Abstract
Diabetes increases the risk of poststroke cognitive impairment (PSCI). Greater hemorrhagic transformation (HT) after stroke is associated with vasoregression and cognitive decline in male diabetic rats. Iron chelator deferoxamine (DFX) prevents vasoregression and improves outcomes. Although diabetic female rats develop greater HT, its impact on poststroke cerebrovascularization and cognitive outcomes remained unknown. We hypothesized that diabetes mediates pathological neovascularization, and DFX attenuates poststroke cerebrovascular remodeling and improves neurological outcomes in female diabetic rats. Female control and diabetic animals were treated with DFX or vehicle for 7 days after stroke. Vascular indices, microglial activation, and blood-brain barrier (BBB) integrity were evaluated on day 14. Results from diabetic female rats were partially compared with our previously published findings in male counterparts. Hemin-induced programmed cell death was studied in male and female brain microvascular endothelial cell lines (BMVEC). There was no vasoregression after stroke in either control or diabetic female animals. DFX prevented diabetes-mediated gliovascular remodeling and compromised BBB integrity while improving memory function in diabetes. Comparisons of female and male rats indicated sex differences in cognitive and vascular outcomes. Hemin mediated ferroptosis in both male and female BMVECs. DFX improved survival but had differential effects on ferroptosis signaling in female and male cells. These results suggest that stroke and associated HT do not affect cerebrovascularization in diabetic female rats, but iron chelation may provide a novel therapeutic strategy in the prevention of poststroke memory impairment in females with diabetes via the preservation of gliovascular integrity and improvement of endothelial cell survival.
NEW & NOTEWORTHY The current study shows for the first time that diabetes does not promote aberrant cerebrovascularization in female rats. This contrasts with what we reported in male animals in various diabetes models. Deferoxamine preserved recognition memory function in diabetic female animals after stroke. The effect(s) of stroke and deferoxamine on cerebrovascular density and microglial activation also appear(s) to be different in female diabetic rats. Lastly, deferoxamine exerts detrimental effects on animals and BMVECs under control conditions.
Keywords: diabetes, female, ferroptosis, poststroke cognitive impairment, stroke
INTRODUCTION
Type 2 diabetes has long been associated with an increased risk of neurovascular and neurodegenerative diseases including ischemic stroke as well as Alzheimer’s disease and related dementias (ADRD) (1, 2), but these neurological complications of diabetes are only recently gaining attention (2). Poststroke cognitive impairment (PSCI) is a leading ADRD. With recent advancements leading to a decline in stroke mortality, stroke has become a disease of survivors who suffer from functional and cognitive deficits. The rate of PSCI almost doubled between 1990 and 2000 (3). More than 30% of the 800,000 annual acute ischemic stroke victims have diabetes, putting them at a higher risk for physical and cognitive disability (4). Based on the growing evidence that iron accumulation in the brain parenchyma contributes to the pathophysiology of many neurodegenerative diseases (5–7) and that patients with diabetes are more likely to develop hemorrhagic transformation (HT), we investigated the effect of iron chelation on ischemic stroke recovery. We showed that iron chelation with deferoxamine (DFX) improves long-term sensorimotor and cognitive functions in male diabetic rats (8). Women suffer from poorer outcomes, greater disability, and mortality, as well as increased risk of PSCI (9, 10). We have reported greater HT in diabetic female rats (11). Whether HT negatively impacts stroke recovery in females, especially in comorbid conditions, is unknown. Clinical studies did not address sex differences (12–14), and, to the best of our knowledge, there are no publications investigating the interaction of HT and sex on stroke recovery in preclinical models.
Although stroke and vascular contributions to cognitive impairment/dementia (VCID) are not considered traditional microvascular complications of diabetes, increasing evidence suggests that microvascular dysfunction that occurs early in diabetes is a key player. The neurovascular unit (NVU) concept, which has emerged from the failures of clinical studies focusing on neuroprotection for stroke therapies, provides a framework to incorporate other brain cells and blood vessels into neurovascular protection and restoration research (15, 16). We have shown that diabetes promotes excessive pathological neovascularization in the brain in male rats (17, 18), and an ischemic injury layered on this pathology causes greater HT and vascular rarefaction that is associated with poor recovery (19–22). We have also shown that diabetic female rats are more prone to HT, especially after a thromboembolic stroke (11). Accordingly, we sought to determine the impact of stroke and iron chelation on cerebral vascularization in diabetic female rats. The working hypothesis was that DFX treatment will prevent cerebral vasoregression and gliovascular remodeling after stroke in female diabetic rats and this will be associated with improved functional outcomes.
MATERIAL AND METHODS
Study Design and Animal Groups
All experiments were performed on female Wistar rats (Envigo) at the Augusta University (Augusta, GA) before the investigators relocated to the Medical University of South Carolina (Charleston, SC). Animals were housed under 12-h:12-h light/dark cycle (6:00 am–6:00 pm) with food and water available ad libitum. Control rats (8–10 wk old) were received 2 wk before stroke surgery. Diabetic rats were procured at 4 wk of age, and diabetes was induced as described below. Studies, conducted by the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals, were approved and monitored by the Institutional Animal Care and Use Committee at Augusta University and adhered to the current ARRIVE guidelines. All behavioral testing and data analyses were conducted in a blinded manner. Study design, number of animals that entered and finished the study, mortality rate, metabolic profiles, and end points measured are shown in Fig. 1. Male animal data used in sex comparisons were from our previous publication (8).
Figure 1.
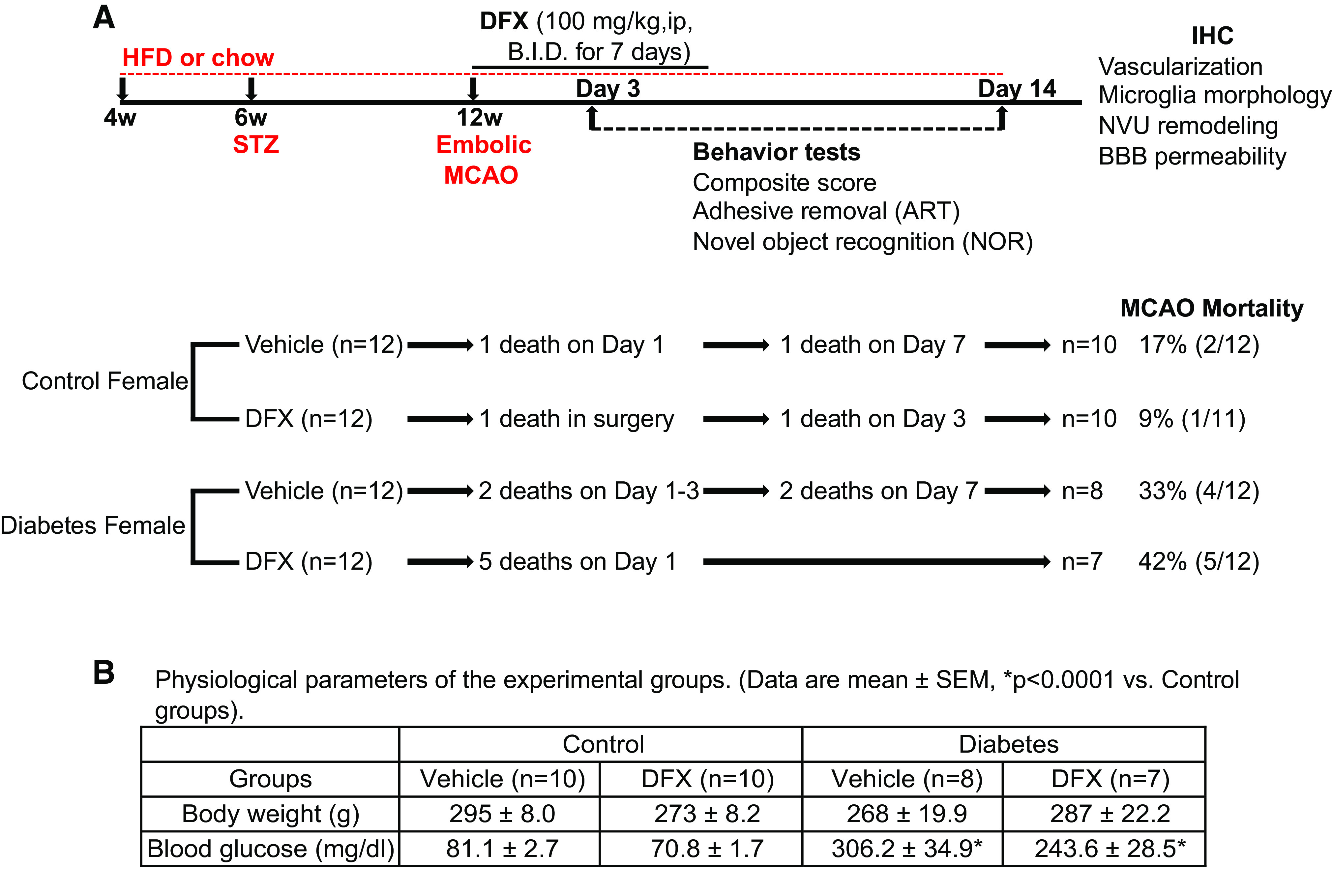
A: schematic time line and description of the experimental design, animal numbers per group, mortality rate, and end points measured. B: physiological parameters of the study groups. BBB, blood brain barrier; DFX, deferoxamine; HFD, high-fat diet; IHC, immunohistochemistry; MCAO, middle cerebral artery occlusion; NVU, neurovascular unit; STZ, streptozotocin.
Induction of Diabetes
Animals were procured at 4 wk of age and were immediately put on a high-fat diet (HFD, 45% fat, Research Diets). A low dose of streptozotocin (STZ, 35 mg/kg ip, Cayman Chemicals) was injected at 6 wk of age. If blood glucose was not above 150 mg/dL at 5 days postinjection, a second dose (20 mg/kg) was administered. Blood glucose levels were measured before noon twice a week from tail vein samples using a commercially available glucometer (Freestyle, Abbott Diabetes Care). The overall average blood glucose level is reported in Fig. 1B. Control rats were maintained on a regular chow diet.
Thromboembolic Stroke Surgery
Stroke was induced by embolic middle cerebral artery occlusion (MCAO), as we reported (11), when all the animals reached 10–12 wk of age. All surgeries were completed before noon and performed by one surgeon. Rats were randomly assigned in a block size of two rats per cage to four different groups: control + vehicle, control + DFX, diabetes + vehicle, and diabetes + DFX. For thromboembolic occlusion, arterial blood from a donor rat was supplemented with human fibrinogen (2 mg/mL) and immediately withdrawn into 20 cm of polyethylene (PE)-50 tubing to clot at room temperature for 6 h and subsequently kept at 4°C for 18 h. The PE-50 tube containing the clot was cut into 5-cm-long pieces. The clots were then transferred to a Petri dish containing sterilized saline and left for further retraction at room temperature for 4 h. A single 4 ± 0.5-cm-long clot was transferred to a PTFE Sub-lite catheter (Braintree Scientific, Braintree, MA) for surgery. Animals were anesthetized with 5% isoflurane and maintained with 2% isoflurane in 70% N2-30% O2 using a facemask. The PTFE sub-lite catheter containing the clot was inserted up to the origin of MCA through the stump of the external carotid artery (ECA), and the clot was gently injected with 100-µL sterile saline. The catheter was removed immediately after embolization. Laser-Doppler imaging with a scanning system (PIM3, Perimed) was used to confirm a successful occlusion and ensure similar levels of cerebral blood flow reduction in all groups. DFX (100 mg/kg ip, Sigma-Aldrich) or vehicle was given every 12 h for 7 days starting 3 h after the surgery based on our previous studies with the suture occlusion and embolic stroke models. Our suture occlusion model studies showed that HT starts by 2 h after reperfusion. Our embolic stroke studies showed that spontaneous resolution starts by 2 h after occlusion and CBF recovers to normal levels by 6 h after occlusion. The estrus cycle was carefully monitored by vaginal swab and surgery was performed in the diestrus phase. In the first 5 days of the postoperative period, blood glucose and body weight were monitored daily for all animals.
Behavioral Tests
Animals were acclimated to the behavior room for 1 wk before surgery. Neurobehavioral tests were recorded and scored by one investigator blinded to the experimental groups to achieve consistency in scoring (20, 23, 24). Tests included Bederson’s score, beam walk, adhesive removal test (ART), and novel object recognition (NOR) before and after stroke on days 3 and 14 as we reported (8, 21, 22, 24) (Fig. 2). A composite neurological score was reported as the sum of Bederson’s score (11) and the beam walk score (25) on a 1–14 scale, with a higher score indicating an improved outcome. For ART, contact and removal latency of the adhesive paper dot was recorded. For each day, the average was taken from three trials with a maximum removal latency of 180 s/trial. Cognitive function was assessed with the NOR task. Animals were habituated to test apparatus for 4 days before baseline testing for 10 min. On the day of testing, the animal underwent three phases: acclimation, familiarization, and novel. The time spent exploring familiar and novel objects was recorded. Recognition index [RI: total time (in s) spent exploring novel object (TN) divided by the total time spent exploring both novel and familiar (TF) objects; RI = TN/(TN + TF)] and discrimination index 2 [d2 = (TN − TF)/(TN + TF)] were calculated. NOR test was administered at baseline and day 14 after stroke surgery. The following inclusion criteria were applied to all animals in the study: RI must be between 0.40 and 0.60 in the A/A session, and the animals must explore objects for a minimum of 30 s within the 5 min trial. All animals met the criteria.
Figure 2.
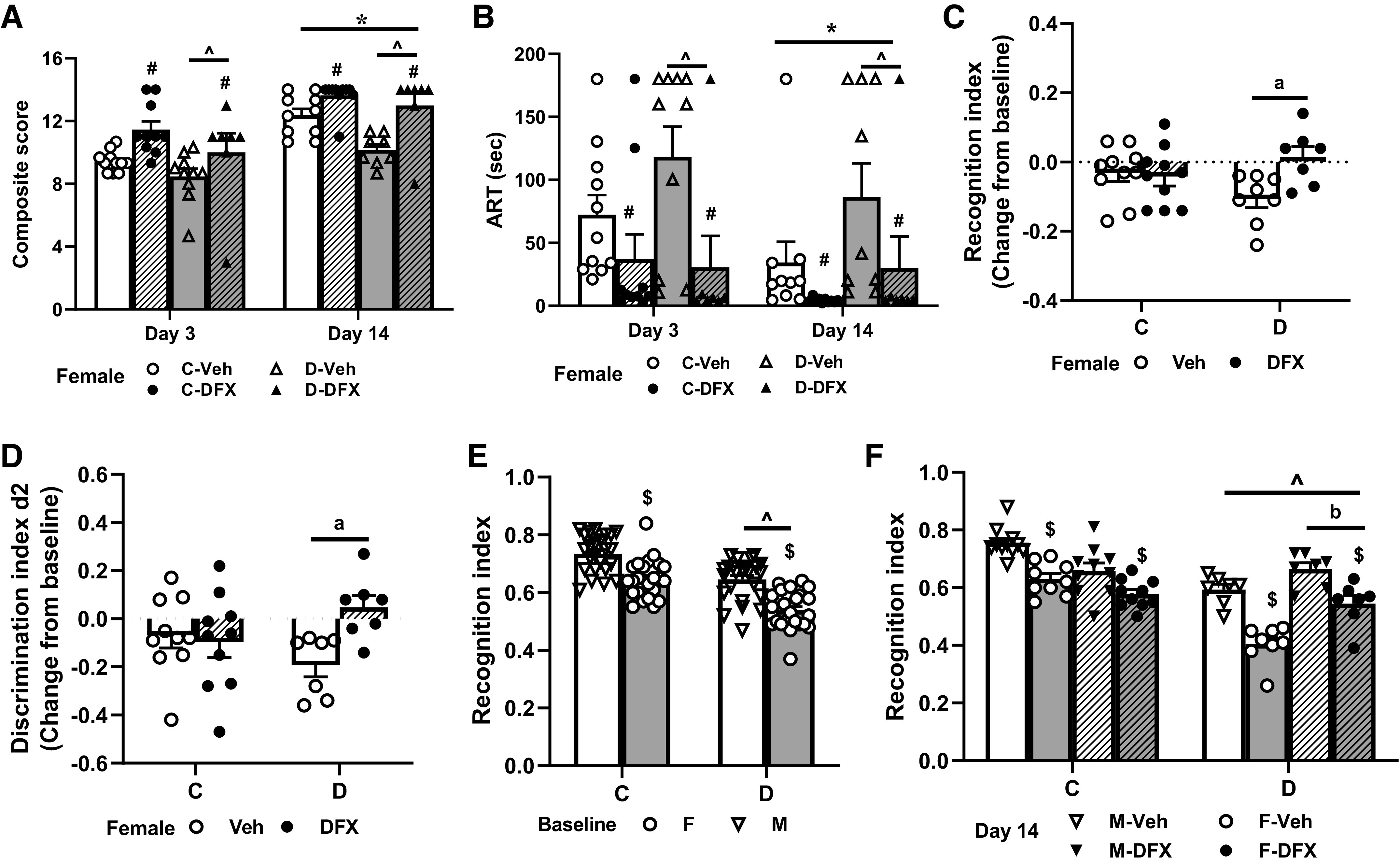
DFX recovered stroke-induced sensorimotor deficits in both diabetic and control female rats and improved cognitive function after stroke in diabetic animals. Treatment (DFX), disease (diabetes), time, and sex effects are indicated by #, ^, *, and $ signs, respectively. Any interaction is indicated by a letter. A: composite score was lower in diabetes vehicle animals on both days 3 and 14, and DFX treatment enhanced recovery in both control and diabetic groups (*P < 0.0001 vs. day 3, ^P = 0.001 vs. C, #P < 0.0001 vs. Veh). B: ART results showed that DFX-mediated improvement was more significant in diabetic animals on day 3, whereas deficits remained high in diabetes vehicle animals by day 14 (*P = 0.07 vs. day 3, ^P = 0.04 vs. C, #P = 0.0005 vs. Veh). C and D: NOR test indicated a disease and treatment interaction such that DFX improved recognition index (RI) and discrimination index (d2) only in diabetic animals (aP = 0.02). E: female animals in both control and diabetic cohorts had lower RI at baseline ($P < 0.0001 vs. M). RI was lower in diabetic rats compared with control rats (^P < 0.0001 vs. C). F: poststroke RI was lower in diabetic animals (^P < 0.0001 vs. C). DFX lowered RI in both female and male control animals, whereas it improved RI in diabetic cohorts (interaction, bP < 0.01). There was also a sex effect with RI being lower in control, as well as in diabetic female vehicle- and DFX-treated rats than male counterparts ($P < 0.001 vs. M). Results are shown as means ± SE and scattered graphs with individual data points. Male animal results were cited from previous publication (8). For A, B, and F, three-way ANOVA was used. For C, D, and E, two-way ANOVA analyses were performed. ART, adhesive removal test; C, control; D, diabetes; DFX, deferoxamine; F, female; M, male; NOR, novel object recognition; Veh, vehicle.
Assessment of Vascularization
Vascularization indices (vascular volume, surface area, and branch density) were measured as we previously reported (17, 22). Rats were injected with 500 µL of 50 mg/mL FITC-dextran (MW, 2,000,000, Sigma-Aldrich) under isoflurane anesthesia via the jugular vein before euthanasia. FITC perfused throughout the animal for a minimum of 10 min. Before termination plasma was obtained for metabolic analysis. Animals were euthanized with isoflurane inhalation overdose and thoracotomy. Brains were stored in 4% paraformaldehyde for 24–48 h followed by 30% sucrose in PBS. Brains were sectioned using a cryostat, and confocal images were obtained by the Zeiss 760 confocal microscope using 100-µm sections. Specifically, Z-stacked images were obtained from regions of interest (ROI) in the cortex and striatum (bregma −1 to +1) as shown in Fig. 2. This region was chosen because of its proximity to MCA and its branches that supply the frontal motor cortex and is the location of hemorrhage in diabetic animals (17, 23) and vasoregression (8) in male diabetic animals. Images from three ROIs in the ipsilateral cortical and striatal areas in three different sections were captured. The mean value from these images was calculated, and each animal had a total of nine unique images. The Z-stack referenced an image size of 1.984 µm, 512 × 512 pixels, and 20× magnification. Raw images were then imported into Volocity 6.0 (Improvision) where they were reconstructed into 3 D images to determine the surface area and vascular volume. Surface area indicates the absolute surface area of the total vasculature. Percentage of vascular volume indicates vascular volume ratio in comparison to total volume. Absence or poor FITC signal in the infarcted area were similar in all groups suggesting similar infarct severity. Branch density was calculated with FIJI (NIH) by creating a binary image, skeletonizing this image, and averaging the number of branches over the longest/shortest path multiplied by 100%. For each image, 8–10 measurements were averaged after being sorted by longest and shortest path. Identical threshold parameters were used across all the sections.
Assessment of Neurovascular Remodeling
Aquaporin 4 (AQP4) polarity was measured as an index of the NVU remodeling (24). Free-floating 20-µm cryostat sections were incubated with AQP4 antibody (1:250, Santa Cruz, sc-32739) at 4°C overnight (24). Following the primary incubation, sections were incubated with a fluorescent-conjugated secondary antibody (1:1,000, Thermo Fisher) for 1 h at room temperature. Z-stacked images were captured for AQP4 and FITC-dextran-filled vessels for 10 slices. To determine the total area of AQP4 immunoreactivity, images were captured at a low threshold. To determine the area of AQP4 immunoreactivity in astrocyte endfeet, the same areas were imaged at a high threshold. AQP4 polarity was calculated as follows: area of total AQP4 immunoreactivity − area of endfeet AQP4 immunoreactivity = unpolarized AQP4 immunoreactivity.
Assessment of Microglia Morphology and Activation
Ionized calcium-binding adaptor molecule 1 (Iba1; 1:500; Wako Pure Chemical, 019–19741) was used to measure reactive microglia/macrophages in the cortex and striatum (24). Microglia were imaged at ×20 and ×60 magnification using Cytation software (BioTek) and converted to binary followed by skeletonization using FIJI software. The AnalyzeSkeleton plugin was used to analyze the number of microglia process end points/cell and summed microglia process length/cell. All data that had two or fewer end points were discarded, and the sum of all end points was used for the number of microglia process end points/cell. To determine the summed microglia process length/cell, the sum of all branch lengths was used after branch lengths less than 0.1 were removed. FIJI was also used to measure cell swelling and number of protrusions per cell. Cell swelling was measured using the freehand selection tool to draw around the cell soma. One investigator counted the number of protrusions from the soma of each microglia.
To evaluate the activation status of microglia, three 20-µm sections (200 µm apart) from the start, middle, and end of the brain region (bregma −1 to +1 as in vascularization studies) were processed with Iba1 antibody (1:100), as well as proinflammatory markers including TNF-α (1:100, NBP2-34539, Novus Biologicals) and anti-inflammatory marker arginase 1 (1:100, 661291IG, Proteintech Group). Slides were imaged with a ×20 objective using an Olympus IX73 microscope (Olympus).
Assessment of Blood-Brain Barrier Permeability
For each animal, three 20-µm sections that were 200 µm apart were stained with IgG primary antibody (1:250, No. 559073, BD Bioscience) and imaged with a ×20 objective using a Zeiss Axioplan 2 Imaging (Carl Zeiss Micro-imaging). MetaMorph Image Analysis Software (Molecular Devices) was used to analyze staining intensity in three ROIs in the cortex and striatum by determining the percent threshold area. Identical threshold parameters were used across all sections. The threshold was averaged for each animal ipsilaterally. Results were expressed as IgG threshold.
Brain Microvascular Endothelial Cell Culture and Treatments
Male (HBEC-5i, American Type Culture Collection, CRL 3245) and female (hCMEC/D3, kind gift from Dr. J. Zastre at the UGA College of Pharmacy) derived human brain microvascular endothelial cells (BMVECs) were cultured as reported (26–30). Cells were used up to eight passages. Cells were cultured in normal glucose (NG) and diabetes-mimicking media that contained high-glucose (HG, 25 mM) and sodium palmitate (P, 50 µM) in a 1:1 ratio in MCDB-131 complete medium and M199 medium for 2 days. On the third day, all cells were starved in Dulbecco’s modification of Eagle’s medium containing 1% penicillin-streptomycin but no serum. After 6-h starvation, hypoxia was induced by treatment with 200 µM cobalt (II) chloride hexahydrate for 24 h (31, 32). mRNA and protein expression of regulated cell death markers were measured for assessment of apoptosis, necroptosis, and ferroptosis. Primers (Thermo Fisher Scientific) were designed as follows: caspase 3, forward 5′- TGGAATGTCCTGGGACACCG-3′ and reverse 5′- ACCGAGATGTCATTCCAGTGC-3′; receptor interacting serine/threonine kinase 3-RIPK3, forward 5′- ACCTTCCAGCCTGATGTCGT-3′ and reverse 5′- TGACCTCCCTGGATATCGCC-3′; iron responsive element binding protein 2-IREB2, forward 5′- ACGCCCCAAAAGCAAGGATAC-3′ and reverse 5′- GCAGAACATCATACTTGGTGCC-3′; with GAPDH forward 5′- AATGGGCAGCCGTTAGGAAA-3′ and reverse 5′- GCGCCCAATACGACCAAATC-3′. In some experiments, the susceptibility of male and female cells to ferroptosis was investigated. For this purpose, cells grown in a regular medium were serum starved for 4 h and then incubated with vehicle, ferroptosis-inducer erastin (20 µM, Cayman Chemicals), or erastin plus ferroptosis inhibitor ferrostatin-1 (Fer-1, 1 µM, Tocris Biotech) for 24 h. Protein expression of IREB2, glutathione peroxidase 4 (GPX4), ferritin heavy chain (FHC), nuclear factor erythroid 2-related factor 2 (NRF2), and acyl-CoA synthetase long-chain family member 4 (ACSL4) were measured. In additional experiments, cells were treated with 50 µM hemin, an oxidized product of hemoglobin, in the presence and absence of Fer-1 or 100 µM DFX (added 30 min before hemin) for 6 h to mimic the in vivo experiments. Cell lysates were collected and prepared for Western blot analysis. In all experiments, similar passage numbers were used in both cell lines that were cultured in parallel.
Western Blot Analysis
Cell lysates of BMVECs were assessed for ferroptosis markers. Primary antibodies included anti-GPX4 (Abcam, ab22604), anti-IREB2 (Abcam, ab181153), anti-FHC (Abcam, ab65080), and anti-ACSL4 (Invitrogen, PA5-27137) at 1:1,000 dilution or anti-β-actin (Sigma, A2854) at 1:3,000 dilution. After washing, membranes were incubated for 1 h at 20°C with appropriate secondary antibodies (1:3,000) and were treated with the enhanced chemiluminescent reagent. The signals were monitored on GE AI 680 imager. Relative band intensity was determined by densitometry software and normalized with β-actin protein.
Lipid Peroxidation Assay
Immunofluorescence staining of 4-hydroxynonenal (4HNE) was used as a marker of lipid peroxidation. Cells cultured on slides were incubated with hemin and hemin plus Fer-1 followed by incubation with primary anti-4HNE antibody (1:100, Abcam, ab46545) and fluorochrome-labeled secondary antibody. Slides were imaged on an Olympus IX73 microscope (Olympus).
Cell Viability Assay
Cell viability was measured by CytoSelect Cell Viability and Cytotoxicity assay kit (Cell BioLabs) using two different methods per the manufacturer’s recommendations. Percent change in cell viability from the control group was plotted.
Data Analysis
Power analysis was made at α = 0.05. Based on the hemoglobin index data for HT in our past studies (control, 1.5 ± 3.0; and diabetic, 13 ± 4.0, means ± SD), a sample size of eight per group was predicted to provide at least 85% power to detect the effect of disease on bleeding and 40% extra were added because of the increased mortality with diabetes (n = 12/group). Neurobehavioral tests (composite score and ART) were analyzed using disease (control vs. diabetes) × treatment (DFX vs. vehicle) and time (day 3 vs. day 14) with three-way ANOVA. Cognition data (RI and d2) were analyzed as change from baseline at day 14 using a two-way ANOVA (disease × treatment). Vascularization, blood-brain barrier (BBB) permeability, NVU remodeling, and microglia morphology in the cortex or striatum were analyzed similarly using two-way ANOVA. Vascularization indices between control and diabetic female rats subjected to sham surgery were compared by Student’s t test. To investigate sex and treatment (DFX) interactions, new ANOVA analyses (two-way, Figs. 2E, 3, insets, and 4; or three-way, Fig. 2F) were performed using female data from this study and male data from our published study (8). Cell culture studies with DFX were analyzed using a 2 DFX (yes vs. no) × 2 hemin (yes vs. no) ANOVA. Cell culture studies with Fer-1 that included three groups were analyzed by one-way ANOVA. A Tukey’s test was used for post hoc analyses. Significance was determined at a type I error rate of 5%. GraphPad Prism version 8.1.2 was used for all analyses. Results are given as means ± SE. For three-way ANOVA comparisons, significance levels are given in the figure legend. Significant treatment (DFX), disease (diabetes), time, and sex effects are indicated by #, ^, *, and $ signs, respectively. Any significant interaction is indicated by a letter throughout the figures. For two-way ANOVA comparisons, ANOVA tables are shown on each figure.
Figure 3.
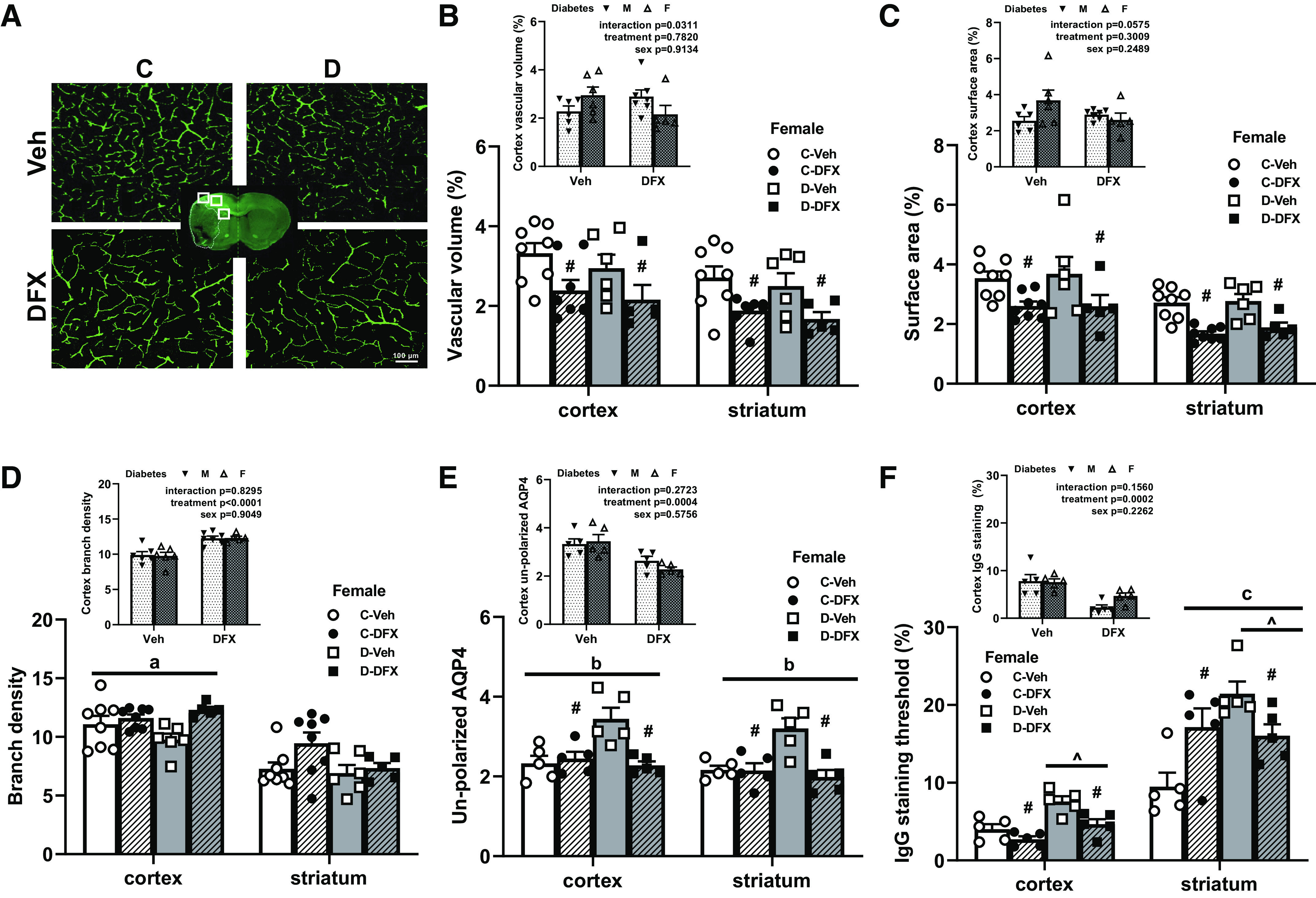
Diabetes caused greater poststroke NVU remodeling and BBB disruption but not vasoregression in female rats. DFX and diabetes effects are indicated by # and ^, respectively. Any interaction is indicated by a letter. A: representative images of FITC-filled vasculature acquired from the ipsilateral cortex. There was no difference between control and diabetic female rats treated with vehicle. DFX treatment lowered vascular volume (B) and surface area (C) both in the cortex and striatum in both control and diabetic groups (#P < 0.01). Comparison of female diabetic rats to male diabetic animals from our previously published work showed an interaction indicating DFX effects both sexes differently (B and C, insets). D: there was a trend for DFX increasing branch density in the cortex of diabetic rats with no effect in controls (aP = 0.07). There was no difference among groups in the striatum. Comparison of male and female diabetic rats showed a clear treatment effect in both sexes (D, inset). E: unpolarized AQP4 was increased in untreated diabetic animals, suggesting NVU remodeling in both cortex and striatum. DFX lowered this index only in diabetic rats resulting in a disease by treatment interaction (bP = 0.005). DFX was effective in both sexes (E, inset). F: IgG staining was greater in diabetic rats in both cortex (^P < 0.003) and striatum (^P < 0.01). DFX lowered IgG staining in the cortex in both control and diabetic animals (#P < 0.003). In the striatum, there was a diabetes and DFX interaction resulting from DFX increasing IgG staining in controls and lowering it in diabetics (cP = 0.003). DFX was effective in both sexes (F, inset). BBB disruption was seen in diabetes + vehicle animals, whereas DFX prevented both (E and F). Results are shown as means ± SE and scattered graphs with individual data points. All panels were analyzed two-way ANOVA. BBB, blood-brain barrier; C, control; D, diabetes; DFX, deferoxamine; F, female; M, male; NVU, neurovascular unit; Veh, vehicle.
Figure 4.
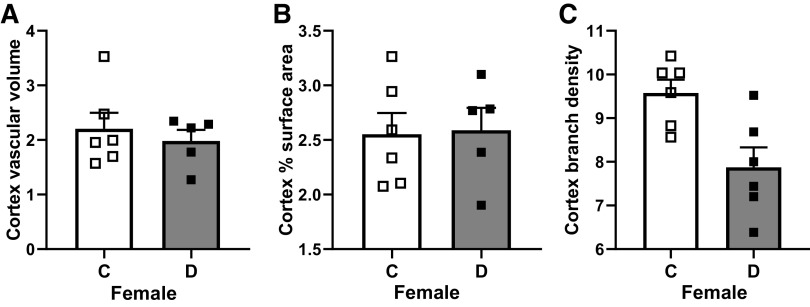
Diabetes does not impact cerebral vascularization patterns in naive (no stroke) female animals. A–C: cortical vascular volume, surface area, and branch density were similar between control and diabetic female rats. Results are shown as means ± SE and scattered graphs with individual data points. Data were analyzed by Student’s t test. C, control; D, diabetes.
RESULTS
Deferoxamine Prevents Stroke-Mediated Cognitive Decline
Mortality in the acute phase of stroke (day 1) was higher in the diabetic groups after thromboembolic stroke (Fig. 1), which was expected as we have previously shown (20). On day 1, there were six deaths in the diabetic cohorts and only 1 death in the control group (death due to surgery not included). Diabetes + vehicle rats showed greater motor deficits as indicated by the lower composite scores on either day 3 or day 14 (Fig. 2A). Although most control vehicle rats recovered, diabetes vehicle rats showed little improvement by day 14. DFX improved deficits in both cohorts with the largest improvement in diabetic rats on day 14 (Fig. 2A). ART, a test that depends on fine sensorimotor skills, showed similar deficits in both groups on day 3, which were improved by DFX (Fig. 2B). By day 14, control vehicle animals showed improvement, but there was high variability in diabetic animals resulting in a trend for time effect (Fig. 2B, *P = 0.07). DFX treatment was effective in normalizing ART scores in both groups. Working memory, measured by NOR (RI and d2) and analyzed as a change from baseline on day 14, showed that there was a diabetes and DFX interaction such that DFX had no effect on controls but improved RI (Fig. 2C) and d2 (Fig. 2D) indices in diabetic animals (aP = 0.02). When compared with the male cohorts in a previous study (8), female rats showed a general lower RI at baseline. There was a disease effect with RI being lower in diabetic cohorts (Fig. 2E). After stroke, RI was significantly lower in female and male diabetic untreated animals than in control counterparts (Fig. 2F). There was also a sex effect with RI being lower in female rats, and this effect was greater in the diabetic cohort. Interestingly, DFX treatment worsened the RI index in control animals. In diabetic cohorts, DFX prevented a further decline in males and improved cognition in females.
Vasoregression Is Not Found in Diabetic Female Animals after Stroke
At day 14 after stroke surgery, there was no difference in vascular volume or surface area in the cortex or striatum of control or diabetic female rats (Fig. 3, B and C). Interestingly, DFX treatment lowered both indices in both control and diabetic female animals (#P < 0.01). DFX did not affect cortical branch density in controls, but there was a trend for restored levels in the diabetic cohort (Fig. 3D, aP = 0.07). There was no difference between the groups in the striatum. Since these results were different from our published results in male rats (8), we next compared the data from diabetic female rats to our published data with diabetic male rats to investigate potential sex differences (Fig. 3, insets). There was a sex and treatment interaction that indicated that DFX lowered vascular volume and surface area indices in females but increased it in males (Fig. 3B, inset). On the other hand, DFX increased branch density in both sexes (Fig. 3D, inset). To clarify the impact of diabetes alone on the vascularization in the brains of female rats, vascular volume, surface area, and branch density were measured in both control and diabetic naive female animals without any treatment, and there was no significant difference (Fig. 4).
Deferoxamine Treatment Attenuates Poststroke Gliovascular Remodeling in Diabetes
There was a significant increase in unpolarized AQP4, an index of gliovascular remodeling in the diabetes group (Fig. 3E). DFX treatment prevented this in diabetic but not control rats in the cortex resulting in an interaction (bP = 0.005). Results in the striatum were similar. BBB permeability was greater in the diabetes vehicle group, especially in the striatum (Fig. 3F). DFX was effective in improving BBB integrity in the cortex in both groups. In the striatum, there was a disease and treatment interaction resulting in greater permeability in controls and lower permeability in diabetic animals with DFX treatment (Fig. 3F, cP = 0.003). We next compared the diabetic female data set from this study to the diabetic male data set from our earlier results (8). In both sexes, DFX was effective in lowering gliovascular remodeling and BBB permeability (Fig. 3, E and F, insets).
Deferoxamine Treatment Does Not Attenuate Poststroke Microglial Activation in Diabetes
Microglial cell body size in the cortex area was increased in the diabetes vehicle group, indicative of microglia activation (Fig. 5A). DFX did not affect this response in diabetes, whereas it increased it in controls (aP < 0.01). Other morphology indices including the number of protrusions, end points, and total branch length were lower in the diabetes vehicle group suggesting an activated amoeboid morphology (Fig. 5, B–D). There was diabetes and DFX interaction such that DFX treatment had no effect on these measures in diabetes but lowered them in controls, again suggesting activation of the microglia with the treatment (bP < 0.001). A similar effect was observed in the striatum. A comparison of diabetic female animals to our historical data in male animals treated with vehicle and DFX suggested that there was no sex difference in vehicle cohorts, but DFX increased branching in males, indicative of restoration of ramified morphology with no effect in females (Fig. 5, B–D, insets). The pro- and anti-inflammatory status of microglia were assessed by expression levels of TNF-α and arginase 1 in Iba1 positive cells. Qualitatively TNF-α staining was more profound in the diabetes vehicle group, whereas DFX prevented this response (Fig. 5E). Arginase 1 staining was also greater in diabetes, but DFX had no effect (Fig. 5F). However, arginase-1 colocalization in microglia became more evident in control DFX samples suggesting activation of anti-inflammatory phenotype.
Figure 5.
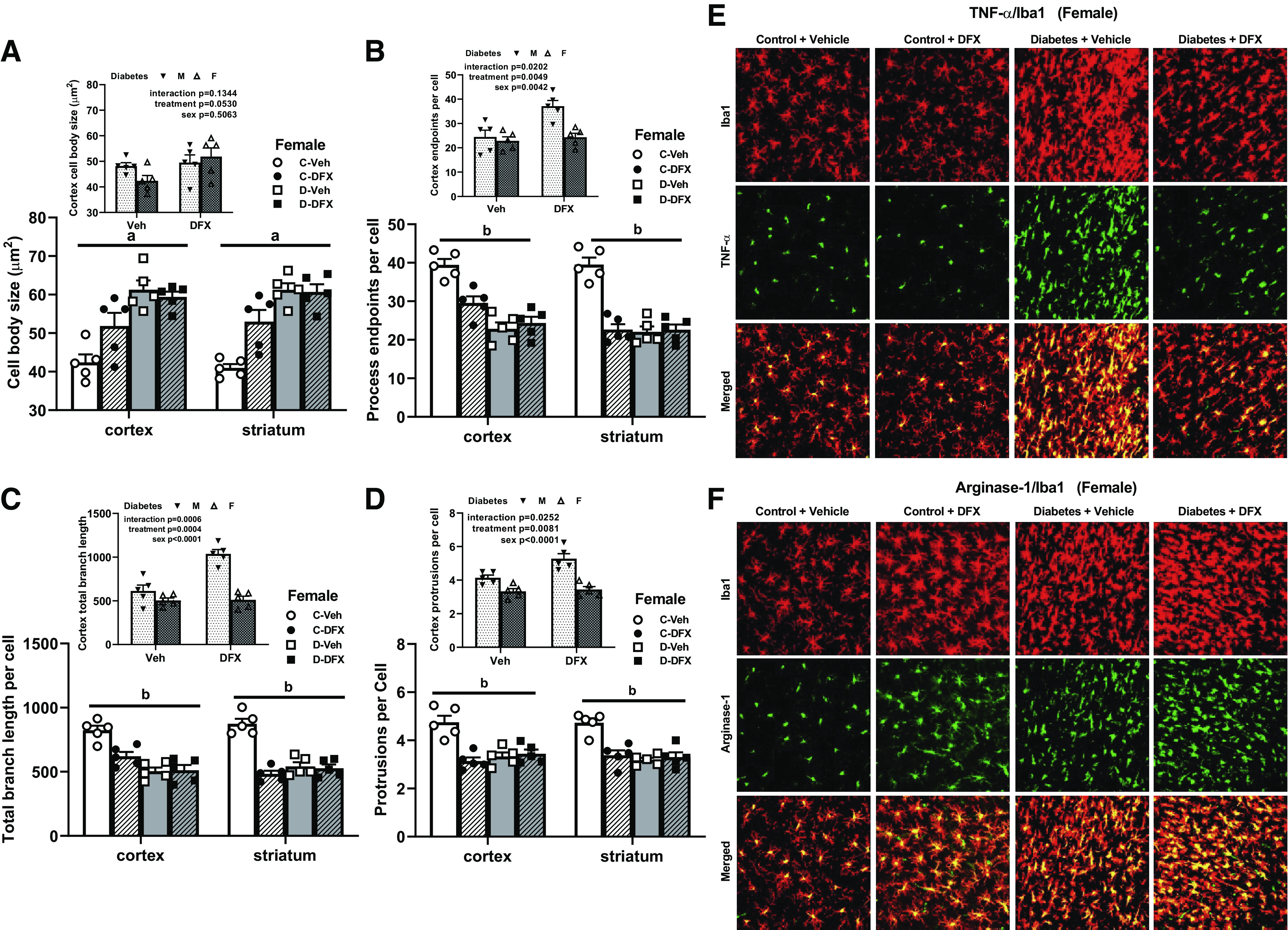
Poststroke microglia activation was greater in diabetic female animals. A: cell body size was increased in both cortex and striatum of diabetic animals indicating activation of microglia. DFX increased cell body size in controls and did not affect diabetic animals either in cortex or striatum (aP < 0.01). Comparison of male and female diabetic rats indicated an increase in body size with DFX in both groups (A, inset). Process end points per cell (B), total branch length (C), and protrusions (D) were all lower in diabetic animals, indicating activation consistent with the loss of ramified morphology. DFX decreased these indices in controls and had no effect in diabetic animals (bP < 0.001). Comparison of male and female diabetic rats suggested that DFX reverses these morphological changes in male but not male diabetic rats (B–D, insets). E and F: representative images of colocalization of microglia marker Iba1 with TNF-α or arginase 1 suggested that diabetes increases both proinflammatory and anti-inflammatory markers, respectively. DFX prevented TNF-α increase but had no effect on arginase 1. In the control group, DFX appeared to increase anti-inflammatory arginase 1. Results are shown as means ± SE and scattered graphs with individual data points. All panels were analyzed two-way ANOVA. C, control; D, diabetes; DFX, deferoxamine; F, female; M, male; Veh, vehicle.
Deferoxamine prevents ferroptotic cell death in both female and male BMVECs.
In contrast to our findings in male diabetic animals (8, 22), we did not see a decrease in vascularization after stroke in female diabetic rats. So, we first looked at the expression of markers of different cell death pathways in female versus male cell lines under diabetes (HG + P) and stroke (hypoxia)-like conditions. There was a twofold increase in IREB2 expression, a marker of ferroptosis, in female cells as opposed to a robust (∼10-fold) increase in male cells (Fig. 6, A and B); please note the scale difference in IREB2 for female and male cells. This confirmed our past findings in primary BMVECs from control Wistar and diabetic Goto-Kakizaki (GK) rats which showed greater ferroptosis in cells isolated from male diabetic animals (8). We next compared ferroptosis in both cell lines in more detail. Erastin, an inducer of ferroptosis, did not significantly change the expression of FHC (an important regulator of ferroptosis by binding free iron) in either cell line but ferroptosis inhibitor Fer-1 increased FHC in male cells (Fig. 6C). GPX4 is a phospholipid hydroperoxidase that protects cells against membrane lipid peroxidation, a key feature of ferroptosis. In female cells, there was no change, but erastin significantly decreased the expression of GPX4, which was reversed by Fer-1 treatment, in male cells (Fig. 6D). IREB2 levels were greater in male cells. Erastin did not cause a further increase, but ferrostatin-1 lowered it to female levels (Fig. 6E).
Figure 6.
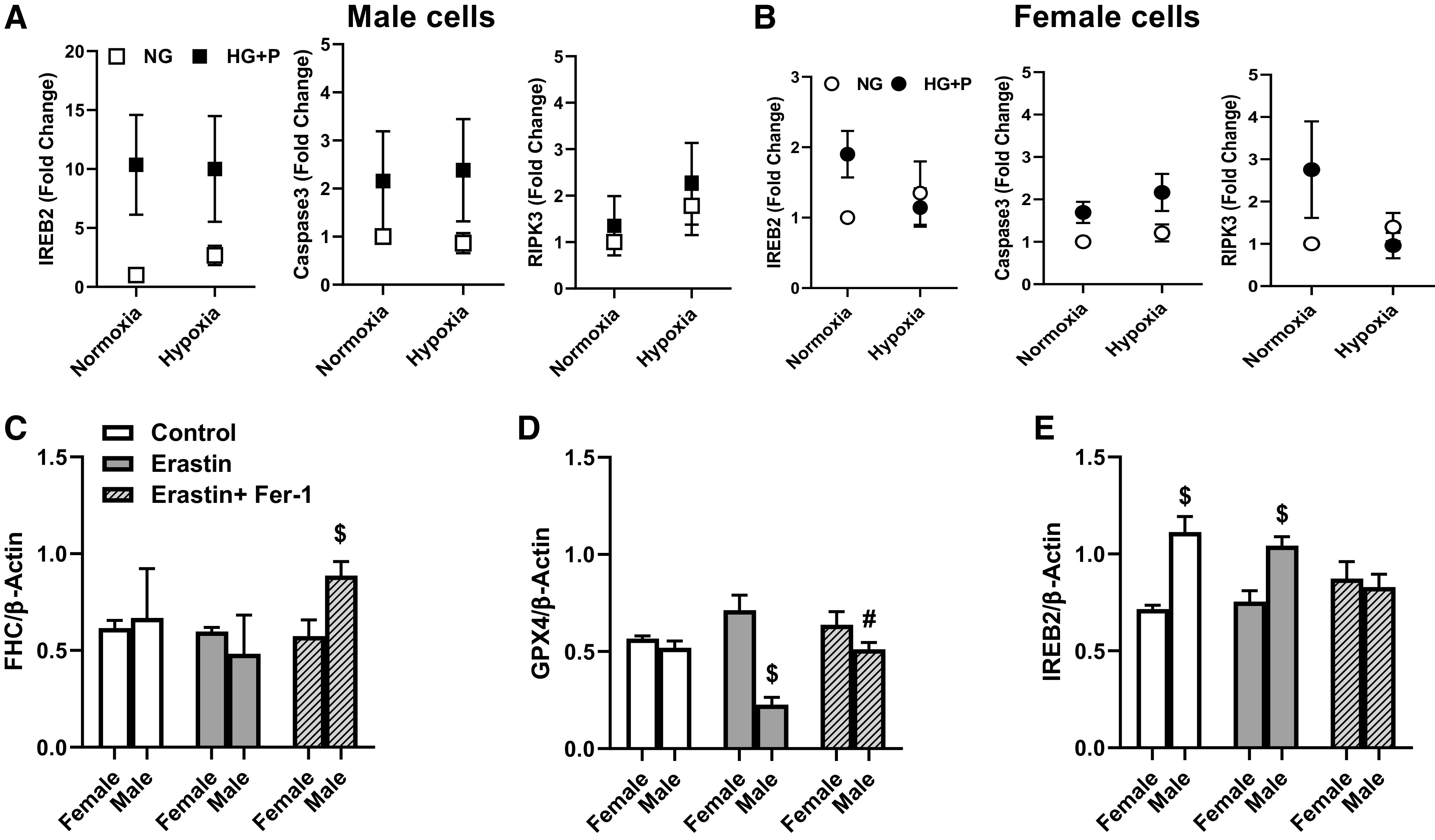
Diabetes-like conditions increased IREB2 expression to a greater extent in male cells (A) compared with female cells (B), as well as to markers of apoptosis (caspase 3) and necroptosis (RIPK3), especially under hypoxia environment. C: treatment of cells with ferroptosis inducer erastin did not affect FHC in either female or male cells, but there was an increase in FHC in male cells treated with erastin plus ferroptosis inhibitor Fer-1 ($P < 0.05). D: GPX4 levels were similar in control cells. Erastin lowered GPX4 in male cells ($P = 0.04) but did not affect female cells. Fer-1 recovered GPDX4 levels in male cells (#P = 0.0041 vs. erastin). E: IREB2 levels were greater in male cells in control and erastin groups ($P = 0.001). Fer-1 lowered IREB2 in male cells to levels observed in female cells. Results are shown as means ± SE of 3 individual experiments in triplicate. For C–E, three-way ANOVA was used. FHC, ferritin heavy chain; GPX4, glutathione peroxidase 4; IREB2: iron responsive element-binding protein 2; RIPK3, receptor interacting serin/threonine kinase 3.
We next used hemin, a product of hemoglobin, to mimic in vivo HT conditions and measured lipid peroxidation, a key feature of ferroptosis. In both cell lines, hemin increased lipid peroxidation (Figs. 7A and 8A). To complement in vivo studies, we investigated the effect of DFX on hemin-induced ferroptosis. In female cells, hemin lowered cell survival and DFX improved survival under control or hemin conditions (Fig. 7B). DFX decreased GPX4 and NRF2 under control conditions but prevented hemin-induced decreases in these protective proteins, resulting in a treatment (DFX) and intervention (hemin) interaction (Fig. 7, C and D). Hemin increased FHC but DFX had no effect (Fig. 7E). There were no changes in ACSL4 levels in female cells (Fig. 7F). In male cells, DFX prevented the hemin-induced decrease in cell viability (Fig. 8B). This was accompanied by increases in protective GPX4 and NRF2 and prevention of hemin-induced increase in ACSL4 (Fig. 8, C, D, and F). Hemin increased FHC (Fig. 8E) and DFX did not affect this response.
Figure 7.
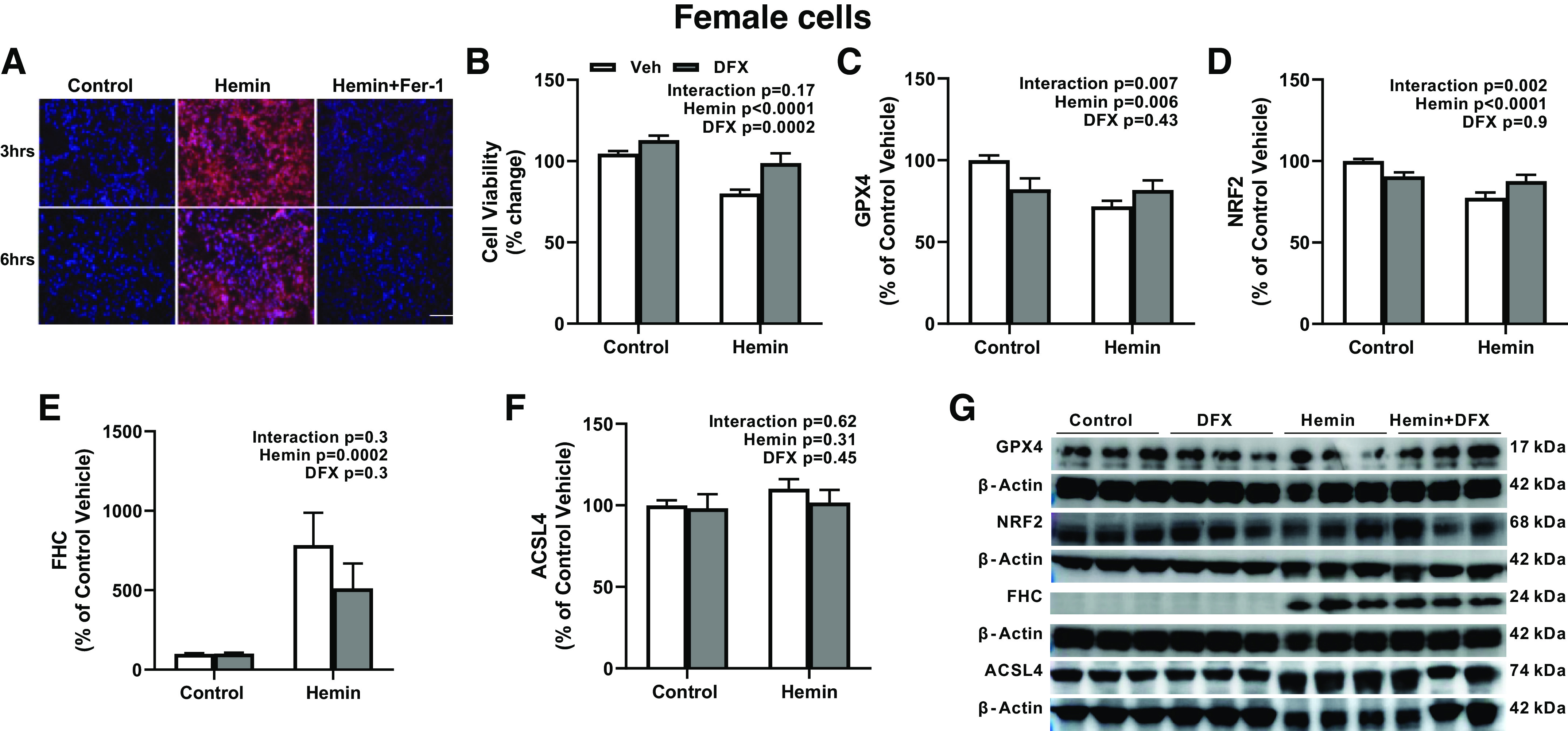
Hemin stimulates ferroptosis in female human immortalized BMVEC line (hCMEC/D3). A: representative images showing increased lipid peroxidation by hemin, which was prevented by Fer-1. B: hemin lowered cell survival. DFX improved survival in both control and hemin-treated cells. Hemin decreased GPX4 (C) and NRF2 (D) levels but increased FHC (E) levels. DFX treatment had differential effects by decreasing GPX4 and NRF2 levels in control cells but increasing those in hemin-treated cells. DFX did not affect the FHC level. ACSL4 level (F) had no significant change in all groups. Representative images (G) were obtained from three individual experiments in triplets. Results are shown as means ± SE of 3 individual experiments in triplicate. All panels were analyzed with two-way ANOVA. ACSL4, acyl-CoA synthetase long-chain family member 4; DFX, deferoxamine; GPX4, glutathione peroxidase 4; FHC, ferritin heavy chain; IHC, immunohistochemistry; NRF2, nuclear factor erythroid 2-related factor 2.
Figure 8.
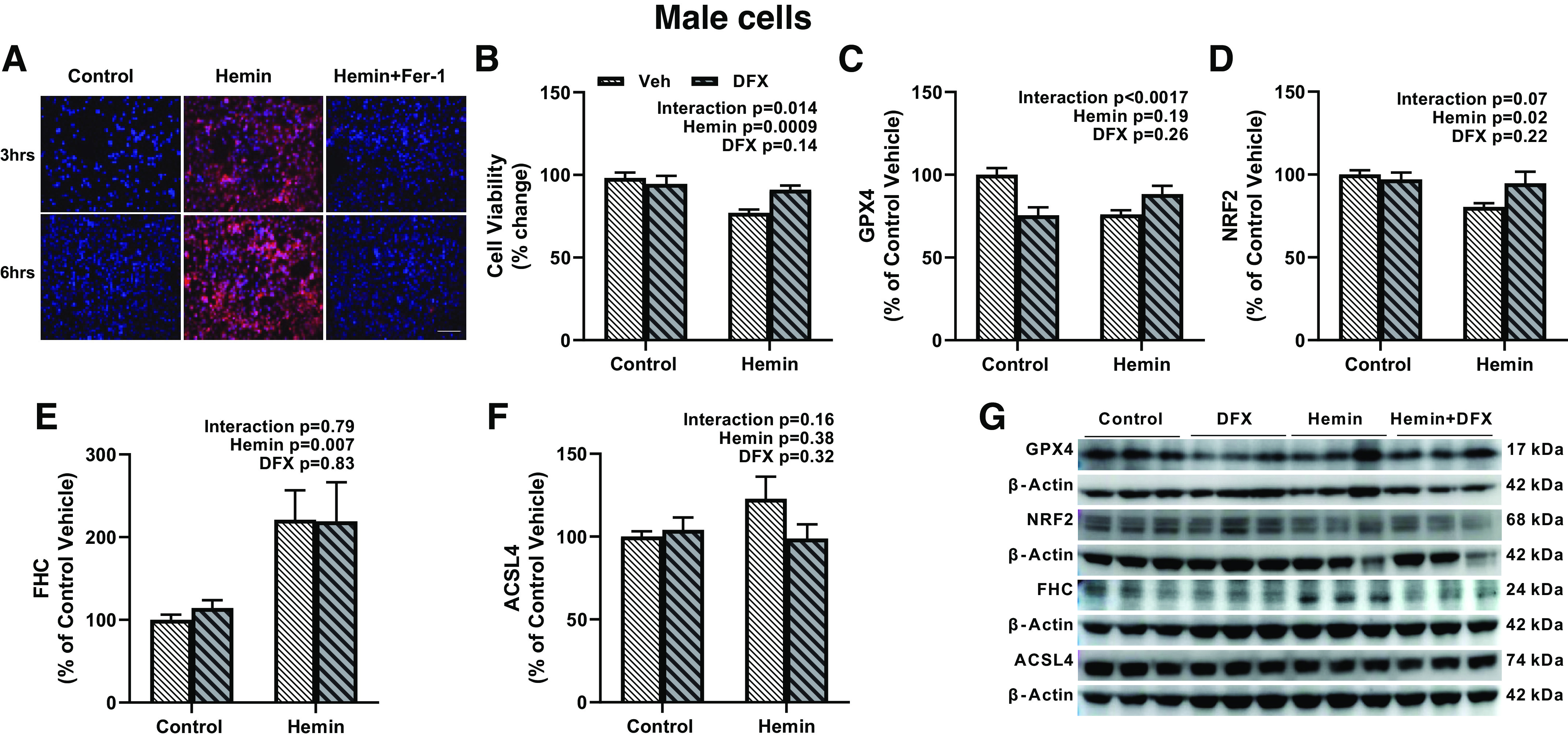
Hemin stimulates ferroptosis in male human immortalized BMVEC line (HBEC-5i). A: representative images showing increased lipid peroxidation by hemin, which was prevented by Fer-1. B: hemin lowered cell viability. DFX treatment improved viability in the hemin but not control groups. Hemin decreased GPX4 (C) and NRF2 (D) levels but increased FHC levels (E). Please note the difference in y-axis in IHC levels in E to the same in Fig. 7E, which shows a more robust FHC expression in female cells. DFX treatment had differential effects by decreasing GPX4 and NRF2 levels in control cells but increasing these protective mechanisms in hemin-treated cells. There was no effect on FHC. F: there was no significant change in ACSL4 expression. G: representative images were obtained from 3 individual experiments in triplets. Results are shown as means ± SE of 3 individual experiments in triplicate. All panels were analyzed with two-way ANOVA. ACSL4, acyl-CoA synthetase long-chain family member 4; DFX, deferoxamine; GPX4, glutathione peroxidase 4; FHC, ferritin heavy chain; IHC, immunohistochemistry; NRF2, nuclear factor erythroid 2-related factor 2.
DISCUSSION
Several clinical facts provided a translational rationale for this preclinical study. First, diabetes increases the risk and severity of neurovascular and neurodegenerative diseases such as ischemic stroke and ADRD including PSCI (1, 2, 33). Second, diabetes increases the risk of vascular injury, bleeding into the brain, after ischemic stroke (13, 14). Third, dysregulation of iron homeostasis in the brain is implicated in neurological diseases including ADRD and stroke (5, 34–36). In a series of studies integrating these observations into preclinical models, we showed that in male diabetic animals, 1) there is significant cerebral vasoregression and endothelial cell death after stroke (19, 22); 2) vascular loss correlates with the degree of vascular injury, i.e., presence of bleeding into the brain and poor functional outcomes (22, 23); and 3) iron chelation with DFX prevents vasoregression and associated cognitive decline (8), suggesting that iron chelation may be a viable therapeutic option. We also reported that diabetes negates cerebrovascular protection in young female rats and causes severe HT after ischemic stroke (11). Numerous pieces of evidence have emerged demonstrating how diagnostic tools or therapeutic targets identified in men may not work for women (37, 38). Women suffer disproportionately from diabetes, as well as ischemic stroke and PSCI. Inadequate inclusion of female and diabetic animals in preclinical studies has limited our understanding of the underlying and interacting mechanisms in the development and progression of cognitive decline in the diabetes (38). The current study addressed this important gap and asked the questions: 1) How does diabetes impact cerebrovascularization in female rats? 2) Does diabetes contribute to cerebral vasoregression and neurovascular remodeling after ischemic stroke as occurs in males? 3) Does DFX prevent vasoregression and improve outcomes in female rats? and 4) Are there differences in BMVEC survival and cell death pathways between female and male cells?
Brain health is directly affected by cardiovascular and metabolic health (15). Cerebrovascular function and integrity are critical for not only much-needed blood delivery to the brain but also trophic coupling between the cells of the NVU (16) and angiogenesis (39). We focused on vascularization and structural changes at the vasoglial interface for multiple reasons. First, microvascular dysfunction is a common pathology between diabetes and dementia. Second, all neurocentric approaches have failed to identify any therapies for stroke or cognitive impairment. In contrast to our hypothesis that DFX will prevent vasoregression and improve outcomes in female diabetic rats, we did not observe any differences in vascularization indices in the cortex and striatum before or after stroke between control and diabetic animals. However, diabetic animals showed baseline cognitive deficits, which further declined after stroke, and experienced greater and sustained physical deficits (22, 23). One can argue that bleeding into the brain may not be a factor in mediating vascular cell death, and vascularization status may not be associated with a poor recovery in female rats. Another possibility is that vascularization may be affected in other brain regions. We have previously reported lower vascular surface area in the dentate gyrus of diabetic female rats as compared with control rats after thromboembolic occlusion of MCA (40). It is also possible that although there is no change in vascular volume, surviving endothelial cells may lose their integrity and/or change phenotype. Studies suggest that cells that escape stress-induced cell death undergo endothelial mesenchymal transformation (EndMT) or senescence (41, 42). There are sex-specific differences in the endothelial cell biology/pathology (43, 44). BMVECs from female animals are more resistant to cell death (44), supporting the idea that female cells can develop EndMT or senescence if they survive. Cells that acquire these phenotypes lose their barrier and trophic functions and become proinflammatory (42). Two recent reviews provide an interesting synthesis of the link between iron dysregulation, senescence, and ferroptotic neuronal cell death in the brain (5, 45). The diabetic microenvironment promotes senescence (46). Our findings showing that there is greater permeability, gliovascular remodeling, and proinflammatory microglia activation in diabetic animals support this argument and warrant further studies on the nature of microvascular phenotypic changes.
Since diabetes causes greater HT after stroke and increasing evidence suggests that iron can be detrimental and play an important role in neurodegenerative diseases (7, 34, 36), we used iron chelation by DFX as a therapeutic strategy. From a mechanistic perspective, DFX prevented stroke-induced gliovascular remodeling and BBB disruption in diabetic animals. In contrast to our previous study in males, we did not see an effect of DFX on microglial morphology in diabetic rats. From a functional perspective, these changes were associated with better motor and cognitive function. Interestingly, DFX caused a decrease in all vascularization parameters in both groups. Since these animals also showed better functional outcomes, one can speculate that this decrease in vascularization indices may not be pathological but adaptive to improve vascular integrity/phenotype, which remains to be tested. We took advantage of the existing vascularization data in our published study on male animals and performed new analyses to investigate potential sex differences. The current findings and past results collectively suggest that there are sex and preexisting comorbid disease status differences in vascular changes in response to DFX. Although the phase II i-DEF trial failed to show any potential beneficial effect of DFX in patients with intracerebral hemorrhage, long-term cognitive outcomes remain unknown (47). There remains great interest in the use and effective delivery of iron chelators in neurodegenerative disorders (35, 48). Several findings of this study are important to highlight. First, DFX lowered recognition memory in both female and male control rats. Second, DFX decreased microglia ramification indices in control animals, suggesting that it promotes a proinflammatory phenotype in controls. Finally, in vitro findings showed an interaction in which DFX lowered protective NRF2 and GPX4 under control conditions but improved the levels of these proteins under hemin-induced stress conditions. This data set collectively suggests that iron chelation under normal conditions can be detrimental. The sex and disease interactions we discussed above should be considered in studies moving forward with these therapeutics.
Ferroptosis is gaining attention in neurodegenerative diseases and brain injury (5, 49, 50). This nonapoptotic iron-dependent form of cell death occurs when cellular antioxidant defense systems are overwhelmed. It is accepted that cell death must be inhibited by DFX to call it ferroptosis. We have shown that iron promotes robust ferroptosis in primary male BMVECs isolated from diabetic animals, and DFX was effective in improving cell viability in this group (8). A recent article also reported iron-mediated ROS-dependent changes in the endothelial cell genome that is associated with senescence and ferroptosis, and DFX-conjugated nanoparticles prevented these responses (51). Since we did not observe vasoregression in diabetic female rats after stroke, we first compared cell death under conditions that mimic stroke and diabetes. Male cells showed a greater increase in IREB2 expression than female cells. Based on this, we focused on ferroptosis markers in cells exposed to erastin, an inducer of ferroptosis, and hemin, a product of the oxidation of heme in the blood, to mimic HT. Our results show that ferroptosis occurs and DFX improves cell survival in both cell lines, but there are differences in some of the readouts of ferroptosis. Although hemin induced robust lipid peroxidation in both cell lines, changes in other markers of ferroptosis were not as robust, suggesting the activation of other pathways that we did not investigate. ACSL4 was increased with hemin in male but not in female cells. GSH levels did not change in male cells but decreased in female cells after the hemin challenge. These subtle differences may affect the time line of ferroptosis development in vitro and in vivo, possibly explaining why there is no vasoregression in diabetic female rats after stroke.
We recognize the limitations of our study. To be comparable with earlier studies with male rats, we followed the animals only for 14 days. Longer monitoring is desirable but higher mortality and poor overall conditions necessitating early termination often result in a low number of animals completing the study (5, 8). In this study, some animals were not perfused well with FITC at termination, and there was no signal to analyze the vasculature. In the diabetic group, some brains were extremely fragile for tissue processing because of the ischemic injury. Collectively, these led to an uneven number of animals in some of the histological assessments. Another limitation is that we did not measure infarct size or HT in this study. Based on the images of the FITC-filled vessels that show very faint or black areas in the infarcted region, we believe the infarct severity was comparable between groups. Only adult animals were used in this study. A combination of chronological aging and diabetes is likely to be even deadlier and may cause even few animals at the end of the study. However, the findings provide evidence that even at a relatively young age, diabetes worsens stroke recovery. We compared vascularization indices measured in diabetic female rats in this study to our previous results in diabetic male rats, which is encouraged to incorporate sex as a biological variable without the need to repeat studies in both sexes. We report only recognition memory deficits after stroke and other domains of cognition need to be evaluated. Lastly, we measured only vascularization indices in the brains and cell death pathways in BMVECs. We did not evaluate endothelial cell phenotype. Whether and to what extent microvessels undergo EndMT and/or senescence remain important questions to answer because the cerebrovascular disease is a common pathology between diabetes and dementia.
Nevertheless, the current study addresses an important problem. Clinical trials showed that glycemic control does not prevent cognitive decline (2, 52), suggesting hyperglycemia is not the sole factor. With the projected increase in the number of people with diabetes and diabetes increasing the risk of stroke and cognitive impairment in both younger male and female individuals (53), there is an urgent need to understand the underlying vascular mechanisms linking diabetes to dementia to develop mechanism-based effective therapies. As depicted in Fig. 9, our results suggest that iron chelation may provide a novel therapeutic strategy in the prevention of PSCI in both sexes. However, the underlying mechanisms may be different. Although it preserves gliovascular integrity and improves endothelial survival in females with diabetes, it inhibits vasoregression and microglial activation in males with diabetes.
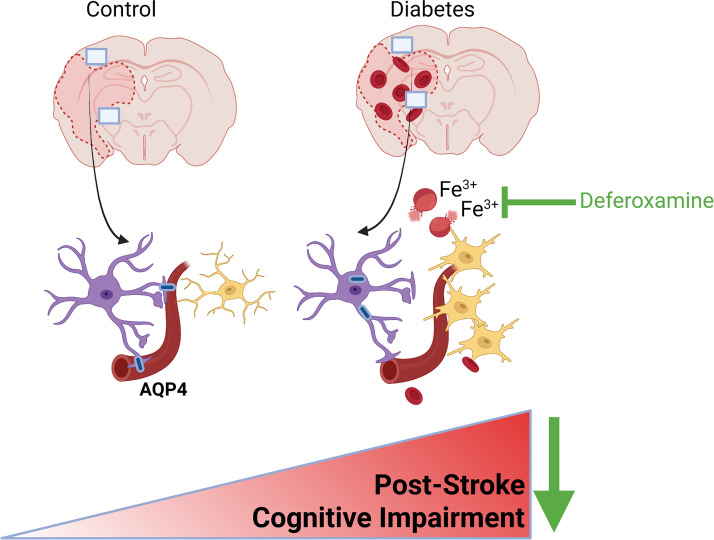
Figure 9.
Summary of findings. Our results show that diabetes does not affect cortical and striatal vascularization in female diabetic rats, and there is no vasoregression in these regions after cerebral ischemia. Current findings indicate disease and sex-specific effects of DFX on control and diabetic female and male animals, as well as on ferroptosis signaling pathways in male and female microvascular endothelial cells. These suggest that iron chelation may provide a novel therapeutic strategy in the prevention of PSCI via preservation of gliovascular integrity and improvement of endothelial cell survival in females with diabetes and via the inhibition of vasoregression and microglial activation in males with diabetes. DFX, deferoxamine; PSCI, poststroke cognitive impairment.
DATA AVAILABILITY
Data will be made available upon reasonable request.
GRANTS
This study was supported by Veterans Affairs (VA) Merit Review Grant BX000347, VA Senior Research Career Scientist Award IK6 BX004471, National Institutes of Health (NIH) Grants RF1 NS083559 (formerly R01 NS083559) and R01 NS104573 (multi-PI, S.C.F. as co-PI; to A.E.), Diabetic Complications Research Consortium Awards 17AU3831/18AU3903 supported by NIH Grant DK076169/115255 (to W.L.), and American Heart Association Postdoctoral Fellowship Post831316 (to R.C.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.C.F. and A.E. conceived and designed research; W.L., Y.A., R.C., S.J., R.A.W., M.A., and G.D. performed experiments; W.L., Y.A., R.C., S.J., R.A.W., M.A., G.D., and A.E. analyzed data; W.L., Y.A., R.A.W., M.A., G.D., and A.E. interpreted results of experiments; W.L., Y.A., R.C., S.J., and A.E. prepared figures; W.L., Y.A., and A.E. drafted manuscript; W.L., Y.A., S.C.F., and A.E. edited and revised manuscript; W.L., Y.A., R.C., S.J., R.A.W., M.A., G.D., S.C.F., and A.E. approved final version of manuscript.
REFERENCES
- 1. Srikanth V, Sinclair AJ, Hill-Briggs F, Moran C, Biessels GJ. Type 2 diabetes and cognitive dysfunction-towards effective management of both comorbidities. Lancet Diabetes Endocrinol 8: 535–545, 2020. doi: 10.1016/S2213-8587(20)30118-2. [DOI] [PubMed] [Google Scholar]
- 2. van Sloten TT, Sedaghat S, Carnethon MR, Launer LJ, Stehouwer CDA. Cerebral microvascular complications of type 2 diabetes: stroke, cognitive dysfunction, and depression. Lancet Diabetes Endocrinol 8: 325–336, 2020. doi: 10.1016/S2213-8587(19)30405-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ukraintseva S, Sloan F, Arbeev K, Yashin A. Increasing rates of dementia at time of declining mortality from stroke. Stroke 37: 1155–1159, 2006. doi: 10.1161/01.STR.0000217971.88034.e9. [DOI] [PubMed] [Google Scholar]
- 4. Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, Elkind MSV, Evenson KR, Ferguson JF, Gupta DK, Khan SS, Kissela BM, Knutson KL, Lee CD, Lewis TT, Liu J, Loop MS, Lutsey PL, Ma J, Mackey J, Martin SS, Matchar DB, Mussolino ME, Navaneethan SD, Perak AM, Roth GA; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2021 Update: a report from the American Heart Association. Circulation. 143: e254–e743, 2021. doi: 10.1161/CIR.0000000000000950. [DOI] [PubMed] [Google Scholar]
- 5. Derry PJ, Hegde ML, Jackson GR, Kayed R, Tour JM, Tsai A-L, Kent TA. Revisiting the intersection of amyloid, pathologically modified tau and iron in Alzheimer's disease from a ferroptosis perspective. Prog Neurobiol 184: 101716, 2020. doi: 10.1016/j.pneurobio.2019.101716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Morris G, Berk M, Carvalho AF, Maes M, Walker AJ, Puri BK. Why should neuroscientists worry about iron? The emerging role of ferroptosis in the pathophysiology of neuroprogressive diseases. Behav Brain Res 341: 154–175, 2018. doi: 10.1016/j.bbr.2017.12.036. [DOI] [PubMed] [Google Scholar]
- 7. Wilkinson DA, Pandey AS, Thompson BG, Keep RF, Hua Y, Xi G. Injury mechanisms in acute intracerebral hemorrhage. Neuropharmacology 134: 240–248, 2018. doi: 10.1016/j.neuropharm.2017.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Abdul Y, Li W, Ward R, Abdelsaid M, Hafez S, Dong G, Jamil S, Wolf V, Johnson MH, Fagan SC, Ergul A. Deferoxamine treatment prevents post-stroke vasoregression and neurovascular unit remodeling leading to improved functional outcomes in type 2 male diabetic rats: role of endothelial ferroptosis. Transl Stroke Res 12: 615–630, 2021. doi: 10.1007/s12975-020-00844-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chauhan A, Moser H, McCullough LD. Sex differences in ischaemic stroke: potential cellular mechanisms. Clin Sci (Lond) 131: 533–552, 2017. doi: 10.1042/CS20160841. [DOI] [PubMed] [Google Scholar]
- 10. Manwani B, McCullough LD. Sexual dimorphism in ischemic stroke: lessons from the laboratory. Womens Health (Lond) 7: 319–339, 2011. doi: 10.2217/whe.11.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li W, Ward R, Valenzuela JP, Dong G, Fagan SC, Ergul A. Diabetes worsens functional outcomes in young female rats: comparison of stroke models, tissue plasminogen activator effects, and sexes. Transl Stroke Res 8: 429–439, 2017. doi: 10.1007/s12975-017-0525-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Khatri P, Wechsler LR, Broderick JP. Intracranial hemorrhage associated with revascularization therapies. Stroke 38: 431–440, 2007. doi: 10.1161/01.STR.0000254524.23708.c9. [DOI] [PubMed] [Google Scholar]
- 13. Mankovsky BN, Ziegler D. Stroke in patients with diabetes mellitus. Diabetes Metab Res Rev 20: 268–287, 2004. doi: 10.1002/dmrr.490. [DOI] [PubMed] [Google Scholar]
- 14. Nannetti L, Paci M, Baccini M, Rinaldi LA, Taiti PG. Recovery from stroke in patients with diabetes mellitus. J Diabetes Complications 23: 249–254, 2009. doi: 10.1016/j.jdiacomp.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 15. Gorelick PB, Furie KL, Iadecola C, Smith EE, Waddy SP, Lloyd-Jones DM, Bae H-J, Bauman MA, Dichgans M, Duncan PW, Girgus M, Howard VJ, Lazar RM, Seshadri S, Testai FD, van Gaal S, Yaffe K, Wasiak H, Zerna C; American Heart Association/American Stroke Association. Defining optimal brain health in adults: a presidential advisory from the American Heart Association/American Stroke Association. Stroke 48: e284–e303, 2017. doi: 10.1161/STR.0000000000000148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Iadecola C. The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron 96: 17–42, 2017. doi: 10.1016/j.neuron.2017.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Prakash R, Johnson M, Fagan SC, Ergul A. Cerebral neovascularization and remodeling patterns in two different models of type 2 diabetes. PLoS One 8: e56264, 2013. doi: 10.1371/journal.pone.0056264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Prakash R, Somanath PR, El-Remessy AB, Kelly-Cobbs A, Stern JE, Dore-Duffy P, Johnson M, Fagan SC, Ergul A. Enhanced cerebral but not peripheral angiogenesis in the Goto-Kakizaki model of type 2 diabetes involves VEGF and peroxynitrite signaling. Diabetes 61: 1533–1542, 2012. doi: 10.2337/db11-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Abdelsaid M, Prakash R, Li W, Coucha M, Hafez S, Johnson MH, Fagan SC, Ergul A. Metformin treatment in the period after stroke prevents nitrative stress and restores angiogenic signaling in the brain in diabetes. Diabetes 64: 1804–1817, 2015. doi: 10.2337/db14-1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jackson L, Dong G, Althomali W, Sayed MA, Eldahshan W, Baban B, Johnson MH, Filosa J, Fagan SC, Ergul A. Delayed administration of angiotensin II type 2 receptor (AT2R) agonist compound 21 prevents the development of post-stroke cognitive impairment in diabetes through the modulation of microglia polarization. Transl Stroke Res 11: 762–775, 2020. doi: 10.1007/s12975-019-00752-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jackson L, Dumanli S, Johnson MH, Fagan SC, Ergul A. Microglia knockdown reduces inflammation and preserves cognition in diabetic animals after experimental stroke. J Neuroinflammation 17: 137, 2020. doi: 10.1186/s12974-020-01815-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Prakash R, Li W, Qu Z, Johnson MA, Fagan SC, Ergul A. Vascularization pattern after ischemic stroke is different in control versus diabetic rats: relevance to stroke recovery. Stroke 44: 2875–2882, 2013. doi: 10.1161/STROKEAHA.113.001660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li W, Valenzuela JP, Ward R, Abdelbary M, Dong G, Fagan SC, Ergul A. Post-stroke neovascularization and functional outcomes differ in diabetes depending on severity of injury and sex: Potential link to hemorrhagic transformation. Exp Neurol 311: 106–114, 2019. doi: 10.1016/j.expneurol.2018.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ward R, Li W, Abdul Y, Jackson L, Dong G, Jamil S, Filosa J, Fagan SC, Ergul A. NLRP3 inflammasome inhibition with MCC950 improves diabetes-mediated cognitive impairment and vasoneuronal remodeling after ischemia. Pharmacol Res 142: 237–250, 2019. doi: 10.1016/j.phrs.2019.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Feeney DM, Gonzalez A, Law WA. Amphetamine, haloperidol, and experience interact to affect rate of recovery after motor cortex injury. Science 217: 855–857, 1982. doi: 10.1126/science.7100929. [DOI] [PubMed] [Google Scholar]
- 26. Eigenmann DE, Xue G, Kim KS, Moses AV, Hamburger M, Oufir M. Comparative study of four immortalized human brain capillary endothelial cell lines, hCMEC/D3, hBMEC, TY10, and BB19, and optimization of culture conditions, for an in vitro blood-brain barrier model for drug permeability studies. Fluids Barriers CNS 10: 33, 2013. doi: 10.1186/2045-8118-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Poller B, Gutmann H, Krähenbühl S, Weksler B, Romero I, Couraud P-O, Tuffin G, Drewe J, Huwyler J. The human brain endothelial cell line hCMEC/D3 as a human blood-brain barrier model for drug transport studies. J Neurochem 107: 1358–1368, 2008. doi: 10.1111/j.1471-4159.2008.05730.x. [DOI] [PubMed] [Google Scholar]
- 28. Puech C, Hodin S, Forest V, He Z, Mismetti P, Delavenne X, Perek N. Assessment of HBEC-5i endothelial cell line cultivated in astrocyte conditioned medium as a human blood-brain barrier model for ABC drug transport studies. Int J Pharm 551: 281–289, 2018. doi: 10.1016/j.ijpharm.2018.09.040. [DOI] [PubMed] [Google Scholar]
- 29. Wassmer SC, de Souza JB, Frère C, Candal FJ, Juhan-Vague I, Grau GE. TGF-β1 released from activated platelets can induce TNF-stimulated human brain endothelium apoptosis: a new mechanism for microvascular lesion during cerebral malaria. J Immunol 176: 1180–1184, 2006. doi: 10.4049/jimmunol.176.2.1180. [DOI] [PubMed] [Google Scholar]
- 30. Weksler B, Romero IA, Couraud PO. The hCMEC/D3 cell line as a model of the human blood brain barrier. Fluids Barriers CNS 10: 16, 2013. doi: 10.1186/2045-8118-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wu D, Yotnda P. Induction and testing of hypoxia in cell culture. J Vis Exp 54: 2899, 2011. doi: 10.3791/2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Miyamoto N, Maki T, Shindo A, Liang AC, Maeda M, Egawa N, Itoh K, Lo EK, Lok J, Ihara M, Arai K. Astrocytes promote oligodendrogenesis after white matter damage via brain-derived neurotrophic factor. J Neurosci 35: 14002–14008, 2015. doi: 10.1523/JNEUROSCI.1592-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Biessels GJ, Despa F. Cognitive decline and dementia in diabetes mellitus: mechanisms and clinical implications. Nat Rev Endocrinol 14: 591–604, 2018. doi: 10.1038/s41574-018-0048-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Devos D, Cabantchik ZI, Moreau C, Danel V, Mahoney-Sanchez L, Bouchaoui H, Gouel F, Rolland A-S, Duce JA, Devedjian J-C; FAIRPARK-II and FAIRALS-II studygroups. Conservative iron chelation for neurodegenerative diseases such as Parkinson's disease and amyotrophic lateral sclerosis. J Neural Transm (Vienna) 127: 189–203, 2020. doi: 10.1007/s00702-019-02138-1. [DOI] [PubMed] [Google Scholar]
- 35. Farr AC, Xiong MP. Challenges and opportunities of deferoxamine delivery for treatment of Alzheimer's disease, Parkinson's disease, and intracerebral hemorrhage. Mol Pharm 18: 593–609, 2021. doi: 10.1021/acs.molpharmaceut.0c00474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yan N, Zhang J. Iron metabolism, ferroptosis, and the links with Alzheimer's disease. Front Neurosci 13: 1443, 2019. doi: 10.3389/fnins.2019.01443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shansky RM, Murphy AZ. Considering sex as a biological variable will require a global shift in science culture. Nat Neurosci 24: 457–464, 2021. doi: 10.1038/s41593-021-00806-8. [DOI] [PubMed] [Google Scholar]
- 38. Waters A, Society For Women's Health Research Alzheimer's Disease N, Laitner MH; Society for Women's Health Research Alzheimer's Disease Network. Biological sex differences in Alzheimer's preclinical research: a call to action. Alzheimers Dement (N Y) 7: e12111, 2021. doi: 10.1002/trc2.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J Neurosci 26: 13007–13016, 2006. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ward R, Valenzuela JP, Li W, Dong G, Fagan SC, Ergul A. Poststroke cognitive impairment and hippocampal neurovascular remodeling: the impact of diabetes and sex. Am J Physiol Heart Circ Physiol 315: H1402–H1413, 2018. doi: 10.1152/ajpheart.00390.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Derada Troletti C, de Goede P, Kamermans A, de Vries HE. Molecular alterations of the blood-brain barrier under inflammatory conditions: The role of endothelial to mesenchymal transition. Biochim Biophys Acta 1862: 452–460, 2016. doi: 10.1016/j.bbadis.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 42. Acosta JC, Banito A, Wuestefeld T, Georgilis A, Janich P, Morton JP, Athineos D, Kang T-W, Lasitschka F, Andrulis M, Pascual G, Morris KJ, Khan S, Jin H, Dharmalingam G, Snijders AP, Carroll T, Capper D, Pritchard C, Inman GJ, Longerich T, Sansom OJ, Benitah SA, Zender L, Gil J. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat Cell Biol 15: 978–990, 2013. doi: 10.1038/ncb2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Huxley VH, Kemp SS, Schramm C, Sieveking S, Bingaman S, Yu Y, Zaniletti I, Stockard K, Wang J. Sex differences influencing micro- and macrovascular endothelial phenotype in vitro. J Physiol 596: 3929–3949, 2018. doi: 10.1113/JP276048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gupta NC, Davis CM, Nelson JW, Young JM, Alkayed NJ. Soluble epoxide hydrolase: sex differences and role in endothelial cell survival. Arterioscler Thromb Vasc Biol 32: 1936–1942, 2012. doi: 10.1161/ATVBAHA.112.251520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Masaldan S, Belaidi AA, Ayton S, Bush AI. Cellular senescence and iron dyshomeostasis in Alzheimer's disease. Pharmaceuticals (Basel) 12: 93, 2019. doi: 10.3390/ph12020093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Palmer AK, Tchkonia T, LeBrasseur NK, Chini EN, Xu M, Kirkland JL. Cellular senescence in type 2 diabetes: a therapeutic opportunity. Diabetes 64: 2289–2298, 2015. doi: 10.2337/db14-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Selim M, Foster LD, Moy CS, Xi G, Hill MD, Morgenstern LB, Greenberg SM, James ML, Singh V, Clark WM, Norton C, Palesch YY, Yeatts SD, i-DEF Investigators. Deferoxamine mesylate in patients with intracerebral haemorrhage (i-DEF): a multicentre, randomised, placebo-controlled, double-blind phase 2 trial. Lancet Neurol 18: 428–438, 2019. doi: 10.1016/S1474-4422(19)30069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kosyakovsky J, Fine J, Frey W, Hanson L. Mechanisms of intranasal deferoxamine in neurodegenerative and neurovascular disease. Pharmaceuticals (Basel) 14: 95, 2021. doi: 10.3390/ph14020095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B, Stockwell BR. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149: 1060–1072, 2012. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Magtanong L, Dixon SJ. Ferroptosis and brain injury. Dev Neurosci 40: 382–395, 2018. doi: 10.1159/000496922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dharmalingam P, Talakatta G, Mitra J, Wang H, Derry PJ, Nilewski LG, McHugh EA, Fabian RH, Mendoza K, Vasquez V, Hegde PM, Kakadiaris E, Roy T, Boldogh I, Hegde VL, Mitra S, Tour JM, Kent TA, Hegde ML. Pervasive genomic damage in experimental intracerebral hemorrhage: therapeutic potential of a mechanistic-based carbon nanoparticle. ACS Nano 14: 2827–2846, 2020. doi: 10.1021/acsnano.9b05821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zimering MB, Knight J, Ge L, Bahn G, Investigators V; VADT Investigators. Predictors of cognitive decline in older adult type 2 diabetes from the veterans affairs diabetes trial. Front Endocrinol (Lausanne) 7: 123, 2016. doi: 10.3389/fendo.2016.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. D'Adamo E, Caprio S. Type 2 diabetes in youth: epidemiology and pathophysiology. Diabetes care 34, Suppl 2: S161–S165, 2011. doi: 10.2337/dc11-s212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon reasonable request.