Abstract
Coronavirus disease 2019 (COVID-19) is reported to have long-term effects on cardiovascular health and physical functioning, even in the nonhospitalized population. The physiological mechanisms underlying these long-term consequences are however less well described. We compared cardiovascular risk factors, arterial stiffness, and physical functioning in nonhospitalized patients with COVID-19, at a median of 6 mo postinfection, versus age- and sex-matched controls. Cardiovascular risk was assessed using blood pressure and biomarker concentrations (amino-terminal pro-B-type-natriuretic-peptide, high-sensitive cardiac troponin I, C-reactive protein), and arterial stiffness was assessed using carotid-femoral pulse wave velocity. Physical functioning was evaluated using accelerometry, handgrip strength, gait speed and questionnaires on fatigue, perceived general health status, and health-related quality of life (hrQoL). We included 101 former patients with COVID-19 (aged 59 [interquartile range, 55–65] yr, 58% male) and 101 controls. At 175 [126–235] days postinfection, 32% of the COVID-19 group reported residual symptoms, notably fatigue, and 7% required post-COVID-19 care. We found no differences in blood pressure, biomarker concentrations, or arterial stiffness between both groups. Former patients with COVID-19 showed a higher handgrip strength (43 [33–52] vs. 38 [30–48] kg, P = 0.004) and less sleeping time (8.8 [7.7–9.4] vs. 9.8 [8.9–10.3] h/day, P < 0.001) and reported fatigue more often than controls. Accelerometry-based habitual physical activity levels, gait speed, perception of general health status, and hrQoL were not different between groups. In conclusion, one in three nonhospitalized patients with COVID-19 reports residual symptoms at a median of 6 mo postinfection, but we were unable to relate these symptoms to increases in cardiovascular risk factors, arterial stiffness, or physical dysfunction.
NEW & NOTEWORTHY We examined cardiovascular and physical functioning outcomes in nonhospitalized patients with COVID-19, at a median of 6 mo postinfection. When compared with matched controls, minor differences in physical functioning were found, but objective measures of cardiovascular risk and arterial stiffness did not differ between groups. However, one in three former patients with COVID-19 reported residual symptoms, notably fatigue. Follow-up studies should investigate the origins of residual symptoms and their long-term consequences in former, nonhospitalized patients with COVID-19.
Keywords: cardiovascular health, COVID-19, long-term effects, nonhospitalized, physical functioning
INTRODUCTION
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causing coronavirus disease 2019 (COVID-19) has led to a global pandemic. As of November 2022, the World Health Organization reported a total of over 600 million cases and 6.5 million deaths (1). Besides respiratory involvement, COVID-19 can exhibit systemic effects in the acute phase, including affecting the cardiovascular system. Angiotensin-converting enzyme 2-mediated SARS-CoV-2 entry of myocardial and endothelial cells may result in myocardial injury and endothelial dysfunction (2–4). This may result in myocarditis, arrhythmias, acute coronary syndrome, stroke, venous thromboembolism, or heart failure (2–4).
These signs of cardiovascular involvement may persist and lead to long-term symptoms and syndromes including chest pain, shortness of breath, palpitations, myocardial and vascular inflammation, arrhythmias, and thromboembolism (5–9). Postacute effects on physical functioning have also been reported, demonstrating that patients may suffer from fatigue, joint pain, muscle weakness, and a decreased health-related quality of life (hrQoL) months after the infection (5, 6, 8, 10–12). These long-term effects have mostly been investigated in hospitalized populations, whereas the majority of patients with COVID-19 recover at home (13–15). Nonetheless, postacute cardiovascular events, such as dysrhythmias, ischemic heart disease, and heart failure have also been described in patients who were not hospitalized (9).
The underlying mechanisms of postacute effects in nonhospitalized patients with COVID-19 remain incompletely understood. Physiological measurements may help us to understand the origin of these effects. To our knowledge, literature on this subject is limited. Although postacute cardiac biomarker concentrations were reported once, demonstrating an increase of cardiac troponin I, the sample size of this study was small and follow-up duration was limited to 1 mo (16). Studies on the long-term effects on arterial stiffness mostly showed an increase after COVID-19 (17–23). However, findings were discrepant across studies, and not all study samples were representative of the general, nonhospitalized population (17–21). Furthermore, physical functioning after COVID-19 in nonhospitalized patients was reported to be decreased, but these findings were based exclusively on patients suffering from long-term COVID-19 (24–26). Moreover, most studies focused on a specific outcome and did not take multiple relevant parameters into account. Thus, an integrative approach to studying the long-term effects of COVID-19 on physiological parameters in the general, nonhospitalized population is currently lacking.
Therefore, we performed a comprehensive physiological assessment of cardiovascular health and physical functioning in patients with postacute COVID-19 who recovered at home. Given the current literature on postacute cardiovascular events, physical dysfunction, symptoms, and physiological parameters, we hypothesized to observe lower cardiovascular health and physical functioning in nonhospitalized, patients with postacute COVID-19 compared with age- and sex-matched controls.
METHODS
Study Design and Population
In this cross-sectional study, male and female adult volunteers were recruited from the Nijmegen Exercise Study, a cohort consisting of participants of Dutch mass-participation exercise events (International Nijmegen Four Days Marches and the Seven Hills Run) and their family members and friends (27, 28). An inclusion criterion for the COVID-19 group was evidence of a positive polymerase chain reaction (PCR) test for SARS-CoV-2, whereas an exclusion criterion was hospitalization due to the SARS-CoV-2 infection. We aimed to include participants ∼6 mo after SARS-CoV-2 infection. An age- and sex-matched control group was recruited from the same cohort. Controls were only included if they had never had signs, symptoms, or suspicions of a SARS-CoV-2 infection nor a lifetime positive test of any sort for SARS-CoV-2 before study participation. Dutch language proficiency and residency were inclusion criteria for both groups. Randomization was not applicable to the current study design. Primary outcome assessors were not blinded to the participant group. Recruitment of both the COVID-19 and control group took place in May 2021. Participants from both groups were invited for a single visit to our research center at the Radboud University Medical Center (Radboudumc, Nijmegen, The Netherlands) between May and September 2021. Thereafter, a personalized link was sent to every participant for completion of various online questionnaires. Attrition was not applicable to our study because of the cross-sectional study design without follow-up period. The local Medical Research Ethics Committee provided approval (NL36743.091.11), and the study was conducted in accordance with the Declaration of Helsinki. All participants provided written informed consent.
Measurements
COVID-19 characteristics.
The COVID-19 group was asked to report the date of onset and duration of their illness, their vaccination status and vaccination dates, and the symptoms they experienced at the time of infection and at the time of inclusion. Furthermore, they were asked whether they required post-COVID-19 care from physiotherapist, general practitioner, psychologist, occupational therapist, or medical specialist.
Cardiovascular risk factors.
Height (m) and weight (kg; measured with Seca, Hamburg, Germany) were assessed and body mass index (BMI, kg/m2) was calculated. Noninvasive left brachial blood pressure (mmHg) and heart rate (beats/min) were measured twice after 5 min of rest in a supine position (M3, OMRON Healthcare Europe, Hoofddorp, The Netherlands). The average values of the two measurements were used for analysis. Venous blood was drawn from the antecubital vein (8.5 mL, BD Vacutainer SST II Advance) and coagulated for 45–60 min before being centrifuged at 3,000 revolutions/min for 10 min at 4°C. Serum was then transferred to 2-mL microtubes and stored at −80°C until analysis. The following biomarkers were analyzed: full cholesterol profile [total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), and triglycerides (in mmol/L)]; glucose hexokinase (mmol/L); insulin (µIU/mL); creatinine (µmol/L); high-sensitive cardiac troponin I (hs-cTnI; ng/L); amino-terminal pro-B-type natriuretic peptide (NT-proBNP; pmol/L); and C-reactive protein (CRP; mg/L). Analyses were performed batchwise on Atellica (and IMMULITE 2000 for insulin) analyzers (Siemens Healthcare Diagnostics, Tarrytown, NY) in the Gelderse Vallei Hospital (Ede, The Netherlands). Smoking behavior and history of cardiovascular disease (CVD) and cardiovascular risk factors including hypertension, hypercholesterolemia, diabetes mellitus, myocardial infarction, stroke, thrombosis, heart failure, and resuscitation were inquired via questionnaires.
Arterial stiffness.
Arterial stiffness was assessed by noninvasive, image-free ultrasound technology with the ARTSENS Plus (Healthcare Technology Innovation Center, Indian Institute of Technology Madras, Chennai, India) (29–32). Simultaneous recording of carotid artery distensibility and cuff-based femoral artery blood pressure facilitated assessment of carotid-femoral pulse wave velocity (cf-PWV; m/s), a parameter of central stiffness (33). Furthermore, local carotid artery stiffness was quantified using the dimensionless stiffness index β and pressure strain elasticity epsilon (kPa) (34).
Physical functioning.
Ambulant physical activity monitoring (8 day, 24 h) was performed with an activPAL3 micro-accelerometer (PAL Technologies, Glasgow, UK) (35, 36). During this period, participants were requested to keep a sleep/wake diary to enable automated analysis. Data were extracted via PALbatch (PAL Software Suite, version 8, PAL Technologies) and analyzed using a modified version of the script by Winkler et al. (37, 38) in SAS (Statistical Analysis System, RRID:SCR_008567, version 9.4; SAS Inst., Cary, NC) to compute daily light intensity (LIPA) and moderate-to-vigorous physical activity (MVPA) duration (min/day), sleeping and sitting time (h/day), and step count (steps/day). Peak handgrip strength (kg) of the nondominant hand was measured three times separated by 1 min intervals using the Jamar hydraulic hand dynamometer, following the method described by Webb et al. (39). The single highest value was used in the analysis. Gait speed (km/h) was assessed twice over a 4 m stretch with 2-m acceleration and deceleration zones on either side to ensure a stable pace. The fastest try was used in the analysis. This method follows the 4-m gait speed protocol as described in the short physical performance battery (40). Questionnaires were used to assess fatigue (Fatigue Severity Scale, high score indicating more fatigue), general health status (Short Form 12, SF-12, high score indicating a high level of general health status), and hrQoL (EQ-5D-5L, high score indicating a low hrQoL) (41–43).
Statistical Analysis
No power analysis was conducted as all Nijmegen Exercise Study participants with a positive PCR test were eligible for study participation. Biomarker concentrations were log-transformed. Outlier analysis was performed by visual inspection using box plots. Outliers were defined as data points smaller than 1.5 times the interquartile range below Q1 or greater than 1.5 times the interquartile range above Q3, and of which the value was not within the physiological range. All parameters were tested for normality using the Shapiro–Wilk test. Normally distributed continuous variables were reported as means (SD) and compared between the COVID-19 and control group using independent sample t tests, whereas non-Gaussian-distributed continuous variables were reported as median [Q1–Q3] and compared between groups using the Mann–Whitney U test. Categorical variables were reported as numbers (%) and compared using Fisher’s exact test. A subgroup analysis was performed among patients with COVID-19 with residual symptoms versus their age- and sex-matched controls. P values < 0.05 were considered significant. Statistical analyses were performed in RStudio (RRID:SCR_000432, version 4.1.2), and figures were created using packages ggplot2 and cowplot.
RESULTS
Study Population
In total, 6,220 participants of the Nijmegen Exercise Study were invited to participate. From a group of 1,406 interested individuals, all participants (N = 101) with a PCR-proven SARS-CoV-2 infection who were not hospitalized because of their infection were included. From the remaining group of interested individuals, we used one-to-one matching to select N = 101 age- and sex-matched controls without signs, symptoms, or suspicions of a SARS-CoV-2 infection, nor a lifetime positive test of any sort for SARS-CoV-2. This led to a total of N = 202 participants being included in this study. Participants were 58 [54–65] yr old, 118 (58%) were male, and 84 (42%) were female. No outliers were excluded.
COVID-19 characteristics.
The median time between infection and inclusion was 175 [126–235] days (Supplemental Fig. S1; see https://doi.org/10.6084/m9.figshare.20501760). Five participants (7%) had been vaccinated once before becoming infected; none of the participants had completed the primary vaccination series before infection. The majority of the COVID-19 group (76%) was infected before February 2021, when the original SARS-CoV-2 variant was dominant in The Netherlands (44). Out of the 96 participants in the COVID-19 group who completed the questionnaires, 75 participants (78%) had been vaccinated at least once before inclusion. Ninety-four (98%) participants experienced symptoms at the time of infection, of which fatigue (80%), muscle pain (65%), dry cough (64%), headache (61%), and fever (58%) were most commonly reported. Most symptoms were of minor severity (Supplemental Table S1; see https://doi.org/10.6084/m9.figshare.20517747). Thirty-one (32%) participants reported residual symptoms at the time of inclusion of which fatigue, dysosmia/dysgeusia, concentration issues, feeling of weakness and headache were most commonly reported (Supplemental Fig. S2; see https://doi.org/10.6084/m9.figshare.20501769). Seven participants (7%) required post-COVID-19 care of a physiotherapist (N = 5), general practitioner (N = 4), psychologist (N = 3), medical specialist (N = 2), or occupational therapist (N = 2).
Cardiovascular risk factors.
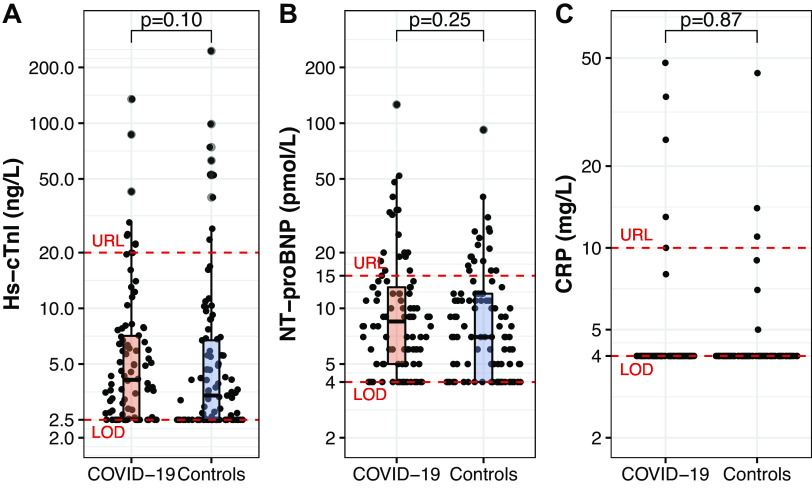
History of CVD and smoking behavior were not different between groups (Table 1). BMI (24.2 [22.3–25.6] vs. 24.7 [22.9–26.5] kg/m2, P = 0.17), systolic blood pressure (134 [124–148] vs. 134 [124–146] mmHg, P = 0.82), diastolic blood pressure [83 (10) vs. 81 (9) mmHg, P = 0.10], and heart rate (58 [52–64] vs. 58 [53–64] beats/min, P = 0.49) were not different between the COVID-19 and control groups. Similarly, serum concentrations of lipid levels, glucose, insulin, and creatinine were not different between groups (Table 1). Concentrations of hs-cTnI, NT-proBNP, and CRP, but also the prevalence for these biomarkers exceeding the assay-specific upper reference limit, were not different between the COVID-19 and control group (Fig. 1).
Table 1.
Comparison of cardiovascular risk factors in the COVID-19 vs. control group
| Cardiovascular Risk Factors | COVID-19 Group |
Control Group |
P Value |
|---|---|---|---|
| N | 101 | 101 | |
| History of cardiovascular disease, n (%)* | |||
| Myocardial infarction | 4 (4) | 3 (3) | 0.72 |
| Heart failure | 2 (2) | 1 (1) | 0.62 |
| Stroke | 2 (2) | 3 (3) | >0.99 |
| Thrombosis | 2 (2) | 2 (2) | >0.99 |
| Hypertension | 17 (18) | 18 (18) | >0.99 |
| Hypercholesterolemia | 14 (15) | 13 (13) | 0.84 |
| Diabetes mellitus | 4 (4) | 4 (4) | >0.99 |
| Resuscitation | 1 (1) | 1 (1) | >0.99 |
| Smoking, n (%)** | 0.45 | ||
| Current | 6 (6) | 3 (3) | |
| Former | 32 (33) | 38 (39) | |
| Never | 58 (60) | 57 (58) | |
| Cholesterol, mmol/L | |||
| Total | 5.2 [4.8–5.9] | 5.1 [4.6–5.9] | 0.31 |
| HDL | 1.6 [1.3–1.8] | 1.6 [1.2–1.8] | 0.28 |
| LDL | 3.1 [2.6–3.6] | 3.0 [2.5–3.7] | 0.65 |
| Triglycerides, mmol/L | 1.1 [0.8–1.3] | 1.0 [0.8–1.5] | 0.96 |
| Glucose, mmol/L | 5.0 [4.8–5.3] | 4.9 [4.7–5.2] | 0.36 |
| Insulin, µIU/mL | 4.1 [2.0–7.1] | 3.8 [2.0–5.6] | 0.24 |
| Creatinine, µmol/L | 80 (SD 13) | 81 (SD 14) | 0.53 |
Values are n (%), medians [Q1–Q3], or means (SD); N, number of patients. *Data were missing for this factor and based on N = 96 (COVID-19 group) and N = 99 (control group). **Data were missing for this factor and based on N = 96 (COVID-19 group) and N = 98 (control group). Differences between groups were assessed with a Fisher’s exact test, Mann–Whitney U test or independent sample t test. COVID-19, coronavirus disease 2019; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Figure 1.
Biomarker concentrations of high-sensitive cardiac troponin I (hs-cTnI; A), amino-terminal pro-B-type-natriuretic peptide (NT-proBNP; B), and C-reactive protein (CRP; C) in the COVID-19 (N = 94) and control (N = 101) groups. Each dot represents an individual data point, whereas the boxplots represent group statistics (Q1, median, Q3; whiskers extending up to 1.5 times the interquartile range). Dashed red lines indicate the upper reference limit (URL) and the limit of detection (LOD). Differences between groups were assessed with a Mann–Whitney U test.
Arterial stiffness.
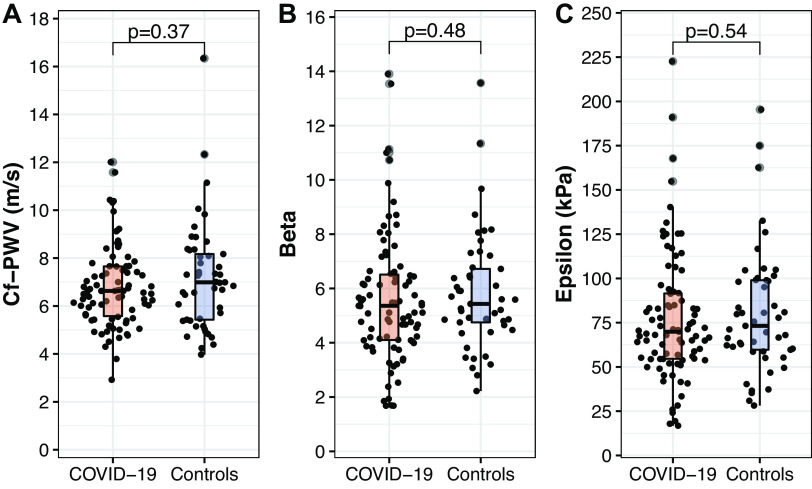
As time constraints restricted us to perform these measurements in the full cohort, arterial stiffness was successfully assessed in 87 former patients with COVID-19 and 49 controls. Participant characteristics differed neither between participants with versus without arterial stiffness measurements in the COVID-19 and control group, nor between patients with COVID-19 and controls with an arterial stiffness measurement (data not presented). No differences in cf-PWV or local arterial stiffness (β, epsilon) were found between the COVID-19 and control group (Fig. 2).
Figure 2.
Arterial stiffness parameters expressed as carotid-femoral pulse wave velocity (cf-PWV, m/s; A), stiffness index β (dimensionless; B), and pressure strain elasticity epsilon (kPa; C) for the COVID-19 (N = 87) and control (N = 49) groups. Each dot represents an individual data point, whereas box plots represent group statistics (Q1, median, Q3; whiskers extending up to 1.5 times the interquartile range). Differences between groups were assessed with a Mann–Whitney U test.
Physical functioning.
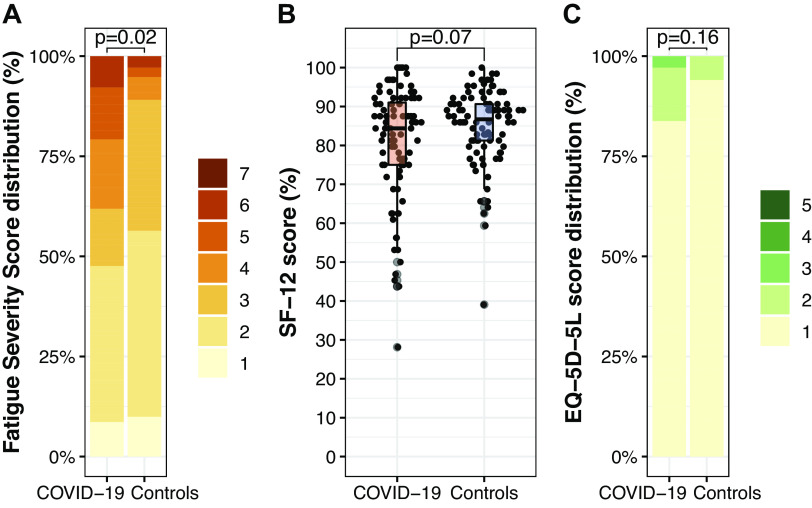
Habitual physical activity characteristics were not different between groups, except for sleeping time [1.0 h/day lower in the COVID-19 versus control group (Fig. 3)]. Step count was not different between the COVID-19 and control group (13,266 [10,600–16,131] vs. 14,024 [11,393–17,197] steps/day, P = 0.42). The COVID-19 group had a higher handgrip strength compared with the control group (43 [33–52] vs. 38 [30–48] kg, P = 0.007), whereas gait speed did not differ between groups (5.5 [5.1–6.2] vs. 5.6 [5.3–6.2] km/h, P = 0.26). Self-reported level of fatigue was higher in the COVID-19 group, whereas the perceived general health status was lower in the COVID-19 versus control group but did not reach statistical significance (P = 0.07, Fig. 4). HrQoL did not differ between groups (Fig. 4).
Figure 3.
Habitual physical activity patterns expressed as sleeping time (A), sitting time (B), time spent in light-intensity physical activity (LIPA; C), and time spent in moderate-to-vigorous physical activity (MVPA; D) for the COVID-19 (N = 101) and control (N = 101) groups. Each dot represents an individual data point, whereas box plots represent group statistics (Q1, median, Q3; whiskers extending up to 1.5 times the interquartile range). Differences between groups were assessed with a Mann–Whitney U test for A, C, and D and an independent sample t test for B.
Figure 4.
Self-reported outcomes of the COVID-19 (N = 96) and control (N = 98) groups: level of fatigue (Fatigue Severity Scale), range 1–7, high score indicating a high level of fatigue (A), general health status [pooled Short Form 12 (SF-12) score], range 0%–100%, high score indicating a high level of general health status (B), and health-related quality of life (hrQoL; EQ-5D-5L), range 1–5, high score indicating a low hrQoL (C). Each dot represents an individual data point, whereas the box plot represents group statistics (Q1, median, Q3; whiskers extending up to 1.5 times the interquartile range). Differences between groups were assessed with a Fisher’s exact test for A and C and a Mann–Whitney U test for B.
Impact of residual symptoms.
Subgroup analyses among patients with COVID-19 (N = 31) with residual symptoms versus their age- and sex-matched controls (N = 31) largely confirmed our primary findings in that no differences were observed in biomarker concentrations and physical functioning outcomes between groups. A lower cf-PWV and perceived general health status were found in the patients with COVID-19 with residual symptoms (Supplemental Table S2; see https://doi.org/10.6084/m9.figshare.20501745).
DISCUSSION
The purpose of this study was to examine the long-term effects of COVID-19 on physiological parameters of cardiovascular health and physical functioning in individuals who recovered at home. We observed no significant differences in traditional cardiovascular risk factors (e.g., BMI, blood pressure), cardiovascular biomarkers (e.g., hs-cTnI, NT-proBNP, CRP), or arterial stiffness between groups. Objective measures of habitual physical activity characteristics were mostly comparable between groups, but the COVID-19 group showed a higher handgrip strength, lower sleeping time, and a higher level of fatigue than the control group. These findings suggest that objective outcomes such as cardiovascular risk factors, biomarker concentrations, and physical activity characteristics are not different between nonhospitalized patients with COVID-19 at a median of 6 mo following infection compared with their age- and sex-matched noninfected peers, despite a high prevalence of residual symptoms and poorer subjective outcomes in the COVID-19 versus control group.
In contrast to our hypothesis, traditional cardiovascular risk factors and cardiovascular biomarker concentrations were comparable between the COVID-19 and control group. To our knowledge, literature on cardiac biomarker concentrations in postacute, nonhospitalized patients with COVID-19 is limited to one study that reported elevated cardiac troponin I levels in 21% of the participants at 28 days after the infection (16). Notably, this study also reported a decline in biomarker concentrations between days 1 and 28. This decline over the course of 1 mo may explain why we did not find elevated hs-cTnI levels in our COVID-19 group, as previously elevated levels could have normalized over a median follow-up of 6 mo.
Indicators of central and local arterial stiffness were comparable between our COVID-19 and control group. As these arterial stiffness parameters are established and independent predictors for future cardiovascular morbidity and mortality (45–48), our data suggest no indication of increased cardiovascular risk within our COVID-19 cohort of relatively healthy nonhospitalized individuals. Furthermore, the subgroup analysis between participants with COVID-19 with residual symptoms and their matched controls showed a lower cf-PWV in the COVID-19 group, opposite of what was expected. However, these results were based on N = 28 COVID-19 and N = 15 control participants and should therefore be interpreted with caution. Follow-up studies with a larger sample size are warranted to further investigate this. Our findings of no differences in arterial stiffness in COVID-19 versus control participants are in line with some (18) but in contrast with other studies (17, 20, 21). The main differences between these studies and our study are that our study had a longer median follow-up duration, included participants of all ages in contrast to young individuals only, and included approximately four times as many participants. Two other studies using the same study population of nonhospitalized individuals reported increased arterial stiffness at 4 and 12 mo post-COVID-19, respectively (22, 23). Their study sample resembled our cohort in terms of age, sample size, and follow-up duration, yet showed results conflicting with our findings. A possible explanation for this could be that they included patients visiting a dedicated post-COVID-19 outpatient clinic. This suggests that these patients were affected more strongly by the disease than our general group of nonhospitalized participants with COVID-19. Moreover, three of these studies suggest a transient increase in arterial stiffness after COVID-19 that declines with time (19, 21, 22). Our finding that arterial stiffness was not different between COVID-19 and control participants may therefore also be due to a normalization of values over the median follow-up of 6 mo.
We found no differences in various markers of cardiovascular risk between the COVID-19 and control group. These findings are contradictory to observations in a large American cohort study (N = 153,760 subjects), in which COVID-19 survivors had an increased incidence of cerebrovascular and thrombotic disorders, dysrhythmias, and inflammatory or ischemic heart disease during 12 mo of follow-up (9). There are several explanations for these discrepant outcomes. First, baseline characteristics were different across studies as participants in the American cohort were older, more often smokers, male, black, or obese and more often had diabetes, hypertension, or hyperlipidemia compared with our Dutch cohort. The higher prevalence of cardiovascular risk factors in the American cohort may have contributed to the increased CVD risk during follow-up, even after correction for confounding factors, as COVID-19 may have acted as a catalyst to express and deteriorate underlying disease. Second, participants in our study were highly physically active, demonstrated by the high daily step count (13,499 [10,924–16,854] steps/day) and a weekly exercise volume (729 [589–904] min) that well exceeds the international guidelines on physical activity (49, 50). Physical activity is known to reduce the risk of cardiovascular and chronic diseases (49–51), and previous studies have demonstrated a protective effect of a physically active lifestyle on COVID-19-related outcomes (52). Our study sample might therefore not be representative of the general population, underestimating the long-term consequences of COVID-19 in those who were not hospitalized. Finally, it may be possible that the impact of different SARS-CoV-2 variants on health outcomes was assessed (53), although the similar timelines of both studies make this explanation less likely.
Habitual physical activity characteristics, such as time spent sedentary, LIPA, and MVPA, were not different between the COVID-19 and control group. These findings are contradictory to some studies (24–26, 54) assessing physical activity levels of former, nonhospitalized patients with COVID-19. It is important to note that studies demonstrating a decline in physical activity patterns included participants who explicitly suffered from long COVID-19 or post-COVID-19 syndrome (24–26) or used subjective measures for physical activity (54). In contrast, the majority (68%) of our sample did not experience long-term symptoms and is therefore more likely to represent the general, nonhospitalized population. Subgroup analysis among individuals with residual symptoms further confirmed our observation that activity patterns were not different between the COVID-19 and control group.
We found a small but significantly higher handgrip strength in the COVID-19 group compared with the control group. This finding is in contrast with previous observations of muscle weakness being a prevalent long-term consequence of COVID-19 in both hospitalized and nonhospitalized patients (6, 12, 55). Nonetheless, handgrip strength of both groups was within the normal range (39) and not associated with differences in physical function (e.g., gait speed). Moreover, clinical significance of handgrip strength is primarily described in frail, elderly individuals while we examined a relatively younger population.
A lower sleeping time and a higher level of fatigue were found in the COVID-19 versus control group. These findings align with the literature, as sleeping disturbances and fatigue have been previously reported as long-term consequences of COVID-19 in multiple studies (5, 6, 12). Fatigue was also the most frequently reported symptom among participants with residual symptoms in our study.
An important finding of our study is the discrepancy between objective cardiovascular and physical functioning measurements and subjective outcomes. The long-term effects of COVID-19 on cardiovascular health and physical functioning may be limited in physically active patients who recovered at home, whereas residual symptoms are reported by one out of three participants, with fatigue being most often mentioned. Noteworthy is that this subgroup with residual symptoms reports a lower perceived general health status, which suggests that these participants are truly affected in their daily life. Follow-up studies are needed to investigate the origin of these residual symptoms, which may be caused by persistent infections, pulmonary injury, diaphragm dysfunction, or other pathophysiological pathways that we did not assess in the current study. Investigating how residual symptoms evolve across the years after COVID-19 and/or following renewed infection(s) will contribute to a more thorough understanding of the long-term consequences of COVID-19 and the corresponding healthcare needs.
Limitations
This study has some limitations. First, we did not perform cardiac imaging, so the long-term impact of COVID-19 on cardiac structure and function in nonhospitalized individuals requires further study. Second, arterial stiffness data were only available in a subset of our cohort. Nevertheless, participant characteristics of individuals with and without an arterial stiffness measurement were comparable, suggesting that these findings were likely unbiased, which was further reinforced by a similar distribution of the arterial stiffness parameters within the COVID-19 and control group. Third, we cannot exclude the possibility that controls were never infected with SARS-CoV-2 as we did not perform serological testing for antibodies. Some controls may have experienced an asymptomatic infection, but the impact of such misclassification on our findings is expected to be minimal given our large sample size. Fourth, we aimed to include participants with COVID-19 ∼6 mo after infection. Although the range of time between infection and inclusion was large (19–462 days), subanalyses revealed that this variation did not impact our main outcomes (data not shown).
Conclusions
No major differences in physiological parameters of cardiovascular health and physical functioning were found between nonhospitalized patients with COVID-19 at a median of 6 mo postinfection and age- and sex-matched controls. Nevertheless, a lower sleeping time and higher level of fatigue were found in former patients with COVID-19, in combination with a high prevalence of residual symptoms and a corresponding lower perception of general health status. Future research should focus on exploring the pathophysiological origins of residual symptoms to further unravel the long-term consequences of COVID-19 in those recovered at home.
DATA AVAILABILITY
Data will be made available upon reasonable request.
SUPPLEMENTAL DATA
Supplemental Table S1: https://doi.org/10.6084/m9.figshare.20517747.
Supplemental Table S2: https://doi.org/10.6084/m9.figshare.20501745.
Supplemental Fig. S1: https://doi.org/10.6084/m9.figshare.20501760.
Supplemental Fig. S2: https://doi.org/10.6084/m9.figshare.20501769.
GRANTS
This project was funded by Dutch Heart Foundation Grant 2020T063.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.M.v.d.S., E.A.B., M.K., G.-J.G., F.H.R., D.H.J.T., and T.M.H.E. conceived and designed research; K.M.v.d.S., E.A.B., Y.A.W.H., D.H.J.T., and T.M.H.E. performed experiments; K.M.v.d.S., E.A.B., D.H.J.T., and T.M.H.E. analyzed data; K.M.v.d.S., E.A.B., Y.A.W.H., D.H.J.T., and T.M.H.E. interpreted results of experiments; K.M.v.d.S. prepared figures; K.M.v.d.S., E.A.B., D.H.J.T., and T.M.H.E. drafted manuscript; E.A.B., T.J.S., J.J., M.K., G.-J.G., F.H.R., Y.A.W.H., D.H.J.T., and T.M.H.E. edited and revised manuscript; K.M.v.d.S., E.A.B., T.J.S., J.J., M.K., G.-J.G., F.H.R., Y.A.W.H., D.H.J.T., and T.M.H.E. approved final version of manuscript.
ACKNOWLEDGMENTS
We sincerely thank all personnel involved in the data collection and Siemens Healthcare Diagnostics for providing the assay kits for the biomarker analyses.
REFERENCES
- 1.World Health Organization. WHO Coronavirus (COVID-19) Dashboard (Online). https://covid19.who.int/ [2022 Jan 10].
- 2.Task Force for the management of COVID-19 of the European Society of Cardiology. European Society of Cardiology guidance for the diagnosis and management of cardiovascular disease during the COVID-19 pandemic: part 1—epidemiology, pathophysiology, and diagnosis. Eur Heart J 43: 1033–1058, 2021. [Erratum in Eur Heart J, 2021]. doi: 10.1093/eurheartj/ehab696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cascella M, Rajnik M, Aleem A, Dulebohn SC, Di Napoli R. Features, evaluation, and treatment of coronavirus (COVID-19). In: StatPearls. Treasure Island, FL: StatPearls Publishing LLC., 2021. [PubMed] [Google Scholar]
- 4. Hendren NS, Drazner MH, Bozkurt B, Cooper LT. Description and proposed management of the acute COVID-19 cardiovascular syndrome. Circulation 141: 1903–1914, 2020. doi: 10.1161/CIRCULATIONAHA.120.047349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, Cook JR, Nordvig AS, Shalev D, Sehrawat TS, Ahluwalia N, Bikdeli B, Dietz D, Der-Nigoghossian C, Liyanage-Don N, Rosner GF, Bernstein EJ, Mohan S, Beckley AA, Seres DS, Choueiri TK, Uriel N, Ausiello JC, Accili D, Freedberg DE, Baldwin M, Schwartz A, Brodie D, Garcia CK, Elkind MSV, Connors JM, Bilezikian JP, Landry DW, Wan EY. Post-acute COVID-19 syndrome. Nat Med 27: 601–615, 2021. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature 594: 259–264, 2021. doi: 10.1038/s41586-021-03553-9. [DOI] [PubMed] [Google Scholar]
- 7. Willems LH, Nagy M, Ten Cate H, Spronk HMH, Groh LA, Leentjens J, Janssen NAF, Netea MG, Thijssen DHJ, Hannink G, Van Petersen AS, Warlé MC. Sustained inflammation, coagulation activation and elevated endothelin-1 levels without macrovascular dysfunction at 3 months after COVID-19. Thromb Res 209: 106–114, 2022. doi: 10.1016/j.thromres.2021.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Crook H, Raza S, Nowell J, Young M, Edison P. Long covid—mechanisms, risk factors, and management. BMJ 374: n1648, 2021. [Erratum in: BMJ 374: n1944, 2021]. doi: 10.1136/bmj.n1648. [DOI] [PubMed] [Google Scholar]
- 9. Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med 28: 583–590, 2022. doi: 10.1038/s41591-022-01689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Augustin M, Schommers P, Stecher M, Dewald F, Gieselmann L, Gruell H, Horn C, Vanshylla K, Cristanziano VD, Osebold L, Roventa M, Riaz T, Tschernoster N, Altmueller J, Rose L, Salomon S, Priesner V, Luers JC, Albus C, Rosenkranz S, Gathof B, Fätkenheuer G, Hallek M, Klein F, Suárez I, Lehmann C. Post-COVID syndrome in non-hospitalised patients with COVID-19: a longitudinal prospective cohort study. Lancet Reg Health Eur 6: 100122, 2021. doi: 10.1016/j.lanepe.2021.100122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fernández-De-Las-Peñas C, Palacios-Ceña D, Gómez-Mayordomo V, Florencio LL, Cuadrado ML, Plaza-Manzano G, Navarro-Santana M. Prevalence of post-COVID-19 symptoms in hospitalized and non-hospitalized COVID-19 survivors: a systematic review and meta-analysis. Eur J Intern Med 92: 55–70, 2021. doi: 10.1016/j.ejim.2021.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang L, Yao Q, Gu X, Wang Q, Ren L, Wang Y, Hu P, Guo L, Liu M, Xu J, Zhang X, Qu Y, Fan Y, Li X, Li C, Yu T, Xia J, Wei M, Chen L, Li Y, Xiao F, Liu D, Wang J, Wang X, Cao B. 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet 398: 747–758, 2021. [Erratum in Lancet 399: 1778, 2022]. doi: 10.1016/S0140-6736(21)01755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mahajan S, Caraballo C, Li S-X, Dong Y, Chen L, Huston SK, Srinivasan R, Redlich CA, Ko AI, Faust JS, Forman HP, Krumholz HM. SARS-CoV-2 infection hospitalization rate and infection fatality rate among the non-congregate population in Connecticut. Am J Med 134: 812–816.e2, 2021. doi: 10.1016/j.amjmed.2021.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Salje H, Tran Kiem C, Lefrancq N, Courtejoie N, Bosetti P, Paireau J, Andronico A, Hozé N, Richet J, Dubost C-L, Le Strat Y, Lessler J, Levy-Bruhl D, Fontanet A, Opatowski L, Boelle P-Y, Cauchemez S. Estimating the burden of SARS-CoV-2 in France. Science 369: 208–211, 2020. [Erratum in Science 368: eabd4246, 2020]. doi: 10.1126/science.abc3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Alkodaymi MS, Omrani OA, Fawzy NA, Shaar BA, Almamlouk R, Riaz M, Obeidat M, Obeidat Y, Gerberi D, Taha RM, Kashour Z, Kashour T, Berbari EF, Alkattan K, Tleyjeh IM. Prevalence of post-acute COVID-19 syndrome symptoms at different follow-up periods: a systematic review and meta-analysis. Clin Microbiol Infect 28: 657–666, 2022. doi: 10.1016/j.cmi.2022.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Velavan TP, Kuk S, Linh LTK, Lamsfus Calle C, Lalremruata A, Pallerla SR, Kreidenweiss A, Held J, Esen M, Gabor J, Neurohr EM, Shamsrizi P, Fathi A, Biecker E, Berg CP, Ramharter M, Addo MM, Kreuels B, Kremsner PG. Longitudinal monitoring of laboratory markers characterizes hospitalized and ambulatory COVID-19 patients. Sci Rep 11: 14471, 2021. doi: 10.1038/s41598-021-93950-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ratchford SM, Stickford JL, Province VM, Stute N, Augenreich MA, Koontz LK, Bobo LK, Stickford ASL. Vascular alterations among young adults with SARS-CoV-2. Am J Physiol Heart Circ Physiol 320: H404–H410, 2021. doi: 10.1152/ajpheart.00897.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nandadeva D, Young BE, Stephens BY, Grotle A-K, Skow RJ, Middleton AJ, Haseltine FP, Fadel PJ. Blunted peripheral but not cerebral vasodilator function in young otherwise healthy adults with persistent symptoms following COVID-19. Am J Physiol Heart Circ Physiol 321: H479–H484, 2021. doi: 10.1152/ajpheart.00368.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nandadeva D, Skow RJ, Grotle A-K, Stephens BY, Young BE, Fadel PJ. Impact of COVID-19 on ambulatory blood pressure in young adults: a cross-sectional analysis investigating time since diagnosis. J Appl Physiol (1985) 133: 183–190, 2022. doi: 10.1152/japplphysiol.00216.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Szeghy RE, Province VM, Stute NL, Augenreich MA, Koontz LK, Stickford JL, Stickford ASL, Ratchford SM. Carotid stiffness, intima–media thickness and aortic augmentation index among adults with SARS‐CoV‐2. Exp Physiol 107: 694–707, 2022. doi: 10.1113/EP089481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Szeghy RE, Stute NL, Province VM, Augenreich MA, Stickford JL, Stickford ASL, Ratchford SM. Six-month longitudinal tracking of arterial stiffness and blood pressure in young adults following SARS-CoV-2 infection. J Appl Physiol (1985) 132: 1297–1309, 2022. doi: 10.1152/japplphysiol.00793.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ikonomidis I, Lambadiari V, Mitrakou A, Kountouri A, Katogiannis K, Thymis J, Korakas E, Pavlidis G, Kazakou P, Panagopoulos G, Andreadou I, Chania C, Raptis A, Bamias A, Thomas K, Kazakou P, Grigoropoulou S, Kavatha D, Antoniadou A, Dimopoulos MA, Filippatos G. Myocardial work and vascular dysfunction are partially improved at 12 months after COVID‐19 infection. Eur J Heart Fail 24: 727–729, 2022. doi: 10.1002/ejhf.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lambadiari V, Mitrakou A, Kountouri A, Thymis J, Katogiannis K, Korakas E, Varlamos C, Andreadou I, Tsoumani M, Triantafyllidi H, Bamias A, Thomas K, Kazakou P, Grigoropoulou S, Kavatha D, Antoniadou A, Dimopoulos MA, Ikonomidis I. Association of COVID‐19 with impaired endothelial glycocalyx, vascular function and myocardial deformation 4 months after infection. Eur J Heart Fail 23: 1916–1926, 2021. doi: 10.1002/ejhf.2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tanriverdi A, Savci S, Kahraman BO, Ozpelit E. Extrapulmonary features of post-COVID-19 patients: muscle function, physical activity, mood, and sleep quality. Ir J Med Sci 191: 969–975, 2022. doi: 10.1007/s11845-021-02667-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Delbressine JM, Machado FVC, Goërtz YMJ, Van Herck M, Meys R, Houben-Wilke S, Burtin C, Franssen FME, Spies Y, Vijlbrief H, van 't Hul AJ, Janssen DJA, Spruit MA, Vaes AW. The impact of post-COVID-19 syndrome on self-reported physical activity. Int J Environ Res Public Health 18: 6017, 2021. doi: 10.3390/ijerph18116017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Humphreys H, Kilby L, Kudiersky N, Copeland R. Long COVID and the role of physical activity: a qualitative study. BMJ Open 11: e047632, 2021. doi: 10.1136/bmjopen-2020-047632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Maessen MFH, Eijsvogels TMH, Verheggen RJHM, Hopman MTE, Verbeek ALM, Vegt FD. Entering a new era of body indices: the feasibility of a body shape index and body roundness index to identify cardiovascular health status. PLoS One 9: e107212, 2014. doi: 10.1371/journal.pone.0107212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bakker EA, Hopman MTE, Lee D-C, Verbeek ALM, Thijssen DHJ, Eijsvogels TMH. Correlates of Total and domain-specific Sedentary behavior: a cross-sectional study in Dutch adults. BMC Public Health 20: 220, 2020. doi: 10.1186/s12889-020-8316-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Joseph J, Nabeel PM, Rao SR, Venkatachalam R, Shah MI, Kaur P. Assessment of Carotid Arterial Stiffness in Community Settings With ARTSENS®. IEEE J Transl Eng Health Med 9: 1900111, 2021. doi: 10.1109/JTEHM.2020.3042386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nabeel PM, Raj KV, Joseph J. Image-free ultrasound for local and regional vascular stiffness assessment: the ARTSENS Plus. J Hypertens 40: 1537–1544, 2022. doi: 10.1097/HJH.0000000000003181. [DOI] [PubMed] [Google Scholar]
- 31. Joseph J, Radhakrishnan R, Kusmakar S, Thrivikraman AS, Sivaprakasam M. Technical validation of ARTSENS-an image free device for evaluation of vascular stiffness. IEEE J Transl Eng Health Med 3: 1900213, 2015. doi: 10.1109/JTEHM.2015.2431471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Joseph J, Kiran R, Nabeel PM, Shah MI, Bhaskar A, Ganesh C, Seshadri S, Sivaprakasam M. ARTSENS® Pen—portable easy-to-use device for carotid stiffness measurement: technology validation and clinical-utility assessment. Biomed Phys Eng Express 6: 025013, 2020. doi: 10.1088/2057-1976/ab74ff. [DOI] [PubMed] [Google Scholar]
- 33. Mitchell GF, Hwang S-J, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation 121: 505–511, 2010. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nabeel PM, Kiran VR, Joseph J, Abhidev VV, Sivaprakasam M. Local pulse wave velocity: theory, methods, advancements, and clinical applications. IEEE Rev Biomed Eng 13: 74–112, 2020. doi: 10.1109/RBME.2019.2931587. [DOI] [PubMed] [Google Scholar]
- 35. Lee LFR, Dall PM. Concurrent agreement between ActiGraph® and activPAL® in measuring moderate to vigorous intensity physical activity for adults. Med Eng Phys 74: 82–88, 2019. doi: 10.1016/j.medengphy.2019.09.018. [DOI] [PubMed] [Google Scholar]
- 36. Klenk J, Büchele G, Lindemann U, Kaufmann S, Peter R, Laszlo R, Kobel S, Rothenbacher D. Concurrent validity of activPAL and activPAL3 accelerometers in older adults. J Aging Phys Act 24: 444–450, 2016. doi: 10.1123/japa.2015-0178. [DOI] [PubMed] [Google Scholar]
- 37. Winkler EA, Bodicoat DH, Healy GN, Bakrania K, Yates T, Owen N, Dunstan DW, Edwardson CL. Identifying adults' valid waking wear time by automated estimation in activPAL data collected with a 24 h wear protocol. Physiol Meas 37: 1653–1668, 2016. doi: 10.1088/0967-3334/37/10/1653. [DOI] [PubMed] [Google Scholar]
- 38. Bakker EA, Van Bakel BMA, Aengevaeren WRM, Meindersma EP, Snoek JA, Waskowsky WM, Van Kuijk AA, Jacobs MMLM, Hopman MTE, Thijssen DHJ, Eijsvogels TMH. Sedentary behaviour in cardiovascular disease patients: risk group identification and the impact of cardiac rehabilitation. Int J Cardiol 326: 194–201, 2021. doi: 10.1016/j.ijcard.2020.11.014. [DOI] [PubMed] [Google Scholar]
- 39. Webb AR, Newman LA, Taylor M, Keogh JB. Hand grip dynamometry as a predictor of postoperative complications reappraisal using age standardized grip strengths. JPEN J Parenter Enteral Nutr 13: 30–33, 1989. doi: 10.1177/014860718901300130. [DOI] [PubMed] [Google Scholar]
- 40. Freire AN, Guerra RO, Alvarado B, Guralnik JM, Zunzunegui MV. Validity and reliability of the short physical performance battery in two diverse older adult populations in Quebec and Brazil. J Aging Health 24: 863–878, 2012. doi: 10.1177/0898264312438551. [DOI] [PubMed] [Google Scholar]
- 41. Rietberg MB, Van Wegen EEH, Kwakkel G. Measuring fatigue in patients with multiple sclerosis: reproducibility, responsiveness and concurrent validity of three Dutch self-report questionnaires. Disabil Rehabil 32: 1870–1876, 2010. [Erratum in Disabil Rehabil 33: 1298, 2011]. doi: 10.3109/09638281003734458. [DOI] [PubMed] [Google Scholar]
- 42.EuroQol Group. EuroQol–a new facility for the measurement of health-related quality of life. Health Policy 16: 199–208, 1990. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 43. Cheak-Zamora NC, Wyrwich KW, McBride TD. Reliability and validity of the SF-12v2 in the medical expenditure panel survey. Qual Life Res 18: 727–735, 2009. doi: 10.1007/s11136-009-9483-1. [DOI] [PubMed] [Google Scholar]
- 44.National Institute for Public Health and the Environment. Variants of the Coronavirus SARS-CoV-2 (Online). Bilthoven: National Institute for Public Health and the Environment, 2022. https://www.rivm.nl/en/coronavirus-covid-19/virus/variants [last accessed 26 January 2022]. [Google Scholar]
- 45. Yu S, McEniery CM. Central versus peripheral artery stiffening and cardiovascular risk. Arterioscler Thromb Vasc Biol 40: 1028–1033, 2020. doi: 10.1161/ATVBAHA.120.313128. [DOI] [PubMed] [Google Scholar]
- 46. Willeit P, Kaptoge S, Welsh P, Butterworth AS, Chowdhury R, Spackman SA, et al. Natriuretic peptides and integrated risk assessment for cardiovascular disease: an individual-participant-data meta-analysis. Lancet Diabetes Endocrinol 4: 840–849, 2016. doi: 10.1016/S2213-8587(16)30196-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Willeit P, Welsh P, Evans JDW, Tschiderer L, Boachie C, Jukema JW, Ford I, Trompet S, Stott DJ, Kearney PM, Mooijaart SP, Kiechl S, Di Angelantonio E, Sattar N. High-sensitivity cardiac troponin concentration and risk of first-ever cardiovascular outcomes in 154,052 participants. J Am Coll Cardiol 70: 558–568, 2017. doi: 10.1016/j.jacc.2017.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, Hegele RA, Krauss RM, Raal FJ, Schunkert H, Watts GF, Borén J, Fazio S, Horton JD, Masana L, Nicholls SJ, Nordestgaard BG, van de Sluis B, Taskinen M-R, Tokgözoglu L, Landmesser U, Laufs U, Wiklund O, Stock JK, Chapman MJ, Catapano AL. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J 38: 2459–2472, 2017. doi: 10.1093/eurheartj/ehx144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gezondheidsraad. Beweegrichtlijnen 2017 (Online). Den Haag: Gezondheidsraad, 2017. https://www.gezondheidsraad.nl/documenten/adviezen/2017/08/22/beweegrichtlijnen-2017 [last accessed 17 August 2022]. [Google Scholar]
- 50.World Health Organization. WHO Guidelines on Physical Activity and Sedentary Behaviour. Geneva: World Health Organization, 2020. [PubMed] [Google Scholar]
- 51.2018 Physical Activity Guidelines Advisory Committee. 2018 Physical Activity Guidelines Advisory Committee Scientific Report. Washington, DC: U.S. Department of Health and Human Services, 2018. [Google Scholar]
- 52. Ezzatvar Y, Ramírez-Vélez R, Izquierdo M, Garcia-Hermoso A. Physical activity and risk of infection, severity and mortality of COVID-19: a systematic review and non-linear dose–response meta-analysis of data from 1 853 610 adults. Br J Sports Med 56: 1188–1193, 2022. doi: 10.1136/bjsports-2022-105733. [DOI] [PubMed] [Google Scholar]
- 53. Lin L, Liu Y, Tang X, He D. The Disease Severity and Clinical Outcomes of the SARS-CoV-2 Variants of Concern. Frontiers in Public Health 9: 775224, 2021. doi: 10.3389/fpubh.2021.775224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Beauchamp MK, Joshi D, McMillan J, Erbas Oz U, Griffith LE, Basta NE, Kirkland S, Wolfson C, Raina P; Canadian Longitudinal Study on Aging (CLSA) Team. Assessment of functional mobility after COVID-19 in adults aged 50 years or older in the Canadian longitudinal study on aging. JAMA Netw Open 5: e2146168, 2022. [Erratum in JAMA Netw Open 5: e220927, 2022]. doi: 10.1001/jamanetworkopen.2021.46168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Paneroni M, Simonelli C, Saleri M, Bertacchini L, Venturelli M, Troosters T, Ambrosino N, Vitacca M. Muscle strength and physical performance in patients without previous disabilities recovering from COVID-19 pneumonia. Am J Phys Med Rehabil 100: 105–109, 2021. doi: 10.1097/PHM.0000000000001641. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table S1: https://doi.org/10.6084/m9.figshare.20517747.
Supplemental Table S2: https://doi.org/10.6084/m9.figshare.20501745.
Supplemental Fig. S1: https://doi.org/10.6084/m9.figshare.20501760.
Supplemental Fig. S2: https://doi.org/10.6084/m9.figshare.20501769.
Data Availability Statement
Data will be made available upon reasonable request.