PURPOSE
The CHOICE-01 study investigated the efficacy and safety of toripalimab in combination with chemotherapy as a first-line treatment for advanced non–small-cell lung cancer (NSCLC).
PATIENTS AND METHODS
Patients (N = 465) with treatment-naive, advanced NSCLC without EGFR/ALK mutations were randomly assigned 2:1 to receive toripalimab 240 mg (n = 309) or placebo (n = 156) once every 3 weeks in combination with chemotherapy for 4-6 cycles, followed by the maintenance of toripalimab or placebo once every 3 weeks plus standard care. Stratification factors included programmed death ligand-1 expression status, histology, and smoking status. The primary end point was progression-free survival (PFS) by investigator per RECIST v1.1. Secondary end points included overall survival and safety.
RESULTS
At the final PFS analysis, PFS was significantly longer in the toripalimab arm than in the placebo arm (median PFS, 8.4 v 5.6 months, hazard ratio = 0.49; 95% CI, 0.39 to 0.61; two-sided P < .0001). At the interim OS analysis, the toripalimab arm had a significantly longer OS than the placebo arm (median OS not reached v 17.1 months, hazard ratio = 0.69; 95% CI, 0.53 to 0.92; two-sided P = .0099). The incidence of grade ≥ 3 adverse events was similar between the two arms. Treatment effects were similar regardless of programmed death ligand-1 status. Genomic analysis using whole-exome sequencing from 394 available tumor samples revealed that patients with high tumor mutational burden were associated with significantly better PFS in the toripalimab arm (median PFS 13.1 v 5.5 months, interaction P = .026). Notably, patients with mutations in the focal adhesion-PI3K-Akt signaling pathway achieved significantly better PFS and OS in the toripalimab arm (interaction P values ≤ .001).
CONCLUSION
Toripalimab plus chemotherapy significantly improves PFS and OS in patients with treatment-naive advanced NSCLC while having a manageable safety profile. Subgroup analysis showed the OS benefit was mainly driven by the nonsquamous subpopulation.
INTRODUCTION
Non–small-cell lung cancer (NSCLC) accounts for about 85% of all lung cancers.1 It is a leading cause of cancer-related death and has the highest incidence rate among all cancers worldwide.2 About a third of new cases and cancer deaths come from China alone.3 For patients with advanced NSCLC without driver mutations, traditional platinum-based chemotherapy has been the standard first-line treatment despite unsatisfactory clinical benefit.
CONTEXT
Key Objective
The CHOICE-01 study aims to investigate the efficacy and safety of toripalimab in combination with chemotherapy as first-line treatment for advanced non–small-cell lung cancer (NSCLC) and identify predictive biomarkers by whole-exome sequencing.
Knowledge Generated
Toripalimab plus chemotherapy significantly improved progression-free survival and overall survival over chemotherapy alone in patients with advanced NSCLC while having a manageable safety profile. Sequencing results revealed that patients with high tumor mutational burden (≥ 10 mutations/Mbp), or mutations in FA-PI3K-Akt, IL-7 signaling pathway or SWI/SNF complex were associated with significantly better progression-free survival in the combination arm than in the placebo arm. Notably, patients with mutations in the FA-PI3K-Akt pathway also achieved significantly better overall survival benefits from the combination.
Relevance
This study supports the use of toripalimab in combination with chemotherapy as a front-line treatment option for patients with advanced NSCLC without EGFR/ALK driver mutations and provides new perspectives on patient selection on the basis of tumor genetic alternations.
Immune checkpoint inhibitors, represented by programmed death-1 (PD-1) or programmed death ligand-1 (PD-L1) antibodies, have dramatically altered the therapeutic landscape in the management of advanced NSCLC because of superior efficacy compared with traditional chemotherapy. Large-scale clinical trials have supported anti–PD-1/PD-L1 combined with chemotherapy as the standard first-line treatment for advanced NSCLC without driver mutations.4-6 However, predictive biomarkers to identify beneficial patient populations need to be further explored. Therefore, rigorously designed large-scale clinical trials of first-line chemoimmunotherapy in NSCLC that harbor comprehensive and integrated end point analyses and biomarker explorations are still warranted.
Toripalimab, a humanized IgG4K monoclonal antibody specific for human PD-1,7-11 engaged a differential domain on PD-1 than nivolumab and pembrolizumab by a crystal structure analysis.12 In a preclinical study, toripalimab promoted stronger antigen-specific interferon-γ production than nivolumab.13 Early clinical trials of toripalimab have also exhibited promising antitumor activities in advanced NSCLC.14,15 In this randomized, double-blind, placebo-controlled, phase III study (CHOICE-01), we compared the efficacy and safety of toripalimab plus chemotherapy versus placebo plus chemotherapy as the first-line treatment for patients with advanced NSCLC without EGFR/ALK driver mutations. Known biomarkers including PD-L1 and tumor mutational burden (TMB) as well as novel genomic alterations were evaluated as predictive biomarkers for survival.
PATIENTS AND METHODS
Study Design
CHOICE-01 was a multicenter, randomized, double-blind, placebo-controlled, phase III study conducted in 59 medical centers across China. Eligible patients must have treatment-naive, locally advanced (stage IIIB or IIIC) or metastatic NSCLC, or completed neoadjuvant/adjuvant therapy ≥ 6 months before enrollment. Nonsquamous NSCLC patients with EGFR or ALK driver mutations were excluded. The full eligibility criteria are available in the study Protocol (online only). This trial is registered with ClinicalTrials.gov (identifier: NCT03856411).
Study Oversight
The trial protocol and amendments were approved by institutional review board at each participating site. All patients provided written informed consent before enrollment. This trial was conducted in full conformance with the ICH E6 guidelines for Good Clinical Practice and the principles of the Declaration of Helsinki. An independent data monitoring committee (iDMC) reviewed the safety and efficacy results at prespecified analyses. All analyses for iDMC's review were prepared by an independent third party.
Random Assignment
A permuted block was used to generate the randomization allocation sequence. Random assignment was stratified on the basis of the baseline PD-L1 expression status (tumor cell [TC] < 1% v TC ≥ 1%), histology (squamous v nonsquamous), and smoking status (frequent smoker [≥ 20 pack years] v infrequent or nonsmoker).
Random allocation sequences for patients and investigational drugs were generated and maintained by an independent unblinded statistician from a third-party vendor. All other personnel involved in the study were blinded.
Procedures
Patients were randomly assigned in a 2:1 ratio to receive either toripalimab or placebo plus chemotherapy. Treatment regimens for squamous NSCLC included nab-paclitaxel 100 mg/m2 intravenously (IV) days 1, 8, and 15 once every 3 weeks + carboplatin AUC five IV once every 3 weeks plus toripalimab or placebo 240 mg IV once every 3 weeks for 4-6 cycles, followed by maintenance of toripalimab or placebo. Treatment regimens for nonsquamous NSCLC included pemetrexed 500 mg/m2 IV + cisplatin 75 mg/m2 IV or carboplatin AUC five IV once every 3 weeks plus toripalimab or placebo 240 mg IV once every 3 weeks for 4-6 cycles, followed by pemetrexed plus toripalimab or placebo maintenance.
Treatment continued until disease progression, death, unacceptable toxicity, investigator decision, withdrawal, or completion of 2 years of treatment, whichever occurred first. At investigator-determined progressive disease, patients were unblinded and patients from the placebo arm were allowed to crossover to toripalimab monotherapy.
Tumor assessments were performed at baseline, every 6 weeks during the first 12 months, and every 9 weeks thereafter. Clinical response was assessed by investigators and a blinded independent central review (BICR) according to the RECIST v1.1 criteria. Computed tomography/magnetic resonance imaging were used for screening/baseline evaluation within 28 days before the first dose. The tumor evaluations included chest, abdomen, pelvis, and all the known sites of disease, and the same radiologic examination method used at screening/baseline were used during subsequent follow-up assessments. The tumor was evaluated once every 6 weeks in the first 12 months and once every 9 weeks in year 2 and beyond using RECIST 1.1 and iRECIST criteria until progression of disease, intolerable toxicity, inability to continue to benefit from the investigational treatment as judged by investigators, withdrawal of informed consent, or death, whichever comes first. If more than one method were used at screening, the most accurate method was used for recording the data according to RECIST v1.1, and this method was used again in all subsequent evaluations. Safety and tolerability were assessed in all patients who received any amount of the study treatment up to 90 days after the last dose of study drug or the start of new anticancer therapy, whichever occurred first. All adverse events were defined and graded according to the National Cancer Institute-Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 5.0.
Archival or fresh tumor biopsy samples were obtained from patients before treatment. PD-L1 expression was evaluated by immunohistochemistry staining with JS311 antibody using a validated assay in a central laboratory (MEDx, Suzhou, China). A cross-correlation study showed similar PD-L1 staining patterns and scores of JS311 with 22C3, 28-8, and SP263 antibodies using biopsy samples from patients with NSCLC.14 PD-L1 positivity was defined as TC ≥ 1%.
Whole-exome sequencing was performed on tumor biopsies and matched peripheral blood mononuclear cell samples in a central laboratory (OrigiMed, Shanghai, China). Genomic alterations including microsatellite stability status, single base substitutions, short and long insertions/deletions (INDELs), copy-number variants, gene rearrangements, and fusions were assessed. The TMB was determined by analyzing somatic mutations including coding base substitution and INDELs per megabase (Mb). A cutoff point of TMB-high (TMB-H) was defined as ≥ 10 mutations/Mb, the threshold used by FoundationOne CDx assay, which was approved by the US Food and Drug Administration as a companion diagnostic of pembrolizumab for the treatment of adult and pediatric patients with unresectable or metastatic solid tumors.
End Points
The primary end point was progression-free survival (PFS) assessed by the investigator. Secondary end points included PFS by BIRC, ORR, overall survival (OS), and safety. The full list of end points is available in the study protocol.
Statistical Analysis
The sample size of the study was determined on the basis of the following assumption: A total of 450 patients were needed to observe 356 PFS events to detect a hazard ratio (HR), 0.7 with 85% power at an overall two-sided significance level of 0.05. The overall type I error rate was controlled by the Pocock boundary as approximated by the Lan-DeMets alpha spending function. PFS, ORR, and OS were tested hierarchically. The trial had 83% power to detect a HR of 0.68 for OS (median 22.1 v 15 months) using a two-sided alpha of 0.05. The stopping boundaries for the OS analyses were computed using the O'Brien Fleming boundary approximated by the Lan-DeMets alpha spending function.
Efficacy was analyzed among the intention-to-treat (ITT) population, which included all randomly assigned patients. The two-sided log-rank test was used as the primary analysis to compare the PFS between the two treatment arms. The HR for PFS was estimated with the use of the stratified Cox proportional hazards model. The Kaplan-Meier method was applied to estimate the median PFS for each treatment arm. The Brookmeyer Crowley method, using log-log transformation to the survival function, was used to construct the 95% CI for the median PFS for each treatment arm. The methods outlined for PFS analyses were also used for the OS and duration of response (DoR) analyses. A test for interaction was conducted using Cox proportional hazards regression models to evaluate subgroup differences.
Safety analyses were performed on the safety population, which included all randomly assigned patients who received any amount of the study drug. One randomly assigned patient was excluded from the safety population as the patient did not receive any study treatment. The details of statistical analyses are available in the statistical analysis plan.
The prespecified interim and final PFS analyses were conducted on November 17, 2020, and October 31, 2021 respectively. At the interim PFS analysis, the iDMC reported that the efficacy boundaries for PFS and ORR were crossed. At the final PFS analysis, the efficacy boundary for OS was crossed.
Data in this study were analyzed using SAS version 9.4 developed by SAS Institute.
RESULTS
Patients and Treatment
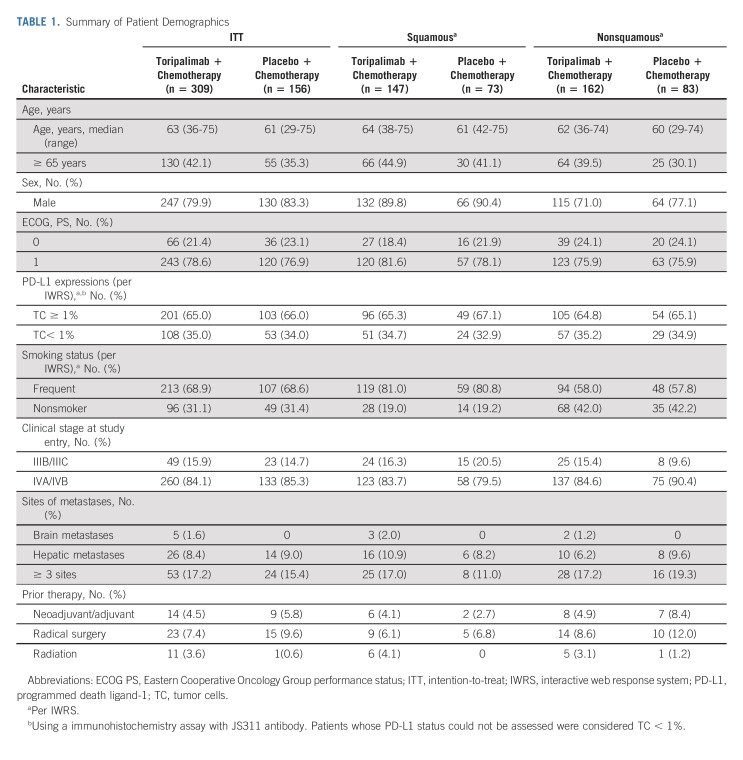
From April 2, 2019, to August 5, 2020, 835 patients were screened from 59 participating centers. A total of 465 patients were successfully enrolled and randomly assigned in a 2:1 ratio to the toripalimab plus chemotherapy arm (n = 309) or placebo plus chemotherapy arm (n = 156), stratified by PD-L1 expression status, histology (squamous v nonsquamous), and smoking status (Fig 1). The primary reasons for screening failures were not meeting inclusion criteria or meeting exclusion criteria (n = 305) and withdrawal of consent (n = 57; Data Supplement, online only). The baseline demographic and disease characteristics were generally balanced between the two treatment arms (Table 1 and Data Supplement).
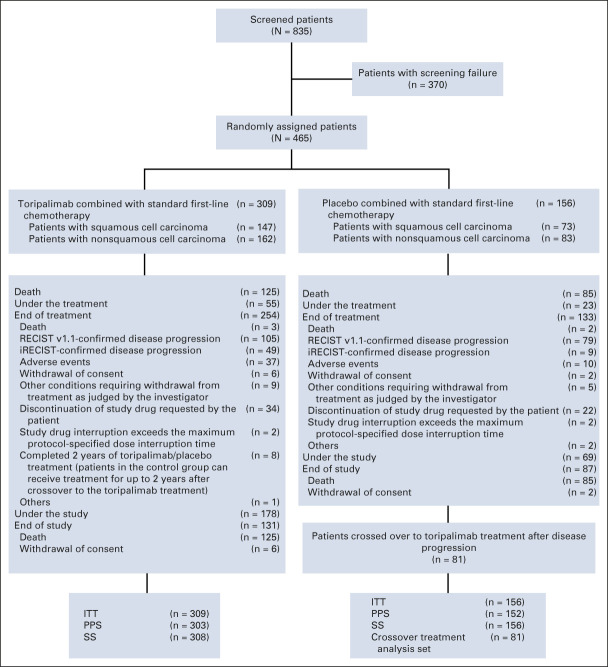
FIG 1.
CONSORT diagram of the CHOICE-01 study. From April 2, 2019, to August 5, 2020, a total of 835 patients were screened. Of these, 370/835 patients (44.3%) failed screening, mainly because of inclusion/exclusion criteria not met (305/370; 82.4%) or withdrawal of the informed consent (57/370; 15.4%). A total of 465 patients were successfully screened and randomly assigned 2:1 to the toripalimab plus chemotherapy arm (n = 309) or placebo plus chemotherapy arm (n = 156), stratified by PD-L1 expression status, histology (squamous v nonsquamous), and smoking status. By the cutoff date, 55 (17.8%) patients in the toripalimab arm and 23 (14.7%) in the placebo arm remained on the study treatment. ITT, intention-to-treat; PD-L1, programmed death ligand-1; PPS, per protocol set; SS, safety set.
TABLE 1.
Summary of Patient Demographics
By the data cutoff date (October 31, 2021), among the ITT population, 13.3% and 65.4% patients in the toripalimab arm and placebo arm, respectively, received subsequent anti–PD-1/PD-L1 treatment (Data Supplement).
PFS
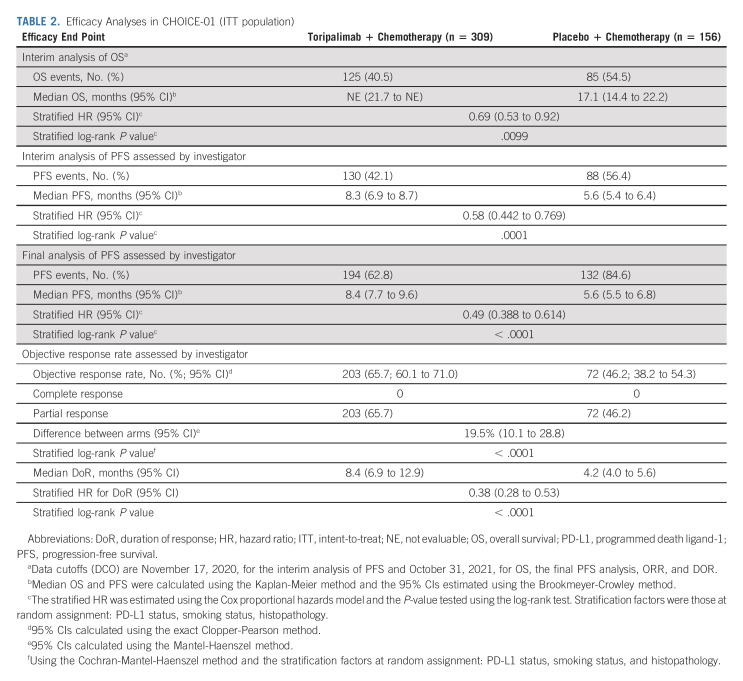
The interim analysis of PFS was conducted with a median follow-up time of 7.1 months and 46.9% of events, on the data cutoff date of November 17, 2020. In the ITT population, toripalimab in combination with chemotherapy decreased the risk of disease progression or death by 42% when compared with placebo plus chemotherapy (median PFS 8.3 v 5.6 months; HR, 0.58; 95% CI, 0.44 to 0.77; P = .0001) as assessed by the investigator (Table 2, Data Supplement). The interim PFS result had crossed the prespecified efficacy boundary. The improvement was supported by the BIRC-determined PFS (Data Supplement).
TABLE 2.
Efficacy Analyses in CHOICE-01 (ITT population)
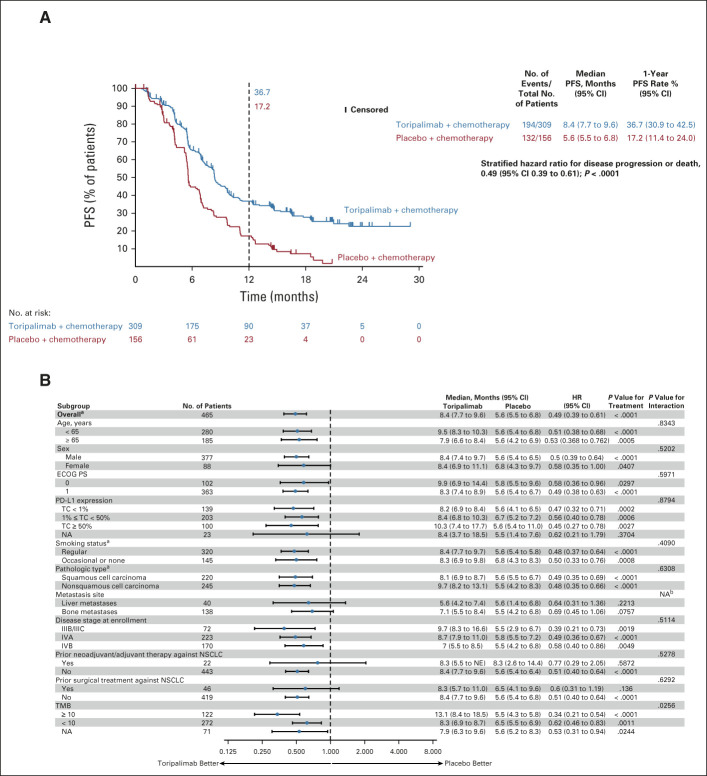
The final PFS analysis (data cutoff October 31, 2021) with 70.1% of events further supported the findings of the interim analysis with an improvement in the HR: median PFS 8.4 versus 5.6 months, HR, 0.49 (95% CI, 0.39 to 0.61), P < .0001. The 1-year PFS rates were 36.7% versus 17.2% in the two arms (Fig 2A and Data Supplement). The BICR-determined PFS was consistent with the investigator-determined result (Data Supplement).
FIG 2.
(A) Kaplan-Meier-estimated PFS curves as assessed by the investigator according to RECIST v1.1 in the intention-to-treat population on the data cutoff date October 31, 2021, are shown to compare the toripalimab plus chemotherapy arm with the placebo plus chemotherapy arm as first-line treatment for patients with advanced NSCLC. Censored patients are marked with “┃” in the graph. Numbers of patients at risk at indicated time points shown below x-axis. Number of events, median PFS, 1-year PFS rates, and stratified HR for PFS are shown to the right of Kaplan-Meier curves. (B) PFS in key subgroups. All HRs were computed using the Cox proportional hazards model. All P values were two-sided with no adjustment of multiplicity. The P values of comparing the Kaplan-Meier curves were computed using the log-rank test stratified by the baseline PD-L1 expression status, histology, and smoking status. The P values of testing the interaction of the subgroup variables with the treatment (B) were computed using the Cox proportional hazards regression model with the treatment arm, the subgroup variable, and their interaction as the covariates. aP values were stratified by the factors used for randomization except the subgroup variable itself. bNA,not applicable due to overlapping metastases to liver and bone. HR, hazard ratio; NA, not available; NSCLC, non–small-cell lung cancer; PD-L1, programmed death ligand-1; PFS, progression-free survival.
The PFS treatment effect favored the toripalimab arm across all major subgroups (Figs 2B and Data Supplement).
OS
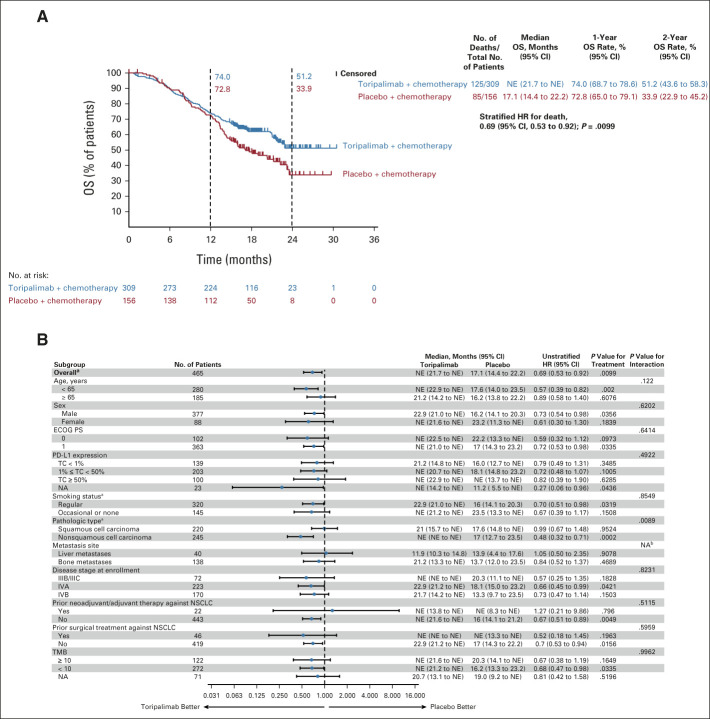
At the first interim OS analysis, OS was deemed immature with only 17.8% of events (Data Supplement). The median OS was not estimable in either arm. At the second interim OS analysis, with a median follow-up of 16.2 months and 45.2% of events, the stratified HR for death in the ITT population was 0.69 (95% CI, 0.53 to 0.92), P = .0099, which crossed the prespecified efficacy boundary of 0.0245 (two-sided, on the basis of the 80.2% information fraction). The median OS has not been obtained (95% CI, 21.7 to NE) in the toripalimab arm and was 17.1 months (95% CI, 14.4 to 22.2) in the placebo arm (Fig 3A and Data Supplement). The 1-year and 2-year OS rates were 74.0% versus 72.8% and 51.2% versus 33.9% in the two arms, respectively (Fig 3A).
FIG 3.
(A) OS in the intention-to-treat population. Kaplan-Meier estimates of OS in the intention-to-treat population on the data cutoff date October 31, 2021, are shown to compare the toripalimab plus chemotherapy arm with the placebo plus chemotherapy arm as first-line treatment for patients with advanced NSCLC. Censored patients are marked with “┃” in the graph. Numbers of patients at risk at indicated time points are shown below the x-axis. Number of events, median OS, 1-year and 2-year OS rates, and stratified HR for death are shown to the right of Kaplan-Meier curves. (B) Overall survival in key subgroups. All HRs were computed using the Cox proportional hazards model. All P values were two-sided with no adjustment of multiplicity. The P values of comparing the Kaplan-Meier curves were computed using the log-rank test stratified by the baseline PD-L1 expression status, histology, and smoking status. The P values of testing the interaction of the subgroup variables with the treatment (B) were computed using the Cox proportional hazards regression model with the treatment arm, the subgroup variable, and their interaction as the covariates. aP values were stratified by the factors used for randomization except the subgroup variable itself. bNA, not applicable due to overlapping metastases to liver and bone. HR, hazard ratio; NA, not available; NE, not evaluable; NSCLC, non–small-cell lung cancer; OS, overall survival; PD-L1, programmed death ligand-1.
The treatment effect on OS was generally consistent across major subgroups and favored the toripalimab combination (Fig 3B). A substantial benefit could be seen in patients with nonsquamous NSCLC, HR, 0.48 (95% CI, 0.32 to 0.71). In patients with squamous cell carcinoma, OS analysis showed no significant difference, HR, 0.99 (95% CI, 0.67 to 1.48; Fig 3B and Data Supplement).
Antitumor Response
The toripalimab chemotherapy combination arm had significantly better ORR and DoR than the placebo arm at the interim (Data Supplement) and final PFS (Data Supplement) analyses. As of October 31, 2021, the investigator-assessed ORR per RECIST v1.1 was higher in the toripalimab arm than in the placebo arm, 65.7% versus 46.2%, P < .0001 (Table 2 and Data Supplement). The median DoR was 8.4 versus 4.2 months, HR, 0.38 (95% CI, 0.28 to 0.53; Table 2 and Data Supplement). Similar BICR-determined ORR and DoR results were observed (Data Supplement).
Adverse Events
As of October 31, 2021, the median toripalimab exposure duration was slightly longer than placebo exposure (6.6 v 5.0 months). Concomitant toripalimab administration did not affect the completion of the planned chemotherapy (Data Supplement).
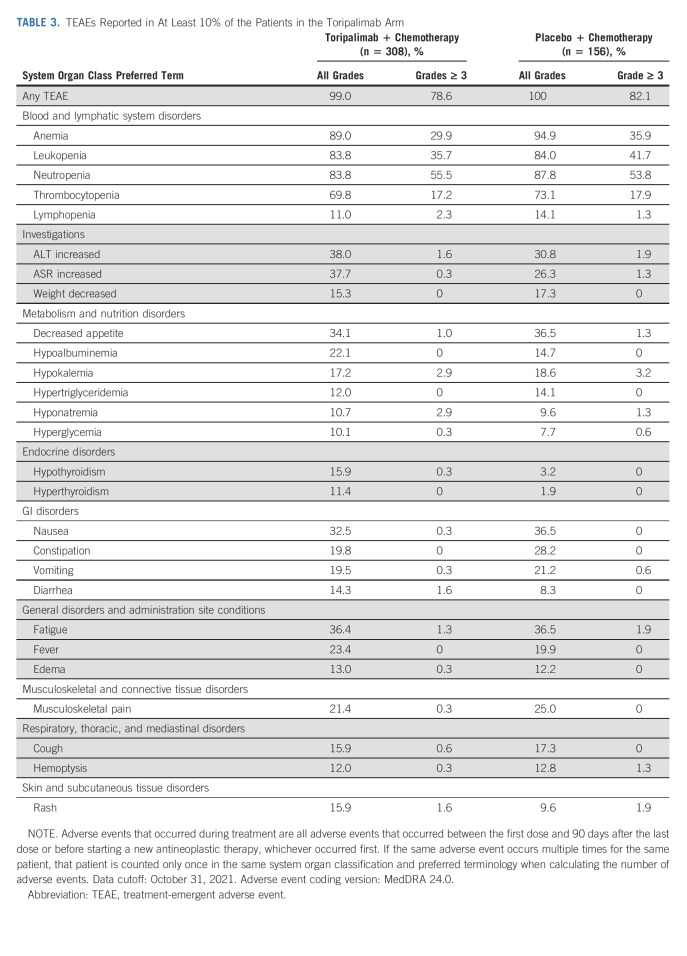
By the cutoff date, all but one patient experienced at least one treatment-emergent adverse event (TEAE; Data Supplement). Similar incidences of grade ≥ 3 TEAEs (78.6% v 82.1%) occurred in the toripalimab arm and the placebo arm. Fatal TEAEs (5.5% v 2.6%), serious adverse events (44.8% v 35.3%), infusion-related reactions (2.6% v 1.3%), and TEAEs leading to discontinuation of toripalimab or placebo (14.3% v 3.2%) were more frequent in the toripalimab arm (Data Supplement).
The most common adverse events are listed in Table 3. Adverse events that occurred more commonly in the toripalimab arm included thyroid disease, diarrhea, edema, pneumonitis, and rash. The majority of these AEs are consistent with the development of immune-related adverse events (irAEs) in patients treated with a checkpoint inhibitor, as previously reported.16
TABLE 3.
TEAEs Reported in At Least 10% of the Patients in the Toripalimab Arm
irAEs, as assessed by blinded investigators, were more frequent in the toripalimab arm than in the placebo arm (49.4% v 21.2%; Data Supplement). The most common irAEs were listed in the Data Supplement, among which 15.6% of the patients in the toripalimab arm and 3.2% of the patients in the placebo arm experienced grade ≥ 3 irAEs.
Biomarker Analysis
Genomic analysis using whole-exome sequencing was performed on tumor biopsies and paired PBMCs, and the results were acquired from 394 patients. Overlapping yet distinctive mutation profiles were observed from two histologic subtypes in this study (Data Supplement).
The median TMB value was 6.6 mutations/Mb in the cohort. Tumor tissues from 122 (26.2%) patients harbored more than 10 mutations/Mb, of which 72.1% were also PD-L1 TC ≥ 1% (Data Supplement). TMB-H patients had better ORR in the toripalimab arm than those in the placebo arm (72.7% v 46.7%), similar to the response rates observed in the two arms in the ITT population (65.7% v 46.2%). Compared with TMB-low patients (median PFS: 8.3 v 6.5 months; HR, 0.62; 95% CI, 0.46 to 0.83), TMB-H patients in the toripalimab arm had more significant PFS benefit than those in the placebo arm (median PFS: 13.1 v 5.5 months, HR, 0.34; 95% CI, 0.21 to 0.54; interaction P = .026; Data Supplement). By contrast, similar OS benefits were observed in both TMB subgroups (interaction P = .9962; Data Supplement).
Further analysis revealed gene mutations that are significantly interacting with the treatment effects (Data Supplement), including RB1, KEAP1, and SMARCA4. Patients harboring SMARCA4 mutations, especially in the nonsquamous subgroup (n = 33), achieved significantly better PFS in the toripalimab arm than patients in the placebo arm (median PFS: 9.9 v 2.9 months, Data Supplement). By contrast, patients with RB1 mutations (n = 21) in the squamous subgroup correlated with worse PFS in the toripalimab arm than in the placebo arm (median PFS 4.2 v 8.2 months, Data Supplement), indicating that they might not benefit from the combination therapy.
Gene Set Enrichment Analysis was performed on significantly interacted genes to identify over-represented biologic pathways (Data Supplement). Interestingly, focal adhesion (FA) or focal adhesion: PI3K-Akt-mTOR signaling pathway with shared genes including COL3A1, COL6A3, FLT1, FLNC, HGF, IRS1, IRS2, ITGA4, ITGA8, and KDR appeared as one of the most enriched pathways for treatment effects. Patients carrying mutations in this pathway demonstrated significantly better PFS and OS from the toripalimab combination therapy than chemotherapy alone (interaction P values ≤ .001; Data Supplement). In addition, patients with alterations in downstream genes of IL-7 signaling pathways (HGF, IRS1, IRS2, and SMARCA4), or in the chromatin remodeling SWI/SNF complex (SMARCA4, SMARCA2, and PBRM1), had favorable PFS in the toripalimab arm (Data Supplement).
The findings were further validated using three publicly available NSCLC data sets with immunotherapy treatments.17-19 Notably, the validation sets confirmed that the patients with altered FA-PI3K-Akt pathway had significantly better PFS than patients with wild-type genes (Data Supplement).
DISCUSSION
In CHOICE-01 study, we compared the efficacy and safety of toripalimab versus placebo in combination with chemotherapy for the first-line treatment of advanced NSCLC. At the prespecified PFS and OS analyses, our results demonstrated that the addition of toripalimab to the standard first-line chemotherapy resulted in statistically significant and clinically meaningful improvements in both PFS and OS than chemotherapy alone, irrespective of PD-L1 expression.
For nonsquamous NSCLC, the OS curves separated early and remained clearly separated throughout with a HR of 0.48 (95% CI, 0.32 to 0.71). In patients with squamous cell carcinoma, OS analysis did not demonstrate a difference between the treatment arms.
The OS curve of the placebo arm illustrated a long plateau period, which may be explained by the extensive crossover and subsequent use of PD-1/L1 inhibitors. More patients from the placebo arm received subsequent anti–PD-1/L1 treatment in CHOICE-01 (65.4%) than previous first-line NSCLC trials, including KEYNOTE-189 (41.3%), KEYNOTE-407 (31.7%), and IMpower-130 (59.2%). Furthermore, a high crossover rate of 71.2% was observed in the squamous subgroup. In CHOICE-01 study, the median OS was 17.1 months in the placebo arm, which is longer than historical results of 11-14 months of chemotherapy alone in NSCLC.4-6 The extensive crossover may explain the prolonged median OS and contribute to the lack of OS benefit seen in the squamous subgroup.
In squamous patients, the OS curves crossed at approximately 15 months with subsequent improvement in the toripalimab arm. Given the significant improvement of PFS observed in both squamous and nonsquamous NSCLC, it is unlikely that PFS results contributed to the initial crossing of the OS curves.
Previous studies have shown that PD-L1 or TMB-H was correlated with favorable responses to single-agent anti–PD-1/PD-L1 therapy in certain solid tumors.20-22 However, these two biomarkers could not present valuable differentiation in terms of chemoimmunotherapy combinations.6,11,23-26 In the current trial, the PFS and survival benefits of toripalimab plus chemotherapy were independent of PD-L1 expression. By contrast, a positive interaction was observed between TMB and toripalimab treatment. Compared with TMB-low patients, TMB-H patients in the toripalimab arm had more favorable PFS than those in the placebo arm.
One intriguing finding of this study is the identification of pathway alterations that were associated with better PFS and/or OS, which have not been reported previously. Disruption of the focal adhesion-PI3K-Akt signaling pathway may increase immune surveillance by overcoming the fibrotic and immunosuppressive tumor microenvironment and sensitize tumor to immunotherapy,27 whereas patients with downstream gene alterations in IL-7 signaling axis and chromatin remodeling complex such as loss of SMARCA4 have been reported to show a favorable response from immune checkpoint inhibitor therapy in solid tumors.28,29,30 An in silico data analysis of TCGA lung adenocarcinoma study also showed higher signature score of CD8 T cells in patients with mutations in focal adhesion-PI3K-Akt or IL-7 signing pathways (Data Supplement). Notably, using three publicly available immunotherapy-treated NSCLC data sets, patients with altered focal adhesion-PI3K-Akt pathway had significantly better PFS than patients with wild-type genes17-19 (Data Supplement).
CHOICE-01 study recruited patients solely from China. We speculate that the findings from CHOICE-01 could be extrapolated to Western patients with NSCLC for the following reasons. First, evidence of similar treatment effects between patients with NSCLC in the West and China is supported by the similar PFS results from CHOICE-01, KEYNOTE-189, and KEYNOTE-407, which is not affected by crossover. Second, a meta-analysis conducted by the US Food and Drug Administration to assess whether the treatment effect is consistent across Asian and non-Asian patients with NSCLC treated with checkpoint inhibitors found that while Asians have a better prognosis overall, the treatment effect of the checkpoint inhibitors was similar in Asians and non-Asians.31 Thus, the efficacy of the immunochemotherapy combination did not differ by race.
In conclusion, the addition of toripalimab to chemotherapy in treatment-naive patients with advanced NSCLC results in superior PFS and OS than chemotherapy alone while having a manageable safety profile. These results support the use of toripalimab with chemotherapy as a first-line therapy for patients with advanced NSCLC without EGFR/ALK mutations.
ACKNOWLEDGMENT
The authors thank the patients who participated in this study and their families.
APPENDIX 1. SUPPLEMENTARY METHODS
Whole-Exome Sequencing Assay
Whole-exome sequencing was performed with the SureSelect Human All Exon V6 kit (Agilent) on tumor biopsies and matched peripheral blood mononuclear cells (PBMC) samples in a central lab (OrigiMed, Shanghai, China). Briefly, a 4-μm section of a hematoxylin and eosin–stained slide of an formalin-fixed paraffin-embedded (FFPE) sample underwent pathologist review to ensure each sample has nucleated cellularity > 80% and tumor content no < 20%. DNA was extracted from unstained FFPE sections using the QIAamp DNA FFPE Tissue Kit (Qiagen) and quantified using the Qubit dsDNA HS Assay Kit (Life Technologies). Enough unstained FFPE sections were used to extract no < 200 ng DNA. After DNA extraction, 1 ng DNA was subjected to polymerase chain reaction (PCR) to amplify housekeeping gene fragments using a set of primers (synthesized by Invitrogen, Life Technologies), producing fragment sizes from 100 to 300 bp. PCR products were analyzed on 1.5% agarose gels and assessed for the DNA quality score depending on the relative signal intensity product band compared with the positive control. DNA samples yield < 50 ng, or the DNA quality score of 0 will be flagged as at risk; 50-500 ng of dsDNA in 50-µL water in microTUBEs is fragmented to approximately 250 bp by sonication (Covaris LE220). Sample library preparation was performed using the KAPA Hyper Prep Kit (KAPA Biosystems), and sample libraries were sized on a LabChip GX Touch HT (Perkin Elmer). Target enrichment was done by using the NGS-based hybrid capture method, as per the manufacturer's protocol (Integrated DNA Technologies), using the pool of customized individually synthesized probes plus xGen Exome Research Panel (Integrated DNA Technologies), targeting nearly 20,000 genes with in total 52.3 Mb of the human genome. Postcapture libraries were quantified using the Qubit dsDNA HS Assay Kit and then evaluated using the DNA High Sensitivity Reagent Kit on LabChip GX Touch HT (Perkin Elmer). The enriched libraries were further pooled together for sequencing on Illumina NovaSeq 6000 with 2 × 150 bp paired-end reads and approximately 500× sequencing depth for FFPE tumor slides or 150× for matched PBMC samples.
Somatic Short Variant and Fusion Analysis
Raw sequence data were processed using a customized analysis pipeline. Alignment of raw reads to the human genome reference sequence (hg19) was performed with the Burrows-Wheeler Aligner (BWA, v0.6.2), followed by PCR duplicates removal using Picard (version 1.47).32 Single-nucleotide variant calling was performed using GATK (v3.1-1) and subsequently called by MUTECT (v1.7). Short insertion/deletions (S-indels) were calibrated for alignment using ABRA (v0.97) and then called by PINDEL (v0.2.5a8). The raw calls of single-nucleotide variants and S-indel were further selected as follows: (1) Minimum five reads were required to support alternative calling, (2) variants with read depths < 30×, with strand bias larger than 10%, or with VAF < 0.5% were removed, and (3) common SNPs defined as those from dbSNP database (version 147), or frequency over 1.5% of Exome Sequencing Project 6500 (ESP6500) or over 1.5% of 1000 genome project, were also excluded for further consideration. Mutations with cancer coverage less than normal coverage are removed. Variants with mutation rate > 3% in matched PBMC samples or near repeat regions were also removed.
For detection of gene rearrangements, paired-end reads with the abnormal insert size of over 2,000 bp aligned to the same chromosome or aligned to different chromosomes were collected and used as discordant reads. The group consisting of discordant reads with a distance < 500 bp formed a cluster, and paired clusters were obtained according to the pairing relationship. Consistent breakpoints from the paired-end discordant reads within a cluster were identified to establish potential rearrangement breakpoints. The breakpoints were double confirmed by BLAT, and the corresponding discordant reads were filtered for those uniquely mapped to the genome reference to constitute rearrangement supported reads. The resulting chimeric read candidates were genome annotated.
Gene Set/Pathway Over representation Analysis
Gene set/pathway enrichment analysis was performed on genes whose alterations are significantly interacting with toripalimab treatment by univariate analysis with nominal P ≤ .05. Hypergeometric test from enricher function in R (package: clusterProfiler), followed with multiple testing adjustment, was used to assess the significance of gene sets compiled by MSigDB database v7.5.1.,33 including hallmark gene sets, canonical pathways, and oncogenic signature gene sets. Top significant gene sets were selected on the basis of P value of .05 and false discovery rate of 0.2.
Zhijie Wang
Speakers' Bureau: Roche China, MSD
Lin Wu
Employment: AstraZeneca, Roche, Bristol Myers Squibb, MSD, Pfizer, Lilly, Boehringer Ingelheim, Merck, Innovent Biologics, Hengrui Pharmaceutical
Qiming Wang
Consulting or Advisory Role: Hansoh Pharma
Research Funding: Hansoh Pharma (Inst)
Tong Zhou
Speakers' Bureau: AstraZeneca, Roche China, Novartis
Xiongwen Tang
Employment: TopAlliance BioSciences Inc
Stock and Other Ownership Interests: TopAlliance BioSciences Inc
Jianjun Yu
Employment: TopAlliance BioSciences Inc, Predicine
Stock and Other Ownership Interests: TopAlliance BioSciences Inc, Predicine
Ellen Maher
Employment: TopAlliance BioSciences Inc
Travel, Accommodations, Expenses: TopAlliance BioSciences Inc
Hui Feng
Employment: Shanghai Junshi BioSciences, TopAlliance BioSciences Inc
Leadership: Shanghai Junshi BioSciences, TopAlliance BioSciences Inc
Stock and Other Ownership Interests: Shanghai Junshi BioSciences
Patents, Royalties, Other Intellectual Property: Shanghai Junshi BioSciences Inc patent
Travel, Accommodations, Expenses: Shanghai Junshi BioSciences
Sheng Yao
Employment: TopAlliance BioSciences Inc, Shanghai Junshi BioSciences
Leadership: Shanghai Junshi BioSciences
Stock and Other Ownership Interests: Shanghai Junshi BioSciences
Patents, Royalties, Other Intellectual Property: Patent applications as employee of TopAlliance Biosciences Inc
Patricia Keegan
Employment: TopAlliance Biosciences
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented at the WCLC 2021, virtual, September 13, 2021 (abstr 220); and at the ASCO monthly plenary, virtual, March 15, 2022.
SUPPORT
This study is sponsored by Shanghai Junshi Biosciences. This work was supported by National key research and development project (2019YFC1315700) to J.W., CAMS Innovation Fund for Medical Sciences (2021-1-I2M-012) to Z.W., the National Natural Sciences Foundation of China (81871889 and 82072586) to Z.W., Beijing Natural Science Foundation (7212084) to Z.W., Aiyou Foundation (KY201701) to J.W., National Major Science & Technology Major Projects (2017ZX09302009) to H.F., and Shanghai Science and Technology Committee Technology Grant (17431900700) to H.F.
CLINICAL TRIAL INFORMATION
NCT03856411 (CHOICE-01)
Z.W. and L.W. contributed equally to this work.
DATA SHARING STATEMENT
Data generated in this study are available within the article or supplementary data files.
AUTHOR CONTRIBUTIONS
Conception and design: Zhijie Wang, Ying Cheng, Xiaoli Zhu, Yingyi Wang, Xiongwen Tang, Hui Feng, Sheng Yao, Jie Wang
Financial support: Zhijie Wang, Jie Wang
Administrative support: Patricia Keegan
Provision of study materials or patients: Zhijie Wang, Lin Wu, Baolan Li, Ying Cheng, Xiaoling Li, Xicheng Wang, Liang Han, Xiaohong Wu, Yun Fan, Yan Yu, Dongqing Lv, Jianhua Shi, Jianjin Huang, Shaozhang Zhou, Baohui Han, Guogui Sun, Qisen Guo, Xiaoli Zhu, Sheng Hu, Wei Zhang, Qiming Wang, Yuming Jia, Ziping Wang, Yong Song, Jingxun Wu, Meiqi Shi, Xingya Li, Zhigang Han, Yunpeng Liu, Zhuang Yu, An-Wen Liu, Xiuwen Wang, Caicun Zhou, Diansheng Zhong, Liyun Miao, Zhihong Zhang, Hui Zhao, Jun Yang, Dong Wang, Yingyi Wang, Qiang Li, Xiaodong Zhang, Mei Ji, Zhenzhou Yang, Jiuwei Cui, Beili Gao, Hu Liu, Lei Nie, Mei He, Shi Jin, Wei Gu, Yongqian Shu, Tong Zhou, Jian Feng, Xinmei Yang, Cheng Huang, Bo Zhu, Yu Yao, Jianjun Yu, Hui Feng, Sheng Yao
Collection and assembly of data: Zhijie Wang, Lin Wu, Baolan Li, Ying Cheng, Xiaoling Li, Xicheng Wang, Liang Han, Xiaohong Wu, Yun Fan, Yan Yu, Dongqing Lv, Jianhua Shi, Jianjin Huang, Shaozhang Zhou, Baohui Han, Guogui Sun, Qisen Guo, Youxin Ji, Xiaoli Zhu, Sheng Hu, Wei Zhang, Qiming Wang, Yuming Jia, Ziping Wang, Yong Song, Jingxun Wu, Meiqi Shi, Xingya Li, Zhigang Han, Yunpeng Liu, Zhuang Yu, An-Wen Liu, Xiuwen Wang, Caicun Zhou, Diansheng Zhong, Liyun Miao, Zhihong Zhang, Hui Zhao, Jun Yang, Dong Wang, Yingyi Wang, Qiang Li, Xiaodong Zhang, Mei Ji, Zhenzhou Yang, Jiuwei Cui, Beili Gao, Buhai Wang, Hu Liu, Lei Nie, Mei He, Shi Jin, Wei Gu, Yongqian Shu, Tong Zhou, Jian Feng, Xinmei Yang, Cheng Huang, Bo Zhu, Yu Yao, Jianjun Yu, Hui Feng, Sheng Yao, Jie Wang
Data analysis and interpretation: Zhijie Wang, Lin Wu, Baolan Li, Ying Cheng, Xiaoling Li, Xicheng Wang, Liang Han, Xiaohong Wu, Yun Fan, Yan Yu, Dongqing Lv, Jianhua Shi, Jianjin Huang, Baohui Han, Guogui Sun, Xiaoli Zhu, Sheng Hu, Wei Zhang, Yuming Jia, Ziping Wang, Yong Song, Jingxun Wu, Meiqi Shi, Xingya Li, Zhigang Han, Yunpeng Liu, Zhuang Yu, An-Wen Liu, Caicun Zhou, Diansheng Zhong, Liyun Miao, Zhihong Zhang, Hui Zhao, Jun Yang, Dong Wang, Yingyi Wang, Qiang Li, Xiaodong Zhang, Mei Ji, Zhenzhou Yang, Jiuwei Cui, Beili Gao, Buhai Wang, Hu Liu, Lei Nie, Mei He, Shi Jin, Wei Gu, Yongqian Shu, Tong Zhou, Jian Feng, Xinmei Yang, Cheng Huang, Bo Zhu, Yu Yao, Xiongwen Tang, Jianjun Yu, Ellen Maher, Hui Feng, Sheng Yao, Patricia Keegan, Jie Wang
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Toripalimab Plus Chemotherapy for Patients With Treatment-Naive Advanced Non–Small-Cell Lung Cancer: A Multicenter Randomized Phase III Trial (CHOICE-01)
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Zhijie Wang
Speakers' Bureau: Roche China, MSD
Lin Wu
Employment: AstraZeneca, Roche, Bristol Myers Squibb, MSD, Pfizer, Lilly, Boehringer Ingelheim, Merck, Innovent Biologics, Hengrui Pharmaceutical
Qiming Wang
Consulting or Advisory Role: Hansoh Pharma
Research Funding: Hansoh Pharma (Inst)
Tong Zhou
Speakers' Bureau: AstraZeneca, Roche China, Novartis
Xiongwen Tang
Employment: TopAlliance BioSciences Inc
Stock and Other Ownership Interests: TopAlliance BioSciences Inc
Jianjun Yu
Employment: TopAlliance BioSciences Inc, Predicine
Stock and Other Ownership Interests: TopAlliance BioSciences Inc, Predicine
Ellen Maher
Employment: TopAlliance BioSciences Inc
Travel, Accommodations, Expenses: TopAlliance BioSciences Inc
Hui Feng
Employment: Shanghai Junshi BioSciences, TopAlliance BioSciences Inc
Leadership: Shanghai Junshi BioSciences, TopAlliance BioSciences Inc
Stock and Other Ownership Interests: Shanghai Junshi BioSciences
Patents, Royalties, Other Intellectual Property: Shanghai Junshi BioSciences Inc patent
Travel, Accommodations, Expenses: Shanghai Junshi BioSciences
Sheng Yao
Employment: TopAlliance BioSciences Inc, Shanghai Junshi BioSciences
Leadership: Shanghai Junshi BioSciences
Stock and Other Ownership Interests: Shanghai Junshi BioSciences
Patents, Royalties, Other Intellectual Property: Patent applications as employee of TopAlliance Biosciences Inc
Patricia Keegan
Employment: TopAlliance Biosciences
No other potential conflicts of interest were reported.
REFERENCES
- 1.Molina JR, Yang P, Cassivi SD, et al. : Non-small cell lung cancer: Epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc 83:584-594, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL, et al. : Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209-249, 2021 [DOI] [PubMed] [Google Scholar]
- 3.Qiu H, Cao S, Xu R: Cancer incidence, mortality, and burden in China: A time-trend analysis and comparison with the United States and United Kingdom based on the global epidemiological data released in 2020. Cancer Commun (Lond) 41:1037-1048, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.West H, McCleod M, Hussein M, et al. : Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 20:924-937, 2019 [DOI] [PubMed] [Google Scholar]
- 5.Paz-Ares L, Luft A, Vicente D, et al. : Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med 379:2040-2051, 2018 [DOI] [PubMed] [Google Scholar]
- 6.Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. : Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 378:2078-2092, 2018 [DOI] [PubMed] [Google Scholar]
- 7.Wei XL, Ren C, Wang FH, et al. : A phase I study of toripalimab, an anti-PD-1 antibody, in patients with refractory malignant solid tumors. Cancer Commun (Lond) 40:345-354, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang B, Chi Z, Chen Y, et al. : Safety, efficacy, and biomarker analysis of toripalimab in previously treated advanced melanoma: Results of the POLARIS-01 multicenter phase II trial. Clin Cancer Res 26:4250-4259, 2020 [DOI] [PubMed] [Google Scholar]
- 9.Wang FH, Wei XL, Feng J, et al. : Efficacy, safety, and correlative biomarkers of toripalimab in previously treated recurrent or metastatic nasopharyngeal carcinoma: A phase II clinical trial (POLARIS-02). J Clin Oncol 39:704-712, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheng X, Chen H, Hu B, et al. : Recombinant humanized anti-PD-1 monoclonal antibody toripalimab in patients with metastatic urothelial carcinoma: Results of an open-label phase II clinical study Polaris-03. J Clin Oncol 38, 2020. (suppl 6; abstr 5040) [Google Scholar]
- 11.Mai H-Q, Chen Q-Y, Chen D, et al. : Toripalimab or placebo plus chemotherapy as first-line treatment in advanced nasopharyngeal carcinoma: A multicenter randomized phase 3 trial. Nat Med 27:1536-1543, 2021 [DOI] [PubMed] [Google Scholar]
- 12.Liu H, Guo L, Zhang J, et al. : Glycosylation-independent binding of monoclonal antibody toripalimab to FG loop of PD-1 for tumor immune checkpoint therapy. MAbs 11:681-690, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu J, Wang F, Dong LH, et al. : Preclinical evaluation of the efficacy, pharmacokinetics and immunogenicity of JS-001, a programmed cell death protein-1 (PD-1) monoclonal antibody. Acta Pharmacol Sin 38:710-718, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Z, Ying J, Xu J, et al. : Safety, antitumor activity, and pharmacokinetics of toripalimab, a programmed cell death 1 inhibitor, in patients with advanced non-small cell lung cancer: A phase 1 trial. JAMA Netw Open 3:e2013770, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang J, Dong L, Yang S, et al. : Safety and clinical efficacy of toripalimab, a PD-1 mAb, in patients with advanced or recurrent malignancies in a phase I study. Eur J Cancer 130:182-192, 2020 [DOI] [PubMed] [Google Scholar]
- 16.Ramos-Casals M, Brahmer JR, Callahan MK, et al. : Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers 6:38, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hellmann MD, Nathanson T, Rizvi H, et al. : Genomic features of response to combination immunotherapy in patients with advanced non-small-cell lung cancer. Cancer Cell 33:843-852 e4, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rizvi NA, Hellmann MD, Snyder A, et al. : Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348:124-128, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miao D, Margolis CA, Vokes NI, et al. : Genomic correlates of response to immune checkpoint blockade in microsatellite-stable solid tumors. Nat Genet 50:1271-1281, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brahmer J, Reckamp KL, Baas P, et al. : Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 373:123-135, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rizvi NA, Mazières J, Planchard D, et al. : Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): A phase 2, single-arm trial. Lancet Oncol 16:257-265, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang F, Wei XL, Wang FH, et al. : Safety, efficacy and tumor mutational burden as a biomarker of overall survival benefit in chemo-refractory gastric cancer treated with toripalimab, a PD-1 antibody in phase Ib/II clinical trial NCT02915432. Ann Oncol 30:1479-1486, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang ZX, Cui C, Yao J, et al. : Toripalimab plus chemotherapy in treatment-naive, advanced esophageal squamous cell carcinoma (JUPITER-06): A multi-center phase 3 trial. Cancer Cell 40:277-288 e3, 2022 [DOI] [PubMed] [Google Scholar]
- 24.Nishio M, Barlesi F, West H, et al. : Atezolizumab plus chemotherapy for first-line treatment of nonsquamous NSCLC: Results from the randomized phase 3 IMpower132 trial. J Thorac Oncol 16:653-664, 2021 [DOI] [PubMed] [Google Scholar]
- 25.Gadgeel S, Rodriguez-Abreu D, Speranza G, et al. : Updated analysis from KEYNOTE-189: Pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non-small-cell lung cancer. J Clin Oncol 38:1505-1517, 2020 [DOI] [PubMed] [Google Scholar]
- 26.Paz-Ares L, Vicente D, Tafreshi A, et al. : A randomized, placebo-controlled trial of pembrolizumab plus chemotherapy in patients with metastatic squamous NSCLC: Protocol-specified final analysis of KEYNOTE-407. J Thorac Oncol 15:1657-1669, 2020 [DOI] [PubMed] [Google Scholar]
- 27.Jiang H, Hegde S, Knolhoff BL, et al. : Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat Med 22:851-860, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jian M, Yunjia Z, Zhiying D, et al. : Interleukin 7 receptor activates PI3K/Akt/mTOR signaling pathway via downregulation of Beclin-1 in lung cancer. Mol Carcinog 58:358-365, 2019 [DOI] [PubMed] [Google Scholar]
- 29.Miao D, Margolis CA, Gao W, et al. : Genomic correlates of response to immune checkpoint therapies in clear cell renal cell carcinoma. Science 359:801-806, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou H, Liu J, Zhang Y, et al. : PBRM1 mutation and preliminary response to immune checkpoint blockade treatment in non-small cell lung cancer. NPJ Precis Oncol 4:6, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang E, Gong Y, Vallejo JJ, et al. : FDA analysis of outcomes in Asian patients (pts) with metastatic non-small cell lung cancer (mNSCLC) receiving immune checkpoint inhibitors (ICI). J Clin Oncol 37, 2019. (suppl 15; abstr e20690) [Google Scholar]
- 32.Gene Set Enrichment Analysis: Molecular signatures database. https://www.gsea-msigdb.org/gsea/msigdb/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data generated in this study are available within the article or supplementary data files.