PURPOSE
Commonly used first-line (1L) treatments for mantle cell lymphoma include high-dose cytarabine-based induction followed by autologous stem-cell transplant (ASCT) for younger patients and several chemoimmunotherapy regimens for older patients. Continuous debates exist on the role of ASCT in younger patients and maintenance rituximab (MR) after bendamustine plus rituximab (BR).
METHODS
Retrospective data from 4,216 patients with mantle cell lymphoma in the Flatiron Health electronic record-derived deidentified database diagnosed between 2011 and 2021, mostly in US community oncology settings, were evaluated for treatment patterns and outcomes. The efficacy findings with ASCT and MR were validated in an independent cohort of 1,168 patients from 12 academic centers.
RESULTS
Among 3,614 patients with documented 1L treatment, BR was the most used. Among 1,265 patients age < 65 years, 30.5% received cytarabine-based induction and 23.5% received ASCT. There was no significant association between ASCT and real-world time to next treatment (hazard ratio [HR], 0.84; 95% CI, 0.68 to 1.03; P = .10) or overall survival (HR, 0.86; 95% CI, 0.63 to 1.18; P = .4) among ASCT-eligible patients. Among MR-eligible patients, MR after BR versus BR alone was associated with a longer real-world time to next treatment (HR, 1.96; 95% CI, 1.61 to 2.38; P < .001) and overall survival (HR, 1.51; 95% CI, 1.19 to 1.92; P < .001). The efficacy findings were consistent in the validation cohort.
CONCLUSION
In this large cohort of patients treated primarily in the US community setting, only one in four young patients received cytarabine or ASCT consolidation, suggesting the need to develop treatments that can be delivered effectively in routine clinical practice. Together with the validation cohort, data support future clinical trials exploring regimens without ASCT consolidation in young patients, whereas MR should be considered for patients after 1L BR and rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone.
INTRODUCTION
Mantle cell lymphoma (MCL) has a heterogenous, often aggressive clinical presentation.1,2 For patients age < 65 years, current guidelines recommend induction with a high-dose cytarabine-containing chemoimmunotherapy regimen followed by consolidation with autologous hematopoietic stem-cell transplant (ASCT) and maintenance with rituximab (MR).3,4 For patients age ≥ 65 years who cannot tolerate intensive regimens, recommended treatments include bendamustine plus rituximab (BR), rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP), and bortezomib-based bortezomib, rituximab, cyclophosphamide, doxorubicin, and prednisone (VR-CAP).3,4 Current guidelines acknowledge lack of evidence from prospective trials for the use of MR after BR.3-5
CONTEXT
Key Objective
We leveraged two independent sources of real-world evidence covering both community and academic practices to evaluate the treatment patterns and outcomes of first-line mantle cell lymphoma treatments, with an emphasis on the role of autologous stem-cell transplant (ASCT) in younger patients and maintenance rituximab after induction with bendamustine plus rituximab (BR).
Knowledge Generated
Our analysis showed that ASCT was underutilized in the US community setting. In patients considered ASCT-eligible, there was no significant association between the receipt of ASCT and overall survival. Importantly, among patients considered eligible for maintenance, maintenance rituximab after BR was associated with longer survival versus BR alone.
Relevance (J.W. Friedberg)
-
These findings from two large retrospective cohorts complement older prospective clinical trial data in evaluating the role of consolidative ASCT and/or maintenance antibody therapy for patients with mantle cell lymphoma. These data support ongoing prospective trials evaluating the role of ASCT in patients achieving complete response without detectable minimal residual disease.*
*Relevance section written by JCO Editor-in-Chief Jonathan W. Friedberg, MD.
A systematic review of clinical trials of commonly used first-line (1L) MCL regimens6 reported a median progression-free survival (PFS) ranging from 16.6 months with R-CHOP alone7 to 109.2 months with R-CHOP/rituximab plus dexamethasone, cytarabine and platinum followed by ASCT.8 The median overall survival (OS) varied from 40 to 70 months in older patients and from 53 to 152 months in younger patients frequently treated with ASCT.6 Retrospective studies from academic institutions demonstrated similar outcomes to prospective trials among patients treated similarly.9
We evaluated treatment patterns and outcomes in 1L MCL and assessed the impact of ASCT in patients age < 65 years and MR after BR or R-CHOP in two large independent real-world cohorts.
METHODS
Data Collection and Inclusion Criteria
This retrospective study analyzed data for adult patients diagnosed with MCL in the United States from January 2011 to January 2021 captured in the nationwide Flatiron Health electronic health record–derived deidentified database.10-12 Key inclusion criteria included confirmed MCL diagnosis per International Classification of Diseases, Ninth Revision, Clinical Modification diagnosis code 200.48 or International Classification of Diseases, 10th Revision, Clinical Modification code C83.1x, age ≥ 18 years at diagnosis, and ≥ 2 clinic visit records. The longitudinal database contains deidentified patient-level structured and unstructured data, curated via technology-enabled abstraction11 from approximately 280 cancer clinics (approximately 800 sites of care). Most patients were treated in a community oncology setting, defined as community clinics not affiliated with teaching institutions. Institutional Review Board approval of the study protocol was obtained before study conduct and included a waiver of informed consent.
The validation cohort13 was derived from 12 academic centers in the United States and BC Cancer, Canada. Demographic, clinical, and outcomes data were retrospectively collected following Institutional Review Board approval at each center. Additional methods on the Flatiron and validation cohorts are given in the Data Supplement (online only).
Patient Cohorts Analyzed
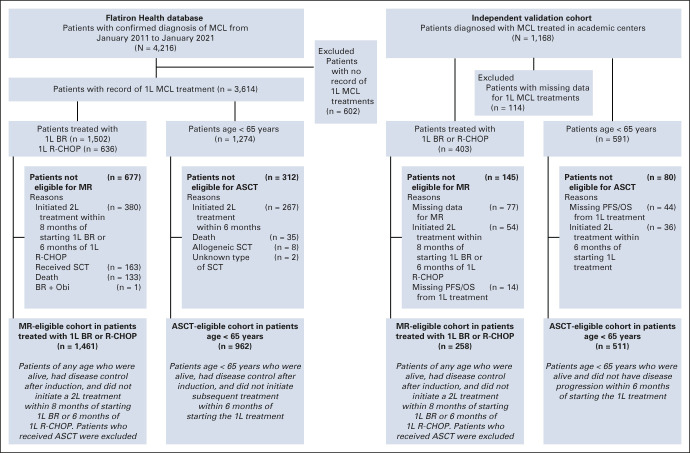
In the Flatiron cohort, 3,614 of 4,216 patients had records of MCL treatment. To study outcomes associated with MR after BR or R-CHOP, and ASCT in young patients, we defined two target study populations: the MR-eligible cohort and ASCT-eligible cohort, which included patients who had disease control after induction treatment, aligning with selection of patients for ASCT and MR in trials and routine practice (Fig 1). As response and progression data were not available in the Flatiron Health database, but information on the initiation of the second-line treatment and death was available, the MR-eligible cohort (n = 1,461) included patients of any age who were alive and did not initiate second-line treatment within 8 months of starting 1L BR or 6 months of 1L R-CHOP; patients who received ASCT were excluded. MR was defined as rituximab monotherapy for ≥ 28 days after 1L rituximab-based induction treatment. The ASCT-eligible cohort (n = 962) included patients age < 65 years who were alive and did not initiate subsequent treatment within 6 months of starting the 1L treatment. Sensitivity analyses were performed using cutoffs of 6, 8, or 10 months (Data Supplement).
FIG 1.
Study flow. 1L, first-line; 2L, second-line; ASCT, autologous stem-cell transplant; BR, bendamustine plus rituximab; MCL, mantle cell lymphoma; MR, maintenance rituximab; Obi, obinutuzumab; OS, overall survival; PFS, progression-free survival; R-CHOP, rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone; SCT, stem-cell transplant.
In the validation cohort (N = 1,168), response and progression data were available, but not data on the initiation of second-line treatment. Therefore, the MR-eligible validation cohort (n = 298) included patients of any age who were alive and did not have disease progression within 8 months of starting 1L BR or 6 months of 1L R-CHOP, and the ASCT-eligible cohort (n = 511; Fig 1) included patients age < 65 years who were alive and did not have disease progression within 6 months of starting 1L treatment.
Outcome Measures
In the Flatiron cohort, we evaluated patient characteristics as documented in the electronic health record, treatment patterns, and real-world time to next treatment (rwTTNT) and OS. rwTTNT, an accepted real-world alternative clinical end point to PFS,14,15 was used in several publications including data from the Flatiron Health database16,17 and others.18 rwTTNT was defined as the time from start of the 1L treatment to subsequent treatment or death, whichever occurred first. In the validation cohort, PFS and OS were evaluated. PFS was defined as the time from start of 1L treatment to disease progression or death, whichever occurred first. OS was defined as the time from start of the 1L treatment to death in both Flatiron and validation cohorts.
Statistical Analyses
Continuous variables were summarized using descriptive statistics; categorical variables were summarized with count and percent. Time-to-event analyses used Kaplan-Meier methods to estimate medians and 3-year rates with associated 95% CIs. The univariate Cox model was used to generate hazard ratios (HRs) with 95% CIs. The log-rank test was used to generate P values. Kaplan-Meier plots were presented up to 60 months because of a smaller number of patients at risk afterward.
Multivariate analyses (MVAs) were conducted to identify predictors of rwTTNT and OS including age, Eastern Cooperative Oncology Group performance status, lactate dehydrogenase (LDH) level, WBC, bulky disease (as per the local oncologist), blastoid/pleomorphic morphology, and the use of MR (no v yes).
RESULTS
Baseline Characteristics and Treatment Patterns
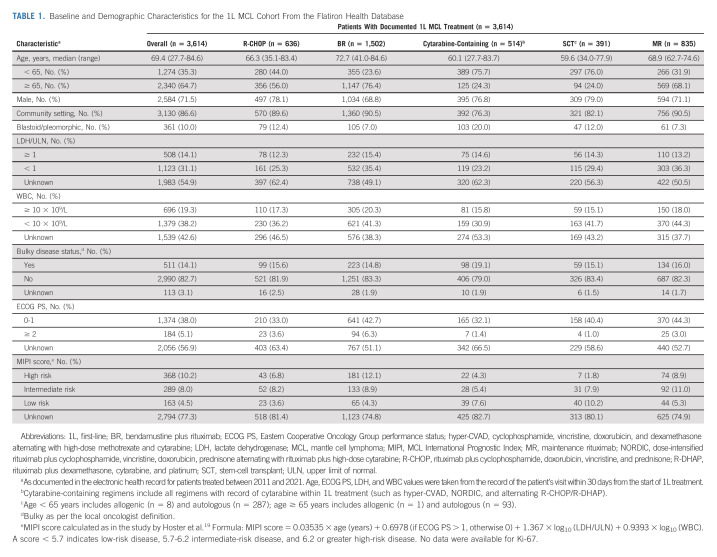
Among 3,614 patients in the Flatiron cohort, the median age at 1L was 69.4 (range, 27.7-84.6) years; 87% of patients were treated in a community oncology setting (Table 1). BR-treated patients were older than those treated with cytarabine-containing regimens or R-CHOP (median age 73, 60, and 66 years, respectively). The MCL International Prognostic Index (MIPI) score was only available for approximately 20% of patients.
TABLE 1.
Baseline and Demographic Characteristics for the 1L MCL Cohort From the Flatiron Health Database
Between 2011 and 2020, in patients age < 65 years (n = 1,265), cytarabine-containing regimens were most frequently used (30.5%), followed by BR (28.0%) and R-CHOP (22.1%); 23.5% of patients received ASCT, and 20.9% received MR. The most common induction regimens before ASCT in patients age < 65 years (n = 287) were R-CHOP (21.3%), BR (19.2%), rituximab plus cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with high-dose methotrexate and cytarabine (18.1%), and dose-intensified rituximab plus cyclophosphamide, vincristine, doxorubicin, prednisone alternating with rituximab plus high-dose cytarabine (16.4%). In patients age ≥ 65 years (n = 2,329), BR was used in 49.0%, R-CHOP in 15.2%, VR-CAP in 1.8%, and MR in 24.3%. In the overall cohort, 120 of 3,614 (3.3%) patients received both ASCT and MR (80 age < 65 years and 40 age ≥ 65 years).
The overall use of R-CHOP decreased from 32.9% in 2011 to 9.7% in 2020, whereas the use of BR increased from 19.7% in 2011 to 52.9% in 2020. The use of MR increased from 14.3% in 2011 to 27.2% in 2019 in patients age < 65 years. The use of cytarabine, ASCT, or MR in patients age ≥ 65 years did not change notably over time (Fig 2 and Data Supplement).
FIG 2.
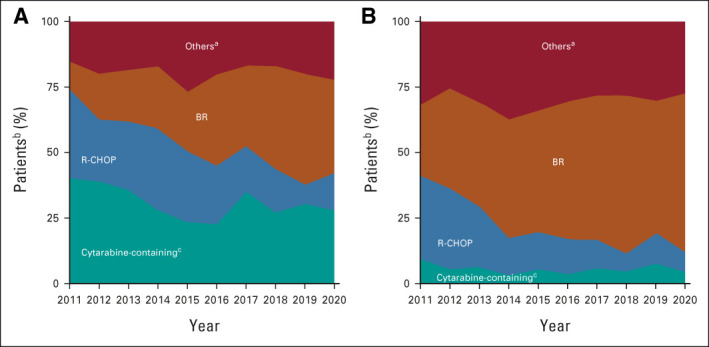
1L mantle cell lymphoma treatment by age and year from the Flatiron cohort: patients age (A) < 65 years (n = 1,265) and (B) ≥ 65 years (n = 2,329). aAmong 3,614 treated patients, other therapies include 9.5% of targeted agents (eg, bortezomib, lenalidomide, BTK inhibitor, and BCL-2 inhibitor), 7.8% of immunotherapy only, 4.7% of clinical trial drugs, 2.4% of chemotherapy only, and additional 1.8% of other types of chemoimmunotherapy. bNumber of patients treated between 2011 and 2020 (different from n = 1,274 in Table 1, which also includes patients treated in 2021). cCytarabine-containing regimens include all regimens with the record of cytarabine within 1L treatment (such as hyper-CVAD, NORDIC, and alternating R-CHOP/R-DHAP). 1L, first-line; BR, bendamustine plus rituximab; hyper-CVAD, cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with high-dose methotrexate and cytarabine; NORDIC, dose-intensified rituximab plus cyclophosphamide, vincristine, doxorubicin, prednisone alternating with rituximab plus high-dose cytarabine; R-CHOP, rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone; R-DHAP, rituximab plus dexamethasone, cytarabine, and platinum.
In the validation cohort (N = 1,168), patients were younger than those in the Flatiron cohort (median age 62 v 69 years; Data Supplement), and the use of ASCT in patients age < 65 years was more frequent (47% v 23.5%).
rwTTNT and OS
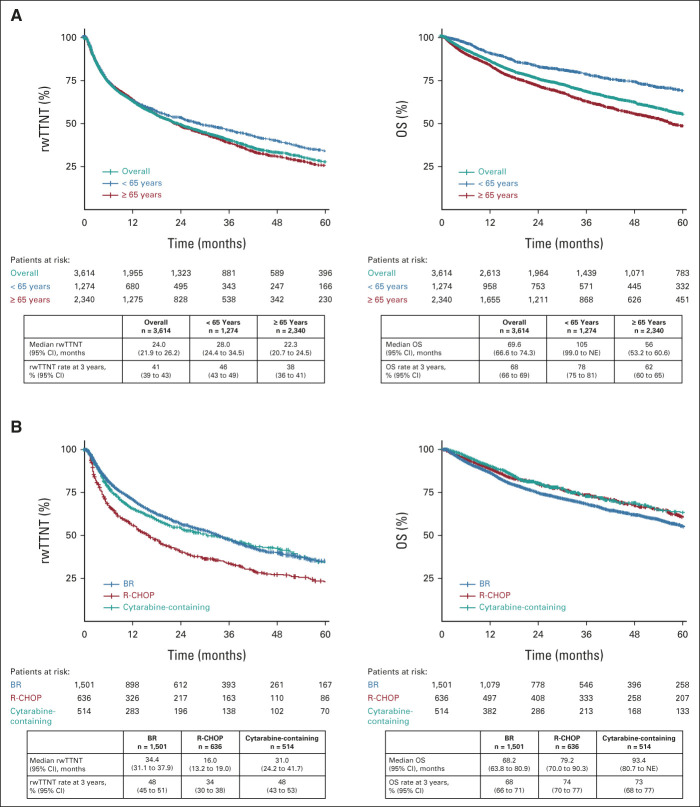
With a median follow-up of 45.5 (range, 0.03-119.4) months, the overall median rwTTNT in the 1L MCL Flatiron cohort (n = 3,614) was 24.0 months (95% CI, 21.9 to 26.2). Patients age < 65 years had better outcomes versus patients age ≥ 65 years with the median rwTTNT of 28.0 months (95% CI, 24.4 to 34.5) and 22.3 months (95% CI, 20.7 to 24.5; Fig 3A).
FIG 3.
rwTTNT and OS in patients with documented first-line mantle cell lymphoma treatment from the Flatiron cohort (A) overall and by age and (B) by treatment (BR, R-CHOP, and cytarabine). BR, bendamustine plus rituximab; NE, not estimable; OS, overall survival; R-CHOP, rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone; rwTTNT, real-world time to next treatment.
Patients treated with BR (n = 1,501) and cytarabine-containing regimens (n = 514) had a similar median rwTTNT of 34.4 months (95% CI, 31.1 to 37.9) and 31.0 months (95% CI, 24.2 to 41.7), respectively, whereas the median rwTTNT with R-CHOP (n = 636) was 16.0 months (95% CI, 13.2 to 19.0). The 3-year OS rate was 68% (95% CI, 66 to 69) in the whole cohort and 68% (95% CI, 66 to 71) with BR, 74% (95% CI, 70 to 77) with R-CHOP, and 73% (95% CI, 68 to 77) with cytarabine-containing regimens (Fig 3B and Data Supplement). MVA of the predictors of rwTTNT and OS in the 1L MCL Flatiron cohort showed that older age, Eastern Cooperative Oncology Group performance status ≥ 2, and high-risk disease features such as LDH/upper limit of normal ≥ 1 versus < 1, WBC ≥ 10 × 109/L, bulky disease, and blastoid and pleomorphic disease were associated with worse real-world outcomes (Data Supplement).
Role of MR After 1L BR and R-CHOP
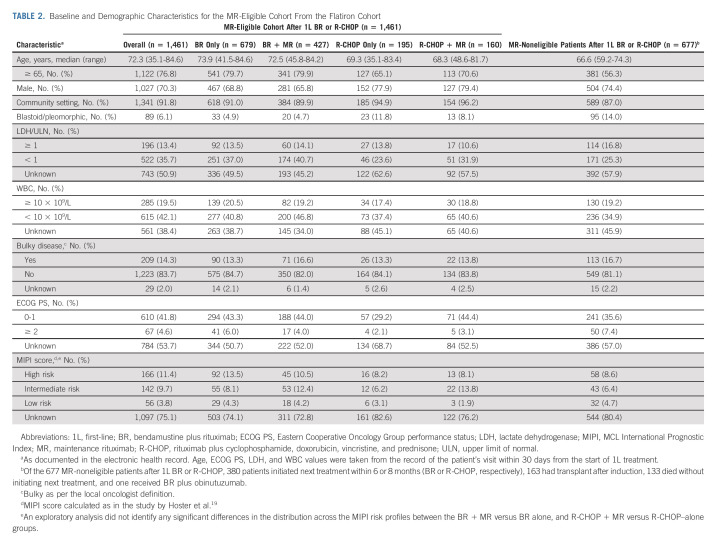
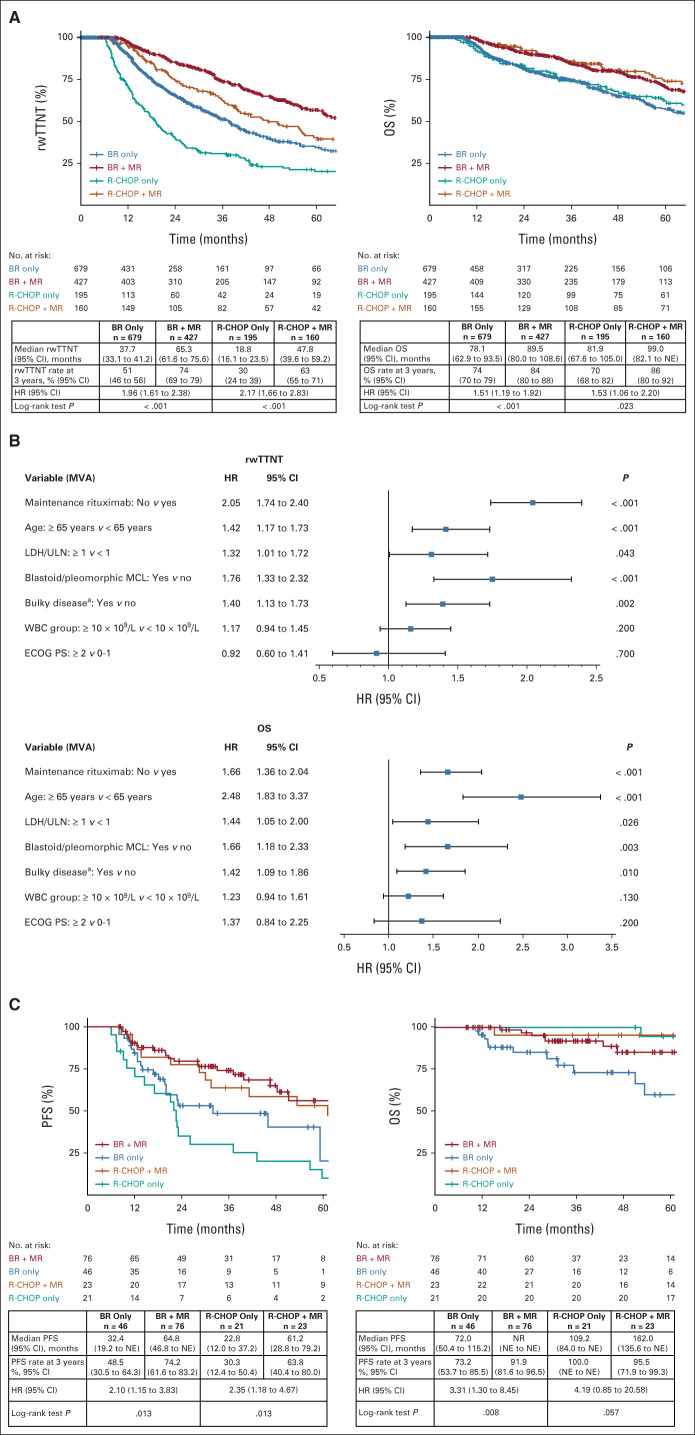
In the Flatiron cohort, of 2,138 patients who received 1L BR or R-CHOP, 1,461 (68.3%) were considered MR-eligible (Fig 1 and Table 2). In the MR-eligible cohort, the median duration of induction therapy was 4.7 months regardless of MR use. The median duration of MR was 19.9 months. MR use after BR versus BR alone was associated with significantly longer rwTTNT (HR, 1.96; 95% CI, 1.61 to 2.38; P < .001) and OS (HR, 1.51; 95% CI, 1.19 to 1.92; P < .001; Fig 4A). The 3-year rwTTNT and OS rates for BR + MR were 74.0% (95% CI, 69 to 79) and 84.0% (95% CI, 80 to 88), respectively, versus 51.0% (95% CI, 46 to 56) and 74.0% (95% CI, 70 to 79) with BR alone. MVA in the MR-eligible cohort revealed that no MR use, age ≥ 65 years, LDH/upper limit of normal ≥ 1, bulky disease, and blastoid/pleomorphic morphology were associated with significantly shorter rwTTNT and OS (Fig 4B).
TABLE 2.
Baseline and Demographic Characteristics for the MR-Eligible Cohort From the Flatiron Cohort
FIG 4.
rwTTNT and OS in the MR-eligible cohort: (A) patients treated with BR or R-CHOP alone, or BR + MR and R-CHOP + MR from the Flatiron cohort; (B) MVA of the predictors of rwTTNT and OS in the MR-eligible cohort from the Flatiron cohort; the MVA used all data, including missing values, as a category for covariates; (C) PFS and OS in the validation cohort. HR was calculated for MR yes versus no in the Flatiron cohort and MR no versus yes in the validation cohort. Median OS should be interpreted with caution as it was reached when few patients were still at risk. a“Bulky” as per local oncologist definition. BR, bendamustine plus rituximab; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; LDH, lactate dehydrogenase; MCL, mantle cell lymphoma; MR, maintenance rituximab; MVA, multivariate analysis; NE, not estimable; NR, not reached; OS, overall survival; PFS, progression-free survival; R-CHOP, rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone; rwTTNT, real-world time to next treatment; ULN, upper limit of normal.
Consistent with the findings from the Flatiron cohort, significantly improved PFS and OS with BR + MR were noted in the MR-eligible patients from the validation cohort (n = 258; Fig 4C). The 3-year PFS and OS rates were 74.2% (95% CI, 61.6 to 83.2) and 91.9% (95% CI, 81.6 to 96.5), respectively, in patients treated with BR + MR, versus 48.5% (95% CI, 30.5 to 64.3) and 73.2% (95% CI, 53.7 to 85.5) with BR alone. The use of MR after 1L R-CHOP improved outcomes versus R-CHOP alone in both Flatiron and validation cohorts (Figs 4A and 4C). The 3-year rwTTNT or PFS rates were numerically higher with BR + MR versus R-CHOP + MR, but the 3-year OS rates were similar.
Role of ASCT in Young Patients (age < 65 years)
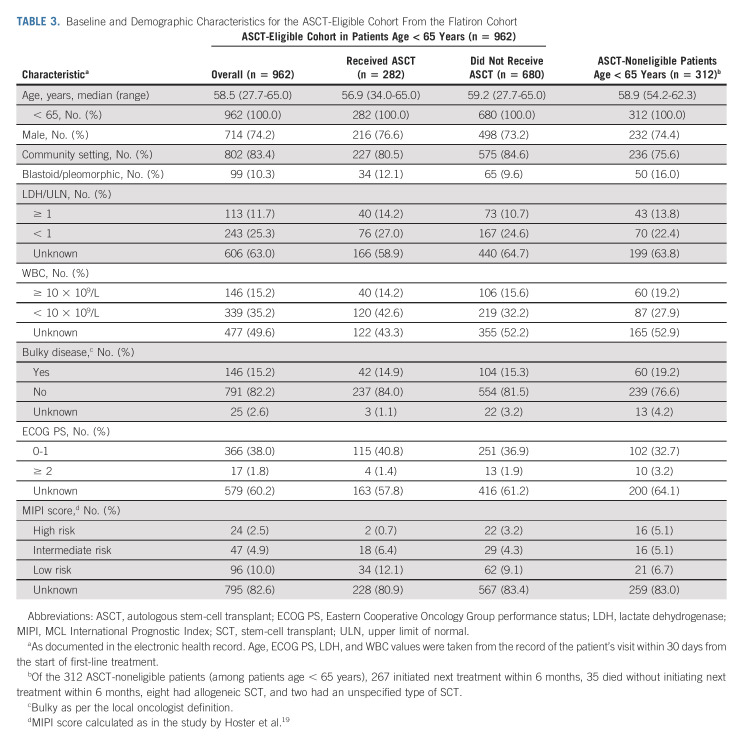
In the Flatiron cohort, among patients age < 65 years, 962 of 1,274 (76%) were considered ASCT-eligible (Fig 1, Table 3). In the ASCT-eligible cohort, there was no significant association between the receipt of ASCT and rwTTNT (HR, 0.84; 95% CI, 0.68 to 1.03; P = .10) or OS (HR, 0.86; 95% CI, 0.63 to 1.18; P = .4; Fig 5A). The 3-year rwTTNT and OS rates were similar between patients who received ASCT (rwTTNT 65% [95% CI, 59 to 71]; OS, 88% [95% CI, 83 to 92]) and those who did not (rwTTNT 59% [95% CI, 55 to 64]; OS, 84% [95% CI, 81 to 88]; Fig 5A). Outcomes by the type of induction treatment in the Flatiron cohort are presented in the Data Supplement.
TABLE 3.
Baseline and Demographic Characteristics for the ASCT-Eligible Cohort From the Flatiron Cohort
FIG 5.
(A) rwTTNT and OS in the ASCT-eligible cohort by ASCT status (yes v no) in the Flatiron cohort; (B) PFS and OS in the validation cohort. Median OS should be interpreted with caution as it was reached when few patients were still at risk. ASCT, autologous stem-cell transplant; HR, hazard ratio; NE, not estimable; NR, not reached; OS, overall survival; PFS, progression-free survival; rwTTNT, real-world time to next treatment.
Similarly, in the validation cohort (n = 511), the 3-year PFS rate for patients who received ASCT (n = 328) was 69.6% (95% CI, 63.6 to 74.8) versus 69.0% (95% CI, 61.2 to 75.6) for patients who did not receive ASCT (n = 183; Fig 5B). The 3-year OS rates were also comparable regardless of ASCT status.
DISCUSSION
In this large US real-world cohort of patients with MCL treated primarily in community-based practices, the rate of ASCT was relatively low (approximately one in four patients age < 65 years). Similarly, low ASCT utilization rates of approximately 10% to approximately 20% in younger patients were also reported in two large US studies.20,21 However, several other studies from the United States, Canada, and Sweden reported much higher rates of ASCT utilization in younger patients (≥ 60%), treated mostly at academic centers.22-24 The different ASCT utilization trends between our study and others suggest that various reasons may affect the decision to undergo ASCT, such as comorbid conditions, limited responses to induction treatments, clinician or patient preference, and access to treatments, including facility type.21,22,24 In addition, socioeconomic factors such as marital status, education level, type of insurance, or income may also play a role in treatment choices.21,24
It is interesting to observe a slight increase of MR use in younger patients between 2017 and 2019 (approximately 17% increase from 2011), which coincided with publication of the phase III clinical evidence supporting MR use in 2017.25 BR, R-CHOP, and VR-CAP were used in almost two thirds of patients age ≥ 65 years. BR was the most commonly used 1L regimen in older patients, increasing from 27.1% to 60.8% between 2011 and 2020, corresponding to a steady decrease in the use of R-CHOP over time and consistent with the clinical evidence supporting BR over R-CHOP in older patients reported in 2013.26 These observations indicate that some of the factors influencing treatment choices might be more amenable to change at the clinical level, such as continuing to improve awareness and communication regarding clinical evidence and treatment guidelines, consultations between physicians working in different settings, and further focus on the development of regimens that can be easily delivered in a broad range of health care settings.
The efficacy outcomes in the Flatiron cohort appeared worse than those in other observational studies, particularly in patients age < 65 years with a median rwTTNT of 28.0 months. A possible explanation is that almost a quarter of young patients initiated subsequent lines of treatment or died within 6 months of starting 1L treatment, a proportion higher than that observed in trials (approximately 10% within the first 6 months),25 reflecting expected differences in patient characteristics and more frequent treatment changes in routine practice. Although specific reasons for changing treatment were not captured in the Flatiron Health database, possible reasons include primary refractory disease, limited response to induction regimen, or treatment intolerance. On the other hand, the efficacy outcomes seen with 1L BR (median rwTTNT of 34.4 months) and 1L R-CHOP (median rwTTNT of 16 months) were consistent with PFS reported in clinical trials.26,27
One of the important findings in our study is that 1L BR + MR was associated with significantly improved rwTTNT and OS versus BR alone, and these findings were validated in an independent cohort. Randomized clinical trials reported that MR after R-CHOP or ASCT improved outcomes,25,28,29 whereas the StiL-NHL7-2008 MAINTAIN trial did not show statistically significant PFS or OS benefit with MR after 1L BR.5 In the MAINTAIN trial, the control BR-only group (no MR) had a median PFS of 4.5 years, which was longer than a median PFS of 3 years published in the StiL-NHL1-2003 study,30 and there might have been limited power to detect a difference between the two study arms given the relatively small sample size.5 Although our findings were based on two separate observational cohorts, there are several other lines of evidence to support MR after BR. For example, in the E1411 trial, patients who received a BR-based induction followed by 2 years of MR achieved a median PFS of roughly 64 months.31 Ongoing BR-based trials in MCL, such as E1411 and SHINE (ClinicalTrials.gov identifier: NCT01776840), evaluating whether the addition of bortezomib/lenalidomide or ibrutinib could further improve the active backbone of BR + MR, will provide further answers.
In addition, our analysis from two independent cohorts revealed no clear rwTTNT or OS benefits associated with the receipt of ASCT among ASCT-eligible patients, regardless of the specific types of induction treatment received (cytarabine-based, BR, or others). Despite the apparent contradiction of these findings with the clinical trial data supporting survival benefit with ASCT,8,32,33 several registry studies reported evidence consistent with our findings. A recent study from the Swedish Lymphoma Registry (n = 592) reported no difference in OS or relative survival in patients treated with MCL2 (ASCT included) versus BR-treated subgroups after adjustment for age at diagnosis, sex, and year of diagnosis.34 A large US real-world analysis showed that the 3-year OS survival rates were largely unchanged at approximately 82%-86% despite increased use of ASCT from approximately 10% to approximately 20% from 2005 to 2017.20 Ongoing randomized trials, such as the European MCL Network Triangle (ClinicalTrials.gov identifier: NCT02858258) and E4151 (ClinicalTrials.gov identifier: NCT03267433), are currently evaluating the role of ASCT, and we must await the results of those studies before drawing final conclusions. However, the strength of the observational data presented here suggests that it may be reasonable to perform clinical trials without a requirement for ASCT in younger patients, so that we can be prepared to move forward with the next set of trials pending completion of the randomized studies.
This retrospective analysis has the strength of large patient numbers reflecting the patient population and practice pattern in routine practice at community and academic centers and the inclusion of an independent validation cohort to confirm the findings. However, these factors do not negate several limitations and potential sources of bias commonly associated with retrospective analyses. First, there were missing data in patient baseline characteristics, which limited the ability to derive the MIPI/combined MIPI and Ki67 scores. Second, tumor response data were not available in the Flatiron cohort, and we had to use rwTTNT as a proxy to define patient eligibility for ASCT or MR. Third, the intention for choosing treatments was not captured in either database. There may be differences in practice at the center level that could not be delineated in the Flatiron cohort. Finally, the use of MR or ASCT was not randomized. Described adjustments do not fully account for patient or disease factors (such as MIPI) influencing the choice of treatment or physician intention at the start of induction therapy or use of MR or ASCT. Therefore, our results are limited by residual bias, including selection and immortal time bias.
Taken together, our findings from two large retrospective cohorts provide additional considerations for the design of future trials evaluating new treatment regimens in MCL. First, there should be a continuous focus on the development of treatments that can be effectively delivered and implemented in routine and community practices. Second, our data suggest that the risk:benefit ratio of ASCT should be carefully weighed for each patient and it may be reasonable to perform future trials without a requirement for ASCT in younger patients. Furthermore, the substantial survival benefit achieved with MR in patients responding to 1L BR, a frequently used chemoimmunotherapy regimen, should be considered routinely in patients with MCL.
ACKNOWLEDGMENT
The authors would like to thank all those involved in the collection of data used in this study. Writing assistance was provided by Ewa Wandzioch of Parexel and funded by Janssen Global Services LLC.
Peter Martin
Consulting or Advisory Role: Janssen, BeiGene, Karyopharm Therapeutics, Kite/Gilead, Verastem, ADC Therapeutics, Bristol Myers Squibb/Celgene, Epizyme, Merck, MorphoSys, Takeda
Research Funding: Karyopharm Therapeutics (Inst)
Jonathon B. Cohen
Consulting or Advisory Role: AbbVie, Janssen, Loxo, Kite/Gilead, AstraZeneca, Aptitude Medical, Adicet Bio, Adaptive Biotechnologies
Research Funding: Celgene (Inst) (Inst), Janssen (Inst), Novartis (Inst), Takeda (Inst), AI Therapeutics (Inst), Genentech (Inst), ASH (Inst), Lymphoma Research Foundation (Inst), Loxo (Inst), BioInvent (Inst), AstraZeneca (Inst)
Michael Wang
Honoraria: Janssen Research & Development, Dava Oncology, OM Pharmaceutical Industries, AstraZeneca, CAHON, Hebei Cancer Prevention Federation, Mumbai Hematology Group, Acerta Pharma, Chinese Anti-Cancer Association, BeiGene, Clinical Care Options, Epizyme, Imedex, Kite, a Gilead Company, Miltenyi Biomedicine, Moffit Cancer Center, Physicians' Education Resource, Breast-Gynecological International Cancer Society, Pharmacyclics/Janssen, Eastern Virginia Medical School, Leukemia & Lymphoma Society, LLC TS Oncology, Medscape, Meeting Minds Experts, OncLive/MJH Life Sciences, Practice Point Communications, First Hospital Zhejiang University, BioInvent
Consulting or Advisory Role: AstraZeneca, Janssen Research & Development, Juno Therapeutics, BioInvent, Pharmacyclics/Janssen, Loxo, Kite, a Gilead Company, InnoCare, Oncternal Therapeutics, CStone Pharmaceuticals, Genentech, BeiGene, DTRM, Epizyme, Miltenyi Biomedicine, VelosBio, Deciphera, Lilly, PeproMene
Research Funding: AstraZeneca, Janssen Research & Development, Pharmacyclics, Kite, a Gilead Company, Juno Therapeutics, BeiGene, Acerta Pharma, Oncternal Therapeutics, BioInvent, Loxo, VelosBio, Celgene, Molecular Templates, Lilly, Innocare, Genmab, Genentech, Vincerx Pharma
Travel, Accommodations, Expenses: Janssen Research & Development, AstraZeneca, Celgene, Dava Oncology, OM Pharmaceutical Industries, Kite, a Gilead Company, Physician Education Resources (PER)
Anita Kumar
Stock and Other Ownership Interests: BridgeBio
Consulting or Advisory Role: Celgene, Kite, a Gilead Company, AstraZeneca/MedImmune, Janssen
Research Funding: AbbVie/Genentech, Adaptive Biotechnologies, Celgene, Seattle Genetics, AstraZeneca/MedImmune, Pharmacyclics
Brian Hill
Honoraria: Pharmacyclics, Gilead Sciences, Genentech, AbbVie, Bayer, AstraZeneca, Novartis, Pfizer, Celgene, Karyopharm Therapeutics, Epizyme, BeiGene, MorphoSys
Consulting or Advisory Role: Novartis, Genentech, AbbVie, Gilead Sciences, Karyopharm Therapeutics, AstraZeneca, Epizyme, MorphoSys, BeiGene
Research Funding: AbbVie (Inst), Karyopharm Therapeutics (Inst), Celgene (Inst), Takeda (Inst), Amgen (Inst), Genentech (Inst), Kite/Gilead (Inst), TG Therapeutics (Inst)
Diego Villa
Honoraria: Roche Canada, Janssen, Gilead Sciences, Acerta Pharma/AstraZeneca, Celgene, AbbVie, BeiGene, Kyowa Kirin International, Sandoz
Consulting or Advisory Role: Roche Canada, Janssen, Gilead Sciences, Acerta Pharma/AstraZeneca, Celgene, AbbVie, BeiGene, Kyowa Kirin International, Sandoz
Research Funding: Roche (Inst), AstraZeneca Canada (Inst)
Brad Kahl
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Consulting or Advisory Role: Celgene, AbbVie, Pharmacyclics, Acerta Pharma, ADC Therapeutics, Genentech, Roche, AstraZeneca, BeiGene, Bayer, MEI Pharma, Kite/Gilead, MorphoSys, Janssen, Celgene (Inst), Incyte, Genmab
Research Funding: Genentech (Inst), Acerta Pharma (Inst), ADC Therapeutics (Inst), Celgene (Inst)
Kami Maddocks
Honoraria: Pharmacyclics, Celgene, Seattle Genetics, MorphoSys, BMS, Karyopharm Therapeutics, Kite, a Gilead Company, ADC Therapeutics, Genmab, Lilly, Genentech, Epizyme, AstraZeneca/Merck, BeiGene, Incyte
Research Funding: Pharmacyclics, Merck, Celgene (Inst)
Natalie S. Grover
Stock and Other Ownership Interests: Sangamo Therapeutics
Honoraria: Kite, a Gilead Company, ADC Therapeutics, Novartis
Research Funding: Genentech
Uncompensated Relationships: Tessa Therapeutics
Keqin Qi
Employment: Janssen
Stock and Other Ownership Interests: Janssen
Lori Parisi
Employment: Johnson & Johnson
Stock and Other Ownership Interests: Johnson & Johnson
Travel, Accommodations, Expenses: Johnson & Johnson
Katherine Daly
Employment: Janssen
Stock and Other Ownership Interests: Janssen, Pfizer
Angeline Zhu
Employment: Janssen Oncology
Stock and Other Ownership Interests: Janssen Oncology
Gilles Salles
Stock and Other Ownership Interests: Owkin
Honoraria: AbbVie, Bayer, Regeneron
Consulting or Advisory Role: Roche/Genentech, Janssen, Novartis, MorphoSys, Epizyme, Genmab, Debiopharm Group, VelosBio, BMS, BeiGene, Incyte, Miltenyi Biotec, Ipsen, AbbVie, Kite/Gilead, Loxo/Lilly, Molecular Partners, Nordic Nanovector, RAPT Therapeutics, Takeda
No other potential conflicts of interest were reported.
See accompanying Oncology Grand Rounds on page 452
PRIOR PRESENTATION
Presented in part at the American Society of Clinical Oncology virtual meeting, June 4-8, 2021 (abstr 7504), and the European Hematology Association virtual meeting, June 13-17, 2021 (abstr EP785).
SUPPORT
Supported by Janssen Research & Development LLC.
P.M. and J.B.C. contributed equally to this work.
AUTHOR CONTRIBUTIONS
Conception and design: Peter Martin, Jonathon B. Cohen, Michael Wang, Brian Hill, Katherine Daly, Angeline Zhu, Gilles Salles
Provision of study materials or patients: Brian Hill, Diego Villa, Brad Kahl
Collection and assembly of data: Peter Martin, Jonathon B. Cohen, Michael Wang, Brian Hill, Diego Villa, Natalie S. Grover, Angeline Zhu
Data analysis and interpretation: Peter Martin, Jonathon B. Cohen, Michael Wang, Anita Kumar, Brian Hill, Diego Villa, Jeffrey M. Switchenko, Brad Kahl, Kami Maddocks, Natalie S. Grover, Keqin Qi, Lori Parisi, Katherine Daly, Angeline Zhu
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Treatment Outcomes and Roles of Transplantation and Maintenance Rituximab in Patients With Previously Untreated Mantle Cell Lymphoma: Results From Large Real-World Cohorts
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Peter Martin
Consulting or Advisory Role: Janssen, BeiGene, Karyopharm Therapeutics, Kite/Gilead, Verastem, ADC Therapeutics, Bristol Myers Squibb/Celgene, Epizyme, Merck, MorphoSys, Takeda
Research Funding: Karyopharm Therapeutics (Inst)
Jonathon B. Cohen
Consulting or Advisory Role: AbbVie, Janssen, Loxo, Kite/Gilead, AstraZeneca, Aptitude Medical, Adicet Bio, Adaptive Biotechnologies
Research Funding: Celgene (Inst) (Inst), Janssen (Inst), Novartis (Inst), Takeda (Inst), AI Therapeutics (Inst), Genentech (Inst), ASH (Inst), Lymphoma Research Foundation (Inst), Loxo (Inst), BioInvent (Inst), AstraZeneca (Inst)
Michael Wang
Honoraria: Janssen Research & Development, Dava Oncology, OM Pharmaceutical Industries, AstraZeneca, CAHON, Hebei Cancer Prevention Federation, Mumbai Hematology Group, Acerta Pharma, Chinese Anti-Cancer Association, BeiGene, Clinical Care Options, Epizyme, Imedex, Kite, a Gilead Company, Miltenyi Biomedicine, Moffit Cancer Center, Physicians' Education Resource, Breast-Gynecological International Cancer Society, Pharmacyclics/Janssen, Eastern Virginia Medical School, Leukemia & Lymphoma Society, LLC TS Oncology, Medscape, Meeting Minds Experts, OncLive/MJH Life Sciences, Practice Point Communications, First Hospital Zhejiang University, BioInvent
Consulting or Advisory Role: AstraZeneca, Janssen Research & Development, Juno Therapeutics, BioInvent, Pharmacyclics/Janssen, Loxo, Kite, a Gilead Company, InnoCare, Oncternal Therapeutics, CStone Pharmaceuticals, Genentech, BeiGene, DTRM, Epizyme, Miltenyi Biomedicine, VelosBio, Deciphera, Lilly, PeproMene
Research Funding: AstraZeneca, Janssen Research & Development, Pharmacyclics, Kite, a Gilead Company, Juno Therapeutics, BeiGene, Acerta Pharma, Oncternal Therapeutics, BioInvent, Loxo, VelosBio, Celgene, Molecular Templates, Lilly, Innocare, Genmab, Genentech, Vincerx Pharma
Travel, Accommodations, Expenses: Janssen Research & Development, AstraZeneca, Celgene, Dava Oncology, OM Pharmaceutical Industries, Kite, a Gilead Company, Physician Education Resources (PER)
Anita Kumar
Stock and Other Ownership Interests: BridgeBio
Consulting or Advisory Role: Celgene, Kite, a Gilead Company, AstraZeneca/MedImmune, Janssen
Research Funding: AbbVie/Genentech, Adaptive Biotechnologies, Celgene, Seattle Genetics, AstraZeneca/MedImmune, Pharmacyclics
Brian Hill
Honoraria: Pharmacyclics, Gilead Sciences, Genentech, AbbVie, Bayer, AstraZeneca, Novartis, Pfizer, Celgene, Karyopharm Therapeutics, Epizyme, BeiGene, MorphoSys
Consulting or Advisory Role: Novartis, Genentech, AbbVie, Gilead Sciences, Karyopharm Therapeutics, AstraZeneca, Epizyme, MorphoSys, BeiGene
Research Funding: AbbVie (Inst), Karyopharm Therapeutics (Inst), Celgene (Inst), Takeda (Inst), Amgen (Inst), Genentech (Inst), Kite/Gilead (Inst), TG Therapeutics (Inst)
Diego Villa
Honoraria: Roche Canada, Janssen, Gilead Sciences, Acerta Pharma/AstraZeneca, Celgene, AbbVie, BeiGene, Kyowa Kirin International, Sandoz
Consulting or Advisory Role: Roche Canada, Janssen, Gilead Sciences, Acerta Pharma/AstraZeneca, Celgene, AbbVie, BeiGene, Kyowa Kirin International, Sandoz
Research Funding: Roche (Inst), AstraZeneca Canada (Inst)
Brad Kahl
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Consulting or Advisory Role: Celgene, AbbVie, Pharmacyclics, Acerta Pharma, ADC Therapeutics, Genentech, Roche, AstraZeneca, BeiGene, Bayer, MEI Pharma, Kite/Gilead, MorphoSys, Janssen, Celgene (Inst), Incyte, Genmab
Research Funding: Genentech (Inst), Acerta Pharma (Inst), ADC Therapeutics (Inst), Celgene (Inst)
Kami Maddocks
Honoraria: Pharmacyclics, Celgene, Seattle Genetics, MorphoSys, BMS, Karyopharm Therapeutics, Kite, a Gilead Company, ADC Therapeutics, Genmab, Lilly, Genentech, Epizyme, AstraZeneca/Merck, BeiGene, Incyte
Research Funding: Pharmacyclics, Merck, Celgene (Inst)
Natalie S. Grover
Stock and Other Ownership Interests: Sangamo Therapeutics
Honoraria: Kite, a Gilead Company, ADC Therapeutics, Novartis
Research Funding: Genentech
Uncompensated Relationships: Tessa Therapeutics
Keqin Qi
Employment: Janssen
Stock and Other Ownership Interests: Janssen
Lori Parisi
Employment: Johnson & Johnson
Stock and Other Ownership Interests: Johnson & Johnson
Travel, Accommodations, Expenses: Johnson & Johnson
Katherine Daly
Employment: Janssen
Stock and Other Ownership Interests: Janssen, Pfizer
Angeline Zhu
Employment: Janssen Oncology
Stock and Other Ownership Interests: Janssen Oncology
Gilles Salles
Stock and Other Ownership Interests: Owkin
Honoraria: AbbVie, Bayer, Regeneron
Consulting or Advisory Role: Roche/Genentech, Janssen, Novartis, MorphoSys, Epizyme, Genmab, Debiopharm Group, VelosBio, BMS, BeiGene, Incyte, Miltenyi Biotec, Ipsen, AbbVie, Kite/Gilead, Loxo/Lilly, Molecular Partners, Nordic Nanovector, RAPT Therapeutics, Takeda
No other potential conflicts of interest were reported.
REFERENCES
- 1.Dreyling M, Campo E, Hermine O, et al. : Newly diagnosed and relapsed mantle cell lymphoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 28:iv62-iv71, 2017 [DOI] [PubMed] [Google Scholar]
- 2.Jain P, Wang M: Mantle cell lymphoma: 2019 update on the diagnosis, pathogenesis, prognostication, and management. Am J Hematol 94:710-725, 2019 [DOI] [PubMed] [Google Scholar]
- 3.McKay P, Leach M, Jackson B, et al. : Guideline for the management of mantle cell lymphoma. Br J Haematol 182:46-62, 2018 [DOI] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) B-Cell Lymphomas/Mantle Cell Lymphoma. Version 3.2022, April 25, 2022. https://www.nccn.org/professionals/physician_gls/pdf/b-cell.pdf [Google Scholar]
- 5.Rummel M, Knauf W, Goerner M, et al. : Two years rituximab maintenance vs. observation after first-line treatment with bendamustine plus rituximab (B-R) in patients with mantle cell lymphoma: First results of a prospective, randomized, multicenter phase II study (a subgroup study of the StiL NHL7-2008 MAINTAIN trial). J Clin Oncol 34, 2016. (suppl 15; abstr 7503) [Google Scholar]
- 6.Monga N, Tam C, Garside J, et al. : Clinical efficacy and safety of first-line treatments in patients with mantle cell lymphoma: A systematic literature review. Crit Rev Oncol Hematol 158:103212, 2021 [DOI] [PubMed] [Google Scholar]
- 7.Howard OM, Gribben JG, Neuberg DS, et al. : Rituximab and CHOP induction therapy for newly diagnosed mantle-cell lymphoma: Molecular complete responses are not predictive of progression-free survival. J Clin Oncol 20:1288-1294, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Hermine O, Hoster E, Walewski J, et al. : Addition of high-dose cytarabine to immunochemotherapy before autologous stem-cell transplantation in patients aged 65 years or younger with mantle cell lymphoma (MCL younger): A randomised, open-label, phase 3 trial of the European Mantle Cell Lymphoma Network. Lancet 388:565-575, 2016 [DOI] [PubMed] [Google Scholar]
- 9.Kumar A, Sha F, Toure A, et al. : Patterns of survival in patients with recurrent mantle cell lymphoma in the modern era: Progressive shortening in response duration and survival after each relapse. Blood Cancer J 9:50, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birnbaum B, Nussbaum N, Seidl-Rathkopf K, et al. : Model-assisted cohort selection with bias analysis for generating large-scale. arXiv. 2020. 10.48550/arXiv.2001.09765 [DOI] [Google Scholar]
- 11.Ma X, Long L, Moon S, et al. : Comparison of population characteristics in real-world clinical oncology databases in the US: Flatiron Health. medRxiv. 2020. 10.1101/2020.03.16.20037143 [DOI] [Google Scholar]
- 12.Zhang Q, Gossai A, Monroe S, et al. : Validation analysis of a composite real-world mortality endpoint for patients with cancer in the United States. Health Serv Res 56:1281-1287, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karmali R, Switchenko JM, Goyal S, et al. : Multi-center analysis of practice patterns and outcomes of younger and older patients with mantle cell lymphoma in the rituximab era. Am J Hematol 96:1374-1384, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Griffith SD, Miksad RA, Calkins G, et al. : Characterizing the feasibility and performance of real-world tumor progression end points and their association with overall survival in a large advanced non-small-cell lung cancer data set. JCO Clin Cancer Inform 3:1-13, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walker B, Boyd M, Aguilar K, et al. : Comparisons of real-world time-to-event end points in oncology research. JCO Clin Cancer Inform 5:45-46, 2021 [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Appius A, Pattipaka T, et al. : Real-world management of patients with epidermal growth factor receptor (EGFR) mutation-positive non-small-cell lung cancer in the USA. PLoS One 14:e0209709, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stewart M, Norden AD, Dreyer N, et al. : An exploratory analysis of real-world end points for assessing outcomes among immunotherapy-treated patients with advanced non-small-cell lung cancer. JCO Clin Cancer Inform 3:1-15, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drakaki A, Dhillon PK, Wakelee H, et al. : Association of baseline systemic corticosteroid use with overall survival and time to next treatment in patients receiving immune checkpoint inhibitor therapy in real-world US oncology practice for advanced non-small cell lung cancer, melanoma, or urothelial carcinoma. Oncoimmunology 9:1824645, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoster E, Dreyling M, Klapper W, et al. : A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood 111:558-565, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Riedell PA, Hamadani M, Ahn KW, et al. : Outcomes and utilization trends of front-line autologous hematopoietic cell transplantation for mantle cell lymphoma. Transplant Cell Ther 27:911.e1-911.e7, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sawalha Y, Radivoyevitch T, Jia X, et al. : The impact of socioeconomic disparities on the use of upfront autologous stem cell transplantation for mantle cell lymphoma. Leuk Lymphoma 63:335-343, 2022 [DOI] [PubMed] [Google Scholar]
- 22.Gerson JN, Handorf E, Villa D, et al. : Survival outcomes of younger patients with mantle cell lymphoma treated in the rituximab era. J Clin Oncol 37:471-480, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Villa D, Sehn LH, Savage KJ, et al. : Bendamustine and rituximab as induction therapy in both transplant-eligible and -ineligible patients with mantle cell lymphoma. Blood Adv 4:3486-3494, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glimelius I, Smedby KE, Albertsson-Lindblad A, et al. : Unmarried or less-educated patients with mantle cell lymphoma are less likely to undergo a transplant, leading to lower survival. Blood Adv 5:1638-1647, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le Gouill S, Thieblemont C, Oberic L, et al. : Rituximab after autologous stem-cell transplantation in mantle-cell lymphoma. N Engl J Med 377:1250-1260, 2017 [DOI] [PubMed] [Google Scholar]
- 26.Rummel MJ, Niederle N, Maschmeyer G, et al. : Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: An open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet 381:1203-1210, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Robak T, Huang H, Jin J, et al. : Bortezomib-based therapy for newly diagnosed mantle-cell lymphoma. N Engl J Med 372:944-953, 2015 [DOI] [PubMed] [Google Scholar]
- 28.Kluin-Nelemans HC, Hoster E, Hermine O, et al. : Treatment of older patients with mantle cell lymphoma (MCL): Long-term follow-up of the randomized European MCL elderly trial. J Clin Oncol 38:248-256, 2020 [DOI] [PubMed] [Google Scholar]
- 29.Kluin-Nelemans HC, Hoster E, Hermine O, et al. : Treatment of older patients with mantle-cell lymphoma. N Engl J Med 367:520-531, 2012 [DOI] [PubMed] [Google Scholar]
- 30.Rummel M, Kaiser U, Balser C, et al. : Bendamustine plus rituximab versus fludarabine plus rituximab for patients with relapsed indolent and mantle-cell lymphomas: A multicentre, randomised, open-label, non-inferiority phase 3 trial. Lancet Oncol 17:57-66, 2016 [DOI] [PubMed] [Google Scholar]
- 31.Smith MR, Jegede O, Martin P, et al. : ECOG-ACRIN E1411 randomized phase 2 trial of bendamustine-rituximab (BR)-based induction followed by rituximab (R) ± lenalidomide (L) consolidation for mantle cell lymphoma: Effect of adding bortezomib to front-line BR induction on PFS. J Clin Oncol 39, 2021. (suppl 15; abstr 7503) [Google Scholar]
- 32.Dreyling M, Lenz G, Hoster E, et al. : Early consolidation by myeloablative radiochemotherapy followed by autologous stem cell transplantation in first remission significantly prolongs progression-free survival in mantle-cell lymphoma: Results of a prospective randomized trial of the European MCL Network. Blood 105:2677-2684, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Zoellner AK, Unterhalt M, Stilgenbauer S, et al. : Long-term survival of patients with mantle cell lymphoma after autologous haematopoietic stem-cell transplantation in first remission: A post-hoc analysis of an open-label, multicentre, randomised, phase 3 trial. Lancet Haematol 8:e648-e657, 2021 [DOI] [PubMed] [Google Scholar]
- 34.Lindblad A, Palsdottir T, Glimelius I, et al. : Real-world outcome in mantle cell lymphoma—A study of relative and overall survival in patients primarily treated with R-bendamustine, R-CHOP or the NORDIC MCL2 regimen in Sweden 2007-2017. HemaSphere 4:72, 2020. (suppl 1) [Google Scholar]