PURPOSE
In the phase II ELOQUENT-3 trial (ClinicalTrials.gov identifier: NCT02654132), elotuzumab combined with pomalidomide/dexamethasone (EPd) significantly improved progression-free survival (PFS) versus pomalidomide/dexamethasone (Pd) in patients with relapsed/refractory multiple myeloma (RRMM) previously treated with lenalidomide and a proteasome inhibitor (PI). Here, we present the final overall survival (OS) results.
METHODS
Patients with RRMM who had received ≥ 2 prior lines of therapy, with disease refractory to last therapy and either refractory or relapsed and refractory to lenalidomide and a PI were randomly assigned (1:1) to receive EPd or Pd. The primary end point was PFS per investigator assessment. ORR and OS were secondary end points planned to be tested hierarchically.
RESULTS
A total of 117 patients were randomly assigned to EPd (n = 60) and Pd (n = 57). Among treated patients (EPd 60, Pd 55), there were 37 (61.7%) deaths in the EPd group and 41 (74.5%) in the Pd group, most commonly because of disease progression (EPd 41.7%, Pd 49.1%). Median (95% CI) OS was significantly improved with EPd (29.8 [22.9 to 45.7] months) versus Pd (17.4 [13.8 to 27.7] months), with a hazard ratio of 0.59 (95% CI, 0.37 to 0.93; P = .0217). OS benefit with EPd was observed in most patient subgroups. The safety profile of EPd was consistent with prior reports with no new safety signals detected.
CONCLUSION
EPd demonstrated a statistically significant improvement in OS versus Pd in patients with RRMM previously treated with lenalidomide and a PI who had disease refractory to last therapy. In this setting, ELOQUENT-3 is the first randomized study of a triplet regimen incorporating a monoclonal antibody and Pd to improve both PFS and OS significantly.
INTRODUCTION
Despite substantial improvements in multiple myeloma (MM) therapies in the past 10-15 years, the 5-year relative survival rate is 55.6%.1 MM is mostly incurable and associated with multiple relapses.2 Extending survival in patients with relapsed/refractory MM (RRMM) is particularly challenging for those who have received multiple lines of therapy and are refractory to an immunomodulatory drug and a proteasome inhibitor (PI).3 As most patients will eventually develop disease that is relapsed or refractory to lenalidomide and PIs, additional treatments are needed. Combination regimens with monoclonal antibodies have offered another treatment option for RRMM.
CONTEXT
Key Objective
Multiple myeloma (MM) is associated with multiple relapses, and most patients will eventually become relapsed or refractory to lenalidomide and proteasome inhibitors (PIs). Regimens improving survival among these patients are needed. The primary analysis of ELOQUENT-3 showed elotuzumab combined with pomalidomide/dexamethasone (EPd) significantly improved progression-free survival versus pomalidomide/dexamethasone (Pd) in patients with relapsed/refractory MM previously treated with lenalidomide and a PI. This study reports the final overall survival (OS) results.
Knowledge Generated
EPd significantly improved median OS versus Pd. Improved OS with EPd was also observed in most patient subgroups.
Relevance
EPd is a triplet consisting of a monoclonal antibody and Pd that has shown significant OS benefit in a randomized study for patients with relapsed/refractory MM who received at least two prior therapies including lenalidomide and a PI. These results demonstrate that EPd can provide benefits in the third-line or later setting.
Elotuzumab is a humanized immunoglobulin G1 immunostimulatory monoclonal antibody that binds to signaling lymphocytic activation molecule F7, a glycoprotein highly expressed on the surface of MM cells and natural killer cells.4,5 The mechanism of action of elotuzumab includes natural killer cell–mediated antibody-dependent cellular cytotoxicity on MM cells, direct activation of natural killer cells, and macrophage-mediated killing of MM cells.4-9 In the setting of newly diagnosed MM (NDMM), treatment combinations including elotuzumab have not led to improved efficacy.10,11 In the phase III ELOQUENT-1 study, elotuzumab plus lenalidomide/dexamethasone (ERd) did not improve progression-free survival (PFS) compared with lenalidomide/dexamethasone (Rd) in patients with NDMM not eligible for stem-cell transplantation.12 Elotuzumab-based combinations have, however, led to improved outcomes in patients with RRMM, despite elotuzumab showing limited single-agent activity in this setting.13 In the phase III ELOQUENT-2 trial, ERd significantly improved PFS compared with Rd in patients with RRMM who had one to three prior lines of therapy.14 The overall survival (OS) analysis of ELOQUENT-2 showed that ERd also significantly improved OS versus Rd, with a hazard ratio (HR) of 0.82 (P = .0408).15
Pomalidomide, like lenalidomide, is an immunomodulatory agent that exerts potent, direct tumoricidal and immune-enhancing effects via binding to cereblon, a component in the E3 ubiquitin ligase complex, and subsequent proteasomal degradation of the transcription factors Ikaros and Aiolos.16,17 However, pomalidomide is distinct from lenalidomide in its substrate degradation kinetics and gene modulation profile.16-19 Additionally, pomalidomide has shown antiproliferative activity in lenalidomide-resistant MM cell lines.17 Preclinical studies in mice have shown that the combination of elotuzumab plus pomalidomide/dexamethasone (EPd) has synergistic antimyeloma effects, and it was hypothesized that a similar effect would be observed in patients with RRMM.20 ELOQUENT-3 is a phase II trial evaluating the efficacy and safety of EPd compared with pomalidomide/dexamethasone (Pd) in patients with RRMM previously treated with lenalidomide and a PI.20 The primary analysis of ELOQUENT-3 showed EPd significantly improved PFS compared with Pd (HR, 0.54 [95% CI, 0.34 to 0.86]; two-sided stratified log-rank P = .008).20 Additionally, grade 3 or 4 adverse events (AEs), serious AEs (SAEs), and AEs leading to discontinuation were less frequent with EPd than Pd. On the basis of these results, EPd was approved in several regions including the United States, European Union, Japan, and Switzerland for adults with RRMM who have received ≥ 2 prior therapies including lenalidomide and a PI.21-24
The preliminary OS analysis (minimum follow-up of 9.1 months) of ELOQUENT-3 showed a trend toward improved OS with EPd versus Pd, although the data were still immature.20 A subsequent unplanned interim analysis (minimum follow-up of 18.3 months) continued to show an OS trend in favor of EPd.25 Here, we report the final OS analysis, with a minimum follow-up of 45 months from ELOQUENT-3.
METHODS
Trial Design and Patients
ELOQUENT-3 is a multicenter, randomized, controlled, open-label, phase II trial. The study design has been described previously.20 This trial was conducted in accordance with the International Conference on Harmonisation Good Clinical Practice guidelines. The Protocol (online only) was approved by the institutional review board or independent ethics committee at each participating trial center before the start of the trial. All patients provided written informed consent.
Patients age 18 years or older with measurable MM, an Eastern Cooperative Oncology Group performance status score of 0-2, and ≥ 2 prior lines of therapy were eligible for this study. Eligible patients had disease that was refractory (progression while receiving treatment or within 60 days after discontinuation) or relapsed and refractory (progression within 6 months after treatment discontinuation after achieving ≥ partial response) to lenalidomide and a PI. Eligible patients were also refractory to their most recent prior therapy. Patients with active plasma cell leukemia, creatinine clearance < 45 mL/min, or who had previously been treated with pomalidomide were excluded from the study.
Random Assignment and Treatment
Patients were randomly assigned in a 1:1 ratio to receive EPd or Pd, with random assignment stratified according to the number of prior lines of therapy (2 or 3 v ≥ 4) and International Staging System disease stage at enrollment (I or II v III).
Treatment was administered in 28-day cycles until disease progression, unacceptable toxicity, or withdrawal of consent. Patients in the EPd group received elotuzumab 10 mg/kg intravenously once daily on days 1, 8, 15, and 22 during cycles 1 and 2, and at a dose of 20 mg/kg once daily on day 1 of each cycle thereafter. Patients in both treatment groups received pomalidomide 4 mg orally once daily on days 1 through 21 of each cycle. Patients received oral dexamethasone 40 mg (or 20 mg in patients age older than 75 years) once weekly, except on days of elotuzumab administration, when patients in the EPd group received both oral (28 mg [or 8 mg in patients age older than 75 years]) and intravenous (8 mg) dexamethasone.
End Points
The primary end point was investigator-assessed PFS per International Myeloma Working Group consensus criteria. The secondary end points were ORR and OS.
Statistical Analysis
A hierarchical testing procedure was used to control the experiment-wise type I error at a two-sided 0.20 level and conducted in the following sequence: PFS, ORR, and OS. In the primary analysis, both PFS and ORR were statistically significant20,26; therefore, the entire two-sided α of .20 was passed down to OS. The final analysis of OS in all randomly assigned patients was to be conducted after 78 deaths had been observed. Given the sample size of 78 deaths, the OS study has 75% power to detect the HR of 0.64 with a type I error of 0.2. Haybittle–Peto α spending was chosen to account for the two previous descriptive analyses of OS with very little α spent since there was no intention to stop the study early with respect to previous OS results. Kaplan-Meier analysis was conducted to estimate OS distributions and test for the difference between the treatment groups. Statistical significance of treatment difference in OS was to be claimed if the two-sided stratified log-rank P value was smaller than .20. A stratified Cox proportional hazards model was used to estimate HR. In key patient subgroups, an unstratified Cox proportional hazards model was used to estimate HR.
RESULTS
Baseline Patient Demographics and Disease Characteristics
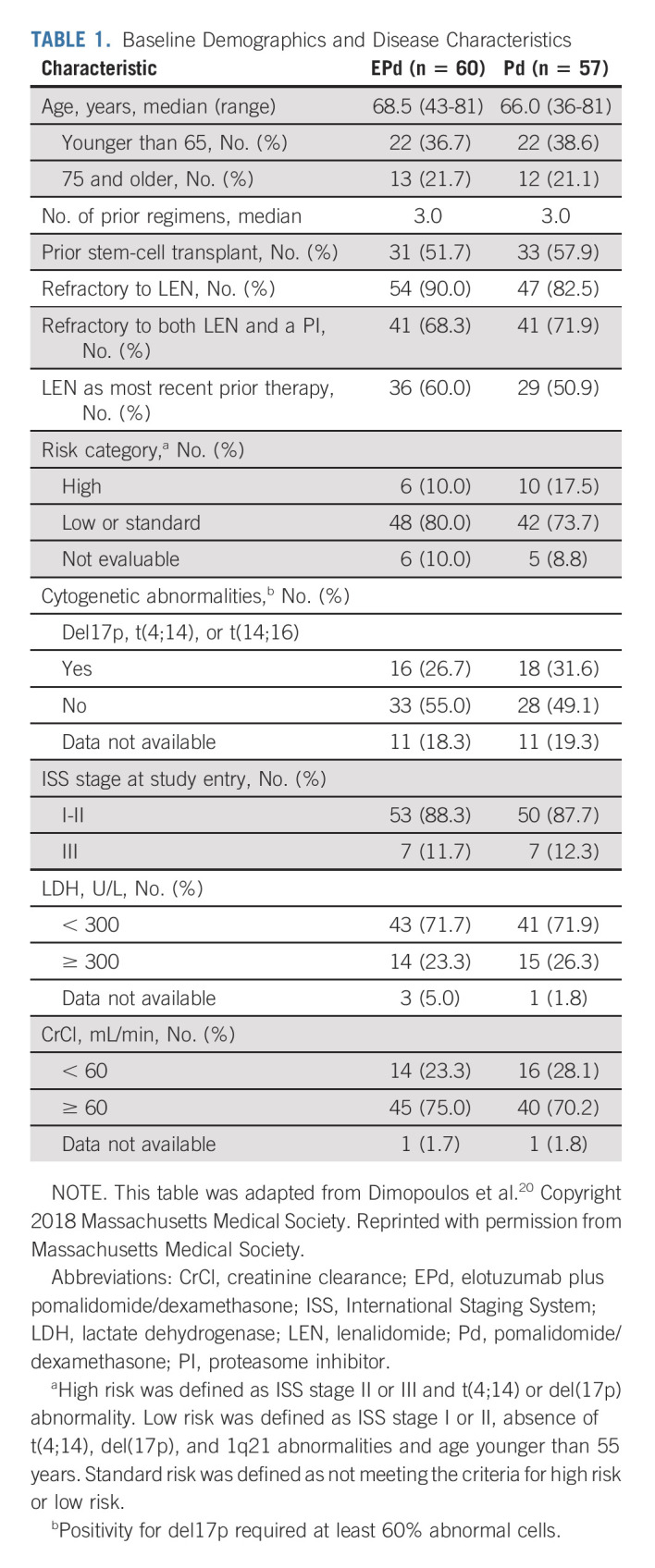
Patients were enrolled from March 2016 through April 2017. In total, 60 patients were randomly assigned to receive EPd and 57 to receive Pd; all 60 patients in the EPd group and 55 in the Pd group were treated (Fig 1). The baseline demographic and disease characteristics were generally balanced between groups and were previously reported at primary analysis20 (Table 1). The median age was 68.5 years in the EPd group and 66.0 years in the Pd group. Patients in each group had received a median of three prior lines of therapy; 68.3% of patients in the EPd group and 71.9% in the Pd group had disease refractory to both lenalidomide and a PI. More patients in the EPd group received lenalidomide as last line of therapy while patients in the Pd group exhibited a slightly higher rate of high-risk disease and slightly worse renal function.
FIG 1.
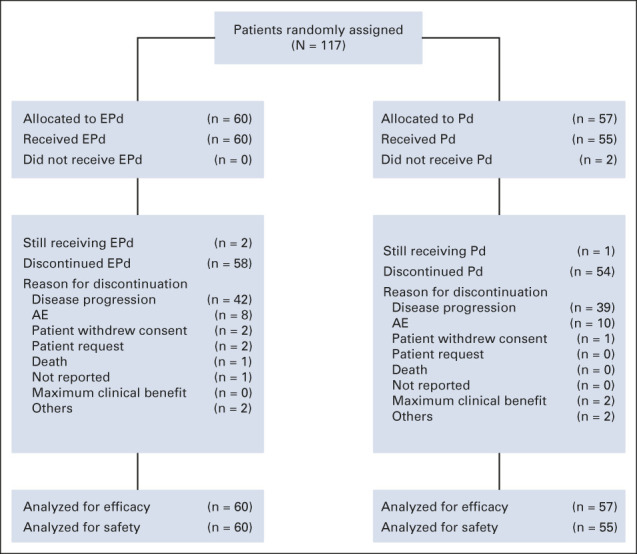
Patient disposition (CONSORT diagram). AE, adverse event; EPd, elotuzumab plus pomalidomide/dexamethasone; Pd, pomalidomide/dexamethasone.
TABLE 1.
Baseline Demographics and Disease Characteristics
Patient Disposition and Exposure
Patient disposition is shown in Figure 1. Treatment was discontinued in 58 (96.7%) patients receiving EPd and 54 (98.2%) receiving Pd. The most common reason for treatment discontinuation in both groups was disease progression, which occurred in 42 (70.0%) and 39 (70.9%) patients in the EPd and Pd groups, respectively. Patients in the EPd group received a median (range) of 9.0 (1-53) treatment cycles while those in the Pd group received 5.0 (1-50). The majority (80.0%) of patients in the EPd group achieved ≥ 90% relative dose intensity (RDI) of elotuzumab. Pomalidomide RDI was balanced between the two groups, with 51.7% of patients in the EPd group and 49.1% in the Pd group achieving an RDI of ≥ 90%. For patients age 75 years and younger (n = 93), 40.8% in the EPd group and 45.5% in the Pd group achieved a dexamethasone RDI of ≥ 90%. For patients age older than 75 years (n = 22), 63.6% and 54.5% in the EPd and Pd groups, respectively, achieved a dexamethasone RDI of ≥ 90%.
Overall Survival
At data cutoff (January 11, 2021), after a minimum follow-up of 45 months, 78 deaths had occurred (37 in the EPd group and 41 in the Pd group). The most common cause of death among treated patients in both groups was disease progression (EPd, 41.7%; Pd, 49.1%). The Kaplan-Meier curves for OS in the EPd and Pd groups displayed early and sustained separation (Fig 2) and demonstrated a statistically significant difference in OS between EPd and Pd (two-sided stratified log-rank P = .0217). The median (95% CI) OS was 29.8 (22.9 to 45.7) months with EPd and 17.4 (13.8 to 27.7) months with Pd. The HR for OS was 0.59 (95% CI, 0.37 to 0.93), corresponding to a 41% reduction in the risk of death with EPd versus Pd. OS rates were higher with EPd than Pd at 1 year (79% v 68%), 2 years (63% v 44%), and 3 years (39% v 29%).
FIG 2.
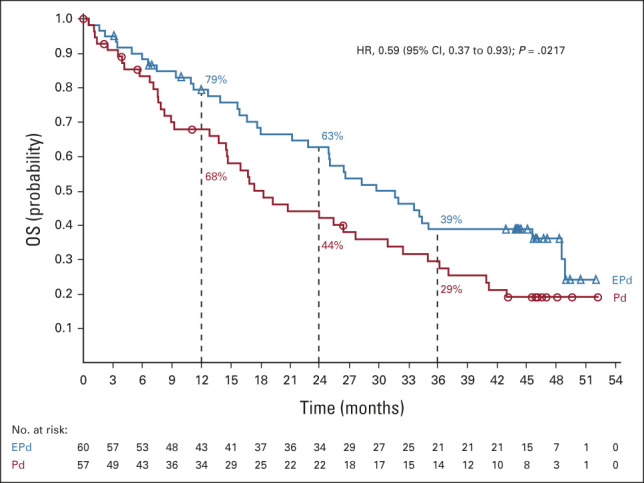
OS (all randomly assigned patients). EPd, elotuzumab plus pomalidomide/dexamethasone; HR, hazard ratio; OS, overall survival; Pd, pomalidomide/dexamethasone.
Subgroup Analyses of OS
The OS benefit observed with EPd was consistent across most subgroups, although sample sizes were small (Fig 3A). Notably, a trend toward improved OS with EPd versus Pd was observed in patients age 75 years and older (median, 34.4 v 14.7 months; HR, 0.36 [95% CI, 0.13 to 1.01]), patients with disease refractory to both lenalidomide and a PI (median, 28.3 v 17.4 months; HR, 0.74 [95% CI, 0.44 to 1.25]), patients with ≥ 4 prior lines of therapy (median, 29.8 v 16.0 months; HR, 0.42 [95% CI, 0.20 to 0.89]), and patients who had received lenalidomide as their most recent prior line of therapy (median, 32.0 v 20.8 months; HR, 0.55 [95% CI, 0.29 to 1.04]) (Fig 3B). Although the benefit of EPd over Pd was not observed in patients who had received prior stem-cell transplant (median OS, 26.6 v 27.7 months; HR, 1.05 [95% CI, 0.58 to 1.90]), this appears to be confounded by favorable risk characteristics of patients receiving Pd within this subgroup, with a higher proportion in the Pd group displaying normal baseline lactate dehydrogenase levels (81.8%) than in the EPd group (61.3%). When adjusted using multivariate analysis, improved OS was confirmed with EPd versus Pd among patients who had received prior stem-cell transplant and those who did not.
FIG 3.
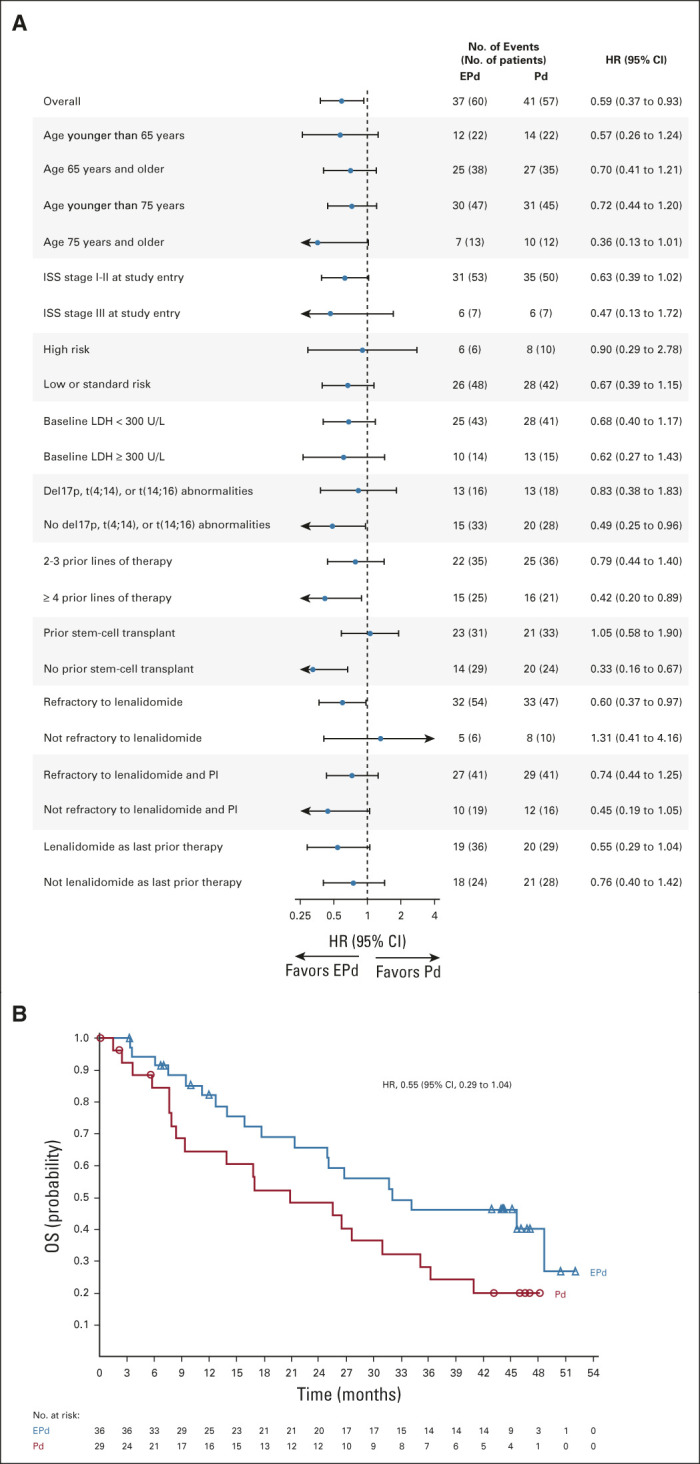
(A) OS in key patient subgroups. (B) Overall survival of patients receiving lenalidomide as their most recent prior line of therapy. NOTE: In (A), HR for the overall population was based on analysis stratified by ISS stage at study entry (I-II v III) and number of prior lines of therapy (2-3 v ≥ 4) at random assignment. HRs for the individual subgroups were based on unstratified analysis. EPd, elotuzumab plus pomalidomide/dexamethasone; HR, hazard ratio; ISS, International Staging System; LDH, lactate dehydrogenase; OS, overall survival; Pd, pomalidomide/dexamethasone; PI, proteasome inhibitor.
Subsequent Therapy
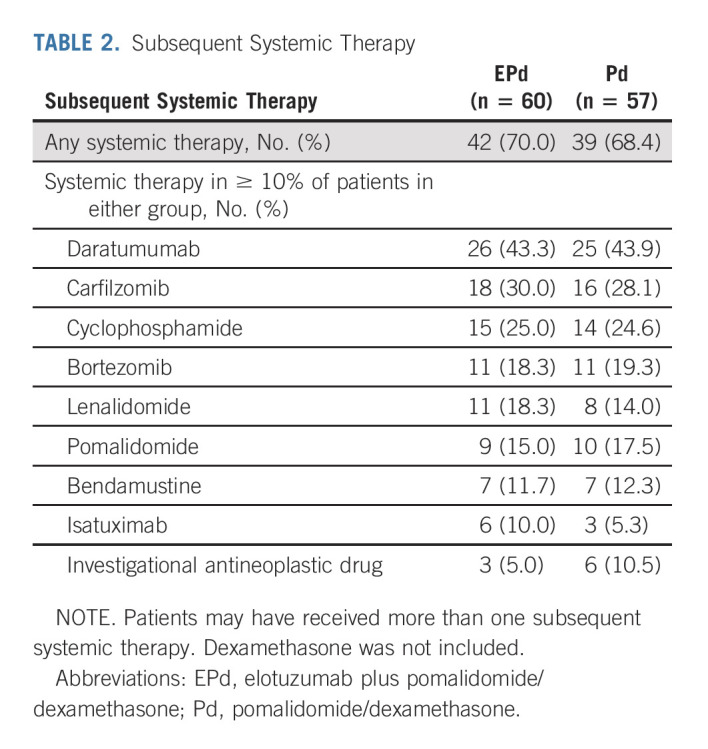
The types and frequency of subsequent therapies were similar in the EPd and Pd groups (Table 2). The most common subsequent systemic therapies received were daratumumab (EPd, 43.3%; Pd, 43.9%), carfilzomib (EPd, 30.0%; Pd, 28.1%), and cyclophosphamide (EPd, 25.0%; Pd, 24.6%). Among 26 (43.3%) patients in the EPd group and 25 (43.9%) patients in the Pd group who received daratumumab as a subsequent therapy, the OS benefit with EPd over Pd was numerically consistent with the overall study population (median, 33.6 v 26.5 months; HR, 0.76 [95% CI, 0.39 to 1.48]).
TABLE 2.
Subsequent Systemic Therapy
Safety
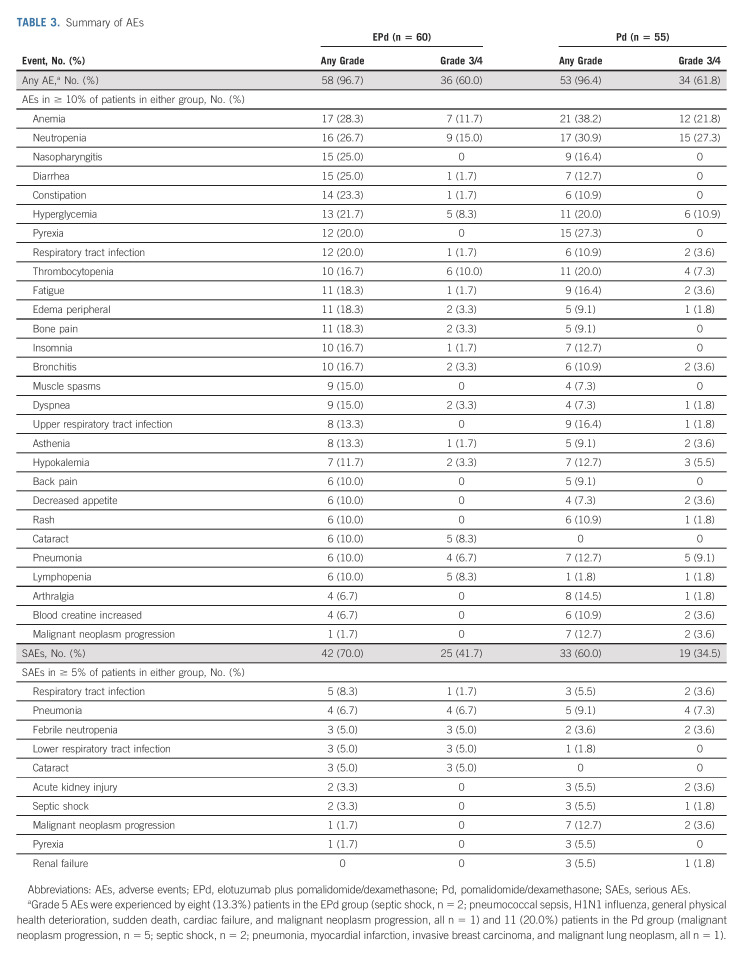
The most common any-grade AEs were anemia (EPd, 28.3%; Pd, 38.2%) and neutropenia (EPd, 26.7%; Pd, 30.9%) (Table 3). The most common grade 3/4 AEs were neutropenia (EPd, 15.0%; Pd, 27.3%) and anemia (EPd, 11.7%; Pd, 21.8%). Any-grade SAEs occurred in 70.0% of patients in the EPd group and 60.0% in the Pd group. The most common SAEs were respiratory tract infection (EPd, 8.3%; Pd, 5.5%) and pneumonia (EPd, 6.7%; Pd, 9.1%). Infections occurred in 70.0% of patients treated with EPd and 65.5% treated with Pd (25.0% and 21.8% were grade 3/4, respectively), while the exposure-adjusted infection rate was 196.1 per 100 patient-years in the EPd group and 234.2 per 100 patient-years in the Pd group. The most common any-grade AEs related to study treatment were neutropenia (EPd, 20%; Pd, 21.8%) and hyperglycemia (EPd, 20%; Pd, 12.7%). Second primary malignancies occurred in 6.7% (n = 4) of patients in the EPd group (prostate cancer, n = 2; pancreatic adenocarcinoma, n = 1; basal cell carcinoma, n = 1) and 3.6% (n = 2) in the Pd group (cholangiocarcinoma, n = 1; invasive breast carcinoma, n = 1). Two patients treated with EPd experienced infusion-related reactions (one grade 1 and one grade 2) that occurred during the first treatment cycle. AEs leading to treatment discontinuation occurred in 18.3% of patients treated with EPd and 23.6% treated with Pd while grade 3/4 AEs leading to discontinuation occurred in 11.7% and 10.9%, respectively. Infections leading to treatment discontinuation occurred in five patients (8.3%) in the EPd group and one patient (1.8%) in the Pd group. There were no treatment-related deaths in this study.
TABLE 3.
Summary of AEs
DISCUSSION
In this final analysis of OS from ELOQUENT-3 (minimum follow-up of 45 months), OS was significantly improved with EPd versus Pd in patients with RRMM who received at least two prior therapies including lenalidomide and a PI. The median OS was prolonged by over 12 months, and the risk of death was reduced by 41% with EPd versus Pd. Additionally, the safety profile of EPd was consistent with previous reports, and no new safety signals were detected.20,25
The prolongation of OS was consistent with the PFS benefit with EPd previously observed in this study.20,25 The Kaplan-Meier curves for OS showed early separation in favor of EPd, which was maintained throughout the duration of follow-up. The types and frequency of subsequent therapies received were balanced between treatment groups, suggesting that the effect on OS was primarily due to the addition of elotuzumab to Pd. Exploratory subgroup analyses suggested improved OS with EPd in subgroups generally associated with poor outcomes, including patients age 75 years and older, patients with disease refractory to lenalidomide and a PI, patients who received ≥ 4 lines of prior systemic therapy, and patients who had received lenalidomide as their most recent prior line of therapy.
Overall, the findings in ELOQUENT-3 complement the final OS results from ELOQUENT-2, which showed that ERd significantly improved OS compared with Rd in patients with one to three prior lines of therapy.15 Although elotuzumab-based combinations have been effective in patients with RRMM, data from ELOQUENT-1 show that ERd did not improve PFS or ORR in patients with NDMM not eligible for transplantation.12 Similar results were reported in the ENDURANCE trial, in which carfilzomib plus Rd did not improve PFS compared with bortezomib plus Rd in patients with NDMM, despite being approved for patients with RRMM.27 It is not clear why these regimens were effective in the relapsed/refractory setting but not in the frontline setting. Further investigation to determine optimal treatment sequencing is warranted.
To our knowledge, EPd is currently the only triplet consisting of a monoclonal antibody and Pd that has shown a significant OS benefit in a randomized study for patients with RRMM who received at least two prior therapies including lenalidomide and a PI. This may be, in part, because ELOQUENT-3 has the longest median follow-up duration of any randomized study investigating a monoclonal antibody–containing triplet regimen. In EQUULEUS, the registrational, noncomparative, phase Ib study of daratumumab plus Pd, the median OS was 17.5 months after a median follow-up of 13.1 months.28 To date, final OS data with daratumumab-based or isatuximab-based combinations have not been reported and are expected to be published in the future.29-32
The known safety profile and tolerability of EPd were maintained over long-term follow-up.20,25 Patients treated with EPd experienced fewer treatment discontinuations compared with patients treated with Pd despite longer treatment duration for EPd. Patients in the EPd group generally experienced fewer hematologic AEs including lower rates of anemia, neutropenia, and thrombocytopenia than patients in the Pd group. The addition of elotuzumab to Pd generally did not lead to an increase in the incidence of grade 3/4 AEs compared with Pd alone.
A limitation of this study was the small sample size. As a result, findings from the subgroup analyses of OS are limited and should be interpreted with caution. Additionally, as daratumumab was not yet approved in earlier lines of therapy at the time of this study, just three patients received daratumumab as a prior therapy, which precluded the analysis of outcomes with EPd after daratumumab. A substantial proportion of patients in the current RRMM population will have been exposed to daratumumab as well as lenalidomide and a PI.33 Daratumumab has been shown to deplete natural killer cells in patients with RRMM,34,35 which may affect the efficacy of subsequent treatments such as elotuzumab.36 Elotuzumab, however, has been shown to inhibit myeloma cell growth in vivo in the absence of functional natural killer cells and to exert comparable antitumor effects through natural killer cells and macrophages.9 Further exploration of the use of EPd in daratumumab-refractory patients is, therefore, warranted. Data from registries and observational studies such as MAMMOTH may shed light on the use of elotuzumab after daratumumab37 and optimal treatment sequencing, as well as translating these results to real-world practice.38
In conclusion, EPd demonstrated a statistically significant reduction in the risk of death versus Pd in patients with RRMM previously treated with lenalidomide and a PI, and a gain in median OS of 1 year. ELOQUENT-3 is the first randomized study of a triplet regimen incorporating a monoclonal antibody and Pd in this setting to show both PFS and OS benefits.
ACKNOWLEDGMENT
The authors thank the patients and families who made this study possible and the clinical study teams who participated in the trial. This study was supported by Bristol Myers Squibb and AbbVie Biotherapeutics. Professional medical writing support for this manuscript was provided by Richard Sora, PhD, of Caudex, funded by Bristol Myers Squibb.
Mihaela Popa McKiver
Employment: Briston Myers Squibb
Stock and Other Ownership Interests: Briston Myers Squibb
Ying-Ming Jou
Employment: Bristol Myers Squibb/Celgene
Stock and Other Ownership Interests: Bristol Myers Squibb/Celgene
Paul G. Richardson
Consulting or Advisory Role: Takeda, Karyopharm Therapeutics, Oncopeptides, Sanofi, Jazz Pharmaceuticals, Secura Bio, Bristol Myers Squibb/Celgene, GlaxoSmithKline, Regeneron, AstraZeneca
Research Funding: Celgene (Inst), Takeda (Inst), Bristol Myers Squibb (Inst), Oncopeptides (Inst), Karyopharm Therapeutics (Inst)
Dominik Dytfeld
Honoraria: Janssen, Celgene/Bristol Myers Squibb, Amgen, Takeda
Consulting or Advisory Role: Amgen, Janssen, Celgene/Bristol Myers Squibb, Takeda
Research Funding: Janssen, Celgene
Xavier Leleu
Honoraria: Janssen-Cilag, Celgene, Amgen, Novartis, Bristol Myers Squibb, Takeda, Sanofi, AbbVie, Merck, Roche, Karyopharm Therapeutics, CARsgen Therapeutics, Oncopeptides, GlaxoSmithKline
Consulting or Advisory Role: Janssen-Cilag, Celgene, Amgen, Takeda, Bristol Myers Squibb, Novartis, Merck, Gilead Sciences, AbbVie, Roche, Karyopharm Therapeutics, Oncopeptides, CARsgen Therapeutics, GlaxoSmithKline
Travel, Accommodations, Expenses: Takeda
David Yao
Employment: Bristol Myers Squibb, Janssen
Stock and Other Ownership Interests: Bristol Myers Squibb
Travel, Accommodations, Expenses: Bristol Myers Squibb, Janssen
Meletios A. Dimopoulos
Honoraria: Amgen, Takeda, Janssen-Cilag, Bristol Myers Squibb, Beigene
Consulting or Advisory Role: Amgen, Janssen-Cilag, Takeda, Bristol Myers Squibb, Beigene
Richard LeBlanc
Consulting or Advisory Role: Janssen, BMS Canada, Amgen, Sanofi, FORUS Therapeutics
Prianka Das
Employment: Bristol Myers Squibb/Celgene
Stock and Other Ownership Interests: Bristol Myers Squibb/Celgene (Inst)
Philippe Moreau
Honoraria: Celgene, Janssen-Cilag, Amgen, GlaxoSmithKline, AbbVie, Sanofi, Oncopeptides
Consulting or Advisory Role: Celgene, Janssen, Amgen, GlaxoSmithKline, Sanofi, AbbVie, Oncopeptides
Marc S. Raab
Honoraria: AbbVie, Bristol Myers Squibb/Celgene, Takeda, GlaxoSmithKline
Consulting or Advisory Role: Bristol Myers Squibb/Celgene (Inst), Amgen (Inst), GlaxoSmithKline (Inst), Janssen (Inst), Sanofi (Inst), Pfizer (Inst)
Research Funding: Bristol Myers Squibb/Celgene (Inst), Janssen (Inst), Sanofi (Inst), Novartis (Inst)
Travel, Accommodations, Expenses: AbbVie, Bristol Myers Squibb/Celgene, Takeda, GlaxoSmithKline, Amgen, Janssen, Sanofi, Pfizer
Jesús San-Miguel
Consulting or Advisory Role: Amgen (Inst), Celgene (Inst), Takeda (Inst), Bristol Myers Squibb (Inst), MSD (Inst), Novartis (Inst), Sanofi (Inst), Janssen (Inst), Roche (Inst), AbbVie (Inst), GlaxoSmithKline (Inst), Karyopharm Therapeutics (Inst), Secura Bio (Inst), Regeneron (Inst), Haemalogix (Inst)
No other potential conflicts of interest were reported
PRIOR PRESENTATION
Presented at the 19th International Myeloma Workshop (IMW), September 8-11, 2021, Vienna, Austria.
SUPPORT
Supported by Bristol Myers Squibb and AbbVie Biotherapeutics.
CLINICAL TRIAL INFORMATION
NCT02654132 (ELOQUENT-3)
DATA SHARING STATEMENT
Bristol Myers Squibb policy on data sharing may be found at https://www.bms.com/researchers-and-partners/clinical-trials-and-research/disclosure-commitment.html.
AUTHOR CONTRIBUTIONS
Conception and design: Meletios A. Dimopoulos, Marc S. Raab, Paul G. Richardson, Jesús San-Miguel
Provision of study materials or patients: Meletios A. Dimopoulos, Sebastian Grosicki, Philippe Moreau, Naoki Takezako, Mitsuo Hori, Xavier Leleu, Richard LeBlanc, Kenshi Suzuki, Marc S. Raab, Jesús San-Miguel
Collection and assembly of data: Meletios A. Dimopoulos, Sebastian Grosicki, Philippe Moreau, Naoki Takezako, Mitsuo Hori, Xavier Leleu, Richard LeBlanc, Kenshi Suzuki, Marc S. Raab, Jesús San‐Miguel
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Elotuzumab Plus Pomalidomide and Dexamethasone for Relapsed/Refractory Multiple Myeloma: Final Overall Survival Analysis From the Randomized Phase II ELOQUENT-3 Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Mihaela Popa McKiver
Employment: Briston Myers Squibb
Stock and Other Ownership Interests: Briston Myers Squibb
Ying-Ming Jou
Employment: Bristol Myers Squibb/Celgene
Stock and Other Ownership Interests: Bristol Myers Squibb/Celgene
Paul G. Richardson
Consulting or Advisory Role: Takeda, Karyopharm Therapeutics, Oncopeptides, Sanofi, Jazz Pharmaceuticals, Secura Bio, Bristol Myers Squibb/Celgene, GlaxoSmithKline, Regeneron, AstraZeneca
Research Funding: Celgene (Inst), Takeda (Inst), Bristol Myers Squibb (Inst), Oncopeptides (Inst), Karyopharm Therapeutics (Inst)
Dominik Dytfeld
Honoraria: Janssen, Celgene/Bristol Myers Squibb, Amgen, Takeda
Consulting or Advisory Role: Amgen, Janssen, Celgene/Bristol Myers Squibb, Takeda
Research Funding: Janssen, Celgene
Xavier Leleu
Honoraria: Janssen-Cilag, Celgene, Amgen, Novartis, Bristol Myers Squibb, Takeda, Sanofi, AbbVie, Merck, Roche, Karyopharm Therapeutics, CARsgen Therapeutics, Oncopeptides, GlaxoSmithKline
Consulting or Advisory Role: Janssen-Cilag, Celgene, Amgen, Takeda, Bristol Myers Squibb, Novartis, Merck, Gilead Sciences, AbbVie, Roche, Karyopharm Therapeutics, Oncopeptides, CARsgen Therapeutics, GlaxoSmithKline
Travel, Accommodations, Expenses: Takeda
David Yao
Employment: Bristol Myers Squibb, Janssen
Stock and Other Ownership Interests: Bristol Myers Squibb
Travel, Accommodations, Expenses: Bristol Myers Squibb, Janssen
Meletios A. Dimopoulos
Honoraria: Amgen, Takeda, Janssen-Cilag, Bristol Myers Squibb, Beigene
Consulting or Advisory Role: Amgen, Janssen-Cilag, Takeda, Bristol Myers Squibb, Beigene
Richard LeBlanc
Consulting or Advisory Role: Janssen, BMS Canada, Amgen, Sanofi, FORUS Therapeutics
Prianka Das
Employment: Bristol Myers Squibb/Celgene
Stock and Other Ownership Interests: Bristol Myers Squibb/Celgene (Inst)
Philippe Moreau
Honoraria: Celgene, Janssen-Cilag, Amgen, GlaxoSmithKline, AbbVie, Sanofi, Oncopeptides
Consulting or Advisory Role: Celgene, Janssen, Amgen, GlaxoSmithKline, Sanofi, AbbVie, Oncopeptides
Marc S. Raab
Honoraria: AbbVie, Bristol Myers Squibb/Celgene, Takeda, GlaxoSmithKline
Consulting or Advisory Role: Bristol Myers Squibb/Celgene (Inst), Amgen (Inst), GlaxoSmithKline (Inst), Janssen (Inst), Sanofi (Inst), Pfizer (Inst)
Research Funding: Bristol Myers Squibb/Celgene (Inst), Janssen (Inst), Sanofi (Inst), Novartis (Inst)
Travel, Accommodations, Expenses: AbbVie, Bristol Myers Squibb/Celgene, Takeda, GlaxoSmithKline, Amgen, Janssen, Sanofi, Pfizer
Jesús San-Miguel
Consulting or Advisory Role: Amgen (Inst), Celgene (Inst), Takeda (Inst), Bristol Myers Squibb (Inst), MSD (Inst), Novartis (Inst), Sanofi (Inst), Janssen (Inst), Roche (Inst), AbbVie (Inst), GlaxoSmithKline (Inst), Karyopharm Therapeutics (Inst), Secura Bio (Inst), Regeneron (Inst), Haemalogix (Inst)
No other potential conflicts of interest were reported
REFERENCES
- 1.Howlader N, Noone A, Krapcho M, et al. : SEER Cancer Statistics Review, 1975-2018. Bethesda, MD, National Cancer Institute, 2021 [Google Scholar]
- 2.Cornell RF, Kassim AA: Evolving paradigms in the treatment of relapsed/refractory multiple myeloma: Increased options and increased complexity. Bone Marrow Transplant 51:479-491, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar SK, Dimopoulos MA, Kastritis E, et al. : Natural history of relapsed myeloma, refractory to immunomodulatory drugs and proteasome inhibitors: A multicenter IMWG study. Leukemia 31:2443-2448, 2017 [DOI] [PubMed] [Google Scholar]
- 4.Hsi ED, Steinle R, Balasa B, et al. : CS1, a potential new therapeutic antibody target for the treatment of multiple myeloma. Clin Cancer Res 14:2775-2784, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tai YT, Dillon M, Song W, et al. : Anti-CS1 humanized monoclonal antibody HuLuc63 inhibits myeloma cell adhesion and induces antibody-dependent cellular cytotoxicity in the bone marrow milieu. Blood 112:1329-1337, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balasa B, Yun R, Belmar NA, et al. : Elotuzumab enhances natural killer cell activation and myeloma cell killing through interleukin-2 and TNF-alpha pathways. Cancer Immunol Immunother 64:61-73, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collins SM, Bakan CE, Swartzel GD, et al. : Elotuzumab directly enhances NK cell cytotoxicity against myeloma via CS1 ligation: Evidence for augmented NK cell function complementing ADCC. Cancer Immunol Immunother 62:1841-1849, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pazina T, James AM, MacFarlane AW, et al. : The anti-SLAMF7 antibody elotuzumab mediates NK cell activation through both CD16-dependent and -independent mechanisms. Oncoimmunology 6:e1339853, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurdi AT, Glavey SV, Bezman NA, et al. : Antibody-dependent cellular phagocytosis by macrophages is a novel mechanism of action of elotuzumab. Mol Cancer Ther 17:1454-1463, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldschmidt H, Mai EK, Bertsch U, et al. : Elotuzumab in combination with lenalidomide, bortezomib, dexamethasone and autologous transplantation for newly-diagnosed multiple myeloma: Results from the randomized phase III GMMG-HD6 trial. Blood 138:486, 2021. 33824974 [Google Scholar]
- 11.Usmani SZ, Hoering A, Ailawadhi S, et al. : Bortezomib, lenalidomide, and dexamethasone with or without elotuzumab in patients with untreated, high-risk multiple myeloma (SWOG-1211): Primary analysis of a randomised, phase 2 trial. Lancet Haematol 8:e45-e54, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dimopoulos MA, Richardson PG, Bahlis NJ, et al. : Addition of elotuzumab to lenalidomide and dexamethasone for patients with newly diagnosed, transplantation ineligible multiple myeloma (ELOQUENT-1): An open-label, multicentre, randomised, phase 3 trial. Lancet Haematol 9:e403-e414, 2022 [DOI] [PubMed] [Google Scholar]
- 13.Zonder JA, Mohrbacher AF, Singhal S, et al. : A phase 1, multicenter, open-label, dose escalation study of elotuzumab in patients with advanced multiple myeloma. Blood 120:552-559, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lonial S, Dimopoulos M, Palumbo A, et al. : Elotuzumab therapy for relapsed or refractory multiple myeloma. N Engl J Med 373:621-631, 2015 [DOI] [PubMed] [Google Scholar]
- 15.Dimopoulos MA, Lonial S, White D, et al. : Elotuzumab, lenalidomide, and dexamethasone in RRMM: Final overall survival results from the phase 3 randomized ELOQUENT-2 study. Blood Cancer J 10:91, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bjorklund CC, Lu L, Kang J, et al. : Rate of CRL4(CRBN) substrate Ikaros and Aiolos degradation underlies differential activity of lenalidomide and pomalidomide in multiple myeloma cells by regulation of c-Myc and IRF4. Blood Cancer J 5:e354, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lopez-Girona A, Mendy D, Ito T, et al. : Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia 26:2326-2335, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ocio EM, Fernández-Lázaro D, San-Segundo L, et al. : In vivo murine model of acquired resistance in myeloma reveals differential mechanisms for lenalidomide and pomalidomide in combination with dexamethasone. Leukemia 29:705-714, 2015 [DOI] [PubMed] [Google Scholar]
- 19.Sehgal K, Das R, Zhang L, et al. : Clinical and pharmacodynamic analysis of pomalidomide dosing strategies in myeloma: Impact of immune activation and cereblon targets. Blood 125:4042-4051, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dimopoulos MA, Dytfeld D, Grosicki S, et al. : Elotuzumab plus pomalidomide and dexamethasone for multiple myeloma. N Engl J Med 379:1811-1822, 2018 [DOI] [PubMed] [Google Scholar]
- 21.Empliciti® (Elotuzumab) [package insert]. Princeton, NJ : Bristol Myers Squibb; 2022
- 22.European Medicines Agency : Empliciti Summary of Product Characteristics. London, United Kingdom, European Medicines Agency, 2019 [Google Scholar]
- 23.Bristol Myers Squibb SA : Empliciti® (Elotuzumab) Product Information (Switzerland). 2019. https://www.swissmedicinfo.ch/ [Google Scholar]
- 24.Japan Pharmaceuticals and Medical Devices Agency : New Drugs Approved in FY 2019. Tokyo, Japan: Japan Pharmaceuticals and Medical Devices Agency, 2019 [Google Scholar]
- 25.Dimopoulos MA, Dytfeld D, Grosicki S, et al. : Elotuzumab plus pomalidomide and dexamethasone for relapsed/refractory multiple myeloma: Efficacy results after additional follow-up of the phase 2, randomized ELOQUENT-3 study. European Hematology Association (EHA) Annual Meeting; Amsterdam, the Netherlands (ed PS1370), 2019 [Google Scholar]
- 26.Dimopoulos MA, Dytfeld D, Grosicki S, et al. : Elotuzumab plus pomalidomide/dexamethasone (EPd) vs Pd for treatment of relapsed/refractory multiple myeloma (RRMM): Results from the phase 2, randomized open-label ELOQUENT-3 study. HemaSphere 3:626-627, 2018. (abstr PS1379) [Google Scholar]
- 27.Kumar SK, Jacobus SJ, Cohen AD, et al. : Carfilzomib or bortezomib in combination with lenalidomide and dexamethasone for patients with newly diagnosed multiple myeloma without intention for immediate autologous stem-cell transplantation (ENDURANCE): A multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol 21:1317-1330, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chari A, Suvannasankha A, Fay JW, et al. : Daratumumab plus pomalidomide and dexamethasone in relapsed and/or refractory multiple myeloma. Blood 130:974-981, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dimopoulos MA, Terpos E, Boccadoro M, et al. : Daratumumab plus pomalidomide and dexamethasone versus pomalidomide and dexamethasone alone in previously treated multiple myeloma (APOLLO): An open-label, randomised, phase 3 trial. Lancet Oncol 22:801-812, 2021 [DOI] [PubMed] [Google Scholar]
- 30.Richardson PG, Perrot A, San-Miguel JF, et al. : Updates from ICARIA-MM, a phase 3 study of isatuximab (Isa) plus pomalidomide and low-dose dexamethasone (Pd) versus Pd in relapsed and refractory multiple myeloma (RRMM). J Clin Oncol 39:8017, 2021 [Google Scholar]
- 31.Mateos MV, Sonneveld P, Hungria V, et al. : Daratumumab, bortezomib, and dexamethasone versus bortezomib and dexamethasone in patients with previously treated multiple myeloma: Three-year follow-up of CASTOR. Clin Lymphoma Myeloma Leuk 20:509-518, 2020 [DOI] [PubMed] [Google Scholar]
- 32.Bahlis NJ, Dimopoulos MA, White DJ, et al. : Daratumumab plus lenalidomide and dexamethasone in relapsed/refractory multiple myeloma: Extended follow-up of POLLUX, a randomized, open-label, phase 3 study. Leukemia 34:1875-1884, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rajkumar SV, Kumar S: Multiple myeloma current treatment algorithms. Blood Cancer J 10:94, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Casneuf T, Xu XS, Adams HC, 3rd, et al. : Effects of daratumumab on natural killer cells and impact on clinical outcomes in relapsed or refractory multiple myeloma. Blood Adv 1:2105-2114, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y, Zhang Y, Hughes T, et al. : Fratricide of NK cells in daratumumab therapy for multiple myeloma overcome by ex vivo-expanded autologous NK cells. Clin Cancer Res 24:4006-4017, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoylman E, Brown A, Perissinotti AJ, et al. : Optimal sequence of daratumumab and elotuzumab in relapsed and refractory multiple myeloma. Leuk Lymphoma 61:691-698, 2020 [DOI] [PubMed] [Google Scholar]
- 37.Gandhi UH, Cornell RF, Lakshman A, et al. : Outcomes of patients with multiple myeloma refractory to CD38-targeted monoclonal antibody therapy. Leukemia 33:2266-2275, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Richardson PG, San Miguel JF, Moreau P, et al. : Interpreting clinical trial data in multiple myeloma: Translating findings to the real-world setting. Blood Cancer J 8:109, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Bristol Myers Squibb policy on data sharing may be found at https://www.bms.com/researchers-and-partners/clinical-trials-and-research/disclosure-commitment.html.