells (all-treated population). The intention-to-treat (ITT) population comprised all enrolled (leukapheresed) patients. Time-to-event end points were analyzed using the Kaplan-Meier method. Comparisons across subgroups used the Kruskal-Wallis test; if significant, Dunn's post hoc test was used to compare between groups. Further statistical analysis details are given in the Data Supplement.
Propensity Score Matching to Examine Impact of Prior Bendamustine Use
Exploratory post hoc propensity score matching25,26 was performed to descriptively compare results among patients on the basis of prior bendamustine use after balancing for key characteristics: age, baseline tumor burden, Eastern Cooperative Oncology Group performance status, simplified MCL international prognostic index score, number of prior lines of chemotherapy, prior acalabrutinib, prior ibrutinib, and POD24 status. Four subgroup comparisons were performed: no use before leukapheresis versus use within 6 months, > 6 months, within 12 months, and > 12 months.26
RESULTS
Patients
As previously reported, 74 patients were enrolled and leukapheresed.22 KTE-X19 was successfully manufactured for 71 patients (96%); 68 received KTE-X19. As of July 24, 2021 (data cutoff), the median follow-up was 35.6 (range, 25.9-56.3) months. Baseline characteristics of the all-treated and ITT populations have been reported.22 High-risk features were common. Baseline characteristics between subgroups are described in the Data Supplement.
Updated Analysis in the All-Treated Population
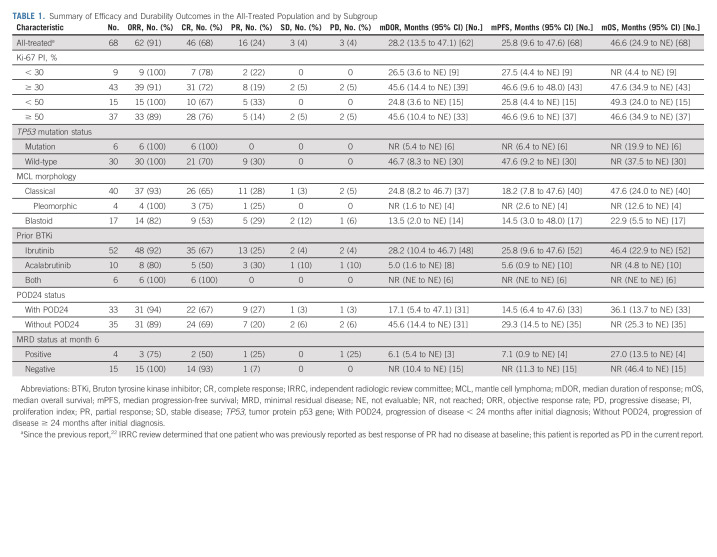
The ORR (IRRC) in the all-treated population was 91% (95% CI, 81.8 to 96.7); CR and PR rates were 68% (95% CI, 55.2 to 78.5) and 24% (95% CI, 14.1 to 35.4), respectively (Table 1). Twenty-five patients converted to CR after initial stable disease or PR (median time to response conversion, 2.3 months). Responses were durable; the median DOR was 28.2 months among the 62 responders (Fig 1A). At data cutoff, 37% of treated patients remained in ongoing response (all CR). Thirteen patients relapsed after month 6. The median DOR was 46.7 months among patients with CR (n = 46) and 2.2 months in patients with PR (n = 16). In the first 28 patients treated (median follow-up, 51.1 months), the median DOR was 36.5 months in the 26 responders; 29% remained in ongoing response.
TABLE 1.
Summary of Efficacy and Durability Outcomes in the All-Treated Population and by Subgroup
FIG 1.
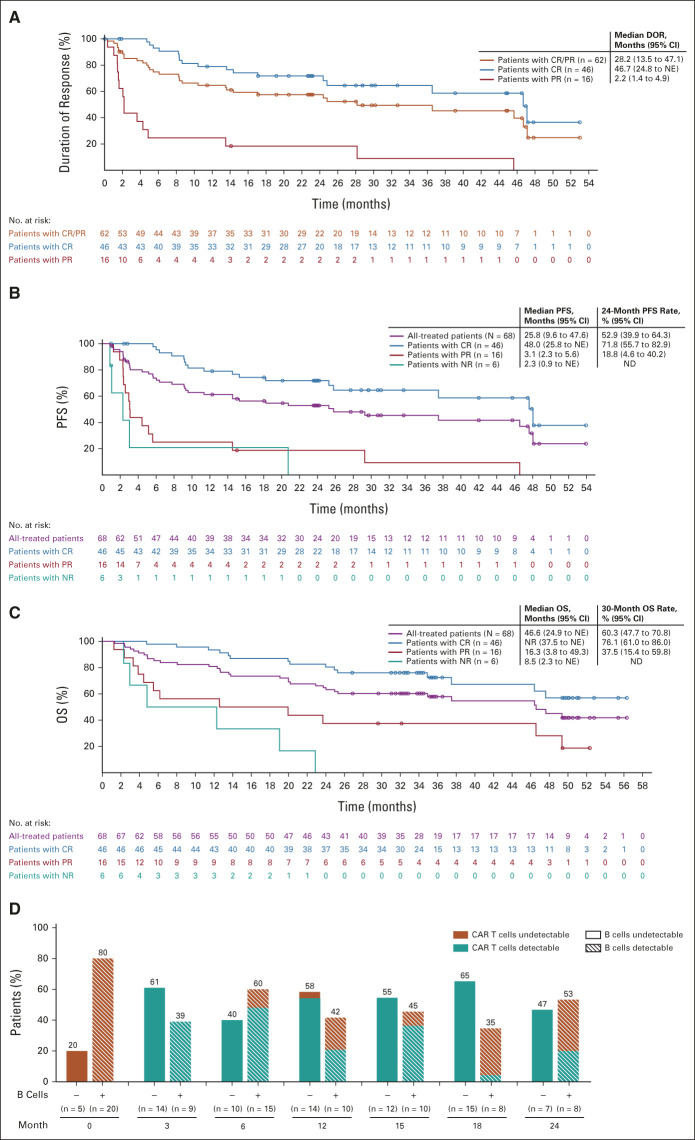
Outcomes for patients in the all-treated population (N = 68) stratified by response and CAR T-cell persistence and B-cell aplasia over time in patients with ongoing response. Responses were assessed by an independent radiologic review committee. (A) DOR. (B) PFS. (C) OS. (D) The proportion of patients with or without detectable B cells and with or without detectable anti-CD19 CAR T cells are shown for all patients in ongoing response at data cutoff. CAR, chimeric antigen receptor; CR, complete response; DOR, duration of response; ND, no data; NE, not estimable; NR, no response; OS, overall survival; PFS, progression-free survival; PR, partial response.
The median PFS in the all-treated population was 25.8 months (Fig 1B). In patients whose best response was CR, PR, and no response, the median PFS was 48.0, 3.1, and 2.3 months, respectively. With 58 of 62 responding patients (94%) achieving response at the postinfusion week 4 visit, PFS by response type was also assessed by a landmark analysis at the week 4 visit, which yielded a similar trend; the median PFS in patients with CR, PR, and no response at the landmark was 46.7, 13.6, and 11.0 months, respectively (Data Supplement). Cumulative incidence of relapse analysis during follow-up confirmed that patients whose best response was CR were less likely to relapse than those with PR or no response (P < .0001; Data Supplement). Only three patients relapsed past 24 months. The median OS in the all-treated population was 46.6 months (Fig 1C). In patients whose best response was CR, PR, and no response, the median OS was not reached (NR), 16.3, and 8.5 months, respectively.
Of 19 MRD-assessable patients at month 6, 15 (79%) were MRD-negative, with an ORR of 100%. Of the four MRD-positive patients (21%), two achieved CR, one achieved PR, and one had progressive disease. At data cutoff, DOR, PFS, and OS medians among the MRD-positive patients were 6.1, 7.1, and 27.0 months, respectively (Table 1). By contrast, among MRD-negative patients, DOR, PFS, and OS medians were NR at data cutoff; 60% remained in ongoing response.
In the ITT population, the ORR was 84% (95% CI, 73.4 to 91.3), with a 62% CR rate (95% CI, 50.1 to 73.2) and a 22% PR rate (95% CI, 12.9 to 32.7). The median PFS was 24.0 months, and the median OS was 47.4 months (24-month PFS rate, 49%; 30-month OS rate, 56%).
No new safety signals were observed in ZUMA-2 with longer follow-up (Data Supplement). Only 3% of all treatment-emergent AEs of interest occurred since the previous data cutoff,22 with any-grade AEs in 18 patients (26%) and grade ≥ 3 in 14 (21%). The most frequent grade ≥ 3 AE of interest was neutropenia (one [1%] grade 3 and seven [10%] grade 4). Grade ≥ 3 serious AEs occurred in seven patients (10%; Data Supplement), including one patient with grade 3 encephalopathy unrelated to KTE-X19 and two patients with KTE-X19–related serious AEs: one with grade 3 pneumonia and grade 3 upper respiratory tract infection and one with grade 3 influenza, indicating that infectious disease might have been observed with longer follow-up. Since the previous analysis, no patients experienced cytokine release syndrome (CRS) or tumor lysis syndrome. Three new fatal AEs occurred (none considered KTE-X19–related): salmonella bacteremia (beginning 24.9 months postinfusion) and two secondary malignancies (myelodysplastic syndrome and acute myeloid leukemia; beginning 25.2 and 37.5 months postinfusion, respectively). There were no KTE-X19–related secondary malignancies or replication-competent retrovirus cases. Twenty-six patients (38%) received intravenous immunoglobulin.
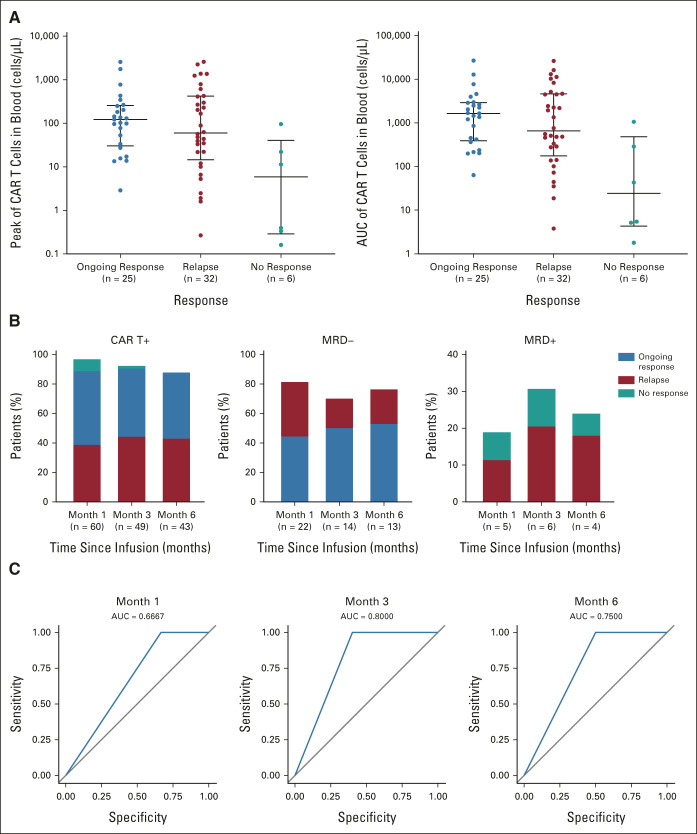
CAR T-cell levels peaked at a median of 15 days postinfusion.22 Median CAR T-cell levels plateaued at 0.19-0.34 cells/μL from months 6 to 18 (Data Supplement). Peak CAR T-cell expansion was highest in patients with ongoing responses versus those who relapsed at 24 months or nonresponding patients (Fig 2A). Among evaluable patients in ongoing response at months 18 and 24, B cells were detectable in 35% and 53% and gene-marked CAR T cells were detectable in 70% and 67%, respectively (Fig 1D). In contrast to CAR T-cell detectability, MRD negativity at months 1, 3, and 6 was associated with durable response (Fig 2B). Area under the receiver operating characteristic analysis further demonstrated the pronounced predictive performance of MRD measured at months 3 and 6 in estimating relapse potential (Fig 2C). Of six patients who were MRD-positive at month 3, four relapsed and two did not respond. Among the four patients who relapsed, relapses occurred at 2.6, 3.1, 5.2, and 6.5 months. Of the four patients who were MRD-positive at month 6, two were MRD-positive at month 3; they relapsed at 6.5 and 29.7 months, respectively.
FIG 2.
CAR T-cell persistence over time by response at data cutoff in patients who had evaluable samples: (A) peak CAR T-cell expansion and AUC (horizontal lines represent the median and interquartile range); (B) frequency of patients with ongoing response, relapse, or no response, by CAR presence (CAR T+), MRD negativity (MRD–), and MRD positivity (MRD+); and (C) ROC curves of true-positive (sensitivity) versus false-positive (specificity) rates for MRD predictability at months 1, 3, and 6 of relapse and nonresponse. AUC, area under the curve; CAR, chimeric antigen receptor; MRD, minimal residual disease; ROC, receiver operating characteristic.
In the six patients reported to have grade 4 neurologic events (NEs),22 three with concurrent grade 4 CRS, significantly higher peak serum levels of interferon gamma (IFN-γ), tumor necrosis factor alpha, monocyte chemoattractant protein-1, interleukin (IL)-2, and IL-6 were observed versus those without NEs, with lack of reversion to baseline levels of IL-6 by day 28 (Data Supplement).
Impact of Prior BTKi Exposure
Among patients treated with prior ibrutinib (n = 52), prior acalabrutinib (n = 10), and both (n = 6), the ORR was 92%, 80%, and 100%, respectively (Table 1). DOR, PFS, and OS medians (Table 1 and the Data Supplement) and safety (Data Supplement) in each subgroup were largely similar to those of the all-treated population. Higher peak CAR T-cell levels and area under the curve (AUC) were found in patients who had received ibrutinib only versus acalabrutinib only (Data Supplement). Peak immunomodulatory and proinflammatory cytokine levels trended higher in patients with prior ibrutinib versus those with prior acalabrutinib, particularly for IFN-γ and IL-6 (Data Supplement).
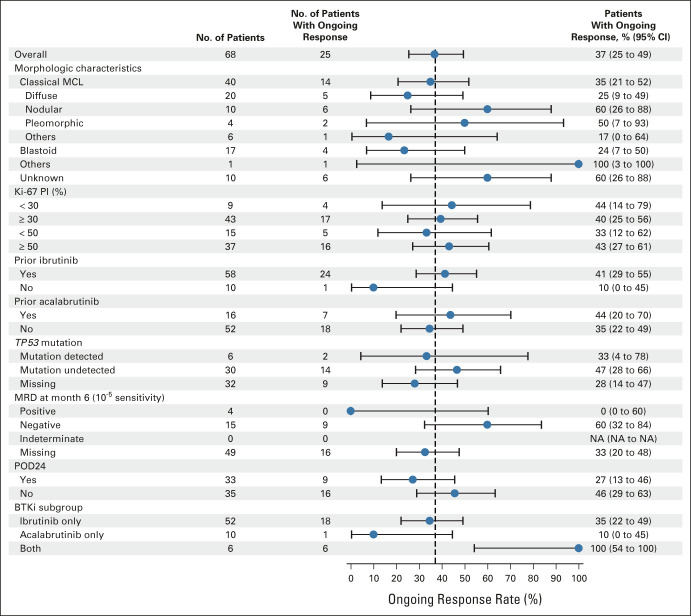
Impact of High-Risk Disease Characteristics
In high-risk subgroups, ORRs were generally consistent (Table 1 and the Data Supplement); ongoing responses at data cutoff are reported in Figure 3. The median DOR exceeded 24 months or was NR in most subgroups and was 17.1 months in patients with POD24. PFS and OS medians were similar across most subgroups but trended lower among patients with POD24 (Table 1 and the Data Supplement). Some differences were noted with blastoid MCL or TP53 mutation although patient numbers were limited.
FIG 3.
Subgroup analysis of ongoing response in the all-treated population (N = 68). BTKi, Bruton tyrosine kinase inhibitor; MCL, mantle cell lymphoma; MRD, minimal residual disease; NA, not available; PI, proliferation index; POD24, progression of disease < 24 months after initial diagnosis; TP53, tumor protein p53 gene.
Any-grade and grade ≥ 3 AE rates were similar across subgroups (Data Supplement). Grade ≥ 3 NEs were numerically higher in patients with Ki-67 PI < 30% versus those with Ki-67 PI ≥ 30% (67% v 30%) and in patients with classical versus blastoid morphology (38% v 18%). Grade ≥ 3 CRS was numerically higher in patients with versus without TP53 mutation (33% v 7%) although numbers are small.
Peak CAR T-cell levels in blood were comparable between Ki-67 PI < 50% and Ki-67 PI ≥ 50% groups (Data Supplement) and between TP53-mutated and wild-type groups (Data Supplement). Patients with POD24 trended toward lower median peak CAR T-cell levels (53.4 cells/μL [range, 0.2-2,566] v 112.4 cells/μL [range, 0.2-2,589]) and median AUC values (583.4 cells/μL × day [range, 1.8-27,744] v 1,588.3 cells/μL × day [range, 3.8-27,239]) than those without POD24 (Data Supplement), as did patients with Ki-67 PI < 30% versus those with Ki-67 ≥ 30% (Data Supplement). Patients with a blastoid morphology had slightly lower median peak CAR T-cell and AUC levels than those with a classical or pleomorphic morphology, suggesting that inflammatory characteristics of blastoid MCL may affect robust CAR T-cell expansion (Data Supplement). Pharmacodynamic profiles in high-risk subgroups and pharmacokinetic/pharmacodynamic profiles in BTKi subgroups are provided in the Data Supplement.
KTE-X19 T-cell memory phenotypes in the product were generally comparable in the all-treated population and across prognostic subgroups (Data Supplement). In line with findings that robust CAR T-cell products yielding higher expansion potential and improved treatment outcomes harbor more naive T-cell populations,27 a trend was observed where higher numbers of total infused naive (CCR7+CD45RA+) or naive and central memory (CCR7+CD45RA–) T cells were associated with ongoing response in patients without POD24 versus those with POD24 (Data Supplement). Similarly, trends toward fewer terminally differentiated effector T-cell phenotypes were observed in the Ki-67 PI < 30% subgroup.
Impact of Timing of Prior Bendamustine Exposure
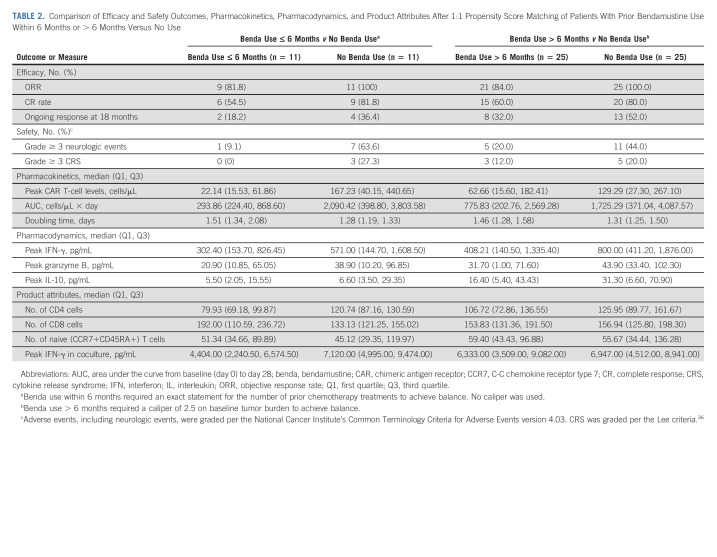
More than half of treated patients (n = 37 [54%]) received prior bendamustine,22 and strong outcomes continued to be observed in the overall population (Table 1). The median time from last exposure to KTE-X19 infusion was 20.9 months (range, 1.0-70.3 months; Data Supplement). In those with and without prior bendamustine, the ORR was 84% (CR rate, 58%) and 100% (CR rate, 77%), respectively. At data cutoff, 29% and 48% of patients, respectively, remained in ongoing response. In patients with and without prior bendamustine, the median DOR was 28.2 months and 46.7 months, respectively, but the two DOR curves were not statistically significantly different (Data Supplement). Given reports of the potential for bendamustine-containing treatments to reduce T-cell number and function21 and the frequent use of bendamustine in MCL,4 we conducted an exploratory, hypothesis-testing, post hoc evaluation of the impact of timing of prior bendamustine exposure on KTE-X19 in a small subset of patients. Data regarding cumulative prior bendamustine doses for patients were not available. Patients with prior bendamustine within 6 months of apheresis had lower peak CAR T-cell levels postinfusion versus patients with prior bendamustine more than 6 months preapheresis or corresponding patients without prior bendamustine (in matched patients [Table 2] and in all patients [Fig 4]). Patients with prior bendamustine within 6 months had lower numbers of CD4+ T cells in product, levels of peak effector serum biomarkers, doubling time, and incidence of grade ≥ 3 CRS and NEs. These trends were not pronounced for patients with prior bendamustine within 12 months (Data Supplement). The observations from this small exploratory post hoc analysis may indicate that patients could benefit from longer time spans between prior bendamustine and cell therapy.
TABLE 2.
Comparison of Efficacy and Safety Outcomes, Pharmacokinetics, Pharmacodynamics, and Product Attributes After 1:1 Propensity Score Matching of Patients With Prior Bendamustine Use Within 6 Months or > 6 Months Versus No Use
FIG 4.
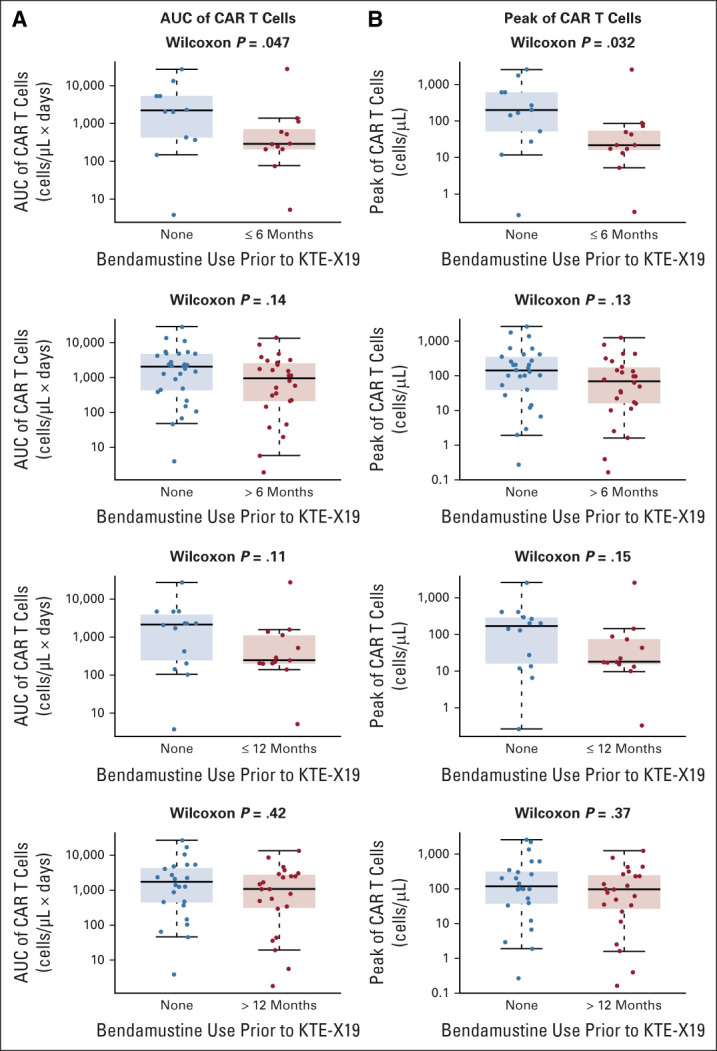
Comparison of (A) AUC and (B) peak of CAR T-cell pharmacokinetics in all evaluable patients with prior bendamustine use (within 6 months, > 12 months, within 12 months, or > 12 months) versus no use. The median is represented by the horizontal line within each box, and the 25th and the 75th percentiles are represented by the lower and upper borders of each box. AUC, area under the curve; benda, bendamustine; CAR, chimeric antigen receptor.
DISCUSSION
After nearly a 3-year follow-up (median, 35.6 months) for patients in ZUMA-2, ORR with KTE-X19 in patients with relapsed/refractory MCL remained consistently high at 91% (68% CR). Responses were durable, with a median DOR of 28.2 months; 37% of treated patients had ongoing responses (all CR), and late relapse potential was low, with only three patients relapsing past 24 months. Despite patients being predominantly high-risk and heavily pretreated,22 the median OS was 46.6 months. Among MRD-assessable patients at month 6, 79% were MRD-negative, with an ORR of 100%. Three of the MRD-assessable patients relapsed around 6 months and all were MRD-positive, suggesting that MRD monitoring past 6 months is important and could facilitate the prediction of late relapse. In addition, DOR, PFS, and OS were NR in patients with MRD negativity at 6 months, suggesting that MRD negativity may predict for a longer response duration, although sample size was limited. Long-term safety was manageable, with only 3% of AEs of interest occurring during this longer follow-up, few late-onset events, and no new CRS.
Response and OS benefits were favorable regardless of the prior BTKi type. Ongoing efficacy trended lower in patients with prior acalabrutinib exposure. Although small sample sizes limit interpretation, the observed differences may reflect increased CAR T-cell differentiation and sustained effector function with ibrutinib.28,29 The mechanistic basis for this difference remains under investigation and is hypothesized to be attributed to ibrutinib-mediated Th2-cell suppression and polarization toward Th1 phenotypes.29-31
Subgroups defined by MCL morphology, Ki-67 PI, TP53 mutation, or POD24 drew clinical benefit from KTE-X19, with ongoing response rates generally comparable with the all-treated population. Blastoid morphology is known to be an important pretreatment risk determinant in MCL.32 Despite relatively lower postinfusion CAR T-cell levels in patients with blastoid MCL, the median OS was 22.9 months, which appears to be favorable in the context of median OS reported in the literature for this subgroup receiving other therapies (eg, 12.8 months with ibrutinib33 and 14.5 months with chemotherapy13). Similarly, a 94% ORR and a 67% CR rate were observed in patients with POD24 who typically have poorer prognoses, despite lower CAR T-cell expansion and higher proinflammatory cytokine levels, including IL-6 and ferritin. A noteworthy limitation of these data is that many subgroups evaluated contain low patient numbers and should be considered exploratory. Further investigation is warranted, but these preliminary findings suggest that KTE-X19 might have the potential to provide meaningful benefit to patients with high-risk disease, including those with POD24, who have few effective treatment options.
Bendamustine-containing treatments are a standard in MCL management.4 In ZUMA-2, KTE-X19 was successfully manufactured for 96% of patients and administered to 92%, of whom 54% had received prior bendamustine.22 Here, 91% of all-treated patients experienced objective response (68% with CR), clearly demonstrating the clinical benefit of KTE-X19. Although sample sizes were small in this exploratory analysis, a poorer pharmacokinetic profile and reduced product doubling time with bendamustine use within 6 months of apheresis were observed. This observation is consistent with other analyses suggesting attenuated T-cell fitness among patients with B-cell hematologic malignancies, including MCL, after exposure to bendamustine and rituximab.4,21,34 The impact on CAR T-cell expansion was less pronounced with extended time between bendamustine exposure and apheresis. Although the generalizability of our analysis was limited by the small numbers of patients and absence of cumulative bendamustine dose data, our findings suggest that bendamustine use shortly before leukapheresis requires careful consideration because of its effects on patient T-cell fitness and potential impact on CAR T-cell expansion. Although patients with prior bendamustine had similar outcomes as the overall ZUMA-2 population, to maximize the benefit of KTE-X19, a potentially curative therapy, it may be advantageous to consider administering KTE-X19 before or in place of bendamustine-containing treatments. Further analyses are warranted to better elucidate the influence of bendamustine on cell therapy in relapsed/refractory MCL.
KTE-X19 demonstrated a manageable toxicity profile with no new safety signals in this long-term analysis. With longer follow-up, AE rates markedly decreased; only 3% of all treatment-emergent AEs of interest reported in ZUMA-2 occurred since the previous report.22 The three new fatal AEs (one salmonella bacteremia and two secondary malignancies [myelodysplastic syndrome and acute myeloid leukemia]) occurred > 2 years after KTE-X19 infusion and were not considered KTE-X19–related. The observation of the KTE-X19–related infectious events of pneumonia and upper respiratory tract infection in one patient and influenza in another patient (all grade 3) may be reflective of compromised immunity in the study population related to B-cell aplasia and previous therapies.
CAR T-cell levels persisted at varying levels through long-term follow-up, and this persistence was coupled with B-cell recovery in most patients. At 24 months, CAR T cells were detectable in 11 of the 25 ongoing responders (44%), supporting previous reports that long-term persistence of functional CAR T cells is not required for durable responses.22,35
In summary, these longer-term ZUMA-2 data demonstrate that a single infusion of KTE-X19 resulted in high rates of durable responses in relapsed/refractory MCL across patients with high-risk disease characteristics, with manageable long-term safety. Collectively, these findings confirm the durable benefits of KTE-X19 and support future investigations of CD19-directed CAR T-cell therapy in patients with high-risk MCL in earlier treatment lines.
ACKNOWLEDGMENT
We thank the patients who participated in this trial and their families, caregivers, and friends and the study investigators, coordinators, and health care staff at each study site. We also thank Anne Kerber, formerly employed by Kite, a Gilead Company, for her contributions to the study and the manuscript. We thank Saran Vardhanabhuti for his contribution to the bendamustine analyses. Medical writing support was provided by Nexus Global Group Science, funded by Kite, a Gilead Company.
Michael Wang
Honoraria: Janssen Research & Development, Dava Oncology, OM Pharmaceutical Industries, AstraZeneca, CAHON, Hebei Cancer Prevention Federation, Mumbai Hematology Group, Acerta Pharma, Chinese Anti-Cancer Association, BeiGene, Chinese Medical Association, Clinical Care Options, Epizyme, Imedex, Kite, a Gilead company, Miltenyi Biomedicine, Moffit Cancer Center, Newbridge Pharmaceuticals, Physicans' Education Resource, Scripps Health, The First Affiliated Hospital of Zhejiang University, Breast-Gynecological International Cancer Society, Pharmacyclics/Janssen
Consulting or Advisory Role: AstraZeneca, Janssen Research & Development, Juno Therapeutics, Bioinvent, Pharmacyclics/Janssen, Pulse Biosciences, Guidepoint Global, Loxo, Kite, a Gilead company, InnoCare, Oncternal Therapeutics, CStone Pharmaceuticals, Genentech, Bayer, BeiGene, DTRM, Epizyme, Miltenyi Biomedicine, VelosBio
Research Funding: AstraZeneca, Janssen Research & Development, Pharmacyclics, Kite, a Gilead company, Juno Therapeutics, BeiGene, Acerta Pharma, Oncternal Therapeutics, Bioinvent, Loxo, VelosBio, Celgene, Molecular Templates, Lilly, InnoCare
Travel, Accommodations, Expenses: Janssen Research & Development, AstraZeneca, Celgene, Dava Oncology, OM Pharmaceutical Industries
Javier Munoz
Honoraria: Kyowa Hakko Kirin, Seattle Genetics, Targeted Oncology, Onc view, Curio Science, Physicans' Education Resource
Consulting or Advisory Role: Kite, a Gilead company, Pfizer, Pharmacyclics, Bayer, Alexion Pharmaceuticals, Bristol Myers Squibb, Janssen, Seattle Genetics, Gilead Sciences, Kyowa Hakko Kirin, Juno Therapeutics, Genentech, Celgene, BeiGene, Fosun Kite, Innovent Biologics, Debiopharm Group, Karyopharm Therapeutics, Genmab, ADC Therapeutics, Epizyme, Servier, Novartis, MorphoSys
Speakers' Bureau: Kite, a Gilead company, Bayer, Pharmacyclics/Janssen, AstraZeneca, Gilead Sciences, Seattle Genetics, Kyowa Hakko Kirin, Acrotech Biopharma, BeiGene, BeiGene, Verastem, Celgene, AbbVie/Genentech
Research Funding: Kite, a Gilead company, Celgene, Portola Pharmaceuticals, Incyte, Genentech/AbbVie, Pharmacyclics/Janssen, Seattle Genetics, Millennium
Andre Goy
Employment: Regional Cancer Care Associates, OM Pharmaceutical Industries
Leadership: COTA, Genomic Testing Cooperative, Resilience Care
Stock and Other Ownership Interests: COTA, Genomic Testing Cooperative, Resilience Care
Consulting or Advisory Role: Elsevier, Kite, a Gilead company (Inst), AstraZeneca, Gilead Sciences, Resilience Care, OncLive Peer Exchange, Physicans' Education Resource, Janssen, Vincera Pharma/Vincerx Pharma
Speakers' Bureau: Bristol Myers Squibb/Celgene
Research Funding: Kite/Gilead (Inst), Acerta Pharma (Inst), AstraZeneca (Inst), Celgene (Inst), Constellation Pharmaceuticals (Inst), Genentech/Roche (Inst), Infinity Pharmaceuticals (Inst), Infinity/Verastem (Inst), Janssen (Inst), Karyopharm Therapeutics (Inst), Pharmacyclics (Inst)
Expert Testimony: AstraZeneca
Travel, Accommodations, Expenses: Physicans' Education Resource
Other Relationship: AstraZeneca, MorphoSys/Incyte
Frederick L. Locke
Consulting or Advisory Role: Novartis, Celgene, Calibr, Alimera Sciences, Cellular Biomedicine Group, Gerson Lehrman Group, EcoR1 Capital, Amgen, Bluebird Bio, Bristol Myers Squibb, Iovance Biotherapeutics, Legend Biotech, Cowen, Kite, a Gilead company, Umoja Biopharma, Takeda, Sana Biotechnology, Daiichi Sankyo/UCB Japan, Bristol Myers Squibb/Celgene, Janssen
Research Funding: Kite, a Gilead company (Inst), Alimera Sciences (Inst), Novartis (Inst), Bluebird Bio (Inst), Bristol Myers Squibb/Celgene (Inst)
Patents, Royalties, Other Intellectual Property: Double Mutant Survivin Vaccine. US010414810B2 (Inst), CAR T Cells With Enhanced Metabolic Fitness. Serial Number: 62/939,727 (Inst), Methods of Enhancing CAR T Cell Therapies. Serial Number: 62/892,292 (Inst), Evolutionary Dynamics of Non-Hodgkin Lymphoma CAR-T cell therapy. Serial Number: 62/879,534 (Inst)
Travel, Accommodations, Expenses: Kite, a Gilead company
Caron A. Jacobson
Honoraria: Kite/Gilead, Novartis, Celgene, Bristol Myers Squibb, Bluebird Bio, Epizyme, Instill Bio, Lonza, Ipsen, Abintus Bio
Consulting or Advisory Role: Kite/Gilead, Novartis, Celgene, Bristol Myers Squibb, Lonza, Ipsen, Instill Bio, Bluebird Bio, Epizyme, Abintus Bio
Research Funding: Pfizer, Kite, a Gilead company
Brian T. Hill
Honoraria: Pharmacyclics, Gilead Sciences, Genentech, AbbVie, Bayer, AstraZeneca, Novartis, Pfizer, Celgene, Karyopharm Therapeutics, Epizyme, BeiGene, Novartis, MorphoSys
Consulting or Advisory Role: Novartis, Genentech, AbbVie, Gilead Sciences, Karyopharm Therapeutics, AstraZeneca, Epizyme, MorphoSys, BeiGene
Research Funding: AbbVie (Inst), Karyopharm Therapeutics (Inst), Celgene (Inst), Takeda (Inst), Amgen (Inst), Genentech (Inst), Kite/Gilead (Inst), TG Therapeutics (Inst)
John M. Timmerman
Honoraria: Kite/Gilead
Consulting or Advisory Role: Kite/Gilead, DAVA Oncology
Research Funding: Bristol Myers Squibb, ImmunGene, Kite, a Gilead company, Merck
Travel, Accommodations, Expenses: Bristol Myers Squibb
Houston Holmes
Leadership: Exuma Biotech
Consulting or Advisory Role: Kite/Gilead, Celgene, Rigel, AstraZeneca, Bayer, Genentech, Janssen, CRISPR Therapeutics, TG Therapeutics, ADC Therapeutics, Epizyme, Karyopharm Therapeutics, Bristol Myers Squibb/Celgene/Juno
Speakers' Bureau: Kite, a Gilead company, Seattle Genetics, Rigel, Dova Pharmaceuticals, Karyopharm Therapeutics
Research Funding: Kite, a Gilead company (Inst), Unum Therapeutics (Inst), Juno Therapeutics (Inst), Novartis (Inst), Genentech (Inst), Janssen (Inst), Celgene (Inst), Caribou Biosciences (Inst), Adicet Bio (Inst), Incyte (Inst), Autolus (Inst), Viracta Therapeutics (Inst), Bristol Myers Squibb/Celgene (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/183906
Samantha Jaglowski
Consulting or Advisory Role: Novartis, Kite/Gilead, Juno Therapeutics, CRISPR Therapeutics, Takeda
Research Funding: Novartis, Kite/Gilead, Unum Therapeutics
Ian W. Flinn
Consulting or Advisory Role: AbbVie (Inst), Seattle Genetics (Inst), TG Therapeutics (Inst), Verastem (Inst), Roche (Inst), Gilead Sciences (Inst), Kite, a Gilead company (Inst), Janssen (Inst), BeiGene (Inst), Takeda (Inst), AstraZeneca (Inst), Juno Therapeutics (Inst), Unum Therapeutics (Inst), MorphoSys (Inst), Nurix (Inst), Shanghai Yingli Pharmaceuticals (Inst), Genentech (Inst), Great Point Partners (Inst), Iksuda Therapeutics (Inst), Novartis (Inst), Pharmacyclics (Inst), Century Therapeutics (Inst), Hutchison MediPharma (Inst), Servier (Inst), Vincerx Pharma (Inst), Genmab (Inst), InnoCare (Inst)
Research Funding: Acerta Pharma (Inst), Agios (Inst), Celgene (Inst), Constellation Pharmaceuticals (Inst), Genentech (Inst), Gilead Sciences (Inst), Incyte (Inst), Infinity Pharmaceuticals (Inst), Janssen (Inst), Kite, a Gilead company (Inst), Novartis (Inst), Pharmacyclics (Inst), Portola Pharmaceuticals (Inst), Roche (Inst), TG Therapeutics (Inst), Trillium Therapeutics (Inst), AbbVie (Inst), ArQule (Inst), BeiGene (Inst), Curis (Inst), FORMA Therapeutics (Inst), Forty Seven (Inst), Merck (Inst), Pfizer (Inst), Verastem (Inst), AstraZeneca (Inst), Unum Therapeutics (Inst), MorphoSys (Inst), Seattle Genetics (Inst), IGM Biosciences (Inst), Loxo (Inst), Rhizen Pharmaceuticals (Inst), Triact Therapeutics (Inst), Bristol Myers Squibb (Inst), CALGB (Inst), CTI (Inst), Fate Therapeutics (Inst), Millennium (Inst), TCR2 Therapeutics (Inst), Tessa Therapeutics (Inst), City of Hope (Inst), CALIBR (Inst), Bio-Path Holdings, Inc (Inst), Nurix (Inst), InnoCare (Inst), Myeloid Therapeutics (Inst)
Peter A. McSweeney
Employment: CBC Medical Group
Consulting or Advisory Role: Kite/Gilead, Gamida Cell, TG Therapeutics
Speakers' Bureau: Kite, a Gilead company
Research Funding: Novartis, Autolus, AlloVir, Kite, a Gilead company
Patents, Royalties, Other Intellectual Property: Royalties < $2,000 US dollars from the Fred Hutchinson Cancer Research Center for monoclonal antibodies against canine CD34
David B. Miklos
Honoraria: Janssen, Fosun Kite Biotechnology
Consulting or Advisory Role: Adaptive Biotechnologies, Juno/Celgene, Pharmacyclics, Janssen
Research Funding: Pharmacyclics, Novartis, Roche/Genentech, Kite, a Gilead company, Adaptive Biotechnologies, Alimera Sciences, Precision Biosciences, Adicet Bio
Patents, Royalties, Other Intellectual Property: Patent held with Pharmacyclics supporting ibrutinib for cGVHD (no royalty claim)
John M. Pagel
Employment: Loxo
Leadership: Loxo
Stock and Other Ownership Interests: Loxo
Consulting or Advisory Role: Gilead Sciences, AstraZeneca, Actinium Pharmaceuticals, BeiGene, Loxo, MEI Pharma, TG Therapeutics, MorphoSys, Epizyme
Marie José Kersten
Honoraria: Novartis, Kite, a Gilead company, Roche
Consulting or Advisory Role: Novartis, Kite, a Gilead Company, Miltenyi Biotec (Inst), Takeda (Inst)
Research Funding: Kite, a Gilead company (Inst)
Travel, Accommodations, Expenses: Novartis, Kite, a Gilead Company, Roche, Celgene
Krimo Bouabdallah
Honoraria: Roche, Takeda Science Foundation, AbbVie, Kite/Gilead
Consulting or Advisory Role: Roche, Takeda, Kite/Gilead
Travel, Accommodations, Expenses: Roche, Takeda
Max S. Topp
Consulting or Advisory Role: Regeneron, Roche, Novartis, Kite/Gilead, Kite/Gilead
Research Funding: Regeneron (Inst), Kite, a Gilead company (Inst), Roche (Inst)
Roch Houot
Honoraria: Bristol Myers Squibb/Celgene, MSD, Kite/Gilead, Roche, Novartis, Janssen
Consulting or Advisory Role: Kite/Gilead
Amer Beitinjaneh
Consulting or Advisory Role: Kite, a Gilead company
Research Funding: Atara Biotherapeutics, Kite/Gilead
Weimin Peng
Employment: Gilead Sciences
Stock and Other Ownership Interests: Gilead Sciences
Rhine R. Shen
Employment: Kite, a Gilead company
Stock and Other Ownership Interests: Kite/Gilead
Patents, Royalties, Other Intellectual Property: Atara Biotherapeutics
Rubina Siddiqi
Employment: Kite, a Gilead company, Amgen
Stock and Other Ownership Interests: Kite, a Gilead company, Amgen
Ioana Kloos
Employment: Kite, a Gilead company
Stock and Other Ownership Interests: Kite, a Gilead company
Patrick M. Reagan
Consulting or Advisory Role: Kite, a Gilead company
Research Funding: Seattle Genetics, Genentech/Roche
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at ASCO Virtual Annual Meeting, May 29-31, 2020; ASCO Virtual Annual Meeting, June 4-8, 2021; and American Society of Hematology Virtual Meeting, December 5-8, 2020.
CLINICAL TRIAL INFORMATION
NCT02601313 (ZUMA-2)
DATA SHARING STATEMENT
Kite is committed to sharing clinical trial data with external medical experts and scientific researchers in the interest of advancing public health, and access can be requested by contacting medinfo@kitepharma.com.
AUTHOR CONTRIBUTIONS
Conception and design: Michael Wang, Andre Goy, Frederick L. Locke, David B. Miklos, Max S. Topp, Weimin Peng, Rhine R. Shen, Patrick M. Reagan
Administrative support: Javier Munoz
Provision of study materials or patients: Javier Munoz, Frederick L. Locke, Caron A. Jacobson, Brian T. Hill, Samantha Jaglowski, David B. Miklos, John M. Pagel, Krimo Bouabdallah, Max S. Topp
Collection and assembly of data: Michael Wang, Javier Munoz, Andre Goy, Frederick L. Locke, Caron A. Jacobson, Brian T. Hill, John M. Timmerman, Houston Holmes, Samantha Jaglowski, Ian W. Flinn, Peter A. McSweeney, David B. Miklos, John M. Pagel, Marie José Kersten, Krimo Bouabdallah, Rashmi Khanal, Max S. Topp, Roch Houot, Amer Beitinjaneh, Rhine R. Shen, Ioana Kloos, Patrick M. Reagan
Data analysis and interpretation: Javier Munoz, Andre Goy, Frederick L. Locke, Caron A. Jacobson, John M. Timmerman, Houston Holmes, Samantha Jaglowski, Ian W. Flinn, Peter A. McSweeney, David B. Miklos, John M. Pagel, Marie José Kersten, Max S. Topp, Roch Houot, Amer Beitinjaneh, Weimin Peng, Xiang Fang, Rhine R. Shen, Rubina Siddiqi, Ioana Kloos, Patrick M. Reagan
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Three-Year Follow-Up of KTE-X19 in Patients With Relapsed/Refractory Mantle Cell Lymphoma, Including High-Risk Subgroups, in the ZUMA-2 Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Michael Wang
Honoraria: Janssen Research & Development, Dava Oncology, OM Pharmaceutical Industries, AstraZeneca, CAHON, Hebei Cancer Prevention Federation, Mumbai Hematology Group, Acerta Pharma, Chinese Anti-Cancer Association, BeiGene, Chinese Medical Association, Clinical Care Options, Epizyme, Imedex, Kite, a Gilead company, Miltenyi Biomedicine, Moffit Cancer Center, Newbridge Pharmaceuticals, Physicans' Education Resource, Scripps Health, The First Affiliated Hospital of Zhejiang University, Breast-Gynecological International Cancer Society, Pharmacyclics/Janssen
Consulting or Advisory Role: AstraZeneca, Janssen Research & Development, Juno Therapeutics, Bioinvent, Pharmacyclics/Janssen, Pulse Biosciences, Guidepoint Global, Loxo, Kite, a Gilead company, InnoCare, Oncternal Therapeutics, CStone Pharmaceuticals, Genentech, Bayer, BeiGene, DTRM, Epizyme, Miltenyi Biomedicine, VelosBio
Research Funding: AstraZeneca, Janssen Research & Development, Pharmacyclics, Kite, a Gilead company, Juno Therapeutics, BeiGene, Acerta Pharma, Oncternal Therapeutics, Bioinvent, Loxo, VelosBio, Celgene, Molecular Templates, Lilly, InnoCare
Travel, Accommodations, Expenses: Janssen Research & Development, AstraZeneca, Celgene, Dava Oncology, OM Pharmaceutical Industries
Javier Munoz
Honoraria: Kyowa Hakko Kirin, Seattle Genetics, Targeted Oncology, Onc view, Curio Science, Physicans' Education Resource
Consulting or Advisory Role: Kite, a Gilead company, Pfizer, Pharmacyclics, Bayer, Alexion Pharmaceuticals, Bristol Myers Squibb, Janssen, Seattle Genetics, Gilead Sciences, Kyowa Hakko Kirin, Juno Therapeutics, Genentech, Celgene, BeiGene, Fosun Kite, Innovent Biologics, Debiopharm Group, Karyopharm Therapeutics, Genmab, ADC Therapeutics, Epizyme, Servier, Novartis, MorphoSys
Speakers' Bureau: Kite, a Gilead company, Bayer, Pharmacyclics/Janssen, AstraZeneca, Gilead Sciences, Seattle Genetics, Kyowa Hakko Kirin, Acrotech Biopharma, BeiGene, BeiGene, Verastem, Celgene, AbbVie/Genentech
Research Funding: Kite, a Gilead company, Celgene, Portola Pharmaceuticals, Incyte, Genentech/AbbVie, Pharmacyclics/Janssen, Seattle Genetics, Millennium
Andre Goy
Employment: Regional Cancer Care Associates, OM Pharmaceutical Industries
Leadership: COTA, Genomic Testing Cooperative, Resilience Care
Stock and Other Ownership Interests: COTA, Genomic Testing Cooperative, Resilience Care
Consulting or Advisory Role: Elsevier, Kite, a Gilead company (Inst), AstraZeneca, Gilead Sciences, Resilience Care, OncLive Peer Exchange, Physicans' Education Resource, Janssen, Vincera Pharma/Vincerx Pharma
Speakers' Bureau: Bristol Myers Squibb/Celgene
Research Funding: Kite/Gilead (Inst), Acerta Pharma (Inst), AstraZeneca (Inst), Celgene (Inst), Constellation Pharmaceuticals (Inst), Genentech/Roche (Inst), Infinity Pharmaceuticals (Inst), Infinity/Verastem (Inst), Janssen (Inst), Karyopharm Therapeutics (Inst), Pharmacyclics (Inst)
Expert Testimony: AstraZeneca
Travel, Accommodations, Expenses: Physicans' Education Resource
Other Relationship: AstraZeneca, MorphoSys/Incyte
Frederick L. Locke
Consulting or Advisory Role: Novartis, Celgene, Calibr, Alimera Sciences, Cellular Biomedicine Group, Gerson Lehrman Group, EcoR1 Capital, Amgen, Bluebird Bio, Bristol Myers Squibb, Iovance Biotherapeutics, Legend Biotech, Cowen, Kite, a Gilead company, Umoja Biopharma, Takeda, Sana Biotechnology, Daiichi Sankyo/UCB Japan, Bristol Myers Squibb/Celgene, Janssen
Research Funding: Kite, a Gilead company (Inst), Alimera Sciences (Inst), Novartis (Inst), Bluebird Bio (Inst), Bristol Myers Squibb/Celgene (Inst)
Patents, Royalties, Other Intellectual Property: Double Mutant Survivin Vaccine. US010414810B2 (Inst), CAR T Cells With Enhanced Metabolic Fitness. Serial Number: 62/939,727 (Inst), Methods of Enhancing CAR T Cell Therapies. Serial Number: 62/892,292 (Inst), Evolutionary Dynamics of Non-Hodgkin Lymphoma CAR-T cell therapy. Serial Number: 62/879,534 (Inst)
Travel, Accommodations, Expenses: Kite, a Gilead company
Caron A. Jacobson
Honoraria: Kite/Gilead, Novartis, Celgene, Bristol Myers Squibb, Bluebird Bio, Epizyme, Instill Bio, Lonza, Ipsen, Abintus Bio
Consulting or Advisory Role: Kite/Gilead, Novartis, Celgene, Bristol Myers Squibb, Lonza, Ipsen, Instill Bio, Bluebird Bio, Epizyme, Abintus Bio
Research Funding: Pfizer, Kite, a Gilead company
Brian T. Hill
Honoraria: Pharmacyclics, Gilead Sciences, Genentech, AbbVie, Bayer, AstraZeneca, Novartis, Pfizer, Celgene, Karyopharm Therapeutics, Epizyme, BeiGene, Novartis, MorphoSys
Consulting or Advisory Role: Novartis, Genentech, AbbVie, Gilead Sciences, Karyopharm Therapeutics, AstraZeneca, Epizyme, MorphoSys, BeiGene
Research Funding: AbbVie (Inst), Karyopharm Therapeutics (Inst), Celgene (Inst), Takeda (Inst), Amgen (Inst), Genentech (Inst), Kite/Gilead (Inst), TG Therapeutics (Inst)
John M. Timmerman
Honoraria: Kite/Gilead
Consulting or Advisory Role: Kite/Gilead, DAVA Oncology
Research Funding: Bristol Myers Squibb, ImmunGene, Kite, a Gilead company, Merck
Travel, Accommodations, Expenses: Bristol Myers Squibb
Houston Holmes
Leadership: Exuma Biotech
Consulting or Advisory Role: Kite/Gilead, Celgene, Rigel, AstraZeneca, Bayer, Genentech, Janssen, CRISPR Therapeutics, TG Therapeutics, ADC Therapeutics, Epizyme, Karyopharm Therapeutics, Bristol Myers Squibb/Celgene/Juno
Speakers' Bureau: Kite, a Gilead company, Seattle Genetics, Rigel, Dova Pharmaceuticals, Karyopharm Therapeutics
Research Funding: Kite, a Gilead company (Inst), Unum Therapeutics (Inst), Juno Therapeutics (Inst), Novartis (Inst), Genentech (Inst), Janssen (Inst), Celgene (Inst), Caribou Biosciences (Inst), Adicet Bio (Inst), Incyte (Inst), Autolus (Inst), Viracta Therapeutics (Inst), Bristol Myers Squibb/Celgene (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/183906
Samantha Jaglowski
Consulting or Advisory Role: Novartis, Kite/Gilead, Juno Therapeutics, CRISPR Therapeutics, Takeda
Research Funding: Novartis, Kite/Gilead, Unum Therapeutics
Ian W. Flinn
Consulting or Advisory Role: AbbVie (Inst), Seattle Genetics (Inst), TG Therapeutics (Inst), Verastem (Inst), Roche (Inst), Gilead Sciences (Inst), Kite, a Gilead company (Inst), Janssen (Inst), BeiGene (Inst), Takeda (Inst), AstraZeneca (Inst), Juno Therapeutics (Inst), Unum Therapeutics (Inst), MorphoSys (Inst), Nurix (Inst), Shanghai Yingli Pharmaceuticals (Inst), Genentech (Inst), Great Point Partners (Inst), Iksuda Therapeutics (Inst), Novartis (Inst), Pharmacyclics (Inst), Century Therapeutics (Inst), Hutchison MediPharma (Inst), Servier (Inst), Vincerx Pharma (Inst), Genmab (Inst), InnoCare (Inst)
Research Funding: Acerta Pharma (Inst), Agios (Inst), Celgene (Inst), Constellation Pharmaceuticals (Inst), Genentech (Inst), Gilead Sciences (Inst), Incyte (Inst), Infinity Pharmaceuticals (Inst), Janssen (Inst), Kite, a Gilead company (Inst), Novartis (Inst), Pharmacyclics (Inst), Portola Pharmaceuticals (Inst), Roche (Inst), TG Therapeutics (Inst), Trillium Therapeutics (Inst), AbbVie (Inst), ArQule (Inst), BeiGene (Inst), Curis (Inst), FORMA Therapeutics (Inst), Forty Seven (Inst), Merck (Inst), Pfizer (Inst), Verastem (Inst), AstraZeneca (Inst), Unum Therapeutics (Inst), MorphoSys (Inst), Seattle Genetics (Inst), IGM Biosciences (Inst), Loxo (Inst), Rhizen Pharmaceuticals (Inst), Triact Therapeutics (Inst), Bristol Myers Squibb (Inst), CALGB (Inst), CTI (Inst), Fate Therapeutics (Inst), Millennium (Inst), TCR2 Therapeutics (Inst), Tessa Therapeutics (Inst), City of Hope (Inst), CALIBR (Inst), Bio-Path Holdings, Inc (Inst), Nurix (Inst), InnoCare (Inst), Myeloid Therapeutics (Inst)
Peter A. McSweeney
Employment: CBC Medical Group
Consulting or Advisory Role: Kite/Gilead, Gamida Cell, TG Therapeutics
Speakers' Bureau: Kite, a Gilead company
Research Funding: Novartis, Autolus, AlloVir, Kite, a Gilead company
Patents, Royalties, Other Intellectual Property: Royalties < $2,000 US dollars from the Fred Hutchinson Cancer Research Center for monoclonal antibodies against canine CD34
David B. Miklos
Honoraria: Janssen, Fosun Kite Biotechnology
Consulting or Advisory Role: Adaptive Biotechnologies, Juno/Celgene, Pharmacyclics, Janssen
Research Funding: Pharmacyclics, Novartis, Roche/Genentech, Kite, a Gilead company, Adaptive Biotechnologies, Alimera Sciences, Precision Biosciences, Adicet Bio
Patents, Royalties, Other Intellectual Property: Patent held with Pharmacyclics supporting ibrutinib for cGVHD (no royalty claim)
John M. Pagel
Employment: Loxo
Leadership: Loxo
Stock and Other Ownership Interests: Loxo
Consulting or Advisory Role: Gilead Sciences, AstraZeneca, Actinium Pharmaceuticals, BeiGene, Loxo, MEI Pharma, TG Therapeutics, MorphoSys, Epizyme
Marie José Kersten
Honoraria: Novartis, Kite, a Gilead company, Roche
Consulting or Advisory Role: Novartis, Kite, a Gilead Company, Miltenyi Biotec (Inst), Takeda (Inst)
Research Funding: Kite, a Gilead company (Inst)
Travel, Accommodations, Expenses: Novartis, Kite, a Gilead Company, Roche, Celgene
Krimo Bouabdallah
Honoraria: Roche, Takeda Science Foundation, AbbVie, Kite/Gilead
Consulting or Advisory Role: Roche, Takeda, Kite/Gilead
Travel, Accommodations, Expenses: Roche, Takeda
Max S. Topp
Consulting or Advisory Role: Regeneron, Roche, Novartis, Kite/Gilead, Kite/Gilead
Research Funding: Regeneron (Inst), Kite, a Gilead company (Inst), Roche (Inst)
Roch Houot
Honoraria: Bristol Myers Squibb/Celgene, MSD, Kite/Gilead, Roche, Novartis, Janssen
Consulting or Advisory Role: Kite/Gilead
Amer Beitinjaneh
Consulting or Advisory Role: Kite, a Gilead company
Research Funding: Atara Biotherapeutics, Kite/Gilead
Weimin Peng
Employment: Gilead Sciences
Stock and Other Ownership Interests: Gilead Sciences
Rhine R. Shen
Employment: Kite, a Gilead company
Stock and Other Ownership Interests: Kite/Gilead
Patents, Royalties, Other Intellectual Property: Atara Biotherapeutics
Rubina Siddiqi
Employment: Kite, a Gilead company, Amgen
Stock and Other Ownership Interests: Kite, a Gilead company, Amgen
Ioana Kloos
Employment: Kite, a Gilead company
Stock and Other Ownership Interests: Kite, a Gilead company
Patrick M. Reagan
Consulting or Advisory Role: Kite, a Gilead company
Research Funding: Seattle Genetics, Genentech/Roche
No other potential conflicts of interest were reported.
REFERENCES
- 1.Jain P, Wang M: Mantle cell lymphoma: 2019 update on the diagnosis, pathogenesis, prognostication, and management. Am J Hematol 94:710-725, 2019 [DOI] [PubMed] [Google Scholar]
- 2.Dreyling M, Campo E, Hermine O, et al. : Newly diagnosed and relapsed mantle cell lymphoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 28:iv62-iv71, 2017 [DOI] [PubMed] [Google Scholar]
- 3.Rummel MJ, Niederle N, Maschmeyer G, et al. : Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: An open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet 381:1203-1210, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Villa D, Sehn LH, Savage KJ, et al. : Bendamustine and rituximab as induction therapy in both transplant-eligible and -ineligible patients with mantle cell lymphoma. Blood Adv 4:3486-3494, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCulloch R, Visco C, Eyre TA, et al. : Efficacy of R-BAC in relapsed, refractory mantle cell lymphoma post BTK inhibitor therapy. Br J Haematol 189:684-688, 2020 [DOI] [PubMed] [Google Scholar]
- 6.Jain P, Dreyling M, Seymour JF, et al. : High-risk mantle cell lymphoma: Definition, current challenges, and management. J Clin Oncol 38:4302-4316, 2020 [DOI] [PubMed] [Google Scholar]
- 7.Silkenstedt E, Linton K, Dreyling M: Mantle cell lymphoma—Advances in molecular biology, prognostication and treatment approaches. Br J Haematol 195:162-173, 2021 [DOI] [PubMed] [Google Scholar]
- 8.Cheah CY, Chihara D, Romaguera JE, et al. : Patients with mantle cell lymphoma failing ibrutinib are unlikely to respond to salvage chemotherapy and have poor outcomes. Ann Oncol 26:1175-1179, 2015 [DOI] [PubMed] [Google Scholar]
- 9.Jain P, Kanagal-Shamanna R, Zhang S, et al. : Long-term outcomes and mutation profiling of patients with mantle cell lymphoma (MCL) who discontinued ibrutinib. Br J Haematol 183:578-587, 2018 [DOI] [PubMed] [Google Scholar]
- 10.Martin P, Maddocks K, Leonard JP, et al. : Postibrutinib outcomes in patients with mantle cell lymphoma. Blood 127:1559-1563, 2016 [DOI] [PubMed] [Google Scholar]
- 11.Epperla N, Hamadani M, Cashen AF, et al. : Predictive factors and outcomes for ibrutinib therapy in relapsed/refractory mantle cell lymphoma—A “real world” study. Hematol Oncol 35:528-535, 2017 [DOI] [PubMed] [Google Scholar]
- 12.Hess G, Dreyling M, Oberic L, et al. : KTE-X19 versus standard of care for relapsed/refractory mantle cell lymphoma previously treated with bruton tyrosine kinase inhibitors: Real-world evidence from Europe. Presented at European Hematology Association, Virtual, June 9-17, 2021 (abstr EP786) [Google Scholar]
- 13.Bernard M, Gressin R, Lefrere F, et al. : Blastic variant of mantle cell lymphoma: A rare but highly aggressive subtype. Leukemia 15:1785-1791, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Dreyling M, Klapper W, Rule S: Blastoid and pleomorphic mantle cell lymphoma: Still a diagnostic and therapeutic challenge. Blood 132:2722-2729, 2018 [DOI] [PubMed] [Google Scholar]
- 15.Katzenberger T, Petzoldt C, Holler S, et al. : The Ki67 proliferation index is a quantitative indicator of clinical risk in mantle cell lymphoma. Blood 107:3407, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Stefancikova L, Moulis M, Fabian P, et al. : Loss of the p53 tumor suppressor activity is associated with negative prognosis of mantle cell lymphoma. Int J Oncol 36:699-706, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Aukema SM, Hoster E, Rosenwald A, et al. : Expression of TP53 is associated with the outcome of MCL independent of MIPI and Ki-67 in trials of the European MCL Network. Blood 131:417-420, 2018 [DOI] [PubMed] [Google Scholar]
- 18.Eskelund CW, Dimopoulos K, Kolstad A, et al. : Detailed long-term follow-up of patients who relapsed after the nordic mantle cell lymphoma trials: MCL2 and MCL3. Hemasphere 5:e510, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Visco C, Tisi MC, Evangelista A, et al. : Time to progression of mantle cell lymphoma after high-dose cytarabine-based regimens defines patients risk for death. Br J Haematol 185:940-944, 2019 [DOI] [PubMed] [Google Scholar]
- 20.Dreyling M, Hoster E, Unterhalt M, et al. : Clinical outcome of mantle cell lymphoma patients with high risk biology (high Ki-67, blastic MCL, or high p53 expression). Blood 134, 2019. (abstr 3996) [Google Scholar]
- 21.Duell J, Lukic DS, Karg M, et al. : Functionally defective T cells after chemotherapy of B-cell malignancies can be activated by the tetravalent bispecific CD19/CD3 antibody AFM11. J Immunother 42:180-188, 2019 [DOI] [PubMed] [Google Scholar]
- 22.Wang M, Munoz J, Goy A, et al. : KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med 382:1331-1342, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matasar MJ, Czuczman MS, Rodriguez MA, et al. : Ofatumumab in combination with ICE or DHAP chemotherapy in relapsed or refractory intermediate grade B-cell lymphoma. Blood 122:499-506, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheson BD, Fisher RI, Barrington SF, et al. : Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J Clin Oncol 32:3059-3068, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenbaum PR, Rubin DB: The central role of the propensity score in observational studies for causal effects. Biometrika 70:41-55, 1983 [Google Scholar]
- 26.Austin PC: An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 46:399-424, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Locke FL, Rossi JM, Neelapu SS, et al. : Tumor burden, inflammation, and product attributes determine outcomes of axicabtagene ciloleucel in large B-cell lymphoma. Blood Adv 4:4898-4911, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fraietta JA, Beckwith KA, Patel PR, et al. : Ibrutinib enhances chimeric antigen receptor T-cell engraftment and efficacy in leukemia. Blood 127:1117-1127, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Long M, Beckwith K, Do P, et al. : Ibrutinib treatment improves T cell number and function in CLL patients. J Clin Invest 127:3052-3064, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dubovsky JA, Beckwith KA, Natarajan G, et al. : Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood 122:2539-2549, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruella M, Kenderian SS, Shestova O, et al. : The addition of the BTK inhibitor ibrutinib to anti-CD19 chimeric antigen receptor T cells (CART19) improves responses against mantle cell lymphoma. Clin Cancer Res 22:2684-2696, 2016 [DOI] [PubMed] [Google Scholar]
- 32.Linton K, Dreyling M: EHA endorsement of ESMO clinical practice guidelines for newly diagnosed and relapsed mantle cell lymphoma. Hemasphere 4:e464, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rule S, Dreyling M, Goy A, et al. : Outcomes in 370 patients with mantle cell lymphoma treated with ibrutinib: A pooled analysis from three open-label studies. Br J Haematol 179:430-438, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hiddemann W, Barbui AM, Canales MA, et al. : Immunochemotherapy with obinutuzumab or rituximab for previously untreated follicular lymphoma in the GALLIUM study: Influence of chemotherapy on efficacy and safety. J Clin Oncol 36:2395-2404, 2018 [DOI] [PubMed] [Google Scholar]
- 35.Locke FL, Ghobadi A, Jacobson CA, et al. : Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): A single-arm, multicentre, phase 1-2 trial. Lancet Oncol 20:31-42, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee DW, Gardner R, Porter DL, et al. : Current concepts in the diagnosis and management of cytokine release syndrome. Blood 124:188-195, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Kite is committed to sharing clinical trial data with external medical experts and scientific researchers in the interest of advancing public health, and access can be requested by contacting medinfo@kitepharma.com.