PURPOSE:
CNS metastases are associated with decreased survival and quality of life for patients with metastatic breast cancer (MBC). Team-based care can optimize outcomes. IMPACT the Brain is a care coordination program that aims to improve access to team-based care for patients with MBC and CNS metastases.
MATERIALS AND METHODS:
Patients with MBC and CNS metastases were eligible for enrollment in this care coordination program. A team of specialists supported a dedicated program coordinator who provided navigation, education, specialty referral, and clinical trial screening. A unique intake form developed for the program created personalized, coordinated, and expedited specialty referrals. Patient-reported outcomes and caregiver burden assessments were collected on a voluntary basis throughout enrollment. Data were analyzed using descriptive statistics.
RESULTS:
Sixty patients were referred, and 53 were enrolled (88%). The median time to program enrollment was 1 day (range, 0-11) and to first visit was 5 days (range, 0-25). On the basis of the program intake form, 47 referrals were made across six specialties, most commonly physical medicine and rehabilitation (n = 10), radiation oncology (n = 10), and neuropsychology (n = 10). Nineteen patients (36%) consented to enroll in clinical trials.
CONCLUSION:
A tailored team-based care coordination program for patients with MBC and CNS metastases is feasible. Use of a unique intake screening form by a dedicated program coordinator resulted in faster time to first patient visit, enabled access to subspecialist care, and supported enrollment in clinical trials. Future research should focus on intervention development using PRO data collected in this care coordination program.
INTRODUCTION
Patients with metastatic breast cancer (MBC) and CNS metastases to the brain and the leptomeninges have complex care needs. The presence of CNS metastases is associated with decreased survival and quality of life for these patients.1-3 Although more patients are being diagnosed with CNS metastases because of improvement in diagnostic studies, the presence of brain metastases and leptomeningeal disease is historically an exclusion criterion in many clinical trials, limiting the evidence base for care delivery.4-6
The primary approach to management of CNS metastases is aimed at local control of disease, which typically involves either surgical or radiosurgical techniques.7,8 However, with the expansion of systemic therapy options for patients with MBC, treatment of brain metastases has become increasingly individualized.9 A team-based approach is essential to determine the sequence and prioritization of therapies given the spectrum of options for care in this setting. Furthermore, the advancement of systemic therapy options for patients with MBC and CNS metastases has been associated with longer survival.9,10 This has potential consequences regarding functional and quality-of-life outcomes for patients, whether from the disease itself or its therapies. As a result, optimal management for patients requires multidisciplinary, team-based cancer care.
IMPACT (Improving Metastatic breast cancer Patient Access to Coordinated Treatment) the Brain is a care coordination program at the University of Michigan Rogel Cancer Center that seeks to improve care of patients with breast cancer and CNS metastases through a personalized, multiteam care delivery model. The goal of this program is to improve care by achieving a faster time to patient intake, enabling access to personalized subspecialist care on the basis of a patient-tailored needs assessment, and improving awareness and enrollment in clinical trials for patients across different departments and specialties.
MATERIALS AND METHODS
Program Organization
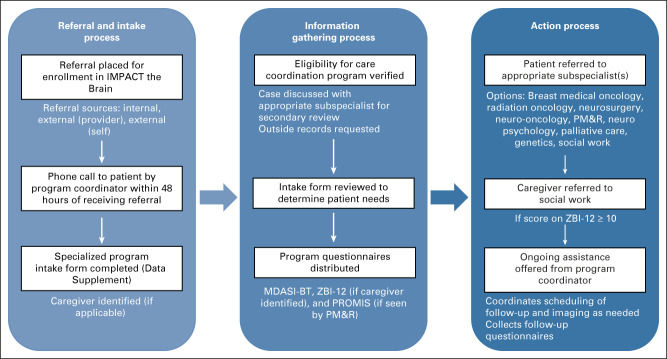
Patients with MBC with CNS metastases were eligible for enrollment in IMPACT the Brain. This team-based care coordination program was conducted according to the institutional review board guidelines. As outlined in Figure 1, a referral initiated the intake process, led by the program's registered nursing coordinator with experience in oncology care. The program coordinator completed an intake screening form designed specifically for this program with each patient via telephone. Demographic information was collected along with information about symptoms, home life, and patient interest in subspecialist care at the University of Michigan. Each participating subspecialty was highlighted in questions included in this form (Appendix Fig A1, online only). If eligible, a postintake case review occurred between the program coordinator and subspecialist team to ensure that the appropriate referrals were requested on the basis of the intake screening form. The team of subspecialists involved in this program included breast medical oncology, radiation oncology, neurosurgery, neuro-oncology, palliative care, genetics, rehabilitation psychology/neuropsychology, and physical medicine and rehabilitation (PM&R).
FIG 1.
Team-based approach to intake and follow-up for patients enrolled in IMPACT the brain. MDASI-BT, MD Anderson Symptom Inventory Brain Tumor; PM&R, physical medicine and rehabilitation; PROMIS, Patient-Reported Outcome Measurement Information System; ZBI-12, Short Form Zarit Burden Interview.
After the intake process, the program coordinator ensured that the requested subspecialist appointments were scheduled and completed. In addition, the coordinator followed up with each patient to assist with subsequent needs. If the patient identified a caregiver during the intake process, the program coordinator followed up with the caregiver and offered assistance to the caregiver as well. Bimonthly team meetings, which included the program coordinator and each participating subspecialist, were held to review patient progress and adjust program processes. As one example, the team meeting identified challenges in patient survey completion, which led to up-front notices to clinicians to remind the patient at their next visit. Each member of the team was responsible for submitting agenda items in advance of the meeting to encourage program functioning. Postevaluation reviews (initiated by the program coordinator either virtually or through electronic communication) were held for patients after they were seen by the necessary subspecialists, allowing for a directed discussion regarding patient progress and treatment course.
Data Collection
Patient demographic, referral, and follow-up data were collected in a prospective database. Additional information was extracted from the University of Michigan's electronic medical record. The following information was obtained for all enrolled patients: age, sex, race/ethnicity, human epidermal growth factor receptor 2 status, presence of extracranial metastatic disease, distance from home to the University, program referral source, date of program intake, date of first visit at the Rogel Cancer Center, and participation of a patient caregiver. The subsequent subspecialist referrals made and patient enrollment in clinical trials were tracked during program participation. Patient-reported outcome (PRO) measures, including the MD Anderson Symptom Inventory Brain Tumor (MDASI-BT), the PROMIS Cancer Function Brief 3D Profile (PROMIS), and the Short Form Zarit Burden Interview (ZBI-12) for caregivers, were collected on a voluntary basis during program enrollment. The PROMIS profile was collected at the first visit for patients seen by PM&R. It measures physical function, fatigue, and social participation in patients with cancer; 50.0 is the population mean and higher scores indicate a greater degree of a trait.11,12 The MDASI-BT is collected at baseline for all patients enrolled in the care coordination program and then every 6 months during program enrollment. It measures severity of cancer-related symptoms including interference with daily life. A score > 4 represents moderate symptoms, and a score > 6 is consistent with severe symptoms. The mean of the interference items can be used to represent overall symptom distress.13-15 If a patient identifies a caregiver and gives permission to contact them, the ZBI-12 is sent to the caregiver to evaluate for caregiver burden. A total score ≥ 10 on the ZBI-12 suggests mild caregiver burden while a score over 20 suggests high burden.16,17
Statistical Analysis
Data were analyzed using descriptive statistics, including frequencies for categorical data and means (standard deviations [SDs]), medians, ranges, and T-scores for continuous data.
RESULTS
Patient Demographics
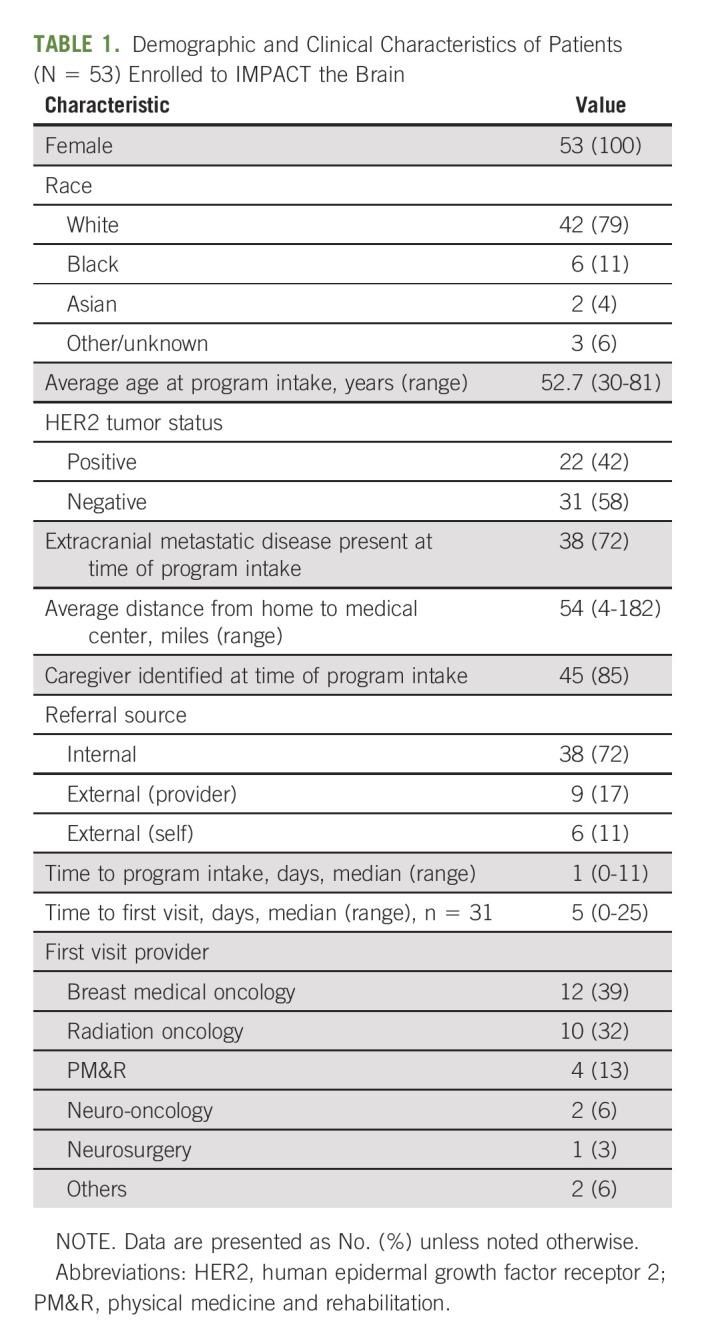
Between May 2020 and February 2022, 60 patients were referred and 53 patients (88%) were enrolled. Two patients died before completion of the intake process while one patient did not quality for enrollment because of absence of confirmed CNS disease. Of the remaining four patients who did not enroll, two declined to participate and two patients were unable to be reached after the referral was placed. The demographic information for enrolled participants is outlined in Table 1. All patients were female, and the average age at time of program intake was 52.7 years. Most patients were White (79%), and almost half (42%) had human epidermal growth factor receptor 2–positive disease. In addition to having CNS metastases, many patients had non-CNS metastatic disease at enrollment (72%). Almost all patients (85%) identified a primary caregiver during intake. The pattern for referrals and initial visit is described in Table 1. Most referrals came from within the University of Michigan; less than one-quarter of patients were referred by external providers. The first visit was typically conducted with breast medical oncology, followed by radiation oncology, and the median time to first visit was 5 days. If a patient was already following with a specialist of the care coordination team when enrolled in IMPACT the Brain, time to first visit was not calculated.
TABLE 1.
Demographic and Clinical Characteristics of Patients (N = 53) Enrolled to IMPACT the Brain
Subspecialist Referral Pattern and Clinical Trial Enrollment
The program coordinator made 47 referrals to subspecialists on the basis of the intake screening form used during program enrollment. Patients were frequently referred to radiation oncology (n = 10, 32%), PM&R (n = 10, 32%), and neuropsychology (n = 10, 32%), followed by social work (n = 6, 19%), breast medical oncology (n = 5, 16%), and neuro-oncology (n = 5, 16%). No new palliative care referrals were made (two patients had an existing palliative care provider). Nineteen patients (36%) consented to enroll in clinical trials—14 consents were for interventional studies, and nine consents were for noninterventional studies. Patients referred to PM&R were seen an average of 3.6 times (range, 1-8 visits). Treatment prescribed by the PM&R physicians included physical therapy (six times), medication management (six), home exercise program (five), occupational therapy (five), interventional pain procedure (four), orthotic (three), subspecialist referral outside of IMPACT (three), speech-language pathology (three), psychosocial support (two), diagnostic tests (two), and durable medical equipment (two).
Patient-Reported Outcomes
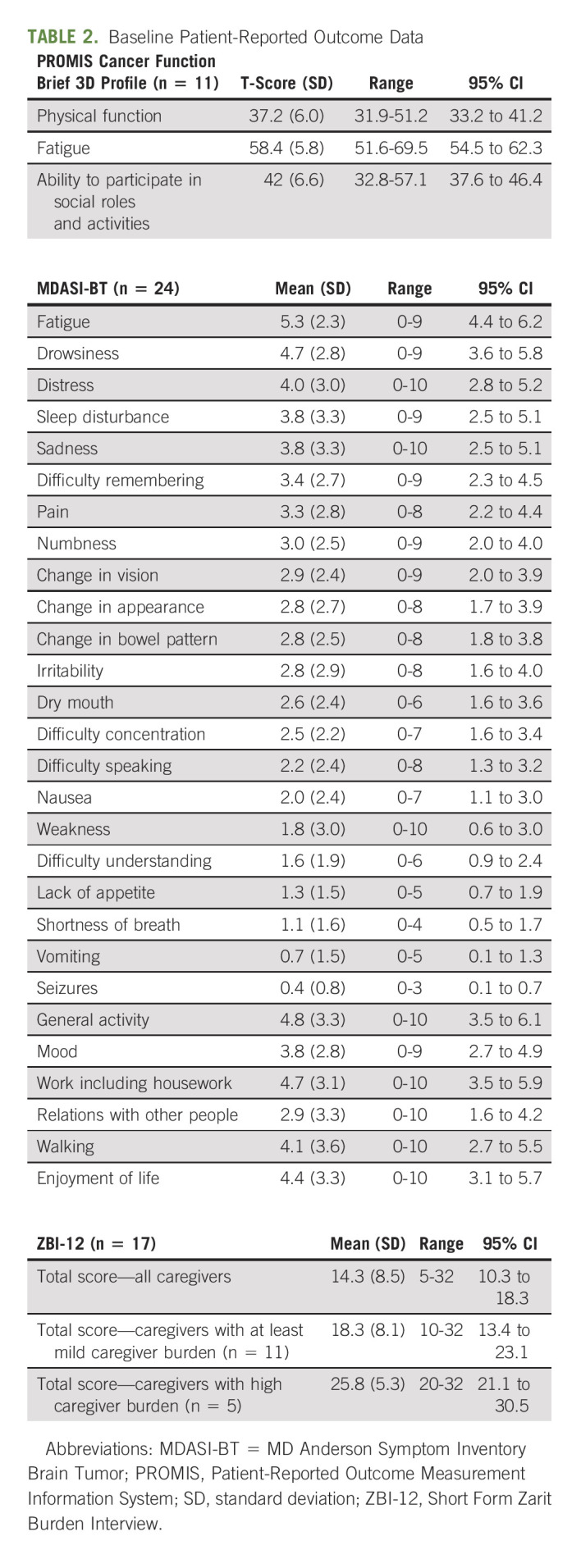
Table 2 describes the results from the baseline PRO measures. A total of 52 surveys were collected from 28 patients and 17 caregivers. The average PROMIS T-scores (n = 11) were 37.2 for physical function (range, 31.9-51.2), 58.4 for fatigue (51.6-69.5), and 42 for ability to participate in social roles and activities (32.8-57.1). For physical functioning, patients reported greatest difficulty with performing heavy housework and completing tasks because of fatigue. They also experienced trouble completing important work and participating in family activities. Unfortunately, longitudinal PROMIS data were not obtainable for most patients because of clinic infrastructure reductions, so changes in function were not recorded. The mean symptom severity score reported on the MDASI-BT (n = 24) was 2.7 (SD 1.2). Fatigue was the most severe symptom recorded, followed by drowsiness and distress. The mean interference score was 4.1 (SD 3.2); five patients (21%) reported moderate and six patients (25%) reported severe overall symptom distress. Seventeen caregivers completed the ZBI-12, and the average total score was 14.3. Of submitted questionnaires, 11 caregivers had a score of ≥ 10, and almost half (n = 5, 45.5%) had a score ≥ 20.
TABLE 2.
Baseline Patient-Reported Outcome Data
DISCUSSION
Despite barriers to cancer care coordination, the importance of multidisciplinary evaluation in the care of patients with brain metastases has been recognized by numerous governing medical bodies.18-21 Team science research suggests that a multiteam system, defined as a collective of two or more interdependent teams working together to achieve shared goals and commonly referred to as a team of teams can provide the highly coordinated care required of patients with cancer.22,23 IMPACT the Brain uniquely models the team of team principles by coordinating up-front screening and communication among a team of health care providers to tailor care to individual patients. Rather than a stand-alone clinic, this approach was developed to complement our multidisciplinary brain tumor clinic and weekly brain tumor board and optimize the current multidisciplinary approach standardized by other brain metastases clinics.24-26
Although there are benefits associated with multiteam systems, challenges occur when communication breaks down. Transactive memory systems, group-level knowledge sharing systems with shared awareness about each member's knowledge responsibilities, have been suggested to overcome these challenges.23,27 Transactive memory relies on three core components: differentiation, credibility, and coordination, each of which was incorporated during the design of IMPACT the Brain. This program comprises multiple subspecialists who focus on their specific areas of expertise, demonstrating differentiation, but has fostered credibility between the subspecialists by coordinating regular team meetings and patient case reviews.23,28,29 Additionally, a feedback loops exists where outcomes from the individual subspecialist's patient evaluation are communicated back to the group through postevaluation reviews, which allows for a collective discussion to help inform and guide the patient's care, fostering further credibility between the providers.
On the basis of our experience, enrollment in IMPACT the Brain enables access to subspecialist care for patients with MBC and brain metastases, particularly for specialties that may be underrepresented in multidisciplinary clinics (such as PM&R and neuropsychology). This is reflected in the utilization of PM&R by participants in our program: 13% of patients enrolled in IMPACT the Brain had their first visit with PM&R, compared with a previous study that found only 4.1% of oncology patients used consultation with PM&R throughout the study period.30 Although there are gaps in the literature regarding subspecialty referral and utilization in this population, including whether access to coordinated subspecialty care leads to improved clinical outcomes, systematically gathering these data through our multiteam system can serve as a platform for future pragmatic interventional research.31
The COVID-19 pandemic has posed unique challenges to the delivery of cancer care.32 When this program was initially designed, the goal was to enhance external provider referrals through physician outreach. However, when the program opened in March 2020, we experienced a low volume of external referrals, as there was a global decrease in external referrals and access to our institution during the pandemic. On the other hand, this also prompted our institution to expand the virtual care program through access and delivery of telehealth by our subspecialty teams. Given that the average distance to the University for our patients is 54 miles, with almost 20% of patients traveling over 100 miles, the delivery of virtual care provides valuable access for patients enrolled in the program.33
For a subset of women with MBC and CNS metastases, progression in the CNS has become the major life-limiting problem.10,34 As such, there is concern regarding whether a longitudinal care coordination program is feasible for this population. The results from enrollment in IMPACT the Brain demonstrated that it is feasible—most referred patients enrolled, and only two patients were deceased before program intake. The feasibility is, in part, due to the role of the nurse program coordinator, who provides the final component of a transactive memory system—coordination. The nurse program coordinator, acting as the main point of contact, combines input from all team members to ensure that accurate information is exchanged within the multiteam system and additional information is obtained when needed. This facilitates short time to program intake, communication across team members, and arrangement of subsequent follow-up.35 Other studies have highlighted delays in care because of long referral wait times while our team-based program with dedicated coordinator support had an average of less than 1 week to first visit for patients requiring additional subspecialist care.36-38 In comparison, for all patients seeking to establish care with a specialist at the University of Michigan, the average wait time for the first visit is estimated at 2-4 weeks. This estimate depends on several variables, including which specialty is being requested and if the patient is new to the medical system. However, enrollment in IMPACT the Brain clearly leads to a shorter time to first visit when compared with the average wait time across specialties at the University of Michigan.
Fewer than 5% of adult patients with cancer are estimated to enroll in clinical trials.39,40 For patients with primary brain tumors, one study found that only 21% participated in clinical trials despite the limited benefit of available standard therapies.41 Participation is likely less for patients with CNS metastases, as the presence of brain metastases excludes or restricts patient enrollment in many clinical trials.42-44 This limits the evidence for care delivery for patients with MBC and CNS metastases. Both ASCO and the US Food and Drug Administration have recognized the need to include this subset of patients in research.19,45 On the basis of clinical trial involvement in almost 40% of patients, enrollment in IMPACT the Brain clearly supports participation in clinical research. Patients were included in both interventional and noninterventional trials, which supported knowledge gains and opportunities that are often not available or offered to this cohort of patients.
In addition to decreased survival, poor health-related quality of life has been documented for patients with CNS metastases, particularly for patients with metastatic lung cancer.46,47 There are fewer published quality-of-life investigations for patients with MBC and CNS metastases.48 The PROs obtained in our program provide baseline data to bridge this knowledge gap, a necessary step to inform subsequent interventions. The baseline PROs suggest that patients struggle particularly with reduced physical function and increased fatigue compared with the general population. These symptoms caused at least moderate distress for half of patients, indicating their significant impact. Addressing functional decline through increased PM&R referrals should be considered a standard of care in this population. Caregivers may also need assistance, with the majority reporting at least mild degrees of caregiver burden. The importance of including caregivers in patient cancer care has previously been recognized; our care coordination program specifically documents caregiver information and goes further to include metrics of caregiver well-being.49-51 Following these metrics longitudinally will allow us to identify vulnerable patients and caregivers and intervene when necessary. Furthermore, this multiteam care coordination program contributes to current knowledge by providing data for a population of patients with historically limited information in terms of health care utilization, clinical trial participation, and PRO data.
Although benefits were observed with participation in this program, there are limitations to note. First, this program was designed without the presence of a matched control group, so conclusions were drawn on the basis of comparison with data published in studies of similar patient populations. Patients were not enrolled to a standard-of-care arm because of the relatively rare nature of this disease and limited number of patients eligible for enrollment in this single-site program. However, future work could benefit from affirming these findings by expanding program enrollment to include a standard-of-care arm. In addition, although we found that it was feasible to collect data from patient-reported outcomes, the completion of questionnaires was voluntary in the program. As a result, the completion rate was lower than in clinical trials where completion is required as part of enrollment. This has motivated us to further examine the barriers to implementing PRO assessments in this patient population, and future directions of the program will focus on incorporating changes to facilitate completion.
In conclusion, use of a multiteam system that leverages the principles of transactive memory to provide longitudinal, coordinated, tailored care for patients with MBC and CNS metastases is feasible. Administration of a unique intake screening form by a dedicated program coordinator resulted in faster time to first patient visit, enabled access to subspecialist care, and supported enrollment in clinical trials. This innovative team-based approach allows for the integration of cancer treatment with supportive oncology, encourages involvement in clinical trials for a group of patients historically underrepresented in research, and provides baseline data for development of future interventional studies. Distribution of the unique program intake form at other institutions could allow for implementation of multidisciplinary care elsewhere.
Although this program demonstrated that it is feasible to collect PRO data, improvements can be made to facilitate completion for this population. In the future, longitudinal acquisition of PRO data can identify important quality-of-life metrics toward this personalized approach to team-based care to further optimize patient care. Additionally, the high degree of caregiver burden identified in this cohort speaks to the need for care coordination programs to identify interventions that mitigate the degree of burden experienced by caregivers of patients with MBC and CNS metastases.
APPENDIX
FIG A1.
Program intake screen form. Personalized program intake screening form developed for IMPACT the Brain, this form is completed over the telephone with each new participant. As indicated in the form, answers to questions 5-12 and 14-15 prompt referral to various subspecialists participating in this team-based care coordination program. Questions 9-10 were adapted from the PHQ-9 and 11-12 were adapted from the GAD-7.52,53 GAD-7, Generalized Anxiety Disorder-7; PHQ-9, Patient Health Questionnaire-9.
Leigh K. Swartz
Employment: TriMed, Integra LifeSciences
Consulting or Advisory Role: LEK
Sofia Merajver
Employment: Inheret, LLC
Leadership: Inheret, LLC
Stock and Other Ownership Interests: Inheret, LLC
Christopher R. Friese
Research Funding: Merck (Inst), NCCN/Pfizer (Inst)
Other Relationship: Patient-Centered Outcomes Research Institute (PCORI), National Cancer Institute
Michelle M. Kim
Consulting or Advisory Role: Blue Earth Diagnostics (Inst)
Research Funding: EpicentRx (Inst), Blue Earth Diagnostics (Inst)
Travel, Accommodations, Expenses: Capital Health
Aki Morikawa
Consulting or Advisory Role: Eisai, Lilly, Seattle Genetics
Research Funding: Lilly (Inst), Merrimack (Inst), Novartis (Inst), Genentech/Roche (Inst), Millenium Pharamceuticals (Inst), Eisai (Inst), Seattle Genetics (Inst), Pfizer (Inst), Tempus (Inst), Molecular Templates (Inst), Dantari Pharmaceuticals (Inst), Suzhou Zanrong Pharma Limited (Inst), Merck Sharp & Dohme Corp (Inst), F. Hoffmann-La Roche Ltd, (Inst)
Other Relationship: Taiho Pharmaceutical
Uncompensated Relationships: Puma Biotechnology
No other potential conflicts of interest were reported.
SUPPORT
Supported by National Comprehensive Cancer Network through a grant provided by Pfizer Inc.
PRIOR PRESENTATION
Presented [in part] at the ASCO Quality Care Symposium, Boston, Massachusetts, United States, September 24th through September 25th, 2021. Presented [in part] at the Society for NeuroOncology Third Annual Conference on Brain Metastases, held virtually, August 19th through August 20th, 2021.
AUTHOR CONTRIBUTIONS
Conception and design: Leigh K. Swartz, Kait Verbal, Christopher R. Friese, Ayano Kiyota, Sean R. Smith, Nicolette Gabel, Michelle M. Kim, Aki Morikawa
Administrative support: Kait Verbal, Christopher R. Friese
Provision of study materials or patients: Kait Verbal, Michelle M. Kim, Aki Morikawa
Collection and assembly of data: Nicole M. Grogan Fleege, Donna Pierce-Gjeldum, Kait Verbal, Ayano Kiyota, Jason Heth, Denise Leung, Sean R. Smith, Michelle M. Kim, Aki Morikawa
Data analysis and interpretation: Nicole M. Grogan Fleege, Sofia Merajver, Christopher R. Friese, Ayano Kiyota, Denise Leung, Sean R. Smith, Nicolette Gabel, Michelle M. Kim, Aki Morikawa
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
IMPACT the Brain: A Team-Based Approach to Management of Metastatic Breast Cancer With CNS Metastases
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Leigh K. Swartz
Employment: TriMed, Integra LifeSciences
Consulting or Advisory Role: LEK
Sofia Merajver
Employment: Inheret, LLC
Leadership: Inheret, LLC
Stock and Other Ownership Interests: Inheret, LLC
Christopher R. Friese
Research Funding: Merck (Inst), NCCN/Pfizer (Inst)
Other Relationship: Patient-Centered Outcomes Research Institute (PCORI), National Cancer Institute
Michelle M. Kim
Consulting or Advisory Role: Blue Earth Diagnostics (Inst)
Research Funding: EpicentRx (Inst), Blue Earth Diagnostics (Inst)
Travel, Accommodations, Expenses: Capital Health
Aki Morikawa
Consulting or Advisory Role: Eisai, Lilly, Seattle Genetics
Research Funding: Lilly (Inst), Merrimack (Inst), Novartis (Inst), Genentech/Roche (Inst), Millenium Pharamceuticals (Inst), Eisai (Inst), Seattle Genetics (Inst), Pfizer (Inst), Tempus (Inst), Molecular Templates (Inst), Dantari Pharmaceuticals (Inst), Suzhou Zanrong Pharma Limited (Inst), Merck Sharp & Dohme Corp (Inst), F. Hoffmann-La Roche Ltd, (Inst)
Other Relationship: Taiho Pharmaceutical
Uncompensated Relationships: Puma Biotechnology
No other potential conflicts of interest were reported.
REFERENCES
- 1. Achrol AS, Rennert RC, Anders C, et al. Brain metastases. Nat Rev Dis Primers. 2019;5:5. doi: 10.1038/s41572-018-0055-y. [DOI] [PubMed] [Google Scholar]
- 2. Witzel I, Laakmann E, Weide R, et al. Treatment and outcomes of patients in the brain metastases in Breast Cancer Network Registry. Eur J Cancer. 2018;102:1–9. doi: 10.1016/j.ejca.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 3. Morikawa A, Jordan L, Rozner R, et al. Characteristics and outcomes of patients with breast cancer with leptomeningeal metastasis. Clin Breast Cancer. 2017;17:23–28. doi: 10.1016/j.clbc.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lin NU, Prowell T, Tan AR, et al. Modernizing clinical trial eligibility criteria: Recommendations of the American Society of Clinical Oncology-Friends of Cancer Research Brain Metastases Working Group. J Clin Oncol. 2017;35:3760–3773. doi: 10.1200/JCO.2017.74.0761. [DOI] [PubMed] [Google Scholar]
- 5. Frisk G, Svensson T, Bäcklund LM, et al. Incidence and time trends of brain metastases admissions among breast cancer patients in Sweden. Br J Cancer. 2012;106:1850–1853. doi: 10.1038/bjc.2012.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cagney DN, Martin AM, Catalano PJ, et al. Incidence and prognosis of patients with brain metastases at diagnosis of systemic malignancy: A population-based study. Neuro Oncol. 2017;19:1511–1521. doi: 10.1093/neuonc/nox077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sawaya R, Hammoud M, Schoppa D, et al. Neurosurgical outcomes in a modern series of 400 craniotomies for treatment of parenchymal tumors. Neurosurgery. 1998;42:1044–1055. doi: 10.1097/00006123-199805000-00054. discussion 1055-1056. [DOI] [PubMed] [Google Scholar]
- 8. Soon YY, Tham IW, Lim KH, et al. Surgery or radiosurgery plus whole brain radiotherapy versus surgery or radiosurgery alone for brain metastases. Cochrane Database Syst Rev. 2014;2014:CD009454. doi: 10.1002/14651858.CD009454.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Park YH, Park MJ, Ji SH, et al. Trastuzumab treatment improves brain metastasis outcomes through control and durable prolongation of systemic extracranial disease in HER2-overexpressing breast cancer patients. Br J Cancer. 2009;100:894–900. doi: 10.1038/sj.bjc.6604941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dawood S, Broglio K, Esteva FJ, et al. Defining prognosis for women with breast cancer and CNS metastases by HER2 status. Ann Oncol. 2008;19:1242–1248. doi: 10.1093/annonc/mdn036. [DOI] [PubMed] [Google Scholar]
- 11. Smith SR, Vargo M, Zucker D, et al. Psychometric characteristics and validity of the PROMIS Cancer Function Brief 3D Profile. Arch Phys Med Rehabil. 2022;103:S146–S161. doi: 10.1016/j.apmr.2020.12.027. [DOI] [PubMed] [Google Scholar]
- 12. Smith SR, Vargo M, Zucker DS, et al. Responsiveness and interpretation of the PROMIS Cancer Function Brief 3D Profile. Cancer. 2022;128:3217–3223. doi: 10.1002/cncr.34376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Armstrong TS, Gning I, Mendoza TR, et al. Clinical utility of the MDASI-BT in patients with brain metastases. J Pain Symptom Manage. 2009;37:331–340. doi: 10.1016/j.jpainsymman.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 14. Armstrong TS, Mendoza T, Gning I, et al. Validation of the M.D. Anderson Symptom Inventory Brain Tumor Module (MDASI-BT) J Neurooncol. 2006;80:27–35. doi: 10.1007/s11060-006-9135-z. [DOI] [PubMed] [Google Scholar]
- 15. Armstrong TS, Vera-Bolanos E, Gning I, et al. The impact of symptom interference using the MD Anderson Symptom Inventory-Brain Tumor Module (MDASI-BT) on prediction of recurrence in primary brain tumor patients. Cancer. 2011;117:3222–3228. doi: 10.1002/cncr.25892. [DOI] [PubMed] [Google Scholar]
- 16. Higginson IJ, Gao W, Jackson D, et al. Short-form Zarit Caregiver Burden Interviews were valid in advanced conditions. J Clin Epidemiol. 2010;63:535–542. doi: 10.1016/j.jclinepi.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 17. Bédard M, Molloy DW, Squire L, et al. The Zarit Burden Interview: A new short version and screening version. Gerontologist. 2001;41:652–657. doi: 10.1093/geront/41.5.652. [DOI] [PubMed] [Google Scholar]
- 18. Le Rhun E, Guckenberger M, Smits M, et al. EANO-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up of patients with brain metastasis from solid tumours. Ann Oncol. 2021;32:1332–1347. doi: 10.1016/j.annonc.2021.07.016. [DOI] [PubMed] [Google Scholar]
- 19. Vogelbaum MA, Brown PD, Messersmith H, et al. Treatment for brain metastases: ASCO-SNO-ASTRO guideline. J Clin Oncol. 2022;40:492–516. doi: 10.1200/JCO.21.02314. [DOI] [PubMed] [Google Scholar]
- 20. Walsh J, Harrison JD, Young JM, et al. What are the current barriers to effective cancer care coordination? A qualitative study. BMC Health Serv Res. 2010;10:132. doi: 10.1186/1472-6963-10-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gorin SS, Haggstrom D, Han PKJ, et al. Cancer care coordination: A systematic review and meta-analysis of over 30 years of empirical studies. Ann Behav Med. 2017;51:532–546. doi: 10.1007/s12160-017-9876-2. [DOI] [PubMed] [Google Scholar]
- 22. Davison RB, Hollenbeck JR, Barnes CM, et al. Coordinated action in multiteam systems. J Appl Psychol. 2012;97:808–824. doi: 10.1037/a0026682. [DOI] [PubMed] [Google Scholar]
- 23. Henry E, Silva A, Tarlov E, et al. Delivering coordinated cancer care by building transactive memory in a team of teams. J Oncol Pract. 2016;12:992–999. doi: 10.1200/JOP.2016.013730. [DOI] [PubMed] [Google Scholar]
- 24. Rajpurohit A, Purandare N, Moiyadi A, et al. Multidisciplinary brain metastasis clinic: Is it effective and worthwhile? Ecancermedicalscience. 2020;14:1136. doi: 10.3332/ecancer.2020.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Danielson B, Fairchild A. Beyond palliative radiotherapy: A pilot multidisciplinary brain metastases clinic. Support Care Cancer. 2012;20:773–781. doi: 10.1007/s00520-011-1149-1. [DOI] [PubMed] [Google Scholar]
- 26. McKee MJ, Keith K, Deal AM, et al. A multidisciplinary breast cancer brain metastases clinic: The University of North Carolina Experience. Oncologist. 2016;21:16–20. doi: 10.1634/theoncologist.2015-0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Peltokorpi V, Hood AC. Communication in theory and research on transactive memory systems: A literature review. Top Cogn Sci. 2019;11:644–667. doi: 10.1111/tops.12359. [DOI] [PubMed] [Google Scholar]
- 28. Sánchez-Manzanares M, Rico R, Gil F, et al. Transactive memory in decision-making teams: Implications for team effectiveness [in Spanish] Psicothema. 2006;18:750–756. [PubMed] [Google Scholar]
- 29. Lewis K. Measuring transactive memory systems in the field: Scale development and validation. J Appl Psychol. 2003;88:587–604. doi: 10.1037/0021-9010.88.4.587. [DOI] [PubMed] [Google Scholar]
- 30. Kumar P, Casarett D, Corcoran A, et al. Utilization of supportive and palliative care services among oncology outpatients at one academic cancer center: Determinants of use and barriers to access. J Palliat Med. 2012;15:923–930. doi: 10.1089/jpm.2011.0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Khan F, Amatya B, Ng L, et al. Multidisciplinary rehabilitation after primary brain tumour treatment. Cochrane Database Syst Rev. 2013;1:Cd009509. doi: 10.1002/14651858.CD009509.pub2. [DOI] [PubMed] [Google Scholar]
- 32. Bakouny Z, Hawley JE, Choueiri TK, et al. COVID-19 and cancer: Current challenges and perspectives. Cancer Cell. 2020;38:629–646. doi: 10.1016/j.ccell.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chang PJ, Jay GM, Kalpakjian C, et al. Patient and provider-reported satisfaction of cancer rehabilitation telemedicine visits during the COVID-19 pandemic. PM R. 2021;13:1362–1368. doi: 10.1002/pmrj.12552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: An accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30:419–425. doi: 10.1200/JCO.2011.38.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. DeChurch LA, Marks MA. Leadership in multiteam systems. J Appl Psychol. 2006;91:311–329. doi: 10.1037/0021-9010.91.2.311. [DOI] [PubMed] [Google Scholar]
- 36. Vidaver RM, Shershneva MB, Hetzel SJ, et al. Typical time to treatment of patients with lung cancer in a multisite, US-based study. JCO Oncol Pract. 2016;12:e643–e653. doi: 10.1200/JOP.2015.009605. [DOI] [PubMed] [Google Scholar]
- 37. Benn BS, Parikh M, Tsau PH, et al. Using a dedicated interventional pulmonology practice decreases wait time before treatment initiation for new lung cancer diagnoses. Lung. 2019;197:249–255. doi: 10.1007/s00408-019-00207-6. [DOI] [PubMed] [Google Scholar]
- 38. Potter S, Govindarajulu S, Shere M, et al. Referral patterns, cancer diagnoses, and waiting times after introduction of two week wait rule for breast cancer: Prospective cohort study. BMJ. 2007;335:288. doi: 10.1136/bmj.39258.688553.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Unger JM, Cook E, Tai E, et al. The role of clinical trial participation in cancer research: Barriers, evidence, and strategies. Am Soc Clin Oncol Ed Book. 2016;35:185–198. doi: 10.14694/EDBK_156686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lara PN, Jr, Higdon R, Lim N, et al. Prospective evaluation of cancer clinical trial accrual patterns: Identifying potential barriers to enrollment. J Clin Oncol. 2001;19:1728–1733. doi: 10.1200/JCO.2001.19.6.1728. [DOI] [PubMed] [Google Scholar]
- 41. Bates AJ, Couillard SA, Arons DF, et al. HOUT-15. Brain tumor patient and caregiver survey on clinical trials: Identifying attitudes and barriers to patient participation. Neuro Oncol. 2017;19(suppl 6):vi109. [Google Scholar]
- 42. Corbett K, Sharma A, Pond GR, et al. Central nervous system-specific outcomes of phase 3 randomized clinical trials in patients with advanced breast cancer, lung cancer, and melanoma. JAMA Oncol. 2021;7:1062–1064. doi: 10.1001/jamaoncol.2021.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kim AE, Wang GM, Waite KA, et al. Cross-sectional survey of patients, caregivers, and physicians on diagnosis and treatment of brain metastases. Neurooncol Pract. 2021;8:662–673. doi: 10.1093/nop/npab042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tan AC, Boggs DH, Lee EQ, et al. Clinical trial eligibility criteria and recently approved cancer therapies for patients with brain metastases. Front Oncol. 2021;11:780379. doi: 10.3389/fonc.2021.780379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.US Department of Health and Human Services; Food and Drug Administration . Cancer Clinical Trial Eligibility Criteria: Brain Metastases Guidance for Industry. 2020. https://www.fda.gov/media/121317/download [Google Scholar]
- 46. Walker MS, Wong W, Ravelo A, et al. Effect of brain metastasis on patient-reported outcomes in advanced NSCLC treated in real-world community oncology settings. Clin Lung Cancer. 2018;19:139–147. doi: 10.1016/j.cllc.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 47. Astrup G, Kaasa S, Aass NK, et al. Patient-reported outcomes in cancer patients newly diagnosed with brain metastases. Ann Oncol. 2021;32:S1076–S1083. [Google Scholar]
- 48. Wu A, Colón GR, Lim M. Quality of life and role of palliative and supportive care for patients with brain metastases and caregivers: A review. Front Neurol. 2022;13:806344. doi: 10.3389/fneur.2022.806344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Alewine C, Ahmad M, Shea R. Caregiver exclusion in the age of COVID: Fighting cancer with half the team. J Clin Oncol. 2021;39:1687–1688. doi: 10.1200/JCO.21.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Applebaum AJ, Kent EE, Lichtenthal WG. Documentation of caregivers as a standard of care. J Clin Oncol. 2021;39:1955–1958. doi: 10.1200/JCO.21.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Litzelman K, Kent EE, Mollica M, et al. How does caregiver well-being relate to perceived quality of care in patients with cancer? Exploring associations and pathways. J Clin Oncol. 2016;34:3554–3561. doi: 10.1200/JCO.2016.67.3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Spitzer RL, Kroenke K, Williams JB, et al. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch Intern Med. 2006;166:1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 53. Costantini L, Pasquarella C, Odone A, et al. Screening for depression in primary care with Patient Health Questionnaire-9 (PHQ-9): A systematic review. J Affect Disord. 2021;279:473–483. doi: 10.1016/j.jad.2020.09.131. [DOI] [PubMed] [Google Scholar]