Abstract
The assembly of pilus colonization factor antigen III (CFA/III) of enterotoxigenic Escherichia coli (ETEC) requires the processing of CFA/III major pilin (CofA) by a prepilin peptidase (CofP), similar to other type IV pilus formation systems. CofA is produced initially as a 26.5-kDa preform pilin (prepilin) and then processed to a 20.5-kDa mature pilin by CofP which is predicted to be localized in the inner membrane. In the present experiment, we determined the nucleotide sequence of the whole region for CFA/III formation and identified a cluster of 14 genes, including cofA and cofP. Several proteins encoded by cof genes were similar to previously described proteins, such as the toxin-coregulated pili of Vibrio cholerae and the bundle-forming pili of enteropathogenic E. coli. The G+C content of the cof gene cluster was 37%, which was significantly lower than the average for the E. coli genome (50%). The introduction of a recombinant plasmid containing the cof gene cluster into the E. coli K-12 strain conferred CFA/III biogenesis and the ability of adhesion to the human colon carcinoma cell line Caco-2. This is the first report of a complete nucleotide sequence of the type IV pili found in human ETEC, and our results provide a useful model for studying the molecular mechanism of CFA/III biogenesis and the role of CFA/III in ETEC infection.
Enterotoxigenic Escherichia coli (ETEC) is a major cause of diarrhea in children and travelers in developing countries. The ability of ETEC to adhere to and colonize the intestinal epithelium is an essential step for pathogenicity in addition to the ability to produce heat-labile enterotoxin (LT) and/or heat-stable enterotoxin (ST) (23). The colonizing ability of human ETEC depends on the presence of colonization factors (CFs) on the surface of the cells, which usually form pili (or fimbriae). Several types of colonization factor antigens (CFAs) and putative colonization factors (PCFs) have been identified on the basis of antigenic specificity and/or N-terminal amino acid sequence of the major subunit (pilin), e.g., CFA/I, CFA/II, CFA/III, CFA/IV, PCFO148, PCFO159, PCFO166, antigen 2230, and antigen 8786 (7, 23). Among these, CFA/II and CFA/IV are heterogeneous and consist of a complex of different antigens named coli surface (CS) antigens. CFA/II is composed of CS1, CS2, and CS3, which are present in different permutations. Similarly, CFA/IV is composed of CS4, CS5, and CS6. Epidemiologic studies indicated that CFA/I- or CFA/II-carrying ETEC strains seem to be the most prevalent and a wide variation in CFs was found in different parts of the world (24, 27, 44). According to our survey, 8% of ETEC strains isolated from patients with travelers' diarrhea in Japan were found to carry CFA/III (12, 13).
The best-characterized pilus genes which usually consist of operons are K88 and K99 of ETEC in animals and pap pili and type 1 pili of uropathogenic E. coli (22, 33). These operons contain 8 to 11 genes encoding the proteins involved in regulation of expression, major pilin, minor pilin (adhesin), periplasmic transportor, outer membrane channel, and so on. Up to now, the operons for the biosynthesis of CFA/I, CS1, CS2, CS3, and CS6 of ETEC in humans have been sequenced and characterized (6, 14, 16, 30, 48).
We have previously isolated a 55-kb plasmid controlling the expression of CFA/III from E. coli 260-1 after it was marked with ampicillin-resistant transposon Tn3, and a 17.4-kb region of the Tn3-marked plasmid was found to be responsible for CFA/III formation (32). We also reported the nucleotide sequences of cofA and cofP encoding major pilin and prepilin peptidase, respectively, and the evidence that CofA is produced initially as a 26.5-kDa preform pilin (prepilin) and then processed to a 20.5-kDa mature pilin by cleavage between Gly-30 and Met-31 residues by CofP which is predicted to be localized in the inner membrane (39–41). The N-terminal 30-amino-acid sequence of the mature CofA is highly hydrophobic and has homology (about 70 to 75% identity) with the type IV class B pilin family such as TcpA for toxin-coregulated pili (TCP) of Vibrio cholerae, BfpA for bundle-forming pili (BFP) of enteropathogenic E. coli (EPEC), and LngA for long pilus (longus) of ETEC (9, 10, 39, 40). They are produced as precursors which are processed at a highly conserved consensus cleavage site (QXG↓F[M]T[S]LXE) located close to their N termini.
We report here the entire nucleotide sequence of the region encoding the genes for CFA/III formation and evidence that the cof gene cluster is similar to the tcp gene cluster for TCP of V. cholerae and bfp gene cluster for BFP of EPEC and demonstrate CFA/III biogenesis in the E. coli K-12 strain.
MATERIALS AND METHODS
Bacterial strains, plasmids, and bacteriophages.
E. coli strains, plasmids, and bacteriophages used in this study are listed in Table 1. E. coli 260-1 was used for the analysis and cloning of the CFA/III genes. A 55-kb plasmid controlling the expression of CFA/III was isolated by marking with the ampicillin-resistant transposon Tn3, resulting in pSH1001 (32). After construction of enzyme (ClaI)-deleted derivative plasmids, the 17.4-kb region in pSH1134 was found to be responsible for CFA/III formation on E. coli HB101 (32). pTT202 and pTT206 carry cofA (major pilin gene) and cofP (prepilin peptidase gene), respectively (40, 41). Cloning vectors pMW119 and pACYC184 belong to different compatibility groups, and they can multiply simultaneously in the same host.
TABLE 1.
Bacterial strains, plasmids, and bacteriophages used in this study
| Strain, plasmid, or bacteriophage | Descriptiona | Reference or source |
|---|---|---|
| E. coli | ||
| 260-1 | LT CFA/III | 12 |
| 31-10 | LT CFA/III | 13 |
| 31-10P | CFA/III-negative spontaneous derivative of strain 31-10; LT | This study |
| HB101 | Host for recombinant plasmids | Laboratory stock |
| JM109 | Host for recombinant M13 bacteriophages | Laboratory stock |
| Plasmid | ||
| pSH1134 | 32.5-kb plasmid containing CFA/III gene cluster and Tn3 insertion; Apr | 32 |
| pTT201 | pMW119 (AccI and BamHI sites) containing 8.6-kb ClaI1-BamHI1 fragment from pTT202; Apr | 40 |
| pTT202 | pMW119 (AccI site) containing 11.5-kb ClaI1-ClaI2 fragment from pSH1134; Apr | 32, 40 |
| pTT206 | pACYC184 (BamHI site) containing 12.4-kb BamHI1-BamHI2 fragment from pSH1134; Cmr | 32, 41 |
| pTT222 | pACYC184 (EcoRI site) containing 5.2-kb EcoRI2-EcoRI3 fragment from pTT206; Tcr | 41 |
| pTT224 | pACYC184 (SalI and BamHI sites) containing 7.6-kb SalI4-BamHI2 fragment from pTT206; Tcr | 41 |
| pTT237 | pMW119 (KpnI and AccI sites) containing 9.0-kb KpnI-ClaI2 fragment from pTT202; Apr | This study |
| pMW119 | Cloning vector; AprlacZ | Nippon Gene |
| pACYC184 | Cloning vector; Cmr Tcr | Nippon Gene |
| Bacteriophage | ||
| M13mp18 | Cloning vector; lacZ | Laboratory stock |
| M13mp19 | Cloning vector; lacZ | Laboratory stock |
Apr, Cmr, and Tcr, ampicillin, chloramphenicol, and tetracycline resistance, respectively.
Bacterial culture conditions.
E. coli strains were routinely grown in Luria-Bertani medium, supplemented with appropriate antibiotics (31). For the optimal expression of CFA/III, E. coli strains were grown on CFA agar plates at 37°C for 20 h (5). 2xYT medium was used for E. coli JM109 to propagate phages (31). Antibiotics were added at the following concentrations: ampicillin, 50 μg/ml; chloramphenicol, 25 μg/ml; and tetracycline, 15 μg/ml.
Enzymes and chemicals.
Restriction endonucleases, exonuclease III, bacterial alkaline phosphatase, T4 DNA polymerase, and T4 DNA ligase were purchased from Takara Shuzo Co., Ltd. (Kyoto, Japan). [α-32P]dCTP was obtained from Amersham Japan Co., Ltd. (Tokyo, Japan). Other chemicals were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan).
General cloning techniques.
Plasmid DNA was extracted from E. coli strains by the alkaline lysis method (31). Digestion of DNA with restriction enzymes, gel electrophoresis, ligation, and transformation were performed using standard procedures (31).
DNA sequencing.
Suitable restriction fragments were subcloned into M13mp18 and M13mp19 and then digested by exonuclease III to generate a series of nested deletions from each clone. The single-stranded DNA templates were prepared according to the standard procedure (31), and the nucleotide sequences were determined by the dideoxy-chain termination method (31) with a 7-DEAZA sequencing kit (Takara Shuzo Co., Ltd., Kyoto, Japan).
Preparation of the periplasmic extract.
E. coli strains on CFA agar plates were harvested in phosphate-buffered saline (PBS), and then the cells were collected by centrifugation at 12,000 × g for 5 min. To prepare the periplasmic extract, the cells were treated with polymyxin B (5,000 U/ml in PBS) at 37°C for 10 min and centrifuged at 12,000 × g for 5 min. The supernatant obtained was used as the periplasmic fraction.
SDS-PAGE and Western blot analysis.
Whole-cell lysates and periplasmic extracts were denatured by boiling for 5 min in a running buffer containing 2% sodium dodecyl sulfate, 1% 2-mercaptoethanol, and 50 mM Tris-HCl (pH 7.5). Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis with 12.5% acrylamide (31). The proteins in the gels were electrophoretically transferred to Immobilon-P membranes (Millipore, Bedford, Mass.) using a semidry blotting apparatus and analyzed by Western blotting (31). Membranes were blocked for 1 h in Tris-buffered saline with 0.05% Tween-20 (TBS-T) containing 5% skim milk. The blocked membranes were incubated for 1 h with a 1:1,000 dilution of rabbit antiserum against purified CFA/III (13) in TBS-T, washed with TBS-T, and incubated for 1 h with a 1:1,000 dilution of peroxidase-conjugated goat anti-rabbit immunoglobulin G (Cappel Laboratories, West Chester, Pa.) in TBS-T containing 5% skim milk. Following another wash with TBS-T, the enzyme activity was detected with the substrate of 4-chloro-1-naphthol.
CFA/III detection.
E. coli strains on CFA agar plates were harvested in PBS. A 10-μl sample of the bacterial suspension (ca. 108 bacteria/ml) was mixed with 10 μl of anti-CFA/III antiserum on a glass slide. The mixture was gently rotated for 2 min, and then bacterial agglutination was observed by the naked eye (13). Pilus formation on the cells was also observed with a transmission electron microscope after staining with 1% (wt/vol) ammonium phosphotungstate (pH 7.0) as described previously (12).
Bacterial adhesion assay.
Caco-2, a human colonic carcinoma cell line, was used. Caco-2 cells were maintained in Dulbecco modified Eagle medium (Life Technologies, Inc., Rockville, Md.) supplemented with 10% fetal calf serum (Life Technologies, Inc., Rockville, Md.) at 37°C in 5% CO2. Caco-2 cells were seeded onto the glass coverslips in six-well tissue culture plates at a concentration of about 105 cells/cm2. The cultures were used at postconfluence after 15 days of incubation, which is the condition for well-mature Caco-2 cells, as previously described (3, 45). Prior to the adhesion assay, Caco-2 cells were washed in PBS (pH 7.0). A suspension of about 106 bacteria/ml (grown on CFA agar) in the culture medium containing 1% d-mannose was prepared, 2 ml of the suspension was added to the washed Caco-2 cells, and the mixture was incubated for 3 h at 37°C in 5% CO2. The samples were washed three times with PBS (pH 7.0), fixed in methanol, stained with 10% Giemsa solution, and examined by oil immersion light microscopy to assess bacterial adherence. The adhesion indices were presented as the percentage of Caco-2 cells with at least one adhering bacterium (index 1) and the average number of bacteria/cell (index 2) by counting 10 randomly chosen fields in three separate experiments.
DNA and protein data analyses.
The analyses of nucleotide and deduced amino acid sequences were performed with GENETYX-MAC version 8.0 (Software Development Co., Ltd., Tokyo, Japan) and the multialignment FASTA program from the Genetics Computer Group (University of Wisconsin, Madison, Wis.) sequence analysis software package. Computer-assisted open reading frame (ORF) search was performed by the following criteria: an ORF would encode a polypeptide of 100 or more translated amino acids; ATG as the translational initiation codon; and an E. coli consensus ribosome-binding site (RBS), which was located at an optimal distance upstream of the ATG (29).
Nucleotide sequence accession number.
The nucleotide sequence reported here will appear in the EMBL, GenBank, and DDBJ nucleotide sequence databases under accession number AB049751.
RESULTS
Region of cof genes for CFA/III formation.
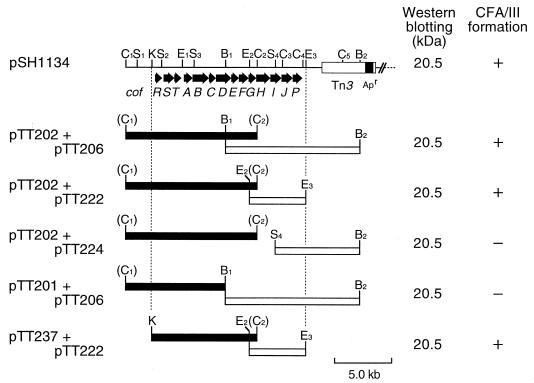
E. coli HB101 harboring both pTT202 (carrying cofA) and pTT206 (carrying cofP) was agglutinated with anti-CFA/III antiserum, and pilus formation on the cells was also observed (32). Moreover, the whole-cell extract was revealed to produce a 20.5-kDa protein (pilin) which was identical to the purified CFA/III on Western blot analysis (40). To define the minimum region responsible for CFA/III formation, we subcloned various restriction fragments of pTT202 and pTT206 into vector plasmid pMW119 and pACYC184, and a series of plasmids were introduced into E. coli HB101. As shown in Fig. 1, E. coli HB101 harboring pTT202 and pTT222 or harboring pTT237 and pTT222 produced the 20.5-kDa processed pilin, and the CFA/III formation on the cells was observed. These results suggested that the region needed for CFA/III formation was restricted to the 14-kb region between the KpnI site and the EcoRI3 site in pSH1134.
FIG. 1.
Restriction maps of pSH1134 and CFA/III clones and their expression. pSH1134, pTT202, and pTT206 were digested with the appropriate restriction endonucleases and the fragments (solid and open boxes) were cloned into pMW119 and pACYC184, respectively. The cloned genes were expressed under the control of their own promoter or promoter on the cloning vector. The proposed organization of the cof gene cluster is illustrated in the upper part of the figure. The values represent the molecular mass (in kilodaltons) on Western blot analysis with anti-CFA/III antiserum. The symbols (+ and −) on the right side show the results of CFA/III formation. + and − represent the formation and the nonformation of CFA/III, respectively. Restriction endonuclease sites: B, BamHI; C, ClaI; E, EcoRI; K, KpnI; and S, SalI.
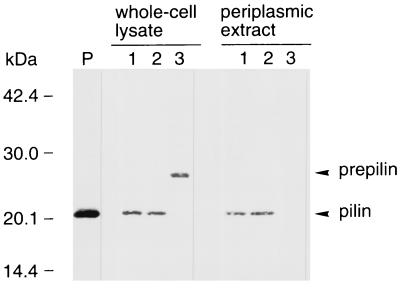
Western blot analysis of CofA.
E. coli HB101 harboring pTT202 and pTT224 or harboring pTT201 and pTT206 produced the 20.5-kDa processed pilin, but no pilus formation was observed on the cells (Fig. 1). To determine the location of the expressed antigen (pilin) in E. coli HB101, we attempted Western blot analysis of CofA. As shown in Fig. 2, a 20.5-kDa protein (pilin) was detected in the periplasm. On the other hand, whole-cell lysates of E. coli HB101 harboring only pTT202 contained the 26.5-kDa prepilin, but no cross-reacting materials (26.5- or 20.5-kDa protein) were detected in the periplasm.
FIG. 2.
Western blot analysis of E. coli HB101 whole-cell lysates (left) and periplasmic extracts (right) using anti-CFA/III antiserum. Lane P, purified CFA/III; lane 1, E. coli HB101 harboring pTT202 and pTT224; lane 2, E. coli HB101 harboring pTT201 and pTT206; lane 3, E. coli HB101 harboring pTT202. The prepilin and processed pilin bands are indicated by arrowheads. Molecular mass markers are noted on the left side.
Nucleotide sequence of cof gene cluster.
We determined the nucleotide sequence of the 14-kb KpnI-EcoRI3 region in pSH1134. The sequencing analysis revealed the presence of 14 tandemly arranged potential ORFs with the same transcriptional orientation, which may constitute an operon (Fig. 1). This gene cluster contained the previously reported cofA and cofP encoding major pilin and prepilin peptidase, respectively. The G+C content of the cof gene cluster was 37%, which was significantly lower than normally found in E. coli (50%). This low G+C content is common for virulence-associated genes of E. coli. The potential promoter sequences corresponding to the −35 (TTTACA, nucleotide positions 535 to 540) and −10 (TACTAT, nucleotide positions 558 to 563) regions were found upstream of cofR, the first gene in the cof gene cluster. These sequences have a high degree of identity to the ς70 promoter −35 (TTGACA) and −10 (TATAAT) regions for E. coli RNA polymerase (11). The spacing of 17 nucleotides between the two regions is optimal. There is no potential promoter sequence downstream of cofR. A potential stem-loop structure which acts as a transcriptional terminator was observed between cofA and cofB (nucleotide positions 3562 to 3595) with the structural free energy ΔG (25°C) of −23.3 kcal/mol (40, 43). Other CF operons also have stem-loop structures downstream of the gene encoding the major pilin (2, 15). This is considered a regulatory mechanism for overexpression of the major pilin gene relative to other genes in the operons. With the exception of cofP, all the genes were preceded by the consensus RBS (29). Although cofP lacks a consensus RBS, cofP is preceded by a nucleotide sequence (GATTA) similar to the proposed RBS of the E. coli sdaA gene (38, 41).
Properties of cof genes and deduced proteins.
The major features of the cof genes and deduced proteins are summarized in Table 2.
TABLE 2.
Features of the cof genes and deduced proteins
| Gene
|
Deduced protein
|
||||||
|---|---|---|---|---|---|---|---|
| Name | Position (start→stop) | Length (bp) | G+C content (%) | Length (amino acids) | Size (kDa) | Predicted function or location | Closest homology by FASTA (% identity, overlap [no. of amino acids]) |
| cofR | 635→935 | 300 | 33.2 | 100 | 11.7 | Regulation | Serovar Typhimurium PefB (53.8, 80); ETEC FaeB (48.1, 79) |
| cofS | 1228→2077 | 849 | 39.2 | 283 | 32.0 | Regulation | ETEC FapR (25.0, 260); ETEC CfaD (24.6, 264) |
| cofT | 2092→2533 | 441 | 43.8 | 147 | 16.7 | Unknown | Serovar Typhi IagB (41.2, 136); EPEC BfpH (31.2, 138) |
| cofA | 2836→3550 | 714 | 42.3 | 238 | 25.3 | Type IV pilin | ETEC LngA (78.2, 238); V. cholerae TcpA (34.7, 225) |
| cofB | 3613→5182 | 1,569 | 36.7 | 523 | 57.1 | Type IV pilin-like protein | V. cholerae TcpB (22.1, 77) |
| cofC | 5201→5612 | 411 | 34.2 | 137 | 15.7 | Unknown | EPEC BfpG (26.9, 134); V. cholerae TcpQ (24.3, 70) |
| cofD | 5632→7087 | 1,455 | 35.4 | 485 | 54.2 | Outer membrane lipoprotein | EPEC BfpB (24.3, 437); V. cholerae TcpC (22.5, 485) |
| cofE | 7091→7649 | 558 | 36.0 | 186 | 21.8 | Inner membrane | None |
| cofF | 7653→8478 | 825 | 32.8 | 275 | 31.2 | Inner membrane | V. cholerae TcpD (24.4, 270) |
| cofG | 8468→8951 | 483 | 39.2 | 161 | 17.7 | Unknown | None |
| cofH | 9151→10483 | 1,332 | 39.0 | 444 | 50.0 | Inner membrane nucleotide-binding protein | V. cholerae TcpT (44.2, 441); EPEC BfpD (26.9, 431) |
| cofI | 10469→11492 | 1,023 | 36.6 | 341 | 37.8 | Inner membrane | V. cholerae TcpE (38.0, 337); EPEC BfpE (23.6, 343) |
| cofJ | 11508→12552 | 1,044 | 35.4 | 348 | 39.6 | Unknown | None |
| cofP | 12557→13376 | 819 | 35.5 | 273 | 30.5 | Prepilin peptidase | V. cholerae TcpJ (35.7, 266); EPEC BfpP (31.7, 262) |
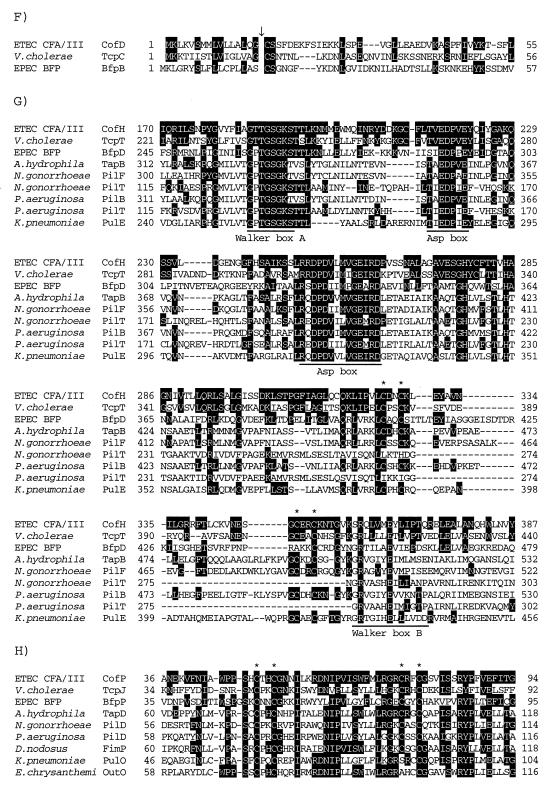
cofR encodes a 100-amino-acid protein (11,739 Da) lacking a signal peptide. The deduced amino acid sequence of CofR is homologous with several bacterial proteins such as PefB (53.8% identity) of Salmonella enterica serovar Typhimurium, FaeB (48.1% identity) for K88 of animal ETEC, AfaA (46.8% identity) for afimbrial adhesin (AFA-III) of uropathogenic and diarrhea-associated E. coli, and PapB (46.3% identity) for P pili of uropathogenic E. coli which have been reported as positive regulators concerning the biogenesis of the pili (Fig. 3A).
FIG. 3.
Partial alignment of the deduced amino acid sequences of the Cof proteins with those of known proteins. Identical amino acids are indicated by a black background. Gaps introduced for alignment are represented by dashes. (A) Alignment of the deduced amino acid sequences of CofR, Salmonella serovar Typhimurium PefB (GenBank accession no. L08613), ETEC FaeB (Z11709), E. coli AfaA (X76688), and E. coli PapB (X03391). (B) Alignment of the deduced amino acid sequences of CofS, ETEC FapR (X53494), ETEC CfaD (M55609), ETEC Rns (J04166), V. cholerae ToxT (X64098), EPEC PerA (Z48561), enteroaggregative E. coli (EAggEC) AggR (Z32523), S. dysenteriae VirF (X58464), and serovar Typhimurium SirC (AF134856) in the region containing the DNA-binding domain (underline). (C) Alignment of the deduced amino acid sequences of CofT, E. coli Gene X (X07264), Salmonella serovar Typhi IagB (X80892), S. flexneri IpgF (L04309), EPEC BfpH (Z68186), and E. coli Slt (M69185). Three motifs conserved in putative lytic transglycosylases are underlined. (D) Alignment of the deduced amino acid sequences of CofA, ETEC LngA (AF004308), V. cholerae TcpA (X64098), EPEC BfpA (Z68186), A. hydrophila TapA (U20255), N. gonorrhoeae PilE (X66144), P. aeruginosa PilA (M14849), Dichelobacter nodosus FimA (X52405), and Moraxella bovis TfpQ (M59712) in the N-terminal region. The cleavage site of type IV pilins is shown by a downward arrow. The conserved glycine, leucine, and glutamic acid residues are marked by asterisks. (E) Alignment of the deduced amino acid sequences of CofB, ETEC LngB, and V. cholerae TcpB (X64098) in the N-terminal region. The type IV pilin-like cleavage site is shown by a downward arrow. The conserved glycine, leucine, and glutamic acid residues are marked by asterisks. (F) Alignment of the deduced amino acid sequences of CofD, V. cholerae TcpC (X64098), and EPEC BfpB (Z68186) in the N-terminal region. The lipoprotein-cleavage site is shown by a downward arrow. (G) Alignment of the deduced amino acid sequences of CofH, V. cholerae TcpT (X64098), EPEC BfpD (Z68186), A. hydrophila TapB (U20255), N. gonorrhoeae PilF (U32588), N. gonorrhoeae PilT (S72391), P. aeruginosa PilB (M32066), P. aeruginosa PilT (M55524), and K. pneumoniae PulE (M32613) in the region containing the nucleotide-binding domain. The Walker box A, Asp boxes, and Walker box B are underlined. The conserved CXXC motifs are marked by asterisks. (H) Alignment of the deduced amino acid sequences of CofP, V. cholerae TcpJ (M74708), EPEC BfpP (Z68186), A. hydrophila TapD (U20255), N. gonorrhoeae PilD (U32588), P. aeruginosa PilD (M32066), D. nodosus FimP (U17138), K. pneumoniae PulO (M32613), and Erwinia chrysanthemi OutO (L02214) in the region containing the conserved CXXC motifs (asterisks).
cofS encodes a 283-amino-acid protein (32,049 Da) lacking a signal peptide. The deduced amino acid sequence of CofS has homology with a number of bacterial transcriptional regulators belonging to the family of proteins represented by AraC, the regulator of the E. coli and serovar Typhimurium arabinose operons (8), e.g., FapR (25.0% identity) for 987P of animal ETEC, CfaD (24.6% identity) for CFA/I, Rns (23.7% identity) for CS1 and CS2 of CFA/II, and ToxT (20.1% identity) for TCP of V. cholerae. All members of the AraC family are most homologous in the C terminal region of the sequence. This region contains a helix-turn-helix motif associated with the DNA-binding activity (Fig. 3B).
cofT encodes a 147-amino-acid protein (16,717 Da). CofT has a potential signal peptide of 20 amino acids which is the most hydrophobic region of the protein. Consequently, because the mature CofT is markedly hydrophilic, it may be in the periplasm or exported out of the cell. The deduced amino acid sequence of CofT has homology with the product of gene X (51.4% identity) for the conjugative transfer of the IncF plasmids (F, R1, and R100) of E. coli, IagB (41.2% identity) of Salmonella enterica serovar Typhi, IpgF (35.2% identity) of Shigella flexneri, BfpH (31.2% identity) for BFP of EPEC, and Slt (31.5% identity) of E. coli (Fig. 3C). In serovar Typhi and S. flexneri, IagB and IpgF are involved in the invasion of the eukaryotic host cells by the bacterial cells (1, 21). The E. coli slt gene encodes a 70-kDa soluble lytic transglycosylase (4). X-ray crystallography revealed that the C-terminal region of Slt has a three-dimensional structure similar to those of hen egg-white lysozyme and bacteriophage T4 lysozyme, associated with a peptidoglycan-lytic activity (42).
cofA encodes a 238-amino-acid protein (25,309 Da) which is the major pilin belonging to the type IV class B pilin family as reported previously (39, 40). The signal peptide of CofA is 30 amino acids long. The N-terminal 30-amino-acid sequence of the mature CofA is the most conserved and hydrophobic region of the protein (Fig. 3D).
cofB encodes a 523-amino-acid protein (57,089 Da). The N-terminal amino acid sequence of CofB is similar to that of type IV pilin. The completely conserved glycine, leucine, and glutamic acid residues in the N terminus of type IV prepilins appear at the 5th, 8th, and 10th amino acids of CofB, respectively. It is likely that the Gly-5–Phe-6 junction of CofB is cleaved by the type IV prepilin peptidase (CofP). The deduced amino acid sequence of CofB has homology with LngB for Longus of ETEC (9) and TcpB (22.1% identity) for TCP of V. cholerae (Fig. 3E).
cofC encodes a 137-amino-acid protein (15,658 Da) containing a typical signal peptide of 25 amino acids. The mature CofC is markedly hydrophilic, suggesting that it may be localized in the periplasm or exported out of the cell. The deduced amino acid sequence of CofC is homologous with BfpG (26.9% identity) for BFP and TcpQ (24.3% identity) for TCP.
cofD encodes 485-amino-acid protein (54,236 Da). Analysis of the deduced amino acid sequence of CofD revealed that the N-terminal sequence conforms to the lipoprotein signal peptidase recognition site, including the presence of the essential cysteine residue to which a glyceride fatty acid lipid would be attached (Fig. 3F). CofD is homologous with BfpB (24.3% identity) for BFP and TcpC (22.5% identity) for TCP. The BfpB and TcpC have been reported as outer membrane lipoproteins related to the biogenesis of BFP and TCP, respectively (25, 28).
cofE encodes a 186-amino-acid protein (21,773 Da). CofE has a markedly hydrophobic C terminus (amino acid positions 159 to 186) which may function as a potential membrane-anchoring domain. No known protein containing an amino acid sequence with significant similarity to that of CofE was found in the GenBank database.
cofF encodes a 175-amino-acid protein (31,188 Da). CofF has a markedly hydrophobic region (amino acid positions 21 to 42) which may be a membrane-spanning domain. The deduced amino acid sequence of CofF is homologous with TcpD (24.4% identity) for TCP of V. cholerae.
cofG encodes a 161-amino-acid protein (17,681 Da). CofG has a typical signal peptide of 25 amino acids. Consequently, because the mature CofG is markedly hydrophilic, it may be in the periplasm or exported out of the cell. No known protein similar to the CofG could be found in the GenBank database.
cofH encodes a 444-amino-acid protein (50,009 Da) lacking a signal peptide. The deduced amino acid sequence of CofH is homologous with several bacterial proteins such as TcpT (44.2% identity) for TCP, BfpD (26.9% identity) for BFP, TapB (28.3% identity) of Aeromonas hydrophila, PilF (27.6% identity) of Neisseria gonorrhoeae, PilB (28.0% identity) of Pseudomonas aeruginosa, and PulE (27.6% identity) of Klebsiella pneumoniae, which are related to the biogenesis of the type IV pili and extracellular protein secretion (26). These proteins carry conserved Walker boxes A and B, Asp boxes, and two pairs of cysteine residues (CXXC motifs) associated with a nucleotide-binding activity (47). They may act in the energy fueling steps of the biogenesis of the type VI pili and the protein secretion system (Fig. 3G).
cofI encodes a 341-amino-acid protein (37,847 Da) lacking a signal peptide. CofI may be an integral cytoplasmic membrane protein since it has three putative transmembrane domains (amino acid positions 108 to 131, 164 to 184, and 316 to 332). The deduced amino acid sequence of CofI is homologous with several bacterial proteins related to the biogenesis of the type IV pili including TcpE (38.0% identity) for TCP, BfpE (23.6% identity) for BFP, and PilC (22.3% identity) of P. aeruginosa.
cofJ encodes a 348-amino-acid protein (39,579 Da). The N terminus of CofJ conforms to a typical signal peptide of 22 amino acids. No known protein similar to the CofJ could be found in the GenBank database.
cofP encodes a 273-amino-acid protein (30,533 Da) which is the type IV prepilin peptidase of the CFA/III as reported previously (41). The type IV prepilin peptidases, including CofP, are homologous over the entire amino acid sequence. Notably, two pairs of cysteine residues (CXXC motifs) that have been shown to be required for the enzymatic activity of P. aeruginosa PilD (36, 37) are present in all type IV prepilin peptidases (Fig. 3H).
Expression of cof gene cluster in E. coli HB101 and adhesive function of CFA/III.
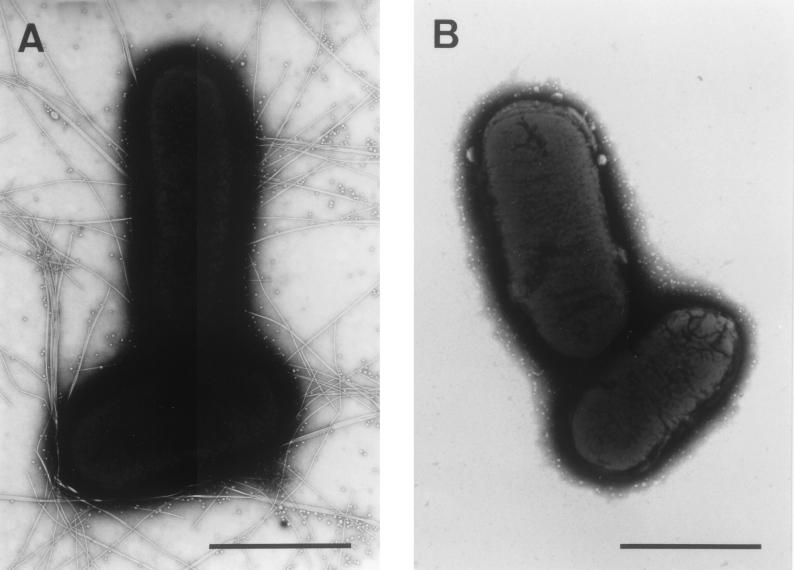
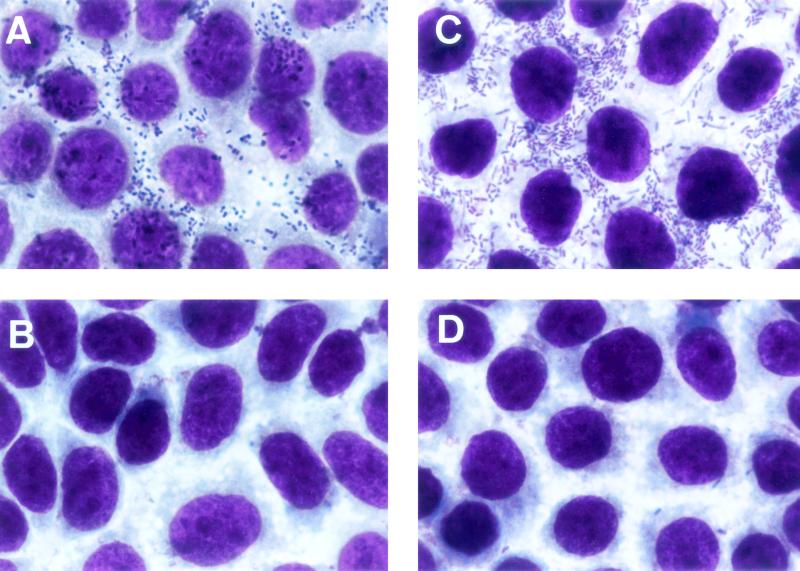
To examine whether the 14-kb KpnI-EcoRI3 region contains all of the information needed for the biogenesis of the functional CFA/III, E. coli HB101 harboring pTT237 and pTT222 was observed by electron microscopy and tested for the ability to adhere to the Caco-2 cells, an established cell culture model for ETEC colonization. As shown in Fig. 4, E. coli HB101 harboring pTT237 and pTT222 produced long rod-like pili with a diameter of 7 nm, but E. coli HB101 harboring pMW119 and pACYC184 did not produce pili as expected. The ability of E. coli strains to adhere to the Caco-2 cells is shown in Fig. 5. The wild-type strain (E. coli 31-10) and E. coli HB101 harboring pTT237 and pTT222 adhered to the Caco-2 cells with indices (index 1) of 84.8 and 89.4% and with the average numbers of bacteria/cell (index 2) of 22.8 and 54.6, respectively. On the other hand, E. coli 31-10P and E. coli HB101 harboring pMW119 and pACYC184 showed no adherence to the Caco-2 cells with indices (index 1) of 5.6 and 4.8% and with the average numbers of bacteria/cell (index 2) of 0.07 and 0.08, respectively. These results suggest that the sequenced region contains all information required for the formation of a functional CFA/III on the surface of E. coli HB101.
FIG. 4.
Electron micrographs of E. coli HB101 after growth on CFA agar plates at 37°C for 20 h. (A) E. coli HB101 harboring pTT237 and pTT222. (B) E. coli HB101 harboring pMW119 and pACYC184. Bar, 1 μm.
FIG. 5.
Micrographs showing adhesion of E. coli strains to Caco-2 cells. (A) ETEC 31-10 (CFA/III-positive strain). (B) ETEC 31-10P (CFA/III-negative strain). (C) E. coli HB101 harboring pTT237 and pTT222. (D) E. coli HB101 harboring pMW119 and pACYC184.
DISCUSSION
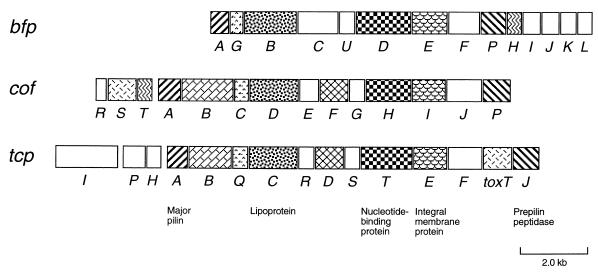
We report here the nucleotide sequence of the minimal region (14-kb KpnI-EcoRI3 region) for CFA/III formation of ETEC. This region contains 14 cof genes which are thought to constitute an operon. Several proteins encoded by the cof genes are homologous with the proteins involved in the BFP biogenesis of EPEC and the TCP biogenesis of V. cholerae (19, 34, 35). The gene organization of the cof genes was compared to those of the bfp and tcp operons (Fig. 6). Both the bfp and the tcp operons are also comprised of 14 genes. The organizations of these gene clusters have some similarity to each other. Especially, the relative positions of the cofA, cofB, cofC, cofD, cofF, cofH, cofI, and cofP genes are conserved in both cof and tcp gene clusters. The major pilin genes (cofA and tcpA) are located in the upstream regions, and the prepilin peptidase genes (cofP and tcpJ) are located at the last positions of these gene clusters. The conservation of gene organizations and the similarity of amino acid sequences suggest that CFA/III and TCP biogenesis systems have evolved from a common ancestral gene system.
FIG. 6.
Genetic organizations of cof, bfp, and tcp gene clusters. All genes except tcpI are transcribed rightward. The homologous genes are indicated by the same shading patterns. Predicted similar functions are indicated in the bottom of the figure.
Pilus operons are generally shown to encode one or two positive regulatory proteins (local regulators) (7, 22). The cof gene cluster also contains two genes (cofR and cofS) encoding regulatory proteins, which are located upstream of the major pilin gene (cofA). These gene products probably act as transcriptional activators of the cof gene cluster, but their precise modes of action are not yet known.
Many virulence gene clusters appear to have been imported as a unit into bacteria that may not have previously been pathogenic (17, 23). This is deduced from their unusual G+C content and/or the presence of insertion sequence flanking them. The G+C content of the cof gene cluster is 37%, which is significantly lower than the average for E. coli (50%). A region homologous with part of the sequence of the transposable element IS630 (20) is observed downstream of the cof gene cluster (nucleotide positions 13587 to 13651). Recent studies (17, 18, 46) of V. cholerae have shown that the tcp gene cluster is located on a Vibrio pathogenicity island which includes the genes of lysogenic filamentous phage (VPIΦ), and TCP functions as a receptor for cholera toxin phage (CTXΦ). This information suggests the interesting possibilities that the cof gene cluster might have been transferred into E. coli via phage(s) or plasmid(s) from another unknown organism and that CFA/III might function as a receptor for unknown phage(s).
In our earlier report (41), we found a close relation between the processing of prepilin and CFA/III pilus formation. However, E. coli HB101 harboring pTT202 and pTT224 or harboring pTT201 and pTT206 produced 20.5-kDa processed pilin in the periplasm, but no pilus formation was observed on the cells. The gene lacking in these plasmids may be required for the pilus formation on the cells. The cofD lacking in pTT201 and pTT206 is homologous with tcpC and bfpB encoding outer membrane lipoproteins for TCP and BFP biogenesis, respectively (25, 28). The protein products of tcpC and bfpB are required for each pilus formation. The genes lacking in pTT202 and pTT224 are cofH and cofI. The CofH and CofI are homologous with the nucleotide-binding proteins and the integral membrane proteins, respectively, related to other type IV pilus biogenesis. Although further study is needed, these cof gene products may have an important role for the pilus formation, probably via lack of the basal apparatus of the pili. We also found that CFA/III itself possessed adhesive function on human colonic epithelial cells. This is in agreement with the previous findings in the suckling mice experiment (12). CFA/III is a complex extracellular organelle involved with several proteins such as minor pilin (adhesin), periplasmic transporter, outer membrane channel, and regulatory protein and is characterized as gene clusters similar to other CFs and type IV pili. Therefore, further studies on the functions of cof gene products are in progress in our laboratory. This knowledge should help in the development of an ideal pilus vaccine against ETEC diarrhea.
ACKNOWLEDGMENTS
We thank Hideo Shinagawa (Department of Molecular Microbiology, Research Institute for Microbial Diseases, Osaka University) for his helpful discussions. We also thank Roy H. Doi (Section of Molecular and Cellular Biology, University of California, Davis) for critical reading of the manuscript.
This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (grant no. 11770144 to T.T.), a Grant for International Health Cooperation Research from the Ministry of Health, Labor, and Welfare of Japan, and the “Research for the Future” Program of the Japan Society for the Promotion of Sciences (grant no. JSPS-RFTF 97L00704 to T.H.).
REFERENCES
- 1.Allaoui A, Ménard R, Sansonetti P J, Parsot C. Characterization of the Shigella flexneri ipgD and ipgF genes, which are located in the proximal part of the mxi locus. Infect Immun. 1993;61:1707–1714. doi: 10.1128/iai.61.5.1707-1714.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Båga M, Göransson M, Normark S, Uhlin B E. Transcriptional activation of a Pap pilus virulence operon from uropathogenic Escherichia coli. EMBO J. 1985;4:3887–3893. doi: 10.1002/j.1460-2075.1985.tb04162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Darfeulle-Michaud A, Aubel D, Chauviere G, Rich C, Bourges M, Servin A, Joly B. Adhesion of enterotoxigenic Escherichia coli to the human colon carcinoma cell line Caco-2 in culture. Infect Immun. 1990;58:893–902. doi: 10.1128/iai.58.4.893-902.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dijkstra A J, Keck W. Peptidoglycan as a barrier to transenvelope transport. J Bacteriol. 1996;178:5555–5562. doi: 10.1128/jb.178.19.5555-5562.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evans D G, Evans D J, Jr, Tjoa W. Hemagglutination of human group A erythrocytes by enterotoxigenic Escherichia coli isolated from adults with diarrhea: correlation with colonization factor. Infect Immun. 1977;18:330–337. doi: 10.1128/iai.18.2.330-337.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Froehlich B J, Karakashian A, Sakellaris H, Scott J R. Genes for CS2 pili of enterotoxigenic Escherichia coli and their interchangeability with those for CS1 pili. Infect Immun. 1995;63:4849–4856. doi: 10.1128/iai.63.12.4849-4856.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaastra W, Svennerholm A-M. Colonization factors of human enterotoxigenic Escherichia coli (ETEC) Trends Microbiol. 1996;4:444–452. doi: 10.1016/0966-842x(96)10068-8. [DOI] [PubMed] [Google Scholar]
- 8.Gallegos M-T, Michán C, Ramos J L. The XylS/AraC family of regulators. Nucleic Acids Res. 1993;21:807–810. doi: 10.1093/nar/21.4.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Girón J A, Gómez-Duarte O G, Jarvis K G, Kaper J B. Longus pilus of enterotoxigenic Escherichia coli and its relatedness to other type-4 pili. Gene. 1997;192:39–43. doi: 10.1016/s0378-1119(97)00039-5. [DOI] [PubMed] [Google Scholar]
- 10.Gómez-Duarte O G, Ruiz-Tagle A, Gómez D C, Viboud G I, Jarvis K G, Kaper J B, Girón J A. Identification of lngA, the structural gene of longus type IV pilus of enterotoxigenic Escherichia coli. Microbiology. 1999;145:1809–1816. doi: 10.1099/13500872-145-7-1809. [DOI] [PubMed] [Google Scholar]
- 11.Harley C B, Reynolds R P. Analysis of E. coli promoter sequences. Nucleic Acids Res. 1987;15:2343–2361. doi: 10.1093/nar/15.5.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Honda T, Arita M, Miwatani T. Characterization of new hydrophobic pili of human enterotoxigenic Escherichia coli: a possible new colonization factor. Infect Immun. 1984;43:959–965. doi: 10.1128/iai.43.3.959-965.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Honda T, Wetprasit N, Arita M, Miwatani T. Production and characterization of monoclonal antibodies to a pilus colonization factor (colonization factor antigen III) of human enterotoxigenic Escherichia coli. Infect Immun. 1989;57:3452–3457. doi: 10.1128/iai.57.11.3452-3457.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jalajakumari M B, Thomas C J, Halter R, Manning P A. Genes for biosynthesis and assembly of CS3 pili of CFA/II enterotoxigenic Escherichia coli: novel regulation of pilus production by bypassing an amber codon. Mol Microbiol. 1989;3:1685–1695. doi: 10.1111/j.1365-2958.1989.tb00154.x. [DOI] [PubMed] [Google Scholar]
- 15.Jordi B J A M, op den Camp I E L, de Haan L A M, van der Zeijst B A M, Gaastra W. Differential decay of RNA of the CFA/I fimbrial operon and control of relative gene expression. J Bacteriol. 1993;175:7976–7981. doi: 10.1128/jb.175.24.7976-7981.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jordi B J A M, Willshaw G A, van der Zeijst B A M, Gaastra W. The complete nucleotide sequence of region 1 of the CFA/I fimbrial operon of human enterotoxigenic Escherichia coli. DNA Sequence. 1992;2:257–263. doi: 10.3109/10425179209020811. [DOI] [PubMed] [Google Scholar]
- 17.Karaolis D K R, Johnson J A, Bailey C C, Boedeker E C, Kaper J B, Reeves P R. A Vibrio cholerae pathogenicity island associated with epidemic and pandemic strains. Proc Natl Acad Sci USA. 1998;95:3134–3139. doi: 10.1073/pnas.95.6.3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karaolis D K R, Somara S, Maneval D R, Jr, Johnson J A, Kaper J B. A bacteriophage encoding a pathogenicity island, a type-IV pilus and a phage receptor in cholera bacteria. Nature. 1999;399:375–379. doi: 10.1038/20715. [DOI] [PubMed] [Google Scholar]
- 19.Manning P A. The tcp gene cluster of Vibrio cholerae. Gene. 1997;192:63–70. doi: 10.1016/s0378-1119(97)00036-x. [DOI] [PubMed] [Google Scholar]
- 20.Matsutani S, Ohtsubo H, Maeda Y, Ohtsubo E. Isolation and characterization of IS elements repeated in the bacterial chromosome. J Mol Biol. 1987;196:445–455. doi: 10.1016/0022-2836(87)90023-4. [DOI] [PubMed] [Google Scholar]
- 21.Miras I, Hermant D, Arricau N, Popoff M Y. Nucleotide sequence of iagA and iagB genes involved in invasion of HeLa cells by Salmonella enterica subsp. enterica ser. Typhi Res Microbiol. 1995;146:17–20. doi: 10.1016/0923-2508(96)80267-1. [DOI] [PubMed] [Google Scholar]
- 22.Mol O, Oudega B. Molecular and structural aspects of fimbriae biosynthesis and assembly in Escherichia coli. FEMS Microbiol Rev. 1996;19:25–52. doi: 10.1111/j.1574-6976.1996.tb00252.x. [DOI] [PubMed] [Google Scholar]
- 23.Nataro J P, Kaper J B. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998;11:142–201. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nirdnoy W, Serichantalergs O, Cravioto A, Lebron C, Wolf M, Hoge C W, Svennerholm A-M, Taylor D N, Echeverria P. Distribution of colorization factor antigens among enterotoxigenic Escherichia coli strains isolated from patients with diarrhea in Nepal, Indonesia, Peru, and Thailand. J Clin Microbiol. 1997;35:527–530. doi: 10.1128/jcm.35.2.527-530.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogierman M A, Manning P A. TCP pilus biosynthesis in Vibrio cholerae O1: gene sequence of tcpC encoding an outer membrane lipoprotein. FEMS Microbiol Lett. 1992;97:179–184. doi: 10.1016/0378-1097(92)90383-y. [DOI] [PubMed] [Google Scholar]
- 26.Pugsley A P. The complete general secretory pathway in gram-negative bacteria. Microbiol Rev. 1993;57:50–108. doi: 10.1128/mr.57.1.50-108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qadri F, Das S K, Faruque A S G, Fuchs G J, Albert M J, Sack R B, Svennerholm A-M. Prevalence of toxin types and colonization factors in enterotoxigenic Escherichia coli isolated during a 2-year period from diarrheal patients in Bangladesh. J Clin Microbiol. 2000;38:27–31. doi: 10.1128/jcm.38.1.27-31.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramer S W, Bieber D, Schoolnik G K. BfpB, an outer membrane lipoprotein required for the biogenesis of bundle-forming pili in enteropathogenic Escherichia coli. J Bacteriol. 1996;178:6555–6563. doi: 10.1128/jb.178.22.6555-6563.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rudd K E, Schneider T D. Compilation of E. coli ribosome binding sites. In: Miller J H, editor. A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related organisms. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. pp. 17.19–17.45. [Google Scholar]
- 30.Sakellaris H, Scott J R. New tools in an old trade: CS1 pilus morphogenesis. Mol Microbiol. 1998;30:681–687. doi: 10.1046/j.1365-2958.1998.01088.x. [DOI] [PubMed] [Google Scholar]
- 31.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 32.Shinagawa H, Taniguchi T, Yamaguchi O, Yamamoto K, Honda T. Cloning of the genes that control formation of the fimbrial colonization factor antigen III (CFA/III) from an enterotoxigenic Escherichia coli. Microbiol Immunol. 1993;37:689–694. doi: 10.1111/j.1348-0421.1993.tb01693.x. [DOI] [PubMed] [Google Scholar]
- 33.Smyth C J, Marron M B, Twohig J M G J, Smith S G J. Fimbrial adhesins: similarities and variations in structure and biogenesis. FEMS Immunol Med Microbiol. 1996;16:127–139. doi: 10.1111/j.1574-695X.1996.tb00129.x. [DOI] [PubMed] [Google Scholar]
- 34.Sohel I, Puente J L, Ramer S W, Bieber D, Wu C-Y, Schoolnik G K. Enteropathogenic Escherichia coli: identification of a gene cluster coding for bundle-forming pilus morphogenesis. J Bacteriol. 1996;178:2613–2628. doi: 10.1128/jb.178.9.2613-2628.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stone K D, Zhang H-Z, Carlson L K, Donnenberg M S. A cluster of fourteen genes from enteropathogenic Escherichia coli is sufficient for the biogenesis of a type IV pilus. Mol Microbiol. 1996;20:325–337. doi: 10.1111/j.1365-2958.1996.tb02620.x. [DOI] [PubMed] [Google Scholar]
- 36.Strom M S, Bergman P, Lory S. Identification of active-site cysteines in the conserved domain of PilD, the bifunctional type IV pilin leader peptidase/N-methyltransferase of Pseudomonas aeruginosa. J Biol Chem. 1993;268:15788–15794. [PubMed] [Google Scholar]
- 37.Strom M S, Nunn D N, Lory S. A single bifunctional enzyme, PilD, catalyzes cleavage and N-methylation of proteins belonging to the type IV pilin family. Proc Natl Acad Sci USA. 1993;90:2404–2408. doi: 10.1073/pnas.90.6.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Su H, Lang B F, Newman E B. l-Serine degradation in Escherichia coli K-12: cloning and sequencing of the sdaA gene. J Bacteriol. 1989;171:5095–5102. doi: 10.1128/jb.171.9.5095-5102.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taniguchi T, Arita M, Sato M, Yamamoto K, Miwatani T, Honda T. Evidence that the N-terminal amino acid sequence of pilus colonization factor antigen III produced by human enterotoxigenic Escherichia coli is similar to that of TcpA pilin of Vibrio cholerae. J Infect Dis. 1994;170:1049–1050. doi: 10.1093/infdis/170.4.1049. [DOI] [PubMed] [Google Scholar]
- 40.Taniguchi T, Fujino Y, Yamamoto K, Miwatani T, Honda T. Sequencing of the gene encoding the major pilin of pilus colonization factor antigen III (CFA/III) of human enterotoxigenic Escherichia coli and evidence that CFA/III is related to type IV pili. Infect Immun. 1995;63:724–728. doi: 10.1128/iai.63.2.724-728.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taniguchi T, Yasuda Y, Tochikubo K, Yamamoto K, Honda T. The gene encoding the prepilin peptidase involved in biosynthesis of pilus colonization factor antigen III (CFA/III) of human enterotoxigenic Escherichia coli. Microbiol Immunol. 1999;43:853–861. doi: 10.1111/j.1348-0421.1999.tb01220.x. [DOI] [PubMed] [Google Scholar]
- 42.Thunnissen A-M W H, Dijkstra A J, Kalk K H, Rozeboom H J, Engel H, Keck W, Dijkstra B W. Doughnut-shaped structure of a bacterial muramidase revealed by X-ray crystallography. Nature. 1994;367:750–753. doi: 10.1038/367750a0. [DOI] [PubMed] [Google Scholar]
- 43.Tinoco I, Jr, Borer P N, Dengler B, Levine M D, Uhlenbeck O C, Crothers D M, Gralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973;246:40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- 44.Viboud G I, Jouve M J, Binsztein N, Vergara M, Rivas M, Quiroga M, Svennerholm A-M. Prospective cohort study of enterotoxigenic Escherichia coli infections in Argentinean children. J Clin Microbiol. 1999;37:2829–2833. doi: 10.1128/jcm.37.9.2829-2833.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Viboud G I, McConnell M M, Helander A, Svennerholm A-M. Binding enterotoxigenic Escherichia coli expressing different colonization factors to tissue-cultured Caco-2 cells and to isolated human enterocytes. Microb Pathog. 1996;21:139–147. doi: 10.1006/mpat.1996.0049. [DOI] [PubMed] [Google Scholar]
- 46.Walder M K, Mekalanos J J. Lysogenic conversion by a filamentous phage encoding cholera toxin. Science. 1996;272:1910–1914. doi: 10.1126/science.272.5270.1910. [DOI] [PubMed] [Google Scholar]
- 47.Walker J E, Saraste M, Runswick M J, Gay N J. Distantly related sequences in the α- and β-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;8:945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wolf M K, de Haan L A M, Cassels F J, Willshaw G A, Warren R, Boedeker E C, Gaastra W. The CS6 colonization factor of human enterotoxigenic Escherichia coli contains two heterologous major subunits. FEMS Microbiol Lett. 1997;148:35–42. doi: 10.1111/j.1574-6968.1997.tb10263.x. [DOI] [PubMed] [Google Scholar]