Abstract
Purpose:
The transplantation of pancreatic islets is a promising cell replacement therapy for type 1 diabetes. Subcutaneous islet transplantation is currently under investigation as a means to circumvent problems associated with standard intra-hepatic islet transplantation. As modifications are being developed to improve the efficacy of subcutaneous islet transplantation, it is important to have robust methods to assess engraftment. Experimentally, ATP-dependent bioluminescence imaging using luciferase reporter genes has been effective for non-invasively tracking engraftment. However, it was heretofore unknown if the bioluminescence of subcutaneously transplanted luciferase-expressing islet grafts correlates with diabetes reversal, a primary outcome of transplantation.
Procedures:
A retrospective analysis was conducted using data obtained from subcutaneous islet transplantations in Lewis rats. The analysis included transplantations from our laboratory in which islet donors were transgenic rats ubiquitously expressing luciferase and recipients were wild type, streptozotocin-induced diabetic rats. Data from 79 bioluminescence scans were obtained from 27 islet transplantations during the post-transplant observation period (up to 6 weeks). The bioluminescence intensity of the subcutaneously transplanted grafts, captured after the intravenous administration of luciferin, was correlated with diabetes reversal.
Results:
After subcutaneous transplantation, islet bioluminescence decreased over time, dropping >50% from 1 to 3 weeks post-transplant. Bioluminescence intensity in the early post-transplant phase (1–2 weeks) correlated with the subsequent reversal of diabetes; based on optimized bioluminescence cutoff values, the bioluminescence intensity of islets at 1 and 2 weeks predicted successful transplantations. However, intensity in the late post-transplant phase (≥4 weeks) did not reflect transplantation outcomes.
Conclusions:
Early-phase bioluminescence imaging of luciferase-expressing islets could serve as a useful tool to predict the success of subcutaneous islet transplantations by preceding changes in glucose homeostasis.
Keywords: Luciferase, Bioluminescence imaging, Subcutaneous islet transplantation, Diabetes, Type 1 diabetes
Introduction
Beta cell replacement therapy is a promising treatment option for patients with type 1 diabetes (T1D). Currently, this is accomplished by transplanting donor islets to the liver via the portal vein [1]. However, the shortage of donor organs has restricted the wide application of this therapy. Insulin-producing beta-like cells derived from stem cells provide a possible solution to address the islet shortage [2], but the site of transplantation must permit close monitoring of the transplanted cells and easy removal due to their potential for malignancy [3]. Given these limitations, the liver is not a viable option for transplantation, and subcutaneous (SC) tissue is the only site currently allowed for the transplantation of stem cell-derived beta-like cells in ongoing clinical trials.
Importantly, the SC site is also the optimal location for the implantation of macroencapsulated islets and stem cell-derived beta-like cells. As T1D is an autoimmune disease, extensive measures are required to protect transplanted cells from autoimmunity. Macroencapsulation has been introduced as a potential approach to permit transplantation in the absence of immunosuppression [4–5]. However, SC tissue is poorly vascularized, which presents a barrier to the supply of nutrients and oxygen (O2) to transplanted cells. Thus, it is important to optimize methods for transplantation at this site, and in fact, many methods have been explored to improve this critical drawback [6–10].
To develop the optimal SC transplantation strategy, the in vivo evaluation of islet engraftment is necessary. The reduction of blood glucose levels in diabetic animals (i.e., reversal of diabetes) is the gold standard for evaluating transplantation success. In addition to monitoring blood glucose, multiple modalities have been developed to better understand the dynamic changes in islet grafts. In particular, non-invasive imaging modalities, including computerized tomography (CT), magnetic resonance imaging (MRI) [11], positron emission tomography (PET) [12], single-photon emission computed tomography (SPECT) [13], and ultrasonography [14], have been increasingly used to track islet graft mass and function. Each has its pros and cons, but most notably, CT and MRI have high spatial resolution for anatomical imaging [11], whereas PET and SPECT better reflect functionality.
Luciferase (LUC)-based bioluminescence imaging (BLI) is frequently used to monitor transplanted cells under experimental conditions. In this technique, a LUC reporter gene is introduced to cells, and the LUC protein catalyzes the oxidation of the substrate luciferin, injected just prior to imaging, that emits bioluminescence in the presence of co-factors (magnesium, O2, ATP, and exogenous luciferin) [15–16], which is captured for quantification [17]. Because the bioluminescence is O2- and ATP-dependent, its intensity reflects the metabolic activity of targeted cells.
In earlier studies, LUC-expressing murine islets were transplanted into the kidney capsule or liver of wild type mice, and the bioluminescence of the grafts was captured [18]; however, their intensity fell sharply to <50% within 2 months [19–21]. In another study, SC-transplanted LUC-expressing adipocytes had a 50% drop in bioluminescence within 3 weeks after transplantation in rats [22]. In our previous work, O2-supplemented LUC-expressing islets transplanted into the SC tissue of syngeneic rats showed a stronger bioluminescent signal than non-oxygenated islets [23–24]. The bioluminescence of islet grafts measured at 1 week post-transplant was significantly higher in recipients that received a 3-day O2 inhalation therapy compared to that in recipients that did not. Subsequent blood glucose changes indicated that the O2 inhalation group also had more favorable outcomes than the control group. This suggests that BLI could predict the success of SC islet transplantations before the confirmation of diabetes reversal defined in blood glucose changes.
Because LUC-based BLI theoretically reflects functional engraftment, we hypothesized that the bioluminescence intensity of SC-transplanted islet grafts would correlate with the reversal of diabetes. However, this correlation has not been clearly elucidated. Therefore, to address this knowledge gap, we retrospectively analyzed changes in the bioluminescence of SC-transplanted LUC-transgenic (Tg) islets in diabetic rats.
Materials and Methods
Study design
A retrospective analysis was performed using LUC-based BLI scans of rats obtained after SC islet transplantation (79 scans from 27 animals). The analysis included data from islet transplantations that were performed in our laboratory in the last 3 years. Inclusion criteria were: (1) studies in which the islets were isolated from LUC-Tg donor rats; (2) recipients were wild type (LUC-negative), streptozotocin (STZ)-induced diabetic rats; (3) islets were transplanted into pre-vascularized SC sites; and (4) LUC-based BLI scans were obtained at least twice within 6 weeks of transplantation. The present analysis also includes 24 BLI scans analyzed in our previous publications [24–25]. The use of animals and animal procedures performed in this study were approved by the City of Hope Beckman Research Institute Institutional Animal Care and Use Committee.
Preparation of LUC-Tg islets
The LUC-Tg Lewis (LEW) strain was developed and provided by Dr. Eiji Kobayashi [26], and maintained in the Animal Resources Center of Beckman Research Institute of City of Hope. In these rats, expression of the LUC reporter gene is driven by the ubiquitously expressed ROSA26 promoter [26]. Islets were isolated from the pancreases of male LUC-Tg LEW rats weighing 350–550 g using our standard procedure [27].
Islet transplantation
Islet recipients were wild type female LEW rats weighing 180–200 g and rendered diabetic by a single intravenous injection of STZ (60 mg/kg, Sigma-Aldrich). Diabetes was confirmed as elevated non-fasting blood glucose >400 mg/dL. Prior to transplantation, a pre-vascularized SC graft bed was prepared on each recipient’s dorsal area using a lyophilized agarose disc impregnated with basic fibroblast growth factor, as previously described [24]. A designated number of isolated islets was prepared in polyethylene tubing (PE-50) to transplant into the pre-vascularized capsule [24]. Six hundred islets were transplanted into most recipients as standard treatment (n=24); to enhance islet transplantation outcomes, some rats received more islets or post-transplant O2 treatment (described below).
Treatments to improve islet transplantation outcomes
To improve islet transplantation outcomes, two established methods were applied: (1) transplantation of more than the standard number of islets (>600) and (2) oxygenation of the graft. Transplantation with >600 islets contained 750 islets (n=1); 1,200 islets (n=1); or 1,500 islets (n=1). The oxygenation of grafts was performed only for 600-islet transplantations via O2 inhalation therapy, administered to recipient rats for 3 consecutive days post-transplant (n=13) [24]. Recipients were housed in O2-controlled cages with 50% O2 during this period. Collectively, the analysis included the following transplantations into pre-vascularized SC capsules: 600 islets (standard, n=11), >600 islets (n=3), and 600 islets with oxygenation (n=13).
Observation of transplant outcomes in islet recipients
The non-fasting blood glucose levels of islet recipients were monitored weekly for 4 to 6 weeks after SC islet transplantation. Diabetes reversal, a primary outcome of islet transplantation, was indicated by reduced blood glucose levels (<200 mg/dL). If recipient rats exhibited diabetes reversal, their SC islet graft was removed under general anesthesia to confirm recurrent diabetes (blood glucose >400 mg/dL), indicating that euglycemia was graft-dependent.
In vivo BLI of LUC-Tg islet grafts
BLI scans of LUC-Tg islet grafts were performed as described [23–24]. Animals underwent 2 to 4 scans (average 2.9) over a span of 4 to 6 weeks following SC transplantation. To capture the bioluminescence of each islet graft, a 2.3 cm-diameter area (beyond the 1.2 cm-diameter area of the pre-vascularized SC region) was scanned at 1, 3, and 5 min after the intravenous (i.v.) injection of luciferin via the tail vein (15 mg/kg of body weight; PerkinElmer, Waltham, MA, USA). The bioluminescent signal was captured using the Lago X platform (Spectral Instruments Imaging, Tucson, AZ, USA) over 60 seconds per image. The average intensity was calculated and expressed in photons/second.
Immunostaining of islet grafts
To assess LUC expression in the grafts over the post-transplantation period, paraffin-embedded sections of the excised grafts were subjected to immunofluorescence staining. After standard rehydration and blocking procedures, slides were incubated with primary antibodies (rabbit anti-LUC 1:100 [GTX125849; GeneTex, Irvine, CA, USA] and guinea pig anti-insulin 1:100 [A0564; Agilent Dako, Santa Clara, CA, USA]) at 4°C overnight. Next, the slides were incubated with secondary antibodies (donkey anti-guinea pig 488 1:1000 and donkey anti-rabbit Cy3 1:2000 [Jackson ImmunoResearch Laboratories, West Grove, PA, USA]) at room temperature for 2 h, followed by nuclear staining with 4′,6 diamino-2-phenylindole (DAPI). Images were captured on a Zeiss Axio-Observer-Z1 microscope with Zeiss Zen software (Leica Microsystems, Buffalo Grove, IL, USA).
Statistics
Data are reported as mean ± standard error. For statistical comparisons between two groups, Student’s t-tests were performed. The associations between two variables were assessed using regression analysis. Receiver operating characteristic (ROC) curves were used to determine the optimal cutoff of the bioluminescent signal (AUC_1–5min) to predict transplantation outcomes. The optimal cutoff was defined as the value that yielded the highest Youden Index (= sensitivity + specificity − 1) [29]. To quantitatively analyze the post-transplant changes in blood glucose in individual rats, the area under the curve (AUC) for weeks 0–4 was calculated (Glucose AUC_0–4W) [28]. Statistical analysis was performed using JMP 9 (SAS Institute, Cary, NC, USA). P < 0.05 was considered statistically significant.
Results
The bioluminescence of SC-transplanted LUC-Tg islets decreases in post-transplant observation
Each BLI examination consisted of three images captured at 1, 3, and 5 min after i.v. injection of luciferin via the tail vein. Analysis of the overall bioluminescence intensity curve for all 79 scans demonstrated an exponential decrease over time after luciferin injection (Suppl. Fig.1a). To quantitatively analyze bioluminescence, we calculated the AUC of the bioluminescence intensity between 1 and 5 min (AUC_1–5min). AUC_1–5min values for the full dataset of 79 scans demonstrated a linear correlation with bioluminescence intensities at 1 min (R2=0.9867, p< 0.0001), 3 min (R2=0.9951, p<0.0001), and 5 min (R2=0.9738, p<0.0001) (Suppl. Fig.1b–d). Fig.1a shows representative BLI images obtained from the same rat 1 min after luciferin injection at 1, 2, and 3 weeks post-SC transplantation. Further analysis of all 79 scans demonstrated that the bioluminescent signals significantly decreased over the observation period of 4–6 weeks (Fig.1b). Histological analysis of the resected LUC-Tg islet grafts (>4 weeks after the transplantation) revealed that LUC protein expression was well-maintained (Fig.1c). This suggests that the decline in bioluminescence reflects a reduction in graft mass, not the attenuation of the LUC reporter.
Figure 1: The bioluminescence of SC-transplanted LUC-Tg islets decreases over time.
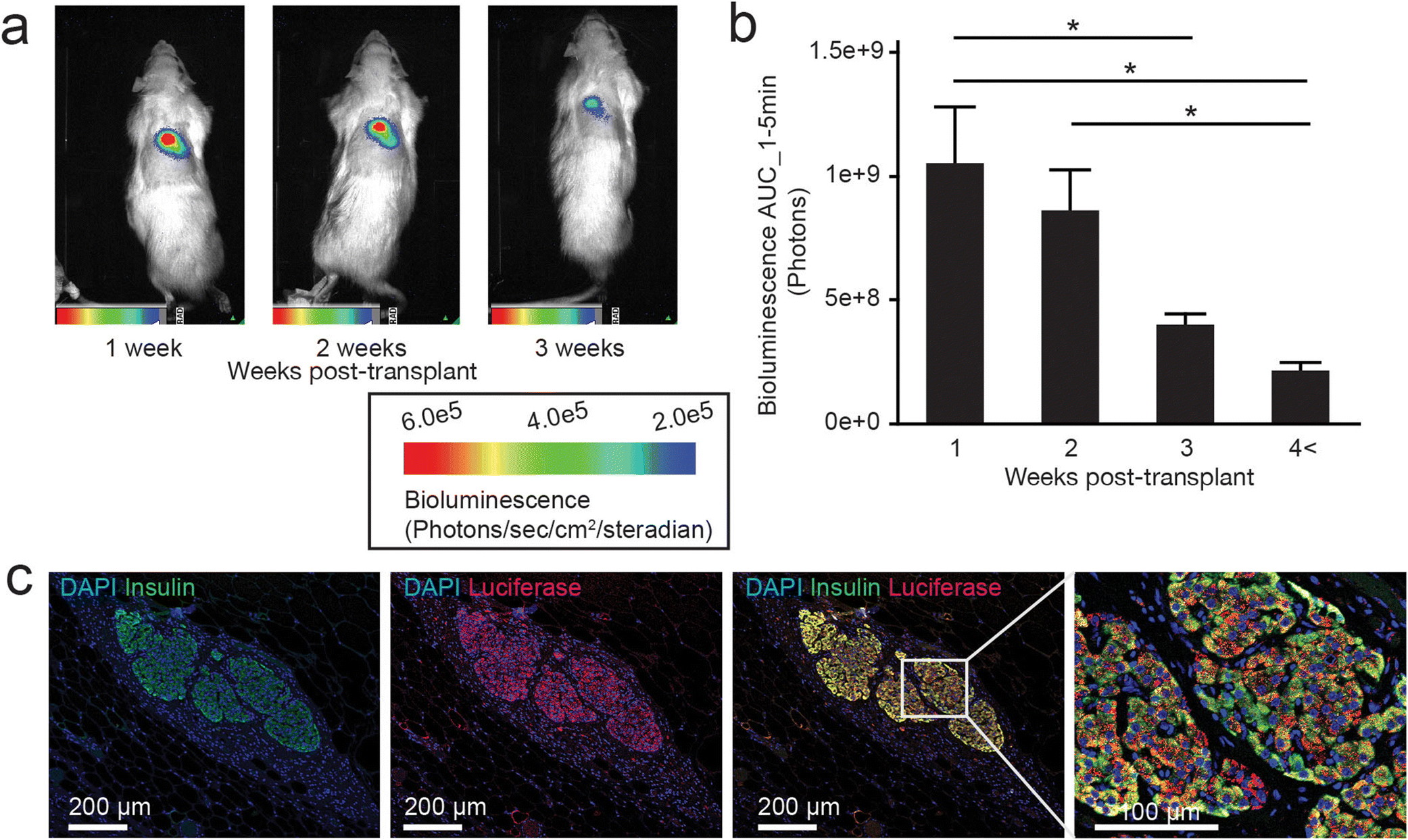
(a) Representative BLI scans obtained from the same rat at 1, 2, and 3 weeks into the post-transplant observation period, 1 min after i.v. luciferin injection. (b) Analysis of the AUC of bioluminescence intensity (AUC_1–5min) at each post-transplant period: 1 week (n=26), 2 weeks (n=23), 3 weeks (n=20), and ≥4 weeks (n=10). AUC_1–5min was calculated using data obtained 1, 3, and 5 min after i.v. luciferin injection. * p<0.05. (c) Representative histological assessment of the LUC protein expression in islet grafts collected 5 weeks after transplantation. From left to right: insulin staining for graft detection; LUC staining; and double staining of insulin and LUC at two magnifications.
Strong bioluminescence of LUC-Tg islet grafts, especially in the early post-transplant phase, is associated with diabetes reversal
Of the 27 rats that received SC islet transplantations, 9 recipients exhibited diabetes reversal, a primary outcome of islet transplantation, and 18 recipients did not (Fig.2a). Rats that exhibited reversal of diabetes achieved euglycemia after 2.9 ± 0.4 weeks post-SC transplantation (see blood glucose changes in Suppl. Fig.2). When we compared the BLI scans of LUC-Tg islet grafts in rats with vs. without diabetes reversal, the AUC_1–5min values from 1, 2, and 3 weeks post-transplant were significantly higher in rats with diabetes reversal than in rats that remained diabetic (p<0.0001, p=0.0016, and p=0.0079, respectively) (Fig.2b). However, the AUC_1–5min values for ≥4 weeks were not significantly different between groups (p=0.9808). Similar trends were demonstrated regardless of O2 treatment (Suppl. Fig.3). In addition, we validated the correlations between early-phase bioluminescence and post-transplant outcomes using a quantitative metric of blood glucose control, Glucose AUC_0–4W, which correlated with bioluminescent signals measured at 1 week post-transplant (R2=0.3616, p=0.0012; Suppl. Fig.4).
Figure 2: The bioluminescence of LUC-Tg islet grafts is higher in animals with diabetes reversal.
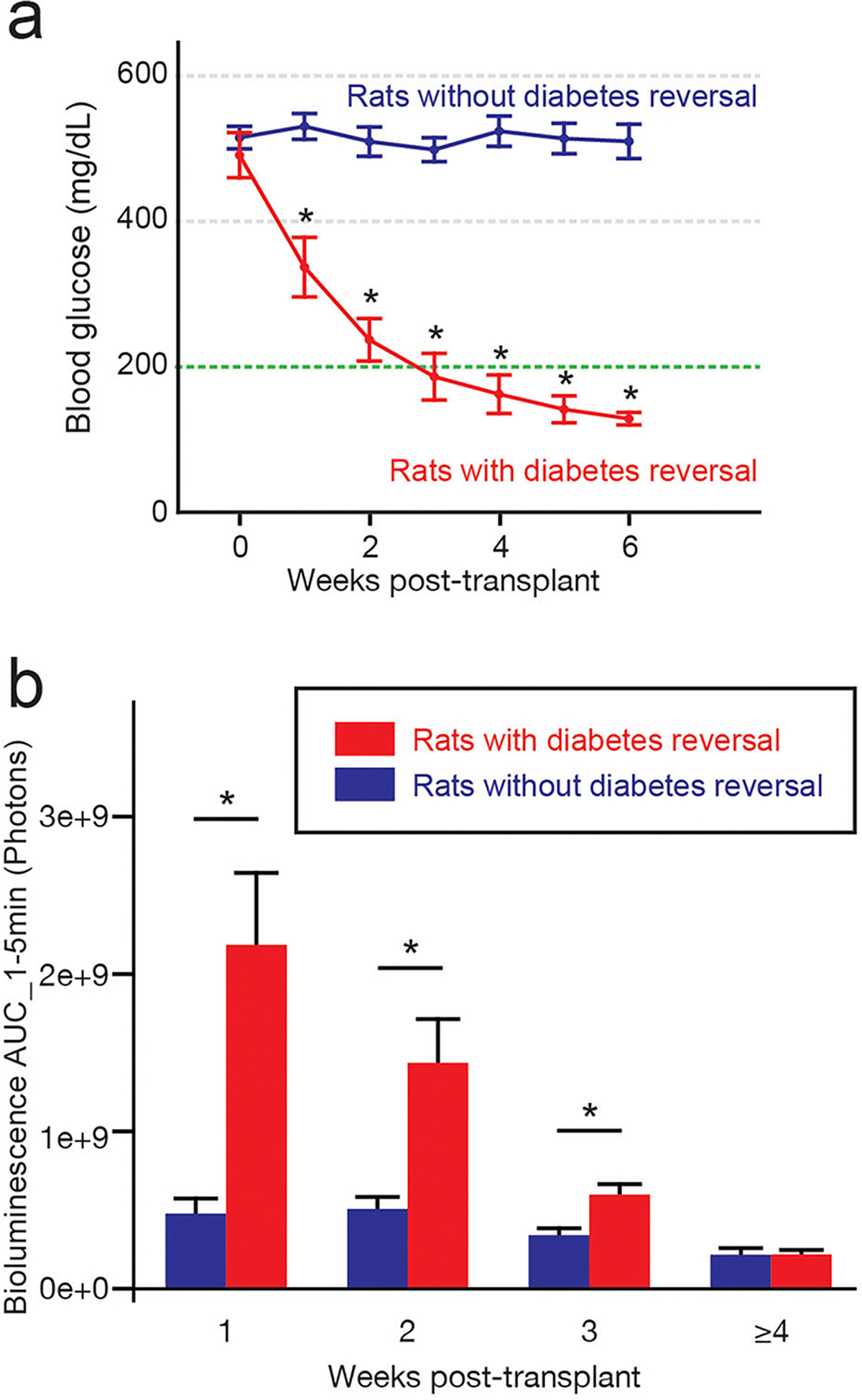
(a) Weekly blood glucose levels of rats that exhibited diabetes reversal (n=9) and rats that did not (n=18). Rats with diabetes reversal showed a significant decrease in blood glucose at 1 week through the end of the observation period (6 weeks). * p<0.05, compared to rats without reversal. Blood glucose data after islet resection was not included. (b) Bioluminescence of LUC-Tg islets in rats with versus without diabetes reversal at each post-transplant period: 1 week (n=26), 2 weeks (n=23), 3 weeks (n=20), and ≥4 weeks (n=10). * p<0.05.
The bioluminescence of LUC-Tg islets predicts reversal of diabetes after SC transplantation
We further examined if the bioluminescence of islet grafts in the early phase (1–2 weeks) post-SC transplantation could predict the reversal of diabetes. We evaluated the AUC_1–5min values of the islet grafts at 1 and 2 weeks post-SC transplantation and used ROC curves to determine the optimal cutoff values to predict diabetes reversal (Suppl. Fig.5): 7.79e+8 photons at 1 week and 1.02e+9 photons at 2 weeks. Importantly, 60% of recipients with bioluminescent signals above the cutoff at 1 week post-transplant exhibited subsequent euglycemia; conversely, only 16.7% of recipients with bioluminescent signals below the cutoff became euglycemic (Fig.3a, χ2=4.5962, p=0.0320). Similarly, all recipients with bioluminescent signals above the cutoff at 2 weeks post-transplant became euglycemic, whereas only 23.5% of those with signals below the cutoff became euglycemic (Fig.3b, χ2=8.4067, p=0.0037). These analyses indicate that the bioluminescence of LUC-Tg islets can potentially be used to predict successful SC transplantation before the confirmation of diabetes reversal in glucose homeostasis.
Figure 3: The bioluminescence of LUC-Tg islets in the early post-transplant phase predicts transplantation outcomes.
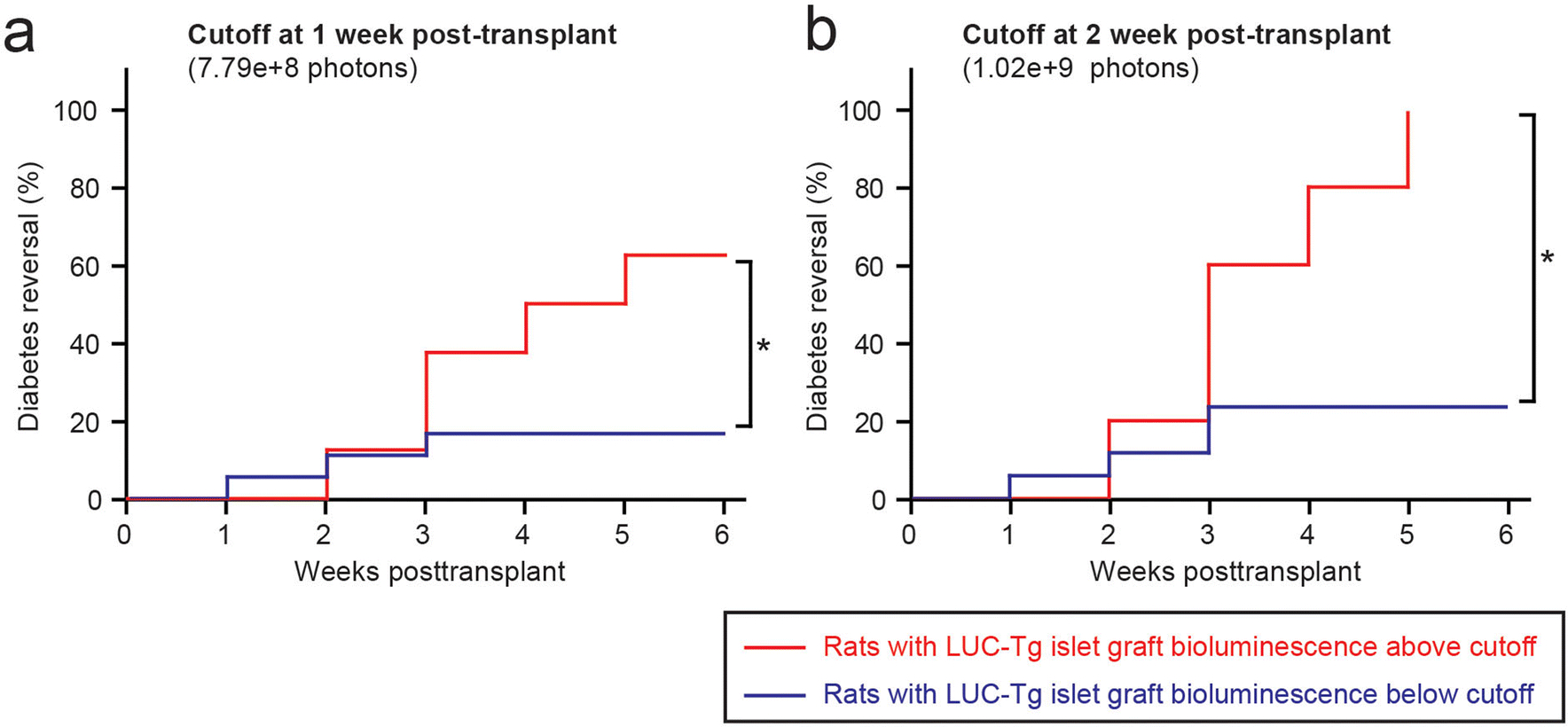
(a) Cumulative curves of diabetes reversal in rats bearing LUC-Tg islets with bioluminescence above versus below the cutoff at 1 week post-transplant. (b) Cumulative curves of diabetes reversal in rats bearing LUC-Tg islets with bioluminescence above versus below the cutoff at 2 weeks post-transplant. * p<0.05.
Discussion
In this study, we investigated if the bioluminescence of SC-transplanted LUC-expressing islet grafts correlates with diabetes reversal. We demonstrated that the bioluminescence of the LUC-Tg grafts in the early post-transplant phase (1–2 weeks) predicted transplantation outcomes in diabetic recipient rats.
The in vivo examination of LUC-based bioluminescence is advantageous in islet transplantation studies because the light emission process is ATP- and O2-dependent. As islet survival and function are also sensitive to the O2 environment [30–31], and insulin secretion from beta cells involves ATP-sensitive K+ channels on the cell membrane [32], the bioluminescent signal of LUC-expressing islet grafts presumably reflects their survival and function in vivo. However, the interpretation of LUC-based bioluminescence data obtained in SC islet transplantation studies must take into account several important considerations.
First, the kinetics and biodistribution of luciferin vary depending upon the administration route and kidney function of the islet recipients. Two major injection methods have been reported: i.v. and intraperitoneal (i.p.). Once a LUC-expressing graft is vascularized (~7 days after transplantation) [22], its bioluminescence reflects its viability, as well as the dynamics of the luciferin solution in systemic circulation. The dynamics of i.v.-injected luciferin are well-documented and consistent among individuals [16], enabling the accurate assessment of graft viability. In contrast, i.p.-injected luciferin takes 10–20 min to distribute to the organs [18, 20], which can result in inconsistent bioluminescence curves. In diabetes research, changes in luciferin dynamics due to diabetic nephropathy, which could potentially induce luciferin retention in systemic circulation, is also a concern. During the 4- to 6-week observation period in this study, the washout ratio of the bioluminescence curve was consistent (Suppl. Fig.6). However, this consistency might not be applicable to future studies that require longer observation period, which will require further testing and optimization.
Second, the reduction in bioluminescence should be carefully interpreted. We demonstrated that at 3 weeks post-SC transplantation, the bioluminescence intensities of the LUC-Tg islet grafts were reduced to ~1/3 of their intensities at 1 week, even in rats with diabetes reversal. One possible explanation for the rapid decay in graft bioluminescence is the loss of LUC expression in the engrafted cells. However, unlike adenovirus and cytomegalovirus promoters, which demonstrate decreased expression over time [33], the ROSA26 promoter used to drive LUC expression in our study exhibits stable and ubiquitous long-term expression [34–35]. Critically, we showed that LUC expression was maintained over 4 weeks in our SC transplantations, suggesting that the decreased bioluminescence reflects a reduction in islet graft mass. Because we confirmed that the reversal of diabetes was islet graft-dependent (showing recurrent diabetes after graft removal), this finding may indicate that only a limited fraction of the transplanted islets contributes to blood glucose control.
Furthermore, the bioluminescence emitted from the graft may be blocked by surrounding soft tissues, as the hemoglobin and melanin present in SC tissues and skin can absorb LUC bioluminescence [17, 36]. Indeed, our analysis of bioluminescence (AUC_1–5 min) as a function of the number of islets transplanted (600–1500) indicated that some bioluminescence was absorbed by surrounding tissues (Suppl. Fig.7). Therefore, the reduction in bioluminescence intensity is not simply proportional to the loss of islet graft viability. Nevertheless, islet graft viability in the earlier stages of transplantation must be improved, and we have shown that LUC-based BLI could be a useful tool to track islet grafts non-invasively.
Finally, the non-cell type-specific nature of LUC expression in the grafts should be considered. In this study, islets were isolated for transplantation from rats that expressed LUC globally [26]. This is important to note because isolated islets include variable amounts of acinar cells and adipose tissue. Thus, the bioluminescent signals from the LUC-Tg islets may have been, in part, from non-islet cells. Such non-specific signals could be mitigated by using Tg models in which LUC is expressed under the control of beta cell-specific promoters, such as the insulin I promoter [18].
In summary, we characterized the LUC-based bioluminescence of SC-transplanted islets in diabetic rats, with respect to transplantation outcomes. Early-phase BLI of SC-transplanted islet grafts could be used to predict subsequent diabetes reversal; therefore, it may represent a promising, non-invasive alternative to conventional assessments of diabetic animals, such as long-term blood glucose observation.
Supplementary Material
Acknowledgments:
We thank Dr. Eiji Kobayashi for providing the LUC-Tg Lewis rat strain. We also thank Drs. Hsun Teresa Ku, Jeffrey Isenberg, and Kerin Higa for their critical reading and editing of the manuscript.
Funding
This study was supported by a grant from the Nora Eccles Treadwell Foundation (Title of Grant: CURE OF DIABETES, Grant Period: July 1, 2012–June 30, 2020, P.I.: Yoko Mullen, MD, PhD).
Footnotes
Disclosure: The authors declare no conflicts of interest.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Shapiro AM, Ricordi C, Hering BJ, et al. (2006) International trial of the Edmonton protocol for islet transplantation. N Engl J Med 355:1318–1330. [DOI] [PubMed] [Google Scholar]
- 2.Rezania A, Bruin JE, Arora P, et al. (2014) Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol 32:1121–1133. [DOI] [PubMed] [Google Scholar]
- 3.Chhabra P, Brayman KL (2013) Stem cell therapy to cure type 1 diabetes: from hype to hope. Stem Cells Transl Med 2:328–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soon-Shiong P, Heintz RE, Merideth N, et al. (1994) Insulin independence in a type 1 diabetic patient after encapsulated islet transplantation. Lancet 343:950–951. [DOI] [PubMed] [Google Scholar]
- 5.de Vos P, Spasojevic M, Faas MM (2010) Treatment of diabetes with encapsulated islets. Adv Exp Med Biol 670:38–53. [DOI] [PubMed] [Google Scholar]
- 6.Luan NM, Iwata H (2014) Long-term allogeneic islet graft survival in prevascularized subcutaneous sites without immunosuppressive treatment. Am J Transplant 14:1533–1542. [DOI] [PubMed] [Google Scholar]
- 7.Kawakami Y, Iwata H, Gu Y, et al. (2000) Modified subcutaneous tissue with neovascularization is useful as the site for pancreatic islet transplantation. Cell Transplant 9:729–732. [DOI] [PubMed] [Google Scholar]
- 8.Gibly RF, Zhang X, Graham ML, et al. (2011) Extrahepatic islet transplantation with microporous polymer scaffolds in syngeneic mouse and allogeneic porcine models. Biomaterials 32:9677–9684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Golocheikine A, Tiriveedhi V, Angaswamy N, Benshoff N, Sabarinathan R, Mohanakumar T (2010) Cooperative signaling for angiogenesis and neovascularization by VEGF and HGF following islet transplantation. Transplantation 90:725–731. [DOI] [PubMed] [Google Scholar]
- 10.Brady AC, Martino MM, Pedraza E, et al. (2013) Proangiogenic hydrogels within macroporous scaffolds enhance islet engraftment in an extrahepatic site. Tissue Eng Part A 19:2544–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evgenov NV, Medarova Z, Dai G, Bonner-Weir S, Moore A (2006) In vivo imaging of islet transplantation. Nat Med 12:144–148. [DOI] [PubMed] [Google Scholar]
- 12.Li J, Rawson J, Chea J, et al. (2019) Evaluation of [(68)Ga]DO3A-VS-Cys(40)-Exendin-4 as a PET Probe for Imaging Human Transplanted Islets in the Liver. Sci Rep 9:5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Kroon I, Andralojc K, Willekens SM, et al. (2016) Noninvasive Imaging of Islet Transplants with 111In-Exendin-3 SPECT/CT. J Nucl Med 57:799–804. [DOI] [PubMed] [Google Scholar]
- 14.Sakata N, Sax N, Yoshimatsu G, et al. (2015) Enhanced ultrasonography using a nano/microbubble contrast agent for islet transplantation. Am J Transplant 15:1531–1542. [DOI] [PubMed] [Google Scholar]
- 15.Morciano G, Sarti AC, Marchi S, et al. (2017) Use of luciferase probes to measure ATP in living cells and animals. Nat Protoc 12:1542–1562. [DOI] [PubMed] [Google Scholar]
- 16.Berger F, Paulmurugan R, Bhaumik S, Gambhir SS (2008) Uptake kinetics and biodistribution of 14C-D-luciferin--a radiolabeled substrate for the firefly luciferase catalyzed bioluminescence reaction: impact on bioluminescence based reporter gene imaging. Eur J Nucl Med Mol Imaging 35:2275–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Contag CH, Bachmann MH (2002) Advances in in vivo bioluminescence imaging of gene expression. Annu Rev Biomed Eng 4:235–260. [DOI] [PubMed] [Google Scholar]
- 18.Virostko J, Radhika A, Poffenberger G, et al. (2010) Bioluminescence imaging in mouse models quantifies beta cell mass in the pancreas and after islet transplantation. Mol Imaging Biol 12:42–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen X, Zhang X, Larson CS, Baker MS, Kaufman DB (2006) In vivo bioluminescence imaging of transplanted islets and early detection of graft rejection. Transplantation 81:1421–1427. [DOI] [PubMed] [Google Scholar]
- 20.Fowler M, Virostko J, Chen Z, et al. (2005) Assessment of pancreatic islet mass after islet transplantation using in vivo bioluminescence imaging. Transplantation 79:768–776. [DOI] [PubMed] [Google Scholar]
- 21.Cao YA, Bachmann MH, Beilhack A, et al. (2005) Molecular imaging using labeled donor tissues reveals patterns of engraftment, rejection, and survival in transplantation. Transplantation 80:134–139. [DOI] [PubMed] [Google Scholar]
- 22.Sunaga A, Sugawara Y, Katsuragi-Tomioka Y, Kobayashi E (2013) The fate of nonvascularized fat grafts: histological and bioluminescent study. Plast Reconstr Surg Glob Open 1:e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Komatsu H, Cook CA, Gonzalez N, et al. (2018) Oxygen transporter for the hypoxic transplantation site. Biofabrication 11:015011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Komatsu H, Rawson J, Barriga A, et al. (2018) Posttransplant oxygen inhalation improves the outcome of subcutaneous islet transplantation: A promising clinical alternative to the conventional intrahepatic site. Am J Transplant 18:832–842. [DOI] [PubMed] [Google Scholar]
- 25.Komatsu H, Gonzalez N, Salgado M, et al. (2020) A subcutaneous pancreatic islet transplantation platform using a clinically applicable, biodegradable Vicryl mesh scaffold - an experimental study. Transpl Int 33:806–818. [DOI] [PubMed] [Google Scholar]
- 26.Hakamata Y, Murakami T, Kobayashi E (2006) “Firefly rats” as an organ/cellular source for long-term in vivo bioluminescent imaging. Transplantation 81:1179–1184. [DOI] [PubMed] [Google Scholar]
- 27.Ito T, Itakura S, Todorov I, et al. (2010) Mesenchymal stem cell and islet co-transplantation promotes graft revascularization and function. Transplantation 89:1438–1445. [DOI] [PubMed] [Google Scholar]
- 28.Salgado M, Gonzalez N, Medrano L, et al. (2020) Semi-Automated Assessment of Human Islet Viability Predicts Transplantation Outcomes in a Diabetic Mouse Model. Cell Transplant 29:963689720919444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Youden WJ (1950) Index for rating diagnostic tests. Cancer 3:32–35. [DOI] [PubMed] [Google Scholar]
- 30.Dionne KE, Colton CK, Yarmush ML (1993) Effect of hypoxia on insulin secretion by isolated rat and canine islets of Langerhans. Diabetes 42:12–21. [DOI] [PubMed] [Google Scholar]
- 31.Komatsu H, Kang D, Medrano L, et al. (2016) Isolated human islets require hyperoxia to maintain islet mass, metabolism, and function. Biochem Biophys Res Commun 470:534–538. [DOI] [PubMed] [Google Scholar]
- 32.Koster JC, Permutt MA, Nichols CG (2005) Diabetes and insulin secretion: the ATP-sensitive K+ channel (K ATP) connection. Diabetes 54:3065–3072. [DOI] [PubMed] [Google Scholar]
- 33.Wang P, Medarova Z, Moore A (2011) Molecular imaging: a promising tool to monitor islet transplantation. J Transplant 2011:202915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zambrowicz BP, Imamoto A, Fiering S, Herzenberg LA, Kerr WG, Soriano P (1997) Disruption of overlapping transcripts in the ROSA beta geo 26 gene trap strain leads to widespread expression of beta-galactosidase in mouse embryos and hematopoietic cells. Proc Natl Acad Sci U S A 94:3789–3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gross JB, Hanken J, Oglesby E, Marsh-Armstrong N (2006) Use of a ROSA26:GFP transgenic line for long-term Xenopus fate-mapping studies. J Anat 209:401–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Edinger M, Cao YA, Hornig YS, et al. (2002) Advancing animal models of neoplasia through in vivo bioluminescence imaging. Eur J Cancer 38:2128–2136. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


