Abstract
Background:
Face shields protect healthcare workers (HCWs) from fluid and large droplet contamination. Their effect on smaller aerosolized particles is unknown.
Materials & methods:
An ultrasonic atomizer was used to simulate particle sizes equivalent to human breathing and forceful cough. Particles were measured at positions correlating to anesthetic personnel in relation to a patient inside an operating theatre environment. The effect of the application of face shields on HCW exposure was measured.
Results & Conclusion:
Significant reductions in particle concentrations were measured after the application of vented and enclosed face shields. Face shields appear to reduce the concentration of aerosolized particles that HCWs are exposed to, thereby potentially conferring further protection against exposure to aerosolized particles in an operating theatre environment.
Keywords: aerosol generating procedure, aerosolized particles, contamination, COVID-19, face shield, personal protective equipment (PPE)
Plain language summary
Face shields protect health workers from splash contamination. We do not know if they protect against smaller invisible aerosol drops that can carry diseases like coronavirus 2019/COVID-19. The authors tested whether face shields can stop floating droplets using different types of face shields. This included one that was designed and made by a 3D printer, and traditional face shields. The shields were tested in a hospital operating room. A machine was designed that made invisible saltwater droplets. A monitor was used to measure the droplets present at a doctor's or nurse's mouth and then if this changed when a face shield was used. The face shield might be helpful in stopping health workers from catching diseases by stopping the flow of aerosol drops.
The COVID-19 pandemic, caused by the SARS-CoV-2 virus, remains a continuing international health crisis. Current personal protective equipment (PPE) guidelines are established on the assumption that the primary mechanism of transmission is via larger respiratory droplets (>5 μm diameter) when they contact a recipient's mucosal surface [1], however, the aerosol transmission of smaller droplet particles (<5 μm) also plays a role and both routes likely represent a continuum for transmissible respiratory infections [2]. Studies suggest respiratory droplets may be aerosolized and carried in a gas cloud with horizontal trajectories beyond 2 m during normal speaking, coughing or sneezing [2–5]. In human influenza models, aerosol inoculation is associated with increased disease severity, lower respiratory tract infection and higher transmissibility [6].
Aerosol-generating procedures (AGPs) are a source of anxiety for healthcare workers (HCWs) due to increasing acknowledgment of the risk of aerosolized transmission [3,7]. HCWs are advised to utilize aerosol precautions at the time of AGPs, which may generate turbulent multiphase gas clouds that remain suspended for hours with the ability to cause longer-range infectious transmission. There is an ongoing debate with regard to the appropriate level of PPE for AGPs given the lack of current evidence, though N95 masks and P2 respirators have now become mainstays for protection against aerosolized particles [3,7].
Whether intubation is considered an AGP has become controversial. A recent study on aerosol sampling during routine clinical practice suggests that anesthesia procedures such as intubation may not be associated with the presumed high risk of aerosol generation, and may be even less risky than a single volitional cough [8]. However, this contrasts previous retrospective evidence [9,10] and a recent observational study that demonstrated that tracheal intubation, extubation and positive pressure bag-mask ventilation contributed to large spikes in aerosol release [11]. This is supported by Tran et al.‘s systematic review [10]. Furthermore, in an international self-reported registry study that included 1718 clinicians who performed one or more intubations in COVID-19 patients, 8.4% developed symptoms of COVID-19, with 3% subsequently confirmed to be COVID-19 positive [12]. Another variable is the clinical setting, as the operating theater environment differs due to the presence of positive pressure laminar flow ventilation. Variables such as ventilation, temperature, humidity and air exchange have been shown to affect the aerosol transmission of infection [13].
While more robust evidence is gathered, airborne precautions are universally recommended when AGPs are performed as a precautionary approach toward the safety of HCWs. The current WHO policy is in keeping with the recommendations of the Infection Control Expert Group (ICEG), endorsed by the Australian Health Protection Principal Committee [14] and includes the use of a particulate filter respirator (N95/P2) or equivalent; a long-sleeved, fluid-resistant gown; eye protection (e.g., goggles, visor, face shield); and gloves and shoe covers for any HCW treating suspected or confirmed COVID-19 patients undergoing AGPs [15].
Viral load in COVID-19 may correlate with infectivity, morbidity and mortality [16,17]. Infected symptomatic patients with high viral load and respiratory symptoms are thought to have increased work in breathing and increased closing capacity and generate increased amounts of pathogenic aerosols thus increasing the risk of viral transmission [18].
Novel COVID-inspired devices such as “intubating boxes” have elucidated the danger of assuming the protectiveness of PPE. Simpson et al. demonstrated potential increased exposure to concentrated streams of aerosolized particles that subsequently traveled along the path of least resistance toward the laryngoscopist's own airway when using “intubating boxes” [19]. This study laid bare the need for further research to test other assumptions on how aerosolized particles are dispersed.
In Australia, face shields for medical use are categorized as class 1 medical devices under the Therapeutic Goods Administration (TGA). Standard hospital-issued face shields consist of an impermeable plastic screen with a supporting headband, designed for single-disposable use. One example is the Halyard Health® Guardall face shield (Figure 1A). Such a practical approach means the upper aspect is enclosed, invariably to provide large droplet and splash protection arising from above the brow line. To date, there are no stringent guidelines on what constitutes an approved face shield in terms of material composition or size of the protective screen [20], and no mandatory Australian testing standard to evaluate efficacy. However, it is generally accepted that face shields' mainstay of protection is against front-on splash from liquids or large droplets [20,21]. They have never been associated with a means of protection against smaller-sized airborne suspended droplets and particles.
Figure 1. . Enclosed Face shields.

(A) Halyard® Guardall. (B) Victorian Government Supply Chain FS-2041, versus Vented Face Shields. (C) PRUSA RC3. (D) Victorian State Supply Chain FS-202004-38.
During the height of the global COVID-19 pandemic, the authors’ institute, along with many worldwide, suffered from PPE shortages, including face shields, due to increased use, distribution, manufacturer supply issues, transportation restrictions and stockpiling. Other generic face shields from parallel overseas sources, like that pictured in Figure 1B, are now a commonplace version of face shields following the traditional enclosed design. Along with concerns regarding “PPE burn” and crisis-level supplies of PPE, reusable alternatives were also sought. One example our center explored was the Prusa Protective Faceshield (RC3, Prusa Research, Prague, Czech Republic), an open-sourced 3D-printed face shield frame to which a plastic polycarbonate sheet could be readily attached and replaced (Figure 1C). Through the help of the Centre for Additive Manufacturing at RMIT University in Melbourne, 300 Prusa RC3 shields were printed, assembled and distributed for use in the operating theater at Box Hill Hospital, Eastern Health (Melbourne, Australia). The shield frames were printed on the university 3D printing farm of forty Zortrax M200plus FDM printers using Z-Ultrat ABS 1.75 mm filament (Zortrax, Poland). Print parameters include 50% infill, 4 shells, line width of 0.4 mm, layer height of 0.190 mm and printing temperature of 260°C. A stacked set of four frames took 17 h to print per printer. Further details on the Prusa RC3 face shield, including specifications and file download for this shield, are available from the website per Creative Commons license at www.prusaprinters.org/prints/25857-prusa-protective-face-shield-rc3.
Apart from reusability, another difference compared with traditional shields used prior to COVID was that the PRUSA design incorporated an unenclosed/vented superior aspect of the headband. Since then, commercially manufactured TGA-approved reusable shields made of tougher polycarbonates have also become available throughout Australian hospitals, which similarly follow this vented-top design (Figure 1D).
The change to vented shields, however, raises certain clinical questions. First, following Simpson's study on intubation boxes, questions were raised about whether enclosed face shields might similarly trap aerosolized particles within the confines of the space within the shield. If so, do vented shields prevent this by allowing the escape of a potential plume of aerosol into the surrounding atmosphere, or are their effects deleterious in that they allow for increased particle load to enter through the vented superior gap? Another assumption is that face shields have no effects whatsoever on dispersed aerosolized particles, as particles should permeate throughout the environment and as such, move in and around any barriers including face shields. Surprisingly, to our knowledge, apart from the study by Lindsley et al. who demonstrated some benefit of face shields from aerosolization from a cough simulator [21], there is scant to no evidence to support or dispel this.
The primary aim of this study was to evaluate the effect (if any) face shields have on the exposure of HCWs to aerosolized particles within a theater environment and, secondarily, to determine any differences in particle concentrations behind open-vented versus enclosed shields. The study was conducted in collaboration with the Department of Mechanical and Aerospace Engineering at Monash University. This work helps build on the currently available body of evidence for the appropriate use of PPE for HCWs performing AGPs, not just for the purpose of COVID-19, but for preparedness for future aerosol-transmitted pathogens.
Materials & methods
This was a single-center, prospective, in situ simulation study performed at a major metropolitan teaching hospital in Melbourne, Australia. The study was approved by the Eastern Health Human Research Ethics Committee (HREC/LNR20/034).
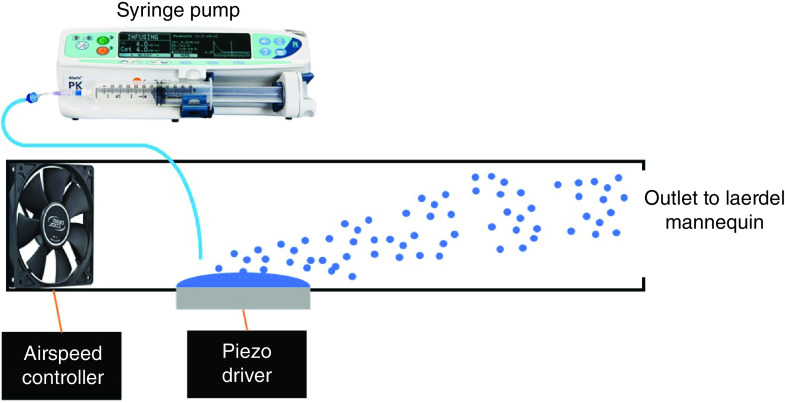
The simulation was performed in operating room number five of the Box Hill Hospital operating suite, which is a 7 m × 8 m theater room compliant with Australian Health Regulations Standards. Under normal operating room ventilation conditions, the airflow is rated at 20 air changes.hour-1 through positive laminar flow. The operating theater receives 50% return air through HEPA filters and has a discharge velocity of 1.2 m.s-1. The operating theater was vacated prior to testing and baseline measurements of air particles were measured to ensure adequate control conditions and exclude external particle contamination. A Laerdal® Airway Management mannequin (Laerdal Medical AS, Stavanger, Norway) was positioned on the operating table in a standard intubating position. An 875kHz ultrasonic atomizer and airflow control unit designed by the Monash University Aerospace Engineering Department allowed the uniform nebulization of sterile 0.9% saline (Figure 2). This device generated aerosolized droplets with diameters ranging from 0.3 to 5 μm, realistically reproducing particle sizes produced by a human cough [22]. Larger-sized droplets from 5 to 10 μm were also produced by the aerosol generator but to a much lesser degree as detected by the particle counter. Aerosol only exited via the mannequin's mouth and nose. Airspeed was controlled by regulating fan speed and monitored with a hot wire anemometer. This approach allowed the mass of aerosol generated and the delivery rate to be independently tuned. This is similar to aerosols produced by particle generators used by the industry for quantitative N95 mask fit testing.
Figure 2. . Aerosol generation setup.
The velocity of airflow was measured using a hot wire anemometer (Modern Device, Wind Sensor Rev. P). The setup generated 71 l.min-1 at the level of the nebulizer, which correlates to peak flow rate when talking [23]. Further velocities were also measured at the level of the Laerdel® mannequin head and chest region. The maximum volumetric output of the nebulizer was 650 ml.hr-1. In this study, fluid delivery of 50 ml.hr-1 was maintained, however, due to losses, particularly of larger droplets within the mannequin airway, the actual volumetric output was below this rate.
Foam mannequin heads attached to Mayo surgical stands were positioned at standard distances and heights away from the Laerdel® mannequin reflective of a laryngoscopist/anesthetist (position 1) and airway assistant's (position 2) intubating positions, and particle count was performed directly in front of the central upper lip at each site (Figure 3). The foam heads were positioned over the edge of the Mayfield tables to ensure aerosolized particles underneath were not obscured by the stands.
Figure 3. . Representation of study (courtesy of BioRender.com) and in vivo setup in operating theater for position 1.
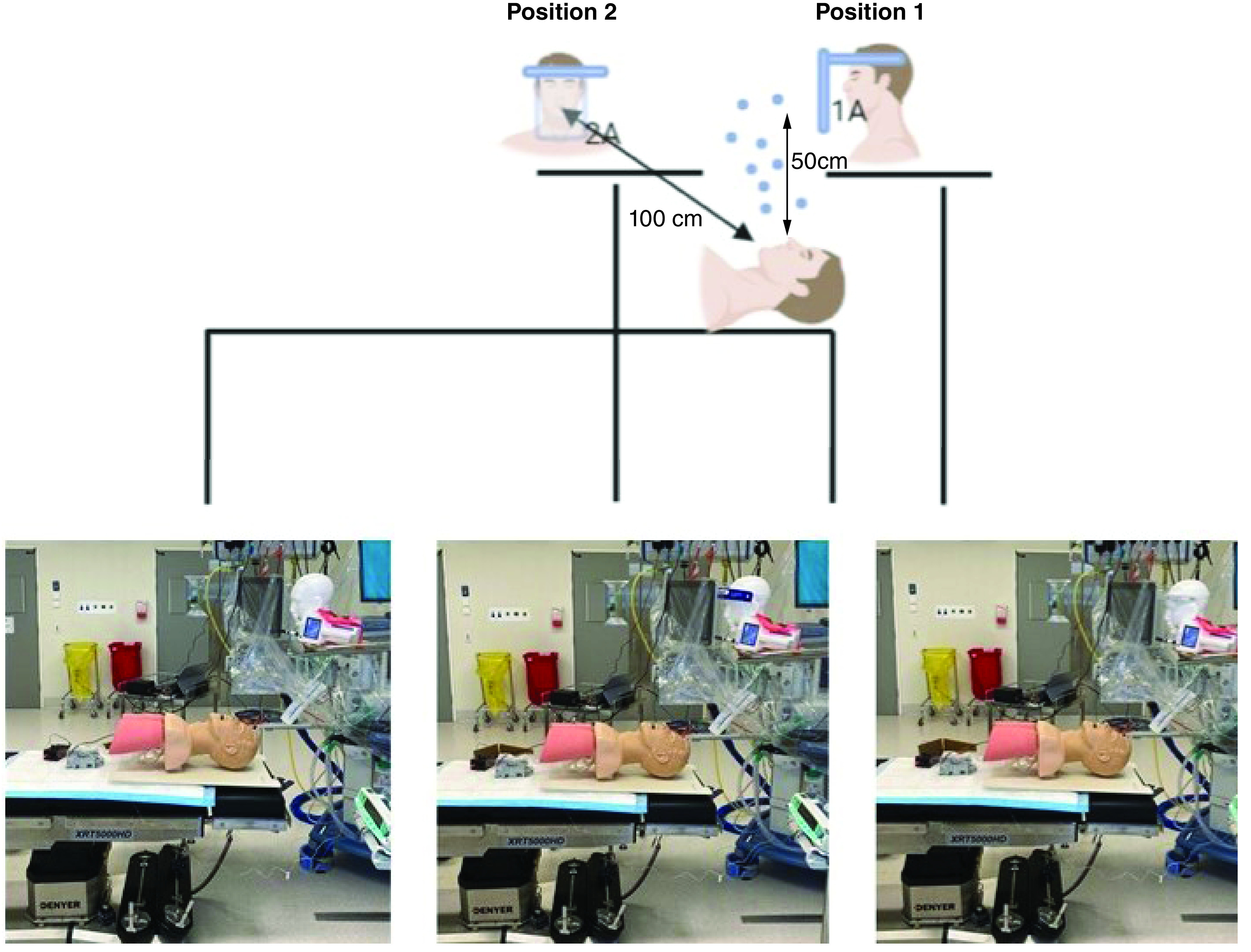
Points of aerosol particle testing. Position 1: laryngoscopist; Position 2: assistant; 1A: particle count 50 cm above aerosol source; 2A: particle count 100 cm above and caudal to aerosol source.
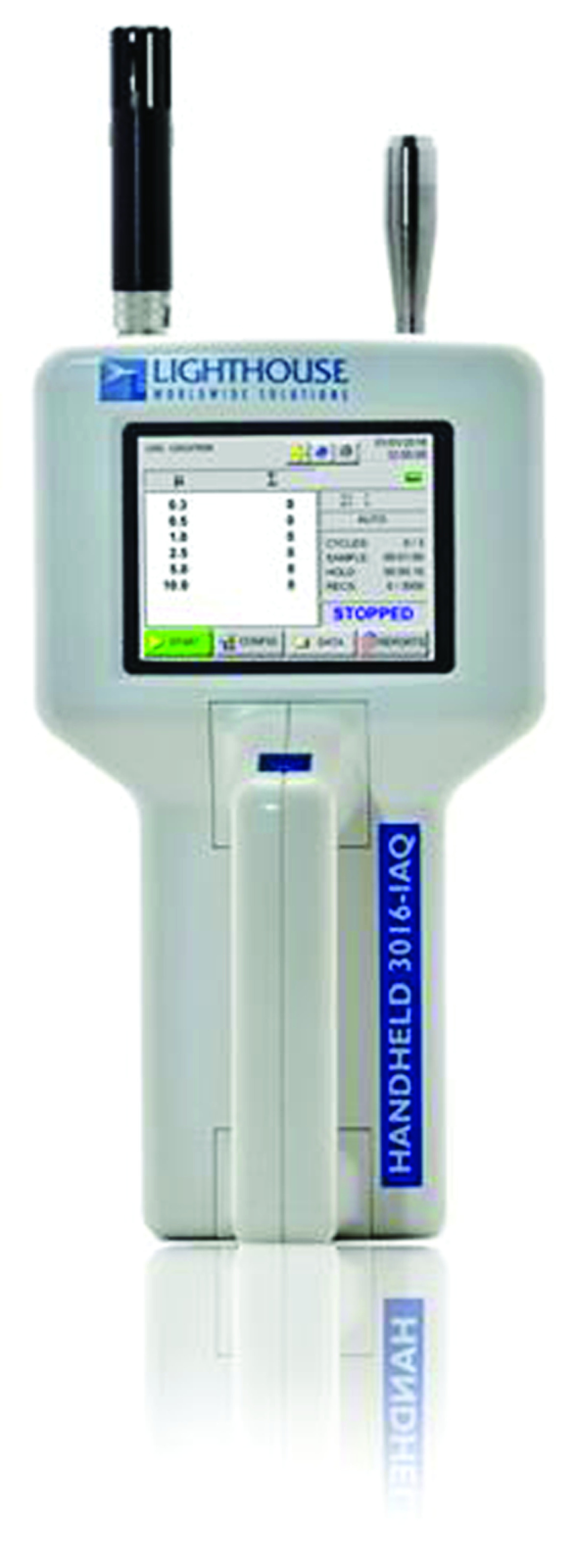
A Lighthouse 3016-IAQ particle counter (CA, USA; Figure 4) was used to measure particles of 0.3, 0.5, 1.0 and 2.5 μm, consistent with the range of smaller droplet particle sizes that were of interest in this study. The same device was used by Simpson et al. in their study on particles and intubating boxes [19]. Particle sizes of 0.3 μm are used as a standard to measure the effectiveness of high-efficiency particulate arrestance (HEPA) filters [24]. Fennelly et al.‘s study of infectious aerosols found that smaller droplets can remain airborne indefinitely in the atmosphere and contaminate the lower respiratory tract in humans [25]. The airborne particle counter flow rate was set to 2.83 l.min-1, with cumulative air particle counts recorded in 15-s sweeps. The room was kept clear of all personnel during testing to prevent aerosol particle contamination from external sources.
Figure 4. . Lighthouse 3016-IAQ Particle Counter (CA, USA).
Measurement of particles was first performed at position 1 in the absence of a face shield. Aerosolization of saline was commenced and a rapid increase in aerosol density was ensured over a 4-min period until a steady state was established, which was calculated to assume a density of 1.045 g.ml-1 for 0.9% saline solution. Readings were then further logged for another 5 min. The room was then “rested” for 20 min to allow 6–8 air changes. Baseline readings confirmed a return to pretesting levels of aerosolized particles which, due to the efficiency of the operating theatre levels, essentially resulted in negligible readings of the presence of any aerosolized particles. The test was then repeated with the application of Face Shield 1 and Face Shield 2. Shield 1 was a Victorian Supply Chain FS-2041 Face Shield, which represented a variety of enclosed shields (Figure 1B). Shield 2 was a PRUSA RC3 3D-printed Face Shield, which represented a variety of open-top/vented shields (Figure 1C). The shields were placed above the brow line of the foam mannequins and the plastic shielding was adjusted to be horizontally 5 cm away from the inlet port of the Lighthouse 3106IAQ.
Sample size consisted of 21 measurements of the size ranges indicated, taken in 15 s intervals. Given the observed large reduction in particle counts, even assuming a significance of 0.0001, very high statistical power in excess of 99% was achieved. Statistical analyses included one-way analysis of variance (ANOVA) with Tukey post-hoc testing between no shield and face shield results, with a statistical significance p-value of <0.0001. Statistical analyses were performed using GraphPad Prism v9.
Results
Figure 5A demonstrates the significant and uniform production of particles generated by the aerosol generation device, with a clear washout of particles from the positive pressure ventilation provided within the operating theater, providing stable and reproducible aerosol generation for analysis.
Figure 5. . Results.
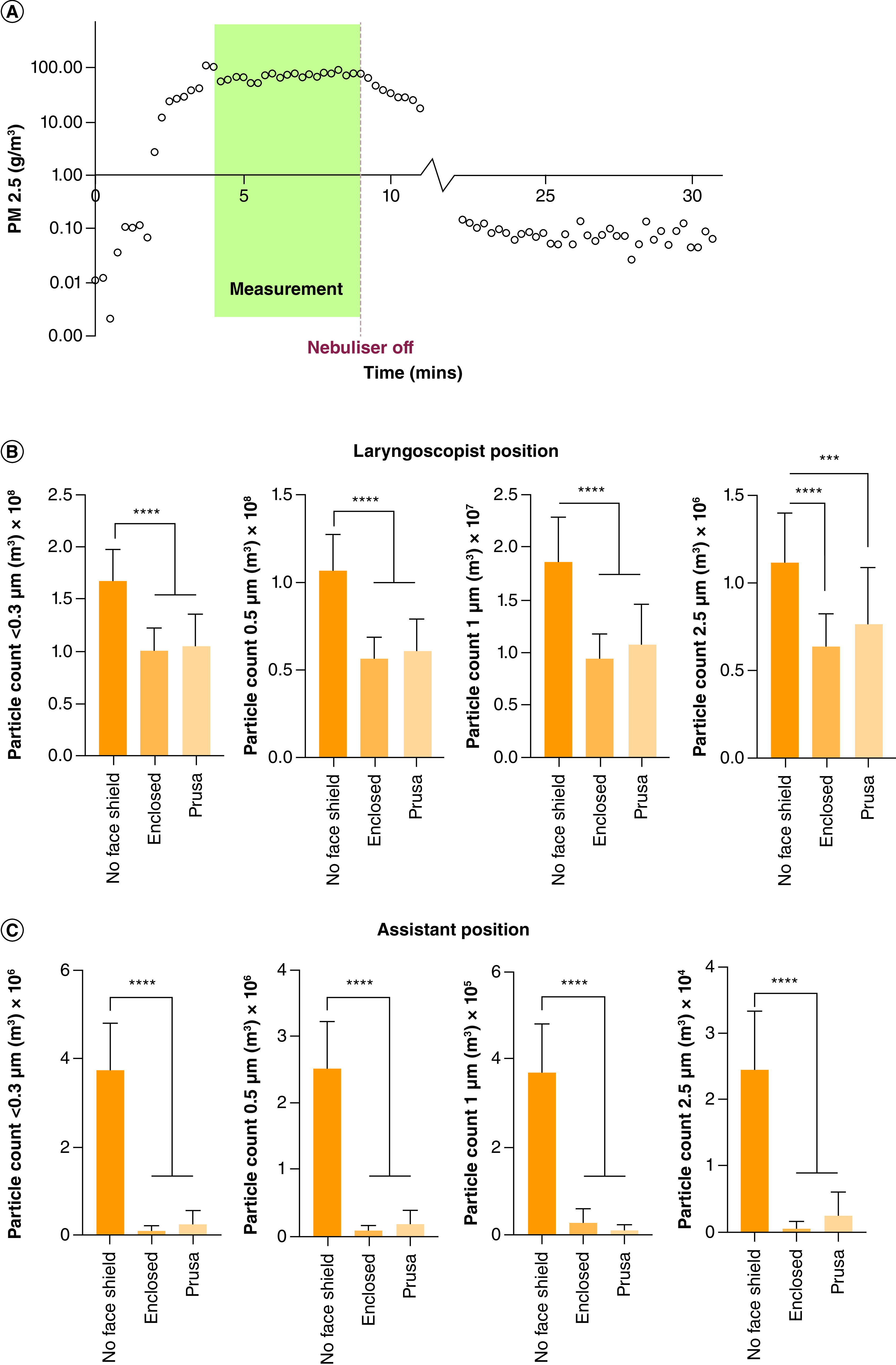
(A) Fine particle mass (PM2.5) recorded cumulatively over 15-s intervals at position 1 without a face shield. The readings used for the statistical analysis are indicated in green. Also evident is the ramp-up time, approximately 4 min, and the required rest time of 15–20 min. (B & C) Average number of particles measured at positions 1 and 2.
Y-axis: averaged particle count per cubic meter for each particle size (0.3, 0.5, 1, 2.5 μm).
****p value < 0.0001; ***p value = 0.0003.
Of note, position 2 (assistant's position) is exposed to a lower particle count than position 1 (laryngoscopist's position) due to the proximity to the patient's face and the downward laminar flow provided by the operating theater circulation. The findings, as shown in Figure 5B & C indicate a statistically significant reduction in aerosol exposure with the application of face shield use in both positions across all four particle sizes.
With the use of a face shield at position 1, a laryngoscopist could therefore expect to see a 40% reduction in aerosol exposure as shown in Figure 5B (95% CI: 0.41 × 108–0.83 × 108, p < 0.0001). At position 2, there is a 95% reduction in aerosol exposure through the use of a face shield (95% CI: 3.017 × 106–3.977 × 106, p < 0.0001). No significant difference was found between vented and enclosed shields at either position tested.
The effect of good circulation on the dispersion of aerosolized particles must also be acknowledged, especially with the positive pressure laminar flow ventilation and high air exchanges per h, which also help reduce aerosol exposure. As the laminar flow moved directly down onto the operation bed, the movement of particles upward under the face shields was significantly reduced. The measured airflow at important sites is shown in Table 1.
Table 1. . Air speed at key locations within the operating theater.
| Location | Speed and direction |
| Over patient face | 0.224 m.s-1 downward |
| Over patient face in line with laryngoscopist's head | 0.422 m.s-1 downward |
| Over patient chest | 0.287 m.s-1 downward |
| Over patient chest in line with assistant's head | 0.556 m.s-1 downward |
| Behind laryngoscopist's enclosed face shield | 0.0853 m.s-1 sideways |
Discussion
Face shields have long been part of accepted PPE against large droplet and splash contamination. However, they are not used specifically for smaller aerosolized droplet and particle protection. As we emerge from the COVID-19 crisis, many local centers have now been supplied with vented-top reusable/washable face shields alongside enclosed disposable face shields. This study demonstrates that face shields may reduce HCW exposure to aerosolized particles in sizes deemed to cause infectious transfer, at least in an operating room environment, and both vented and enclosed face shields provide similar minimization in exposed aerosol concentrations. Although it may seem logical that any barrier is useful in diverting aerosolized particles away from one's face, given the unexpected results that emerged from the intubation boxes study, we believe these results are useful in demonstrating that this was not the case with face shields. We, however, acknowledge that airflow dynamics play a significant role in particle spread, and the results we achieved in an operating theater with highly effective room clearance may confer additional benefits compared with other clinical environments and ventilation, and therefore possible accumulation of aerosolized particles under the shield may be also be seen as with the use of intubation boxes in other settings.
Another important observation was the significant drop in total particles at a further distance from the source of aerosolization, correlating with Lindsley's studies [8,19]. Although the concentration of particles we generated far exceeds that produced by an infected patient's cough, this was done to ensure sufficient particle numbers above effective theater ventilation elimination so as to detect a difference if one existed. Also, with the viral load being an implication of infection [16,17], these data support the suggestion that any ancillary staff present in the theater during AGPs not directly involved with intubation should distance themselves as far from the source as possible to further decrease exposure.
We also would like to comment on the incidental subjective finding among our clinicians that vented top face shields provided the benefit of significantly reduced fogging from exhaled breaths, and as such, provided a perceived advantage especially when worn for a prolonged period. However, vented face shields do potentially expose the wearer to splash exposure from droplets entering from above the brow line. HCWs should therefore consider this in the presence of a significant risk of splash contamination and either utilize additional enclosed protective eyewear in combination with vented shields or return to the use of a traditional enclosed face shield.
We do not suggest that face shields are a replacement for N95/P2 respirators, which remain the gold-standard of protection against aerosolized particles. Nor can we comment on whether a simple measured reduction in the concentration of potentially infective particles near a HCW's face achieved by wearing a face shield confers a clinical reduction in risk of transmission to such airborne pathogens, as infectivity is a complex process in which multiple factors come into play. Although attempts were made to provide a validated means of generating particles similar to the size of that produced by an infectious individual coughing at normal breath rates, a more forceful cough with increased velocities of aerosolized particles may affect spread more so than what we were able to achieve. Further limitations of our study include that it was conducted in a sterile and stationary setting, without normal movements of persons within the operating theater, which may affect the dispersion of particles throughout the room. We did not assess accumulated concentrations of particles over a long duration, however, demonstration of the steady state of our generated particles, which as mentioned, far exceeds that produced by human breathing and coughing, demonstrated how effective operating theater ventilation systems are at the clearance of aerosolized particles. Continuous air exchanges within the operating theater provide protection by preventing aerosolized particles from having time to disperse evenly throughout the room, including behind HCWs’ face shields. Furthermore, we cannot comment on the effectiveness of face shields against aerosolized particles in a closed room environment with a lack of laminar flow ventilation, such as in general wards, outpatient clinics, emergency departments or nonhospital settings.
Conclusion
Face shields are a simple, cheap and generally readily available form of PPE for HCWs. At least in an operative theater environment, face shields, regardless of having enclosed or vented designs, not only provide protection from direct splash contamination but may provide a measurable degree of minimization of aerosolized particle exposure. In doing so, face shields may confer additional benefits in possibly reducing the transmission of airborne respiratory illnesses to HCWs.
There has been exponential growth in our understanding of how to protect HCWs from aerosolized pathogens like COVID-19 since it first appeared. By building on our knowledge base of PPE, we will be better prepared to address possible future threats from aerosolized pathogens. N95 masks/P2 respirators will remain the mainstay of protection for HCWs. However, understanding that by supplementing masks with something as simple as a face shield, HCWs may lower the concentration of particles around the facial area. This in turn may provide some added protection against pathogen infection, though further studies are needed to determine a clinical correlation.
Summary points.
Face shields as personal protective equipment (PPE) are designed to protect healthcare workers (HCWs) against droplet transmission. Their effect on aerosolized particles, either protective or deleterious, has not been investigated.
Clinical face shields, though traditionally enclosed above the brow line, are now available with vented/open tops.
3D printing using simple fused deposition modelling can readily provide HCWs with clinical face shields. The most well-known during the COVID-19 pandemic is the PRUSA RC3, designed for open access use, and developed during the COVID-19 pandemic when supply chain disruptions led to reduced availability of clinical face shields.
A study during COVID-19 demonstrated that devices called “intubation boxes” initially designed to reduce aerosolized particles exposure to HCWs performing airway procedures may actually increase the risk by concentrating particles toward the HCW.
As such, this study was designed to determine if face shields either protect HCWs from aerosol or potentially harm them as with intubation boxes.
This study looked specifically at the operating room environment, which has specific laminar airflow ventilation.
Both types of face shields (open/vented or enclosed) perform similarly in significantly reducing the aerosolized particles reaching the face of a HCW.
This could potentially further help reduce the risk of HCW contamination from airborne viruses from their patients, though the use of N95 masks/P2 respirators remains the mainstay form of protection against airborne respiratory pathogens.
In the operating theater, face shields may provide a significant reduction in exposure to aerosolized particles.
This protection was observed regardless of the type of face shield (vented or enclosed).
Acknowledgments
The authors acknowledge Jason Chuen (Director of Vascular Surgery) and Jasamine Coles-Black of Austin Health's 3Dmedlabs, and COVID-SOS, for crowdsourcing and coordinating access to 3D-printed face shields at the height of the pandemic, and to the Centre of Additive Manufacturing RMIT University, for providing the PRUSA RC3 face shields both for this project and during the COVID-19 crisis. Thanks to PRUSA3D.com for the design and dissemination of their 3D-printed face shields during the COVID-19 pandemic.
Footnotes
Author contributions
I Chao, J Brenker, M Desselle, A Bernard and T Alan were involved in conceptualization, study design and methodology. Funding was organized by I Chao, D Wong, C Low and J Coles-Black. S Lee, D Wong, C Low, Z Keon-Cohen and J Coles-Black were responsible for the literature review. I Chao, S Lee and J Brenker were involved in the data collection and curation, with supervision by T Alan and Z Keon-Cohen. I Chao, J Brenker, D Wong, M Desselle, A Bernard and T Alan were involved in the formal analysis. The original draft was written by I Chao, S Lee, D Wong, C Low and Z Keon-Cohen, with critical review, editing and final approval of the manuscript by all authors.
Financial & competing interests disclosure
The Lighthouse 3016-IAQ particle counter was purchased by the Department of Anaesthesia Box Hill Hospital Special Research Fund. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.WHO. Transmission of SARS-CoV-2: implications for infection prevention precautions. (2020). www.who.int/publications/i/item/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations (Accessed 14 September 2020).
- 2.Bahl P, Doolan C, De Silva C et al. Airborne or droplet precautions for health workers treating COVID-19? J. Infect. Dis. 225(9), 1561–1568 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacIntyre CR, Ananda-Rajah M, Nicholls M et al. Current COVID-19 guidelines for respiratory protection of heath care workers are inadequate. Med. J. Aus. 213(6), 251–252 (2020). [DOI] [PubMed] [Google Scholar]; •• This paper was one of the main triggers for the current research question.
- 4.Bourouiba L. Turbulent Gas Clouds and Respiratory Pathogen Emissions: Potential Implications for Reducing Transmission of COVID-19. JAMA 323(18), 1837–1838 (2020). [DOI] [PubMed] [Google Scholar]; •• This paper was one of the main triggers for the current research question.
- 5.Stadnytskyi V, Bax CE, Bax A et al. The airborne lifetime of small speech droplets and their potential importance in SARS-CoV-2 transmission. Proc. Natl Acad. Sci. USA 117(22), 11875–11877 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindsley WG, Pearce TA, Hudnall J et al. Quantity and size distribution of cough-generated aerosol particles produced by influenza patients during and after illness. J. Occup. Environ. Hyg. 9(7), 443–449 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]; • This paper was the most in-depth and clinically relevant study on aerosol particles and provided a framework for the current simulation.
- 7.Morawska L, Milton DK. It is Time to Address Airborne Transmission of COVID-19. Clin. Infect. Dis. 71(9), 2311–2313 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown J, Gregson FKA, Shrimpton A et al. A quantitative evaluation of aerosol generation during tracheal intubation and extubation. Anaesthesia 76(2), 174–181 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies A, Thomson G, Walker J et al. A review of the risks and disease transmission associated with aerosol generating medical procedures. J. Infect. Prev. 10(4), 122–126 (2009). [Google Scholar]
- 10.Tran K, Cimon K, Severn M et al. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLOS ONE 7(4), e35797 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dhillon RS, Rowin WA, Humphries RS et al. Aerosolisation during tracheal intubation and extubation in an operating theatre setting. Anaesthesia 76, 182–188 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang JW, Li Y, Eames I et al. Factors involved in the aerosol transmission of infection and control of ventilation in healthcare premises. J. Hosp. Infect. 64(2), 100–114 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El-Boghdadly K, Wong DJN, Owen R et al. Risks to healthcare workers following tracheal intubation of patients with COVID-19: a prospective international multicentre cohort study. Anaesthesia 75(11), 1437–1447 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peng PWH, Ho PL, Hota SS. Outbreak of a new coronavirus: what anaesthetists should know. Br. J. Anaest. 124(5), 497–501 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Australian Government Department of Health and Aged Care. Guidance on the use of personal protective equipment (PPE) for health care workiers in the context of COVID-19. Australia. www.health.gov.au/resources/publications/guidance-on-the-use-of-personal-protective-equipment-ppe-in-hospitals-during-the-covid-19-outbreak (Accessed 13 October 2020). [Google Scholar]
- 16.Pan Y, Zhang D, Ynag P et al. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect. Dis. 20(4), 411–412 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu F, Yan L, Wang N et al. Quantitative detection and viral load analysis of SARS-CoV-2 in infected patients. Clin. Infect. Dis. 71(15), 793–798 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson NM, Norton A, Young FP et al. Airborne transmission of severe acute respiratory syndrome coronavirus-2 to healthcare workers: a narrative review. Anaesthesia 75(8), 1086–1095 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simpson JP, Wong DN, Verco L et al. Measurement of airborne particle exposure during simulated tracheal intubation using various proposed aerosol containment devices during the COVID-19 pandemic. Anaesthesia 75(12), 1587–1595 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This paper was one of the main triggers for the current research question.
- 20.Roberge RJ. Face shields for infection control: a review. Journal of occupational and environmental hygiene. J. Occup. Environ. Hyg. 13(4), 235–242 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindsley WG, Noti JD, Blachere FM et al. Efficacy of face shields against cough aerosol droplets from a cough simulator. J. Occup. Environ. Hyg. 11(8), 509–518 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fok TF, Al-Essa M, Monkman S et al. Pulmonary deposition of salbutamol aerosol delivered by metered dose inhaler, jet nebulizer, and ultrasonic nebulizer in mechanically ventilated rabbits. Pediatr. Res. 42(5), 721–727 (1997). [DOI] [PubMed] [Google Scholar]
- 23.Gupta JK, Lin CH, Chen Q. Characterizing exhaled airflow from breathing and talking. Indoor air. Indoor Air 20(1), 31–39 (2010). [DOI] [PubMed] [Google Scholar]
- 24.Morris SN, Fader AN, Milad MP et al. Regarding understanding the ‘scope’ of the problem: why laparoscopy is considered safe during the COVID-19 pandemic. J. Minim. Invasive Gynecol. 27(4), 789–791 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fennelly K. Particle size of infectious aerosols: implications for infection control. Lancet Respir. Med. 8(9), 914–924 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]