Abstract
WHO has declared human mpox (formerly known as monkeypox) a global public health emergency since July, 2022. When case numbers were increasing, so did clinicians' exposures to new elements of the disease. Additionally, the burden of mpox is particularly apparent in immunocompromised patients, who can have more variable and severe manifestations of disease across organ systems. In this Grand Round, we report novel and severe oculocutaneous manifestations of mpox in this population, which are both sight and life threatening. Specifically, we highlight two patients with mpox and AIDS who had refractory skin necrosis that progressed to either ocular compromise or panfacial gangrene, or both. Both patients ultimately died due to systemic complications of their infections. Through clinical analogies, we show how past experiences with related orthopoxviruses, such as variola virus (smallpox) and vaccinia virus, can add useful context for understanding and treating these new disease states. We suspect that in patients who are immunocompromised, monkeypox virus can clinically evolve not only via viraemia but also through direct intradermal spread. We propose that intradermal spread occurs by a process clinically and immunologically analogous to progressive vaccinia, a complication previously seen after conventional smallpox vaccination. We share evidence in support of this theory and implications regarding early management and post-exposure prophylaxis for at-risk populations. Content note: this Grand Round contains graphic images of mpox lesions of the eyes and face.
Introduction
Monkeypox virus is one of many zoonotic viruses in the genus Orthopoxvirus. Although discovered in 1958, monkeypox virus was identified exclusively in non-human hosts until 1970, when the first human infection was reported in the Democratic Republic of Congo, formerly Zaire.1 Historically, sporadic disease has been limited to central and west Africa, particularly in the Democratic Republic of Congo.2 The threat of mpox (formerly known as monkeypox) outside of endemic areas gained worldwide attention following a 2003 outbreak in the mid-western USA.2 A global re-emergence of mpox since May, 2022 has again brought this disease to the forefront. As of January, 2023 nearly 30 000 cases of mpox have been confirmed in the USA alone and over 80 000 cases have been confirmed worldwide.3, 4
Classic clinical features of human mpox include vesiculopapular or pseudopustular skin rash, lymphadenopathy, and fever.2 As case numbers increased, so did our awareness of the breadth of disease across organ systems. Mpox-related ophthalmic disease, for example, is presently rare (occurring in <1% of patients with mpox) when compared with preceding outbreaks but has the potential to cause permanent visual impairment and blindness.4, 5, 6, 7 The US Centers for Disease Control and Prevention (CDC) now classifies the eyes as a so-called special hazard anatomic site.7 Additionally, immunocompromised patients, such as those with AIDS, have shown increased variability and severity of mpox. Reports of severe mpox in this population include atypical findings of more than 100 cutaneous lesions, necrotic or treatment-resistant lesions, haemodynamic instability, or secondary sepsis.8
Monkeypox virus is closely related to other orthopoxviruses, namely variola virus (responsible for smallpox), vaccinia virus (the main component of the smallpox vaccine), and cowpox virus.4 When compared at the nucleotide scale, studies have shown that any two orthopoxviruses share 96% of their core genomes.9 Because of high genetic and antigenic similarity, vaccination with vaccinia virus not only confers immunity against variola virus but also provides approximately 85% co-immunity against monkeypox virus.4, 10 The parallels between these viruses, however, are best appreciated through shared clinical characteristics of disease. In general, both smallpox and mpox cause fevers and cutaneous lesions that evolve from vesicles to pustules, although mpox is typically more localised, less severe, and self-limiting. Inoculation with vaccinia virus leads to similar skin findings, typically confined to the site of vaccination.
In the Grand Round that follows, we present two similar case reports of patients with novel and severe oculocutaneous manifestations of mpox. These patients were cared for at Bellevue Hospital, which is situated in a large urban centre in New York (NY, USA). We reconcile our clinical findings, which include both ophthalmic and cutaneous manifestations, with historical variants of orthopoxviral disease caused by smallpox and vaccinia. In doing so, we seek to provide useful clinical context for understanding the development and management of severe mpox during the current outbreak.
Case report one
A 33-year-old man with a 15-year history of HIV (not on highly active antiretroviral therapy [HAART]), AIDS for at least 3 years (CD4+ 55 cells per μL, viral load 74 210 copies per mL), untreated latent syphilis, chronic hepatitis C, and Mycobacterium avium complex presented with 3 weeks of progressive facial rash, inability to open the eyes, and periorbital oedema. His early symptoms included a vesiculopapular eruption of the malar and nasal areas (figure 1A ). The patient was unvaccinated for orthopoxviruses and was monogamous with a male partner.
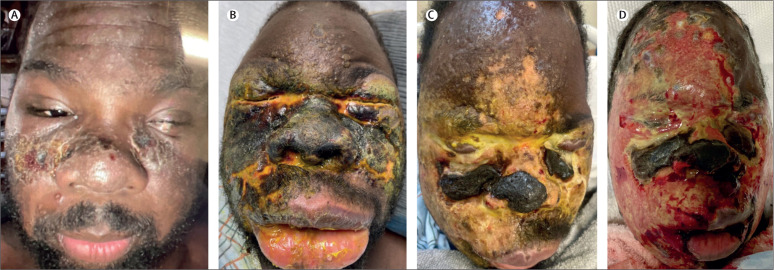
Figure 1.
Chronological progression of mpox facial rash (patient one)
(A) 1 week after symptom onset: ulcerated vesiculopapular rash involving the malar areas and nasal bridge. Surrounding umbilicated papules. Left-sided periorbital oedema. Image courtesy of patient submission. (B) 3 weeks after symptom onset: confluent necrotic facial rash sparing the forehead with overlying honey-colored exudate. Upper and lower eyelids are oedematous, fibrotic, and immobile. There is substantial angio-oedema of the lips. (C) 7 weeks after symptom onset: prominent eschars of the nasal and malar aspects. Increased purulent exudation overlying necrotic skin. Progressive ulceration of the eyelids and distortion of periorbital contour. (D) 11 weeks after symptom onset: panfacial skin sloughing with obfuscation of baseline features. Patient passed away 1 week later.
On initial presentation to the emergency department, the patient was noted to have a confluent necrotic skin rash spanning the bilateral periorbital, nasal, malar, and submalar areas with overlying honey-colored exudate (figure 1B). Distinct umbilicated papules were noted on the forehead, torso, and extremities. Direct examination of the eyes was unsuccessful due to dense eschar of the eyelids, which rendered them immobile. A CT scan revealed intact ocular structures bilaterally (figure 2A ). Swabs collected from multiple sites were analysed by qualitative PCR and consistently came back positive for non-variola Orthopoxvirus DNA (table ). A clinical diagnosis of disseminated monkeypox virus was established. Before confirmatory testing, the patient was started on HAART, pneumocystis prophylaxis, and oral tecovirimat (600 mg twice daily for 2 weeks). The patient underwent punch biopsies of the glabellar region, which showed a histological pattern consistent with viral infection and extensive necrosis. These biopsies showed that the patient had predominantly neutrophilic infiltration with rare lymphocytes. The patient was admitted for 2 weeks and discharged from our institution with mild cutaneous improvement.
Figure 2.
Axial CT scans of the orbits (patient one)
(A) 3 weeks after symptom onset: extensive facial oedema with intact ocular structures bilaterally. (B) 7 weeks after symptom onset: the right globe is ruptured with disorganised contents. The left cornea shows an anterior defect. (C) 11 weeks after symptom onset: bilateral ruptured globes with disorganised contents.
Table.
Sample sites positive for non-variola Orthopoxvirus DNA in two patients
| Sample sites swabbed (approximate days since onset of initial lesions) | |
|---|---|
| Patient one | Unspecified face (17 days); nose (20 days); mouth and lip (49 days); left crystalline lens* (70 days); right upper arm (77 days) |
| Patient two | Back and abdomen (6 days); right eyelid and penis (53 days); right ear (84 days); left hand and right upper arm (98 days) |
Interval swab samples from various sites were collected for each patient throughout their disease course. All specimens were sent for qualitative non-variola Orthopoxvirus PCR (Northwell Health Laboratories, Hempstead, NY, USA) and yielded positive results.
Specimen possibly contaminated by eyelids during extrusion
2 weeks later, the patient presented to our emergency department again with worsened skin necrosis and substantial eyelid oedema. The patient had prominent eschars of the nasal and malar regions with substantial overlying purulence (figure 1C). Baseline facial features were markedly diminished. Repeat head imaging revealed right globe rupture and an anterior chamber defect of the left globe (figure 2B). The right eye showed no light perception; and there was bare light perception in the left eye. An ophthalmic exam of the right eye with retractors revealed only necrotic tissue and no identifiable structures. Examination of the left eye revealed a large, full thickness, central corneal defect that was plugged by prolapsed iris. Left globe preservation was attempted with cyanoacrylate glue but was unsuccessful. During subsequent examination, the patient's crystalline lens spontaneously extruded from the left eye. On repeat orbital imaging, both eyes appeared collapsed with disorganised contents (figure 2C).
A total 5-week hospital stay was complicated by similar necrotic lesions on the patient's torso and extremities. The patient was continued on HAART, switched to intravenous tecovirimat (200 mg twice daily), and received two doses of vaccinia immunoglobulin and broad-spectrum antibiotics (intravenous vancomycin and cefepime). Skin care included antibiotic ointments, emollients, and frequent dressing changes. Despite these measures, the patient's facial gangrene progressed, and he had widespread skin sloughing (figure 1D). The patient ultimately died due to haemorrhagic shock from bleeding rectal ulcers, presumably caused by monkeypox virus and secondary renal failure. Autopsy revealed ulcerations of multiple organs.
Case report two
A 45-year-old man with a 17-year history of HIV (not on HAART), and AIDS for at least one year (CD4+ count 29 cells per μL, viral load 42 674 copies per mL), developed vesiculopapular lesions of the torso, arms, neck, and genitals over 1 week. The patient was unvaccinated for orthopoxviruses and had multiple male sexual partners in the month before symptom onset. With suspected mpox, and at high risk for severe disease, the patient was started on empiric tecovirimat (600 mg twice daily for 2 weeks) and administered a non-replicative vaccine against monkeypox virus (JYNNEOS; Bavarian Nordic, Kvistgaard, Denmark). The patient declined initiation of HAART. Swab specimens of the patient's initial lesions were sent for qualitative PCR, which returned positive for non-variola Orthopoxvirus DNA, corroborating a clinical diagnosis of mpox.
Approximately 1 month later, the patient was examined at an emergency department, where he was noted to have ulcerated papules with necrotic centres of the abdomen and face, including the right upper eyelid and the left malar region (figure 3A , day 1). He was admitted to the hospital the next week after evident progression of his facial lesions, which had become large shallow ulcers with expanded central necrosis (figure 3A, day 8). The patient had also developed periorbital oedema concerning for superimposed preseptal cellulitis. By the following week, the patient's right eyelid lesion had expanded further and began to restrict lid function. No ocular surface involvement was noted, and the patient was started on trifluridine 1% eye drops for prophylaxis and agreed to start HAART. During the next 2 weeks, despite treatment with oral tecovirimat (600 mg twice daily), HAART, and broad-spectrum antibiotics (intravenous daptomycin and levofloxacin), discrete lesions progressed to a necrotic rash of the face, expanding outward from initial foci and becoming confluent (figure 3A, days 11–27). Qualitative PCR analysis of swabs from multiple skin sites consistently returned positive for non-variola Orthopoxvirus DNA (table), compatible with disseminated mpox. The patient received a dose of intravenous vaccinia immunoglobulin but his skin necrosis continued to progress.
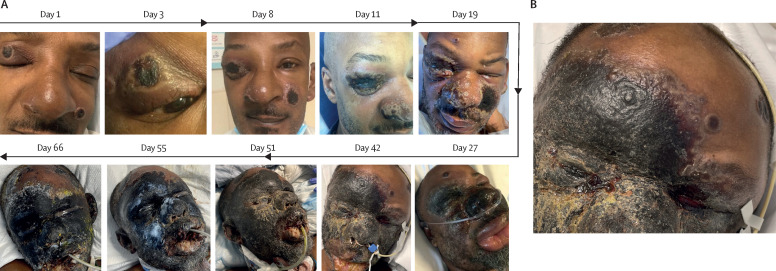
Figure 3.
Chronological progression of mpox facial lesions (patient two)
(A) Contiguous and centrifugal progression of facial skin necrosis from initial foci of mpox lesions. There is gradual development of panfacial gangrene. Day 1 marks the first date necrotic facial lesions were observed on clinical examination. (B) Areas of confluent necrosis are bounded by a raised skin edge containing vesicles.
The patient subsequently developed pharyngeal oedema and was transferred to our tertiary-care center for airway monitoring. Upon arrival, confluent gangrene of the face was apparent (figure 3A, day 42). A raised skin edge containing vesicles was noted along the borders of necrotic skin (figure 3B). There were similar areas of expansive skin necrosis on the torso and extremities. The patient was administered a single dose of intravenous cidofovir (5 mg/kg) with probenecid, and tecovirimat was changed to intravenous formulation (200 mg twice daily). Punch biopsy showed histology consistent with monkeypox virus infection (figure 4A ) and a striking absence of T-lymphocytic infiltrate (figure 4B). The patient soon developed an unstable airway, and surgical tracheostomy was performed. Two more doses of vaccinia immunoglobulin were administered after counsel with the US CDC, again without improvement. In the context of paradoxically worse lesions on HAART, concern was raised for possible immune reconstitution inflammatory syndrome, although CD4+ percentage had only increased by three points. The patient received a short course of prednisone (100 mg via nasogastric tube for 4 days) that was subsequently discontinued after his condition continued to progress. Hospital course was complicated by Sapovirus gastroenteritis, metabolic acidosis, multifactorial renal failure, and severe anaemia. The patient subsequently developed multifocal pneumonia and became ventilator-dependent, dying 15 weeks after the onset of his mpox symptoms.
Figure 4.
Skin histology (patient two)
The skin histology of patient one showed similar findings, but slides were unavailable for publication. (A) There is reticular alteration, ballooning, and necrosis of the epidermis with many keratinocytes exhibiting hyperchromatic or ground-glass nuclei. There is abundant karyorrhectic debris with numerous neutrophils (H&E staining, 200× magnification). (B) Pan T-cell marker CD3+ highlights only rare lymphocytes within the underlying dermis (100× magnification).
Discussion
In patients who are unvaccinated, previously reported ophthalmic manifestations of mpox include periorbital vesicular rash (25% of all patients with ophthalmic involvement), conjunctivitis (30% of patients), ulcerative keratitis (4–8% of patients), and blepharitis (30% of patients).11, 12, 13 Corneal involvement is particularly worrisome because it carries the risk of scarring and permanent vision loss. Previous reports of conjunctivitis, including those from the US CDC, support autoinoculation as a dominant mechanism for localisation to the ocular surface.7 Evidence also exists for viral passage from plasma into conjunctival secretions.14
Historical experiences show that both smallpox and vaccinia can cause analogous ocular disease.4 Ophthalmic complications of smallpox (variolous ophthalmia) have included conjunctivitis, pustular eyelid rash, periorbital oedema, uveitis, and progressive corneal ulceration, although we could find no examples of periorbital gangrene.4, 15, 16, 17 As early as 1903, the mechanism for ocular involvement was debated: keratitis sometimes preceded or presented simultaneously with cutaneous eruption, supporting bloodstream transmission, whereas an obvious decrease in eye involvement with rigorous hand hygiene favored autoinoculation.15 As is the case with monkeypox virus, a mixed mechanism might have been involved.4 The smallpox literature also provides numerous examples of ulcerative keratopathy progressing to corneal perforation and phthisis.15, 16 Although physical examination was restricted in the first case report that we presented, we suspect that autoinoculation of the ocular surface with monkeypox virus initially produced an ulcerative keratitis. Profound immunocompromise then permitted uninhibited viral replication in corneal tissue, resulting in progressive ulceration, corneal perforation, and globe compromise.
Ocular vaccinia, a condition that can develop following conventional smallpox vaccination, provides another example of eye infection from an orthopoxvirus.16 In ocular vaccinia, vaccinia virus is translocated to the eyelids and ocular surface through accidental autoinoculation from the vaccination site. This autoinoculation most commonly causes a vesiculopustular eyelid rash with blepharoconjunctivitis.17 Vaccinia virus can also replicate successfully in the corneal epithelium and stromal keratocytes and, like variola virus, cause ulcerative keratitis and perforation.17, 18 Similar to its relatives, variola virus and vaccinia virus, monkeypox virus appears capable of locally inoculating and replicating within corneal tissue. In addition to more common presentations of mpox-related ophthalmic disease, providers should be aware of the potential for restrictive periorbital gangrene, corneal perforation, and globe compromise.
According to US CDC guidelines, ocular involvement of mpox is an indication for tecovirimat therapy.7 Additionally, keratitis caused by both monkeypox virus and vaccinia virus has successfully been treated with topical trifluridine 1%, although no clinical trial standard exists.4, 19 Trifluridine has, however, shown activity against orthopoxviruses in vitro.20 For patients with eyelid lesions, trifluridine has also been used as post-exposure prophylaxis against autoinoculation of the ocular surface.16 Additional treatment measures for ulcerative keratopathy should include topical antibiotic prophylaxis and surface lubrication. It should be noted that although studies were conflicting, vaccinia immunoglobulin was contraindicated for patients with isolated vaccinia keratitis due to its potential to increase corneal scarring.4 The possibility of corneal scarring could also exist if vaccinia immunoglobulin is used to treat keratitis secondary to monkeypox virus. The US CDC therefore advises caution when considering vaccinia immunoglobulin for this population.7 In summary, anterior segment eye disease secondary to monkeypox virus might respond well to trifluridine 1% eye drops, topical antibiotic prophylaxis, ocular lubricants, and systemic tecovirimat according to US CDC guidelines.4, 7 To decrease the risk of autoinoculation of the ocular surface, patients with mpox should practice hand hygiene, avoid touching their eyes, and not use contact lenses.21
As evidenced by our case reports, baseline immunocompromise has the potential to augment the mpox disease state.22 US CDC data support that mpox patients co-infected with HIV are more likely to require hospital-level care (8% of patients) than those who are otherwise immunocompetent (3% of patients).22 Patients with HIV who have CD4+ counts lower than 200 cells per μL are at particularly high risk for severe disease.23 We believe that the case reports presented in this Grand Round show the first reported examples of mpox progressing to confluent facial gangrene in patients who are immunocompromised. Other case reports from a US CDC series spanning from August to October, 2022 include two patients with AIDS who were not on antiretroviral therapy (CD4+ count <10 cells per μL) and who developed coalescent and necrotic lesions of the back, genitals, and extremities despite treatment with either tecovirimat or vaccinia immunoglobulin, or both.8, 24 One other report describes a man infected with monkeypox virus with previously undiagnosed and treatment-naive HIV (CD4+ count 127 cells per μL) who ultimately developed nasal necrosis.25
These case reports of progressively necrotic skin lesions can again be understood through past clinical experiences with related orthopoxviruses. Because naturally occurring smallpox was eradicated before our modern understanding of the immune system, our knowledge of the smallpox immune response is incomplete.26 Again, vaccinia virus provides a reasonable comparison. It is well known that orthopoxviruses have a particular affinity to replicate in the skin.26 Aside from obvious cutaneous eruptions, this affinity is best appreciated by the technique of scarification, or the rapid and repeated puncture of the skin with a bifurcated needle during conventional smallpox vaccination. By inoculating the skin with infectious vaccinia virus, localised intra-epidermal viral replication ensues, which patients who are immunocompetent can contain with a satisfactory immune response.26 Innate immune cells such as macrophages and neutrophils first work to slow viral spread. This initial immune response is followed by a cell-mediated response involving cytotoxic T lymphocytes, which target infected cells. This process causes the so-called Jennerian pustule, which is characteristic of successful immunisation and confines the vaccinia virus to the localised site of inoculation, preventing the development of viraemia.27
Progressive vaccinia, also known as vaccinia gangrenosum, is a rare clinical entity first recognised in 1893.27 Progressive vaccinia is an obdurate and progressive expansion of the conventional smallpox vaccination lesion caused by unrestricted cutaneous vaccinia virus proliferation. As the vaccinia virus spreads into the deep tissues it causes profound cellular necrosis. Vesicles do not progress to pustules and an enlarging ulcer with a central eschar develops instead.27 Many patients with progressive vaccinia develop bacterial superinfection, and death can occur weeks to months following vaccination.27 Although not always present, many patients also develop metastatic foci of cutaneous gangrene throughout the body, even at otherwise intact skin sites, presumably via the progression to viraemia.27 This disease process occurs exclusively in patients who are immunocompromised, particularly in patients with preconditions marked by deficiencies in cell-mediated immunity.27 These populations do not have sufficient T-lymphocyte responses to eliminate infected skin cells and prevent secondary viral spread.27, 28 Histologically, involved sites show tissue necrosis, viral cytopathic effects, and a striking absence of lymphocytic infiltration.29, 30
Nearly all cases of progressive vaccinia occurred during the 1950s to early 1980s, either in infants with congenital immunodeficiencies or adults with acquired immunodeficiencies.27 The condition was invariably fatal until the introduction of vaccinia immunoglobulin, which decreased the case fatality rate to 25–50%.28 Although there was little overlap between the eras of HIV and smallpox, there are three examples in the military literature from 1987 to 1991 of patients with advanced AIDS undergoing conventional smallpox vaccination only to subsequently develop progressive vaccinia-like illnesses.27 These individuals developed expansively ulcerative or necrotic skin lesions that were both localised and metastatic to their vaccination sites.31, 32 Common to these patients were CD4+ counts lower than 50 cells per μL.27 It was later observed that patients with HIV who underwent conventional smallpox vaccination did not develop progressive vaccinia if CD4+ counts were higher than 200 cells per μL, underscoring the effect of substantial cell-mediated deficiency.33 Since these case reports, there have been only few confirmed accounts of progressive vaccinia in the literature. In 2009, a seemingly healthy member of the US Marine Corps with undiagnosed acute myelogenous leukaemia received a replicative smallpox vaccine (ACAM2000; Acambis, Cambridge, MA, USA) and soon after developed an expansile skin lesion at the inoculation site during chemotherapy.34 In 2015, a patient from Colombia with AIDS (CD4+ count 11 cells per μL) not on HAART contracted bovine vaccinia virus after milking cattle and developed disseminated ulcers of the face and extremities, which resolved with antiretroviral therapy alone.35
In considering the case reports presented in this Grand Round, we suggest that patients with mpox and underlying cell-mediated deficiencies can develop a profound and contiguously spreading skin necrosis by a process analogous to progressive vaccinia. To our knowledge, we are the first to report this association. Instead of cutaneous scarification, sentinel mpox lesions themselves serve as the initial inocula, providing monkeypox virus access to surrounding tissue. Low CD4+ counts preclude adequate cell-mediated responses, which increases the proclivity of monkeypox virus to infect, spread to, and destroy adjacent skin cells. Additionally, the previously shown ability of monkeypox virus to suppress host T-cell responses could have a synergistic effect.36 The result is an expansile cellular necrosis that manifests clinically as progressive gangrene rather than a pustular response. This proposed intradermal dissemination of monkeypox virus is best illustrated by the centrifugal progression of necrotic tissue from early lesions seen in our two patients (Figure 1, Figure 3). These examples also show areas of confluent necrosis bounded by a raised skin edge containing vesicles, another characteristic finding previously seen in patients with progressive vaccinia (figure 3B).27 Analogous to progressive vaccinia, the skin histology of both our patients also showed necrosis, viral cytopathic effects, and a paucity of T-lymphocytes (figure 4). Except for inoculated vaccinia as the causative agent, these clinical characteristics bear striking resemblance to the case definition criteria for progressive vaccinia, as standardised in the Brighton Collaboration and US CDC surveillance guidelines for adverse reactions.37, 38 We therefore propose that like progressive vaccinia, monkeypox virus can clinically evolve in patients who are immunocompromised not only via viraemia but also through contiguous intradermal spread.
It should also be noted that both our patients' biopsies were obtained after their skin lesions had progressed substantially despite HAART. The histological findings for both these patients, however, are inconsistent with known examples of immune reconstitution inflammatory syndrome. To the contrary, tissues affected by immune reconstitution inflammatory syndrome are often marked by lymphocytic infiltration and granulomas, although histopathological criteria have not yet been conventionalised.39, 40 Despite a brief steroid trial for the second patient, we hypothesise that immune reconstitution inflammatory syndrome is not the central disease process for our patients, in view of the evidence for primary intradermal progression of monkeypox virus. Caution should be taken when considering the empiric use of steroids for similar progressive skin manifestations of mpox in patients who are immunocompromised.
Historically, most patients with progressive vaccinia were treated with vaccinia immunoglobulin and immune reconstitution. Although vaccinia immunoglobulin was observationally successful when administered early for progressive vaccinia, no placebo-controlled trials exist to confirm its efficacy.27 Of note, the previously discussed patient with acute myelogenous leukaemia who developed progressive vaccinia was successfully treated with a regimen that included tecovirimat, brincidofovir, and high doses of vaccinia immunoglobulin.34 These experiences suggest that such therapies could also be effective in treating progressive gangrene secondary to monkeypox virus infection by limiting intradermal spread. Tecovirimat in particular has shown a broad ability in vitro and in vivo to inhibit the spread of multiple orthopoxviruses, including vaccinia virus, monkeypox virus, cowpox virus, and variola virus.41 Tecovirimat is currently the first-line treatment for patients with mpox, including those with HIV.42 Although our patients were initiated on multimodal treatments such as antiretrovirals, tecovirimat, cidofovir, or vaccinia immunoglobulin, their symptoms did not remit, probably due to a combination of overwhelming immunosuppression, bacterial superinfection, and delays in care.
In general, to prevent the development of progressive skin necrosis, ocular compromise, and other forms of severe mpox (eg, >100 cutaneous lesions, treatment-resistant lesions, haemodynamic instability, or secondary sepsis), patients with advanced HIV and other immunodeficiencies should be started on tecovirimat early after suspected diagnosis, even before the results of confirmatory testing.24 According to US CDC guidelines, if severe disease does develop, therapy can be escalated to include therapeutics such as vaccinia immunoglobulin, cidofovir, or brincidofovir if clinically appropriate.8 Tecovirimat therapy can also be extended up to 90 days in these populations and should remain the mainstay of treatment.8 It must be emphasised that these measures are not substitutes for immune reconstitution, including prompt initiation of antiretroviral therapies when appropriate.
Previous studies have also suggested that vaccinia immunoglobulin can be used as post-exposure prophylaxis against the complications of replicative smallpox vaccines, including progressive vaccinia and accidental autoinoculation of the eyes or mouth.43 Although no placebo-controlled studies exist to support the efficacy of vaccinia immunoglobulin in this context, side-effects of use have been observationally minimal.43 In 2016, post-exposure therapy with oral tecovirimat and vaccinia immunoglobulin successfully averted progressive vaccinia in another patient with undiagnosed acute myelogenous leukaemia who underwent replicative smallpox vaccination (ACAM2000; Acambis, Cambridge, MA, USA) and subsequent chemotherapy.44 Multimodal post-exposure prophylaxis with tecovirimat and vaccinia immunoglobulin could additionally benefit patients with mpox who are immunocompromised in preventing severe disease, although the efficacy of vaccinia immunoglobulin for these populations is still undetermined.4
Vaccination against monkeypox virus as both pre-exposure and post-exposure (pre-symptomatic) prophylaxis is also important in preventing or mitigating infection in those at high risk of infection, including men who have sex with men and individuals who are immunocompromised.45, 46 Although immunogenicity might be low in individuals who are immunocompromised, a vaccine containing a non-replicative strain of vaccinia virus (ie, MVA-BN, such as JYNNEOS; Bavarian Nordic, Kvistgaard, Denmark) is safe for these populations and should be considered adjunctively within 14 days of possible exposure to monkeypox virus.47 For patients with HIV, a non-replicative vaccine can be administered regardless of CD4+ count or viral load.42, 48
Furthermore, given the clinical possibility for direct intradermal spread of monkeypox virus, we hypothesise that gangrenous tissues themselves could serve as viral reservoirs for re-inoculation of the bloodstream or autoinoculation of distant sites, prolonging the disease state. Early application of topical antivirals (eg, tecovirimat 1%) or debridement of necrotic tissue, or both, could therefore have a role in reducing the viral burden in a patient who is immunocompromised. These therapies have been used previously to treat the skin lesions of progressive vaccinia.28, 49
In the context of the current mpox outbreak, novel manifestations of human mpox can parallel past clinical experiences with smallpox and vaccinia. Our Grand Round emphasises that special attention must be given to patients who are immunocompromised, because this population can develop progressive monkeypox, which could require early interventions. Clinicians must therefore remain vigilant to the variety of this disease as it unfolds and the specialised resources available for treatment.
Search strategy and selection criteria
For this Grand Round, we considered evidence from the latest peer-reviewed manuscripts across the medical literature, including those from the US Centers for Disease Control and Prevention and WHO. We searched PubMed for manuscripts from inception to Nov 5, 2022 with variations of “monkeypox,” “smallpox,” or “vaccinia” in combination with variations of “keratitis,” “ophthalmic,” “necrosis,” “gangrene,” and “immunocompromised.” No language restriction was applied. Further relevant manuscripts were found by examining the works cited by our selected references.
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
Written consent permitting case descriptions and inclusion of clinical media was obtained for all cited individuals. We thank these parties and the staff of the Ophthalmology and Dermatopathology divisions of NYU Langone Medical Center (New York, NY) for their contributions.
Contributors
SC performed an initial literature search, conceptualised the manuscript, and contributed to writing the original draft. AG, ES, SG, JS, and AO also contributed to writing the original draft. ES, SG, LS, and JS reviewed and edited the draft. AG, ES, and SG compiled the figures. AO and LS also provided supervision, review, and editing of the manuscript. SM was involved in data curation. All authors had access to all underlying data, and each verified its accuracy.
References
- 1.Ladnyj ID, Ziegler P, Kima E. A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bull World Health Organ. 1972;46:593–597. [PMC free article] [PubMed] [Google Scholar]
- 2.Kaler J, Hussain A, Flores G, Kheiri S, Desrosiers D. Monkeypox: a comprehensive review of transmission, pathogenesis, and manifestation. Cureus. 2022;14 doi: 10.7759/cureus.26531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention 2022 outbreak cases and data. 2022. https://www.cdc.gov/poxvirus/monkeypox/response/2022/index.html
- 4.Kaufman AR, Chodosh J, Pineda R., 2nd Monkeypox virus and ophthalmology—a primer on the 2022 monkeypox outbreak and monkeypox-related ophthalmic disease. JAMA Ophthalmol. 2022 doi: 10.1001/jamaophthalmol.2022.4567. published online Nov 3. [DOI] [PubMed] [Google Scholar]
- 5.Abdelaal A, Serhan HA, Mahmoud MA, Rodriguez-Morales AJ, Sah R. Ophthalmic manifestations of monkeypox virus. Eye. 2022 doi: 10.1038/s41433-022-02195-z. published online July 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thornhill JP, Barkati S, Walmsley S, et al. Monkeypox virus infection in humans across 16 countries—April-June 2022. N Engl J Med. 2022;387:679–691. doi: 10.1056/NEJMoa2207323. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention Interim clinical considerations for management of ocular monkeypox. 2022. https://www.cdc.gov/poxvirus/monkeypox/clinicians/ocular-infection.html
- 8.Rao A, Schrod C, Alarcon J, Dretler A. Situational update for clinicians about severe monkeypox virus infections. 2022. https://emergency.cdc.gov/coca/ppt/2022/100622_slides.pdf
- 9.Hendrickson RC, Wang C, Hatcher EL, Lefkowitz EJ. Orthopoxvirus genome evolution: the role of gene loss. Viruses. 2010;2:1933–1967. doi: 10.3390/v2091933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiang Y, Lane RK. In: Encyclopedia of virology. 4th edn. Bamford DH, Zuckerman M, editors. Academic Press; Cambridge, MA: 2021. Vaccinia virus (Poxviridae) pp. 854–859. [Google Scholar]
- 11.Ogoina D, Iroezindu M, James HI, et al. Clinical course and outcome of human monkeypox in Nigeria. Clin Infect Dis. 2020;71:e210–e214. doi: 10.1093/cid/ciaa143. [DOI] [PubMed] [Google Scholar]
- 12.Jezek Z, Grab B, Szczeniowski M, Paluku KM, Mutombo M. Clinico-epidemiological features of monkeypox patients with an animal or human source of infection. Bull World Health Organ. 1988;66:459–464. [PMC free article] [PubMed] [Google Scholar]
- 13.Damon IK. Status of human monkeypox: clinical disease, epidemiology and research. Vaccine. 2011;29(suppl 4):D54–D59. doi: 10.1016/j.vaccine.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 14.Mazzotta V, Mondi A, Carletti F, et al. Ocular involvement in monkeypox: description of an unusual presentation during the current outbreak. J Infect. 2022;85:573–607. doi: 10.1016/j.jinf.2022.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baker AR. Eye Complications of smallpox. JAMA. 1903;41:645–648. [Google Scholar]
- 16.Semba RD. The ocular complications of smallpox and smallpox immunization. Arch Ophthalmol. 2003;121:715–719. doi: 10.1001/archopht.121.5.715. [DOI] [PubMed] [Google Scholar]
- 17.Brauner SC, Pavan-Langston D. Smallpox, vaccinia, and the eye. Int Ophthalmol Clin. 2006;46:11–25. doi: 10.1097/00004397-200604620-00004. [DOI] [PubMed] [Google Scholar]
- 18.Larsen IV, Clausius H, Kolb AW, Brandt CR. Both CD8+ and CD4+ T cells contribute to corneal clouding and viral clearance following vaccinia virus infection in C57BL/6 mice. J Virol. 2016;90:6557–6572. doi: 10.1128/JVI.00570-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hughes C, McCollum A, Pukuta E, et al. Ocular complications associated with acute monkeypox virus infection, DRC. Int J Infect Dis. 2014;21:276–277. [Google Scholar]
- 20.Kern ER. In vitro activity of potential anti-poxvirus agents. Antiviral Res. 2003;57:35–40. doi: 10.1016/S0166-3542(02)00198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cash-Goldwasser S, Labuda SM, McCormick DW, et al. Ocular monkeypox—United States, July-September 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1343–1347. doi: 10.15585/mmwr.mm7142e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curran KG. HIV and sexually transmitted infections among persons with monkeypox — eight U.S. jurisdictions, May 17–July 22, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1141–1147. doi: 10.15585/mmwr.mm7136a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vasan A. CDC advisory severe manifestations of human monkeypox among people who are immunocompromised due to HIV or other conditions. 2022. https://www1.nyc.gov/assets/doh/downloads/pdf/han/advisory/2022/cdc-monkeypox-immunocompromised.pdf
- 24.Miller MJ. Severe monkeypox in hospitalized patients—United States, August 10–October 10, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1412–1417. doi: 10.15585/mmwr.mm7144e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boesecke C, Monin MB, van Bremen K, Schlabe S, Hoffmann C. Severe monkeypox-virus infection in undiagnosed advanced HIV infection. Infection. 2022;50:1633–1634. doi: 10.1007/s15010-022-01901-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Engler RJM, Kenner J, Leung DYM. Smallpox vaccination: risk considerations for patients with atopic dermatitis. J Allergy Clin Immunol. 2002;110:357–365. doi: 10.1067/mai.2002.128052. [DOI] [PubMed] [Google Scholar]
- 27.Bray M, Wright ME. Progressive vaccinia. Clin Infect Dis. 2003;36:766–774. doi: 10.1086/374244. [DOI] [PubMed] [Google Scholar]
- 28.Henderson DA, Borio LL, Grabenstein JD. In: Vaccines. 5th edn. Plotkin SA, Orenstein WA, Offit PA, editors. W.B. Saunders; Philadelphia, PA: 2008. Chapter 30—smallpox and vaccinia; pp. 773–803. [Google Scholar]
- 29.Dixon MF. Progressive vaccinia complicating lymphosarcoma. J Pathol. 1970;100:53–67. doi: 10.1002/path.1711000107. [DOI] [PubMed] [Google Scholar]
- 30.Fenner F, Henderson DA, Arita I, Jezek Z, Ladnyi ID. In: Smallpox and its eradication. Fenner F, Henderson DA, Arita I, Jezek Z, Ladnyi ID, editors. World Health Organization; Geneva: 1988. The pathogenesis, pathology, and immunology of smallpox and vaccinia; p. 141. [Google Scholar]
- 31.Redfield RR, Wright DC, James WD, Jones TS, Brown C, Burke DS. Disseminated vaccinia in a military recruit with human immunodeficiency virus (HIV) disease. N Engl J Med. 1987;316:673–676. doi: 10.1056/NEJM198703123161106. [DOI] [PubMed] [Google Scholar]
- 32.Guillaume JC, Saiag P, Wechsler J, Lescs MC, Roujeau JC. Vaccinia from recombinant virus expressing HIV genes. Lancet. 1991;337:1034–1035. doi: 10.1016/0140-6736(91)92689-y. [DOI] [PubMed] [Google Scholar]
- 33.Tasker SA, Schnepf GA, Lim M, et al. Unintended smallpox vaccination of HIV-1-infected individuals in the United States military. Clin Infect Dis. 2004;38:1320–1322. doi: 10.1086/420938. [DOI] [PubMed] [Google Scholar]
- 34.Centers for Disease Control and Prevention (CDC) Progressive vaccinia in a military smallpox vaccinee—United States, 2009. MMWR Morb Mortal Wkly Rep. 2009;58:532–536. [PubMed] [Google Scholar]
- 35.Laiton-Donato K, Ávila-Robayo P, Páez-Martinez A, et al. Progressive vaccinia acquired through zoonotic transmission in a patient with HIV/AIDS, Colombia. Emerg Infect Dis. 2020;26:601–605. doi: 10.3201/eid2603.191365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hammarlund E, Dasgupta A, Pinilla C, Norori P, Früh K, Slifka MK. Monkeypox virus evades antiviral CD4+ and CD8+ T cell responses by suppressing cognate T cell activation. Proc Natl Acad Sci USA. 2008;105:14567–14572. doi: 10.1073/pnas.0800589105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nell P, Kohl KS, Graham PL, et al. Progressive vaccinia as an adverse event following exposure to vaccinia virus: case definition and guidelines of data collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25:5735–5744. doi: 10.1016/j.vaccine.2007.02.088. [DOI] [PubMed] [Google Scholar]
- 38.Casey C, Vellozzi C, Mootrey GT, et al. Surveillance guidelines for smallpox vaccine (vaccinia) adverse reactions. MMWR Recomm Rep. 2006;55:1–16. [PubMed] [Google Scholar]
- 39.Rushing EJ, Liappis A, Smirniotopoulos JD, et al. Immune reconstitution inflammatory syndrome of the brain: case illustrations of a challenging entity. J Neuropathol Exp Neurol. 2008;67:819–827. doi: 10.1097/NEN.0b013e318181b4da. [DOI] [PubMed] [Google Scholar]
- 40.French MA, Price P, Stone SF. Immune restoration disease after antiretroviral therapy. AIDS. 2004;18:1615–1627. doi: 10.1097/01.aids.0000131375.21070.06. [DOI] [PubMed] [Google Scholar]
- 41.Yang G, Pevear DC, Davies MH, et al. An orally bioavailable antipoxvirus compound (ST-246) inhibits extracellular virus formation and protects mice from lethal orthopoxvirus Challenge. J Virol. 2005;79:13139–13149. doi: 10.1128/JVI.79.20.13139-13149.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O'Shea J, Filardo TD, Morris SB, Weiser J, Petersen B, Brooks JT. Interim guidance for prevention and treatment of monkeypox in persons with HIV infection—United States, August 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1023–1028. doi: 10.15585/mmwr.mm7132e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hopkins RJ, Lane JM. Clinical efficacy of intramuscular vaccinia immune globulin: a literature review. Clin Infect Dis. 2004;39:819–826. doi: 10.1086/422999. [DOI] [PubMed] [Google Scholar]
- 44.Lindholm DA, Fisher RD, Montgomery JR, et al. Preemptive tecovirimat use in an active duty service member who presented with acute myeloid leukemia after smallpox vaccination. Clin Infect Dis. 2019;69:2205–2207. doi: 10.1093/cid/ciz286. [DOI] [PubMed] [Google Scholar]
- 45.WHO Vaccines and immunization for monkeypox: interim guidance, 16 November 2022. 2022. https://www.who.int/publications-detail-redirect/WHO-MPX-Immunization
- 46.Petersen E, Zumla A, Hui DS, et al. Vaccination for monkeypox prevention in persons with high-risk sexual behaviours to control on-going outbreak of monkeypox virus clade 3. Int J Infect Dis. 2022;122:569–571. doi: 10.1016/j.ijid.2022.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rao AK, Petersen BW, Whitehill F, et al. Use of JYNNEOS (smallpox and monkeypox vaccine, live, nonreplicating) for preexposure vaccination of persons at risk for occupational exposure to orthopoxviruses: recommendations of the advisory committee on immunization practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:734–742. doi: 10.15585/mmwr.mm7122e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dyer M, Fine SM, Vail RM, et al. Monkeypox Vaccination in Adults With HIV. 2022. https://www.hivguidelines.org/hiv-care/monkeypox/
- 49.Lederman ER, Davidson W, Groff HL, et al. Progressive vaccinia: case description and laboratory-guided therapy with vaccinia immune globulin, ST-246, and CMX001. J Infect Dis. 2012;206:1372–1385. doi: 10.1093/infdis/jis510. [DOI] [PMC free article] [PubMed] [Google Scholar]