Abstract
Modifications of mucosal phospholipids have been detected in samples from patients with Helicobacter pylori-positive gastritis. These alterations appear secondary to increased phospholipase A2 activity (PLA2). The cytosolic form of this enzyme (cPLA2), normally involved in cellular signaling and growth, has been implicated in cancer pathogenesis. The aim of this study was to investigate cPLA2 expression and PLA2 activity in the gastric mucosae of patients with and without H. pylori infection. In gastric biopsies from 10 H. pylori-positive patients, cPLA2 levels, levels of mRNA as determined by reverse transcriptase PCR, levels of protein as determined by immunohistochemistry, and total PLA2 activity were higher than in 10 H. pylori-negative gastritis patients. To clarify whether H. pylori had a direct effect on the cellular expression of cPLA2, we studied cPLA2 expression in vitro with different human epithelial cell lines, one from a patient with larynx carcinoma (i.e., HEp-2 cells) and two from patients with gastric adenocarcinoma (i.e., AGS and MKN 28 cells), incubated with different H. pylori strains. The levels of cPLA2, mRNA, and protein expression were unchanged in Hep-2 cells independently of cellular adhesion or invasion of the bacteria. Moreover, no change in cPLA2 protein expression was observed in AGS or MKN 28 cells treated with wild-type H. pylori. In conclusion, our study shows increased cPLA2 expression and PLA2 activity in the gastric mucosae of patients with H. pylori infection and no change in epithelial cell lines exposed to H. pylori.
Helicobacter pylori infection of the gastric mucosa is present worldwide and may be associated with several pathologic alterations, including gastric cancer (30). The relationship between this organism and the development of gastric cancer has been postulated mainly on the basis of epidemiological investigations and animal models of H. pylori infection (13, 15, 17, 21, 39, 40, 53). This relationship is further supported by the finding that some patients with H. pylori infection show the genetic abnormalities of dysplasia and metaplasia in mucosal areas before the development of carcinoma (35, 50). However, the molecular mechanisms underlying the multistep process of gastric carcinogenesis related to H. pylori infection remain undefined (57).
It has been shown that H. pylori damages the gastric barrier function and induces a dramatic change in mucosal phospholipid composition (34). This is likely due to a local increase in phospholipase A2 (PLA2) activity (4, 27). The cytosolic form (cPLA2), but not the secretory form, of this enzyme is involved in cellular signaling and growth (2, 25, 26, 28, 31, 38, 51) and has recently been implicated in the pathogenesis of malignant transformation (11, 22, 32, 48, 49, 55, 56). Furthermore, many human tumors have been reported to exhibit increased synthesis of prostaglandins, the formation of which is dependent on an increase in cPLA2 activity (16, 18, 19, 29).
In this study, we analyzed cPLA2 expression in gastric-mucosal biopsies from patients with chronic gastritis, with or without H. pylori infection. We showed increases in gastric levels of both cPLA2 mRNA and protein expression and an increase in PLA2 activity in H. pylori-positive patients with respect to levels in H. pylori-negative patients. However, cPLA2 expression in a number of epithelial cell lines exposed to a variety of H. pylori strains remained unchanged.
MATERIALS AND METHODS
Patients.
Ten H. pylori-positive patients with duodenal ulcers and 10 H. pylori-negative patients with chronic gastritis, with ages ranging between 20 and 65 years, were recruited. Entry criteria were the absence of antisecretory or antibiotic drug therapy in the previous month, the absence of anticoagulant drug therapy in the previous week, and the absence of severe associated diseases (such as hepatic, renal, or cardiovascular diseases). During upper gastrointestinal endoscopy, each patient had 12 biopsies taken from the gastric antrum. Four biopsies were fixed immediately in buffered formalin for morphological and immunohistochemical examinations, four were stored frozen at −20°C until the time of detection of PLA2 activity, and four were stored frozen at −80°C until RNA extraction. Serum samples of each patient were stored frozen until the detection of anti-CagA antibody by commercial Western blotting (Helico Blot 2.0; Genelabs Diagnostics, Singapore). H. pylori status was assessed by the concordance of the results of a breath test and Giemsa staining. Histopathological diagnosis by hematoxylin and eosin staining was performed according to the updated Sidney system (12).
Informed consent was obtained from all subjects, and the protocol was approved by the local ethics committee (University of Naples School of Medicine).
Cell culture.
HEp-2 cells from a patent with human larynx carcinoma were obtained from the American Type Culture Collection (ATCC CCL 23) and were grown on tissue culture plasticware in basal Eagle's medium supplemented with Hanks' salts, 50 mM l-glutamine, 0.075% sodium bicarbonate, and 15% fetal bovine serum (FBS; Sigma Chemical Co., S. Louis, Mo.). The cells were incubated at 37°C in 5% CO2 and with 99% humidity, as described previously (54).
Gastric epithelial cells derived from a patient with a poorly differentiated human gastric adenocarcinoma (i.e., AGS cell line) (3) or from a patient with a well-differentiated human gastric adenocarcinoma (i.e., MKN 28 cell line) (44, 45) were grown in Dulbecco's modified Eagle's medium (DMEM; Life Technologies Inc., Rockville, Md.) supplemented with 10% FBS and 1% antibiotic–antimycotic solution (Gibco BRL Laboratories, Grand Island, N.Y.) at 37°C in a humidified atmosphere of 5% CO2 in air.
Bacterial infection.
HEp-2 cells were incubated for 3 h with three different H. pylori strains designated ATCC 51652 (obtained from a patient of the Mayo Clinic with severe active chronic gastritis and a duodenal ulcer), MC199 (obtained from a patient of the Mayo Clinic with a large gastric ulcer secondary to a carcinoid tumor), and MC31 (quality-control strain used for diagnostic testing at the Mayo Clinic) (54). Cells were also incubated with one strain of Shigella flexneri (SW1) (obtained from a patient with clinically invasive disease) and, as a reference, a noninvasive Escherichia coli strain (ATCC 35218). AGS or MKN 28 cells were incubated from 3 to 18 h with H. pylori (ATCC 51652). For cocolture experiments, all bacterial strains were grown in 5% CO2 with 99% humidity for 48 h on sheep blood agar. Just before use, the bacteria were harvested from the agar plate, washed in Gey's solution, and resuspended at 1.5 × 108 to 3 × 108 cells/ml in antibiotic-free DMEM supplemented with 10% FBS or Eagle's medium supplemented with 15% FBS as appropriate.
PLA2 activity.
Total PLA2 activity was calculated according to the release of arachidonic acid in a reaction mixture containing [3H]arachidonate-labeled E. coli membranes (about 30,000 cpm), 40 μmol of Tris-HCl buffer (pH 7.5), 5 μmol of CaCl2, and 20 μg of gastric-mucosa proteins. The reaction mixture was incubated at 37°C in a shaking water bath for 3 h, and the reaction was stopped by the addition of methanol-chloroform (2/1, vol/vol). Lipids were extracted according to the Bligh and Dyer procedure (6) and separated by thin-layer chromatography. Radiolabeled products were identified with a radioactivity scanner, scraped off, and counted with a liquid scintillation counter, and their radioactivities are expressed as percentages of the total radioactivity.
Preliminary experiments with different amounts of proteins, labeled substrates, and incubation times were performed to set up optimal conditions (data not shown).
RT-PCR of human biopsies.
The gastric biopsy specimens were homogenized, and total RNA was extracted using TRIzol reagent (Gibco BRL Laboratories). The quantity and quality of extracted total RNA were analyzed on denaturing 1% agarose gel. Subsequently, first-strand complementary DNA was synthesized using 1 μg of RNA and 200 U of reverse transcriptase (RT) (SuperScript RT; Gibco BRL). The reaction profile was 37°C for 10 min, followed by 42°C for 60 min. To control for contamination by genomic DNA, all RNA samples were run in duplicate with and without the addition of RT. Aliquots of the cDNAs were PCR coamplified simultaneously with the following specific primers for cPLA2 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an internal control: for cPLA2, 5′ CTCATGCCCAGACCTACGATT 3′ (forward) and 5′ TAATACGACTCACTATAGGGCGTCAGGTTTGAC 3′ (reverse), and for GAPDH, 5′ CACCATCTTCCAGGAGCCAG 3′ (forward) and 5′ TCACGCCACAGTTTCCCGGA 3′ (reverse).
PCR conditions were as follows: denaturation at 94°C for 1 min, annealing at 53°C for 1 min, and extension at 72°C for 1 min. The reaction proceeded for 32 cycles; the GAPDH primers were added to the reaction mixture after 10 cycles. At the end of the process, a 10-min extension step was included. The amplification conditions were established to obtain the linear reaction necessary for semiquantitative analysis. The amplified products were quantified by densitometric scanning, and the intensities of cPLA2 bands were related to that of GAPDH in each sample.
Immunohistochemistry.
For each sample, 4-μm-thick serial sections from paraffin blocks were cut, dewaxed, and rehydrated. The endogenous peroxidase was inhibited by incubation with 3% H2O2 in methanol (20 min at room temperature). To reduce nonspecific background staining, the slides were incubated with 5% goat serum (15 min at room temperature). To enhance immunostaining, sections were treated with an antigen retrieval solution (10 mM citric acid monohydrate [pH 6.0], adjusted with 2 N NaOH) and heated three times in a microwave oven at high power for 5 min. Finally, the slides were incubated overnight at 4°C in a moist chamber with a mouse anti-human cPLA2 monoclonal antibody (Santa Cruz Biotechnology, Santa Cruz, Calif.; dilution, 1:100). The avidin-biotin-peroxidase complex procedure (ABC standard; Vector Laboratories, Burlingame, Calif.) was then performed according to the method of Hsu et al. (20). Peroxidase activity was detected with diaminobenzidine as the substrate. Finally, sections were weakly counterstained with Harris' hematoxylin and mounted with a synthetic medium was used as a coverslip. Two independent approaches were used to confirm the specificity of the immunohistochemical signal: (i) serial dilution of the primary antibody was carried out until the signal disappeared and (ii) nonimmune mouse immunoglobulin G (IgG) was used instead of primary antibody, which failed to reveal relevant staining.
A case of colon adenocarcinoma was used as a positive control. Sections were considered positively stained only when the relevant cytoplasmic staining for cPLA2 was unequivocal.
The degree of immunopositivity was evaluated semiquantitatively. A total of 400 cells was counted in random fields from representative areas of the lesions, and the immunoreactive cells were roughly assessed, expressed as percentages, and scored as follows: when 0 to 5% of cells were reactive, they were considered negative; when 5 to 25% of cells were reactive, they were considered to have low positivity; when 25 to 50% of cells were reactive, they were considered to have moderate positivity; and when >50% of cells were reactive, they were considered to have high positivity.
Northern blot analysis from cultured HEp-2 cells.
Total RNA from either untreated HEp-2 cells or HEp-2 cells treated for 3 h with three different strains of H. pylori, one strain of S. flexneri, and one strain of E. coli was extracted using TRIzol reagent. The amount and quality of RNA extracted were evaluated by ethidium bromide staining after running a denaturing agarose gel. For each sample, 20 μg of RNA was loaded onto a denaturing 1.2% agarose gel and subsequently blotted onto a nylon membrane (Hybond; Amersham). After 4 h of prehybridization, hybridization was carried out using the radioactively labeled PCR products corresponding to cPLA2. To normalize the amount of RNA loaded in each lane, the same filter was hybridized with a GAPDH probe, obtained by radioactive labeling of the 360-bp PCR product. The bands obtained by autoradiography were densitometrically scanned, and the intensities of the cPLA2 bands were related to that of GAPDH.
Western blot analysis.
HEp-2, AGS, or MKN 28 cells either not exposed or exposed to bacterial strains were lysed with a modified radioimmunoprecipitation assay buffer in the presence of protease inhibitors and freshly prepared phenylmethylsulfonyl fluoride. Protein concentration was determined by a modified Bradford method (Biorad assay kit) using bovine serum albumin as the standard. Equivalent amounts of protein (25 μg) were loaded in each lane and separated by electrophoresis on sodium dodecyl sulfate–7.5% polyacrylamide gels. After electrophoresis for 150 min at 94 V, the proteins were electroblotted to a nitrocellulose membrane at 22 V.
The cPLA protein was identified using a specific mouse anti-human cPLA2 monoclonal antibody (diluted 1/1,000; Santa Cruz) as the primary antibody and an anti-mouse IgG antibody (diluted 1/5,000) as the secondary antibody. Visualization was obtained with an ECL kit (Amersham Pharmacia Biotechnology).
Acridine orange internalization assay.
HEp-2 cells were trypsinized, washed in FBS, and counted. Approximately 106 cells were placed in each well of a Lab-Tek Permanox chamber slide (Nunc, Inc., Naperville, Ill.) with 100 μl of BME and incubated overnight to allow cells to attach. One hundred-microliter aliquots of the bacterial suspension (1.5 × 108 to 3 × 108 cells/ml) were added to chamber wells of duplicate slides and incubated for 3 h. After incubation, the chambers were gently washed three times with Hanks' balanced salt solution (HBSS) to remove any bacteria not adhering to the HEp-2 cells, and the chambers were thus removed.
Cells on the chamber slides were stained with 0.01% acridine orange in Gey's solution for 45 s at room temperature and washed with HBSS. The cells were then stained with 0.05% crystal violet in 0.155 M NaCl for 45 s and washed with HBSS. Crystal violet does not penetrate the HEp-2 cells and cannot quench internalized acridine orange-stained bacteria. The slides were examined at a magnification of ×1,000 with switching between fluorescent and phase-contrast optics. In each well, 50 HEp-2 cells were examined to determine the number with visible adherent and internalized bacteria.
Northern and Western analyses and an acridine orange internalization assay were performed in triplicate for each bacterial isolate, and the means and standard deviations were determined.
Statistical analysis.
The unpaired t test was used to compare RT-PCR levels of cPLA2 and PLA2 activities in H. pylori-positive and H. pylori-negative patients. The differences in the densitometric values of Northern and Western blot analyses were evaluated by a one-way analysis of variance test.
RESULTS
In vivo studies.
The H. pylori-negative patients had nonactive chronic gastritis; of these, four had mild or moderate atrophy restricted to the antrum. The H. pylori-positive patients had mild or moderate active gastritis associated with peptic disease; 6 out of 10 showed mild-to-moderate atrophy predominantly restricted to the antrum. Positivity for anti-CagA antibody, detected by serum Western blot analysis, was present in 7 out of 10 H. pylori-positive patients.
The inflammatory infiltrate consisted of neutrophils and lymphocytes in H. pylori-positive patients and lymphocytes in H. pylori-negative patients.
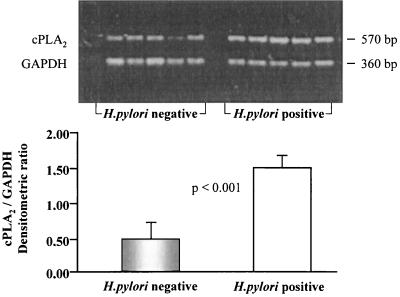
Semiquantitative RT-PCR was performed by coamplifying cPLA2 with GAPDH as an internal control. The results of the analysis of two bands of 360 and 570 bp, corresponding to GAPDH and cPLA2, respectively, are shown at the top of Fig. 1. The intensities of the bands were quantified by densitometric scanning, and the relative ratio of the intensity of the cPLA2 band to that of the GAPDH band was determined and is reported in the histogram at the bottom of Fig. 1. The bands corresponding to cPLA2 in H. pylori-positive patients were two- or threefold more intense than those in H. pylori-negative subjects (P < 0.001).
FIG. 1.
Expression of cPLA2 determined by RT-PCR analysis in vivo. Shown is a representative RT-PCR analysis of cPLA2 performed on gastric-mucosal specimens from patients with chronic gastritis who were infected (n = 5) and not infected (n = 5) with H. pylori. GAPDH expression was used as the internal control. The sizes of the amplified bands of 570 and 360 bp are indicated at the side. The histogram at the bottom reports the densitometric values of the cPLA2/GAPDH ratios grouped by H. pylori status (unpaired t test, P < 0.001).
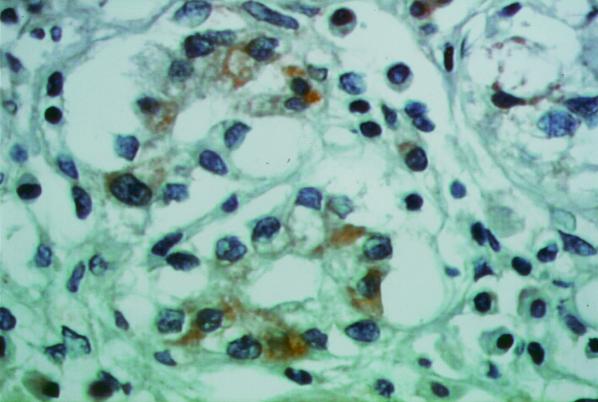
The immunohistochemical expression of cPLA2 showed a relevant cytosolic positivity (in the Golgi and cytoplasmic patterns) that was restricted to epithelial cells only in the samples from H. pylori-infected patients (Fig. 2); H. pylori-negative patients showed only focal cPLA2 immunoreactivity in a Golgi pattern (Fig. 3). No immunoreactivity for cPLA2 was observed when nonimmune mouse IgG was used as the primary antibody (data not shown).
FIG. 2.
Immunohistochemical expression of cPLA2. Shown is a tissue section from a patient with H. pylori-positive chronic gastritis showing strong, definite immunostaining for cPLA2 and Golgi and cytoplasmic patterns, with sporadic membrane reinforcement of the signal (ABC standard; magnification, ×400).
FIG. 3.
Immunohistochemical expression of cPLA2. Shown is a tissue section from a patient with H. pylori-negative chronic gastritis showing only a focal positivity for cPLA2 in glandular epithelial cells, with a Golgi distribution pattern (avidin-biotin-peroxidase complex standard; magnification, ×400).
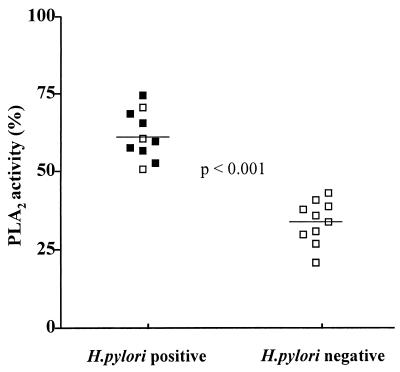
Total PLA2 activity, calculated as a percentage of arachidonic acid released, was significantly higher in H. pylori-positive than in H. pylori-negative patients (61.1% ± 7.9% versus 32.9% ± 6.6% P < 0.001) (Fig. 4).
FIG. 4.
PLA2 activity. Total PLA2 activity was calculated as a percentage of the amount of arachidonic acid released in H. pylori-positive (■, anti-CagA positive) and H. pylori-negative (□) patients (unpaired t test; P < 0.001).
In vitro studies.
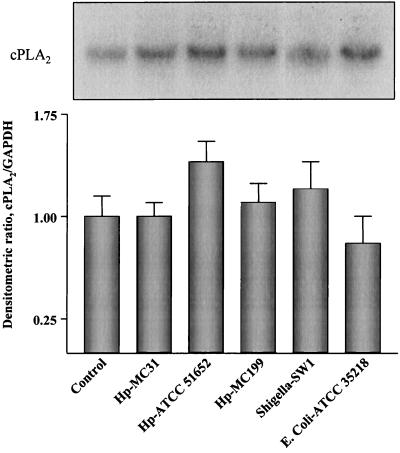
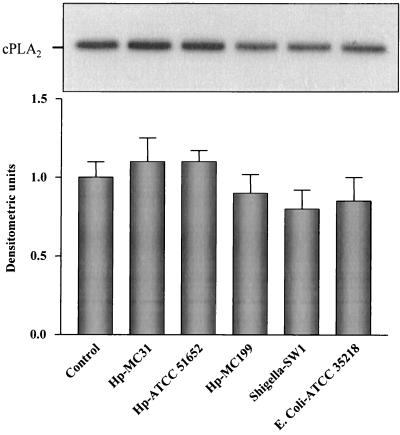
To assess whether H. pylori directly affected cPLA2 expression under conditions independent of systemic factors, we used an in vitro system consisting of HEp-2 cells exposed to different H. pylori strains. We also used S. flexeneri and E. coli to assess whether any effect on cPLA2 expression was specifically related to H. pylori. No significant difference in the level of cPLA2 mRNA expression was found in cells treated with H. pylori or other bacterial strains with respect to that in control, untreated cells (Fig. 5). To correlate the steady-state mRNA levels obtained with the levels of cPLA2 protein expression, Western blot analysis was performed. As shown in Fig. 6, a band corresponding to a protein with an apparent molecular mass of 110 kDa was detected. Similarly to the results with mRNA previously shown, the intensity of this band did not change, whatever the invasiveness of the bacteria in the coculture system. To examine adherence and internalization of the bacterial strains, HEp-2 cells were studied both morphologically and by the acridine orange assay (54). Three hours after infection, the percentage of HEp-2 cells associated with or invaded by each of the bacterial strains was determined. The results are shown in Table 1 and indicate that two of the H. pylori strains (ATCC 51652 and MC199) demonstrated substantial cellular invasion (98 and 12%, respectively) and association (100 and 74%, respectively) of HEp-2 cells. H. pylori strain MC31 and S. flexneri strain SW1 adhered to cells (34 and 24% of cells, respectively) and invaded cells to a lesser degree (with both strains invading 6% of cells). No adherence or penetration of HEp-2 cells was observed for the E. coli strain used (ATCC 35218).
FIG. 5.
cPLA2 mRNA expression in HEp-2 cells in vitro. Northern blot analysis was carried out on total RNA extracted from HEp-2 cells either not infected or infected with the various bacterial strains indicated. The top panel shows a representative autoradiograph from three experiments, with the bands corresponding to cPLA2 mRNA (20 μg of RNA was loaded in each lane). The histogram in the bottom panel reports the means ± standard deviations of the densitometric values of the cPLA2/GAPDH ratios obtained from three independent experiments. Hp-MC31, H. pylori quality-control strain used for diagnostic testing at the Mayo Clinic; Hp-ATCC 51652, H. pylori strain obtained from a patient of the Mayo Clinic with severe active chronic gastritis and a duodenal ulcer; Hp-MC199, H. pylori strain obtained from a patient of the Mayo Clinic with a large gastric ulcer secondary to a carcinoid tumor; Shigella-SW1, strain obtained from a patient with clinically invasive disease; E. coli-ATCC 35218, a noninvasive bacterium.
FIG. 6.
Expression of cPLA2 protein in HEp-2 cells in vitro. Western blot analysis was performed on cell lysates (25 μg) from HEp-2 cells either mock infected (control) or infected with the bacterial strains indicated. The top panel shows a representative autoradiograph from three experiments; the band corresponds to cPLA2, and the protein migrates with an apparent molecular mass of 110 kDa. The histogram at the bottom reports the mean ± standard deviation of densitometric values for the cPLA2 bands obtained from three independent experiments. Hp-MC31, H. pylori quality-control strain used for diagnostic testing at the Mayo Clinic; Hp-ATCC 51652, H. pylori strain obtained from a patient of the Mayo Clinic with severe active chronic gastritis and a duodenal ulcer; Hp-MC199, H. pylori strain obtained from a patient of the Mayo Clinic with a large gastric ulcer secondary to a carcinoid tumor; Shigella-SW1, strain obtained from a patient with clinically invasive disease; E. coli-ATCC 35218, a noninvasive bacterium.
TABLE 1.
Cellular association and invasion by bacteria
| Bacterial strain | % of HEp-2 cells showing:
|
|
|---|---|---|
| Cellular association with bacteria | Cellular invasion by bacteria | |
| H. pylori ATCC 51652 | 100 | 98 |
| H. pylori MC199 | 74 | 12 |
| H. pylori MC31 | 34 | 6 |
| S. flexneri SW1 | 24 | 6 |
| E. coli ATCC 35218 | 0 | 0 |
To rule out cell line-specific abnormalities, we also studied the role of H. pylori in cPLA2 protein expression by Western blot analysis of human gastric epithelial cells derived from poorly differentiated (i.e., AGS cells) or well-differentiated (i.e., MKN 28 cells) gastric adenocarcinomas. The incubation of AGS or MKN 28 cells with wild-type H. pylori for up to 18 h did not exert any significant effect on cPLA2 protein expression compared with that in control untreated cells (Fig. 7).
FIG. 7.
Expression of cPLA2 protein in AGS and MKN 28 cells in vitro. Western blot analysis was performed on cell lysates (25 μg) from AGS and MKN 28 cells incubated for 3, 6, or 18 h with H. pylori strain ATCC 51652 (obtained from a patient of the Mayo Clinic with severe active chronic gastritis and a duodenal ulcer) or with serum-free DMEM supplemented with 10% FBS (control). A representative autoradiograph from three independent experiments showing a cPLA2-immunoreactive band with an apparent molecular mass of 110 kDa is shown.
DISCUSSION
PLA2 is an important enzyme and is expressed in several types of human cells (1, 22, 24, 25, 28, 31, 37). This wide distribution has been correlated with the important role it plays in several metabolic pathways, particularly the transduction of cell growth signals (2, 25, 26, 28, 31, 38, 46, 51). The expression of cPLA2 in cells of the intestinal tract has been thoroughly investigated (23, 36) and has also been correlated with the development of several inflammatory diseases (7, 41, 51, 52). Studies with cellular systems have shown that the increase in cellular eicosanoid production, promoted by cytokines and agents causing cell damage, is at least in part due to the activation of cPLA2 and the elevation of its cellular levels (18, 19, 29). It has also been found that cPLA2 is a target of antiinflammatory glucocorticoid drugs that attenuate eicosanoid synthesis in a number of different cell types (33). Substantial evidence thus indicates that cPLA2 may be an important component in the cascade of events leading to the production of the proinflammatory and injurious mediators of disease states (7, 52). Moreover, elevated eicosanoid production has been observed in a number of tumor cells and is likely to contribute to the altered growth conditions leading to cell transformation (18). In line with this evidence are the results of studies in which cPLA2 levels have been correlated with the activation of Ras, a protooncogene with well-established involvement in tumorigenesis and metastasis in several animal and human model systems (9, 14, 16). This study was undertaken to investigate cPLA2 tissue levels and activity in gastric mucosa infected with H. pylori and to assess whether any such increase might reflect the invasive nature of the organism. The results reported in this study show that in human gastric mucosal biopsies the levels of mRNA and protein expression of cPLA2 were higher in H. pylori-positive patients than in H. pylori-negative patients (Fig. 1 to 3). Moreover, we found an increase in PLA2 enzymatic activity in H. pylori-infected gastric mucosa compared with that in noninfected mucosa (Fig. 4). This is in agreement with a recent report by Pomorski et al., who found that activation of cPLA2 was responsible for the increased production of PGE2 and arachidonic acid in AGS cells exposed to H. pylori in vitro (42).
An increase in the expression of cPLA2 has been described to occur in a variety of tumors of the gastrointestinal tract (11, 22, 31, 48, 49, 55, 56). Therefore, our finding suggests that cPLA2 up-regulation may play a role in the development of H. pylori-related gastric cancer. In partial support of this hypothesis, we observed by immunohistochemistry enhanced cPLA2 positivity in metaplastic areas of patients with H. pylori-positive gastritis and in patients with gastric adenocarcinoma (data not shown). To investigate whether up-regulation of cPLA2 expression was directly related to H. pylori infection and bacterial invasiveness, we set up an in vitro model system consisting of HEp-2 cells exposed to H. pylori cell suspensions. The Hep-2 cell line has been extensively used to assess the effects of H. pylori in epithelial cells in vitro (43, 54). Using several H. pylori and other bacterial strains with a wide spectrum of invasiveness, we demonstrated cellular adhesion and invasion. However, we did not detect any change in either cPLA2 mRNA or protein levels (Fig. 5 to 6), even when longer incubation times were used. The longer incubation times frequently resulted in HEp-2 lysis and the lifting of the HEp-2 monolayers (unpublished observation). We therefore conclude that infection of Hep-2 cells by H. pylori as well as by other bacterial strains does not promote cPLA2 expression.
To exclude cell line-specific effects, we studied cPLA2 protein expression in AGS or MKN 28 gastric epithelial cells treated with a wild-type H. pylori strain and found no significant change versus what occurred in control, untreated cells. Based on these results, we postulate that systemic events specifically related to H. pylori infection, such as inflammatory reaction and host immune response, are needed for the up-regulation of cPLA2 to occur in vivo (5, 8, 10).
The increased cPLA2 activity described by Pomorski et al. in AGS cells exposed to H. pylori is not in contrast with the results of our in vitro studies. In fact, an increase in cPLA2 activity may occur through mechanisms independent of up-regulation of cPLA2 expression, e.g., in the intracellular calcium-mediated pathway (25, 28, 47).
In conclusion, our study shows that cPLA2 expression and PLA2 activity are up-regulated in patients with H. pylori gastritis. The increase in cPLA2 expression in vivo but not in vitro suggests that systemic events specifically related to H. pylori infection may play a role in this up-regulation. We postulate that up-regulation of cPLA2 might be involved in the cascade of H. pylori-related events implicated in gastric carcinogenesis.
REFERENCES
- 1.Ackermann E J, Kempner E S, Dennis E A. Ca2+-independent cytosolic phospholipase A2 from macrophage-like p388D1 cells. J Biol Chem. 1994;269:9227–9233. [PubMed] [Google Scholar]
- 2.Amandi-Burgermeister E, Tibes U, Kaiser B M, Friebe W G, Scheuer W V. Suppression of cytokine synthesis, integrin expression and chronic inflammation by inhibitors of cytosolic phospholipase A2. Eur J Pharmacol. 1997;326:237–250. doi: 10.1016/s0014-2999(97)85419-2. [DOI] [PubMed] [Google Scholar]
- 3.Barranco S C, Townsend C M, Jr, Casartelli C, Macik B G, Burger N L, Boerwinkle W R, Gourley W K. Establishment and characterization of an in vitro model system for human adenocarcinoma of the stomach. Cancer Res. 1983;43:1703–1709. [PubMed] [Google Scholar]
- 4.Berstad K, Sjodahl R, Berstad A. Phosholipase A2 activity in gastric juice from patients with active and H. pylori-eradicated healed duodenal ulcer. Aliment Pharmacol Ther. 1994;8:175–180. doi: 10.1111/j.1365-2036.1994.tb00275.x. [DOI] [PubMed] [Google Scholar]
- 5.Blaser M J. Hypothesis on the pathogenesis and natural history of Helicobacter pylori-induced inflammation. Gastroenterology. 1992;107:720–727. doi: 10.1016/0016-5085(92)90126-j. [DOI] [PubMed] [Google Scholar]
- 6.Bligh E G, Dyer W J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 7.Bomalaski J S, Fallon M, Turner R A, Crooke S T, Meunier P C, Clark M A. Identification and isolation of phospholipase A2 activating protein in human rheumatoid arthritis synovial fluid: induction of eicosanoid synthesis and an inflammatory response in joints injected in vivo. J Lab Clin Med. 1990;116:814–825. [PubMed] [Google Scholar]
- 8.Crabtree J E. Gastric mucosal inflammatory response to Helicobacter pylori. Aliment Pharmacol Ther. 1996;10:29–37. doi: 10.1046/j.1365-2036.1996.22164003.x. [DOI] [PubMed] [Google Scholar]
- 9.Davis T W, Boghaert E R, Guthridge C J, Steiner M R, Zimmer S G. The effect of group II phospholipase A2 on RAS-induced metastasis. In: Honn K V, editor. Eicosanoids and other bioactive lipids in cancer, inflammation and radiation injury. New York, N.Y: Plenum Press; 1977. pp. 9–17. [DOI] [PubMed] [Google Scholar]
- 10.D'Elios M M, Manghetti M, De Carli M, Costa F, Baldari C T, Burroni D, Telford J L, Romagnani S, Del Prete G. Th1 effector cells specific for Helicobacter pylori in the gastric antrum of patients with peptic ulcer disease. J Immunol. 1997;158:962–967. [PubMed] [Google Scholar]
- 11.Dimberg J, Samuelsson A, Hugander A, Soderkvist P. Gene expression of cyclooxygenase-2, group II and cytosolic phospholipase A2 in human colorectal cancer. Anticancer Res. 1988;18:3283–3287. [PubMed] [Google Scholar]
- 12.Dixon M F, Genta R M, Yardley J H, Correa P the participants in the International Workshop on the Histopathology of Gastritis, Houston 1994. Classification and grading of gastritis. Am J Surg Pathol. 1996;20:1161–1118. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 13.The Eurogast Study Group. An international association between Helicobacter pylori infection and gastric cancer. Lancet. 1993;341:1359–1362. [PubMed] [Google Scholar]
- 14.Field J K, Spandidos D A. The role of ras and myc oncogenes in human solid tumors and their relevance on diagnosis and prognosis. Anticancer Res. 1990;10:1–22. [PubMed] [Google Scholar]
- 15.Forman D, Newell D G, Fullerton F. Association between infection with Helicobacter pylori and risk of gastric cancer: evidence from prospective investigation. BMJ. 1991;302:1302–1305. doi: 10.1136/bmj.302.6788.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heasly L E, Thaler S, Nicks M, Price B, Skorecki K, Nemenoff R A. Induction of cytosolic phospholipase A2 by oncogenic ras in human non-small cell lung cancer. J Biol Chem. 1997;272:14501–14504. doi: 10.1074/jbc.272.23.14501. [DOI] [PubMed] [Google Scholar]
- 17.Honda S, Fujioka T, Tokieda M, Nishizono A, Nasu M. Development of Helicobacter pylori-induced gastric carcinoma in Mongolian gerbils. Cancer Res. 1998;58:4255–4259. [PubMed] [Google Scholar]
- 18.Honn K V, Bockman R S, Marnett L J. Prostaglandin and cancer: a review of tumor initiation through tumor metastasis. Prostaglandins. 1981;21:833–864. doi: 10.1016/0090-6980(81)90240-9. [DOI] [PubMed] [Google Scholar]
- 19.Hori T, Shibamoto S, Hayakawa M. Stimulation of prostaglandin production by hepatocyte growth factor in human gastric carcinoma cells. FEBS Lett. 1993;334:331–334. doi: 10.1016/0014-5793(93)80705-y. [DOI] [PubMed] [Google Scholar]
- 20.Hsu S M, Raine L, Faine L, Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase technique: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981;29:577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- 21.International Agency for Research on Cancer Working Group on the Evaluation of Carcinogenic Risks to Humans. Schistosomes, liver flukes, and Helicobacter pylori: views and expert opinions of an IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, France: IARC; 1994. Helicobacter pylori; pp. 177–240. [Google Scholar]
- 22.Kiyohara H, Egami H, Kako H, Shibata Y, Murata K, Ohshima S, Sei K, Suko S, Kurano R, Ogawa M. Immunohistochemical localization of group II phospholipase A2 in human pancreatic carcinomas. Int J Pancreatol. 1993;13:49–57. doi: 10.1007/BF02795199. [DOI] [PubMed] [Google Scholar]
- 23.Kiyohara H, Egami H, Shibata Y, Murata K, Ohshima S, Ogawa M. Light microscopy immunohistochemical analysis of the distribution of group II phospholipase A2 in human digestive organs. J Histochem Cytochem. 1992;40:1659–1664. doi: 10.1177/40.11.1431054. [DOI] [PubMed] [Google Scholar]
- 24.Kramer R M, Roberts E F, Manetta J V, Sportsman J R, Jakubowski J A. Ca2+-sensitive cytosolic phospholipase A2 (cPLA2) in human platelets. J Lipid Mediat. 1993;6:209–216. [PubMed] [Google Scholar]
- 25.Kramer R M, Sharp J D. Structure, function and regulation of Ca2+-sensitive cytosolic phopspholipase A2 (cPLA2) FEBS Lett. 1997;410:49–53. doi: 10.1016/s0014-5793(97)00322-0. [DOI] [PubMed] [Google Scholar]
- 26.Kuwata H, Nakatani Y, Murakami M, Kudo I. Cytosolic phospholipase A2 is required for cytokine-induced expression of type IIA secretory phospholipase A2 that mediates optimal cyclooxygenase-2-dependent delayed prostaglandin E2 generation in rat 3Y1 fibroblasts. J Biol Chem. 1998;273:1733–1740. doi: 10.1074/jbc.273.3.1733. [DOI] [PubMed] [Google Scholar]
- 27.Langton S R, Cesareo S D. Helicobacter pylori-associated phospholipase A2 activity: a factor in peptic ulcer production? J Clin Pathol. 1992;45:221–224. doi: 10.1136/jcp.45.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mansbach C M., II Phospholipases: old enzymes with new meaning. Gastroenterology. 1990;98:1369–1382. doi: 10.1016/0016-5085(90)90359-9. [DOI] [PubMed] [Google Scholar]
- 29.Marnett L J. Aspirin and the potential role of prostaglandins in colon cancer. Perspectives in cancer research. Cancer Res. 1992;52:5575–5589. [PubMed] [Google Scholar]
- 30.Marshall B J. Helicobacter pylori. Am J Gastroenterol. 1994;89:116–128. [PubMed] [Google Scholar]
- 31.Mukherjee A B, Miele L, Pattabiraman N. Phospholipase A2 enzymes: regulation and physiological role. Biochem Pharmacol. 1994;48:1–10. doi: 10.1016/0006-2952(94)90216-x. [DOI] [PubMed] [Google Scholar]
- 32.Murata K, Egami H, Kiyohara H, Oshima S, Kurizaki T, Ogawa M. Expression of group-II phospholipase A2 in malignant and non-malignant human gastric mucosa. Br J Cancer. 1993;68:103–111. doi: 10.1038/bjc.1993.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakano T, Ohara O, Teraoka H, Arita H. Glucocorticoids suppress group II phospholipase A2 production by blocking mRNA synthesis and post-transcriptional expression. J Biol Chem. 1990;265:12745–12748. [PubMed] [Google Scholar]
- 34.Nardone G, D'Armiento F P, Corso G, Coscione P, Budillon G. Lipids of human gastric mucosa: effect of Helicobacter pylori infection and non alcoholic cirrhosis. Gastroenterology. 1994;107:362–368. doi: 10.1016/0016-5085(94)90160-0. [DOI] [PubMed] [Google Scholar]
- 35.Nardone G, Staibano S, Rocco A, Mezza E, D'Armiento F P, Insabato L, Coppola A, Salvatore G, Lucariello A, Figura N, De Rosa G, Budillon G. Effect of Helicobacter pylori infection and its eradication on cell proliferation, DNA status, and oncogene expression in patients with chronic gastritis. Gut. 1999;44:789–799. doi: 10.1136/gut.44.6.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nevalainen T, Gronroos J M, Kallajoki M. Expression of group II phospholipase A2 in the human gastrointestinal tract. Lab Investig. 1995;72:201–208. [PubMed] [Google Scholar]
- 37.Nevalainen T J, Marki F, Kortesuo P T, Grutter M G, Di Marco S, Shmitz A. Synovial type (group II) phospholipase A2 in cartilage. J Rheumatol. 1993;20:325–330. [PubMed] [Google Scholar]
- 38.Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992;258:607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- 39.Nomura A, Stemmermann G N, Chyou P H, Kato I, Perez-Perez G, Blaser M J. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N Engl J Med. 1991;325:1132–1136. doi: 10.1056/NEJM199110173251604. [DOI] [PubMed] [Google Scholar]
- 40.Parsonnet J, Friedman G D, Oremtreich N, Vogelman H. Risk for gastric cancer in people with CagA positive or CagA negative Helicobacter pylori infection. Gut. 1997;40:297–301. doi: 10.1136/gut.40.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peterson J W, Dickey W D, Saini S S, Gourley W, Klimpel G R, Chopra A K. Phospholipase A2 activating protein and idiopathic inflammatory bowel disease. Gut. 1996;39:698–704. doi: 10.1136/gut.39.5.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pomorski T, Meyer T F, Naumann M. Helicobacter pylori-induced prostaglandin E2 synthesis involves activation of cytosolic phospholipase A2 in epithelial cells. J Biol Chem. 2001;5:804–810. doi: 10.1074/jbc.M003819200. [DOI] [PubMed] [Google Scholar]
- 43.Ricci V, Galmiche A, Doye A, Necchi V, Solcia E, Boquet P. High cell sensitivity to Helicobacter pylori VacA toxin depends on a GPI-anchored protein and is not blocked by inhibition of the clathrin-mediated pathway of endocytosis. Mol Biol Cell. 2000;11:3897–3909. doi: 10.1091/mbc.11.11.3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Romano M, Razandi M, Sekhon S, Krause W J, Ivey K J. Human cell line for the study of damage to gastric epitelial cells in vitro. J Lab Clin Med. 1988;111:430–440. [PubMed] [Google Scholar]
- 45.Romano M, Ricci V, Memoli A, Tuccillo C, Di Popolo A, Sommi P, Acquaviva A M, Del Vecchio Blanco C, Bruni C B, Zarrilli R. Helicobacter pylori up-regulates cyclooxygenase-2 mRNA expression and prostaglandin E2 synthesis in MKN 28 gastric mucosal cells in vitro. J Biol Chem. 1998;273:28560–28563. doi: 10.1074/jbc.273.44.28560. [DOI] [PubMed] [Google Scholar]
- 46.Sharp J D, White D. Cytosolic PLA2: mRNA levels and potential for transcriptional regulation. J Lipid Mediat. 1993;8:183–189. [PubMed] [Google Scholar]
- 47.Shier W T. Activation of high levels of endogenous phospholipase A2 in cultured cells. Proc Natl Acad Sci USA. 1979;76:195–199. doi: 10.1073/pnas.76.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soydan A S, Gaffen J D, Weech P K, Tremblay N M, Kargman S, O'Neil G, Bennett A, Tavares I A. Cytosolic phospholipase A2, cyclooxygenases and arachidonate in human stomach tumors. Eur J Cancer. 1997;33:1508–1512. doi: 10.1016/s0959-8049(97)00168-8. [DOI] [PubMed] [Google Scholar]
- 49.Soydan A S, Tavares I A, Weech P K, Tremblay N M, Bennett A. High molecular weight phospholipase A2 and fatty acid in human colon tumours and associated normal tissue. Eur J Cancer. 1996;32A:1781–1787. doi: 10.1016/0959-8049(96)00166-9. [DOI] [PubMed] [Google Scholar]
- 50.Tahara E. Molecular mechanism of stomach carcinogenesis. J Cancer Res Clin Oncol. 1993;119:265–272. doi: 10.1007/BF01212724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takakuwa T, Endo S, Inada K, Kasai T, Yamada Y, Ogawa M. Assessment of inflammatory cytokines, nitrate/nitrite, type II phospholipase A2, and soluble adhesion molecules in systemic inflammatory response syndrome. Res Commun Mol Pathol Pharmacol. 1997;98:43–52. [PubMed] [Google Scholar]
- 52.Vadas P, Browning J, Edelson J, Pruzanski W. Extracellular phospholipase A2 expression and inflammation: the relationship with associated disease states. J Lipid Mediat. 1993;8:1–30. [PubMed] [Google Scholar]
- 53.Watanabe T, Tada M, Nagai H, Sasaki S, Nakao M. Helicobacter pylori infection induces gastric cancer in Mongolian gerbils. Gastroenterology. 1998;115:642–648. doi: 10.1016/s0016-5085(98)70143-x. [DOI] [PubMed] [Google Scholar]
- 54.Wilkinson S M, Uhl J R, Kline B C, Cockerill F R., III Assessment of invasion frequencies of cultured HEp-2 cells by clinical isolates of Helicobacter pylori using an acridine orange assay. J Clin Pathol. 1998;51:127–133. doi: 10.1136/jcp.51.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamashita S, Ogawa M, Abe T, Yamashita J, Sakamoto K, Niwa H, Yamamura K. Group II phospholipase A2 in invasive gastric cancer cell line is induced by interleukin 6. Biochem Biophys Res Commun. 1994;198:878–884. doi: 10.1006/bbrc.1994.1125. [DOI] [PubMed] [Google Scholar]
- 56.Yamashita S, Yamashita J, Sakamoto K, Inada K, Nakashima Y, Murata K, Saishoji T, Nomura K, Ogawa M. Increased expression of membrane-associated phospholipase A shows malignant potential of human breast cancer cells. Cancer. 1993;71:3058–3064. doi: 10.1002/1097-0142(19930515)71:10<3058::aid-cncr2820711028>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 57.Zarrilli R, Ricci V, Romano M. Molecular response of gastric epithelial cells to Helicobacter pylori-induced cell damage. Cell Microbiol. 1999;1:93–99. doi: 10.1046/j.1462-5822.1999.00018.x. [DOI] [PubMed] [Google Scholar]