ABSTRACT
Serodiagnosis of strongyloidiasis is usually performed by ELISA for the detection of IgG antibodies due to its high sensitivity and practicality, but its main limitation is a constant source of S. stercoralis antigens. The use of S. venezuelensis as a heterologous source of antigens has facilitated several published studies on the serodiagnosis and epidemiology of human strongyloidiasis. The main objective of this study was to evaluate the diagnostic accuracy of surface cuticle antigens of infective larvae of S. venezuelensis extracted with CTAB detergent (L3-CTAB) in comparison with soluble somatic extracts (L3-SSE) using a panel of sera from immunocompetent and immunocompromised individuals, at three different cut-offs. ROC curve analysis showed that L3-CTAB had an AUC of 0.9926. At the first cut-off value (OD 450 nm = 0.214), sensitivity and specificity were 100% and 90.11%, respectively, with a diagnostic accuracy of 0.93. At a second cut-off value (OD 450 nm = 0.286), sensitivity and specificity were 70% and 100%, respectively, with a diagnostic accuracy of 0.91. However, at an alternative third cut-off value (OD 450 nm = 0.589), sensitivity and specificity were 95% and 97.8%, respectively, with a diagnostic accuracy of 0.97. Using L3-CTAB as an antigenic source, the seropositivity rate in immunocompromised patients was 28.13% (9/32) whereas a seropositivity rate of 34.38% (11/32) was found when L3-SSE was used in ELISA. Therefore, the L3-CTAB is simple and practical to obtain and was found to be highly sensitive and specific.
Keywords: Strongyloidiasis, Strongyloides venezuelensis, Surface, Enzyme-linked immunosorbent assay, Serodiagnosis
INTRODUCTION
Strongyloidiasis is an intestinal parasitic infection caused by nematode Strongyloides stercoralis with a worldwide distribution, especially in tropical and subtropical regions. Human strongyloidiasis is still considered a neglected tropical disease and it is estimated that approximately 600,000,000 individuals are infected worldwide 1 .
In immunocompetent individuals, Strongyloides infection is usually chronic and sometimes even asymptomatic, but fatal cases can occur in patients infected with human T-cell lymphotropic virus type-1 (HTLV-1), patients receiving corticosteroids or other immunosuppressive therapies, where regular mechanisms of immunity are impaired or altered, allowing the parasite to reproduce massively with episodes of autoinfection. This critical condition, known as hyperinfection syndrome, is mainly characterized by the spread of the parasite to other organs, increasing the mortality rate from 60% to 87% 2 .
Definitive diagnosis of strongyloidiasis is routinely made by microscopic observation of larvae in stool samples, but it may be difficult due to low or irregular larval excretion, resulting in false-negative results 3,4 . In order to increase the sensitivity of the diagnosis, different immunoserological tests were developed and applied for the serodiagnosis of Strongyloides infection. Among them, enzyme-linked immunosorbent assay (ELISA) is the most widely used test for detecting IgG antibodies whose main advantages are its high sensitivity and great utility in seroepidemiological studies 5,6 . Most of these ELISAs use antigenic extracts from other Strongyloides species, such as S. ratti and S. venezuelensis, due to limitations in obtaining antigens from S. stercoralis 4,7 .
Several antigenic extracts from S. ratti or S. venezuelensis have been evaluated in order to obtain better values of sensitivity and specificity, and these include total soluble somatic extracts 8,9 , alkaline somatic extracts 10 , and detergent somatic extracts 9,11 . Although maximum sensitivity can be obtained with these types of antigenic extracts, their main limitation is the cross-reactivity with other types of helminths. On the other hand, excretory-secretory products from infective larvae (iL3) of S. venezuelensi obtained under in vitro conditions have shown to have excellent values of sensitivity and specificity and almost no cross-reactivity, but their production is very time consuming 12 . During their migration through host tissues, the cuticle surface of Strongyloides larvae is in direct contact with the immune system, being the main target for the immune response. Therefore, cuticle surface proteins may be an important antigenic source for the serodiagnosis of strongyloidiasis. Several authors have described the use of the cationic detergent cetyltrimethylammonium bromide (CTAB) to extract the cuticle from different nematode parasites 13-17 . This study aimed to evaluate the surface extract from S. venezuelensis iL3 obtained by treatment with CTAB detergent for the detection of IgG antibodies in serum samples from immunocompetent and immunocompromised patients with strongyloidiasis.
MATERIALS AND METHODS
Serum samples
A total of 163 serum samples from immunocompetent (n = 131) and immunocompromised (n = 32) individuals were obtained at the Hospital das Clinicas da Faculdade de Medicina da Universidade de Sao Paulo, Sao Paulo State, Brazil (HC-FMUSP), after signing the informed consent form (Research Ethics Committee of HC-FMUSP, Protocol Nº 5.638.310, CAAE Nº 61636722000000068). This study used a convenience sample, made up of serum samples belonging to the Laboratorio de Investigacao Medica 06 (LIM06), which were collected and used in previous studies.
Serum samples of immunocompetent individuals were composed of 40 patients with positive parasitological analysis for S. stercoralis (Group 1); 48 serum samples from individuals with negative parasitological analysis (Group 2); 43 serum samples from patients with positive parasitological analysis for other intestinal parasites (Group 3), including infection by Ascaris lumbricoides (n = 8), Trichuris trichiura (n = 8), hookworms (n = 8), Enterobius vermicularis (n = 1), Hymenolepis nana (n = 11), Schistosoma mansoni (n = 6), and Giardia intestinalis (n = 1).
Serum samples of immunocompromised individuals with positive parasitological analysis for S. stercoralis (Group 4) were composed of transplant candidate patients (n = 15), post-transplanted patients (n = 5), patients with cancer (n = 6), patients infected with HTLV-1 (n = 3), and HIV patients (n = 3). Parasitological diagnosis of all these samples was based on the modified Baermann’s method, the spontaneous sedimentation technique 18 and agar plate culture 19 .
Production of iL3 from S. venezuelensis
S. venezuelensis iL3 were obtained from 48 h charcoal fecal cultures from male Wistar rats (Rattus norvegicus) who were experimentally infected at 30 days of age. These iL3 were recovered by using a modified Baermann’s technique; the larvae were treated with sodium hypochlorite 0.25% for 5 min and then washed four times with sterile distilled water by centrifugation at 4,000 x g for 1 min at room temperature in the Eppendorf Centrifuge model 5427 R (Eppendorf® SE, Germany), and then stored at -20 °C until use 12 . The rats received sterilized food and water ad libitum and were handled in compliance with the animal ethics guidelines adopted by the Animal Research Ethics Committee of FMUSP (protocol Nº 0356A).
Soluble somatic extract from S. venezuelensis iL3
A soluble somatic extract (L3-SSE) was prepared according to the procedure described by Corral et al. 20 . Briefly, approximately 200,000 washed iL3 were resuspended in 1 mL of 25 mm Tris–HCl, pH 7.5, containing a protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA), and sonicated on ice using five cycles, each with a pulse of 20 s, with a Portable Ultrasonic Cell Disruptor (Model UCD-P01, Biobase®, Shandong, China). The mixture was centrifuged at 12,400 x g for 30 min at 4 °C and the supernatant was collected and stored at -20 °C until use.
Extraction of surface cuticle from S. venezuelensis iL3 with CTAB detergent
Approximately 200 mg (dry weight) of iL3 were resuspended in 1 mL of phosphate-buffered saline 0.01 M, pH 7.2 (PBS) containing 0.25% CTAB detergent and incubated overnight at 4 °C. The suspension was centrifuged at 12,400 x g for 10 min and the supernatant was stored at -20 °C. This antigenic extract was named L3-CTAB. The protein content from both types of antigenic extracts was quantified by the commercial kit Pierce™ BCA Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA).
ELISA
ELISA for the detection of IgG antibodies was based on the procedure described by Roldán Gonzáles et al. 12 . Briefly, the Costar® 96-well flat bottom microtitration polystyrene plates (Sigma-Aldrich Chemical Co., St. Louis, MO, USA) were coated (100 μL/well) with each type of the antigenic extracts at a concentration of 10 μg/mL, diluted in carbonate-bicarbonate buffer (0.06 M, pH 9.6) and incubated overnight at 4 °C. After three manual washes (300 μL/well) of 5 min each with PBS containing 0.1% Tween-20 (PBS-T), the plates were blocked with 5% nonfat milk (Molico®, Nestlé, Brazil) diluted in PBS-T for 45 min at 37 °C. The plates were washed with PBS-T as described above, and serum samples were tested in duplicate (100 μL/well), diluted 1:200 in blocking solution and incubated for 45 min at 37 °C. After three washings with PBS-T, peroxidase-conjugated goat anti-human IgG (Fc specific) antibodies (Sigma-Aldrich Chemical Co., St. Louis, MO, USA) were diluted 1:10,000 in blocking solution, added to the plates (100 μL/well) and incubated for 45 min at 37 °C. After three washings with PBS-T, tetramethylbenzidine chromogen solution (Thermo Fischer Scientific, Waltham, MA, USA) was added (100 μL/well) and incubated for 7 min at 37 °C; the reaction was stopped with 2N sulfuric acid (50 μL/well). The plates were finally read at 450 nm using an ELISA reader (Thermo Fischer Scientific, Waltham, MA, USA) and the results were expressed in optical density (OD) units. All assays were monitored by including a positive (pool of serum samples from Group 1) and negative (pool of serum samples from Group 2) control sera as well as a blank without any serum sample. Since the structure of polystyrene ELISA plates are not perfect and minimal pipetting errors may occur during the assay, a 10% variation in the results from positive and negative control sera was tolerated as an internal control in each assay.
Statistical analysis
Statistical analyses were performed using the GraphPad Prism software, version 8.0 (Graph Pad Software Inc., San Diego, USA). In order to evaluate the diagnostic accuracy of each antigenic extract, sera from Group 1 (cases) were used to calculate the diagnostic sensitivity and sera from Groups 2 and 3 (controls) were used to calculate the diagnostic specificity following the receiver operating characteristic (ROC) curve analysis with a 95% confidence interval (CI), in which cut-off values for 100% sensitivity or 100% specificity were calculated. Alternatively, maximum possible sensitivity and specificity were also calculated. Other measures of diagnostic accuracy such as area under the ROC curve (AUC), positive predictive values (PPV) and negative predictive values (NPV), likelihood ratio and overall accuracy were calculated 21 . The D’Agostino–Pearson’s normality test and Paired t-test with statistical significance at p < 0.05 were used to compare the ELISA OD results obtained in each of the four groups of sera, using L3-CTAB and L3-SSE.
RESULTS
The protein content resulting from CTAB detergent extraction of 200 mg of dried S. venezuelensis iL3 was approximately between 1 and 1.3 mg/mL, with no signs of larval degradation.
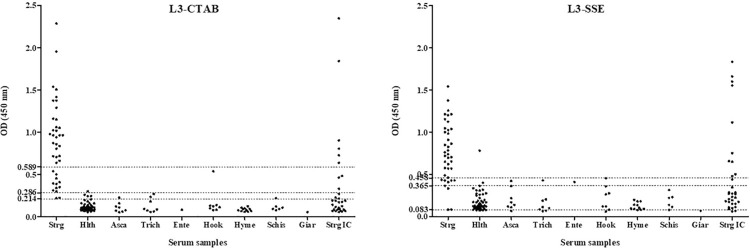
ROC curve analysis showed that L3-CTAB had an AUC of 0.99 (95% CI = 0.98 – 1.0, P < 0.0001), and L3-SSE had an AUC of 0.95 (95% CI = 0.89 – 1.0, P < 0.0001). Sera from Group 1 were slightly more reactive to L3-CTAB (range from 0.219 to 2.29) when compared to L3-SSE (range from 0.084 to 1.54), but this difference was not statistically significant (t = 1.60; P = 0.117). Sera from Group 2 were less reactive to L3-CTAB (range from 0.057 to 0.302) than L3-SSE (range from 0.073 to 0.402), and this difference was statistically significant (t = 4.11; P = 0.0002). Sera from Group 3 showed a comparable range of reactivity to both antigenic extracts; however, L3-CTAB had a lower OD mean (0.118; range from 0.054 to 0.539) than L3-SSE (0.176; range from 0.058 to 0.455), and this difference was statistically significant (t = 4.25; P = 0.0001).
When cut-off values for 100% sensitivity (L3-CTAB = 0.214; L3-SSE = 0.083) were chosen, L3-CTAB achieved a specificity of 90.11%, whereas L3-SSE achieved a poor specificity of 15.38%; L3-CTAB achieved a PPV of 81.63%, whereas L3-SSE achieved a PPV of 34.19%, and the diagnostic accuracy was 0.93 and 0.41 for L3-CTAB and L3-SSE, respectively (Table 1). With regard to cross-reactivity, five sera (11.63%) from Group 3 were positive for L3-CTAB, whereas 34 sera (79.07%) from this group were positive for L3-SSE (Figure 1).
Table 1. Diagnostic accuracy of L3-CTAB and L3-SSE from infective larvae of S. venezuelensis .
| Diagnostic parameters | L3-CTAB | L3-SSE | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Cut-off | 1 | 2 | 3 | 1 | 2 | 3 |
| OD (450 nm) | 0.214 | 0.286 | 0.589 | 0.083 | 0.365 | 0.458 |
| % Sensitivity | 100 | 95 | 70 | 100 | 92.5 | 77.5 |
| % Specificity | 90.11 | 97.8 | 100 | 15.38 | 94.51 | 100 |
| Likelihood ratio | 10.11 | 43.22 | ∞ | 1.18 | 16.83 | ∞ |
| % Positive predictive value | 81.63 | 95 | 100 | 34.19 | 88.10 | 100 |
| % Negative predictive value | 100 | 97.8 | 88.35 | 100 | 96.63 | 91 |
| Diagnostic accuracy | 0.93 | 0.97 | 0.91 | 0.41 | 0.94 | 0.93 |
| % Cross-reactivity | 11.63 | 2.33 | 0 | 79.07 | 9.30 | 0 |
L3-CTAB = Surface cuticle antigens from infective larvae of S. venezuelensis extracted with CTAB detergent; L3-SSE = Soluble somatic extract from infective larvae of S. venezuelensis; ∞ = undefined.
Figure 1. Reactivity of sera (expressed in ELISA OD at 450 nm) from immunocompetent individuals with S. stercoralis infection (Group 1; Strg), healthy (Group 2; Hlth), with other parasitosis [Group 3, including those infected with A. lumbricoides (Asca), T. trichiura (Tric), E. vermicularis (Ente), hookworms (Hook), H. nana (Hyme), S. mansoni (Schi), and G. intestinalis (Giar)], and immunocompromised with strongyloidiasis (Group 4; Strg IC) against the surface cuticle antigens extracted with CTAB detergent (L3-CTAB) or the soluble somatic extract (L3-SSE) from infective larvae of S. venezuelensis by ELISA. Each graphic shows the cut-off values (Y axis).
In order to eliminate all the cross-reactivity, cut-off values for 100% specificity (L3-CTAB = 0.589; L3-SSE = 0.458) were chosen. In these conditions, L3-CTAB achieved a sensitivity of 70%, whereas L3-SSE achieved a sensitivity of 77.5%. Even though both antigenic extracts achieved 100% PPV, L3-SSE showed a better NPV when compared with L3-CTAB. Diagnostic accuracy using this cut-off was 0.91 and 0.93 for L3-CTAB and L3-SSE, respectively (Table 1).
New cut-off values were calculated in order to obtain the maximum possible values for both sensitivity and specificity (L3-CTAB = 0.286; L3-SSE = 0.365). In these new conditions, L3-CTAB achieved a sensitivity and specificity of 95% and 97.83%, whereas L3-SSE achieved a sensitivity and specificity of 92.5% and 93.48%, respectively. The values of PPV and NPV of L3-CTAB were better than those of L3-SSE as well as its diagnostic accuracy (Table 1). With regard to cross-reactivity, one serum sample (2.33%) from Group 3 was positive for L3-CTAB whereas four sera (9.30%) from this group were positive for L3-SSE (Figure 1). Using the cut-off values described above, L3-CTAB was not as sensitive as L3-SSE in detecting positive cases in sera from immunocompromised individuals (Group 4), finding low positivity (9 out of 32, 28.13%) when compared to L3-SSE (11 out of 32, 34.38%). Although some sera from this group were more reactive to L3-CTAB, the OD mean of L3-SSE (0.476; range from 0.062 to 1.84) was higher than L3-CTAB (0.360; range from 0.058 to 2.35) and statistically significant (t = 2.78; P = 0.0092).
DISCUSSION
Human strongyloidiasis is still underdiagnosed and underestimated, resulting in a serious public health problem worldwide. Although the detection of antibodies may not be useful for post-treatment monitoring, the detection of IgG antibodies by ELISA is preferred due to its high sensitivity, reproducibility, and its capacity to work with a high number of samples, being a powerful tool for clinical diagnosis and seroepidemiological studies 7 .
Usually, ELISA classifies a sample as positive or negative using a cut-off value and the most used method to choose this value is the ROC curve analysis, where the AUC determines the inherent ability of the test to discriminate between the diseased and healthy individuals 21 . In fact, one of the main advantages of the ROC curve is that it provides a wide range of different cut-off values to confer greater sensitivity or specificity to the test. In certain situations, it is more convenient to prioritize high sensitivity (i.e., a screening test for epidemiological studies) or high specificity (i.e., a diagnostic test to confirm a disease) and the choice of the cut-off value will depend on the objective of the study.
In this study, the diagnostic accuracy of surface cuticle antigens of S. venezuelensis iL3 obtained by simple incubation with the CTAB cationic detergent (L3-CTAB) was evaluated through ELISA in a panel of serum samples from immunocompetent individuals using three different cut-off values, showing better results when compared to a saline somatic extract (L3-SSE). However, both antigenic extracts were unable to detect all serum samples from the immunocompromised patients with strongyloidiasis, obtaining low values of seropositivity. Several authors have described that the lower sensitivity of serological tests found in immunocompromised patients may reflect a decreased level of antibody production, especially in patients with HTLV-1 infection, HIV infection, and hematologic malignancies 22-24 . Thus, serological tests for antibody detection may not be useful screening tests for identifying strongyloidiasis in this type of population.
Pritchard et al. 13 were the first to demonstrate that the CTAB cationic detergent was capable of extracting the surface cuticle antigens from live adult parasites of the nematode Nematospiroides dubius. Likewise, several authors have followed this procedure to extract the surface cuticle antigens of live parasites from Trichinella spiralis 15-17 and S. ratti 14 . Nevertheless, the main limitation of this procedure is the use of live parasites in a cell culture medium or PBS at 37 °C for extracting the surface cuticle antigens, since these physiological conditions may stimulate the release of E/S products by live parasites, thus contaminating the extraction of the surface antigens. Our procedure differs from these previous authors in the use of frozen larvae, since live S. venezuelensis larvae were capable of releasing considerable amounts of E/S products when incubated in PBS for 48 h at 37 °C 12 . This modification guarantees that our CTAB antigenic extract contains only proteins from the surface of the larvae. Murrell et al. 25 have described that the infective larvae from S. ratti and T. spiralis have a negatively charged surface that binds cationized ferritin and ruthenium red; this fact could have facilitated an active extraction with the CTAB cationic detergent, resulting in considerable quantities of material from the larval surface.
Limitations
Some limitations of this study can be evaluated. Firstly, the possibility that S. stercoralis infection was not diagnosed in Groups 2 and 3 as part of a polyparasitism, since helminth infections are common in individuals from Latin America. Secondly, the low sensitivity in detecting anti-Strongyloides IgG antibodies in immunocompromised individuals. Since most cases of hyperinfection and/or disseminated disease occur in immunocompromised patients, other complementary strategies, such as the detection of larvae or parasite DNA or its antigens in bronchoalveolar lavage or feces, could improve diagnosis in this group of patients. Finally, for a better evaluation of the L3-CTAB, it is necessary to increase the number of analyzed samples.
CONCLUSION
Our procedure is simple and practical to carry out and does not require additional expensive reagents or equipment. Furthermore, the possibility of obtaining proteins from the surface of the parasite using this detergent opens the way for future studies on the biology, biochemistry and proteomics of the surface of S. venezuelensis and its interaction with the host. Future studies will be necessary to determine which proteins make up the surface cuticle of S. venezuelensis iL3.
Footnotes
ERRATUM: Rev Inst Med Trop Sao Paulo. 2023;65:e1
http://doi.org/10.1590/S1678-9946202365001
On page 1, Abstract:
Where it reads:
At a second cut-off value (OD 450 nm = 0.286), sensitivity and specificity were 70% and 100%, respectively, with a diagnostic accuracy of 0.91. However, at an alternative third cut-off value (OD 450 nm = 0.589), sensitivity and specificity were 95% and 97.8%, respectively, with a diagnostic accuracy of 0.97.
Should be read:
At a second cut-off value (OD 450 nm = 0.589), sensitivity and specificity were 70% and 100%, respectively, with a diagnostic accuracy of 0.91. However, at an alternative third cut-off value (OD 450 nm = 0.286), sensitivity and specificity were 95% and 97.8%, respectively, with a diagnostic accuracy of 0.97.
REFERENCES
- 1.Buonfrate D, Bisanzio D, Giorli G, Odermatt P, Fürst T, Greenaway C, et al. The global prevalence of Strongyloides stercoralis infection. Pathogens. 2020;9:468. doi: 10.3390/pathogens9060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vasquez-Rios G, Pineda-Reyes R, Pineda-Reyes J, Marin R, Ruiz EF, Terashima A. Strongyloides stercoralis hyperinfection syndrome: a deeper understanding of a neglected disease. J Parasit Dis. 2019;43:167–175. doi: 10.1007/s12639-019-01090-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marcos LA, Terashima A, Canales M, Gotuzzo E. Update on strongyloidiasis in the immunocompromised host. Curr Infect Dis Rep. 2011;13:35–46. doi: 10.1007/s11908-010-0150-z. [DOI] [PubMed] [Google Scholar]
- 4.Requena-Méndez A, Chiodini P, Bisoffi Z, Buonfrate D, Gotuzzo E, Muñoz J. The laboratory diagnosis and follow up of strongyloidiasis: a systematic review. PLoS Negl Trop Dis. 2013;7:e2002. doi: 10.1371/journal.pntd.0002002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nunes JB, Emídio TC, Marques MJ, Caldas IS, Souza RL, Kanamura HY, et al. Seroepidemiological aspects of human infection by Strongyloides stercoralis in Alfenas, southern Minas Gerais, Brazil. Rev Soc Bras Med Trop. 2018;51:855–859. doi: 10.1590/0037-8682-0090-2018. [DOI] [PubMed] [Google Scholar]
- 6.Casado L, Rodriguez-Guardado A, Boga JA, Fernández-Suarez J, Martínez-Camblor P, Rodríguez-Perez M, et al. Use of serology in a systematic screening programme for strongyloidiasis in an immigrant population. Int J Infect Dis. 2019;88:60–64. doi: 10.1016/j.ijid.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Kalantari N, Chehrazi M, Ghaffari S, Gorgani-Firouzjaee T. Serological assays for the diagnosis of Strongyloides stercoralis infection: a systematic review and meta-analysis of diagnostic test accuracy. Trans R Soc Trop Med Hyg. 2020;114:459–469. doi: 10.1093/trstmh/trz135. [DOI] [PubMed] [Google Scholar]
- 8.Eamudomkarn C, Sithithaworn P, Sithithaworn J, Kaewkes S, Sripa B, Itoh M. Comparative evaluation of Strongyloides ratti and S. stercoralis larval antigen for diagnosis of strongyloidiasis in an endemic area of opisthorchiasis. Parasitol Res. 2015;114:2543–2551. doi: 10.1007/s00436-015-4458-3. [DOI] [PubMed] [Google Scholar]
- 9.Corral MA, Paula FM, Gottardi M, Meisel DM, Chieffi PP, Gryschek RC. Membrane fractions from Strongyloides venezuelensis in the immunodiagnosis of human strongyloidiasis. Rev Inst Med Trop Sao Paulo. 2015;57:77–80. doi: 10.1590/S0036-46652015000100011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Machado ER, Ueta MT, Gonçalves-Pires MR, Oliveira JB, Faccioli LH, Costa-Cruz JM. Strongyloides venezuelensis alkaline extract for the diagnosis of human strongyloidiasis by enzyme-linked immunosorbent assay. Mem Inst Oswaldo Cruz. 2003;98:849–851. doi: 10.1590/s0074-02762003000600024. [DOI] [PubMed] [Google Scholar]
- 11.Feliciano ND, Gonzaga HT, Gonçalves-Pires MR, Gonçalves AL, Rodrigues RM, Ueta MT, et al. Hydrophobic fractions from Strongyloides venezuelensis for use in the human immunodiagnosis of strongyloidiasis. Diagn Microbiol Infect Dis. 2010;67:153–161. doi: 10.1016/j.diagmicrobio.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 12.Roldán Gonzáles WH, Meisel DM, Paula FM, Gryschek RC. Diagnostic accuracy of somatic and excretory-secretory antigens from Strongyloides venezuelensis infective larvae for the immunodiagnosis of human strongyloidiasis. Parasitology. 2021;148:1522–1527. doi: 10.1017/S0031182021001207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pritchard DI, Crawford CR, Duce IR, Behnke JM. Antigen stripping from the nematode epicuticle using the cationic detergent cetyltrimethylammonium bromide (CTAB) Parasite Immunol. 1985;7:575–585. doi: 10.1111/j.1365-3024.1985.tb00101.x. [DOI] [PubMed] [Google Scholar]
- 14.Northern C, Grove DI, Warton A, Lovegrove FT. Surface labelling of Strongyloides ratti: stage-specificity and cross-reactivity with S. stercoralis. Clin Exp Immunol. 1989;75:487–492. [PMC free article] [PubMed] [Google Scholar]
- 15.Grencis RK, Crawford C, Pritchard DI, Behnke JM, Wakelin D. Immunization of mice with surface antigens from the muscle larvae of Trichinella spiralis. Parasite Immunol. 1986;8:587–596. doi: 10.1111/j.1365-3024.1986.tb00872.x. [DOI] [PubMed] [Google Scholar]
- 16.Cui J, Liu RD, Wang L, Zhang X, Jiang P, Liu MY, et al. Proteomic analysis of surface proteins of Trichinella spiralis muscle larvae by two-dimensional gel electrophoresis and mass spectrometry. Parasit Vectors. 2013;6:355. doi: 10.1186/1756-3305-6-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu RD, Cui J, Liu XL, Jiang P, Sun GG, Zhang X, et al. Comparative proteomic analysis of surface proteins of Trichinella spiralis muscle larvae and intestinal infective larvae. Acta Trop. 2015;150:79–86. doi: 10.1016/j.actatropica.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Garcia LS. Diagnostic medical parasitology. 4. Washington: American Society for Microbiology; 2001. [Google Scholar]
- 19.Koga K, Kasuya S, Khamboonruang C, Sukhavat K, Ieda M, Takatsuka N, et al. A modified agar plate method for detection of Strongyloides stercoralis. Am J Trop Med Hyg. 1991;45:518–521. doi: 10.4269/ajtmh.1991.45.518. [DOI] [PubMed] [Google Scholar]
- 20.Corral MA, Paula FM, Meisel DM, Castilho VL, Gonçalves EM, Levy D, et al. Potential immunological markers for diagnosis of human strongyloidiasis using heterologous antigens. Parasitology. 2017;144:124–130. doi: 10.1017/S0031182016001645. [DOI] [PubMed] [Google Scholar]
- 21.Eusebi P. Diagnostic accuracy measures. Cerebrovasc Dis. 2013;36:267–272. doi: 10.1159/000353863. [DOI] [PubMed] [Google Scholar]
- 22.Ramanathan R, Nutman T. Strongyloides stercoralis infection in the immunocompromised host. Curr Infect Dis Rep. 2008;10:105–110. doi: 10.1007/s11908-008-0019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luvira V, Trakulhun K, Mungthin M, Naaglor T, Chantawat N, Pakdee W, et al. Comparative diagnosis of strongyloidiasis in immunocompromised patients. Am J Trop Med Hyg. 2016;95:401–404. doi: 10.4269/ajtmh.16-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vazquez Guillamet LJ, Saul Z, Miljkovich G, Vilchez GA, Mendonca N, Gourineni V, et al. Strongyloides stercoralis infection among human immunodeficiency virus (HIV)-infected patients in the United States of America: a case report and review of literature. Am J Case Rep. 2017;18:339–346. doi: 10.12659/AJCR.902626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murrell KD, Graham CE, McGreevy M. Strongyloides ratti and Trichinella spiralis: net charge of epicuticle. Exp Parasitol. 1983;55:331–339. doi: 10.1016/0014-4894(83)90030-9. [DOI] [PubMed] [Google Scholar]