Abstract
The sequestration of Plasmodium falciparum-infected erythrocytes (pRBC) away from the peripheral circulation is a property of all field isolates. Here we have examined the pRBC of 111 fresh clinical isolates from children with malaria for a number of adhesive features in order to study their possible coexpression and association with severity of disease. A large number of adhesion assays were performed studying rosetting, giant rosetting, and binding to CD36, intercellular adhesion molecule 1, platelet endothelial cell adhesion molecule 1, thrombospondin, heparin, blood group A, and immunoglobulins. Suspension assays were performed at the actual parasitemia of the isolate, while all the static adhesion assays were carried out at an equal adjusted parasitemia. The ability to bind to multiple receptors, as well as the ability to form rosettes and giant rosettes, was found to be more frequent among isolates from children with severe versus mild malaria (P = 0.0015). Rosettes and giant rosettes were more frequent for children with severe malaria, and the cell aggregates were larger and tighter, than for those with mild disease (P = 0.0023). Binding of immunoglobulins (97% of isolates) and of heparin (81% of isolates) to infected erythrocytes was common, and binding to heparin and blood group A was associated with severity of disease (P = 0.011 and P = 0.031, respectively). These results support the idea that isolates that bind to multiple receptors are involved in the causation of severe malaria and that several receptor-ligand interactions work synergistically in bringing about severe disease.
The sequestration of Plasmodium falciparum-infected erythrocytes (pRBC) away from the peripheral circulation is a property of all field isolates (15, 18). Still, it has been suggested that the parasites may be of a specific adhesive phenotype when bound in large numbers in particular organs and may thereby cause severe disease (1, 2, 14, 28). Rosetting, i.e., the binding of uninfected erythrocytes to the pRBC, and autoagglutination, i.e., the binding of pRBC to pRBC, are other features commonly seen in field isolates. The aggregates resulting from rosetting and autoagglutination may block capillary blood flow and have previously been shown to be associated with severe malaria (9, 13, 27).
pRBC of parasites that both adhere to the vascular endothelium and form rosettes (or autoagglutinates) are frequently found in patients with severe malaria (13, 27). It could therefore be expected that a parasite that binds to multiple erythrocyte and endothelial receptors causes more obstruction than one that only adheres with single-receptor specificity in the microvasculature.
The ligand, for rosetting as well as cytoadherence, on the pRBC is P. falciparum erythrocyte membrane protein 1 (PfEMP1), a protein expressed by the parasite and exported to the membrane of the infected erythrocyte (7, 24). Several receptors for PfEMP1, including CD36, thrombospondin (TSP), intercellular adhesion molecule-1 (ICAM-1), platelet endothelial adhesion molecule 1 (PECAM-1/CD31), heparan-sulfate-like glycosaminoglycans, and blood group sugars, have been identified on the human endothelium and on uninfected erythrocytes (4, 5, 6, 8, 19, 26).
A linkage between rosetting and other major adhesive phenotypes has been uncovered with an in vitro-cloned parasite (FCR3S1.2) where the pRBC were found to bind to multiple endothelial and erythrocyte receptors (12, 26). Similarly, the parasite strain ITG was found to adhere in a synergistic fashion to CD36 and ICAM-1 when the latter receptor was available together with CD36 (17). Taken together, these findings have led us to examine the pRBC of 111 fresh clinical isolates of children with malaria for a number of adhesive features in order to study their possible coexpression and association with the severity of the disease.
MATERIALS AND METHODS
Study area.
The study was carried out at Kilifi District Hospital and adjacent dispensaries, situated 60 km north of Mombasa on the Kenyan coast. The hospital is equipped with a high-dependency ward to treat children with life-threatening illnesses. However, most children admitted to hospital are treated in the general pediatric ward. Following the long and short rains, the area has prolonged seasonal transmission of P. falciparum by Anopheles gambiae sensu lato complex (16).
Collection and culture of clinical isolates.
Parasite samples were collected between December 1998 and February 1999 and between June and August 2000 from children with a primary diagnosis of malaria attending or admitted to Kilifi District Hospital or adjacent dispensaries. The samples were collected at admission, before antimalarial treatment was started. An algorithm for taking and processing patient blood was developed so that the origin of the sample remained unknown until the study was completed. For this study, hospitalized children were defined as severe cases non ultra descriptus (NUD) if they were found to be prostrated or hyperparasitemic (>20%) but did not meet the criteria of severe anemia or cerebral malaria. Others suffered from severe anemia (hemoglobin concentration of <5 g/dl) or had cerebral malaria (defined as a Blantyre coma score of <2 or as being comatose with simultaneous inability to localize a painful stimulus). Cases were considered nonsevere if patients were judged by the examining clinician as having uncomplicated disease not meeting any of the above criteria. Patients with nonsevere cases were recruited from the hospital general pediatric ward, the outpatient clinic, and dispensaries in the Kilifi area. The mean age of patients with mild malaria was 3.5 years, and that of the group with severe malaria was 3.9 years. After clinical judgment, the patients enrolled in the study could be divided into two groups: one with severe malaria, comprising 55 individuals, and one with mild malaria, comprising 56 children (Table 1). For inclusion, parasitemia of 4% or more was required. After parental consent had been obtained, 2 to 5 ml of blood was taken into a tube containing heparin (Leo Laboratories). Parasites were prepared from acute samples as follows. To remove mononuclear cells, the cell pellet was resuspended in 1 volume of RPMI 1640 (pH 7.2; containing 25 μg of gentamicin sulfate/ml, 1 mM glutamine, and 4 mg of d-glucose/ml [Gibco BRL]) and layered onto a cushion of Lymphoprep (Nycomed). Following centrifugation at 3,000 × g for 10 min, the cell pellet was washed in RPMI 1640. To remove granulocytes, cells were mixed with 4 volumes of 70% Plasmagel (Bellon) diluted in RPMI 1640, and the erythrocytes were allowed to settle for 15 min at 37°C. Following a wash in RPMI 1640, the erythrocytes were cultured in RPMI 1640 in the presence of 10% European, malaria-naïve AB positive serum until they matured into pigmented trophozoites (25).
TABLE 1.
Classification of the different severity groups
| Severity group | Clinical status | n | Mean age (yr) |
|---|---|---|---|
| Mild | Uncomplicated disease | 45 | 3.5 |
| Severe anemia | Hb < 5 g/dl | 21 | 3.4 |
| Cerebral malaria | Blantyre coma score of <2 | 11 | 4.3 |
| Severe NUDa | Hyperparasitemia of >20%, prostration | 18 | 4.0 |
Severely ill patients who did not meet the criteria for severe anemia or cerebral malaria.
Cell lines.
Mouse L cells expressing PECAM-1/CD31 and Chinese hamster ovary cells (CHO cells) transfected with CD36 or ICAM-1 were grown as previously described (23).
Estimation of parasitemia, rosette formation, and giant rosetting.
The parasites were stained with one drop (10 μg/ml) of acridine orange to determine parasitemia and then counted in a Nikon Optiphot (Tokyo, Japan) UV microscope using a 10× ocular and a 40× lens. An additional parasitemia assessment using Giemsa-stained blood films was made in parallel for each isolate. The level of spontanous rosette formation was calculated as the number of rosette-forming late-stage pRBC per total number of late-stage pRBC. Spontanously formed giant rosettes were defined as three or more pRBC adherent to each other, regardless of the presence or absence of bound uninfected erythrocytes. This is an important difference from autoagglutination, for which only agglutinates of infected RBC not containing any uninfected erythrocytes are scored. A rosette was considered small if the number of bound uninfected erythrocytes (RBCs) ranged from 2 to 5, medium if the number was 6 to 10, and large if it was 11 or more. Rosettes were characterized by density, as loose or tight, depending on the appearance of the rosette and the manner in which the RBCs were bound to the pRBC (Fig. 1) (10). Similarly, giant rosettes were defined as small if the number of pRBC ranged from 3 to 5, medium if the number was 6 to 10, and large if it was 11 or more.
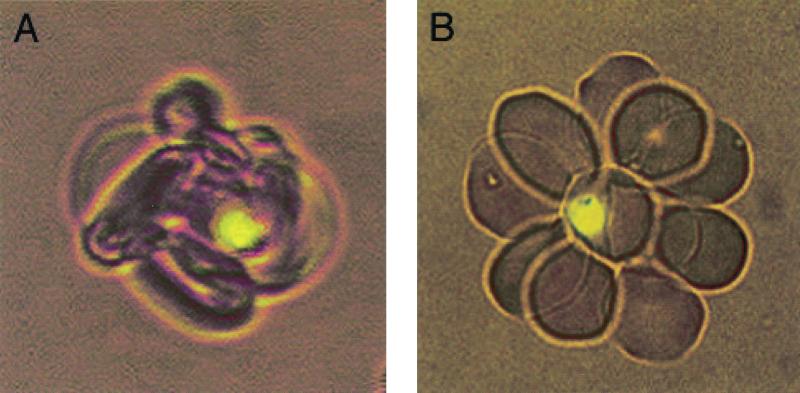
FIG. 1.
Difference in appearance between a rosette scored as tight (A) and a rosette scored as loose (B).
Suspension fluorescence assays for immunoglobulin, heparin, and PECAM-1/CD31 binding.
Immunoglobulin binding capacity was assessed using fluorescein isothiocyanate (FITC)-conjugated sheep anti-human immunoglobulin antibodies (Swedish Institute for Infectious Disease Control. Solna, Sweden) as described previously (23). Heparin binding was determined accordingly, but with soluble FITC-labeled heparin directly bound to the pRBC (5). Soluble(s) recombinant human PECAM-1/CD31 was purchased from R&D Systems (Minneapolis, Minn.) and subsequently labeled with the fluorophore Alexa 488 (Molecular Probes, Eugene, Oreg.) according to the instructions provided in the Alexa 488 labeling kit. In brief, 30 μl of live pRBC was washed twice and resuspended in RPMI 1640, pH 6.8, to the original volume before the soluble fluorescent CD31 was added at a final concentration of 100 μg/ml. After 30 min of incubation at room temperature, protected from light (with gentle resuspension after 15 min), the pRBC were washed three times with RPMI 1640, pH 6.8 (12a). Parasites were stained with ethidium bromide as described previously, and the fluorescence rate was assessed by counting the number of surface fluorescent pRBC per total number of pRBC in a Nikon UV microscope, using a 10× ocular and a 40× lens (23).
Static cytoadherence assays for PECAM-1/CD31, CD36, and ICAM-1.
The cytoadherence assays were performed essentially as described previously (23). Isolates with parasitemia of more than 6% were diluted (maintaining 2% hematocrit), resulting in parasitemia between 4 and 6% in all the isolates tested.
Static TSP adhesion assay.
Soluble TSP purified from platelets was purchased from Gibco BRL and spotted onto plastic dishes at a concentration of 50 μg/ml in 50 mM Tris buffer (with 150 mM NaCl and 1 mM CaCl2 [pH 7.4]) and incubated for 1 h. Dishes were washed once in 50 mM Tris buffer and blocked with 1% bovine serum albumin in 50 mM Tris for 1 h at 37°C. After two subsequent washes in 50 mM Tris, fresh parasite culture was added at 2% hematocrit in RPMI medium and further processed as described above for the static cytoadherence assays. The number of bound pRBC per 30 fields (equivalent to 1 mm2) was counted in a light microscope using a 10× ocular and an oil immersion lens with a magnification of ×100.
FACS analysis.
The fluorescence-activated cell sorter (FACS) assay was performed like the suspension assay for immunoglobulin, heparin, and PECAM-1/CD31 binding described above. In the final step the labeled erythrocytes were stained with ethidium bromide, diluted in 1 ml of RPMI 1640 (pH 6.8), and subsequently processed in the FACS.
Data analysis.
Data were stored, formatted, and analyzed in Microsoft Excel (Microsoft Corp.). Statistical analyses were performed in StatView 4.5 (Abacus Concepts, Inc.). Data were analyzed by the Mann-Whitney U test, Spearman's rank correlation coefficient test, and Fisher's exact test as indicated.
RESULTS
Originally, 125 samples meeting our inclusion criteria of parasitemia of >4 % were obtained, but 14 of the isolates did not grow satisfactorily and were excluded. Eventually, 111 samples grew well, showing mature, healthy-looking trophozoites; of these, 55 were from severe and 56 from mild cases. Not all of the assays were performed on every sample. We found overall high binding rates for the parameters studied. Ninety-eight percent of the isolates bound to CD36 expressed on CHO cells (102 of 104; range, 0 to 1,250 pRBC/100 cells; mean, 236 pRBC/100 cells), and 88% bound to PECAM-1/CD31 expressed on L cells (88 of 100; range, 0 to 1,706 pRBC/100 cells; mean, 188 pRBC/100 cells). In general, adhesion to ICAM-1 expressed on CHO cells was frequent, at 98% (57 of 58; range, 0 to 277 pRBC/100 cells; mean, 46.7 pRBC/100 cells), but the magnitude of binding was low compared to those for CD36 and PECAM-1/CD31. Ninety percent of the isolates adhered to TSP (62 of 69; range, 0 to 2,649 pRBC/mm2; mean, 560 pRBC/mm2), and the binding was uniform and strong.
Immunoglobulin binding was detected in 97% of isolates (108 of 111; range, 0 to 72%; mean, 15.6%), heparin binding in 81% (35 of 43; range, 0 to 60%; mean, 11%), and sPECAM-1/CD31 binding in 53% (58 of 110; range, 0 to 69%, mean, 5.4%). The fluorescence intensity varied slightly between samples, but also between the receptors, with sPECAM-1/CD31 giving the strongest reactivity. In the cases of immunoglobulin, sCD31, and blood group A binding, FACS analyses were run in parallel with the assays read in the microscope, and very good agreement was found; r = 0.85, 0.72, and 0.70 respectively. Rosetting was detected in 84% of isolates (93 of 111; range, 0 to 93%; mean, 17.1%), and giant rosetting was detected in 55% (61 of 111; range, 0 to 78%; mean, 5%).
Correlations between the parameters studied.
When subjected to further analysis, rosetting and giant rosetting were found to correlate with each other (r = 0.625; P < 0.0001). All the other parameters showed correlation coefficients (r) below 0.5.
Association between the parameters studied and disease severity.
Separate analysis of the parameters investigated revealed an association with disease severity for many of the adhesive interactions displayed by the pRBC. The most pronounced correlations were with parasitemia, giant rosetting, and rosetting, but there were also correlations with blood group A and heparin binding, whereas immunoglobulin binding did not show any significant correlation with severity (Table 2). We also found that the rosettes were larger and tighter, and that the giant rosettes were larger, in the group of children with severe malaria than in to the mild-disease group (Table 2). Binding to static PECAM-1/CD31, ICAM-1, CD36, and TSP did not exhibit a correlation with severity; on the contrary, for these parameters, we found a nonsignificant association with mild disease (Table 2). Binding in the static assays was in all cases lower for isolates from cerebral malaria and severe malaria NUD cases than for those from mild cases.
TABLE 2.
Summary of data for each of the parameters studied and comparison between groupsa
| Parameter | Total mean | Mean, mild group | Mean, severe group | Severe vs mild group (P) | Mean, cerebral malaria group | Cerebral vs mild group (P) | Mean, anemia group | Anemia vs mild group (P) | Mean NUD group | NUD vs mild group (P) |
|---|---|---|---|---|---|---|---|---|---|---|
| Parasitemia (%) | 10.7 | 6.8 | 14.8 | <0.001 | 10.9 | 0.1 | 14.3 | <0.001 | 18 | <0.001 |
| Rosetting (%) | 17.1 | 12.9 | 21.4 | 0.010 | 17.2 | 0.923 | 29.7 | 0.001 | 16.9 | 0.092 |
| Giant rosetting (%) | 6 | 2.3 | 9.8 | <0.001 | 9.3 | 0.194 | 13.2 | <0.001 | 8.3 | 0.001 |
| sIgb binding | 15.6 | 13.2 | 18.0 | 0.315 | 17.6 | 0.498 | 21.6 | 0.086 | 15.9 | 0.949 |
| Soluble heparin binding (%) | 10.4 | 4.7 | 14.3 | 0.011 | 14.7 | 0.148 | 18.0 | 0.047 | 9.4 | 0.030 |
| Soluble blood group A binding (%) | 3.2 | 1.8 | 5.4 | 0.031 | 5.9 | 0.014 | 3.1 | 0.145 | 7.0 | 0.346 |
| sCD31 binding (%) | 5.4 | 2.5 | 8.4 | 0.060 | 3.5 | 0.704 | 9.4 | 0.121 | 10.5 | 0.080 |
| CD31 binding (pRBC/100 target cells) | 188 | 209 | 167 | 0.989 | 112 | 0.228 | 172 | 0.775 | 196 | 0.626 |
| CD36 binding (pRBC/100 target cells) | 236 | 267 | 205 | 0.336 | 215 | 0.293 | 189 | 0.554 | 193 | 0.336 |
| ICAM-1 binding (pRBC/100 target cells) | 48 | 57 | 31 | 0.114 | 30 | 0.285 | 48 | 0.662 | 21 | 0.126 |
| TSP binding (pRBC/mm2) | 560 | 620 | 467 | 0.140 | 364 | 0.246 | 584 | 0.256 | 378 | 0.181 |
P values are from the Mann-Whitney U test.
sIg, soluble immunoglobulin.
Multiadhesion and severity.
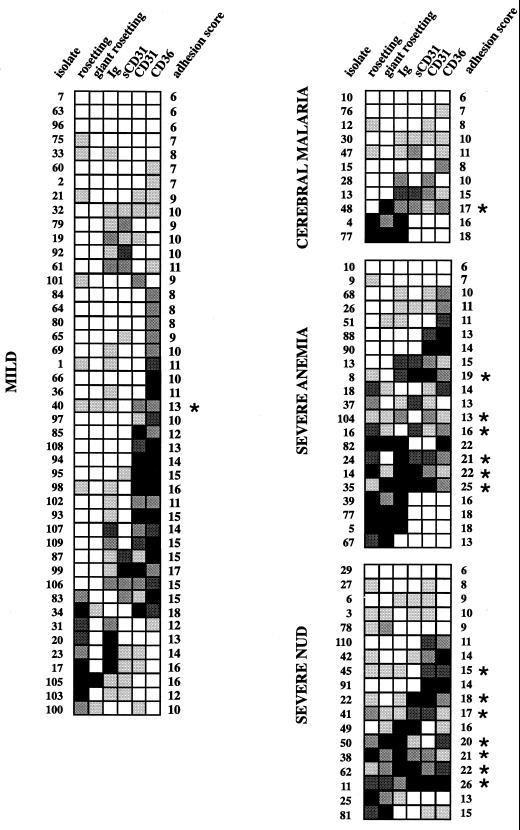
When we analyzed the adhesion data, it became apparent that binding to several receptors was more frequent in the severe group than in the mild group. As an example, six of the isolates from the severe group (isolates 11, 14, 35, 38, 41, and 62) bound strongly to all of the receptors studied. Collected data were analyzed in such a way as to obtain the highest number of parameters possible in the highest number of isolates. Since not all assays were performed on all isolates, this resulted in a total of 92 isolates analyzed for rosetting, giant rosetting, and binding to immunoglobulin, sPECAM-1/CD31, PECAM-1/CD31, and CD36. To be able to compare adhesion scores of the isolates, we arbitrarily transformed the binding data for each receptor into categories ranging from 1 to 5, where 1 represents low or no binding and 5 represents high binding (Fig. 2). The limits within each category were chosen to approximate normal distribution when the limits for each group were set. We subsequently added together the numeric values of the seven binding parameters. For example, isolate 1 has an adhesion score of 11 (2 + 1 + 2 + 1 + 1 + 4) (Fig. 2). The figures were summarized for each isolate and group (mild and severe) and compared using the Mann-Whitney U test. The mean adhesion score for the mild group was 11.222, and that for the severe group was 14.000; the difference was highly significant (P < 0.0085). To further analyze the adhesion of parasites from patients with severe or mild malaria, we employed Fisher's exact test to compare the number of isolates within each group that had a score of >1 for more than four of the six binding parameters. Fourteen of the 47 isolates from the group with severe malaria were multiadhesive, while only 1 out of 45 isolates from the children with mild malaria bound to more than four of the receptors (P = 0.0015). The frequency of very high binders, isolates that were given the number 5 in a given binding category (Fig. 2), was also significantly higher in the severe group (47 of 282) than in the mild group (26 of 270; P = 0.034 by Fisher's exact test). The number of isolates with adhesion scores for ICAM-1, TSP, and blood group A binding was too low to be included in the multiadhesion analysis. However, incorporation of these three parameters revealed that they strengthened the tendency toward multiadhesion in the severe group, although, due to the very low number of complete isolates in the severe group, this finding was not statistically significant. Similarly, heparin contributed to multiadhesion in a smaller group of seven parameters, and the difference between groups with mild and severe malaria was statistically significant (P = 0.0185).
FIG. 2.
Mosaic representing the frequency and magnitude of adhesion. Squares □, ░, ▒, ▓, and █ indicate adhesion magnitude and correspond to scores of 1 to 5, respectively, where 1 represents low or no binding and 5 indicates high binding. For different parameters, scores represent different percentages of pRBC involved, as follows. For rosetting, 1 = 0 to 9%; 2 = 9 to 18%; 3 = 18 to 27%; 4 = 27 to 36%; 5 = >36%. For giant rosetting, 1 = 0 to 4%; 2 = 4 to 8%; 3 = 8 to 12%; 4 = 12 to 16%; 5 = >16%. For immunoglobulin binding, 1 = 0 to 7%; 2 = 7 to 14%; 3 = 14 to 21%; 4 = 21 to 28%; 5 = >28%. For heparin and sCD31 binding, 1 = 0 to 6%; 2 = 6 to 12%; 3 = 12 to 18%; 4 = 18 to 24%; 5 = >24%. For the number of pRBC bound to CD31- and CD36-transfected L or CHO cells, 0 to 100 pRBC/100 transfectants; 2 = 100 to 200/100 transfectants; 3 = 200 to 300/100 transfectants; 4 = 300 to 400/100 transfectants; 5 = >400/100 transfectants. Asterisks indicate isolates with binding scores of >1 in more than four categories.
The parameters were subjected to further analysis in which some related parameters were omitted. When either rosetting or giant rosetting was omitted, there was still a significant association with disease severity. The same result was found when either immunoglobulin binding or heparin binding or both were excluded. When only one of the two PECAM-1/CD31 binding assays was counted, the difference between the mild and severe groups was still statistically significant (data not shown).
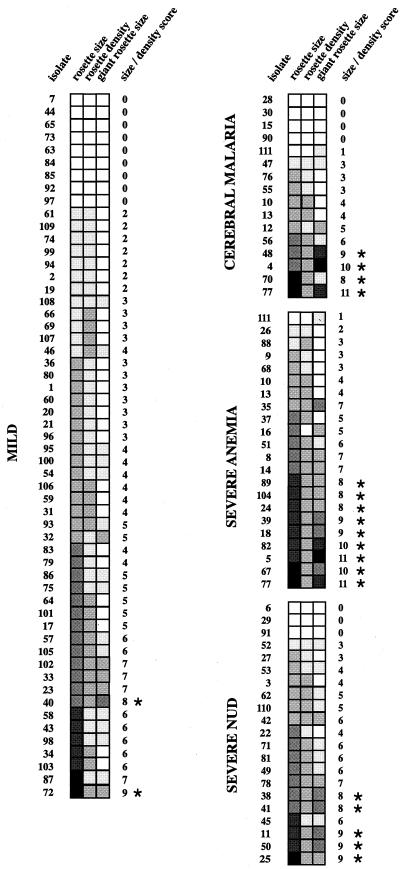
Rosetting size and density and giant rosette size.
The description of rosettes and giant rosettes according to size and density provides further important information about the characteristics of the isolates. The size and density of the rosettes and giant rosettes of the isolates were therefore also compared between the two groups. The means of the size-density scores were 3.661 and 5.400 for the mild and severe groups, respectively, and were significantly different (P = 0.0023 by the Mann-Whitney U test) (Fig. 3). High scores were found in clusters of isolates very much corresponding to those with high adhesion scores. Isolates with a total rosette-giant rosette size-density score of >7 for an individual isolate were significantly more common in the severe group (17 of 55) than in the mild group (2 of 56; P = 0.0010 by Fisher's exact test) (Fig. 3).
FIG. 3.
Mosaic illustrating the frequency, size, and density of rosettes and the frequency and size of giant rosettes. Squares □, ░, ▒, , ▓, and █ correspond to scores of 0 to 5, respectively, and represent magnitude of size and density where 0 indicates no rosettes or no giant rosettes, 5 indicates large size, and 2 indicates high density. The limits within each parameter are as follows. For rosetting and giant rosetting size, 1 = 2 to 5 RBC/pRBC (small); 3 = 6 to 10 RBC/pRBC (medium), and 5 = ≥11 RBC/pRBC (large). For rosetting density (see Fig. 1), 1 = loose; 2 = tight. Since many of the samples contained rosettes or giant rosettes that fell between these groups, catagories 2 (2 to 10 RBC/pRBC; small-medium) and 4 (5 to 15 pRBC/RBC; medium-large) were added. Asterisks indicate a total size-density score of >7.
Cerebral malaria and severe anemia versus uncomplicated disease.
Of the children with severe malaria analyzed for multiadhesion, 23% (11 of 47) were determined to have cerebral malaria, 45% (21 of 47) to have severe anemia, and 38% (18 of 47) to be suffering from unspecified severe disease (severe NUD), with some overlap between the groups (isolates 10, 13, and 77) (Fig. 2). In the group of patients with severe anemia there was significantly higher binding (mean adhesion score, 15.150, score for uncomplicated malaria, 11.222; P = 0.003) and more multiadhesive isolates (6 of 21 compared to 1 of 45 in the uncomplicated group; P = 0.0089). The isolates from the group designated as unspecified severe (see Materials and Methods) also showed a significantly higher adhesive profile (mean adhesion score = 14.222, P = 0.0415) and were more multiadhesive than those from mild cases (7 of 18 compared to 1 of 45; P = 0.002). However, in the cerebral malaria group the mean adhesion score was 11.500 (P = 0.8756) and only one isolate was multiadhesive (1 of 11 versus 1 of 45; P = 0.3797).
Analysis of rosette size and density and of giant rosette size revealed a similar pattern; in the severe anemia group, rosettes were significantly larger and tighter, and giant rosettes were larger, than in the uncomplicated group (P = 0.0005). The same picture emerged when we compared the unspecified severe group with the uncomplicated group (P = 0.0193), and the cerebral group with the uncomplicated group (P = 0.8443).
DISCUSSION
The results of this investigation suggest that pRBC of isolates that adhere to multiple receptors and that form large and tight rosettes (or giant rosettes) are associated with severe malaria. Binding of immunoglobulins and of heparin to infected, erythrocytes was also common and associated with severe disease. Yet, rather than focusing on one receptor specificity underlying severe disease, we propose that several receptor-ligand interactions may work synergistically in bringing about severe malaria.
We have here used a model for estimating multiadhesion in which several adhesive phenomena are investigated, the results of which are added to produce an adhesion score. Some of the parameters may partial overlap, i.e., immunoglobulin binding and rosetting, heparin binding and rosetting, although the difference between the mild and severe groups was still significant when either of the parameters was omitted from the calculations of the adhesion scores (5, 11). This could be due to the fact that the molecular mechanisms underlying phenomena such as rosetting and giant rosetting exhibit a high degree of complexity and are likely based on multiple receptor-ligand interactions.
It remains unknown whether multiadhesion of the pRBC found in the patients with severe malaria is due to the coexpression of multiple binding events in clonal populations of parasites, or if the observation reflects multiclonal infection, where each clone infecting the patient adheres with a distinct receptor specificity. In previous investigations it has been shown that some wild isolates recognize more than one receptor, and there have also been several lines of evidence indicating that this is a property of the individual pRBC rather than a reflection of pRBC subpopulations within an isolate (17, 29). Recently we have found that one of the major parasite-derived polypeptides present on the surface of pRBC, PfEMP1 mediates binding to several independent host receptors (11, 12). Thus it is possible that isolates from children with severe malaria indeed carry clones of parasites that bind to multiple receptors through a single PfEMP1 species.
It was interesting to find a predominance of large and tight rosettes and large giant rosettes in the group of severely ill patients. This may reflect synergism between different rosetting receptors or may be due to blood group differences in the patient material, since it has been shown that RBC carrying blood groups A and B form larger rosettes than those solely dependent on heparan sulfate-like GAGs or CR1 (6, 10, 22). Rosetting and its association with disease severity have also been investigated in several other previous studies, and contradicting pictures have emerged. Studies from The Gambia, Gabon, Thailand, and Kenya (9, 13, 27, 30) have shown a higher frequency of rosettes in isolates taken from patients with severe malaria than in those from patients with mild disease, whereas work from Papua New Guinea and Malawi (3, 20) has not. This could possibly be explained by geographical variations of isolates and hemoglobinopathies among the population, the extent of treatment prior to hospitalization, or, perhaps even more important, how the patients were clinically classified. Further, fresh isolates have not always been used in all investigations, although it has been demonstrated that the pRBC of freeze-thawed isolates change their phenotype upon reculturing (21).
An important issue in all studies dealing with receptor-specific adhesion in malaria is whether the pRBC obtained from the peripheral circulation are really representative of those sequestered pRBC causing the symptoms. Early-stage rings are found in the periphery, and the patient is likely to be infected with heterogeneous isolates that vary regarding to the adhesive properties of the ligand expressed (PfEMP1). In this study we found that the 12 patients suffering from cerebral malaria had a significantly lower adhesive profile, both with the individual parameters and with multiadhesion, suggesting that we analyzed the pRBC that did not sequester and cause the symptoms. This supports previous observations that in cerebral malaria the adhesive parasites are sequestered, whereas only subclones of nonadhesive immature forms are found in the peripheral circulation. On the other hand, this may also indicate the lack of a true association between cerebral malaria and the adhesive profile of the parasite isolate. More difficult to explain is the significantly higher adhesive profile seen in the severe anemia group. It is possible that multiadhesive pRBC are also expressing different PfEMP-1 molecules scattered on the erythrocyte surface in a denser fashion, which would make them more immunogenic and facilitate removal by the immune system, but then why are they not sequestered? Another explanation might be that the pRBC are really prone to cause cerebral malaria, but severe anemia counteracts sequestration by enhanced rheological effects on the circulation. In this way there could be a balance between anemia and cerebral disease, i.e., a certain degree of anemia will prevent cerebral disease by preventing sequestration. The third group of severely ill patients, those with unspecified severe disease, is more difficult to account for; this group comprises the severely ill patients who did not meet the criteria for either neurological deficit or severe anemia. We can only speculate what would have happened to these children if they had been left untreated, though this group also displayed significantly higher adhesion scores and more multiadhesion than patients with uncomplicated cases.
Thus we may under- or overscore binding to certain receptors. There is also a level of uncontrolled variation in the clinical definition of disease, as it can only take into account signs and symptoms at presentation. Some individuals who arrive at hospital with uncomplicated malaria may have gone on to develop cerebral involvement in the absence of treatment. Therefore, a proportion of potentially severely ill patients will be classified as having mild malaria. Thus, a certain level of insecurity lies in the rather artificial division between children with severe or mild malaria.
Previous thinking on the relationship between severity and adhesion has tended to emphasize the potential role of a single, specific adhesive phenotype. For example, the findings presented here imply that children who carry pRBC that bind blood group A or heparin, as well as those that form rosettes and giant rosettes, are prone to come down with severe malaria. The picture that emerges from this study is complex and implies that several nonadhesive factors also are important for the pathogenesis of severe disease. Yet the data presented also suggest that any receptor-binding specificity might be of importance in the presence of the right set of coreceptors. The ability of the parasite to display adhesive features that will act in a synergistic fashion seems to promote sequestration and play a role in the pathogenesis of severe malaria.
ACKNOWLEDGMENTS
This paper is published with the permission of the Director of KEMRI. We thank all the staff at KEMRI/Centre for Geographic Medicine Research Coast, Kilifi, Kenya, for critical input and an excellent collaboration.
We also thank Johan Lindbäck at the Swedish Institute for Infectious Disease Control, Epidemiology Unit, for valuable help with the statistical analyses.
This work was supported by grants from the Swedish International Development Authority SIDA-SAREC, the Karolinska Institutet, the Swedish Medical Research Council, KEMRI, and the Wellcome Trust.
A. Heddini and F. Pettersson contributed equally to this work.
REFERENCES
- 1.Aikawa M. Human cerebral malaria. Am J Trop Med Hyg. 1988;39:3–10. doi: 10.4269/ajtmh.1988.39.3. [DOI] [PubMed] [Google Scholar]
- 2.Aikawa M, Iseki M, Barnwell J W, Taylor D, Oo M M, Howard R J. The pathology of human cerebral malaria. Am J Trop Med Hyg. 1990;43(Suppl.):30–37. doi: 10.4269/ajtmh.1990.43.30. [DOI] [PubMed] [Google Scholar]
- 3.Al-Yaman F, Genton B, Mokela D, Raiko A, Kati S, Rogerson S, Reeder J, Alpers M. Human cerebral malaria: lack of significant association between erythrocyte rosetting and disease severity. Trans R Soc Trop Med Hyg. 1995;89:55–58. doi: 10.1016/0035-9203(95)90658-4. [DOI] [PubMed] [Google Scholar]
- 4.Barnwell J W, Asch A S, Nachman R L, Yamaya M, Aikawa M, Ingravallo P. A human 88-kD membrane glycoprotein (CD36) functions in vitro as a receptor for a cytoadherence ligand on Plasmodium falciparum-infected erythrocytes. J Clin Investig. 1989;84:765–772. doi: 10.1172/JCI114234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barragan A, Fernandez V, Chen Q, von Euler A, Wahlgren M, Spillmann D. The duffy-binding-like domain 1 of Plasmodium falciparumerythrocyte membrane protein 1 (PfEMP1) is a heparan sulfate ligand that requires 12 mers for binding. Blood. 2000;95:3594–3599. [PubMed] [Google Scholar]
- 6.Barragan A, Kremsner P G, Wahlgren M, Carlson J. Blood group A antigen is a coreceptor in Plasmodium falciparumrosetting. Infect Immun. 2000;68:2971–2975. doi: 10.1128/iai.68.5.2971-2975.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baruch D I, Pasloske B L, Singh H B, Bi X, Ma X C, Feldman M, Taraschi T F, Howard R J. Cloning the P. falciparumgene endcoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell. 1995;82:77–87. doi: 10.1016/0092-8674(95)90054-3. [DOI] [PubMed] [Google Scholar]
- 8.Berendt A R, Simmons D L, Tansey J, Newbold C I, Marsh K. Intercellular adhesion molecule-1 is an endothelial cell adhesion receptor for Plasmodium falciparum. Nature. 1989;341:57–59. doi: 10.1038/341057a0. [DOI] [PubMed] [Google Scholar]
- 9.Carlson J, Helmby H, Hill A V S, Brewster D, Greenwood B M, Wahlgren M. Human cerebral malaria: association with erythrocyte rosetting and lack of anti-rosetting antibodies. Lancet. 1990;336:1457–1460. doi: 10.1016/0140-6736(90)93174-n. [DOI] [PubMed] [Google Scholar]
- 10.Carlson J, Wahlgren M. Plasmodium falciparumerythrocyte rosetting is mediated by promiscuous lectin-like interactions. J Exp Med. 1992;176:1311–1317. doi: 10.1084/jem.176.5.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Q, Heddini A, Barragan A, Fernandez V, Pearce F, Wahlgren M. The semiconserved head structure of Plasmodium falciparumerythrocyte membrane protein 1 mediates binding to multiple independent host receptors. J Exp Med. 2000;192:1–9. doi: 10.1084/jem.192.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandez V, Treutiger C J, Nash G B, Wahlgren M. Multiple adhesive phenotypes linked to rosetting-binding of erythrocytes in Plasmodium falciparummalaria. Infect Immun. 1998;66:2969–2975. doi: 10.1128/iai.66.6.2969-2975.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12a.Heddini, A., et al. Plasmodium falciparum-infected erythrocytes bind soluble PECAM-1/CD31 and the receptor is frequently recognized by clinical isolates. Am. J. Trop. Med. Hyg., in press. [DOI] [PubMed]
- 13.Kun J F J, Schmidt-Ott R J, Lehman L G, Lell B, Luckner D, Greve B, Matousek P, Kremsner P G. Merozoite surface antigen 1 and 2 genotypes and rosetting Plasmodium falciparum in severe and mild malaria in Lambaréné, Gabon. Trans R Soc Trop Med Hyg. 1998;92:110–114. doi: 10.1016/s0035-9203(98)90979-8. [DOI] [PubMed] [Google Scholar]
- 14.MacPherson G G, Warrell M J, White N J, Looareesuwan S, Warrell D A. Human cerebral malaria. A quantitative ultrastructural analysis of parasitized erythrocyte sequestration. Am J Pathol. 1985;119:385–401. [PMC free article] [PubMed] [Google Scholar]
- 15.Marsh K. Clinical features of malaria. In: Wahlgren M, Pearlman P, editors. Malaria, Molecular and clinical aspects. Amsterdam, The Netherlands: Harwood Academic Publishers; 1999. pp. 87–117. [Google Scholar]
- 16.Mbogo C N M, Snow R W, Kabiru E W, Ouma J H, Githure J I, Marsh K, Beier J C. Low-level Plasmodium falciparumtransmission and the incidence of severe malaria infections on the Kenyan coast. Am J Trop Med Hyg. 1993;49:245–253. doi: 10.4269/ajtmh.1993.49.245. [DOI] [PubMed] [Google Scholar]
- 17.McCormick C J, Craig A, Roberts D, Newbold C I, Berendt A R. Intercellular adhesion molecule-1 and CD36 synergise to mediate adherence of Plasmodium falciparum-infected erythrocytes to cultured human microvascular endothelial cells. J Clin Investig. 1997;100:2521–2529. doi: 10.1172/JCI119794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller L H, Good M F, Milon G. Malaria pathogenesis. Science. 1994;264:1878–1883. doi: 10.1126/science.8009217. [DOI] [PubMed] [Google Scholar]
- 19.Roberts D D, Sherwood J A, Spitalnik S L, Patton L J, Howard R J, Dixit V M, Frazier W A, Miller L H, Ginsburg V. Thrombospondin binds falciparum malaria parasitized erythrocytes and may mediate cytoadherence. Nature. 1985;318:64–66. doi: 10.1038/318064a0. [DOI] [PubMed] [Google Scholar]
- 20.Rogerson S, Tembenu R, Dobano C, Plitt S, Taylor T, Molyneux M. Cytoadherence characteristics of Plasmodium falciparum-infected erythrocytes from Malawian children with severe and uncomplicated malaria. Am J Trop Med Hyg. 1999;61:467–472. doi: 10.4269/ajtmh.1999.61.467. [DOI] [PubMed] [Google Scholar]
- 21.Rowe A, Obeiro J, Newbold C I, Marsh K. Plasmodium falciparumrosetting is associated with malaria severity in Kenya. Infect Immun. 1995;63:2323–2326. doi: 10.1128/iai.63.6.2323-2326.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rowe A J, Moulds J M, Newbold C I, Miller L H. Plasmodium falciparumrosetting mediated by a parasite-variant erythrocyte membrane protein and complement-receptor 1. Nature. 1997;388:292–295. doi: 10.1038/40888. [DOI] [PubMed] [Google Scholar]
- 23.Schlichtherle M, Wahlgren M, Perlmann H, Scherf A, editors. Methods in malaria research. 3rd ed. Manassas. Va: American Type Culture Collection; 2000. [Google Scholar]
- 24.Su X, Heatwole V M, Wertheimer S P, Guinet F, Herrfeldt J A, Peterson D S, Ravetch J A, Wellems T E. The large diverse gene family var encodes proteins involved in cytoadherence and antigenic variation of Plasmodium falciparum-infected erythrocytes. Cell. 1995;82:89–100. doi: 10.1016/0092-8674(95)90055-1. [DOI] [PubMed] [Google Scholar]
- 25.Trager W, Jensen J B. Human malaria parasites in continuous culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 26.Treutiger C J, Heddini A, Fernandez V, Muller W A, Wahlgren M. PECAM-1/CD31, an endothelial receptor for binding Plasmodium falciparum-infected erythrocytes. Nat Med. 1997;3:1405–1408. doi: 10.1038/nm1297-1405. [DOI] [PubMed] [Google Scholar]
- 27.Treutiger C J, Hedlund I, Helmby H, Carlson J, Jepson A, Twumasi P, Kwiatkowski D, Greenwood B M, Wahlgren M. Rosette formation in Plasmodium falciparumisolates and anti-rosette activity of sera from Gambians with cerebral or uncomplicated malaria. Am J Trop Med Hyg. 1992;46:503–510. doi: 10.4269/ajtmh.1992.46.503. [DOI] [PubMed] [Google Scholar]
- 28.Turner G, Morrison H, Jones M, Davis T, Gatter K, Buley I, Looaresuwan S, Nagachinta B, Gatter K, Newbold C, White N, Berendt A. An immunochemical study of the pathology of fatal malaria: evidence for widespread endothelial activation and a potential role for ICAM-1 in cerebral sequestration. Am J Pathol. 1994;145:1057–1069. [PMC free article] [PubMed] [Google Scholar]
- 29.Udomsangpetch R, Reinhardt P H, Schollaardt T, Elliott J F, Kubes P, Ho M. Promiscuity of clinical Plasmodium falciparumisolates for multiple adhesion molecules under flow conditions. J Immunol. 1997;158:4359–4364. [PubMed] [Google Scholar]
- 30.Udomsangpetch R, Taylor B J, Looareesuwan S, White N J, Elliott J F, Ho M. Receptor specificity of clinical Plasmodium falciparumisolates: non-adherence to cell-bound E-selectin and vascular cell adhesion molecule-1. Blood. 1996;88:2754–2760. [PubMed] [Google Scholar]